| Declaration of potential conflict of interest of authors/collaborators of Update of the Brazilian Guidelines on Nuclear Cardiology - 2020If the last three years the author/developer of the Update: | |||||||
|---|---|---|---|---|---|---|---|
| Names Members of the Update | Participated in clinical studies and/or experimental trials supported by pharmaceutical or equipment related to the guideline in question | Has spoken at events or activities sponsored by industry related to the guideline in question | It was (is) advisory board member or director of a pharmaceutical or equipment | Committees participated in completion of research sponsored by industry | Personal or institutional aid received from industry | Produced scientific papers in journals sponsored by industry | It shares the industry |
| Barbara Juarez Amorim | No | No | No | No | No | No | No |
| Claudio Tinoco Mesquita | NIH | Bayer | No | No | Pfizer | No | No |
| Gabriel Blacher Grossman | No | No | No | No | No | No | No |
| João Vicente Vitola | No | No | No | No | No | No | No |
| José Claudio Meneghetti | No | No | No | No | No | No | No |
| José Roberto Nolasco de Araújo | No | No | No | No | No | No | No |
| Lara Cristiane Terra Ferreira Carreira | No | No | No | No | No | No | No |
| Luiz Eduardo Mastrocola | No | No | No | No | No | No | No |
| Rafael Willain Lopes | No | No | No | No | No | No | No |
| Ronaldo de Souza Leao Lima | No | No | No | No | No | No | No |
| Simone Cristina Soares Brandão | No | No | No | No | No | No | No |
| William Azem Chalela | No | No | No | No | No | No | No |
List of Abbreviations and Acronyms
HED - 11C - meta-hydroxyephedrine labeled with Carbon-11
PIB-11C - PET - pittsburgh B compound labeled with carbon-11 by PET imaging
MIBG-123I - metaiodobenzylguanidine labeled with iodine 123
13NH3 - ammonia labeled with Nitrogen-13
H2O-15O - water labeled with Oxygen-15
FDG-18F - fluorodeoxyglucose labeled with Fluorine-18
FDG-18F - PET/TC - fluorodeoxyglucose labeled with fluorine-18 by hybrid imaging (positron emission tomography coupled with computerized tomography)
Sodium fluoride-18F - fluorine-18 labeled Sodium Fluoride for PET Amyloid Imaging
201Hg - mercury-201
201Tl - thallium-201
82Rb - rubidium-82
82Sr - strontium-82
99mTc - technetium-99m
MIBI-99mTc - technetium-99m-labeled SESTAMIBI or MIBI
Pyrophosphate-99mTc - technetium-99m-labeled pyrophosphate
ACEI - angiotensin converting enzyme inhibitors
ACS - acute coronary syndrome
Aden - adenosine
ADMIRE-HF - AdreView Myocardial Imaging for Risk Evaluation in HF
AF - atrial fibrillation
AHA - American Heart Association
AL - light chain immunoglobulin
ALARA - as low as reasonably achievable
AMI - acute myocardial infarction
angio-CT - angiotomography of coronary arteries
ARB - angiotensin receptor blockers
ATP III - Adult Treatment Panel, from the Program for Detection, Evaluation, and Treatment of High Cholesterol in Adults
AUC - area under the curve
AVB - atrioventricular blockage
BMI - body mass index
BNP - B-natriuretic peptide
CA - cardiac amyloidosis
CABG - coronary artery bypass graft
CC - coronary calcium
CAD - coronary artery disease
CCA - coronary cineangiography
CFR - coronary flow reserve
CHF - congestive heart failure
CIED - cardiac implantable electronic devices
CMR - cardiac magnetic resonance
CONFIRM - Coronary CT Angiography Evaluation for Clinical Outcomes: an International Multicenter Registry
COURAGE - Clinical Outcomes Utilizing Revascularization and Aggressive druG Evaluation Trial
CPU - chest pain unit
CRP - C reactive protein
CRT - cardiac resynchronization therapy
CS - calcium score
CTX - cardiotoxicity
CV - cardiovascular
Cx - circumflex coronary artery
CZT - cadmium zinc telluride semiconductors
DDD - artificial pacemaker stimulation mode
DG1 - diagonal 1 coronary artery
Dipy. - dipyridamole
DM - diabetes mellitus
Dobut. - dobutamine
DS - duke score
ECG - 12-lead electrocardiogram
ECHO - echocardiogram
EDV - end diastolic volume
ERASE Chest Pain -The Emergency Room Assessment of Sestamibi for Evaluation of Chest Pain Trial
ESV - end systolic volume
ET - exercise testing
FAME - Fractional Flow Reserve versus Angiography for Guidance of PCI in Patients with Multivessel Coronary Artery Disease
FBP - filtered back-projection
FDA - food and drug administration
FDG-6-P - fluorodeoxyglucose - 6 - phosphate
FFA - free fatty acids
FFR - fractional flow reserve
FRS - Framingham risk score
Gated-SPECT - myocardial perfusion imaging by single photon emission computed tomography technique synchronized with electrocardiogram
HBP - high blood pressure
HF - heart failure
HFpEF - heart failure with preserved ejection fraction
HFrEF - heart failure with reduced ejection fraction
HMR - heart to mediastinum ratio
HR - heart rate
IAEA - International Atomic Energy Agency
ICD - implantable cardioverter defibrillator
ICNC - International Conference of Nuclear Cardiology
IE - infectious endocarditis
IFR - instantaneous flow reserve/instantaneous wave-free ratio
INCAPS - IAEA Nuclear Cardiology Protocols Cross-Sectional Study
ISCHEMIA - International Study of Comparative Health Effectiveness with Medical and Invasive Approaches
IV - intravenously/intravenous
keV - kilo-electron volts
LAD - left anterior descending coronary artery
LAFB - left anterior fascicular block
LBBB - left bundle branch block
LV - left ventricle
LVAD / VAD - left ventricular assist device / ventricular assist devices
LVEF - left ventricular ejection fraction
MBF - myocardial blood flow
MBFR - myocardial blood flow reserve
MBq - megabequerel
mCi - milicurie
MET - metabolic equivalent.
MFR - myocardial flow reserve
MIBI / SESTAMIBI - 2-methoxy-isobutyl-isonitrile
MPS - myocardial perfusion scintigraphy
MR - magnetic resonance
MRS - myocardial revascularization surgery
mSv - millisieverts
MVO2 - myocardial oxygen consumption
NaI - sodium iodine
NE - norepinephrine
NPV - negative predictive value
NSTEMI - non-ST segment elevation myocardial infarction
NSVT - nonsustained ventricular tachycardia
NYHA HF - New York Heart Association Heart Failure Class
OMT - optimized medical therapy
OR - odds ratio
OSEM - ordered subset expectation maximization
PAREPET - prediction of arrhythmic events with positron emission tomography
PARR-2 - PET and Recovery after Revascularization study
PCI - percutaneous coronary intervention
PET - positron emission tomography
PET/CT - positron emission tomography coupled with computed tomography (hybrid imaging)
PET/MR - positron emission tomography coupled with magnetic resonance (hybrid imaging)
PM - pacemaker
PREMIER - Performance of Rest Myocardial Perfusion Imaging in the Management of Acute Chest Pain in the Emergency Room in Developing Nations
PROCAM - PROSpective CArdiovascular Munster Study
PROMISE - Prospective Multicenter Imaging Study for Evaluation of Chest Pain
RCA - right coronary artery
Regad. - regadenoson
RESCUE - Randomized Evaluation of patients with Stable angina Comparing diagnostic Examinations
ROC - receiver operating characteristics
ROI - regions of interest
ROMICAT II - rule out myocardial infarction by cardiac computed tomography
RV - right ventricle/ventricular
SBC - Brazilian Society of Cardiology
SBMN - Brazilian Society of Nuclear Medicine
SBP - systolic blood pressure
SCORE - Systematic Coronary Risk Evaluation Study
SDS - summed difference score
Shining / Shine Through - residual activity effect
SPECT - myocardial perfusion imaging by single photon emission computed tomography
SRS - summed rest/redistribution score
SSS - summed stress score
STEMI - ST segment elevation myocardial infarction
STICH - Surgical Treatment for Ischemic Heart Failure study
SUS - Brazil’s public Single Health System (acronym in Portuguese)
SUV - standard uptake value
TIA - transient ischemic attack
TID - transient ischemic dilatation
TOF - time of flight
TTR - transthyretin
TTR CA - transthyretin cardiac amyloidosis
UA - unstable angina
USA - United States of America
VAD - ventricular assist devices
VF - ventricular fibrillation
VT - ventricular tachycardia
WR - myocardial washout rate
1. Introduction
Nuclear cardiology is a non-anatomical, physiological imaging method. The use of radioactive or radiopharmaceutical substances makes it possible to study several physiopathological mechanisms of cardiovascular disease in vivo. Via this imaging technique, it is also possible to visualize and accompany an instituted therapy’s physiological effects on cardiac function, on the cellular and biochemical level. Of all the applications of nuclear medicine in cardiology, scintigraphy or myocardial perfusion imaging with technetium-99m-labeled radiopharmaceuticals synchronized with electrocardiogram (Gated-SPECT), is the most common exam in clinical practice. For this reason, this technique will be the most discussed in these Guidelines.
Recent years have, however, seen a growing concern among the scientific community regarding rational and optimized use of ionizing radiation in medicine. Cardiovascular imaging, moreover, encompasses all functional and anatomical imaging techniques and should, in this context, be used rationally and cost-effectively. Other applications of nuclear medicine in cardiology have also emerged and gained prominence during the past decades, especially positron emission tomography (PET) for the study of coronary flow reserve, cardiac sympathetic activity, and inflammatory/infectious processes, and cardiac amyloidosis (CA). All of these aspects have been taken into consideration and will be covered in detail in the chapters developed herein.
Guidelines recommendations are highly valuable tools for medical activity of the highest quality. The objective is to support and aid doctors in making decisions regarding their patients, by elaborating orientations which may be useful as part of the decision-making process. No Guidelines, however, should be replaced by the abilities, experience, and clinical judgments of specialized professionals who are have the final say in their decisions concerning each individual patient.
In general, whenever possible and applicable, classifications of recommendation have been adopted for indicating cardiac scintigraphy, supported by levels of evidence, in accordance with the recommendations established by classical cardiology guidelines (Table 1).
Table 1.
Classes of recommendation and levels of evidence
| Classes of recommendation |
| Class I - Conditions for which there is conclusive evidence or, in the absence of conclusive evidence, general consensus that the procedure is safe and useful/effective |
| Class II - Conditions for which there are conflicting evidence and/or divergent opinions regarding the procedure's safety and usefulness/effectiveness |
| Class IIA - Weight or evidence/opinion in favor of the procedure. The majority of studies/experts approve. |
| Class IIB - Safety and usefulness/effectiveness less well established, with no prevailing opinions in favor |
| Class III - Conditions for which there is evidence and/or consensus that the procedure is not useful/effective and could, in some cases, be harmful |
| Levels of Evidence |
| Level A - Data obtained from multiple concordant large randomized trials and/or robust meta-analysis of randomized clinical trials |
| Level B - Data obtained from less robust meta-analysis, from a single randomized trial, or from non-randomized (observational) trials |
| Level C - Data obtained through consensus of expert opinion |
Based on current evidence, this document, which does not function as a substitute, practically and objectively adds important data to and updates the Brazilian Cardiology Society’s (SBC) First Guidelines and Update on Nuclear Cardiology, both of which were published by the Brazilian Archives of Cardiology (Arquivos Brasileiros de Cardiologia), in 2002 and 2005, respectively.
As in the previously mentioned documents, those who participated in the elaboration of these Guidelines are considered specialists in their respective areas and were, for this reason, chosen to develop the chapters thereon. The committed involvement of all colleagues representing the SBC and the Brazilian Society of Nuclear Medicine (SBMN) have made the elaboration of these new update of Brazilian Guidelines on Nuclear Cardiology possible. It is our hope that they will be of great use, especially to Cardiologists and Nuclear Medicine and Clinical Physicians in Brazil. The Organizing Committee appreciates the collaboration of all those involved.
2. Addendum to the ISCHEMIA Study*
At the time of publication of this guideline, the International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) had not been published yet, although the main findings were presented on November 16, 2019 at the American Heart Association (AHA) annual congress in Philadelphia, USA, available on the study’s website. Considering its importance for medical decision-making and the potential implications for nuclear cardiology, a few relevant concerns should be highlighted on the findings available so far:
The main objective of the ISCHEMIA study was to assess whether patients (P) with at least moderate ischemia on a functional examination would benefit from myocardial revascularization (coronary artery bypass grafting or percutaneous coronary intervention) added to optimal medical therapy). Were randomized 5,179 patients with stable CAD and myocardial ischemia documented by one of many different methods (myocardial perfusion scintigraphy, stress echocardiography, cardiac magnetic resonance imaging, exercise testing not associated with cardiac imaging). These noninvasive methods were used to define the etiology of chest pain and for cardiovascular risk stratification, a management approach established in clinical practice that is not invalidated by the findings of the study. Prior knowledge indicates that patients with lower ischemic burden have a better prognosis than individuals with larger and more intense ischemia;
The ISCHEMIA trial demonstrated no benefit of myocardial revascularization (Invasive Group - IG) versus optimal medical therapy (OMT) to reduce the major outcomes of “death” and “acute myocardial infarction.” Despite the methodological differences, these results were somewhat similar to those of the COURAGE study. It is noteworthy that the mortality curves began to separate after two years of medical follow-up, apparently benefiting the IG and potential long-term implications, which justified the increased clinical follow-up of P, underway at the moment. Note that the IG had an improved quality of life assessment, reduced frequency of angina and lower use of specific medication compared to the OMT group;
The ISCHEMIA trial is one of the most relevant studies on stable CAD, with important messages for clinical practice. The validity of the results is emphasized for the population sample evaluated in the study and for the definitions of ischemia and its severity levels employed. However, for exclusion situations, such as P with left main disease, recent acute coronary syndrome, angioplasty in the previous 12 months, ejection fraction < 35% and progressive or unstable symptoms, prior knowledge remains unchanged. Both CAD and ischemic heart disease represent a broad spectrum of patients, with inherent heterogeneity and important prognostic implications (extensive evidence base in the literature and described in detail in the current guideline). Were excluded from the trial an impressive number of P that had at least moderate angina and ischemia in the absence of coronary obstructions, showing the diversity of the disease and the value of functional assessment;
The main question is whether the ISCHEMIA study has properly evaluated a significant number of P with moderate/severe ischemia, aiming to determine whether myocardial revascularization adds prognostic benefit to these patients, as documented by scintigraphy, which was not the exclusive method of documentation. There was the inclusion (randomization) of cases with nonexistent or mild ischemia (12% of the total randomized), which is surprising for a study that was initially intended to include only patients with moderate to severe ischemia. There was also a change in the criteria for inclusion of P with severe ischemia in the study, with a significant number based on the results of exercise testing, without imaging, a decision made after the study was in progress. From this change, the percentage of these P that would effectively have severe myocardial ischemia on scintigraphy is questioned;
Therefore, the Editorial Board of this guideline believes that the definitive analysis of the results will only be possible after the formal publication of the trial results.
3. The Application of Nuclear Medicine Techniques to Justify Financial Resources Available for Attending Cardiology Patients in Brazil
3.1. Introduction
Cardiovascular diseases are the main cause of death in Brazil, and they are responsible for 30% of deaths worldwide every year.1 They are responsible for approximately 8% of total healthcare costs in Brazil, a figure which has been increasingly annually, in parallel with population aging.1 Teich and Araújo estimated that in 2011, approximately 200,000 events associated with acute coronary syndromes occurred in Brazil, entailing a massive impact of 3.88 billion Brazilian reals, considering only hospital and indirect costs, associated with loss of productivity.2 Considering these findings, it has been demonstrated that (preventive) measures play a crucial role in reducing morbidity and mortality, and they should be a priority in national healthcare policy design, as they have profound additional impacts on reducing costs and maintaining productivity. Another significant point, however, which has contributed to reducing the outcome of “cardiovascular death” and to justifying expenses, involves the use of tools which make accurate diagnosis of a determined condition possible (?) and which aid and guide the conduct of physicians, based on these results. Myocardial perfusion scintigraphy (MPS) plays a significant role in justifying financial resources for attending patients with established or potential cardiovascular disease.
3.2. Cost-Effectiveness in Comparison with Cardiac Catheterization
One of the main fundaments of MPS is its good ability to identify low-risk patients, who do not require invasive intervention, in spite of established coronary disease, such as anatomical lesions on coronary angiography.3 Observational studies in the 1990’s have demonstrated that MPS was able to identify high- and low-risk groups, resulting in reduced costs for patients with coronary artery disease (CAD) and avoiding procedures that are not associated with improved patient health outcomes. A major prospective study carried out in the United States of America (USA) recruited 11,372 patients with stable angina, who were referred to either MPS or cardiac catheterization. Patients were adjusted by clinical risk, and the costs of direct cardiac catheterization (aggressive strategy) were compared to initial scintigraphy followed by selective catheterization in high-risk patients (conservative strategy). Although both strategies had similar adverse outcomes, such as cardiac death and non-fatal myocardial infarction, revascularization rates were higher (between 13% and 50%) in patients who underwent catheterization directly.4 This reflex of revascularizing anatomical lesions which do not determine ischemia led to unnecessary associated medical costs of around 5,000 dollars per patient in this study.4 Currently, the use of medical resources for conditions that do not have consequences for patients or that could be managed conservatively is known as “overtreatment.”5 The study of the impact of MPS on reducing costs has shown that its main function is to prevent patients who have low or moderate risks on single photon emission computed tomography (SPECT) from being treated with unnecessary catheterizations and revascularizations. Similarly to this North American study, Underwood et al.6 have demonstrated that strategies which incorporate myocardial scintigraphy to evaluate patients with stable coronary diseases are both cheaper than and as effective as strategies involving invasive anatomical assessment.6 Cerci et al.7 evaluated the impact of diagnostic exams on patients with CAD in different scenarios within Brazil’s public Single Health System (SUS, acronym in Portuguese). The study’s most relevant finding is that, although non-invasive functional tests are the most frequently solicited exams for evaluating patients with suspected or known CAD, the majority of healthcare costs for these patients are related to procedures/invasive treatment. In other words, in the Brazilian context, the costs of diagnostic exams continue to be significantly lower than those of invasive and therapeutic procedures. In this manner, it seems logical to affirm that, if scintigraphy exams are made available to patients attended by the SUS, there will be a similar impact on the reduction of healthcare costs, which has been the case in the USA and some countries in Europe. Another relevant piece of data from this study refers to the fact that the majority of patients who were revascularized had not undergone tests to document ischemic burden; only anatomical diagnostic techniques had been applied.7
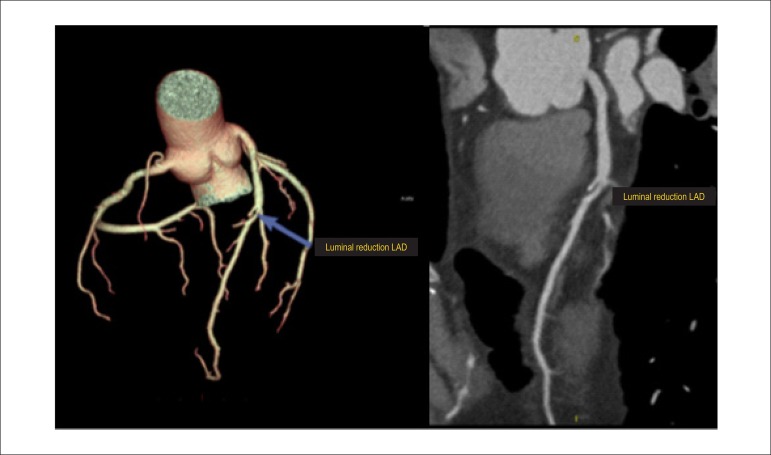
3.3. Cost-Effectiveness of Myocardial Perfusion Scintigraphy in Relation to Coronary Angiotomography
Angiotomography (angio-CT) of coronary arteries offers very accurate, non-invasive anatomical assessment, and it has proved to be an excellent technique for ruling out obstructive coronary disease in low- to intermediate-risk patients. Angio-CT, however, has presented results similar to those of cardiac catheterization in relation to triggering a higher number of myocardial revascularizations, which do not necessarily (means) reduced cardiovascular outcomes. In a recent meta-analysis comparing angio-CT to functional methods, no differences were observed regarding the outcomes of death or cardiac hospitalization, but there was a 29% reduction in the number of non-fatal infarctions. On the other hand, the use of this method was associated with 33% and 86% higher rates of invasive coronary angiography and myocardial revascularization, respectively. It is not known whether the reduction in non-fatal infarctions may be attributed to the higher number of revascularizations, which is (unlikely) considering in light of other studies on stable CAD, or to the higher use of statins and aspirin associated with the recognition of anatomical coronary lesions.8 With the objective of elucidating the role of angio-CT on cost-effectiveness of approaches to stable CAD in comparison with myocardial scintigraphy, the Randomized Evaluation of Patients With Stable Angina Comparing Diagnostic Examinations (RESCUE) study, which is being developed, is expected to compare these strategies in a prospective, randomized manner.9
The authors of a recent meta-analysis published by the American Heart Association (AHA)/Circulation, have reinforced 2 important aspects of cost-effectiveness:10
The importance of performing appropriate exams as a way of (ensuring) their cost-effectiveness, especially techniques like MPS.
The results of appropriate exams should effectively lead to appropriate decision making in clinical conduct and patient management.
4. Indications for Myocardial Perfusion Scintigraphy
Over the past years, different medical societies have published criteria for defining scenarios in which myocardial scintigraphy may be adequately utilized. In addition to traditional classification of recommendation and levels of evidence, more recent criteria on appropriate MPS exam referral have been suggested, dividing indications into appropriate, possibly appropriate, and rarely appropriate, resulting from the application of scores constructed based on clinical scenarios and specific methodologies.11 In this classification, indications with scores from 1 to 3 are considering rarely appropriate; 4 to 6, possibly appropriate; and 7 to 9, appropriate. Published documents are based on evidence from American and European Guidelines, as well as the recently published Brazilian Guidelines on stable coronary disease.12-15
Regardless of classification type, there is consensus that symptomatic patients with intermediate risks of ischemic heart disease are the ones who most benefit from MPS in terms of diagnostic and prognostic evaluation. The exam should preferably be performed in association with physical exercise in patients with sufficient physical and clinical conditions (estimated ability for activities of daily living with metabolic expenditure greater than 5 METs), in order to measure their functional capacity, hemodynamic responses (heart rate and blood pressure behavior), stress-induced arrhythmias, and other responses. It is recommended that patients with complete left bundle branch block, regardless of functional ability, undergo MPS under pharmacological stress (dipyridamole or adenosine). In the same manner, regardless of pretest probability of ischemic heart disease, patients with low functional ability or uninterpretable electrocardiogram (ECG) are indicated to undergo MPS. On the other hand, patients with low probability of ischemic heart disease, higher functional ability, and interpretable ECG are not indicated for MPS (Table 2).
Table 2.
Indication criteria for myocardial perfusion scintigraphy in symptomatic patients
| Assessment of patients with non-acute chest pain or ischemic equivalent | Score |
|---|---|
| Low pretest probability of CAD, with interpretable resting ECG and ability to exercise | 3 |
| Low pretest probability of CAD, with uninterpretable resting ECG or inability to exercise | 7 |
| Intermediate pretest probability of CAD, with interpretable resting ECG and ability to exercise | 7 |
| Intermediate pretest probability of CAD, with uninterpretable resting ECG or inability to exercise | 9 |
| High pretest probability of CAD, regardless of interpretable resting ECG and ability to exercise | 8 |
ACS: acute coronary syndrome; CAD: coronary artery disease; ECG: 12-lead electrocardiogram.
In patients with heart failure (HF) and left ventricular systolic dysfunction or recent-onset atrial fibrillation (AF), ventricular tachycardia (VT) or syncope, the indication for MPS is appropriate or possibly appropriate, unless the patient in question is low risk or has low pretest probability. Asymptomatic patients with no history of ischemic heart disease and without abnormal exercise testing (ET) generally do not benefit from undergoing MPS. In specific situations, in patients with high calcium scores (greater than or equal to 400), diabetes, chronic renal insufficiency, or a prevalent family history of ischemic heart disease, performing MPS may aggregate value to the medical decision-making process, with satisfactory cost-effectiveness. Asymptomatic patients with abnormal stress ECG who are re-stratified with the use of prognostic scores, such as the Duke score, may also benefit from complementary investigation via MPS, especially if their risk scores are intermediate or high (Table 3). Diverse examples of clinical situations cited in Table 3 may also be found in the section on integration of diagnostic modalities.
Table 3.
Indication criteria for myocardial perfusion scintigraphy in asymptomatic patients and/or patients with prior exams
| Asymptomatic patients - detection of CAD/risk stratification | Score |
|---|---|
| Low risk (ATP III criteria) | 1 |
| Intermediate risk (ATP III criteria) - interpretable ECG | 3 |
| Intermediate risk (ATP III criteria) - uninterpretable ECG | 5 |
| High risk (ATP III criteria) | 7 |
| High risk and calcium score (Agatston) between 100 and 400 | 7 |
| Calcium score (Agatston) > 400 | 7 |
| Low-risk Duke score (> +5) | 2 |
| Intermediate-risk Duke score (between -11 and + 5) | 7 |
| High-risk Duke score (< -11) | 8 |
Agatston: score that defines the presence and quantity of calcium in coronary arteries, characterizing atherosclerosis; ATP III: Adult Treatment Panel, from the program for detection, evaluation, and treatment of high cholesterol in adults; CAD: coronary artery disease.
When patients have established ischemic heart disease and are asymptomatic, early myocardial perfusion studies with radiopharmaceuticals should be avoided following percutaneous coronary intervention and/or myocardial revascularization surgery procedures. In the event of percutaneous coronary intervention and myocardial revascularization surgery, the application of MPS has been observed to have a favorable cost-benefit ratio for follow up after more than 2 and 5 years, respectively, even in asymptomatic patients. Symptomatic patients with specific clinical conditions (or equivalent manifestations) may benefit from the exam before this period (Table 4).
Table 4.
Indication criteria for myocardial perfusion scintigraphy in patients who have undergone revascularization procedures (CABG or PCI)
| Previous percutaneous revascularization or surgical procedures | Score |
|---|---|
| Symptomatic | 8 |
| Asymptomatic, CABG less than 5 years prior | 5 |
| Asymptomatic, CABG 5 or more years prior | 7 |
| Asymptomatic, percutaneous revascularization less than 2 years prior | 3 |
| Asymptomatic, percutaneous revascularization 2 or more years prior | 6 |
CABG: myocardial revascularization surgery; PCI percutaneous coronary intervention.
For patients with previous exams who manifest new symptoms or who require assessment of the repercussion of diagnosed intermediate lesions and characterization of arteries with obstructive lesions “responsible” for a larger myocardial area at risk, as well as patients with multivascular diseases, the indication for MPS is classified as appropriate or possibly appropriate. In patients with established coronary disease and worsening symptoms, MPS may aid in quantifying ischemic burden (extent and intensity of defects) and in determining medical management. In clinically stable patients with previous exams performed more than 2 years prior, MPS may be appropriate (Table 5).
Table 5.
