Abstract
Background: Injuries to digital nerves are common with trauma to the hand, often requiring surgery. Surgical management of these injuries can be performed using several techniques: direct repair (neurorrhaphy), autograft, allograft, and conduit repair. In light of increasing the availability and use of various digital nerve repair techniques, a new systematic review and meta-analysis was undertaken to comparatively review the available evidence to determine any differences in outcomes to better guide treatment in cases with digital nerve gaps. Methods: Current literature on sensory outcomes of various digital nerve repair techniques was reviewed using static 2-point discrimination (S2PD), moving 2-point discrimination (M2PD), Semmes-Weinstein monofilament testing (SWMF), and complication rates as outcomes of interest. After inclusion and exclusion criteria were applied, 15 articles were reviewed and 625 nerve repairs were analyzed. Results: The average gap length for allograft repair, autograft repair, and conduit repair was 15.4, 24.7, and 13.4 mm, respectively. For S2PD outcomes, autograft repair was statistically superior to all other forms of repair. Allograft trended higher than neurorrhaphy and conduit repair, but results were not statistically significant. For SWMF outcomes, autograft repair was statistically superior to conduit repair and neurorrhaphy; it was statistically comparable with allograft repair. Allograft performed statistically superior to conduit repair relative to M2PD. Conclusions: Based on the current updated meta-analysis using newer data and techniques, we found that all available techniques have reasonable outcomes. Yet when managing a digital nerve injury with a gap, thereby excluding direct neurorrhaphy, both autograft and allograft performed comparably and were superior to conduit repair.
Keywords: keywords, digital nerve, repair, sensory outcomes, direct suture, allograft, basic science, autograft, conduit
Introduction
Injuries to the digital nerves are common with trauma to the hand. These injuries can result in numbness and impairment of the hand. Furthermore, the impact of the nerve injury may also lead to partial or permanent disability, change in profession, time loss from work, and other economic impacts to the patient.1-3 Surgery is often indicated to repair injured digital nerves.
Surgical management of digital nerve injuries includes several techniques, such as direct suture repair (neurorrhaphy) and the use of autografts, allografts, and synthetic conduits. In lesions with a gap smaller than 5 mm, direct, tension-free, end-to-end neurorrhaphy has traditionally been the preferred repair method. When direct repair is not possible, grafting techniques and conduit repair are often implemented. When autograft repair is selected, typical sources include the sural nerve, medial antebrachial cutaneous nerve, and posterior interosseous nerve. However, the use of autologous nerve grafting carries the risk, time, and cost of additional harvest surgery with secondary donor site morbidity, including sensory loss, neuroma, and scar formation. More recently, processed nerve allografts have become available. These allografts provide decellularized and predegenerated human nerve tissue for use in restoring nerve continuity. Potential complications associated with the allograft include infectious disease transmission. Finally, synthetic conduits have become a commonly used technique for nerve repair with a gap. Advantages of conduit repair over autograft repair include elimination of harvest surgery and subsequently the absence of donor site morbidity and potential protective effects against neuroma formation.4
Previous meta-analyses have been conducted to compare sensory outcomes on the various surgical repair techniques for digital nerve lesions. In 2012, Mermans et al5 reviewed 34 articles comparing direct suture (neurorrhaphy) repair, autograft repair, and conduit repair and concluded that the type of operation for digital nerve repair does not influence the sensory outcome. A meta-analysis and systematic review by Paprottka et al4 in 2013 reviewed 87 publications comparing direct suture (neurorrhaphy) repair, autograft repair, conduit repair, vein graft repair, end-to-side repair, and replantation repairs and concluded that, for sensory outcomes, no surgical method was superior to another for digital nerve repair. However, neither of these prior meta-analyses considered or compared allograft nerve repair.
In light of the increasing availability and use of various digital nerve repair constructs, including allograft options of late, an updated meta-analysis was undertaken to comparatively review the available evidence to determine differences in outcomes.
Methods
A systematic literature review was performed. PubMed was searched using keywords “digital nerve,” “repair,” “sensory outcomes,” “direct suture,” “autograft,” “allograft,” “avance,” “conduit,” and “nerve tube.” The search was kept broad to capture all the relevant literature. All articles in print or Epub ahead of print were captured. The search was restricted to articles published between 1975 and 2018.
