Abstract
Background
A variety of medicinal products prepared from secondary tubers of Harpagophytum procumbens subsp. procumbens (Burch.) DC.ex Meisn. (Devil’s Claw) and H. zeyheri are marketed in Africa, Europe, the United States, South America and elsewhere, where they are used for inflammatory and musculoskeletal conditions such as arthritis, lower back pain, rheumatism and neuralgia, etc. While clinical studies conducted over the last twenty years support the general safety of such products, infrequent gastrointestinal disturbances (diarrhea, nausea, vomiting, abdominal pain), headache, vertigo and hypersensitivity (allergic) reactions (rash, hives and face swelling) have been documented. Sex-related differences occur in the health conditions for which Devil’s Claw products are used, so it is likely that usage is similarly sex-related and so might be side effects and potential toxicities. However toxicologic studies of Devil’s Claw products have been conducted primarily with male animals. To address this deficit, we report toxicological studies in female and male rats of several H. procumbens (HP) aqueous-alcohol extracts chemically analyzed by UPLC-MS.
Methods
Female and male Sprague Dawley rats were studied for one and three months in groups differing by consumption of diets without and with HP extracts at a 7–10-fold human equivalent dose (HED). Sera were analyzed for blood chemistry, and heart, liver, lung, kidney, stomach, and small and large intestine tissues were examined for histopathology. Treatment group differences for blood chemistry were analyzed by ANOVA with Dunnett’s test and significant group differences for endpoints with marginal distributional properties were verified using the Kruskal-Wallis test. Group differences for histopathology were tested using Chi Square analysis.
Results
Significant group by sex-related differences in blood chemistry were detected in both studies. Additionally, several sex-related differences occurred between the studies. However, significant histopathology effects associated with the consumption of the extracts were not detected.
Conclusion
Toxicologic analysis of Devil’s Claw extracts cause significant sex-related effects in blood chemistry. However, in our judgement, none of the observed effects suggest serious toxicity at these doses and durations. Subsequent toxicologic and clinical studies of H. procumbens and other medicines with similar properties should explore in greater detail the basis and consequences of potential sex-related effects.
Keywords: Harpagophytum procumbens, Devil’s claw, Inflammation, Musculoskeletal, Toxicology, Rats, Sex-related
Background
H. procumbens is an important traditional medicinal plant of sub-Sarahan Africa first used by the San and Khoi peoples. It is a weedy, perennial tuberous plant with fruits having numerous long spines with hooks, giving rise to the colloquial name of the genus, Devil’s Claw. Secondary tubers are processed into extracts used throughout Africa, Europe and the Americas for treatment of musculoskeletal, inflammatory and other health problems that cause a major source of pain, loss of function and disability for many humans. Individuals with these conditions frequently employ botanicals as primary or supplementary medical treatments, however, scientific evidence of safety and efficacy of most such practices is limited. Devil’s Claw products containing H. procumbens and/or H. zeyheri are widely used to treat inflammatory, immunological and musculoskeletal conditions such as arthritis, lower back pain, rheumatism and neuralgia, as well as other conditions [1–5]. The limited clinical studies include reports of occasional gastrointestinal, kidney and cardiovascular effects but their causes have not been investigated [1, 5]. Concern about potential toxicities is accentuated by the variety of products, none of which are chemically well defined [1–3]; and that most reported preclinical toxicologic studies have been conducted with male animals, despite evidence that females experience higher inflammatory and immune system responses than males [6–8].
Sex-related differences occur in the incidence of conditions for which Devil’s Claw products are used, e.g. osteoarthritis, which has an inflammatory component [9–11] and whose prevalence increases with age, especially when comparing women of post-menopausal age with men of comparable age [8, 10–15]. These findings have led to the hypothesis that decreasing levels of circulating estrogen following menopause are associated with increased production of inflammatory cytokines [8, 10–14, 16–19]. Similar sex-related effects may occur with other conditions. Addressing such sex-related differences is important for understanding the safety and effectiveness of Devil’s Claw products and the many other medical treatments for inflammatory and immune system conditions. To help redress the lack of sex-related toxicologic data of Devil’s Claw products, we conducted one- and three-month studies in female and male adult rats of aqueous-alcoholic H. procumbens extracts characterized chemically by UPLC-MS for anti-inflammatory secondary metabolites.
Methods
Preparation and characterization of extracts
Dried H. procumbens secondary tubers were sourced by Parceval Herbal Products, LLC (Wellington, South Africa) in compliance with the South Africa Biodiversity Act. Mr. Ulrich Feiter confirmed identity of the plant materials. A botanical voucher (NBG 1488135-0) has been deposited at the Compton Herbarium, South African National Botanical Institute, Kirstenbosch Botanical Garden, Cape Town, South Africa. The dried, milled, plant material was processed as described in U.S. Patent 6280737B1(example 2): Approximately 100 kg was suspended in ~10x weight purified water at 85 °C for two hours, allowed to cool to room temperature and the residue pressed to recover supernatant; the residue was washed with approximately 4x initial weight of purified water (at 85 °C) for two hours and cooled to room temperature, then again pressed and the supernatant recovered. The combined supernatants were filtered through 10 μM and 1 μM filters and vacuum dried at 50 °C to approximately 50% moisture. This ‘primary extract’ was added slowly to 96% v/v ethanol (approximately 300 kg) while stirring, and a precipitate was allowed to form over two hours. The supernatant was carefully removed and concentrated under vacuum. A portion was further treated with water saturated n-butanol as described by U.S. Patent 6280737B1 (example 5). Two independent “primary’ aqueous – ethanolic extracts having 5.5% harpagoside (2014 HP) and 3.3% harpagoside (2016 HP) were studied as well as the 2016 HP extract further purified with water saturated n-butanol, which resuled in a five-fold enrichment of harpagoside.
