Abstract
Objective
To evaluate the expression of a set of miRNAs to identify differentially expressed miRNAs that might be considered reliable biomarkers on Diabetic Retinopathy (DR) blood samples.
Results
Expression levels of MiR-320a, MiR-342-3p, MiR-155, MiR-99a, MiR-29a and MiR-27b were analyzed in 60 healthy controls, 48 Diabetes Melitus (DM) without DR patients and 62 DR patients by qRT-PCR. MiR-320a was shown to be downregulated in the plasma of DR patients compared with DM patients without DR and healthy subjects. Target genes were predicted using miRWalk3.0, miR targeting data and target gene interaction data were imported to Cytoscape to visualize and merge networks and top ranked predicted genes were run through Ontology Genes to perform enrichment analysis on gene sets and classification system to identify biological processes and reactome pathways associated with DR. Highly scored target genes of miR-320a were categorized for various biological processes, including negative regulation of cell aging, negative regulation of cellular protein metabolic process and regulation of cellular response to stress that are critical to the development of DR. Our findings suggest that MiR-320a may have a role in the pathogenesis of DR and may represent novel biomarkers for this disease.
Keywords: MicroRNA, Diabetic Retinopathy, Circulating microRNAs, Biomarker
Introduction
Diabetic retinopathy (DR), a leading cause of acquired vision impairment and blindness among working-age adults, is a frequent microvascular complication of diabetes melitus (DM) [1–4]. Traditional risk factors for DR include longer diabetes duration, dyslipidemia, high blood pressure and poor blood glucose control [5], but epidemiological data suggest that differential genetic susceptibility may be related to this chronic complication [6] and epigenetics mechanisms, such as non-coding RNAs, are supposed to mediate the interplay between genetic and environmental factors.
Predicting the clinical course of the disease is often difficult for many DM patients highlighting the necessity of development of sensitive, specific and widely available clinical laboratory-based monitoring testes for this condition and the importance of improving our knowledge of the pathogenesis of DR [7]. A biomarker would allow potential early treatment of DM patients who are at high risk of developing DR, could help to predict the progression of DR to vision threatening DR or the identification of low risk people, but until date, no ideal biomarkers for identifying or predicting DR have been determined.
MicroRNAs are single stranded, short length (21 to 23 nucleotides) non-coding RNA molecules that regulate post-transcriptional gene expression by binding to the complementary sites of targets mRNA and have important functions in gene regulatory networks [8–10]. In the past few years, scientists have found that miRNA can be rapidly released from tissues into the circulation with the development of a pathology and aberrant expression of circulating miRNAs has been detected in a wide range of pathological conditions including cancer [11, 12], diabetes [13, 14], cardiovascular [15–18] and neurodegenerative [19] diseases. They were recently demonstrated to be transported between cells as well as circulate in body fluids [20, 21] and these findings have inspired a great using extracellular circulating miRNAs as non-invasive biomarkers for molecular diagnostics, disease stratification and prognostics.
Recent studies have detected miRNAs in the blood or vitreous humor of DR patients, suggesting that miRNA may be involved in DR and that some miRNAs may be biomarkers for DR [22–26], but many of them focused on miRNAs expressed in cells or animal models, so the global miRNA pattern in the sera of plasma samples of DR patients has not been determined.
Herein, we investigated expression levels of miR-320a, miR-342-3p, miR-155, miR-99a, miR-29a and miR-27b in healthy people, DM without DR patients and DR patients by qRT-PCR aiming to find specific miRNAs that could serve as reliable and reproducible biomarkers for DR. This candidate microRNAs were selected from a review of previously published studies and were also chosen based on using prior related experiments. We also integrated differentially expressed miRNAs to their target genes and categorized target genes for biological processes involved in the pathogenesis of DR.
Main text
Study subjects
A total of 170 patients, divided in three groups (60 healthy controls, 48 DM without DR patients and 62 DR patients) participated in this study and Additional file 1: Table S1 shows some clinicopathological characteristics of the recruited subjects. Universidade Estadual de Santa Cruz, Ilhéus, Bahia, Brazil Ethics Commitee approved the written consent that was taken from all the participants. The subjects were identified and classified by certified ophthalmologist that conducted fundus fluorescein angiography at CENOE (Clinica Especializada de Olhos, Ilhéus, Bahia, Brazil).
