Abstract
Spinal cord injury (SCI) usually leads to acute neuronal death and delayed secondary degeneration, resulting in sensory dysfunction, paralysis, and chronic pain. Excessive excitation is one of the critical factors leading to secondary neural damage initiated by various insults. KCNQ/Kv7 channels are highly expressed in spinal neurons and axons and play an important role in controlling their excitability. Enhancing KCNQ channel activity by using its specific opener retigabine could thus be a plausible treatment strategy to reduce the pathology after SCI. We produced contusive SCI at T10 in adult male rats, which then received 10 consecutive days’ treatment with retigabine or vehicle starting 3 hours or 3 days after contusion. Two different concentrations and two different delivery methods were applied. Delivery of retigabine via Alzet osmotic pumps, but not intraperitoneal injections 3 hours after contusion, promoted recovery of locomotor function. Remarkably, retigabine delivery in both methods significantly attenuated the development of mechanical stimuli–induced hyperreflexia and spontaneous pain; however, no significant difference in the thermal threshold was observed. Although retigabine delivered 3 days after contusion significantly attenuated the development of mechanical hypersensitivity and spontaneous pain, the locomotor function is not improved by the delayed treatments. Finally, we found that early application of retigabine attenuates the inflammatory activity in the spinal cord and increases the survival of white matter after SCI. Our results suggest that decreasing neuronal excitability by targeting KCNQ/Kv7 channels at acute stage aids the recovery of locomotor function and attenuates the development of neuropathic pain after SCI.
SIGNIFICANCE STATEMENT
Several pharmacological interventions have been proposed for spinal cord injury (SCI) treatment, but none have been shown to be both effective and safe in clinical trials. Necrotic neuronal death and chronic pain are often the cost of pathological neural excitation after SCI. We show that early, brief application of retigabine could aid locomotor and sensory neurobehavioral recovery after SCI, supporting the use of this drug in the clinic to promote motor and sensory function in patients with SCI.
Introduction
The spinal cord, which contains neurons and major bundles of axons, is critical for transmitting impulses between the brain and the peripheral nervous system. However, the vertebrate-protected spinal cord can be damaged by vehicle accidents, sports-related accidents, war injuries, and tumors, which result in 17,000 acute spinal cord injury (SCI) cases every year and approximately 300,000 sustained traumatic patients with SCI in the United States (Rabinstein, 2018). SCI usually results in acute neuronal death and secondary degeneration of spinal tissues, followed by a chronic stage in which cavities, cysts, and scar tissue is formed, leaving the individual with paralysis, autonomic problems, sensory dysfunction, and chronic pain for the rest of their life (Anderson and Hall, 1993). Treatment after SCI offers a major biomedical challenge. It is well known that some of the pathological consequences in the spinal cord after initial injury are caused by multiple factors that induce secondary degeneration. Many ascending sensory and descending motor tracts in the spinal cord often remain intact after initial traumatic injury (Anderson and Hall, 1993). The initial continuity of the residual white matter together with knowledge of the factors involved in secondary injury processes suggests that early application of pharmacological treatments that counteract the secondary cascade could minimize their pathological consequences and thus promote the survival and functional recovery of spinal tissue.
A widespread event in both initial and secondary injury after SCI is cell depolarization and consequent intracellular Ca2+ overloading (through voltage-gated Ca2+ channels and ligand-gated Ca2+-permeable ion channels) (Ross et al., 1999; Hall and Springer, 2004), which causes excitotoxicity and expands the lesion both horizontally and rostrocaudally to cause further neuronal degeneration and axonal demyelination. For example, the blockade of persistent inward Na+ currents and glutamate release with riluzole provides a powerful neuroprotective effect in SCI animals (Lips et al., 2000; Schwartz and Fehlings, 2001; Nógrádi et al., 2007). L-type Ca2+ channels are expressed in both spinal cord motor neurons and subgroups of interneurons (Morisset and Nagy, 1996; Carlin et al., 2000; Collins et al., 2001), which modulate neuronal excitability directly (depolarizing neurons) or indirectly (enhancing neurotransmission). Nimodipine, an L-type Ca2+ channel blocker, has been demonstrated to promote spinal cord function of SCI rats (Fehlings et al., 1989). Therefore, minimizing persistent depolarization is a plausible strategy to treat SCI-induced dysfunctions.
KCNQ channels belong to the Kv7 subfamily (Kv7.1-Kv7.5) (Jentsch, 2000), and Kv7.2-Kv7.5 channels are present in sensory neurons, spinal cord interneurons, motor neurons, and various axons (Alaburda et al., 2002; Passmore et al., 2003; Devaux et al., 2004) and play an important role in stabilizing resting membrane potentials. Importantly, retigabine (ezogabine), a specific KCNQ channel activator (selectively targeting Kv7.2-Kv7.5 channels) approved by the U.S. Food and Drug Administration to treat partial epilepsies, hyperpolarizes primary afferent fibers (Rivera-Arconada and Lopez-Garcia, 2005) and reduces the transmission of Aδ- and C-fiber activity to dorsal horn neurons (Passmore et al., 2003). Also, retigabine decreases sensory Aδ- and C-fiber discharges induced by heat stimulation in an isolated rat skin-nerve preparation. Conversely, a KCNQ/Kv7 channel inhibitor, XE991, significantly increases the peripheral Aδ discharge produced by both thermal and mechanical stimulation in vivo (Brown and Passmore, 2009).
Recent work shows that chronic spontaneous activity occurs in primary nociceptors of rats after SCI (Bedi et al., 2010). Delivery of retigabine causes a reversible hyperpolarization, suppresses spontaneous activity (both in vivo and in vitro), and relieves signs of chronic SCI pain (Wu et al., 2017). The KCNQ channel activator retigabine dose dependently prevents both serum withdrawal–induced and N-methyl-D-aspartate-induced neurodegeneration in vitro (Boscia et al., 2006). Retigabine pretreatment affords neuroprotective effects for chemotherapy-induced peripheral neuropathy (Nodera et al., 2011; Li et al., 2019). Also, open KCNQ channels protect neurons from cerebral ischemia (Rekling, 2003). Since excessive neuronal excitation is an important factor for secondary degeneration after SCI, reducing the activity of neurons by opening KCNQ/Kv7 channels may protect spinal neurons and axons from degeneration after SCI, thereby promoting recovery of motor and sensory function. In this study, we report that repeated application of retigabine to open these channels at the acute stage promotes neurobehavioral recovery after SCI.
Materials and Methods
All procedures conformed to the guidelines of the International Association for the Study of Pain and followed the Guide for the Care and Use of Laboratory animals. The protocols were approved by the animal care and use committee of the University of Texas Medical Branch at Galveston. Male adult Sprague-Dawley rats (200–300 g) were housed two per cage (with wood chip bedding) in an animal facility with a controlled environment (21 ± 1°C, 12-hour dark/light reversed cycle) and had free access to standard rat chow and drinking water. The rats were purchased from Charles River, and a 1-week habituation period was applied before the experiment.
Spinal Cord Injury
SCI in humans generally results from contusion of the spinal cord. Thus, a spinal cord contusion model was used. Considering the variability in spinal cord damage induced by different contusion models (Young, 2002), contusion was performed using Infinite Horizon impactor (Precision Systems & Instrumentation, Lexington, KY), which creates a reliable contusive injury to the exposed spinal cord by rapidly applying a force-defined impact (Scheff et al., 2003). Animals were anesthetized with a mixture of acepromazine (0.75 mg/kg), xylazine (20 mg/kg), and ketamine (80 mg/kg), followed by laminectomy and moderate spinal contusion (150 kdyne with 1-second dwell time) at T10. Sham animals underwent a laminectomy without spinal impact. The muscles were sutured over the spine before stapling the skin flaps with wound clips. SCI/laminectomy animals were then returned to their cages, which were placed on heating pads (∼37°C). Postinjured animals received twice-daily injections of analgesic (buprenorphine; 0.02 mg/kg, i.p.) for 5 days and prophylactic antibiotics (Baytril, 2.5 mg/kg, i.p.) for 10 days. The bladder was manually evacuated twice daily until recovery of bladder function. Rats were checked for any behaviors of spontaneous pain, which include extensive grooming, marked inactivity, or autotomy. Any animals exhibiting severe signs of spontaneous pain were euthanized immediately.
