Abstract
Background: We have reported that bioactive sutures coated with bone marrow–derived mesenchymal stem cells (BMSCs) enhance tendon repair strength in an in vivo rat model. We have additionally shown that growth differentiation factor 8 (GDF-8, also known as myostatin) simulates tenogenesis in BMSCs in vitro. The purpose of this study was to determine the possibility of BMSC-coated bioactive sutures treated with GDF-8 to increase tendon repair strength in an in vivo rabbit tendon repair model. Methods: Rabbit BMSCs were grown and seeded on to 4-0 Ethibond sutures and treated with GDF-8. New Zealand white rabbits’ bilateral Achilles tendons were transected and randomized to experimental (BMSC-coated bioactive sutures treated with GDF-8) or plain suture repaired control groups. Tendons were harvested at 4 and 7 days after the surgery and subjected to tensile mechanical testing and quantitative polymerase chain reaction. Results: There were distinguishing differences of collagen and matrix metalloproteinase RNA level between the control and experimental groups in the early repair periods (day 4 and day 7). However, there were no significant differences between the experimental and control groups in force to 1-mm or 2-mm gap formation or stiffness at 4 or 7 days following surgery. Conclusions: BMSC-coated bioactive sutures with GDF-8 do not appear to affect in vivo rabbit tendon healing within the first week following repair despite an increased presence of quantifiable RNA level of collagen. GDF-8’s treatment efficacy of the early tendon repair remains to be defined.
Keywords: tendon repair, myostatin, stem cells, bone marrow derived, in vivo
Introduction
Flexor tendon injuries in the hand are quite common. Although new suture techniques and postoperative rehabilitation protocols have improved clinical outcomes following these injuries,11,18,23,26 complications such as adhesions and repair ruptures have not been solved entirely.24,35 To reduce the development of adhesions, early tendon gliding is necessary. However, early motion may promote gap formation or repair ruptures in the early healing process. These issues are considered related to the tendon’s intrinsically poor capacity for healing as a result of its hypocellular nature.28 Several researches have done several in vitro studies on bone marrow–derived mesenchymal stem cells (BMSCs) for tendon healing.9,20,21,34,36 They found that BMSC-seeded gel patch transplantation has the potential to enhance flexor tendon healing in vitro and that platelet-rich plasma and growth differentiation factor 5 (GDF-5) could enhance the effect.9,20,34 They also showed that BMSCs could survive up to 2 weeks in vitro, organize along the collagen fibers on the tendon sections, and express a marker of tendon phenotype, tenomodulin.21 Using an in vivo canine model, Amadio et al lacerated and repaired flexor digitorum profundus tendons. Histologically, tendons treated with BMSCs and stimulated with GDF-5 had a smooth surface with intrinsic healing.36 Uysal et al made the same finding using adipose-derived stem cells for rabbit’s Achilles tendon repair (n = 6).29
In our previous studies, we have reported that BMSCs seeded on to commercial suture can infiltrate and repopulate the acellular zone within the injured tendon while remaining metabolically active.31,32 We have also shown that tendons repaired using the BMSC-coated sutures have greater strength than control tendons (without BMSCs) in an in vivo rat model.33 Furthermore, we have focused on the potential beneficial effects of GDF-8 (also known as myostatin), regarded as a regulator of the structure and function of tendon tissue by inducing the expression of type I collagen and increasing the proliferation of tendon fibroblasts.14 We have previously shown that GDF-8 stimulates tenogenesis in BMSCs in vitro.13 Based on these earlier studies, we hypothesized that GDF-8 may stimulate differentiation of BMSCs toward a tenocyte lineage and increase their ability to activate repair of injured tendons using our bioactive sutures.
The purpose of the current study was to determine the effect of BMSC-coated bioactive sutures treated with GDF-8 on tendon strength in an in vivo rabbit tendon repair model.
Materials and Methods
Isolation and Treatment of Rabbit BMSCs
Rabbit bone marrow stem cells were purchased from Cyagen (Cat#: RBXMX-01001). According to the manufacturer’s protocol, the cells were grown in OriCell™MSC Growth Medium (Cat#: GUXMX-9001) and changed every 3 days. When the cells were approximately 80% to 90% confluent, they were dissociated with Trypsin-EDTA and passaged.
