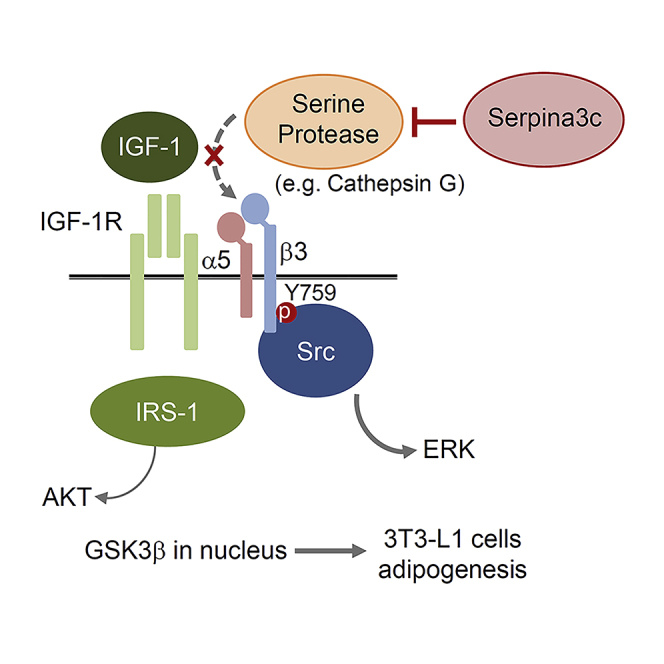
Summary
Preadipocyte differentiation can be induced upon a hormonal treatment, and various factors secreted by the cells may contribute to adipogenesis. In this study, RNA-seq revealed Serpina3c as a critical factor regulating the signaling network during adipogenesis. Serpina3c is a secretory protein and is highly expressed in fat tissues. Knockdown of Serpina3c decreased adipogenesis by attenuating the mitotic clonal expansion of 3T3-L1 cells. These cells exhibited decreases in integrin α5, which abolished the phosphorylation of integrin β3. We found that Serpina3c inhibits a serine protease that regulates integrin α5 degradation. Knockdown of Serpina3c disrupted integrin-mediated insulin growth factor 1 (IGF-1) signaling and ERK activation. Serpina3c-mediated regulation of integrin-IGF-1 signaling is also associated with AKT activation, which affects the nuclear translocation of GSK3β. Altogether, our results indicate that Serpina3c secreted from differentiating adipocytes inhibits serine proteases to modulate integrin/IGF-1-mediated ERK and AKT signaling and thus is a critical factor contributing to adipogenesis.
Subject Areas: Molecular Biology, Developmental Biology, Transcriptomics
Graphical Abstract
Highlights
-
•
RNA-seq revealed Serpina3c as a critical factor regulating adipogenesis
-
•
Knockdown of Serpina3c attenuated the mitotic clonal expansion of 3T3-L1 cells
-
•
Knockdown of Serpina3c leads to the degradation of integrin α5
-
•
Serpina3c regulates integrin-mediated IGF-1 signaling and ERK/AKT activation
Molecular Biology; Developmental Biology; Transcriptomics
Introduction
Obesity is a global disease associated with metabolic disorders such as diabetes, hypertension, and cardiovascular disease (Kopelman, 2000). Studies of the complex process by which adipocytes differentiate from preadipocyte precursors (i.e., adipogenesis) are important for understanding the underlying mechanisms of this disease (Gregoire et al., 1998). The 3T3-L1 preadipocyte cell line is an in vitro model widely used to study the molecular mechanisms of adipocyte differentiation (MacDougald and Lane, 1995). When proliferating 3T3-L1 preadipocytes become confluent in culture dishes, they enter G1 growth-arrest phase (Tang et al., 2003b). Upon treatment with hormonal cocktail (a mixture of 3-isobutyl-1-methylxanthine, dexamethasone, and insulin, hereafter called MDI) and fetal bovine serum (FBS), growth-arrested preadipocytes reenter the cell cycle and initiate differentiation via serial expression of adipogenic genes such as those encoding CCAAT/enhancer binding protein (C/EBP) β, C/EBPα, and peroxisome proliferator-activated receptor γ (PPARγ) (Lin and Lane, 1994, Tang et al., 2003a). This resumed cell cycle is called mitotic clonal expansion, and the process sustains during the first 2–3 days of differentiation and is essential for 3T3-L1 differentiation because an inhibition of this step completely blocks adipogenesis (Tang et al., 2003b).
In our previous studies to identify important differentiation factors secreted by clonally expanding cells, we observed that the differentiation program was accelerated in adipocytes treated with conditioned medium (CM) relative to those treated with MDI (Choi et al., 2014). Moreover, the CM-treated cells expressed adipocyte-specific genes before undergoing clonal expansion (Choi et al., 2014). Thus, the aim of the present study was to reveal the factors in CM responsible for promoting adipogenesis. To this end, we began by screening gene expression changes in cells treated with CM. We subsequently identified the serine protease Serpina3c as a protein regulating 3T3-L1 preadipocyte differentiation. Serpina3c is a member of the Serpin superfamily, and some Serpin family proteins have been implicated in adipocyte differentiation and obesity in the proteomic study (Zvonic et al., 2007); however, their precise physiological roles are unknown. Serpina3c is classified in the serpin A clade, which comprises 11 genes (SERPINA1 and SERPINA3 to -12) and 2 pseudogenes (SERPINA2 and SERPINA13P) in humans and 9 genes (Serpina3a, -b, -c, and -f to -n) in mice (Heit et al., 2013). Serpina3 is a known inhibitor of chymotrypsin and cathepsin G (Law et al., 2006) and is reported to be expressed in the blood, liver, kidney, and lung (Heit et al., 2013). We reveal here that Serpina3c is highly expressed in adipose tissue of mice and has an important role in adipocyte differentiation. This effect was closely associated with ERK and AKT activation via insulin-like growth factor 1 (IGF-1) signaling, which involves a complex formed by the IGF-1 receptor (IGF-1R) and the integrin α5-β3 heterodimer (Tahimic et al., 2013). We further demonstrate that Serpina3c inhibits a serine protease that degrades integrin α5. Thus, Serpina3c is a critical factor involved in the early signaling pathway of adipogenesis, and inhibition of Serpina3c may offer beneficial effects in the treatment of obesity.
