Abstract
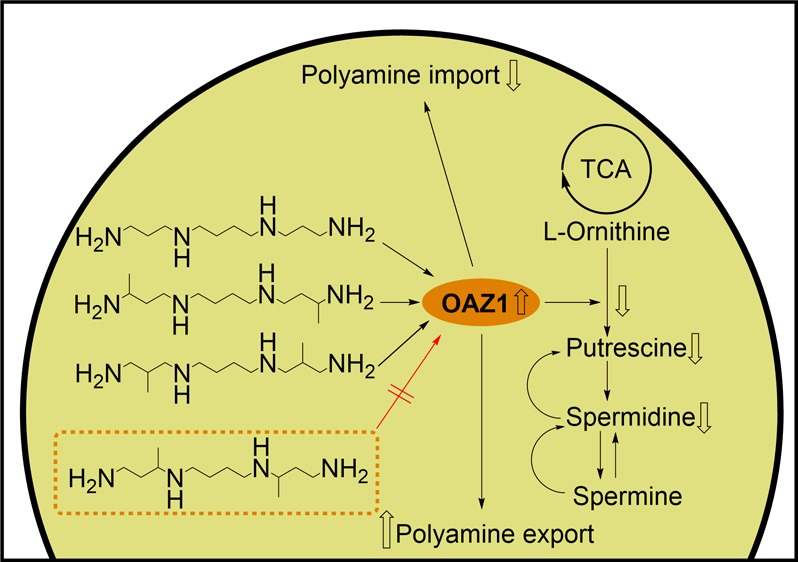
The biogenic polyamines, spermine (Spm) and spermidine, are organic polycations present in millimolar concentrations in all eukaryotic cells participating in the regulation of vital cellular functions including proliferation and differentiation. The design and biochemical evaluation of polyamine analogues are cornerstones of polyamine research. Here we synthesized and studied novel C-methylated Spm analogues: 2,11-dimethylspermine (2,11-Me2Spm), 3,10-dimethylspermine (3,10-Me2Spm), 2-methylspermine, and 2,2-dimethylspermine. The tested analogues overcame growth arrest induced by a 72 h treatment with α-difluoromethylornithine, an ornithine decarboxylase (ODC) inhibitor, and entered into DU145 cells via the polyamine transporter. 3,10-Me2Spm was a poor substrate of spermine oxidase and spermidine/spermine-N1-acetyltransferase (SSAT) when compared with 2,11-Me2Spm, thus resembling 1,12-dimethylspermine, which lacks the substrate properties required for the SSAT reaction. The antizyme (OAZ1)-mediated downregulation of ODC and inhibition of polyamine transport are crucial in the maintenance of polyamine homeostasis. Interestingly, 3,10-Me2Spm was found to be the first Spm analogue that did not induce OAZ1 and, consequently, was a weak downregulator of ODC activity in DU145 cells.
Introduction
The biogenic polyamines, spermine (Spm, 1), spermidine (Spd), and their precursor putrescine (Put), are present in micromolar to millimolar concentrations in all eukaryotic cells, which a priori determines the diversity of their functions, many of which are vitally important.1,2 The investigation of the individual cellular functions of Spm and Spd is complicated by the ability of polyamines to replace each other in many functions; furthermore, Spm is readily interconverted into Spd.1−4
Spd is vitally important because in addition to its many cellular functions, it is the sole donor of the aminobutyl group for the hypusination of a specific Lys residue of eukaryotic translation initiation factor 5A (eIF5A, which is an important elongation factor). This post-translational modification is crucial to allow this protein to function in protein synthesis by promoting the translation of polyproline motifs and some other ribosome-pausing sites.5 Respectively, when cellular polyamine levels decrease, the hypusination of eIF5a is affected last, as demonstrated in a mutant strain of S. cerevisiae, which has a limited pool of Spd.6 This milestone finding explains why Spd, but not Spm, plays a key role in supporting the growth of cells with chronic polyamine deficiency.
In contrast, such an exclusive function of Spm at the cellular level is not known. The deletion of the spermine synthase gene (SMS), or inhibition of the enzyme, exerts no dramatic effect on cell growth, but affect the cellular response to some drugs,7−9 oxidative stress, or UV irradiation.10 However, Snyder-Robinson’s syndrome is a very rare disease attributable to mutations in the human SMS gene (at chromosome Xp22.11) leading to a major decrease in the amounts of Spm and an increase in the Spd contents of tissues including the brain. The disease is characterized by intellectual disability, osteoporosis, hypotonia, speech abnormalities, and seizures.11 Treatment of patient-derived lymphoblast cell lines with Spm returned their Spd/Spm-ratio within the range of wild type cells,12 but as the authors discussed, a simple dietary supplementation of Spm would not be a therapeutic option. However, it is clear that Spm and consequently an appropriate Spd/Spm ratio is required for the normal physiological function of brain and other tissues.
Genetically modified microorganisms and cell lines2,4 as well as transgenic animals13 have been widely used to disclose the individual functions of Spm and Spd as well as the biological properties of polyamines in general. In these experiments, the use of metabolically stable, functionally active mimetics of polyamines is beneficial to avoid Spm and Spd interconversions, as first demonstrated in the case of spermidine/spermine N1-acetyltransferase (SSAT)-transgenic rats. Catabolically stable 1-methylspermidine (1-MeSpd) prevented the development of acute pancreatitis and death of the animals with induced SSAT, the rate-limiting enzyme of Spd catabolism.14 Later, the investigation of the biological properties of other C-methylated Spd analogues demonstrated that all of these analogues mimicked Spd in reverting the growth inhibition caused by a 3 day treatment with the polyamine biosynthesis inhibitor α-difluoromethylornithine (DFMO) and were transported into cells via the polyamine transporter. However, the enzymes of polyamine catabolism differently recognized C-methylated Spd analogues.15 Thus only 3-methylspermidine (3-MeSpd) is metabolically stable, whereas 1-methylspermidine (1-MeSpd) is not a substrate of SSAT but is converted into 1-methylspermine in DU145 cells.15,16 Only 1-MeSpd and 2-methylspermidine (2-MeSpd), but not 3-MeSpd and 8-methylspermidine (8-MeSpd), support the growth of the cells after prolonged DFMO treatment, although 1-MeSpd, 2-MeSpd, and 3-MeSpd, but not 8-MeSpd, are the substrates of deoxyhypusine synthase (DHS).17,18 Interestingly, among these Spd analogues, only 2-MeSpd is unable to downregulate ODC in DU145 cells.15 Therefore, the biochemical properties of C-methylated Spd analogues can be adjusted by simply moving the methyl group along the polyamine backbone, and these analogues have demonstrated a good potential for studying the enzymes of polyamine metabolism and the cellular functions of Spd.19
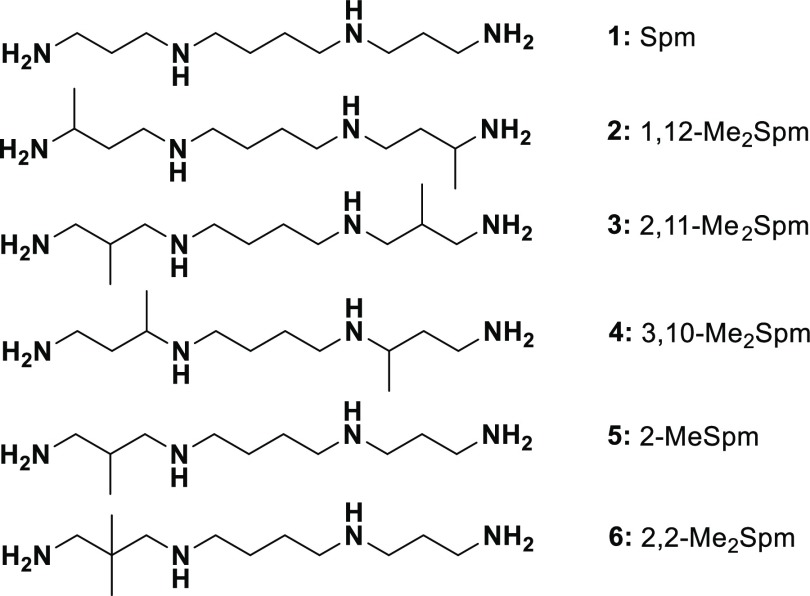
Here we have synthesized and investigated the biological properties of novel bis-methylated derivatives of Spm (Figure 1), assuming that the biochemical properties of these analogues would be determined by the position of methyl substituent(s). All of the analogues tested competed with Spm for transport into DU145 cells. The substrate properties of 1,12-Me2Spm (2), 2,11-Me2Spm (3), and 3,10-Me2Spm (4) toward SSAT, spermine oxidase (SMOX), and bovine plasma semicarbazide-sensitive amino oxidase (SSAO) depended on the position of the methyl groups. Among these compounds, 4 did not induce antizyme (OAZ1), and it was only a weak downregulator of ODC. Thus, to the best of our knowledge, 4 is the first functionally active mimetic of Spm that does not interfere with OAZ1-dependent regulatory pathways. Surprisingly, among the C-methylated Spd derivatives, only 2-MeSpd possesses the same set of properties,15 indicating the complexity and peculiarities in the regulation of OAZ1 biosynthesis by the analogues of Spm and Spd.
Figure 1.
Structures of C-methylated spermine analogues and the natural polyamine Spm.
Results and Discussion
Chemistry
There are various synthetic strategies for building the backbone, that is, C–N bonds, of biogenic polyamines, their analogues, and derivatives in solution,20,21 and some involve the adoption of a solid-phase technique.22 The development of these synthetic methods makes it possible to obtain a wide spectrum of quite complex polyamine analogues possessing antitumor,23 antiparasite,24 and antibacterial25,26 activities. Recently, a number of polyamine derivatives have been designed and synthesized to deliver biomolecules into cells using the polyamine transporter.27−29 These synthetic routes also proved to be suitable for the preparation of novel effective inhibitors of the cellular transport of both Spm and Spd.30 On the basis of these data and our previous synthetic experience,15,31−33 convergent syntheses were used to obtain the previously unknown symmetric compounds 3 and 4 (Schemes 1 and 2). The preparation of the novel compounds 2-MeSpm (5) and 2,2-Me2Spm (6) involved a stepwise elongation of the aminomethylene backbone (Scheme 3).
Scheme 1. Synthesis of the Novel Spermine Analogues 3 and 4 from Amino Alcohols.
Reagents and conditions: (a) CbzCl, H2O, NaHCO3, THF. (b) (1) MsCl, Et3N, C6H6, Et2O; (2) LiN3, MeOH, Δ. (c) (1) Ph3P, THF, Δ; (2) 40% aq H2NCH3, THF. (d) NsCl, CH2Cl2, Et3N. (e) (1) J(CH2)4J, DMF, K2CO3, 45 °C; (2) PhSH, DMF, K2CO3. (f) H2/Pd, AcOH, MeOH.
Scheme 2. Synthesis of Novel Spermine Analogue 3 from Dinitrile.
Reagents and conditions: (a) 21: CH2=C(CH3)CN, 90 °C. 22: CH3CH=CHCN, 90 °C. (b) Boc2O, THF. (c) LiAlH4, THF, −5 to −2 °C. (d) HCl, EtOH.
Scheme 3. Synthesis of Novel Spermine Analogues 5 and 6.
