Abstract
Objective:
Fetal cardiac intervention (FCI) has been performed at our center in selected fetuses with complex congenital heart disease since 2000. Most interventions are performed in fetuses with a ductus arteriosus (DA)-dependent circulation. Indomethacin promotes closure of the DA in newborns and in fetal life, a potentially life threatening complication in fetuses with ductus-dependent congenital heart disease.
Methods:
We reviewed our experience with FCI with a focus on the frequency, features, and clinical course of ductal constriction. Fetuses undergoing FCI receive comprehensive pre- and postoperative cardiac and cerebral ultrasound evaluation, approximately 24 hours before and after the procedure, including imaging of DA flow and Doppler assessment of the umbilical artery and vein, ductus venosus, and, since 2004, the middle cerebral artery.
Results:
Among 113 fetuses that underwent FCI, 24 of which were older than 28 0/7 weeks gestation, 2 were found to have DA constriction due to indomethacin therapy within 24 hours of intervention. Both of these were 30-week fetuses with hypoplastic left heart syndrome and restrictive or intact atrial septum. The DA was stenotic by spectral and color Doppler, and middle cerebral and umbilical artery pulsatility indexes were depressed. After discontinuation of indomethacin, the Doppler indices improved or normalized.
Conclusion:
Close echocardiographic monitoring of fetal Doppler flow velocities is very important after fetal intervention and indomethacin treatment, as the consequences of DA constriction in a fetus with hypoplastic left heart syndrome are potentially lethal. Sonographic evaluation should include measurement of cerebral and umbilical arterial flow velocities as well as color and spectral Doppler interrogation of the DA.
Key Words: Fetal therapy, Ductal constriction, Middle cerebral artery Doppler, Indocin, Hypoplastic left heart syndrome
Introduction
Indomethacin and other non-steroidal anti-inflammatory drugs (NSAIDs) have been shown to promote closure of the ductus arteriosus (DA) in newborns as well as in fetal life [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15]. In newborns, indomethacin is used therapeutically to promote DA closure mostly in premature infants to reverse the adverse hemodynamic effects of a moderate or large DA [2]. In pregnancy, indomethacin is used to prevent uterine contractions after fetal therapeutic procedures and to suppress premature labor [3, 4, 6−10, 13, 14]. However, maternal ingestion of NSAIDs for pregnancy-related musculoskeletal pain during an otherwise healthy pregnancy may cause in utero ductal constriction or closure, with potentially devastating consequences for the fetus. DA constriction can be tolerated in a structurally normal fetal heart, but in a fetus with DA-dependent systemic blood flow, it is potentially lethal.
Supported by contributions from the Kenrose Kitchen Table Foundation.
Fetal cardiac intervention (FCI) has been carried out at Children's Hospital Boston and Brigham & Women's Hospital since 2000 for aortic stenosis with evolving hypoplastic left heart syndrome (HLHS), HLHS with an intact or highly restrictive atrial septum, pulmonary atresia with evolving hypoplastic right heart, and other structural anomalies causing fetal hydrops [16, 17, 18, 19, 20, 21]. In most fetuses undergoing FCI, systemic blood flow is completely or partially dependent on flow through the DA. Previous adverse events observed in fetuses undergoing FCI include fetal demise, fetal hemodynamic instability, fetal hemopericardium, and maternal mirror syndrome in 2 cases with severe fetal hydrops [17, 18, 20, 21, 22].
Maternal administration of indomethacin is standard tocolytic therapy in the peri-FCI period. However, the frequency, features, and outcomes of indomethacin associated DA constriction in fetuses undergoing FCI have not been characterized. In this study, we reviewed our experience with FCI to assess the frequency and manifestation of DA constriction in a series of 113 fetuses that underwent FCI for the aforementioned indications in the fetal cardiac intervention program at Children's Hospital Boston and the Brigham & Women's Hospital.