Indication criteria for myocardial perfusion scintigraphy for risk stratification and prognostic assessment of patients with proven stable coronary artery disease and/or prior exams
| Asymptomatic patients or patients with stable symptoms - previously "normal" stress imaging exams | Score |
|---|---|
| Intermediate/high risk (ATP III) - stress imaging exam ≥ 2 years prior | 6 |
| Asymptomatic patients or patients with stable symptoms - CCA or abnormal imaging exams, without prior CABG | |
| Previously "unclear," "contradictory," or "borderline" non-invasive assessment - obstructive CAD as initial concern | 8 |
| New, recent, or progressive symptoms | |
| Normal CCA or normal stress imaging exam | 6 |
| Coronary cineangiography (invasive or non-invasive) | |
| Coronary stenosis or anatomical abnormality whose significance is unclear | 9 |
ACS: acute coronary syndrome; ATP III: Adult Treatment Panel, from the program for detection, evaluation, and treatment of high cholesterol in adults; CAD: coronary artery disease; CCA: coronary cineangiography; CABG: myocardial revascularization surgery.
In patients who present acute chest pain, with clinical suspicion of acute coronary syndrome (ACS), normal or uninterpretable ECG (old left bundle branch block or pacemaker) and normal biomarkers, resting myocardial scintigraphy may exclude acute cardiovascular events with a high degree of safety (high negative predictive value [NPV]), allowing patients to be discharged from the emergency room. If the exam is normal, investigation may continue with outpatient tests involving physical or pharmacological stress, whether associated or non-associated with non-invasive imaging, and even anatomical assessment via coronary angio-CT, in specific conditions. For patients with ACS who are clinically stable, with neither recurring chest pain nor HF, and who have not undergone any invasive exam, MPS is useful for detecting presence and extent of myocardial ischemia (Table 6).
Table 6.
Indication criteria for myocardial perfusion scintigraphy in patients with acute chest pain or post-acute coronary syndrome
| Assessment of patients with acute chest pain | Score |
|---|---|
| Resting image only | |
| Possible ACS - ECG without ischemic alterations or LBBB or pacemaker; low-risk TIMI score; borderline, minimally elevated, or negative troponin | 8 |
| Possible ACS - ECG without ischemic alterations or LBBB or pacemaker; high-risk TIMI score; borderline, minimally elevated, or negative troponin | 7 / 8 |
| Possible ACS - ECG without ischemic alterations or LBBB or pacemaker; negative initial troponin. Recent (up to 2 hours) or evolving chest pain | 7 |
| Assessment of post-ACS patients (infarction with or without elevated ST segment) | |
| Stable, post-AMI patients, with ST segment elevation, for assessment of ischemia; cardiac catheterization not performed | 8 |
| Stable, post-AMI patients, without ST segment elevation, for assessment of ischemia; cardiac catheterization not performed | 9 |
ACS: acute coronary syndrome; AMI: acute myocardial infarction; CAD: coronary artery disease; ECG: 12-lead electrocardiogram; LBBB: left bundle branch block.
Indications for MPS to assess pre-operative risk of non-cardiac surgeries and vascular surgeries have also been recently revised.16 Patients who will undergo low-risk surgeries do not need to undergo MPS. If the surgery is not low-risk, functional capacity is the factor that determines whether MPS will be necessary. In patients with functional capacity estimated at greater than or equal to 4 METs, without cardiac symptoms, regardless of clinical or surgical risk, non-invasive assessment of myocardial ischemia is generally not recommended. However, for patients with low functional capacity and elevated clinical/surgical risks, there is an indication to perform MPS under pharmacological stress. The following are considered clinical risks: history of ischemic heart disease, congestive heart failure (CHF), cerebrovascular disease, diabetes mellitus (DM), and renal insufficiency (creatinine > 2.0 mg/dl). In the absence of these risk factors, regardless of functional capacity, surgery may be performed without complementary functional exams (Table 7).
Table 7.
Indication criteria for myocardial perfusion scintigraphy for pre-operative assessment of non-cardiac surgeries
| Pre-operative assessment of non-cardiac surgeries | Score |
|---|---|
| Low-risk surgery | 1 |
| Intermediate-risk surgery or vascular surgery Functional capacity greater than or equal to 4 METs |
1 |
| Intermediate-risk surgery or vascular surgery Functional capacity unknown or less than 4 METs No clinical risk factors |
1 |
| Intermediate-risk surgery Functional capacity unknown or less than 4 METs One or more clinical risk factors |
7 |
| Vascular surgery Functional capacity unknown or less than 4 METs One or more clinical risk factors |
8 |
MET: metabolic equivalent.
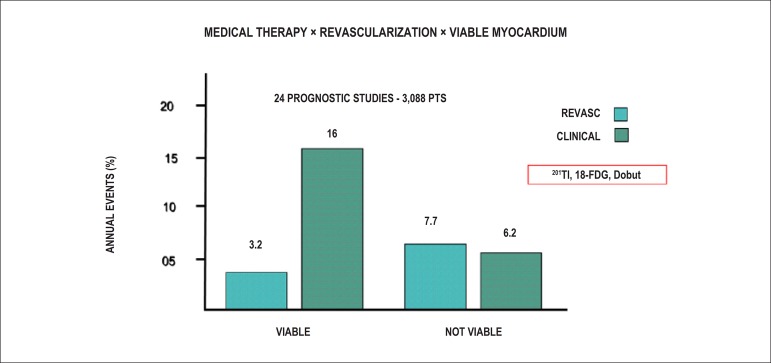
In patients with accentuated left ventricular dysfunction who are eligible for myocardial revascularization, assessment of myocardial viability may aid selection of patients who will benefit from this treatment (Table 8).
Table 8.
Indication criteria for myocardial perfusion scintigraphy for assessment of myocardial viability
| Assessment of myocardial viability | Score |
|---|---|
| Accentuated left ventricular dysfunction | 9 |
| Eligible for myocardial revascularization | 9 |
MPS is, therefore, an appropriate indication in diverse clinical manifestations of ischemic heart disease, from acute manifestations in the emergency room to diagnostic investigation of stable patients, aiding in therapeutic decision making through various tools which make it possible to define disease severity, as well as in pre-operative assessment in specific situations and in defining the benefits of revascularization for patients with significant myocardial viability. It is worth noting that, for diagnostic investigation, patients with intermediate probability of ischemic heart disease are those who most benefit from MPS and that it is rarely appropriate in patients with low probability.
5. Myocardial Perfusion Scintigraphy Methods - types of Cardiovascular Stress
5.1. Radiopharmaceuticals Used to Perform Myocardial Perfusion Scintigraphy
In Brazil, the main radiopharmaceuticals available for obtaining images of the myocardium are thallium-201 (201Tl) and those labeled with technetium-99m (99mTc), which mainly include 2-methoxy-isobutyl-isonitrile, known as Sestamibi (or MIBI), and tetrofosmin. Given that these are the most widely used, the specific methods used for acquiring images with them will be presented.
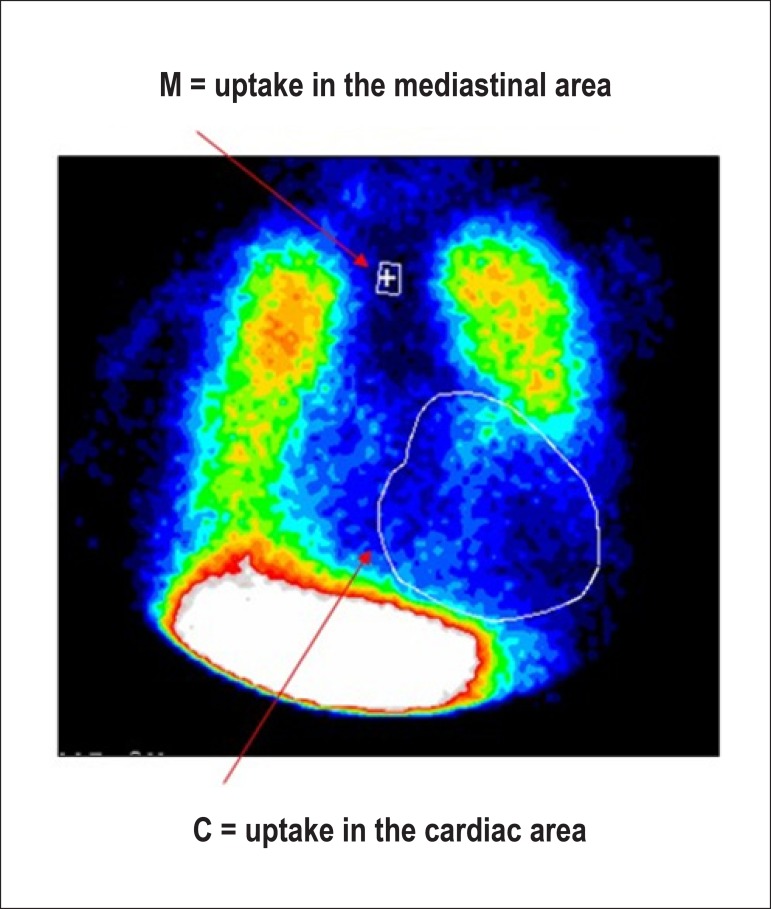
Thallium-201 or 201Tl 17 is a monovalent cation with biological properties analogous to those of potassium. It is both intracellular and absent in scar tissue, and it is thus designated for differentiating ischemic myocardium from fibrosis. It has a physical half-life of 73 hours, and it decays by electron capture to mercury-201 (201Hg), and the photons emitted for imaging are primarily x-rays (of 201Hg itself) between 68 and 80 kilo-electron volts (keV), in addition to lower quantities of gamma radiation in the energy range of 135 keV and 166 KeV. Upon intravenous injection, initial myocardial uptake is proportional to regional blood flow, depending on the integrity of the cellular membrane. It penetrates the cellular membrane via active transport, involving energy expenditure (Na+/K+ATPase system), with a high first-pass extraction fraction in the myocardium (the proportion of 201Tl which is extracted from blood and absorbed by myocytes), of around 70% to 85%.
Maximum concentration of thallium-201 in the myocardium occurs approximately 5 minutes after injection, which is generally administered during peak exercise or clinical and/or electrocardiographic alterations triggered during an ET or a pharmacological test. It presents rapid disappearance or clearance from the intravascular compartment. Following initial distribution of the radioisotope throughout the myocardium, related to blood flow, the phenomenon of redistribution begins 10 to 15 minutes after injection. This is dependent on clearance or washout of thallium-201 from the myocardium, which no longer depends on blood flow but rather on the concentration gradient between myocytes and blood levels. Redistribution of thallium-201 is quicker in normal myocardium than in ischemic myocardium, resulting in different activities in these tissues (differential “washout”).
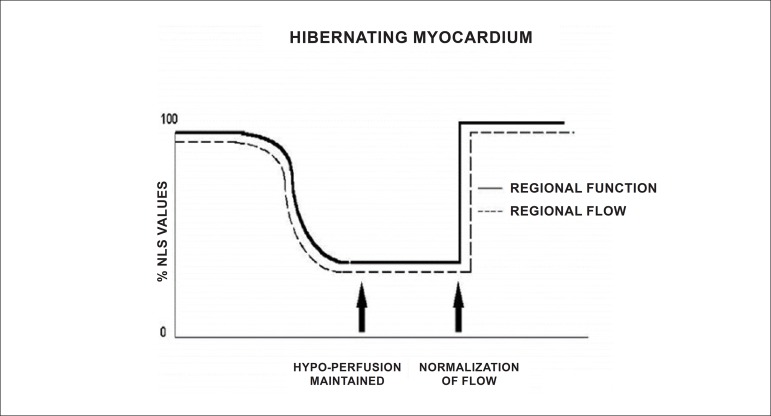
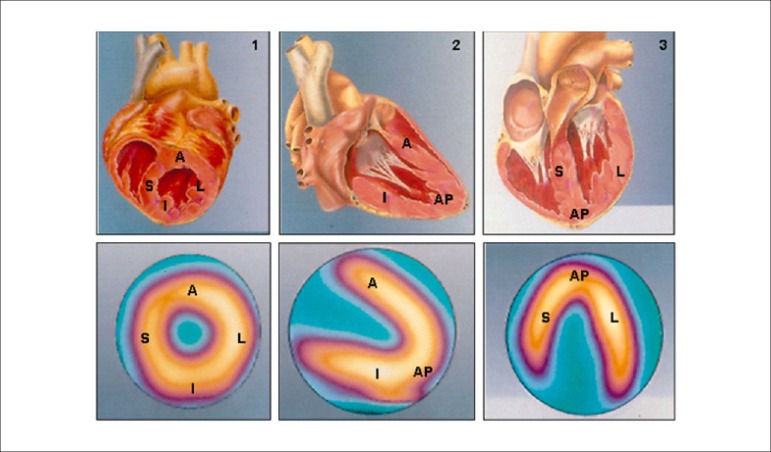
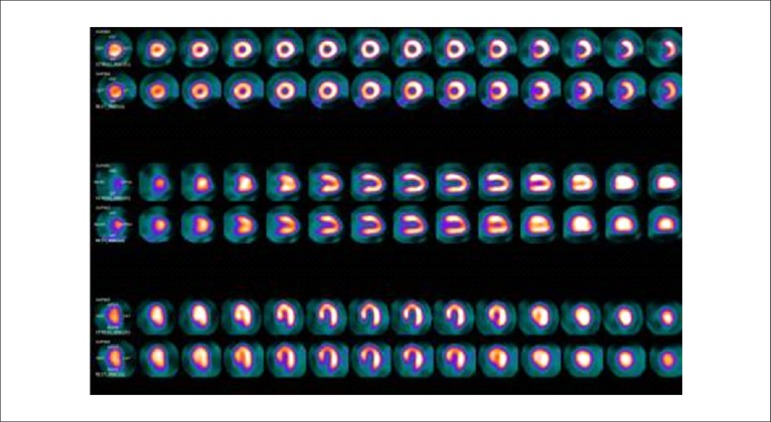
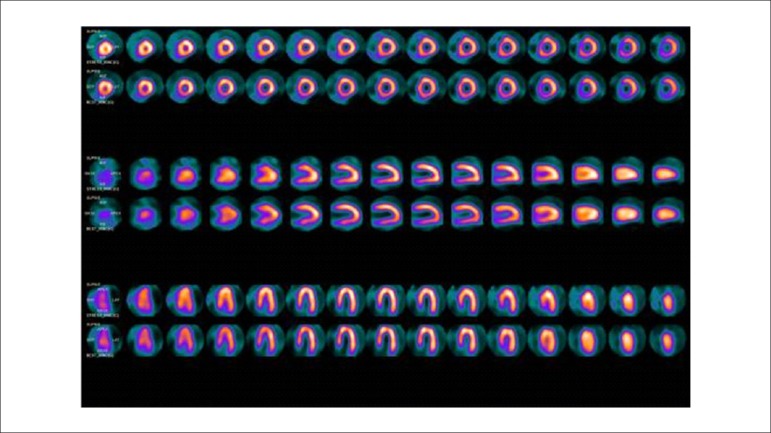
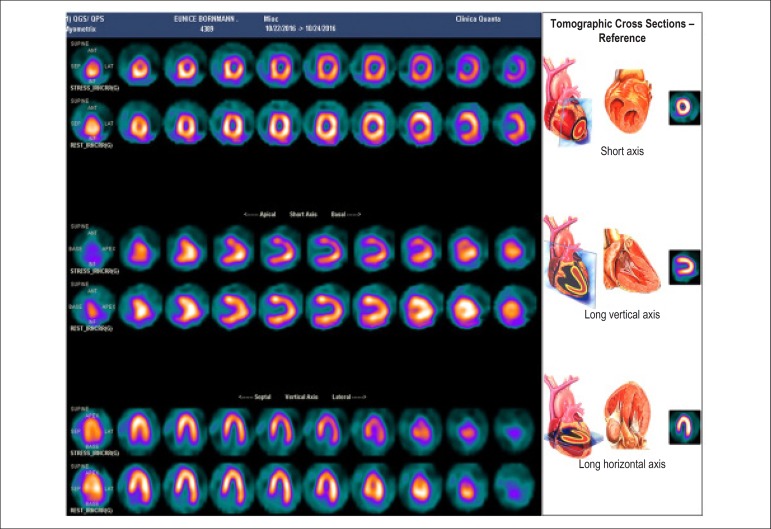
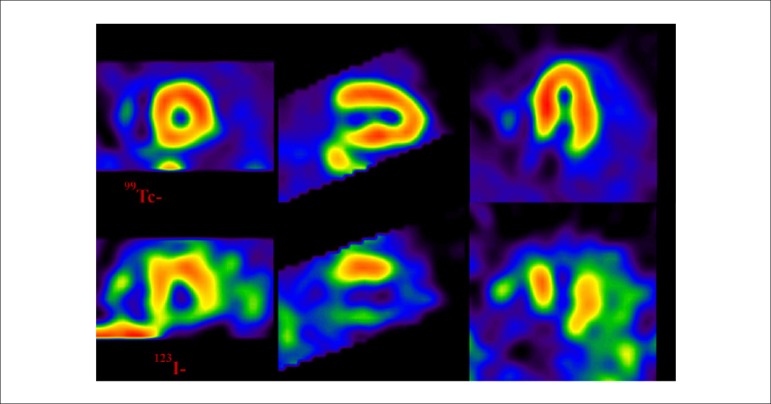
Due to the characteristics described and the ability to evaluate the integrity of the cellular membrane, thallium-201 has the additional property of studying myocardial viability, predominantly related to hibernating myocardium (Figure 1).18-20 This represents the condition of resting left ventricular dysfunction, resulting from chronic hypoperfusion in myocardial regions where, although the myocytes have remained viable (alive), they have chronically depressed contractile function. Hibernation may also be seen as a “flow-contraction” agreement process, where metabolism remains dependent on residual myocardial flow in a manner sufficient for minimum substrate supply and inhibitory substance removal. Therefore, the condition of hibernation, notwithstanding reduced resting coronary flow, is not necessarily associated with the presence of chronic ischemia, given that oxygen supply and consumption ratio may be preserved.21,22
Figure 1.
Hibernation represented as persistent decrease of blood flow and contractile function. Recovery of function is immediate following restoration of coronary flow. %: percent values; NLS: normals.
Source: Adapted from Dilszian.277
Technetium-99m-labeled SESTAMIBI or MIBI (MIBI- 99mTc):23,24 The most frequently used marker for myocardial perfusion studies, is 2-methoxy-isobutyl-isonitrile, a stable, lipophilic, cationic compound belonging to the isonitrile family, which has the property of crossing cellular (sarcolemmal) membranes and binding to myocyte mitochondria through the mechanism of passive diffusion, depending on the electrochemical transmembrane gradient. It therefore involves no energy expenditure. It has a lower first-pass extraction fraction in the myocardium than thallium-201, of approximately 60%.25
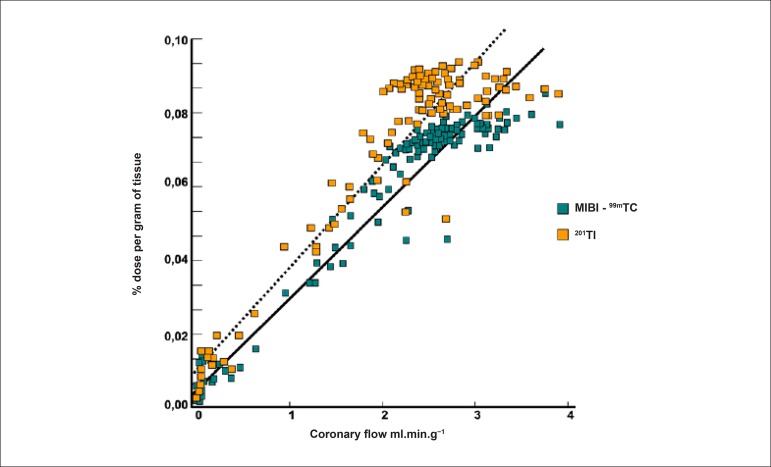
It does not expressively present the phenomenon of redistribution, largely remaining retained within mitochondria. This property makes it necessary to deliver 2 separate injections of the radiopharmaceutical, 1 during the resting and 1 during the stress phase. This may be done either on the same day or on different days. As MIBI is not radioactive, it must be labeled with technetium-99m (99mTc), which has a physical half-life of 6 hours and emits gamma photons in the energy range of 140 keV (photopeak). Similarly to thallium-201, initial myocardial uptake is proportional to regional blood flow, depending on the integrity of the cellular membrane. In this manner, a linear relationship is observed between the intravenous dose per gram of myocardium and blood flow per minute (Figure 2), starting at minimal flow ranges of approximately 2.0 to 2.5 milliliters per gram.minute-1, values normally found in maximum exercise testings. When very high coronary flow are reached, generally over 3.0 mililiters per gram.minute -1, the linear relationship between this variable and myocardial uptake is lost, with decreased blood extraction of the radiopharmaceutical, in a phenomenon known as “roll off”.26-28 Nonetheless, owing to higher energy emission (higher photopeak), measured in keV, it presents higher quality images, in comparison with thallium-201. Finally, the elimination of MIBI-99mTc takes place through the hepatobiliary system, whereas elimination of thallium-201 is mainly achieved through the renal system. Regarding other isonitriles approved by the FDA for assessment of obstructive CAD, only tetrofosmin, whose properties are similar to those of MIBI-99mTc, has been made available for clinical use.
Figure 2.
Linear association between intravenous dose per gram of myocardium and blood flow per minute, using the radiopharmaceuticals 201Tl and MIBI-99mTc. Once coronary flow exceeds 2.5 ml.min.g-1, a loss of linear relationship is observed (phenomenon of “roll off”).
Source: Adapted from Berman DS.116
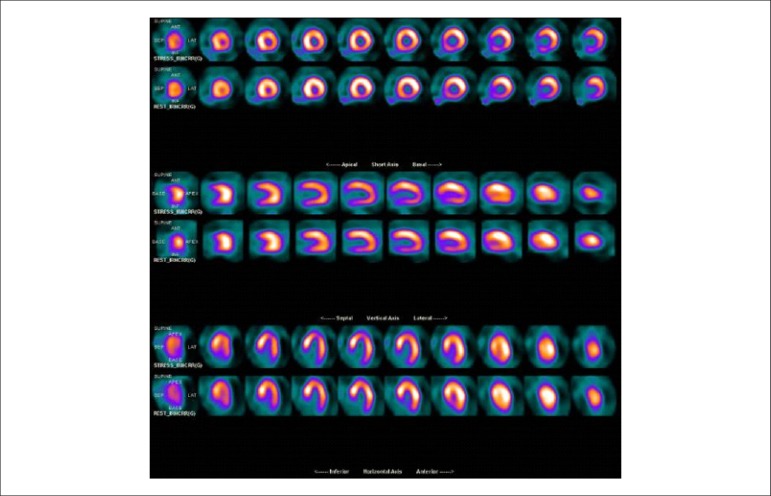
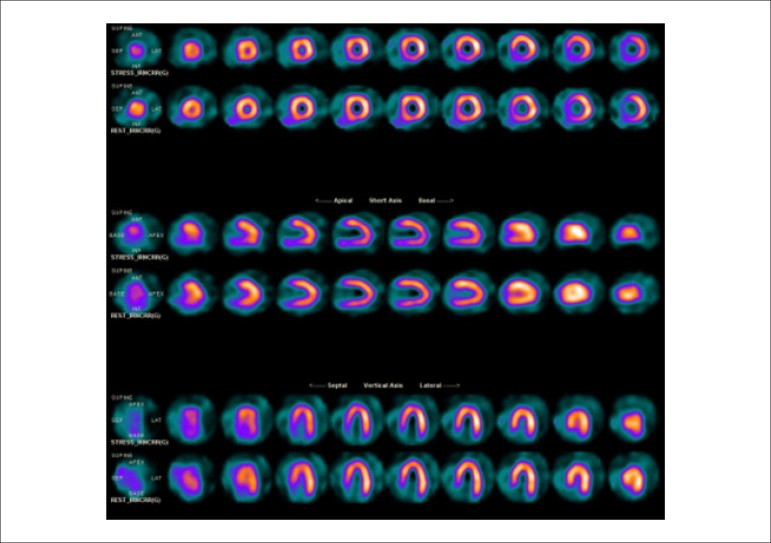
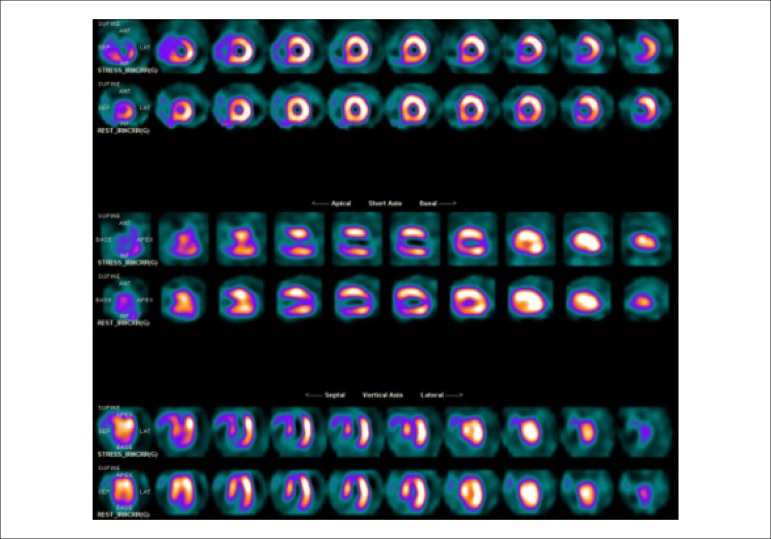
5.2. Myocardial Perfusion Scintigraphy with Tomography Imaging (SPECT)
Technological evolution of computerized systems has made it possible to divide the myocardium of the left ventricle (LV) into tomographic slices measuring only a few millimeters. In conventional gamma cameras (with iodide sodium crystals) the size of a pixel (the smallest component of a digital image) is 6.4 mm, and in CZT (cadmium zinc telluride semiconductors) technology it is 4 mm, representing related cross sections and, consequently, the method’s spatial resolution.29-31 The resulting images facilitate the separation of nearby regions, improving contrast resolution and allowing for better detection of differences in concentrations of radioactivity in the myocardium. The SPECT technique also allows for detection of ischemic regions, even those that are small in size, i.e., approximately 2% of LV mass, in tissue with relatively normal tracer concentration.