Reagents and conditions: (a) H2N(CH2)4OH, THF, 20 °C. (b) CbzCl, H2O, NaHCO3, THF. (c) (1) MsCl, Et3N, C6H6, Et2O; (2) 29: H2NCH2CH(CH3)CH2NH2, THF, 20 °C. 30: H2NCH2C(CH3)2CH2NH2, THF, 20 °C. (d) H2/Pd, AcOH, MeOH.
The synthesis of 3 and 4 started with commercially available 3-amino-2-methylpropanol-1 (7) and 4-aminobutanol-2 (8), which were primarily converted into N-Cbz-aminoalcohols 9 and 10 and then to the corresponding mesylates. Mesylates without isolation were treated with LiN3, and azides 11 and 12 were reduced to amines 13 and 14 (Scheme 1).
It is noteworthy that the use of 40% aq CH3NH2 to convert intermediate iminophosphoranes into amines (Staudinger reaction) proved to be superior than the traditionally used aq NH4OH.34 Amines 13 and 14 were converted to nosylates 15 and 16, which were alkylated with 1,4-diiodobutane in DMF in the presence of K2CO3. The alkylation was a fairly clean procedure, but for the successful isolation of 17 and 18 by column chromatography on SiO2, it was essential to remove any traces of unreacted nosylates 15 and 16. For this purpose, 15–20 mol % of BnBr was added to the reaction mixture before a one-pot removal of the nosyl protecting group with PhSH. These steps consequently allowed us to obtain pure bis-Cbz-tetraamines 17 and 18 in high yield. The subsequent removal of the Cbz groups by the catalytic hydrogenation and recrystallization of tetrahydrochlorides yielded target compounds 3 and 4 in overall yields of 21 and 18% (each in eight steps), as calculated from starting materials 7 and 8, respectively. It is noteworthy that whereas bis-Ns-Put alkylation with the corresponding N-Cbz-alkyl bromides is one step shorter, the use of 1,4-diiodobutane and nosylates 15 and 16 is preferable. This nosylate route provided higher yields and reduced the complexity of the reaction mixture in the stage of alkylation.
An alternative preparation of 3 and 4 started with metacrylonitrile and crotononitrile, respectively. It is more practical to perform a Michael addition of 19 and 20 to metacrylonitrile and crotononitrile, respectively (Scheme 2), than to introduce two equivalents of nitriles in the reaction with Put.
As expected, the addition of 20 to crotononitrile in the absence of a catalyst was faster than the addition of 19 to metacrylonitrile, and the reaction was virtually completed within 15 h at 90 °C (reaction was monitored by NMR). On the contrary, the reaction of 19 with metacrylonitrile required heating for 96 h to 90 °C. Compounds 21 and 22 are oily liquids (bp ca. 150 °C/0.2 Torr), and their final purification was performed using flash chromatography on SiO2. The reduction of bis-Boc-diaminodinitriles 23 and 24 to bis-Boc-tetraamines with LiAlH4 was not as straightforward as previously described35 for the reduction of N4,N8-bis-(tert-butyloxycarbonyl)-2-methyl-8-amino-4-azaoctanenitrile and N4,N8-bis-(tert-butyloxycarbonyl)-3-methyl-8-amino-4-azaoctanenitrile to bis-Boc-derivatives of the corresponding C-methylated Spd’s, which smoothly proceeded in Et2O at −5 to 0 °C. Nonetheless, it was found that neither 23 nor 24 was soluble in Et2O, whereas the reduction of 23 with LiAlH4 in THF made it possible to obtain bis-Boc-tetraamine 25 in only 15% yield because of the competing retro-Michael reaction leading to N4,N8-bis-(tert-butyloxycarbonyl)-2-methyl-1,8-diamino-4-azaoctane (16%) and N1,N4-bis-(tert-butyloxycarbonyl)-1,4-diaminobutane as the main product. The subsequent deprotection of 25 and the recrystallization of tetrahydrochloride afforded the desired 3 in an overall yield of 6.5% (four steps). The reduction of 24 with LiAlH4 in THF at −5 to 0 °C was unsuccessful, and the target compound N4,N9-bis-(tert-butyloxycarbonyl)-3,10-dimethyl-1,12-diamino-4,9-diazadodecane was not formed. Here again, the main product was N1,N4-bis-(tert-butyloxycarbonyl)-1,4-diaminobutane, whereas the second byproduct, N4,N8-bis-(tert-butyloxycarbonyl)-3-methyl-1,8-diamino-4-azaoctane, was isolated from the reaction mixture in only a 5% yield.
The reduction of 21 and 22 with H2/Ni-Raney gave target compounds 3 and 4 in good yield, but the products contained minor impurities, and thus the purification of preparative amounts of 3 and 4 was rather laborious. Using this synthetic scheme, we managed to obtain 3 and 4 at only 90–92% purity (data not shown). Thus the shorter “nitrile approach” in our hands was not very productive for the syntheses of 3 and 4.
The syntheses of unsymmetric 5 and 6 were performed in five steps each (Scheme 3) by the subsequent elongation of the polyamine backbone starting with 3-[N-(benzyloxycarbonyl)]amino-1-propyl methanesulfonate (26), which was treated with an excess of 4-aminobutanol-1 in THF at 20 °C to produce 27. The secondary amino group of 27 was protected with the Cbz group to give 28, which was converted to the corresponding methanesulfonate and treated with an excess of 1,3-diamino-2-methylpropane or 1,3-diamino-2,2-dimethylpropane in THF without isolation to produce bis-Cbz-tetraamines 29 and 30 in good yield. It should be noted that the 13C NMR spectra of compounds 28, 29, and 30 (the same is true for bis-Boc-derivatives 23, 24, and 25) were very complicated at 20 °C due to the existence of Cbz and Boc rotamers. Finally, the removal of Cbz groups resulted in the preparation of target compounds 5 and 6 in overall yields of 38 and 33% (each in five steps), as calculated from the starting methanesulfonate 26.
The above procedures allowed us to synthesize the previously unknown compounds 3–6 at >95% purity and thus created the possibility to study the cellular effects of these analogues and their interactions with the enzymes of polyamine metabolism.
Biological Evaluation
Uptake of the Analogues and Their Effects on Intracellular Pools of Polyamines
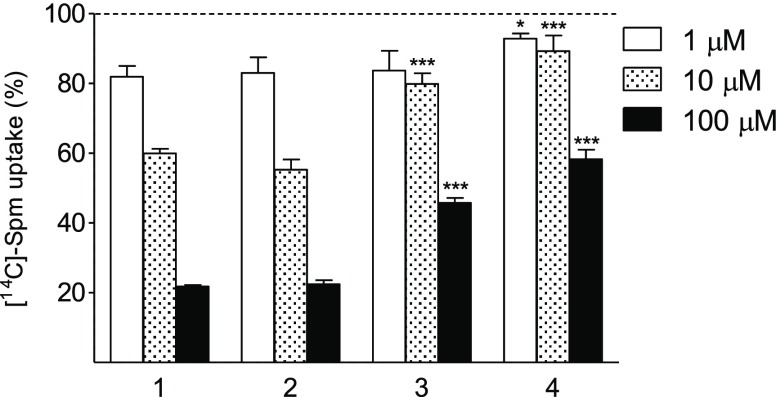
The study of the biochemical potential of novel bis-methylated analogues of 1 (Figure 1) was started with the investigation of their ability to be taken up by cells. All three analogues of 1 competed for uptake with [14C]-labeled 1 in DU145 prostate cancer cells. However, 3 and 4 were much weaker competitors than 2 (Figure 2). The competition efficacy of the analogues decreased as the methyl substituent was positioned closer to the secondary amino group, indicating that it is an important determinant in polyamine transport, a finding that is in agreement with previous observations for 3-MeSpd.15
Figure 2.
Uptake competition of compounds 1, 2, 3, and 4 (10 min) with [14C]-Spm (10 μM) in DU145 cells. Data are means ± SD, n = 3. * and *** refer to the statistical significance of p < 0.05 and p < 0.001, respectively, as compared with the corresponding concentration of 1.
All three analogues reduced the intracellular levels of the natural polyamines, whereas the total polyamine level (natural polyamines + analogue) remained almost unchanged (Table 1). Interestingly, after 6 h of incubation of DU145 cells with bis-methylated analogues of 1 in the absence of aminoguanidine (AG), an inhibitor of SSAO, the intracellular amount of 4 was almost half of that of 3 and about one-third of that of 2 (Table 1). Apparently, after 6 h, about one half of 4 had been converted into 3-MeSpd; after 3 days of incubation, the conversion of 4 into 3-MeSpd had reached nearly 80% (Table 1). In the presence of AG, the analogues accumulated intracellularly equally well and at levels close to that of 1 in the control samples. The transformation of 4 into 3-MeSpd was reduced but not completely prevented by AG, suggesting that 4 is catabolized not only by SSAO but also by some other enzyme. These unforeseen results prompted us to conduct comparative studies to elucidate the substrate properties of 2, 3, and 4 toward enzymes involved in polyamine catabolism.
Table 1. Polyamine Pools in DU145 Cells Treated for 6 h or 3 days with 100 μM of the Analogues with or without 1 mM AGa.
| Put | Spd | Spm | N1-AcSpd | analogue | MeSpd | |
|---|---|---|---|---|---|---|
| 6 h | ||||||
| control | 40 ± 2 | 134 ± 7 | 106 ± 4 | <3 | ||
| 2 | 15 ± 2*** | 105 ± 10*** | 84 ± 8*** | 5 ± 1 | 70 ± 8 | nd |
| 3 | 10 ± 1*** | 99 ± 8*** | 76 ± 5*** | 4 ± 0 | 59 ± 1 | nd |
| 4 | 27 ± 2*** | 79 ± 4*** | 80 ± 4*** | 3 ± 0 | 26 ± 1 | 34 ± 4 |
| 3 days | ||||||
| control | 19 ± 2 | 123 ± 3 | 102 ± 4 | 3 ± 0 | ||
| 2 | <3*** | 13 ± 2*** | 27 ± 3*** | <3 | 168 ± 18 | 4 ± 1 |
| 3 | <3*** | 13 ± 0*** | 40 ± 3*** | nd | 147 ± 7 | 20 ± 1 |
| 4 | 12 ± 1** | 34 ± 2*** | 69 ± 4*** | <3 | 29 ± 2 | 116 ± 5 |
| AG | 16 ± 3 | 175 ± 5 | 157 ± 8 | 3 ± 1 | ||
| AG+2 | nd | 3 ± 1*** | 19 ± 1*** | <3 | 217 ± 6 | 10 ± 1 |
| AG+3 | nd | <3*** | 33 ± 2*** | nd | 222 ± 15 | nd |
| AG+4 | nd | 31 ± 2*** | 68 ± 5*** | nd | 191 ± 18 | 44 ± 3 |
Units are pmol/μg DNA. Data are means ± SD, n = 3. ** and *** refer to statistical significance of p < 0.01 and p < 0.001, respectively, as compared with the control sample. nd, not detectable.
Interaction of Bis-Methylated Spm Analogues with the Enzymes Involved in Polyamine Catabolism
The study of the biochemical properties of novel bis-methylated derivatives of 1 was continued by investigating their interactions with recombinant SSAT and SMOX, which are the rate-limiting enzymes of Spm and Spd catabolism, and also with Cu2+-dependent bovine plasma SSAO, which is capable of utilizing both Spm and Spd as substrates. It was observed that the structure/activity relationships are unique for each enzyme and the substrate properties of bis-methylated analogues of 1 depended on the position of the methyl groups.
