Abstract
Background: This study was designed to quantify the performance of the pediatric Patient-Reported Outcome Measurement Information System (PROMIS) when delivered as part of routine care to children with upper extremity (UE) fractures. Methods: This cross-sectional study analyzed 964 new pediatric patients presenting with an UE fracture. All patients completed PROMIS computer adaptive tests for pain interference, peer relationships, UE function, and mobility domains at clinic registration. PROMIS was completed by parent-proxy (n = 418) for 5- to 7-year-olds and self-reported by 8- to 10-year-olds (n = 546). PROMIS score distributions were defined, and Pearson correlations assessed the interrelation between PROMIS domains. Student’s t tests compared mean PROMIS scores between parent-proxy and self-completion groups. Results: UE scores indicated the greatest average impairment of all PROMIS domains. However, 13% of patients reached the UE score ceiling indicating maximal UE function. UE scores and mobility scores had a strong positive correlation while UE scores had a moderate negative correlation with pain interference. In all patients, peer relationships were, at most, very weakly correlated with any other PROMIS domain. After grouping by fracture type, parent-proxy completion estimated worse UE function, more pain interference, and worse peer relationship. Conclusions: Pediatric PROMIS UE function scores capture impairment from UE fractures but do have a strong positive correlation with pediatric PROMIS Mobility, which assesses lower extremity function. Among children with UE fractures, parent-proxy completion of pediatric PROMIS appears associated with worse scores on most PROMIS domains.
Keywords: pediatric, upper extremity, fracture, patient-reported outcomes, PROMIS
Introduction
Patient-reported outcome measures (PROMs) have become increasingly emphasized in orthopedic surgery. They are used in clinical care, research, and are now being considered as a factor influencing reimbursement.5 In pediatrics, examples of commonly used, validated PROMs include the Pediatric Outcomes Data Collection Instrument (PODCI), Child Health Questionnaire (CHQ), DISABKIDS Chronic Generic Measure (DCGM), KINDL-R, and Pediatric Quality of Life Inventory (PedsQL). All of these PROMs contain physical function domains and no single consensus measure has emerged.13 Recently, the Patient-Reported Outcome Measurement Information System (PROMIS) was developed by the National Institutes of Health (NIH) as a means of standardizing patient-reported outcome scores and measuring health-related quality of life.4 PROMIS is not disease specific, which improves its applicability and positions it as a possible universal outcome measure.
PROMIS assessments are provided in a computer adaptive tests (CAT) format that leverages item response theory to improve survey performance. The pediatric PROMIS Physical Function assessment is comprised of individual mobility and upper extremity (UE) assessments that produce separate component scores.4,9,14,20 In the pediatric population, a self-report version can be completed by the child (8-17 years old) or a parent-proxy version can be completed by a parent or caregiver (for children 5-7 years old).24 PROMIS scores are normalized to a mean score of 50, standard deviation of 10, with a theoretical range of 0 to 100.3 A higher score corresponds to a greater amount of the domain being measured. For example, a higher physical function score corresponds to better physical function, whereas a higher depression score corresponds to greater depression.
Although the adult PROMIS assessments have been extensively studied, there is a paucity of literature regarding the performance of pediatric PROMIS assessments and even less information regarding pediatric PROMIS scores in the context of orthopedic surgery.14,15 Currently, PROMIS has been incorporated into health care clinics for research purposes to further understand the utility of PROMIS scores.17,18 Nonetheless, the performance of pediatric PROMIS assessments following UE injuries remains uncertain. There is a need for additional study to describe and analyze the current performance of PROMIS assessments in a pediatric orthopedic population. This could potentially serve to further refine PROMIS so that it can become a tool confidently utilized to inform clinical care.
This study was designed to assess the performance of pediatric PROMIS Physical Function (mobility, UE), pain interference, and peer relationship components in patients with UE fractures. The aims for this study were to determine the degree to which these 3 PROMIS domains are correlated and to identify any ceiling/floor effects in this population. We also aimed to test the null hypothesis that there would be no difference in score distributions between patient-reported and parent-proxy PROMIS domain scores.
