ABSTRACT
Intestinal parasites affect millions of children globally. We aimed to assess effects of deworming children on nutritional and cognitive outcomes across potential effect modifiers using individual participant data (IPD). We searched multiple databases to 27 March 2018, grey literature, and other sources. We included randomised and quasi randomised trials of deworming compared to placebo or other nutritional interventions with data on baseline infection. We used a random-effects network meta-analysis with IPD and assessed overall quality, following a pre-specified protocol. We received IPD from 19 trials of STH deworming. Overall risk of bias was low. There were no statistically significant subgroup effects across age, sex, nutritional status or infection intensity for each type of STH. These analyses showed that children with moderate or heavy intensity infections, deworming for STH may increase weight gain (very low certainty). The added value of this review is an exploration of effects on growth and cognition in children with moderate to heavy infections as well as replicating prior systematic review results of small effects at the population level. Policy implications are that complementary public health strategies need to be assessed and considered to achieve growth and cognition benefits for children in helminth endemic areas.
KEYWORDS: Deworming, network meta-analysis, systematic review, individual participant data
Introduction
In 2014, over 800 million people were infected with soil-transmitted helminths (STH) and water-borne schistosomes (Pullan et al. 2014). Mass deworming has been described as one of the most cost-effective development interventions (Bundy et al. 2018). The WHO 2017 guidelines recommend annual or biannual mass deworming for children, to be accompanied with hygiene and water interventions (World Health Organization 2017).
Two recent systematic reviews on mass deworming for soil-transmitted helminths concluded there was little to no effect on nutritional status, cognition or school attendance (Taylor‐Robinson et al. 2015; Welch et al. 2016). These reviews did not find evidence of subgroup effects across the intensity of infection, prevalence, nutritional status or sex. In contrast, a different meta-analysis found larger effects for weight in areas with a higher prevalence of STH infections (Croke et al. 2016).
These previous assessments of effect modification assessed the relationship between the effects of deworming to study-level average infection prevalence, which may be confounded by other study-level characteristics (e.g. food security, other NGO programmes). The question of whether deworming has greater effects on child nutritional and cognitive outcomes in areas with higher prevalence and intensity of infections is important for policy to focus deworming where it is most needed and likely to have the largest impact.
We aimed to explore the importance of effect modifiers using individual participant data (IPD). IPD allows all outcomes to be converted to a common metric, allows adjustment for potential confounders and provides better power to assess subgroup effects (Dagne et al. 2016). Because concurrent interventions are often given in studies of deworming, we used a network meta-analysis approach which has greater power to compare different treatments which may not have been directly compared.
Methods
We conducted a systematic review and network meta-analysis of individual participant data (IPD). Network meta-analysis (NMA) is an approach which compares effects of multiple interventions using evidence from trials which have directly compared them as well as estimating indirect effects for interventions which have not been compared to each other in a trial (Salanti 2012). NMA has been described as the ‘next generation’ of evidence synthesis because it increases the precision of effect estimates for direct comparisons and allows comparisons of interventions which may not have been directly compared (Salanti 2012). The use of IPD further strengthens the approach leading to decreases in heterogeneity within comparisons and improving overall network consistency because it accounts for effect modification at the individual participant level and missing data can be addressed (Debray et al. 2018).
We report results according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) extensions for network meta-analysis (NMA) (Hutton et al. 2015) and IPD (Stewart et al. 2015). The protocol was published with the Campbell Collaboration (Welch et al. 2018). Deviations from the protocol are described in Appendix S1.
Search strategy and selection criteria
We included randomised and quasi-randomised controlled trials of deworming for STH (including Ascaris lumbricoides, Trichuris trichiura and hookworm) or schistosomiasis in children 0.5 years to 16 years of age, with a duration of four months or more. We included studies of mass deworming as well as those which screened for infection. We included nutritional co-interventions and comparisons with other deworming or nutritional interventions. We excluded studies with less than 100 participants since the Advisory Board and the team felt the additional effort to trace these studies would have a limited contribution to the evidence network. We excluded studies that did not measure baseline infection intensity of STH.
We searched up to 27 March 2018 in MEDLINE, CINAHL, LILACS, EMBASE, the Cochrane Library, Econlit, Internet Documents in Economics Access Service, Public Affairs Information Service, Social Services Abstracts, Global Health CABI and CAB Abstracts (Appendix S2). We searched grey literature, websites, asked experts, contacted authors and screened 299 included studies from relevant systematic reviews. There were no language limits.
Two independent reviewers screened titles and abstracts. Conflicts were discussed and resolved with VW or MG. All potentially eligible studies were retrieved and screened in duplicate in full text.
The first authors of all eligible studies were invited to join the Trialists’ Collaborative in December 2016. In case of no reply, we contacted all authors and their institutions. Data collection was closed in March 2018. Authors signed a data-sharing agreement. IPD were checked for completeness. We verified any questions with the authors. Data were prepared in an excel spreadsheet which included outcomes, covariates and study design variables. We replicated the baseline and end of study analyses and calculated the standardised mean difference between the publication and our replication (Austin 2009). To assess the influence of studies which we were unable to retrieve, we compared average findings for weight gain using aggregate data from our prior review (Welch et al. 2016).
Data analysis
Two independent reviewers collected details on participants, interventions, outcomes, study design, setting and duration (Table 1) using a pre-tested form.
Table 1.
Characteristics of included studies.