According to the guidelines from Global Diabetic Retinopathy Project Group [27], DR was diagnosed after routine fundus examination and fundus fluorescence angiogryphy examination. Patients with DM suffering from any form of hemangioma, small bleeding points, formation of new blood vessels, vitreous hemorrhage or secondary retinal detachment in the retina were classified as DR patients. Patients with diabetic ketosis, atherosclerotic disease and cardiac arrhythmias, trauma surgery, acute or chronic infection, hepatic disease and other endocrine metabolic diseases were excluded.
Blood samples, RNA isolation and cDNA synthesis
Venous blood samples (5 mL) were collected from each donor in BD vacutainers dipotassium EDTA anticoagulant. Plasma fraction was separated by centrifugation. Plasma sample of 300 µL was mixed with 900 µL Trizol LS (Invitrogen) and RNA isolation was performed according to the manufacturer’s instructions. A NanoDrop 1000 (Thermo Scientific) was used to measure RNA concentration. Only RNA samples with a 260/280 ratio of ≥ 1.8 were included. 500 ng of total RNA was reverse transcribed using miR-specific primers and Taqman miRNA Reverse Transcription Kit (Applied Biosystem) in a scaled down volume of 15 µL RT reaction, according to the manufacturer’s instructions [28].
Quantitative real-time PCR
Taqman MicroRNA assays (Applied Biosystems) and a QuantStudio3 Instrument (ThermoFisher Scientific) were used to measure expression levels of individual miRNAs by RT-qPCR. RT-qPCR amplification mixtures contained 20 ƞg template cDNA, 10 µL Taqman master mix (Applied Biosystems) and probes for MiR-320a (Assay ID: 002277), MiR-342-3p (Assay ID: 002260), MiR-155 (Assay ID: 002287), MiR-99a (Assay ID: 000435), MiR-29a (Assay ID: 002112) and MiR-27b (Assay ID: 000409) in a final volume of 20 µL. The PCR conditions were: incubation for 10 min at 95 °C, followed by 40 cycles of 10 s at 95 °C and 1 min at 60 °C. The Ct values for RT-qPCR were determined using the QuantStudio™ Design & Analysis Software (Applied Biosystems) and the single-threshold method. PCR reactions were performed in a duplicate and experiments with coefficients of variation greater than 5% or that displayed unusual amplification curves were excluded from further analysis. A no-template control (NTC) and no reverse transcription controls (No-RT) were also included. The mean cycle threshold (Ct) values from duplicate measurements were used to calculate expression of target gene, with normalization to an internal control miR-328-3p (Assay ID: 000543), which might be considered steady internal reference gene in expression studies on DR plasma samples [28], using 2 − ΔCt formula [29–31] and present as fold change.
Computational prediction of potential miRNA targets
Target genes were predicted using miRWalk3.0 (http://mirwalk.umm.uni-heidelberg.de/). The miRWalk platform is based on predicted mRNA targets and integrates the predicted targets from various prediction tools: miRDB, TargetScan and miRTarbase. We setted filter for this tool with minimum score of 0.85. The miR targeting data and target gene interaction data were imported to Cytoscape, which was used to visualize and merge networks. Top ranked predicted genes were run through Ontology Genes (http://geneontology.org/) to perform enrichment analysis on gene sets and classification system to identify biological processes and reactome pathways associated with DR.
Statistical analysis
Parametric data of all three groups were analyzed using one-way ANOVA with Tukey’s post hoc. All data were analyzed using the Prism 5.01 computer software (GraphPad, San Diego, CA, USA). Statistical differences were considered to be significant at p < 0.05.
Results
Demographic and clinical profile of study subjects
The clinical characteristics of the patients are shown in Additional file 1: Table S1. Briefly, there were no significant differences in age and body mass index (BMI) between the three groups patients. Patients with DR were more often male and had a longer duration of diabetes compared to patients without DR. Moreover, daily insulin use was more frequent among patients with DR than in those without this complication.