Delivery of Retigabine
Two delivery methods were used in this study. Considering that the half-life of retigabine is 8–11 hours (Luszczki, 2009), SCI rats were given retigabine or vehicle twice daily by intraperitoneal injection for 10 consecutive days starting 3 hours or 3 days after contusion or received constant infusion via an osmotic minipump. Our previous study indicates that i.p. injection of 10 mg/kg retigabine is effective in attenuating SCI-induced chronic pain. Although the threshold concentration for inhibiting neuronal activity via intraperitoneally injected retigabine is controversial (Xu et al., 2010; Hayashi et al., 2014), at least one study indicates that 2.5 mg/kg retigabine (i.p.) inhibits Complete Freund's adjuvant-induced mechanical allodynia (Xu et al., 2010). Thus, the concentrations at 10 mg/kg and 3 mg/kg were used for i.p. injection. In total, 750 µg/kg per hour for osmotic pump was used because the amount is close to 10 mg/kg of i.p. injections and is easy for calculation. Osmotic minipumps (Alzet 2002, 0.5 μl/h; Alzet Corp., Cupertino, CA) were filled with 0.9% saline (vehicle) or retigabine in 0.9% saline with a syringe. The prefilled osmotic pumps were then placed in saline at 37°C overnight. Preloaded and primed pumps were then implanted subcutaneously in the flank, followed by spinal cord contusion/laminectomy. Retigabine or saline was delivered by pumps 3 hours or 3 days after impact for 10 days. Retigabine concentration in the blood reached its peak at 90 minutes after intraperitoneal injection (Luszczki, 2009). It will take more than 3 hours for the osmotic minipumps to deliver (750 μg/kg per hour) the accumulated amount of 2.5 mg/kg retigabine. To quickly establish EC50 in the time course that is comparable to intraperitoneal injection, a booster dose (3 mg/kg retigabine, i.p.) was administered immediately before perfusion.
Behavioral Tests
Reflex Sensitivity Tests.
A standard 5-day sequence of tests for hindlimb reflex sensitivity was performed before impact and then after retigabine/vehicle treatments, as described previously (Bedi et al., 2010). The reflex tests were performed under red light. The experimenters who collected reflex data were blinded to any drug treatment. Before each behavioral test, animals were allowed to habituate for 20 minutes in each of the testing chambers over a 5-day period. Below-level thermal sensitivity (heat) was tested using the Hargreaves radiant heat method (Plantar Analgesia Meter; IITC, Woodland Hills, CA), in which the withdrawal latency of the hindpaws was measured. To prevent possible tissue injury, the stimulus was terminated if no withdrawal occurred within 20 seconds. Both hindpaws were tested. The test sequence was performed three times at 20-minute intervals. The mean of six latency measurements (three from each hindpaw) was used for each data point for each animal. A series of calibrated von Frey filaments (Stoelting, Wood Dale, IL) was used to test below-level mechanical sensitivity by stimulating the glabrous surface of the hindpaws (Hulsebosch et al., 2000). Thresholds were determined with the “up-down” method (Chaplan et al., 1994). Each hindpaw received only one test series.
Conditioned Place Preference Test for Ongoing Pain.
Conditioned place preference (CPP) tests were performed as previously described (Yang et al., 2014). Briefly, rats (SCI animals accepted brief vehicle and SCI animals received retigabine) were allowed to adjust to the CPP device on day 1 after a 3-day conditioning phase in which animals received twice-daily conditioning injections. In the analgesic session, the rats received conditioning anesthetic [retigabine (10 mg/kg, i.p.)]. At 5 minutes after retigabine injection, animals were kept in the nonpreferred white chamber for 1 hour. In the other daily session (nonanalgesic session), rats received identical amount of vehicle (saline, intraperitoneally). At 5 minutes after vehicle injection, animals were kept in the preferred black chamber for 1 hour. At 1 day after the conditioning session, animals without any injection were introduced into the central open gray chamber. The time that the animal spent in each of the three chambers (gray, white, and black) was recorded for 15 minutes. Animals that spent more time in the white chamber (paired with analgesic injection during conditioning phase) than they did in the black chamber (paired with saline injection during conditioning phase) during the testing phase were considered to have chronic spontaneous ongoing pain (i.e., if the time in white minus time in black was positive).
Basso, Beattie, and Bresnahan Locomotor Scoring.
To detect the effects of SCI on locomotor function, the 21-point Basso, Beattie, and Bresnahan (BBB) locomotor test was used (Basso et al., 1995). Rats were placed in an open-field container, and their locomotor behavior was observed and scored in white light (Basso et al., 1995). Testing was performed daily for the first 5 days and then weekly thereafter for 5–7 weeks after contusion. Animals with a BBB score of 1 or more at day 1 after contusion were excluded from the SCI group.
Horizontal Ladder.
At 1 week before injury, a training session was performed. Rats were trained daily to run across a horizontal ladder that was 1 m long with a testing field of 0.8 m. The testing field contained 10 randomly allocated rungs that were spaced 3–8 cm apart. Each session consisted of three ladder crossings. On the final day of the training session, tests were videotaped. The videotapes were viewed in slow motion so that the total number of hindlimb misses and foot slips during the course can be quantified and a basal score can be assigned to each animal tested. If the entire paw went below the rung during the testing, a score of 1 was assigned. This test was then performed once a week for 5 weeks, starting on day 14 after contusion.
Western Blotting
After behavioral tests (42 days after contusion), animals were deeply anesthetized with Beuthanasia (Merck, Kenilworth, NJ) and perfused with ice-cold PBS. The L4 and L5 of the spinal cords from each rat were removed and immediately placed in 1.5-ml Eppendorf tubes on dry ice. Tissues were homogenized in 500 μl of lysis buffer (Radioimmunoprecipitation assay; Teknova) containing a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). After homogenization, samples were sonicated three times (10-second pulses) and centrifuged at 14,000 rpm for 10 minutes at 4°C. The protein concentration of lysates was determined by the BCA assay (BCA protein assay Kit; Pierce). Samples were prepared for SDS-PAGE (4%–20% Tris-HCl; Bio-Rad) by 1:1 dilution with Laemmli sample buffer, and 30 μg of protein was loaded in each well. After electrophoresis, the gel was transferred to a Polyvinylidene difluoride membrane and blocked with 10% nonfat dry milk in PBS + 0.1% Tween 20 prior to incubation with antibody against GFAP (AB5541; Millipore), Iba-1 (Ionized calcium binding adaptor molecule 1; 016-20001; JUJIFILM Wako Chemicals), pSTAT3 (9211; Cell Signaling Technology, MA), p38 (9212; Cell Signaling Technology), and β-actin (ab6276; Abcam, MA) overnight at 4°C. The membrane was incubated with horseradish peroxidase–conjugated anti-rabbit or anti-mouse IgG (Jackson ImmnuoResearch, PA) for 1 hour at room temperature and developed using the ECL kit (Pierce). Protein expression was quantified by optical density using Image J software (National Institutes of Health). Color molecular weight standards were run on each gel, and β-actin was detected as a loading control.
Eriochrome Cyanine Histochemistry
Iron-eriochrome cyanine R (EC) was performed on spinal cord sections to differentiate gray and white matter using a protocol adapted from previously published methods (Rabchevsky et al., 2001; James et al., 2011). After all behavior tests (42 days after contusion), animals were euthanized with Beuthanasia (Merck) and perfused with cold PBS followed by 4% paraformaldehyde solution (Sigma-Aldrich). The spinal cords were then extracted (∼10 mm), with the lesion site located centrally, and postfixed in 4% paraformaldehyde overnight. Tissues were then incubated in 30% sucrose at 4°C for 24 hours. The spinal cords at the lesion site were embedded in cryoembedding media (Optimal cutting temperature; Sakura Finetek, Torrance, CA) and then stored at −80°C. The tissues were cut into transverse sections at 20-μm thicknesses and were mounted on a series of positive charge slides with 200-μm intervals between sections. The section of each animal with the largest cavity was defined as the lesion epicenter. The quantification of the lesion volume was analyzed from 3 mm caudal/rostral to the epicenter. Sections were dehydrated, cleared, rehydrated, and then stained. Stained sections were viewed using a Nikon microscope and quantified using NIS Elements software.
