Abstract
Purpose:
Triple-negative breast cancer is characterized by fast progression with high possible for metastasis and poor survival. Dysfunction of microRNAs plays an important role in the initiation and progression of cancer. Our previous microRNA-seq data indicated the downregulation of miR-331-3p in triple-negative breast cancer tissues compared with that of the noncancer tissues. However, the function of miR-331-3p in triple-negative breast cancer remains largely unknown. Herein, the involvement of miR-331-3p in triple-negative breast cancer was investigated and the therapeutic potential of miR-331-3p was also explored.
Methods:
Real-time quantitative polymerase chain reaction was performed to detect the expression of miR-331-3p in triple-negative breast cancer tissues and cell lines. The cell proliferation was determined by the cell counting kit-8 assay. Apoptosis of triple-negative breast cancer cells was examined by annexin V/propidium iodide staining. miRDB database was used to predict the potential targets of miR-331-3p. Western blot was performed to examine the expression of the target protein.
Results:
miR-331-3p was significantly downregulated in triple-negative breast cancer tissues and cell line. Lower miR-331-3p expression was significantly correlated with the tumor size, TNM stage, and lymph node metastasis of patients with triple-negative breast cancer. Functional experiments showed that the overexpression of miR-331-3p inhibited the proliferation and increased apoptosis of triple-negative breast cancer cells. Neuropilin-2 was identified as a target of miR-331-3p, which harbored binding site of miR-331-3p in its 3′-untranslated region. Overexpression of miR-331-3p decreased the messenger RNA and protein levels of neuropilin-2 in triple-negative breast cancer cells. Restoration of neuropilin-2 partially reversed the inhibitory effects of miR-331-3p on the proliferation of triple-negative breast cancer cells.
Conclusions:
Our results demonstrated the novel function of miR-331-3p/neuropilin-2 signaling in regulating the malignant behaviors of triple-negative breast cancer cells, which suggested miR-331-3p as a potential target for the treatment of triple-negative breast cancer.
Keywords: miR-331-3p, TNBC, NRP2
Introduction
Breast cancer has been considered as the most common malignancy and is a major cause of cancer-related mortality among females worldwide.1-3 Attributed to the progress in the early diagnosis and follow-up on effective treatments, the prognosis of patients with breast cancer has been greatly improved. However, the incidence and mortality rate of breast cancer is still increasing in developing countries.4 Triple-negative breast cancer (TNBC) is a subtype of breast cancer accounting for 15% of breast cancer cases. Triple-negative breast cancer is characterized by the lack of estrogen receptor, progesterone receptor, and low expression of human epidermal growth factor receptor 2.5,6 Despite high sensitivity toward chemotherapy, TNBC shows more aggressive clinical behaviors and poorer prognosis than other subtypes of breast cancer. Thus, it is urgent to explore the molecular mechanisms underlying the progression of TNBC.
MicroRNAs (miRNAs) are characterized as small, single-stranded noncoding RNAs with the length of 19 to 22 nucleotides.7,8 Increasing evidence has indicated that miRNAs negatively regulated gene expression via binding to the 3′-untranslated region (UTR) of target messenger RNAs (mRNAs), leading to the mRNA degradation or translation inhibition.9 Dysfunction of miRNAs has been found to be associated with the initiation and progression of TNBC.10,11 For example, miR-589 served as a tumor suppressor in TNBC by targeting the metastasis-associated protein 2.12 Recent study showed that the overexpression of miR-29b-3p promoted the progression of TNBC via downregulating TNF Receptor Associated Factor 3 (TRAF3) and activating nuclear factor-kappa B signaling.13 Interestingly, an increasing body of evidence demonstrated the tumor suppressive potential of miR-331-3p in multiple cancers.14-17 Highly expressed miR-331-3p suppressed the epithelial–mesenchymal transition in non-small cell lung cancer.18 The serum expression of miR-331-3p was significantly decreased in patients with esophageal adenocarcinoma with recurrence compared to those without.19 The tumor suppressive capacity of miR-331-3p was also demonstrated in colorectal cancer, where overexpressed miR-331-3p inhibited the cell proliferation and accelerated apoptosis.16 These results demonstrated the antitumor effects of miR-331-3p in the development of cancers; however, the expression and biological function of miR-331-3p in TNBC has not yet been studied.
Neuropilin-2 (NRP2) is a member of the neuropilin family of receptor proteins that modulates various cellular physiological conditions including angiogenesis, migration, and proliferation.20,21 The overexpression of NRP2 has been found in cancers and serves as an important prognostic marker for a worse clinical outcome in patients with prostate cancer.22-24 Downregulation of NRP2 inhibits cancer progression and might benefit the outcome of patients with cancer.
In this study, we found that the expression of miR-331-3p was decreased in TNBC tissues. Overexpression of miR-331-3p suppressed the proliferation and induced apoptosis of TNBC cells. Further mechanism study identified NRP2 as a target of miR-331-3p, which was negatively regulated by miR-331-3p. Our findings demonstrated the novel function of miR-331-3p/NRP2 axis in regulating the malignant behaviors of TNBC cells, indicating the potential of miR-331-3p in the treatment of TNBC.