The experiments were carried out at fourth passage. Recombinant myostatin (R&D Systems, Minneapolis, Minnesota) was dissolved into the serum-free media at a final concentration of 500 ng/mL which we found to be the suitable concentration for tenogenesis in our previous in vitro studies.13
Cell Seeding on 4-0 Ethibond Suture
4-0 Ethibond Excel braided polyester sutures (Ethicon Inc, Somerville, New Jersey) were cut into 15 cm segments and coated with 25 ng/mL ICAM (Invitrogen) in 1× PBS for 9 hours on ice, followed by 10 µg/mL poly-l-lysine (Sigma) and 10 ng/mL collagen 4 (Sigma) overnight at 37°C. These conditions were previously identified as suitable for cellular adhesion in our previous studies.13,14,31,32,33 The sutures were then rinsed with warm PBS and transferred to ultra-low attachment sterile polystyrene culture dishes (Corning) at cell density of 4 × 106 cells in 0.5 mL of media, and incubated for 3 days at 37°C in a humidified chamber enriched with 5% CO2.31
Surgical Procedure
The animal experimental protocol was approved by the Animal Care and Use Committee at our institution. All institutional and national guidelines for the care and use of laboratory animals were followed. Eleven- to 13-week-old (3-4 kg) male New Zealand white rabbits (Charles River Laboratories, Inc, Wilmington, Massachusetts) were used in the protocol. Eighteen rabbits were divided into 2 groups for assessment at 4 (n = 9) and 7 (n = 9) days following surgical treatment. The animals were internally controlled by performing the surgery on the bilateral hindlimbs in a blinded, randomized fashion.
The animals were induced with subcutaneous injections of ketamine (35 mg/kg body weight) and xylazine (5 mg/kg body weight) and maintained on isoflurane inhalational anesthesia. A 3-cm longitudinal skin incision was made along the posterior aspect of the hind limb. The dissection was performed down to the paratenon overlying the Achilles tendon, in line with the skin incision. The tendon was gently dissected and care was taken to minimize dissection of the anterior aspect of the tendon to prevent additional vascular insult to the tendon. The tendon was incised transversely with a sharp scalpel 1 cm from the osteotendinous junction and repaired with epitendinous suture using 3-0 Prolene (Ethicon Inc, Somerville, New Jersey) and Kessler stitch using 4-0 Ethibond sutures. We then detached the distal insertion of the Achilles tendon to off-load the tendon repair. The distal attachment of the Achilles’ tendons was detached together with its enthesis and approximately 5 mm of bone from the calcaneum. The procedure was repeated for the contralateral limb and the tendons were randomized to repair with either experimental (BMSC-coated bioactive sutures treated with GDF-8) or plain suture control groups. Once both repairs were completed, the wounds were closed with 4-0 Prolene. To standardize the repairs: (1) the surgeon was blinded to which limb was treated with which suture; (2) the same surgeon performed all the surgical procedures; and (3) left and right limbs were dissected in succession with the identical technique. After surgery, the animals were allowed unrestricted activity and received food and water ad libitum.
Tendon Harvest
The rabbits underwent pentobarbital euthanasia at the designated time points (4 and 7 days). Through the previous skin incisions, dissection was performed proximal and distal to the repair site. The gastrocnemius muscle was transected through the muscle belly well proximal to the tendon repair. The harvested specimens were then immersed in PBS and immediately frozen to −20°C until biomechanical analysis was performed.
RNA Isolation and Real-Time Reverse Transcription PCR
Five rabbits each at day 4 and day 7 were used for analysis of gene expression. Every sample was tested in triplicate for each gene. Total RNA was extracted from the tissue by Trizol reagent (Life Technologies, Carlsbad, California) according to the manufacturer’s instructions. Two micrograms of total RNA was reverse transcribed into complementary DNA (cDNA) by High Capacity cDNA Reverse Transcription Kits (Applied Biosystems, Foster City, California). Finally, real-time polymerase chain reaction (PCR) was performed using the ABI 7900 HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). The relative gene expression levels were presented as 2−ΔCt, whereΔCt = Cttarget gene – CtGAPDH.