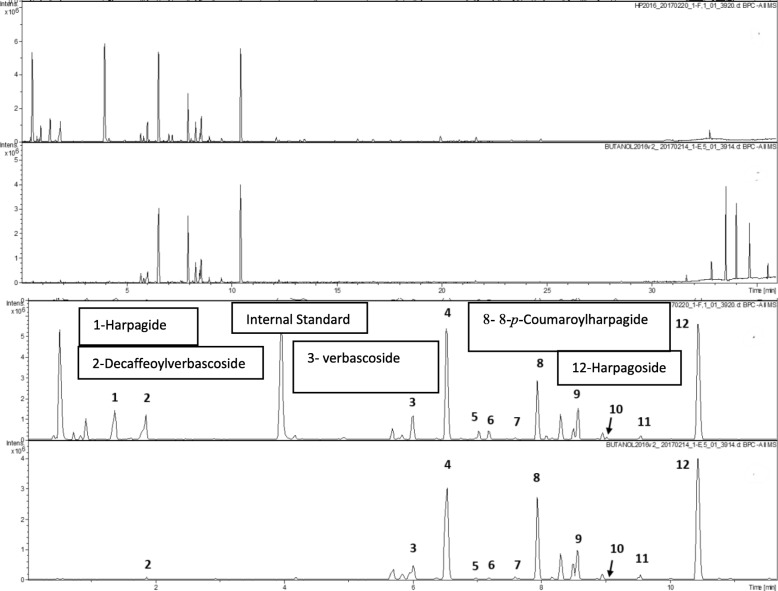
All extracts were analyzed by UPLC (with diode detection) - TOF-MS plus MS/MS (with lockspray ionization operated in the ESI negative mode) for candidate bioactive secondary metabolites characteristic of H. procumbens [3] (Fig. 1). LC-MS analyses were performed on a Bruker maXis impact quadrupole-time-of-flight mass spectrometer coupled to a Waters ACQUITY UPLC system. Separation was achieved on a Waters C18 column (2.1 × 100 mm, BEH C18 column with 1.7-um particles) using a linear gradient and mobile phase A (0.1% formic acid) and B (B: acetonitrile). Gradient condition: B increased from 5 to 70% over 30 min, then to 95% over 3 min, held at 95% for 3 min, then returned to 5% for equilibrium. The flow rate was 0.56 mL/min and the column temperature was 60 °C. Mass spectrometry was performed in the negative electrospray ionization mode with the nebulization gas pressure at 43.5 psi, dry gas of 12 L/min, dry temperature of 250 C and a capillary voltage of 4000 V. Mass spectral data were collected from 100 and 1500 m/z and were auto-calibrated using sodium formate after data acquisition. Quantitation of harpagoside concentration was performed using UV at 280 nm. Standard curve was generated using authentic harpagoside standard of 6 different concentrations. Harpagoside in the samples was quantified using the peak area at UV280 nm and the calibration curve. The concentrations of the harpagoside was then used calculated the amount of harpagoside in the samples. All extracts fulfill the requirement of European Pharmacopoeia that Devil’s Claw products contain no less than 1.2% harpagoside, and their compositions qualitatively resemble Devil’s Claw products marketed in Europe and the U.S. (data not shown).
Fig. 1.
UPLC-DAD-MS analysis of metabolites in aqueous-ethanolic and butanolic extracts of H. procumbens (top two panels, total elution profile); bottom two panels, portion of the gradient (min. 1–15) in which iridoid and phenylethanoid/phenylpropanoid anti-inflammatory metabolites elute. Marked peaks were identified by comparison with commercial analytical standards
Study design and treatment
Adult Sprague Dawley rats were housed in a University of Missouri vivarium under the care of licensed veterinarians with a protocol (#8654) approved by the University of Missouri Institutional Animal Care and Use Committee. Animals were acclimated for seven days, and 12:12 light and dark cycles were maintained with lights on at 0700 and off at 1900. All animals were monitored twice daily for health status and were singly housed. Animals were euthanized at 0900 with Ketamine/xylazine, 73 and 17 mg/kg followed terminal heart stick and cervical dislocation or induction of bilateral pneumothoraces as secondary means of assuring euthanasia. There are no deviations from the standards and regulations promulgated under the Animal Welfare Act.
One-month study: Male rats were obtained from Dr. Kathy Timms (University of Missouri) and female rats were purchased from Charles River (Raleigh, USA). Animals randomly assigned to groups of six males and females each were daily fed rodent chow with a peanut butter ‘treat’ used to deliver the HP extract. Control animals were provided chow and treats without HP extract, while treatment animals consumed chow and treats containing HP extract such that the daily consumption of harpagoside in the extract was approximately 1 g/kg/day. (This is between 7.5x to 10x the human equivalent dose (HED) reported in most human clinical studies.) Male rats were fasted overnight for chow to induce them to eat the treat, but female rats ate the treat without fasting. Fasted animals were provided food at 0800 to ensure complete treat intake. After 30 days, rats were anesthetized, and plasma collected by heart stick; the animals were euthanized and selected organs (heart, liver, kidney, lungs, stomach, large and small intestine) were collected and fixed in 4% paraformaldehyde for histopathology.
Three-month study: Male rats were obtained from Dr. Jonathan Green (University of Missouri) and females were purchased from Charles River (Raleigh). Males were randomly assigned to two groups of six animals (treat without extract or treat with 2014HP at 10x the HED). Females were randomly assigned to two groups of eight (treat without extract or treat with 2014HP at 10x the HED) and fasted overnight for chow if they did not regularly eat the treats. Fasted animals were provided food at 0800 to ensure complete treat intake. Blood was collected via saphenous vein every month and after 90 days of HP extract consumption rats were anesthetized and plasma collected by heart stick; the animals were euthanized and organs (heart, liver, kidney, lungs, stomach, large and small intestine) were collected and fixed in 4% paraformaldehyde for histopathology.
Hematology and histopathology analyses
Sera and tissue sections were analyzed for blood chemistry and histopathology by the University of Missouri Veterinary Medical Diagnostic Laboratory (VMDL). Blood chemistry was measured by Beckman Coulter AU480 Chemistry Analyzer with the Ion Selective Electrode (ISE) measuring method. For calcium measurement Arsenazo, OSR65117, was used. Creatinine reagent OSR6678 was used for creatinine measurement. For Glucose OSR6621 was used. OSR6222 was used for inorganic phosphate measurement. Urea Nitrogen (Bun) reagent OSR6634 was used for urea nitrogen.
The visceral organs including lung, liver, kidney, heart, stomach, small and large intestines were collected and fixed in 10% neutral-buffered formalin. For histopathological examination by a board-certified veterinary pathologist, the fixed tissues were trimmed, routinely processed, sectioned at 4 μm, and stained with hematoxylin and eosin. The pathologist was blinded to the definition of each treatment group. Pathologic lesion from each organ were separately scored from 0 to 3 (0: none, 1: mild, 2: moderate, 3: severe).
