Abstract
Background:
Functional deficits and health-related impairments are common after an Achilles tendon rupture (ATR). Rehabilitation protocols vary greatly, and few studies have allowed loading in combination with ankle motion immediately after surgery (ie, early functional mobilization [EFM]). It is unclear whether EFM may counteract the negative impact of ankle immobilization after an ATR.
Purpose:
The primary aim of this study was to assess the efficacy of EFM compared with standard treatment (ie, 2 weeks of unloading in a plaster cast followed by 4 weeks of weightbearing in an orthosis) regarding patient-reported and functional outcomes in patients with an ATR after acute operative repair. The secondary aim was to explore whether the occurrence of deep venous thrombosis (DVT) during the 2 postoperative treatments affected outcomes.
Study Design:
Randomized controlled trial; Level of evidence, 1.
Methods:
A total of 135 patients who underwent ATR repair, randomized to either EFM, including immediate postoperative loading and ankle motion, or standard treatment, were evaluated with functional tests and 5 self-administered outcome questionnaires at 6 and 12 months postoperatively.
Results:
At 6 months, the EFM group scored higher on the RAND 36-Item Health Survey (RAND-36) questionnaire subscales of general health and vitality (P < .05) compared with the control group. No significant differences between the groups were found on disease-specific questionnaires (Achilles tendon Total Rupture Score [ATRS] and Foot and Ankle Outcome Score [FAOS]). At 12 months, no significant differences on any of the patient-reported outcome measures or the functional heel-rise test were seen between the groups. The RAND-36 subscale of general health, however, exhibited higher values in the EFM group (82.6 ± 16.9) than the control group (77.1 ± 17.0) (P = .051) at 12 months after the injury. Patients sustaining DVT postoperatively had lower self-reported outcomes on the ATRS, FAOS, and RAND-36 questionnaires at 6 and 12 months compared with patients not having sustained DVT (all P < .05).
Conclusion:
This study demonstrated that an accelerated postoperative protocol with immediate loading and ankle motion resulted in better general health and vitality at 6 months. However, there were no differences between the groups in the recovery of heel-rise function. Future studies should focus on the means to reduce the risk of DVT to improve patient outcomes after ATR.
Registration:
NCT02318472 (ClinicalTrials.gov identifier).
Keywords: Achilles tendon, rupture, surgical repair, early functional mobilization, patient-reported outcome measures, heel-rise test
Functional deficits, decreased strength, and health-related impairments after an Achilles tendon rupture (ATR) are common in patients treated both surgically and nonsurgically.5,8,25,33,35,36,43 Early functional mobilization (EFM) compared with cast immobilization is reported to enhance short- and long-term outcomes after an ATR.11,30 The deficits associated with an ATR and ankle immobilization may occur from excessive tendon elongation,24,41 loss of calf muscle function,22,32,44 or sustaining deep venous thrombosis (DVT) postoperatively.17,26 DVT has been demonstrated as a predictor of suboptimal functional outcomes at 1 year after ATR repair.2 It is, however, still unclear whether EFM may counteract the negative impact of ankle immobilization indirectly (eg, via the reduction of DVT) or directly via enhanced calf muscle function.
Functional mobilization protocols vary greatly, and few studies have allowed loading in combination with ankle motion immediately after surgery.12,28,39 Functional mobilization, aiming to minimize long-term calf muscle atrophy and strength deficits,11 should be performed early after an injury, which may be less of a concern when an ATR is surgically repaired.25,29,36,45 Whether immediate postoperative weightbearing affects the degree of tendon elongation in the short and long term has, however, not been established. In addition to weightbearing, increased ankle motion may exert beneficial effects on calf muscle activation7 and tendon recovery9,18,39,47 as well as minimize the incidence of DVT.42 Calf muscle activation compresses the veins in the calf and increases blood flow, which in turn may decrease the risk of DVT10,14,16,42,46 after an ATR. Utilizing a postoperative protocol that combines immediate weightbearing and ankle movement might further improve both the short- and long-term outcomes in patients after an ATR.
Therefore, in this randomized controlled trial, patients receiving EFM were instructed to bear weight as tolerated immediately postoperatively in an ankle dynamic orthosis that also allowed partial ankle joint motion. The control group received standard treatment including 2 weeks of immobilization in a below-knee plaster cast, which was followed by weightbearing in an ankle-stable orthosis.
The primary aim of this randomized controlled trial was to assess the efficacy of postoperative EFM compared with standard treatment with regard to the outcomes of disease-specific and general health questionnaires as well as functional tests in patients with an ATR. EFM during the initial 6 weeks was compared with standard treatment, which included 2 weeks in a plaster cast followed by 4 weeks of immobilization in an ankle-stable orthosis. The secondary aim was to explore whether the occurrence of DVT during the 2 postoperative treatments affected the 6- and 12-month patient-reported and functional outcomes after ATR.
Methods
Ethical approval was obtained from the regional ethical committee in Stockholm, Sweden. The study was registered on ClinicalTrials.gov (NCT02318472). All participants received oral and written information about the study procedure and provided written informed consent before surgery.
Patients
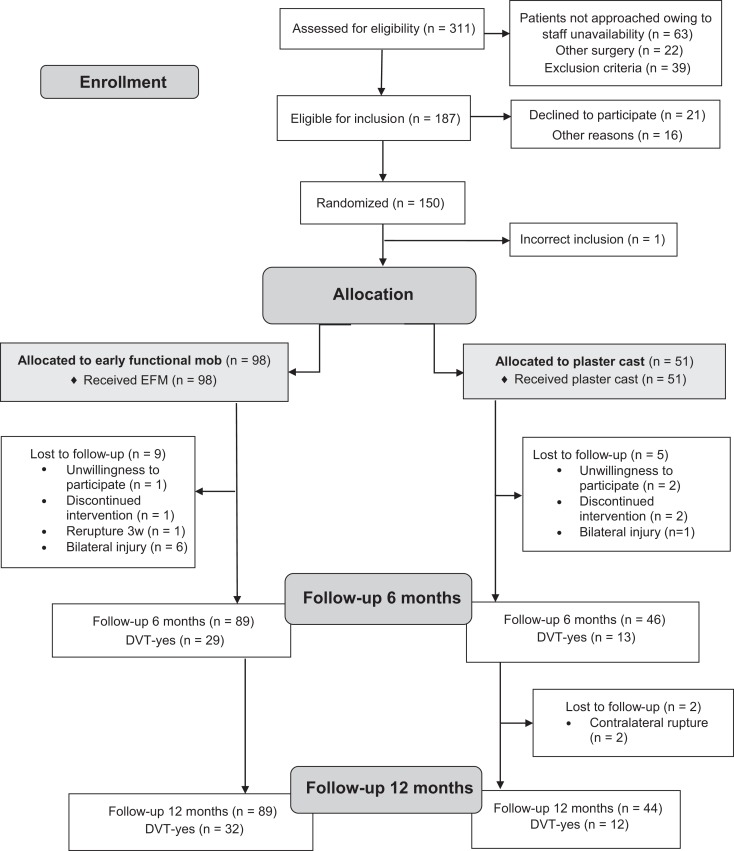
Between December 2013 and February 2018, there were 311 patients with an acute ATR who were screened for eligibility at Karolinska University Hospital, Södersjukhuset, and Danderyd Hospital in Stockholm. Of these, 150 patients (114 men, 36 women) were enrolled and randomized postoperatively. Randomization was achieved using consecutively numbered sealed envelopes produced by a biostatistician and opened after surgery (Figure 1). Enrollment and exclusion criteria are reported elsewhere.3
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) flowchart. DVT, deep venous thrombosis; EFM, early functional mobilization.