Data Analysis
Animal numbers in each experiment were determined by power analysis, published reports, and past experience. All data were presented as means ± S.E.M. Data were analyzed with Prism 8.0 (Graphpad, La Jolla, CA) and Sigmaplot 14 (Systat software, San Jose, CA). The data were first tested for normality of distribution and homogeneity of variance. If any data did not meet these requirements, nonparametric equivalent analyses were used. Repeated measures two-way ANOVA followed by Sidak’s multiple comparison tests, one-way ANOVA with repeated measures followed by Bonferroni post hoc tests, or unpaired t test (two-tailed) were used to analyze the significance difference among animal groups. BBB scoring was compared between groups on days −1, 1, 2, 3, 7, 14, 21, 28, 35, and 42. The hindlimb foot slips were compared between groups on days −1, 14, 21, 28, 35, and 42. The α level is 0.05 for all statistical tests; P < 0.05 was deemed statistically significant. The n in all experiments is the number of animals used. Statistically significant differences were illustrated in each figure (*P < 0.05; **P < 0.01; ***P < 0.001).
Results
Brief Retigabine Delivery at Acute Stage Attenuates the Development of Mechanical Hyperreflexia and Spontaneous Ongoing Pain after SCI.
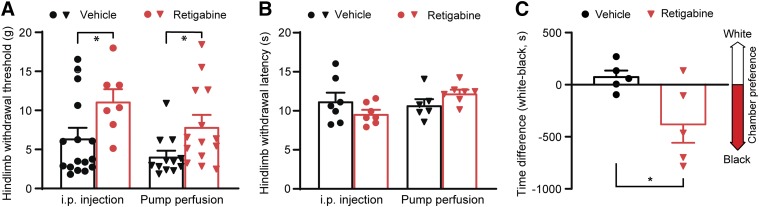
It is reported that retigabine reverses established SCI-induced reflex hypersensitivity (Wu et al., 2017). We observed the effect of early, brief delivery of retigabine (3 hours after contusion for 10 consecutive days) on the development of pain-like behavior after SCI. Thermal and mechanical sensitivity of hindpaws was tested. Remarkably, retigabine significantly mitigated the development of mechanical stimuli–induced hindlimb hyperreflexia (Fig. 1A) in all delivery methods (i.p., 3 mg/kg, twice daily, and Alzet osmotic pump, 750 μg/kg per hour) although thermal (heat) threshold values between retigabine and vehicle groups were not altered (Fig. 1B). Spontaneous ongoing pain also occurs in patients with SCI; thus, we performed the CPP test 35 days after SCI to determine whether retigabine alters the development of SCI-induced spontaneous ongoing pain as compared with their vehicle counterparts (Yang et al., 2014; Wu et al., 2017). After the conditioning phase, in which the conditioned analgesic treatment was paired with placement of animals in the innately less-preferred white chamber, the SCI animals received vehicle, but not SCI animals received retigabine via osmotic pumps starting 3 hours after spinal cord contusion for 10 consecutive days, spent more time in the white chamber during testing phase (Fig. 1C). These results indicate that brief delivery of retigabine at acute stages of SCI mitigates the development of signs of chronic pain.
Fig. 1.
The effects of early, brief application of retigabine (starting 3 hours after contusion for 10 days) on development of pain behaviors after SCI. (A) Mechanical hypersensitivity of hindpaws was measured 28 days after initial traumatic spinal cord injury. Each dot per column represents one animal. P = 0.033, t(21) = 2.278, for groups with intraperitoneal injection (3 mg/kg, twice daily); P = 0.0324, t(24) = 2.271, for groups with osmotic pump perfusion (750 μg/kg per hour). Two-tailed unpaired t test. (B) Heat hypersensitivity of hindpaws was measured 28 days after initial traumatic spinal cord injury. Each dot per column represents one animal. P = 0.2058, t(12) = 1.338, for groups with intraperitoneal injection (3 mg/kg, twice daily); P = 0.1112, t(11) = 1.732, for groups with osmotic pump perfusion (750 μg/kg per hour). Two-tailed unpaired t test. *P < 0.05. (C) Spontaneous pain was evaluated with CPP tests 35 days after contusion. Rats were treated with retigabine via osmotic pump (750 μg/kg per hour). Each dot per column represents one animal. P = 0.0385, t(8) = 2.474; two-tailed unpaired t test. *P < 0.05.
Early Osmotic Pump Delivery but Not Intraperitoneal Injection of Retigabine Promotes Both BBB Score and Horizontal Ladder Performance after SCI.
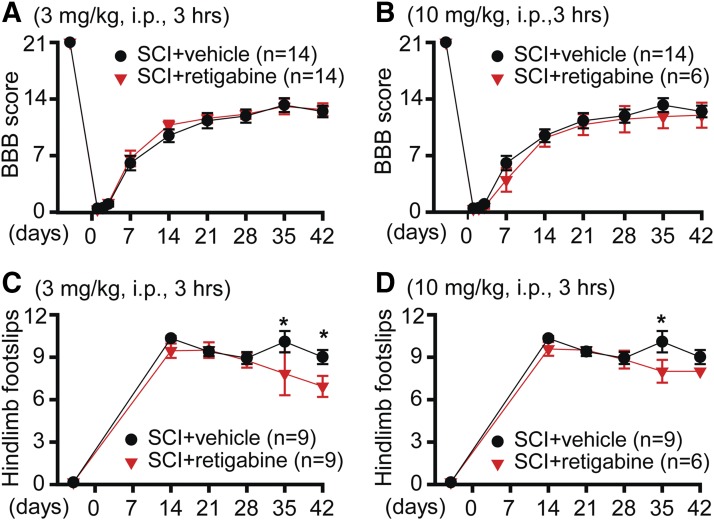
Locomotor behavioral tests are important tools for evaluating the therapeutic efficacy of medicine after SCI. The effect of retigabine on locomotor function after SCI was observed at first. Both BBB 21-point open-field locomotion score and horizontal ladder test were used to evaluate the locomotor function of retigabine- and vehicle-treated SCI rats. Retigabine was delivered 3 hours after contusion for 10 consecutive days. Two different concentrations (3 and 10 mg/kg, i.p., twice daily) were used. Neither 3 (Fig. 2A) nor 10 mg/kg (Fig. 2B) retigabine showed a significant effect on BBB scoring. However, the hindlimb foot slips tested with horizontal ladder showed significant improvement at some later time points after contusion (Fig. 2, C and D).
Fig. 2.
