Abstract
Background/Aims:
Increased cerebrospinal fluid (CSF) tau, decreased CSF amyloid-β42 (Aβ42) and the apolipoprotein E gene (APOE) ε4 allele predict progression from mild cognitive impairment (MCI) to Alzheimer's disease (AD). Here, we investigated these markers to assess their predictive value and influence on the rate of disease progression.
Methods:
Using ELISA, we measured the CSF biomarkers in 47 AD patients, 58 patients with MCI and 35 healthy control subjects. Twenty-eight MCI patients revisited the clinic and half of them progressed to AD during a period of 3–12 years.
Results:
The expected changes in CSF total (T)-tau, phosphorylated (P)-tau and Aβ42 levels were found in AD, confirming the diagnostic value of these biomarkers. We were also able to corroborate an increased risk for progression from MCI to AD with elevated CSF T-tau and P-tau and with the presence of the APOE ε4/ε4 genotype, but not with decreased Aβ42. Finally, for the first time we demonstrated that MCI subjects with high CSF T-tau or P-tau and APOE ε4 homozygosity progressed faster from MCI to AD. Conclusions: CSF T-tau and P-tau as well as the APOE ε4/ε4 genotype are robust predictors of AD and are also associated with a more rapid progression from MCI to AD.
Key Words: Alzheimer's disease, Mild cognitive impairment, Biomarkers, Cerebrospinal fluid, Tau, Phospho-tau, Amyloid-β, Apolipoprotein E, Disease progression
Introduction
Two types of protein deposits, plaques and tangles, characterize the neuropathology of Alzheimer's disease (AD). Whereas the extracellular plaques mainly consist of aggregated amyloid-β with 40 (Aβ40) or 42 (Aβ42) amino acids, the intraneuronal tangles are composed of extensively phosphorylated aggregated tau protein. Levels of total (T)-tau, phosphorylated (P)-tau and Aβ42 can be measured in the cerebrospinal fluid (CSF), where a combination of increased T-tau or P-tau and decreased Aβ42 levels indicates AD. A meta-analysis of 12 studies on CSF biomarkers calculated the combination of T-tau and Aβ42 to have a sensitivity of 89% and a specificity of 90% for AD [1].
This biochemical CSF signature is also predictive of subjects who will progress from mild cognitive impairment (MCI) to AD. A longitudinal study of MCI patients followed over 4–6 years calculated a sensitivity of 95% and a specificity of 83% for disease progression based on the combination of T-tau and Aβ42 CSF levels [2].
Both MCI and AD patients carrying the ε4 allele of the apolipoprotein E gene (APOE), a major genetic risk factor for late-onset AD, have a further decrease in CSF Aβ42 [3], and in several studies, they were also found to have a more pronounced increase in CSF tau levels relative to those not carrying the ε4 allele [3, 4, 5, 6]. Moreover, MCI patients with the APOE ε4 allele have been described to be more likely to progress to AD as compared to those without the APOE ε4 allele [7].
In this study, we measured CSF T-tau, P-tau, Aβ342 and Aβ40 in a cohort of AD, MCI and control subjects with and without APOE ε4. We investigated the effects of diagnoses and APOE genotypes on CSF biomarker levels and assessed the predictive values of APOE and CSF biomarkers for disease progression. In particular, we wanted to explore if these biochemical and genetic markers can predict time to progression from MCI to AD.
Subjects and Methods
Clinical Samples
The CSF samples included were collected at the Department of Geriatrics at the Karolinska University Hospital in Huddinge, Sweden. The AD and MCI patients were selected consecutively from among those patients who underwent a lumbar puncture as a part of their dementia investigation between 1993 and 2001. Forty-seven of these patients were clinically diagnosed as having AD according to the NINCDS-ADRDA criteria [8], and 58 had been diagnosed with MCI according to the criteria of Petersen et al. [9]. In addition, 35 subjects (mainly spouses of patients) with no history of memory complaints and no measurable cognitive deficiencies were included as healthy controls (table 1). In order to study APOE ε4-related effects more effectively, carriers of the APOE ε2 allele were excluded. Thus, only subjects with APOE ε3/ε3, APOE ε3/ε4 and APOE ε4/ε4 were included in this study.
Table 1.