In the present study, those patients who attended the 6-month follow-up were included. A total of 135 patients, aged between 18 and 75 years, with an acute unilateral ATR surgically repaired within 1 week of injury met the criteria for this study (Figure 1).
Study Procedure
All surgeons were instructed to use the same standardized surgical technique: the modified Kessler suture technique.17 Directly after surgery, patients were randomized into 1 of 2 groups with a ratio of 2 (intervention) to 1 (control).3,47 The intervention group received EFM in a dynamic orthosis (VACOped; OPED) with adjustable range of motion of the ankle joint. The orthosis was initially set with a range of motion of 15° to 30° of plantarflexion, and patients could bear weight as tolerated. At 2 weeks, the range of motion was increased to 5° to 30° of plantarflexion for the following 4 weeks. Full weightbearing was allowed. The patients were encouraged to perform unloaded plantarflexion exercises immediately postoperatively (from neutral and free plantarflexion) for 1 hour per day without the orthosis.
Patients in the control group received standard treatment with immobilization in a below-knee plaster cast for 2 weeks and were nonweightbearing with crutches. After removal of the cast, at 2 weeks postoperatively, the patients received a stable orthosis (Aircast AirSelect Elite; DJO) fixed at the ankle joint. Full weightbearing was allowed. The orthosis was provided with 3 heel wedges that were gradually removed during the following 4 weeks. After the 2-week follow-up visit, both groups were instructed to perform unloaded plantarflexion exercises several times per day without the orthosis.
At 6 weeks, the orthosis was discontinued in both groups, and patients were advised to wear normal shoes with a heel lift on the injured side for another month. Both groups were given the same rehabilitation protocol, and patients were advised to contact a physical therapist in primary care for supervised rehabilitation.
Evaluations at Follow-up
Patient-Reported Outcomes
Questionnaires were sent home to the patients before the follow-up visit and were returned at the follow-up visits at 6 and 12 months. Patients filled out 5 self-administered questionnaires at 6 and 12 months postoperatively. The Achilles tendon Total Rupture Score (ATRS)34 consists of 10 items and is scored from 0 (“worst”) to 10 (“best”). A total score of 100 is computed. A low score indicates more symptoms and greater limitations in physical activity after treatment of ATR. The Physical Activity Scale19,38 is used to evaluate physical activity before and after an injury. It is scored from 1 (no physical activity) to 6 (heavy physical exercise several times per week). The RAND 36-Item Health Survey (RAND-36)20,21 is a health-related quality of life questionnaire that consists of 36 items on physical and psychosocial health. It is divided into 8 subscales and scored on a verbal rating scale with different scoring alternatives. A higher subscale score indicates better health status. The Foot and Ankle Outcome Score (FAOS)37 consists of 42 items evaluating foot- and ankle-related symptoms, pain, function in activities of daily living (ADL) and in sports and recreation (Sports/Rec), and quality of life in relation to foot problems (QoL). The questionnaire is divided into 5 subscales, and the score ranges from 0 (worst) to 100 (best). The Tampa Scale of Kinesiophobia, Swedish version,27 comprises 17 items and is scored on a 4-point Likert scale with alternatives ranging from 1 (strongly disagree) to 4 (strongly agree). A total sum of the 17 items is calculated, with scores ranging from 17 to 68. A high score (>37) is defined as kinesiophobia.27
Functional Outcomes
Functional evaluations were performed at the follow-up visits at 6 and 12 months at the physical therapy department. All tests were performed by 1 physical therapist (S.A.) not involved in the patients’ rehabilitation but not blinded to treatment regimens.
A calf muscle endurance heel-rise test was performed as described earlier.40 The Musclelab linear encoder (Ergotest) was used for data collection. The tests were performed in the same order, and the uninjured side was tested first. Standardized footwear was used. Patients warmed up on a stationary bike for 5 minutes, followed by 10 repetitions of 2-leg heel rises before testing. During the heel-rise test, verbal encouragement was given. The patient was instructed to go as high as possible on each heel rise with a straight knee. A metronome was used for a standardized frequency of 30 heel rises per minute. The test was terminated when the patient stopped or could not maintain frequency.
At the 12-month follow-up, 2 different jump tests were added. The first test was the vertical jump test as a measure of explosive strength in the lower extremity.15 Patients rated their perceived fear, pain, and discomfort when jumping from 0 to 10 on a visual analog scale. The other test was the side hop test in which the patient performed 10 jumps sideways on 1 leg for time.23 The uninjured leg was tested first. For the vertical jump test, the best of 3 attempts was counted, and for the side hop test, only 1 trial was performed. The jump tests were performed after the heel-rise test.
For the functional assessments, 120 patients were evaluated at the 6-month follow-up, at a mean time of 6.4 ± 0.7 months after surgery, and 123 patients were evaluated at the 12-month follow-up, at a mean time of 12.4 ± 0.5 months after surgery.
Assessment of DVT
All patients were screened for DVT in the injured leg using unilateral compression duplex ultrasound at 2 and 6 weeks postoperatively.3 A trained nurse or an experienced ultrasonographer, blinded to the treatment regimens, performed all the compression duplex ultrasound scans using a CX50 ultrasound machine (Philips).3
Statistical Analysis
This study was initially powered for the risk of DVT with EFM, and inclusion was performed with a 2:1 ratio.3 All descriptive statistics as well as the analyses were conducted in SPSS (Version 25.0; IBM). Descriptive data were reported as the mean, standard deviation, and frequency. The continuous variables (heel-rise test) were scrutinized for severely skewed distributions or outliers. However, no skewness was found, and thus, differences between the groups in these variables were analyzed with the parametric Student t test. The nonparametric Mann-Whitney U test was used to compare differences between the groups in the outcome questionnaires. A paired Student t test was used for comparisons of the heel-rise variables between the 6- and 12-month follow-up visits and the Wilcoxon signed-rank test for comparing paired ordinal data. Comparisons for categorical variables between the groups were performed with the Pearson chi-square test. A 2-way analysis of variance was conducted to examine whether there was an effect of treatment and the occurrence of DVT on variables from the heel-rise test and ATRS. The limb symmetry index (LSI) was used to compare the groups on the heel-rise test. The LSI was defined as the ratio between the injured and uninjured limbs, expressed as a percentage (LSI = injured/uninjured × 100). The level of significance was P ≤ .05 for all analyses.