Methods
Data were reviewed for all fetuses that underwent attempted FCI from 2000 (first case) through 2008. According to the FCI protocol, every fetus received a comprehensive pre- and postoperative cardiac and cerebral ultrasound evaluation, approximately 24 h prior to and 24 h after the procedure. This included color imaging of the DA flow and Doppler assessment of the umbilical artery (UA) and vein, ductus venosus, and, routinely since 2004, the middle cerebral artery (MCA). UA and MCA Doppler spectra were traced and the maximum (systolic), minimum (end-diastolic), and mean velocities measured; the pulsatility index was calculated as: PI = (systolic velocity − diastolic velocity)/mean velocity.
FCI was performed using a percutaneous approach or with a limited laparotomy without hysterotomy, as previously reported [16, 18, 19]. By protocol, mothers of fetuses <32 weeks gestational age received rectal indomethacin 50 mg preoperatively followed by 3 oral doses of 25 mg every 8 h starting immediately after the procedure.
Due to the rarity of DA constriction, no statistical analysis was performed and data are reported in descriptive fashion. Data are presented as median and range.
Results
From 2000 to 2008, 113 fetuses underwent attempted FCI. This included 70 fetuses with severe aortic stenosis and evolving HLHS in which balloon dilation of the aortic valve was performed at a median age of 23 weeks (20–32 weeks), 22 fetuses with HLHS and intact or restrictive atrial septum in which balloon dilation or stenting of the atrial septum was performed at a median age of 29 weeks (23–34 weeks), 11 fetuses with pulmonary atresia and intact ventricular septum in which pulmonary valve opening and dilation was performed at a median age of 24 weeks (21–28 weeks) [16, 17, 18, 19, 20], and 9 fetuses with hydrops in which aortic valve and/or atrial septal dilation was performed at a median of 29 weeks (23–32 weeks). Overall, 24 fetuses underwent a FCI at greater than 28 0/7 weeks: 10 for HLHS with intact atrial septum, 7 for aortic stenosis with evolving HLHS, and 7 for hydrops and structural heart disease. In 3 fetuses ≥32 0/7 weeks gestation at the time of intervention, indomethacin was not used.
Altogether, we identified 2 cases of DA constriction resulting from indomethacin given for tocolysis during the first 24 h after FCI, both after atrial septoplasty for HLHS with an intact or highly restrictive atrial septum in fetuses that were 30 weeks' gestational age at the time of intervention. One mother was an 18-year-old gravida 1 who was carrying a fetus with HLHS, aortic atresia, and a restrictive atrial septal defect. The other mother was a 21-year-old gravida 1 who was carrying a fetus with HLHS, aortic atresia, an intact atrial septum, and an obstructed levoatrial-cardinal vein. In both cases, FCI was technically successful and the atrial septum was opened, in one case with balloon dilation alone and in the other with atrial septal stenting [16, 17]. In both cases, the mother and fetus tolerated the procedure well. Both mothers received rectal indomethacin according to our protocol (50 mg preoperatively followed by 3 oral doses of 25 mg every 8 h starting immediately after the procedure).
In both cases, fetal echocardiograms performed by protocol within 24 h after the procedure revealed abnormal MCA and UA Doppler flow patterns (table 1, fig. 1, 2). MCA and UA maximum and mean velocities were depressed, indicative of decreased flow and minimum diastolic velocities were unchanged or elevated, resulting in substantial reduction in the pulsatility index (fig. 3). Umbilical venous mean velocities were unchanged, but flow reversal in the ductus venosus was observed in 1 case. There was mild right ventricular dysfunction in both fetuses, with mild (n = 1) or moderate (n = 1) tricuspid valve regurgitation. Neither fetus had evidence of hydrops. On further evaluation, the DA was noted to be anatomically narrowed with prominent color Doppler aliasing indicating high Doppler flow velocity due to stenosis.
Table 1.