Demographics, CSF levels and APOE genotypes of the subjects
| Controls | MCI | AD | Stable MCI | Progressors | |
|---|---|---|---|---|---|
| Demographics | |||||
| Number | 35 | 58 | 47 | 14 | 14 |
| Mean age, years | 57.0 ± 8.1 | 62.9 ± 8.2 | 71.7 ± 8.1 | 61.3 ± 7.4 | 66.0 ± 6.3 |
| Females, n (%) | 23 (66) | 30 (52) | 21 (45) | 9 (64) | 6 (42) |
| CSF biomarker levels | |||||
| Aβ40, pM | 1,331 ± 448 | 1,432 ± 534 | 1,412 ± 519 | 1,230 ± 607 | 1,629 ± 567 |
| Aβ42, pM | 98 ± 47 | 108 ± 72 | 66 ± 34 | 88 ± 48 | 91 ± 71 |
| T-tau, ng/l | 248 ± 166 | 373 ± 302 | 619 ± 291 | 252 ± 216 | 621 ± 418 |
| P-tau, ng/l | 41 ± 14 | 51 ± 29 | 63 ± 25 | 38 ± 16 | 74 ± 45 |
| APOE genotype, n (%) | |||||
| ε3/ε3 | 10 (23) | 19 (44) | 14 (33) | 6 (43) | 4 (29) |
| ε3/ε4 | 20 (35) | 19 (33) | 19 (33) | 7 (50) | 3 (21) |
| ε4/ε4 | 5 (13) | 20 (51) | 14 (36) | 1 (7) | 7 (50) |
| ε4 frequency | (43) | (56) | (50) | (32) | (61) |
Biomarker levels are shown as mean values ± SD.
After a standard lumbar puncture, each CSF sample was aliquoted and stored at −80°C until quantification. The APOE genotypes were determined by restriction fragment length polymorphism analysis of DNA isolated from whole blood drawn at the initial visit [10].
A total of 28 MCI patients revisited the clinic (table 1). Of these, 14 had remained stable with MCI for at least 3 years (follow-up time: range 3–12 years, mean ± SD 5.6 ± 2.7 years), whereas 14 had progressed to AD (follow-up time: range 1–8 years, mean ± SD 3.4 ± 2.1 years). There were no significant differences in age or sex distribution, in APOE genotypes or in levels of T-tau, P-tau, Aβ342 and Aβ40 between the MCI patients who revisited the clinic and those who did not (data not shown).
The study was approved by the local ethical committee.
Quantification of CSF Aβ and Tau
Levels of T-tau and P-tau (phosphorylated at Thr181) were measured using Innotest hTau Ag and Innotest Phospho-Tau(181P) ELISA kits, respectively (Innogenetics, Ghent, Belgium) [11, 12]. Levels of Aβ42 and Aβ40 were analyzed using a well-characterized ELISA with BNT77 (mouse IgA anti-Aβ 11–28; Takeda, Osaka, Japan) and horseradish peroxidase-conjugated detector antibodies (BA27 IgG2 mouse anti-Aβ40 and BC05 IgG1 mouse anti-Aβ42), as previously described [13].
Statistical Analyses
To compare CSF biomarker levels across the diagnostic groups, Kruskal-Wallis ANOVA was used. Sensitivity and specificity were calculated using The Discriminant Analysis function in the Statistica software (StatSoft Inc., Tulsa, Okla., USA). A priori classification probabilities for the groups were set as equal. Kruskal-Wallis ANOVA or the Mann-Whitney U test were also used to compare biomarker levels between the groups with different APOE genotypes. Moreover, the Mann-Whitney U test was used for comparison of biomarker levels between disease progressors and nonprogressors. The relative risk for disease progression with the different APOE genotypes was calculated by a standard method [14]. In order to calculate the relative risk with different T-tau and P-tau levels, subjects were divided into tertiles based on their CSF levels, whereby the incidence of AD was compared between the highest and the lowest tertile.
For incidence analysis, the MCI subjects who revisited the clinic were divided into 2 groups. For analysis of CSF T-tau, P-tau and Aβ42 levels, the patients were divided into 2 groups of equal sizes depending on the levels. For APOE, 1 group included APOE ε4 homozygotes and 1 group consisted of APOE ε3/ε4 heterozygotes combined with APOE ε3 homozygotes. The incidence of AD over time in the various groups was compared by a log-rank test.