Mechanical Analysis
The specimens were thawed to room temperature on the day of mechanical testing. Tensile testing was performed on an Instron 5944 materials testing system using a 2 KN load cell (Instron Corp, Norwood, Massachusetts). The load cell accuracy was verified to be within 0.5% for loads from 0.2% to 100% full scale. A universal joint was incorporated into the setup to reduce off axis loading. The proximal end of the tendon was bonded to 100-grit sandpaper using cyanoacrylate (CA) glue and then attached to the upper grip on the test machine. The calcaneus was attached directly to the base grip. Prior to testing, spherical markers (2 mm diameter) were attached approximately 7 mm proximal and distal to the repair site using CA glue (Figure 1). Marker separation (gap formation) was monitored throughout testing with a model acA1600-20gm CCD camera (Basler Inc, Exton, Pennsylvania) fitted with a Zoom 7000 lens (Navitar Inc, Rochester, New York). The accuracy of this measurement method is approximately 0.06 mm (root-mean-square).
Figure 1.
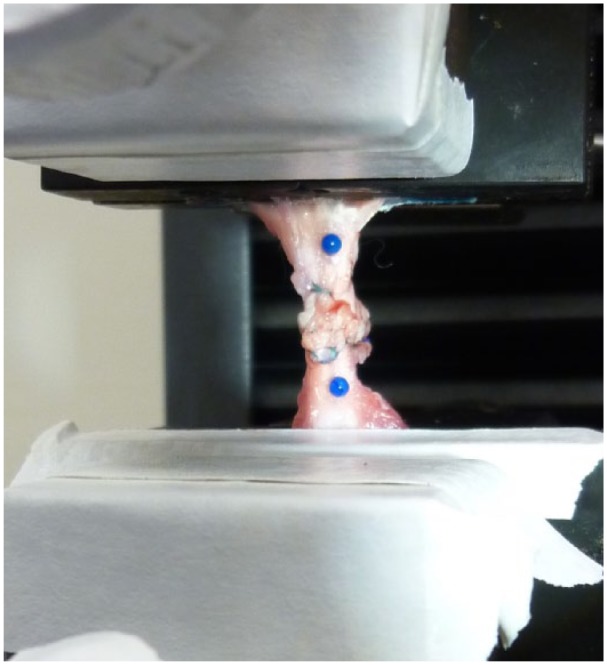
Repaired tendon mounted to the materials testing machine. Spherical markers were attached proximal and distal to the repair site to monitor gap formation during tensile loading.
Due to the possibility of the relatively weak nature of the repaired tendons, there was concern that preconditioning the specimens could lead to premature failure. Consequently, preconditioning was excluded from the testing protocol. Following the application of a 2 N preload, specimens were pulled at a rate of 10 mm/min until catastrophic failure. Force and image data were synchronized and recorded at 10 Hz. Outcome measures included force to 1-mm and 2-mm gap formation and stiffness, defined as the maximum slope of the loading curve prior to 2-mm gap formation.
Statistical Analysis
It was determined from preliminary testing of rats at the day 4 time point that a group sample size of 9 would be required to show a 30% difference in force to 1 mm of tendon repair site gap formation with a power of 0.8. Data were reported as mean ± SD. Comparisons between the experimental and control groups were performed using GraphPad Prism 7 Mann-Whitney test with significance set at P < .05.
Results
The sample size was n = 9 for specimens harvested at both day 4 and day 7. All specimens failed at the tendon repair site by suture pullout. Several specimens (Day 4, Control: n = 1; Day 7, Control: n = 2; Day 7, Experimental: n = 1) reached peak load prior to 2 mm of repair site gap formation. These specimens were excluded from the analysis of force to 2-mm gap formation.
For specimens harvested 4 days following surgery, no significant differences were observed in force to 1 mm (Control: 10.0 ± 2.1 N vs Experimental: 11.0 ± 3.3 N; P = .305) or 2 mm (Control: 18.1 ± 7.1 N vs Experimental: 19.6 ± 6.0 N; P = .343) gap formation. Similarly, no difference in repair stiffness was found between tests groups at day 4 (Control: 9.9 ± 6.0 N/mm vs Experimental: 10.6 ± 3.9 N/mm; P = .602) (Figure 2). The same result was seen at day 7. No differences in force to 1 mm (Control: 14.5 ± 4.4 N vs Experimental: 11.3 ± 2.1 N; P = .102) or 2 mm (Control: 22.1 ± 6.7 N vs Experimental: 20.9 ± 4.0 N; P = .641) gap formation, or repair stiffness (Control: 14.6 ± 5.4 N/mm vs Experimental: 10.3 ± 2.6 N/mm; P = .074) were found between test groups for specimens harvested 7 days following surgery (Figure 3).