Results
Serpina3c Is Expressed and Secreted by Differentiating Adipocytes
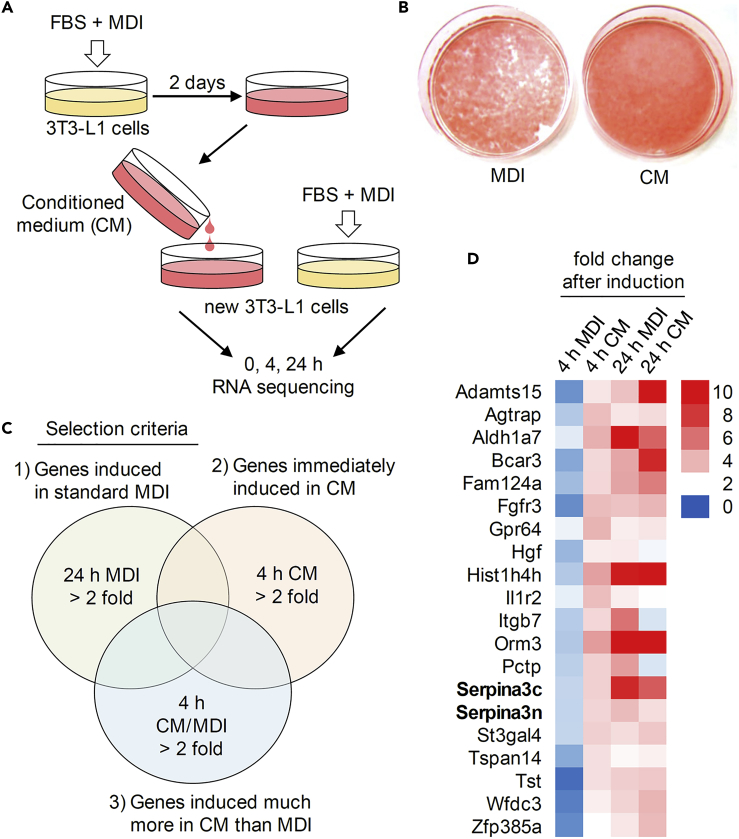
3T3-L1 preadipocytes were cultured to confluence in Dulbecco's modified Eagle's medium supplemented with 10% calf serum. Two days later, the cells were treated with MDI and FBS to induce cell-cycle reentry and preadipocyte differentiation. On differentiation day 2, the medium (i.e., CM) was collected and used to treat 3T3-L1 preadipocytes. Cells were then harvested 0, 4, or 24 h after treatment with CM (or MDI as a control) for RNA sequencing (Figure 1A). As previously observed (Choi et al., 2014), the terminal differentiation of CM-treated 3T3-L1 adipocytes was accelerated as evidenced by Oil Red O staining (Figure 1B). To identify genes associated with this acceleration, we focused on genes (1) upregulated >2-fold by MDI and FBS after 24 h of differentiation, (2) induced immediately (at 4 h of differentiation) by CM, and (3) with a CM/MDI ratio >2-fold at 4 h of differentiation (Figure 1C). We thought that these criteria would meet a critical condition to find factors that are up-regulated by CM, that is, by the factors secreted by differentiating adipocytes. This enables us to identify ~20 candidate genes (Figure 1D; the total gene expression dataset is available in Data S1). Of these, Serpina3c and Serpina3n have attracted immediate attention, because these are predicted as secreting molecules whose roles are not clarified in adipogenesis yet.
Figure 1.
Gene Expression Profile in Differentiating 3T3-L1 Cells Treated with CM
(A) A schematic model of CM preparation using a 3T3-L1 preadipocyte differentiation system. Confluent growth-arrested 3T3-L1 preadipocytes were induced to differentiate with MDI and FBS. After 2 days, cell-exposed medium was collected to treat other preadipocytes. Differentiating cells were harvested after 0, 4, and 24 h for RNA sequencing. MDI + FBS-treated cells were used as a control group.
(B) CM accelerated 3T3-L1 adipocyte differentiation as shown by Oil Red O staining at day 5 of induction.
(C) Strategy for the identification of CM-containing factors via RNA sequencing data.
(D) Heatmap showing relative expression of 20 genes identified from RNA sequencing data. Red, high expression; blue, low expression. Fold changes of the total genes are presented in Data S1. These RNA sequencing data using MDI and CM are publicly available from Gene Expression Omnibus (GEO: GSE144130).