| Study | Country | Design | Type of Cluster (number of clusters) | Sample size (children per cluster) | Age range (years) Recruitment |
Interventions | Frequency of deworming, Study duration | Compliance with STH deworming |
|---|---|---|---|---|---|---|---|---|
| Beasley 1999 | Tanzania | RCT SAT |
357 | 7 to 12 community |
1. Albendazole + Praziquantel 2. Placebo |
5 months, 5 months |
Not reported | |
| Beasley Tanbase 1999 (thesis) |
Tanzania | RCT SAT |
217 | 6 to 15 community |
1. Albendazole + Praziquantel + iron, 2. Placebo |
4 months, 4 months |
100% (full course of 10 doses of iron given on a daily basis was received over 4 weeks due to absenteeism). | |
| Ebenezer 2013 | Sri Lanka | cRCT MDA |
Schools (100) |
1570 (20) |
8 to 15 schools |
1. Mebendazole + iron tablet 2. Placebo |
6 months, 6 months |
94–98% with deworming, 80% with iron tablets |
| Friis 2003 | Kenya | RCT MDA |
915 | 8 to 18 schools |
1. Albendazole + praziquantel 2. Albendazole + praziquantel + micronutrient tablet 3. micronutrient tablet 4. Placebo |
8 months, 8 months |
Not reported | |
| Huong 2007 | Vietnam | RCT MDA |
426 | 6 to 9 schools |
1. Mebendazole + unfortified noodles 2. Mebendazole + iron fortified noodles 3. Mebendazole + iron tablet 4. iron-fortified noodles 5. Placebo + unfortified noodles |
3 months, 6 months |
93–99% consumed noodles and iron tablets | |
| Liu 2017 | China | cRCT MDA |
Townships (112) | 2179 (20) |
9 to 12 Town-ships |
1. Albendazole 2. Control (no deworming) |
6 months, 12 months |
52% fully compliant, 76% took at least one out of two albendazole pills in both rounds |
| Ndibazza 2013 | Uganda | RCT MDA |
2016 | 1 to 2 community |
1. Albendazole 2. Placebo |
3 months, 45 months |
19–22% received all 16 doses (over 5 years) | |
| Nga 2009 | Vietnam | RCT MDA |
510 | 6 to 8 schools |
1. Albendazole + unfortified biscuit 2. Albendazole + micronutrient fortified biscuit 3. micronutrient fortified biscuit 4. Placebo + unfortified biscuit |
4 months, 4 months |
94% compliant with consuming biscuits | |
| Olds 1999 | Kenya (note: data from Kenya site, not from China or Philippines sites) |
RCT MDA |
371 | 6 to 17 villages |
1. Albendazole 2. Praziquantel 3. Albendazole + Praziquantel 4. Placebo |
6 months, 12 months |
Not reported | |
| Rohner 2010 | Côte d’Ivoire | RCT MDA |
311 | 6 to 14 schools |
1. Iron fortified biscuits 2. Albendazole + Praziquantel 3. IPT 4. Albendazole + Praziquantel + Iron fortified biscuits 5. Albendazole + Praziquantel + IPT 6. IPT + Iron fortified biscuits 7. Albendazole + Praziquantel + IF + IPT 8. Placebo + unfortified biscuits |
3 months, 6 months |
94% for anthelminthics | |
| Solon 2003 | Philippines | RCT MDA |
831 | 6 to 17 schools |
1. Albendazole + unfortified beverage 2. Albendazole + micronutrient fortified beverage 3. micronutrient fortified beverage 4. Placebo + unfortified beverage |
4 months, 4 months |
Not reported | |
| Stoltzfus 1997 | Tanzania | cRCT MDA |
Schools (12) |
4034 | 5 to 19 schools |
1. Mebendazole (2/year) 2. Mebendazole (3/year) 3. Placebo |
6 months (1) 4 months (2), 12 months |
Not reported |
| Stoltzfus 2004 | Tanzania | cRCT MDA |
House-holds (451) | 463 | 0.5 to 6 House-holds |
1. Mebendazole (high) 2. Mebendazole (high) + iron supplement 3. iron supplement 4. Placebo |
3 months, 12 months |
Not reported |
| Yap 2014 | China | RCT SAT |
194 | 9 to 12 schools |
1. Albendazole 2. Placebo |
6 months, 6 months |
92%, field investigators directly observed consumption | |
| Kirwan 2009 | Nigeria | RCT MDA |
1367 | 1 to 4 Pre-school in villages |
1. Albendazole 2. Placebo |
4 months, 14 months |
Not reported. Consumption was directly observed | |
| Hall 2006 | Vietnam | cRCT MDA |
Schools (80) |
2916 | 8 to 9 schools |
1. Albendazole + vitamin A 2. Placebo + vitamin A |
6 months, 12 months |
Not reported |
| Miguel 2004 | Kenya | cRCT MDA |
Schools (75) |
15881 | 6 to 18 schools |
1. Albendazole 2. Albendazole + Praziquantel 3. Control |
6 months, 3 years |
96% |
| Rousham 1994 | Bangladesh | cRCT MDA |
Villages (7) |
124 | 2 to 6 villages |
1. Mebendazole 2. Placebo |
2 months, 12 months |
Not reported |
| Wiria 2013* | Indonesia | cRCT MDA |
House-holds (954) | 1854 | 2 to 16 villages |
1. Albendazole 2. Placebo |
3 months, 21 months |
78% with antheminthic |
MDA: Mass drug administration, SAT: Screen and Treat, cRCT: cluster randomised trial, RCT: randomised controlled trial.
References of included studies in Appendix S3 Additional References in Supporting Information.
*For Wiria 2013, Of 4004 people randomized, only 1854 met our age range (6 months to 16 years), and of those 1854, only 738 had baseline data for weight and height.
The primary outcomes were weight (kilograms), height (centimetres), haemoglobin (g/L) and cognitive outcomes. We chose to use weight and height in kg and cm, respectively, because these would have the greatest precision to detect differences. To adjust for differences in the importance of a given weight and height change for different ages, sex, anaemia and nutritional status, we adjusted for these factors in our model, as well as infection intensity of each STH. We did not include harms since these have been assessed in previous reviews and are not considered serious (World Health Organization 2017). We intended to assess plasma ferritin but only six studies reported this outcome, thus we decided not to conduct these analyses which the advisory board felt could be misleading.
We used multiple imputations for missing data, based on the assumption that data are missing at random (Debray et al. 2018; Groenwold, Moons, and Vandenbroucke 2014; Jakobsen et al. 2017) For studies with less than 50% missing data, we used multiple imputation for baseline and endline and created five complete datasets using Proc MI in SAS9.4/STAT (SAS Institute Inc., Cary, NC, USA). All model estimates and standard errors were obtained by fitting the model to each of these five imputed datasets and aggregating results across them using Rubin’s Rule which incorporates uncertainty due to imputation. Proc MIANALYZE in SAS 9.4/STAT was used to obtain the overall estimates across imputed datasets. We used WHO Anthro 3.2.2 to calculate BMI-for-age, and height-for-age z-scores. Anaemia was defined according to age and sex using WHO standards and corrected for altitude, if needed (World Health Organization 2011).
Risk of bias was appraised by outcome with the Cochrane Risk of Bias tool in duplicate. A funnel plot was constructed for comparisons with more than 10 studies.
We used a frequentist approach for random-effects NMA. The covariates were identified with the advisory group: age, sex, baseline weight, height, haemoglobin, and infection intensity. Random effect General Linear Mixed Models (GLMM) were conducted with two random intercepts considered in the model: random effect ‘trial’ accounts for the response variables of patients within a given trial being correlated; and random effect ‘Patient’s clusters’ which accounts for the correlation of responses between any patients from the same clusters (such as villages, schools or households) within a given trial. We expected a connected network of trials to allow direct and indirect comparisons based on our previous review (Welch et al. 2016). We used the GLIMMIX procedure in SAS 9.4/STAT (SAS Institute Inc., Cary, NC, USA) for the GLMM NMA, considering models that account for multi-arm trials and adjust for covariates. Results are summarised as point estimates with 95% confidence intervals for the outcomes of weight (kg), height (cm), and haemoglobin (g/L). Data were analysed as intention to treat.
The transitivity assumption was assessed by comparing the distribution of effect modifiers for each comparison (Salanti 2012). The evidence network was designed with the advisory board, research team and trial authors based on clinical judgement and other evidence. The consistency assumption was assessed by evaluating the heterogeneity of direct comparisons (using visual inspection of forest plots and I2) (Higgins and Thompson 2002) and comparing the effect estimates for direct evidence (aggregate), direct evidence (IPD) and indirect + direct evidence (NMA).
We planned subgroup analyses across a priori defined variables of individual-level baseline intensity of infection with A. lumbricoides, T. trichiura and hookworm, stunting, undernutrition, anaemia, age, sex, and socioeconomic status (Welch et al. 2018).
We conducted planned sensitivity analyses restricting to studies with low risk of bias for allocation concealment and without imputation (i.e. complete case analysis).