Figure 2.
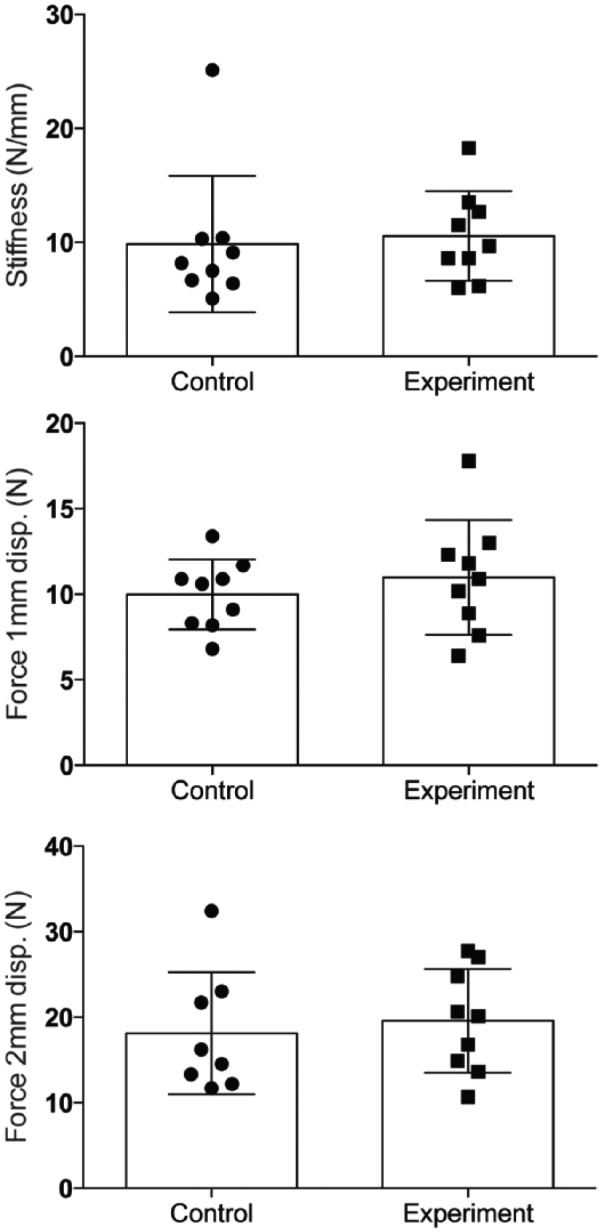
Force required to produce 1-mm and 2-mm repair site gap, and stiffness for specimens harvested 4 days following surgery. Data are presented as mean ± SD.
Figure 3.
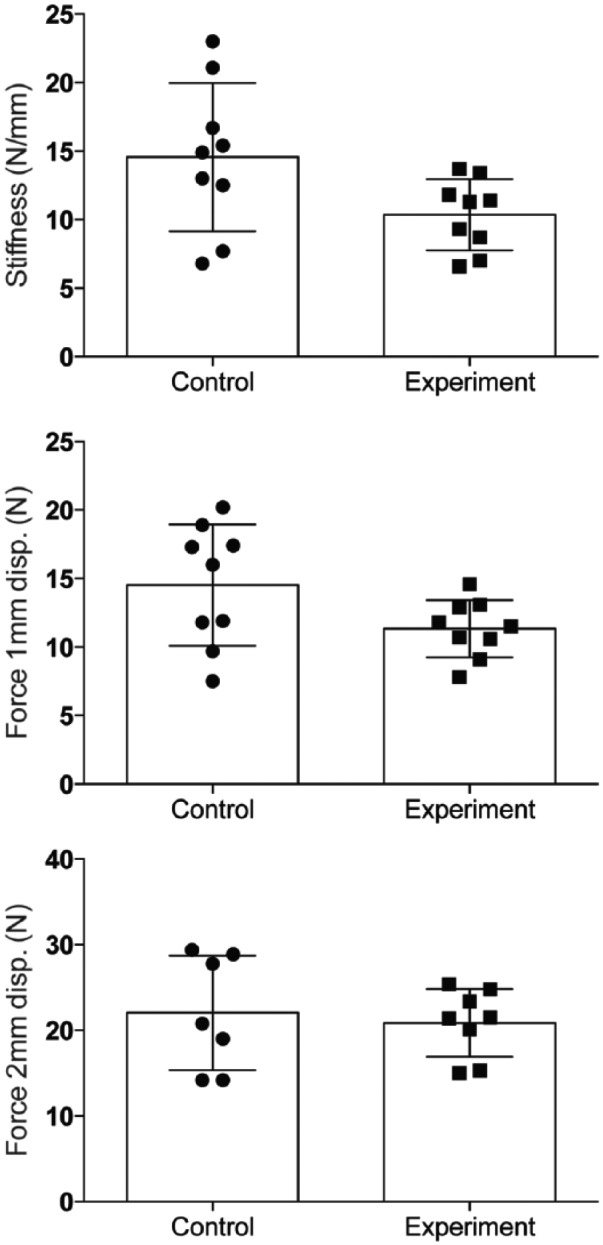
Force required to produce 1-mm and 2-mm repair site gaps, and stiffness for specimens harvested 7 days following surgery. Data are presented as mean ± SD.