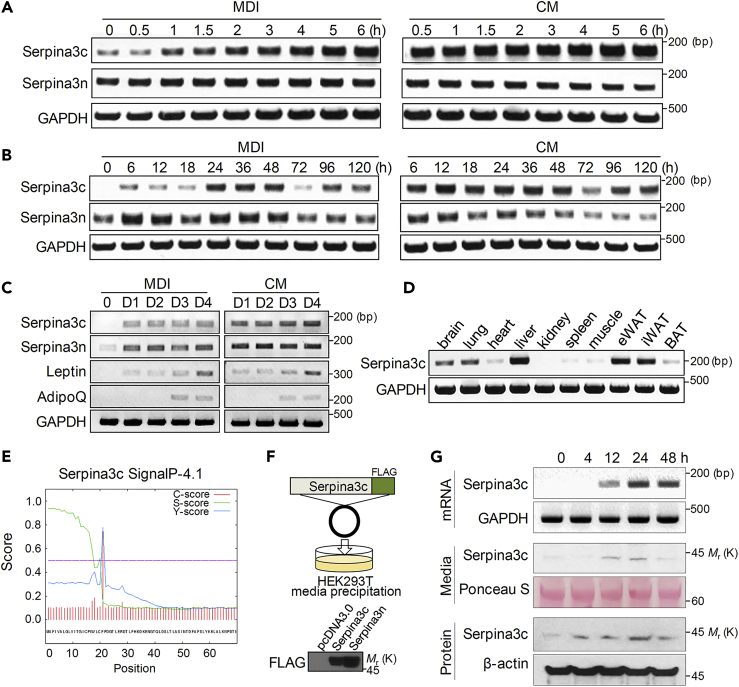
We further examined the expression of Serpina3c and Serpina3n during the course of 3T3-L1 differentiation. In cells treated with MDI, Serpina3c expression gradually increased, peaking at 24–48 h, whereas the expression of Serpina3n did not change markedly (Figures 2A and 2B). By contrast, the expression of Serpina3c rapidly reached the highest level soon after CM treatment and maintained this level during the whole differentiation process (Figures 2A and 2B). During the late stage of adipogenesis, the expression levels of adipokines such as leptin and adiponectin were not affected by Serpina3c (Figure 2C). Serpina3c mRNA expression was high in white adipose tissues and liver but much lower in other mouse tissues (Figure 2D). These data are in accordance with a role for Serpina3c in adipocyte differentiation and the rapid progression of adipogenesis induced by CM.
Figure 2.
General Characterization of Serpina3c in Adipocytes
(A–C) Serpina3c expression patterns induced by MDI or CM treatment in 3T3-L1 adipocytes. Cell lysate were prepared at various times, (A) 0∼6 h or (B) 0∼120 h after treatment for RT-PCR analysis of Serpina3c, Serpina3n, and Gapdh. (C) Also, the expression of adipokines, Leptin and AdipoQ, was compared with those of Serpina3c and Serpina3n.
(D) Tissue distribution of Serpina3c by RT-PCR in C57BL/6 male mice.
(E) Amino acid sequence of Serpina3c was analyzed by SignalP-4.1 prediction (www.cbs.dtu.dk/services/SignalP-4.1/) showing the N-terminal signal peptide for secretory proteins.
(F) Overexpression vector was generated by inserting mouse Serpina3c (or Serpina3n) encoding a C-terminal FLAG tag into pcDNA3.0. HEK293T cells were transfected with this construct, and 2 days later, the medium was collected, precipitated by cold acetone, and subjected to western blot analysis to detect the FLAG-tagged protein secreted from cells. pcDNA3.0 was used as a negative control.
(G) Serpina3c expression and secretion patterns induced by MDI in 3T3 L1 adipocytes. RT-PCR was performed to detect expression of Serpina3c mRNA after treatment. Western blot analysis was performed to investigate expression and secretion of Serpina3c protein in precipitated media or cell lysate after treatment.
Serpina3c (also known as alpha 1 proteinase inhibitor antitrypsin [Heit et al., 2013, Law et al., 2006]) is predicted to be a secretory protein with a signal peptide on its N terminus (Figure 2E). To confirm that it is a secretory protein, we generated Serpina3c and Serpina3n overexpression vectors with FLAG tags and transfected them into HEK293T cells. Serpina3c and -3n proteins were detected in the medium from transfected cells (Figure 2F), indicating that they are indeed secreted from cells. In addition, the secretion of Serpina3c protein was paralleled with intracellular expression of Serpina3c (Figure 2G).
Knockdown of Serpina3c Inhibits Mitotic Clonal Expansion and Adipocyte Differentiation
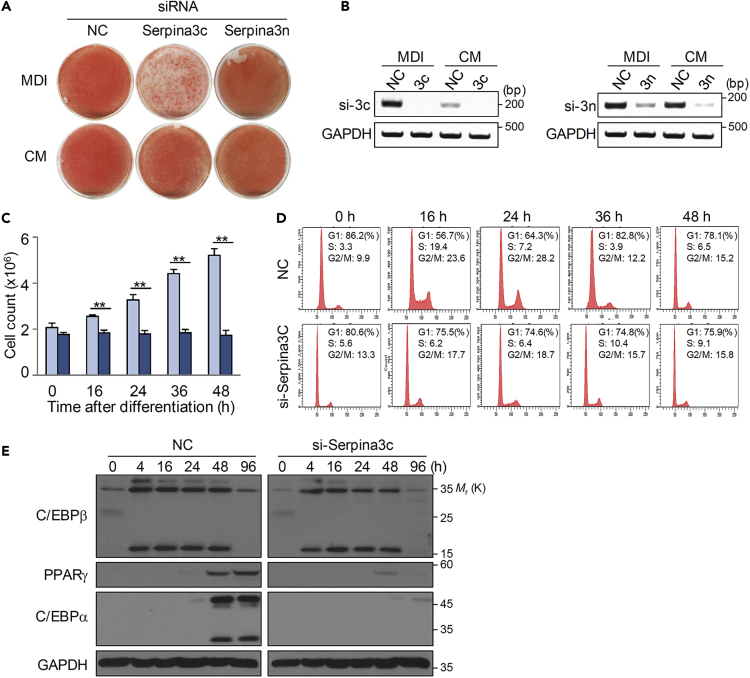
To clarify the role of Serpina3c or Serpina3n in adipogenesis, we knocked down expression via RNA interference. Although Serpina3c and Serpina3n are located on the same chromosome and encode proteins with similar amino acid sequences, only knockdown of Serpina3c affected 3T3-L1 adipogenesis (Figures 3A and 3B). Interestingly, the effect of Serpina3c knockdown was observed in cells treated with MDI but not with CM, indicating that CM may already contain Serpina3c secreted by differentiating cells. In other words, differentiating 3T3-L1 cells with MDI secrete Serpina3c into the medium (i.e., CM), then CM-treated 3T3-L1 cells no longer require Serpina3c expression.