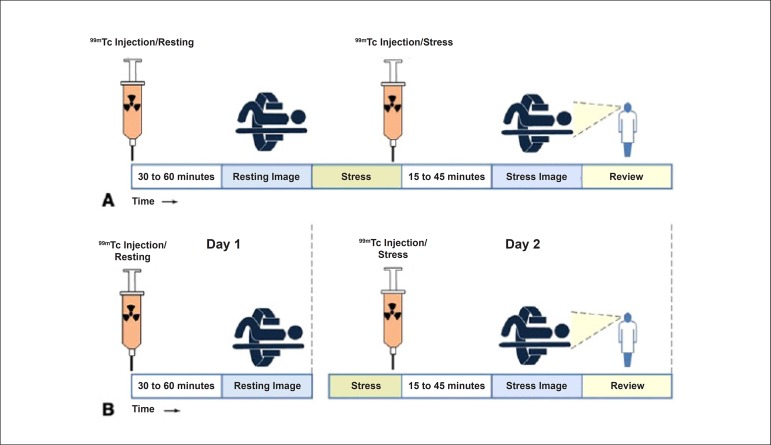
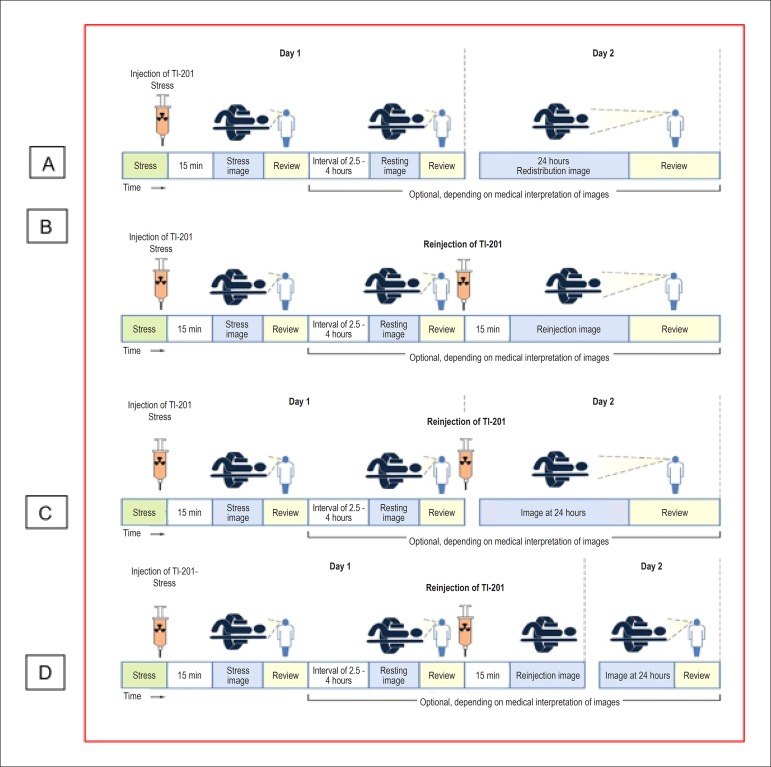
Protocols: The preferred means of obtaining perfusion images of the myocardium and LV function with tracers labeled with technetium-99m (99mTc) is known as the “1-day protocol” (Figure 3A), made up of 2 stages, (resting-stress or stress-resting). During the first step, the injected dose of MIBI-99mTc, measured in millicuries (mCi) or megabecquerels (mBq), is three times lower than the dose administered during the second phase, thus avoiding the residual activity effect or “shining through” phenomenon. Another option is the “2-day protocol” (Figure 3B), where in each phase is performed on a separate day. In this case, similar doses and acquisition parameters are used. It is important to emphasize that, in situations where stress images are taken before resting ones, even if the perfusion images are normal, it is nevertheless important to obtain resting images, except in specific cases, given that analysis of LV function in both situations may provide relevant information, including the possibility of detecting patients with homogenous tracer distribution due to balanced severe coronary diseases. Furthermore, the detection of transient LV dilatation may also be useful in this case, and this requires that both phases be performed. However, in asymptomatic patients who have intermediate/low risks and no clinical evidence of CAD, who have undergone the stress phase as the initial MPS stage and whose perfusion images are normal, it is possible to dispense with the resting phase, in what is known as the “stress only protocol.” In this situation, recent studies have provided evidence that the test’s prognostic value is maintained and that diagnostic ability is similar to the costs of high sensitivity. Furthermore, the patient receives a lower dose of radioactive activity, and total exam time is reduced.32,33
Figure 3.
Perfusion image acquisition and myocardial function with the radiopharmaceuticals sestamibi (MIBI) or tetrofosmin labeled with technetium-99m or 99mTc: “one-day” protocol (A) and “two-day” protocol (B). The legends “99mTc Injection/Resting” and “99mTc Injection/Stress” represent administration of the radiopharmaceutical MIBI-99mTc during both stages, with dosage measured in millicuries (mCi), established in accordance with equipment and acquisition model used, as well as patient weight. In Protocol A, the stress dose is 3 times higher than the resting dose; in Protocol B, the resting and stress doses are similar, considering an interval of 24 hours between image acquisition.
5.3. Myocardial Perfusion Scintigraphy with Tomographic Images Synchronized with Electrocardiogram (Gated-SPECT)34-41
Cardiac images should be acquired synchronized with patient ECG, allowing for additional analysis of ventricular function, simultaneous with myocardial perfusion evaluation. This information adds data to the medical decision-making process within known incremental prognostic values, and it improves test accuracy, especially regarding specificity values. Considering this aspect, in situations where there are doubts between persistent perfusion defects and/or artifacts (due to breast or diaphragmatic attenuation), analysis of ventricular wall motility and thickness may contribute to differentiating these two causes. When apparent reduced relative uptake of a radiopharmaceutical is due to an artifact, the motility and systolic thickness of this wall are normal.
The estimated results of left ventricular ejection fraction (LVEF) that are conventionally considered normal vary according to technique and methodology employed. With the Gated-SPECT technique, this value is ≥ 50% for both sexes; there are few references with differentiated values for men and women, in addition to different established limits of normality. Due to specific aspects related to methodologies used to calculate LVEF, values found in individuals who are shorter and individuals with smaller ventricular cavities and/or hypertrophic ventricles, especially in women, may be overestimated, at times exceeding values of 75% to 80%.
Calculations of LVEF and ventricular volumes obtained by Gated-SPECT may be utilized for prognostic stratification. LVEF < 45% and end systolic volume (ESV) > 70 ml are associated with increased risks of cardiac death.42,43 This analysis may be carried out either while resting or under stress; it should preferably be done during both steps, however, considering the possibility of detecting transient LV dysfunctions induced by physical exercise or pharmacological stress.
Cardiac arrhythmias pose difficulties to the acquisition of ECG-synchronized images and may significantly influence the results obtained for ejection fraction and produce artifacts in myocardial perfusion images. There is a technically defined time window for RR interval variation, generally around 20%, after which point heartbeats are rejected. This situation means that if there is an arrhythmia which produces variations between RR intervals above these established limits, such as persistent AF, the corresponding data from that specific cardiac cycle will be rejected, and there will consequently be lower counting statistics. In these cases, images should be acquired without ECG synchronization in order to avoid the occurrence of artifacts.
5.4. Cardiovascular Stress
The basic principle of using cardiovascular stress associated with myocardial perfusion images consists of creating heterogeneity in blood flow between vascular territories irrigated by normal coronary arteries with significant obstructive stenoses.44,45 The use of myocardial perfusion agents makes it possible to visualize this heterogeneity in regional blood flow. In practice, of all existing cardiovascular stressors, only ET and pharmacological tests have been used.
Both stress modalities, physical exercise and pharmacological vasodilation, have similar sensitivity and specificity for the detection of CAD via analysis of perfusion images.46-48
Physical stress: ET is the associated method of choice for diagnostic and prognostic values, which have already been established in conformity with clinical, hemodynamic, and electrocardiographic variables obtained during exercise, which add incremental data to myocardial perfusion study. Stress tests have a higher chance of revealing abnormalities in patients with more severe and extensive obstructive arterial disease. Chest pain and/or decreased systolic blood pressure (SBP) during low levels of exercise are highly important findings that are associated with adverse prognoses and multivessel coronary disease. Other markers of unfavorable prognosis include high-magnitude ST segment depression, with a horizontal or downsloping aspect, which may appear early during low workloads or be characterized by late recovery after stress has ceased, present in multiple leads, among others (Table 9).
Table 9.
Exercise testing parameters associated with unfavorable prognosis and multivessel coronary disease.
| • ECG: |
| - ST-segment depression ≥ 2 mm, with descending morphology and early appearance (metabolic load < 5 - 6 METs), involving multiple leads, usually lasting for ≥ 5 minutes of recovery |
| - Exercise-induced ST-segment elevations |
| - Reproducible, symptomatic, or sustained ventricular tachycardia (> 30 s) |
| • Metabolic load < 5 - 6 METs* |
| • Chronotropic incompetence |
| • Systolic blood pressure: inability to reach values ≥ 120 mmHg, or sustained decrease ≥ 10 mmHg, or fall below resting values during progressive exercise |
| • Symptoms: angina pectoris when performing a lower workload , generally during the beginning of exercise, when conventional protocols are applied |
ECG: electrocardiogram; MET: metabolic equivalent. (*1 MET = oxygen consumption in supine resting conditions, equivalent to 3.5 mL.kg-1.min-1)
Some studies have incorporated stress test variables into diagnostic and prognostic scores.49 The most widely used in our context is the Duke prognostic score. Using Cox’s regression analysis, Mark DB et al. proposed50 and validated51 this score for use with the exercise treadmill test and the Bruce protocol. It is calculated by the following formula:
or
The angina index has a value of 0 (zero) if there are no symptoms during exercise, 1 (one) if non-limiting chest pain occurs, and 2 (two) if the pain is impeditive (growing intensity) as exercise proceeds. In accordance with the results of the regression equation, patients are classified as follows:
High-risk group: patients with scores ≤ -11, with an annual cardiovascular mortality rate ≥ 5%.
-
Low-risk group: patients with scores ≥ 5, with an annual cardiovascular mortality rate < 1%. In clinical practice, when patients are considered high-risk, this reinforces a priori the indication for invasive study with the aim of managing and directing medical treatment, be it interventional or not, while always taking the possibility of improving morbimortality and quality of life into account. In patients with intermediates results, i.e., scores between > -11 and < +5, in order to reclassify risk, complementary exams associated with imaging, such as the following, may be required:
- Myocardial perfusion scintigraphy (MPS) with ET or vasodilators.
- Vasodilator stress cardiac magnetic resonance (technique associated with inability to exercise).
- Doppler echocardiogram under stress or specific conditions.
- Computerized angiotomography of coronary arteries.
Finally, in patients considered low-risk, medical management is related to prevention measures. On the other hand, based on a growing base of evidence, these methods,52 especially MPS, have become of paramount importance for quantifying ischemic area, even in patients who are considered high-risk, with the aim of assisting and directing the medical approach to be adopted,53-58 notwithstanding the unavailability of information from randomized clinical trials such as the “Ischemia Study,” which will be able to assist in better management of patients with extensive areas of the myocardium at risk.59
Furthermore, emphasis given to exercise as the primary stress-producing agent of choice within the cardiovascular system has become clear, given that it is the most physiological method for triggering myocardial ischemia, based on sympathetic stimulation and the increase in the main determinants of myocardial oxygen consumption (MVO2), such as HR, blood pressure, and myocardial contractility. Likewise, exercise leads to coronary vasodilation through biochemical mechanisms, resulting in increased blood flow to the myocardium and greater oxygen supply, thus meeting the necessary demands imposed during the application of extreme effort. This ability to increase coronary blood flow, which reaches three to four times baseline values during peak exercise, in the absence of significant obstructive coronary lesions, conceptually represents the phenomenon known as “coronary reserve,” considered the main characteristic of MPS with radiopharmaceuticals. Moreover, with respect to the limitations and contraindications of this methodology,60 joint analysis of both stress test and cardiac imaging exams will play a fundamental role in the medical decision-making process, albeit in view of previous clinical information or pretest probability of obstructive CAD.
With relation to the main methodological aspects, the following stand out:
Prior venous access in an arm, in a “Y” shape (separate routes), for radiopharmaceutical injection during peak exercise and subsequent flush with saline solution, respectively.
Safety criteria for administering and interrupting stress should be in accordance with established guidelines, reinforcing the need for a maximum test.61
Following intravenous administration of the radiopharmaceutical, stimulate continuation of stress for 1 more minute.
When using MIBI-99mTc (absolute preference in Brazil), image acquisition follows conventional protocols (30 to 60 minutes after stopping stress). Variations in initial acquisition time depend on patient type (obesity, prior abdominal surgery, prominent extracardiac activity in the resting images phase.
When using thallium-201, considering the phenomenon of redistribution, images should be taken 10 to 15 minutes after stopping stress.
Pharmacological tests: Represent excellent alternatives for evaluating patients with physical limitations or clinical impediments to undergoing efficacious exercise testing . The most frequent conditions are found in Table 10. They represent around 20% to 30% of all cases of scintigraphy referral and approximately 50% of elderly patients.62 The drugs used in these circumstances are dipyridamole, adenosine or regadenoson, and dobutamine. These drugs induce maximum vasodilation and increase coronary flow, allowing for assessment of coronary reserve, with diagnostic and prognostic power similar to that of exercise,63,64 which has recently been extended to elderly patients and women.65,66
Table 10.
Main indications for use of pharmacological stress in patients with contraindications or limitations to undergoing exercise stress24,46
| • Motor sequelae from cerebral vascular insufficiency and degenerative or inflammatory musculoskeletal pathologies |
| • Compensated congestive heart failure |
| • Chronic pulmonary obstructive disease with important functional restriction, but without recent hyperresponsiveness |
| • Low functional capacity |
| • Other non-cardiac conditions that result in an inability to exercise efficiently |
| • Severe arterial hypertension |
| • Complex ventricular arrhythmias triggered by effort |
| • Pre-operative cardiological assessment for major abdominal vascular surgery |
| • Presence of left bundle branch intraventricular conduction disorders |
| • Risk stratification for recent evolution of myocardial infarction |
| • Use of drugs that interfere with oxygen consumption elevation |
| • Presence of artificial electric stimulation |
In cases of left His bundle branch block or artificial pacemaker with ventricular stimulation, the first option is a pharmacological test with dipyridamole or adenosine, with the aim of avoiding what are known as false-positive results (alterations in relative radiopharmaceutical uptake, in the absence of obstructive lesions). These are caused by atypical movement of the interventricular septum, which occurs in these situations and is accentuated when myocardial scintigraphy is performed with ET. Reduced radiopharmaceutical uptake is often observed in these patients and is most frequently related to the septal region, which may be exacerbated by the stress test, as increased HR increases paradoxical septal motion and, consequently, reduces perfusion in this wall.67,68
Primary vasodilators: Dipyridamole, adenosine, and regadenoson (not available for routine clinical practice in Brazil) provoke a significant increase in coronary flow in normal arteries and a small or nonexistent increase in arteries with functionally significant stenosis, thus resulting in relative heterogeneity of flow between LV walls. During maximum vasodilatation, when the radioisotope is injected, the difference in relative radiopharmaceutical uptake in LV walls will also be observed, making it possible to diagnose coronary disease:
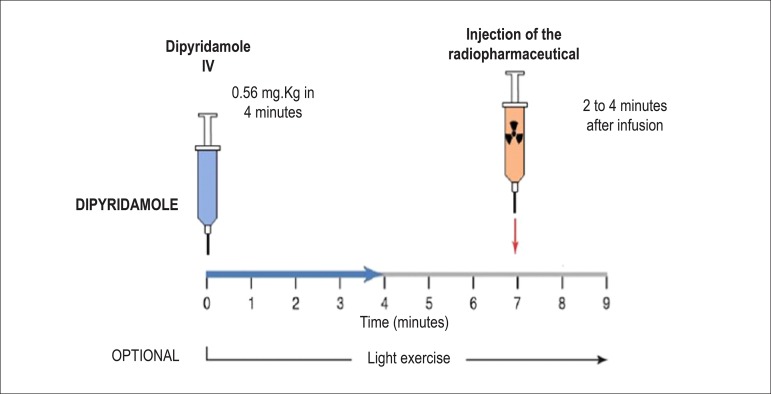
Dipyridamole: the total dose of dipyridamole is 0.56 mg.kg-1 up to a maximum dose of 60 mg or 6 vials (a 2-ml vial = 10 mg), administered intravenously (IV), preferably with a 4-minute infusion pump, diluted in 50 ml of saline solution (SS). It may, alternatively, be injected manually (with a 20-ml syringe), using the same dilution. Alternatively, a more elevated dose of 0.84 mg.kg-1 may be used in select cases. The radiopharmaceutical is administered IV during hyperemia or maximum vasodilation, 2 to 4 minutes after the end of dipyridamole infusion (Figure 4). Dipyridamole inhibits the action of the enzyme adenosine deaminase, wich degrades endogenous adenosine, in addition to blocking reuptake of adenosine into the cellular membrane, with a consequent increase in extracellular concentration and resulting coronary vasodilation. Its biological half-life is approximately 45 minutes.
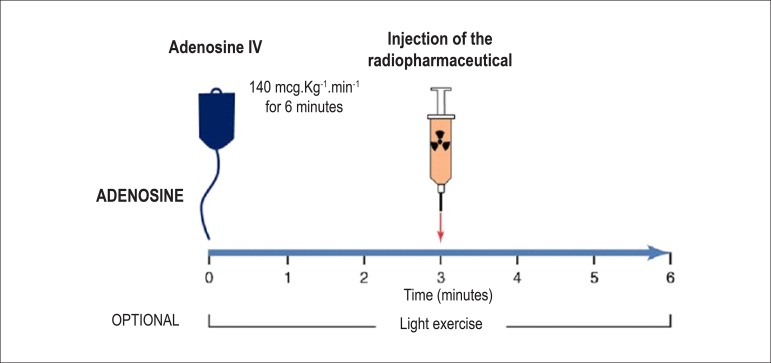
Adenosine: The usual dose is 140 µg.kg-1.min-1, and it must mandatorily be administered via a 6-minute continuous infusion pump, diluted in 50 ml of SS, with the injection of the radiopharmaceutical administered during the third minute via a different intravenous access (Figure 5). It is, also, possible to inject the solution for 4 minutes, in which case the radiopharmaceutical is administered during the second minute.69 Because xanthines block the vasodilation effect, patients should be instructed to suspend them for 24 hours before a scheduled exam with dipyridamole or 12 hours before a scheduled exam with adenosine, in addition to any other drug or product, food, or drink that contains methylxanthines or theophyllines, including coffee, tea, soft drinks, chocolate, energy drinks, compound analgesics containing caffeine, especially for treatment of muscular pain or migraines, et al. Reference lists are available for consultation.70 Adenosine induces coronary vasodilation via specific activation of A2A receptors in the cellular membrane, resulting in increased coronary flow up to 4- or 5-fold resting values.
Figure 4.
Myocardial perfusion scintigraphy associated with injection of dipyridamole. The moment of maximum vasodilation or coronary hyperemia occurs between 2 and 4 minutes after completing intravenous dipyridamole administration (blue arrow, 4 minutes), at which point the radiopharmaceutical (99mTc-tetrofosmin or MIBI-99mTc, orange arrow) is injected. Clinical observation should be continuous throughout the exam, registering blood pressure, heart rate, and electrocardiogram every 2 minutes or in accordance with medical decision, with a typical total exam time of 9 to 10 minutes.24,46
Figure 5.
Myocardial perfusion scintigraphy associated with injection of adenosine. The need for continuous intravenous administration is due to the drug’s ultrashort plasma half-life (2 to 10 seconds), with the aim of maintaining coronary hyperemia, which reaches its peak close to the third minute. At this moment, the radiopharmaceutical (MIBI-99mTc) is injected. After completing the solution at 6 minutes, frequent monitoring of blood pressure, heart rate, and electrocardiographic registers is maintained for a variable time of 4 to 6 minutes
Accuracy for detecting CAD with the use of MPS is comparable between both drugs. It is worth reiterating that, in exams using dipyridamole and adenosine, modifications in the ST segment occur relatively infrequently, even in patients with obstructive CAD (lower sensibility). In some instances, only the relative difference in flow observed in patients with different degrees of luminal obstruction and coronary reserve will determine perfusion defects, and ischemia will not necessarily be present. For this condition, collateral circulation is necessary, which causes coronary steal, with consequent alterations in contractility. Nevertheless, the sensitivity of scintigraphy images associated with the use of pharmacological agents or stress tests is similar. Adverse effects or “paraeffects” of using these drugs23,71 occur in approximately 50% of patients with dipyridamole and in up to 80% of patients with adenosine. Common side effects include headache, dizziness, flushed face, feeling hot, chest pain, ST alterations and others (Tables 11 and 12).72 These manifestations generally do not last long, and in most cases they may be reversed by administering intravenous aminophylline at 1 to 2 mg.kg-1 or 72 mg (3 ml) to 240 mg (10 ml or 1 vial) 2 minutes after injecting the radiotracer, when MPS is associated with dipyridamole. When adenosine is used, there is no need to inject an antagonist, given its ultrashort half-life, from 2 - 10 seconds, the recommendation being simply to interrupt the infusion. When it is not medically possible to perform either the physical stress or the pharmacological dilation modality with dipyridamole or adenosine, intravenous administration of dobutamine solution may be the best option for assessing coronary reserve flow, with regards to increased MVO2. Contraindications to dipyridamole and adenosine use are listed in Table 13.
Table 11.
Adverse effects or "paraeffects" related to intravenous administration of dipyridamole for performance of myocardial perfusion scintigraphy24,46
| Adverse effects or paraeffects | % |
|---|---|
| Chest pain | 20 |
| Headache | 12 |
| Dizziness | 12 |
| Alterations in ST | 8 |
| Ventricular extrasystoles | 5 |
| Nausea | 5 |
| Arterial hypotension | 5 |
| Facial flushing | 3 |
| Atrioventricular blockage | 2 |
| Fatal or non-fatal myocardial infarction | Extremely rare |
| Any minor event | 50 |
Table 12.
Adverse effects or "paraeffects" related to intravenous adenosine administration via infusion pump for performance of myocardial perfusion scintigraphy24,46
| Adverse effects or paraeffects | % |
|---|---|
| Facial flushing | 35 to 40 |
| Chest pain | 25 to 30 |
| Shortness of breath | 20 |
| Dizziness | 7 |
| Nausea | 5 |
| Symptoms of hypotension | 5 |
| Atrioventricular blockage | 8 |
| Alterations in ST | 5 - 7 |
| Atrial fibrillation | Case reports |
| Convulsions | Case reports |
| Hemorrhagic/ischemic stroke | Case reports |
| Any minor event | 80 |
Table 13.
| Absolute |
| • Bronchospastic disease during activity, recent hyperreactivity (< 3 months), status asthmaticus |
| • Second- or third-degree atrioventricular blockage, in the absence of a pacemaker |
| • Arterial hypotension (systolic blood pressure less than 90 mmHg) |
| • Recent transient ischemic attack or cerebrovascular accident (< 2 months) |
| • Recent use (less than 24 hours) of dipyridamole in patients who are to receive adenosine |
| Relative |
| • History of reactive pulmonary disease, with no recent crises (> 3 months) |
| • Sinus node disease |
| • Severe sinus bradycardia |
| • Severe bilateral carotid disease |
It is, finally, important to stress that, with both dipyridamole and adenosine, no significant increases are observed in MVO2, which, in clinical practice, is translated as the product of heart rate (HR) × systolic blood pressure (SBP), or the double product. During pharmacological stimulation, SBP values generally drop by around 10% while HR increases by approximately the same proportion, with no consequent increase in MVO2.
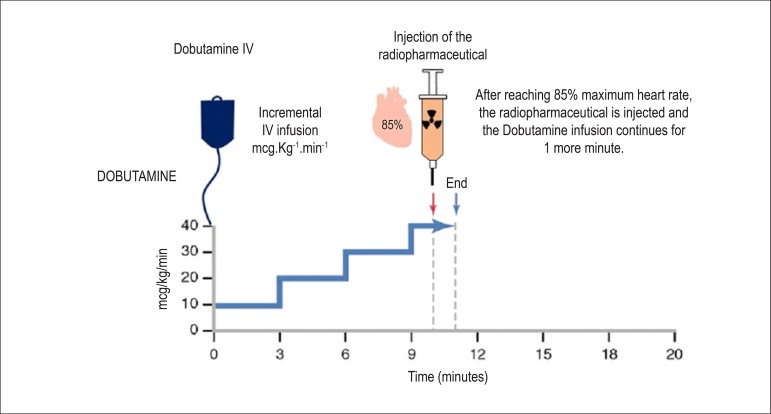
Drugs that promote elevated myocardial oxygen consumption: These drugs represent an alternative for patients who cannot undergo ET or pharmacological stress with dipyridamole or adenosine. Examples include patients who have contraindications or limitations for stress test, as well as pulmonary obstructive disease with recent crises of bronchial hyperreactivity, arterial hypotension (SBP < 90 mmHg), and significant obstructive carotid artery lesions on both sides. This is also an alternative modality in patients indicated for dipyridamole or adenosine who have ingested substances derived from caffeine or methylxanthines (competitive antagonists) over the past 24 and 12 hours, respectively. The most commonly used is dobutamine, which acts on beta-1 (β-1) adrenergic receptors, with chronotropic and inotropic stimulation, depending on the infused dose, in addition to direct effects on beta-2 (β-2) receptors, with peripheral vasodilation response. This results in an increase in cardiac output, HR, and SBP, leading to an increase in MVO2 and, consequently, in coronary vasodilation. Protocol: The protocol begins with venous administration of the solution (250 mg of dobutamine diluted in 250 ml of saline solution - 1 mg per 1 ml) via infusion pump at a dose of 10 ug.kg-1.min-1 for 3 minutes (first step), followed by 20 µg.kg-1.min-1 for 3 minutes (second step), adding 10 µg.kg-1.min-1 every 3 minutes (third and fourth steps) until the maximum dose of 40 µg.kg-1.min-1 has been reached (Figure 6).73,74 In patients who have not reached submaximal HR and who do not have evidence of ischemia, it is possible to associate intravenous atropine (0.25 to 2 mg) and perform isometric stress with hand grip maneuvers (e.g., compressing a tennis ball). A Brazilian study has demonstrated that early use of atropine (following the first phase of dobutamine infusion) is safe and that it reduces infusion time and complaints during stress, without affecting diagnostic precision.75 Furthermore, the presence of perfusion defects induced by pharmacological vasodilatation and motility abnormalities triggered by stress aggregate incremental prognostic value to the test, which has recently been validated with the use of ultrarapid cameras (CZT technology).76 Contraindications to dobutamine use may be found in Table 14. Patients on betablockers should stop taking these medications for 48 to 72 hours before the test. Special attention should be given to patients with bronchospasm undergoing MPS with dobutamine, whose plasma half-life is around 2 to 3 minutes, considering that its antagonist is metoprolol at an intravenous dose of 5 mg and that it is contraindicated in the presence of pulmonary obstructive disease. The most frequent adverse events or paraeffects associated with administration of dobutamine solution are listed in Table 15. To reverse them, in addition to metoprolol, other intravenous short-acting betablockers, such as esmolol (0.5 mg.kg), which is available, should be injected after the first minute of radiotracer injection.
Figure 6.
Myocardial perfusion scintigraphy associated with intravenous administration of dobutamine solution (250 mg or 1 vial diluted in 250 ml of saline solution). It may begin with an alternative initial dose of 5 mcg.kg-1.min-1, for 3 minutes, with sequentially increasing doses every 3 minutes, up to 40 mcg.kg-1.min-1 or until 85% of maximum heart rate has been reached (explained in the figure and the text), at which point the radiopharmaceutical (MIBI-99mTc or 99mTc-tetrofosmin) is injected. In the event of inadequate increase in heart rate and in the absence of contraindications (glaucoma, prostatic hypertrophy), atropine is additionally recommended, either early on or starting at the third step.