The mean force to 1-mm and 2-mm gap formation tended to increase for both control and experimental groups as time following repair increased. A similar upward trend was observed in mean stiffness for the control group. However, the mean stiffness for the experimental group was very similar for specimens harvested 4 and 7 days following surgery.
The expression of Collagen1a2 and Collagen2a1, a marker for connective tissues, was increased in the experimental groups at both 4 days (Figure 4) and 7 days (Figure 5). In addition, the messenger RNA (mRNA) level of matrix metalloproteinase (MMP1 and MMP3, markers of tissue turnover) was also increased for the experimental groups at day 4 (Figure 4) and day 7 (Figure 5) (P < .05).
Figure 4.
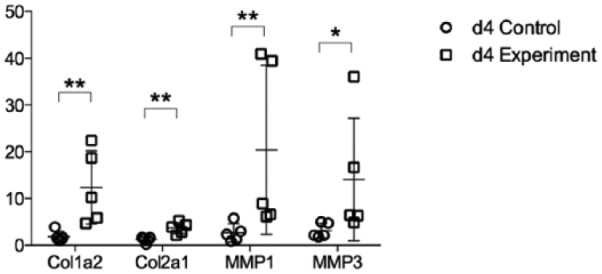
Col1a2, Col2a1, MMP1, and MMP3 messenger RNA expression at 4 days after surgery. Data are presented as mean ± SD. Col = collagen; MMP = matrix metalloproteinase.
*P < 0.05, **P < 0.01.
Figure 5.
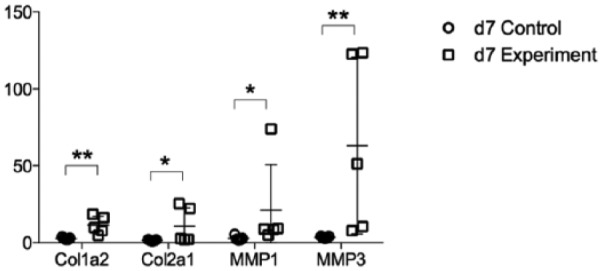
Col1a2, Col2a1, MMP1, and MMP3 messenger RNA expression at 7 days after surgery. Data are presented as mean ± SD. Col = collagen; MMP = matrix metalloproteinase.
*P < 0.05, **P < 0.01.