Comparison of miRNA levels between study groups
Expression levels of MiR-320a, MiR-342-3p, MiR-155, MiR-99a, MiR-29a and MiR-27b were analyzed and, as shown in Fig. 1a, qRT-PCR analysis showed that circulating plasma level of miR-320a was profoundly downregulated in patients with DR compared to healthy subjects and DM patients (< 0.0001). Patients with DR had approximately five-fold lower levels of miR-320a in comparison to healthy subjects and DM patients without DR, which are not significantly different between them. No significant differences were observed for MiR-342-3p, MiR-155, MiR-99a, MiR-29a and MiR-27b expression (Fig. 1b–f).
Fig. 1.
Circulating levels of miR-320a (a), miR-342-3p (b), MiR-29a (c), MiR-99a (d), MiR-27b (e) and MiR-155 (f) in healthy control subjects, diabetic patients without DR and diabetic patients with DR, evaluated by Taqman real-time PCR (arbitrary units). Data are represented graphically as the mean ± SEM of 48 to 62 subjects/group. *< 0.0001
Target gene prediction of miR-320a
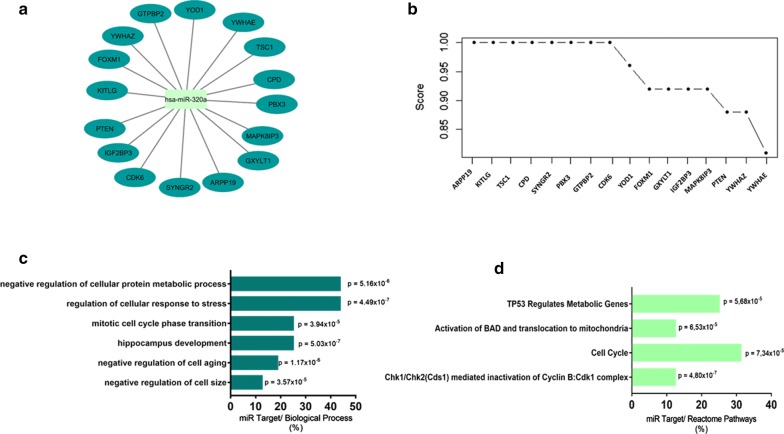
As the miR-320a presented low expression in DR patients, with significant difference compared to the control group, we performed the in silico prediction to identify target genes possibly modulated by this miRNA in patients with DR. Based on analysis using miRWalk (integrating the miRDB, TargetScan and MiRTarbase), we observe that 16 genes are modulated (Fig. 2a) and were organized according to the significance presented by score in miRWAlk (Fig. 2b). From the analysis of functional enrichment, 6 significant biological processes (Fig. 2c) and 4 Reactome pathways (Fig. 2d) were found, which may indicate this miRNA involvement in important genes modulation in DR. The genes related to biological processes and reactome pathways are listed in Table 1.
Fig. 2.
Target gene prediction with biology process and reactome pathways of miR-320a. a Interaction networks of miR-320 and target genes, based on analysis using miRWalk and b correlation between level significance by score. For this genes c Biological processes and d Reactome pathways with p-values were determined
Table 1.
Biologic Process and reactome pathways for MiR-320a target prediction
| Biology process | Acession | Genes |
|---|---|---|
| Negative regulation of cell size | GO:0045792 | PTEN, TSC1 |
| Negative regulation of cell aging | GO:0090344 | PTEN, CDK6, FOXM1 |
| Hippocampus development | GO:0021766 | YWHAE, PTEN, TSC1, CDK6 |
| Mitotic cell cycle phase transition | GO:0044772 | YWHAE, CDK6, FOXM1, ARPP19 |
| Regulation of cellular response to stress | GO:0080135 | YWHAE, PTEN, CDK6, FOXM1, YOD1, MAPK8IP3, TSC1 |
| Negative regulation of cellular protein metabolic process | GO:0032269 | YWHAE, PTEN, ARPP19, YOD1, FOXM1, IGF2BP3, TSC1 |
| Reactome pathways | ||
| Chk1/Chk2(Cds1) mediated inactivation of Cyclin B:Cdk1 complex | R-HSA-75035.4 | YWHAE, YWHAZ |
| Cell cycle | R-HSA-1640170.3 | YWHAE, CD6, ARPP19, FOXM1, YWHAZ |
| Activation of BAD and translocation to mitochondria | R-HSA-111447.2 | YWHAE, YWHAZ |
| Tp53 regulates metabolic genes | R-HSA-5628897.4 | YWHAE, YWHAZ, PTEN, TSC1 |
Discussion
Non-invasive and reliable biomarkers are needed to predict the risk of developing DM and its complications. Several researches have been focused on searching molecules involved in the pathogenic mechanisms at the basis of the development of DR [32, 33]. Circulating miRNAs have been largely addressed and investigated as non-invasive potential biomarkers in several diseases, including metabolic disorders [34]. Our experiment initially discovered that the levels of miR-320a are significantly down-regulated in the DR group comparing with those in the DM without DR and healthy control group.
