Abstract
Although preeclampsia is a common and serious complication of pregnancy, insight into its pathobiology and diagnosis is lacking. Circulating plasma exosomes, which contain RNA and other molecules and have recently become accessible for diagnostics, may be informative in this regard. We tested the hypothesis that preeclampsia may affect the miRNA cargo within circulating maternal blood exosomes. We collected plasma from 60 pregnant women at term, including 20 women with pregnancy complicated by preeclampsia, and 20 women with fetal growth restriction (FGR) and 20 with healthy pregnancy, serving as controls. We isolated exosomes from the maternal plasma by continuous density gradient ultracentrifugation. Our main outcome variable was exosomal miRNA cargo, analyzed by qPCR-based TaqMan Advanced miRNA assay in a card format, and the expression of differentially expressed exosomal miRNA in whole plasma from the same participants. We found that 7 miRNA species were differentially expressed in exosomes from women with preeclampsia and those from controls. In contrast, there was no significant difference in exosomal miRNA expression between women with FGR and controls. The results were not affected by fetal sex. Only one of the preeclampsia-related, differentially expressed exosomal miRNAs was significantly different in whole plasma miRNA analysis. We concluded that unlike whole plasma miRNA, exosomes extracted from the plasma of women with preeclampsia exhibit a unique miRNA profile, suggesting that plasma exosomal miRNA could provide insight into the pathophysiology of preeclampsia, and may play a role in disease diagnostics.
Keywords: Pregnancy, preeclampsia, fetal growth, exosomes, microRNA
Graphical Abstract
INTRODUCTION
Human placental dysfunction has been associated with common complications of pregnancy, primarily preeclampsia and fetal growth restriction (FGR). The overall incidence of preeclampsia is 3–5% of all pregnancies, with a higher incidence in certain populations across the globe.1, 2 FGR occurs in 5–8% of all pregnancies in developed countries, and its incidence is even higher in low-income countries.3 Both diseases entail significant fetal and maternal morbidity and mortality.4, 5 Newborns surviving these pregnancies are at a higher risk of short- and long-term diseases during infancy, childhood, and adulthood.4, 6
Attempts to achieve a better understanding of placental or endothelial cell dysfunction leading to preeclampsia have been hampered by insufficient in vivo models, inaccurate diagnostic tests, and limited therapeutic and preventive approaches.7–9 Diagnostic imaging by sonography or MRI has markedly improved in the last decade10, 11, yet their impact on elucidating disease pathobiology or on diagnostics in the context of these conditions has been limited.12, 13
The search for blood tests that can be harnessed for understanding diseases of human pregnancy has been bolstered by the identification of circulating placental proteins that may aid in disease diagnosis even prior to full clinical manifestation. Several studies have confirmed that the ratio of blood sFlt-1/PlGF is elevated in the second and third trimesters of pregnancy in preeclampsia, and this assay is gradually entering clinical practice. PAPP-A14 has been shown to be lower in first trimester maternal serum prior to the development of preeclampsia. Other maternal blood analytes are being validated for clinical practice.14, 15
In addition to circulating proteins, the blood is known to contain extracellular vesicles, (EVs) released from diverse cell types.16–18 Recent technological advances that enable the isolation of EVs, separation of their subtypes, and analysis of their cargo have markedly accelerated research in this area.19, 20 Many bodily cell types, including placental trophoblasts19, 20, have been shown to release EVs.16 Among the three main EV types, namely exosomes, microvesicles and apoptotic bodies, most information is available on exosomes, which measure 30–150 nm in diameter and are known to contain proteins, mRNAs, lncRNAs, miRNAs, and lipids.17, 21–24 Exosomes are formed as intraluminal vesicles within multivesicular bodies, which fuse with the cell membrane during exocytosis.16–18
Unlike non-vesicular, circulating molecules, packaging within exosomes (or other vesicles) may protect the cargo from degradation, and allow more precise delivery to target cells.25 Therefore, isolation of plasma exosomes and analysis of their cargo may provide a unique view into relevant diseases or define new diagnostic or prognostic potential for diverse disorders.21, 26, 27
In the present work, we pursued circulating exosomes, isolated from women with preeclampsia and compared to exosomes from women with uncomplicated term pregnancies or with pregnancies that were complicated by FGR. We hypothesized that the miRNA profile in exosomes isolated from the peripheral blood of healthy women or FGR is different from that of exosomes from women with preeclampsia. We also assessed whether differences in exosomal miRNA expression could be found by analysis of total plasma miRNA.
METHODS
Note that additional detailed methods are included in the supplement section. The authors declare that all supporting data are available within the article (and in the online-only Data Supplement).
Participants
The protocol for all studies included here was approved by the Institutional Review Board of the University of Pittsburgh (#PRO11060400, approved annually since July 1, 2011). A total of 60 pregnant women were recruited to this case-control study. We included 20 women with mild or severe preeclampsia, 20 women with FGR, and 20 healthy participants serving as controls. All participants were recruited as a part of our Perinatal Biology Lab biobank at Magee-Womens Research Institute. All participants delivered at Magee-Womens Hospital of the University of Pittsburgh Medical Center between July 2013 and Dec. 2018. The clinical diagnosis of mild or severe preeclampsia was based on criteria established by the International Society for the Study of Hypertension in Pregnancy28 and as previously published by our own institution.29–31 FGR was defined as we have previously detailed.32 Demographic information is shown in Table 1.
Table 1.