Results
Most injuries were sports related, and the most common causes of injury were football (26%; 35/135) and badminton (16%; 22/135), while 7 injuries (5%) were not sports related. Moreover, demographic and clinical characteristics are presented in Table 1. There were no significant differences in patient characteristics between the groups (all P > .05).
Table 1.
Demographic and Clinical Characteristics of Participantsa
| EFM (n = 89) | Control (n = 46) | |
|---|---|---|
| Age, y | 39.4 ± 8.1 | 39.7 ± 8.0 |
| Male sex, n (%) | 67 (75) | 35 (76) |
| Body mass index, kg/m2 | 24.9 ± 2.6 | 25.0 ± 2.5 |
| Nicotine use, n (%) | 19 (21) | 6 (13) |
| Left side injured, n (%) | 46 (52) | 25 (54) |
| Time to surgery, d | 3.7 ± 1.7 | 3.6 ± 1.6 |
| Infection, n (%) | 1 (1) | 1 (2) |
| DVT, n (%) | 34 (39) | 14 (30) |
| PAS score before injury | 4.7 ± 1.0 | 4.6 ± 1.0 |
aValues are reported as mean ± SD unless otherwise specified. DVT, deep venous thrombosis; EFM, early functional mobilization; PAS, Physical Activity Scale.
Outcomes by Treatment Group
Patient-Reported Outcomes
At 6 months, the mean ATRS value was 65.1 ± 19.2 in the EFM group and 59.1 ± 21.4 in the control group (P = .134). Also at 6 months, the EFM group scored significantly higher on the RAND-36 subscales of general health (P = .012) and vitality (P = .022) compared with the control group. Moreover, the EFM group exhibited higher values on the RAND-36 subscale of role functioning/physical at 6 months, but the data were not statistically significant (P = .051) (Table 2). No other significant differences in patient-reported outcomes were seen at 6 months.
Table 2.
Patient-Reported Outcomes by Treatment Groupa
| 6 mo | 12 mo | P Valueb | ||||||
|---|---|---|---|---|---|---|---|---|
| EFM | Control | P Value | EFM | Control | P Value | EFM | Control | |
| ATRS | 65.1 ± 19.2 (n = 77) | 59.1 ± 21.4 (n = 40) | .134 | 80.3 ± 15.9 (n = 76) | 81.1 ± 15.7 (n = 41) | .846 | <.001 | <.001 |
| PAS | 4.0 ± 1.1 (n = 73) | 3.9 ± 1.1 (n = 40) | .618 | 4.2 ± 1.1 (n = 75) | 4.3 ± 1.1 (n = 40) | .598 | .195 | .036 |
| FAOS pain | 88.3 ± 11.8 (n = 77) | 86.6 ± 12.7 (n = 40) | .498 | 93.2 ± 9.5 (n = 76) | 94.5 ± 8.1 (n = 41) | .594 | <.001 | .002 |
| FAOS symptoms | 81.2 ± 15.1 | 77.3 ± 17.1 | .245 | 87.3 ± 13.1 | 85.1 ± 15.8 | .576 | .003 | .009 |
| FAOS ADL | 93.8 ± 8.8 | 93.0 ± 6.9 | .191 | 96.7 ± 6.4 | 97.1 ± 5.5 | .840 | <.001 | <.001 |
| FAOS Sports/Rec | 69.9 ± 20.9 | 64.9 ± 19.4 | .104 | 81.8 ± 16.3 | 81.6 ± 15.3 | .777 | <.001 | <.001 |
| FAOS QoL | 60.3 ± 17.1 | 56.6 ± 17.7 | .294 | 72.8 ± 18.3 | 72.6 ± 15.8 | .776 | <.001 | <.001 |
| RAND-36 PF | 84.0 ± 13.3 (n = 77) | 83.0 ± 12.4 (n = 40) | .440 | 90.4 ± 12.5 (n = 75) | 90.6 ± 11.2 (n = 41) | .816 | <.001 | .001 |
| RAND-36 RP | 78.6 ± 33.4 | 65.6 ± 37.0 | .051 | 88.3 ± 26.7 | 89.6 ± 25.0 | .639 | .073 | .001 |
| RAND-36 BP | 84.6 ± 16.4 (n = 76) | 82.9 ± 17.5 (n = 39) | .642 | 90.4 ± 16.6 | 92.5 ± 9.0 | .865 | .012 | .003 |
| RAND-36 GH | 83.2 ± 14.1 | 74.8 ± 18.2 | .012 | 82.6 ± 16.9 | 77.1 ± 17.0 | .051 | .485 | .568 |
| RAND-36 VT | 70.4 ± 18.5 | 61.0 ± 20.4 | .022 | 69.0 ± 20.4 | 64.9 ± 20.2 | .258 | .275 | .357 |
| RAND-36 SF | 88.9 ± 15.8 | 84.2 ± 19.1 | .125 | 91.8 ± 16.9 | 88.5 ± 20.3 | .569 | .090 | .480 |
| RAND-36 RE | 83.1 ± 33.2 | 88.4 ± 27.8 | .577 | 93.8 ± 20.3 | 87.0 ± 30.7 | .282 | .030 | .388 |
| RAND-36 MH | 82.9 ± 11.9 | 80.3 ± 15.5 | .721 | 82.5 ± 13.5 | 81.0 ± 15.3 | .785 | .714 | .921 |
| TSK-SV | 31.8 ± 7.3 (n = 68) | 30.8 ± 6.2 (n = 38) | .792 | 28.9 ± 7.4 (n = 73) | 30.3 ± 7.0 (n = 37) | .258 | .002 | .268 |
aValues are reported as mean ± SD. Bolded values indicate statistical significance (P < .05). ADL, activities of daily living; ATRS, Achilles tendon Total Rupture Score; BP, bodily pain; EFM, early functional mobilization; FAOS, Foot and Ankle Outcome Score; GH, general health; MH, mental health; PAS, Physical Activity Scale; PF, physical functioning; QoL, quality of life; RAND-36, RAND 36-Item Health Survey; RE, role functioning/emotional; RP, role functioning/physical; SF, social functioning; Sports/rec, sports and recreation; TSK-SV, Tampa Scale of Kinesiophobia, Swedish version; VT, vitality.
bChange from 6 to 12 months with the Wilcoxon signed-rank test for comparison of paired observations.