The effects of early, repeated intraperitoneal administration of retigabine on the recovery of locomotor function after SCI. (A) BBB scoring was performed −1, 1, 2, 3, 7, 14, 21, 28, 35, and 42 days after contusion. SCI animals received either 3 mg/kg retigabine (i.p., twice daily) or identical vehicle 3 hours after contusion for 10 consecutive days. Repeated measures two-way ANOVA followed by Sidak’s multiple comparison test. Treatment F(1, 26) = 0.1714, P = 0.6822; time F(9, 234) = 445.7, P < 0.0001; interaction F(9, 234) = 0.4473, P = 0.9081. Baseline, P > 0.999; 1 day, P > 0.999; 2 days, P > 0.999; 3 days, P > 0.999; 7 days, P = 0.9992; 14 days, P = 0.7635; 21 days, P > 0.999; 28 days, P > 0.999; 35 days, P > 0.999; 42 days, P > 0.999. (B) BBB scoring was performed −1, 1, 2, 3, 7, 14, 21, 28, 35, and 42 days after contusion. SCI animals received either 10 mg/kg retigabine (i.p., twice daily) or identical vehicle 3 hours after contusion for 10 consecutive days. Repeated measures two-way ANOVA followed by Sidak’s multiple comparison test. Treatment F(1, 18) = 0.3431, P = 0.5653; time F(9, 162) = 252.4, P < 0.0001; interaction F(9, 162) = 0.6304, P = 0.7699. Baseline, P > 0.999; 1 day, P > 0.999; 2 days, P > 0.999; 3 days, P > 0.999; 7 days, P = 0.6373; 14 days, P > 0.999; 21 days, P > 0.999; 28 days, P > 0.999; 35 days, P = 0.9471; 42 days, P > 0.999. (C) Horizontal ladder test was performed −1, 14, 21, 28, 35, and 42 days after contusion. SCI animals received either 3 mg/kg retigabine (i.p., twice daily) or identical vehicle 3 hours after contusion for 10 consecutive days. Repeated measures two-way ANOVA followed by Sidak’s multiple comparison test. Treatment F(1, 16) = 4.432, P = 0.0516; time F(5, 80) = 150.6, P < 0.0001; interaction F(5, 80) = 2.545, P = 0.035. Baseline, P > 0.999; 14 days, P = 0.8821; 21 days, P = 0.9904; 28 days, P = 0.9994; 35 days, P = 0.0133; 42 days, P = 0.0333. (D) Horizontal ladder test was performed −1, 14, 21, 28, 35, and 42 days after contusion. SCI animals received either 10 mg/kg retigabine (i.p., twice daily) or identical vehicle 3 hours after contusion for 10 consecutive days. Repeated measures two-way ANOVA followed by Sidak’s multiple comparison test. Treatment F(1, 13) = 7.821, P = 0.0151; time F(5, 65) = 145.9, P < 0.0001; interaction F(5, 65) = 2.352, P = 0.0503. Baseline, P > 0.999; 14 days, P = 0.1383; 21 days, P > 0.9999; 28 days, P > 0.9999; 35 days, P = 0.0057; 42 days, P = 0.1423. *P < 0.05.
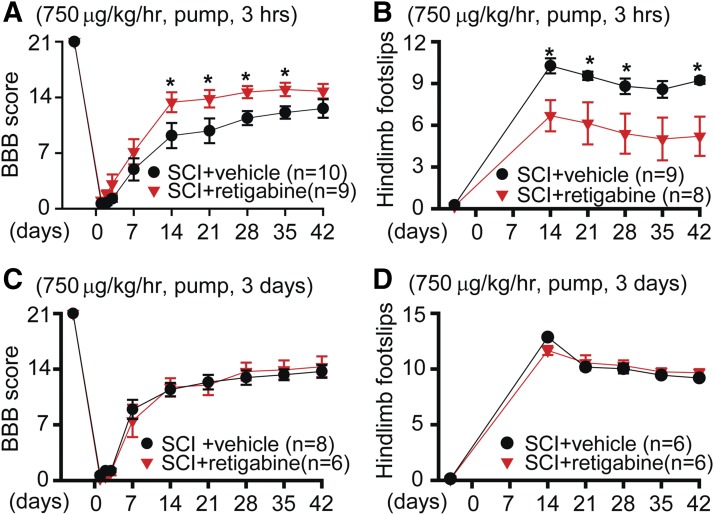
Considering that intraperitoneal injection of retigabine does not maintain the blood concentration of this medicine at a stable level, which probably diminishes its neuronal protective effect in vivo, we thus applied retigabine (750 μg/kg per hour) with the Alzet osmotic minipump starting 3 hours after contusion for 10 days, which maintained a stable retigabine concentration in animals as compared with intraperitoneal injection. In contrast with outcomes after intraperitoneal injection, retigabine delivered via osmotic pumps (750 μg/kg per hour) promoted recovery of locomotor function, which was measured with both BBB scoring (Fig. 3A) and horizontal ladders (Fig. 3B). These data strongly suggest that consistently decreasing neuronal excitability is important for the recovery of locomotor function after SCI.
Fig. 3.
The effects of early and delayed osmotic pump delivery of retigabine on recovery of locomotor function after SCI. (A) BBB scoring was performed −1, 1, 2, 3, 7, 14, 21, 28, 35, and 42 days after contusion. SCI animals received either 750 μg/kg per hour retigabine or identical vehicle 3 hours after contusion for 10 consecutive days. Repeated measures two-way ANOVA followed by Sidak’s multiple comparison test. Treatment F(1, 17) = 4.083, P = 0.0593; time F(9, 153) = 219.1, P < 0.0001; interaction F(9, 153) = 2.548, P = 0.0094. Baseline, P > 0.999; 1 day, P > 0.999; 2 days, P = 0.9967; 3 days, P = 0.8643; 7 days, P = 0.6936; 14 days, P = 0.0286; 21 days, P = 0.0402; 28 days, P = 0.0452; 35 days, P = 0.0482; 42 days, P = 0.7149. (B) Horizontal ladder test was performed −1, 14, 21, 28, 35, and 42 days after contusion. SCI animals received either 750 μg/kg per hour retigabine or identical vehicle 3 hours after contusion for 10 consecutive days. Repeated measures two-way ANOVA followed by Sidak’s multiple comparison test. Treatment F(1, 11) = 5.805, P = 0.0347; time F(5, 55) = 54.12, P < 0.0001; interaction F(5, 55) = 3.105, P = 0.0154. Baseline, P > 0.999; 14 days, P = 0.0148; 21 days, P = 0.0358; 28 days, P = 0.0408; 35 days, P = 0.0991; 42 days, P = 0.0451. (C) BBB scoring was performed −1, 1, 2, 3, 7, 14, 21, 28, 35, and 42 days after contusion. SCI animals received either 750 μg/kg per hour retigabine or identical vehicle 3 days after contusion for 10 consecutive days. Repeated measures two-way ANOVA followed by Sidak’s multiple comparison test. Treatment F(1, 11) = 0.00807, P = 0.93; time F(9, 99) = 270.7, P < 0.0001; interaction F(9, 99) = 0.6681, P = 0.7358. Baseline, P > 0.9999; 1 day, P > 0.9999; 2 days, P = 0.9996; 3 days, P > 0.9999; 7 days, P = 0.9235; 14 days, P > 0.9999; 21 days, P > 0.999; 28 days, P = 0.9992; 35 days, P > 0.9999; 42 days, P > 0.9999. (D) Horizontal ladder test was performed −1, 14, 21, 28, 35, and 42 days after contusion. SCI animals received either 750 μg/kg per hour retigabine or identical vehicle 3 days after contusion for 10 consecutive days. Repeated measures two-way ANOVA followed by Sidak’s multiple comparison test. Treatment F(1, 10) = 0.01203, P = 0.9148; time F(5, 50) = 539, P < 0.0001; interaction F(5, 50) = 2.862, P = 0.0239. Baseline, P > 0.999; 14 days, P = 0.0856; 21 days, P = 0.9608; 28 days, P = 0.9929; 35 days, P = 0.9884; 42 days, P = 0.9054. *P < 0.05.
Delayed Application of Retigabine (3 Days after Contusion) Alleviates the Development of Chronic Pain but Not Motor Dysfunction after SCI.
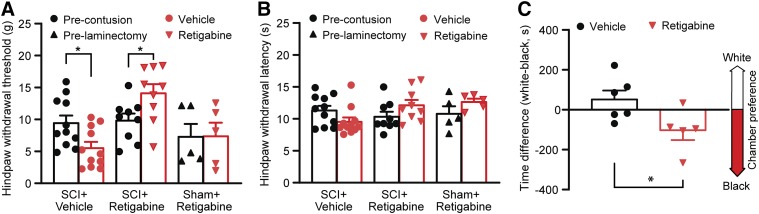
We then delayed the application of retigabine (3 days after contusion) to see whether it also improves motor function after SCI and prevents the development of post-SCI pain. Retigabine (750 μg/kg per hour) was delivered for 10 consecutive days by an Alzet osmotic pump to SCI rats starting 3 days after contusion. The effects of retigabine on locomotor function after SCI were observed. Both the BBB open-field locomotion score and horizontal ladder test were performed to evaluate locomotor function in retigabine- and vehicle-treated SCI rats. Retigabine has no significant effect on BBB scoring (Fig. 3C) and hindlimb foot slips tested with the horizontal ladder as compared with their vehicle-treated counterparts (Fig. 3D).