Group differences for blood chemistry endpoints were analyzed by ANOVA with Dunnett’s test; significant group differences for endpoints with marginal distributional properties were verified using the Kruskal-Wallis test. Hemolysis was confirmed with binary logistic regression to predict hemolysis, Wald chi square statistic. For analyses comparing only control versus the 2014 HP group the Dunnett’s test was not used. Group differences for histopathology endpoints were tested using Chi Square analysis.
Results
Throughout and at the end of the studies, all rats appeared alert, lacking porphyrin secretions and moved about their cages freely. Gross examination indicated all mucus membranes were moist and organs were of normal size and color.
One-month study
No significant differences in histopathology were noted, other than the control animals experienced a higher incidence of heart inflammation (3 rats) than any of the treatment groups (0% of the rats) (Table 1). Blood glucose, urea nitrogen, potassium, anion gap, globulin and phosphate were altered in treatment groups relative to the control group. Sodium was significantly altered between treatment groups (Table 2), suggestive of mild effects upon electrolyte and acid-base balance. There was no significant elevation of liver enzyme levels (alanine transaminase (ALT) or alkaline phosphatase (ALP) diagnostic of hepatotoxicity, but total bilirubin levels increased significantly compared to the controls. Some of this may be due to increased hemolysis in samples during collection.
Table 1.
One Month Study Histopathology Analysis: Test of Group Differences (n = 46; 22 females, 24 males)
| Control (n = 12) |
2014 HP (n = 11) |
2016 HP (n = 11) |
2016 Butanolic HP (n = 12) |
X2 (df) p | ||
|---|---|---|---|---|---|---|
| Scoring: |
None - # (%) Mild - # (%) |
None - # (%) Mild - # (%) |
None - # (%) Mild - # (%) |
None - # (%) Mild - # (%) |
||
| Lung | Inflammation |
12 (100) 0 (0) |
11 (100) 0 (0) |
11 (100) 0 (0) |
12 (100) 0 (0) |
* |
| Edema |
12 (100) 0 (0) |
11 (100) 0 (0) |
11 (100) 0 (0) |
12 (100) 0 (0) |
* | |
| Liver | Degeneration |
12 (100) 0 (0) |
11 (100) 0 (0) |
11 (100) 0 (0) |
12 (100) 0 (0) |
* |
| Inflammation |
12 (100) 0 (0) |
11 (100) 0 (0) |
11 (100) 0 (0) |
12 (100) 0 (0) |
* | |
| Necrosis & hemorrhage |
12 (100) 0 (0) |
10 (91) 1 (9) |
11 (100) 0 (0) |
11 (92) 1 (8) |
2.10 (3) .55 | |
| Fibrosis |
12 (100) 0 (0) |
11 (100) 0 (0) |
11 (100) 0 (0) |
12 (100) 0 (0) |
* | |
| Apoptosis | ** |
** 1 |
** 1 |
** | ** | |
| Kidney | Glomerulonephritis |
12 (100) 0 (0) |
11 (100) 0 (0) |
11 (100) 0 (0) |
12 (100) 0 (0) |
* |
| Tubular degeneration / necrosis |
12 (100) 0 (0) |
9 (82) 2 (18) |
11 (100) 0 (0) |
11 (92) 1 (8) |
4.12 (3) .25 | |
| Interstitial nephritis |
12 (100) 0 (0) |
10 (91) 1 (9) |
11 (100) 0 (0) |
11 (92) 1 (8) |
2.10 (3) .55 | |
| Proteinuria |
10 (83) 2 (17) |
6 (55) 5 (45) |
8 (73) 3 (27) |
7 (58) 5 (42) |
2.80 (3) .42 *** |
|
| Heart | Inflammation |
9 (75) 3 (25) |
11 (100) 0 (0) |
11 (100) 0 (0) |
12 (100) 0 (0) |
9.09 (3) .03 |
| Necrosis |
12 (100) 0 (0) |
11 (100) 0 (0) |
11 (100) 0 (0) |
12 (100) 0 (0) |
* | |
| Fibrosis |
12 (100) 0 (0) |
11 (100) 0 (0) |
11 (100) 0 (0) |
12 (100) 0 (0) |
* | |
| Stomach | Inflammation |
12 (100) 0 (0) |
11 (100) 0 (0) |
11 (100) 0 (0) |
12 (100) 0 (0) |
* |
| Mucosal erosion / ulceration |
12 (100) 0 (0) |
11 (100) 0 (0) |
11 (100) 0 (0) |
12 (100) 0 (0) |
* | |
| Small Intestine | Inflammation |
12 (100) 0 (0) |
11 (100) 0 (0) |
11 (100) 0 (0) |
12 (100) 0 (0) |
* |
| Mucosal erosion / ulceration |
12 (100) 0 (0) |
11 (100) 0 (0) |
11 (100) 0 (0) |
12 (100) 0 (0) |
* | |
| Large Intestine | Inflammation |
12 (100) 0 (0) |
11 (100) 0 (0) |
11 (100) 0 (0) |
12 (100) 0 (0) |
* |
| Mucosal erosion / ulceration |
12 (100) 0 (0) |
11 (100) 0 (0) |
11 (100) 0 (0) |
12 (100) 0 (0) |
* | |
# Data included in the attached Excel file
* Chi-square test statistic of differences in level of response between groups not computed due to distribution of responses - no variability in level of response
** Liver apoptosis was not recorded for any male animals; only noted as “mild” in 2 female rats; no coding for the other female rats and all the male rats
*** An additional statistical test comparing control and 2014 HP groups. X2 (df) p → 2.25 (1) .13. No significant difference between the two most extreme groups
Table 2.