Initial screening was based on article title and information in article abstracts involving digital nerve suture repair, allograft, autograft, and conduit repair. All articles thought to be relevant to this study were included for review. Inclusion criteria were observational cohort studies and randomized controlled trials that reported at least 2 of the following 4 outcome parameters in patients undergoing surgery for digital nerve lesions: sensory outcomes using static 2-point discrimination (S2PD), moving 2-point discrimination (M2PD), Semmes-Weinstein monofilament testing (SWMF), and complication rates. Exclusion criteria were peripheral nerve lesions not localized to the digital nerves in the hand and surgical techniques that used other repair constructs such as vein grafts or muscle grafts. Studies with pediatric patients were excluded. Studies were then selected for full-text review.
A modified classification system derived from Mackinnon et al was used to group S2PD outcomes. Scores ≤6 mm were considered “excellent,” scores from 6 to 15 mm were considered “good,” and scores >15 mm were considered “poor.” In addition, a modified classification system from Mackinnon et al was used to group M2PD outcomes. Scores ≤3 mm were classified as “excellent,” scores from 4 to 7 mm were classified as “good,” and scores >7 mm were classified as “poor.”6 A modified classification system derived from Imai et al was used to group SWMF outcomes. Patients with scores ≤2.83 were considered “normal” for sensation. Patients with scores from 2.83 to 4.31 were considered to have “diminished light touch,” with scores from 4.31 to 4.56 were considered to have “diminished protective sensation,” with scores from 4.56 to 6.10 were considered to have “loss of protective sensation,” and with scores >6.10 were considered “anesthetic.”7
Standardized data extraction was performed. Categorical outcomes included S2PD scores, M2PD scores, SWMF scores, and presence of complications. The S2PD and SWMF scores were grouped according to a determined classification system (see below) and analyzed using χ² analysis. The M2PD scores were grouped according to a determined classification system but were analyzed using the Fisher exact test (see below). The presence of complications was analyzed with the Fisher exact test.
Results
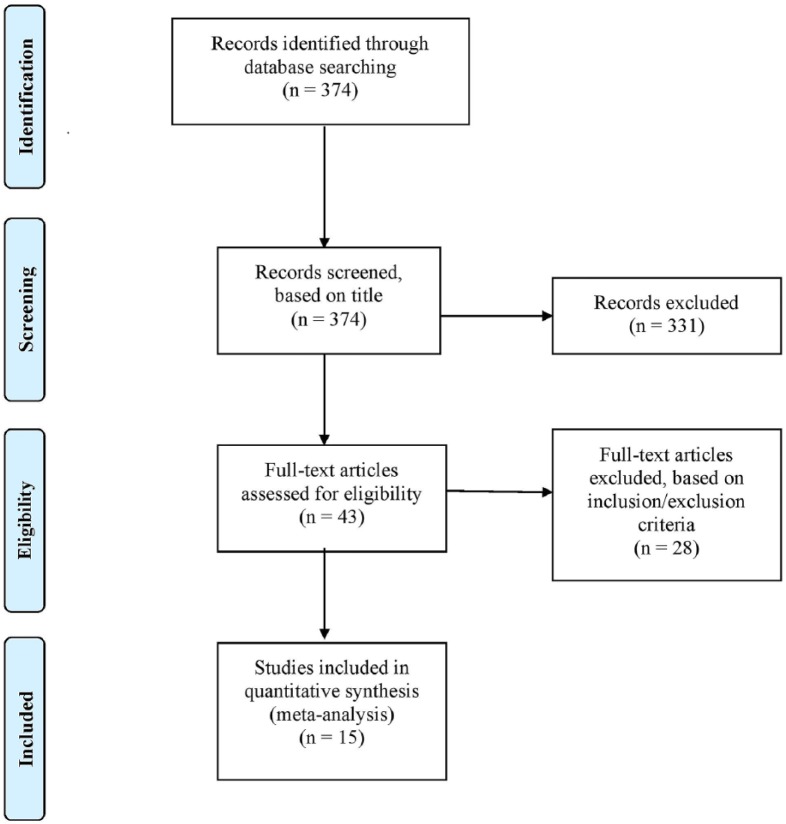
Initial search identified 374 results using keywords. Articles were screened based on title, and 331 were excluded for being irrelevant to the topic or non-English. Forty-three studies were selected for full-text review. Twenty-eight were eliminated for failure to meet inclusion criteria. Thus 15 articles were included in the final data analysis.8-22 Figure 1 details the database search and study selection.