Table 14.
| Absolute |
| • Cardiac arrhythmias including atrial fibrillation and ventricular tachycardia (sustained or non-sustained) |
| • Severe aortic stenosis and hypertrophic obstructive cardiomyopathy |
| • Systolic arterial hypotension (< 90 mmHg), uncontrolled systolic arterial hypertension (systolic > 200 mmHg), severe or stage III hypertension |
| • Unstable angina or recent myocardial infarction |
| • Aneurysms or aortic dissection |
| • Symptomatic vascular cerebral insufficiency |
| • Presence of implanted cardiac defibrillator |
| • Alterations in metabolism of potassium |
| Relative |
| • Abdominal aortic aneurysm (> 5 cm in diameter) |
| • Presence of thrombi in left ventricle |
| • Left ventricular ejection fraction < 25% (due to increased risk of ventricular arrhythmias) |
Table 15.
| Adverse effects | % |
|---|---|
| ST alterations | 33 |
| Precordial pain | 31 |
| Palpitation | 29 |
| Headache | 14 |
| Facial flushing | 14 |
| Dyspnea | 14 |
| Significant arrhythmias (supraventricular and ventricular) | 8 to 10 |
Combined stress: The association of dynamic stress with low workloads (e.g., until the second stage of the Bruce protocol or until feeling light fatigue, equivalent to the number 13 on the subjective Borg stress scale) and vasodilators has been shown to reduce subdiaphragmatic (hepatic) activity and improve the ratio of radiation activity emitted between the target organ and the viscera (background), with consequent improvements in image quality.77 It has similarly shown a decrease in the occurrence of adverse effects resulting from the infusion of dipyridamole or adenosine, as well as the incidence of atrioventricular blockage. This protocol is ideal for patients who are able to exercise but who are using medications that limit increases in HR (betablockers, antiarrhythmic drugs, et al.).
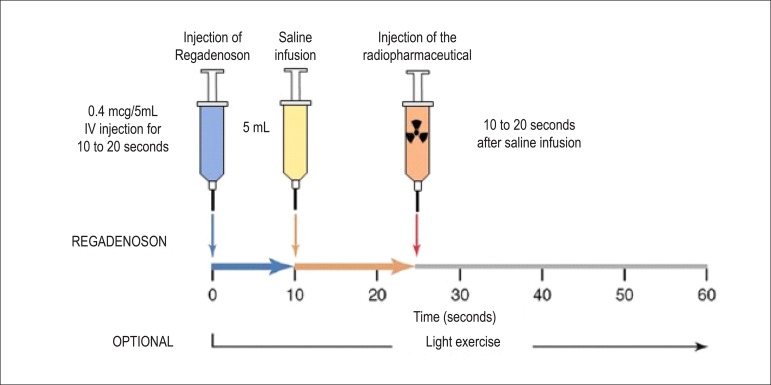
New drugs: There are 3 types of adenosine receptors (Table 16). The use of specific selective antagonists to A2 receptors has shown evidence of adequate coronary hyperemia and lower intensity of systemic effects, especially chest pain and atrioventricular blockage. A double-blind, randomized (regadenoson or adenosine), multicenter study78 involving 784 patients has shown that diagnostic information is similar and that there were no serious adverse effects; regadenoson, however, was tolerated better than adenosine. Second-degree atrioventricular blockage occurred in 3 patients with adenosine and in no patients with regadenoson. Regadenoson’s short biological half-life minimizes and limits the duration of adverse effects, diminishing monitoring time. It is administered via bolus, and it is not necessary to adjust dose to body weight (Figure 7). Its use is promising in patients with chronic obstructive pulmonary disease. The incidence of serious complications79 with the performance of cardiovascular stress is related in Table 17.
Table 16.
Types of existing receptors in the cellular membrane and responses to stimuli
| Type | Resulting effects |
|---|---|
| A1 | Atrioventricular blockage |
| A2a | Coronary artery vasodilation |
| A2b | Peripheral vasodilation, bronchospasm |
| A3 | Bronchospasm |
Figure 7.
Myocardial perfusion scintigraphy associated with intravenous administration of regadenoson, a specific agonist of adenosine A2A receptors in the cellular membrane. Activation of the receptor produces coronary vasodilation with a consequent increase in flow, similar to dipyridamole and adenosine. Maximum plasma concentration is reached 1 to 4 minutes after injection, with a biological half-life of 2 to 4 minutes during the first phase. The intermediate and the late phases follow, with approximate duration of 30 minutes (loss of pharmocodynamic effect) and 2 hours (decline in plasma concentration). The radiopharmaceutical, MIBI-99mTc or Tetrofosmin- 99mTc, is injected at the moment of maximum hyperemia, close to 30 seconds after injection of regadenoson.
Table 17.
Serious adverse events related to cardiovascular stress methods (rate of events observed per 1,000 individuals)79
| Serious events | ET | Dobut | Dipy | Aden | Regad |
|---|---|---|---|---|---|
| Any event | 0.1 - 3.46 | 2.988 | 0.714 - 2.6 | 0.97 | CR |
| Death | 0 to 0.25 | CR | 0.5 | CR | CR |
| VF/VT | 0 to 25.7 | 0.6 - 1.35 | NR | NR | NR |
| AMI | 0.038 | 0.3 - 3 | 1 | 0.108 | CR |
| Cardiac rupture | Unk | CR | NR | NR | NR |
| High-grade AVB / ASY | Unk | NR | CR | CR | CR |
| Bronchospasm | Unk | NR | 1.5 | 0.76 | CR |
| Stroke/TIA | Unk | CR | NR | NR | CR |
| AF | Unk | 5 - 40 | NR | NR | CR |
| Seizure | Unk | CR | NR | 1.5 | CR |
Aden: adenosine; AVB: atrioventricular blockage; AF: atrial fibrillation; AMI: acute myocardial infarction; ASY: asystole; CR: case report; Dipy: dipyridamole; Dobut: dobutamine; ET: exercise testing; NR: not reported; Regad: regadenoson; TIA: transient ischemic attack; Unk: Unknown; VF/VT: ventricular fibrillation/ventricular tachycardia.
5.5. Image Generation and Perfusion Defects in Myocardial Scintigraphy with Radioisotopes
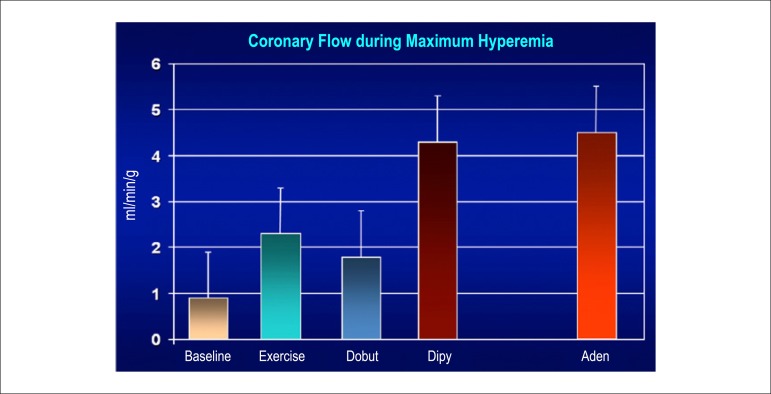
Resting coronary flow is 1 ml.g.min-1, increased 3- to 5-fold during maximal vasodilation or hyperemia, under physical or pharmacological stress (Figure 8).28 In the presence of obstructive coronary lesions, resting coronary flow decreases when luminal narrowing is greater than 80%, due to exhaustion of the coronary reserve. When physical or pharmacological stress are applied, early exhaustion of the coronary reserve is observed, and it then exhibits a drop, generally beginning with lesions with luminal narrowing of 50%.80 This information has currently been validated based on invasive measures of coronary flow reserve (CFR), fractional flow reserve (FFR), and instantaneous flow reserve (IFR), considered “standard” for characterizing myocardial ischemia; some have also been reproduced by non-invasive PET methods.81-86 Tests with pharmacological stimulation using dipyridamole or adenosine associated with MPS are considered frequently to result in coronary flows in the range of 4 ml per gram of myocardium per minute,87-89 generating homogenous relative uptake patterns of the radioisotopes in the myocardium, and scintigraphy images are considered normal when the coronary arteries are free of atherosclerotic processes. There are, however, specific situations in which patients with balanced multivessel disease (lesions in 3 arteries with similar coronary reserve) in which perfusion images appear with apparently homogeneous radiopharmaceutical distribution.90
Figure 8.
Effects of different types of stress methods on coronary flow elevation and values reached during maximum hyperemia. Baseline: resting coronary flow, 1 ml.min.g-1; Exercise: reaching values 2.5 to 3.5 times baseline coronary flow value; Dobut (dobutamine): reaching values around 2.0 to 2.5 times baseline coronary flow value; Dipy (dipyridamole) and Aden (adenosine): reaching values as high as 5.0 times baseline coronary flow value.28
From the conceptual point of view, it is necessary to comprehend that the generation of scintigraphy images is based on relative radiopharmaceutical uptake, which is injected intravenously during physical exercise or pharmacological test, predominantly in the LV myocardium. Comparison of radiopharmaceutical uptake between ventricular walls is expressed in images based on a scale of colors, created by specific computer programs, which, in addition to allowing for subjective analysis of perfusion, make semi-quantitative and quantitative evaluation of affected myocardial area possible.
5.6. Possible Scintigraphy Imaging Results, Using Qualitative, Semi-quantitative, and Quantitative Analyses
Visual or qualitative analysis: By simply inspecting images resulting from perfusion tomography and ventricular function exams (Gated-SPECT technique), it is possible to assess blood flow and regional contractility of the LV myocardium indirectly. Tomography images are reconstructed as multiple slices along the anatomical axis of the LV, defined as corresponding regions and respective relations with coronary territory. The slices are taken on the short, long vertical, and long horizontal axes (Figure 9). Characterization of uptake of the radiopharmaceutical MIBI-99mTc or Tetrofosmin-99mTc during both exam stages (resting and stress, 1 day protocol) and thallium-201 during the stress and redistribution phases focuses on the anterior, septal, inferior , lateral, and apical regions of the LV (Figure 9). The short-axis projection uses transverse tomographic slices of the LV, sweeping from the apex or distal portion, through the middle of the cavity, to the basal portion. All regions and subdivisions are numerically identified, in accordance with the established scoring system, with the aim of standardizing segmentary analysis of the LV myocardium for perfusion study. Division into 17 segments has consensually been accepted, resulting in less interpretation subjectivity (Figure 10 and Table 18). Different radiopharmaceutical uptake and retention patterns allow for differentiation of normal, ischemic, and fibrotic tissues. The normal myocardium has similar uptake during both the stress and resting/redistribution phases, whereas the ischemic myocardium shows reduced relative uptake in stress images and normal uptake during resting/redistribution. Fibrotic tissue, on the other hand, shows reduced relative uptake during both study phases. If fibrotic tissue coexists with an ischemic yet viable myocardium, reduced relative uptake will be observed during the stress phase, with partial improvement during the resting/redistribution phase. Hibernating myocardium will also show persistent reduced uptake, or be it, reduced uptake that is similar in both the stress and the resting phases. To differentiate it from fibrotic tissue, it is possible to perform assessment of viable myocardium with thallium-201, in which case it is sometimes necessary to add another phase or stage, namely that of late redistribution or reinjection, interpreted in the same manner.
Figure 9.
Two-dimensional reconstruction of scintigraphy images representing normal perfusion patterns (lower images), in line with minor axis (1), vertical long axis (2), and horizontal long axis (3) cross sections and their respective corresponding anatomical cross sections (upper images). A: anterior; AP: apical; I: inferior; L: lateral; S: septal. Adapted from Mastrocola LE.195
Figure 10.
Numerical segmentation model of the left ventricular myocardium in 17 parts, considering tomographic slices of the minor and long vertical axes (distal or apical, middle, and basal or proximal portions), representing the myocardial regions; furthermore, correspondence of segments may be seen as presented in the polar map, which represents radiopharmaceutical distribution throughout the left ventricular myocardium in the form of a polar map, whose center corresponds to the apex and whose peripheries correspond to the basal portions. The correspondence between the numerical classifications and their respective segments is described in Table 18.
Table 18.
Numerical classification of segmentary division of the left ventricular myocardium into 17 parts, in cross sections (slices ?) of the minor and long-vertical axes
| Regions/walls | Distal/apical portion | Middle portion | Proximal/basal portion |
|---|---|---|---|
| Anterior | 13 | 7 | 1 |
| Anteroseptal | - | 8 | 2 |
| Inferoseptal | - | 9 | 3 |
| Septal | 14 | - | - |
| Inferior | 15 | 10 | 4 |
| Inferolateral | - | 11 | 5 |
| Anterolateral | - | 12 | 6 |
| Lateral | 16 | - | - |
| Apex | 17 | - | - |
Obs: apical region includes the apex and distal or apical portion of all walls (13,14,15,16).
Semiquantitative analysis: With the aim of numerically assessing the intensity of radiopharmaceutical uptake (perfusion), within the established standards (17-segment model), specific scores have been developed: a) perfusion - considers the following numerical scale: 0 = normal; 1 = mildly reduced radiopharmaceutical uptake; 2 = moderately reduced uptake; 3 = severely reduced uptake; 4 = absence of radiopharmaceutical uptake. Scores of 3 or 4 are normally associated with coronary stenosis of > 90%. Therefore, the higher the number of affected segments is; the more extensive the process; the higher the summed scores, and the greater the severity will be. This has an unquestionable prognostic value for patients with CAD. The following calculations are achieved by the sum of values attributed to each segment: the sum of the values attributed to each segment during the stress phase is known as the “summed stress score” (SSS); this is repeated during the baseline or redistribution phase to obtain the “summed rest/redistribution score” (SRS). The difference between the SSS and the SRS is known as the “summed difference score” (SDS). According to Hachamovitch et al.56,57 numerical SSS values < 4 are considered normal; between 4 and 8, mildly abnormal; between 9 and 13, moderately abnormal; and > 13, severely abnormal. It is worth emphasizing that SSS values < 4, which may not necessarily be zero, are understood as normal, because there are myocardial regions which show lower radiopharmaceutical concentrations in and of themselves and may, consequently, receive values other than zero.
Quantitative analysis: Polar maps are two- or three-dimensional reconstructions of the LV, initially elaborated with the proposal of encompassing relative radiopharmaceutical distribution throughout the heart in a single image. They are shown in circular form, resembling a target, for which reason they are also known as “bull’s eye plots.” Radiopharmaceutical uptake, which is representative of perfusion, is shown on a color scale, with the LV apex occupying the center of the target, while basal regions of the heart are represented by the outermost circle of the target (Figure 10). Programs capable of reconstructing these images also allow for percentage quantification of areas with reduced uptake by comparing the images to a databank of normal individuals of the same age and sex. Perfusion defects may also be quantified by the number of pixels in a determined region and by existing standard deviations in relation to normal perfusion areas.
We may also obtain polar maps with parameters relative to ventricular function, such as LV wall motility and systolic thickness. These methods of quantitative analysis serve as complements to assist in qualitative or semiqualitative visual analysis.
Evaluation of ventricular function with perfusion agents: In a manner analogous to that described for perfusion study, segmentary contractile analysis of the LV makes use of motility and systolic thickness scores for each segment, also considering division into 17 segments, visualized in the cross sections of the minor axis (distal, mid-cavity, and proximal regions) and the long vertical axis (anteroapical and inferoapical regions). Numerical values are attributed. Analysis of motility of LV walls is performed directly on the computer monitor, making the subendocardial contour visible. Analysis of systolic thickness should be directed to the color scale chosen for a group of images. When thickness is within normal limits, the color increment is observed toward the bottom of the scale. Furthermore, it is possible to obtain percentage of thickness in each region. For distal and middle slices of the minor axis, as well as for the apex, average normal thickness is around 40%, with a score of zero (0) Thicknesses between 30% and 40% are interpreted as borderline; those between 20% and 30% receive a score of 1 (mild reduction); 10% to 20%, a score of 2 (moderate to severe reduction); and less than 10%, a score of 3 (absence of thickness). In the proximal (or basal) cross section of the minor axis, thickness of around 20% is considered normal. A score of 1 is not used, but rather only scores of 2 and 3. Abnormalities in motility and thickness generally go hand-in-hand, with slight differences in gradation between the two (Table 19) and in the resulting sums. In some cases, we may observe discrepancy between results, for instance, following revascularization surgery and in the presence of left bundle branch block, in which the motility of the interventricular septum is compromised, whereas thickness is not.
Table 19.
Segmentary analysis of left ventricle motility and systolic thickness by single photon emission computed tomography synchronized with ECG (Gated-SPECT)
| Score (points) | Motility | Thickness |
|---|---|---|
| 0 | Normal | Normal |
| 1 | Mild hypokinesis | Mild reduction |
| 2 | Moderate hypokinesis | Moderate/severe reduction |
| 3 | Severe hypokinesis | Absence of thickness |
| 4 | Akinesis | - |
| 5 | Dyskinesis | - |
Whenever possible, analysis of ventricular function should be performed at the baseline phase and after stress, with the purpose of detecting additionally indicative alterations in stunned or hibernating myocardium. The validated scores, which have been previously described, are recommended. Regarding the effect of global analysis of LV systolic function, LVEF is the parameter with the best reliability, and scores predominantly concentrate on segmentary analysis.
Furthermore, the subjective or qualitative assessment of images regarding results related to myocardial perfusion study, which have been previously described (homogenous distribution or normal uptake of the radiopharmaceutical in the myocardium; transient low uptake suggestive of ischemia; fixed low uptake suggestive of fibrosis; partially reversible low uptake suggestive of ischemia associated with fibrosis), should take the presence of the following types of artifacts into account:
Technical artifacts, resulting from inadequate image processing.
Motion artifacts.
Attenuation artifacts, due to interposition of mammary or diaphragmatic tissue (intestinal handles), which are factors that interfere with the specificity of the exam.
6. Current Utilization of Myocardial Perfusion and Ventricular Function Studies with Radiopharmaceuticals as Part of the Medical Decision-Making Process
MPS with the injection of radiopharmaceuticals associated with ET or the administration of coronary vasodilators is an established method for diagnosis and risk stratification of obstructive CAD,91-100 with the aim of guiding more effective clinical management of patients as part of the medical decision-making process.101,102 It currently integrates other non-invasive cardiovascular imaging techniques, such as Doppler echocardiography with color flow mapping, CS, coronary angio-CT, PET, and cardiac magnetic resonance (MR), to characterize risk and functional expression of atherosclerotic disease.103 Accuracy of method was, until recently, based on invasive coronary cineangiography, considered the standard for this comparison. The following stand out as highly relevant aspects, considered of paramount importance to the modality:
Obtaining variables and parameters that are fundamental to incremental prognostic characterization of CAD, such as electrocardiographic response to exercise, functional capacity , chronotropic response, blood pressure, et al. Of all forms of stress associated to MPS, the exercise testing is, without a doubt, the one that adds the greatest amount of information.104-106
When analyzing perfusion images, the possibility of quantifying area of myocardial ischemia or myocardium at risk has advanced a great deal over the past decades, undoubtedly participating in risk stratification and medical decision making for stable CAD, where it provides assistance for the choice between maintaining clinical treatment and interventional treatment.107 Even in the absence of randomized studies published to date, which might reaffirm this information (the Ischemia Study - report to the Addendum of this guideline),108 the evidence which has currently been accumulated and made available documents better evolution in patients with severe ischemic burden who undergo myocardial revascularization.109-112
When analyzing ventricular function images, indirect observation of thickness and motility of the LV walls and comparison of resting and exercise ejection fractions greatly improve the method’s specificity for characterizing true ischemia and aggregate incremental prognostic value with the definition of markers of severity, such as transient ischemic dilatation (TID), representing ventricular dysfunction and / or subendocardic ischemia induced by applied stress.
The ability to infer coronary flow reserve under applied stress or stimulus with elevated accuracy, superior to other conventional methods, with the exception of PET, is the most important physiological parameter for characterization of ischemia and the medical decision-making process, currently available in clinical practice with direct invasive measures of FFR and instantaneous wave-free ratio (IFR). Software currently in development for calculating coronary reserve in association with SPECT methodology and other non-invasive methods will likely aggregate unquestionable value to appropriate clinical or interventional treatment choices, in the near future, encompassing not only obstructive atherosclerotic disease, but also physiopathological conditions, including microvascular disease and endothelial dysfunction in the scenario of ischemic heart disease.85,113-115
The main applications with the best cost-effectiveness are shown in patients with intermediate pre-test probability of CAD, estimated based on the integration of clinical variables which have been established and documented in Brazilian and international guidelines, with their respective recommendations and levels of evidence (Tables 20 and 22 and Figure 11). Ideal diagnostic and prognostic capacities have for decades been considered with regard to severe coronary lesions. Nonetheless, exercise testing are indicated as the ideal and preferred association for myocardial scintigraphy, considering the physiological nature of the form of applied exercise and the established clinical value of the variables obtained during and after work.116-120
Table 20.
Recommendations for cardiovascular risk assessment, considering the presence or absence of known risk factors. European Guidelines on cardiovascular disease prevention in clinical practice119
| Recommendations | Class of recommendation | Level of evidence |
|---|---|---|
| CV risk assessment in individuals with family history of premature CV disease, family history of dyslipidemia, major risk factors (smoking, HBP, DM, raised lipid levels), or specific comorbidities that increase CV risk. | I | C |
| Repeat risk assessment every 5 years; repeat more often in individuals with risks close to levels which treatment is mandatory | I | C |
| Consider CV risk assessment in men > age 40 and women > age 50 or post-menopausal with no known risk factors | IIb | C |
| CV risk assessment in men < age 40 and women < age 50 with no known risk factors is not recommended | III | C |
C: level of evidence based on consensus of expert opinion and/or small studies, registries, or retrospective studies; CV: cardiovascular; DM: diabetes mellitus; HBP: High blood pressure; I, IIb, and III: class of recommendation.
Table 22.
Percent probability of obstructive coronary artery disease, considering the presence of chest pain, sex, and age. Comparison between LR and HR patients. Adapted from Gibbons RJ et al. and the Brazilian Cardiology Society's Third Guidelines on Exercise Testing117,119
| Age | Non-anginal chest pain | Atypical angina | Typical angina | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |||||||
| LR | HR | LR | HR | LR | HR | LR | HR | LR | HR | LR | HR | |
| 35 | 3 | 35 | 1 | 19 | 8 | 59 | 2 | 39 | 30 | 86 | 10 | 78 |
| 45 | 9 | 47 | 2 | 22 | 21 | 70 | 5 | 43 | 51 | 92 | 20 | 79 |
| 55 | 23 | 59 | 4 | 25 | 45 | 79 | 10 | 47 | 80 | 95 | 38 | 82 |
| 65 | 49 | 69 | 9 | 29 | 71 | 86 | 20 | 51 | 93 | 97 | 56 | 84 |
CAD: coronary artery disease; HR: high risk (smoking, diabetes, or dyslipidemia); LR: low risk (without smoking, diabetes, or dyslipidemia).
Figure 11.
Calculation of 10-year risk of fatal cardiovascular event, in populations of countries with high cardiovascular risk, considering the risk factors of age, sex, smoking, total cholesterol, and systolic blood pressure. Cart from the SCORE (Systematic Coronary Risk Evaluation) Study. The color scale varying from red to green corresponds to percent risk over 10 years, described in the upper part of the Table. Adapted from Piepoli MF et al.120
Pharmacological tests performed in nuclear cardiology represent good alternatives for assessing patients with physical limitations or clinical limitations to undergoing efficient exercise testings. They include approximately 20% to 30% of all cases referred for scintigraphy and approximately 50% of elderly patients.121 In these circumstances, the drugs utilized are dipyridamole, adenosine,69,122,123 and regadenoson.124 (Additional details described in Methodology.)
Similarly, in practice, when comparing conventional algorithms used for established the probability of CAD and major adverse events in stable chest pain patients or asymptomatic patients, such as Framingham Risk Score (FRS), PROCAM, SCORE, Diamond Forrester,125 or Global Risk;126 the estimated prevalence (EP) of the disease is observed to be significantly higher than the observed prevalence (OP), when coronary angio-CT is used to characterize luminal obstruction, ≥ 50% and ≥ 70%, respectively. In this situation, an international multicenter study (CONFIRM)127 of 14,048 consecutive patients with clinical suspicion of coronary obstructive atherosclerosis who underwent angio-CT showed that, in all age and sex categories, guidelines for calculating probability overestimated prevalence in the general population (51% EP × 18% OP for lesions ≥ 50% and 42% EP × 10% OP for obstructions ≥ 70%, p < 0.001), directed by accentuated differences between patients with typical angina (86% EP × 29% OP for lesions ≥ 50%) and atypical angina (47% EP × 15% OP for lesions > 50%). Considering this information to be true, more evidence has arisen within the literature in the search for new markers which might aggregate value and assist in more objective and realistic restratification of cardiovascular risk, with specific guidelines128 dealing with critical questions regarding, for instance, what types of evidence will contribute to risk assessment or reclassification when new markers are added to traditional scores, with emphasis on functional capacity and CS (Table 23).
Table 23.
New markers for cardiovascular risk stratification
| • High-sensitivity CRP |
| • Apolipoprotein B |
| • Glomerular filtration rate |
| • Microalbuminuria |
| • Ankle-brachial index |
| • Family history |
| • Functional capacity |
| • Mean carotid intima thickness |
| • Coronary calcium score |
CRP: C-reactive protein.
6.1. The Application of Bayes’ Theorem to Analysis of Myocardial Perfusion Images with Radiopharmaceuticals
Even when isolated analysis of images is used to describe perfusion findings, interpret data, and write reports, medical comments and conclusions should be the result of the integration of all available pretest clinical data and data obtained during the performance of the stress or associated stimulus test within the denominated incremental prognostic value. In this sense, Bayes’ theory of conditional probability or the application of Bayesian principles assists in decision making by establishing that the risk of an event occurring after a test is influenced by the sensitivity and specificity of an applied method, as well as the pretest prevalence of the disease, all of which are incorporated into the estimation of post-test probability for characterization of myocardial ischemia and, consequently, CAD (Figure 12).129,130
Figure 12.
Formula for calculating post-test probability of a disease according to Bayes’ theorem.
In this way, the diagnostic ability of a test is related to the population type selected, and it is may create tendencies or biases. For example, the selective referral of patients with “positive,” “altered,” or “ischemic” results for coronary cineangiography studies, in conjunction with few referrals of individuals with negative results, increases the chance of false-positive results with respect to true-negative results. This would be an equivocal methodology for evaluating the accuracy of a test, artificially decreasing the method’s specificity or its ability to select healthy individuals within a population.131,132 On the other hand, sensitivity will expressively increase in patients referred with a high prevalence of symptoms.
Many possibilities may be present for medical management within different prevalences of clinically estimated CAD, emphasizing that the diagnostic power of conventional exercise testing or tests associated with MPS is at a maximum when the pretest probability of CAD is intermediate. However, for a given pretest probability, the post-test probability increases progressively with the severity of the alterations found, such as the amount of myocardium at risk or the sum of extent and intensity (ischemic burden) of perfusion modifications in the perfusion images with radiopharmaceuticals. In the extreme case of a study with severe abnormalities, post-test probability will be elevated regardless of pretest probability (Figure 13).130
Figure 13.
Importance of amount of myocardium at risk (extension and intensity of ischemia) on myocardial perfusion imaging with radiopharmaceuticals (99mTc-sestamibi or thallium-201) to post-test probability of coronary artery disease (CAD). For a given pretest probability (50% indicated in the graph), the post-test probabilities will be significantly higher according to imaging findings. With the condition of high-risk ischemia or > 20% extent of ischemic myocardium, the clinical implications for decision making become practically independent of pre-test probability of CAD.