One Month Study Blood Chemistry Group ANOVA
| Mean (sd) | |||||
|---|---|---|---|---|---|
| Measure | F, p value (df 3, 42) | Control | 2014 HP | 2016 HP | 2016 Butanolic HP |
| Glucose (mg/dL) | 3.38, .03 | 297.92 (61.40) | 248.27 (43.67) * (p = .048) | 239.82 (34.52) * (p = .02) | 253.42 (48.58) |
| Urea Nitrogen (mg/dL) | 15.50, <.001 | 20.67 (3.45) | 20.82 (2.27) | 14.82 (1.72) * (p < .001) | 19.00 (1.48) |
| Creatinine (mg/dL) *** | 1.33, .28 | ||||
| Sodium (mEq/L) | 3.28, .03 | 142.00 (2.37) | 143.18 (1.78) ** (p = .017) | 140.36 (1.69) ** (p = .017) | 141.75 (2.45) |
| Potassium (mEq/L) | 11.83, <.001 | 4.82 (0.37) | 5.66 (0.86) | 6.95 (0.65) * (p < .001) | 6.53 (1.47) * (p < .001) |
| Chloride (mEq/L) | 1.34, .27 | ||||
| Bicarbonate (mEq/L) | 1.87, .15 | ||||
| Anion Gap (mEq/L) | 4.42, .009 | 18.00 (1.54) | 20.46 (1.13) * (p = .003) | 19.73 (1.79) * (p = .049) | 19.67 (2.10) (p = .052) |
| Albumin (g/dL) | 0.09, .97 | ||||
| Total Protein (g/dL) | 0.63, .60 | ||||
| Globulin (g/dL) | 3.53, .023 | 2.77 (0.22) | 2.58 (0.20) | 2.52 (0.19) * (p = .009) | 2.62 (0.15) |
| Calcium (mg/dL) | 0.06, .98 | ||||
| Phosphorus (mg/dL) | 3.89, .015 | 7.04 (0.65) | 7.74 (0.66) * (p = .035) | 7.87 (0.60) * (p = .009) | 7.68 (0.66) |
| Cholesterol (mg/dL) | 0.06, .98 | ||||
| Total Bilirubin (mg/dL)*** | 25.08, <.001 | 0.10 (0.00) | 0.21 (0.05) * (p < .001) | 0.23 (0.05) * (p < .001) | 0.23 (0.05) * (p < .001) |
| ALT (U/L) | 1.29, .29 | ||||
| ALP (U/L) | 1.76, .17 | ||||
| GGT (U/L) | All values were “< 3” – no analyses conducted | ||||
| CK (U/L) **** | 2.75, .06 | 334.58 (143.97) | 467.55 (277.07) | 246.73 (114.43) | 242.42 (265.53) |
| X2, p value (df 3) |
Control % Slight Hemolysis |
2014 HP % Slight Hemolysis |
2016 HP % Slight Hemolysis |
Butanolic HP % Slight Hemolysis |
|
| Hemolysis (Mild to moderate hemolysis may cause false increases in direct bilirubin and AST measurement. Marked hemolysis can adversely affect all chemistry tests.) | 0.32, .96 | 25% | 18% | 18% | 17% |
* Intervention group(s) was significantly (p < .05) different from control group
** Intervention groups were significantly different from each other
***Based on distributional properties of the outcome measure, group differences were also tested using nonparametric Kruskal-Wallis Test
Creatinine X2 = 4.04, df 3, p = .257
Total Bilirubin X2 = 30.34, df 3, p < .001
**** Note – when outlier value (Data_id = 34) was excluded from the analysis, F (3, 41) = 1.81, p = .16
Significant sex-related differences occurred for the majority of the measures and group by sex interactions were determined to be significant for sodium, potassium, and phosphate levels (Table 3). With group by sex interactions, the differences between the groups varied between male or female data analysis. Sodium for females showed no significant group differences, but for males the control group differed significantly from the 2016 HP and Butanolic HP groups. For potassium, females differed between 2016 HP and the control group. The male control group differed significantly from each of the HP groups. Females in the 2016 HP and Butanolic HP groups exhibited increased phosphate compared to control, while males did not (Table 4). When considering just the sex and group main effects independently, the bicarbonate level elevation in males is indicative of acidosis and may explain the anion gap differences between the control and the treatment group. Additional analysis of other outcome measures is provided in Additional file 1.
Table 3.
One Month Study Blood Chemistry Group by Sex ANOVA
| Measure | GROUP main effect F, p value (df 3, 38) |
SEX main effect F, p value (df 1, 38) |
GROUP X SEX interaction F, p value (df 3, 38) |
|---|---|---|---|
| Glucose (mg/dL) | 4.97, .005 | 17.50, <.001 | 1.05, .38 |
| Urea Nitrogen (mg/dL) | 16.15, <.001 | 0.06, .81 | 1.43, .25 |
| Creatinine (mg/dL) | 1.91, .15 | 30.79, <.001 | 1.22, .31 |
| Sodium (mEq/L) | 4.54, .01 | 8.97, .005 | 4.47, .01 * |
| Potassium (mEq/L) | 78.88, <.001 | 154.09, <.001 | 31.05, <.001 * |
| Chloride (mEq/L) | 1.83, .16 | 21.08, <.001 | 0.03, .99 |
| Bicarbonate (mEq/L) | 3.78, .02 | 33.07, <.001 | 2.36, .09 |
| Anion Gap (mEq/L) | 4.77, .01 | 2.11, .15 | 1.99, .13 |
| Albumin (g/dL) | 0.27, .85 | 190.31, <.001 | 0.38, .77 |
| Total Protein (g/dL) | 1.81, .16 | 125.86, <.001 | 1.32, .28 |
| Globulin (g/dL) | 4.42, .01 | 13.13, .001 | 1.90, .15 |
| Calcium (mg/dL) | 0.14, .94 | 69.77, <.001 | 0.24, .87 |
| Phosphorus (mg/dL) | 5.00, .005 | 2.31, .14 | 4.24, .01 * |
| Cholesterol (mg/dL) | 0.03, .99 | 24.10, <.001 | 0.35, .79 |
| Total Bilirubin (mg/dL) | 33.00, <.001 | 10.81, .002 | 1.95, .14 |
| ALT (U/L) | 2.98, .04 | 45.48, <.001 | 0.74, .53 |
| ALP (U/L) | 1.86, .15 | 2.04, .16 | 2.61, .07 |
| GGT (U/L) | All values were “< 3” – no analyses conducted | ||
| CK (U/L) | 3.70, .02 | 3.79, .06 | 2.80, .053 |
|
Overall Model Test - X2 = 9.21, df 7, p = .24 Binary logistic regression to predict hemolysis. Wald X2 statistic |
|||
| Hemolysis (Mild to moderate hemolysis may cause false increases in direct bilirubin and AST measurement. Marked hemolysis can adversely affect all chemistry tests.) | X2 = 1.90, df 3, p = .59 | X2 = 0.0, df 1, p = .99 | X2 = 0.1, df 3, p = 1.0 |
*Only Sodium, Potassium and Phosphorus are significant for group by sex interactions
Table 4.
