Abstract
Background
Osteoarthritis (OA) is characterized by the formation and deposition of calcium-containing crystals in joint tissues, but the underlying mechanisms are poorly understood. The gasotransmitter hydrogen sulfide (H2S) has been implicated in mineralization but has never been studied in OA. Here, we investigated the role of the H2S-producing enzyme 3-mercaptopyruvate sulfurtransferase (3-MST) in cartilage calcification and OA development.
Methods
3-MST expression was analyzed in cartilage from patients with different OA degrees, and in cartilage stimulated with hydroxyapatite (HA) crystals. The modulation of 3-MST expression in vivo was studied in the meniscectomy (MNX) model of murine OA, by comparing sham-operated to MNX knee cartilage. The role of 3-MST was investigated by quantifying joint calcification and cartilage degradation in WT and 3-MST−/− meniscectomized knees. Chondrocyte mineralization in vitro was measured in WT and 3-MST−/− cells. Finally, the effect of oxidative stress on 3-MST expression and chondrocyte mineralization was investigated.
Results
3-MST expression in human cartilage negatively correlated with calcification and OA severity, and diminished upon HA stimulation. In accordance, cartilage from menisectomized OA knees revealed decreased 3-MST if compared to sham-operated healthy knees. Moreover, 3-MST−/− mice showed exacerbated joint calcification and OA severity if compared to WT mice. In vitro, genetic or pharmacologic inhibition of 3-MST in chondrocytes resulted in enhanced mineralization and IL-6 secretion. Finally, oxidative stress decreased 3-MST expression and increased chondrocyte mineralization, maybe via induction of pro-mineralizing genes.
Conclusion
3-MST-generated H2S protects against joint calcification and experimental OA. Enhancing H2S production in chondrocytes may represent a potential disease modifier to treat OA.
Keywords: Calcium-containing crystals, Osteoarthritis, Animal model, Hydrogen sulfide, Chondrocyte calcification
Background
Osteoarthritis (OA) is the most common joint disease affecting millions of people [1]. It is characterized by cartilage degradation, subchondral bone sclerosis and synovitis [2]. In addition, calcium-containing crystals within joint structures are another prominent features of OA, and they participate in its initiation and progression. These crystals were found in 50% up to 100% of synovial fluid [3] and cartilage [4] from OA patients undergoing joint replacement. Two families of calcium-containing crystals were identified in OA: basic calcium phosphate (BCP) (e.g., hydroxyapatite (HA)), and calcium pyrophosphate dihydrate (CPPD) [5]. In vitro, BCP crystals induced catabolic and inflammatory responses [6, 7]. When injected into mice knees, they caused mild synovitis but severe cartilage damage, resembling human OA features [8]. In our recent study, we observed BCP calcific deposits in joints following meniscectomy. Moreover, we found reciprocal crosstalk between BCP and IL-6 production [9], and a positive correlation between these two entities and the severity of cartilage degradation. Thus, we hypothesize that inhibiting crystal formation and deposition in the joint could be of therapeutic value for OA treatment.
Hydrogen sulfide (H2S) is an endogenous gasotransmitter in our body, together with nitric oxide (NO) and carbon monoxide (CO) [10]. In mammalian tissues, H2S is generated by three different enzymes: cystathionine beta-synthase (CBS), cystathionine gamma-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST). These enzymes use cysteine as a substrate to produce H2S [11, 12], and their expression is tissue-specific. H2S showed biological effects [13] that can be of relevance in OA, such as reduced pro-inflammatory responses [14], reduced mineralization [15–18], improved anabolic/catabolic balance [19, 20], and decreased oxidative stress (reactive oxygen species (ROS) production) [21, 22]. H2S signaling occurs in part through post-translational modification (namely, S-sulfhydration) of specific cysteine residues in target proteins with the potential to alter their function [23]. In addition, H2S oxidation leads to sulfite (SO32−), thiosulfate (S2O32−) [24], and sulfate (SO42−) generation in the mitochondria [12], which could themselves mediate H2S effects.
Current therapeutic approaches for OA are either symptomatic or surgical. Therefore, there is a medical need for interventions that target the pathological processes of OA. We hypothesized that H2S can prevent calcium-containing crystals deposition in the joint, and subsequently OA progression. In particular, we demonstrated that activation of the 3-MST/H2S axis improved outcomes in both human and experimental OA.
Methods
Mice and experimental osteoarthritis
3-MST KO (n = 8) [25] and WT female mice (n = 8), 8-weeks old, on a C57BL/6 background, were subjected to medial meniscectomy (MNX) of the right knee, while the contralateral knee was sham-operated as control [26]. Two months after, mice were sacrificed, blood collected, and serum obtained by 15 min centrifugation at 15000×g, and knees fixed in 10% formalin.
MicroCT-scan
MicroCT-scans analysis was performed using a SkyScan 1076® X-ray μCT scanning system (SkyScan, Belgium) and the following parameters: 18 μm resolution, 60 kV, 167 μA, 0.4° rotation step over 360°, 0.5 mm Aluminum filter, 1180 ms exposure time. Ex vivo samples acquisition was made using formol fixed knees. Images were reconstructed using NRecon Version 1.6.6.0 (Skyscan, Belgium) considering the following parameters: gray-values = 0.0000–0.105867, ring artifact reduction = 3, beam hardening correction = 40% [27]. Newly formed calcific deposits at the site of the removed medial meniscus were considered as Volumes-Of-Interest (VOI) for the quantitative analysis of new formation volume (mm3) and new formation crystal content (μg) by CTAnalyzer V.1.10.
Mouse knee histology
Knees were decalcified in EDTA for 20 days and embedded in paraffin. Sagittal sections (5 μm thick, 3 sections/mouse, spaced 70 μM apart) of the medial compartment were stained with Safranin-O and counterstained with fast green/iron hematoxylin. Blinded OARSI score (0–24 score) [28] for cartilage damage and Safranin-O loss was assessed by two independent observers.
Thiosulfate measurement
Serum was delipidized with dichloromethane and centrifuged. The supernatant was derivatized with monobromobimane, acetonitrile, and HEPES/EDTA buffer (pH 8) for 30 min in the dark. Methanosulfonic acid was added to stop the reaction and proteins removed by centrifugation. Thiosulfate was determined by HPLC [29, 30]: Waters-2695 module, fluorescence detector (excitation wavelength of 380 nm, the emission wavelength of 480 nm) and a reverse-phase column. The eluants were PIPES (10 mM, pH 6.6) and methanol (gradient). Concentrations were calculated by integrating the area under the curve.
Human cartilage explants
Human cartilage (tibia and femur) from 15 OA patients undergoing knee replacement (Kellgren-Lawrence K/L score 1 to 4, age 64.69 years ± 10.58) was obtained from the Otto-von-Guericke University (Magdeburg-D). Patients were grouped into low (K/L 1–2 and OARSI 2–3, age 70.25 ± 5.37 years), medium (K/L 3 and OARSI 3–4, age 65.25 ± 14.88 years), and high (K/L 4 and OARSI 5, age 59.8 ± 9.33 years) OA grade. Full-thickness cartilage explants were fixed in 4%PFA and embedded in paraffin. Five-micrometer-thick sagittal sections were cut for further immunohistochemical and calcification analysis.