Source: Adapted from Udelson JE et al.130
Furthermore, not only Bayesian analysis, but also statistical techniques that use multivariate analysis to estimate post-test risk may also provide important diagnostic information, with the following advantages: they do not require the tests to be independent of each another or the diagnostic indexes (sensitivity and specificity) to remain constant in populations with different disease prevalences. Thus, in the condition of continuous-scale diagnostic tests, changes in percentages of sensitivity and specificity should be taken into consideration when cutoff values for classifying individuals with and without a disease vary. Some results may even be expressed as the sum of sensitivity and specificity for an “optimal” cutoff value. However, owing to the fact that an optimal cutoff value is not relevant to a specific application, it is recommendable to plot these indexes under a range or scale of values of interest, generally distributed under a receiver operating characteristics (ROC) curve, expressed in a 2-axis graph, where the y axis represents sensitivity and the x axis = 1 − specificity, for variable cutoff values (Figure 14).133
Figure 14.
Hypothetical examples of ROC curves, with: the area under the curve representing maximum or perfect diagnostic accuracy of the standard utilized (curve A; AUC = 1); “real” area under the curve representing good efficiency of the method used, often found in clinical practice (curve B; AUC = 0.85); the 45-degree diagonal line corresponding to random chance (curve C; AUC = 0.50), with the area under the ROC demonstrating the averages of diagnostic accuracy across a spectrum of cutoff values. On rare occasions, the estimated AUC is less than 0.5, indicating that the test being evaluated performs worse than random chance. Adapted from Zou KH et al.133
6.2. Value of the Diagnosis-Prognosis Binomial to Integrated Assessment of Perfusion Images
The presence of transient or reversible defects in radiopharmaceutical uptake reflect ischemia, which is in itself associated with greater incidence of future events, when comparing normal images or images with persistent perfusion defects. Thus, in patients with suspected or proven chronic coronary disease, estimation of the quantity of myocardium at risk as assessed by semi-quantitative and quantitative analyses, extent, intensity, and degree of reversibility of existing defects, as well as measures of LVEF following physical or pharmacological stress, have prognostic value, indicating risk of events during clinical follow up.134-138 Other scintigraphy markers of severity may stand out, such as apparent transient dilation of the LV, induced or accentuated by exercise or pharmacological tests,139,140 which may translate to extensive subendocardial ischemia, in addition to high pulmonary uptake, translating to LV dysfunction. Furthermore, increased uptake in the walls of the right ventricle (RV) in multi-arterial patients whose lesions are predominantly in the left coronary territory, may suggest an imbalance in perfusion between ventricles.141,142
Considering the scope and accumulated experience of MPS with radioisotopes in diverse clinical scenarios relating to CAD, guidelines and consensuses have suggested the main applications based on levels of evidence in the literature, and created scores that numerically classify indications as inappropriate; possible, but questionable; and appropriate143,144 (additional details described in the Indications section).
6.3. Radiopharmaceuticals for Performance of Myocardial Perfusion Scintigraphy and Image Generation and Perfusion Defects
Nuclear cardiology is connected to the assessment of cardiovascular physiology, currently encompassing metabolism, innervation, myocardial perfusion, ventricular function, and synchronism. It has a capability for early detection of cardiovascular physiopathological alterations, allowing for interventions which may interrupt or revert the disease condition before structural alterations are established in a definitive, evolutive, and irreversible manner. To represent cardiac physiology, images are formed using the principle of radiotracers or tracers,29 in which the exchange of stable atoms with their isotopes does not alter the biological properties of the organism where the images are being obtained.
Radioactive labeling is performed with minimal quantities of chemical substances, resulting in a radiopharmaceutical that may be used to truly represent physiological or biochemical state of unlabeled molecules . In this manner, alterations to the physiology being evaluated and toxicity effects do not occur. These characteristics are different from other imaging methods which use elevated concentrations of chemical substances to create sufficient contrast and, consequently, obtain images of the functional situation and anatomical aspects of the organ under study.145 The images in this specialty are digital; they either use “pixels” as units of measurement for resolution or are transformed into a digital matrix, emphasizing that “pixel” values of images of the ventricular myocardium are directly proportional to physiological cardiovascular properties. Physical phenomena such as the “Compton scattering effect,” the “photoelectric effect,” and geometric distortions should, however, be considered,146 given that they tend to interfere with direct proportionality, in a manner that is decreasing as equipment and image reconstruction techniques technologically evolve. Furthermore, another factor related to acceptance and preference of nuclear cardiology for detecting myocardial perfusion defects is the elevated, superior resolution contrast (allowing for differentiation between normal and decreased perfusion) in comparison with other imaging methods,147,148 even considering lower spatial resolution. There is also a peculiar aspect, namely, that the myocardium (organ of interest) appears emphasized due to the greater brightness in comparison with underlying structures (background) and, consequently, provides excellent signaling, which facilitates the development of integrated, computerized algorithms for SPECT and PET techniques. These programs, which automatically process and objectively quantify images, have good comprehension, and they are well validated and internationally utilized.149-151 From the conceptual point of view, it is necessary to grasp that scintigraphy image generation is based on relative uptake of the radiopharmaceutical in the myocardium of the LV, when it is injected intravenously during physical exercise or pharmacological tests. The comparison of radiopharmaceutical uptake between ventricular walls is expressed in images based on a color scale, created by specific computer programs which, in addition to allowing for subjective analysis of perfusion, make it possible to conduct semi-quantitative and quantitative evaluation of affected myocardial area. During visual evaluation of scintigraphy images, the following are taken into consideration: homogenous distribution patterns or normal radiopharmaceutical uptake in the myocardium, transient low uptake suggestive of ischemia, fixed low uptake suggestive of fibrosis, and partially reversible low uptake suggestive of ischemia associated with fibrosis24,152 (Examples are provided in the Methodology and Tutorial Cases sections).
7. Evaluation of Patients with Potential Acute Coronary Syndrome - Algorithms in the Chest Pain Unit
7.1. Introduction
Continuous chest pain is one of the most common symptoms in emergency units, accounting for approximately 8 million annual visits in the USA.153 Although approximately 50% of patients are admitted for diagnostic definition, only 30% of visits will correspond to the condition of acute coronary syndrome (ACS), 2% to 4% of whom will be inappropriately discharged from the hospital (Figure 15), leading to serious risks of severe events, in addition to legal-medical problems. Considering these implications, as well as hesitation to discharge patients with acute myocardial infarction (AMI), assessment of patients with atypical chest pain in emergency unit has emphasized admission for posterior clarification and risk stratification. With the development of more sensitive cardiac biomarkers in conjunction with more precise non-invasive exams and validated clinical parameters, early identification of low-risk patients has been carried out more rapidly. In this process of diagnostic and prognostic assessment, the following play an important role: resting ECG, cardiac enzymes, and non-invasive exams such as ET, MPS, Doppler echocardiogram, and coronary angio-CT, in addition to cardiac resonance in specific cases. The choice of recommended imaging method should be based on the procedures available, local institutional experience, and present clinical situation. The exam with the highest diagnostic accuracy and negative predictive value (NPV) will offer more precise risk stratification, which is fundamental to decision making regarding need for hospital admission or safe discharge from the emergency unit.
Figure 15.
Chest pain spectrum in emergency units, with clinical implications, forms of presentation of acute coronary syndrome and available methods for investigation and risk stratification. Cor: coronary; Myo: myocardium. NSTEMI: non-ST-segment-elevation myocardial infarction; STEMI: ST-segment-elevation myocardial infarction.
Source: Adapted from Amsterdam EA.154
In addition to the 2 physiopathological conditions described (Non-ST segment Elevation Myocardial Infarction - NSTEMI and ST segment elevation myocardial infarction - STEMI), unstable angina also stands out, which does not feature myocardial necrosis as an initial consequence.155,156 Nevertheless, unstable plaque and evolutive phenomena of erosion and rupture may progress to infarction and related complications, such as severe arrhythmias, ventricular dysfunction, and death. Conditions of vasospasm, in epicardial coronary arteries or with microvascular disease, have additionally been implicated in ACS without thrombosis and myocardial infarction, in the absence of obstructive lesions.157-159 It is, finally, important to emphasize that, in patients with documented ACS and intermediate- to high-risk patients, invasive coronary cineangiography and percutaneous revascularization represent the most frequent forms of initial assessment, and non-invasive imaging methods are reserved for clinically stable situations and low- to intermediate-risk patients, with the aim of reclassifying risk, diagnosis, and stratification in the post-event phase.160,161
7.2. Goals for Evaluating Acute Chest Pain and Participation of Non-invasive Methods in Assessing ACS154,162,163
Precise diagnosis for appropriate conduct in UA or AMI, whether with clinical treatment or invasive strategy via catheterization and angioplasty.
Early, safe discharge from the hospital if clinical data and exams show no abnormalities. Probability of severe cardiac events < 1% over 30 days of evolution following discharge from the emergency unit or hospital.
Following serial evaluation of ECG, without modifications, in addition to normal cardiac enzymes and clinical situation characterized as low- to intermediate-risk, non-invasive functional exams may play an important role in risk stratification of patients with acute chest pain. The choice of MPS, cardiac resonance, or angio-CT will depend on the objective and the clinical question to be answered.
Exercise testing: constitutes an important strategy for assessing patients with suspected ACS following stabilization, and it aids prognosis and medical management . Patients with chest pain in the emergency room, once they have been identified as low-risk, may undergo ET, a normal result of which confers low annual risk of cardiovascular events, allowing for earlier and safer discharge from the hospital.164 Brazilian and international guidelines recommend ET as a first-choice exam for risk stratification in patients who are able to exercise, as the procedure is low-cost and widely available, and it has a low rate of complications, similar to that of tests conducted in normal conditions.165 A treadmill or a cycle ergometer may be used, following appropriate protocols for the patient’s clinical conditions, such as the ramp protocol or the modified Naughton or Bruce protocol. Logistics related to performing ET in the emergency unit may, however, be compromised as a result of unavailable operational personnel or infrastructure during certain periods (e.g. weekends or night shifts).
Summary of indications for ET in ACS (characterize low-risk after initial clinical stratification)
Baseline ECG and biomarkers (necrosis) without alterations.
Absence of symptoms (precordial pain or dyspnea).
Hemodynamic stability and adequate conditions for physical effort.
If ET results are normal and the patient has shown good functional capacity, other procedures may be unnecessary, in virtue of the test’s high NPV.165
Summary of Recommendations and Evidence
Class of recommendation I. Level of evidence: B
Low-risk (clinical and ECG) patients with normal biomarkers should be referred for exercise test after 9 to 12 hours. Within the routines of chest pain units, these exams may be used as discharge criteria.
If it is not possible to perform ET or if ECG is uninterpretable, the patient may undergo provocative tests for ischemia associated with non-invasive imaging.
Doppler echocardiogram (ECHO): This is fundamental for evaluating patients with acute chest pain166-168 and evolving ACS, initially considering LVEF, segmentary contractile alterations, and the presence of thrombi, in addition to mechanical complications (rupture of interventricular septum or papillary muscles) that result in severe events, such as cardiorespiratory arrest. Moreover, this method may also evaluate chest pain with non-coronary etiology, such as pericardial disease, hypertrophic cardiomyopathy, aortic dissection in the presence of renal insufficiency that makes it impossible to perform angio-CT, and others. In addition to assessing the presence and extent of ventricular dysfunction, it is able to quantify severity of valvular abnormalities that may be present and associated with ischemic etiology.
Summary of Recommendations and Evidence
Recommendation class I
Transthoracic ECHO is indicated when there is clinical suspicion of aortic and pericardial diseases, pulmonary embolism, and valvulopathies (level of evidence: C).
In cases with complications resulting from unstable ACS, such as interventricular communication and mitral insufficiency (level of evidence: C).
Stress echocardiography is considered an alternative to exercise testing in patients who cannot exercise (level of evidence: B).
Recommendation class IIa
Patients suffering from chest pain - resting ECG to determine whether or not pain is of ischemic origin (level of evidence: B).
Patients with uncomplicated anterior wall AMI, with the objective of determining the exact size of the ischemic lesion (level of evidence: B).
In stable patients with evolving ACS, echocardiography associated with pharmacological stress before hospital discharge may identify induced ischemia and assist in risk stratification and medical management of immediate follow-up (6 to 12 weeks), especially if LVEF values are below 40%.
Coronary angiotomography: Many studies have shown that coronary angio-CT is an important tool for evaluating acute chest pain, especially in low- to intermediate-risk patients.169-172 It is a safe procedure for diagnosing ACS, and it is able to reduce intra-hospital follow-up time and contribute to cost reduction. In the Rule Out Myocardial Infarction by Cardiac Computed Tomography II (ROMICAT II) Study, duration of hospital stay was significantly lower in patients stratified via angio-CT in comparison with the group submitted to conventional evaluation (23.2 ± 37 hours vs. 30.8 ± 28 hours).173 There was also a significant increase in percentage of patients discharged from the emergency unit in the group stratified with this method (46.7% vs. 12.4% p < 0.001), in spite of higher costs associated with angio-CT and the greater tendency to refer patients for catheterization and revascularizations.
Based on recent publications, low- to intermediate-risk patients with acute chest pain, non-diagnostic ECG, and negative markers of necrosis have Class-I recommendation and level of evidence A for undergoing angio-CT, especially considering the method’s NPV. There are, nevertheless, limitations in the presence of STEMI and NSTEMI (Figure 16) (with the exception of coronary dissection) and known CAD or prior revascularization where the existence of intracoronary prostheses (stents) and calcium may negatively influence the exam’s specificity for its proposed aim, leaving the possibilities of functional evaluation and global repercussion. Finally, it is necessary to consider exposure to elevated doses of radiation and lower image quality for the exclusion of pulmonary embolism, aortic dissection, or ACS (triple rule-out).174
Figure 16.
Clinical scenarios of patients who present with chest pain at emergency units. Situations of ACS diagnosed as STEMI and UA/NSTEMI correspond to orientations established by pertinent guidelines. In the condition of possible or suspected ACS, the previously described sequences of diagnostic investigation and stratification are recommended. ACS: acute coronary syndrome; CAD: coronary artery disease; MPS: myocardial perfusion scintigraphy; NL: normal; NSTEMI: non-STsegment elevation acute myocardial infarction; STEMI: ST-segment elevation acute myocardial infarction; UA: unstable angina.
Myocardial perfusion scintigraphy (MPS): Within the scope of its applications (See the Indications chapter), the following stand out: indirect evaluation of coronary reserve and consequent estimation of functional significance of coronary stenoses, evaluation of the efficacy of therapeutic interventions, and stratification of ACS risk. One of the principal indications for MPS during the first 12 hours of symptom onset is to decide whether or not to hospitalize a patient with chest pain and suspected CAD, when ECG was normal or had non-specific alterations. Resting MPS, when it is performed in an early phase of attendance and considered low-risk, determines a low index of future cardiac events. Recent studies have demonstrated that, when ACS is suspected, the use of resting perfusion images with radiopharmaceuticals175,176 is also associated with shorter hospital stays and lower costs, and it is additionally able to reduce unnecessary hospitalizations.177,178 Furthermore, numerous observational studies have demonstrated a high NPV for normal resting perfusion images, with the objective of ruling out AMI or short-term cardiac events.179 In a study by Schaeffer et al.,180 479 patients underwent resting MPS and were followed for 16 months. Of these patients, 434 had “normal” resting MPS, and 45 had “abnormal” resting MPS. In the normal group, only 3 patients (0.7%) had severe cardiac events, showing a NPV of 99.3%.180 Equally, multiple evaluations have demonstrated the efficacy and safety of MPS with SPECT for assessing patients with chest pain in the emergency unit.181-183 Population samples involved, however, were more heterogeneous and had higher numbers of risk factors for CAD. MPS effectively foresaw which patients would require coronary angiography. Nabi et al. related that 38.3% of patients with abnormal MPS underwent revascularization, while 0.9% of patients with normal MPS subsequently underwent coronary intervention. Of patients with myocardial area at risk (ischemia) involving > 10% extent in perfusion images, 55% underwent revascularization.182,183
The study known as the PREMIER trial by N Better et al.184 evaluated the performance of resting MPS in investigating chest pain in the emergency room with 356 low- to intermediate-risk patients, from 8 developing countries, including 2 Brazilian centers. The primary outcome considered included the compound events of death, non-fatal AMI, recurring angina, and coronary revascularization over 30 days, and the results reaffirmed the association between normal images and a good NPV (99.3%) for severe events (death or AMI).184 Moreover, it is worth highlighting that the presence of resting perfusion alterations (abnormal scintigraphy) was the only variable independently associated with the primary outcome (adjusted OR = 8.19, 95% CI: 4.10-16.40, p = 0.0001), with even higher expression when only patients who received injections during episodes of pain were considered (adjusted OR = 17.35). On the other hand, results considered high-risk indicated worse prognoses for future cardiac events (death, AMI, myocardial revascularization surgery, or percutaneous intervention).185,186
The Emergency Room Assessment of Sestamibi for Evaluation of Chest Pain (ERASE) Study, which evaluated patients with ACS and normal or non-diagnostic ECG who were still in the emergency room, observed admission rates of 54% in patients who underwent MPS and 63% in other patients, suggesting that the initial strategy of resting scintigraphy is satisfactory, based on the fact that it demonstrates good risk stratification capability.184,186
International guidelines recommend the use of resting myocardial perfusion images for acute chest pain as a class I recommendation with level of evidence A for risk stratification of patients with suspected ACS and non-diagnostic ECG.187,188
Time of radiopharmaceutical injection: The main applications of MPS within the first hours of a patient’s arrival at the hospital are:
Radiopharmaceutical injection (technetium-99m-labeled sestamibi / MIBI or tetrofosmin, also known as 99mTc-sestamibi and 99mTc-tetrofosmin), while resting, during an episode of chest pain, with normal or non-specific ECG, with the objective of rapid diagnostic definition.
Radiopharmaceutical injection while resting, in the absence of chest pain, with normal or non-specific ECG, when the symptom has ceased less than 6 hours prior, but preferably within the 2 preceding hours. Wackers et al.179 have demonstrated that, in cases where injections are administered up to 6 hours after pain, in ACS, there is an 84% incidence of perfusion abnormalities; this decreases to 19% when intravenous radiopharmaceutical administration occurred between 12 and 18 hours after the last episode of pain. Kontos et al.176 found no reduction in sensitivity for identifying patients who evolved to AMI or revascularization when the injection was administered during the moment of pain or up to 6 hours after the cessation of the symptom.
Taken as a whole, perfusion imaging with radiopharmaceuticals for evaluation of acute chest pain does not present any formal contraindications; it is well tolerated by most patients, and the quantitative assessment of information about ischemia is of special importance to the clinical decision-making process.
PET: In a retrospective study conducted with more than 7,000 patients who presented at the emergency unit with chest pain,189 92.5% of patients with positive stress or resting PET were diagnosed with ACS, by cardiac catheterization, electrocardiographic alterations, or positive cardiac biomarkers. In patients with reversible perfusion defects while resting or under stress (positive PET) who underwent cardiac catheterization, 87% were considered to have significant CAD. For patients without reversible perfusion abnormalities whose exams were classified as negative, no deaths were informed during the 30-day follow-up period. PET has a significantly better spatial resolution and higher sensitivity when compared to MPS. In Brazil, the elevated costs and low availability of this technology for patients with ACS are, however, limiting factors to its routine use.
UA and NSTEMI: Patients whose clinical conditions indicate a high risk of AMI or UA should undergo hemodynamic study. For those with low to intermediate risks whose unstable angina (UA) has been clinically stabilized, MPS has shown an important value for risk stratification.190 Additionally, it assists in diagnosis and medical management. It is recommended within the first hours of the patient’s arrival at the hospital. Cases with a history of chest pain, whose biochemical markers, nevertheless, show no alterations, with normal or non-diagnostic ECG are considered candidates. When patients are symptomatic or immediately following cessation of symptoms, intravenous administration of Sestamibi-99mTc or Tetrofosmin-99mTc occurs, preferably while resting, followed by image acquisition directly after or up to 6 hours following radiopharmaceutical injection. To perform MPS associated with physical stress or vasodilator drugs in appropriate patients (those with low to intermediate risks), symptoms should be under control or angina should be stabilized for at least 48/72 hours.191 Patients without ischemia or infarction and preserved LV function have good prognosis and they may be managed conservatively, while patients with significant ischemia induced during associated tests should be referred for invasive exams.
The simultaneous information provided by myocardial perfusion and ventricular function via scintigraphy synchronized with ECG (Gated-SPECT) is of fundamental importance, given that both the absolute LVEF value and the extent and intensity of perfusion defects have prognostic value for the occurrence of future cardiac events.
Finally, with the advent of chest pain units and emergency units for the evaluation of patients with suspected ACS and with new tools which have become available, such as clinical risk scores, biomarkers, or multimodalities (non-invasive exams), algorithms have been proposed to support investigation and treatment of different clinical presentation scenarios (Figure 16). Their implementation aims to improve cost-effectiveness and to lower morbidity and mortality in the management of this subpopulation, within the spectrum of ischemic heart disease.
Recommendations and Evidence
Class I
Stress and resting MPS as an alternative to cases with limitations to ET (level of evidence: C).
Class II
Patients suffering from chest pain may be evaluated via resting MPS to determine whether the pain is of ischemic origin or not (level of evidence: A).
STEMI: Coronary cineangiography is a priority indication for initially attending patients with ACS and ST-segment elevation, seeing that coronary reperfusion is the primary objective. However, in cases in which clinical condition, ECG, and biochemical markers are inconclusive, MPS may aggregate incremental diagnostic and prognostic value. These situations are generally characterized by atypical clinical conditions in patients with non-specific electrocardiographic alterations in the ST segment, left bundle branch block, and, mainly, in those who are attended before or after the onset of the condition, while they are already outside of the ideal period for dosage of biochemical markers.
Recommendations and evidence for stress and resting MPS following STEMI
Class I
Before being discharged from the hospital, in stable patients who have not undergone coronary cineangiography for risk assessment and therapeutic decision making (level of evidence B).
Complementary evaluation following coronary cineangiography, in cases where there are doubts, with the aim of defining and quantifying ischemia for eventual myocardial revascularization (level of evidence B).
8. Positron Emission Tomography in Cardiology
8.1. Introduction
Myocardial perfusion defects evaluated with the use of radiopharmaceuticals and induced by stress are well established as a technique with diagnostic and prognostic capability for the identification of flow-limiting coronary diseases. In MPS, interpretation has mainly been qualitative, semiquantitative, and quantitative, assessing regional perfusion in relative terms.95,102,121,192-196
8.2. Basic Principles of Positron Emission and Main Indications
PET consists of a specific method in nuclear medicine. It is different from the widely used gamma camera or Anger camera employed in MPS for the technique commonly known as SPECT. PET, differently from SPECT, uses emitters of positrons, particles similar to electrons (except for the fact that they have a positive electric charge), with very short half-lives. The principle of PET consists of the detection of 2 photons (gamma rays) that are emitted in diametrically opposite directions to occasion annihilation of the positron upon encountering an electron in the periphery of the atom. This detection occurs through a series of crystals arranged throughout the 360 degrees of a ring-shaped detector surrounding the patient. The detection of the 2 photons emitted in diametrically opposite directions, at exactly 180 degrees, with an existing coincidence circuit in the PET equipment, making it different from SPECT, which uses single photons.197
Since PET cameras have incorporated electronic collimation, mechanical collimators made of lead have not been made necessary, allowing for greater sensitivity than in SPECT systems. The sensitivity of current 3D PET systems is 5 times greater than of the older 2D PET ones. On the other hand, there is more attenuation in PET studies than in SPECT, making attenuation correction necessary to the reconstruction of PET images. The most current systems, which have a resource known as time of flight (TOF), are based on the speed of light to localize the annihilation event in a much smaller directional ray than in conventional PET cameras, resulting in increased spatial resolution.
This method has already been established as the standard for assessing myocardial viability (See the Myocardial Viability section), with the use of a glucose analogue labeled with fluorine-18 (18F-FDG), a technique which, although it is not widely used in Brazilian clinical practice, is widely viable in most nuclear medicine centers where PET is available in Brazil. Its use for the assessment of myocardial perfusion is not necessarily a new technique, as it dates back to more than 30 years ago and has since been evolving.198-200 Nonetheless, its clinical use has remained restricted for many years owing to its methodological complexity, high operational costs, and low availability of devices and tracers. Recent technological advances have reduced the costs, and its increasingly frequent use in oncology has resulted in increased equipment availability. Nowadays, non-invasive estimation of absolute coronary flow and flow reserve with this technique has become possible and has been validated.197 The scenario is contrary in Brazil, however. In spite of a small amount of experience using research protocols, this method is not available in clinical practice. Even though PET cameras are distributed throughout the country, there is a lack of other radiotracers for the modality as well as a lack of economic viability.
8.3. Radioactive Tracers for Use in Positron Emission Tomography
Different available radiotracers make it possible to identify vasoactive, metabolic, or neurological processes that are present in diverse cardiomyopathies and atherosclerosis, on the molecular level. The images acquired allow for evaluation of the cardiovascular system on different levels, including: perfusion,201-203 metabolism,204-207 sympathetic innervation,208,209 and inflammation;210-212 depending on the tracer utilized. Myocardial perfusion studies using PET may be performed using different tracers, each of which possesses specific characteristics, advantages, and disadvantages (Table 24).
Table 24.
Main characteristics of perfusion myocardial radiotracers labeled with positron emitters
| Rubidium-82 (82Rb) | Ammonia labeled with Nitrogen-13 (13NH3) | Water labeled with Oxygen-15 (15O-H2O) |
18F-Flurpiridaz | |
|---|---|---|---|---|
| Physical half-life | 1.27 min | 9.97 min | 2.04 min | 110 min |
| Extraction fraction (flow) | 40% to 70% | 94% to 98% | 95% to 100% | > 90% |
| Means of production | 82-strontium/rubidium (82Sr/Rb) generator | Cyclotron | Cyclotron | Cyclotron |
| Advantages | • Commercially available in the form of a generator • Capable of evaluating flow quantitatively • Short half-life for quick tests • Low radiation |
• High contrast resolution • Capable of evaluating flow quantitatively • Potential for use with exercise |
• Ideal for quantification of flow • Short half-life for quick tests • Low radiation |
• High contrast resolution • Capable of evaluating flow quantitatively • Potential for use with exercise • May be distributed by central production |
| Disadvantages | • Short half-life does not allow for exercise • Lower resolution |
• Requires a local cyclotron • Heterogeneity of distribution |
• Requires a local cyclotron • Short half-life does not allow for exercise |
• Not commercially available |
Rubidium-82 (82Rb) and ammonia labeled with nitrogen-13 (13NH3) have been approved for clinical use by the Food and Drug Administration (FDA), and they are the most commonly used in the USA. On the other hand, water labeled with oxygen-15 (15O-H2O) has been used mainly for research in the USA, as it diffuses freely between blood and the myocardium, which makes it ideal for quantitative flow measures. In South America and Brazil, associated costs, especially with tracers, have limited their use. Initial experience with myocardial perfusion using PET and 82Rb have been conducted at the Heart Institute of São Paulo (InCor, acronym in Portuguese).213 As 82Rb is the only one of these tracers produced in a generator system, from strontium-82, it has an advantage in relation to the others whose production depends on a cyclotron. These generators may be transported, and, specifically in case of Brazil, they may be imported especially for this purpose. Their short physical half-life of 76 seconds constitutes another favorable aspect, given that it implies very low dosimetry for the patient, with estimated exposure lower than 2 milliSieverts (mSv), in a stress and resting protocol, including tomography for attenuation correction. On the other hand, stress with exercise becomes unviable, considering the elevated costs, making it possible only in high-volume centers that perform around 40 exams weekly.214 Payment tables for medical procedures in Brazil, to date, restrict the payment of PET for oncological indications, which complicates its use for cardiology. The use of 13NH3 requires a cyclotron, the installation of which has extremely high costs, but it provides high contrast images, due to its high first-pass extraction fraction. It offers good accuracy for absolute measure of myocardial blood flow (MBF), and its relatively long half-life of approximately 10 minutes, makes physical exercise viable.215
18F-flurpiridaz is a new tracer. Although its production requires a cyclotron, its labeling with fluoride-18 makes it possible to utilize the production and distribution systems that are already widely available for oncological use of PET. Due to its relatively long half-life of 110 minutes, it is appropriate for associated use with an exercise testing, and its high first-pass extraction fraction also makes it ideal for flow quantification. Its use is currently being evaluated in a phase-III study.216-220
8.4. Use of PET for Assessment of Myocardial Ischemia
PET has advantages over conventional SPECT, including higher spatial resolution and contrast rate, higher sensitivity than the tracers classically used in MPS (thallium-201 and technetium-99m-labeled radiopharmaceuticals), and higher specificity, considering the attenuation correction system based on coupled CT, which results in better capability to differentiate true perfusion defects from attenuation artifacts.201,221-223 These advantages are notably applied to some special populations, such as obese patients and women with voluminous breasts, in whom gamma ray attenuation in soft tissue may be a factor of greater importance to the final quality of cardiac images.