Materials and Methods
Tissue Samples
Paired TNBC tissues and corresponding adjacent normal tissues were collected from 50 patients who underwent surgical resection. These patients did not receive chemotherapy or radiotherapy before surgery. Tissues were stored at liquid nitrogen before further experiments. Written informed consents were received from all patients.
Cell Culture and Transfection
Human TNBC cell lines including MDA-MB-231, BT-549, MDA-MB-468, and HCC1937, and human normal breast epithelial cell MCF-10A were obtained from the Chinese Academy of Sciences (Shanghai, China). All cells were cultured in Dulbecco Modified Eagle Medium (Gibco, Grand Island, New York) containing 10% fetal bovine serum (Gibco) with 1% of penicillin–streptomycin (Sigma-Aldrich, St Louis, Missouri). Cells were maintained in a humidified incubator containing 5% CO2 at 37°C.
The miR-331-3p mimics and miRNA-control were purchased from GeneCopoeia (Guangzhou, China). Cell transfection was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, California) according to the manufacturer’s instructions. After transfection for 48 hours, cells were harvested for further analysis.
Real-Time Quantitative Polymerase Chain Reaction
Total RNA was extracted from tissues or cells using Trizol reagent (Invitrogen) following the manufacturer’s protocol. The RNA concentration was measured using the NanoDrop 2000 (NanoDrop Technologies; Thermo Fisher Scientific, Inc, Carlsbad, CA, USA). RNA was converted into complementary DNA (cDNA) using the miRNA cDNA Synthesis Kit (Tiangen, Beijing, China) according to the guidelines. The level of miR-331-3p was determined using the miRNA qPCR detection kit (CoWin Biosciences Co, Ltd, Beijing, China) on the Light Cycler 96 System (Roche Applied Science, Shanghai, China). The polymerase chain reactions were performed as follows: 95°C for 30 seconds followed by 40 cycles of 95°C for 10 seconds and 60°C for 30 seconds. The relative expression of miR-331-3p was normalized to that of U6 RNA and calculated using the 2−ΔΔCq method.
Western Blot
Cells were washed twice with precold phosphate-buffered saline (PBS) and lysed in Radioimmunoprecipitation assay buffer (RIPA) buffer (Beyotime, Shanghai, China) on ice for 15 minutes. The protein concentration was quantified with the bicinchoninic acid assay method (Beyotime). Proteins were separated by 15% of sodium dodecyl sulphate-polyacrylamide gel electrophoresis and subsequently transferred to the polyvinylidene difluoride membrane (Merck KGaA, Darmstadt, Germany). The membrane was blocked with 5% nonfat milk followed by incubating with primary antibody against NRP2 (ab185710; Abcam) or glyceraldehyde 3-phosphate dehydrogenase (ab181602; Abcam, Shanghai, China) at 4°C overnight. After washing twice with Tris-buffered Saline+Tween 20 (TBST), the membrane was incubated with horseradish peroxidase–conjugated secondary antibody for 1 hour at room temperature. The signals were visualized with the enhanced chemiluminescence reagent kit (Beyotime) according to the manufacturer’s instructions.
Cell Counting Kit-8 Assay
Triple-negative breast cancer cells transfected with miR-331-3p mimics or negative control miRNA were plated into 96-well plate with the density of 2000 cells per well. The CCK-8 solution (Solarbio Science & Technology Co, Ltd, Beijing, China) was added into the medium at the indicated time points and incubated for additional 4 hours at 37°C with 5% CO2. The absorbance of each well at 450 nm was determined with the microplate reader (Bio-Rad, Hercules, California).
Cell Apoptosis
The apoptosis of TNBC cells was determined using the Annexin V-Fluorescein Isothiocyanate (FITC) apoptosis detection kit (BioLegend, San Diego, California). Briefly, cells transfected with miR-331-3p mimics or control miRNA were collected and washed twice with precold PBS. Cells were then resuspended in binding buffer and stained with Annexin V-FITC and propidium iodide for 15 minutes in the dark. The apoptosis ratio was analyzed with the flow cytometer (FACScan; BD Biosciences, Franklin Lakes, New Jersey).
Luciferase Reporter Assay
The fragments of NRP2 3′-UTR containing the wild-type or mutated binding sites of miR-331-3p were amplified and inserted into the pmiR-RB-Report luciferase vector (Promega Corporation, Madison, Wisconsin). Cells were cotransfected with miR-331-3p mimics or control miRNA with wild-type or mutant pmiR-NRP2-3′-UTR. After transfection for 48 hours, cells were harvested and the luciferase activity was determined with the Dual-Luciferase reporter system (Promega Corporation) according to the manufacturer’s instructions. The luciferase activity of Renilla was detected for the normalization.
Statistical Analysis
Results were presented as mean ± standard deviation. The statistical analysis was determined with the GraphPad Prism 7.0 software (San Diego, CA, USA). The comparison between groups was analyzed by 2-tailed Student t test or 1-way analysis of variance followed by Tukey post hoc test. The analysis for expression of miR-331-3p or NRP2 in TNBC tissues and adjacent normal tissues was determined by the paired t test. The association between the level of miR-331-3p and the clinical features of patients with TNBC was determined using the χ2 test. The correlation between the expression of miR-331-3p and NRP2 was detected with Spearman correlation test. P < .05 was considered as statistically significant.