Discussion
Several authors have explored the introduction of pluripotent mesenchymal stem cells and/or growth factors to a tendon repair site to accelerate healing and increase the early strength of repair. BMSCs may differentiate along the tenocytic lineage when stimulated by exogenous factors.10,27 Growth factors, including GDF-8, GDF-5, epidermal growth factor, and basic fibroblast growth factor, have been shown to promote BMSCs to differentiate into tenocytes.3,4,22 Some authors have successfully shown the use of BMSCs and growth factors for tendon repair.9,21 Our previous studies demonstrated the feasibility of the sutures coated with BMSCs in treating tendon injuries in a rat model and specifically that tendons repaired with these sutures displayed statistically greater strength in comparison with plain suture repairs.31-33
In terms of growth factors, GDF-5 has been identified as important factor during early tendon healing.1 GDF-6 and GDF-7 may be involved in matrix remodeling and may play a role in tissue regeneration of tendons, although less so in early tendon healing.7,30 We have focused on GDF-8 because it has been recognized as a regulator of the structure and function of tendon tissue. Mendias et al demonstrated that compared with wild-type control animals, the tendons of GDF-8-deficient mice are 40% smaller, have a 45% reduction in fibroblast density, and have marked reductions in the expression of type I collagen and extracellular matrix production genes scleraxis and tenomodulin.14 GDF-8 seems to be more important for tendon physiology than the related molecules GDF-5, GDF-6, and GDF-7 because mice lacking one of these proteins have mild phenotypes.15-17 We have already showed that GDF-8 has the ability to increase BMSCs growth and differentiation toward a tenocyte lineage in our previous in vitro study.13 Therefore, in our current study we tested the hypothesis that GDF-8 stimulates BMSCs to differentiate down a tenocyte lineage increasing the repair potential of injured tendons with bioactive sutures using an in vivo rabbit tendon injury model. Although ultimate load has been assessed in some studies,4,8,12 we believe that the force to generate 1 mm and 2 mm gapping is the most clinically relevant parameter as previously described in some literature19,26 because ultimate load can be due to many factors, including the strength of the suture knot. In addition, ultimate load may occur beyond 2 mm of repair site gap formation, when the repair has already clinically failed.
In this study, there were no significant differences between experimental and control groups for the force to 1-mm and 2-mm repair site gap formation and stiffness.
However, GDF-8 did increase the expression of collagen mRNA and MMP mRNA in the rabbit tendons. This proves the concept of GDF-8 stimulating fibroblast proliferation and collagen production, increasing the mass and cross-sectional area of the healing tissue despite the lack of an improvement in mechanical strength. This suggests that GDF-8 enhances the effect of BMSCs on tendon healing at the molecular level at these early time points following repair in vivo, but the biomechanical strength has not shown clinically significant improvement. Our findings support another study suggesting that GDF-8 does not improve the strength of healing tendons.5 In addition, Eliasson et al described that GDF-8 plays a greater role in maintaining intact tendon properties than in tendon healing because the gene expression for GDF-8 was much higher in intact tendons than in the healing callus tissue.6 Although we have already found that GDF-8 stimulates BMSCs to differentiate into tenocytes in vitro, the results of this current in vivo study may suggest that those tenocytes contribute to tendon maintenance due to the GDF-8 rather than to the early strength of the tendon repair. Our results also imply that GDF-8 may even reduce or blunt the effect of BMSCs on tendon healing because, as demonstrated by our previous study, BMSCs accelerated tendon healing in the in vivo rat model. To further clarify the effect of GDF-8 on BMSCs used in tendon repair, we are further investigating the strength of the tendon repaired by control and BMSC-coated sutures without GDF-8 using the same rabbit tendon model.
It is still unclear and worth further consideration why GDF-8–treated BMSC-coated suture repair failed to increase the tendon healing strength in the in vivo tendon repair model despite its ability to stimulate BMSCs to differentiate into tenocytes in vitro and ex vivo.
There are some limitations of this study. Our sample size is relatively small. However, because it was determined from preliminary testing that a group sample size of 9 would be required to show a 30% difference in force at 1 mm of tendon repair site gapping, we believe that our results indeed demonstrate that GDF-8 does not enhance the effect of BMSCs on the tendon repair. Second, we evaluated the tendon healing only at relatively early time points (4 and 7 days). In our previous rat model, the bioactive cell-coated sutures enhanced repair strength at 7 to 10 days, but there was no significant effect at later stages. Longer follow-up may be needed, but we believe that assessing tendon healing at early time points is critical in the understanding of tendon healing because they span the nadir in repair strength, regardless of the number and configuration of core and epitendinous sutures.25 Similarly, it was found in our previous in vivo rat model that the benefit of the bioactive sutures was seen most significantly in the earlier time points.
In conclusion, GDF-8 does not appear to enhance the effect of BMSCs on tendon healing in vivo. On the contrary, it may actually impair the effect of BMSCs on tendon healing, encouraging additional investigation. It is important to address if stem cell and regenerative medicine is able to deliver sustainable benefit and one of the current challenges is the persistence of a cross-border market in untested and potentially ineffective therapies as described by Cossu et al.2 We believe that our results add an important contribution to this challenge.
Acknowledgments
This work was supported by the Stanford Department of Orthopaedic Surgery, the Whiting family, Craig and Nikki Johnson, Kent Thiry, and Denise O’Leary.