Materials and Methods
This cross-sectional study analyzed data from 964 pediatric (5-10 years old) patient visits to the offices of a tertiary orthopedic center. New patients who presented with UE fractures between June 1, 2016, and June 1, 2017, were eligible for inclusion. Our Institutional Review Board deemed this study exempt from full review as only de-identified data were used in this study.
Our department administers PROMIS as part of routine clinical care at all office visits. All patients were provided a computer tablet (iPad mini, Apple, Cupertino, California) at check-in that automatically delivered the following PROMIS CATs: Physical Function-Upper Extremity v1.0, Physical Function-Mobility v1.0, PROMIS Pain Interference-v1.0, and PROMIS Peer Relationships-v1.0. Resulting PROMIS scores were automatically uploaded into the patient’s electronic health record upon assessment completion. Consistent with the recommended administration of PROMIS pediatric CATs, for patients aged 5 to 7 years, the parent/guardian was instructed to complete the PROMIS parent-proxy CATs. Patients aged 8 to 10 years were instructed to complete the PROMIS pediatric self-report CATs on their own.
Our institution’s electronic medical record and administrative databases were queried for age, sex, race, provider visited, International Classification of Disease, Tenth Revision (ICD-10) codes, and PROMIS domain scores (pain interference, UE function, peer relationships, and mobility) at the time of the initial visit. The PROMIS domains comprise specific groups of questions that inquire about a given health domain. For example, the pediatric UE function assessment includes statements such as, “I could open a jar by myself,” and “I could pull open heavy doors.” Each statement then allows a Likert style answer with options ranging from “with no trouble” to “not able to do so.” The type of primary fracture was defined by ICD-10 code. The following ICD-10 codes were used to define each fracture group: humeral shaft (S42.2, S42.3), distal humerus (S42.4), proximal forearm (S52.0, S52.1, S52.2, S52.3), distal forearm (S52.5, S52.6), unspecified forearm (S52.9), wrist/hand (S62.0, S62.1, S62.2, S62.3, S62.5, S62.6, S62.9).
Statistical Analysis
Patients were grouped according to PROMIS being completed by parent-proxy (n = 418) or self-report (n = 546). Univariate descriptive statistics characterized PROMIS scores and the frequency of patients with each UE fracture type. For each group, Pearson correlations assessed the degree of interrelation between PROMIS domains.
The percentage of patients reaching the ceiling and floor scores were calculated as the percentage of patients reaching the highest (ceiling) and lowest (floor) scores within each PROMIS domain. These percentages defined the floor and ceiling effects for each domain. Correlation coefficients (r) were interpreted as recommended by Evans: 0.00-0.19 very weak, 0.20-0.39 weak, 0.40-0.59 moderate, 0.60-0.79 strong, 0.80-1.00 very strong.12
To assess the impact of parent-proxy completion versus self-report on absolute PROMIS scores, student’s t tests compared mean PROMIS scores between the groups stratified by fracture type. The unspecified forearm and humeral shaft fracture groups were excluded from the subgroup analysis as they contained too few patients to draw reasonable conclusions.
Results
After applying the inclusion criteria, 964 patients contributed data that were analyzed (Table 1). For the self-report surveys, UE function scores indicated the greatest impairment of all PROMIS domains (mean: 33, SD: ±11) (Table 2). However, at presentation, 8.3% reached the ceiling UE score indicating maximal UE function. There were also ceiling effects in mobility (15%) and peer relationship (17%) scores and a floor effect in pain interference (7%). UE scores showed a strong positive correlation with mobility (r = 0.62) (Figure 1), and a moderate negative correlation with pain interference (r = −0.58) (Table 3). Pain interference and mobility scores also had a moderate negative correlation (r = −0.43). Peer relationships, at most, showed a very weak positive correlation with any other PROMIS domain (r < 0.15).
Table 1.