SSAT
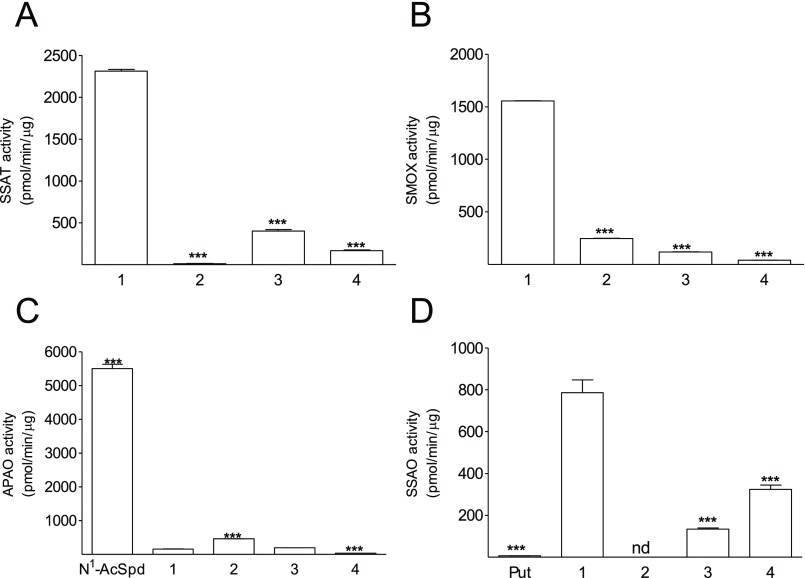
Mouse recombinant SSAT did not acetylate 2 (Figure 3A), whereas 3 and 4 were found to be approximately 7 and 12 times less preferred substrates of the enzyme than its natural substrate 1 (Figure 3A). The kinetic parameters for 3 were Vmax = 895 ± 18 pmol/min/μg protein, Km = 52 ± 5 μM and those for 4 were Vmax = 136 ± 5 pmol/min/μg protein, Km = 60 ± 11 μM (Figure S1). Compounds 2 and 4 were found to be poor inhibitors of the enzyme, achieving 80 and 75% inhibition at 500 μM, respectively, when the concentration of 1, used in this assay as a substrate, was 50 μM (Figure S2). The inability of 2 to participate in the SSAT-catalyzed reaction may be attributed to the steric effect of the methyl group in the α-position of the amino group, which prevents the compound’s acetylation. The poor substrate properties of 4, having a methyl group in the γ-position of the acetylated amino group, were unexpected. However, the X-ray structure of SSAT with the binary complex, Spm-HS-CoA, clearly revealed that in the active center of the enzyme, there was no evidence of any special group being responsible for the deprotonation of the terminal amino group of 1.36 Deprotonation is achieved by a “proton wire” formed with water molecules coordinated by the substrate amino groups and the side chains of Asp93 and Glu92. This “proton wire” covers not only the N-1 amino group but also the adjacent N-4 amino group. Hence, we hypothesize that the presence of a methyl group at the third position of 4 can affect the “proton wire”, and it also introduces hydrophobic contacts between 4 and the side chains of Leu128 and Trp154 (that are positioned close to the secondary (N-4) amino group), and these can restrict the N1-acetylation of 4.
Figure 3.
Analogues as substrates for (A) mouse recombinant SSAT (5 mM analogues), (B) human recombinant SMOX (1 mM analogues), (C) human recombinant APAO (2 mM analogues), and (D) bovine plasma SSAO (5 mM analogues) in vitro. Saturating concentrations of the natural substrates were used, and the analogues were tested at the same concentrations. Data are means ± SD, n = 3. *** refers to statistical significance of p < 0.001 as compared with Spm, respectively. nd, not detectable.
SMOX, APAO, and SSAO
Previously, we have demonstrated that the (S,S)-diastereomer of 2 is an approximately two-fold better substrate of SMOX than compound 1 (its natural substrate), whereas the (R,R)-diastereomer of 2 has virtually no substrate properties.37 The ability of racemic 2, 3, and 4 to serve as substrates for SMOX decreased as the methyl substituent was positioned closer to the secondary (N-4) amino group (Figure 3B). With human recombinant SMOX, the kinetic parameters for 3 were Vmax = 124 ± 3 pmol/min/μg protein, Km = 121 ± 11 μM (Figure S1); for 4, it was not possible to determine the kinetic parameters due to its poor substrate properties. As with 1, the analogues were poor substrates for human recombinant acetylpolyamine oxidase (APAO), an enzyme that is structurally close to SMOX but uses acetylated polyamines as substrates (Figure 3C). Recently, we have synthesized N1-acetylated derivatives of 1-MeSpd, 2-MeSpd, 3-MeSpd, and 8-MeSpd and studied their interaction with APAO. It was observed that N1-Ac-2-MeSpd and N1-Ac-8-MeSpd, but not N1-Ac-3-MeSpd, could act as substrates of the enzyme.38 Thus the structure–activity relationships for 2, 3, 4, and N1-acetylated derivatives of 1-MeSpd, 2-MeSpd, 3-MeSpd, and 8-MeSpd were rather similar for both enzymes. This is likely because the methyl group on the third position of the polyamine backbone may restrict the proton splitting at the C-3 carbon atom and influence the formation of the Schiff base, a key intermediate of the oxidation reactions catalyzed by APAO and SMOX. The opposite dependence of the substrate properties on the position of the methyl substituent took place in the case of SSAO, where the substrate properties of 4 were already ∼30% of that of 1. Furthermore, 4 was more efficiently degraded by bovine plasma SSAO than 3, whereas 2 was resistant toward the enzyme (Figure 3D). It should be noted that in a recent comprehensive study, no degradation of Spd and Spm was detected in human serum under cell culture conditions but only in serum from ruminant sources.39 However, a membrane-bound enzyme (VAP-1; gene name AOC3) with similar substrate specificity as bovine plasma SSAO has been identified in the human vasculature, adipocytes, chondrocytes, and odontoblasts, and a soluble form of this enzyme has also been described.40
The ability of 2, 3, and 4 to compete in SMOX, APAO, and SSAO reactions with their natural substrates was studied using a fixed 50 μM concentration of Spm, N1-acetylspermidine (N1-Ac-Spd), and Spm, respectively. Compound 4 achieved 70% inhibition of SMOX at 50 μM, whereas 3 also displayed 70% inhibition of the enzyme, albeit at 500 μM concentration, indicating that 4 has a much lower affinity for the enzyme than compound 3 (Figure S2). In contrast, compound 3 achieved 80% inhibition of SSAO at 50 μM, whereas 2 and 4 were less effective (Figure S2). Compound 4 achieved 45% inhibition of APAO at 50 μM, whereas 2 and 3 were much less effective (Figure S2).
Taken together, our data demonstrate that the interaction and substrate and inhibitory properties of bis-methylated analogues of 1 toward the enzymes of polyamine catabolism may be regulated by a simple movement of the methyl group along the polyamine analogue backbone.
Effects on Growth of DU145 Cells and on the Intracellular Activities of the Enzymes Involved in Polyamine Metabolism
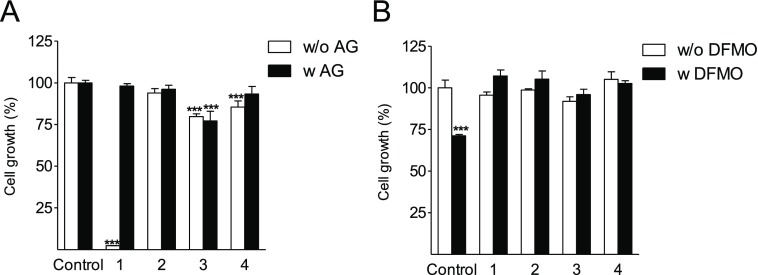
Despite being substrates for SSAO (see above), 2, 3, and 4 did not cause marked cytotoxicity in DU145 cells, and the cells could be cultured in either the presence or absence of AG, an inhibitor of SSAO (Figure 4A). In contrast, the natural polyamine 1 was very toxic in the absence of AG. All of the analogues were able to overcome the growth arrest caused by a 3 day treatment with the ODC inhibitor DFMO (Figure 4B), indicating that they are recognized as polyamine mimetics. However, it should be noted that because they are analogues of 1 and are capable of depleting intracellular natural polyamines, it is apparent that compounds 2, 3, and 4 do not support the growth of cells under conditions of chronic polyamine deprivations because they cannot act as precursors for hypusine synthesis.
Figure 4.
Growth of DU145 cells treated with 100 μM analogues for 3 days (A) with or without 1 mM AG and (B) with or without 5 mM DFMO (supplemented with 1 mM AG). Data are means ± SD, n = 3. *** refers to statistical significance of p < 0.001 as compared with the control sample.
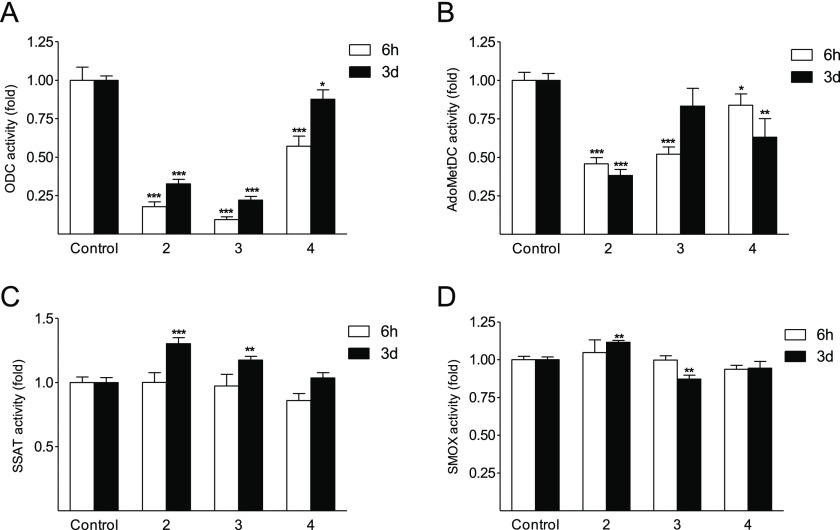
Compound 4 was clearly the least efficient downregulator of ODC, whereas both 3 and 2 displayed similar and much more potent downregulatory effects compared with that of 4 (Figure 5A). In contrast, 3 and 4 were less potent than 2 at inhibiting AdoMetDC activity (Figure 5B). The studied bis-methylated analogues of 1 elicited only minor changes in SSAT and SMOX activities in DU145 cells (Figure 5C,D).
Figure 5.
Activities of (A) ODC, (B) AdoMetDC, (C) SSAT, and (D) SMOX in DU145 cells treated with 100 μM of the analogues for 6 h or 3 days. Data are means ± SD, n = 3. *, **, and *** refer to the statistical significance of p < 0.05, p < 0.01, and p < 0.001 as compared with the control sample, respectively.