Although motor function was not improved by delayed retigabine treatment via osmotic pump (750 μg/kg per hour, starting 3 days after contusion for 10 days), the development of mechanical hypersensitivity after SCI was attenuated. As compared with the vehicle-treated SCI animals, the hindpaw withdrawal threshold to mechanical stimulation (von Frey) but not hindpaw withdrawal delay to heat was obviously higher in the retigabine-treated SCI animals 28 days after contusion (Fig. 4, A and B). We also tested the spontaneous pain in SCI rats treated with delayed retigabine/vehicle. The CPP test was performed 35 days after SCI. As compared with the vehicle-treated SCI animals, the development of preference for the conditioned analgesic injection–paired white chamber was significantly attenuated by the delayed, brief retigabine treatment (Fig. 4C). These results indicate that delayed administration of retigabine after SCI can still mitigate the development of SCI chronic pain.
Fig. 4.
The effects of delayed application of retigabine (750 μg/kg per hour, starting 3 days after contusion for 10 days) via osmotic pump on development of SCI-induced hyperreflexia behaviors. (A) Mechanical sensitivity of hindpaws was tested 28 days after initial traumatic spinal cord injury. Each dot per column represents one animal. P = 0.026, U = 26 for SCI + vehicle groups, Mann-Whitney rank sum test; P = 0.03, t(16) = 2.376, for SCI + retigabine groups; P = 0.9787, t(8) = 0.0275, for sham groups. Two-tailed unpaired t test. (B) Heat hypersensitivity of hindpaws was measured 28 days after initial traumatic spinal cord injury. Each dot per column represents one animal. P = 0.0853, t(20) = 1.811, for SCI + vehicle groups; P = 0.138, t(16) = 1.561, for SCI + retigabine groups; P = 0.1848, t(8) = 1.451, for sham groups. Two-tailed unpair t test. (C) CPP tests were performed 35 days after contusion. Each dot per column represents one animal. P = 0.0412, t(9) = 2.381, unpaired t test (two-tailed). *P < 0.05.
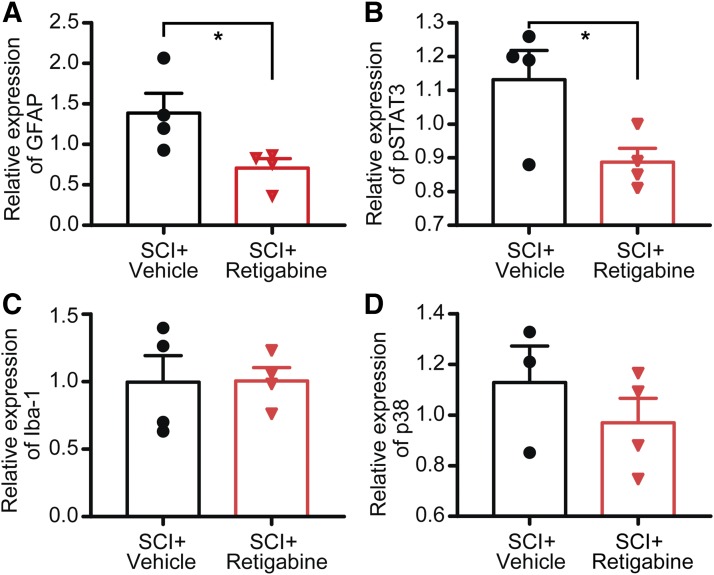
Early, Brief Delivery of Retigabine after Contusion Prevents the Activation of Glial Cells in the Spinal Cord.
Glial cells are activated after spinal cord injury at, below, and above the level of the lesion site of the spinal cord (Hains and Waxman, 2006; Carlton et al., 2009). We found that the expression level of GFAP and pSTAT3 at the lesion site of the spinal cord in SCI groups treated with retigabine (750 μg/kg per hour, osmotic pump, starting 3 hours after contusion for 10 days) were significantly decreased as compared with those of the vehicle-treated SCI groups (Fig. 5, A and B). However, we did not observe a significant difference in the expression level of Iba-1, a marker of microglia, in the lesion site between retigabine- and vehicle-treated groups (Fig. 5C). Considering that the direct role of Iba-1 in chronic pain genesis is unclear, we thus used an additional marker that is functionally linked to neuropathic pain development, phosphorylated-P38 MAPK (Jin et al., 2003). Again, no significant differences in P38 MAPK expression levels were found between brief retigabine- and vehicle-treated animals (Fig. 5D).
Fig. 5.
The effects of early retigabine application by osmotic pump (750 μg/kg per hour, starting 3 hours after contusion for 10 days) on SCI-induced activation of glial cells in the L4/L5 spinal cords. (A) The effect of treatments on SCI-induced GFAP expression. GFAP was normalized to β-actin. P = 0.0451, t(6) = 2.523, unpaired t test (two-tailed). (B) The effect of treatments on SCI-induced pSTAT3 expression. pSTAT3 was normalized to β-actin. P = 0.0416, t(6) = 2.583, unpaired t test (two-tailed). (C) The effect of treatments on SCI-induced Iba-1 expression. Iba-1 was normalized to β-actin. P = 0.9692, t(6) = 0.04028, unpaired t test. (D) The effect of treatments on SCI-induced expression of phosphorylated MAPK 38. P = 0.9654, t(5) = 0.3787, unpaired t test (two-tailed). Dots in each column represent an animal. *P < 0.05.
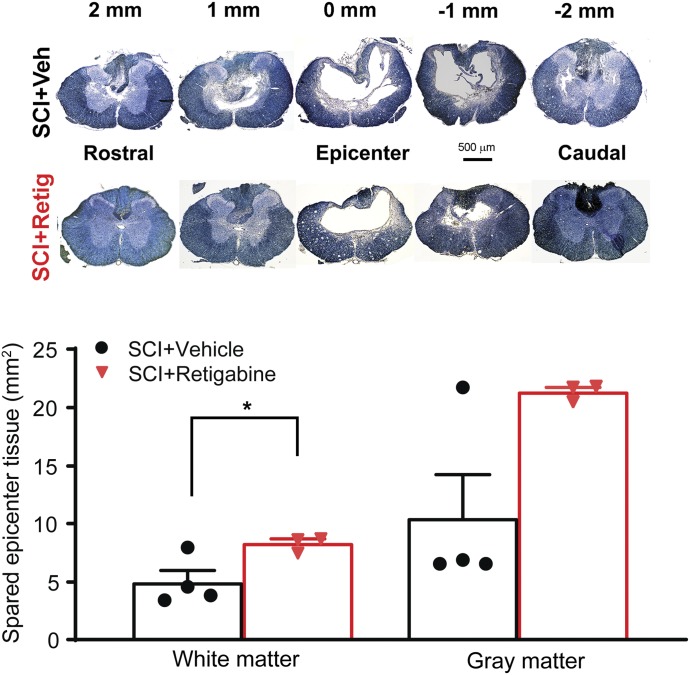
Early, Brief Delivery of Retigabine after Contusion Attenuates the Necrosis of the Spinal Cord Neurons.
Morphologic evidence was also collected histologically by measuring lesion volume in the spinal cord. The most important outcome was increased survival of spinal neurons and axons. Based on the result from motor function tests, we emphasized the data from animals treated with retigabine via osmotic pump 3 hours after contusion for 10 days. We measured the volume of spared white matter and gray matter at the injury site by EC staining. As shown in Fig. 6, we observed more surviving spinal tissue in the retigabine-treated animals after spinal contusion as compared with that from the vehicle-treated rats. The areas of spared white matter were significantly preserved in the retigabine (SCI + retigabine) group than in the control (SCI + vehicle) group. However, no significant differences in spared gray matter were found between brief retigabine- and vehicle-treated animals (Fig. 6). These results indicate that early retigabine treatment decreases the degeneration of spinal tissues after SCI.
Fig. 6.