Relevant demographic data for all participants (n=60).
| Group | FGR (n=20) | Healthy (n=20) | Preeclampsia (n=20) | p-value (ANOVA) |
|---|---|---|---|---|
| Maternal age (year) | 31 (29–32) | 31 (28–33) | 28 (25–32) | 0.33 |
| Number of pregnancies | 2.2 (1.3–3.0) | 2.4 (1.8–3.1) | 2.3 (1.4–3.2) | 0.81 |
| Primigravida (%) | 50 | 20 | 40 | 0.13 |
| Number of living children | 0.7 (0–1.4) | 0.95 (0.6–1.3) | 0.95 (0.2–1.7) | 0.8 |
| Gestational age at delivery | 37 (36.1–38)* | 39 (38.9–39.4) | 37 (36.1–38.1)* | 0.0002 |
| Pre-pregnancy BMI | 24.8 (15–38)* | 29.3 (18–45) | 30.9 (18–39) | 0.03 |
| Delivery BMI | 29.9 (20–44)* | 35.1 (23–46) | 37.3 (26–40) | 0.008 |
| Neonatal weight (gms) | 2372 (2117–2586)* | 3587 (3371–3644) | 3131 (3040–3564) | 0.0001 |
| Placental weight | 542 (422–624)* | 727 (663–791) | 684 (499–712) | 0.0008 |
Data are mean and 95% confidence interval.
Denotes significantly different from the control by posthoc testing at p<0.05.
Plasma sample collection
We collected 10 ml of blood in Na-EDTA tubes during routine blood testing, performed when each participant was admitted for labor and delivery at our hospital. Each blood sample was centrifuged at 1500 g for 10 min at 4°C, and the supernatant was again centrifuged at the same conditions to remove any trace of blood cells. The plasma was frozen in −80°C. We have previously shown that the EVs remain stable in these conditions when not repeatedly thawed and frozen.20
Statistics
All analyses were done using the statistical computing software R or Prism 7.0. The clinical data were compared using one-way ANOVA with a post hoc Dunnett test, with p<0.05 denoting significance.
For analysis of RT-qPCR TaqMan Array Card data, we explored three different normalization methods. First, we normalized the Ct values by the median expression value of miRNAs that were relatively abundant (mean Ct values <35) and reliably detected (present in at least 80% of the samples from each group). We removed C19MC miRNAs when calculating the medians, because of their high sample-to-sample variability that we discovered in plasma C19MC miRNA expression.33, 34 Second, we normalized the data using the housekeeping gene method, where we identified four candidate housekeeping miRNAs based on the method described by Vandesompele et al.35 and implemented in the Bioconductor package NormqPCR by Perkins et al.36 Third, we normalized our data using the stably expressed miR-21–5p. We compared the three normalization methods, we found that they are consistent and led to similar results (Fig S2).
The heatmap for C19MC miRNAs was based on normalized and log2-transformed miRNA expression in the plasma exosomes, maternal plasma, and placental smRNAseq libraries. The small RNA libraries were aligned against the human reference genome (GRCh38), using Bowtie.37 The aligned sequences were annotated using the human miRNA database maintained at miRBase v21.38 The miRNA counts were then normalized by library size and log2 transformed.
The comparison of miRNA expression among the three conditions was based on the normalized Ct values based on the median method, described above and commonly used for RNA arrays or RNAseq data. After normalization, for each miRNA, we performed both a two-sample t test, comparing either the control sample group and the FGR group or the control samples and the preeclampsia samples. We also performed and a Wilcoxon rank sum test between each 2 groups to further bolster our analysis. All p-values were adjusted using Benjamini and Hochberg’s method to control the false discovery rate.39
RESULTS
Clinical Characteristics
We included data and specimens from 60 pregnant women and separated these into three groups: those with FGR; those with preeclampsia; and uncomplicated pregnancies, serving as control (n=20 in each group). Baseline demographic and clinical variables are provided in Table 1. As expected, mean gestational age at delivery was less than that of the FGR and preeclampsia groups, but neonatal and placental weight were not significantly different between the preeclampsia and control group.
Analysis of plasma exosomes and level of plasma exosomal miRNA
We isolated plasma exosomes using iodixanol (OptiPrep)-based continuous density gradient ultracentrifugation, currently a gold-standard approach for isolation of exosomes. Exosomes from all groups expressed the characteristic tetraspanin proteins CD63 and TSG101 and the expected NTA profile (Fig. 1A–B). As shown in Fig. 1C–D, we found that concentration of plasma exosomes from women with FGR and preeclampsia was 1.47-fold and 1.45-fold higher, respectively, compared to healthy controls (p <0.05). Interestingly, the mean diameter of exosomes derived from participants with preeclampsia (107.5 nm) was larger than that of controls (84.9 nm, Fig. 1D, p< 0.05).
Fig. 1: Isolation and characterization of plasma exosomes.
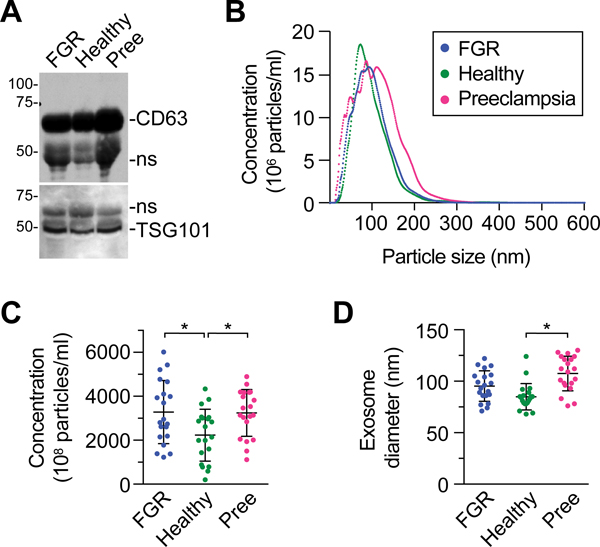
(A) A representative western blot showing the expression of exosomal tetraspanins CD63 and TSG101. As exosomes do not express standard proteins used for loading control, samples are normalized by loading 20 ug of protein. Non-specific (ns) bands are marked. (B) The profile of plasma exosome concentration across a range of particle sizes, determined using NTA as described in Methods. (C) The concentration of exosomes in plasma samples from the three conditions (expressed as mean exosome number x 108/ml plasma: FGR: 3282, Healthy: 2232, Preeclampsia: 3242). (D) The distribution of exosome diameter in plasma samples from the three conditions. A and B are representative experiments (n=3). C and D include samples from all participants (n=20 in each condition, and * denotes p <0.05.