Significant improvements (all P < .05) from 6 to 12 months were observed in self-reported function, pain, and QoL in both groups (Table 2). At 12 months, no significant differences in any of the patient-reported outcomes were seen between the groups. The RAND-36 subscale of general health, however, exhibited higher values in the EFM group (82.6 ± 16.9) than the control group (77.1 ± 17.0) (P = .051) at 12 months after injury.
Functional Outcomes
There were no significant differences (all P > .05) between the EFM and control groups on the heel-rise test at neither 6 or 12 months postoperatively or on the jump tests (Table 3). The injured side was significantly improved (P < .01) between the 6- and 12-month follow-up visits for all variables in the heel-rise test but was still significantly worse than the healthy side (Appendix Table A1).
Table 3.
Functional Outcomes by Treatment Groupa
| 6 mo | 12 mo | |||||
|---|---|---|---|---|---|---|
| EFM (n = 78) | Control (n = 42) | P Value | EFM (n = 83) | Control (n = 40) | P Value | |
| Total concentric work, J | ||||||
| Injured side | 1382.4 ± 654.4 (0.0-3760.9) | 1531.1 ± 651.6 (45.1-2630.8) | 1776.1 ± 677.8 (176.2-3602.6) | 2077.8 ± 744.4 (272.6-4373.2) | ||
| Uninjured side | 2367.7 ± 647.6 (1120.9-3966.3) | 2596.7 ± 817.5 (880.4-4376.5) | 2446.1 ± 728.6 (916.0-4338.8) | 2675.6 ± 798.9 (1094.0-4895.7) | ||
| LSI, % | 58.6 ± 23.0 (0.0-104.3) | 58.6 ± 21.2 (5.1-100.1) | .999 | 74.4 ± 23.0 (7.0-121.6) | 77.7 ± 19.5 (24.9-119.6) | .433 |
| No. of heel rises | ||||||
| Injured side | 22.0 ± 8.3 (0-54) | 23.4 ± 8.6 (3-57) | 25.7 ± 8.1 (4-50) | 27.7 ± 9.3 (12-59) | ||
| Uninjured side | 27.6 ± 6.9 (14-46) | 29.2 ± 8.8 (15-59) | 28.0 ± 7.2 (15-49) | 29.3 ± 8.8 (16-60) | ||
| LSI, % | 80.3 ± 22.1 (0.0-140.9) | 80.0 ± 21.5 (16.7-122.7) | .954 | 92.6 ± 20.5 (13.3-135.1) | 95.3 ± 20.7 (63.3-172.7) | .508 |
| Maximum height of heel rise, cm | ||||||
| Injured side | 9.9 ± 2.5 (0.0-15.3) | 10.0 ± 2.9 (2.3-14.7) | 11.0 ± 2.4 (5.9-15.6) | 11.9 ± 2.3 (4.1-16.3) | ||
| Uninjured side | 13.7 ± 1.8 (10.1-17.5) | 13.7 ± 2.1 (9.1-17.5) | 14.0 ± 2.0 (9.2-19.6) | 14.3 ± 1.9 (11.0-18.7) | ||
| LSI, % | 72.3 ± 18.1 (0.0-123.3) | 73.3 ± 19.5 (23.0-107.4) | .776 | 79.2 ± 15.9 (36.9-127.9) | 83.4 ± 12.8 (33.6-108.4) | .151 |
| Vertical jump test, cm | ||||||
| Injured side | 24.3 ± 6.5 | 23.6 ± 6.4 | ||||
| Uninjured side | 27.5 ± 6.9 | 27.3 ± 6.2 | ||||
| LSI, % | 89.2 ± 14.6 | 86.9 ± 15.1 | .431 | |||
| Side hop test, s | ||||||
| Injured side | 4.7 ± 1.5 | 4.9 ± 1.6 | ||||
| Uninjured side | 4.7 ± 1.5 | 4.7 ± 1.7 | ||||
| LSI, % | 100.3 ± 12.3 | 103.1 ± 13.4 | .260 | |||
aValues are reported as mean ± SD or mean ± SD (range). EFM, early functional mobilization; LSI, limb symmetry index (injured/uninjured × 100).
4-Group Analysis
Because the incidence of DVT in both treatment groups was high postoperatively,3 a secondary analysis with a 4-group (treatment group × DVT) design was performed to evaluate the effect of treatment group and DVT on outcomes. There were no significant interaction effects of group (EFM vs control) and DVT for any of the patient-reported or functional outcomes at 6 or 12 months (P > .05).
Outcomes by DVT
Patient-Reported Outcomes
Patients who experienced DVT exhibited significantly lower self-reported outcomes on the ATRS, FAOS subscales of ADL and QoL, and RAND-36 subscales of physical functioning and role functioning/emotional at 6 months compared with patients not having sustained DVT (all P < .05) (Table 4).
Table 4.