Results
miR-331-3p Was Downregulated in TNBC Tissues and Cell Lines
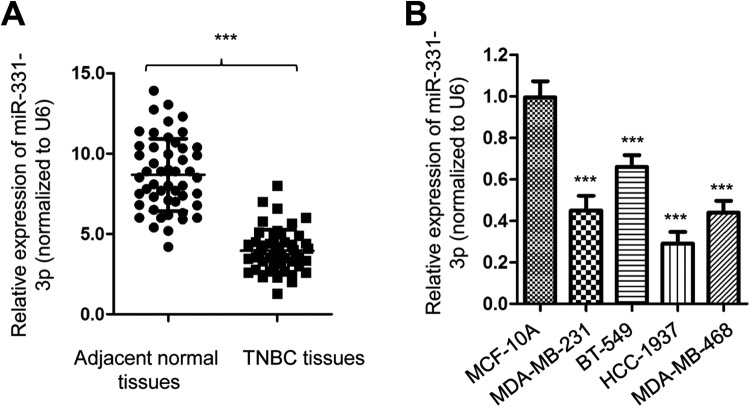
To investigate the role of miR-331-3p in TNBC, the expression level of miR-331-3p was analyzed in 50 paired TNBC tissues and adjacent normal tissues by real-time quantitative polymerase chain reaction (RT-qPCR). The result showed that the level of miR-331-3p was significantly lower in TNBC tissues than that of the nontumor tissues (Figure 1A). Additionally, the expression of miR-331-3p in TNBC cell lines including MDA-MB-231, BT-549, MDA-MB-468, and HCC1937 was also obviously downregulated compared with that of the normal breast epithelial MCF-10A cells (Figure 1B). These results indicated the downregulation of miR-331-3p in TNBC.
Figure 1.
miR-331-3p was decreased in TNBC. A, Relative miR-331-3p expressions measured by RT-qPCR in TNBC tissues and paired adjacent normal tissues. B, Analysis of miR-331-3p expression level in TNBC cells compared with MCF-10A cells. RT-qPCR indicates real-time quantitative polymerase chain reaction; TNBC, triple-negative breast cancer.
To further determine the clinical meaning of miR-331-3p underexpression in TNBC, these 50 patients with TNBC enrolled in this study were divided into low-miR-331-3p and high-miR-331-3p expression group based on the median value of miR-331-3p expression. The data showed that low miR-331-3p expression was significantly correlated with the tumor size, TNM stage, and lymph node metastasis of patients with TNBC (Table 1). These results demonstrated the downregulation of miR-331-3p might play a role in the malignancy of TNBC.
Table 1.
Association Between the Level of miR-331-3p and Clinicopathological Characteristics of Patients With TNBC.
| Clinical Features | Total | MiR-331-3p Expression | P Value | |
|---|---|---|---|---|
| Low | High | |||
| Age, years | .445 | |||
| <45 | 18 | 12 | 6 | |
| ≥45 | 32 | 20 | 12 | |
| Tumor size (cm) | .022 | |||
| ≥4 | 15 | 12 | 3 | |
| <4 | 35 | 20 | 15 | |
| TNM stage | .015 | |||
| I-II | 25 | 13 | 12 | |
| III-IV | 25 | 19 | 6 | |
| Lymph node metastasis | .005 | |||
| Positive | 34 | 24 | 10 | |
| Negative | 16 | 8 | 8 | |
Abbreviation: TNBC, triple-negative breast cancer.
Overexpression of miR-331-3p Inhibited the Proliferation of TNBC Cells
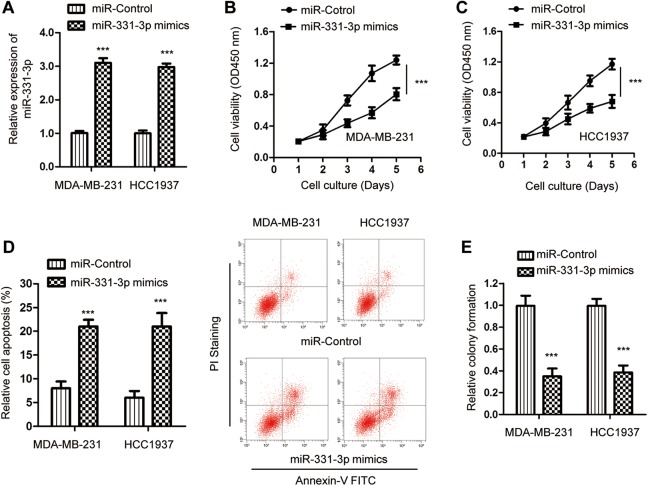
To determine the function of miR-331-3p in the development of TNBC, miR-331-3p was overexpressed by transfecting miR-331-3p mimics into both MDA-MB-231 and HCC1937 cells. The overexpression of miR-331-3p was confirmed by RT-qPCR assay (Figure 2A). The influence of miR-331-3p on the proliferation of TNBC cells was evaluated by the CCK-8 assay. Overexpression of miR-331-3p significantly decreased the proliferation of both MDA-MB-231 and HCC1937 cells compared with the cells expressing negative control miRNA (Figure 2B and C). To detect whether the growth defects of TNBC cells with miR-331-3p was associated with cell apoptosis, the apoptotic rate of cells with or without overexpressed miR-331-3p was assessed by fluorescence-activated cell sorting analysis. The result indicated that transfection of miR-331-3p markedly increased the apoptosis percentage of both MDA-MB-231 and HCC1937 cells (Figure 2D). Meanwhile, the soft agar colony formation was also performed with TNBC cells expressing miR-331-3p mimics or miR-control. As indicated in Figure 2E, the overexpression of miR-331-3p significantly decreased the number of colonies, confirming the proliferation-repressing function of miR-331-3p. Collectively, these data suggested the tumor suppressive role of miR-331-3p in modulating the growth of TNBC cells.