We elected to use TaqMan qPCR technology to interrogate miRNA levels in exosomes after assessing the sensitivity of next generation small RNA sequencing when applied to plasma miRNA. We assessed the sensitivity on the basis of the exosomal expression of C19MC miRNAs, which are known (by sensitive qPCR) to be expressed in maternal plasma exosomes but absent in plasma exosomes from non-pregnant women.34, 40 Even after several miRNAseq experiments we could not consistently detect C19MC miRNAs in maternal plasma exosomes. For example, we found that, out of four maternal plasma exosome miRNAseq libraries, C19MC miRNAs were reliably detected in only one library (Fig. S1A). In contrast, we detected numerous C19MC miRNAs when qPCR-based TaqMan cards were used (Fig. S1B).
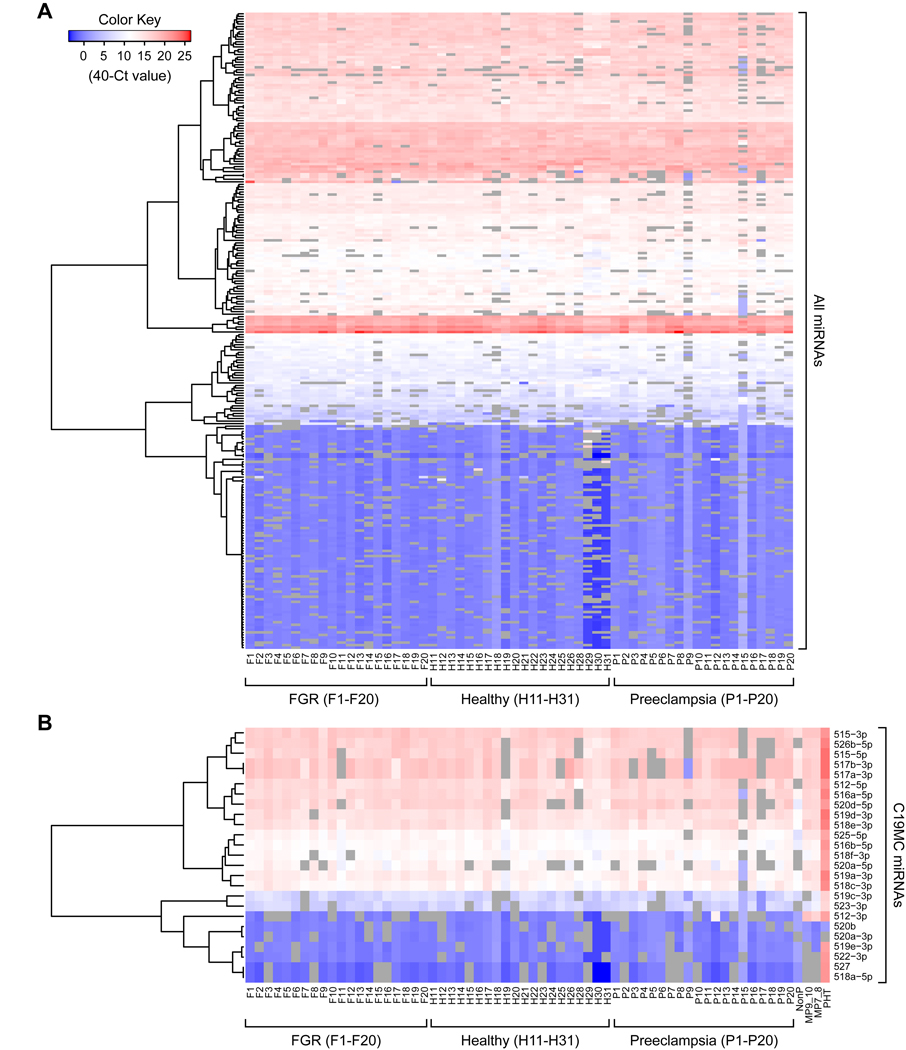
Using qPCR-based TaqMan cards, we identified 133 miRNAs that were detected in at least 80% of the samples from each group with a mean Ct value lower than 35 (based on our criteria and normalization as defined in Methods). All three normalization methods yielded similar results (Fig. S2). When we analyzed all 60 samples for discrete miRNA expression clustering, no notable expression pattern across the three paradigms was identified (Fig. 2A). Similarly, the placenta-specific C19MC miRNAs did not yield any discrete pattern among the three paradigms.
Fig. 2: Heatmap of the log2 -normalized expression of all miRNAs in the 60 participants.
(A) All detectable miRNAs. (B) Detectable C19MC miRNAs. The columns are clustered by using unsupervised hierarchical agglomerative clustering. The lack of a distinctive miRNA expression pattern among the three groups is noted.
To further validate our analysis we assessed the Spearman’s correlation between exosomal C19MC miRNA expression, measured by Taqman cards, and C19MC miRNA expression in placenta measured by small RNA seq, as we previously analyzed and reported.20, 33, 34 We found that the differences in the correlation coefficients were significant (p <0.05, Fig. 3). These data provide further support the reliability of our TaqMan-based analysis of exosomal miRNAs.
Fig. 3: The correlation between detectable C19MC miRNA in plasma exosomes and in the placenta.