For HA crystal stimulation experiment, cartilage (tibia and femur) from 4 OA patients (mean age 72 ± 10 years) undergoing knee replacement (K/L score = 4) was obtained from the Orthopedic Department (CHUV, Lausanne-CH). Six-millimeter-diameter disks (3 disks/patient) were dissected from macroscopically intact cartilage using a dermal punch. In order to match for location across treatment groups, each disk was divided into two equal parts, and each half was stimulated or not with 500 μg/ml HA crystals for 24 h in DMEM+ 1%P/S + 50 μg/ml L-ascorbic acid 2-phosphate. Cartilage was fixed in 4%PFA for immunohistochemical analysis.
Human cartilage histology and quantification of calcification
For each patient, three sections of full-thickness cartilage were stained with Von Kossa/Safranin-Orange staining (Sigma). Pictures were taken using a Zeiss Axiovert microscope and Zen software at × 2.5 magnification, in order to have the whole cartilage section depicted on the picture. Images were then converted into a grayscale. The total cartilage area (100%) was marked in the image using ImageJ (NIH Image). The percentage (%) of calcified cartilage over the total cartilage area was identified using a threshold for black and white. The mean value of the three sections/patient was calculated. Four to 5 patients were analyzed for each K/L-OARSI group.
Immunohistochemical analysis
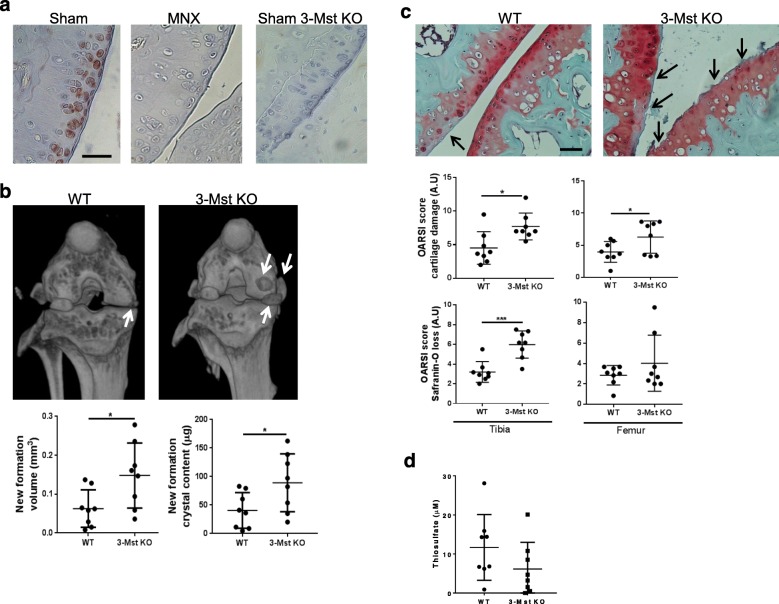
3-MST expression was evaluated using an anti-3-MST rabbit polyclonal antibody (Novusbio NBP1-82617) on paraffin sections. The antibody was demonstrated to be specific, as a negative staining was obtained both when 3-MST IHC was performed without the primary antibody (data not shown) and when 3-MST IHC was performed on knee sections from 3-MST KO mice (Fig. 2a, Sham 3-MST KO).
Fig. 2.
3-MST deficiency exacerbates joint calcification and cartilage damage in experimental OA. a Representative immunohistochemical analysis of 3-MST expression in the knee section from sham-operated and MNX WT mice. A knee section from a sham-operated 3-Mst KO mouse was used to prove the specificity of the 3-MST antibody. Scale bars 150 μm. b Representative micro-CT scan images of WT and 3-MST KO MNX murine knee joints two months after surgery. White arrows show calcified periarticular deposits in MNX WT knees and their exacerbation in 3-MST KO mice. Graphs show CTAnalyzer quantitative analysis of new formation volume (mm3) and new formation crystal content (μg) in WT and 3-MST KO MNX knees. c Representative histologies of WT and 3-MST KO MNX knees, stained with Safranin-O. Black arrows show increased cartilage degradation in 3-MST KO mice. Scale bars 150 μm. Graphs show femoral scoring of cartilage damage and Safranin-O loss, accordingly to OARSI method. d Thiosulfate measurement in the serum of WT and 3-MST KO mice. Mice number WT n = 8, 3-MST KO n = 8
Analysis of 3-MST expression in sham-operated versus meniscectomized murine knees was made by evaluation of positivity in histological sections. In humans, for each patient, three sections of full-thickness cartilage were stained with the 3-MST Ab. Pictures of three fields per section were taken using a Zeiss Axiovert microscope and Zen software at × 10 magnification. The total number of cells and the number of 3-MST-positive cells were counted in each field, and the percentage (%) of 3-MST-positive cells was calculated. The mean value of the three fields was calculated for each section and the mean value of the three sections/patient was calculated. Four to 5 patients were analyzed for each K/L-OARSI group.
Hydroxyapatite crystals and secondary calciprotein particles
Hydroxyapatite crystals (HA) crystals were synthesized, characterized [31], and sonicated for 5 min in sterile PBS prior to experiment. Secondary calciprotein particles (CPP) were synthesized as previously described [15]. Briefly, 10% FBS, 3.5 mM phosphate (2.14 mM Na2HPO4, 1.36 mM NaH2PO4, Sigma), 1 mM calcium (CaCl2, Sigma), 1%P/S, and 1%L-Glutamine where added to DMEM. This medium was stored at 37 °C for 7 days to generate secondary CPP and then centrifuged at 25000×g for 2 h at 4 °C. Calcium content was measured in the resuspended pellet by the QuantiChrom™ Calcium Kit.
Murine articular chondrocytes isolation
Primary knee immature chondrocytes were isolated from 5 to 7 days old mice [9] and amplified for 7 days in DMEM+ 1%P/S + 10%FBS to reach chondrocytic differentiation [32]. For calcification studies, chondrocytes were cultivated for 24 h in DMEM+ 1%P/S + 10%FBS, supplemented with secondary calciprotein particles (CPP-50 μg/ml calcium) to induce calcification and were concomitantly treated with 0.4% DMSO, 500 μM H2O2 (Sigma-Aldrich, dissolved in culture medium), 1 mM N-acetylcysteine NAC (Sigma-Aldrich, dissolved in DMSO), or a combination of those. For qRT-PCR studies, separate plates were used and chondrocytes were cultivated for 4 h in DMEM+ 1%P/S only, supplemented with 0.4% DMSO or 50 μM of the 3-MST inhibitor (compound 3 [33], dissolved in DMSO, kindly provided by Prof. Kenjiro Hanaoka, University of Tokyo). For alkaline phosphatase activity, separate plates were used and chondrocytes were cultivated for 6 h in DMEM+ 1%P/S only, supplemented with 0.4% DMSO, 50 μM of the 3-MST inhibitor, 500 μM H2O2, or a combination of those.