The objective of evaluating myocardial perfusion via PET is to detect physiologically significant coronary stenoses, aiding clinical management of patients with known or suspected CAD and patients who, although they have no known diseases, possess risk factors, in order to evaluate atherosclerosis progression. Other objectives include determining the cause of ischemic symptoms to recommend clinical treatment or revascularization, estimating potential for future adverse events, and improving patient survival. One of its strengths is that it is the non-invasive modality of choice for accurately quantifying MBF. It allows for quantification in absolute terms of ml.min per gram of myocardium in stress and resting phases. The ratio between the 2 flows is known as the myocardial flow reserve (MFR), a valuable parameter that makes it possible to overcome one of the currently existing limitations to conventional perfusion imaging with SPECT when evaluating patients with multivessel CAD.
Results of invasive studies (FAME-1 and FAME-2) that analyzed FFR demonstrated its value in evaluating functional significance of single-vessel stenoses.224,225 Some studies have provided evidence of a correlation between regional MFR and FFR measured invasively without comparing them directly, however.113,226
Quantitative PET measures of MBF in absolute terms represent a paradigm change in the evaluation and management of patients with CAD, with a disassociation from the anatomical gold standard of coronary cineangiography, which had previously been established for decades, and a return to functional assessment. These measures additionally make it possible to expand the use of perfusion imaging within the current scenario, with the aim of detecting flow-limiting epicardial lesions, for earlier stages of atherosclerosis, microvascular dysfunction (Figure 17), and evaluation of balanced flow reductions in triple-vessel disease. They also offer an opportunity to monitor responses to changes in lifestyle or risk factors and therapeutic interventions.227,228
Figure 17.
A 53-year old patient, of pardo race, with chronic renal insufficiency, undergoing hemodialysis, and left ventricular hypertrophy. Pre-renal transplant evaluation. Counterclockwise: A) PET perfusion with rubidium-82 with no segmentary defects in uptake between different walls of the left ventricle. B) Coronary tomography with evidence of parietal calcium in the anterior descending and circumflex arteries, but no obstructive lesions. C) Quantification of myocardial blood flow and flow reserve, widely reduced throughout all coronary territories (In the bar graph, coronary flow values < 2.0 ml.min-1.gram-1 of myocardium are considered abnormal; values between 2.0 and 2.5 ml.min-1.gram-1 are considered in the gray zone; and values > 2.5 ml.min-1.gram-1 are considered normal), notwithstanding the absence of obstructive epicardial disease, are indicative of microvascular disease. LAD: left anterior descending; Cx: circunflex; RCA: rigth coronary artery.
Source - INCOR - FMUSP - SP.
Two recent meta-analyses evaluating methodology have indicated that PET has superior accuracy in comparison with SPECT. The first meta-analysis compared PET with SPECT synchronized with ECG and associated with attenuation correction. In analysis with a ROC curve, the area under the curve for PET and SPECT was, respectively, 0.95 and 0.90 (p < 0.0001), showing a small superiority for PET. The second meta-analysis indicated 82Rb as the most used tracer, resulting in higher sensitivity for PET. Specificity, on the other hand, although superior, was not statistically significant.222
Regarding prognosis, similarly to SPECT, for which data are abundant, robust, and well established, normal myocardial perfusion with PET is indicative of good prognosis, with cardiac events varying between 0.09% and 0.9% during 1 year of follow-up, depending on the population analyzed. On the other hand, adverse events increase with the extent of perfusion defects on PET. A recently published register including more than 7,000 patients demonstrated that the hazard ratio of cardiac death increased with every 10% increment of extent of perfusion defects, classified as mild, moderate, and severe, respectively, hazard ratio: 2.3 (95% CI: 1.4-3.8; p = 0.001); hazard ratio: 4.2 (95% CI: 2.3-7.5; p < 0.001), and hazard ratio: 4.9 (95% CI: 2.5-9.6; p < 0.0001), in relation to a normal exam.221
8.5. Patient Preparation, Types of Stress, and Dosimetry
Preparations include a 6-hour water-only fast. Patients should avoid caffeine and foods or medications containing xanthines (theophylline, theobromine) for at least 24 hours. Generally speaking, stress protocols are generic for all types of perfusion agents, bearing similarities to those of MPS with SPECT, with specific differences in accordance with acquisition protocols.
Current dosimetry for studies with rubidium-82 (82Rb) in adults, considering maximum administered activity per 60-mCi dose, may vary from 1.1 to 3.5 mSv of total effective dose. With the current advances in instrumentation of PET cameras, studies with good diagnostic quality may be acquired with injected activities that vary from 20 to 40 mCi per resting and stress dose, resulting in even lower exposure. In studies with ammonia labeled with nitrogen-13 (13NH3), the habitual activity is 10 to 20 mCi per dose (which corresponds to 1.48 mSv per dose). Doses of up to 25 to 30 mCi may be used in patients with high body mass index (BMI), with relatively lower dosimetry as a function of its shorter half-life and the low energy of its positron.
The evolution of imaging systems has allowed for the development of PET/CT capable of performing hybrid imaging, or be it, using CT not only for attenuation correction but also for quantification of CS and acquisition of coronary angio-CT, in addition to allowing for the fusion of these images, facilitating the integration of anatomical and functional information. Another great advance is that of PET systems incorporated to PET/MR. This new hybrid imaging modality has enormous potential for structural-functional evaluation, tissue characterization, and reduced exposure to radiation.229-231 This, thus, amplifies the possibility of developing new studies, with the aim of expanding data on clinical applications and diagnostic and prognostic benefits of PET in studies of more diverse cardiovascular conditions.
Objective measures of coronary flow reserve will certainly be able to be extended to the MPS-SPECT method, providing evidence of nuclear medicine’s ability to carry out quantifications of MBF and allowing for additional parameters for evaluating perfusion, as well as myocardial reserve, with a resulting impact on clinical management of patients, which will objectively orient decisions about revascularization (Figure 18).232
Figure 18.
Coronary flow reserve (CFR). Left: In patients with high CRF, there were no statistically significant differences in adjusted rates of annual events, notwithstanding apparent reduction observed in patients who underwent angioplasty or coronary artery bypass graft (CABG). Right: In patients with low CRF, both procedures showed significant benefits in reducing events.
Source: Adapted from Taqueti VR et al.232
Provided that availability barriers are overcome and costs of both imaging systems and tracers are reduced, especially in developing countries such as Brazil, the growing application of this new methodology has a promising outlook in cardiology.
9. Integrating Diagnostic Modalities in Cardiology - Tutorial Cases
9.1. Introduction
Technological evolution has facilitated the development of excellent tools for both establishing diagnosis and estimating prognosis of CAD. These advances allow us to evaluate different aspects of anatomy and physiology of the heart non-invasively, with great accuracy. Most importantly, today we are able to rely on methods which help establish the best course of treatment in the most diverse clinical situations of patients with suspected or known diseases, whether they are symptomatic or asymptomatic. This wide range of alternatives presents an additional challenge to doctors, namely, that of defining the best strategy and the most rational complementary sequence of evaluation possible, regarding use of resources for diverse clinical situations, guaranteeing not only the highest accuracy in evaluation, but also the best benefits, considering healthcare costs. Doctors generally should seek to orient initial investigation with the aim of using the lowest number of diagnostic exams for an effective evaluation. However, in this era of multimodalities, it has become necessary to perform more than 1 exam in order to make the best therapeutic decision. Combined assessment of different phenomena of the heart is often necessary, for instance, to define physiological repercussions of an anatomical lesion.
Two questions linked to the bases of medical semiology and to the essence of medicine ask, “Who is the patient?” and “What information is the doctor looking for?” In this approach, the application of good techniques, such as anamnesis and complete physical examination, has become clear in clinical medicine, enabling doctors to formulate their initial patient profiles and to establish the most probable diagnostic hypotheses. Joint estimation of the pre-test probability of the disease233 and knowledge regarding the accuracy of a test to determine post-test probability of a true or false result (Bayes’ Theorem) are implicit and no less important. The application of this basic principle, associated with knowledge regarding what different diagnostic tools may offer, allows for the elaboration of better investigation strategies. Confirming or excluding the presence of CAD from the anatomical point of view or, alternatively, investigating the physiological repercussions of myocardial ischemia via stress tests have distinct implications for patient management. Whether to look for one response or another will depend on the patient at hand and the question the doctor wishes to answer.
9.2. Integrating Physiology (Exercise Testing and Nuclear Cardiology) and Anatomy (Calcium Score and Coronary Angiotomography)
Exercise testing (ET), also known as ergometric test, stress or exercise tests, showing evidence of good performance (high workload) with normal results and MPS showing absence of ischemia do not represent absence of CAD indeed . In the presence of CAD, however, these findings are associated with better prognosis in relation to patients with ischemia, given that their use is extremely useful for risk stratification of patients with or without this disease. On the other hand, it is important to know that methodologies based on the anatomy of coronary arteries, such as coronary angio-CT, may also stratify risk, but the presence of atherosclerosis detected by this modality does not necessarily imply poor prognosis or, much less, mean that the patient will necessarily benefit from myocardial revascularization procedures. It may merely represent that prognosis is worse that that of an individual without atherosclerosis. It is, thus, worth reaffirming that it is necessary for doctors to possess global knowledge of their patients and also to delineate clearly the investigation strategy for the question they are seeking to answer. Basic knowledge regarding advantages and disadvantages of available procedures are implicit, making the absolute most of the technological evolutions that have occurred in recent years. Initially speaking, all modalities which have been covered may be used for diagnosis and prognosis. It is, however, evident that they all have strengths and limitations, which are not necessarily uniform for all patients; or be it, there are determined patient characteristics which may make one test superior or inferior to another (Table 25).
Table 25.
Main advantages and disadvantages of exercise testing (ET), myocardial perfusion scintigraphy (MPS), and coronary angiotomography (angio-CT) for assessment of coronary artery disease (CAD)
| Advantages | Disadvantages | |
|---|---|---|
| ET | • Widely available • Relatively low complexity • Relatively low cost • Does not involve radiation |
• Requires ability to exercise • ECG may be uninterpretable • Limited accuracy • Does not detect initial CAD |
| MPS | • Localizes and quantifies ischemia • Evaluates perfusion and LV function associated with exercise • Evaluates ischemia in patients unable to exercise • Makes it possible to monitor treatment |
• Technological complexity • Uses radiation • Attenuation artifacts • Does not detect initial CAD |
| Angio-CT | • Excludes CAD with great accuracy • Detects CAD in its initial phase • Allows for anatomical evaluation (e.g., anomalous coronaries) • Quick exam |
• May overestimate obstructions • Limited use for known CAD • Limited for physiological aspects • Uses radiation |
ECG: electrocardiogram; LV: left ventricle. Source: Adapted from Vitola JV.234
Once the doctor has answered the 2 initial questions, “Who is the patient?” and “What is the main diagnostic hypothesis?” he or she needs to answer the third question: “What is the most appropriate test for this patient and for the question I want to answer?” To answer this question, it is essential to know the main advantages and disadvantages of the exams, integrating the results of Bayes’ theorem and, thus, defining post-test probability. It is, moreover, necessary to identify when continuous or complementary analysis will be required in order to obtain additional information for better patient management (Figure 19).
Figure 19.
The importance of integrating information about different diagnostic modalities in an era of multimodalities. Generally speaking, the diagnostic modalities most used in Brazil are: exercise testing, echocardiogram, myocardial perfusion scintigraphy, angiotomography to evaluate coronary anatomy and calcium score, cardiac magnetic resonance, and, finally, invasive coronary cineangiography (cardiac catheterization). In this scenario, the doctor is central to establishing the best strategy and should ask the following questions: (1) What is my patient’s clinical profile? (2) What information am I looking for regarding the clinical hypotheses I have raised? (3) What test will provide this information? (4) Will I need further information to make a decision and manage this patient?
Source: Vitola JV.234
Of the techniques that have been covered, angio-CT is the most recent, showing great technological evolution, notably over the past 10 years. Data from important clinical studies have consolidated and recognized the value of this modality, as well as how to integrate it with other available tools. Before the advent of angio-CT, studies of patient anatomy presented greater difficulties, as it was mainly obtained via cardiac catheterization, with all the limitations, complications, and costs associated with invasive interventions. The development of a non-invasive, relatively simple and quick imaging technique has, in recent years, made it possible to recover the role of anatomical evaluation of the heart as a diagnostic and prognostic tool, thus integrating angio-CT into the multimodality scenario. Tests that allow for evaluation of cardiac physiology, such as ET and nuclear cardiology with MPS, have been routinely utilized for many decades, and, as they are not invasive, they have been employed for a large number of patients with suspected or known CAD. A great deal has been learned about these tools’ capabilities for diagnosis and, especially, for establishing prognosis and defining better courses of treatment.
Since the beginning of ET, initially in the 1950’s, followed by MPS in the 1970’s, information obtained has emphasized the great value of physiology, especially for evaluated coronary reserve flow, with highly consistent data for stratifying risk of cardiac death in patients with known or suspected CAD. Different physiological variables assist in characterizing patients as low-, intermediate-, or high-risk. The results of these tests, however, are not always in agreement, when comparing physiological tests to each another (ET with MPS) or to anatomical tests (angio-CT and catheterization). Potential “disagreements” and situations that may generate doubts are more common when comparing anatomy and physiology, especially at this moment, with the expanded use of angio-CT.
The scope of this text is not to revise details in relation to the variables involved, but rather to integrate information obtained from different available non-invasive tools, especially the interrelations between MPS, angio-CT, and ET. Summarily, the main variables of ET that represent high risk are: low functional capacity, greater magnitude of ST depression, occurrence in multiple leads, descending ST segment, ST-segment elevation in leads without Q waves, depressed chronotropic response, drop in blood pressure during stress, the presence of complex ventricular arrhythmia, manifestations of angina during low workloads, among others. In MPS, the principal markers of severity are: extensive perfusion defects with severe intensity, especially transient defects (transient reduced uptake) in more than 1 territory and mixed fibrosis patterns associated with ischemia (persistent reduced uptake associated with transient reduced uptake), stress-induced LV dilation, tracer uptake in the RV and the lungs, low LVEF, and LV with or without transient dilation associated with stress.
Conversely, aspects associated with low risk on the ET are represented by high functional capacity, absence of important ST-segment abnormalities or stress angina, good hemodynamic response with appropriate increase in HR and blood pressure, and absence of complex ventricular arrhythmias. In relation to MPS, markers of good prognosis are associated with normal myocardial perfusion and preserved LV ventricular function. Most of the time, especially in the most severe cases, diagnostic modalities are in agreement, or be it, a patient with high-risk ET findings will, likely, show significant MPS defects, corresponding to coronary anatomy compatible with advanced CAD. There are, however, different scenarios in which disagreeing results present challenges to better patient management.
The cases subsequently exposed are intended to integrate clinical data with the use of multimodalities, extending discussions within the medical decision-making process for understanding, interpreting, and suggesting conduct for dealing with agreements and, especially, disagreements. Figure 20 illustrates a concept in which the doctor begins evaluation using medical procedures of anamnesis and physical examination during the initial phase (I), formulating the main diagnostic hypotheses, which are fundamental aspects for defining the best investigation strategy. Subsequently, depending on the questions formulated, relatively simple or more basic diagnostic tests are obtained (II), such as: resting ECG, ET, resting ECHO and, eventually, CS. Subsequently, in accordance with the need for additional information and depending on the diagnostic hypotheses considered, the doctor may consider the application of more advanced non-invasive imaging techniques (III), such as angio-CT, MPS, and, potentially, cardiac magnetic resonance. Applying scientific knowledge regarding diagnostic and prognostic value, to both basic tests and more advanced non-invasive imaging methods, it is possible to establish filters for selecting patients who will really require invasive testing, such as cardiac catheterization (IV), notably with the aim of planning for myocardial revascularization.
Figure 20.
Concept of a rational strategy for evaluating and integrating modalities in a logical sequence of investigation of stable patients. It begins with the best rationalization for formulating diagnostic hypotheses (I), going on to the most basic tests (II), such as ECG/ET, ECHO, CS, continuing, as necessary, to more advanced non-invasive imaging methods (III), such as angio-CT, MPS, and CMR. The non-invasive tests, whether basic or advanced, should serve as “filters” for invasive testing, i.e. cardiac catheterization (IV), which should serve in planning advanced treatment only in patients under consideration for myocardial revascularization.234
9.3. Practical Examples of Integration of Modalities
1. Patient with abnormal ET, Duke score characterizing intermediate risk, and normal MPS
Clinical history: female, age 50, with hypertension, dyslipidemia, atypical symptoms, and borderline ET. Referred for MPS (Figures 21 and 22).
Figure 21.
Case 1 - Electrocardiogram tracing during peak stress and initial recovery with alterations (explained in the text).
Figure 22.
Case 1 - Myocardial perfusion scintigraphy within normality. Images acquired with dedicated cardiac equipment (gamma camera), equipped with solid cadmium-zinc-tellurium detectors.
Findings: The patient exercised for 8.5 minutes on the Bruce protocol, with satisfactory HR and blood pressure responses, reproducing the ET findings that motivated referral for MPS, with ST-segment depression varying from 1 to 1.5 mm after 80 mm on the J point, with aspect varying from slow ascending to horizontal, in multiple leads (Figure 21). She denied having precordial pain, but there was a manifestation of cervical and mandibular discomfort. Calculation of the Duke Score, , resulted in intermediate risk, both considering the symptom as angina and not considering it as angina . MPS was within normality.
Comments: This is one of the most common situations in nuclear cardiology laboratories. The first questions to be formulated refer to the risk defined by the ET. Abnormal responses may characterize low, intermediate, or high risks. For MPS, the best indication is for intermediate risk, which was the case with this patient. It should ideally be associated with physical exercise instead of pharmacological alternatives; when this is normal, the patient is stratified as low risk and, in most cases, the exam will indicate a probability of death lower than 1% per year, implying conservative medical management. At this moment, investigation may cease, based on the conclusion that the patient’s symptoms are not related to significant myocardial ischemia and that he or she requires prevention with the objective of controlling hypertension and dyslipidemia. Other findings in clinical practice include patients with functional capacity similar to the case described but with higher magnitude of ST-segment depression, resulting in a high-risk Duke score. Due to the absence of angina during stress and good functional capacity, however, the doctor may suspect that the calculation is overestimating the risk via ET. Such findings may be observed more frequently in patients with hypertension, possibly related to myocardial hypertrophy. In Brazil, Vitola et al. studied patients with high-risk Duke scores and MPS results, finding perfusion abnormalities in 70% of these individuals.235 However, the other 30% showed normal MPS, and it was demonstrated that these patients had excellent prognosis. Thus, in specific cases, even in the presence of high risks characterized by the same score, the application of multimodalities, such as the association of physical stress with non-invasive MPS imaging, are appropriate before proceeding to investigation via catheterization. Furthermore, it may also be possible to utilize angio-CT in some cases, considering its high NPV, with the aim of clarifying diagnosis and excluding important CAD, especially in young patients, where the probability of a “false-positive” result is higher.
2. Patient with normal ET and abnormal MPS
Clinical history: male, age 36, long-standing DM, insulin dependent, obese and hypertensive. Atypical symptoms, notably related to fatigue during stress. Referred for MPS to investigate ischemia, following ET which was normal but which had low sensitivity owing to electric axis deviation to the left, suggestive of left anterior fascicular block (LAFB) on resting ECG.
Findings: Resting ECG characterized LAFB (Figure 23). Time in the Bruce protocol was 10 minutes, with neither angina nor ST-segment alterations (Figure 24). Perfusion images showed transient reduced uptake, with large extension and moderate to severe intensity, suggestive of ischemia, involving predominantly the inferolateral, lateral, anterior, and anteroseptal walls of the LV, extending to the apex (Figure 25). Observe how the LV cavity dilate after physical exercise, with the appearance of diffuse hypokinesis and a drop in LVEF from 55% to 45% when comparing both stages.
Figure 23.
Case 2 - Resting electrocardiogram suggestive of left anterior fascicular block.
Figure 24.
Case 2 - Electrocardiogram obtained during immediate recovery, representing peak effort, heart rate of 144 bpm, with total workload performed considered satisfactory, in addition to the absence of ischemic ST-segment alterations.
Figure 25.
Case 2 - Myocardial perfusion scintigraphy showing important myocardial perfusion abnormalities, with multivessel ischemia and transient left ventricular cavity dilatation, representing high-risk indicators. Images acquired with dedicated cardiac equipment (gamma camera), equipped with conventional sodium iodide crystals.
Comments: Given a normal ET, with a high workload or good performance, with neither angina nor ST-segment alterations, patients are generally considered to have low post-test risk, but this is not always the case, as can be seen here. Nor does a normal ET represent the absence of CAD, as potentially shown by the presence of calcium in the coronary arteries on angio-CT or by ischemia detected by a more sensitive technique, such as MPS. In accordance with the evolution of medical knowledge in this era of multimodalities, restratification, even of patients with low risk on exercise testing, has become possible, as an exception. These possibilities should be considered more frequently in patients with family history of early CAD, DM, or multiple combined risk factors, and especially in those with high clinical risk (Framingham score) or LAFB on resting ECG. The case presented exemplifies precisely this scenario of a clinically high-risk patient (multiple risk factors, including DM), with LAFB on resting ECG and low risks on ET, who was restratified to a higher level of risk via perfusion imaging, due to the presence of important ischemia and LV dysfunction during stress, which are high-risk indicators. In this condition, when these tests are in disagreement, in a young, symptomatic patient (probable ischemic equivalent) with high clinical risk confirmed on an imaging exam, referral for coronary cineangiography is supported as part of medical management.
3. Patient in pre-operative evaluation for non-cardiac surgery, with mild abnormalities on MPS, high calcium score, and non-obstructive CAD
Clinical history: male, age 65, hypertensive, obese (BMI = 45), stroke 5 years prior, asymptomatic, in pre-operative evaluation for cholecystectomy and bariatric surgery. Interpretable resting ECG, unable to exercise. Referred for MPS with dipyridamole as initial investigation exam.
Findings: ECG tracings show no modification during and after intravenous administration of dipyridamole. Perfusion images reveal mild defects (small extension) in radiopharmaceutical uptake in the inferior and inferolateral/lateral walls and in the LV apex (the latter being transient), with preserved LV function (Figure 26). Considering the 2 protocol series of image acquisition, resting and under pharmacological stimulation, interpretation is limited due to the significant obesity. The finding may even represent an attenuation artifact. With the patient in an asymptomatic conditions, with the reported alterations in perfusion and preserved LV function, additional information is required before making the important decision of approving the patient for surgery, and anatomical evaluation via angio-CT is thus recommended. The findings indicate a CS of 1,621 measured by the Agatston score, corresponding to the 96% percentile, when compared to individuals of the same sex, age, and race.236 Moreover, there is evidence of non-obstructive lesions (< 30%) in all coronaries and an absence of significant obstructions > 50% (Figure 27).
Figure 26.
Case 3 - Myocardial perfusion scintigraphy showing mild alterations in myocardial perfusion, with analysis limited by significant obesity (grade III). Images acquired with dedicated cardiac equipment (gamma camera), equipped with conventional sodium iodide crystals.
Figure 27.
Case 3 - Angio-CT showing vascular calcifications involving coronary arteries and ascending and descending aorta.
Comments: In the presence of multiple risk factors, considering the patient’s age and stroke history, the probability of CAD is intermediate to high. Pre-operative risk stratification is necessary, and, although the resting ECG was interpretable, the patient was unable to perform exercise. MPS with dipyridamole is well indicated, given that normal results could lead to the patient being approved for surgery. On the other hand, faced with abnormalities in perfusion and/or function, with indicators of high risk, there is a sufficient base of evidence for indicating catheterization. In this case, however, the result showed normal LV function and mild alterations in perfusion, with the possible presence of artifacts or results of small vessel CAD (microcirculation) and/or endothelial dysfunction. The angio-CT findings indicated high CS compatible with a high atherosclerotic burden and poor long-term prognosis,237 which was not surprising given the profile of this patient whose coronary calcium (CC) was in the 96% percentile, meaning that 96% of individuals of the same age, sex, and race had lower coronary calcification indexes than the case described. Nonetheless, the contrasting anatomical evaluation revealed non-obstructive coronary lesions (< 30%), which reinforces the possibility that the patient might have small vessel CAD, which already bears physiological repercussions, implying more aggressive clinical management. The absence of significant obstructive lesions or high-risk anatomy serves as an additional filters for avoiding invasive examination (catheterization) and confirming that surgical risk would not be prohibitive. The most appropriate form of management for this patient, likewise, appears to gear toward aggressive preventative measures, with risk-factor control and follow up, in addition to medical treatment of CAD, which does not, however, require myocardial revascularization. Bariatric surgery itself may perhaps assist in controlling these risk factors.
4. Patient with elevated CS normal MPS and ET
Clinical history: male, age 52, asymptomatic, diagnosed with DM 5 years prior, hypertensive and dyslipidemic. Calculated CS.
Findings: CS resulted in a high Agatston score of 1,143, in the 99% percentile (Figure 28). MPS with physical exercise was indicated. Patient underwent stress in the Bruce protocol for 10 minutes, reaching HR of 158 bpm (94% of the recommended maximum HR), with no clinical, electrocardiographic, or hemodynamic alterations. MPS (with a CZT camera) showed homogenous radiopharmaceutical distribution in the LV walls (Figure 29), as well as normal LV systolic function.
Figure 28.
Case 4 - Angio-CT imaging shows elevated calcification index in coronary arteries.
Figure 29.
Case 4 - Myocardial perfusion scintigraphy within normality. Images acquired with dedicated cardiac equipment, equipped with solid cadmium-zinc-tellurium detectors.