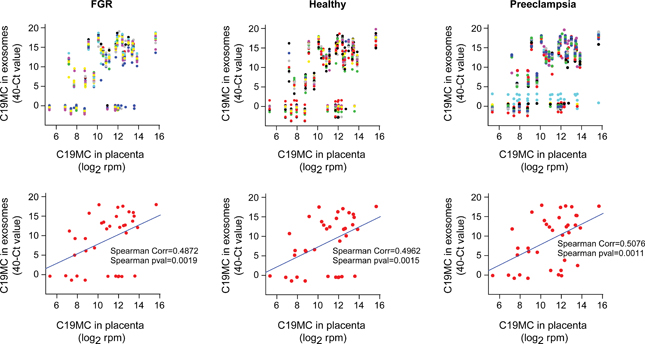
The expression of exosomal miRNAs (measured by TaqMan Cards) was normalized by the median, while expression of the placental miRNA (measured by smRNAseq) was normalized by library size then log2 – transformed data (see Methods), and expressed as log2 rpm (reads per million). In the upper panels each dot column represents a unique miRNA, where each dot’s x-coordinate equals the mean miRNA expression in the placenta, and the y-coordinate equals miRNA-specific expression in each exosome sample. In the lower panels each dot represents a unique miRNA, where each x-coordinate equals the mean miRNA expression in the placenta, and the y-coordinate equals the mean miRNA expression in all exosome samples from the corresponding group. The Spearman’s correlation coefficients and significance are shown.
To better visualize differences among the three clinical paradigms in our dataset, we used classic multidimensional scaling (cMDS). As shown in Fig. S3, and consistent with the heatmap (Fig. 2), no clear separation was observed among the three clinical groups. Because two participants with preeclampsia (#P9 and #P15) seemed to have a deviant MDS pattern, we (TP, a placental pathologist) evaluated the histopathology of all preeclamptic placentas included in our study. Indeed, the placenta of participant #P9 was the only one to exhibit histopathological evidence of placental abruption, with retroplacental hemorrhage and decidual necrosis. Participant #P15 exhibited the most severe case of extensive distal villous hypoplasia and severe hypoxic damage. Nonetheless, to avoid post-analysis bias, we included these samples in all subsequent assays.
Identification of differentially-expressed exosomal miRNAs
To identify discrete miRNA species that were differentially expressed among the three clinical groups, we first validated that normalization using the median approach provided similar results in pairwise comparisons across the three clinical groups. As shown in Fig. S4, we found no significant differences between each two clinical groups. Using the same approach, we compared all miRNAs that were expressed in plasma exosomes among the three clinical groups. As shown in Fig. 4A, seven miRNAs were differentially expressed in plasma exosomes between healthy and preeclamptic participants, and none of the miRNAs were different between participants with FGR and the other two groups. Notably, the expression of miR-486–3p in the preeclampsia samples formed two distinct clusters. The differences within the miR-486–3p group were not explained by the available clinical information and did not meet our criteria for differentially expressed miRNA. The odds ratios for preeclampsia for a change in one Ct value (2-fold) for each of the seven miRNAs are provided in Fig. 4B.
Fig. 4: A comparison of differentially expressed miRNA across the three conditions.
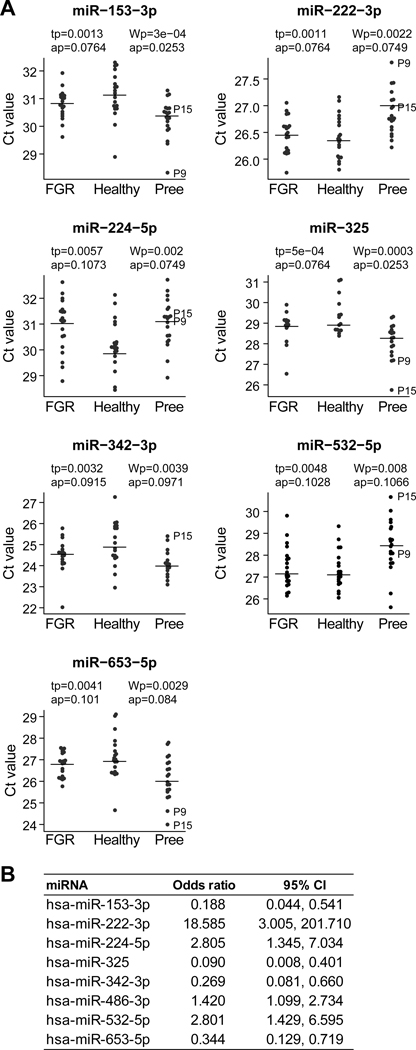
(A) The y-axis is the expression of miRNAs in Ct value, normalized to the median as detailed in Methods. The p-values and adjusted p-values for both the t-test and Wilcoxon rank sum test are shown. Note that higher Ct value represents lower expression. (B) The odds ratios and 95% confidence intervals for preeclampsia (vs healthy controls) for an increase of 1 Ct value (reduced miRNA expression by 2-fold).
We also examined the relationship between fetal sex and miRNA expression based on two methods: (a) median miRNA expression in all 60 participants (irrespective of group), based on t test or Wilcoxon’s rank sum test, after adjusting for FDR control, there were no sex-dependent differences (p=0.34 and p=0.66, respectively). (b) for each miRNA, we compared two nested models, where a base model has the patient group as the only independent variable, and the complex model has both the patient group and sex as independent variables. Using F test comparing the base model against the complex model with or without interaction, after adjusting for FDR control, the p values were 0.94 (complex model without group interaction) and 0.99 (complex model with group interaction). We concluded that there was no difference in exosomal miRNA expression between female or male fetuses. Similarly, we found no correlation between difference in exosomal miRNA expression and participants body mass index (BMI, Table 1) based on pre-pregnancy BMI or BMI at delivery (false discovery rate controlled at 0.05).
Comparison of differentially expressed miRNA in plasma exosomes and whole plasma
In the plasma, miRNAs are found not only in exosomes but also in other vesicles or bound by plasma non-vesicular proteins.41–43 To assess whether the expression differences in exosomal miRNAs could have been detected using whole plasma samples, we compared the expression levels of the seven differentially expressed exosomal miRNAs in whole plasma from the same patients. We found that the whole plasma expression of miR-153–3p, miR-653–5p, and miR-325 was very low (Ct value = 38), and that only miR-222–3p exhibited a significant difference between preeclampsia and control (Fig. 5).