Crystal detection in articular chondrocyte cultures
For Alizarin Red staining, cells were fixed in 10% formol for 30 min and calcium-containing crystals stained by applying 2% Alizarin red solution (pH 5.3) for 1 h [34]. After washings with tap water, pictures were taken. For calcium content quantification, separate plates were used. Cell monolayers were decalcified with 0.6 M HCl for 24 h. The following day, calcium content was quantified by the QuantiChrom™ Calcium Kit (BioAssay Systems) by reding absorbance at 612 nm using the Spectramax M5e reader (Molecular Devices).
IL-6 quantification in articular chondrocyte cultures
Cell supernatants from the cells used for the measurement of calcium content were assayed using murine IL-6 ELISA kit (eBioscience) and by reading absorbance at 450 nm and 570 nm using the Spectramax M5e reader.
Alkaline phosphatase (Alp) activity in articular chondrocyte cultures
Supernatant was removed, chondrocytes lysed in 0.01% SDS (dissolved in water), and alkaline phosphatase (Alp) activity was measured in cell lysate using a p-Nitrophenyl Phosphate assay (Alpl Assay Kit, Abcam, ab83369) and by reading absorbance at 405 nm.
H2S detection in articular chondrocyte cultures
Primary murine chondrocytes (106cells/condition) were treated for 6 h with 50 μM 3-MST inhibitor or 0.4% vehicle (DMSO). They were then resuspended in fluorescence-activated cell sorting (FACS) buffer (5%FCS, 5 mM EDTA in PBS) and the H2S fluorescent probe P3 added (10 μM, [35]). FACS analysis was performed right after with a UV laser (LSRII SORP cytometer, BD Biosciences) and data processed by FACS Diva (BD Biosciences) and FlowJoX (Tree Star).
LDH measurement in articular chondrocyte cultures
Measurement of the leakage of components from the cytoplasm into the surrounding culture medium has been widely accepted as a valid method to estimate the number of non-viable cells. Lactate dehydrogenase (LDH) in the supernatant was measured using the fluorimetric method CytoTox-ONE™ Homogeneous Membrane Integrity Assay (Promega), by recording fluorescence at an excitation wavelength of 560 nm and an emission wavelength of 590 nm. Culture medium from wells without cells was used as a negative control (0% cytotoxicity), while medium from wells with lysed cells (1% Triton X-100) was used as a positive control (100% cytotoxicity). The percent cytotoxicity of each experimental wells was then calculated as follows: Percent cytotoxicity = [(Experimental value)-(Culture medium value)]/[(Positive control value)-(Culture medium value)]× 100.
ATDC5 chondrogenic cell line
For qRT-PCR analysis, ATDC5 cells (from ATCC cell line) were cultured in DMEM/F12 (1%P/S only) and treated for 4 h with different combinations of 0.4% DMSO (Sigma-Aldrich), 50 μl/ml CPP, 50 μM 3-MST inhibitor, and 500 μM H2O2. For reactive oxygen species (ROS) measurement, separate plates were used and ATDC5 were cultured in DMEM/F12 without phenol red and FBS and cells were treated with the same conditions described above, or with 10 ng/ml mouse recombinant IL-6 (Gibco, dissolved in DMSO) where indicated.
ROS level measurement in ATDC5 cells
Mitochondrial ROS level was measured with Red Mitochondrial Superoxide Indicator (MitoSOX, Life Technologies). Briefly, ATDC5 in half area 96-wells clear bottom black plate were stimulated or not for 1 h with 50 μl/ml CPP and treated or not with vehicle 0.4% DMSO, 50 μM 3-MST inhibitor, 50 μM H2O2, 1 mM NAC, or 10 ng/ml IL-6 in DMEM/F12 without phenol red. After stimulation, cells were loaded 30 min with 5 μM MitoSOX, and fluorescence intensity measured (excitation wavelength of 510 nm, emission wavelength of 580 nm) using the Spectrax M5e reader.
Wells with cells-DMEM/F12 only, as well as wells with MitoSOX-DMEM/F12 only, were also included in order to measure cells and MitoSOX autofluorescence, respectively. These background values were then subtracted to the experimental wells values, and the obtained results were plotted as MitoSOX signal (A.U).
Real-time PCR analysis in articular chondrocyte cultures and ATDC5 cells
Cells were lysed in TRIzol reagent (Thermo Scientific) in a ratio of 500 μl TRIzol every 106 cells. RNA was extracted (RNA Clean and Concentrator5, Zymoresearch), reverse transcribed (Superscript II, Invitrogen), and quantitative Real Time-PCR (qRT-PCR) with gene-specific primers (Table 1) using the LightCycler480®system (Roche Applied Science) was performed. Each reaction mix was composed by 3.75 μl LightCycler 480 SYBR Green I Master (Roche) + 0.75 μl of 5 μM primer pair specific for each gene+ 0.5 μl of LightCycler Water (Roche) + 2.5 μl of 20 ng/μl cDNA. Wells with RNase/DNase-free water instead of cDNA were included for each amplified gene as a negative control.
Table 1.
Murine gene specific primers for qRT-PCR
| Gene | Forward primer (5′ ➔ 3′) | Reverse primer (5′➔ 3′) |
|---|---|---|
| mAnk | TGT CAA CCT CTT CGT GTC CC | GAC AAA ACA GAG CGT CAG CG |
| mAlpl | TTG TGC CAG AGA AAG AGA GAG | GTT TCA GGG CAT TTT TCA AGG T |
| mAnx5 | CCT CAC GAC TCT ACG ATG CC | AGC CTG GAA CAA TGC CTG AG |
| mPit-1 | CTC TCC GCT GCT TTC TGG TA | AGA GGT TGA TTC CGA TTG TGC |
| mPit-2 | AAA CGC TAA TGG CTG GGG AA | AAC CAG GAG GCG ACA ATC TT |
| m3-Mst | CTG GGA AAC GGG GAG CG | GCT CGG AAA AGT TGC GGG |
| mTbp | CTT GAA ATC ATC CCT GCG AG | CGC TTT CAT TAA ATT CTT GAT GGT C |
| mGapdh | CTC ATG ACC ACA GTC CAT GC | CAC ATT GGG GGT AGG AAC AC |
Data was normalized against Tbp and Gapdh references genes, with fold induction of transcripts calculated against control cells.
Statistical analysis
For human ex vivo experiments, values represent means ± SD and 4 to 5 patients per group were analyzed. For in vitro experiments, values represent means ± SD of triplicates. For each readout, three independent experiments were performed. For in vivo experiments, eight mice per group were used.
