Abstract
This cross-sectional study evaluates the use of race in estimating glomerular filtration rate and whether height and weight could substitute for race using pooled data from 10 studies of people with and without chronic kidney disease.
Glomerular filtration rate (GFR) is critically important for determining drug dosing as well as prognosis and treatment in patients with kidney disease. Despite its importance, we rarely measure it directly. Instead, we use serum creatinine level to estimate GFR (eGFRcr). Because serum creatinine is determined by diet and muscle mass as well as GFR, we use age, sex, race (African American vs non–African American), height, or weight to adjust the estimation of GFR.1,2,3
Using race in the equation to estimate GFR is problematic because race is a social rather than a biological construct.4 People self-define their race in different ways, and many people are of mixed race, making any single category flawed. We sought to compare estimated GFR with vs without including patient race in the analysis using a data set that had been previously used to develop the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation,3 the guideline-recommended GFR-estimating equation for adults, and to determine whether height and weight might substitute for race.
Methods
The CKD-EPI equation was developed using a pooled data set from 10 studies of people with and without chronic kidney disease, all of whom had measured GFR values using urinary clearance of iothalamate and serum creatinine traceable to an international reference standard.3 Race was classified as African American or other and assigned by the study participants or the investigator. Performance of the equation was evaluated using root mean square error (RMSE) and bias. RMSE was computed for the regression of measured GFR (mGFR) on eGFRcr on a logarithmic scale. Bias was computed as the median value for the difference between eGFRcr and mGFR (eGFRcr − mGFR). We compared median bias and RMSE between equations using Wilcoxon signed rank tests. Analyses were performed from May through June 2019 using SAS software version 9.4M6 (SAS Institute). The Tufts Health Sciences Institutional Review Board deemed the study exempt from review owing to the use of deidentified data.
Results
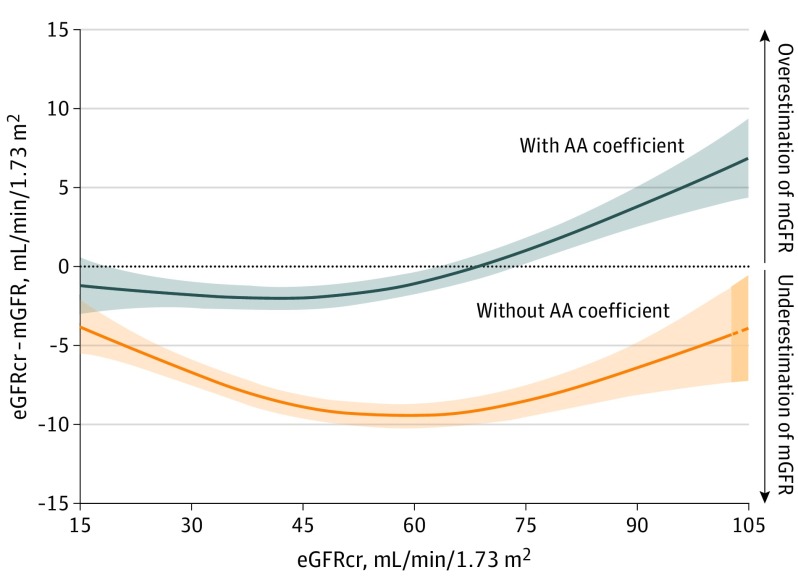
Among the 8254 participants in the development data set, 2601 (31.5%) were African American, 3606 (43.6%) were women, and the mean (SD) age and mGFR were 47 (15) years and 68 (40) mL/min/1.73 m2, respectively. As shown in the Figure, eliminating the race coefficient in the CKD-EPI equation was associated with a systematic error in the evaluation of African American individuals, an underestimation of mGFR throughout the range of the eGFRcr values. As shown in the Table, a new equation without race was associated with worse performance, more so in African American individuals than in non–African American individuals (equation 2). Inclusion of height and weight in addition to race did not meaningfully decrease the association of race with eGFRcr (coefficient, 1.15 vs 1.16) nor meaningfully improve performance (equation 3). Even when height and weight were included, eliminating race was associated with worse equation performance, more so in African American individuals than in non–African American individuals (equation 4).
Figure. Difference Between Estimated and Measured Glomerular Filtration Rate (GFR) With and Without the African American (AA) Coefficient Across a Range of Estimated GFR Values.
Data from 2601 AA participants from the Chronic Kidney Disease Epidemiology Collaboration development and internal validation sample were analyzed. Measured GFR (mGFR) values were obtained using urinary clearance of iothalamate. Estimated GFR based on serum creatinine (eGFRcr) values were computed using serum creatinine traceable to an international reference standard. We computed eGFRcr values with (gray) and without (orange) the application of the AA coefficient and removed values less than the 2.5 percentile and greater than the 97.5 percentile of these distributions, leaving 2463 participants for the analysis. Model plotted represents the generalized additive models for eGFRcr (mL/min/1.73 m2) on the difference between mGFR and eGFRcr (mL/min/1.73 m2) (excluding an additional 190 participants from the plot). The colored area along the lines represents 95% CIs of the estimate. The dashed line and darker orange shading indicate extrapolation of the model beyond the available data.