Figure 2.
Overexpression of miR-331-3p inhibited the growth of TNBC cells. A, Both MDA-MB-231 and HCC-1937 cells were transfected with miR-331-3p mimics or miR-control. The expression of miR-331-3p was detected by RT-qPCR. B and C, CCK-8 assay was applied to determine the activity of TNBC cells after transfection of miR-331-3p. D, Overexpression of miR-331-3p increased the apoptosis of TNBC cells. E, Transfection of miR-331-3p inhibited the colony formation of MDA-MB-231 and HCC-1937 cells. CCK-8 indicates cell counting kit-8; RT-qPCR, real-time quantitative polymerase chain reaction; TNBC, triple-negative breast cancer.
Neuropilin-2 Was a Target of miR-331-3p in TNBC Cells
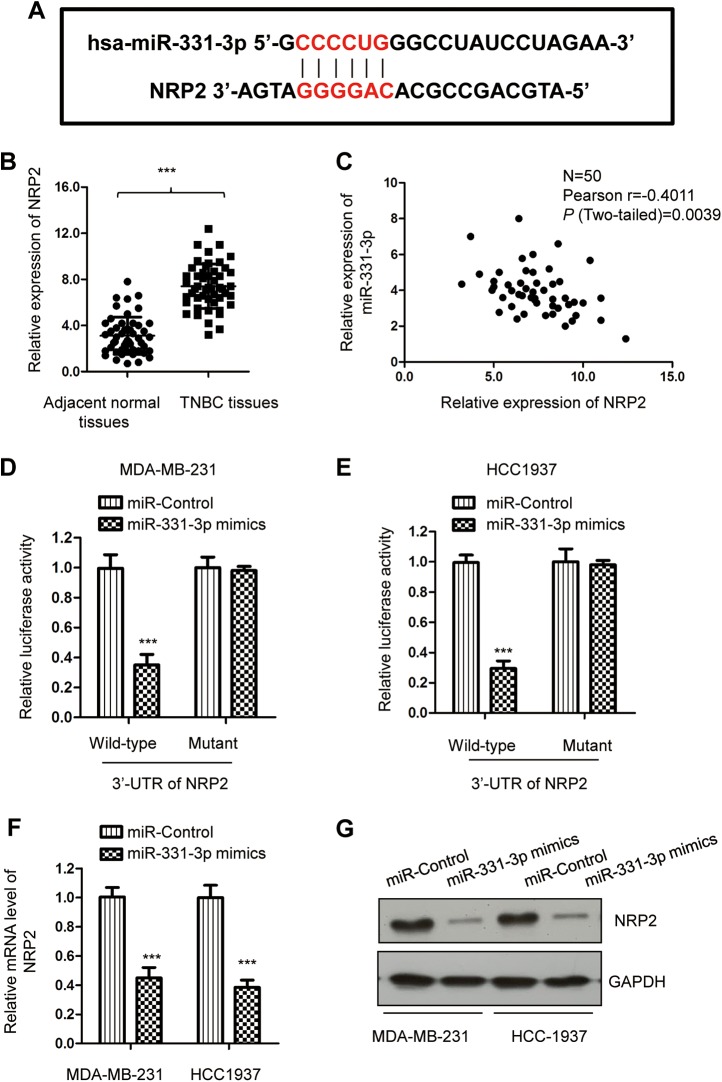
To explore the underlying mechanism by which miR-331-3p regulated TNBC cell proliferation, the potential targets of miR-331-3p were predicted using the bioinformatics tools (http://www.mirdb.org/). Notably, complementary binding sites of miR-331-3p was found in the 3′-UTR of NRP2 (Figure 3A). To further investigate the relationship between miR-331-3p and NRP2, the expression of NRP2 in TNBC tissues and matched adjacent normal tissues was determined by RT-qPCR. As indicated in Figure 3B, the mRNA level of NRP2 was significantly increased in TNBC tissues in comparison with the noncancerous tissues. Furthermore, the correlation between the expression of NRP2 and miR-331-3p was also analyzed using the Spearman correlation test. The data showed that the level of NRP2 was inversely correlated with that of miR-331-3p in TNBC tissues (Figure 3C).