Data was analyzed with GraphPad Prism software. The variation between data sets was evaluated using Student’s t test or one-way or two-way ANOVA test, where appropriate. Bonferroni correction was used as a post hoc analysis in case of multiple comparisons. Correlation between parameters was evaluated using the correlation test and expressed by the Pearson correlation coefficient (− 1 < r < 1). Differences were considered statistically significant at *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Results
3-MST expression in human cartilage negatively correlates with OA severity and chondrocyte calcification, and it is downregulated by HA crystals
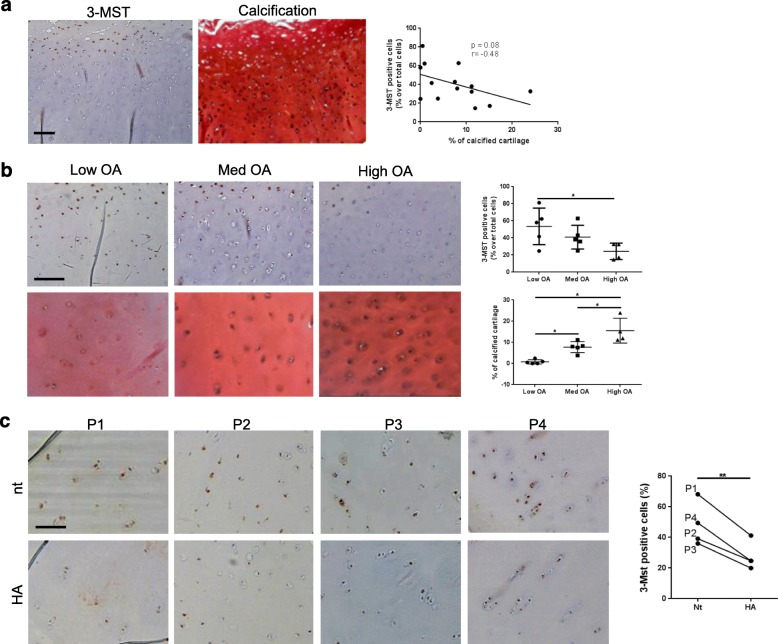
Immunohistochemistry on human OA cartilage (Fig. 1a) revealed high 3-MST expression by chondrocytes in the superficial area of cartilage and low expression in intermediate-deep layers. In contrast, chondrocyte calcification was present in deep cartilage and negative in the superficial zone. Thus, we found a trend (p = 0.08) towards an inverse correlation (r = − 0.48) between the two parameters (graph Fig. 1a). When specimens were divided into low, medium, or high OA, 3-MST expression was decreased by 20–30% in medium and high OA (Fig. 1b), while chondrocyte calcification was increased as assessed by von Kossa staining (Fig. 1b). We next stimulated cartilage explants with HA crystals for 24 h (Fig. 1c). 3-MST expression was significantly inhibited by HA crystals in four patients, although at different degrees (Fig. 1c). Altogether, these results indicate that chondrocyte calcification increases during OA progression and negatively impacts on 3-MST expression proportionally to disease severity.
Fig. 1.
Cartilage 3-MST expression is negatively correlated with OA severity and chondrocyte calcification and downmodulated by HA crystals. a 3-MST immunohistochemical staining in cartilage explants from end-stage osteoarthritis patients and consecutive sections stained with von Kossa/Safranin-O staining for calcium-containing crystals. For each staining, one representative picture from one out of 14 patients is shown. Scale bars 200 μm. The graph shows the correlation between the % of 3-MST-positive cells and the % of calcified cartilage in the different patients. n = 14 patients. b Immunohistochemical staining of 3-MST in cartilage from individuals with low, medium, and high stage osteoarthritis and von Kossa/Safranin-O staining for calcium-containing crystals in consecutive sections. Pictures from one representative patient out of 5 patients per group are shown. Scale bars 200 μm. The graphs show the % of 3-MST-positive cells and the % of calcified cartilage. Three fields were counted per patient and the mean plotted in the graph. n = 14 patients. c 3-MST immunohistochemistry of human cartilage explants stimulated 24 h with HA crystals (HA 500 μg/ml) or not (Nt). Scale bars 200 μm. The graph shows the % of 3-MST-positive cells in Nt vs HA-treated explants in each patient. Lines connect the Nt condition and the HA condition for each patient. n = 4 patients
3-MST regulates joint calcification and cartilage damage in experimental OA
To investigate the role of 3-MST in the pathogenesis of osteoarthritis, we subjected 3-MST deficient mice (3-MST KO) to meniscectomy. As in human OA samples, we observed that 3-MST expression was higher in sham-operated healthy knees than in osteoarthritic MNX knees (Fig. 2a). Two months post-surgery, CT-scans evidenced increased calcification in 3-MST KO knees if compared to WT knees (Fig. 2b, white arrows). Quantitative analysis of calcifications revealed that both their volume and their overall crystal content were significantly higher in 3-MST KO mice (graphs Fig. 2b). In parallel, cartilage damage (fissures and fibrillations, black arrows) as well as proteoglycan loss (reduced Safranin-O staining) were exacerbated in 3-MST KO joints compared to WT joints, as mirrored by both histological analysis and OARSI scores (Fig. 2c). Finally, serum thiosulfate level was higher in WT mice than in 3-MST KO mice (Fig. 2d).
3-MST regulates chondrocyte mineralization and IL-6 secretion in vitro
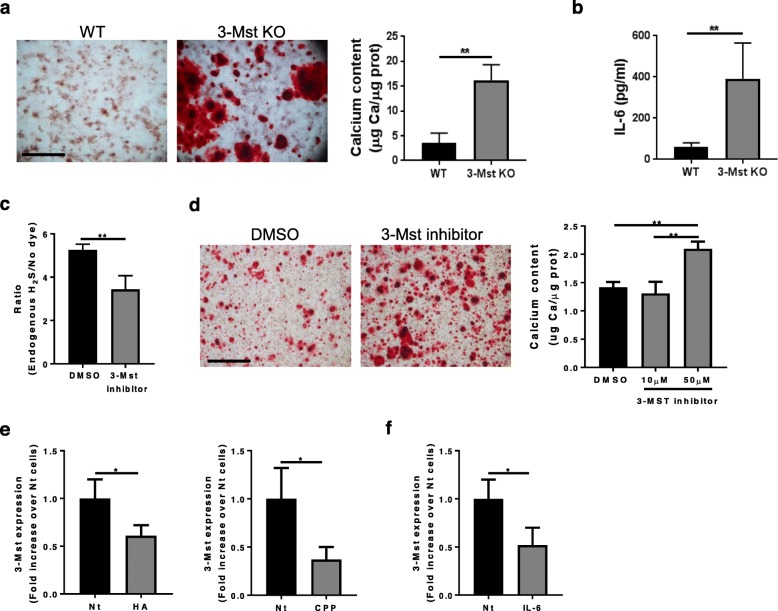
After 24 h in the presence of CPP, 3-MST KO chondrocytes had exacerbated mineralization as demonstrated by Alizarin red staining (Fig. 3a) and by quantification of calcium content in the cell monolayer (graph Fig. 3a). In parallel, we found that 3-MST KO cells secreted higher levels of basal IL-6 than WT cells (Fig. 3b). This would support our previous finding that IL-6 sustains mineralization in chondrocytes [9]. As the second approach to lower 3-MST activity, we treated WT chondrocytes with a pharmacological 3-MST inhibitor. Firstly, we confirmed by FACS that the 3-MST inhibitor significantly decreased H2S production by chondrocytes (Fig. 3c). In accordance with the findings in 3-MST KO chondrocytes (Fig. 3a), treatment with the 3-MST inhibitor triggered chondrocyte mineralization (Fig. 3d). Conversely, stimulation of chondrocytes with crystals (HA or CPP) caused 3-MST downregulation (Fig. 3e). Accordingly, the addition of IL-6 also decreased 3-MST expression by 2-fold (Fig. 3f). Altogether these results suggest that two of the main OA triggers (calcium-containing crystals and IL-6) negatively affect the endogenous generation of H2S by 3-MST and vice-versa.