Comments: This situation has occurred more frequently in clinical practice, to the extent that CS has gone on to be incorporated as a screening method for CAD and risk stratification in the subgroup of asymptomatic patients (DM and intermediate Framingham score). This disagreement between results is understandable given that the presence of atherosclerosis will not necessarily result in ischemia detected by functional methods. For instance, an ET may indicate low risk according to the Duke score in a patient who has performed only 5 minutes of exercise in the Bruce protocol but who showed neither ST alterations nor angina. It is intuitive to grasp that, in the presence of coronary disease (most cases with high CS), this does not represent exactly the same low risks as in a patient without CAD (absence of coronary calcification or zero CS). Regarding these facts, there is extensive literature on the prognostic value of CS, with long follow-up periods (> 15 years).238 In this manner, it is feasible to expect the group characterized as low-risk by the Duke score to be heterogeneous, and patients should thus be treated individually, considering the intensity of prevention. In the case demonstrated, as the patient has DM, there had already been an indication for statin use, with the very high CS (in the 99% percentile) reallocating the patient into an even higher risk within the group with DM. As part of data revision which has been occurring over the past 20 years, cases have been found which showed normal but which, nonetheless, presented coronary events during medium-term evolution, in a manner similar to the discrepancies recently observed between ET and CT. Likewise, a recent study by Chang et al.239 observed the same discrepancies in patients with low-risk Duke scores from ET and CS > 400. They evaluated 946 patients with the Framingham score, classifying the majority as intermediate-risk (estimated 11.1% average for events over 10 years) and, basically, asymptomatic, as evaluated by ET and CS. The average Duke score was 8.4, categorized as low-risk (≥ 5). Stress tests were positive or altered in 12.3% of patients, while CS > 100 were found in 54.2% of patients. MPS was abnormal in 10.9% of the same population. It was demonstrated that CS restratified risk for patients with low-risk Duke scores, identifying individuals with atherosclerosis and higher propensity for events. Furthermore, a current register known as CONFIRM has accumulated data that definitively suggest that, in the presence of non-obstructive CAD,240 evolution may be worse in patients without CAD. It has, thus, become evident that the anatomical technique with angio-CT is identifying coronary atherosclerosis earlier. This set of information definitively represents a change of paradigm in the medical decision-making process. In this situation where CAD is identified, medical management will be geared toward more aggressive prevention of modifiable risk factors and minute observation of possible symptoms that translate to disease instability. In the absence of ischemia, revascularization procedures should not be considered.
5. Patient with high CS and abnormal MPS
Clinical history: female, age 68, asymptomatic, with intermediate-risk Framingham score. Performed CS for risk restratification.
Images: reproduced with permission of Vitola JV.234
Findings: The resulting CS was high, at 1,282, according to the Agatston score, placing this patient in the 99% percentile (Figure 30). With this finding, functional evaluation was indicated, using MPS with MIBI-99mTc associated with exercise. The patient exercised for 7.5 minutes in the Bruce protocol, showing ST-segment depression of up to 3 mm during peak stress, with a varying aspect which tended toward descending in multiple leads, without symptoms (Figure 31). With these findings, the Duke score , or , resulting in classification as intermediate risk. In the perfusion images, the presence of transient reduced uptake was evidently observed, involving the middle and distal portions of the anteroseptal and anterior walls and apex of the LV, with accentuated intensity and medium extent, compatible with significant ischemia in the territory of the anterior descending artery (Figure 32). Furthermore, mild transient dilation of the LV cavity was observed during stress, in addition to radiopharmaceutical uptake in the RV wall, which are high-risk markers.
Figure 30.
Case 5 - Angio-CT revealing severe calcification in coronary arteries.
Figure 31.
Case 5 - Electrocardiogram tracing demonstrating ischemic electrocardiographic alterations in multiple leads, with submaximal heart rate levels. Detailed explanation in the text.
Figure 32.
Case 5 - Myocardial perfusion scintigraphy demonstrating significant ischemia in the territory of the anterior descending artery. Images acquired with dedicated cardiac equipment (gamma camera), equipped with conventional sodium iodide crystals.
Comments: Evidence in the literature has supported the use of CS for risk restratification in patients who have intermediate clinical risks (using, for instance the Framingham or the global risk score), but who are in asymptomatic phases. The higher the CS, the higher the risk will be; not coincidentally, the probability of silent ischemia will also be higher, and this, in turn, increases the patient risks even further. Data have demonstrated that, when CS values are between 400 and 999, the probability of perfusion defects reaches up to 29%, and when values are > 1,000, the probability increases to 39%.241 Brazilian data from Cerci et al. have reported similar information, with an ischemia prevalence of 34% in patients with CC over 400.242 Within medical orientation, rigorous preventative measures have shown evident benefits in individuals with high CS. Caution, however, is recommended when indicating revascularization procedures, emphasizing the absence of formal indication, consensus, or evidence regarding benefits based solely on CS results. On the other hand, recommendations exist for individuals with high-risk anatomy, at least moderate ischemic burden (in terms of extent and intensity), and the presence of symptoms refractory to clinical treatment.14 Notwithstanding indications documented in guidelines, levels of evidence demonstrating the benefits of myocardial revascularization with the aim of reducing mortality in patients with stable CAD, based both on information about anatomy243,244 and ischemia quantification (retrospective data),245 have been questioned, considering the absence of randomized studies published to date. With this in mind, what is known as the ISCHEMIA study246 was designed (report to the addendum of thi guideline), randomizing patients who have at least moderate ischemia (more than 10% of the myocardium affected by ischemia of significant intensity or severity) from 400 centers worldwide into 2 treatment scenarios: “optimized clinical treatment” versus “optimized clinical treatment associated with revascularization of ischemic territory,” excluding patients with left main trunk lesion > 50% on angio-CT. The study objective was to attempt to identify subgroups where revascularization benefits stable patients, filling in this important gap in current scientific evidence. Considering the available information (current evidence and guidelines), it seems appropriate to investigate ischemia in patients with CS over 400 in the attempt to identify individuals with high ischemic burdens (extent and intensity of perfusion defects), who may benefit from invasive strategies.
6. Patient with abnormal ET, normal MPS, and normal coronary angio-CT results
Clinical history: male, age 33, atypical chest pain, recent onset of DM, high blood pressure (HBP) and family history of early CAD, ET resulting in intermediate-risk Duke score (+3).
Findings: The stress phase of the ET revealed high estimated metabolic expenditure (11 METs), with 9 minutes in the Ellestad protocol. ST-segment depression of up to 1.5 mm (measured in the J point), with a descending aspect (Figure 33), were observed in multiple leads, during the recovery phase only, and they were sustained until the end of this phase. The Duke score was +3 (intermediate-risk), and MPS showed homogenous radiopharmaceutical distribution throughout the walls of the LV, considered within normal limits (Figure 34). Due to persistence of symptoms during evolution, coronary angio-CT was solicited 2 months later, showing an absence of obstructive lesions and a CS of zero (Figure 35).
Figure 33.
Case 6 - Electrocardiogram tracing showing electrocardiographic alterations (ST depression), especially during the recovery phase of the exercise c test.
Figure 34.
Case 6 - Myocardial perfusion scintigraphy within normality, with two image acquisition series, resting and stress, with dedicated cardiac equipment (gamma camera), equipped with conventional sodium iodide crystals.
Figure 35.
Case 6 - Coronary angio-CT within normality.
Comments: In patients with intermediate probability of CAD, the indicated methods (MPS or angio-CT) are additional possibilities for investigation. At least 2 randomized studies have evaluated these strategies:
The PROMISE study,247 which included 10,003 individuals with suspected CAD, with 25 months of follow up regarding the primary outcome (composed of death, AMI, and hospitalization for UA), showed similar evolution in both randomization groups (A) functional tests, including MPS, 5,007 patients with 3% events versus B) angio-CT, 4,996 patients with 3.3% events (p = 0.75). There was, however, a higher number of revascularizations in the group that began with anatomical evaluation.
The International Atomic Energy Agency study248 demonstrated that the initial strategy of angio-CT entails the solicitation of additional diagnostic methods, including MPS itself or direct catheterization. These findings could have been foreseen due to the differences in information between both techniques, as coronary angio-CT is more sensitive for detection of anatomical diseases without important physiological impact. Like Case 1, the situation presented in Case 6 is common in routine coronary angio-CT. In cases where ET shows intermediate or low risk and, especially, in those where the patient has intermediate or low pre-test probability, angio-CT has one of its most precise indications. The main diagnostic virtue of angio-CT is its high NPV which essentially excludes CAD. Thus, if the probability of disease is intermediate or low, the chance of excluding it is greater, and the test shows better benefits. In the case described, as symptoms persisted, complementary evaluation with angio-CT was highly useful to the medical decision-making process. Furthermore, prognosis in a patient without CAD on angio-CT is excellent, with nearly zero risk of AMI and coronary events for up to 5 years,249 owing to its high NPV and to the fact that characteristic evolution of CAD habitually progresses slowly, with individual variations, and this lowers the chances of an individual developing CAD culminating in a coronary event over a period of 5 years. Integrated analysis of these exams, in the case therefore, infers an excellent prognosis, notwithstanding the altered ET, and its rules out CAD quite safely, in the same manner that MPS had already excluded the presence of myocardial ischemia. On the other hand, if the present case had been associated with a high probability of CAD or inadequate technical conditions (e.g. high ventricular response atrial fibrillation), the scenario could have been different, given that, for a patient with high atherosclerotic burden, the positive predictive value of angio-CT is limited. Furthermore, artifacts caused by an unfavorable situation (AF) may severely impair diagnostic accuracy. In these cases, MPS would be a better form of evaluation.
7. Patient with artificial electric pacemaker, abnormal MPS and normal angio-CT
Clinical history: female, age 49, diagnosed with Chagas heart disease, using an artificial pacemaker, pain during effort, type II DM, non-insulin-dependent, diagnosed 4 years prior.
Findings: MPS performed with dipyridamole, considering the presence of artificial pacemaker stimulation (Figure 36) suggestive of DDD mode (resting ECG with atrial spikes, without clear visualization of ventricular command). Perfusion defects associated with pharmacological stress were characterized as moderate intensity and medium extent, involving the inferior (mediobasal portion) and inferolateral walls and the apex of the LV (Figure 37), and they were partially transient (predominance of ischemia). Angio-CT showed left dominant coronary circulation, with no signs of atherosclerosis. The presence of atrioventricular pacemaker electrodes limited assessment of the image via angio-CT.
Figure 36.
Case 7 - Electrocardiogram tracing of patient with artificial pacemaker.
Figure 37.
Case 7 - Myocardial perfusion scintigraphy showing significant perfusion abnormalities (notably following administration of dipyridamole, with partial improvement while resting) and dilation of the left ventricle. Image acquired with dedicated cardiac equipment (gamma camera), equipped with conventional sodium iodide crystals.
Comments: Returning to the basic and appropriate principles of questions about pre-test probability and characterization of severity based on MPS findings, it is necessary to give special emphasis to the synergy between methods in this case. Symptomatic female patients with DM generally have an intermediate probability of obstructive CAD, mainly depending on the duration and aggressiveness of DM. On the other hand, they also have a high prevalence of endothelial dysfunction and microvascular disease, which may cause alterations in myocardial perfusion.250 There also exists the condition of Chagas heart disease, which features angina as a manifestation in the absence of obstructive epicardial coronary disease. The doubt which the doctor likely faces upon receiving the MPS results is the following: “What is the chance of obstructive CAD? And of endothelial dysfunction?” This is due not only to the diagnostic question, but also to the therapeutic implications, such as aggressiveness in reducing low density lipoprotein (LDL) cholesterol and the use of acetylsalicylic acid (ASA), for example. Another question is, “Does the perfusion modification in the apex represent an alteration related to Chagas heart disease?” Other questions similarly arise regarding the possibility of silent infarction related to CAD or a defect associated with artificial electrical stimulation resulting in customary atypical movement in the interventricular septum (component of an artifact). In this scenario, a safe and non-invasive way to exclude CAD is to perform angio-CT, which showed normal results in this case. It is important to underline the additional incremental prognostic value of angio-CT in this scenario, given that prognosis for this patient who does not have atherosclerosis, with mild ischemia (likely due to endothelial dysfunction), is considerably better than it would be were there conditions of mild ischemia in a patient suffering from uni- or bi-arterial obstructive CAD, or even in a patient with multivessel non-obstructive CAD.251 Other considerations refer to the possibility of MPS artifacts, not only related to the pacemaker in this case, but mainly to attenuation artifacts, when attenuation correction is not available, or when the prone position is not routinely used, in addition to the previously described Chagas heart disease. If an artifact is highly suspected, corroborated by the presence of systolic thickness of LV walls without alterations, angio-CT may avoid unnecessary catheterization, even in patients with higher probabilities of CAD. Within Brazilian experience, in a laboratory with a high nuclear cardiology volume, only 24% of patients with mild myocardial ischemia on MPS referred for angio-CT have obstructive CAD. The majority are women (58.8%), 33% of whom have non-obstructive CAD and 43% of whom do not have CAD (International Conference of Nuclear Cardiology - ICNC 2017).252
8. Patient with abnormal angio-CT and normal MPS
Clinical history: male, age 51, atypical symptoms, active, with positive family history for early CAD.
Findings: Angio-CT showed a CS of 1,445 on the Agatston score (99% percentile); significant obstructive CAD involving the left anterior descending artery in its distal portion (> 70%), with occlusion of the first diagonal branch, which receives collateral circulation; and non-obstructive CAD in the circumflex and right coronary arteries (Figure 38). MPS with perfusion and LV function were considered within normal limits (Figure 39), and ET revealed optimal physical performance (estimated metabolic expenditure of 18 METs) and normal electrocardiographic, clinical, and hemodynamic responses.
Figure 38.
Case 8 - Angio-CT showing significant obstructive alterations and evidence of advanced coronary artery disease. DG1: diagonal 1 coronary artery branch; LAD: left anterior descending artery; RCA: right coronary artery.
Figure 39.
Case 8 - Myocardial perfusion scintigraphy within normality. Images acquired with dedicated cardiac equipment, equipped with solid cadmium-zinc-tellurium detectors.
Comments: This is a challenging clinical situation, which expresses a fundamental example of integration of non-invasive anatomical and physiological modalities with the goal of avoiding unnecessary revascularization procedures. Performing angio-CT as an initial exam had the objective of excluding obstructive CAD in a young patient with intermediate pre-test probability. Meta-analysis of recent studies has demonstrated a probable benefit of this initial anatomic strategy in this scenario, with reduced AMI, when compared to initial functional test,253 recently incorporated into guidelines in the United Kingdom.254 This investigation, however, leads to an increase in the number of invasive procedures and revascularizations, with the risk of these procedures not being appropriate.245,246 Thus, in the described scenario, with evident anatomy of obstructive CAD, which nevertheless does not meet the criteria for high risk (left main coronary lesion or triple-vessel lesions involving affected areas proximal to the left anterior descending artery), the management considered most appropriate is certainly ischemia quantification, considering that revascularization would be indicated in the presence of at least moderate ischemic burden.241 In this specific case, the patient should be clinically treated, with an aggressive secondary prevention approach, with close monitoring of modifiable risk factors and special attention to the manifestation of symptoms, postponing revascularization, at least in this moment where there is a lack of evidence regarding its benefits.
9. Patient with abnormal angio-CT and abnormal MPS
Clinical history: male, age 51, with atypical chest pain (not always related to effort). Dyslipidemia and family history of early CAD (Both his father and brother had AMI resulting in death at the age of 53). Referred for coronary angio-CT to rule out obstructive CAD as the cause of the symptoms.
Findings: Angio-CT showed a CS of 12.2 (Agatston score, 67% percentile), mild, non-calcified atherosclerosis in the left main coronary and partially calcified atheromatous plaque in the middle third of the left anterior descending branch (Figure 40), resulting in moderate to significant luminal reduction (60% to 70%). The patient was referred for MPS associated with physical stress, exercising for 11 minutes in the Bruce protocol, with no significant ST-segment alterations (Figure 41) and without reproducing the symptoms. MPS images showed mild transient reduced uptake (ischemia) in the anteroseptal and septal walls and the apex of the LV (Figure 42).
Figure 40.
Case 9 - Angio-CT demonstrating significant obstructive luminal lesion, with the absence of calcification in coronary arteries. LAD left anterior descending artery.
Figure 41.
Case 9 - Electrocardiogram tracing obtained during immediate recovery period, with no significant alterations.
Figure 42.
Case 9 - Myocardial perfusion scintigraphy demonstrating transient reduced uptake, characterized by mild intensity and small extent, suggestive of ischemia in the anteroseptal and septal walls and the apex. Images acquired with appropriate cardiac equipment (gamma camera), equipped with conventional sodium iodide crystals.
Comments: Considering that a male patient with stable chest pain is characterized as having an intermediate pre-test probability of CAD, the routine non-invasive methods for diagnostic and prognostic evaluation are indicated. If the resting ECG is normal and the patient has informed ability to exercise (performing daily activities with estimated metabolic expenditure of > 5 METs), the ET is then the consensual indication, provided that its limitations are taken into account. In the case in question, the early family history of CAD stands out. This fundamental clinical information is not always incorporated into traditional methods of estimating pre-test probability. In this context, coronary angio-CT was chosen, in part to rule out obstructive CAD (high NPV), which is present in only 23% of symptomatic patients within the same probability range, according to the CONFIRM register.240 This register demonstrates lower observed prevalence of 50% to 70% obstructive lesions on angio-CT, in comparison with the expected prevalence calculated by conventional algorithms, establishing the concept that the routine algorithms for characterizing pre-test or expected probability of events during long follow-up periods, such as the Framingham, PROCAM, Diamond Forrester, SCORE, and Global Risk; overestimate CAD. This is also the case with detection of early, non-obstructive CAD (present in 34% of patients in this register), especially in patients with family history. This investigation strategy has already been shown to be effective and likely to reduce AMI,252 as previously discussed in this section; it is, however, necessary to be careful with excessive interventions. This was precisely the role of functional evaluation via MPS in this case. Detection and quantification of ischemia are fundamental for determining patient management, given that the presence of moderate to severe ischemia alone would justify a more invasive strategy, such as revascularization, in the absence of refractory angina. This case was thus started on optimal clinical treatment, similar to that of patients included in the COURAGE study.
10. Patient with abnormal ET, normal MPS, and abnormal angio-CT
Clinical history: female, age 67, with fatigue related to effort. Hypertensive ex-smoker, diagnosed with diabetes 1 year prior. Referred for MPS following abnormal ET with intermediate risk.
Findings: The patient exercised for 9 minutes in the Bruce protocol, reaching a HR of 136 bpm (89% maximum HR predicted based on age), triggering stress arrhythmias (ventricular and supraventricular extrasystole, in addition to periods of nonsustained ventricular tachycardia [NSVT]). ST-segment depression reached 3 mm in multiple leads (Figure 43), but the patient was asymptomatic. Duke Score = - 6, characterized as intermediate risk. On MPS, there was an absence of signs of ischemia (Figure 44). Considering the clinical profile and the finding of complex ventricular arrhythmia (NSVT), concomitant with descending ST-segment depression, in spite of normal perfusion on MPS, the clinical option was to perform an angio-CT, which showed advanced atherosclerosis (Figure 45) with a CS of 829 on the Agatston score (97% distribution percentile) and non-obstructive lesions (< 30%) in multiple vessels.
Figure 43.
Case 10 - Electrocardiogram tracing demonstrates ischemic ST-segment alterations and episodes of supraventricular arrhythmia, in addition to nonsustained ventricular tachycardia.
Figure 44.
Case 10 - Myocardial perfusion scintigraphy with homogenous radiopharmaceutical distribution throughout the walls of the left ventricle, considered within the limits of normal. Images acquired with dedicated cardiac equipment (gamma camera), equipped with conventional sodium iodide crystals. Reproduced with the permission of Vitola JV.234
Figure 45.
Case 10 - Coronary angio-CT showing significant coronary atherosclerosis, with multivascular calcification. Reproduced with the permission of Vitola JV.234
Comments: In this patient, analysis of ET plays an important role in case management. MPS study with the radiopharmaceutical MIBI-99mTc showed no abnormalities, which, in itself, determines excellent short-term prognosis. The presence of ventricular tachyarrhythmia during stress, however, adds a risk that is not, in practice, incorporated into risk prognosis by the Duke score. Furthermore, it limits analysis of the ST segment and may give rise to diagnostic doubts. One study which stands out in the literature verified that the inclusion of ventricular arrhythmia as a variable in the Duke score during ET increased its restratification potential in 30% of patients.23 Once again, questions may arise related to the lower sensitivity of MPS, which was apparently normal in this case (a false-negative result?). Another question is, “Should differential diagnoses such as cardiomyopathy, specific conduction tissue disease, among others, be additionally considered?” Although functionally severe obstructive CAD is improbable when MPS is normal, anatomical data from angio-CT complement and clarify many of these doubts which arose due to the conflicting results of the two functional tests. One alternative would be to perform CS, but, particularly in symptomatic patients, more detailed evaluated of anatomy via angio-CT, which quantifies degree of obstruction and determines the presence and extent of non-calcified atherosclerosis, adds incremental prognostic value.256 In this specific case, the presence of non-obstructive atherosclerosis, even with normal perfusion, denotes worse prognosis than in patients with normal perfusion and the absence of atherosclerosis.257 Moreover, the presence of atherosclerosis in multiple segments, as in the present case, confers a prognosis similar to that of uniarterial obstructive CAD.250 In conclusion, clinical translation of such findings could be resumed in the following manner: there are no indications that the ET alterations are secondary to ischemia, and there are thus no accrued benefits to coronary intervention, whether percutaneous or surgical revascularization. Orientation should be toward aggressive treatment of atherosclerosis to reach lipid goals recommended in current guidelines, in addition to strict control of other modifiable risk factors. The patient’s current profile confers medium- and long-term prognosis similar to that of a patient with obstructive CAD in a single coronary artery.
11. Patient unable to exercise, abnormal MPS associated with pharmacological stimulus with dipyridamole and angio-CT showing non-obstructive CAD, ischemia suggestive of microcirculatory abnormalities
Clinical history: female, age 68, with stress fatigue. Hypertensive and obese, diagnosed with diabetes 8 years prior. Referred for MPS due to difficulty performing physical exercise test.
Findings: During the attempted test with physical exercise, the patient exercised for only 6 minutes in the Bruce protocol, with a peak HR of 115 bpm (75.6% the expected upper limit, based on age). The stress phase was discontinued due to fatigue and calf-muscle pain, also establishing suspected chronotropic incompetence. As an alternative, the protocol was initiated with dipyridamole, and it was considered altered due to ST-segment depression of 1.0 mm, in 2 leads, following completion of intravenous administration (Figure 46). MPS was considered abnormal due to transient reduced uptake suggestive of ischemia, involving the anterior and anterolateral (predominantly in the middle distal portion) walls of the LV, with moderate intensity and medium extent, characterized as mild to moderate ischemic burden, in addition to preserved LV function (Figure 47). Considering the high clinical risk profile, the findings of probable chronotropic incompetence, ST-segment alterations with dipyridamole, and ischemia in the anterior descending territory; the option was to complement with angio-CT, which showed non-calcified and non-obstructive atherosclerosis, with a CS of zero and a mild lesion (< 30%) in the anterior descending branch.
Figure 46.
Case 11 - Electrocardiogram tracing with ischemic ST-segment alterations following administration of dipyridamole.
Figure 47.
Case 11 - Myocardial perfusion scintigraphy (MPS) demonstrating moderate ischemic burden in the anterior wall, extending to the anterolateral wall of the left ventricle. Images acquired with dedicated cardiac equipment (gamma camera), equipped with conventional sodium iodide crystals.
Comments: This is a classic example of a symptomatic patient with a combination of factors which, in association, may result in phenomena of endothelial and microcirculatory dysfunction, with the consequent condition of myocardial ischemia. This physiopathological condition, little over a decade ago, would have led to coronary cineangiography study in order to rule out obstructive CAD. As a consequence, “normal” coronary arteries were often observed, in what were known as “white catheterizations.” With the findings described in specific populations, especially in female patients, the recent use of the term “ischemic heart disease” has gone on to express the conditions of obstructive atherosclerosis, endothelial dysfunction, and microvascular dysfunction more adequately. A recent review published by Pepine et al. in 2015 258 described important differences in the CAD spectrum in both sexes, pointing out that symptomatic women have a lower prevalence of obstructive CAD than men with the same symptoms. On the other hand, they tend to have more microvascular dysfunction, plaque erosion, and thrombus formation. In this specific case, the following factors stand out: female sex, obesity, DM, altered functional tests (both the post-dipyridamole ECG and perfusion imaging via MPS), and non-obstructive CAD on angio-CT.
Based on this combination of individual characteristics, especially with endothelial dysfunction and reduced coronary flow reserve (CFR), associated with non-obstructive CAD, the risk of cardiovascular events is significantly higher, and it is similar to that of individuals who have obstructive CAD, but who are not indicated for revascularization.259 Thus, as the prevalence of obstructive CAD is lower in women, angio-CT has been growing as a preferential diagnostic method for ruling out obstructive CAD in patients with intermediate probability, especially when there are limits to the physical exercise test.260 In the case in question, the combination of functional (ischemia) and anatomical (non-obstructive CAD) data was essential to the diagnosis of endothelial dysfunction and to guiding therapeutic management.
12. Abnormal ET characterized as intermediate-risk and abnormal MPS with high-risk indicators
Clinical history: male, age 69, with precordial pain during greater efforts for 4 months. Hypertensive, ex-smoker, referred for MPS due to altered ET, with intermediate Duke score.
Findings: patient exercised for 9.5 minutes in the Bruce protocol, reporting non-limiting anginal pain during peak exercise, with descending ST-segment depression of 1.0 mm, measured at the J point, in multiple leads, with prolonged duration, during the recovery phase (Duke score = +0.5) (Figure 48). Perfusion imaging showed transient reduced uptake in the septum and apex of the LV, which was exercise-induced, characterized by severe intensity and medium extent (moderate ischemic burden), associated with the component of persistent reduced uptake in the described territory. Apical and septal akinesis (predominantly distal) were also observed following exercise, as well as apparent transient dilation of the LV cavity and uptake of MIBI-99mTc in RV walls, which are additional markers of severity (Figure 49).
Figure 48.
Case 12 - Electrocardiogram showing prolonged ischemic alterations during the late recovery phase.
Figure 49.
Case 12 - Myocardial perfusion scintigraphy (MPS) showing important ischemic findings (transient, stress-induced reduced uptake), with high-risk indicators, involving the territory of the left anterior descending artery (LAD). Images acquired with dedicated cardiac equipment (gamma camera), equipped with conventional sodium iodide crystals.
Comments: This is an example of a case where functional methods are in agreement regarding detection of ischemia. Cardiac imaging, here, provides additional information to the ET (intermediate-risk Duke score), which is known as “incremental prognostic value.” Quantification of ischemia via MPS restratifies this patient as high-risk. In this situation, revascularization procedures may be considered based on current evidence, although the results of the ISCHEMIA study (ISCHEMIA study Addendum - report to item 2 of this guideline) have not been yet published. This case is complementary to the discussion elaborated in Case 1, where non-invasive images via MPS added prognostic value and guided patient management.
10. Evaluation of Myocardial viability via Myocardial Perfusion Scintigraphy
10.1. Introduction
In some patients with chronic CAD and ventricular dysfunction, revascularization may significantly improve symptoms, ventricular function, and mortality. Physiopathological conditions and acute and chronic mechanisms of adaptation to temporary reduction of coronary flow include stunning, hibernation, and preconditioning, either separately or coexisting in the same patient.261 Evaluation of myocardial viability is, consequently, important to the therapeutic decision-making process for patients with ventricular dysfunction (class of recommendation I, level of evidence B) without angina,262-266 given that functional improvement will not occur in the presence of fibrosis. Several non-invasive imaging techniques are available for detection of viable myocardium, including stress echocardiography with dobutamine, cardiac resonance, and nuclear imaging with SPECT or PET. These methods evaluate different myocardial characteristics and, thus, present variations in sensitivity and specificity.261 In the SPECT technique, the use of thallium-201 (201Tl) is the established method for evaluating myocardial perfusion and the integrity of the cellular membrane. In comparison with other radiopharmaceuticals and available techniques, this has become the choice for determining viability.267,268
Additionally and in conjunction with these non-invasive techniques, whose aim is to obtain functional and/or anatomical information, constituting the basis of the physiopathological approach to underlying HF, multiple detector CT is also appropriate.