Doppler flow data prior to and following FCI in 2 fetuses with DA constriction
| Variable | Patient 1 |
Patient 2 |
||||
|---|---|---|---|---|---|---|
| pre-intervention | 24 h postintervention | 48 h postintervention | pre-intervention | 24 h postintervention | 48 h postintervention | |
| DA diameter, mm | 3.5 | 2.0 | 3.5 | 4.3 | 2.1 | 3.8 |
| DA velocity, m/s | 1 | 3.2 | 1.5 | 1 | 2.6 | 1 |
| DA gradient, mm Hg | 4 | 41 | 9 | 4 | 27 | 4 |
| MCA velocity, cm/s | ||||||
| Maximum | 26.7 | 20.2 | 11.9 | 32.1 | 18.3 | 34 |
| Minimum | 7.8 | 10 | 4.8 | 6.9 | 5.3 | 6.1 |
| Mean | 14.8 | 13 | 7.57 | 15.2 | 11.1 | 16.9 |
| MCA pulsatility index | 1.28 | 0.77 | 0.94 | 1.66 | 1.17 | 1.65 |
| UA velocity, cm/s | ||||||
| Maximum | 29.4 | 14 | 32.4 | 50.8 | 48.1 | 54.1 |
| Minimum | 4.5 | 8 | 5.1 | 11.5 | 17.2 | 17.2 |
| Mean | 15.5 | 11.2 | 16.9 | 32.8 | 34.2 | 33.8 |
| UA pulsatility index | 1.61 | 0.54 | 1.6 | 1.2 | 0.9 | 1.09 |
Fig. 1.
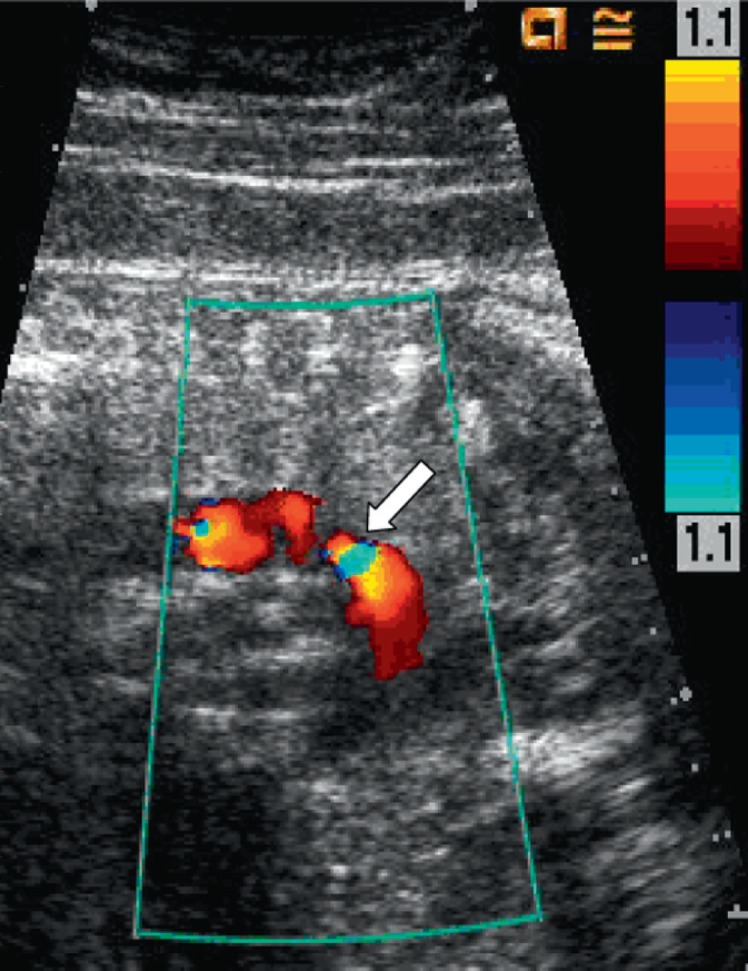
DA constriction with color Doppler aliasing (arrow)
Fig. 2.