Fig. 3.
Endogenously H2S produced by 3-MST regulates chondrocytes calcification and IL-6 secretion and vice versa. a Alizarin red staining of WT and 3-MST KO chondrocytes cultured with CPP for 24 h. Pictures represent triplicates from one experiment of three independent experiments. Graph represents calcium content in the cell monolayer, expressed in μg Ca/μg protein. n = 3. b IL-6 secretion in cell supernatant of WT and 3-MST KO chondrocytes from point (a). n = 3. c FACS analysis of endogenous H2S production by WT chondrocytes treated for 6 h with vehicle (DMSO) or 50 μM 3-MST inhibitor and incubated with P3 probe. n = 3. d Alizarin red staining of WT chondrocytes cultured in CPP for 24 h in the presence or absence of 50 μM 3-MST inhibitor. Pictures represent triplicates from one experiment of three independent experiments. Graph represents calcium content in the cell monolayer, expressed in μg Ca/μg protein. n = 3. e qRT-PCR for 3-Mst gene expression in WT chondrocytes stimulated or not with 500 μg/ml HA crystals or CPP for 4 h, or with (f) 10 ng/ml IL-6. n = 3
Oxidative stress regulates 3-MST expression and mineralization in chondrocytes
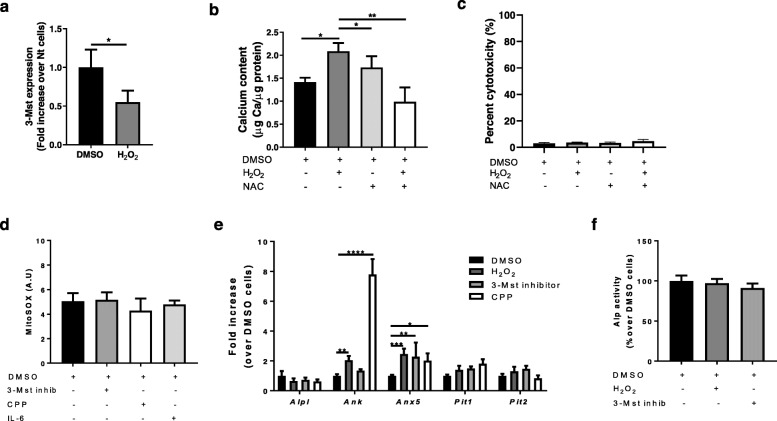
Oxidative stress has been implicated in the progression of OA via different mechanisms [36], but never via a direct role in chondrocyte calcification. We found that H2O2 stimulation led to significantly decreased 3-MST expression (Fig. 4a) in chondrocyte, but concomitantly increased calcification (Fig. 4b), while the ROS scavenger NAC reverted the latter effect. Cell viability was not affected in any conditions (Fig. 4c). Inversely, neither promoters of calcification (CPP and IL-6) nor H2S inhibition (3-MST inhibitor) altered mitochondrial ROS production by chondrocytes (Fig. 4d). Taken together, these results support a deleterious role of ROS upstream to chondrocyte calcification and inflammation, likely mediated by inhibition of 3-MST-generated H2S.
Fig. 4.
Oxidative stress regulates 3-MST expression, mineralization, and inflammation in chondrocytes. a qRT-PCR for 3-Mst gene expression in WT chondrocytes stimulated or not with 500 μM H2O2 for 4 h. n = 3. b Calcium content in chondrocytes monolayer incubated for 24 h with CPP and treated with vehicle (DMSO) or 500 μM H2O2 or with 1 mM NAC or with a combination of them. Calcium content is expressed in μg Calcium/μg protein. c LDH release in cell supernatant of chondrocytes from point (b). n = 3. d Mitochondrial ROS production (MitoSOX) in chondrocytes treated with vehicle (DMSO), or 50 μM 3-MST inhibitor or CPP or 10 ng/ml IL-6 for 1 h. n = 3. e qRT-PCR of the indicated genes in chondrocytes stimulated with vehicle (DMSO), or 500 μM H2O2, or 50 μM 3-MST inhibitor or CPP for 4 h. n = 3. f Alp activity in chondrocytes lysates treated with vehicle, or 500 μM H2O2, or 50 μM 3-MST inhibitor for 6 h. n = 3
Finally, we assessed the expression of genes involved in the calcification process in chondrocyte cultured in presence of CPP, or H2O2, or 3-MST inhibitor. H2O2 significantly increased Ank and Anx5 expression, as the pro-calcifying stimulus CPP and the 3-MST inhibitor did (Fig. 4e). This strengthens the hypothesis that H2O2 may induce chondrocyte calcification via inhibition of endogenous H2S production. Alpl expression (Fig. 4e) and activity (Fig. 4f), and Pit1 and Pit2 expression were not modulated in all conditions.
Discussion
A large body of evidence supports the idea that calcium-containing crystals are active players in the initiation and progression of OA [5, 9, 37, 38]. However, the mechanisms of cartilage calcification are largely unknown and to date, there is no treatment that can prevent crystal deposition or dissolve already formed calcifications.
Here, we have demonstrated that the 3-MST/H2S pathway is involved in cartilage calcification and OA progression. Decreasing the endogenous level of H2S in chondrocytes, either genetically by 3-MST deficiency or pharmacologically by an 3-MST inhibitor, led to exacerbated mineralization in vitro (Fig. 3a, d). A proof of concept of the protective role of 3-MST-generated H2S was given by the in vivo MNX model, where 3-MST-deficient mice were affected by joint calcification and cartilage degradation (Fig. 2b, c) more severely than WT mice. Another evidence was that in knee cartilage from both MNX mice (Fig. 2a) and OA patients (Fig. 1b) we found an inverse correlation between 3-MST expression, and the extent of calcification as well as OA severity.
3-MST deficiency in humans is responsible for a rare inheritable disorder called mercaptolactate-cysteine disulfiduria (MCDU) [39]. MCDU patients are not only mainly affected by mental retardation [25], but can also exhibit skeletal abnormalities (high forehead, arachnodactyly, genum valgum, and joint hyperflexibility (Orphanet:1035)). Other studies support a protective role of H2S in pathological mineralization. H2S decreased vascular calcification [15, 17, 18, 40], while inhibition of CSE activity caused the opposite effect [18]. The H2S-donor sodium thiosulfate (STS) inhibited knee joint calcification during experimental OA [37]. On the other hand, some studies demonstrated the pro-mineralizing effect of H2S. H2S induced physiological mineralization of human periodontal [41] and mesenchymal [42] stem cells. CBS deficiency was associated with human [43] and murine [44] osteoporosis. Furthermore, the H2S-donor GYY4137 stimulated bone formation in vivo [45, 46].
While all these studies investigated the CBS/H2S or the CSE/H2S pathway in mineralization, to our knowledge, we are the first to highlight the importance of the 3-MST/H2S pathway in this context. We will discuss here below the mechanisms by which lack of 3-MST-generated H2S could facilitate chondrocyte calcification and OA progression and the time-course of the events.