Figure 1.
Chart of search method and study selection.
Demographic data for each nerve repair type are given in Table 1. The mean age of patients undergoing neurorrhaphy, allograft repair, autograft repair, and conduit repair was 36.4, 36.2, 33.8, and 38.6 years, respectively. The mean follow-up for neurorrhaphy, allograft repair, autograft repair, and conduit repair was 13.3, 9.4, 23.2, and 21.1 months, respectively. The average gap length for allograft repair, autograft repair, and conduit repair was 15.4, 24.7, and 13.4 mm, respectively.
Table 1.
Demographics by Repair Type..
| Demographics for data evaluating outcomes after neurorrhaphy | ||||||||
|---|---|---|---|---|---|---|---|---|
| Article | Year | Intervention | Design | No. of subjects | Nerves repaired | Mean age, y | Mean FU, mo | |
| He et al8 | 2015 | Neurorrhaphy vs allograft | Prospective | 81 | 123 | 36.9 | 6 | |
| Bulut et al9 | 2016 | Neurorrhaphy | Retrospective | 63 | 96 | 36.4 | 21.4 | |
| Arnaout et al10 | 2014 | Neurorrhaphy with protective conduit vs without protection | Prospective | 24 | 27 | 38 | 6 | |
| Hirasawa et al11 | 1985 | Neurorrhaphy | Prospective | 12 | 12 | 29.9 | 35 | |
| Total | — | — | — | 180 | 258 | — | — | |
| Weighted mean values | — | — | — | — | — | 36.4 | 13.3 | |
| Demographics for data evaluating outcomes after allograft repair | ||||||||
| Article | Year | Intervention | Design | No. of subjects | Nerves repaired | Gap length, mm | Mean age, y | Mean FU, mo |
| He et al8 | 2015 | Allograft vs neurorrhaphy | Prospective | 72 | 95 | 18 | 33 | 6 |
| Rinker et al12 | 2015 | Allograft | Retrospective | 24 | 33 | 11 | 43 | 16 |
| Means et al13 | 2016 | Allograft vs conduit | Prospective | 5 | 6 | 12.8 | 42 | 12 |
| Taras et al14 | 2013 | Allograft | Prospective | 14 | 18 | 11 | 39 | 15 |
| Total | — | — | — | 115 | 152 | — | — | — |
| Weighted mean values | — | — | — | — | — | 15.4 | 36.2 | 9.4 |
| Demographics for data evaluating outcomes after autograft repair | ||||||||
| Article | Year | Intervention | Design | No. of subjects | Nerves repaired | Gap length, mm | Mean age, y | Mean FU, mo |
| Chevrollier et al15 | 2014 | Autograft | Retrospective | 15 | 15 | 37.5 | 41.3 | 26.9 |
| Chen et al16 | 2012 | Pedicle autograft vs sural and MABC autograft | Prospective | 16 | 16 | 24.7 | 33 | 21.9 |
| Chen et al12 | 2012 | Sural and MABC autograft vs pedicle autograft | Prospective | 27 | 27 | 23.6 | 32.1 | 22.3 |
| Chen et al13 | 2013 | Proper digital nerve autograft vs sural autograft | Prospective | 17 | 21 | 23 | 31.9 | 25 |
| Chen et al13 | 2013 | Sural autograft vs proper digital nerve autograft | Prospective | 31 | 31 | 24 | 31 | 23 |
| Pilanci et al18 | 2014 | Autograft | Prospective | 15 | 15 | 18.1 | 38.5 | 20.7 |
| Total | — | — | — | 121 | 125 | — | — | — |
| Weighted mean values | — | — | — | — | — | 24.7 | 33.8 | 23.2 |
| Demographics for data evaluating outcomes after conduit repair | ||||||||
| Article | Year | Intervention | Design | No. of subjects | Nerves repaired | Gap length, mm | Mean age, y | Mean FU, mo |
| Means et al13 | 2016 | Conduit vs allograft | Prospective | 7 | 9 | 12.2 | 38 | 6 |
| Bushnell et al19 | 2008 | Conduit | Prospective | 9 | 9 | 20 | 35.4 | 15 |
| Lohmeyer et al20 | 2014 | Conduit | Prospective | 35 | 40 | 12.3 | 37.9 | 12 |
| Schmauss21 | 2014 | Conduit | Retrospective | 16 | 20 | 13.7 | 43.2 | 58 |
| Lohmeyer et al22 | 2009 | Conduit | Retrospective | 12 | 12 | 12.5 | 37.3 | 12 |
| Total | — | — | — | 79 | 90 | — | — | — |
| Weighted mean values | — | — | — | — | — | 13.4 | 38.6 | 21.1 |
Note. FU = follow-up; MABC = medial antebrachial cutaneous nerve.