Results
Increased Tau and Decreased Aβ42 in CSF of AD Patients
AD patients displayed increased CSF T-tau and P-tau levels compared to MCI and control subjects, whereas levels of Aβ42 were decreased (p < 0.01 for all). There was no difference in Aβ40 levels between the diagnostic groups (table 1, fig. 1).
Fig. 1.
Levels of CSF biomarkers in AD patients, individuals with MCI and healthy controls (C). Levels of T-tau (a) and P-tau (b) were increased, whereas levels of Aβ42 (c) were decreased in AD patients compared to controls and MCI subjects (p < 0.01 for all). d Levels of Aβ40 did not differ between the diagnostic groups.
Sensitivity and specificity were calculated for the various CSF biomarkers. All measures that differed significantly between AD patients and controls, i.e. T-tau, P-tau and Aβ42, displayed sensitivities and specificities ranging from 57 to 86%. Based on these analyses, cutoff levels for the biomarkers were determined as follows: 438 ng/l for T-tau, 51 ng/l for P-tau and 82 pM for Aβ42. When combined, the 3 measures distinguished AD patients from controls with 85% sensitivity and 83% specificity.
Effects of APOE on CSF Biomarkers
For AD patients, there were no overall differences in T-tau or P-tau CSF levels between the different APOE genotypes. However, both T-tau and P-tau levels were elevated in controls (p < 0.05 and p < 0.001, respectively) and MCI subjects (p < 0.05 and p = 0.06, respectively) with the APOE ε4/ε4 genotype. In each diagnostic group, individuals homozygous for the APOE ε4 allele had significantly lower levels of CSF Aβ42 compared to individuals with the other APOE genotypes (MCI: p < 0.001; controls and AD: p < 0.05). Interestingly, for AD patients there was also a nonsignificant trend towards decreased Aβ40 levels among APOE ε4/ε4 homozygotes (p = 0.05). However, the APOE ε4 allele did not influence Aβ40 levels among MCI subjects and controls (data not shown).
Surprisingly, in neither of the diagnostic groups were there any significant differences in the levels of T-tau, P-tau or Aβ42 for APOE ε3/ε4 heterozygotes compared to individuals homozygous for APOE ε3. However, the biomarker levels in the APOE ε4 homozygotes were significantly altered compared to both APOE ε3/ε3 and APOE ε3/ε4 carriers (data not shown).
Elevated CSF Tau Levels and the APOE ε4/ε4 Genotype Predict Progression from MCI to AD
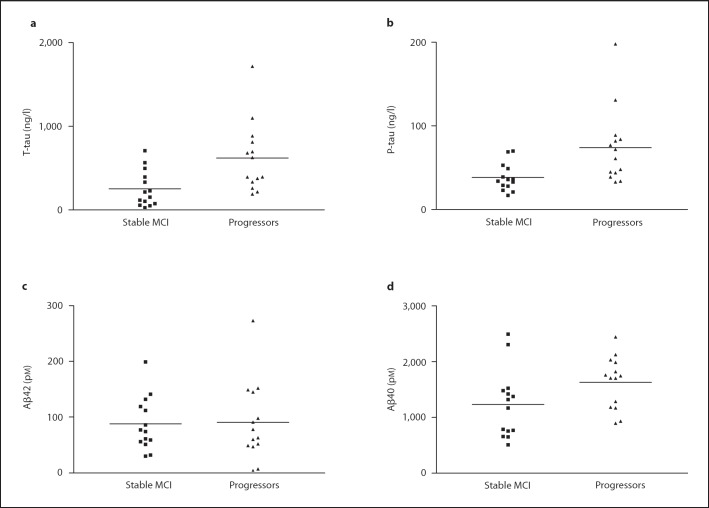
Of the 28 MCI patients who revisited the clinic during the follow-up period of 3–12 years, 14 had progressed to AD whereas 14 had remained stable at MCI (table 1). The average baseline CSF T-tau and P-tau levels for the progressors were significantly higher than the levels among nonprogressors (p < 0.01 for both; table 1, fig. 2).
Fig. 2.