Antizyme-Related Effects of the Analogues
The described differences in the effects of bis-methylated analogues of 1 on the activity of ODC (the key and rate-limiting enzyme of polyamine biosynthesis) in DU145 cells were the most interesting and unexpected findings. Hence, we decided to study these effects in more detail, assuming them to be related to the induction of antizyme (OAZ1). The short-living OAZ1 protein is one of the key regulators of polyamine homeostasis. OAZ1 binds to the ODC subunit and directs it to the 26S proteasome, triggering a rapid decline in intracellular ODC activity. Additionally, OAZ1 is known to block the transport of extracellular polyamines into the cell and to increase polyamine efflux.41 The biosynthesis of OAZ1 is induced by 1, Spd, and their analogues. We initially investigated the ability of bis-methylated analogues of 1 to induce OAZ1 by exploiting an indirect method where we compared the uptake of the analogue in the absence and presence of cycloheximide (CHX). CHX inhibits protein synthesis, including the synthesis of short-living OAZ1. If the analogue was able to induce OAZ1,then OAZ1 would downregulate the uptake of the analogue in the absence of CHX. Interestingly, the uptakes of 1, 2, and 3 were each increased after the incubation of cells with CHX, unlike that of 4, indicating that 4 might be a poor inducer of OAZ1 (Figure 6A). The results of the indirect assay were confirmed by an OAZ1 immunoblot experiment, which confirmed the lack of OAZ1 induction by compound 4 (Figure 6C). The ability of 2, 3, and 4 to downregulate ODC was studied after 6 h of incubation of DU145 cells using 0.1, 10, 25, 50, and 100 μM concentrations of the analogues in the media. For comparison, the effects of 1 were studied at the same concentrations (Figure 6B). It was clear that 1, 2, and 3 already at 10 μM decreased the intracellular activity of ODC by as much as 80%, whereas at the same concentration, 4 decreased the ODC activity only ∼5%, and even at a 100 μM concentration it decreased the ODC activity by only 55% (Figure 6B). Thus the poor downregulatory activity of 4 cannot be associated with its lower intracellular concentration due to its partial conversion into 3-MeSpd (see the previous paragraph) because at a concentration as low as 10 μM, compounds 1, 2, and 3, were able to downregulate ODC by 80%. Previously, we have studied the ability of different methylated Spd analogues to downregulate ODC activity in DU145 cells and demonstrated that only 2-MeSpd was a poor downregulator.15 At the moment, it is unclear why 2-MeSpd and 4 exhibit different effects from the other methylated Spd’s and bis-methylated analogues of 1. We cannot exclude the possibility that the position of the methyl group is essential for the stabilization of the pseudoknot in the mammalian OAZ1 mRNA, located just after the termination codon of ORF1; this is known to be necessary for the +1 frameshift of OAZ1 mRNA, which is required for the biosynthesis of full-length OAZ1.41,42 Whatever the reason, as far as we are aware, 4 is the first functionally active Spm mimetic that is unable to induce OAZ1. This analogue might be a useful tool for studying the regulation of the induction of OAZ1 biosynthesis by polyamines.
Figure 6.
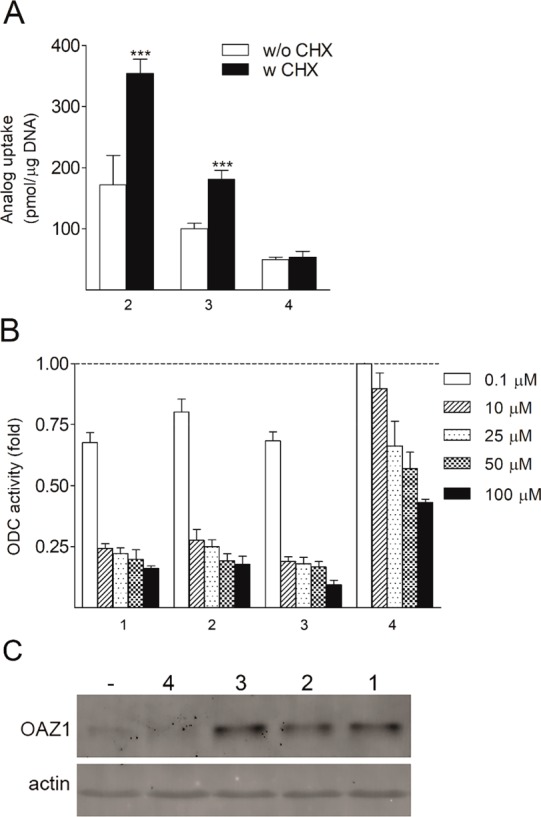
(A) Analogue uptake (6 h, 100 μM) in DU145 cells in the presence and absence of the protein synthesis inhibitor CHX (10 μg/mL). Data are means ± SD, n = 3. *** refers to the statistical significance of p < 0.001 as compared with samples without CHX. (B) ODC activity in DU145 cells treated with 0.1, 10, 25, 50, or 100 μM analogues for 6 h. (C) Induction of OAZ1 protein by the analogues (100 μM) after 4 h of treatment in the presence of MG132 (25 μM), an inhibitor of proteasomal degradation.
Conclusions
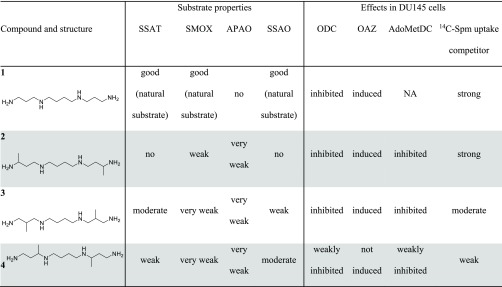
Our data demonstrate that the electrostatic interactions of properly positioned amino groups of 2, 3, and 4 with cellular targets and the enzymes of polyamine metabolism are not the only mode to allow recognition of the analogue and Spm. The correct spatial organization of the key part(s) of the analogue is of crucial importance, and the recognition is affected by the presence of even a comparatively small methyl group. Moreover, by changing the position of the methyl group in the analogue backbone, it is possible to regulate the biochemical properties of bis-C-methylated analogues of Spm, making them interesting research compounds with which to study the cellular functions of the individual polyamines (Table 2). The present results also provide new insights into the structural limitations that should be taken into consideration during the design of the drugs to treat Snyder–Robinson syndrome. Because 3 and 4 are chiral molecules, it is very likely that the different diastereomers display distinct biological properties, as has been previously demonstrated for compound 2.37
Table 2. Summary of the Biological Properties of the Analogues and the Natural Polyamine Spma.
NA, not available (not measured)
Experimental Section
General Procedures
Flash chromatography was performed on Kieselgel (40–63 μm, Merck, Germany), with eluents being indicated in the text. TLC was carried out on precoated Kieselgel 60 F254 plates (Merck, Germany) for the elution of (A) 4:2:1:2 n-BuOH/AcOH/Py/H2O and (B) 7:3 dioxane/25% NH4OH. The compounds on TLC plates were visualized after a color reaction with ninhydrin or with iodine. Proton (1H) and carbon (13C) NMR spectra were recorded on a Bruker Avance III apparatus (300.13 MHz for 1H; 75.43 MHz for 13C) using CDCl3 or D2O as solvents. Chemical shifts are given in parts per million (ppm) (δ relative residual to solvent peak for 1H and 13C when recorded in CDCl3 or relative to DSS when recorded in D2O). The letter “J” indicates normal 3JHH couplings if not specified. Melting points (mp) were determined in open glass capillaries on a Mel-Temp 3.0 apparatus (Laboratory Devices) and are uncorrected. High-resolution mass spectra (HRMS) were measured on a Bruker micrOTOF II instrument using electrospray ionization (ESI).43 The measurements were done in positive ion mode (interface capillary voltage −4500 V) with a mass range from m/z 50 to m/z 3000; external or internal calibration was done with ESI Tuning Mix (Agilent). A syringe injection was used for solutions in water for hydrochlorides 3–6 and acetonitrile for other compounds (flow rate 5 μL/min). Nitrogen was applied as a dry gas, and the interface temperature was set to 180 °C.
The final compounds 3–6 were ≥95% pure, as evidenced by NMR and analytical HPLC following the known method for polyamines and their analogues.44
Materials
The human prostate cancer cell line DU145 was obtained from American Type Culture Collection. Compound 2 was synthesized as previously described.31 Reference compound 1 was commercially obtained with purity of ≥97% (cat. no. S3256, Sigma-Aldrich). DFMO was obtained from ILEX Oncology. Putrescine dihydrochloride (cat. no. P7505, purity ≥98%), spermidine trihydrochloride (cat. no. S2501, purity ≥98%), and aminoguanidine (cat. no. 396494, purity ≥98%) were purchased from Sigma-Aldrich. [14C]-labeled spermine tetrahydrochloride (specific activity 112 mCi/mmol), l-ornithine (specific activity 57 mCi/mmol), acetyl-CoA (specific activity 60 mCi/mmol), and S-adenosyl-l-methionine (specific activity 54 mCi/mmol) were obtained from GE Healthcare. All solvents were used as purchased without further purification. Methanesulfonyl chloride, benzyl chloroformate, Boc2O, 4-aminobutanol-1, 2,2-dimethyl-1,3-diaminopropane, thiophenol, o-nitrophenylsulfonyl chloride (NsCl), NaCN, triphenylphosphine, and 40% aq methylamine were purchased from Aldrich (USA). 2-Methyl-1,3-diaminopropane was supplied by TCI Europe (Belgium). Crotononitrile, metacrylonitrile, LiAlH4, and 4-aminobutanol-2 (8) were purchased from Acros (Belgium). N-(Benzyloxycarbonyl)-3-amino-1-propyl methanesulfonate was prepared following the published procedure.45 2-Methyl-8-amino-4-azaoctanenitrile and 3-methyl-8-amino-4-azaoctanenitrile were synthesized as previously described.15 The synthesis of 2-methyl-3-aminopropanol-1 was performed starting from 3-aminoisobutyric acid (Acros, Belgium) and is described in the Supporting Information.