Cohort Demographics for Total Study Population.
| N (%) | ||
|---|---|---|
| Age: 5-7 years | Age: 8-10 years | |
| Sex | ||
| Female | 181 (43.3) | 265 (48.5) |
| Male | 237 (56.7) | 281 (51.5) |
| Race | ||
| White/Caucasian | 329 (78.7) | 441 (80.8) |
| Black/African American | 74 (17.7) | 84 (15.4) |
| Other | 15 (3.6) | 21 (3.9) |
| Upper extremity fracture type | ||
| Humeral shaft | 17 (4.1) | 21 (3.9) |
| Distal humerus | 62 (14.8) | 37 (6.8) |
| Proximal forearm | 107 (25.6) | 92 (16.9) |
| Unspecified forearm | 17 (4.1) | 18 (3.3) |
| Distal forearm | 151 (36.1) | 221 (40.5) |
| Wrist and hand | 64 (15.3) | 156 (28.6) |
| PROMIS surveys completed | ||
| Upper extremity | 407 (97.4) | 529 (96.9) |
| Mobility | 418 (100) | 534 (97.8) |
| Peer relationships | 409 (97.8) | 527 (96.5) |
| Pain interference | 411 (98.3) | 532 (97.4) |
Note. PROMIS = Patient-Reported Outcome Measurement Information System.
Table 2.
PROMIS Scores According to Patient Group.
| PROMIS domain |
Mean (SD) |
Range |
Floor effect |
Ceiling effect |
||||
|---|---|---|---|---|---|---|---|---|
| Parent-proxy | Self-administered | Parent-proxy | Self-administered | Parent-proxy | Self-administered | Parent-proxy | Self-administered | |
| Upper extremity | 30 (10) | 33 (11) | 14-56 | 14-57 | 5.20% | 1.32% | 4.90% | 8.30% |
| Mobility | 45 (9) | 44.9 (9) | 22-60 | 23-62 | 0.20% | 0.01% | 14.60% | 14.8% |
| Peer relationship | 50 (10) | 52.5 (10) | 15-66 | 17-66 | 1.20% | 0.57% | 1.70% | 16.70% |
| Pain interference | 54 (8) | 48.7 (8) | 22-78 | 32-74 | 0.20% | 6.80% | 0.50% | 0.38% |
Note. PROMIS = Patient-Reported Outcome Measurement Information System.
Figure 1.
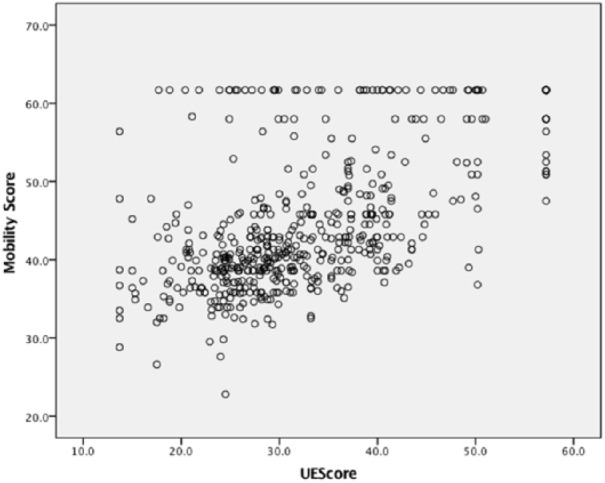
Correlation of mobility and upper extremity scores in 8- to 10-year-old total study population.
Table 3.
Correlations Between PROMIS Scores in 8- to 10-Year-Olds (r Values) in Total Study Population.
| Mobility | Upper extremity | Pain interference | Peer relationships | |
|---|---|---|---|---|
| Mobility | 1 | |||
| Upper extremity | 0.619 | 1 | ||
| Pain interference | −0.428 | −0.582 | 1 | |
| Peer relationships | 0.146 | −0.009 | −0.094 | 1 |
Note. PROMIS = Patient-Reported Outcome Measurement Information System.
For the parent-proxy survey group, UE function scores indicated the greatest impairment of all PROMIS domains (mean: 30, SD: ±10) (Table 2). At presentation, 4.9% reached the ceiling UE score indicating maximal UE function and 5.2% reached the floor UE score indicating minimal UE function. UE scores showed a strong positive correlation with mobility (r = 0.64) (Figure 2), and a moderate negative correlation with pain interference (r = −0.48) (Table 4). Pain interference also had a moderate negative correlation with mobility (r = −0.41). Peer relationships, at most, showed a very weak positive correlation with any other PROMIS domain (r < 0.12).