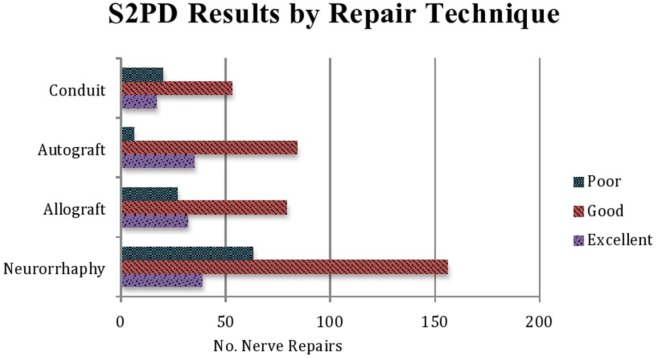
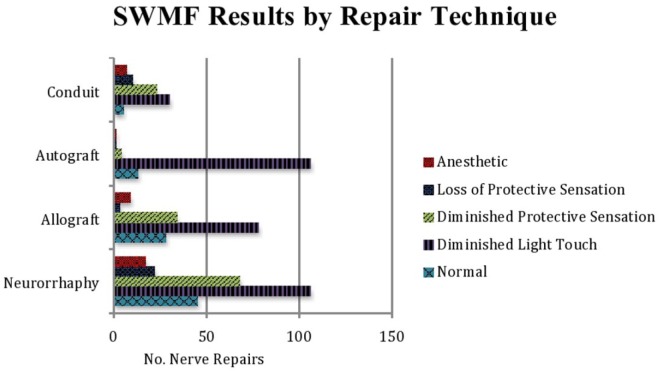
The S2PD, M2PD, and SWMF sensory results are summarized in Table 2 (χ2 = 26.9269; P < .000149) and Figure 2, Table 3 (P < .00001), and Table 4 (χ2 = 93.7396; P < .00001) and Figure 3, respectively.
Table 2.
Comparison of Static 2-Point Discrimination Outcomes Across Surgical Procedures.
| Neurorrhaphy | Allograft | Autograft | Conduit | Total | |
|---|---|---|---|---|---|
| Excellent: ≤6 mm | 39 (15%) | 32 (23%) | 35 (28%) | 17 (19%) | 122 |
| Good: 6-15 mm | 156 (60%) | 79 (57%) | 84 (67%) | 53 (59%) | 373 |
| Poor: >15 mm | 63 (24%) | 27 (20%) | 6 (5%) | 20 (22%) | 116 |
| Total | 258 | 138 | 125 | 90 | 611 |
Note. χ2 = 26.9269; P < .0001.
Figure 2.
Static 2-point discrimination results for each repair technique. Scores ≤6 mm were considered “excellent,” scores from 6 to 15 mm were considered “good,” and scores >15 mm were considered “poor.”
Table 3.
Comparison of Moving 2-Point Discrimination Across Surgical Procedures.
| Neurorrhaphy | Allograft | Conduit | Total | |
|---|---|---|---|---|
| Excellent: ≤3 mm | 8 (67%) | 1 (2%) | 0 (0%) | 9 |
| Good: 4-7 mm | 3 (25%) | 35 (88%) | 6 (67 %) | 44 |
| Poor: >7 mm | 1 (8%) | 4 (10%) | 3 (33%) | 8 |
| Total | 12 | 40 | 9 | 61 |
Note. P < .00001 using the Fisher exact test.