The very first mechanism involved seems to be reduced 3-MST expression/activity by increased oxidative stress. We indeed showed here that hydrogen peroxide (H2O2), a major reactive oxygen species (ROS), was able to decrease 3-Mst expression (Fig. 4a). The effect of ORS on 3-MST can also occur at the post-transcriptional level, as it was shown previously that H2O2 inhibited the activity of mouse recombinant 3-MST and further H2S generation [47]. Subsequently to 3-MST inhibition, we showed that H2O2 exacerbated chondrocyte mineralization (Fig. 4b) while the ROS scavenger NAC reverted this effect. Other studies exist in the literature that supports an important role or ROS in triggering chondrocyte calcification [48] and metalloproteases production [49, 50], ultimately leading to OA progression. The fact that preventing oxidative stress is beneficial in reducing chondrocyte calcification, was also highlighted in a previous study from our group, in which we demonstrated that the H2S metabolite thiosulfate was able to decrease ROS production and calcification in chondrocytes [37]. We therefore hypothesize that increased oxidative stress inhibits 3-MST/H2S ultimately leading to increased chondrocyte calcification. Importantly, while ROS suppressed 3-MST/H2S pathway and induced calcification, we could not found the opposite, that is 3-MST inhibition or calcification trigger (CPP) did not increase mitochondrial ROS production. This could be because the 3-MST function in mitochondria is compensated by another enzyme called rhodanese [51]. Further investigations are needed to determine if decreased 3-MST/H2S impact on total ROS production in chondrocytes.
We next investigated in more details the possible underlying mechanisms by which 3-MST inhibition could exacerbate calcification in chondrocytes, and found that both 3-MST inhibitors (H2O2 and the 3-MST inhibitor itself) caused upregulation of calcification genes such as Ank and Anx5 (Fig. 4e). This is in line with our previous data of Anx5 downregulation by the H2S metabolite thiosulfate [37]. The expression or the activity of other calcification enzymes and channels (Alpl, Pit1, Pit2) were not impacted by H2O2 or the 3-MST inhibitor (Fig. 4e, f).
An additional trigger of chondrocyte calcification is known to be inflammation. In particular, in our study from 2015 [9], we demonstrated that a vicious cycle exists between chondrocyte calcification and the pro-inflammatory cytokine IL-6. In the current study, we demonstrated that 3-MST inhibition (3-MST deficient chondrocytes), led not only to increased calcification but also to increased IL-6 secretion by chondrocytes (Fig. 3b). Conversely, we found that both pro-calcifying factors (HA, CPP) and pro-inflammatory factors (IL-6) led to the downregulation of 3-Mst expression (Fig. 3e, f), thus reducing H2S. This loop is shown in Fig. 5.
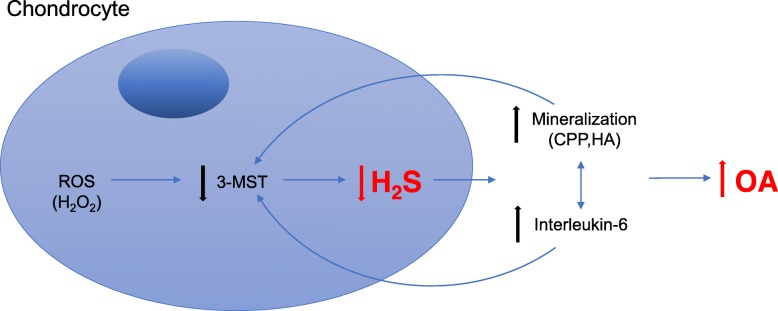
Fig. 5.
Proposed mechanism for 3-MST involvement in osteoarthritic joints. Firstly, reactive oxygen species such as H2O2 decrease 3-MST expression (Fig. 4a). 3-MST inhibition leads to decreased endogenous H2S production (Fig. 3c) which favors increased chondrocyte calcification (Fig. 3a and d), and interleukin-6 secretion (Fig. 3b). An amplification loop exists between mineralization and interleukin-6, which triggers OA progression [9]. Finally, mineralization and interleukin-6 can also cause downregulation of 3-MST, leading to sustained deleterious signal towards disease progression
A thing that remains to be clarified is whether the effects caused by inhibition of the 3-MST (exacerbated calcification and inflammation) are due to decreased levels of H2S or one of its metabolites such as thiosulfate. In 3-MST KO mice, which have decreased serum levels of H2S [25], we also found decreased serum levels of thiosulfate (Fig. 2b). We previously demonstrated that thiosulfate is protective against joint calcification and cartilage degradation in experimental OA, likely due to its anti-inflammatory, antioxidant, and anti-catabolic properties [37].
Finally, further data are needed to determine whether the other H2S producing enzymes have a role in calcification in OA. Although we have already excluded a major role for CBS (because not expressed in cartilage, and because we did not observe any OA phenotype in CBS KO mice, data not shown), it is likely that CSE, which is expressed by chondrocytes [52], may as well have a role in joint calcification.
Conclusions
We have established a key role for the 3-MST/H2S axis in the regulation of pathological chondrocyte calcification in OA. Oxidative stress is an upstream event leading to reduced 3-MST/H2S levels. Impaired 3-MST/H2S levels increase chondrocyte calcification and IL-6 secretion. Moreover, calcium-containing crystals and IL-6 can in turn inhibit 3-MST-mediated H2S production, resulting in even greater mineralization and OA progression (Fig. 5). Whether these phenotypes are due to the lack of H2S, or the lack of one of its metabolites such as thiosulfate, or the accumulation of the 3-MST substrate 3-mercaptopyruvate remains to be investigated. Our results suggest that augmenting H2S production by 3-MST activation may be an approach to treat calcifying disorders.
Acknowledgements
We thank Véronique Chobaz for her excellent technical support. We are grateful to Prof. Thomas Hügle for his suggestions, critical reading, and editing of the manuscript. Hydroxyapatite (HA) crystals were kindly provided by Prof. Christèle Combes (CIRIMAT, INPT-UPS-CNRS, Toulouse, France). Serum thiosulfate measurement was kindly performed by Dr. Andreas Pasch (Calciscon AG, Nidau, Switzerland). 3-MST inhibitor was kindly provided by Prof Kenjiro Hanaoka, University of Tokyo.
Abbreviation
- 3-MST
3-Mercaptosulfur transferase
- CBS
Cystathionine beta-synthase
- CSE
Cystathionine gamma-lyase
- H2S
Hydrogen sulfide
- NO
Nitric oxide
- CO
Carbon monoxide
- BCP
Basic calcium phosphate
- HA
Hydroxyapatite
- CPPD
Calcium pyrophosphate dihydrate
- CPP
Calciprotein particles
- MNX
Meniscectomy
- ROS
Reactive oxygen species
- NAC
N-acetyl cysteine
- VOI
Volume of interests
- OARSI
Osteoarthritis research society international
- K/L
Kellgren-Lawrence
- LDH
Lactate dehydrogenase
- Alpl
Alkaline phosphatase
- Ank
Progressive ankylosis protein homolog
- Anx5
Annexin 5
- Pit1
Phosphate transporter 1
- Pit2
Phosphate transporter 2
- MCDU
Beta-mercaptolactate cysteine disulfiduria
- VSMCs
Vascular smooth muscle cells
- IL-6
Interleukin-6
Authors’ contributions
SN designed, performed, and evaluated most experiments. NC took part in the in vivo experiment. NN originally generated the 3-MST knockout mice. AS, AP, GC, and NB designed the project and evaluated results. JB collected and analyzed human cartilage calcification. DE set up and performed FACS analysis. All co-authors participated in the writing of the manuscript. The authors read and approved the final manuscript.