Figure 3.
miR-331-3p targeted the 3′-UTR of NRP2 and inhibited the expression of NRP2. A, The predicted binding sequences for miR-331-3p within the 3′-UTR of NRP2. Seed sequences are illustrated. B, RT-qPCR result showed the mRNA level of NRP2 was significantly upregulated in TNBC tissues compared to the matched adjacent nontumorous tissues. C, Linear correlation analysis between NRP2 and miR-331-3p expression in TNBC tissues using Spearman correlation analysis. D and E, Overexpression of miR-331-3p markedly decreased the luciferase activity in cells carrying WT 3′-UTR of NRP2 mRNA, while the mutated 3′-UTR of NRP2 was insensitive to miR-331-3p transfection. F and G, The mRNA and protein expression of NRP2 after introducing of miR-331-3p was measured by RT-qPCR and Western blot analysis, respectively. mRNA indicates messenger RNA; NRP2, neuropilin-2; RT-qPCR, real-time quantitative polymerase chain reaction; TNBC, triple-negative breast cancer; UTR, untranslated region; WT, wild-type.
To validate whether NRP2 was a target of miR-331-3p in TNBC, luciferase report assay was performed by transfecting luciferase vector carrying wild-type or mutated 3′-UTR of NRP2 and miR-331-3p mimics. The results demonstrated that overexpression of miR-331-3p significantly decreased the luciferase activity of wild-type but not mutated 3′-UTR of NRP2 (Figure 3D and E). A similar result was also obtained in HEK293 cells (Supplementary Figure 1A). To determine whether the interaction of miR-331-3p with the 3′-UTR of NRP2 affected the mRNA stability of NRP2, RT-qPCR assay was performed with MDA-MB-231 and HCC1937 cells expressing miR-331-3p mimics. As presented in Figure 3F, the overexpression of miR-331-3p obviously reduced the mRNA level of NRP2 in TNBC cells. Consistently, the protein level of NRP2 was also decreased with the transfection of miR-331-3p compared with the control cells (Figure 3G). Taken together, these results suggested that NRP2 was directly targeted and suppressed by miR-331-3p in TNBC cells.
Restoration of NRP2 Reversed the Suppressive Role of miR-331-3p in TNBC
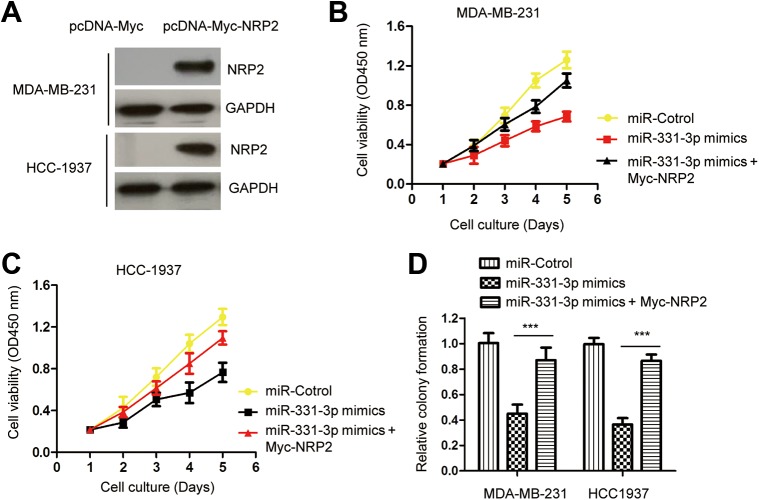
To further determine the functional significance of NRP2 in miR-331-3p-induced phenotype, NRP2 was overexpressed by transfecting pcDNA-Myc-NRP2 into MDA-MB-231 and HCC1937 cells. The expression level of Myc-tagged NRP2 was examined by Western blot with anti-Myc antibody (Figure 4A). The CCK-8 assay revealed that restoration of NRP2 significantly abrogated the inhibitory effect of miR-331-3p on the proliferation of TNBC cells (Figure 4B and C). This finding demonstrated that reconstitution of NRP2 rescued miR-331-3p-mediated inhibition in TNBC cells. To further support this conclusion, colony formation assay was performed by cotransfecting miR-331-3p mimics and Myc-NRP2. As indicated in Figure 4D, the overexpression of miR-331-3p reduced the colony formation of TNBC cells, while reintroducing of NRP2 significantly restored miR-331-3p-medited growth repression of TNBC cells. These results indicated that miR-331-3p inhibited the malignant behaviors of TNBC cells partially via targeting NRP2.
Figure 4.
Reconstitution of NRP2 rescues the miR-331-3p-mediated inhibition of TNBC cells. A, NRP2 expression of TNBC cells with the transfection of Myc-NRP2. B and C, Restoration of NRP2 significantly abrogated the suppressive role of miR-331-3p on the proliferation of TNBC cells. D, Overexpression of NRP2 promoted the colony formation of MDA-MB-231 and HCC1937 cells that carrying miR-331-3p mimics. NRP2 indicates neuropilin-2; TNBC, triple-negative breast cancer.