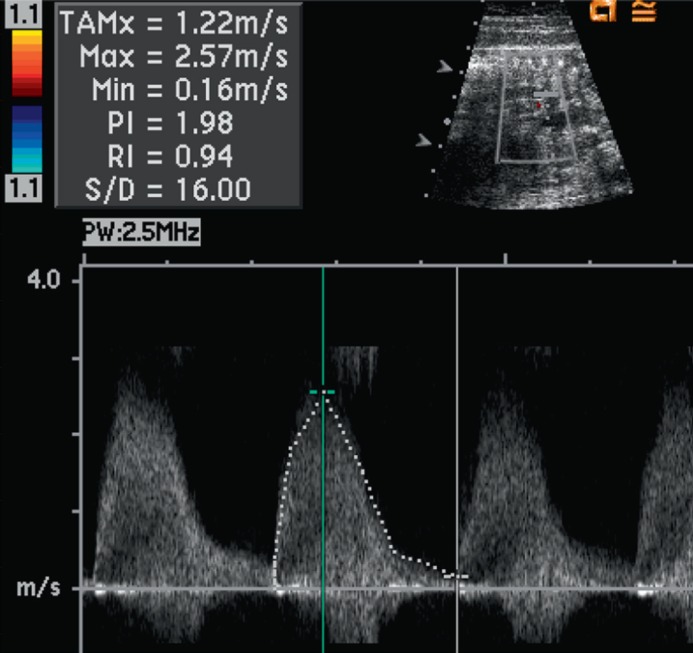
The pulse Doppler signal demonstrates high velocity flow across the DA, with a maximum velocity of 2.57 m/s (normal ductus arteriosus velocity is less than 1 m/s at this gestational age)
Fig. 3.
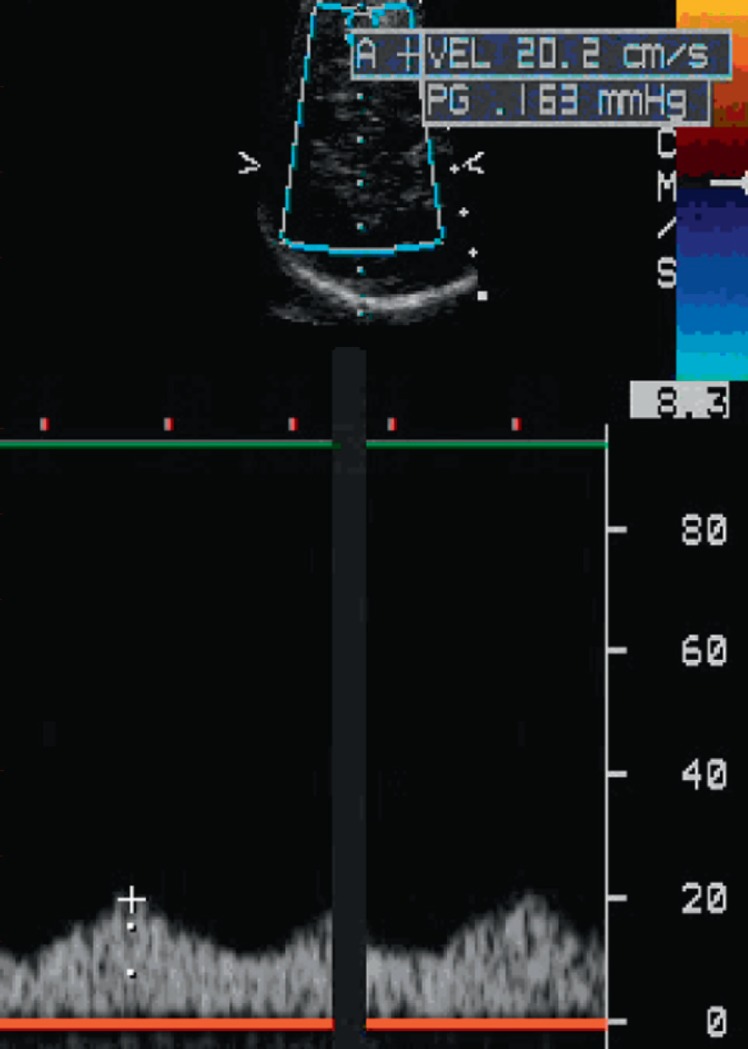
Reduced MCA systolic velocity and decreased pulsatility index of 0.77 in patient 1
The findings of DA constriction were assumed to be secondary to indomethacin and anticipated to be reversible based on previous reports [2, 7, 8, 10, 14, 15]. Both mothers were observed for a further 24 h, and repeat fetal echocardiograms were performed. No additional indomethacin was given. The repeat echocardiograms revealed resolution of the DA constriction and reversal of the Doppler flow patterns towards normal, with partial normalization of MCA and UA flow indices. Right ventricular dysfunction and tricuspid valve regurgitation resolved. The Doppler pattern normalized in the fetus with flow reversal in the ductus venosus. The 2 patients described in this report were liveborn, 1 prematurely at 34 weeks and the other electively at 38 weeks' gestational age. Both patients are currently alive and have undergone the second stage palliative surgery for HLHS.