Figure 3.
Serpina3c Is Required for Mitotic Clonal Expansion during Adipogenesis
(A and B) 3T3-L1 cells were transfected with negative control (NC), Serpina3c, or Serpina3n siRNAs. Differentiation of transfected cells was induced by MDI or CM. Serpina3c depletion abolishes 3T3-L1 differentiation in MDI-treated cells. Oil Red O staining was conducted on day 8 (A) and knockdown of Serpina3c was assessed by RT-PCR (B).
(C) Cell proliferation determined by cell counting at 0, 16, 24, 36, and 48 h after MDI treatment. Data are represented as mean ± SE. p Values less than 0.05 were considered significant, with ∗p < 0.05, ∗∗p < 0.01 as determined by Student's t test.
(D) Cell-cycle analysis by flow cytometry at the indicated time points.
(E) Western blot analysis was performed for differentiation markers of adipocytes in control cells and those with Serpina3c knockdown (si-Serpina3c).
During mitotic clonal expansion, cell numbers normally increase 2- to 3-fold (Lane et al., 1999, Lee et al., 2009). However, this was not observed in cells with Serpina3c knockdown after MDI induction (Figure 3C). These results were confirmed by fluorescence-activated cell sorting analysis. The G1-S2 transition was inhibited in cells with Serpina3c knockdown, whereas more control cells reentered the cell cycle after MDI treatment (Figure 3D). Consistent with a role for Serpina3c in adipogenesis, knockdown of Serpina3c resulted in reduced expression of PPARγ and C/EBPα (Figure 3E). This result suggests that Serpina3c is associated with mitotic clonal expansion in adipogenesis.
Serpina3c Is Involved in Adipogenesis via the Integrin-Mediated IGF-1 Pathway
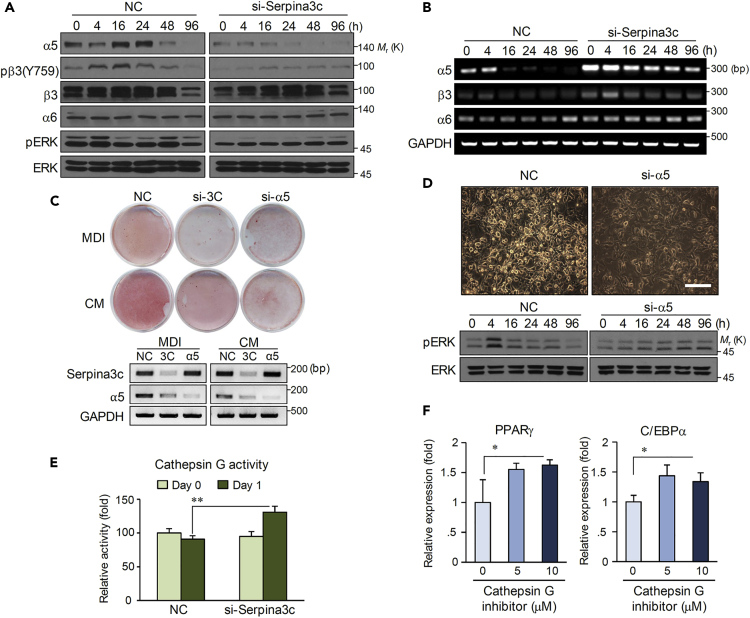
As human SERPINA3 is reported as an inhibitor of cathepsin G (Heit et al., 2013, Law et al., 2006), a type of serine protease known to modulate integrin clustering in neutrophils and induce integrin cleavage in platelets (Raptis et al., 2005, Si-Tahar et al., 1997), we speculated that integrin regulation may be altered in cells with Serpina3c knockdown. Western blotting revealed that integrin α5 but not integrin α6 was decreased by Serpina3c knockdown (Figure 4A). Integrin α5 and β3 heterodimerize and interact with IGF-1R (Sekimoto et al., 2005), and in the presence of IGF-1, Tyr759 of integrin β3 is phosphorylated and binds Src (Cowan et al., 2000, Fagerholm et al., 2004). As shown in Figure 4A, integrin β3 phosphorylation was diminished in cells with Serpina3c knockdown, suggesting that integrin-IGF-1R signaling is altered. Accordingly, phosphorylation of the downstream signaling enzyme ERK, which is required for C/EBPβ phosphorylation and mitotic clonal expansion in adipogenesis (Kim et al., 2007, Tang et al., 2003a), was decreased by Serpina3c knockdown. Notably, the expression of integrin α5 mRNA was increased by Serpina3c knockdown (Figure 4B), indicating that the effect of Serpina3c is not at the level of transcription but rather at the level of protein translation or degradation. Nevertheless, knockdown of integrin α5 mRNA inhibited adipocyte differentiation with MDI. With CM treatment, adipogenesis of si-Serpina3c cells was slightly rescued owing to the intracellular Serpina3c protein in CM. In contrast, knockdown of integrin α5, a downstream molecule of Serpina3c, showed no change between MDI and CM treatment (Figure 4C). Besides transducing signals from the extracellular matrix to the cell, integrin also serves to attach the cell to the extracellular matrix (Gao et al., 2014). Our experiment indicates that knockdown of integrin α5, without altering cellular structure, blocks adipogenesis by inhibiting ERK signaling (Figure 4D). Confocal microscopy also has shown that the cellular structure of integrin α5 knockdown cells was not changed during differentiation (Figure S1).
Figure 4.
Knockdown of Serpina3c Results in Integrin α5-mediated ERK Inactivation
(A) 3T3-L1 cells were transfected with either negative control siRNA (NC) or si-Serpina3c and then differentiated using MDI. Integrin α5, β3, and α6 and phosphorylated integrin β3 and ERK were detected by western blotting at the indicated time points.
(B) RT-PCR was performed to assess the expression of integrin α5, β3, and α6. 3T3-L1 cells were transfected with NC or Serpina3c siRNA, and then differentiation was induced by MDI.