Fig. 5: A comparison of differentially expressed miRNA between plasma exosomes and total plasma.
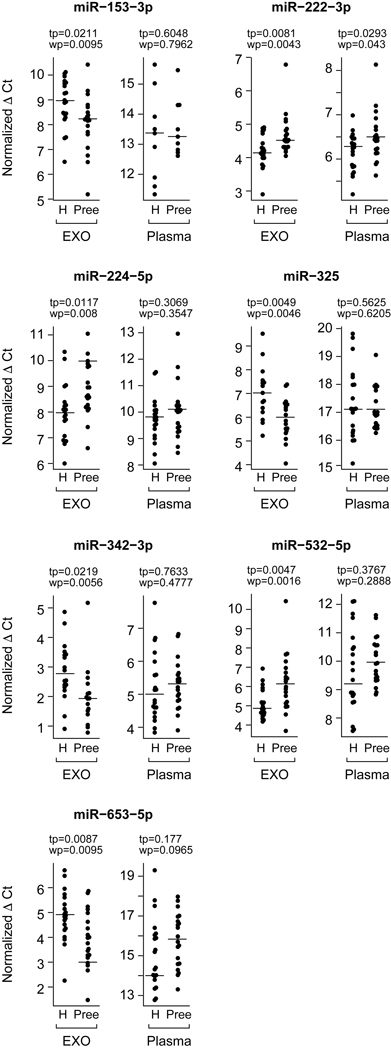
We focused on differentially expressed miRNA (Fig. 4) between preeclampsia and control samples. The y-axis is the expression of miRNAs in Ct value, normalized to the median (see Methods). The p values for both the t-test and Wilcoxon rank sum test are shown. Note that higher Ct value represents lower expression.
DISCUSSION
Although there were no distinct clustering patterns in exosomal miRNA expression among participants with preeclampsia, those with FGR, or controls, we found that several individual exosomal miRNA species were differentially expressed between preeclampsia and control exosomes. These miRNAs may illuminate disease-relevant pathways or contribute to the diagnosis of preeclampsia. For example, two of the seven differentially expressed exosomal miRNAs, miR-153–3p and miR-325–3p, exhibited a 2-fold up-regulation in exosomes from preeclampsia. Zeng et al. suggested that overexpression of miR-153–3p inhibited cell proliferation and invasion and promotes apoptosis.44 Liang et al. showed that miR-153 binds the 3′UTR of HIF1a mRNA and suppressed its expression in association with diminished tube formation in primary human umbilical vein endothelial cells (HUVECs), reduced VEGF-A expression, and angiogenesis.45, 46 Others suggested that miR-325–3p inhibits cell invasion and proliferation in non-small-cell lung cancer, with a similar effect in bladder cancer.47, 48 Among other differentially expressed miRNA, placental miR-342–3p was found to be upregulated in preeclamptic women49, and has been implicated in endothelial cell dysfunction in obese children.50 We also found that, except for one miRNA (miR-222–3p), none of the other miRNAs that were differentially expressed in exosomes exhibited a similar pattern in whole plasma analysis, highlighting the potential benefit of analyzing exosomal miRNA cargo for understanding relevant gestational disease or for diagnostics. Interestingly, we found that two of the samples from pregnancies complicated by preeclampsia (P15 and P9) were outliers by MDS analysis. Further pathological examination confirmed that they exhibited deviant placental histology, which might explain their difference from other samples. When we removed these two outliers and re-tested for differentially expressed miRNAs, we obtained similar results (not shown). Notably, maternal BMI, mode of delivery or fetal sex did not explain our results, and most preeclamptic participants provided the blood sample before treated with magnesium. Lastly, the differences in exosomal miRNA expression are unlikely to be explained by the differences in gestational age, as these expression differences also existed between the preeclampsia and FGR groups, which had the same mean gestational age at delivery.
Initial work in the area of circulating exosomal miRNA in pregnancy focused primarily on vesicle size and preeclampsia51 and on discrete functions of exosomal miRNA52, 53, We found that the concentration of plasma exosomes from preeclampsia and FGR was 1.5-fold higher compared to control samples, as previously reported.54 We also found the mean exosome diameter in preeclampsia (107.5 nm) to be larger than in controls (84.9 nm). The biological significance of these observation remains uncertain. Our data suggest that these small differences and relatively large patient-to-patient variability would make it impractical to use exosome concentration or size for diagnostic purposes.
Recent work by several groups has focused on the prevalence, expression, and potential clinical use of miRNA-containing exosomes during human pregnancy.54–60 Specifically in preeclampsia, PCR-based targeted screening for selected miRNAs showed that levels of miR-210, −136, −494, and −495 were elevated in peripheral blood exosomes in women were diagnosed with this disease.61 In addition, in a small group of four participants, miR-134, −196b, −302c, −346, −376c, −486–3p, −590–5p, and −618 were differentially expressed in women with preeclampsia. The difference from our results may be explained by the small number of participants used in this study. In a targeted PCR-based screen for the expression of selected chromosome 19 miRNA cluster (C19MC) members, miR-517–5p, miR-520a-5p, and miR-525–5p were found to be downregulated in preeclampsia.60 Finally, Salomon et al.54 used pooled DNA libraries to assess the differential expression of exosomal miRNAs that were isolated from the maternal blood and found 12 miRNAs that were differentially regulated between women with preeclampsia and healthy controls, including miR-423–5p, −451a, −107, −486–1-5p, −486–2-5p, −15a, −92–2-3p, −103a-1–3p, −103a-2–3p, −92a, and −126–3p. We did not pool libraries, and thus this procedure and potentially other differences in methodology or analysis may explain the diverse results.