Seven other fetuses with HLHS or evolving HLHS died within 24 h of FCI. Two of these were 30–31 weeks' gestation at intervention; the other 5 were <26 weeks. One of these patients died after delivery for persistent bradycardia, and in another the cause of death was confirmed by fetopsy to be the result of a massive hemopericardium. In the other 5 early fetal deaths (<24 h post procedure), demise occurred prior to the follow-up ultrasound and thus DA constriction was not definitively excluded.
Discussion
In this report, we describe 2 cases of reversible indomethacin-induced DA constriction after FCI for HLHS. Among more than 113 fetuses that have undergone FCI at our institution since 2000 [17, 18, 19, 20, 21], these were the only two in which ductal constriction was observed. Five other fetuses died within 24 h of FCI and did not have definitive exclusion of DA constriction; 2 of these were 30–31 weeks gestation at the time of intervention and the others were <26 weeks. Thus, this appears to be a relatively rare occurrence. Of note, the majority of FCI procedures at our center are performed in the late second trimester, and only 24 of 113 procedures were performed at beyond 28 0/7 weeks. The 2 fetuses that developed DA constriction were at the most mature end of the gestational age spectrum, which is consistent with reported data concerning the risk of indomethacin-induced DA constriction [23].
DA constriction in the fetus is well described, with the majority of cases occurring in one of two clinical situations: (1) in otherwise normal pregnancies where the mother has taken NSAIDs or the cause is unknown [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 24], and (2) in fetuses that have undergone fetal surgical procedures, where the DA has been shown to be reversible [25, 26]. What makes the DA constriction in these two patients particularly significant was their cardiac diagnosis. In HLHS with aortic atresia, the only source of cardiac output is the right ventricle, and all blood flow to the systemic circulation must pass through the DA. Thus, ductal constriction is potentially lethal. As observed in our patients, ductal constriction in HLHS can lead to right ventricular dysfunction, tricuspid regurgitation, and aberrations in peripheral Doppler flow patterns.
In the fetus, the MCA Doppler pattern is a particularly important marker of stress and overall diminished perfusion to the brain and through the umbilical artery, as it may reflect central redistribution in the setting of impaired cardiac output [27, 28, 29]. Baseline abnormalities of MCA flow have been documented in fetuses with HLHS [30, 31]. Although systemic flow cannot be measured in the presence of ductal constriction, it is likely reduced, which in turn can result in acidosis and potentially fetal demise. In our patients, we were faced with two management options. The first was to deliver a 30-week fetus with HLHS, with the attendant high risks of postnatal morbidity and mortality. The alternative was observation, with expected reversal of the ductal constriction, which we chose. Repeat fetal echocardiography 24 h after the diagnosis of ductal constriction revealed the DA to be widely patent with normalization of Doppler indices and cardiac function. Prematurity and associated premature rupture of membranes remain significant limitations to open and fetoscopic fetal intervention [32], although this has not been a major issue in percutaneous FCI such as the procedures performed in these cases. The incidence of DA constriction after the use of indomethacin may be higher in fetuses with more advanced gestational age, but indomethacin remains very effective in achieving uterine quiescence following direct uterine puncture [2, 3, 10, 32]. When considering the use of indomethacin for tocolysis following fetal interventions, it is important to weigh the risks of prematurity against the benefits of utilizing an effective agent for which side effects appear to be reversible.