(C) Cells treated with NC, si-Serpina3c (si-3C), or si-Integrin α5 (si-α5) were stained with Oil Red O on day 6, and depletion of Serpina3c or integrin α5 was assessed by RT-PCR.
(D) Microscopy images of cells 2 days after transfections with NC and si-α5. Cell structure was not changed. Western blot analysis was performed for ERK and phosphorylated ERK. Scale bar, 50 μm.
(E) Cathepsin G activity was measured in 3T3-L1 cells transfected with NC or si-Serpina3c on day 0 or day 1 after differentiation. Data are represented as mean ± SD. p Values less than 0.05 were considered significant, with ∗p < 0.05, ∗∗p < 0.01 as determined by Student's t test.
(F) Gene expression changes of PPARγ and C/EBPα during differentiation after treatment with cathepsin G inhibitor. Data are represented as mean ± SD. p Values less than 0.05 were considered significant, with ∗p < 0.05, ∗∗p < 0.01 as determined by Student's t test.
How does Serpina3c contribute to the maintenance of integrin α5 protein? To this end, we measured cathepsin G activity. During adipogenesis, cathepsin G activity was significantly increased during adipocyte differentiation in cells with Serpina3c knockdown (Figure 4E). Moreover, administration of a synthetic cathepsin G inhibitor during adipogenesis increased PPARγ and C/EBPα expression, suggesting that the inhibition of cathepsin G promotes adipogenesis (Figure 4F). Altogether, these data show that Serpina3c leads to the inhibition of cathepsin G, thereby protecting integrin α5 from degradation.
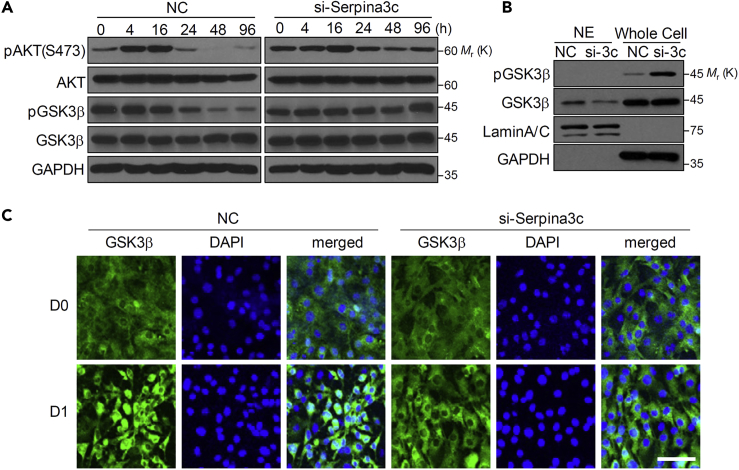
Serpina3c Knockdown Attenuates Nuclear Translocation of GSK3β via Prolonged AKT Activation
We next investigated AKT activation, which is also induced by IGF-1 signaling (Kim et al., 2016). Knockdown of Serpina3c prolonged the increase in AKT phosphorylation during differentiation (Figure 5A). Accordingly, phosphorylation of the downstream target glycogen synthase kinase 3β (GSK3β) was also prolonged (Figure 5A). Phosphorylated GSK3β does not translocate to the nucleus (Tang et al., 2005). Consistent with this, no phosphorylated GSK3β was detected in the nuclear fraction of 3T3-L1 adipocytes, and a decreased amount of nuclear GSK3β was observed in cells with Serpina3c knockdown (Figure 5B), indicating that nuclear translocation was suppressed. The inhibition of GSK3β nuclear translocation by knockdown of Serpina3c was confirmed by immunocytochemistry (Figure 5C). Thus, Serpina3c promotes GSK3β translocation to the nucleus, which is required for the phosphorylation of Ser184 and Thr179 of C/EBPβ that is necessary for adipogenesis (Tang et al., 2005).
Figure 5.
Serpina3c knockdown Induces Prolonged AKT Phosphorylation Resulting in Inhibited GSK3β Translocation
3T3-L1 cells were transfected with NC or si-Serpina3c and then differentiated using MDI.
(A) Protein levels in transfected cells were measured by western blotting.
(B) Nuclear extracts (NE) and whole-cell lysates were prepared 24 h after MDI treatment and phosphorylated (p) GSK3β and GSK3β levels were measured by western blotting. LaminA/C and GAPDH were used as nuclear and cytosol protein marker, respectively.
(C) Cells transfected with NC or si-Serpina3c were induced to differentiate with MDI and fixed for immunocytochemistry against GSKβ 24 h later. Scale bar, 50 μm.
