Abstract
This study uses National Health and Nutrition Examination Survey data to evaluate whether patients enrolled in the clinical trials that support the American College of Cardiology/American Heart Association (ACC/AHA) guideline are representative of the US adult population recommended additional pharmacotherapy by the ACC/AHA guideline.
The 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline for high blood pressure (BP) management lowered thresholds for diagnosing and treating hypertension to 130/80 mm Hg for all adults.1 These recommendations were based primarily on the results of the Systolic Blood Pressure Intervention Trial (SPRINT) and Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial,1,2,3 which recruited patients at increased cardiovascular risk and imposed exclusion criteria related to comorbidities, life expectancy, and likelihood of medication adherence.2,3 In the present study, we used National Health and Nutrition Examination Survey (NHANES) data to evaluate whether patients enrolled in the SPRINT and ACCORD trials are representative of the US adult population who met criteria for additional pharmacotherapy by the ACC/AHA guideline.
Methods
We analyzed pooled NHANES questionnaire, physical examination, and laboratory data from the 2013-2014 and 2015-2016 cycles4 to identify SPRINT and ACCORD trial eligibility criteria (Figure 15,6). Methods for operationalizing NHANES data are included in the Supplement. Missing data were imputed using the fully conditional specification method and 20 imputation sets. Sampling weights were used to provide nationally representative estimates with 95% CIs.
Figure 1. Flowchart of SPRINT and ACCORD Eligibility Criteria Applied to Adult NHANES Participants.
ACC/AHA indicates American College of Cardiology/American Heart Association; ACCORD, Action to Control Cardiovascular Risk in Diabetes trial; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; NHANES, National Health and Nutrition Examination Survey; SBP, systolic blood pressure; SPRINT, Systolic Blood Pressure Intervention Trial; ULN, upper limit of normal.
SI conversion factors: To convert HDL and LDL to mmol/L, divide by 0.0259; creatinine to µmol/L, multiply by 88.4.
Trial eligibility was determined in NHANES participants without and with diabetes using the published inclusion and exclusion criteria from the SPRINT and ACCORD trials, respectively.2,3 While both trials excluded individuals with factors likely to limit medication adherence, only SPRINT specified what these factors might include; thus, SPRINT exclusions were applied to both populations.
aThe 2017 ACC/AHA guideline defines hypertension based on the average of 2 BP readings of 130/80 mm Hg or higher on 2 separate occasions. In NHANES, BP was measured as the mean of 3 measurements obtained at 1-minute intervals during a single medical evaluation.
bDiabetes was defined by self-reported history or a hemoglobin A1c as 6.5% or higher (to convert to proportion of total hemoglobin, multiply by 0.01).
cLife expectancy less than 3 years is estimated based on Lee Index score of 14 or higher because a score of 14 is associated with a median predicted life expectancy of 3.1 years.5
dAnimal fluency test score less than 15. The animal fluency test examines categorical verbal fluency and scores have been shown to discriminate between persons with normal cognitive functioning compared with those with mild cognitive impairment and more severe forms of cognitive impairment, such as Alzheimer disease.6
Analysis began December 2018. We first calculated the number of adults classified as having hypertension and recommended intensified pharmacotherapy according to the ACC/AHA guideline. We then categorized individuals who met criteria for intensified pharmacotherapy into 3 categories based on trial eligibility criteria: (1) trial eligible, (2) not meeting inclusion criteria (owing to young age or lack of cardiovascular risk factors), and (3) meeting at least 1 exclusion criteria. All analyses were conducted using Stata version 14.1 (StataCorp). The National Center for Health Statistics institutional review board reviewed and approved each NHANES cycle, and participants provided written informed consent.4
Results
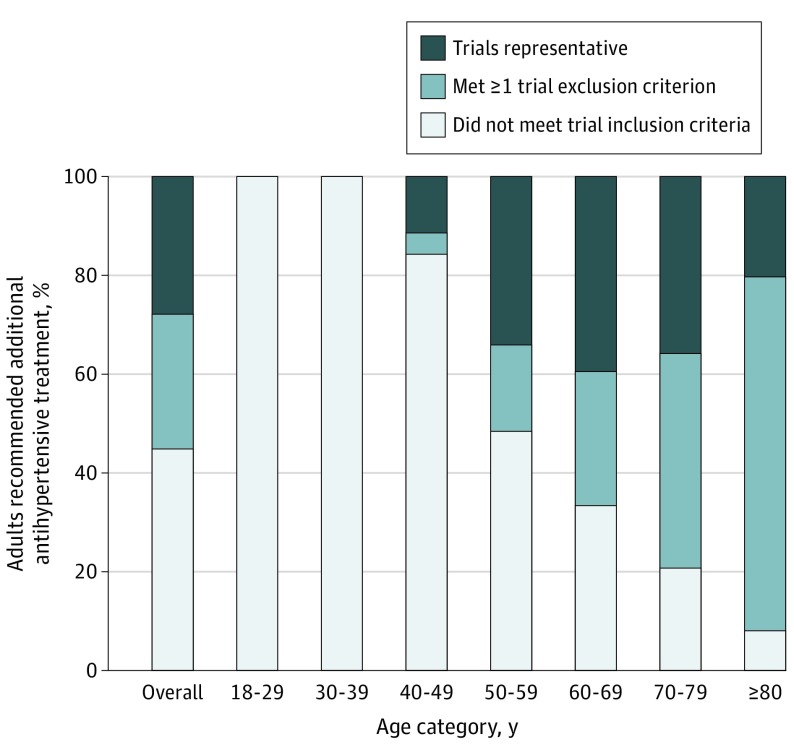