We developed a summary of findings table for STH deworming vs. placebo, and assessed GRADE certainty for each outcome (Puhan et al. 2014).
We met with our advisory board in November 2016, July 2017 and March 2018. Trial authors met twice to discuss results (November 2017, March 2018). This study was approved by the Bruyère Research Institute and SickKids research ethics boards.
The evidence network and composition of the nodes were decided based on clinical and methodological reasons with our advisory board (Giovane et al. 2013).
Results
Search and study identification
We screened 14034 titles and abstracts. Three hundred and forty studies were screened in full-text. Fifty-five studies were considered eligible (Figure 1). Reasons for exclusion at full-text were not randomised or quasi-randomised trial (n = 262), no baseline infection intensity (n = 14) or too short (9 studies were <4 months duration) (Appendix S3).
Figure 1.
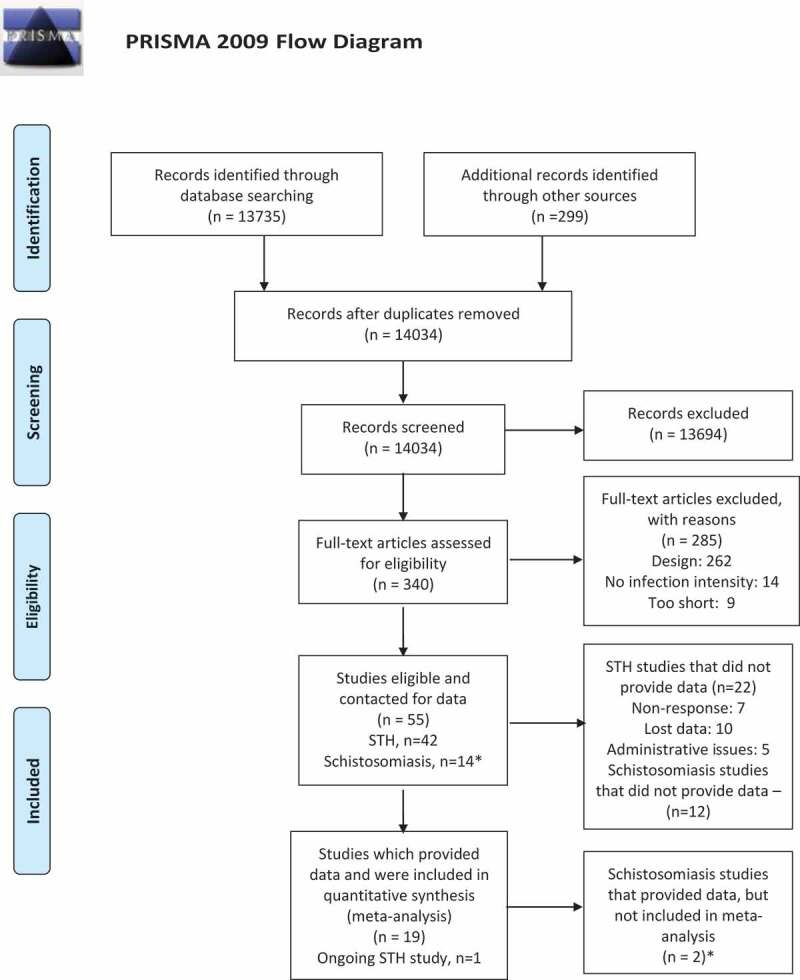
PRISMA Flow chart of searches up to 27 March 2018.
Number of STH studies and Schistosomiasis studies adds to 56 (not 55) since Olds 1999 study is counted as both STH deworming and schistosomiasis deworming because it is a factorial trial.
We obtained data from only two trials of deworming for schistosomiasis (2/14 studies). The advisory board decided not to analyse these since results would not be representative of the totality of the evidence.
We obtained data for 79% of children (31,945 of 40,525) randomised to eligible studies of STH deworming (19 of 42 eligible published trials). For the 22 studies that we could not retrieve, reasons for not providing data were non-response (n = 7 studies), lost data (n = 10 studies) and administrative issues (n = 5). For studies conducted before 2000, we received only 4 of 19 STH deworming studies (21%).
Comparison of studies retrieved with those not retrieved
We used aggregate data from our previous Campbell review to compare studies of deworming for STH vs. placebo for which we received data compared to those for which data were not received. The effects on weight gain were 0.02 kg (−0.04 to 0.08) for studies received vs. 0.17 kg (−0.11, 0.44) for those not received (interaction test for subgroup differences: p = 0.31). Studies for which we did not receive IPD had a larger pooled effect size on weight gain with a wider confidence interval.
Description of studies
The studies were conducted globally (Table 1; Figure 2; S1 Table). Children were a median of 10.8 years old at enrolment (interquartile range: 8.8 to 13.0) according to IPD, median study duration was 12 months (range 4 months to 45 months), median frequency of deworming was every 4 months (range: 2 to 8 months), and 7 out of 19 studies gave a single dose of deworming.
Figure 2.
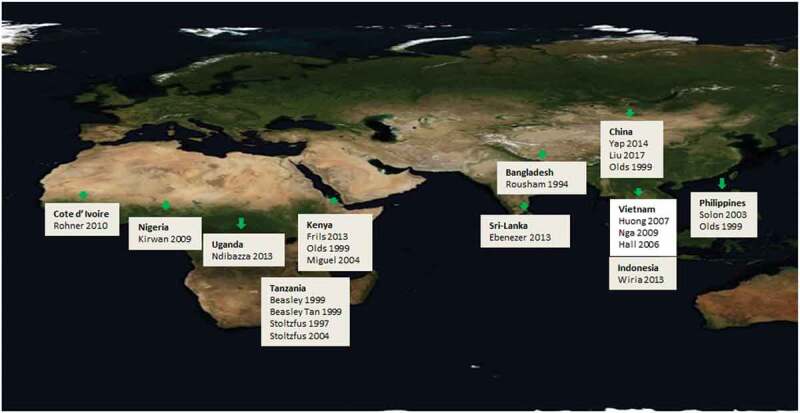
Countries where included studies were conducted.
*Olds 1999 is a single study which was carried out in three sites (Kenya, China and the Philippines); we only received IPD for the Kenya site. Figure source: https://pxhere.com/en/photo/1262215, CC0 Public Domain.
For nutritional status, 16% of the children were below −2 z-scores for BMI-for-age, 33% were stunted and 50% were anaemic (Table 2). The prevalence of infection was 45% for A. lumbricoides (31% light, 13% moderate and 1% heavy infection intensity), 52% for T. trichiura (38% light, 14% moderate and 0% heavy) and 45% for hookworm (38% light, 5% moderate and 2% heavy infection intensity). For the studies which we received, five studies had greater than 50% missing data (Table S2).
Table 2.