Figure 2.
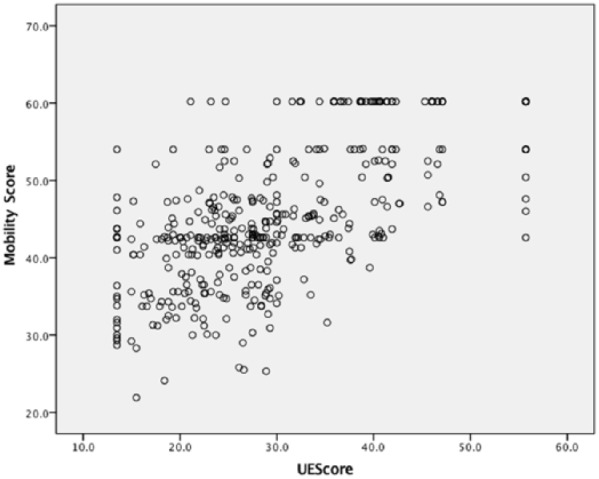
Correlation of mobility and upper extremity scores in 5- to 7-year-old total study population.
Table 4.
Correlations Between PROMIS Scores in 5- to 7-Year-Olds (r Values) in Total Study Population.
| Mobility | Upper extremity | Pain interference | Peer relationships | |
|---|---|---|---|---|
| Mobility | 1 | 0.608 | 0.608 | 0.608 |
| Upper extremity | 0.639 | 1 | 0.608 | 0.608 |
| Pain interference | −0.409 | −0.480 | 1 | 0.608 |
| Peer relationships | 0.118 | −0.097 | 0.015 | 1 |
Note. PROMIS = Patient-Reported Outcome Measurement Information System.
Comparing the two groups defined by administration mode, parent-proxy completion estimated worse UE function (−3.7 points, P < .01), more pain interference (5.1 points, P < .01), and worse peer relationships (−2.5 points, P < .01) but similar mobility scores compared with self-report completion (Table 5).
Table 5.
Test for Significance Between 5 to 7 and 8 to 10 PROMIS Scores in the Study Population.a
| PROMIS domain | Distal humerus |
Proximal forearm |
Distal forearm |
Wrist and hand |
Overall |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| P value | Mean difference (95% CI) | P value | Mean difference (95% CI) | P value | Mean difference (95% CI) | P value | Mean difference (95% CI) | P value | Mean difference (95% CI) | |
| Mobility | 0.239 | 2.39 (−1.62 to 6.40) | 0.292 | −1.34 (−3.85 to 1.16) | 0.884 | −0.14 (−2.05 to 1.77) | 0.508 | −0.81 (−3.24 to 1.61) | 0.75 | −0.19 (−1.33 to 0.96) |
| Pain interference | <0.001 | −6.65 (−10.21 to −3.08) | 0.009 | −3.26 (−5.70 to −0.82) | <0.001 | −5.58 (−7.38 to −3.79) | <0.001 | −6.38 (−8.50 to −4.25) | <0.001 | −5.12 (−6.19 to −4.05) |
| Peer relationships | 0.368 | 2.21 (−2.25 to 6.01) | 0.045 | 2.90 (0.06 to 5.74) | 0.043 | 2.22 (0.07 to 4.38) | 0.121 | 2.45 (−0.65 to 5.55) | <0.001 | 2.49 (1.16 to 3.82) |
| Upper extremity | 0.001 | 7.54 (3.38 to 11.71) | 0.055 | 2.73 (−0.6 to 5.53) | 0.005 | 3.31 (1.00 to 5.62) | 0.125 | 2.28 (−0.64 to 5.20) | <0.001 | 3.74 (2.38 to 5.11) |
Note. PROMIS = Patient-Reported Outcome Measurement Information System; CI = confidence interval.
A positive mean difference indicates a higher domain score in the 5- to 7-year-old study population.