Patient-Reported and Functional Outcomes by Presence of DVTa
| 6 mo | 12 mo | P Valueb | ||||||
|---|---|---|---|---|---|---|---|---|
| No DVT | DVT | P Value | No DVT | DVT | P Value | No DVT | DVT | |
| ATRS | 66.2 ± 19.8 (n = 72) | 57.4 ± 19.4 (n = 44) | .022 | 83.4 ± 15.4 (n = 72) | 75.8 ± 15.8 (n = 44) | .003 | <.001 | <.001 |
| PAS | 4.0 ± 1.1 (n = 71) | 3.9 ± 1.2 (n = 41) | .587 | 4.3 ± 1.1 (n = 71) | 4.1 ± 1.2 (n = 43) | .468 | .031 | .374 |
| FAOS pain | 88.1 ± 12.4 (n = 73) | 86.9 ± 11.6 (n = 44) | .520 | 94.3 ± 8.6 (n = 72) | 92.3 ± 9.6 (n = 44) | .226 | <.001 | .010 |
| FAOS symptoms | 80.9 ± 15.3 | 78.1 ± 16.8 | .384 | 87.2 ± 13.1 | 85.2 ± 15.8 | .546 | .004 | .009 |
| FAOS ADL | 94.1 ± 8.4 | 92.3 ± 7.8 | .046 | 97.6 ± 5.7 | 95.6 ± 6.5 | .006 | <.001 | <.001 |
| FAOS Sports/Rec | 70.9 ± 20.3 | 63.4 ± 20.3 | .087 | 84.4 ± 14.3 | 77.2 ± 17.6 | .035 | <.001 | <.001 |
| FAOS QoL | 61.6 ± 18.4 | 54.5 ± 14.8 | .024 | 76.2 ± 16.5 | 66.8 ± 17.6 | .009 | <.001 | .001 |
| RAND-36 PF | 85.8 ± 12.1 (n = 72) | 80.0 ± 13.6 (n = 44) | .011 | 92.2 ± 10.9 (n = 71) | 87.5 ± 13.3 (n = 44) | .007 | <.001 | <.001 |
| RAND-36 RP | 77.1 ± 35.0 | 68.8 ± 35.0 | .116 | 90.5 ± 24.8 | 85.8 ± 28.2 | .288 | .027 | .003 |
| RAND-36 BP | 84.3 ± 17.2 (n = 71) | 83.1 ± 16.2 (n = 43) | .582 | 92.0 ± 12.6 | 89.7 ± 17.0 | .587 | .007 | .017 |
| RAND-36 GH | 80.5 ± 16.5 | 79.7 ± 15.2 | .540 | 83.8 ± 15.7 | 75.1 ± 17.9 | .003 | .192 | .110 |
| RAND-36 VT | 69.6 ± 18.7 | 62.7 ± 20.3 | .100 | 71.1 ± 18.1 | 61.5 ± 22.6 | .020 | .827 | .837 |
| RAND-36 SF | 89.2 ± 15.9 | 83.9 ± 18.7 | .114 | 90.8 ± 18.8 | 90.2 ± 17.5 | .769 | .350 | .080 |
| RAND-36 RE | 89.8 ± 25.4 | 76.5 ± 38.5 | .042 | 93.9 ± 20.6 | 87.1 ± 29.9 | .184 | .620 | .060 |
| RAND-36 MH | 83.2 ± 13.5 | 80.0 ± 12.8 | .124 | 84.3 ± 13.5 | 78.1 ± 14.6 | .016 | .609 | .795 |
| TSK-SV | 30.6 ± 6.5 (n = 67) | 33.3 ± 7.3 (n = 38) | .082 | 28.3 ± 7.9 (n = 68) | 31.2 ± 5.8 (n = 41) | .013 | .007 | .070 |
aValues are reported as mean ± SD. Bolded values indicate statistical significance (P < .05). ADL, activities of daily living; ATRS, Achilles tendon Total Rupture Score; BP, bodily pain; DVT, deep venous thrombosis; FAOS, Foot and Ankle Outcome Score; GH, general health; MH, mental health; PAS, Physical Activity Scale; PF, physical functioning; QoL, quality of life; RAND-36, RAND 36-Item Health Survey; RE, role functioning/emotional; RP, role functioning/physical; SF, social functioning; Sports/Rec, sports and recreation; TSK-SV, Tampa Scale of Kinesiophobia, Swedish version; VT, vitality.
bChange from 6 to 12 months with the Wilcoxon signed-rank test for comparison of paired observations.
Patients who had sustained DVT demonstrated significantly worse outcomes on the ATRS; FAOS subscales of ADL, Sports/Rec, and QoL; and RAND-36 subscales of physical functioning, general health, vitality, and mental health at 12 months than patients without DVT (all P < .05). At 12 months, patients who had sustained DVT also presented with a higher degree of kinesiophobia than patients without diagnosed DVT (P = .013) (Table 4).
Functional Outcomes
There were no significant differences (P > .05) in functional outcomes (heel-rise and jump tests) between patients with and without DVT (Appendix Table A2).
Discussion
The main finding of this study was that immediate postoperative EFM in patients with ATR resulted in significantly higher scores on general health and vitality at 6 months compared with the control group. EFM did not seem to negatively affect heel-rise function, which indicates that muscle-tendon recovery might not be greatly influenced by differences in treatment protocols.
The observed difference in health-related QoL was linked to differences in mobilization regimens between groups, independent of the occurrence of DVT. Patients suffering DVT during treatment, however, exhibited greater impairments in general health and disease-specific subjective outcomes at 6 and 12 months, independent of the postoperative treatment regimen.
An essential finding of this study was the observation that the EFM group exhibited superior health-related QoL at 6 months, as measured with the RAND-36. Suchak et al43 reported that earlier weightbearing resulted in greater health-related QoL at 6 weeks; however, at the 6-month follow-up, this difference no longer existed. In this earlier study, all patients were nonweightbearing for the first 2 weeks and then randomized to weightbearing or not.43 In our study, however, patients in the EFM group were allowed weightbearing from day 1 postoperatively, which may explain the differences in outcomes at 6 months between the 2 studies. Additionally, a study on nonoperatively treated patients with an ATR reported better health-related QoL in patients allowed to bear weight compared with the nonweightbearing group during treatment.5
The observed difference at 6 months between the EFM and control groups on the RAND-36 subscales of general health and vitality is suggested to depend mainly on the first 2 postoperative weeks in which patients in the EFM group were weightbearing while the controls were nonweightbearing. These findings seem to reflect that direct weightbearing after an injury has essential effects on patients’ more global health conditions. A possible explanation might be that patients have a faster trajectory to recovery with early loading; however, the difference evens out in the long term. The effects of weightbearing on general health and vitality may additionally depend on other factors such as the fear of loading and the number of daily steps taken. Our finding at 6 months of no difference in kinesiophobia between the 2 groups suggests that fear of loading did not affect the difference in outcomes. The number of daily steps taken has been shown to be critical for overall health1 and is known to be positively correlated with increased loading.4 Thus, further studies need to assess whether it is the actual amount of loading or the number of steps taken that relates to improved health and vitality.
The difference in subjective outcomes at 6 months may also be related to the varying degree of ankle motion performed in the 2 groups. The EFM group was allowed partial ankle motion within the orthosis from day 1, which was continued for 6 weeks. The control group, however, received a plaster cast for 2 weeks, followed by 4 weeks of immobilization in an ankle-stable orthosis. Movements of the ankle have been speculated to activate the calf muscle7 and enhance tendon healing.9,18,39,47 The observation, however, of nonsignificant differences at 6 months on the heel-rise test between the EFM and control groups suggests that the ankle motion produced within the EFM group was too small to further improve calf muscle recovery.
Despite the significant differences in RAND-36 scores at 6 months, no major differences between groups were noted in ATRS or functional outcomes. This observation seems to suggest that EFM produces an improvement in the medium term (6 months) limited to general health status, which is not paralleled by better functional enhancements. Presumably, the minor dissimilarities between the 2 rehabilitation protocols, which mainly occurred during the first 2 weeks when patients were in pain within the inflammatory healing phase, are not enough to affect tendon-muscle recovery.