Our study was not designed to interrogate the potential cellular and molecular targets of circulating exosomes during pregnancy. We also did not try to specifically isolate and study placenta-derived exosomes, which have been estimated to constitute 12–25% of all circulating exosomes in term pregnancy.62 Preeclampsia is a systemic disease, in which exosomes from numerous tissue sources may contribute to its pathophysiology, particularly to maternal endothelial cell dysfunction. Future research may require isolation and analysis of exosomes from the placenta, endothelial cells, or other tissues and tracking of their potential targets. Notably, trophoblastic exosomes carrying a potentially aberrant miRNA repertoire might have been overly diluted among exosomes from other sources, thus masking any potential differences in miRNA expression.
The strengths of our approach included (a) the relatively large sample size used in a sensitive, high-throughput screen of plasma exosomal miRNAs when compared to published studies in complicated pregnancies; (b) the inclusion of two relevant control groups, FGR and healthy pregnancies; (c) a comparison of miRNA profiles among isolated exosomes rather than comparison to total plasma miRNA; (d) the rigorous process used for exosome purification, based on “gold standard” technologies and avoiding products of questionable purity and reliability63, 64; (e) the use of several measures for quality control, including NTA analysis for sample purity, analysis of exosomal proteins, and a comparison of the expression ranking of exosomal C19MC miRNAs to their rank in the placenta; (f) data normalization using several approaches and rigorous multi-assay informatic analysis.
In our investigation we included participants with late onset preeclamptic, which likely represents an interaction of placental disease with maternal cardiovascular and metabolic conditions. This corresponds to our focus on circulating maternal plasma exosomes, not only those released from the placenta. Early onset preeclampsia would have required control and FGR participants from earlier gestational ages, which were not targeted by our studies. Our study was limited by the use of TaqMan arrays for our high-throughput screen. While we had to use this approach in order to maintain an acceptable level of sensitivity, it is possible that certain miRNA species were not represented in our arrays. While our study was not designed to identify or test disease biomarkers, it is also possible that including additional samples might have uncovered potential disease-related biomarkers. For example, using normalization by miR-21–5p, the median Ct value standard deviation of the 132 most abundant and reliably detected miRNAs was 0.973. If 5% of all miRNAs were candidate biomarkers with a difference of 2-fold between preeclampsia samples and controls, at a power of 0.8 while controlling for a false discovery rate at 0.05, 32 control and 32 preeclampsia samples would be needed. This number may become relevant if our approach is used for evaluation of exosomal miRNA biomarkers in preeclampsia. Future research may also focus on other types of EVs, such as the recently discovered “exomeres”65, which may harbor cargo that is relevant to preeclampsia.
PERSPECTIVES
Although the general miRNA landscape within circulating maternal plasma exosomes during pregnancy complicated by preeclampsia was similar to that of healthy controls or to pregnancies complicated by fetal growth restriction, the expression of seven miRNA species was significantly different in preeclampsia. This difference was not apparent when the whole plasma miRNA repertoire was analyzed, suggesting that plasma exosomal miRNA could provide insight into the pathophysiology of preeclampsia, and assist in disease diagnostics.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new?
The expression of seven miRNA species in circulating maternal plasma exosomes in preeclampsia is different from healthy controls or from pregnancies complicated by fetal growth restriction. This difference was not apparent when the whole plasma miRNA repertoire was analyzed.
What is relevant?
Plasma exosomal miRNA could provide insight into the pathophysiology of preeclampsia, a common hypertensive disorder of pregnancy, and assist in disease diagnostics.
Summary
Unlike whole blood miRNA, the miRNA cargo within circulating exosomes in pregnant women with preeclampsia is unique, and should be further pursued for mechanistic insights and diagnostic applications.
ACKNOWLEDGEMENTS
The authors thank Tiffany Coon for technical assistance; Lori Rideout for assistance with manuscript preparation; and Bruce Campbell for editing.
SOURCES OF FUNDING
The project was supported by the joint Third Xiangya Hospital/Central South University-University of Pittsburgh Scholar program (to H.L.), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) grants R01HD086325 and R37HD086916 (to Y.S.), the 25 Club of Magee-Womens Hospital (to Y.S.), and the Margaret Ritchie R. Battle Family Charitable Fund (to Y.S.).
Footnotes
DISCLOSURES
Y.S. is a consultant to Illumina, Inc.