1,12-Diamino-2,11-dimethyl-4,9-diazadodecane Tetrahydrochloride (3)
Method A
A suspension of Pd/black in MeOH (ca. 0.25 mL) was added to a solution of 17 (0.5 g, 1.03 mmol) in a mixture of AcOH/MeOH (1:1, 7 mL), and hydrogenation was carried out at atmospheric pressure. The solids were filtered and washed with MeOH, and the combined filtrates were evaporated to dryness in vacuo. The residue was coevaporated with EtOH (2 × 5 mL), redissolved in EtOH (4 mL), and diluted with 5 M HCl (1.0 mL), and the resulting solution was evaporated to dryness in vacuo. The residue was coevaporated with dry EtOH (3 × 5 mL) and recrystallized from a MeOH–EtOH mixture to give 3 (0.28 g, 75%) as colorless crystals: mp 241–242 °C, Rf 0.19 (A), Rf 0.14 (B). 1H NMR (300.13 MHz, D2O): δ 3.22–3.08 (m, 8H, 2*NHCH2CH2 + 1/2 H2NCH2CH(CH3)CH2 + 1/2 H2NCH2CH(CH3)CH2), 3.03 (dd, 2J(H,H) = 12.9 Hz, J = 8.6 Hz, 2H, 1/2 H2NCH2CH(CH3)CH2), 2.92 (dd, 2J(H,H) = 13.1 Hz, J = 8.8 Hz, 2H, 1/2 H2NCH2CH(CH3)), 2.43–2.24 (m, 2H, 2*CH), 1.89–1.72 (m, 4H, 2*NHCH2CH2), 1.15 (d, J = 6.8 Hz, 6H, 2*CH3). 13C NMR (75.43 MHz, D2O): δ 51.29, 48.31, 43.02, 30.06, 23.29, 14.85. HRMS: m/z calculated for C12H30N4 + H+ [M + H+]: 231.2543. Found 231.2545. HPLC analysis (2000 pmol): retention time = 26.25 min; peak area 99.9%; elution buffers and gradients were as described.44
Method B
To a solution of 25 (0.6 g, 1.4 mmol) in MeOH (6 mL) was added HCl/MeOH (1.5 mL, 10 M), and the mixture was stirred for 30 min at 20 °C, followed by evaporation to dryness in vacuo (bath temperature 20 °C). The residue was coevaporated with MeOH (2 × 5 mL), dissolved in a minimum volume of MeOH, and precipitated with an excess of Et2O, which, after drying in vacuo over P2O5/KOH, resulted in crude 3, which was recrystallized from MeOH/EtOH to give 3 (0.4 g, 76%) as colorless crystals: mp 240–242 °C, Rf 0.21 (A), Rf 0.15 (B). 1H NMR (300.13 MHz, D2O): δ 3.21–3.08 (m, 8H, 2*NHCH2CH2 + 1/2 H2NCH2CH(CH3)CH2 + 1/2 H2NCH2CH(CH3)CH2), 3.02 (dd, 2J(H,H) = 12.9 Hz, J = 8.6 Hz, 2H, 1/2 H2NCH2CH(CH3)CH2), 2.92 (dd, 2J(H,H) = 13.1 Hz, J = 8.8 Hz, 2H, 1/2 H2NCH2CH(CH3)), 2.39–2.26 (m, 2H, 2*CH), 1.87–1.70 (m, 4H, 2*NHCH2CH2), 1.15 (d, J = 6.8 Hz, 6H, 2*CH3). 13C NMR (75.43 MHz, D2O): δ 51.26, 48.27, 42.93, 30.01, 23.27, 14.84. HRMS: m/z calculated for C12H30N4 + H+ [M + H+]: 231.2543. Found 231.2547.
1,12-Diamino-3,10-dimethyl-4,9-diazadodecane Tetrahydrochloride (4)
The compound was prepared as described for 3 from 18 (1.8 g, 3.61 mmol), which afforded 4 (1.1 g, 81%), mp 234–235 °C, Rf 0.22 (A), Rf 0.07 (B). 1H NMR (300.13 MHz, D2O): δ 3.50–3.37 (m, 2H, CH), 3.23–3.04 (m, 8H, 2*H2NCH2 + 2*NHCH2), 2.27–2.12 (m, 2H, CH2CH), 2.03–1.87 (m, 2H, CH2CH), 1.85–1.73 (m, 4H, 2*HNCH2CH2), 1.36 (d, J = 6.6 Hz, 6H, 2*CH3). 13C NMR (75.43 MHz, D2O): δ 52.70, 44.85, 36.55, 30.88, 23.70, 15.74. HRMS: m/z calculated for C12H30N4 + H+ [M + H+]: 231.2543. Found 231.2535. HPLC analysis (2000 pmol): retention time = 26.26 min; peak area 99.8%; elution buffers and gradients were as described.44
1,12-Diamino-2-methyl-4,9-diazadodecane Tetrahydrochloride (5)
The compound was prepared as described for 3 from 29 (1.45 g, 3 mmol). After recrystallization from MeOH/EtOH, 5 (0.89 g, 82%) was obtained as colorless crystals, mp 238–239 °C (dec.). Rf 0.21 (A), Rf 0.05 (B). 1H NMR (300.13 MHz, D2O): δ 3.22–3.06 (m, 10H, 1/2 H2NCH2CH(CH3) + 1/2 CH(CH3)CH2NH + 3*NHCH2CH2 + H2NCH2CH2CH2), 3.01 (dd, 2J(H,H) = 12.9 Hz, J = 8.6 Hz, 1H, H2NCH2CH(CH3)CH2), 2.93 (dd, 2J(H,H) = 13.1 Hz, J = 8.8 Hz, 1H, H2NCH2CH), 2.41–2.25 (m, 1H, CH), 2.17–2.03 (m, 2H, H2NCH2CH2), 1.87–1.73 (m, 4H, CH2CH2CH2CH2), 1.15 (d, 3H, CH3, J = 6.8 Hz). 13C NMR (75.43 MHz, D2O): δ 51.29, 48.32, 47.65, 45.20, 43.02, 37.22, 30.06, 24.39, 23.42, 23.27, 14.85. HRMS: m/z calculated for C11H28N4 + H+ [M + H+]: 217.2387. Found 217.2381. HPLC analysis (2000 pmol): retention time = 25.98 min; peak area 99.8%; elution buffers and gradients were as described.44
1,12-Diamino-2,2-dimethyl-4,9-diazadodecane Tetrahydrochloride (6)
The compound was prepared as described for 3 from 30 (1.26 g, 2.53 mmol), which, after recrystallization from MeOH/EtOH, gave 6 (0.8 g, 84%) as colorless crystals, mp 246–247 °C. Rf 0.19 (A), Rf 0.11 (B). 1H NMR (300.13 MHz, D2O): δ 3.19–3.10 (m, 8H, H2NCH2CH2CH2NHCH2 + CH2NHCH2C(CH3)2), 3.09 (s, 2H, NHCH2C(CH3)2), 3.03 (s, 2H, C(CH3)2CH2NH2), 2.15–2.04 (m, 2H, H2NCH2CH2CH2), 1.90–1.72 (m, 4H, NHCH2CH2CH2CH2), 1.17 (s, 6H, 2*CH3). 13C NMR (75.43 MHz, D2O): δ 56.01, 49.16, 47.63 (2C), 45.19, 37.22, 33.25, 24.38, 23.43, 23.02, 22.20. HRMS: m/z calculated for C12H30N4 + H+ [M + H+]: 231.2543. Found 231.2539. HPLC analysis (2000 pmol): retention time = 26.28 min; peak area 100%; elution buffers and gradients were as described.44
N-(Benzyloxycarbonyl)-3-amino-2-methylpropanol-1 (9)
Benzyl chloroformate (9.17 g, 53.7 mmol) was added in five portions within 15 min intervals to a cooled (0 °C) and vigorously stirred mixture of 2-methyl-3-aminopropanol-1 (7) (4.56 g, 51.2 mmol), 2 M Na2CO3 (25 mL), 1 M NaHCO3 (25 mL), and THF (50 mL). Stirring was continued for 2 h at 0 °C and for 6 h at room temperature. The organic layer was separated, and the water layer was extracted with DCM (3 × 25 mL). The combined organic extracts were concentrated in vacuo. The residue was dissolved in DCM (80 mL), washed with H2O (20 mL), 0.5 M H2SO4 (3 × 15 mL), H2O (20 mL), and brine (2 × 20 mL), and dried over anhydrous MgSO4 and filtered. The filtrate solvent was distilled off in vacuo, and crude 9 was triturated with an ether/hexane (1:3) mixture (80 mL) and left overnight at +4 °C. The precipitate was filtered and dried in vacuo at 1 Torr to produce a white solid 9 (10.4 g, 91%). TLC, NMR, and ESI-MS data are presented in the Supporting Information.
N-(Benzyloxycarbonyl)-4-aminobutanol-2 (10)
The compound was prepared from 4-aminobutanol-2 (8) (4.9 g, 55 mmol) and benzyl chloroformate (7.13 mL, 50 mmol) in a mixture of THF (55 mL), 2 M Na2CO3 (25 mL), and 1 M NaHCO3 (25 mL) as described for 9. Crude 10 was triturated with an ether/hexane (1:3) mixture (60 mL) and left overnight at +4 °C. The colorless oil was separated and dried in vacuo at 1 Torr to give 10 (10.1 g, 82%). The oil solidified at +4 °C but slowly self-converted to an oil at 20 °C. TLC, NMR, and ESI-MS data are presented in the Supporting Information.
N-(Benzyloxycarbonyl)-1-amino-2-methyl-3-azidopropane (11)
Methanesulfonyl chloride (2.26 mL, 29 mmol) in dry DCM (15 mL) was added dropwise within 20 min to a stirred and cooled (0 °C) solution of 9 (6.33 g, 28.4 mmol) and Et3N (6.0 mL, 43 mmol) in dry DCM (70 mL). Stirring was continued for 1 h at 0 °C, then for 4 h at 20 °C, and the reaction mixture was poured in a 1 M NaHCO3 (40 mL) solution. The organic layer was separated and washed with 1 M NaHCO3 (2 × 30 mL), H2O (15 mL), 0.5 M H2SO4 (3 × 40 mL), H2O (15 mL), and brine (25 mL). The DCM solution was dried (MgSO4) and concentrated in vacuo to give intermediate N-(benzyloxycarbonyl)-3-amino-2-methyl-1-propyl methanesulfonate (Rf 0.55 (CHCl3/MeOH 97:3)), which, as such, was dissolved in MeOH (25 mL) containing LiN3 (2.45 g, 50 mmol) and refluxed for 1 h. The reaction mixture was concentrated in vacuo, H2O (20 mL) was added to the residue, and the mixture was extracted with CHCl3 (4 × 20 mL). Combined organic extracts were washed with H2O (15 mL) and brine (2 × 20 mL), dried (MgSO4), and evaporated to dryness in vacuo to give 11 (6.10 g, 86%, as calculated from 9) as a viscous colorless oil. TLC, NMR, and ESI-MS data are presented in the Supporting Information.
N-(Benzyloxycarbonyl)-1-amino-3-azidobutane (12)
Intermediate N-(benzyloxycarbonyl)-4-amino-2-butyl methanesulfonate (Rf 0.37 (CHCl3/MeOH 98:2)), was prepared from 10 (6.9 g, 31 mmol), MsCl (2.32 mL, 30 mmol), and Et3N (4.85 mL, 35 mmol) as described for the synthesis of 11. Crude methanesulfonate was refluxed with LiN3 (2.95 g, 60 mmol) in MeOH (40 mL) for 3 h, the reaction mixture was concentrated in vacuo, H2O (30 mL) was added to the residue, and the mixture was extracted with CHCl3 (4 × 20 mL). Combined organic extracts were washed with H2O (15 mL) and brine (2 × 15 mL) and dried (MgSO4). Crude 12 was purified on a silica gel column (120 g) using DCM as an eluent to give 12 (6.25 g, 84%) as a viscous colorless oil. TLC, NMR, and ESI-MS data are presented in the Supporting Information.
N1-(Benzyloxycarbonyl)-1,3-diamino-2-methylpropane (13)
A solution of 11 (6.08 g, 24.5 mmol) and Ph3P (14.11 g, 52.7 mmol) in THF (60 mL) was refluxed for 1 h, cooled to 20 °C, added to 40% aq CH3NH2 (30 mL), and stirred at 20 °C for additional 2 h. The reaction mixture was concentrated in vacuo, the residue was poured into 1.0 M HCl (40 mL), and the solution was extracted with DCM (4 × 20 mL). The aqueous phase was diluted with 5.0 M NaOH (10 mL) and extracted with DCM (4 × 15 mL), and the extract was dried (K2CO3) and evaporated to dryness in vacuo. The residue was purified on a silica gel column (120 g) using a dioxane/25% NH4OH (99:1) mixture as an eluent. The appropriate fractions after concentration and drying in vacuo over P2O5 gave 13 (3.89 g, 68%) as a colorless oil. TLC, NMR, and ESI-MS data are presented in the Supporting Information.