Characteristics of children in the 14 studies used for the base case analyses (see footnote below table for abbreviations).
| Variable | Distribution | sample size |
|---|---|---|
| BMI-for-age | ≤ – 2 | 2259 |
| (z-score) | > −2 | 11696 |
| Height-for-age | ≤ −2 | 4609 |
| (z-score) | > −2 | 9346 |
| Hookworm | 0 (none) | 7700 |
| (epg) | 1–384 | 3169 |
| >384 | 3086 | |
| T. trichiura | 0 (none | 6729 |
| (epg) | 1–288 | 3656 |
| >288 | 3570 | |
| A. lumbricoides | 0 (none) | 7738 |
| (epg) | 1–1776 | 3116 |
| >1776 | 3101 | |
| Anyworm** | 0 | 4721 |
| 1 | 6047 | |
| 2 | 3187 | |
| Anaemia*** | No | 7300 |
| Yes | 6655 | |
| Age at time of treatment | < 5 years | 2448 |
| ≥5 years | 11507 | |
| Sex | Male | 7298 |
| Female | 6657 |
*epg: eggs per gram of stool.
**‘Anyworm’ is a variable indicating children with no detected STH infection of any type of STH, light intensity using WHO cut-offs for each type of STH, or moderate or heavy infection intensity for any type of STH.
***Anaemia cut-points defined on the basis of age and sex using WHO guidelines (World Health Organization 2011).
Risk of bias
The overall risk of bias was low. The risk of attrition bias was high in 7 of 19 studies (Figure 3). A funnel plot for STH deworming vs. placebo did not show evidence of publication bias. We obtained unpublished data on nutritional outcomes from 8 studies suggesting selective outcome reporting is an issue in this area.
Figure 3.
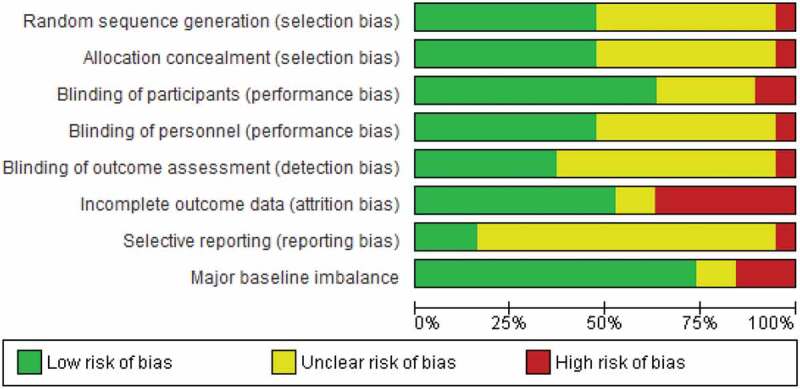
Risk of bias graph.
Evidence network
We decided on the evidence network of six nodes as the base case with our advisory board based on clinical and methodological reasons (Rouse, Chaimani, and Li 2017). This is described as a deviation from our protocol (Appendix S1) since the method for developing the evidence network was not described in our a priori protocol (Welch et al. 2018). We tested whether our findings were robust to using the full evidence network which consisted of 18 nodes for 19 RCTs (Figure S1). The network of six nodes grouped all deworming for STH (of any frequency) together, all micronutrients together and all deworming for schistosomiasis together. The six nodes were: 1) placebo, 2) STH deworming alone, 3) Deworming for schistosomiasis with or without STH deworming, 4) Deworming for schistosomiasis with any type of iron or micronutrient, 5) STH deworming with any type of micronutrient or iron, and 6) iron or micronutrient alone (Figure 4). We analysed and compared results for both the six-node and full evidence network.
Figure 4.
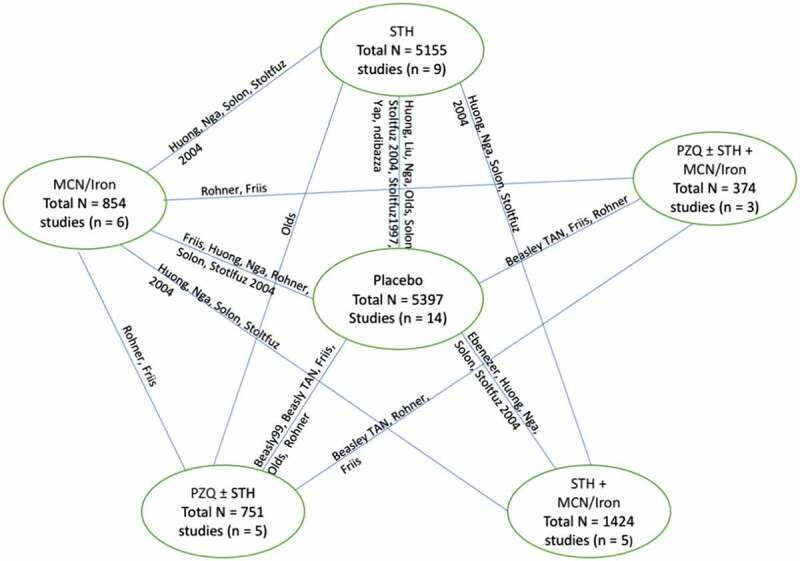
Evidence network, six nodes.
MCN: Micronutrients; PZQ: praziquantel; STH: Soil-transmitted helminths deworming.
Main analysis
In our IPD NMA evidence network, the results for the weight of STH deworming alone vs. placebo was 0.01 kg (95%CI: −0.08, 0.11) (for other comparisons, Figure 5). The effect on height for STH deworming alone vs. placebo was 0.09 cm (95%CI: −0.08 to 0.27) (see Figure 5). For haemoglobin, the effect of STH deworming alone vs. placebo was 0.32 g/L (95%CI: −0.63, 1.26). When deworming for STH was combined with deworming for schistosomiasis or iron/micronutrients, there were increases in haemoglobin vs. placebo. For STH and micronutrients vs. micronutrients alone, the effect was 0.70 g/L (95%CI: −0.72, 2.11; Figure 5). For cognition, studies were not pooled since the advisory board felt that measures were too different in linguistic translation and implementation (Table S3 for measures). For STH deworming alone vs. placebo, there were no clinically meaningful effects observed for short-term attention, school achievement or motor development. One study found improved short-term attention for micronutrient fortification and STH deworming compared to placebo(Nga et al. 2011). These cognition analyses were limited by a few participants.
Figure 5.:
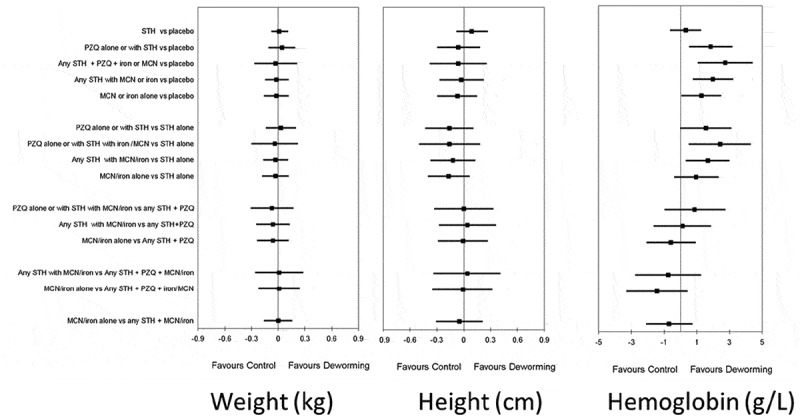
Main effects on weight (in kg), height (in cm) and haemoglobin (in g/L).