Discussion
PROMs are increasingly utilized in the adult orthopedic population as important clinical information.10,22 The NIH developed PROMIS CATs as a standardized measurement of patient-reported outcomes across diseases and specialties.7,8 In orthopedics, PROMIS CATs have been compared to validated legacy PROM instruments and performed favorably across many adult orthopedic conditions.2,23 However, in the pediatric orthopedic population, PROMs such as the PODCI are routinely used and PROMIS CATs are slowly being incorporated alongside them.18,25
Few studies have evaluated pediatric PROMIS assessments in the orthopedic population. Wall et al reported on the long-term outcomes of Huber Opposition Transfer for augmenting hypoplastic thumb.25 In that study, PODCI and PROMIS scores were collected. Wall et al utilized PROMIS CATs to evaluate a specific pediatric orthopedic population similar to how our group analyzed pediatric orthopedic patients presenting with an UE fracture. In this small series (8 self-report PROMIS, 7 parent-proxy PROMIS administrations), their data indicate a similar finding to our study in that parent-proxy administration resulted in poorer perceived physical function but similar pain levels. Our data substantiate this finding and complements Wall’s early data in 15 cases with a considerably larger population of over 950 patients. The current project expands our understanding of pediatric PROMIS assessments by analyzing the degree of correlation between PROMIS assessments in this population.
In adults, the PROMIS physical function item bank is a unidimensional construct, assessing overall physical function. The pediatric PROMIS Physical Function item bank is split into two separate assessments, mobility and UE function. Mobility captures lower extremity function while UE represents UE function. During preliminary pediatric PROMIS testing, these two item banks were kept separate because they were not highly correlated.11 Contrary to this presumed independence, our data indicate a strong correlation between the UE and mobility scores in both parent-proxy and self-report surveys. We cannot definitively explain this concordance. This correlation may be due to an overlap between movements described in the questions of both item banks such that neither is as extremity specific as intended. For example, within the mobility assessment questions include, “I could keep up when I played with other kids,” “I could bend over to pick something up,” and “I could get up from the floor by myself.” These tasks, although intended to measure lower extremity function, likely capture more global function impacted by UE injury. Alternatively, the strong correlation may be secondary to studying a population of children with UE fractures. A painful, immobilized UE may limit the patient’s overall activity level despite any lower extremity injury.
The peer relationships score is used as a proxy for depressive symptoms and isolation in our orthopedic practice. Interestingly, we found that peer relationships are not related to UE, mobility, or pain interference scores after an UE fracture. This is in contrast to the adult population, where depression and social deprivation are correlated with UE physical function.6,16,19 This lack of correlation in the pediatric population may be due to the acute trauma that was studied. The acuteness of these patient’s injury may not make the children feel “different” for a long period of time and subsequently not cause them to associate the injury with their identity. In this case, the child has a temporary condition that will resolve and is unlikely to change their interactions with their peers. For this reason, we were not surprised by the presence of nearly 1 in 5 patients scoring at the ceiling of the peer relationship survey indicating no issues with social interactions.
The parent-proxy item banks should be completed by the parents or caregivers of children ages 5 to 7 years.24 In our study, parent-proxy surveys indicated more pain interference, less UE function, and worse peer relationships for their child than the self-administrated group. These differences were both statistically significant and clinically relevant as they approached and surpassed the suggested Minimal Clinically Important Difference (MCID) (3-5 points) values for these assessments.1,21 Parent-proxy and self-administered surveys produced similar mobility scores. Prior research found that parental responses and child self-reported PROMIS answers were more congruent on objective scales such as physical function as compared to scales of internal experiences (eg, energy, pain interference).23 In contrast to the work by Varni et al, which queried children and parents from pediatric clinics, we have found greater average differences in scores between those surveys completed by parents and those by the children themselves. That prior study also found greater agreement between children and adult answers when the children had chronic pain, which was postulated to have allowed the adults to better understand the children’s feelings over time. In our patient population with acute fractures, it is possible that children suffering an acute trauma creates a novel experience for the child and the minimal time for the parent to observe the impact of the trauma could contribute to the increased discrepancy in scores that we have identified. As the mode of administration in our clinical practice is dictated by patient age, it is possible that the differences in scoring could be influenced by the age of the patient groups. We have attempted to minimize this possibility by analyzing a narrow age window of 5- to 7-year-olds against 8- to 10-year-olds. Also, given the consistency of differences found when examining each fracture location, we do believe that the differential scoring represents a systematic bias associated with the mode of completion.