N1-(Benzyloxycarbonyl)-1,3-diaminobutane (14)
The compound was prepared by refluxing 12 (6.2 g, 25 mmol) and Ph3P (13.1 g, 50 mmol) in THF (60 mL) for 1.5 h, followed by a treatment with 40% aq CH3NH2 (30 mL), as described for 13. The isolation of 12 was performed by column chromatography on silica gel (160 g) using a dioxane/25% NH4OH (97:3) mixture as an eluent. The appropriate fractions were concentrated, and the residue after drying in vacuo over P2O5 afforded 14 (3.5 g, 65%) as a colorless oil. TLC, NMR, and ESI-MS data are presented in the Supporting Information.
N1-(Benzyloxycarbonyl)-N3-(o-nitrophenylsulfonyl)-1,3-diamino-2-methylpropane (15)
To the cooled (0 °C) solution of 13 (3.00 g, 13.5 mmol) and Et3N (2.25 mL, 16 mmol) in dry DCM (40 mL), a solution of NsCl (3.08 g, 13.9 mmol) in dry DCM (20 mL) was added within 30 min with stirring, which was continued for 1 h at 0 °C and then for 3 h at 20 °C. The reaction mixture was diluted with DCM (20 mL), the layers were separated, and the organic layer was washed with 1 M NaHCO3 (4 × 15 mL), H2O (15 mL), 10% citric acid (4 × 15 mL), H2O (15 mL), and brine (2 × 20 mL) and dried (MgSO4). The solvent was distilled off in vacuo, and the residue was dried in vacuo over P2O5 to afford 15 (5.33 g, 97%) as a colorless semisolid substance. TLC, NMR, and ESI-MS data are presented in the Supporting Information.
N1-(Benzyloxycarbonyl)-N3-(2-nitrophenylsulfonyl)-1,3-diaminobutane (16)
The compound was prepared as described for 15 from 14 (3.44 g, 15.5 mmol), Et3N (2.4 mL, 17.3 mmol), and NsCl (3.48 g, 15.7 mmol) in dry DCM that, after drying in vacuo over P2O5, gave 16 (6.14 g, 97%) as a semisolid substance. TLC, NMR, and ESI-MS data are presented in the Supporting Information.
N1,N12-Bis(benzyloxycarbonyl)-1,12-diamino-2,11-dimethyl-4,9-diazaoctane (17)
A mixture of 15 (1.76 g, 4.3 mmol), K2CO3 (1.71 g, 12.4 mmol), and 1,4-diiodobutane (0.62 g, 2 mmol) in dry DMF (10 mL) was stirred for 12 h at 40 °C, followed by the addition of BnBr (0.2 mL, 1.67 mmol) and stirring for 12 h at 20 °C. Then, K2CO3 (0.83 g, 6 mmol) and PhSH (0.85 g, 7.7 mmol) in DMF (2 mL) were added and stirring was continued for an additional 12 h at 20 °C, the salts were separated by centrifugation and washed with DMF (2 × 5 mL), and the combined supernatants were evaporated to dryness in vacuo. The residue was suspended in DCM (25 mL), washed with H2O (2 × 5 mL) and brine (2 × 3 mL), dried (K2CO3), concentrated in vacuo, and purified on silica gel column (60 g) using a mixture of dioxane/25% NH4OH (95:5) as an eluent. The appropriate fractions were concentrated, and the residue, after drying in vacuo over P2O5, afforded 17 (0.55 g, 55%) as a colorless viscous oil. TLC, NMR, and ESI-MS data are presented in the Supporting Information.
N1,N12-Bis(benzyloxycarbonyl)-1,12-diamino-3,10-dimethyl-4,9-diazaoctane (18)
The compound was prepared as described for 17 from 16 (6.15 g, 15.1 mmol), K2CO3 (6.0 g, 43.5 mmol), and 1,4-diiodobutane (2.18 g, 7.03 mmol) in abs DMF. Purification on the silica gel column (160 g) using a dioxane/25% NH4OH (100:1 → 95:5) mixture as an eluent, concentration of the appropriate fractions, and drying the resultant residue in vacuo over P2O5 produced 18 (1.83 g, 52.3%) as a colorless viscous oil. TLC, NMR, and ESI-MS data are presented in the Supporting Information.
2,11-Dimethyl-4,9-diazadodecane-1,12-dinitrile (21)
A mixture of 2-methyl-8-amino-4-azaoctanenitrile (19) (11.3 g, 73 mmol) and metacrylonitrile (2.3 g, 34 mmol) was heated for 96 h at 90 °C and the reaction was monitored by NMR; then, the mixture was distilled to obtain “crude” 21 (5.8 g), bp 145–148 °C/0.2 Torr. Purification on a silica gel column (130 g) using a mixture of dioxane/25% NH4OH (99:1) as an eluent and the subsequent concentration of the appropriate fractions gave (after drying in vacuo over P2O5) 21 (5.0 g, 66%) as a colorless viscous oil. TLC, NMR, and ESI-MS data are presented in the Supporting Information.
3,10-Dimethyl-4,9-diazadodecane-1,12-dinitrile (22)
The compound was prepared as described for 21 from 3-methyl-8-amino-4-azaoctanenitrile (20) (6.56 g, 42 mmol) and crotononitrile (1.34 g, 20 mmol), except that heating was carried out at 90 °C for 15 h. The distillation of the reaction mixture gave “crude” 22 (3.7 g, bp 153–155 °C/0.17 Torr). Purification on a silica gel column (140 g) using a mixture of dioxane/25% NH4OH (95:5) as an eluent and concentrating the appropriate fractions gave (after drying in vacuo over P2O5) 22 (3.53 g, 79%) as a colorless viscous oil. TLC, NMR, and ESI-MS data are presented in the Supporting Information.
N4,N9-Bis(tert-butyloxycarbonyl)-2,11-dimethyl-4,9-diazadodecane-1,12-dinitrile (23)
A solution of 21 (2.6 g, 11.7 mmol) and Boc2O (4.75 g, 21.8 mmol) in dioxane (25 mL) was stirred for 6 h at +20 °C and then concentrated in vacuo. The residue was dissolved in DCM (50 mL), and the solution was subsequently washed with 10% citric acid (3 × 10 mL), H2O (10 mL), 1 M NaHCO3 (10 mL), H2O (10 mL), and brine (2 × 10 mL) and dried (MgSO4). The purification of the crude material on a silica gel column (120 g) using a mixture of CHCl3/MeOH (100:1) as an eluent and the concentration of the appropriate fractions gave (after drying in vacuo over P2O5) 23 (4.2 g, 85%), mp 90–91 °C (EtOAc–hexane). TLC, NMR, and ESI-MS data are presented in the Supporting Information.
N4,N9-Bis(tert-butyloxycarbonyl)-3,10-dimethyl-4,9-diazadodecane-1,12-dinitrile (24)
The compound was prepared as described for 23 from 22 (3.46 g, 15.6 mmol) and Boc2O (6.32 g, 29 mmol) in dioxane (35 mL). The purification of the crude material on a silica gel column (130 g) using a mixture of CHCl3/MeOH (100:1) as an eluent and concentrating the appropriate fractions gave (after drying in vacuo over P2O5) 24 (5.34 g, 87%), mp 81.5–82 °C (EtOAc–hexane). TLC, NMR, and ESI-MS data are presented in the Supporting Information.
N4,N9-Bis(tert-butyloxycarbonyl)-1,12-diamino-2,11-dimethyl-4,9-diazadodecane (25)
A solution of 23 (4.2 g, 10 mmol) in THF (15 mL) was added with mechanical stirring for 20 min to a cooled (−5 °C) suspension of LiAlH4 (1.63 g, 43 mmol) in THF (50 mL), and stirring was continued for 1 h at −5 to −2 °C. The reaction mixture was cooled to −10 °C and carefully subsequently quenched with H2O (2.3 mL), 20% aq NaOH (1.3 mL), H2O (6.2 mL), and 40% aq NaOH (7.5 mL), maintaining a temperature below −5 °C. After warming to 20 °C, the organic layer was separated, and the residue was treated with THF (3 × 25 mL). The combined THF extracts were concentrated in vacuo, and the residue was dissolved in DCM (50 mL), washed with brine (2 × 10 mL), and dried (K2CO3). Purification on a silica gel column (60 g) using a mixture of dioxane/25% NH4OH (99:1) as an eluent and the concentration of the appropriate fractions gave (after drying in vacuo over P2O5) 25 (0.65 g, 15%) as a colorless oil. TLC, NMR, and ESI-MS data are presented in the Supporting Information.
N1-(Benzyloxycarbonyl)-1-amino-4-azaoctanol-8 (27)
A solution of N-Cbz-3-amino-1-propyl methanesulfonate (26)45 (5.74 g, 20 mmol) and 4-aminobutanol-1 (14.24 g, 160 mmol) in dry THF (30 mL) was stirred for 24 h at 20 °C, which was then concentrated at 0.2 Torr. A solution of 2 M NaOH (30 mL) was added to the residue, and the resulting mixture was extracted with DCM (3 × 15 mL); combined organic extracts were washed with H2O (2 × 10 mL) and brine (15 mL) and dried (K2CO3). Purification on a silica gel column (135 g) using a dioxane/25% NH4OH (95:5) mixture as an eluent and the concentration of the appropriate fractions gave (after drying in vacuo over P2O5) 27 (4.1 g, 73%) as a colorless oil. TLC, NMR, and ESI-MS data are presented in the Supporting Information.
N1,N4-Bis(benzyloxycarbonyl)-1-amino-4-azaoctanol-8 (28)
The compound was prepared as described for 9 from 27 (3.98 g, 14.2 mmol) and benzyl chloroformate (2.47 g, 14.5 mmol) in a mixture of THF (35 mL), 2 M Na2CO3 (20 mL), and 1 M NaHCO3 (55 mL), which (after drying in vacuo over P2O5) resulted in 28 (5.64 g, 96%) as a viscous oil. TLC, NMR, and ESI-MS data are presented in the Supporting Information.
N9,N12-Bis(benzyloxycarbonyl)-1,12-diamino-2-methyl-4,9-diazadodecane (29)
Methanesulfonyl chloride (0.7 g, 6 mmol) in dry C6H6 (5 mL) was added dropwise with stirring to a cooled (+4 °C) solution of 28 (2.5 g, 6 mmol) and Et3N (1.1 mL, 8 mmol) in C6H6/Et2O (40 mL, 1:3). Stirring was continued for 1 h at +4 °C and then for 1 h at 20 °C, the precipitate was filtered, and the filtrate was subsequently washed with 1 M NaHCO3 (4 × 5 mL), H2O (5 mL), 0.5 M H2SO4 (3 × 5 mL), H2O (5 mL), and brine (10 mL) and dried (MgSO4). The solvent was evaporated in vacuo to give an intermediate N1,N4-bis(benzyloxycarbonyl)-1-amino-4-aza-8-octylmethanesulfonate (Rf 0.77 (CHCl3/MeOH 97:3)), which was used (without purification) to alkylate 1,3-diamino-2-methylpropane (5.3 g, 60 mmol) in THF (30 mL). The reaction mixture was kept for 16 h at 4 °C, then for 24 h at 20 °C and evaporated to dryness at 0.5 Torr. A solution of 2 M NaOH (5 mL) and DCM (15 mL) was added, the organic layer was separated, and the water phase was extracted with DCM (3 × 5 mL). The combined organic extracts were washed with H2O (5 mL) and brine (10 mL), dried (MgSO4), and filtered. The solvent was removed in vacuo, and the residue was purified on a silica gel column (65 g) using a dioxane/25% NH4OH (95:5) mixture as an eluent. The appropriate fractions were concentrated, and the residue after drying in vacuo over P2O5 gave 29 (1.95 g, 67%, as calculated from 28) as a colorless oil. TLC, NMR, and ESI-MS data are presented in the Supporting Information.