STH: soil-transmitted helminth deworming, PZQ: praziquantel; MCN: micronutrients in any form (supplements or fortified beverage or food); iron in any form (supplement or fortification).
The transitivity and consistency assumptions were considered plausible based on assessing heterogeneity, consistency of direct and indirect estimates, and distribution of covariates across each comparison (Table S4). GRADE quality was assessed as moderate for all outcomes for STH vs placebo, downgraded because we obtained only 46% of eligible studies (79% of randomised children).
Analysis of effects on infection prevalence showed variable effectiveness of the different combinations of STH deworming and schistosomiasis deworming on A. lumbricoides, T. trichiura and hookworm (Figures S2-S4).
Subgroup analyses and effect modification
We planned to use WHO cut-offs for the intensity of infection but there were too few children with moderate and high intensity STH infection (less than 13% for each STH) for the network meta-analysis model. Thus, we decided with the Advisory Board and Trialists Collaborative post-hoc to use two methods to assess the role of infection intensity: 1) subgroup analysis for direct evidence; and 2) NMA using lower cut-offs based on median infection intensity to allow assessment of the gradient of effect. For the analysis of subgroup effects using direct evidence from trials, for STH deworming compared to placebo, the effect for children with moderate or heavy intensity A. lumbricoides infections (WHO cut-offs) was 0.12 kg (95%CI: −0.05, 0.28); for moderate or heavy intensity T. trichiura infections, the effect was 0.11 kg (−0.14, 0.35) and for moderate or heavy intensity hookworm infections, the effect on weight was −0.53 kg (95%CI: −2.09, 1.03). No subgroup tests for interaction were statistically significant (see Figure S5–S13 for weight, height and haemoglobin across each type of STH). However, there were larger effects on weight gain for children with moderate or heavy intensity infections of A. lumbricoides and T. trichiura than children with light or no detected infection.
For the second approach, analysis using the IPD NMA evidence network, tests for interaction were not statistically significant for any subgroup effects for any comparisons across: age, sex, BMI-for-age, height-for-age, anaemia and intensity of any type of STH infection (using cut-offs for intensity of infection for each STH based on the median distribution). There was imprecision for these effect modification analyses across STH intensity. The 95% confidence intervals included potentially important effects of up to 460 g for weight and 7 g/L for haemoglobin for higher intensity infections of A. lumbricoides, T. trichiura or hookworm (defined according to the median of the distribution) for STH deworming vs. placebo (Table 3).
Table 3.
Effect modifier analysis across STH infection intensity for STH deworming vs. placebo.
| Level | Weight (kg) | Height (cm) | Haemoglobin (g/L) | |
|---|---|---|---|---|
| A. lumbricoides | No detected infection | 0.00(−0.11,0.12) | 0.07(−0.14,0.27) | 0.48(−0.69,1.66) |
| Lighter intensity (1–1776 epg) | 0.07(−0.13,0.27) | 0.25(−0.14,0.63) | −0.10(−1.75,1.55) | |
| Higher intensity (>1776 epg) | 0.08(−0.13,0.29) | 0.04(−0.22,0.30) | 0.03(−2.08,2.14) | |
| Hookworm | No detected infection | 0.02(−0.09,0.13) | 0.06(−0.13,0.26) | 0.07(−0.92,1.06) |
| Lighter intensity (1–384 epg) | 0.05(−0.12,0.23) | 0.20(−0.16,0.55) | −0.04(−1.84,1.77) | |
| Higher intensity (>384 epg) | 0.16(−0.13,0.46) | 0.20(−0.13,0.52) | 3.58(0.13,7.02) | |
| T. trichiura | No detected infection | −0.01(−0.12,0.10) | 0.02(−0.18,0.22) | 0.17(−1.01,1.35) |
| Lighter intensity (1–288 epg) | 0.04(−0.11,0.20) | 0.30(−0.04,0.64) | 0.24(−1.23,1.71) | |
| Higher intensity (>288 epg) | 0.17(−0.06,0.41) | 0.07(−0.20,0.34) | 1.33(−1.14,3.81) |
epg: eggs per gram.
Sensitivity analyses
The main effects for each outcome were robust to six sensitivity analyses: 1) unadjusted analysis, 2) complete case analysis with base case studies (n = 14), 3) complete case analysis with five additional studies that had >50% missing data, 4) studies at low risk of bias, 5) full evidence network with 18 nodes, and 6) restricting to studies with a larger effect on reducing STH infection prevalence.
Updating prior meta-analyses with new IPD
We decided post-hoc to compare our results with prior meta-analyses and update prior meta-analyses with previously unpublished data on weight. In comparison to the meta-analysis by Croke et al., when we add data from this IPD to the aggregate data from our previous Campbell review (Welch et al. 2016), our effect estimate for weight is comparable for both fixed and random-effects meta-analyses, though there are difference in the studies we included (Table S5). When we add all studies from Croke et al. to our prior meta-analysis with data from three previously unpublished studies on weight, the effect on weight is 0.10 kg (05%CI: 0.03 to 0.17). In comparison to two prior Cochrane (Taylor‐Robinson et al. 2015) and Campbell (Welch et al. 2016) reviews, our findings are similar, with a smaller effect size for weight and height (Table S6). We conducted post-hoc meta-regression analyses, using aggregate data from all studies included in our prior systematic review as well as previously unpublished data on weight from this review (Welch et al. 2016). There was no statistically significant effect of year of publication, T. trichiura or A. lumbricoides prevalence. Hookworm prevalence was positively associated with weight gain (p = 0.014; Figure 6).
Figure 6.
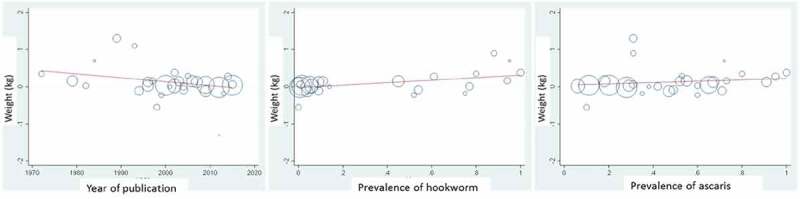
Meta-regression analyses using all STH deworming vs. placebo studies.
Uses aggregate data from all studies.
Discussion
A central issue of the debate about the effects of deworming on child nutritional and cognitive outcomes has been the difficulty in detecting effects from averages across populations where many children are uninfected or lightly infected and thus unlikely to benefit from deworming. Thus, the added value of our study is the assessment of effect modification using individual level data. Analysis of individual level data for children with moderate or heavy intensity infections shows that deworming may increase weight in children with moderate to heavy intensity infections of A. Lumbricoides or T. trichiura (but not hookworm) (very low certainty). These analyses did not show a statistically significant relationship for any other covariates for the effect of deworming on weight, height, haemoglobin or cognition in our network meta-analysis; covariates were age, anaemia, height for age, BMI for age and infection intensity for each type of STH according to the median of the distribution. These effects modification analyses are considered very low certainty since they are based on subgroup analyses and are imprecise. It is uncertain whether these findings would apply to children with heavy intensity infections since these represent less than 20% of the children in these analyses.