Footnotes
Ethical Approval: This study was approved by our institutional review board (APLAC 27441).
Statement of Human and Animal Rights: All institutional and national guidelines for the care and use of laboratory animals were followed.
Statement of Informed Consent: This study involves no human participants, so informed consent was not sought or required.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: J Yao  https://orcid.org/0000-0003-0758-083X
https://orcid.org/0000-0003-0758-083X
References
- 1. Chhabra A, Tsou D, Clark RT, et al. GDF-5 deficiency in mice delays Achilles tendon healing. J Orthop Res. 2003;21:826-835. [DOI] [PubMed] [Google Scholar]
- 2. Cossu G, Birchall M, Brown T, et al. Lancet commission: stem cells and regenerative medicine. Lancet. 2018;391:883-910. [DOI] [PubMed] [Google Scholar]
- 3. Daher RJ, Chahine NO, Razzano P, et al. Tendon repair augmented with a novel circulating stem cell population. Int J Clin Exp Med. 2011;4:214-219. [PMC free article] [PubMed] [Google Scholar]
- 4. Dines JS, Weber L, Razzano P, et al. The effect of growth differentiation factor-5-coated sutures on tendon repair in a rat model. J Shoulder Elbow Surg. 2007;16:(5 suppl):S215-221 [DOI] [PubMed] [Google Scholar]
- 5. Eliasson P, Andersson T, Kulas J, et al. Myostatin in tendon maintenance and repair. Growth Factors. 2009;27:247-254. [DOI] [PubMed] [Google Scholar]
- 6. Eliasson P, Fahlgren A, Aspenberg P. Mechanical load and BMP signaling during tendon repair: a role for follistatin? Clin Orthop Relat Res. 2008;466:1592-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fu SC, Wong YP, Chan BP, et al. The roles of bone morphogenetic protein (BMP) 12 in stimulating the proliferation and matrix production of human patellar tendon fibroblasts. Life Sci. 2003;72:2965-2974. [DOI] [PubMed] [Google Scholar]
- 8. Hamada Y, Katoh S, Hibino N, et al. Effects of monofilament nylon coated with basic fibroblast growth factor on endogenous intrasynovial flexor tendon healing. J Hand Surg Am. 2006;31:530-540. [DOI] [PubMed] [Google Scholar]
- 9. Hayashi M, Zhao C, An KN, et al. The effects of growth and differentiation factor 5 on bone marrow stromal cell transplants in an in vitro tendon healing model. J Hand Surg Br. 2011;36:271-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoffmann A, Pelled G, Turgeman G, et al. Neotendon formation induced by manipulation of the Smad8 signalling pathway in mesenchymal stem cells. J Clin Invest. 2006;116:940-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Komatsu F, Mori R, Uchio Y. Optimum surgical suture material and methods to obtain high tensile strength at knots: problems of conventional knots and the reinforcement effect of adhesive agent. J Orthop Sci. 2006;11:70-74. [DOI] [PubMed] [Google Scholar]
- 12. Koob TJ, Summers AP. Tendon–bridging the gap. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:905-909. [DOI] [PubMed] [Google Scholar]
- 13. Le W, Yao J. The effect of myostatin (GDF-8) on proliferation and tenocyte differentiation of rat bone marrow-derived mesenchymal stem cells. J Hand Surg Asian Pac Vol. 2017;22:200-207. [DOI] [PubMed] [Google Scholar]
- 14. Mendias CL, Bakhurin KI, Faulkner JA. Tendons of myostatin-deficient mice are small, brittle, and hypocellular. Proc Natl Acad Sci U S A. 2008;105:388-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mikic B, Bierwert L, Tsou D. Achilles tendon characterization in GDF-7 deficient mice. J Orthop Res. 2006;24:831-841. [DOI] [PubMed] [Google Scholar]
- 16. Mikic B, Entwistle R, Rossmeier K, et al. Effect of GDF-7 deficiency on tail tendon phenotype in mice. J Orthop Res. 2008;26:834-839. [DOI] [PubMed] [Google Scholar]
- 17. Mikic B, Schalet BJ, Clark RT, et al. GDF-5 deficiency in mice alters the ultrastructure, mechanical properties and composition of the Achilles tendon. J Orthop Res. 2001;19:365-371. [DOI] [PubMed] [Google Scholar]