Serpina3c Increases Adipogenesis by 3T3-L1 Preadipocytes and Is Increased in Animals and Humans with Obesity
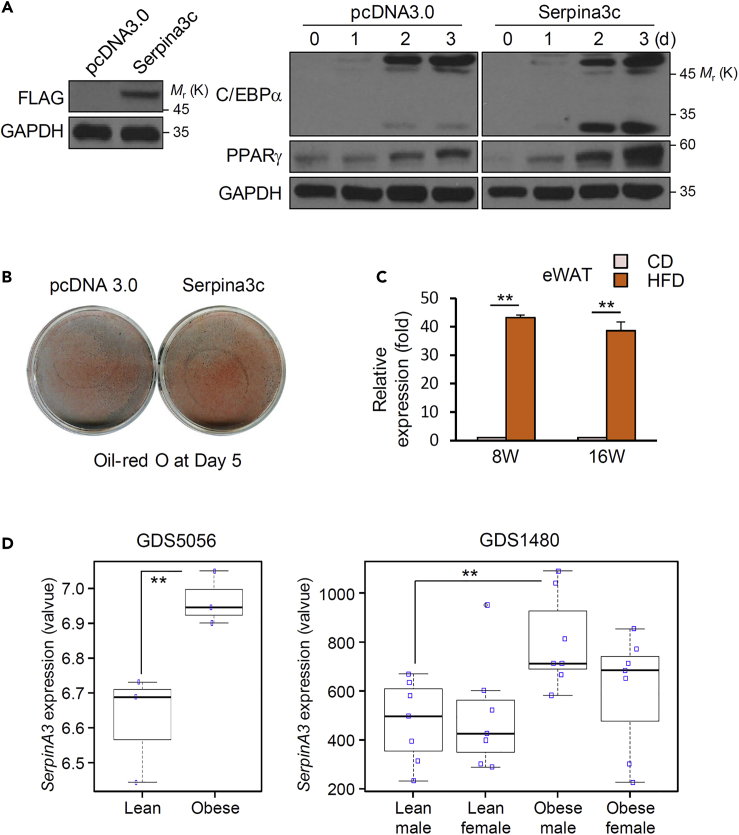
To verify the role of Serpina3c in adipogenesis, the differentiation of adipocytes was examined in cells overexpressing Serpina3c. The expression of C/EBPα and PPARγ during differentiation was increased in 3T3-L1 cells transfected with pcDNA3.0 encoding FLAG-tagged Serpina3c (Figure 6A). Oil Red O staining revealed that the differentiation of these cells was accelerated (Figure 6B). Consistent with an increase of adipogenesis by Serpina3c, we found that the expression of this factor was higher in epididymal white adipose tissues of mice fed a high-fat diet than in those fed a chow diet (Figure 6C). When comparing the expression of Serpina3c by the duration of high-fat diet, the Serpina3c expression in high-fat diet for 8 weeks was slightly higher than that of 16 weeks but was not statistically significant. We also accessed a public repository (NCBI, Gene Expression Omnibus GDS5056 and GDS1480) to obtain expression data from adipose stem cells from morbidly obese and non-obese individuals. The expression levels of SERPINA3, a human ortholog gene of SERPINA3C, was higher in obese individuals than in lean individuals (p < 0.05; Figure 6D). These data show that SerpinA3 family proteins, including Serpina3c, play a critical role in adipocyte differentiation and obesity.
Figure 6.
Serpina3c Overexpression Increases Adipocyte Differentiation
3T3-L1 cells were transfected with a control (pcDNA 3.0) or Serpina3c overexpression vector and induced to differentiate.
(A) Overexpressed Serpina3c was confirmed 2 days after transfection by detection of the FLAG tag from whole-cell lysates. Proteins collected at the indicated times after induction were used to assay the levels of C/EBPα and PPARγ by western blot (GAPDH was used as loading control).
(B) Oil Red O staining on day 5.
(C) Serpina3c expression by quantitative real-time PCR in epididymal WAT tissues from C57BL/6 male mice fed a chow diet (CD) or a high-fat diet (HFD) for 8 or 16 weeks. WAT, white adipose tissue. Data are represented as mean ± SD. p Values less than 0.05 were considered significant, with ∗p < 0.05, ∗∗p < 0.01 as determined by Student's t test.
(D) SERPINA3 gene expression levels in adipose stem cells or preadipocytes from obese and lean individuals, using publicly available repository data (Gene Expression Omnibus accessions GDS5056 and GDS1480). Data are represented as mean ± SD. p Values less than 0.05 were considered significant, with ∗p < 0.05, ∗∗p < 0.01 as determined by Student's t test.
Discussion
The mechanisms of 3T3-L1 differentiation have been investigated since these cells were first cloned by Howard Green (Green and Meuth, 1974). These efforts have identified the roles of transcription factors C/EBPβ, C/EBPα, and PPARγ in adipogenesis (Gregoire et al., 1998, Lin and Lane, 1994), but the complex molecular processes regulating adipocyte differentiation have yet to be fully defined. In the present study, we found that Serpina3c is a regulator of adipogenesis and was responsible for the accelerated differentiation of adipocytes induced by CM (Choi et al., 2014). We further show that its regulation is via modulation of integrin-IGF-1 signaling.
From the RNA sequencing data, the gene expression profile was compared between CM-induced adipocytes and MDI-induced adipocytes, elucidating Serpina3c as a gene expected to be critically involved in adipogenesis. Serpins are the largest and most broadly distributed superfamily of protease inhibitors (Barrett et al., 2001, Irving et al., 2000, Law et al., 2006), nearly ubiquitous in higher eukaryotes and with highly diverse functions. However, the role of serpins in metabolism has not been investigated fully. SerpinB1 was recently found to promote pancreatic β-cell proliferation and contribute to insulin sensitivity (El Ouaamari et al., 2016). With regard to adipocytes, Serpina12 (vaspin [visceral adipose tissue-derived serine protease inhibitor]) is the most well-known serpin, functioning as an insulin-sensitizing adipokine in obesity (Hida et al., 2005). Our data here reveal that Serpina3c stimulates adipocyte differentiation.