Secondly, our network meta-analysis approach allows the comparison of effects for deworming for STH when combined with micronutrients, iron or deworming for schistosomiasis. Combining STH deworming with micronutrients or iron was more effective than placebo for improving haemoglobin, but not more effective than micronutrients or iron alone. Combinations with praziquantel were also more effective at improving haemoglobin than STH deworming alone. There were little to no effects on short-term attention, school performance or development in individual studies, but these were limited by imprecision.
Thirdly, we updated previous meta-analyses by adding previously unpublished data obtained from authors for this review. These analyses reinforce the findings of population-level small effects on average for weight. This included three previously unpublished estimates for weight. This analysis replicated estimates of three prior systematic reviews of an average effect size on the weight of about 100 g and showed that this effect size was not related to the year of publication.
The findings are based on trials that have an overall low risk of bias, and main effects were robust to restricting to the lowest risk of bias studies. We considered the assumptions of transitivity and consistency for network meta-analysis were plausible based on our analyses.
In 2010, the prevalence of all STH infections was below 30% for all regions of the world for each STH which is somewhat lower than the average prevalence of 46% in our sample (Pullan et al. 2014). These averages obscure the fact that some areas may have very high prevalence and heavy intensity infections. We expect that the average effects of about 100 g on weight may apply to areas with similar infection prevalence and intensity levels to children in this analysis but cannot be assumed to apply to areas where the intensity of infection is much higher.
Croke et al. (2016) proposed that the prior Cochrane review was underpowered to detect effects which could be considered cost-effective. With 31,945 children and 19 studies, our current analysis has over 90% power to detect an average effect size of 0.1 kg in the presence of moderate heterogeneity for the comparison of STH deworming vs. placebo (Hedges and Pigott 2001; Tiebel 2008). Croke et al. argued that the average effect size they found of 0.13 kg was cost-effective based on dividing the cost of $0.60 for two annual treatments of deworming by the weight gain, equating to $4.48/kg which is far less than the comparable cost per kg of school meals of $112 to $252/kg (Galloway et al. 2009). We consider the average effect size, based on the totality of evidence, not just the IPD analysis, of 100 g, to be of small practical importance for the typical child in these deworming studies of 7–10 years of age, who gains approximately 2 kg per year based on WHO annual growth charts. For children with moderate or heavy intensity A. lumbricoides infections, the effect on weight was 0.12 kg (95%CI: −0.05 to 0.28) which includes effect sizes of up to 280 g which may be of importance to younger and more vulnerable children. This is smaller than the effects of other nutritional programmes such as school feeding which increases weight gain on average at a population level by about 0.39 kg annually (Kristjansson et al. 2007). Our systematic review cannot predict outcomes or cost-effectiveness for chemoprophylaxis where the intensity of infection is very heavy, since less than 2% of our sample had heavy intensity infections according to WHO cut-offs.
Strengths are a comprehensive search and transparent methodology described in an a priori protocol. We included an expert advisory board with expertise in nutrition, parasitology, economics and methodology and a trialists’ collaborative. We were able to obtain data from 79% of children randomised to eligible deworming studies. We accounted for differences in baseline nutritional status, age, sex, anaemia and infection intensity and also assessed possible effect modification across these factors. The results were robust to a range of sensitivity analyses including complete case analysis and restricting to studies at low risk of bias. We were able to include data from studies which may not have been designed or powered for these outcomes.
A limitation of our analyses is that we were unable to retrieve 22 out of 41 eligible studies (which included 8,580 children), despite, in most cases, the goodwill of the original trialists, due to inability to locate the data, administrative barriers to sharing data or lost datasets. Another limitation is that only 2% of children in these studies had heavy intensity infections defined according to WHO cut-offs, which limited our assessment of effects across intensity, in particular for children with heavy intensity infections. These analyses across the intensity of infection are limited by measurement issues since WHO cut-offs for intensity are arbitrary and their importance may vary according to different contexts. Also, infection intensity was assessed by different methods including Kato-Katz, PCR and other techniques which have different measurement properties. The median study duration of 12 months limits any inferences about long-term effects of mass deworming. Our analysis included 7 studies with a single dose of STH deworming which may have been influenced by re-infection in endemic areas. We were unable to adjust for socioeconomic status, water/hygiene environment, presence of schistosomiasis or malaria since there were insufficient details across studies.
Conclusions
For children with moderate or heavy intensity infections (using WHO cut-offs), deworming may have small effects on weight but not height or haemoglobin (very low certainty evidence). Effects of deworming are uncertain in children with heavy intensity infections. Based on the totality of evidence from three prior systematic reviews and new data from IPD previously unpublished, average effects of mass deworming on child nutritional status and cognition are small at the population level (moderate certainty), thus needs to be complemented by other public health measures. To maximise the societal benefit of research, we strongly support the Open Science movement to reduce limitations in data retrieval for analyses such as these which aim to improve the lives of the most vulnerable populations.
Biographies
Mr. Omar Dewidar is a research assistant at Bruyère Research Institute a graduate student at the School of Epidemiology and Public Health at the University of Ottawa Canada.
Dr Alison Elliot is professor of Tropical Medicine at the London School of Hygiene & Tropical Medicine, a Wellcome Trust Senior Fellow in Clinical Research and an honorary consultant in Tropical Medicine at the University College Hospital, London.
Dr. Elizabeth Ghogomu is a senior research associate at the Methods Centre at Bruyere Research Institute in Ottawa, Canada.
Dr. Simon Cousens is professor of epidemiology and medical statistics at LSHTM, UK.
Ms. Michelle Gaffeyis a research fellow at The Hospital for Sick Children (SickKids), Toronto, Canada
Dr. Paul Arora is assistant professor at the Dalla Lana School of Public Health, University of Toronto, Canada.
Dr. Robert Black is a professor of international health at the Johns Hopkins Bloomberg School of Public Health. He holds a joint appointment in medicine at the Johns Hopkins School of Medicine.
Dr. Mary Christine Castro is executive director of the Nutrition Center of the Philippines and Vice-Chair of Regional Nutrition Committee-NCR.
Ms. Li Chen is a biostatistician at the Cardiovascular Research Methods Centre, University of Ottawa Heart Institute, Canada.
Dr. Vivian A. Welch is director of the Methods Centre at Bruyere Research Institute in Ottawa, Canada and associate professor at the school of epidemiology and public Health, University of Ottawa.
Dr. Alomgir Hossain, PhD is a Scientist at the Ottawa Heart Institute Research Corporation and an Assistant Professor, School of Epidemiology and Public Health, Faculty of Medicine, at the University of Ottawa. Dr. Hossain is also appointed as an Adjunct Professor in the Department of Community Health and Epidemiology, School of Medicine at the University of Saskatchewan.
Dr. Henrik Friis, MD, PhD is former associate professor of epidemiology and current professor of international nutrition and health, University of Copenhagen.
Dr. T. Déirdre Hollingsworth is an infectious disease epidemiologist at Big Data Institute, Li Ka Shing Centre for Health Information and Discovery, University of Oxford, Oxford, UK.
Dr. Sue Horton is a Professor of Health Economics in the School of Public Health and Health Systems at University of Waterloo, Canada.