Tendon elongation, which would be a severe adverse event of using aggressive EFM, has also been found to negatively affect functional outcomes.6,41 Thus, the observation demonstrating no differences in the functional outcome of the LSI heel-rise height between the 2 groups suggests that tendon elongation does not seem to increase because of EFM. This conclusion is substantiated by earlier studies showing that the heel-rise height correlates with Achilles tendon length at 1 year after an ATR41 and that EFM does not seem to include a risk of greater tendon elongation.30
The results at 12 months showing no significant differences in functional outcomes between patients in the EFM and control groups can probably be attributed to the minor differences between the treatment groups in terms of weightbearing status. In this study, all patients from both groups were allowed weightbearing from the third week postoperative. Weightbearing from week 3 in the control group was chosen as the standard of care, which is used in many hospitals. Earlier studies comparing functional mobilization with immobilization most often used 4 to 6 weeks of nonweightbearing for the control group.12,13,31,43
In this study, a further aim was to assess whether the high occurrence of DVT during leg immobilization could affect the outcomes between the 2 treatment groups. The observation that patients who experienced DVT demonstrated significantly lower self-reported outcomes on all questionnaires at 6 months was, however, not related to the postoperative treatment.
This finding suggests that the improved subjective outcomes seen in the EFM group were not affected by a reduction of DVT but rather by more direct factors such as differences in weightbearing and ankle movement between the rehabilitation protocols.
The observation that patients who had sustained DVT postoperatively reported inferior subjective outcomes also at 12 months on the ATRS, FAOS (ADL, Sports/Rec, and QoL), and RAND-36 (physical functioning, general health, vitality, and mental health) demonstrates that DVT is a major predictor for negative outcomes of an ATR. This finding is in agreement with another study2 and furthermore highlights that DVT is an independent factor to address to improve long-term outcomes.
The strength of this study is that the EFM protocol used has been assessed in detail when it comes to weightbearing and the number of daily steps taken during the first 2 postoperative weeks.4
The potential limitations of this study are that there were several orthopaedic surgeons involved in the surgical treatment of the patients and that the physical therapist performing the outcome tests was not blinded to the treatment regimen. Another limitation of the study was that the rehabilitation protocol was not standardized after week 6. These limitations may explain some of the variation in patient outcomes but may also make the results more generalizable in clinical practice.
Conclusion
This study demonstrated that an accelerated postoperative protocol with immediate loading and ankle motion resulted in better general health and vitality at 6 months. However, there were no differences between the groups in the recovery of heel-rise function. Future studies should place an additional focus on the means to reduce the risk of DVT to improve patient outcomes after ATR.
APPENDIX
Table A1.
Side-to-Side Comparison at 6- and 12-Month Follow-upa
| 6 mo | 12 mo | P Valueb | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Injured | Uninjured | P Value | n | Injured | Uninjured | P Value | ||
| Total concentric work, J | |||||||||
| EFM group | 78 | 1382.4 ± 654.4 (0.0-3760.9) | 2367.7 ± 647.6 (1120.9-3966.3) | <.001 | 83 | 1776.1 ± 677.8 (176.2-3602.6) | 2446.1 ± 728.6 (916.0-4338.8) | <.001 | <.001 |
| Control group | 42 | 1531.1 ± 651.6 (45.1-2630.8) | 2596.7 ± 817.5 (880.4-4376.5) | <.001 | 40 | 2077.8 ± 744.4 (272.6-4373.2) | 2675.6 ± 798.9 (1094.0-4895.7) | <.001 | <.001 |
| No. of heel rises | |||||||||
| EFM group | 78 | 22.0 ± 8.3 (0-54) | 27.6 ± 6.9 (14-46) | <.001 | 83 | 25.7 ± 8.1 (4-50) | 28.0 ± 7.2 (15-49) | .001 | <.001 |
| Control group | 42 | 23.4 ± 8.6 (3-57) | 29.2 ± 8.8 (15-59) | <.001 | 40 | 27.7 ± 9.3 (12-59) | 29.3 ± 8.8 (16-60) | .078 | <.001 |
| Maximum height of heel rise, cm | |||||||||
| EFM group | 78 | 9.9 ± 2.5 (0.0-15.3) | 13.7 ± 1.8 (10.1-17.5) | <.001 | 83 | 11.0 ± 2.4 (5.9-15.6) | 14.0 ± 2.0 (9.2-19.6) | <.001 | <.001 |
| Control group | 42 | 10.0 ± 2.9 (2.3-14.7) | 13.7 ± 2.1 (9.1-17.5) | <.001 | 40 | 11.9 ± 2.3 (4.1-16.3) | 14.3 ± 1.9 (11.0-18.7) | <.001 | <.001 |
aValues are reported as mean ± SD (range). Bolded values indicate statistical significance (P < .05). EFM, early functional mobilization.
bComparison of the injured side between the 6- and 12-month follow-up.
Table A2.
Heel-Rise Test Results by Presence of DVTa
| 6 mo | 12 mo | |||||
|---|---|---|---|---|---|---|
| No DVT (n = 77) | DVT (n = 42) | P Value | No DVT (n = 78) | DVT (n = 44) | P Value | |
| Total concentric work, J | ||||||
| Injured side | 1443.0 ± 655.9 (45.1-3760.9) | 1438.7 ± 654.6 (0.0-2819.9) | 1877.1 ± 655.3 (272.6-3347.1) | 1893.0 ± 800.2 (176.2-4373.2) | ||
| Uninjured side | 2430.0 ± 734.6 (880.4-4161.3) | 2477.4 ± 699.0 (1271.1-4376.5) | 2481.1 ± 724.9 (916.0-4338.8) | 2586.5 ± 821.6 (1301.5-4895.7) | ||
| LSI, % | 59.2 ± 21.7 (5.1-104.3) | 58.5 ± 23.1 (0.0-101.2) | .873 | 76.9 ± 21.5 (24.9-119.6) | 73.9 ± 22.0 (7.0-121.6) | .468 |
| No. of heel rises | ||||||
| Injured side | 22.6 ± 8.7 (3-57) | 22.4 ± 8.0 (0-46) | 26.4 ± 8.5 (12-59) | 26.2 ± 8.7 (4-50) | ||
| Uninjured side | 28.4 ± 8.0 (14-59) | 27.7 ± 7.0 (16-45) | 28.4 ± 8.1 (15-60) | 28.6 ± 7.4 (17-46) | ||
| LSI, % | 79.2 ± 19.9 (15.6-117.6) | 82.0 ± 25.3 (0.0-140.9) | .516 | 94.0 ± 19.8 (49.0-172.7) | 92.6 ± 22.2 (13.3-135.1) | .713 |
| Maximum height of heel rise, cm | ||||||
| Injured side | 10.1 ± 2.6 (2.3-15.3) | 9.8 ± 2.7 (0.0-14.2) | 11.5 ± 2.4 (4.1-16.3) | 11.2 ± 2.3 (5.9-15.6) | ||
| Uninjured side | 13.6 ± 1.9 (9.1-17.2) | 13.9 ± 1.9 (9.6-17.5) | 14.0 ± 2.0 (9.2-19.4) | 14.1 ± 1.8 (10.4-19.6) | ||
| LSI, % | 74.0 ± 17.5 (23.0-123.3) | 71.0 ± 19.8 (0.0-105.2) | .391 | 81.7 ± 13.9 (33.6-114.4) | 79.6 ± 15.8 (42.9-127.9) | .458 |
aValues are reported as mean ± SD (range). DVT, deep venous thrombosis; LSI, limb symmetry index (injured/uninjured × 100).