The pediatric PROMIS UE function score appropriately appeared to be the PROMIS scale most affected by an UE fracture. However, there appeared to be a substantial ceiling effect. This ceiling effect has been documented to affect 7% to 11% of adult patients with UE conditions.2 This ceiling effect is a relevant concern as those indicating maximal function at initial presentation will be unable to demonstrate any value of treatment delivered. We are not aware of a consensus tolerance for ceiling effects among orthopedic outcome measures but are concerned when this affects nearly 1 in 10 patients. In adults, the ceiling effect has been linked to a lack of highly demanding UE questions. This is likely compounded when delivering similar questions to children who are remarkably adaptable and often find ways to continue the majority of activities despite an UE cast.
Our study has several limitations. Inherent to our cross-sectional design, we are unable to determine how the scores of these patient-reported health assessments collected at presentation impact treatment outcomes. Second, patients were grouped by the anatomic location of fracture sites without further delineation of fracture severity. To minimize potential bias from differential percentages of fracture types impacting on the overall mean PROMIS score difference between groups, we analyzed each subgroup to assure similar PROMIS score patterns across all fracture groups. Finally, all patients and parents were specifically instructed on who should complete the PROMIS survey when handed the mini-tablet computer but we did not monitor these patients and parents as they completed the PROMIS surveys. Therefore, there was still the potential for parents or other siblings to have completed the PROMIS CATs on behalf of patients aged 8 to 10 years or for the patient aged 5 to 7 years to fill out the PROMIS CATs on behalf of the parent.
This study presents an initial experience with pediatric PROMIS in patients with UE fractures. As anticipated, UE function scores were the most impacted by the fractures but a ceiling effect was present. Our data also indicate that the PROMIS UE and mobility components of physical function are strongly correlated and this may warrant potential survey refinement to increase their independence. Finally, among children with UE fractures, parent-proxy completion of pediatric PROMIS magnifies perceived physical impairment, peer relationships, and estimated pain.
Footnotes
Ethical Approval: This study was reviewed by our institutional review board and deemed exempt without need for written consent due to the use of de-identified data.
Statement of Human and Animal Rights: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Statement of Informed Consent: This study was reviewed by our institutional review board and deemed exempt without need for written consent due to the use of de-identified date.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported in this publication was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). This includes TL1 TR000449 – ICTS Clinical Research Predoctoral Training Program. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
ORCID iDs: WD Gerull  https://orcid.org/0000-0002-7722-6046
https://orcid.org/0000-0002-7722-6046
UC Okoroafor  https://orcid.org/0000-0003-4980-5160
https://orcid.org/0000-0003-4980-5160
References
- 1. Amtmann D, Kim J, Chung H, et al. Minimally important differences for Patient Reported Outcomes Measurement Information System pain interference for individuals with back pain. J Pain Res. 2016;9:251-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beckmann JT, Hung M, Voss MW, et al. Evaluation of the Patient-Reported Outcomes Measurement Information System Upper Extremity Computer Adaptive Test. J Hand Surg Am. 2016;41(7):739-744. [DOI] [PubMed] [Google Scholar]
- 3. Bevans M, Ross A, Cella D. Patient-Reported Outcomes Measurement Information System (PROMIS): efficient, standardized tools to measure self-reported health and quality of life. Nurs Outlook. 2014;62(5):339-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Broderick JE, DeWitt EM, Rothrock N, et al. Advances in patient-reported outcomes: the NIH PROMIS(®) measures. EGEMS (Wash DC). 2013;1(1):1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bushnell BD. Bundled payments in orthopedic surgery. Orthopedics. 2015;38(2):128-135. [DOI] [PubMed] [Google Scholar]