The effects of brief retigabine delivery via osmotic pump (750 μg/kg per hour, starting 3 hours after contusion for 10 days) on SCI-induced tissue loss in the spinal cord. Representative images in the upper panel showing EC staining of spinal cord sections from both retigabine- and vehicle-treated SCI rats. The graph in the lower panel indicates the quantification of the spared gray matter and white matter of spinal cords at the epicenter of the contusion site from vehicle- and retigabine-treated SCI rats. P = 0.045, t(5) = 2.648, for white matter between vehicle and retigabine groups. Unpaired student t test (two-tailed); P = 0.229, U = 2.0, for gray matter between vehicle and retigabine groups. Mann-Whitney rank sum test. Dots in each column represents an animal. *P < 0.05. Retig, retigabine; Veh, vehicle.
Discussion
Injury to the spinal cord leads to a number of deficits, including motor dysfunction and chronic pain. A widespread event in both initial and secondary injury after SCI is neuronal depolarization (Ross et al., 1999; Hall and Springer, 2004), which causes cytotoxicity that leads to further neuronal degeneration, axonal loss, and axonal demyelination. Decreasing neuronal excitability by blocking persistent Na+ currents and glutamate release with riluzole decreases locomotor dysfunction and pain after SCI (Lips et al., 2000; Schwartz and Fehlings, 2001; Nógrádi et al., 2007). KCNQ ion channels are widely expressed in the primary sensory neurons, spinal cord motor neurons, and interneurons and axons (Alaburda et al., 2002; Passmore et al., 2003; Devaux et al., 2004). They play an important role in stabilizing resting membrane potentials. Recent studies indicate that decreasing neuronal excitability at early stages by targeting KCNQ channels attenuates the development of chemotherapy-induced neuronal degeneration and chronic pain (Li et al., 2019), amyotrophic lateral sclerosis–induced motor neuron death (Wainger et al., 2014), and stroke-induced dysfunction (Bierbower et al., 2015; Vigil et al., 2019). The current study shows that neutralizing neuronal excitability by pharmacologically enhancing the activity of KCNQ channels with retigabine could be a useful strategy for attenuating the development of SCI-induced functional and pathological alterations. Enhanced neuronal activity has been associated with elevations in cytosolic calcium concentration induced by calcium influx through voltage-sensitive calcium channels and calcium release from intracellular stores. Although our previous study indicates that the Kv7 channel expression and function in dorsal root ganglion neurons are unchanged after SCI (Wu et al., 2017), we cannot exclude the possibility that the expression and function of Kv7 in the spinal cord motor neurons and interneurons are altered since Ca2+-calmodulin suppresses the trafficking and/or function of Kv7 channels (Alaimo and Villarroel, 2018), which raises the possibility of positive-feedback loops driving neuronal overexcitation. The increased cytosolic Ca2+ concentration led to multiple cellular pathological alterations. For example, increased intracellular Ca2+ triggers mitochondrial Ca2+ overloading, which exaggerates neuronal functions by jeopardizing mitochondrial respiration (Llorente-Folch et al., 2015), producing reactive oxygen species (Adam-Vizi and Starkov, 2010), impairing mitochondrial dynamic via mitophagy (Brady et al., 2007), and inducing apoptosis (Giorgi et al., 2012). These alterations thus ultimately result in the injury-induced secondary degeneration in the spinal cord. In addition, maintaining neuronal membrane potential alone during their enhanced activity consumes more than 90% of energy produced by the cell (Erecińska and Silver, 1994; Erecińska et al., 1994). Neuronal overexcitation may thus lead to energy exhaustion and degeneration because other energy-dependent processes that are critical for cell maintenance, including molecular transportation and ionic exchanging, will suffer the torments of hunger. Thus, although neuronal injury can be induced by different causes in different locations, neuronal hyperpolarization after neuronal injury is likely a shared mechanism that induces neuronal degeneration and plasticity.
Both BBB score and ladder tests were used to detect the motor function of SCI animals. However, their outcomes were not consistent. Ladder tests showed a better recovery in motor function as compared with those from BBB scoring, especially when the retigabine was applied via an osmotic minipump that can maintain the drug concentration at a stable level. It has been demonstrated that BBB scores show low correlation with fiber conductions, especially at the recovery stage, in which BBB scores show no further improving but fiber conduction is still ameliorating (James et al., 2011). In contrast, there is a high correlation between ladder scores and the percentage of conducting fibers (James et al., 2011). Our histology study indicates that early application of retigabine protects white matter after SCI. Preserving even 5%–10% of the axonal population could aid the recovery of locomotor and sensory function (Blight, 1983). Cell depolarization is an important contributor to secondary degeneration after SCI (Ross et al., 1999; Hall and Springer, 2004). Our data suggest that neuronal depolarization occurs at the early stage of the secondary degeneration since the locomotor outcomes resulting from early treatment (3 hours) are better than those from delayed treatment (3 days).
The hyperexcitability of primary nociceptors is critical for the initiation and maintenance of SCI-induced chronic pain (Bedi et al., 2010; Yang et al., 2014). As compared to the central nervous system, an effective vascular permeability barrier is lacking in the dorsal root ganglion (Hirakawa et al., 2004; Abram et al., 2006; Jimenez-Andrade et al., 2008), and their cell bodies are likely to be exposed to the highest concentrations of peripherally applied retigabine; therefore, the effect of retigabine on chronic pain is not that sensitive to the delivery routes and starting time.
Retigabine is used for both the preventive treatment after SCI and the CPP conditioning in this study. Our data indicate that early, brief retigabine but not delayed retigabine attenuates the development of SCI-induced neurobehavioral dysfunction. The CPP tests were performed 4 weeks after contusion; thus, the interference induced by conditional retigabine during CPP tests is minimal, if there is any. Spinal cord injury induces both mechanical and thermal hyperreflexia (Bedi et al., 2010; Wu et al., 2013, 2017; Yang et al., 2014). Acute application of retigabine decreases established heat and mechanical hypersensitivity after SCI (Wu et al., 2017). However, brief application of retigabine at acute stage decreases only the development of mechanical hypersensitivity and not heat hypersensitivity after SCI. Several factors have been shown to be critical for the development of SCI-induced chronic pain, including the transient receptor potential cation channel subfamily V member 1 ion channel plasticity in the primary sensory neurons (Wu et al., 2013), Nav1.3 overexpression and GABAergic alteration in the spinal cord dorsal horn neurons (Hains et al., 2003; Lu et al., 2008; Meisner et al., 2010), glial cell activation in the central nervous system (Hains and Waxman, 2006; Zhao et al., 2007; Detloff et al., 2008; Carlton et al., 2009), and descending antinociceptive serotonergic pathway disruption (Bruce et al., 2002). It is unknown to what extent these factors differentially contribute to mechanical and/or thermal hypersensitivity and how these different factors respond to retigabine treatment. Heat sensitivity is not always altered in parallel with the changes in mechanical sensitivity in chronic neuropathic pain models. For example, mechanical hypersensitivity but not heat hypersensitivity generally develops in paclitaxel-induced peripheral neuropathy, which involves similar mechanisms (except microglial activation in the spinal cord) as SCI (Zheng et al., 2011; Burgos et al., 2012; Zhang et al., 2012; Li et al., 2015, 2018, 2019; Nashawi et al., 2016).
Spinal cord injury results in the invasion of cells of the immune system that can increase the excitability of neurons in the spinal cord (Detloff et al., 2008; Dulin et al., 2013). Although it is not clear how the immune system is activated after SCI, it has been demonstrated that at least neuronal hyperexcitability contributes to the activation of glial cells in the spinal cord (Xie et al., 2009), thus forming a positive loop that worsens the development of both locomotor dysfunction and chronic pain. Both astrocytes and microglial cells are activated after spinal cord injury at, below, and above the level of the lesion site of the spinal cord, which is linked to the development of SCI-induced neurobehavioral dysfunction (Hains and Waxman, 2006; Carlton et al., 2009). Although the expression levels of astrocyte marker GFAP in the lesion site is significantly decreased after retigabine treatment, the changes in the expression levels of microglial markers Iba-1 and phosphorylated P38 MAPK are not significant. One study reports that SCI induces significant upregulation of the phosphorylated STAT3 and contributes to the astrogliosis in the spinal cord (Herrmann et al., 2008). Consistent with the changes in GFAP, the expression level of pSTAT3 in the spinal cord is decreased upon retigabine delivery, which suggests the treatment decreases the inflammatory activity. The early phase of cellular inflammation (10 days postinjury) is correlated with impaired locomotor function, whereas the later phase of cellular inflammation (14–180 days postinjury) does not coincide with altered locomotor function after SCI (Beck et al., 2010). Our study indicates that decreasing neuronal excitability at early phases of SCI attenuates both the expression level of GFAP and inflammatory activity in the spinal cord, which might contribute to the improvement of locomotor function and chronic pain after SCI.