Footnotes
Final revision submitted November 14, 2019; accepted December 3, 2019.
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was supported by grants from the Swedish Research Council for Sport Science, the Stockholm County Council (ALF Project), and the Swedish Research Council and by grant funding and supplies from OPED (to P.W.A.). AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the Stockholm Regional Ethical Committee (No. 2013/1791-31/3).
References
- 1. Althoff T, Sosič R, Hicks JL, King AC, Delp SL, Leskovec J. Large-scale physical activity data reveal worldwide activity inequality. Nature. 2017;547(7663):336–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arverud ED, Anundsson P, Hardell E, et al. Ageing, deep vein thrombosis and male gender predict poor outcome after acute Achilles tendon rupture. Bone Joint J. 2016;98-B(12):1635–1641. [DOI] [PubMed] [Google Scholar]
- 3. Aufwerber S, Heijne A, Edman G, Grävare Silbernagel K, Ackermann PW. Early mobilization does not reduce the risk of deep venous thrombosis after Achilles tendon rupture: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2020;28(1):312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aufwerber S, Heijne A, Grävare Silbernagel K, Ackermann PW. High plantar force loading after Achilles tendon rupture repair with early functional mobilization. Am J Sports Med. 2019;47(4):894–900. [DOI] [PubMed] [Google Scholar]
- 5. Barfod KW, Bencke J, Lauridsen HB, Ban I, Ebskov L, Troelsen A. Nonoperative dynamic treatment of acute Achilles tendon rupture: the influence of early weight-bearing on clinical outcome. A blinded, randomized controlled trial. J Bone Joint Surg Am. 2014;96(18):1497–1503. [DOI] [PubMed] [Google Scholar]
- 6. Baxter JR, Farber DC, Hast MW. Plantarflexor fiber and tendon slack length are strong determinates of simulated single-leg heel raise height. J Biomech. 2019;86:27–33. [DOI] [PubMed] [Google Scholar]
- 7. Booth FW. Physiologic and biochemical effects of immobilization on muscle. Clin Orthop Relat Res. 1987;219:15–20. [PubMed] [Google Scholar]
- 8. Bostick GP, Jomha NM, Suchak AA, Beaupré LA. Factors associated with calf muscle endurance recovery 1 year after Achilles tendon rupture repair. J Orthop Sports Phys Ther. 2010;40(6):345–351. [DOI] [PubMed] [Google Scholar]
- 9. Bring D, Reno C, Renstrom P, Salo P, Hart D, Ackermann P. Prolonged immobilization compromises up-regulation of repair genes after tendon rupture in a rat model. Scand J Med Sci Sports. 2010;20(3):411–417. [DOI] [PubMed] [Google Scholar]
- 10. Broderick BJ, Breathnach O, Condon F, Masterson E, ÓLaighin G. Haemodynamic performance of neuromuscular electrical stimulation (NMES) during recovery from total hip arthroplasty. J Orthop Surg Res. 2013;8(3):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brumann M, Baumbach SF, Mutschler W, Polzer H. Accelerated rehabilitation following Achilles tendon repair after acute rupture: development of an evidence-based treatment protocol. Injury. 2014;45(11):1782–1790. [DOI] [PubMed] [Google Scholar]
- 12. Cetti R, Henriksen LO, Jacobsen KS. A new treatment of ruptured Achilles tendons: a prospective randomized study. Clin Orthop Relat Res. 1994;308:155–165. [PubMed] [Google Scholar]
- 13. Costa ML, MacMillan K, Halliday D, et al. Randomised controlled trials of immediate weight-bearing mobilisation for rupture of the tendo Achillis. J Bone Joint Surg Br. 2006;88(1):69–77. [DOI] [PubMed] [Google Scholar]
- 14. Craik JD, Clark A, Hendry J, et al. The effect of ankle joint immobilization on lower limb venous flow. Foot Ankle Int. 2015;36:18–23. [DOI] [PubMed] [Google Scholar]
- 15. DeSalles PG, Vasconcellos FV, de Salles GF, Fonseca RT, Dantas EH. Validity and reproducibility of the Sargent jump test in the assessment of explosive strength in soccer players. J Hum Kinet. 2012;33:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Domeij-Arverud E, Ackermann PW. Deep venous thrombosis and tendon healing. Adv Exp Med Biol. 2016;920:221–228. [DOI] [PubMed] [Google Scholar]
- 17. Domeij-Arverud E, Labruto F, Latifi A, Nilsson G, Edman G, Ackermann PW. Intermittent pneumatic compression reduces the risk of deep vein thrombosis during postoperative lower limb immobilisation: a prospective randomised trial of acute ruptures of the Achilles tendon. Bone Joint J. 2015;97-B(5):675–680. [DOI] [PubMed] [Google Scholar]
- 18. Eliasson P, Andersson T, Aspenberg P. Rat Achilles tendon healing: mechanical loading and gene expression. J Appl Physiol. 2009;107:399–407. [DOI] [PubMed] [Google Scholar]
- 19. Grimby G. Physical activity and muscle training in the elderly. Acta Med Scand. 1986;711:233–237. [DOI] [PubMed] [Google Scholar]
- 20. Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med. 2001;33(5):350–357. [DOI] [PubMed] [Google Scholar]
- 21. Hays RD, Sherbourne CD, Mazel RM. The RAND 36-item health survey 1.0. Health Econ. 1993;2:217–227. [DOI] [PubMed] [Google Scholar]
- 22. Heikkinen J, Lantto I, Piilonen J, et al. Tendon length, calf muscle atrophy, and strength deficit after acute Achilles tendon rupture: long-term follow-up of patients in a previous study. J Bone Joint Surg Am. 2017;99(18):1509–1515. [DOI] [PubMed] [Google Scholar]
- 23. Itoh H, Kurosaka M, Yoshiya S, Ichihashi N, Mizuno K. Evaluation of functional deficits determined by four different hop tests in patients with anterior cruciate ligament deficiency. Knee Surg Sports Traumatol Arthrosc. 1998;6:241–245. [DOI] [PubMed] [Google Scholar]