We estimated that 107.7 million US adults (95% CI, 99.3 million-116.0 million) would be classified as having hypertension, of which 58.8 million (95% CI, 53.7 million-63.8 million) would be recommended intensified pharmacotherapy by the 2017 ACC/AHA guideline. Of adults recommended intensified pharmacotherapy, 27.9% (95% CI, 25.2%-30.5%) met trial eligibility criteria; 44.9% (95% CI, 42.3%-47.5%) did not meet trial inclusion criteria because of low cardiovascular risk, and 27.3% (95% CI, 24.5%-30.0%) met at least 1 exclusion criteria (Figure 2). Most adults younger than 50 years would not have been included in trials owing to low cardiovascular risk; most older adults met the inclusion criteria for increased cardiovascular risk but also met exclusion criteria.
Figure 2. Representativeness of the Systolic Blood Pressure Intervention Trial and Action to Control Cardiovascular Risk in Diabetes Trial in US Adults Recommended Additional Antihypertensive Pharmacologic Treatment by the ACC/AHA Guideline.
The 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline defines hypertension based on blood pressure (BP) level above 130/80 mm Hg. The ACC/AHA guideline recommends pharmacologic therapy to all patients whose BP level is greater than 140/90 mm Hg and to patients whose BP level is between 130-139/80-89 mm Hg if they have a history of diabetes, are older than 65 years, or have a 10-year cardiovascular disease risk of more than 10% by pooled cohort risk equations. National Health and Nutrition Examination Survey analysis includes individuals with BP level higher than 130/80 mm Hg and those reporting use of BP medications regardless of measured BP. Individuals classified as recommended additional antihypertensive pharmacologic treatment include those not previously taking any antihypertensives and those currently taking antihypertensives but above ACC/AHA guideline goal.
Discussion
Although the 2017 ACC/AHA guideline substantially expanded the number of adults diagnosed as having hypertension and eligible for treatment, the clinical trials underlying new treatment thresholds are representative of less than one-third of the guideline target population. Trials were most representative of adults aged 50 and 69 years, indicating large evidence gaps on the effectiveness of intensive BP treatment in younger adults with low cardiovascular risk and in older adults with multimorbidity. Study limitations include BP measurement during a single visit, self-report of medical comorbidities, and missing data within NHANES.
Given these results, clinicians should be aware that the risk-benefit profile of intensive BP targets in low-risk individuals and younger adults is unknown and recommendations are based on extrapolation of findings from higher-risk populations. Furthermore, older adults with multimorbidity, who were largely excluded from trials, may have a reduced likelihood of benefit owing to competing risks of noncardiovascular death and a potentially increased risk of adverse events associated with polypharmacy. For most individuals addressed in the guidelines and not represented by trials, a patient-centered approach tailoring recommendations by degree of BP elevation, competing risks, and time to benefit is likely preferable to unwavering adoption of strict treatment targets.
eTable. Detailed Trial Inclusion and Exclusion Criteria
References
- 1.Whelton PK, Carey RM, Aronow WS, et al. . 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2018;138(17):e426-e483. [DOI] [PubMed] [Google Scholar]
- 2.Wright JT Jr, Williamson JD, Whelton PK, et al. ; SPRINT Research Group . A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cushman WC, Evans GW, Byington RP, et al. ; ACCORD Study Group . Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575-1585. doi: 10.1056/NEJMoa1001286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention National Health and Nutrition Examination Survey. Updated December 26, 2019. Accessed April 15, 2019. https://www.cdc.gov/nchs/nhanes/index.htm
- 5.Lee SJ, Boscardin WJ, Kirby KA, Covinsky KE. Individualizing life expectancy estimates for older adults using the Gompertz Law of Human Mortality. PLoS One. 2014;9(9):e108540. doi: 10.1371/journal.pone.0108540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grundman M, Petersen RC, Ferris SH, et al. ; Alzheimer’s Disease Cooperative Study . Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61(1):59-66. doi: 10.1001/archneur.61.1.59 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Detailed Trial Inclusion and Exclusion Criteria