Levels of CSF biomarkers at baseline in individuals with MCI progressing to AD and those who remained stable during the follow-up. Both T-tau (a) and P-tau (b) levels were increased at baseline in individuals progressing to AD (p < 0.01 for both). An increase was also observed for Aβ40 (p < 0.05; d), whereas there was no difference in Aβ42 levels (c).
There was no significant difference in CSF Aβ42 baseline levels between those MCI subjects who had progressed to AD and those who had not (table 1, fig. 2c). However, progressors displayed significantly increased CSF Aβ40 levels compared to nonprogressors (p < 0.05; table 1, fig. 2d). Finally, when calculating the Aβ42/Aβ40 ratio, there was no significant difference between progressors and nonprogressors (data not shown).
Eight of the 28 MCI subjects reevaluated at the clinic were APOE ε4 homozygotes. Of these, 7 (i.e. 50% of all progressors) had progressed to AD. Meanwhile, only 1 of the APOE ε4 homozygotes had remained at the MCI stage, representing only 7% of the nonprogressors (table 1).
The relative risk of progressing from MCI to AD for carriers of the APOE ε4/ε4 genotype in comparison to APOE ε3/ε3 was calculated to be 2.2 [confidence interval (CI) 1.0–3.1]. When comparing APOE ε4/ε4 with APOE ε3/ε4, the relative risk was 2.9 (CI 1.2–4.5). No increase in the relative risk was observed when comparing APOE ε3/ε4 with APOE ε3/ε3. The relative risk was also evaluated for CSF T-tau and P-tau by comparing the highest with the lowest tertile of the 28 reevaluated MCI subjects. Higher levels of T-tau were then found to have a relative risk of 7.0 (CI 1.7–38.6), whereas higher P-tau corresponds to a relative risk of 3.5 (CI 1.2–10.1). However, there was no difference in the relative risk between subjects with high and low CSF Aβ42 levels.
We also wanted to estimate the combined risk of having high T-tau or P-tau levels together with the APOE ε4/ε4 genotype. Notably, none of the 8 individuals with T-tau levels below 216 ng/l and with the APOE ε3/ε3 or ε3/ε4 genotypes were found to progress to AD, whereas all 5 APOE ε4/ε4 carriers with T-tau levels over 564 ng/l did progress during the follow-up period.
The sensitivity and specificity for the combined measures of CSF T-tau, P-tau and Aβ42 to identify MCI progressors at baseline were found to be 64 and 79%, respectively. However, when the APOE genotype status was taken into account, the sensitivity increased to 71% and the specificity to 93%.
Elevated CSF Tau and the APOE ε4/ε4 Genotype Are Associated with a More Rapid Conversion to AD
Kaplan-Meier curves and a log-rank test were used in order to estimate the influence of CSF biomarker levels and APOE genotypes on the time to conversion from MCI to AD. The group of MCI subjects with the 50% highest tau levels at baseline was found to progress to AD within a shorter time span compared to the group with the 50% lowest levels. The shorter time to conversion could be seen with both T-tau (p < 0.05; data not shown) and P-tau (p < 0.01; fig. 3). Finally, APOE ε4 homozygosity was also found to be associated with a shorter time to conversion (p < 0.05; data not shown).
Fig. 3.

The MCI subjects who revisited the clinic were divided into 2 groups of equal size according to their levels of CSF P-tau. The group with the higher levels was found to have a significantly higher incidence of AD and a shorter time to progression compared to the group with the lower levels (p < 0.01).
Discussion
The need for reliable predictive biomarkers in AD will become even more pronounced as novel therapeutic strategies are becoming available. To help the clinician decide whether a disease-modifying treatment should be initiated, a useful marker should be indicative also at early disease stages, preferably already before the onset of symptoms.
Increased tau and decreased Aβ42 in CSF have been shown to have sensitivities and specificities of 80–90% in numerous studies [1]. In our study, Aβ42 was found to give the highest sensitivity (85%), whereas T-tau and P-tau had the highest specificities (86 and 83%, respectively). The Aβ40 levels were similar for all diagnostic groups and the Aβ42/Aβ40 ratio did not improve sensitivity. In addition, and in contrast to a previous report, the Aβ42/P-tau ratio did not greatly affect sensitivity and specificity [15].