- 24. Kangas J, Pajala A, Ohtonen P, Leppilahti J. Achilles tendon elongation after rupture repair: a randomized comparison of 2 postoperative regimens. Am J Sports Med. 2007;35(1):59–64. [DOI] [PubMed] [Google Scholar]
- 25. Lantto I, Heikkinen J, Flinkkila T, et al. A prospective randomized trial comparing surgical and nonsurgical treatments of acute Achilles tendon ruptures. Am J Sports Med. 2016;44(9):2406–2414. [DOI] [PubMed] [Google Scholar]
- 26. Lapidus LJ, Rosfors S, Ponzer S, et al. Prolonged thromboprophylaxis with dalteparin after surgical treatment of Achilles tendon rupture: a randomized, placebo-controlled study. J Orthop Trauma. 2007;21(1):52–57. [DOI] [PubMed] [Google Scholar]
- 27. Lundberg MKE, Styf J, Carlsson SG. A psychometric evaluation of the Tampa Scale for Kinesiophobia: from a physiotherapeutic perspective. Physiother Theory Pract. 2004;20(2):121–133. [Google Scholar]
- 28. Maffulli N, Tallon C, Wong J, Peng Lim K, Bleakney R. Early weightbearing and ankle mobilization after open repair of acute midsubstance tears of the Achilles tendon. Am J Sports Med. 2003;31(5):692–700. [DOI] [PubMed] [Google Scholar]
- 29. Maquirriain J. Achilles tendon rupture: avoiding tendon lengthening during surgical repair and rehabilitation. Yale J Biol Med. 2011;84(3):289–300. [PMC free article] [PubMed] [Google Scholar]
- 30. McCormack R, Bovard J. Early functional rehabilitation or cast immobilisation for the postoperative management of acute Achilles tendon rupture? A systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2015;49(20):1329–1335. [DOI] [PubMed] [Google Scholar]
- 31. Mortensen HM, Skov O, Jensen PE. Early motion of the ankle after operative treatment of a rupture of the Achilles tendon: a prospective, randomized clinical and radiographic study. J Bone Joint Surg Am. 1999;81(7):983–990. [DOI] [PubMed] [Google Scholar]
- 32. Mullaney MJ, McHugh MP, Tyler TF, Nicholas SJ, Lee SJ. Weakness in end-range plantar flexion after Achilles tendon repair. Am J Sports Med. 2006;34(7):1120–1125. [DOI] [PubMed] [Google Scholar]
- 33. Nilsson-Helander K, Silbernagel KG, Thomeé R, et al. Acute Achilles tendon rupture: a randomized, controlled study comparing surgical and nonsurgical treatments using validated outcome measures. Am J Sports Med. 2010;38(11):2186–2193. [DOI] [PubMed] [Google Scholar]
- 34. Nilsson-Helander K, Thomeé R, Grävare-Silbernagel K, et al. The Achilles tendon Total Rupture Score (ATRS): development and validation. Am J Sports Med. 2007;35(3):421–426. [DOI] [PubMed] [Google Scholar]
- 35. Olsson N, Nilsson-Helander K, Karlsson J, et al. Major functional deficits persist 2 years after acute Achilles tendon rupture. Knee Surg Sports Traumatol Arthrosc. 2011;19(8):1385–1393. [DOI] [PubMed] [Google Scholar]
- 36. Olsson N, Silbernagel KG, Eriksson BI, et al. Stable surgical repair with accelerated rehabilitation versus nonsurgical treatment for acute Achilles tendon ruptures: a randomized controlled study. Am J Sports Med. 2013;41(12):2867–2876. [DOI] [PubMed] [Google Scholar]
- 37. Roos EM, Brandsson S, Karlsson J. Validation of the Foot and Ankle Outcome Score for ankle ligament reconstruction. Foot Ankle Int. 2001;22(10):788–794. [DOI] [PubMed] [Google Scholar]
- 38. Saltin B, Grimby G. Physiological analysis of middle-aged and old former athletes: comparison with still active athletes of the same ages. Circulation. 1968;38(6):1104–1115. [DOI] [PubMed] [Google Scholar]
- 39. Schepull T, Aspenberg P. Early controlled tension improves the material properties of healing human Achilles tendons after ruptures: a randomized trial. Am J Sports Med. 2013;41(11):2550–2557. [DOI] [PubMed] [Google Scholar]
- 40. Silbernagel KG, Nilsson-Helander K, Thomeé R, Eriksson BI, Karlsson J. A new measurement of heel-rise endurance with the ability to detect functional deficits in patients with Achilles tendon rupture. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):258–264. [DOI] [PubMed] [Google Scholar]
- 41. Silbernagel KG, Steele R, Manal K. Deficits in heel-rise height and Achilles tendon elongation occur in patients recovering from an Achilles tendon rupture. Am J Sports Med. 2012;40(7):1564–1571. [DOI] [PubMed] [Google Scholar]
- 42. Sochart DH, Hardinge K. The relationship of foot and ankle movements to venous return in the lower limb. J Bone Joint Surg Br. 1999;81(4):700–704. [DOI] [PubMed] [Google Scholar]
- 43. Suchak AA, Bostick GP, Beaupré LA, Durand DC, Jomha NM. The influence of early weight-bearing compared with non-weight-bearing after surgical repair of the Achilles tendon. J Bone Joint Surg Am. 2008;90(9):1876–1883. [DOI] [PubMed] [Google Scholar]
- 44. Suydam SM, Buchanan TS, Manal K, Silbernagel KG. Compensatory muscle activation caused by tendon lengthening post-Achilles tendon rupture. Knee Surg Sports Traumatol Arthrosc. 2015;23(3):868–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Twaddle BC, Poon P. Early motion for Achilles tendon ruptures: is surgery important? A randomized, prospective study. Am J Sports Med. 2007;35(12):2033–2038. [DOI] [PubMed] [Google Scholar]
- 46. Valic Z, Buckwalter JB, Clifford PS. Muscle blood flow response to contraction: influence of venous pressure. J Appl Physiol. 2005;98(1):72–76. [DOI] [PubMed] [Google Scholar]
- 47. Valkering KP, Aufwerber S, Ranuccio F, Lunini E, Edman G, Ackermann PW. Functional weight-bearing mobilization after Achilles tendon rupture enhances early healing response: a single-blinded randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017;25(6):1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]