Funding
This work was supported by the Fonds National Suisse de la recherche scientifique (grant 310030-130085/1).
Availability of data and materials
Data are available upon request to authors.
Ethics approval and consent to participate
All animal procedures were in compliance with the European Community guidelines for the use of experimental animals; experimental protocols were approved by the Ethical Committee of the Prefecture of Athens (198177). Animals received a standard rodent laboratory diet. All efforts were made to minimize suffering. Human samples were obtained with the approval of the Centre Hospitalier Universitaire Vaudois ethical committee or by the institutional Review Board of the Faculty of Medicine of the Otto-von-Guericke University (IRB no. 23/16), and written informed consent of patients was obtained.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sen R, Hurley JA. Osteoarthritis. In StatPearls. Treasure Island (FL). StatPearls Publishing LLC; 2018. https://eproofing.springer.com/journals_v2/mainpage.php?token=n8ucue4E67IXQzdZsqCJ2CkvZIo62dcyQCptZB5cJmEUCNytt1oWuw.
- 2.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nalbant S, Martinez JA, Kitumnuaypong T, et al. Synovial fluid features and their relations to osteoarthritis severity: new findings from sequential studies. Osteoarthr Cartil. 2003;11:50–54. doi: 10.1053/joca.2002.0861. [DOI] [PubMed] [Google Scholar]
- 4.Fuerst M, Bertrand J, Lammers L, et al. Calcification of articular cartilage in human osteoarthritis. Arthritis Rheum. 2009;60:2694–2703. doi: 10.1002/art.24774. [DOI] [PubMed] [Google Scholar]
- 5.Ea HK, Liote F. Advances in understanding calcium-containing crystal disease. Curr Opin Rheumatol. 2009;21:150–157. doi: 10.1097/BOR.0b013e3283257ba9. [DOI] [PubMed] [Google Scholar]
- 6.Conway R, McCarthy GM. Calcium-containing crystals and osteoarthritis: an unhealthy alliance. Curr Rheumatol Rep. 2018;20:13. doi: 10.1007/s11926-018-0721-9. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy GM, Dunne A. Calcium crystal deposition diseases - beyond gout. Nat Rev Rheumatol. 2018;14:592–602. doi: 10.1038/s41584-018-0078-5. [DOI] [PubMed] [Google Scholar]
- 8.Easley RA, Patsavas MC, Byrne RH, et al. Spectrophotometric measurement of calcium carbonate saturation states in seawater. Environ Sci Technol. 2013;47:1468–1477. doi: 10.1021/es303631g. [DOI] [PubMed] [Google Scholar]
- 9.Nasi S, So A, Combes C, et al. Interleukin-6 and chondrocyte mineralisation act in tandem to promote experimental osteoarthritis. Ann Rheum Dis. 2016;75:1372–1379. doi: 10.1136/annrheumdis-2015-207487. [DOI] [PubMed] [Google Scholar]
- 10.Polhemus DJ, Lefer DJ. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ Res. 2014;114:730–737. doi: 10.1161/CIRCRESAHA.114.300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes MN, Centelles MN, Moore KP. Making and working with hydrogen sulfide: the chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: a review. Free Radic Biol Med. 2009;47:1346–1353. doi: 10.1016/j.freeradbiomed.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Olson KR. H2S and polysulfide metabolism: conventional and unconventional pathways. Biochem Pharmacol. 2018;149:77–90. doi: 10.1016/j.bcp.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Rose P, Moore PK, Zhu YZ. H2S biosynthesis and catabolism: new insights from molecular studies. Cell Mol Life Sci. 2017;74:1391–1412. doi: 10.1007/s00018-016-2406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gemici B, Wallace JL. Anti-inflammatory and cytoprotective properties of hydrogen sulfide. Methods Enzymol. 2015;555:169–193. doi: 10.1016/bs.mie.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 15.Aghagolzadeh P, Radpour R, Bachtler M, et al. Hydrogen sulfide attenuates calcification of vascular smooth muscle cells via KEAP1/NRF2/NQO1 activation. Atherosclerosis. 2017;265:78–86. doi: 10.1016/j.atherosclerosis.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Lin TH, Tang CH, Hung SY, et al. Upregulation of heme oxygenase-1 inhibits the maturation and mineralization of osteoblasts. J Cell Physiol. 2010;222:757–768. doi: 10.1002/jcp.22008. [DOI] [PubMed] [Google Scholar]
- 17.Wu SY, Pan CS, Geng B, et al. Hydrogen sulfide ameliorates vascular calcification induced by vitamin D3 plus nicotine in rats. Acta Pharmacol Sin. 2006;27:299–306. doi: 10.1111/j.1745-7254.2006.00283.x. [DOI] [PubMed] [Google Scholar]
- 18.Zavaczki E, Jeney V, Agarwal A, et al. Hydrogen sulfide inhibits the calcification and osteoblastic differentiation of vascular smooth muscle cells. Kidney Int. 2011;80:731–739. doi: 10.1038/ki.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burguera EF, Vela-Anero A, Magalhaes J, et al. Effect of hydrogen sulfide sources on inflammation and catabolic markers on interleukin 1beta-stimulated human articular chondrocytes. Osteoarthr Cartil. 2014;22:1026–1035. doi: 10.1016/j.joca.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 20.Vela-Anero A, Hermida-Gomez T, Gato-Calvo L, et al. Long-term effects of hydrogen sulfide on the anabolic-catabolic balance of articular cartilage in vitro. Nitric Oxide. 2017;70:42–50. doi: 10.1016/j.niox.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Spassov SG, Donus R, Ihle PM, et al. Hydrogen sulfide prevents formation of reactive oxygen species through PI3K/Akt signaling and limits ventilator-induced lung injury. Oxidative Med Cell Longev. 2017;2017:3715037. doi: 10.1155/2017/3715037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng D, Dong S, Li T, et al. Exogenous hydrogen sulfide attenuates cardiac fibrosis through reactive oxygen species signal pathways in experimental diabetes mellitus models. Cell Physiol Biochem. 2015;36:917–929. doi: 10.1159/000430266. [DOI] [PubMed] [Google Scholar]
- 23.Zhang D, Du J, Tang C, et al. H2S-induced Sulfhydration: biological function and detection methodology. Front Pharmacol. 2017;8:608. doi: 10.3389/fphar.2017.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marutani E, Yamada M, Ida T, et al. Thiosulfate Mediates Cytoprotective Effects of Hydrogen Sulfide Against Neuronal Ischemia. J Am Heart Assoc. 2015;4(11):1–11. [DOI] [PMC free article] [PubMed]