Some studies have focused on finding an association between altered expression of MiR-320a and DM and DR. MiR-320 was found to regulate IGF-1 and IGF1R69 expression, playing a key role in developing insulin resistance in adipose tissues and endothelial cells [35]. Ling et al. also found that miR-320 augments insulin sensitivity in adipocyte in the insulin resistant condition, targeting PI3-Kp85 during the development of insulin resistance in adipocytes [36]. However, a few studies have focused on analyzing circulating miR-320a expression and the role of this miRNA and its targets in DR remains still unknown.
In the current study, several MiR-320a target genes were identified and top-ranking genes were ARPP19, KITLG, TSC1, CPD, SYGNR2, PBX3, GTPBP2 and CDK6. Among these genes the TSC1 and CDK6 are reported in the literature as important in DM. The TSC1 negatively regulates mammalian target of rapamycin complex 1 (mTORC). TSC–mTOR pathway may result in the development of metabolic diseases and DM complications [37]. Curiously, the CDK6 gene was reported as an inductor of pancreatic β-cell replication and human islets proliferation by Fiaschi-taesch et al. [38] The CDK6 is still suppressed indirectly by upregulation miRNAs in DM and complications of the disease [39, 40].
However, in this study a negative reduction in MiR-320a was identified in patients with DR compared to the control group and the group with DM without DR, what could mean an increase in the CDK6 gene expression [41]. The low MiR-320a expression would lead to a high expression of CDK6, due to a dysregulation in the cell cycle mechanism, since this pathology would cause vascular and cellular changes [2].
Using Gene Ontology (GO) classification system, genes were categorized into several biological processes that are critical in the course of DR, including regulation of cellular response to stress [42, 43], negative regulation of cellular protein metabolism process [44], mitotic cell cycle phase transition [45, 46]. In conclusion, we found a five-fold downregulation of miR-320a in the plasma of patients with DR. Integrated genes were identified for this miRNA and we divided top-ranked genes into biological process that are critical for DR based on total target scores. Despite our results, our data has several limitations. Our experimental context does not allow to infer about the mechanism by which DM duration has a different effect on the circulating miRNAs expression profile in different groups, because we could investigate the expression of only some specific microRNAs. Besides this, we had a small sample size that could make more difficult for us to identify significant relationships from our data. Additionally, the analysis of a bigger number of miRNAs expression could be much more informative about new candidates for DR biomarker use, but we were able to evaluate only six candidate microRNAs that were selected from previously published data and based on prior related experiments. Our findings suggest that miR-320a may have a role in the pathogenesis of DR, but other future studies are needed to investigate if this circulating microRNA has clinical importance or if it would permit an accurate identification of risk factors or prevention of events.
Limitation of the study
The study was conducted only in a single hospital.
Despite computer-based prediction methods are valuable in preliminary identification of miRNA target genes, inherent limitations should be considered when applying the results of these searches to experimental validation.
Supplementary information
Additional file 1: Table S1. Clinical characteristics of the patients.
Acknowledgements
This work was supported by FAPESB (Fundação de Amparo à Pesquisa do Estado da Bahia), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), and UESC (Universidade Estadual de Santa Cruz). We are indebted to all the patients who participated in this research and made it possible and to the CENOE staff who assisted with recruitment and access to medical records.
Abreviations
- MiRs
MicroRNAs
- DM
Diabetes melitus
- DR
Diabetic retinopathy
- EDTA
Ethylenediaminetetraacetic acid
- RNA
Ribonucleic acid
- DNA
Desoxyribonucleic acid
- cDNA
Complementary DNA
- PCR
Polymerase Chain Reaction
- qRT-PCR
Quantitative reverse transcribed PCR
- NTC
no-template control
- No-RT
No reverse transcription
- ANOVA
Analysis of variance
Authors’ contributions
MSGP and MLJ contributed to the collection and selection of samples and clinical data and contributed to the data interpretation. TCG performed and analyzed the experiments. LOSM participated in study design and contributed to the data interpretation. CMK supervised the study and wrote the manuscript. All authors contributed to scientific discussion and approved the final manuscript. All authors read and approved the final manuscript.