Acknowledgments
We thank Dr. Heather Lander for critical reading and editing of the manuscript.
Abbreviations
- BBB
Basso, Beattie, and Bresnahan
- CPP
conditioned place preference
- EC
eriochrome cyanine R
- GFAP
glial fibrillary acidic proteinIba-1 Ionized calcium binding adaptor molecule 1
- Kv
voltage-gated K channel
- MAPK
mitogen-activated protein kinase
- pSTAT3
phosphorylated signal transducer and activator of transcription 3
- SCI
spinal cord injury
Authorship Contributions
Participated in research design: Wu, Yang.
Conducted experiments: Wu, Li, Xie, Xu, Dang, Yang.
Performed data analysis: Wu, Xie, Yang.
Wrote or contributed to the writing of the manuscript: Wu, Yang.
Footnotes
The project was supported by grants from the Department of Defense USAMRAA [W81XWH-14-1-0593 to Q.Y.], Craig H. Neilsen Foundation [383428 to Q.Y.], and National Institutes of Health National Cancer Institute [R01 CA208765 to Q.Y.].
References
- Abram SE, Yi J, Fuchs A, Hogan QH. (2006) Permeability of injured and intact peripheral nerves and dorsal root ganglia. Anesthesiology 105:146–153. [DOI] [PubMed] [Google Scholar]
- Adam-Vizi V, Starkov AA. (2010) Calcium and mitochondrial reactive oxygen species generation: how to read the facts. J Alzheimers Dis 20 (Suppl 2):S413–S426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaburda A, Perrier JF, Hounsgaard J. (2002) An M-like outward current regulates the excitability of spinal motoneurones in the adult turtle. J Physiol 540:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaimo A, Villarroel A. (2018) Calmodulin: a multitasking protein in Kv7.2 potassium channel functions. Biomolecules 8:E57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DK, Hall ED. (1993) Pathophysiology of spinal cord trauma. Ann Emerg Med 22:987–992. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12:1–21. [DOI] [PubMed] [Google Scholar]
- Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ. (2010) Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain 133:433–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi SS, Yang Q, Crook RJ, Du J, Wu Z, Fishman HM, Grill RJ, Carlton SM, Walters ET. (2010) Chronic spontaneous activity generated in the somata of primary nociceptors is associated with pain-related behavior after spinal cord injury. J Neurosci 30:14870–14882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierbower SM, Choveau FS, Lechleiter JD, Shapiro MS. (2015) Augmentation of M-type (KCNQ) potassium channels as a novel strategy to reduce stroke-induced brain injury. J Neurosci 35:2101–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blight AR. (1983) Cellular morphology of chronic spinal cord injury in the cat: analysis of myelinated axons by line-sampling. Neuroscience 10:521–543. [DOI] [PubMed] [Google Scholar]
- Boscia F, Annunziato L, Taglialatela M. (2006) Retigabine and flupirtine exert neuroprotective actions in organotypic hippocampal cultures. Neuropharmacology 51:283–294. [DOI] [PubMed] [Google Scholar]
- Brady NR, Hamacher-Brady A, Yuan H, Gottlieb RA. (2007) The autophagic response to nutrient deprivation in the hl-1 cardiac myocyte is modulated by Bcl-2 and sarco/endoplasmic reticulum calcium stores. FEBS J 274:3184–3197. [DOI] [PubMed] [Google Scholar]
- Brown DA, Passmore GM. (2009) Neural KCNQ (Kv7) channels. Br J Pharmacol 156:1185–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce JC, Oatway MA, Weaver LC. (2002) Chronic pain after clip-compression injury of the rat spinal cord. Exp Neurol 178:33–48. [DOI] [PubMed] [Google Scholar]
- Burgos E, Gómez-Nicola D, Pascual D, Martín MI, Nieto-Sampedro M, Goicoechea C. (2012) Cannabinoid agonist WIN 55,212-2 prevents the development of paclitaxel-induced peripheral neuropathy in rats. Possible involvement of spinal glial cells. Eur J Pharmacol 682:62–72. [DOI] [PubMed] [Google Scholar]
- Carlin KP, Jones KE, Jiang Z, Jordan LM, Brownstone RM. (2000) Dendritic L-type calcium currents in mouse spinal motoneurons: implications for bistability. Eur J Neurosci 12:1635–1646. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Du J, Tan HY, Nesic O, Hargett GL, Bopp AC, Yamani A, Lin Q, Willis WD, Hulsebosch CE. (2009) Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain 147:265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63. [DOI] [PubMed] [Google Scholar]
- Collins DF, Burke D, Gandevia SC. (2001) Large involuntary forces consistent with plateau-like behavior of human motoneurons. J Neurosci 21:4059–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detloff MR, Fisher LC, McGaughy V, Longbrake EE, Popovich PG, Basso DM. (2008) Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp Neurol 212:337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux JJ, Kleopa KA, Cooper EC, Scherer SS. (2004) KCNQ2 is a nodal K+ channel. J Neurosci 24:1236–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulin JN, Karoly ED, Wang Y, Strobel HW, Grill RJ. (2013) Licofelone modulates neuroinflammation and attenuates mechanical hypersensitivity in the chronic phase of spinal cord injury. J Neurosci 33:652–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecińska M, Nelson D, Yudkoff M, Silver IA. (1994) Energetics of the nerve terminal in relation to central nervous system function. Biochem Soc Trans 22:959–965. [DOI] [PubMed] [Google Scholar]
- Erecińska M, Silver IA. (1994) Ions and energy in mammalian brain. Prog Neurobiol 43:37–71. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Tator CH, Linden RD. (1989) The effect of nimodipine and dextran on axonal function and blood flow following experimental spinal cord injury. J Neurosurg 71:403–416. [DOI] [PubMed] [Google Scholar]
- Giorgi C, Baldassari F, Bononi A, Bonora M, De Marchi E, Marchi S, Missiroli S, Patergnani S, Rimessi A, Suski JM, et al. (2012) Mitochondrial Ca(2+) and apoptosis. Cell Calcium 52:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains BC, Klein JP, Saab CY, Craner MJ, Black JA, Waxman SG. (2003) Upregulation of sodium channel Nav1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. J Neurosci 23:8881–8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains BC, Waxman SG. (2006) Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci 26:4308–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED, Springer JE. (2004) Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx 1:80–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Iwata M, Tsuchimori N, Matsumoto T. (2014) Activation of peripheral KCNQ channels attenuates inflammatory pain. Mol Pain 10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV. (2008) STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci 28:7231–7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa H, Okajima S, Nagaoka T, Kubo T, Takamatsu T, Oyamada M. (2004) Regional differences in blood-nerve barrier function and tight-junction protein expression within the rat dorsal root ganglion. Neuroreport 15:405–408. [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE, Xu GY, Perez-Polo JR, Westlund KN, Taylor CP, McAdoo DJ. (2000) Rodent model of chronic central pain after spinal cord contusion injury and effects of gabapentin. J Neurotrauma 17:1205–1217. [DOI] [PubMed] [Google Scholar]
- James ND, Bartus K, Grist J, Bennett DL, McMahon SB, Bradbury EJ. (2011) Conduction failure following spinal cord injury: functional and anatomical changes from acute to chronic stages. J Neurosci 31:18543–18555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch TJ. (2000) Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci 1:21–30. [DOI] [PubMed] [Google Scholar]