One Month Study Blood Chemistry Group ANOVA Tests, separately by Sex
| Sodium | ||||
| Females F = 2.13; df 3, 18; p = .13 | ||||
| Males F = 11.79, df 3, 20; p < .001 | ||||
| Measure | Mean (sd) | |||
| Control | 2014 HP | 2016 HP | 2016 Butanolic HP | |
| Female – Sodium (mEq/L) | 141.33 (3.08) | 144.00 (1.58) | 141.60 (1.52) | 143.67 (2.07) |
| Male – Sodium (mEq/L) | 142.67 (1.37) | 142.50 (1.76) | 139.33 (1.03)** | 139.83 (0.41)** |
| Potassium | ||||
| Females F = 15.69; df 3, 18; p < .001 | ||||
| Males F = 125.07, df 3, 20; p < .001 | ||||
| Measure | Mean (sd) | |||
| Control | 2014 HP | 2016 HP | 2016 Butanolic HP | |
| Female – Potassium (mEq/L) | 4.85 (0.44) | 4.82 (0.33) | 6.36 (0.35) ** | 5.17 (0.48) |
| Male – Potassium (mEq/L) | 4.78 (0.32) | 6.37 (0.29) ** | 7.43 (0.34) ** | 7.90 (0.24) ** |
| Phosphorus | ||||
| Females F = 5.40; df 3, 18; p = .01 | ||||
| Males F = 1.68, df 3, 20; p = .20 | ||||
| Measure | Mean (sd) | |||
| Control | 2014 HP | 2016 HP | 2016 Butanolic HP | |
| Female – Phosphorus (mEq/L) | 6.55 (0.50) | 7.38 (0.65) | 8.18 (0.64) ** | 7.70 (0.92) ** |
| Male – Phosphorus (mEq/L) | 7.53 (0.31) | 8.03 (0.55) | 7.62 (0.45) | 7.65 (0.32) |
**HP group(s) was significantly (p < .05) different from Control Group
We also analyzed 2014 HP and control data from the 1-month study separately to directly compare it with the 3-month study: Out of 20 variables tested (Table 6) in the blood chemistry panel there were a total of 5 group, 11 sex-related and 3 group by sex-related effects in the 1-month study. (Potassium, CK and bicarbonate having group by sex effects).
Table 6.
One Month Study Blood Chemistry Control and 2014HP group by Sex ANOVA (bold – significant differences)
| Serum measure | GROUP main effect | SEX main effect | GROUP X SEX interaction | Special Notes *Report as p < .001 |
|---|---|---|---|---|
| F, p value (df 1,19) | F, p value (df 1, 19) | F, p value (df 1, 19) | ||
| Glucose (mg/dL) | 6.27, .02 | 5.47, .03 | 0.89, .36 | Placebo > 2014 HP & Females < Males |
| Urea Nitrogen (mg/dL) | 0.04, .84 | .12, .74 | 2.03, .17 | No significant interaction |
| Creatinine (mg/dL) | 0.13, .73 | 24.89, <.001 | 0.29, .60 | Females > Males |
| Sodium (mEq/L) | 2.07, .17 | 0.01, .93 | 2.65, .12 | No significant interaction |
| Potassium (mEq/L) | 27.70, <.001 | 25.14, <.001 | 29.88, <.001 |
differences between the groups is different for females and males. Females: Placebo > 2014 HP Males: Placebo < 2014 HP** |
| Chloride (mEq/L) | 0.07, .80 | 7.98, .01 | 0.03, .87 | Females > Males |
| Bicarbonate (mEq/L) | 0.90, .36 | 18.27, <.001 | 6.95, .02 |
differences between the groups is different for females and males. Females: Placebo < 2014 HP Males: Placebo > 2014 HP |
| Anion Gap (mEq/L) | 18.69, <.001 | 0.23, .64 | 2.83, .11 | Placebo < 2014 HP |
| Albumin (g/dL) | 0.17, .69 | 77.95, <.001 | 0.51, .49 | Females > Males |
| Total Protein (g/dL) | 1.42, .25 | 74.89, <.001 | 0.26, .62 | Females > Males |
| Globulin (g/dL) | 6.40, .02 | 16.81, .001 | 0.03, .87 | Placebo > 2014 HP** & Females > Males |
| Calcium (mg/dL) | 0.11, .74 | 45.25, <.001 | 0.91, .35 | Females > Males |
| Phosphorus (mg/dL) | 9.82, .005 | 14.87, .001 | 0.61, .45 | Placebo < 2014 HP & Females < Males |
| Cholesterol (mg/dL) | 0.02, .90 | 11.89, .003 | 0.08, .78 | Females < Males |
| Total Bilirubin (mg/dL) | 66.58, <.001 | 4.29, .052 | 4.29, .052 | Placebo < 2014 HP** |
| ALT (U/L) | 0.65, .43 | 21.51, <.001 | 0.20, .66 | Females < Males |
| ALP (U/L) | 1.04, .32 | 8.09, .01 | 3.00, .10 | Females < Males |
| GGT (U/L) | All values were “< 3” – no analyses conducted | |||
| CK (U/L) | 4.36, .05 | 6.75, .02 | 8.28, .01 |
differences between the groups is different for females and males. Females: Placebo < 2014 HP Males: Placebo > 2014 HP |
| Hemolysis (Mild to moderate hemolysis may cause false increases in direct bilirubin and AST measurement. Marked hemolysis can adversely affect all chemistry tests.) |
Overall Model Test - X2 = 9.04, df 3, p = .03 Binary logistic regression to predict hemolysis. Wald X2 statistic |
|||
| X2 = 0.11, df 1, p = .74 | X2 = 0.0, df 1, p = .99 | X2 = 0.0, df 1, p = 1.0 | ||
**HP group(s) was significantly (p < .05) different from Control Group
Three-month study
The 3-month study was performed with the 2014 HP extract only; therefore, all statistical comparisons of the 3-month study and 1-month study used only data for the 2014 HP extract and relevant control groups. Since the studies were performed at different times, the statistical data is presented separately and differences between the two studies noted.
As with the 1-month study, histopathology analysis found no significant effects for any of the outcome variables tested. Kidney interstitial nephritis, proteinuria and tubular degeneration/necrosis were noted, however, none of these were statistically significant (Additional file 1:). Out of 20 variables tested in the blood chemistry panel there were 1 group, 12 sex-related and 2 group by sex-related effects. Phosphorus and bilirubin were the only variables with a group by sex-related effect. All the significant interactions are listed in bold in Table 5 (3-month).