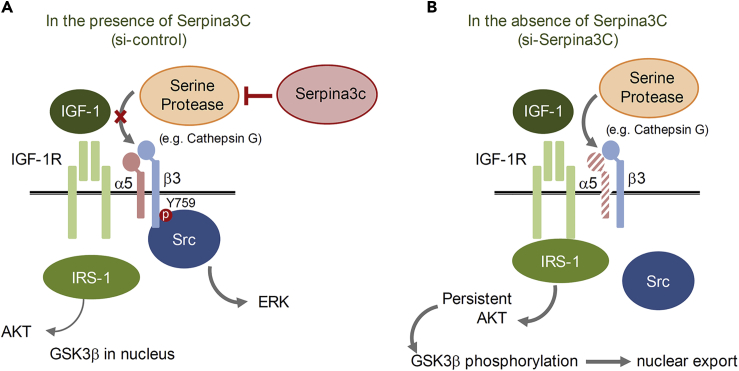
Serpina3c is a secretory protein that can affect extracellular matrix proteins. We show here that it is a serine protease inhibitor, with one target protein being cathepsin G for integrin α5 degradation (Figure 4A). The knockdown of Serpina3c interfered with IGF-1 signaling, disrupting the activation of ERK and AKT. Figure 7 shows a possible mechanism by which Serpina3c controls adipogenesis. IGF-1 receptors form a complex with integrin α5-β3 heterodimers required for activation of the IGF-1 pathway in 3T3-L1 cells. The binding of IGF-1 to its receptor induces the phosphorylation of integrin β3 to bind Src and activate downstream targets, such as ERK. Serine proteases (e.g., cathepsin G) degrade integrin α5 and abolish Src-mediated ERK activation. In the absence of the integrin complex, IGF-1 signals through insulin response substrate 1, which leads to a sustained AKT signaling and the phosphorylation and nuclear export of GSK3β. Thus, Serpina3c is required for activation of ERK and nuclear translocation of GSK3β, which are both are necessary for C/EBPβ phosphorylation and adipogenesis.
Figure 7.
Schematic Illustration of How Serpina3c Regulates Adipogenesis
(A) IGF-1 receptors form a complex with the integrin α5-β3 heterodimer that is required to activate ERK. With ligand binding, integrin β3 is phosphorylated at Tyr759, leading to Src binding and ERK activation. In this situation, Serpina3c blocks any serine proteases, which can degrade integrin α5. Insulin receptor substrate 1 (IRS-1) may transiently induce AKT signaling.
(B) In the absence of Serpina3c, serine proteases degrade integrin α5, and the integrin-IGF-1 receptor complex is not formed, resulting in persistent activation of AKT by IRS-1 and phosphorylation of GSK3β, thereby attenuating adipose differentiation.
In summary, the results from this study provide important advances in understanding the mechanism of adipogenesis. We show that CM induces the transcription of several genes, including Serpina3c, which stimulates adipocyte differentiation without mitotic clonal expansion. Furthermore, we show that integrin in the extracellular matrix contributes to adipocyte differentiation. Specifically, Serpina3c inhibits serine proteases that degrade integrin α5, thereby shifting IGF-1 signaling away from AKT activation toward ERK signaling via the IGF-1-integrin complex. Thus, Serpina3c plays an important role in the transition from mitotic clonal expansion to terminal differentiation and is critical for adipocyte differentiation and adipogenesis.
Limitations of the Study
In this study, we suggested cathepsin G as a candidate serine protease that is inhibited by Serpina3c. However, there is a possibility that other proteases under the inhibition of Serpina3c may exist in addition to cathepsin G. Another limitation is that, in humans, Serpina3 clade is consisted of single gene, SERPINA3, so it is necessary to confirm whether SERPINA3 functions in the same mechanism as SERPINA3C in mice. In addition, further study using SERPINA3C knockout mice should be performed to determine how Serpina3c works in vivo. Finally, further purification of Serpina3c protein is needed to fully clarify the effect of Serpina3c overexpression.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government, Ministry of Science and ICT (MSIT) (NRF-2018R1A5A2025079).
Author Contributions
Y.C., H.J.K., and J.-w.K. designed research; Y.C., H.C., B.K.Y., H.L., J.W.S., H.J.K., and J.-w.K. performed research; Y.C., H.J.K., and J.-w.K. analyzed data; and Y.C. and J.-w.K. wrote the paper.
Declaration of Interest
The authors declare no conflict of interest.
Published: March 27, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100961.
Contributor Information
Hyo Jung Kim, Email: hjkim17@yuhs.ac.
Jae-woo Kim, Email: japol13@yuhs.ac.
Data and Code Availability
The accession numbers for the 3T3-L1 RNA-seq using MDI and CM is GEO: GSE144130. Expression levels of SERPINA3 in human adipose tissue, which is a human ortholog gene of SERPINA3C, were obtained from a public repository (GEO: GDS5056 and GDS 1480). Expression levels of SERPINA3 were compared among groups by Student’s t tests using R version 3.6.0 software (R Development Core Team, Vienna, Austria).
Supplemental Information
References
- Barrett A.J., Rawlings N.D., O'Brien E.A. The MEROPS database as a protease information system. J. Struct. Biol. 2001;134:95–102. doi: 10.1006/jsbi.2000.4332. [DOI] [PubMed] [Google Scholar]
- Choi H., Lee H., Kim T.H., Kim H.J., Lee Y.J., Lee S.J., Yu J.H., Kim D., Kim K.S., Park S.W. G0/G1 switch gene 2 has a critical role in adipocyte differentiation. Cell Death Differ. 2014;21:1071–1080. doi: 10.1038/cdd.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan K.J., Law D.A., Phillips D.R. Identification of Shc as the primary protein binding to the tyrosine-phosphorylated beta(3) subunit of alpha(IIb)beta(3) during outside-in integrin platelet signaling. J. Biol. Chem. 2000;275:36423–36429. doi: 10.1074/jbc.M004068200. [DOI] [PubMed] [Google Scholar]