REFERENCES
- 1.Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre-eclampsia. Lancet. 2016;387:999–1011. [DOI] [PubMed] [Google Scholar]
- 2.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. Am J Obstet Gynecol. 2000;182:198–206. [DOI] [PubMed] [Google Scholar]
- 4.Geva R, Eshel R, Leitner Y, Valevski AF, Harel S. Neuropsychological outcome of children with intrauterine growth restriction: a 9-year prospective study. Pediatrics. 2006;118:91–100. [DOI] [PubMed] [Google Scholar]
- 5.Goffin SM, Derraik JGB, Groom KM, Cutfield WS. Maternal pre-eclampsia and long-term offspring health: Is there a shadow cast? Pregnancy Hypertens. 2018;12:11–15. [DOI] [PubMed] [Google Scholar]
- 6.Bokslag A, van Weissenbruch M, Mol BW, de Groot CJ. Preeclampsia; short and long-term consequences for mother and neonate. Early Hum Dev. 2016;102:47–50. [DOI] [PubMed] [Google Scholar]
- 7.Sibley CP. Treating the dysfunctional placenta. J Endocrinol. 2017;234:R81–r97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kingdom JC, Audette MC, Hobson SR, Windrim RC, Morgen E. A placenta clinic approach to the diagnosis and management of fetal growth restriction. Am J Obstet Gynecol. 2018;218:S803–s817. [DOI] [PubMed] [Google Scholar]
- 9.Lyall F, Robson SC, Bulmer JN. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: relationship to clinical outcome. Hypertension. 2013;62:1046–1054. [DOI] [PubMed] [Google Scholar]
- 10.Markovic S, Fages A, Roussel T, Hadas R, Brandis A, Neeman M, Frydman L. Placental physiology monitored by hyperpolarized dynamic (13)C magnetic resonance. Proc Natl Acad Sci USA. 2018;115:E2429–e2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiserud T, Piaggio G, Carroli G, Widmer M, Carvalho J, Neerup Jensen L, Giordano D, Cecatti JG, Abdel Aleem H, Talegawkar SA, et al. The World Health Organization Fetal Growth Charts: A Multinational Longitudinal Study of Ultrasound Biometric Measurements and Estimated Fetal Weight. PLoS Med. 2017;14:e1002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadji C, Cannie MM, Resta S, Guez D, Abi-Khalil F, De Angelis R, Jani JC. Magnetic resonance imaging for prenatal estimation of birthweight in pregnancy: review of available data, techniques, and future perspectives. Am J Obstet Gynecol. 2019;220:428–439. [DOI] [PubMed] [Google Scholar]
- 13.Ingram E, Morris D, Naish J, Myers J, Johnstone E. MR imaging measurements of altered placental oxygenation in pregnancies complicated by fetal growth restriction. Radiology. 2017;285:953–960. [DOI] [PubMed] [Google Scholar]
- 14.Morris RK, Bilagi A, Devani P, Kilby MD. Association of serum PAPP-A levels in first trimester with small for gestational age and adverse pregnancy outcomes: systematic review and meta-analysis. Prenat Diagn. 2017;37:253–265. [DOI] [PubMed] [Google Scholar]
- 15.Agrawal S, Cerdeira AS, Redman C, Vatish M. Meta-Analysis and systematic review to assess the role of soluble FMS-like yyrosine kinase-1 and placenta growth factor ratio in prediction of preeclampsia: The SaPPPhirE Study. Hypertension. 2018;71:306–316. [DOI] [PubMed] [Google Scholar]
- 16.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–228. [DOI] [PubMed] [Google Scholar]
- 17.Tkach M, Thery C. Communication by extracellular vesicles: Where we are and where we need to go. Cell. 2016;164:1226–1232. [DOI] [PubMed] [Google Scholar]
- 18.Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17. [DOI] [PubMed] [Google Scholar]
- 19.Wu M, Ouyang Y, Wang Z, Zhang R, Huang PH, Chen C, Li H, Li P, Quinn D, Dao M, Suresh S, Sadovsky Y, Huang TJ. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc Natl Acad Sci USA. 2017;114:10584–10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouyang Y, Bayer A, Chu T, Tyurin VA, Kagan VE, Morelli AE, Coyne CB, Sadovsky Y. Isolation of human trophoblastic extracellular vesicles and characterization of their cargo and antiviral activity. Placenta. 2016;47:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, Fissell WH, Patton JG, Rome LH, Burnette DT, Coffey RJ. Reassessment of exosome composition. Cell. 2019;177:428–445. e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB, Ofrecio JM, Wollam J, Hernandez-Carretero A, Fu W, Li P, Olefsky JM. Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell. 2017;171:372–384. e312. [DOI] [PubMed] [Google Scholar]
- 24.Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, Rao TN, Winnay JN, Garcia-Martin R, Grinspoon SK, Gorden P, Kahn CR. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542:450–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Margolis L, Sadovsky Y. The biology of extracellular vesicles: The known unknowns. PLoS Biol. 2019;17:e3000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manier S, Liu CJ, Avet-Loiseau H, Park J, Shi J, Campigotto F, Salem KZ, Huynh D, Glavey SV, Rivotto B, et al. Prognostic role of circulating exosomal miRNAs in multiple myeloma. Blood. 2017;129:2429–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiao X, Fan Z, Chen H, He P, Li Y, Zhang Q, Ke C. Serum and exosomal miR-122 and miR-199a as a biomarker to predict therapeutic efficacy of hepatitis C patients. J Med Virol. 2017;89:1597–1605. [DOI] [PubMed] [Google Scholar]
- 28.Tranquilli AL, Dekker G, Magee L, Roberts J, Sibai BM, Steyn W, Zeeman GG, Brown MA. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens. 2014;4:97–104. [DOI] [PubMed] [Google Scholar]