This is particularly important in older fetuses, such as those treated for HLHS with an intact atrial septum in our experience. In our FCI protocol, indomethacin is administered to mothers of fetuses up to 32 weeks, beyond which the risk of DA constriction is significantly increased [23].
Conclusion
Fetal DA constriction is an important and serious complication of NSAIDs. DA constriction is reversible after discontinuation of the medication. Close echocardiographic monitoring of fetal Doppler flow velocities is extremely important after fetal intervention and administration of NSAIDs. Although DA constriction is an uncommon complication of indomethacin after FCI, the consequences of DA constriction in a fetus with HLHS are potentially lethal. Thus, in evaluating fetuses that have undergone FCI for HLHS, clinicians should be cognizant of the potential for DA constriction and the sonographic findings of a substantially depressed pulsatility index in both the MCA and UA.References
References
- 1.Takami T, Yoda H, Kawakami T, Yamamura H, Nakanishi T, Nakazawa M, Takei Y, Miyajima T, Hoshika A. Usefulness of indomethacin for patent ductus arteriosus in full-term infants. Pediatr Cardiol. 2007;28:46–50. doi: 10.1007/s00246-006-1426-9. [DOI] [PubMed] [Google Scholar]
- 2.Vermillion ST, Newman RR. Recent indometacin tocolysis is not associated with neonatal complications in preterm infants. Am J Obstet Gynecol. 1999;181:1083–1086. doi: 10.1016/s0002-9378(99)70085-2. [DOI] [PubMed] [Google Scholar]
- 3.Norton ME, Merrill J, Cooper BA, Kuller JA, Clyman RI. Neonatal complications after the administration of indometacin for preterm labour. N Engl J Med. 1993;329:1602–1607. doi: 10.1056/NEJM199311253292202. [DOI] [PubMed] [Google Scholar]
- 4.Souter D, Harding J, McCowan L, O'Donnell C, McLeay E, Baxendale H. Antenatal indometacin-adverse fetal effects confirmed. Aust NZ J Obstet Gynecol. 1998;38:357–358. doi: 10.1111/j.1479-828x.1998.tb02949.x. [DOI] [PubMed] [Google Scholar]
- 5.Hammerman C, Glaser J, Kaplan M, Schimmel MS, Ferber B, Eidelman AI. Indometacin tocolysis increases postnatal patent ductus arteriosus severity. Pediatrics. 1998;102:1202–1203. doi: 10.1542/peds.102.5.e56. [DOI] [PubMed] [Google Scholar]
- 6.Eronen M, Pesonen E, Kurki T, Teramo K, Ylikorkala O, Hallman M. Increased incidence of bronchopulmonary dysplasia after antenatal administration of indometacin to prevent preterm labor. J Pediatr. 1994;124:782–788. doi: 10.1016/s0022-3476(05)81374-5. [DOI] [PubMed] [Google Scholar]
- 7.Suarez VR, Thompson LL, Jain V, Olson GL, Hankins GDV, Belfort A, Saade GR. The effect of in utero exposure to indometacin on the need for surgical closure of a patent ductus arteriosus in the neonate. Am J Obstet Gynecol. 2002;187:886–888. doi: 10.1067/mob.2002.127464. [DOI] [PubMed] [Google Scholar]
- 8.Vermillion ST, Scardo JA, Lashus AG, Wiles HB. The effect of indometacin tocolysis on fetal ductus arteriosus constriction with advancing gestational age. Am J Obstet Gynecol. 1997;177:256–261. doi: 10.1016/s0002-9378(97)70184-4. [DOI] [PubMed] [Google Scholar]
- 9.Van Den Veyver IB, Moise KJ., Jr Prostaglandin synthetase inhibitors in pregnancy. Obstet Gynecol Surv. 1993;48:493–502. doi: 10.1097/00006254-199307000-00026. [DOI] [PubMed] [Google Scholar]