- 6. Calfee R, Chu J, Sorensen A, et al. What is the impact of comorbidities on self-rated hand function in patients with symptomatic trapeziometacarpal arthritis? Clin Orthop Relat Res. 2015;473(11):3477-3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63(11):1179-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5)(suppl 1):S3-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cunningham NR, Kashikar-Zuck S, Mara C, et al. Development and validation of the self-reported PROMIS pediatric pain behavior item bank and short form scale. Pain. 2017;158(7):1323-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deshpande PR, Rajan S, Sudeepthi BL, et al. Patient-reported outcomes: a new era in clinical research. Perspect Clin Res. 2011;2(4):137-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DeWitt EM, Stucky BD, Thissen D, et al. Construction of the eight-item patient-reported outcomes measurement information system pediatric physical function scales: built using item response theory. J Clin Epidemiol. 2011;64(7):794-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Evans J. Straightforward Statistics for the Behavioral Sciences. Pacific Grove, CA: Brooks/Cole Publishing; 1996. [Google Scholar]
- 13. Hullmann SE, Ryan JL, Ramsey RR, et al. Measures of general pediatric quality of life: Child Health Questionnaire (CHQ), DISABKIDS Chronic Generic Measure (DCGM), KINDL-R, Pediatric Quality of Life Inventory (PedsQL) 4.0 Generic Core Scales, and Quality of My Life Questionnaire (QoML). Arthritis Care Res (Hoboken). 2011;63 (suppl 11):S420-430. [DOI] [PubMed] [Google Scholar]
- 14. Irwin DE, Gross HE, Stucky BD, et al. Development of six PROMIS pediatrics proxy-report item banks. Health Qual Life Outcomes. 2012;10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Irwin DE, Varni JW, Yeatts K, et al. Cognitive interviewing methodology in the development of a pediatric item bank: a Patient Reported Outcomes Measurement Information System (PROMIS) study. Health Qual Life Outcomes. 2009;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. London DA, Stepan JG, Boyer MI, et al. The impact of depression and pain catastrophization on initial presentation and treatment outcomes for atraumatic hand conditions. J Bone Joint Surg Am. 2014;96(10):806-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morgan EM, Mara CA, Huang B, et al. Establishing clinical meaning and defining important differences for Patient-Reported Outcomes Measurement Information System (PROMIS®) measures in juvenile idiopathic arthritis using standard setting with patients, parents, and providers. Qual Life Res. 2017;26(3):565-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mulcahey MJ, Haley SM, Slavin MD, et al. Ability of PROMIS pediatric measures to detect change in children with cerebral palsy undergoing musculoskeletal surgery. J Pediatr Orthop. 2016;36(7):749-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peters RM, Menendez ME, Mellema JJ, et al. Sleep disturbance and upper-extremity disability. Arch Bone Jt Surg. 2016;4(1):35-40. [PMC free article] [PubMed] [Google Scholar]
- 20. Quinn H, Thissen D, Liu Y, et al. Using item response theory to enrich and expand the PROMIS® pediatric self report banks. Health Qual Life Outcomes. 2014;12:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thissen D, Liu Y, Magnus B, et al. Estimating minimally important difference (MID) in PROMIS pediatric measures using the scale-judgment method. Qual Life Res. 2016;25(1):13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Vliet MM, Maradey JA, Homa KA, et al. The usefulness of patient-reported measures for clinical practice. Plast Reconstr Surg. 2013;132(1):105-112. [DOI] [PubMed] [Google Scholar]
- 23. Varni JW, Thissen D, Stucky BD, et al. Item-level informant discrepancies between children and their parents on the PROMIS(®) pediatric scales. Qual Life Res. 2015;24(8):1921-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Varni JW, Thissen D, Stucky BD, et al. PROMIS® Parent Proxy Report Scales for children ages 5-7 years: an item response theory analysis of differential item functioning across age groups. Qual Life Res. 2014;23(1):349-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wall LB, Patel A, Roberts S, et al. Long-term outcomes of Huber opposition transfer for augmenting hypoplastic thumb function. J Hand Surg Am. 2017;42(8):657-657. [DOI] [PMC free article] [PubMed] [Google Scholar]