N9,N12-Bis(benzyloxycarbonyl)-1,12-diamino-2,2-dimethyl-4,9-diazadodecane (30)
The compound was prepared as described for 29 from 28 (2.9 g, 7 mmol) and 1,3-diamino-2,2-dimethylpropane (7.14 g, 70 mmol) in dry THF (50 mL) and resulted in 30 (1.95 g, 56%, as calculated from 28) as a colorless viscous oil. TLC, NMR, and ESI-MS data are presented in the Supporting Information.
Cell Culture
Cells were cultured in high-glucose Dulbecco’s modified Eagle’s medium (DMEM, Sigma-Aldrich) supplemented with 10% heat-inactivated fetal bovine serum (Sigma-Aldrich), 2 mM l-glutamine, and 50 μg/mL gentamycin (Sigma-Aldrich). The cells were incubated in a humidified atmosphere at +37 °C, 5% CO2. The cells were harvested by trypsinization, washed with phosphate-buffered saline (PBS), pelleted, and stored at −70 °C before analyses. The cell number was measured electronically with the Coulter counter model Z1 (Coulter Electronics). The cells were lysed in a buffer containing 50 mM potassium phosphate buffer pH 7.2, 0.1 mM EDTA, 0.1% Triton X-100, and 0.1 mM dithiothreitol. Samples for polyamine measurement were taken (mixed with 1/10 volumes of 50% sulphosalicylic acid with 100 μM diaminoheptane), and the rest of the lysate was centrifuged for 16 000g for 20 min at +4 °C. The supernatant was used for enzymatic assays of SSAT, ODC, SMOX, and AdoMetDC.
Uptake Experiments
Competition experiments inDU145 cells were done as described.15 In the OAZ1-dependent uptake experiment, the cells were first incubated in the presence or absence of CHX (10 μg/mL, Sigma-Aldrich) for 1 h to prevent OAZ1 induction in response to analogues. Then, the incubation was continued with a 100 μM analog for 4 h in the presence or absence of CHX, and the intracellular concentrations of analogues were measured with HPLC.
Polyamines and Enzyme Activities
Intracellular polyamines and analogues were analyzed with HPLC according to a published method.44 The polyamine sample pellets were dissolved to 0.1 M NaOH, and the amount of DNA was measured using PicoGreen reagent (Invitrogen) according to the manufacturer’s instructions. SSAT and ODC activities were measured as previously described,46,47 respectively. AdoMetDC activity was determined as previously described,48 and SMOX activity was determined as described.16
Experiments with Recombinant/Purified Proteins
Plasmid coding human SMOX was a kind gift from Dr. Carl Porter, Roswell Park Cancer Institute, NY. The recombinant protein was produced and purified as previously published.16 Cloning and producing mouse recombinant SSAT have been previously described.31 Experiments with purified bovine plasma SSAO (Worthington) were carried out as previously described.49
Western Blot
Total protein (30 μg/lane) was run on 16% SDS-PAGE, transferred to PVDF membrane (Immobilon-FL, Millipore), and probed with rabbit polyclonal anti-OAZ1 (a kind gift from Prof. Olli Jänne) and mouse monoclonal antiactin (1:1000, Santa Cruz Biotechnology) antibodies overnight. Secondary antibodies were antirabbit DyLight 649 (1:3500, Thermo Scientific) and antimouse Cy3 ECLPlex (1:15 000, GE Healthcare). The membrane was imaged using a ChemiDoc MP imager (Bio-Rad).
Statistical Analysis
Values are means ± SD. One-way analysis of variance (ANOVA) with Tukey’s post hoc test was used for multiple comparisons with the aid of a software package, GraphPad Prism 5.03 (GraphPad Software). *, **, and *** refer to p values of <0.05, <0.01, and <0.001, respectively.
Acknowledgments
The synthesis of the compounds was supported by the Russian Science Foundation (grant no. 17-74-20049), the Russian Foundation for Basic Research (grant no. 18-54-11008), and enzymatic and cellular experiments by the Academy of Finland (grant nos. 292574 and 315487). We thank Ms. Anne Karppinen (UEF), Ms. Arja Korhonen (UEF), Ms. Tuula Reponen (UEF), and Mr. Sergei Negria (EIMB) for their skillful technical assistance.
Glossary
Abbreviations Used
- AdoMetDC
S-adenosyl-l-methionine decarboxylase
- APAO
acetylpolyamine oxidase
- br s
broad signal
- DMF
N,N-dimethylformamide
- 1-MeSpd
1-methylspermidine (1,8-diamino-5-azanonane)
- 2-MeSpd
2-methylspermidine (1,8-diamino-2-methyl-4-azanoctane)
- 3-MeSpd
3-methylspermidine (1,8-diamino-3-methyl-4-azanoctane)
- 1,12-Me2Spm
1,12-dimethylspermine (2,13-diamino-5,10-diazatetradecane)
- 2,11-Me2Spm
2,11-dimethylspermine (1,12-diamino-2,11-dimethyl-4,9-diazadodecane)
- 3,10-Me2Spm
3,10-dimethylspermine (1,12-diamino-3,10-dimethyl-4,9-diazadodecane)
- OAZ1
antizyme 1
- ODC
ornithine decarboxylase
- Put
putrescine (1,4-diaminobutane)
- Spd
spermidine (1,8-diamino-4-azaoctane)
- Spm
spermine (1,12-diamino-4,9-diazadodecane)
- SMOX
spermine oxidase
- SSAO
semicarbazide-sensitive amine oxidase
- SSAT
spermidine/spermine N1-acetyltransferase
- THF
tetrahydrofuran
- TLC
thin layer chromatography
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jmedchem.9b01666.
Author Contributions
# M.K. and M.T.H. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Miller-Fleming L.; Olin-Sandoval V.; Campbell K.; Ralser M. Remaining mysteries of molecular biology: the role of polyamines in the cell. J. Mol. Biol. 2015, 427, 3389–3406. 10.1016/j.jmb.2015.06.020. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Functions of polyamines in mammals. J. Biol. Chem. 2016, 291, 14904–14912. 10.1074/jbc.R116.731661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani D.; De Bandt J. P.; Cynober L. Aliphatic polyamines in physiology and diseases. Clin. Nutr. 2014, 33, 14–22. 10.1016/j.clnu.2013.09.019. [DOI] [PubMed] [Google Scholar]
- Casero R. A. Jr; Marton L. J. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat. Rev. Drug Discovery 2007, 6, 373–390. 10.1038/nrd2243. [DOI] [PubMed] [Google Scholar]
- Park M. H.; Wolff E. C. Hypusine, a polyamine-derived amino acid critical for eukaryotic translation. J. Biol. Chem. 2018, 293, 18710–18718. 10.1074/jbc.TM118.003341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay M. K.; Park M. H.; Tabor H. Hypusine modification for growth is the major function of spermidine in Saccharomyces cerevisiae polyamine auxotrophs grown in limiting spermidine. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 6554–6559. 10.1073/pnas.0710970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen V. P.; Niiranen K.; Halmekytö M.; Pietilä M.; Diegelman P.; Parkkinen J. J.; Eloranta T.; Porter C. W.; Alhonen L.; Jänne J. Spermine deficiency resulting from targeted disruption of the spermine synthase gene in embryonic stem cells leads to enhanced sensitivity to antiproliferative drugs. Mol. Pharmacol. 2001, 59, 231–238. 10.1124/mol.59.2.231. [DOI] [PubMed] [Google Scholar]
- Mackintosh C. A.; Pegg A. E. Effect of spermine synthase deficiency on polyamine biosynthesis and content in mice and embryonic fibroblasts and the sensitivity of fibroblasts to 1,3-bis(2-chloroethyl)-N-nitrosourea. Biochem. J. 2000, 351, 439–447. 10.1042/bj3510439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeguchi Y.; Mackintosh C. A.; McCloskey D. E.; Pegg A. E. Effect of spermine synthase on the sensitivity of cells to antitumor agents. Biochem. J. 2003, 373, 885–892. 10.1042/bj20030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J.; Gritli-Linde A.; Heby O. Skin fibroblasts from spermine synthase-deficient hemizygous gryo male (Gy/Y) mice overproduce spermidine and exhibit increased resistance to oxidative stress but decreased resistance to UV irradiation. Biochem. J. 2000, 352, 381–387. 10.1042/bj3520381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauren Cason A; Ikeguchi Y.; Skinner C.; Wood T. C; Holden K. R; Lubs H. A; Martinez F.; Simensen R. J; Stevenson R. E; Pegg A. E; et al. X-Linked spermine synthase gene (SMS) defect: the first polyamine deficiency syndrome. Eur. J. Hum. Genet. 2003, 11, 937–944. 10.1038/sj.ejhg.5201072. [DOI] [PubMed] [Google Scholar]
- Murray-Stewart T.; Dunworth M.; Foley J. R.; Schwartz C. E.; Casero R. A. Polyamine homeostasis in Snyder-Robinson syndrome. Med. Sci. 2018, 6, 112. 10.3390/medsci6040112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänne J.; Alhonen L.; Pietilä M.; Keinänen T. A. Genetic approaches to the cellular functions of polyamines in mammals. Eur. J. Biochem. 2004, 271, 877–894. 10.1111/j.1432-1033.2004.04009.x. [DOI] [PubMed] [Google Scholar]
- Räsänen T. L.; Alhonen L.; Sinervirta R.; Keinänen T.; Herzig K.-H.; Suppola S.; Khomutov A. R.; Vepsäläinen J.; Jänne J. A polyamine analogue prevents acute pancreatitis and restores early liver regeneration in transgenic rats with activated polyamine catabolism. J. Biol. Chem. 2002, 277, 39867–39872. 10.1074/jbc.M205967200. [DOI] [PubMed] [Google Scholar]
- Hyvönen M. T.; Keinänen T. A.; Khomutov M.; Simonian A.; Weisell J.; Kochetkov S. N.; Vepsäläinen J.; Alhonen L.; Khomutov A. R. The use of novel C-methylated spermidine derivatives to investigate the regulation of polyamine metabolism. J. Med. Chem. 2011, 54, 4611–4618. 10.1021/jm200293r. [DOI] [PubMed] [Google Scholar]