Table 5.
Three Month Study Blood Chemistry Males and Females Group by Sex ANOVA (bold – significant differences)
| Serum measure | GROUP main effect | SEX main effect | GROUP X SEX interaction | Special Notes *Report as p < .001 |
|---|---|---|---|---|
| F, p value (df 1,24) | F, p value (df 1, 24) | F, p value (df 1, 24) | ||
| Glucose (mg/dL) | 2.24, .15 | 18.31, <.001 | 0.56, .46 | Females < Males |
| Urea Nitrogen (mg/dL) | 3.72, .07 | 0.28, .60 | 0.03, .86 | No significant interaction |
| Creatinine (mg/dL) | 9.10, .006 | 14.41, .001 | 0.01, .92 | Placebo < 2014 HP & Females > Males |
| Sodium (mEq/L) | 0.07, .80 | 4.79, .04 | 0.93, .35 | Females > Males |
| Potassium (mEq/L) (> 10 values coded as missing data) | 0.003, .96 | 74.43, <.001 | 0.46, .50 | Females < Males |
| Chloride (mEq/L) | 0.13, .73 | 11.99, .002 | 1.94, .18 | Females < Males |
| Bicarbonate (mEq/L) | 1.22, .28 | 45.86, <.001 | 0.08, .79 | Females > Males |
| Anion Gap (mEq/L) (missing data coded with group average) | 3.12, .09 | 12.49, .002 | 3.12, .09 | Females < Males |
| Albumin (g/dL) | 1.93, .18 | 98.42, <.001 | 0.52, .48 | Females > Males |
| Total Protein (g/dL) | 4.24, .051 | 55.26, <.001 | 0.46, .50 | Females > Males |
| Globulin (g/dL) | 2.79, .11 | 0.06, .80 | 0.11, .74 | No significant interaction |
| Calcium (mg/dL) | 0.00, .99 | 67.10, <.001 | 0.01, .92 | Females > Males |
| Phosphorus (mg/dL) | 0.26, .61 | 1.70, .21 | 4.81, .04 |
Differences between groups for females and males. Females: Placebo > 2014 HP Males: Placebo < 2014 HP |
| Cholesterol (mg/dL) (outlier coded with group average) | 2.55, .12 | 42.63, <.001 | 2.95, .10 | Females < Males |
| Total Bilirubin (mg/dL) | 17.62, <.001 | 6.51, .02 | 4.94, .04 | Significant main effects and interaction. Significant differences between females and males. For both females and males, Placebo < 2014 HP. This difference was much greater for females than it was for males. (With a significant interaction, do not interpret either significant main effect.) |
| ALT (U/L) | 0.86, .36 | 2.75, .11 | 1.16, .29 | No significant interaction |
| ALP (U/L) | 1.38, .25 | 0.80, .38 | 1.69, .21 | No significant interaction |
| GGT (U/L) | All values were “< 3” – no analyses conducted | |||
| CK (U/L) (outlier value coded with group average) | 0.34, .57 | 5.60, .03 | 0.47, .50 | Females > Males |
| Hemolysis (Mild to moderate hemolysis may cause false increases in direct bilirubin and AST measurement. Marked hemolysis can adversely affect all chemistry tests.) | Overall Model Test - X2 = 9.82, df 3, p = .02Binary logistic regression to predict hemolysis. Wald X2 statistic | The occurrence of hemolysis varied with group and sex. Female rats showed more hemolysis (only 1 male in 3-month data showed slight hemolysis). Hemolysis occurred at a slightly higher rate in the control group (50% of the female rats) compared to the HP group (37.5% of the female rats). | ||
| X2 = 0.00, df 1, p = .99 | X2 = 0.0, df 1, p = 1.00 | X2 = 0.0, df 1, p = .99 | ||
| NOTE - Total Protein: p value could be rounded to p = .05; if we interpret it, Placebo > 2014 HP | ||||
While there were many sex related effects observed in the blood chemistry panel, bilirubin was the only variable that had a significant difference between the control and treatment groups in both 1-month and 3-month time points.
Discussion
A variety of Devil’s Claw products and dietary supplements have been commercially marketed for over 50 years and are widely used for inflammatory health conditions such as arthritis, lower back pain, rheumatism and neuralgia [20]. While clinical studies conducted over the past twenty years support the general safety of such products, infrequent gastrointestinal disturbances (diarrhea, nausea, vomiting, abdominal pain), headache and vertigo, electrolyte imbalance, hypertension, and hypersensitivity (allergic) reactions (rash, hives and face swelling) have been documented [4, 21–25].
Most toxicologic studies of Devil’s Claw materials have used products that were not chemically defined [26–28] and used only male rats. Two recent studies (also with not well-defined Devil’s Claw product) used male and female mice; in these, acute (24 h) and chronic (3 months) treatments caused no significant toxicologic effects [29]. However, mild degenerative changes and fibrin accumulation in lung bronchioles and alveoli were observed as well as degeneration of hepatocytes with accumulated tissue debris in embryos - indicative of liver lesions; and necrosis with lymphocytic perivascular cuffing were observed in the adults. In the embryonic kidney sections, degeneration of proximal and distal convoluted tubules with tissue debris collection in the lumens were observed. In pregnant mice of the same group, necrosis of convoluted tubules and infiltration of mononuclear inflammatory cells along with lymphocytic perivascular cuffing was seen [30]. In none of these studies were sex-related effects evaluated or reported.
In the studies reported here, multiple sex-related effects were observed: Creatine kinase (CK) was differentially altered between the two different study groups and sexes in the 1-month study; in the 3-month study there was no difference between treatment and control group, but a sex-related difference did occur, with females having higher serum CK than males. Several changes in electrolytes were observed, also sex-related. While there were many sex related effects observed in the blood chemistry panel, bilirubin was the only variable that differed between the control and treatment groups in both 1-month and 3-month studies. We speculate that the elevated bilirubin may be due to some hemolysis in the samples and also to Heme oxygenase-1 (HO-1) catalyzed breakdown of heme. We have recently observed induction of HO-1 in rats treated with H. procumbens aqueous-ethanolic extracts [31], and others have also inferred H. procumbens affects HO-1 expression [32].