Dr. Charles King is faculty at Department of Pediatrics, University of California, La Jolla, California
Dr. Thi Le Huong is Director, Institute for Preventive Medicine and Public Health, Hanoi Medical University and Dean, Faculty of Nutrition and Food Safety, Institute for Preventive Medicine and Public Health, Hanoi Medical University
Dr. Chengfang LIU is Associate Professor with Tenure School of Advanced Agricultural Sciences (SAAS) China Center for Agricultural Policy (CCAP), Peking University
Dr. Fabian Rohner is an international nutrition and public health specialist at GroundWork, Switzerland. He focuses on the design and management of impact evaluations and M&E plans for large-scale food fortification and infant and young child nutrition projects.
Dr. Emily Rousham is a reader in global public health at the School of Sport, Exercise and Health Sciences, Loughborough University, Leicestershire, UK.
Ms. Rehana Salaam is a PhD candidate at University of Adelaide and before starting her PhD, Rehana was working as an Assistant Professor, at South Australian Health and Medical Research Institute
Dr. Erliyani Sartonto is a senior researcher at Department of Parasitology, Leiden University Medical Center, Leiden, The Netherlands
Dr. Peter Steinmann is a trained epidemiologist and public health specialist based at Swiss Tropical and Public Health Institute, University of Basel, Basel, Switzerland with a track record of work related to neglected tropical diseases (NTDs), WASH and public health in general of 15 years
Dr. Taniawati Supali is a researcher at the Department Parasitology, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
Dr. Peter Tugwellis Professor of Medicine and Epidemiology & Community Medicine at the University of Ottawa and Director for the Centre for Global Health at Bruyere Research Institute.
Dr. Emily Webb, MMath, MSc, PhD is associate professor in medical statistics and epidemiology at the department of infectious disease epidemiology, LSHTM
Dr. Franck Wieringa is research fellow at UMR204 Nutripass, Institute de Recherche pour le Développement, Montpellier, France
Dr. Pattanee Winnichagoon is Professor at Community/International Nutrition, Institute of Nutrition, Mahidol University, Nakhon Pathom, Thailand
Dr. Maria Yazdanbakhsh is Head of the Department of Parasitology, Leiden University Medical Center, Leiden, The Netherlands
Dr. Zulfiqar A. Bhutta is the Inaugural Robert Harding Chair in Global Child Health at The Hospital for Sick Children (SickKids), Co-Director of the SickKids Centre for Global Child Health and the Founding Director of the Centre of Excellence in Women and Child Health at the Aga Khan University.
Dr. George Wells is Director of the Cardiovascular Research Methods Centre at the University of Ottawa Heart Institute and Professor in the School of Epidemiology, Public Health and Preventive Medicine at the University of Ottawa.
Funding Statement
This research was funded by the Bill and Melinda Gates Foundation [OPP1140742].
Acknowledgments
We would like to thank Celia Holland for being part of our Advisory board and providing comments on interim results. We would like to thank Bishop Beasley, Nilanthi de Silva, Rebecca Stoltzfus and James Tielsch for contributing data. We would like to thank Jessica Trawin, Christine Mathew, who contributed to data collection and preparation; and Jordan Bernick who contributed to the analysis. We would like to thank anonymous peer referees who provided constructive comments that helped us improve on an earlier version of this paper. We would like to acknowledge the Campbell Collaboration International Development Coordinating group which managed the peer review process for the protocol and final review. This systematic review will be co-published with the Campbell Collaboration.
Author contributions
VW, EG, AH, AR, GAW had full access to the data and take responsibility for the accuracy of data analyses.
Concept and design: ZB, MG, SC, VW, GAW, PA, and the Advisory Board (SH, RB, PT, DH, CH)
Acquisition of data: VW, EG, CM, OD, AR and all trial authors (DB, MCC, AE, HF, DH, CK, HL, CL, ES, PS, TS, FR, ER, EW, FW, PW, MY)
Drafting of the manuscript: VW, EG, AR
Critical revision of content: all authors
Statistical analysis: VW, AH, AR, OD
Obtained funding: ZB, MG, SC, GAW, VW.
Author Summary
Mass deworming has been a cornerstone of development programmes for many years. However, recent systematic reviews have shown small effects on nutritional outcomes at a population level and reached discordant conclusions about the importance of the prevalence of infections. We collected individual participant data from all eligible studies to assess whether the small effects at population-level were explained by subgroup effects in vulnerable populations with poorer nutritional status or heavier intensity infections.
We used a network meta-analysis approach which allows the comparison of different combinations of deworming with micronutrients and iron. Our findings suggest that the effects of deworming on weight gain may be larger in children with moderate to heavy intensity infections, based on very low certainty evidence. We also identified new studies and added these to prior meta-analyses, which replicate prior findings of small average effects of deworming on nutritional outcomes and no effect on cognitive outcomes.
A limitation of this review is that almost half of the eligible studies could not be retrieved due to the burden of sharing data and lost data. Thus, we emphatically support the urgent need for much wider adoption of Open Data to facilitate analyses such as these aimed at understanding benefits for the most vulnerable populations.
Disclosure statement
Michelle Gaffey, Robert Black, Deidre Hollingsworth, Sue Horton, Paul Arora, Alison Riddle, Rehana Salam, Simon Cousens, Omar Dewidar have no conflict of interest, financial or otherwise that may influence judgments made in this review. Vivian Welch, Elizabeth Ghogomu, Alomgir Hossain, Zulfi Bhutta, Peter Tugwell and George Wells are authors of the Campbell systematic review and NMA of mass deworming for children (Welch et al. 2016). Vivian Welch is editor‐in‐chief of the Campbell Collaboration.
Registration
The protocol for this systematic review was peer-reviewed and published with the Campbell Collaboration.
Role of funder/sponsors
The funder had no role in the analysis, interpretation or decision to publish
Supplementary material
The Supplemental data for this article can be accessed here.
References
- Austin, P. C. 2009. “Balance Diagnostics for Comparing the Distribution of Baseline Covariates between Treatment Groups in Propensity‐score Matched Samples.” Statistics in Medicine 28 (25): 3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundy, D. A., Appleby L. J., Bradley M., Croke K., Hollingsworth T. D., Pullan R.,H.C. Turner, and N. de Silva. 2018. “100 Years of Mass Deworming Programmes: A Policy Perspective from the World Bank’s Disease Control Priorities Analyses.” Advances in Parasitology 100: Elsevier: 127–154. [DOI] [PubMed] [Google Scholar]
- Croke, K., Hicks J. H., Hsu E., Kremer M., and Miguel E.. 2016. “Does Mass Deworming Affect Child Nutrition. Meta-analysis, Cost-Effectiveness, and Statistical Power.” National Bureau of Economic Research Working Paper Series, 22382.