Table 4.
Comparison of Semmes-Weinstein Monofilament Testing Outcomes Across Surgical Procedures.
| Neurorrhaphy | Allograft | Autograft | Conduit | Total | |
|---|---|---|---|---|---|
| Normal: ≤2.83 | 45 (17%) | 28 (18%) | 13 (10%) | 5 (7%) | 91 |
| Diminished light touch: 2.83-4.31 | 106 (41%) | 78 (51%) | 106 (85%) | 30 (40%) | 320 |
| Diminished protective sensation: 4.31-4.56 | 68 (26%) | 34 (22%) | 4 (3%) | 23 (31%) | 129 |
| Loss of protective sensation: 4.56-6.10 | 22 (9%) | 3 (2%) | 1 (1%) | 10 (13%) | 36 |
| Anesthetic: >6.10 | 17 (7%) | 9 (6%) | 1 (1%) | 7 (9%) | 34 |
| Total | 258 | 152 | 125 | 75 | 610 |
Note. χ2 = 93.7396; P < .00001.
Figure 3.
Semmes-Weinstein monofilament testing results for each repair type. Scores ≤2.83 were considered “normal,” scores from 2.83 to 4.31 were considered “diminished light touch,” scores from 4.31 to 4.56 were considered “diminished protective sensation,” scores from 4.56 to 6.10 were considered “loss of protective sensation,” and scores >6.10 were considered “anesthetic.”
For S2PD outcomes, autograft repair was found to have the highest percentage of repairs with “excellent” sensory outcome followed by allograft repair, conduit repair, and neurorrhaphy (28% vs 23% vs 19% vs 15%). The majority of each repair type, however, resulted in “good” outcomes (neurorrhaphy, 60%; allograft, 57%; autograft, 67%; and conduit, 59%). However, when “excellent” and “good” results were combined for all repair types and compared for S2PD (Supplemental Table S1), the autograft repair was statistically superior to allograft (P < .001), conduit (P < .005), and neurorrhaphy (P < .0001). In contrast, outcomes trended higher for allograft repair among the other repair techniques, but were not statistically significant. Finally, autograft repair was also associated with the lowest percentage of “poor” S2PD outcomes, followed by allograft, conduit, and neurorrhaphy (5% vs 20% vs 22% vs 24%).
Of the 4 studies that included M2PD as a sensory outcome (none of the autograft studies included this outcome measure), most of the neurorrhaphy repairs resulted in “excellent” M2PD results (67%), whereas most of the allograft repairs (88%) and conduit repairs (67%) resulted in “good” M2PD sensory outcomes. Neurorrhaphy also had the lowest percentage of “poor” outcomes, followed by allograft and conduit repair (8% vs 10% vs 33%). However, when “excellent” and “good” results were combined for all repair types and compared for M2PD (Supplemental Table S2), there was no statistical difference between direct repair and allograft repairs (P = .60), whereas both were statistically superior to conduit repair (P < .0001).
For SWMF outcomes, allograft repair reported the highest percentage of “normal” sensation, followed by neurorrhaphy, autograft, and conduit repair (18% vs 17% vs 10% vs 7%). Most SWMF outcomes for each repair technique were in the “diminished light touch” category (85% of autograft repairs, 51% of allograft repairs, 41% of neurorrhaphy, and 40% of conduit repairs). However, when normal sensation, diminished light touch, and diminished protective sensation were combined and then compared with the rate of loss of protective sensation and anesthetic outcomes (Supplemental Table S3), there was no statistical difference between autograft and allograft repairs (P = .052), whereas both autograft and allograft repairs were statistically superior to conduit repair (P < .01). Moreover, allograft showed the highest percentage of “normal” repairs (18%), followed by neurorrhaphy (17%), autograft (10%), and conduit repairs (7%). When “normal” sensory outcomes were compared (Supplemental Table S4), neurorrhaphy (P < .03) and allograft repair (P < .02) were statistically superior to conduit repair. No significant difference (P = .1) was present between autograft and allograft repairs. Autograft repair reported the lowest percentage of “anesthetic” SWMF results, followed by allograft repair, neurorrhaphy, and conduit repair (1% vs 6% vs 7% vs 9%). When a number of “anesthetic” repairs were compared with all other outcomes (Supplemental Table S5), autograft repair showed a statistically significant decrease in the number of “anesthetic” outcomes than did neurorrhaphy (P < .05) and conduit repair (P < .01). There was no significant difference (P = .054) in the number of “anesthetic” outcomes among allograft and autograft repairs.