Discussion
Increasing evidence has identified the key function of miRNAs in the malignancy of cancers by acting as tumor suppressors or oncogenes.25-28 The role of miR-331-3p in tumor development has recently drawn wide attention. It was reported that miR-331-3p suppressed the proliferation of colorectal cancer cells via targeting the HER2 through the PI3K/Akt and Extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) pathways.16 In gastric cancer, miR-331-3p directly targeted E2F1 and induced cell growth defects.29 miR-331-3p was found to attenuate the epithelial–mesenchymal transition by regulating ErbB2 and VAV2 in non-small cell lung cancer.18 These findings suggested the tumor suppressive role of miR-331-3p in the development of cancers. However, little is known about the biological function and molecular mechanism of miR-331-3p in TNBC.
In this study, our results showed the decreased expression of miR-331-3p in TNBC tissues, which was significantly correlated with the advanced progression of patients with TNBC. These findings indicated that the dysfunction of miR-331-3p might play a role in the development of TNBC. Further study is necessary to clarify the underlying mechanism by which miR-331-3p was underexpressed in TNBC. A recent study found that the serum miR-331-3p was associated with the postoperative survival of patients with hepatocellular carcinoma (HCC) and can be regarded as an independent prognostic factor for the patients with HCC.30 To evaluate the clinical significance of miR-331-3p in TNBC, the expression of miR-331-3p with the 5-year overall survival rate of patients with TNBC is an interesting question to be answered. Notably, different from what we obtained in the present study, previous studies also demonstrated the oncogenic function of miR-331-3p in the progression of cancers.31 For example, miR-331-3p was a tumor-promoting miRNA in prostate cancer and a promising biomarker of prostate cancer.32 It was also found that miR-331-3p facilitated the proliferation and metastasis of HCC by targeting PH domain and leucine-rich repeat protein phosphatase.33 These results indicated the tumor-promoting function of miR-331-3p in these cancers. However, in this study, the overexpression of miR-331-3p inhibited the proliferation and induced apoptosis of TNBC cells, suggesting the tumor suppressive role of miR-331-3p in TNBC. In addition to what we have obtained in this study, the inhibitory function of miR-331-3p in the progression of TNBC needs to be confirmed by in vivo study. Considering the role of miR-331-3p in different cancers, the tumor suppressive or oncogenic function of miR-331-3p might be associated with the cancer type.
Neuropilins play a major role in signal transduction due to their ability to interact with multiple tyrosine kinase–associated receptors.34 Neuropilin-2 was found as a coreceptor for Vascular endothelial growth factor-C (VEGF-C) and D and interacted with Ligand-induced vascular endothelial growth factor receptor-3 (VEGFR-3).35-39 Overexpression of NRP2 was primarily associated with increase angiogenesis and tumor cell survival.36,40-42 Therefore, blocking the activity of NRP2 is a promising strategy to inhibit the growth of cancer cells. Neuropilin-2 has been verified as a direct target of miRNAs in human cancers. miR-486-5p inhibited the tumor growth and lymphangiogenesis of colorectal cancer by targeting NRP2.43 It was also showed that miR-15b and miR-152 suppressed the invasion and angiogenesis of glioma cells via modulating the expression of NRP2.44 Additionally, NRP2 was also targeted by miR-1247 and mediated the suppressive role of miR-1247 in pancreatic cancer.45 In this study, our results identified NRP2 as a target of miR-331-3p and negatively regulated by miR-331-3p. To further demonstrate the functional mechanism of NRP2 in TNBC, the effects on VEGF signaling, the downstream target of NRP2, by miR-331-3p encourage future study. Overexpression of NRP2 was found in TNBC tissues, which was, significantly, negatively correlated with that of miR-331-3p. To provide more evidence about the significance of NRP2 in TNBC, the correlation between the expression of NRP2 with the clinical features as well as the prognosis of patients with TNBC needs further investigation. Our results demonstrated the novel mechanism of miR-331-3p/NRP2 axis in the progression of TNBC. Consistently, previous data also indicated the negative regulation of NRP2 by miR-331-3p in cervical cancer and glioblastoma, suggesting the general function of miR-331-3p/NRP2 signaling in the development of cancers.
In conclusions, our results revealed the downregulation of miR-331-3p in TNBC that was associated with the advanced progression of patients with TNBC. miR-331-3p inhibited the malignancy of TNBC at least via targeting NRP2. Therefore, miR-331-3p might be a promising therapeutic target for TNBC.
Supplemental Material
Supplementary_Figure_1 for miR-331-3p Suppresses Cell Proliferation in TNBC Cells by Downregulating NRP2 by Mingchuan Zhao, Mengmeng Zhang, Zhonghua Tao, Jun Cao, Leiping Wang and Xichun Hu in Technology in Cancer Research & Treatment
Acknowledgments
The authors thank the helpful suggestions from the colleagues.
Abbreviations
- cDNA
complementary DNA
- CCK-8
cell counting kit-8
- FITC
fluorescein isothiocyanate
- mRNA
messenger RNA
- miRNA
microRNA
- NRP2
neuropilin-2
- PCR
polymerase chain reaction
- PBS
phosphate-buffered saline
- RT-qPCR
real-time quantitative polymerase chain reaction
- TNBC
triple-negative breast cancer
- UTR
untranslated region.
Authors’ Note: MZ and MZ contributed equally to this work. The cancer tissues collection was approved by the Ethics Committee of Fudan University School of Medicine (FDU2017010114).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Science and Technology Major Project (2020ZX09201-013).