- 10.Moise KJ. Effect of advancing gestational age on the frequency of fetal ductal constriction in association with maternal indometacin use. Am J Obstet Gynecol. 1993;168:1350–1353. doi: 10.1016/s0002-9378(11)90763-7. [DOI] [PubMed] [Google Scholar]
- 11.Ojala R, Ikonen S, Tammela O. Perinatal indomethacin treatment and neonatal complications in preterm infants. Eur J Pediatr. 2000;159:153–155. doi: 10.1007/s004310050040. [DOI] [PubMed] [Google Scholar]
- 12.Loe SM, Sanchez-Ramos L, Kaunitz AM. Assessing the neonatal safety of indomethacin tocolysis: a systematic review with meta-analysis. Obstet Gynecol. 2005;106:173–179. doi: 10.1097/01.AOG.0000168622.56478.df. [DOI] [PubMed] [Google Scholar]
- 13.Cordero L, Nankervis CA, Gardner D, Giannone PJ. The effects of indomethacin tocolysis on the postnatal response of the ductus arteriosus to indomethacin in extremely low birth weight infants. J Perinatol. 2007;27:22–27. doi: 10.1038/sj.jp.7211612. [DOI] [PubMed] [Google Scholar]
- 14.Respondek M, Weil SR, Huhta JC. Fetal echocardiography during indomethacin treatment. Ultrasound Obstet. Gynecol. 1995;5:85–89. doi: 10.1046/j.1469-0705.1995.05020086.x. [DOI] [PubMed] [Google Scholar]
- 15.Takizawa T, Ikeda Y, Togashi H, Yamamoto M, Arishima K, Akahori F, Masaoka T. Inhibitory effect of indomethacin on neonatal lung catabolism of prostaglandin E2: possible mechanism of the reopening of the ductus arteriosus after indomethacin therapy. J Toxicol Sci. 1996;21:243–248. doi: 10.2131/jts.21.4_243. [DOI] [PubMed] [Google Scholar]
- 16.Marshall AC, Van der Velde ME, Tworetzky W, Gomez CA, Wilkins-Haug L, Benson CB, Jennings RW, Lock JE. Creation of an atrial septal defect in utero for fetuses with hypoplastic left heart syndrome and intact or highly restrictive atrial septum. Circulation. 2004;20(110):253–258. doi: 10.1161/01.CIR.0000135471.17922.17. [DOI] [PubMed] [Google Scholar]
- 17.Marshall AC, Levine J, Morash D, Silva V, Lock JE, Benson CB, Wilkins-Haug LE, McElhinney DB, Tworetzky W. Results of in utero atrial septoplasty in fetuses with hypoplastic left heart syndrome. Prenat Diagn. 2008;28:1023–1028. doi: 10.1002/pd.2114. [DOI] [PubMed] [Google Scholar]
- 18.Tworetzky W, McElhinney DB, Marx GR, Benson CB, Brusseau R, Morash D, Louise E, Wilkins-Haug LE, Lock JE, Marshall AC. In utero valvuloplasty for pulmonary valve atresia with hypoplastic right ventricle: techniques and outcomes. Pediatrics. 2009 doi: 10.1542/peds.2008-2014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tworetzky W, Wilkins-Haug L, Jennings RW, van der Velde ME, Marshall AC, Marx GR, Colan SD, Benson CB, Lock JE, Perry SB. Balloon dilation of severe aortic stenosis in the fetus: potential for prevention of hypoplastic left heart syndrome: candidate selection, technique, and results of successful intervention. Circulation. 2004;110:2125–2131. doi: 10.1161/01.CIR.0000144357.29279.54. [DOI] [PubMed] [Google Scholar]
- 20.Mirzrahi-Arnaud A, Tworetzky W, Bulich LA, Wilkins-Haug LE, Marshall AC, Benson CB, Lock JE, McElhinney DB. Pathophysiology, management, and outcome of fetal hemodynamic instability during prenatal cardiac intervention. Pediatr Res. 2007;62:325–330. doi: 10.1203/PDR.0b013e318123fd3a. [DOI] [PubMed] [Google Scholar]