Understanding sex-related differences of anti-inflammatories and analgesics used to treat pain is a major research priority [33]. Analgesics most frequently used for musculoskeletal conditions, such as non-steroidal anti-inflammatory drugs (NSAIDs) cause toxicities in liver, kidney, cardiovascular and gastrointestinal function [34–36]. A recent analysis of patients in a large multicenter randomized clinical trial of celecoxib, naproxen and ibuprofen users for one year, identified males as having higher risk than females for major toxicities in either of the cardiovascular, gastrointestinal, or renal systems [37]. Determining whether well-defined botanicals used for pain management have similar toxicities is critically important.
Past studies of H. procumbens products suggest the anti-inflammatory and analgesic properties are due to inhibition of eicosanoid and nitric oxide (NO) biosynthesis, by altering expression and/or inhibition of COX and LOX and inducible nitric oxide synthase (iNOS), and to altered expression of pro- and anti-inflammatory cytokines and other mediators [2, 38]. Studies of well-defined products such as employed here, can answer whether H. procumbens extracts will inhibit both cyclooxygenases 1/2 (COX) and 5-lipoxygenase (LOX) expression and/or activities. Such dual COX/LOX inhibitors should be more efficacious with fewer side effects than current (single target) NSAIDs, and may reduce the use of opioid painkillers.
Conclusions
Consumption of 7-10x the human equivalent dose of several chemically defined H. procumbens aqueous-ethanolic extracts by female and male Sprague Dawley for one and three months resulted in no significant histopathology of liver heart, kidney, heart, lung or GI tract. However, analysis of blood chemistry indicated significant treatment group by sex interaction differences for sodium, potassium and phosphate of 1-month study rats when considering control and the 2014HP and 2016 Butanolic HP extracts. When considering just the control and 2014HP extract, potassium and CK were significant in group by sex interations. In the 3-month study of 2014 HP extract, phosphorus was the only group by sex outcome that was significantly affected. It appears that blood chemistry and signficant sex-related effects are caused by subtle chemical differences in the HP extracts.
Supplementary information
Additional file 1 a. Independently analyzed additional outcome measures of 1-month study group by sex ANOVA. b. 1-Month Study Histopathology Analysis: Test of Group Differences – Control versus 2014 HP (n = 23; 11 females, 12 males). c. 3-Month Study Histopathology Analysis: Test of Group Differences – Control versus 2014 HP (n = 28; 16 females, 12 males)
Acknowledgements
We thank Ms. Sydney Tyler for help with treatment of rats and sample collection; Dr. Kim Dae Young for histopathology analysis of tissue samples; Dr. Ravi Nistala for advice about the blood chemistry analyses; Dr. Zhentian Lei for HPLC-MS analyses; and the NIH for financial support.
Abbreviations
- AAVLD
American Association of Veterinary Laboratory Diagnosticians
- ALP
Alkaline Phosphatase
- ALT
Alanice Transaminase
- CK
Creatine Kinase
- COX-2
Cyclooxygenase 2
- CRP
C-reactive protein
- Cys-LT
Cysteinyl leukotrienes
- DC
Devils’s Claw
- HED
Human Equivalent Dose
- HP
H. procumbens
- NSAIDs
Non-Steriodal Anti-Inflammatory Drugs
- PGE2
Prostaglandin E2
- VMDL
Veterinary Medical Diagnostic Laboratory
Authors’ contributions
KJ, contributed to experimental design, treatment of rats, collection of samples and analyses, writing of manuscript. AP, contributed to the experimental design, collection of samples and interpretation of results. EAG-B, contributed to collection of samples and analysis. MG, contributed to analysis of results and interpretation. WF, contributed to experimental design, analysis of extracts, analysis of data and writing of manuscript. All authors read and approved of the final version of the manuscript.
Funding
Financial support was provided by Grant R21AT009086 from the NIH/National Center for Complementary and Integrative Health (NCCIH) and the University of Missouri. The contents are solely the responsibility of the authors and do not necessarily reflect the views of the sponsors. Sponsors approved the design of the study but had no role in the collection, analysis and interpretation of the results.
Availability of data and materials
Raw data generated is available at:
10.32469/10355/67307
Ethics approval and consent to participate
Animal use was conducted by protocol (#8654) approved by the University of Missouri Institutional Animal Care and Use Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kirtan Joshi, Email: kvj5kc@health.missouri.edu.
Alan Parrish, Email: parrishar@health.missouri.edu.
Elizabeth A. Grunz-Borgmann, Email: GrunzE@health.missouri.edu
Mary Gerkovich, Email: gerkovichm@umkc.edu.
William R. Folk, Email: folkw@missouri.edu
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12906-019-2789-9.
References
- 1.Harpagophyti radix. 2016 [cited 2018 April 25]; Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/herbal/medicines/herbal_med_000113.jsp&mid=WC0b01ac058001fa1d.