ORCID iD: Xichun Hu https://orcid.org/0000-0002-3488-6394
https://orcid.org/0000-0002-3488-6394
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Anastasiadi Z, Lianos GD, Ignatiadou E, Harissis HV, Mitsis M. Breast cancer in young women: an overview. Updates Surg. 2017;69(3):313–317. [DOI] [PubMed] [Google Scholar]
- 2. Ribnikar D, Ratosa I, Perhavec A, Amir E. General overview and treatment recommendations for young women with breast cancer. Rev Invest Clin. 2017;69(2):77–93. [DOI] [PubMed] [Google Scholar]
- 3. Libson S, Lippman M. A review of clinical aspects of breast cancer. Int Rev Psychiatry. 2014;26(1):4–15. [DOI] [PubMed] [Google Scholar]
- 4. Peart O. Breast intervention and breast cancer treatment options. Radiol Technol. 2015;86(5):535M–558M; quiz 559-562. [PubMed] [Google Scholar]
- 5. Andreopoulou E, Schweber SJ, Sparano JA, McDaid HM. Therapies for triple negative breast cancer. Expert Opin Pharmacother. 2015;16(7):983–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Le Du F, Ueno NT. Targeted therapies in triple-negative breast cancer: failure and future. Womens Health. 2015;11(1):1–5. [DOI] [PubMed] [Google Scholar]
- 7. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. [DOI] [PubMed] [Google Scholar]
- 8. Mohr AM, Mott JL. Overview of microRNA biology. Semin Liver Dis. 2015;35(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. [DOI] [PubMed] [Google Scholar]
- 10. Piasecka D, Braun M, Kordek R, Sadej R, Romanska H. MicroRNAs in regulation of triple-negative breast cancer progression. J Cancer Res Clin Oncol. 2018;144(8):1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nagini S. Breast cancer: current molecular therapeutic targets and new players. Anticancer Agents Med Chem. 2017;17(2):152–163. [DOI] [PubMed] [Google Scholar]
- 12. Chu J. MicroRNA-589 serves as a tumor suppressor microRNA through directly targeting metastasis-associated protein 2 in breast cancer. Oncol Lett. 2019;18(3):2232–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang B, Shetti D, Fan C, Wei K. miR-29b-3p promotes progression of MDA-MB-231 triple-negative breast cancer cells through downregulating TRAF3. Biol Res. 2019;52(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Epis MR, Giles KM, Barker A, Kendrick TS, Leedman PJ. miR-331-3p regulates ERBB-2 expression and androgen receptor signaling in prostate cancer. J Biol Chem. 2009;284(37):24696–24704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu XH, Sun M, Nie FQ, et al. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer. 2014;13:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao D, Sui Y, Zheng X. MiR-331-3p inhibits proliferation and promotes apoptosis by targeting HER2 through the PI3K/Akt and ERK1/2 pathways in colorectal cancer. Oncol Rep. 2016;35(2):1075–1082. [DOI] [PubMed] [Google Scholar]
- 17. Epis MR, Giles KM, Beveridge DJ, et al. miR-331-3p and Aurora Kinase inhibitor II co-treatment suppresses prostate cancer tumorigenesis and progression. Oncotarget. 2017;8(33):55116–55134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li X, Zhu J, Liu Y, Duan C, Chang R, Zhang C. MicroRNA-331-3p inhibits epithelial-mesenchymal transition by targeting ErbB2 and VAV2 through the Rac1/PAK1/beta-catenin axis in non-small-cell lung cancer. Cancer Sci. 2019;110(6):1883–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gu J, Zhang J, Zheng L, Ajani JA, Wu X, Ye Y. Serum miR-331-3p predicts tumor recurrence in esophageal adenocarcinoma. Sci Rep. 2018;8(1):14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sulpice E, Plouet J, Berge M, Allanic D, Tobelem G, Merkulova-Rainon T. Neuropilin-1 and neuropilin-2 act as coreceptors, potentiating proangiogenic activity. Blood. 2008;111(4):2036–2045. [DOI] [PubMed] [Google Scholar]
- 21. Schellenburg S, Schulz A, Poitz DM, Muders MH. Role of neuropilin-2 in the immune system. Mol Immunol. 2017;90:239–244. [DOI] [PubMed] [Google Scholar]
- 22. Cohen T, Herzog Y, Brodzky A, et al. Neuropilin-2 is a novel marker expressed in pancreatic islet cells and endocrine pancreatic tumours. J Pathol. 2002;198(1):77–82. [DOI] [PubMed] [Google Scholar]
- 23. Narazaki M, Segarra M, Tosato G. Neuropilin-2: a new molecular target for antiangiogenic and antitumor strategies. J Natl Cancer Inst. 2008;100(2):81–83. [DOI] [PubMed] [Google Scholar]
- 24. Jubb AM, Sa SM, Ratti N, et al. Neuropilin-2 expression in cancer. Histopathology. 2012;61(3):340–349. [DOI] [PubMed] [Google Scholar]