Moreover, we were able to confirm the previously reported effects of the APOE ε4 allele on the CSF biomarkers for AD. Both controls and individuals with MCI who were homozygous for APOE ε4 displayed increased T-tau and P-tau as well as decreased Aβ42 in CSF compared to subjects with other APOE genotypes.
Significantly altered biomarker levels were seen only for individuals homozygous for APOE ε4, whereas there were no such changes for heterozygous individuals. This observation may be partly related to the relatively low average age of our patients; besides the increased disease risk for APOE ε4 homozygotes it is known that APOE ε4 homozygotes also have a lower age at disease onset [16, 17]. Nevertheless, significant changes for APOE ε4 heterozygotes might also have been detected with a larger sample size.
The combined measure of increased T-tau and decreased Aβ42 has been demonstrated to have a high predictive value to identify those MCI patients who will progress to AD [2, 18]. Accordingly, we found that CSF T-tau and P-tau levels were significantly higher among progressors.
In our study, CSF Aβ42 levels did not differ significantly between progressors and nonprogressors. Somewhat surprisingly, the MCI cases progressing to AD were found to have significantly higher levels of Aβ40. Whereas Aβ40 levels were not reported in one of the previous longitudinal studies [2], the other such study described nonsignificantly increased levels of Aβ40 among progressors [18]. Thus, although the results in our study are overall in accordance with previous studies, the differences with respect to both Aβ42 and Aβ40 cannot easily be explained. The studies were conducted on similar populations and were similarly designed, although we had a longer follow-up period compared to the previous investigations (3–12 years vs. 4–6 years in Hansson et al. [2]).
The APOE ε4 allele has previously been reported as a predictor for AD development [2, 7]. Therefore, we sought to evaluate the impact of APOE ε4 as a prognostic marker compared to the CSF biomarkers. Similarly to previous studies, the APOE ε4 allele was found to strongly predict progression from MCI to AD. We found that the relative risk for disease progression was comparable between APOE ε4 homozygotes and subjects with increased levels of CSF T-tau or P-tau. When looking at the combined measure of increased tau and APOE ε4, it could be concluded that all 5 reinvestigated MCI individuals homozygous for APOE ε4 and with T-tau levels in the highest tertile did indeed progress to AD. Thus, the best sensitivity and specificity for disease conversion among MCI subjects were obtained when combining the 3 CSF biomarkers (T-tau, P-tau and Aβ42) with the APOE genotype status (71% sensitivity and 93% specificity).
Interestingly, higher levels of T-tau or P-tau not only predicted progression from MCI to AD, but were also associated with a shorter time to progression (fig. 3). To our knowledge, such a relationship has not been clearly demonstrated before. These findings may be explained by the longer follow-up period in our study, which also enabled us to distinguish those MCI subjects who progressed to AD after several years with mild cognitive symptoms. Such individuals may have a slower disease variant characterized by an extended prodromal phase where the steady-state levels of CSF tau are relatively low compared to individuals with intense neuronal degeneration and rapid disease progression.
Another type of promising biomarker is represented by the use of positron emission tomography (PET) with various amyloid-binding ligands. In a number of studies utilizing PET with Pittsburgh B compound, AD patients have been identified with high accuracy [19]. In addition, it has been reported that amyloid deposition can be detected in presymptomatic carriers of various AD-causing mutations [20]. This new imaging method thus has large potential as an antecedent AD marker. In one study on 21 MCI patients, all 7 cases found to be progressors during the follow-up period had amyloid retention at baseline which was comparable to that in patients with manifest AD [21]. Ongoing studies will elucidate whether a combined measure of PET-Pittsburgh B compound and any (or a combination of) CSF markers will further increase the predictive value for early identification of AD.
Acknowledgements
The following foundations are acknowledged for their financial support: the Swedish Research Council, the Swedish Brain Foundation, the Swedish Society of Medicine and The Swedish Alzheimer Foundation. We thank Åsa Källén for skilful technical assistance.