Only 4 studies detailed surgical complications. Allograft complications were reported as all minor, consisting of prolonged pain, effusion, or wound exudate production for greater than 2 weeks after operation. All autograft complications were reported as donor site complications. Conduit repair complications consisted of infection and prolonged pain. Neurorrhaphy cases had no complications documented. Overall, the rate and extent of complication reporting were poor and inconsistent, thereby limiting the ability to provide comparative complication outcomes.
Discussion
The choice of surgical repair technique of digital nerve lesions continues to be a point of controversy. Surgical options for repair include neurorrhaphy or use of an autograft, allograft, or synthetic conduit. Prior studies have shown that sensory outcomes for neurorrhaphy, autograft, and conduit techniques are similar.4,5 The aim of this study was to provide an updated systematic review and meta-analysis of the sensory outcomes and complication rates of neurorrhaphy, autograft use, conduit use, and now more recently with allograft repair, in the management of digital nerve lesions. This updated meta-analysis compared sensory outcomes using S2PD, M2PD, and SWMF.
For the S2PD outcome, autograft repair resulted in the greatest chance of “excellent” sensory recovery and the lowest chance of “poor” sensory recovery. Yet, there was no statistical difference between autograft and allograft repairs. Moreover, most of the S2PD outcomes in all repair types result in “excellent” and “good” sensory recovery. Similarly, for SWMF outcomes, there was no difference between autograft and allograft repairs, although allograft had the highest percentage of “normal” sensation at 17% versus 10% with autograft repair. In 2015, He et al reported on the use of allograft versus neurorrhaphy using S2PD as the primary outcome. The study concluded that the allograft technique is comparable with neurorrhaphy in terms of efficacy and safety.6 Results of our study agree with these findings in terms of efficacy. For S2PD outcomes, 75% of neurorrhaphy results were “excellent” or “good,” whereas 80% of allograft results were also “excellent” or “good” (P = .5). Furthermore, in 2016, Means et al compared S2PD outcomes in allograft repair with conduit repair.13 The study concluded that allograft repair resulted in more improved sensory outcome than conduits. In contrast, our study found that although allograft trended toward higher S2PD outcomes relative to conduit, when combining “excellent” and “good” S2PD results, we found no significant difference between allograft and conduit sensory outcomes (P = .7).
Fewer studies reported sensory outcomes for M2PD. No studies that measured autograft techniques included M2PD as an outcome measure. Based on data provided, direct repair and allograft were found to provide the highest chance of “excellent” M2PD outcomes and the lowest chance for “poor” M2PD outcomes in our analysis. Similarly, Means et al concluded that allograft repair resulted in better M2PD outcomes than conduit repair. A subanalysis of our updated meta-analysis agrees with this conclusion—that a higher percentage of allograft repairs results in “excellent” and “good” outcomes than do conduit repairs (P < .0001).
Few complications were reported across all repair techniques. Moreover, when they were reported, they were either minor or inconsistently identified and reported. Therefore, unfortunately, no meaningful data can be drawn on complications in our analysis for the various nerve repair techniques.
Limitations to this study include the inherent nature of meta-analysis relying on the quality of studies from which data were extracted. The study is limited by publication bias for the individual studies analyzed. In addition, not all studies had each of the sensory outcomes and complication rates that we sought to measure. Furthermore, not every study reported S2PD, M2PD, or SWMF outcomes in the same manner. We were forced to use 2 modified classification systems to group the results into categories that were comparable across sensory outcomes.