Funding
No funding was received for this research study.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Ethical approval was obtained from the Ethical Committee of the Universidade Estadual de Santa Cruz, Ilhéus, Bahia, Brazil on 10 January 2017 with ethical number 1.887.879 and research was performed in accordance with the Declaration of Helsinki. All of the study participants were informed about the purpose of the study, about their right to participate or to with draw at any time if they don’t want and their confidentiality. Written consent was taken from participants to participate in this study and approved by the EC. This research is original and not considered in another journal for publication.
Consent to publish
Not applicable.
Competing interests
Authors declared that they have no conflict of interest and approved for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Marcelle SanJuan Ganem Prado, Email: cellesanjuan@yahoo.com.br.
Mirthz Lemos de Jesus, Email: mirthz@hotmail.com.
Thaline Cunha de Goes, Email: thalinecgoes@gmail.com.
Lucilla Silva Oliveira Mendonça, Email: lucilla.s.oliveira@gmail.com.
Carla Martins Kaneto, Email: cmkaneto@uesc.br.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13104-020-05001-9.
References
- 1.Kobrin Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14:179–183. doi: 10.1080/09286580701396720. [DOI] [PubMed] [Google Scholar]
- 2.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 3.Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015;2:17. doi: 10.1186/s40662-015-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solomon SD, Chew E, Duh EJ, Sobrin L, Sun JK, VanderBeek BL, et al. Diabetic retinopathy: a position statement by the American Diabetes Association. Diab Care. 2017;40:412–418. doi: 10.2337/dc16-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ting DSW, Tan KA, Phua V, Tan GSW, Wong CW, Wong TY. Biomarkers of diabetic retinopathy. Curr Diab Rep. 2016 doi: 10.1007/s11892-016-0812-9. [DOI] [PubMed] [Google Scholar]
- 6.Priščáková P, Minárik G, Repiská V. Candidate gene studies of diabetic retinopathy in human. Mol Biol Rep. 2016;43:1327–1345. doi: 10.1007/s11033-016-4075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joglekar MV, Januszewski AS, Jenkins AJ, Hardikar AA. Circulating microRNA biomarkers of diabetic retinopathy. Diabetes. 2016;65:22–24. doi: 10.2337/dbi15-0028. [DOI] [PubMed] [Google Scholar]
- 8.Bhaskaran M, Mohan M. MicroRNAs. Vet Pathol. 2014;51:759–774. doi: 10.1177/0300985813502820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 10.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 11.McDermott AM, Kerin MJ, Miller N. Identification and validation of miRNAs as endogenous controls for RQ-PCR in blood specimens for breast cancer studies. PLoS ONE. 2013;8:1–11. doi: 10.1371/journal.pone.0083718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niu Y, Wu Y, Huang J, Li Q, Kang K, Qu J, et al. Identification of reference genes for circulating microRNA analysis in colorectal cancer. Sci Rep. 2016;6:1–9. doi: 10.1038/srep35611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chien H-Y, Lee T-P, Chen C-Y, Chiu Y-H, Lin Y-C, Lee L-S, et al. Circulating microRNA as a diagnostic marker in populations with type 2 diabetes mellitus and diabetic complications. J Chinese Med Assoc. 2014;78:204–211. doi: 10.1016/j.jcma.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Satake E, Pezzolesi MG, Md Dom ZI, Smiles AM, Niewczas MA, Krolewski AS. Circulating miRNA profiles associated with hyperglycemia in patients with Type 1 diabetes. Diabetes. 2018;67:1013–1023. doi: 10.2337/db17-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaneto CM, Nascimento JS, Moreira MCR, Ludovico ND, Santana AP, Silva RAA, et al. MicroRNA profiling identifies miR-7-5p and miR-26b-5p as differentially expressed in hypertensive patients with left ventricular hypertrophy. Braz J Med Biol Res. 2017;50:1–9. doi: 10.1590/1414-431x20176211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaneto CM, Nascimento JS, Prado MSJG, Mendonça LSO. Circulating miRNAs as biomarkers in cardiovascular diseases. Eur Rev Med Pharmacol Sci. 2019;23:2234–2243. doi: 10.26355/eurrev_201903_17271. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Chen S, Zhang J, Shan S, Chen L, Wang R, et al. Analysis of serum microRNAs as potential biomarker in coronary bifurcation lesion. Dis Markers. 2015 doi: 10.1155/2015/351015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao W, Zhao S-P, Zhao Y-H. MicroRNA-143/-145 in cardiovascular diseases. Biomed Res Int. 2015;2015:1–9. doi: 10.1155/2015/531740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serafin A, Foco L, Blankenburg H, Picard A, Zanigni S, Zanon A, et al. Identification of a set of endogenous reference genes for miRNA expression studies in Parkinson’s disease blood samples. BMC Res Notes. 2014;7:715. doi: 10.1186/1756-0500-7-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guay C, Regazzi R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat Rev Endocrinol. 2013;9:513–521. doi: 10.1038/nrendo.2013.86. [DOI] [PubMed] [Google Scholar]
- 21.Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, et al. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56:1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qing S, Yuan S, Yun C, Hui H, Mao P, Wen F, et al. Serum MiRNA biomarkers serve as a fingerprint for proliferative diabetic retinopathy. Cell Physiol Biochem. 2014;34:1733–1740. doi: 10.1159/000366374. [DOI] [PubMed] [Google Scholar]
- 23.Hirota K, Keino H, Inoue M, Ishida H, Hirakata A. Comparisons of microRNA expression profiles in vitreous humor between eyes with macular hole and eyes with proliferative diabetic retinopathy. Graefe’s Arch Clin Exp Ophthalmol. 2015;253:335–342. doi: 10.1007/s00417-014-2692-5. [DOI] [PubMed] [Google Scholar]
- 24.Gomaa AR, Elsayed ET, Moftah RF. MicroRNA-200b expression in the vitreous humor of patients with proliferative diabetic retinopathy. Ophthalmic Res. 2017;58:168–175. doi: 10.1159/000475671. [DOI] [PubMed] [Google Scholar]
- 25.Ma J, Wang J, Liu Y, Wang C, Duan D, Lu N, et al. Comparisons of serum miRNA expression profiles in patients with diabetic retinopathy and type 2 diabetes mellitus. Clinics. 2017;72:111–115. doi: 10.6061/clinics/2017(02)08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazzeo A, Beltramo E, Lopatina T, Gai C, Trento M, Porta M. Molecular and functional characterization of circulating extracellular vesicles from diabetic patients with and without retinopathy and healthy subjects. Exp Eye Res. 2018;176:69–77. doi: 10.1016/j.exer.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson CP, Ferris FL, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 28.Prado MSJG, de Goes TC, de Jesus ML, Mendonça LSO, Nascimento JS, Kaneto CM. Identification of miR-328-3p as an endogenous reference gene for the normalization of miRNA expression data from patients with diabetic retinopathy. Sci Rep. 2019;9:1–8. doi: 10.1038/s41598-018-37186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Oliveira SA, de Freitas Souza BS, Barreto EPS, Kaneto CM, Neto HA, Azevedo CM, et al. Reduction of galectin-3 expression and liver fibrosis after cell therapy in a mouse model of cirrhosis. Cytotherapy. 2012;14:339–349. doi: 10.3109/14653249.2011.637668. [DOI] [PubMed] [Google Scholar]
- 30.Leal MMT, Costa-Ferro ZSM, Souza BSDF, Azevedo CM, Carvalho TM, Kaneto CM, et al. Early transplantation of bone marrow mononuclear cells promotes neuroprotection and modulation of inflammation after status epilepticus in mice by paracrine mechanisms. Neurochem Res. 2014;39:259–268. doi: 10.1007/s11064-013-1217-7. [DOI] [PubMed] [Google Scholar]