- 29.Schmella MJ, Assibey-Mensah V, Parks WT, Roberts JM, Jeyabalan A, Hubel CA, Catov JM. Plasma concentrations of soluble endoglin in the maternal circulation are associated with maternal vascular malperfusion lesions in the placenta of women with preeclampsia. Placenta. 2019;78:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gandley RE, Althouse A, Jeyabalan A, Bregand-White JM, McGonigal S, Myerski AC, Gallaher M, Powers RW, Hubel CA. Low soluble syndecan-1 precedes preeclampsia. PLoS One. 2016;11:e0157608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hauspurg A, Countouris ME, Jeyabalan A, Hubel CA, Roberts JM, Schwarz EB, Catov JM. Risk of hypertension and abnormal biomarkers in the first year postpartum associated with hypertensive disorders of pregnancy among overweight and obese women. Pregnancy Hypertens. 2019;15:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mouillet JF, Chu T, Hubel CA, Nelson DM, Parks WT, Sadovsky Y. The levels of hypoxia-regulated microRNAs in plasma of pregnant women with fetal growth restriction. Placenta. 2010;31:781–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang G, Mouillet JF, Mishima T, Chu T, Sadovsky E, Coyne CB, Parks WT, Surti U, Sadovsky Y. Expression and trafficking of placental microRNAs at the feto-maternal interface. FASEB J. 2017;31:2760–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paquette AG, Chu T, Wu X, Wang K, Price ND, Sadovsky Y. Distinct communication patterns of trophoblastic miRNA among the maternal-placental-fetal compartments. Placenta. 2018;72–73:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:research0034.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perkins JR, Dawes JM, McMahon SB, Bennett DL, Orengo C, Kohl M. ReadqPCR and NormqPCR: R packages for the reading, quality checking and normalisation of RT-qPCR quantification cycle (Cq) data. BMC Genomics. 2012;13:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42:D68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B (Methodological) 1995;57:289–300. [Google Scholar]
- 40.Dumont TMF, Mouillet JF, Bayer A, Gardner CL, Klimstra WB, Wolf DG, Yagel S, Balmir F, Binstock A, Sanfilippo JS, Coyne CB, Larkin JC, Sadovsky Y. The expression level of C19MC miRNAs in early pregnancy and in response to viral infection. Placenta. 2017;53:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010;38:7248–7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA. 2011;108:5003–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng HF, Yan S, Wu SF. MicroRNA-153–3p suppress cell proliferation and invasion by targeting SNAI1 in melanoma. Biochem Biophys Res Commun. 2017;487:140–145. [DOI] [PubMed] [Google Scholar]
- 45.Liang H, Ge F, Xu Y, Xiao J, Zhou Z, Liu R, Chen C. miR-153 inhibits the migration and the tube formation of endothelial cells by blocking the paracrine of angiopoietin 1 in breast cancer cells. Angiogenesis. 2018;21:849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang H, Xiao J, Zhou Z, Wu J, Ge F, Li Z, Zhang H, Sun J, Li F, Liu R, Chen C. Hypoxia induces miR-153 through the IRE1alpha-XBP1 pathway to fine tune the HIF1alpha/VEGFA axis in breast cancer angiogenesis. Oncogene. 2018;37:1961–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin T, Zhou S, Gao H, Li Y, Sun L. MicroRNA-325 is a potential biomarker and tumor regulator in human bladder cancer. Technol Cancer Res Treat. 2018;17:1533033818790536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao S, Zhao T, Jin H. Expression of MicroRNA-325–3p and its potential functions by targeting HMGB1 in non-small cell lung cancer. Biomed Pharmacother. 2015;70:72–79. [DOI] [PubMed] [Google Scholar]
- 49.Choi SY, Yun J, Lee OJ, Han HS, Yeo MK, Lee MA, Suh KS. MicroRNA expression profiles in placenta with severe preeclampsia using a PNA-based microarray. Placenta. 2013;34:799–804. [DOI] [PubMed] [Google Scholar]
- 50.Khalyfa A, Kheirandish-Gozal L, Bhattacharjee R, Khalyfa AA, Gozal D. Circulating microRNAs as potential biomarkers of endothelial dysfunction in obese children. Chest. 2016;149:786–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Redman CW, Tannetta DS, Dragovic RA, Gardiner C, Southcombe JH, Collett GP, Sargent IL. Review: Does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta. 2012;33:S48–54. [DOI] [PubMed] [Google Scholar]
- 52.Delorme-Axford E, Bayer A, Sadovsky Y, Coyne CB. Autophagy as a mechanism of antiviral defense at the maternal-fetal interface. Autophagy. 2013;9:2173–2174. [DOI] [PubMed] [Google Scholar]
- 53.Ouyang Y, Mouillet JF, Coyne CB, Sadovsky Y. Review: placenta-specific microRNAs in exosomes - good things come in nano-packages. Placenta. 2014;35:S69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salomon C, Guanzon D, Scholz-Romero K, Longo S, Correa P, Illanes SE, Rice GE. Placental exosomes as early biomarker of preeclampsia: potential role of exosomal microRNAs across gestation. J Clin Endocrinol Metab. 2017;102:3182–3194. [DOI] [PubMed] [Google Scholar]
- 55.Sarker S, Scholz-Romero K, Perez A, Illanes SE, Mitchell MD, Rice GE, Salomon C. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J Transl Med. 2014;12:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Escudero CA, Herlitz K, Troncoso F, Acurio J, Aguayo C, Roberts JM, Truong G, Duncombe G, Rice G, Salomon C. Role of extracellular vesicles and microRNAs on dysfunctional angiogenesis during preeclamptic pregnancies. Front Physiol. 2016;7:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Biro O, Alasztics B, Molvarec A, Joo J, Nagy B, Rigo J, Jr. Various levels of circulating exosomal total-miRNA and miR-210 hypoxamiR in different forms of pregnancy hypertension. Pregnancy Hypertens. 2017;10:207–212. [DOI] [PubMed] [Google Scholar]
- 58.Menon R, Debnath C, Lai A, Guanzon D, Bhatnagar S, Kshetrapal PK, Sheller-Miller S, Salomon C. Circulating exosomal mirna profile during term and preterm birth pregnancies: a longitudinal study. Endocrinology. 2019;160:249–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Biro O, Fothi A, Alasztics B, Nagy B, Orban TI, Rigo J, Jr. Circulating exosomal and Argonaute-bound microRNAs in preeclampsia. Gene. 2019;692:138–144. [DOI] [PubMed] [Google Scholar]
- 60.Hromadnikova I, Dvorakova L, Kotlabova K, Krofta L. The prediction of gestational hypertension, preeclampsia and fetal growth restriction via the first trimester screening of plasma exosomal C19MC microRNAs. Int J Mol Sci. 2019;20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Motawi TMK, Sabry D, Maurice NW, Rizk SM. Role of mesenchymal stem cells exosomes derived microRNAs; miR-136, miR-494 and miR-495 in pre-eclampsia diagnosis and evaluation. Arch Biochem Biophys. 2018;659:13–21. [DOI] [PubMed] [Google Scholar]
- 62.Elfeky O, Longo S, Lai A, Rice GE, Salomon C. Influence of maternal BMI on the exosomal profile during gestation and their role on maternal systemic inflammation. Placenta. 2017;50:60–69. [DOI] [PubMed] [Google Scholar]
- 63.Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, Wauben MH, Witwer KW, Thery C. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, Mark MT, Molina H, Martin AB, Bojmar L, et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol. 2018;20:332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.