Conclusions
As there continues to be improvement in surgical technology, technique, and literature on sensory outcomes of digital nerve repair, it is important to re-examine the surgical approaches and their sensory outcomes. Evaluation of current and future sensory outcomes can help to elucidate a superior method of repair. This study was a systematic review and meta-analysis of the most recent literature on digital nerve lesion surgical repair techniques (neurorrhaphy, allograft, autograft, and conduit repair) and their sensory outcomes. Based on the current updated meta-analysis using newer data and techniques in the management of digital nerve injuries with gaps, we found that autograft and allograft repair are comparable, with both providing generally superior sensory outcomes than conduit repair.
Supplemental Material
Supplemental material, Supplemental_table_S1 for Sensory Outcomes in Digital Nerve Repair Techniques: An Updated Meta-analysis and Systematic Review by Zachary J. Herman and Asif M. Ilyas in Hand
Supplemental material, Supplemental_Table_S2 for Sensory Outcomes in Digital Nerve Repair Techniques: An Updated Meta-analysis and Systematic Review by Zachary J. Herman and Asif M. Ilyas in Hand
Supplemental material, Supplemental_Table_S3 for Sensory Outcomes in Digital Nerve Repair Techniques: An Updated Meta-analysis and Systematic Review by Zachary J. Herman and Asif M. Ilyas in Hand
Supplemental material, Supplemental_Table_S4 for Sensory Outcomes in Digital Nerve Repair Techniques: An Updated Meta-analysis and Systematic Review by Zachary J. Herman and Asif M. Ilyas in Hand
Supplemental material, Supplemental_Table_S5 for Sensory Outcomes in Digital Nerve Repair Techniques: An Updated Meta-analysis and Systematic Review by Zachary J. Herman and Asif M. Ilyas in Hand
Footnotes
Supplemental material for this article is available online.
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: No experimentation or procedures were performed on animals or humans during this study.
Statement of Informed Consent: No informed consent was required for this meta-analysis and systematic review.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Jaquet J, Luijsterburg AJ, Kalmijn S, et al. Median, Ulnar, and combined median-Ulnar nerve injuries: functional outcome and return to productivity. J Trauma. 2001;51(4):687-692. [DOI] [PubMed] [Google Scholar]
- 2. Rosberg HE, Carlsson KS, Höjgård S, et al. Injury to the human median and Ulnar nerves in the forearm—analysis of costs for treatment and rehabilitation of 69 patients in southern Sweden. J Hand Surg Br. 2005;30(1):35-39. [DOI] [PubMed] [Google Scholar]
- 3. Renner A, Cserkuti F, Hankiss J. Late results after nerve transplantation on the upper extremities. Handchir Mikrochir Plast Chir. 2004;36(1):13-18. [DOI] [PubMed] [Google Scholar]
- 4. Paprottka FJ, Wolf P, Harder Y, et al. Sensory recovery outcome after digital nerve repair in relation to different reconstructive techniques: meta-analysis and systematic review. Plast Surg Int. 2013;2013:704589-704517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mermans JF, Franssen BB, Serroyen J, et al. Digital nerve injuries: a review of predictors of sensory recovery after microsurgical digital nerve repair. Hand. 2012;7(3):233-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mackinnon SE, Dellon AL. Clinical nerve reconstruction with a bioabsorbable polyglycolic acid tube. Plast Reconstr Surg. 1990;85(3):419-424. [DOI] [PubMed] [Google Scholar]
- 7. Imai H, Tajima T, Natsuma Y. Interpretation of cutaneous pressure threshold (Semmes-Weinstein monofilament measurement) following median nerve repair and sensory reeducation in the adult. Microsurgery. 1989;10(2):142-144. [DOI] [PubMed] [Google Scholar]
- 8. He B, Zhu Q, Chai Y, et al. Safety and efficacy evaluation of a human acellular nerve graft as a digital nerve scaffold: a prospective, multicentre controlled clinical trial. J Tissue Eng Regen Med. 2015;9(3):286-295. [DOI] [PubMed] [Google Scholar]