- 18. Miller B, Dodds SD, deMars A, et al. Flexor tendon repairs: the impact of FiberWire on grasping and locking core sutures. J Hand Surg Am. 2007;32:591-596. [DOI] [PubMed] [Google Scholar]
- 19. Moriya T, Zhao C, An KN, et al. The effect of epitendinous suture technique on gliding resistance during cyclic motion after flexor tendon repair: a cadaveric study. J Hand Surg Am. 2010;35:552-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morizaki Y, Zhao C, An KN, et al. The effects of platelet-rich plasma on bone marrow stromal cell transplants for tendon healing in vitro. J Hand Surg Am. 2010;35:1833-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Omae H, Zhao C, Sun YL, et al. Multilayer tendon slices seeded with bone marrow stromal cells: a novel composite for tendon engineering. J Orthop Res. 2009;27:937-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rohrich RJ, Trott SA, Love M, et al. Mersilene suture as a vehicle for delivery of growth factors in tendon repair. Plast Reconstr Surg. 1999;104:1713-1717. [DOI] [PubMed] [Google Scholar]
- 23. Silfverskiöld KL, May EJ. Flexor tendon repair in zone II with a new suture technique and an early mobilization program combining passive and active flexion. J Hand Surg Am. 1994;19:53-60. [DOI] [PubMed] [Google Scholar]
- 24. Silva MJ, Boyer MI, Gelberman RH. Recent progress in flexor tendon healing. J Orthop Sci. 2002;7:508-514. [DOI] [PubMed] [Google Scholar]
- 25. Strickland JW.Flexor tendon injuries: I. Foundations of treatment. J Am Acad Orthop Surg. 1995;3:44-54. [DOI] [PubMed] [Google Scholar]
- 26. Tanaka T, Amadio PC, Zhao C, et al. Gliding characteristics and gap formation for locking and grasping tendon repairs: a biomechanical study in a human cadaver model. J Hand Surg Am. 2004;29:6-14. [DOI] [PubMed] [Google Scholar]
- 27. Towler DA, Gelberman RH. The alchemy of tendon repair: a primer for the (S)mad scientist. J Clin Invest. 2006;116:863-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tozer S, Duprez D. Tendon and ligament: development, repair and disease. Birth Defects Res C Embryo Today. 2005;75:226-236. [DOI] [PubMed] [Google Scholar]
- 29. Uysal CA, Tobita M, Hyakusoku H, et al. Adipose-derived stem cells enhance primary tendon repair: biomechanical and immunohistochemical evaluation. J Plast Reconstr Aesthet Surg. 2012;65:1712-1719. [DOI] [PubMed] [Google Scholar]
- 30. Wong YP, Fu SC, Cheuk YC, et al. Bone morphogenetic protein 13 stimulates cell proliferation and production of collagen in human patellar tendon fibroblasts. Acta Orthop. 2005;76:421-427. [PubMed] [Google Scholar]
- 31. Yao J, Korotkova T, Riboh J, et al. Bioactive sutures for tendon repair: assessment of a method of delivering pluripotential embryonic cells. J Hand Surg Am. 2008;33 1558–64.f–1564. [DOI] [PubMed] [Google Scholar]
- 32. Yao J, Korotkova T, Smith RL. Viability and proliferation of pluripotential cells delivered to tendon repair sites using bioactive sutures—an in vitro study. J Hand Surg Am. 2011;36:252-258. [DOI] [PubMed] [Google Scholar]
- 33. Yao J, Woon CY, Behn A, et al. The effect of suture coated with mesenchymal stem cells and bioactive substrate on tendon repair strength in a rat model. J Hand Surg Am. 2012;37:1639-1645. [DOI] [PubMed] [Google Scholar]
- 34. Zhao C, Chieh HF, Bakri K, et al. The effects of bone marrow stromal cell transplants on tendon healing in vitro. Med Eng Phys. 2009;31:1271-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao C, Moran SL, Cha SS, et al. An analysis of factors associated with failure of tendon repair in the canine model. J Hand Surg Am. 2007;32:518-525. [DOI] [PubMed] [Google Scholar]
- 36. Zhao C, Ozasa Y, Reisdorf RL, et al. Engineering flexor tendon repair with lubricant, cells, and cytokines in a canine model. Clin Orthop Relat Res. 2014;472:2569-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]