- Järvinen A.; Grigorenko N.; Khomutov A. R.; Hyvönen M. T.; Uimari A.; Vepsäläinen J.; Sinervirta R.; Keinänen T. A.; Vujcic S.; Alhonen L.; Porter C. W.; Jänne J. Metabolic stability of alpha-methylated polyamine derivatives and their use as substitutes for the natural polyamines. J. Biol. Chem. 2005, 280, 6595–6601. 10.1074/jbc.M412788200. [DOI] [PubMed] [Google Scholar]
- Hyvönen M. T.; Khomutov M.; Petit M.; Weisell J.; Kochetkov S. N.; Alhonen L.; Vepsäläinen J.; Khomutov A. R.; Keinänen T. A. Enantiomers of 3-methylspermidine selectively modulate deoxyhypusine synthesis and reveal important determinants for spermidine transport. ACS Chem. Biol. 2015, 10, 1417–1424. 10.1021/cb500938e. [DOI] [PubMed] [Google Scholar]
- Hyvönen M. T.; Keinänen T. A.; Khomutov M.; Simonian A.; Vepsäläinen J.; Park J. H.; Khomutov A. R.; Alhonen L.; Park M. H. Effects of novel C-methylated spermidine analogs on cell growth via hypusination of eukaryotic translation initiation factor 5A. Amino Acids 2012, 42, 685–695. 10.1007/s00726-011-0984-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinänen T. A.; Hyvönen M. T.; Alhonen L.; Vepsäläinen J.; Khomutov A. R. Selective regulation of polyamine metabolism with methylated polyamine analogues. Amino Acids 2014, 46, 605–620. 10.1007/s00726-013-1587-9. [DOI] [PubMed] [Google Scholar]
- Kuksa V.; Buchan R.; Kong Thoo Li P. Synthesis of polyamines, their derivatives, analogues and conjugates. Synthesis 2000, 2000, 1189–1207. 10.1055/s-2000-6405. [DOI] [Google Scholar]
- Karigiannis G.; Papaioannou D. Structure, biological activity and synthesis of polyamine analogues and conjugates. Eur. J. Org. Chem. 2000, 2000, 1841–1863. . [DOI] [Google Scholar]
- Hahn F.; Schepers U. Solid phase chemistry for the directed synthesis of biologically active polyamine analogs, derivatives, and conjugates. Top. Curr. Chem. 2007, 278, 135–208. 10.1007/128_2007_135. [DOI] [Google Scholar]
- Casero R. A. Jr; Woster P. M. Recent advances in the development of polyamine analogues as antitumor agents. J. Med. Chem. 2009, 52, 4551–4573. 10.1021/jm900187v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlinden B. K.; Niemand J.; Snyman J.; Sharma S. K.; Beattie R. J.; Woster P. M.; Birkholtz L. M. Discovery of novel alkylated (bis)urea and (bis)thiourea polyamine analogues with potent antimalarial activities. J. Med. Chem. 2011, 54, 6624–6633. 10.1021/jm200463z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B.; Pachaiyappan B.; Gruber J. D.; Schmidt M. G.; Zhang Y. M.; Woster P. M. Antibacterial diamines targeting bacterial membranes. J. Med. Chem. 2016, 59, 3140–3151. 10.1021/acs.jmedchem.5b01912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borselli D.; Blanchet M.; Bolla J. M.; Muth A.; Skruber K.; Phanstiel O. 4th; Brunel J. M. Motuporamine derivatives as antimicrobial agents and antibiotic enhancers against resistant gram-negative bacteria. ChemBioChem 2017, 18, 276–283. 10.1002/cbic.201600532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reigada C.; Phanstiel O. 4th; Miranda M. R.; Pereira C. A. Targeting polyamine transport in Trypanosoma cruzi. Eur. J. Med. Chem. 2018, 147, 1–6. 10.1016/j.ejmech.2018.01.083. [DOI] [PubMed] [Google Scholar]
- Traquete R.; Ghani R. A.; Phanstiel O.; Wallace H. M. Ant 4,4, a polyamine-anthracene conjugate, induces cell death and recovery in human promyelogenous leukemia cells (HL-60). Amino Acids 2013, 44, 1193–1203. 10.1007/s00726-012-1452-2. [DOI] [PubMed] [Google Scholar]
- Palmer A. J.; Wallace H. M. The polyamine transport system as a target for anticancer drug development. Amino Acids 2010, 38, 415–422. 10.1007/s00726-009-0400-2. [DOI] [PubMed] [Google Scholar]
- Muth A.; Kamel J.; Kaur N.; Shicora A. C.; Ayene I. S.; Gilmour S. K.; Phanstiel O. 4th Development of polyamine transport ligands with improved metabolic stability and selectivity against specific human cancers. J. Med. Chem. 2013, 56, 5819–5828. 10.1021/jm400496a. [DOI] [PubMed] [Google Scholar]
- Järvinen A. J.; Cerrada-Gimenez M.; Grigorenko N. A.; Khomutov A. R.; Vepsäläinen J. J.; Sinervirta R. M.; Keinänen T. A.; Alhonen L. I.; Jänne J. E. n-Methyl polyamines: efficient synthesis and tolerance studies in vivo and in vitro. First evidence for dormant stereospecificity of polyamine oxidase. J. Med. Chem. 2006, 49, 399–406. 10.1021/jm050872h. [DOI] [PubMed] [Google Scholar]
- Weisell J.; Hyvönen M. T.; Häkkinen M. R.; Grigorenko N. A.; Pietilä M.; Lampinen A.; Kochetkov S. N.; Alhonen L.; Vepsäläinen J.; Keinänen T. A.; Khomutov A. R. Synthesis and biological characterization of novel charge-deficient spermine analogs. J. Med. Chem. 2010, 53, 5738–5748. 10.1021/jm100439p. [DOI] [PubMed] [Google Scholar]
- Grigorenko N. A.; Khomutov A. R.; Keinänen T. A.; Järvinen A.; Alhonen L.; Jänne J.; Vepsäläinen J. Synthesis of novel optical isomers of i-methylpolyamines. Tetrahedron 2007, 63, 2257–2262. 10.1016/j.tet.2006.12.065. [DOI] [Google Scholar]
- Zabolotnev D.Engelhardt Institute of Molecular Biology, RAS, Moscow, Russia, Personal Communication, 2018.
- Khomutov A. R.; Weisell J.; Khomutov M. A.; Grigorenko N. A.; Simonian A. R.; Häkkinen M. R.; Keinänen T. A.; Hyvönen M. T.; Alhonen L.; Kochetkov S. N.; Vepsäläinen J. Methylated polyamines as research tools. Methods Mol. Biol. 2011, 720, 449–462. 10.1007/978-1-61779-034-8_29. [DOI] [PubMed] [Google Scholar]
- Montemayor E. J.; Hoffman D. W. The crystal structure of spermidine/spermine N1-acetyltransferase in complex with spermine provides insights into substrate binding and catalysis. Biochemistry 2008, 47, 9145–9153. 10.1021/bi8009357. [DOI] [PubMed] [Google Scholar]
- Hyvönen M. T.; Keinänen T. A.; Cerrada-Gimenez M.; Sinervirta R.; Grigorenko N.; Khomutov A. R.; Vepsäläinen J.; Alhonen L.; Jänne J. Role of hypusinated eukaryotic translation initiation factor 5A in polyamine depletion-induced cytostasis. J. Biol. Chem. 2007, 282, 34700–34706. 10.1074/jbc.M704282200. [DOI] [PubMed] [Google Scholar]
- Khomutov M. A.; Hyvönen M. T.; Simonian A. R.; Weisell J.; Vepsäläinen J.; Alhonen L.; Kochetkov S. N.; Keinänen T. A.; Khomutov A. R. Acetylated derivatives of C-methylated analogues of spermidine: synthesis and interaction with N1-acetylpolyamine oxidase. Mendeleev Commun. 2018, 28, 479–481. 10.1016/j.mencom.2018.09.008. [DOI] [Google Scholar]
- Wang L.; Liu Y.; Qi C.; Shen L.; Wang J.; Liu X.; Zhang N.; Bing T.; Shangguan D. Oxidative degradation of polyamines by serum supplement causes cytotoxicity on cultured cells. Sci. Rep. 2018, 8, 10384. 10.1038/s41598-018-28648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles G. A. Mammalian plasma and tissue-bound semicarbazide-sensitive amine oxidases: biochemical, pharmacological and toxicological aspects. Int. J. Biochem. Cell Biol. 1996, 28, 259–274. 10.1016/1357-2725(95)00130-1. [DOI] [PubMed] [Google Scholar]
- Coffino Ph. Regulation of cellular polyamines by antizyme. Nat. Rev. Mol. Cell Biol. 2001, 2, 188–194. 10.1038/35056508. [DOI] [PubMed] [Google Scholar]
- Matsufuji S.; Matsufuji T.; Miyazaki Y.; Murakami Y.; Atkins J. F.; Gesteland R. F.; Hayashi S. Autoregulatory frameshifting in decoding mammalian ornithine decarboxylase antizyme. Cell 1995, 80, 51–60. 10.1016/0092-8674(95)90450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsedilin A. M.; Fakhrutdinov A. N.; Eremin D. B.; Zalesskiy S. S.; Chizhov A. O.; Kolotyrkina N. G.; Ananikov V. P. How sensitive and accurate are routine NMR and MS measurements?. Mendeleev Commun. 2015, 25, 454–456. 10.1016/j.mencom.2015.11.019. [DOI] [Google Scholar]
- Hyvönen T.; Keinänen T. A.; Khomutov A. R.; Khomutov R. M.; Eloranta T. O. Monitoring of the uptake and metabolism of aminooxy analogues of polyamines in cultured cells by high-performance liquid chromatography. J. Chromatogr., Biomed. Appl. 1992, 574, 17–21. 10.1016/0378-4347(92)80093-6. [DOI] [PubMed] [Google Scholar]
- Khomutov M. A.; Mandal S.; Weisell J.; Saxena N.; Simonian A. R.; Vepsäläinen J.; Madhubala R.; Kochetkov S. N. Novel convenient synthesis of biologically active esters of hydroxylamine. Amino Acids 2010, 38, 509–517. 10.1007/s00726-009-0410-0. [DOI] [PubMed] [Google Scholar]
- Libby P. R. Calf liver nuclear N-acetyltransferases. Purification and properties of two enzymes with both spermidine acetyltransferase and histone acetyltransferase activities. J. Biol. Chem. 1978, 253, 233–237. [PubMed] [Google Scholar]
- Jänne J.; Williams-Ashman H. G. On the purification of L-ornithine decarboxylase from rat prostate and effects of thiol compounds on the enzyme. J. Biol. Chem. 1971, 246, 1725–1732. [PubMed] [Google Scholar]
- Hyvönen M. T.; Howard M. T.; Anderson C. B.; Grigorenko N.; Khomutov A. R.; Vepsäläinen J.; Alhonen L.; Jänne J.; Keinänen T. A. Divergent regulation of the key enzymes of polyamine metabolism by chiral alpha-methylated polyamine analogs. Biochem. J. 2009, 422, 321–328. 10.1042/BJ20090737. [DOI] [PubMed] [Google Scholar]
- Ucal S.; Häkkinen M. R.; Alanne A. L.; Alhonen L.; Vepsäläinen J.; Keinänen T. A.; Hyvönen M. T. Controlling of N-alkylpolyamine analogue metabolism by selective deuteration. Biochem. J. 2018, 475, 663–676. 10.1042/BCJ20170887. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.