- 9. Bulut T, Akgün U, Citlak A, et al. Prognostic factors in sensory recovery after digital nerve repair. Acta Orthop Traumatol Turc. 2016;50(2):157-161. [DOI] [PubMed] [Google Scholar]
- 10. Arnaout A, Fontaine C, Chantelot C. Sensory recovery after primary repair of palmar digital nerves using a Revolnerv® collagen conduit: a prospective series of 27 cases. Chir Main. 2014;33:279-285. [DOI] [PubMed] [Google Scholar]
- 11. Hirasawa Y, Katsumi Y, Tokioka T. Evaluation of sensibility after sensory reconstruction of the thumb. J Bone Joint Surg Br. 1985;67(5):814-819. [DOI] [PubMed] [Google Scholar]
- 12. Rinker B, Ingari J, Greenberg J, et al. Outcomes of short-gap sensory nerve injuries reconstructed with processed nerve allografts from a multicenter registry study. J Reconstr Microsurg. 2015;31(5):384-390. [DOI] [PubMed] [Google Scholar]
- 13. Means KR, Jr, Rinker BD, Higgins JP, et al. A multicenter, prospective, randomized, pilot study of outcomes for digital nerve repair in the hand using hollow conduit compared with processed allograft nerve. Hand. 2016;11(2):144-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taras JS, Amin N, Patel N, et al. Allograft nerve reconstruction for digital nerve loss. J Hand Surg Am. 2013;38:1965-1971. [DOI] [PubMed] [Google Scholar]
- 15. Chevrollier J, Pedeutour B, Dap F, et al. Evaluation of emergency nerve grafting for proper palmar digital nerve defects: a retrospective single centre study. Orthop Traumatol Surg Res. 2014;100(6):605-610. [DOI] [PubMed] [Google Scholar]
- 16. Chen C, Tang P, Zhang X. Reconstruction of proper digital nerve defects in the thumb using a pedicle nerve graft. Plast Reconstr Surg. 2012;130(5):1089-1097. [DOI] [PubMed] [Google Scholar]
- 17. Chen C, Tang P, Zhang X. Finger sensory reconstruction with transfer of the proper digital nerve dorsal branch. J Hand Surg Am. 2013;38(1):82-89. [DOI] [PubMed] [Google Scholar]
- 18. Pilanci O, Ozel A, Basaran K, et al. Is there a profit to use the lateral antebrachial cutaneous nerve as a graft source in digital nerve reconstruction. Microsurgery. 2014;34(5):367-371. [DOI] [PubMed] [Google Scholar]
- 19. Bushnell B, McWilliams A, Whitener G, et al. Early clinical experience with collagen nerve tubes in digital nerve repair. J Hand Surg Am. 2008;33(7):1081-1087. [DOI] [PubMed] [Google Scholar]
- 20. Lohmeyer J, Kern Y, Schmauss D, et al. Prospective clinical study on digital nerve repair with collagen nerve conduits and review of literature. J Reconstr Microsurg. 2014;30(4):227-234. [DOI] [PubMed] [Google Scholar]
- 21. Schmauss D, Finck T, Liodaki E, et al. Is nerve regeneration after reconstruction with collagen nerve conduits terminated after 12 months? The long-term follow-up of two prospective clinical studies. J Reconstr Microsurg. 2014;30(8):561-568. [DOI] [PubMed] [Google Scholar]
- 22. Lohmeyer J, Siemers F, Machens H, et al. The clinical use of artificial nerve conduits for digital nerve repair: a prospective cohort study and literature review. J Reconstr Microsurg. 2009;25(1):55-61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_table_S1 for Sensory Outcomes in Digital Nerve Repair Techniques: An Updated Meta-analysis and Systematic Review by Zachary J. Herman and Asif M. Ilyas in Hand
Supplemental material, Supplemental_Table_S2 for Sensory Outcomes in Digital Nerve Repair Techniques: An Updated Meta-analysis and Systematic Review by Zachary J. Herman and Asif M. Ilyas in Hand
Supplemental material, Supplemental_Table_S3 for Sensory Outcomes in Digital Nerve Repair Techniques: An Updated Meta-analysis and Systematic Review by Zachary J. Herman and Asif M. Ilyas in Hand
Supplemental material, Supplemental_Table_S4 for Sensory Outcomes in Digital Nerve Repair Techniques: An Updated Meta-analysis and Systematic Review by Zachary J. Herman and Asif M. Ilyas in Hand
Supplemental material, Supplemental_Table_S5 for Sensory Outcomes in Digital Nerve Repair Techniques: An Updated Meta-analysis and Systematic Review by Zachary J. Herman and Asif M. Ilyas in Hand