- 31.Souza BSF, Azevedo CM, Lima RS, Kaneto CM, Vasconcelos JF, Guimarães ET, et al. Bone marrow cells migrate to the heart and skeletal muscle and participate in tissue repair after Trypanosoma cruzi infection in mice. Int J Exp Pathol. 2014;95:321–329. doi: 10.1111/iep.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaviarasan K, Jithu M, Arif Mulla M, Sharma T, Sivasankar S, Das UN, et al. Low blood and vitreal BDNF, LXA4 and altered Th1/Th2 cytokine balance are potential risk factors for diabetic retinopathy. Metabolism. 2015;64:958–966. doi: 10.1016/j.metabol.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Simó-Servat O, Simó R, Hernández C. Circulating biomarkers of diabetic retinopathy: an overview based on physiopathology. J Diab Res. 2016;2016:1–13. doi: 10.1155/2016/5263798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pescador N, Pérez-Barba M, Ibarra JM, Corbatón A, Martínez-Larrad MT, Serrano-Ríos M. Serum circulating microRNA profiling for identification of potential Type 2 diabetes and obesity biomarkers. PLoS ONE. 2013;8:21–23. doi: 10.1371/journal.pone.0077251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chakraborty C, Doss CGP, Bandyopadhyay S, Agoramoorthy G. Influence of miRNA in insulin signaling pathway and insulin resistance: micro-molecules with a major role in type-2 diabetes. Wiley Interdiscip Rev RNA. 2014;5:697–712. doi: 10.1002/wrna.1240. [DOI] [PubMed] [Google Scholar]
- 36.Ling HY, Ou HS, Feng SD, Zhang XY, Tuo QH, Chen LX, et al. Changes in microrna (mir) profile and effects of mir-320 in insulin-resistant 3t3-l1 adipocytes. Clin Exp Pharmacol Physiol. 2009;36:32–39. doi: 10.1111/j.1440-1681.2009.05207.x. [DOI] [PubMed] [Google Scholar]
- 37.Inoki K. Role of TSC—mTOR pathway in diabetic nephropathy. Diab Res Clin Prat. 2008;82:59–62. doi: 10.1016/j.diabres.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 38.Fiaschi-taesch NM, Salim F, Kleinberger J, Troxell R, Cozar-castellano I, Selk K, et al. Induction of human β-cell proliferation and engraftment using a single G1/S regulatory molecule, cdk6 nathalie. Diabetes. 2010;59:1926–1936. doi: 10.2337/db09-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kölling M, Kaucsar T, Schauerte C, Hübner A, Dettling A, Park J, et al. Therapeutic miR-21 silencing ameliorates diabetic kidney disease in mice. Mol Ther. 2017;25:165–180. doi: 10.1016/j.ymthe.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hou Q, Zhou L, Tang J, Ma N, Xu A, Tang J, et al. LGR4 is a direct target of MicroRNA-34a and modulates the proliferation and migration of retinal pigment epithelial ARPE-19 cells. PLoS ONE. 2016;15:1–12. doi: 10.1371/journal.pone.0168320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tadano T, Kakuta Y, Hamada S, Shimodaira Y, Kuroha M, Kawakami Y, et al. MicroRNA-320 family is downregulated in colorectal adenoma and affects tumor proliferation by targeting CDK6. World J Gastrointest Oncol. 2016;8:532–542. doi: 10.4251/wjgo.v8.i7.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2011;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kowluru RA, Kanwar M. Oxidative stress and the development of diabetic retinopathy: contributory role of matrix metalloproteinase-2. Free Radic Biol Med. 2010;46:1677–1685. doi: 10.1016/j.freeradbiomed.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kowluru RA, Santos JM, Zhong Q. Sirt1, a negative regulator of matrix metalloproteinase-9 in diabetic retinopathy. Retin Cell Biol. 2014;55:5652–5660. doi: 10.1167/iovs.14-14383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cui C, Li Y, Liu Y. Down-regulation of miR-377 suppresses high glucose and hypoxia- induced angiogenesis and inflammation in human retinal endothelial cells by direct up-regulation of target gene SIRT1. Hum Cell. 2019;32:260–274. doi: 10.1007/s13577-019-00240-w. [DOI] [PubMed] [Google Scholar]
- 46.Kimura I, Honda R, Okai H, Okabe M. Vascular endothelial growth factor promotes cell-cycle transition from G0 to G1 phase in subcultured endothelial cells of diabetic rat thoracic aorta. Jpn J Pharmacol. 2000;83:47–55. doi: 10.1254/jjp.83.47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Clinical characteristics of the patients.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.