- 21.McElhinney DB, Marshall AC, Wilkins-Haug LE, Brown DW, Benson CB, Silva V, Marx GR, Mizrahi-Arnaud A, Lock JE, Tworetzky W. Predictors of technical success and postnatal biventricular outcome after in utero aortic valvuloplasty for aortic stenosis with evolving hypoplastic left heart syndrome. Circulation. 2009;120:1482–1490. doi: 10.1161/CIRCULATIONAHA.109.848994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirzrahi-Arnaud A, Wilkins-Haug LE, Marshall A, Silva V. Maternal mirror syndrome after in utero aortic valve dilation: a case report. Fetal Diagn Ther. 2006;21:439–443. doi: 10.1159/000093887. [DOI] [PubMed] [Google Scholar]
- 23.Rasanen J, Debbs RH, Wood DC, Weiner S, Weil SR, Huhta JC. Human fetal right ventricular ejection force under abnormal loading conditions during the second half of pregnancy. Ultrasound Obstet Gynecol. 1997;10:325–332. doi: 10.1046/j.1469-0705.1997.10050325.x. [DOI] [PubMed] [Google Scholar]
- 24.Soslow JH, Friedberg MK, Silverman NH. Idiopathic premature closure of the ductus arteriosus: an indication for early delivery. Echocardiography. 2008;25:650–652. doi: 10.1111/j.1540-8175.2008.00667.x. [DOI] [PubMed] [Google Scholar]
- 25.Donofrio MT, Bremer YA, Moskowitz WB. Diagnosis and Management of restricted or closed foramen ovale in fetuses with congenital heart disease. Am J Cardiol. 2004;94:1348–1351. doi: 10.1016/j.amjcard.2004.07.133. [DOI] [PubMed] [Google Scholar]
- 26.Donofrio MT. Images in cardiovascular medicine: premature closure of the foramen ovale and ductus arteriosus in a fetus with transposition of the great arteries. Circulation. 2002;105:65–66. doi: 10.1161/hc1102.105232. [DOI] [PubMed] [Google Scholar]
- 27.Larsen LU, Sloth E, Petersen OB, Pedersen TF, Sorensen K, Uldbjerg N. Systolic myocardial velocity alterations in the growth-restricted fetus with cerebroplacental redistribution. Ultrasound Obstet Gynecol. 2009;34:62–67. doi: 10.1002/uog.6375. [DOI] [PubMed] [Google Scholar]
- 28.Turan OM, Turan S, Gungor S, Berg C, Moyano D, Gembruch U, Nicolaides KH, Harman CR, Baschat AA. Progession of Doppler abnormalities in intrauterine growth restriction. Ultrasound Obstet Gynecol. 2008;32:160–167. doi: 10.1002/uog.5386. [DOI] [PubMed] [Google Scholar]
- 29.Mari G, Hanif F, Kruger M, Cosmi E, Santolava-Forgas J, Treadwell MC. Middle cerebral artery peak systolic velocity: a new Doppler parameter in the assessment of growth-restricted fetuses. Ultrasound Obstet Gynecol. 2007;29:310–316. doi: 10.1002/uog.3953. [DOI] [PubMed] [Google Scholar]
- 30.Kaltman JR, Di H, Tian Z, Rychik J. Impact of congenital heart disease on cerebrovascular blood flow dynamics in the fetus. Ultrasound Obstet Gynecol. 2005;25:32–36. doi: 10.1002/uog.1785. [DOI] [PubMed] [Google Scholar]
- 31.McElhinney DB, Benson CB, Brown DW, Wilkins-Haug LE, Marshall AC, Zaccagnini L, Tworetzky W. Cerebral blood flow characteristics and biometry in fetuses undergoing prenatal intervention for aortic stenosis with evolving hypoplastic left heart syndrome. Ultrasound Med Biol. 2009 doi: 10.1016/j.ultrasmedbio.2009.09.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pomini F, Noia G, Mancuso S. Hypothetical role of prostaglandins in the onset of preterm labor after fetal surgery. Fetal Diagn Ther. 2007;22:94–99. doi: 10.1159/000097104. [DOI] [PubMed] [Google Scholar]