Table. Performance of the 2009 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Creatinine Equation With and Without Specification of African American (AA) Race and With and Without Height and Weight.
| Equation Coefficientsa | All (N = 8254)b | AA individuals (n = 2601) | Non-AA individuals (n = 5653) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AA coefficient (95% CI)c | RMSE (95% CI)d | P valuee | RMSE (95% CI)d | P valuee | Median bias (95% CI)f | P valuee | RMSE (95% CI)d | P valuee | Median bias (95% CI)f | P valuee | |
| 1. Scr, age, sex, and race | 1.16 (1.14 to 1.17) | 0.236 (0.229 to 0.242) | NA | 0.243 (0.232 to 0.254) | NA | 0 (−0.5 to 0.6) | NA | 0.232 (0.225 to 0.241) | NA | 0.5 (0.2 to 0.8) | NA |
| 2. Scr, age, and sex | NA | 0.244 (0.238 to 0.251) | <.001 | 0.258 (0.248 to 0.268) | <.001 | −4.0 (−4.5 to −3.5) | <.001 | 0.238 (0.230 to 0.247) | <.001 | 1.4 (1.1 to 1.7) | <.001 |
| 3. Scr, age, sex, race, height, and weight | 1.15 (1.14 to 1.17) | 0.235 (0.229 to 0.242) | .02 | 0.242 (0.232 to 0.253) | <.001 | 0.1 (−0.4 to 0.6) | .01 | 0.232 (0.225 to 0.241) | .95 | −0.5 (−0.8 to −0.2) | .07 |
| 4. Scr, age, sex, height, and weight | NA | 0.243 (0.237 to 0.250) | <.001 | 0.255 (0.245 to 0.265) | <.001 | −3.7 (−4.0 to −3.3) | <.001 | 0.238 (0.230 to 0.246) | <.001 | 1.3 (1.0 to 1.6) | <.001 |
Abbreviations: NA, not applicable; RMSE, root mean square error; Scr, serum creatinine.
Other equations were not considered because they are not more accurate than the CKD-EPI equation in external validation data sets and are not recommended by current guidelines.
Data are from the CKD-EPI pooled development data sets.3 Because of the large sample size and paired comparisons, small differences in RMSE and bias may be statistically significant. Mean (SD) measured glomerular filtration rate (mGFR) was 68 (40) mL/min/1.73 m2); mGFR was measured using urinary clearance of iothalamate. The estimated GFR (eGFR) based on Scr (eGFRcr) values were computed using Scr traceable to an international reference standard.
Coefficient for equation expressed on the multiplicative scale of GFR. If the addition of height and weight to the equation completely accounted for the effect of AA race, the AA coefficient would be very close to 1.00.
RMSE for the regression of mGFR on eGFR computed on a logarithmic scale. RMSE is the square root of the mean of squared differences between mGFR and eGFR; lower RMSE values indicate higher accuracy of the eGFR. For comparison of equations, we compared the paired differences of the squared differences on the log scale.
P values compared with row 1.
Median difference between eGFRcr and mGFR in mL/min/1.73 m2 (no difference between eGFRcr and mGFR would correspond to no bias; a negative value indicates an underestimation of mGFR). Median bias is not reported for the column “all” because it is expected to be approximately 0 in the pooled development data set. For comparison of equations, we compared the paired differences of the differences.
Discussion
The study results show that a strategy of eliminating the African American coefficient from the CKD-EPI equation was associated with a systematic bias toward underestimation of mGFR in African American individuals, which was not overcome by substituting height and weight. In particular, the equation with the African American coefficient was much more accurate at eGFRcr values less than approximately 75 mL/min/1.73 m2. We are concerned that the strategy of eliminating race from the equation as suggested by Eneanya et al5 may have unintended consequences in African American individuals, such as inappropriate early transplant or dialysis initiation, overdiagnosis of CKD, overestimation of the association of the risk of adverse outcomes with reduced GFR, inadequate dosing of drugs excreted by glomerular filtration (eg, some antibiotics and cancer chemotherapy), and limited access to tests (eg, some imaging procedures) and treatments that require a higher level of GFR (eg, metformin, sodium-glucose cotransporter 2 inhibitors, bisphosphonates), including living kidney donation. Better methods are needed to improve the accuracy of GFR assessment without requiring specification of race.
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1-150. [DOI] [PubMed] [Google Scholar]
- 2.Miller WG, Jones GRD. Estimated glomerular filtration rate; laboratory implementation and current global status. Adv Chronic Kidney Dis. 2018;25(1):7-13. doi: 10.1053/j.ackd.2017.09.013 [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Institute of Medicine Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. National Academies Press; 2003. [PubMed] [Google Scholar]
- 5.Eneanya ND, Yang W, Reese PP. Reconsidering the consequences of using race to estimate kidney function. JAMA. 2019;322(2):113-114. doi: 10.1001/jama.2019.5774 [DOI] [PubMed] [Google Scholar]