- El Ouaamari A., Dirice E., Gedeon N., Hu J., Zhou J.Y., Shirakawa J., Hou L., Goodman J., Karampelias C., Qiang G. SerpinB1 promotes pancreatic beta cell proliferation. Cell Metab. 2016;23:194–205. doi: 10.1016/j.cmet.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerholm S.C., Hilden T.J., Gahmberg C.G. P marks the spot: site-specific integrin phosphorylation regulates molecular interactions. Trends Biochem. Sci. 2004;29:504–512. doi: 10.1016/j.tibs.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Gao Y., Liu S., Huang J., Guo W., Chen J., Zhang L., Zhao B., Peng J., Wang A., Wang Y. The ECM-cell interaction of cartilage extracellular matrix on chondrocytes. Biomed. Res. Int. 2014;2014:648459. doi: 10.1155/2014/648459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H., Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974;3:127–133. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- Gregoire F.M., Smas C.M., Sul H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- Heit C., Jackson B.C., McAndrews M., Wright M.W., Thompson D.C., Silverman G.A., Nebert D.W., Vasiliou V. Update of the human and mouse SERPIN gene superfamily. Hum. Genomics. 2013;7:22. doi: 10.1186/1479-7364-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hida K., Wada J., Eguchi J., Zhang H., Baba M., Seida A., Hashimoto I., Okada T., Yasuhara A., Nakatsuka A. Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc. Natl. Acad. Sci. U S A. 2005;102:10610–10615. doi: 10.1073/pnas.0504703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving J.A., Pike R.N., Lesk A.M., Whisstock J.C. Phylogeny of the serpin superfamily: implications of patterns of amino acid conservation for structure and function. Genome Res. 2000;10:1845–1864. doi: 10.1101/gr.gr-1478r. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Cha J.Y., Seok J.W., Choi Y., Yoon B.K., Choi H., Yu J.H., Song S.J., Kim A., Lee H. Dexras1 links glucocorticoids to insulin-like growth factor-1 signaling in adipogenesis. Sci. Rep. 2016;6:28648. doi: 10.1038/srep28648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.W., Tang Q.Q., Li X., Lane M.D. Effect of phosphorylation and S-S bond-induced dimerization on DNA binding and transcriptional activation by C/EBPbeta. Proc. Natl. Acad. Sci. U S A. 2007;104:1800–1804. doi: 10.1073/pnas.0611137104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman P.G. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- Lane M.D., Tang Q.Q., Jiang M.S. Role of the CCAAT enhancer binding proteins (C/EBPs) in adipocyte differentiation. Biochem. Biophys. Res. Commun. 1999;266:677–683. doi: 10.1006/bbrc.1999.1885. [DOI] [PubMed] [Google Scholar]
- Law R.H.P., Zhang Q.W., McGowan S., Buckle A.M., Silverman G.A., Wong W., Rosado C.J., Langendorf C.G., Pike R.N., Bird P.I. An overview of the serpin superfamily. Genome Biol. 2006;7:216. doi: 10.1186/gb-2006-7-5-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Lee Y.J., Choi H., Ko E.H., Kim J.W. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J. Biol. Chem. 2009;284:10601–10609. doi: 10.1074/jbc.M808742200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F.T., Lane M.D. Ccaat/enhancer binding-protein-alpha is sufficient to initiate the 3t3-L1 adipocyte differentiation program. Proc. Natl. Acad. Sci. U S A. 1994;91:8757–8761. doi: 10.1073/pnas.91.19.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougald O.A., Lane M.D. Transcriptional regulation of gene expression during adipocyte differentiation. Annu. Rev. Biochem. 1995;64:345–373. doi: 10.1146/annurev.bi.64.070195.002021. [DOI] [PubMed] [Google Scholar]
- Raptis S.Z., Shapiro S.D., Simmons P.M., Cheng A.M., Pham C.T. Serine protease cathepsin G regulates adhesion-dependent neutrophil effector functions by modulating integrin clustering. Immunity. 2005;22:679–691. doi: 10.1016/j.immuni.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Sekimoto H., Eipper-Mains J., Pond-Tor S., Boney C.M. (alpha)v(beta)3 integrins and Pyk2 mediate insulin-like growth factor I activation of Src and mitogen-activated protein kinase in 3T3-L1 cells. Mol. Endocrinol. 2005;19:1859–1867. doi: 10.1210/me.2004-0481. [DOI] [PubMed] [Google Scholar]
- Si-Tahar M., Pidard D., Balloy V., Moniatte M., Kieffer N., VanDorsselaer A., Chignard M. Human neutrophil elastase proteolytically activates the platelet integrin alpha(IIb)beta(3) through cleavage of the carboxyl terminus of the alpha(IIB) subunit heavy chain - involvement in the potentiation of platelet aggregation. J. Biol. Chem. 1997;272:11636–11647. doi: 10.1074/jbc.272.17.11636. [DOI] [PubMed] [Google Scholar]
- Tahimic C.G., Wang Y., Bikle D.D. Anabolic effects of IGF-1 signaling on the skeleton. Front. Endocrinol. (Lausanne) 2013;4:6. doi: 10.3389/fendo.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q.Q., Gronborg M., Huang H., Kim J.W., Otto T.C., Pandey A., Lane M.D. Sequential phosphorylation of CCAAT enhancer-binding protein beta by MAPK and glycogen synthase kinase 3beta is required for adipogenesis. Proc. Natl. Acad. Sci. U S A. 2005;102:9766–9771. doi: 10.1073/pnas.0503891102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q.Q., Otto T.C., Lane M.D. CCAAT/enhancer-binding protein beta is required for mitotic clonal expansion during adipogenesis. Proc. Natl. Acad. Sci. U S A. 2003;100:850–855. doi: 10.1073/pnas.0337434100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q.Q., Otto T.C., Lane M.D. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc. Natl. Acad. Sci. U S A. 2003;100:44–49. doi: 10.1073/pnas.0137044100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvonic S., Lefevre M., Kilroy G., Floyd Z.E., DeLany J.P., Kheterpal I., Gravois A., Dow R., White A., Wu X.Y. Secretome of primary cultures of human adipose-derived stem cells - modulation of serpins by adipogenesis. Mol. Cell Proteomics. 2007;6:18–28. doi: 10.1074/mcp.M600217-MCP200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The accession numbers for the 3T3-L1 RNA-seq using MDI and CM is GEO: GSE144130. Expression levels of SERPINA3 in human adipose tissue, which is a human ortholog gene of SERPINA3C, were obtained from a public repository (GEO: GDS5056 and GDS 1480). Expression levels of SERPINA3 were compared among groups by Student’s t tests using R version 3.6.0 software (R Development Core Team, Vienna, Austria).