- 25. Kwak PB, Iwasaki S, Tomari Y. The microRNA pathway and cancer. Cancer Sci. 2010;101(11):2309–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Farazi TA, Spitzer JI, Morozov P, Tuschl T. miRNAs in human cancer. J Pathol. 2011;223(2):102–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Asadzadeh Z, Mansoori B, Mohammadi A, et al. microRNAs in cancer stem cells: biology, pathways, and therapeutic opportunities. J Cell Physiol. 2019;234(7):10002–10017. [DOI] [PubMed] [Google Scholar]
- 28. Hosseinahli N, Aghapour M, Duijf PHG, Baradaran B. Treating cancer with microRNA replacement therapy: a literature review. J Cell Physiol. 2018;233(8):5574–5588. [DOI] [PubMed] [Google Scholar]
- 29. Guo X, Guo L, Ji J, et al. miRNA-331-3p directly targets E2F1 and induces growth arrest in human gastric cancer. Biochem Biophys Res Commun. 2010;398(1):1–6. [DOI] [PubMed] [Google Scholar]
- 30. Chen L, Chu F, Cao Y, Shao J, Wang F. Serum miR-182 and miR-331-3p as diagnostic and prognostic markers in patients with hepatocellular carcinoma. Tumour Biol. 2015;36(10):7439–7447. [DOI] [PubMed] [Google Scholar]
- 31. Chen X, Luo H, Li X, et al. miR-331-3p functions as an oncogene by targeting ST7 L in pancreatic cancer. Carcinogenesis. 2018;39(8):1006–1015. [DOI] [PubMed] [Google Scholar]
- 32. Fujii T, Shimada K, Tatsumi Y, Tanaka N, Fujimoto K, Konishi N. Syndecan-1 up-regulates microRNA-331-3p and mediates epithelial-to-mesenchymal transition in prostate cancer. Mol Carcinog. 2016;55(9):1378–1386. [DOI] [PubMed] [Google Scholar]
- 33. Chang RM, Yang H, Fang F, Xu JF, Yang LY. MicroRNA-331-3p promotes proliferation and metastasis of hepatocellular carcinoma by targeting PH domain and leucine-rich repeat protein phosphatase. Hepatology. 2014;60(4):1251–1263. [DOI] [PubMed] [Google Scholar]
- 34. Peng K, Bai Y, Zhu Q, Hu B, Xu Y. Targeting VEGF-neuropilin interactions: a promising antitumor strategy. Drug Discov Today. 2019;24(2):656–664. [DOI] [PubMed] [Google Scholar]
- 35. Favier B, Alam A, Barron P, et al. Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes human endothelial cell survival and migration. Blood. 2006;108(4):1243–1250. [DOI] [PubMed] [Google Scholar]
- 36. Kim WH, Lee SH, Jung MH, et al. Neuropilin2 expressed in gastric cancer endothelial cells increases the proliferation and migration of endothelial cells in response to VEGF. Exp Cell Res. 2009;315(13):2154–2164. [DOI] [PubMed] [Google Scholar]
- 37. Saban MR, Sferra TJ, Davis CA, et al. Neuropilin-VEGF signaling pathway acts as a key modulator of vascular, lymphatic, and inflammatory cell responses of the bladder to intravesical BCG treatment. Am J Physiol Renal Physiol. 2010;299(6):F1245–1256. [DOI] [PubMed] [Google Scholar]
- 38. Xu Y, Yuan L, Mak J, et al. Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. J Cell Biol. 2010;188(1):115–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Djordjevic S, Driscoll PC. Targeting VEGF signalling via the neuropilin co-receptor. Drug Discov Today. 2013;18(9-10):447–455. [DOI] [PubMed] [Google Scholar]
- 40. Geretti E, Klagsbrun M. Neuropilins: novel targets for anti-angiogenesis therapies. Cell Adh Migr. 2007;1(2):56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gray MJ, Van Buren G, Dallas NA, et al. Therapeutic targeting of neuropilin-2 on colorectal carcinoma cells implanted in the murine liver. J Natl Cancer Inst. 2008;100(2):109–120. [DOI] [PubMed] [Google Scholar]
- 42. Yasuoka H, Kodama R, Tsujimoto M, et al. Neuropilin-2 expression in breast cancer: correlation with lymph node metastasis, poor prognosis, and regulation of CXCR4 expression. BMC Cancer. 2009;9:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu C, Li M, Hu Y, et al. miR-486-5p attenuates tumor growth and lymphangiogenesis by targeting neuropilin-2 in colorectal carcinoma. Onco Targets Ther. 2016;9:2865–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zheng X, Chopp M, Lu Y, Buller B, Jiang F. MiR-15b and miR-152 reduce glioma cell invasion and angiogenesis via NRP-2 and MMP-3. Cancer Lett. 2013;329(2):146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shi S, Lu Y, Qin Y, et al. miR-1247 is correlated with prognosis of pancreatic cancer and inhibits cell proliferation by targeting neuropilins. Curr Mol Med. 2014;14(3):316–327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_Figure_1 for miR-331-3p Suppresses Cell Proliferation in TNBC Cells by Downregulating NRP2 by Mingchuan Zhao, Mengmeng Zhang, Zhonghua Tao, Jun Cao, Leiping Wang and Xichun Hu in Technology in Cancer Research & Treatment