- 2.Mncwangi N, et al. Devil's claw-a review of the ethnobotany, phytochemistry and biological activity of Harpagophytum procumbens. J Ethnopharmacol. 2012;143(3):755–771. doi: 10.1016/j.jep.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Mncwangi NP, et al. What the devil is in your phytomedicine? Exploring species substitution in Harpagophytum through chemometric modeling of 1H-NMR and UHPLC-MS datasets. Phytochemistry. 2014;106:104–115. doi: 10.1016/j.phytochem.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Oltean H, et al. Herbal medicine for low-back pain. Cochrane Database Syst Rev. 2014;12:CD004504. doi: 10.1002/14651858.CD004504.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gagnier JJ, et al. Herbal medicine for low Back pain: a Cochrane review. Spine (Phila Pa 1976) 2016;41(2):116–133. doi: 10.1097/BRS.0000000000001310. [DOI] [PubMed] [Google Scholar]
- 6.Marriott I, Huet-Hudson YM. Sexual dimorphism in innate immune responses to infectious organisms. Immunol Res. 2006;34(3):177–192. doi: 10.1385/IR:34:3:177. [DOI] [PubMed] [Google Scholar]
- 7.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28(5):521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 8.Cutolo M, et al. The immunomodulatory effects of estrogens: clinical relevance in immune-mediated rheumatic diseases. Ann N Y Acad Sci. 2010;1193:36–42. doi: 10.1111/j.1749-6632.2009.05383.x. [DOI] [PubMed] [Google Scholar]
- 9.Blagojevic M, et al. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthr Cartil. 2010;18(1):24–33. doi: 10.1016/j.joca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Pereira D, et al. The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthr Cartil. 2011;19(11):1270–1285. doi: 10.1016/j.joca.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Srikanth VK, et al. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthr Cartil. 2005;13(9):769–781. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum. 1998;41(8):1343–1355. doi: 10.1002/1529-0131(199808)41:8<1343::AID-ART3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Felson DT, et al. The incidence and natural history of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1995;38(10):1500–1505. doi: 10.1002/art.1780381017. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence RC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41(5):778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 15.Schiphof D, et al. Factors for pain in patients with different grades of knee osteoarthritis. Arthritis Care Res (Hoboken) 2013;65(5):695–702. doi: 10.1002/acr.21886. [DOI] [PubMed] [Google Scholar]
- 16.Boyan BD, et al. Hormonal modulation of connective tissue homeostasis and sex differences in risk for osteoarthritis of the knee. Biol Sex Differ. 2013;4(1):3. doi: 10.1186/2042-6410-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karsdal MA, et al. The pathogenesis of osteoarthritis involves bone, cartilage and synovial inflammation: may estrogen be a magic bullet? Menopause Int. 2012;18(4):139–146. doi: 10.1258/mi.2012.012025. [DOI] [PubMed] [Google Scholar]
- 18.Pfeilschifter J, et al. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23(1):90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- 19.Razmjou S, et al. Effect of the menopausal transition and physical activity energy expenditure on inflammatory markers: a MONET group study. Menopause. 2016;23(12):1330–1338. doi: 10.1097/GME.0000000000000716. [DOI] [PubMed] [Google Scholar]
- 20.Guo R, et al. Botany, Phytochemistry, pharmacology and toxicity of Strychnos nux-vomica L.: a review. Am J Chin Med. 2018;46(1):1–23. doi: 10.1142/S0192415X18500015. [DOI] [PubMed] [Google Scholar]
- 21.Vlachojannis J, Roufogalis BD, Chrubasik S. Systematic review on the safety of Harpagophytum preparations for osteoarthritic and low back pain. Phytother Res. 2008;22(2):149–152. doi: 10.1002/ptr.2314. [DOI] [PubMed] [Google Scholar]
- 22.Devil's claw root: ulcers and gastrointestinal bleeding? Prescrire Int. 2013;22(144):296. [PubMed]
- 23.Cuspidi C, et al. Systemic hypertension induced by Harpagophytum procumbens (devil's claw): a case report. J Clin Hypertens (Greenwich) 2015;17(11):908–910. doi: 10.1111/jch.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Posadzki P, Watson LK, Ernst E. Adverse effects of herbal medicines: an overview of systematic reviews. Clin Med (Lond) 2013;13(1):7–12. doi: 10.7861/clinmedicine.13-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carvalho RR, et al. Syndrome of inappropriate antidiuretic hormone secretion induced by the phytotherapy Harpagophytum procumbers: case report. J Bras Nefrol. 2017;39(1):79–81. doi: 10.5935/0101-2800.20170013. [DOI] [PubMed] [Google Scholar]
- 26.Newall CA, Anderson LA, Phillipson JD. Herbal medicines : a guide for health-care professionals. 1996. London: Pharmaceutical Press.
- 27.Shaw D, et al. Traditional remedies and food supplements. A 5-year toxicological study (1991-1995) Drug Saf. 1997;17(5):342–356. doi: 10.2165/00002018-199717050-00006. [DOI] [PubMed] [Google Scholar]
- 28.Whitehouse LW, Znamirowska M, Paul CJ. Devil's claw (Harpagophytum procumbens): no evidence for anti-inflammatory activity in the treatment of arthritic disease. Can Med Assoc J. 1983;129(3):249–251. [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Harbi NO. Toxicity studies on Harpagophytum procumbens (Devil's claw) capsules in mice. J Med Plants Res. 2013;7(42):3089–3097. [Google Scholar]
- 30.Davari SA, Miri A, Shahraki E. Teratogenic effects of Harpagophytum procumbens Ethanolic extract in mice and fetuses. Zahedan J Res Med Sci. 2016;18(10):e3481. [Google Scholar]
- 31.Ungerer, G.C., J; Ndam, ; Bekemeier, M; Song, H; Li, R; Siedhold, H; Yang, B; Appenteng, M; Greenlief, M, Miller D; Sun, G; Folk, W; Gu, Z, Harpagophytum procumbens extract ameliorates allodynia and modulates oxidative and antioxidant stress pathways in a rat model of spinal cord injury. Neuromolecular Medicine, 2020. In press. [DOI] [PubMed]
- 32.Parenti C, et al. Involvement of the Heme-Oxygenase pathway in the Antiallodynic and Antihyperalgesic activity of Harpagophytum procumbens in rats. Molecules. 2015;20(9):16758–16769. doi: 10.3390/molecules200916758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.in Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. 2011: Washington (DC). [PubMed]
- 34.Kim S, Joo KW. Electrolyte and acid-base disturbances associated with non-steroidal anti-inflammatory drugs. Electrolyte Blood Press. 2007;5(2):116–125. doi: 10.5049/EBP.2007.5.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmeltzer PA, et al. Liver injury from nonsteroidal anti-inflammatory drugs in the United States. Liver Int. 2016;36(4):603–609. doi: 10.1111/liv.13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knights KM, Mangoni AA, Miners JO. Non-selective nonsteroidal anti-inflammatory drugs and cardiovascular events: is aldosterone the silent partner in crime? Br J Clin Pharmacol. 2006;61(6):738–740. doi: 10.1111/j.1365-2125.2006.02678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solomon DH, et al. Derivation and validation of a major toxicity risk score among nonsteroidal Antiinflammatory drug users based on data from a randomized controlled trial. Arthritis Rheumatol. 2019;71(8):1225–1231. doi: 10.1002/art.40870. [DOI] [PubMed] [Google Scholar]
- 38.Anauate MC, Torres LM, de Mello SB. Effect of isolated fractions of Harpagophytum procumbens D.C. (devil's claw) on COX-1, COX-2 activity and nitric oxide production on whole-blood assay. Phytother Res. 2010;24(9):1365–9. doi: 10.1002/ptr.3124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1 a. Independently analyzed additional outcome measures of 1-month study group by sex ANOVA. b. 1-Month Study Histopathology Analysis: Test of Group Differences – Control versus 2014 HP (n = 23; 11 females, 12 males). c. 3-Month Study Histopathology Analysis: Test of Group Differences – Control versus 2014 HP (n = 28; 16 females, 12 males)
Data Availability Statement
Raw data generated is available at:
10.32469/10355/67307