- 25.Nagahara N, Nagano M, Ito T, et al. Antioxidant enzyme, 3-mercaptopyruvate sulfurtransferase-knockout mice exhibit increased anxiety-like behaviors: a model for human mercaptolactate-cysteine disulfiduria. Sci Rep. 2013;3:1986. doi: 10.1038/srep01986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamekura S, Hoshi K, Shimoaka T, et al. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthr Cartil. 2005;13:632–641. doi: 10.1016/j.joca.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Nasi S, So A, Combes C, et al. Interleukin-6 and chondrocyte mineralisation act in tandem to promote experimental osteoarthritis. Ann Rheum Dis. 2015;75(7):1372–9. [DOI] [PubMed]
- 28.Pritzker KP, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthr Cartil. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Farese S, Stauffer E, Kalicki R, et al. Sodium thiosulfate pharmacokinetics in hemodialysis patients and healthy volunteers. Clin J Am Soc Nephrol. 2011;6:1447–1455. doi: 10.2215/CJN.10241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newton GL, Dorian R, Fahey RC. Analysis of biological thiols: derivatization with monobromobimane and separation by reverse-phase high-performance liquid chromatography. Anal Biochem. 1981;114:383–387. doi: 10.1016/0003-2697(81)90498-x. [DOI] [PubMed] [Google Scholar]
- 31.Prudhommeaux F, Schiltz C, Liote F, et al. Variation in the inflammatory properties of basic calcium phosphate crystals according to crystal type. Arthritis Rheum. 1996;39:1319–1326. doi: 10.1002/art.1780390809. [DOI] [PubMed] [Google Scholar]
- 32.Gosset M, Berenbaum F, Thirion S, Jacques C. Primary culture and phenotyping of murine chondrocytes. Nat Protoc. 2008;3:1253–1260. doi: 10.1038/nprot.2008.95. [DOI] [PubMed] [Google Scholar]
- 33.Hanaoka K, Sasakura K, Suwanai Y, et al. Discovery and mechanistic characterization of selective inhibitors of H2S-producing enzyme: 3-Mercaptopyruvate Sulfurtransferase (3MST) targeting active-site cysteine Persulfide. Sci Rep. 2017;7:40227. doi: 10.1038/srep40227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gregory CA, Gunn WG, Peister A, Prockop DJ. An alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004;329:77–84. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Singha S, Kim D, Moon H, et al. Toward a selective, sensitive, fast-responsive, and biocompatible two-photon probe for hydrogen sulfide in live cells. Anal Chem. 2015;87:1188–1195. doi: 10.1021/ac503806w. [DOI] [PubMed] [Google Scholar]
- 36.Lepetsos P, Papavassiliou AG. ROS/oxidative stress signaling in osteoarthritis. Biochim Biophys Acta. 1862;2016:576–591. doi: 10.1016/j.bbadis.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Nasi S, Ea HK, Liote F, et al. Sodium thiosulfate prevents chondrocyte mineralization and reduces the severity of murine osteoarthritis. PLoS One. 2016;11:e0158196. doi: 10.1371/journal.pone.0158196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stack J, McCarthy G. Basic calcium phosphate crystals and osteoarthritis pathogenesis: novel pathways and potential targets. Curr Opin Rheumatol. 2016;28:122–126. doi: 10.1097/BOR.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 39.Billaut-Laden I, Rat E, Allorge D, et al. Evidence for a functional genetic polymorphism of the human mercaptopyruvate sulfurtransferase (MPST), a cyanide detoxification enzyme. Toxicol Lett. 2006;165:101–111. doi: 10.1016/j.toxlet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Pasch A, Schaffner T, Huynh-Do U, et al. Sodium thiosulfate prevents vascular calcifications in uremic rats. Kidney Int. 2008;74:1444–1453. doi: 10.1038/ki.2008.455. [DOI] [PubMed] [Google Scholar]
- 41.Su Y, Liu D, Liu Y, et al. Physiologic levels of endogenous hydrogen sulfide maintain the proliferation and differentiation capacity of periodontal ligament stem cells. J Periodontol. 2015;86:1276–1286. doi: 10.1902/jop.2015.150240. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Yang R, Liu X, et al. Hydrogen sulfide maintains mesenchymal stem cell function and bone homeostasis via regulation of Ca (2+) channel sulfhydration. Cell Stem Cell. 2014;15:66–78. doi: 10.1016/j.stem.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levasseur R. Bone tissue and hyperhomocysteinemia. Joint Bone Spine. 2009;76:234–240. doi: 10.1016/j.jbspin.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Robert K, Maurin N, Vayssettes C, et al. Cystathionine beta synthase deficiency affects mouse endochondral ossification. Anat Rec A Discov Mol Cell Evol Biol. 2005;282:1–7. doi: 10.1002/ar.a.20145. [DOI] [PubMed] [Google Scholar]
- 45.Grassi F, Tyagi AM, Calvert JW, et al. Hydrogen sulfide is a novel regulator of bone formation implicated in the bone loss induced by estrogen deficiency. J Bone Miner Res. 2016;31:949–963. doi: 10.1002/jbmr.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang X, Chen Y, Lu K, et al. GYY4137 promotes bone formation in a rabbit distraction osteogenesis model: a preliminary report. J Oral Maxillofac Surg. 2015;73:732 e731–732 e736. doi: 10.1016/j.joms.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 47.Modis K, Asimakopoulou A, Coletta C, et al. Oxidative stress suppresses the cellular bioenergetic effect of the 3-mercaptopyruvate sulfurtransferase/hydrogen sulfide pathway. Biochem Biophys Res Commun. 2013;433:401–407. doi: 10.1016/j.bbrc.2013.02.131. [DOI] [PubMed] [Google Scholar]
- 48.Morita K, Miyamoto T, Fujita N, et al. Reactive oxygen species induce chondrocyte hypertrophy in endochondral ossification. J Exp Med. 2007;204:1613–1623. doi: 10.1084/jem.20062525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reed KN, Wilson G, Pearsall A, Grishko VI. The role of mitochondrial reactive oxygen species in cartilage matrix destruction. Mol Cell Biochem. 2014;397:195–201. doi: 10.1007/s11010-014-2187-z. [DOI] [PubMed] [Google Scholar]
- 50.Del Carlo M, Schwartz D, Erickson EA, Loeser RF. Endogenous production of reactive oxygen species is required for stimulation of human articular chondrocyte matrix metalloproteinase production by fibronectin fragments. Free Radic Biol Med. 2007;42:1350–1358. doi: 10.1016/j.freeradbiomed.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagahara N, Tanaka M, Tanaka Y, Ito T. Novel Characterization of Antioxidant Enzyme, 3-Mercaptopyruvate Sulfurtransferase-Knockout Mice: Overexpression of the Evolutionarily-Related Enzyme Rhodanese. Antioxidants (Basel). 2019;8(5):116–225. [DOI] [PMC free article] [PubMed]
- 52.Fox B, Schantz JT, Haigh R, et al. Inducible hydrogen sulfide synthesis in chondrocytes and mesenchymal progenitor cells: is H2S a novel cytoprotective mediator in the inflamed joint? J Cell Mol Med. 2012;16:896–910. doi: 10.1111/j.1582-4934.2011.01357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request to authors.