References
- 1.Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx. 2004;1:213–225. doi: 10.1602/neurorx.1.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansson O, Zetterberg H, Buchhave P, et al. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 3.Prince JA, Zetterberg H, Andreasen N, et al. APOE epsilon4 allele is associated with reduced cerebrospinal fluid levels of Abeta42. Neurology. 2004;62:2116–2118. doi: 10.1212/01.wnl.0000128088.08695.05. [DOI] [PubMed] [Google Scholar]
- 4.Galasko D, Chang L, Motter R, et al. High cerebrospinal fluid tau and low amyloid beta42 levels in the clinical diagnosis of Alzheimer disease and relation to apolipoprotein E genotype. Arch Neurol. 1998;55:937–945. doi: 10.1001/archneur.55.7.937. [DOI] [PubMed] [Google Scholar]
- 5.Tapiola T, Pirttila T, Mehta PD, et al. Relationship between apoE genotype and CSF beta-amyloid (1-42) and tau in patients with probable and definite Alzheimer’s disease. Neurobiol Aging. 2000;21:735–740. doi: 10.1016/s0197-4580(00)00164-0. [DOI] [PubMed] [Google Scholar]
- 6.Sunderland T, Mirza N, Putnam KT, et al. Cerebrospinal fluid beta-amyloid1-42 and tau in control subjects at risk for Alzheimer’s disease: the effect of APOE epsilon4 allele. Biol Psychiatry. 2004;56:670–676. doi: 10.1016/j.biopsych.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 7.Herukka SK, Helisalmi S, Hallikainen M, et al. CSF Abeta42, Tau and phosphorylated Tau, APOE epsilon4 allele and MCI type in progressive MCI. Neurobiol Aging. 2007;28:507–514. doi: 10.1016/j.neurobiolaging.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 8.McKhann G, Drachman D, Folstein M. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 9.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 10.Ingelsson M, Shin Y, Irizarry MC, et al. Curr Protoc Hum Genet. 2003. Genotyping of apolipoprotein E: comparative evaluation of different protocols. Chapter 9:Unit 9.14. [DOI] [PubMed] [Google Scholar]
- 11.Blennow K, Wallin A, Ågren H, et al. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer’s disease? Mol Chem Neuropathol. 1995;26:231–245. doi: 10.1007/BF02815140. [DOI] [PubMed] [Google Scholar]
- 12.Vanmechelen E, Vanderstichele H, Davidsson P, et al. Quantification of tau phosphorylated at threonine 181 in human cerebrospinal fluid: a sandwich ELISA with a synthetic phosphopeptide for standardization. Neurosci Lett. 2000;285:49–52. doi: 10.1016/s0304-3940(00)01036-3. [DOI] [PubMed] [Google Scholar]
- 13.Ingelsson M, Fukumoto H, Newell K, et al. Early Abeta accumulation and progressive synaptic loss, gliosis and tangle formation in AD brain. Neurology. 2004;62:925–931. doi: 10.1212/01.wnl.0000115115.98960.37. [DOI] [PubMed] [Google Scholar]
- 14.Rosner B. Florence KY, Cengage Learning Inc. 2006. Fundamentals of Biostatistics, ed 6. [Google Scholar]
- 15.Maddalena A, Papassotiropoulos A, Muller-Tillmanns B, et al. Biochemical diagnosis of Alzheimer disease by measuring the cerebrospinal fluid ratio of phosphorylated tau protein to beta-amyloid peptide42. Arch Neurol. 2003;60:1202–1206. doi: 10.1001/archneur.60.9.1202. [DOI] [PubMed] [Google Scholar]
- 16.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 17.Sando SB, Melquist S, Cannon A, et al. APOE epsilon 4 lowers age at onset and is a high risk factor for Alzheimer’s disease; a case control study from central Norway. BMC Neurol. 2008;8:9. doi: 10.1186/1471-2377-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansson O, Zetterberg H, Buchhave P, et al. Prediction of Alzheimer’s disease using the CSF Abeta42/Abeta40 ratio in patients with mild cognitive impairment. Dement Geriatr Cogn Disord. 2007;23:316–320. doi: 10.1159/000100926. [DOI] [PubMed] [Google Scholar]
- 19.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 20.Klunk WE, Price JC, Mathis CA, et al. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J Neurosci. 2007;27:6174– 6184. doi: 10.1523/JNEUROSCI.0730-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forsberg A, Engler H, Almkvist O, et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging. 2008;29:1456–1465. doi: 10.1016/j.neurobiolaging.2007.03.029. [DOI] [PubMed] [Google Scholar]