- Dagne, G. A., Brown C. H., Howe G., Kellam S. G., and Liu L.. 2016. “Testing Moderation in Network Meta‐analysis with Individual Participant Data.” Statistics in Medicine 35 (15): 2485–2502. doi: 10.1002/sim.v35.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debray, T. P., Schuit E., Efthimiou O., Reitsma J. B., Ioannidis J. P., Salanti G., Moons K. G., et al. 2018. “An Overview of Methods for Network Meta-analysis Using Individual Participant Data: When Do Benefits Arise?” Statistical Methods in Medical Research 27 (5): 1351–1364. doi: 10.1177/0962280216660741. [DOI] [PubMed] [Google Scholar]
- Galloway, R., Kristjansson E., Gelli A., Meir U., Espejo F., and Bundy D.. 2009. “School Feeding: Outcomes and Costs.” Food and Nutrition Bulletin 30 (2): 171–182. doi: 10.1177/156482650903000209. [DOI] [PubMed] [Google Scholar]
- Giovane, C. D., Vacchi L., Mavridis D., Filippini G., and Salanti G. J.. 2013. “Network Meta‐analysis Models to Account for Variability in Treatment Definitions: Application to Dose Effects.” Statistics in Medicine 32 (1): 25–39. doi: 10.1002/sim.5512. [DOI] [PubMed] [Google Scholar]
- Groenwold, R. H., Moons K. G., and Vandenbroucke J. P.. 2014. “Randomized Trials with Missing Outcome Data: How to Analyze and What to Report.” CMAJ : Canadian Medical Association Journal 186 (15): 1153–1157. doi: 10.1503/cmaj.131353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges, L. V., and Pigott T. D.. 2001. “The Power of Statistical Tests in Meta-analysis.” Psychological Methods 6 (3): 203. doi: 10.1037/1082-989X.6.3.203. [DOI] [PubMed] [Google Scholar]
- Higgins, J. P., and Thompson S. G.. 2002. “Quantifying Heterogeneity in a Meta‐analysis.” Statistics in Medicine 21 (11): 1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Hutton, B., Salanti G., Caldwell D. M., Chaimani A., Schmid C. H., Cameron C., Ioannidis J. P. A., et al. 2015. “The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions: Checklist and Explanations.” Annals of Internal Medicine 162 (11): 777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- Jakobsen, J. C., Gluud C., Wetterslev J., and Winkel P.. 2017. “When and How Should Multiple Imputation Be Used for Handling Missing Data in Randomised Clinical Trials–A Practical Guide with Flowcharts.” BMC Medical Research Methodology 17 (1): 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjansson, E., Robinson V., Petticrew M., MacDonald B., Krasevec J., Janzen L.,T. Greenhalgh, G. Wells, J. MacGowan, A. Farmer, B.J. Shea, A. Mayhew, and P. Tugwell. 2007. “School Feeding for Improving the Physical and Psychosocial Health of Disadvantaged Elementary School Children”. Cochrane Database of Systematic Reviews (Online), no. 1: CD004676. doi: 10.1002/14651858.CD004676.pub2. [DOI] [PubMed] [Google Scholar]
- Nga, T. T., Winichagoon P., Dijkhuizen M. A., Khan N. C., Wasantwisut E., and Wieringa F. T.. 2011. “Decreased Parasite Load and Improved Cognitive Outcomes Caused by Deworming and Consumption of Multi-micronutrient Fortified Biscuits in Rural Vietnamese Schoolchildren.” The American Journal of Tropical Medicine and Hygiene 85 (2): 333–340. doi: 10.4269/ajtmh.2011.10-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhan, M. A., Schünemann H. J., Murad M. H., Li T., Brignardello-Petersen R., Singh J. A., Kessels A. G., et al. 2014. “A GRADE Working Group Approach for Rating the Quality of Treatment Effect Estimates from Network Meta-analysis”. Bmj 349: g5630. doi: 10.1136/bmj.g5630. [DOI] [PubMed] [Google Scholar]
- Pullan, R. L., Smith J. L., Jasrasaria R., and Brooker S. J.. 2014. “Global Numbers of Infection and Disease Burden of Soil Transmitted Helminth Infections in 2010.” Parasites & Vectors 7 (1): 37. doi: 10.1186/1756-3305-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse, B., Chaimani A., and Li T. J. I.. 2017. “Network Meta-analysis: An Introduction for Clinicians.” Internal and Emergency Medicine 12 (1): 103–111. doi: 10.1007/s11739-016-1583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanti, G. 2012. “Indirect and Mixed‐treatment Comparison, Network, or Multiple‐treatments Meta‐analysis: Many Names, Many Benefits, Many Concerns for the Next Generation Evidence Synthesis Tool.” Research Synthesis Methods 3 (2): 80–97. doi: 10.1002/jrsm.1037. [DOI] [PubMed] [Google Scholar]
- Stewart, L. A., Clarke M., Rovers M., Riley R. D., Simmonds M., Stewart G., Tierney J. F., et al. 2015. “Preferred Reporting Items for a Systematic Review and Meta-analysis of Individual Participant Data: The PRISMA-IPD Statement.” Jama 313 (16): 1657–1665. doi: 10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- Taylor‐Robinson, D. C., Maayan N., Soares‐Weiser K., Donegan S., and Garner P.. 2015. “Deworming Drugs for Soil‐transmitted Intestinal Worms in Children: Effects on Nutritional Indicators, Haemoglobin, and School Performance.” Cochrane Database of Systematic Reviews, no. 7. doi: 10.1002/14651858.CD000371.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiebel, J. 2008. “Calculation of Statistical Power in Meta-analysis OSF2008.” doi: 10.17605/OSF.IO/W4XRS. [DOI]
- Welch, V., Ghogomu E., Hossain A., Arora P., Cousens S., Gaffey M.,A. Riddle, R. Salam, P. Tugwell, Z. Bhutta, G. A. Wells. and 2018. “PROTOCOL: Mass Deworming for Improving Health and Cognition of Children in Endemic Helminth Areas: A Systematic Review and Individual Participant Data Network Meta-analysis". Campbell Systematic Reviews 14: 1-46. doi: 10.1002/CL2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch, V. A., E. Ghogomu, A. Hossain, S. Awasthi, Z. Bhutta, C. Cumberbatch, R. Fletcher, J. McGowan, S. Krishnaratne, E. Kristjansson, S. Sohani, S. Suresh, P. Tugwell, H. White and G. Wells. 2016. “Deworming and Adjuvant Interventions for Improving the Developmental Health and Well-being of Children in Low- and Middle-income Countries: A Systematic Review and Network Meta-analysis.” Campbell Systematic Reviews12: 1-383. doi: 10.4073/csr.2016.7 [DOI] [Google Scholar]
- World Health Organization . 2011. “Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity.” Accessed 12September 2018. http://www.who.int/vmnis/indicators/haemoglobin/en/
- World Health Organization . 2017. “Guideline: Preventive Chemotherapy to Control Soil-transmitted Helminth Infections in At-risk Population Groups.” World Health Organization. Accessed 12September 2018 http://apps.who.int/iris/bitstream/handle/10665/258983/9789241550116-eng.pdf;jsessionid=EB785E884EDF96CCC9826D3F9AAE0912?sequence=1 [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- World Health Organization . 2011. “Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity.” Accessed 12September 2018. http://www.who.int/vmnis/indicators/haemoglobin/en/
- World Health Organization . 2017. “Guideline: Preventive Chemotherapy to Control Soil-transmitted Helminth Infections in At-risk Population Groups.” World Health Organization. Accessed 12September 2018 http://apps.who.int/iris/bitstream/handle/10665/258983/9789241550116-eng.pdf;jsessionid=EB785E884EDF96CCC9826D3F9AAE0912?sequence=1 [PubMed]