- Jimenez-Andrade JM, Herrera MB, Ghilardi JR, Vardanyan M, Melemedjian OK, Mantyh PW. (2008) Vascularization of the dorsal root ganglia and peripheral nerve of the mouse: implications for chemical-induced peripheral sensory neuropathies. Mol Pain 4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SX, Zhuang ZY, Woolf CJ, Ji RR. (2003) p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci 23:4017–4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Li J, Zuo Y, Dang D, Frost JA, Yang Q. (2019) Activation of KCNQ channels prevents paclitaxel-induced peripheral neuropathy and associated neuropathic pain. J Pain 20:528–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Adamek P, Zhang H, Tatsui CE, Rhines LD, Mrozkova P, Li Q, Kosturakis AK, Cassidy RM, Harrison DS, et al. (2015) The cancer chemotherapeutic paclitaxel increases human and rodent sensory neuron responses to TRPV1 by activation of TLR4. J Neurosci 35:13487–13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, North RY, Rhines LD, Tatsui CE, Rao G, Edwards DD, Cassidy RM, Harrison DS, Johansson CA, Zhang H, et al. (2018) DRG voltage-gated sodium channel 1.7 is upregulated in paclitaxel-induced neuropathy in rats and in humans with neuropathic pain. J Neurosci 38:1124–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips J, de Haan P, Bodewits P, Vanicky I, Dzoljic M, Jacobs MJ, Kalkman CJ. (2000) Neuroprotective effects of riluzole and ketamine during transient spinal cord ischemia in the rabbit. Anesthesiology 93:1303–1311. [DOI] [PubMed] [Google Scholar]
- Llorente-Folch I, Rueda CB, Pardo B, Szabadkai G, Duchen MR, Satrustegui J. (2015) The regulation of neuronal mitochondrial metabolism by calcium. J Physiol 593:3447–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Zheng J, Xiong L, Zimmermann M, Yang J. (2008) Spinal cord injury-induced attenuation of GABAergic inhibition in spinal dorsal horn circuits is associated with down-regulation of the chloride transporter KCC2 in rat. J Physiol 586:5701–5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luszczki JJ. (2009) Third-generation antiepileptic drugs: mechanisms of action, pharmacokinetics and interactions. Pharmacol Rep 61:197–216. [DOI] [PubMed] [Google Scholar]
- Meisner JG, Marsh AD, Marsh DR. (2010) Loss of GABAergic interneurons in laminae I-III of the spinal cord dorsal horn contributes to reduced GABAergic tone and neuropathic pain after spinal cord injury. J Neurotrauma 27:729–737. [DOI] [PubMed] [Google Scholar]
- Morisset V, Nagy F. (1996) Modulation of regenerative membrane properties by stimulation of metabotropic glutamate receptors in rat deep dorsal horn neurons. J Neurophysiol 76:2794–2798. [DOI] [PubMed] [Google Scholar]
- Nashawi H, Masocha W, Edafiogho IO, Kombian SB. (2016) Paclitaxel causes electrophysiological changes in the anterior cingulate cortex via modulation of the γ-aminobutyric acid-ergic system. Med Princ Pract 25:423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodera H, Spieker A, Sung M, Rutkove S. (2011) Neuroprotective effects of Kv7 channel agonist, retigabine, for cisplatin-induced peripheral neuropathy. Neurosci Lett 505:223–227. [DOI] [PubMed] [Google Scholar]
- Nógrádi A, Szabó A, Pintér S, Vrbová G. (2007) Delayed riluzole treatment is able to rescue injured rat spinal motoneurons. Neuroscience 144:431–438. [DOI] [PubMed] [Google Scholar]
- Passmore GM, Selyanko AA, Mistry M, Al-Qatari M, Marsh SJ, Matthews EA, Dickenson AH, Brown TA, Burbidge SA, Main M, et al. (2003) KCNQ/M currents in sensory neurons: significance for pain therapy. J Neurosci 23:7227–7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabchevsky AG, Fugaccia I, Sullivan PG, Scheff SW. (2001) Cyclosporin A treatment following spinal cord injury to the rat: behavioral effects and stereological assessment of tissue sparing. J Neurotrauma 18:513–522. [DOI] [PubMed] [Google Scholar]
- Rabinstein AA. (2018) Traumatic spinal cord injury. Continuum (Minneap Minn) 24 (2, Spinal Cord Disorders):551–566. [DOI] [PubMed] [Google Scholar]
- Rekling JC. (2003) Neuroprotective effects of anticonvulsants in rat hippocampal slice cultures exposed to oxygen/glucose deprivation. Neurosci Lett 335:167–170. [DOI] [PubMed] [Google Scholar]
- Rivera-Arconada I, Lopez-Garcia JA. (2005) Effects of M-current modulators on the excitability of immature rat spinal sensory and motor neurones. Eur J Neurosci 22:3091–3098. [DOI] [PubMed] [Google Scholar]
- Ross IB, Koyanagi I, Wallace MC, Tator CH. (1999) Autoradiographic [3H]nimodipine distribution after experimental spinal cord injury in rats. J Neurotrauma 16:739–746. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE., Jr. (2003) Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma 20:179–193. [DOI] [PubMed] [Google Scholar]
- Schwartz G, Fehlings MG. (2001) Evaluation of the neuroprotective effects of sodium channel blockers after spinal cord injury: improved behavioral and neuroanatomical recovery with riluzole. J Neurosurg 94(2 Suppl):245–256. [DOI] [PubMed] [Google Scholar]
- Vigil FA, Bozdemir E, Bugay V, Chun SH, Hobbs M, Sanchez I, Hastings SD, Veraza RJ, Holstein DM, Sprague SM, et al. (2019) Prevention of brain damage after traumatic brain injury by pharmacological enhancement of KCNQ (Kv7, “M-type”) K + currents in neurons. J Cereb Blood Flow Metab DOI: 10.1177/0271678X19857818 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainger BJ, Kiskinis E, Mellin C, Wiskow O, Han SS, Sandoe J, Perez NP, Williams LA, Lee S, Boulting G, et al. (2014) Intrinsic membrane hyperexcitability of amyotrophic lateral sclerosis patient-derived motor neurons. Cell Rep 7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Li L, Xie F, Du J, Zuo Y, Frost JA, Carlton SM, Walters ET, Yang Q. (2017) Activation of KCNQ channels suppresses spontaneous activity in dorsal root ganglion neurons and reduces chronic pain after spinal cord injury. J Neurotrauma 34:1260–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Yang Q, Crook RJ, O’Neil RG, Walters ET. (2013) TRPV1 channels make major contributions to behavioral hypersensitivity and spontaneous activity in nociceptors after spinal cord injury. Pain 154:2130–2141. [DOI] [PubMed] [Google Scholar]
- Xie W, Strong JA, Zhang JM. (2009) Early blockade of injured primary sensory afferents reduces glial cell activation in two rat neuropathic pain models. Neuroscience 160:847–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Wu Y, Bi Y, Tan L, Gan Y, Wang K. (2010) Activation of voltage-gated KCNQ/Kv7 channels by anticonvulsant retigabine attenuates mechanical allodynia of inflammatory temporomandibular joint in rats. Mol Pain 6:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Wu Z, Hadden JK, Odem MA, Zuo Y, Crook RJ, Frost JA, Walters ET. (2014) Persistent pain after spinal cord injury is maintained by primary afferent activity. J Neurosci 34:10765–10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young W. (2002) Spinal cord contusion models. Prog Brain Res 137:231–255. [DOI] [PubMed] [Google Scholar]
- Zhang H, Yoon SY, Zhang H, Dougherty PM. (2012) Evidence that spinal astrocytes but not microglia contribute to the pathogenesis of Paclitaxel-induced painful neuropathy. J Pain 13:293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Waxman SG, Hains BC. (2007) Modulation of thalamic nociceptive processing after spinal cord injury through remote activation of thalamic microglia by cysteine cysteine chemokine ligand 21. J Neurosci 27:8893–8902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng FY, Xiao WH, Bennett GJ. (2011) The response of spinal microglia to chemotherapy-evoked painful peripheral neuropathies is distinct from that evoked by traumatic nerve injuries. Neuroscience 176:447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]