However, the selection of imaging modalities, whose purpose is to assess CHF and, specifically, viability of a dysfunctional myocardium, depends on the clinical information that is required for adequate patient management (Table 26), with inherent questions and affirmations, such as:
Table 26.
Clinical situations where nuclear cardiology should be considered a preference for assessing myocardial viability, in the following order of choice: PET with 18F-FDG, resting scintigraphy with thallium-201 and reinjection protocol, and resting scintigraphy with Sestamibi - 99mTc sensitized with oral nitrite
| • Evaluation of extent/localization of dysfunctional myocardium at risk, through significant hypoperfusion (hibernating myocardium) |
| • Clinical situations in which sensitivity is sought for assessment of viability |
| • Contraindications to the use of MR: patients with pacemakers or cardiac defibrillators incompatible with resonance, cerebral clips, cochlear implants, metallic fragments in their eyes, or renal insufficiency |
| • Conditions which limit image acquisition via MR: claustrophobia, irregular cardiac rhythm, dyspnea with inability to remain in dorsal decubitus for prolonged periods |
| • Availability of the method and local expertise |
18F-FDG: fluorodeoxyglucose labeled with fluorine-18; MR: magnetic resonance; PET: positron emission tomography.
Is the etiological cause of the cardiomyopathy in question ischemic or non-ischemic?
In patients considered “ischemic,” the need for revascularization should be evaluated with respect to characterization of the quantity of myocardium at risk/viability.
Evolving assessment of LV function and the possibility of remodeling are mandatory elements for analysis within the clinical decision-making process;269 other elements include secondary mitral regurgitation,270 implantable devices, such as defibrillators and/or resynchronization therapy.271
Moreover, when the etiology of LV dysfunction, considered of the utmost magnitude for therapeutic decision making, is mandatorily under discussion, it has been verified that the majority of patients will have ischemic cardiomyopathy. Data from 24 multicenter studies on CHF, published in high-impact periodicals between 1986 and 2005 and summarized in 2006, including 43,568 individuals, showed a 62% prevalence of CAD. This frequency is probably underestimated to the extent that coronary cineangiography was not performed in all patients.272
10.2. Morphology
It had initially been established that the recovery of ventricular function, when a hibernating myocardium was revascularized, should indicate that structural changes were absent or minimal, as observed in experimental models of stunned myocardium. However, since the beginning of the 1980’s, it has been known that chronically dysfunctional myocardial segments demonstrate distinct morphological changes under microscopy.273
There is a combination of normal, atrophic, and hypertrophic myocytes, with or without evidence of necrosis. Electron microscopy shows loss and/or disorganization of myofilaments and alterations in sarcoplasmic reticulum and mitochondria. These structural changes may contribute to slow functional recovery following revascularization.274
10.3. Evaluation of Viable Myocardium
The differentiation between the presence and absence of viability is highly relevant in patients under consideration for revascularization. Many patients who demonstrate viability associated with severe LV dysfunction may still be candidates for revascularization, but not for cardiac transplant.275
10.4. Physiopathology and Definitions
The term viable myocardium, regardless of the contractile state of the myocardium, should be understood differently in the context of CHF and viability study, given that the main objective is to predict improvement in LV function following revascularization.
Persistent contractile dysfunction of the LV may be related to chronic hibernation and/or stunning, and not merely associated with fibrotic tissue. Revascularization may improve function and survival if the dysfunctional myocardium is still viable. Functional improvement will not occur in the presence of fibrosis.
The initial point for discussion of viability implies regional dysfunction, detected by various non-invasive methods, including echocardiography as an initial line of investigation.
Differentiation between hibernation and stunning may be established based on blood flow to the myocardium. While resting flow is chronically diminished in hibernation, it may still be preserved in the stunned myocardium, with a compromised flow reserve however.19
Clinically speaking, it may not always be feasible to separate the 2 physiopathological conditions; nor is it always necessary, considering that both entities require revascularization in order for there to be improvements in ventricular function. Affected areas are referred to as viable myocardium or myocardium at risk.276
The coexistence of normal myocardium, however, and the formation of subendocardial scarring will not result in improved function, which is now considered “non-jeopardized” area of viable myocardium.
Most experience in assessing viability has been obtained with nuclear imaging, utilizing SPECT and PET, with assessment of perfusion and metabolism. Moreover, using SPECT, cellular and mitochondrial membrane integrity may be characterized.
It has been demonstrated that 40% to 50% of dysfunctional segments without contractile reserve may still have preserved perfusion and metabolism, some of which will recover function following revascularization procedures. The loss of contractile reserve is associated with structural damage characterized by greater severity and fibrosis formation.
Several viability standards have been recognized in areas of contractile dysfunction, for instance: I) any region with > 50% of radiopharmaceutical uptake in resting images; II) any perfusion defect with > 10% increase in uptake of late images.277 It is, however, necessary to emphasize that areas with > 50% uptake often do not improve in function, given that these regions contain mixtures of normal myocardium and non-transmural scarring.
Uptake and retention of tracers (sestamibi and tetrofosmin, labeled with technetium-99m) depends on perfusion, cellular membrane integrity, and mitochondrial function, where radiopharmaceutical uptake of > 50% to 60% in dysfunctional areas is frequently used as a sign of viability, when observed in resting images.
10.5. The Most Frequently Used Protocols24,278,279
Stress and redistribution - two steps or image acquisition series/one injection of 201Tl: This is a conventional technique with image acquisition between 2 and 10 minutes (a maximum of 15 minutes) following injection of 201Tl during peak stress (first step). These images reflect initial distribution of the radioisotope dependent on blood flow and, thus, regional myocardial flow. Two to four hours after initial intravenous administration of the radioisotope, in the resting condition (second step), a new series of images is obtained, representing the “redistribution phase,” related to the continuous exchange of 201Tl throughout the myocardium and extracellular behavior. This protocol has been designed to study ischemia, and it is not sufficient for characterization of viability, given that viable tissues may not exhibit improvement in radiopharmaceutical uptake (reversibility) within conventional time periods for redistribution images, giving the apparent impression of persistent reduced radioisotope uptake or fibrosis.
Stress/redistribution and reinjection - three steps or image acquisition series/two injections of 201Tl (Figure 50B):In addition to the conventional protocol, which contains only 1 injection of 201Tl during stress (at a dose of up to 3.0-3.5 mCi), this includes the reinjection of 201Tl (generally at a dose of 1 mCi) immediately after the redistribution phase, with the aim of elevating blood concentration of the radioisotope, with a new image acquisition series that may vary in terms of time (between 6 to 24 hours). There is evidence that up to 50% of regions with perfusion defects that are apparently “fixed or persistent” improve in terms of relative radioisotope uptake. This information is predictive of improvement in regional function following revascularization. Areas with sustained or severe reduced uptake following reinjection show a low probability of recovering ventricular function. Further variations are demonstrated in Figures 50 C and D.
Stress/redistribution and late imaging - three steps or image acquisition series/one injection of 201Tl (Figure 50A): The addition of late imaging 24 hours after injection of thallium-201 during the stress or distribution phase (first step) allows more time for the phenomenon of redistribution to occur and, consequently, for increase in myocardial uptake of the radiopharmaceutical. This technique shows good predictive value for improvement of ventricular function following revascularization, but suboptimal NPV owing to the technical quality of images obtained after this period, as well as to the fact that some patients do not demonstrate redistribution during very prolonged periods.
Resting/redistribution - two steps or image acquisition series/one injection of 201Tl while resting: This protocol eliminates the stress phase, based on knowledge of the physiopathology of temporal variations in coronary flow in hibernating myocardium or in unstable patients, leading to perfusion defects that may occur in resting studies, with redistribution in late images. Qualitative studies using the resting-redistribution protocol to evaluate efficacy of revascularization have shown that the majority of myocardial regions with reversible defects during pre-operative periods presented post-operative normalization in perfusion and/or improved ventricular function. Some viable regions, however, may not present redistribution, even in images at 24 hours, unless 201Tl blood levels are elevated following reinjection. Practically speaking, most of the clinical information necessary to the medical decision-making process, related to viability study, will have been obtained during the stress-redistribution-reinjection sequence, and late images will generally not be necessary. There is no established consensus regarding the accuracy of Sestamibi - 99mTc while resting for detection of viability, with some studies showing similar uptake for 201Tl and Sestamibi - 99mTc (quantitative assessment) in patients with UA and LV dysfunction. It has also been observed to have satisfactory predictive value for improvement in contractility in the ventricular walls following revascularization, similar to that of 201Tl.280 The administration of nitrates before injection of the radiopharmaceutical with the objective of improving resting myocardial flow seems to improve accuracy for detecting myocardial viability.281
Figure 50.
Imaging protocols with the use of thallium-201 (201Tl) for characterizing viability of the myocardium. A represents the classic sequence for defining ischemia (Day 1), with the injection of a dose of approximately 3 to 4 mCi during stress, without subsequent reinjection, adding new image acquisition 24 hours later (Day 2); B, C, and D emphasize the need for reinjection of 201Tl with an approximate dose of 1 mCi and sequencing as demonstrated in the proposed protocol.
10.6. Positron Emission Tomography282,283
This non-invasive exam is the reference for detecting myocardial viability, as it simultaneously offers information on myocardial perfusion and metabolism. In normal fasting and aerobic metabolism conditions, while resting, long-chain free fatty acids (FFA) represent around 65% to 70% of energy supply to the cardiac muscle, with a lower participation of glucose (15% to 20%),21 whereas, in post-prandial conditions, glucose becomes the preferred substrate. In the presence of ischemia, the oxidative metabolism of FFA is diminished and glucose also becomes the preferred myocardial substrate. In this manner, even if the energy produced by anaerobic glycolysis is not enough to maintain myocardial contractility, it is vital for the preservation of cellular membrane integrity, which is characteristic of dysfunctional, yet viable myocardium. Some positron emitters, such as fluorine-18, labeling a glucose analogue or fluorodeoxyglucose (FDG), may be used to evaluate viable (or hibernating) myocardium, which is defined as metabolically active tissue, in the presence of a coinciding perfusion defect. FDG-18F penetrates the cells of the myocardium by the same transport mechanism as glucose and, following phosphorylation to FDG-6-P, remains in the intracellular medium in proportion to the rate of glycolysis, reflecting the quantity of viable tissue. Patients are typically prepared for imaging with administration of glucose overload and subsequent doses of insulin, prior to intravenous injection of 5 to 15 mCi of FDG-18F. Images are acquired 45 to 90 minutes after the administration of the tracer, lasting, approximately, 15 to 30 minutes. Low-dose CT is required for attenuation correction. The combination of perfusion and metabolism imaging via PET has a sensitivity of 88% and a specificity of 74% for myocardial viability.284 Some differences exist for patients with diabetes, for whom the preferred form of viability evaluation is with 201Tl or agents bound to 99mTc. It is also important to remember that the radioisotope fluorine-18 or 18F is produced in a cyclotron, consisting of the bombardment of enriched water labeled with oxygen-18 or 18O and decaying via positron emission, with an energy range of 511 keV. Its half-life is 110 minutes, allowing for the best spatial resolution among radiotracers used for PET.
When using PET for joint analysis of metabolism with FDG-18F and perfusion (positron emitters are not available for this in clinical practice in Brazil), an excellent predictive value for functional recovery has been observed. Measurements of MBF are typically performed with rubidium-82 (82Rb) or ammonia labeled with nitrogen-13 (13NH3). Based on the concept of hibernating myocardium, segments with reduced perfusion, but with preserved FDG uptake (known as mismatches), are classified as viable, with functional improvements following adequate revascularization. However, when perfusion and FDG uptake are diminished or apparently absent (matches), this reflects an absence of viability (areas of fibrosis), which do not show improvements after revascularization. Finally, the combined approach to blood flow-FDG mismatches has been widely document as a predictor of post-revascularization regional improvements in motility, as well as improvements in symptoms of HF, exercise capacity, and prognosis. The PET and Recovery after Revascularization (PARR-2) study,285 which is still the only randomized prospective study to evaluate the benefits of results of a management strategy assisted by PET in patients with severe LV dysfunction, evaluated 430 randomized individuals for viability study with PET or conventional treatment without the use of PET. Results of primary analysis after 12 months of follow-up have showed a tendency toward improved results in the FDG-assisted PET, which was not statistically significant. On the other hand, post-hoc analysis, including only patients who adhered to the management strategy recommended based on the findings of FDG PET, showed significant improvements in mortality with the PET-assisted approach, when compared to standard care. Di Carli et al.286 demonstrated that small areas of viable myocardium (more than 5%), identified by PET, stratified patients into subgroups of high-risk of cardiac events in a year. Equally, comparative studies on viability between PET and SPECT with 201Tl demonstrated agreement of results in 80% of cases.
10.7. Additional Information Based on Evidence within the Medical Decision-making process for Patients with Congestive Heart Failure, Decreased Left Ventricular Ejection Fraction, and Viable Myocardium
A classic meta-analysis involving 24 studies and 3,088 patients (Left Ventricular Ejection Fraction - LVEF 32% ± 8%), with the use of different imaging methods, demonstrated that patients with viability had significant reductions in mortality when comparing surgical revascularization treatment and clinical treatment, with no benefits for either group in the absence of viability (Figure 51).287
Figure 51.
Prognostic studies in patients with severe chronic coronary artery disease and ventricular dysfunction evaluating late survival with revascularization versus medical therapy following viability study via non-invasive testing. 201Tl: thallium-201; FDG-18F: metabolic imaging with fluorodeoxyglucose labeled with fluorine-18; Dobut: doppler echocardiography associated with intravenous injection of dobutamine solution; Revasc: myocardial revascularization surgery; Pts: patients; Viable: viable myocardium. Adapted from Allman KC, et al.287 Note that in the presence of viable myocardium and clinical treatment, the rate of events was > 4 times (16%) higher than in the same situation with surgical treatment via myocardial revascularization (3.2%); however, in the situation of absence of viable myocardium, the comparison between clinical and surgical treatment showed no significant differences in mortality (6.2% vs. 7.7%, respectively).
In contrast, a recent multicenter prospective randomized study, known as the Surgical Treatment for Ischemic Heart Failure (STICH) study,288 was unable to show benefits regarding mortality between patients randomized into revascularization surgery (CABG), in comparison with optimized medical therapy (OMT), in primary intention-to-treat analysis, for patients with dilated cardiomyopathy (LVEF ≤ 35%) of ischemic etiology (the first of 2 tested hypotheses, involving 99 centers in 22 countries).
SPECT and/or Doppler echocardiography, associated with a low dose of dobutamine, were used to characterize viability, based on established methodological criteria. Of the 1,212 patients included for evaluation, 601 underwent the aforementioned viability studies, comparing 298 receiving medical therapy and surgical revascularization (CABG Group) to 303 patients with OMT. Of the total of 478 patients with viable myocardium, the outcome of death occurred in 178 (37%), in contrast with 58 (51%) of deaths in 114 patients without viability (hazard ratio: 0.64; 95% CI: 0.48-0.86; p = 0.003). However, after adjusting for other baseline variables, the association with mortality was not significant (p = 0.21). There was significant crossover between the 2 groups and the analysis in question was in favor of better results with CABG.
In spite of these negative findings, recognizing biases in critical analysis of the results in this occasion, it is understood that, in the real world, faced with current evidence,289-291 viability study may be of assistance when choosing the best treatment in selected populations, leading to better prognosis in evolution.
11. New Technologies and Future Perspectives for Nuclear Cardiology in Studying Ischemic Heart Disease
Established experience and extensive documentation, which has been accumulated over the past decades, have demonstrated that MPS has satisfactory sensitivity and specificity, emphasizing good NPV for ruling out obstructive CAD. Analysis of 32 studies has shown that SPECT has a sensitivity of 87% and a specificity of 73% for detecting significant angiographic lesions (stenosis > 50%).292 Some limitations to the conventional technique have been observed, such as restricted spatial resolution, reduced counting rates, and attenuation of artifacts. Anger gamma cameras with sodium iodide crystals and photomultipliers, which transform emitted gamma photons into light or scintillation, also have limited temporal resolution in comparison with other imaging methods, such as PET, and they require higher doses of radiotracers, besides to carry out exams with longer image acquisition times. On the other hand, the innumerous advantages of the nuclear method include: a) the utilization of radioisotopes that do not alter the biological properties of the organism being studied; b) high radioactive labeling with a minimal amount of chemical substances, faithfully representing physiology and cellular biochemistry; c) minimal toxicity; d) pixel (smallest component of a digital image) values of myocardial images are directly proportional to parameters inherent in cardiovascular physiology, such as perfusion, function, metabolism, and innervation, attributes which are not shared by other modalities, such as angio-CT, cardiac resonance, and echocardiography; e) another aspect which stands out is the superior contrast resolution for detecting perfusion abnormalities, differentiating normal and hypoperfusion myocardium with great accuracy, facilitating visual and quantitative image analysis.29,293
Evolution of hardware: As exposure to radiation and its long-term deleterious effects have become important concerns on the part of regulatory authorities and scientific societies, new technologies have been introduced to reduce doses of radiotracers in nuclear exams while maintaining image quality and diagnostic accuracy. In this context, new equipment with CZT detectors arose in the first decade of 2000. Differently from traditional Anger gamma cameras, gamma radiation is directly converted to electric pulses upon contact with the CZT detectors, increasing energy resolution and dispensing with photomultipliers, which makes the detectors much finer and lighter. They are also distinct from older conventional cameras in terms of better spatial and energy resolution and the ability to distinguish dispersed radiation, in addition to being more sensitive for detection of emitted photons.294-296
Duvall et al.297 have shown the viability of a reduced-dose protocol and of reduced doses in a study carried out with a dedicated CZT gamma camera (Discovery NM 530c, GE Healthcare), using 5 mCi of 99mTc as a resting dose and 15 mCi of 99mTc as stress dose to label sestamibi or tetrofosmin. There was a significant reduction in exposure to radiation in relation to anterior SPECT protocols, and image quality and accuracy for diagnosing CAD were maintained. Gimelli et al.298 have also evaluated the viability of a stress-resting protocol with a reduced dose, used a CZT camera in a cohort of 137 patients referred for evaluation of CAD, with subsequent coronary cineangiography. Accuracy for identifying coronary lesions was not affected by reduced radioisotope dosage, and high sensitivity and specificity values were obtained. Hindorf et al.299 showed that the ideal patient position should be established when performing myocardial exams with CZT gamma cameras.
In addition to evaluating diagnostic accuracy, it has become important to evaluate prognostic value of new imaging protocols using CZT cameras. Oldan et al.300 compared prognostic value between CZT and conventional gamma cameras, observing that prognostic information provided by CZT technology, regarding the outcome composed of all-case mortality and non-fatal AMI, was similar to that provided by traditional equipment (Anger camera). The same dose, however, was administered to both gamma-camera groups. This fact limited the study’s ability to evaluate prognostic value with reduced-dose protocols, as previously described.
Brazilian researchers301 compared the prognostic values of an ultrafast low-dose protocol with a CZT camera and a traditional protocol with a conventional gamma camera with sodium iodide crystals. By means of a propensity score, 2 groups with equal numbers of patients and similar baseline characteristics were compared. The average dose of radiation in the first group (which underwent the exam with a conventional camera) was estimated at 9.5 mSv, whereas the CZT group had an average exposure dose of around 6 mSv. This dose was lower than the effective average SPECT doses mentioned in previous studies. It was confirmed that the CZT camera protocol showed similar prognostic results when compared to those obtained with traditional SPECT cameras, emphasizing that patients with MPS on CZT had a lower events rate than those who underwent MPS with traditional gamma cameras.
Evolution of software: Filtered back-projection (FBP) is a traditional reconstruction algorithm in LV imaging, which has was used in many SPECT gamma cameras over time. It considers that the radiation emitting object, in this case the heart, is at the same distance from all detectors and that the photons are also uniformly detected at all angles. This supposition, however, leads to a large number of image artifacts, which may be caused by breast attenuation and loss of counting density at higher distance from the heart, for example. FBP, thus, presents limitations that lead to the development of new iterative reconstruction images, which allow for the correction of these inherent artifacts.302
New algorithms have been developed, the most widely used of which is known as ordered subset expectation maximization (OSEM). This iterative reconstruction technique is based on estimated projection of the object studied, with additional comparison between the acquired and estimated projections of the object. The projection of proportion is generated, containing the differences between real and estimated projections of the object. These differences are used to modify initial estimation, and every cycle in this chain is called an iteration. Iterations are carried out until a projection more similar to the real object has been achieved. Iterative reconstruction allows for correction of image artifacts, such as dispersion, attenuation, and noise suppression during the reconstruction process, in order to improve image quality and resolution.303
DePuey et al.304 described the use of OSEM for processing exams with different acquisition periods (7 to 15 minutes) performed with a dual-head gamma camera with high resolution collimators. It was demonstrated that, notwithstanding lower time, image quality was maintained or even improved by the use of these reconstruction methods. Additionally, in a Brazilian study, Lima et al.305 analyzed prognostic accuracy of a new reconstruction algorithm, “Evolution for Cardiac TM,” with reduced dose and acquisition time in a dedicated gamma camera (Ventri, GE Healthcare), with an average dosimetry of 6.2 ± 0.3 mSv. The 2,958 patients who underwent exams were followed for approximately 3 years, and their results demonstrated a very low rate of major events (death or infarction), when imaging was normal in comparison with the group with abnormal imaging exams.
New perspectives: MPS may have some imaging limitations in patients with multivessel CAD, due to loss of comparative perfusion parameters between different areas of the myocardium, considering that image generation is based on relative uptake of coronary flow labeled with radiopharmaceuticals between the walls of the LV, as described in the introduction to these Guidelines. Given this situation, quantification of absolute coronary flow and coronary flow reserve (CFR) has arisen as an important alternative for diagnostic and prognostic evaluation of these patients. Falcão et al.306 demonstrated that evaluation of flow reserve via PET assists in the detection of CAD in patients who have left bundle branch blocks. Patients with apparently normal perfusion and abnormal CFR have higher annual rates of cardiac death, non-fatal myocardial infarction, late revascularization, and hospitalization due to cardiac causes than patients with normal perfusion and normal CFR (6.3% versus 1.4%; p < 0.05).307 Ziadi et al.308 have expanded these results in a prospective study involving 704 patients who underwent injection with rubidium-82 (82Rb) for PET, with the objective of comparing results in patients with reduced or normal CFR and patients with normal or abnormal perfusion exams. Reduced CFR was an independent predictor of major events, including cardiac death and myocardial infarction, adding prognostic value to the perfusion results. Murthy et al.309 studied the predictive power of CFR in 2,783 consecutive patients, observing a 5.6-fold increase in the risk of cardiac death in patients with lower CFR values, compared to patients with higher values. This variable showed incremental prognostic value in comparison to relative analysis of perfusion and LVEF. The prognostic value of myocardial flow reserve (MFR) as a variable goes beyond CAD. This has been demonstrated in patients with ischemic and non-ischemic cardiomyopathy,310 hypertrophic cardiomyopathy,311 and post-cardiac transplant.312
The measure of coronary flow reserve using PET is quite accurate, but as it is expensive, it is not widely available in clinical practice, especially in Brazil. However, as SPECT technology is an easily accessible tool, studies show the possibility of acquiring dynamic images and quantifying MFR with this method, with some limitations, however, when using traditional gamma cameras, including limited temporal resolution.313,314 With the advent of CZT cameras, it has become viable to quantify CFR using this technology.
Wells et al.315 developed a pig model for measuring absolute myocardial blood flow and flow reserve, using three different radioisotopes, namely, 201Tl, Tetrofosmin-99mTc and Sestamibi-99mTc. Following in this research area, Bouallègue et al.316 obtained CFR in 23 patients with known three-vessel disease using a CZT gamma camera, successfully obtaining good correlation with angiographic findings. New discoveries related to dynamic SPECT image acquisition with measurements of CFR are opening new and exciting research fields that will allow for different applications of SPECT to the extent that their results are better validated.
12. Strategies for Reducing Exposure to Radiation
Ionizing radiation refers to radiation with enough energy to “ionize” atoms and molecules during its interaction with matter, in this case, with elements in the human body. Depending on the type and level of energy used, on the duration of exposure, and on the dose absorbed, damage to the organism may occur. Professionals involved in medical exams or therapies that make use of ionizing radiation should be familiar with the basics of what is known as the “as low as reasonably achievable” (ALARA) principle. This stipulates that an individual’s exposure to radiation must be minimized, regardless of reason for exposure. In relation to nuclear imaging, this means obtaining the principal exam information, most commonly MPS, using the minimum amount of radiation necessary to maintain diagnostic quality. This involves not only the choice of radiotracer, but also the best technique and the best protocol that may be adjusted to minimize radiation exposure.
Furthermore, 2 other important principles guide the application of ionizing radiation for medical imaging: “justification” and “optimization.” Justification signifies that a study should be well indicated and justified as adequate, in following with appropriate criteria, as described in these guidelines. The best way to minimize a patient’s exposure to radiation is not to recommend an exam that is not appropriately indicated. This situation, however, is often beyond the control of the doctor responsible for performing the exam and should be understood by the referring doctor, who should have a basic grasp on the scientific literature. It is important to emphasize that the risk of a patient dying due to cardiovascular disease (the leading cause of death in Brazil), without undergoing a well indicated exam, is much higher than any eventual risk owing to radiation exposure. The risks of a patient with suspected coronary disease who has an appropriate indication for an exam are not theoretical, but rather real, and they are higher than the risks resulting from radiation use. The other principle which demands our attention is optimization, which represents adjustments to protocols, including the use of the best technological resources available (modern software and hardware) to perform an exam. This task is the responsibility of a multiprofessional team, made up of physicians, supervising nurses, nursing technicians, radiology technologists, biomedical specialists, biologists trained to manipulate radioactive material, professionals with training in radiopharmacy, and a team of medical physicists. The risks of deleterious effects of radiation involving patients, provided that the multiprofessional team uses the best technique and consistently observes the principles of justification and optimization, are minimal, and they are, at times, the fruit of theoretical elaboration with no consolidated practical base.
The INCAPS Nuclear Cardiology Protocols Cross-Sectional Study (INCAPS), an important, recently conducted international study coordinated by the International Atomic Energy Agency (IAEA), involving 65 countries, including Brazil, verified that radiation exposure may be different when comparing patients living in different countries, given the diversity of implemented protocols in nuclear cardiology practice worldwide.317 Innumerous opportunities have been found to improve the application of nuclear cardiology comprehensively worldwide, basically using the principle of optimization. It has generally been identified that with simple, low-cost orientations, such as adjusting dose to BMI, it is possible to reduce the administered dose of radiation and patient exposure significantly. Latin America is an example,318 where there are opportunities to improve protocols. It has been recommended that the majority of patients undergoing MPS have a maximum estimated radiation exposure of 9 mSv. This goal is feasible to reach when applying the recommendations listed in Table 27, which are strategies supported by the IAEA.24 In the Brazilian centers that participated in the INCAPS study, average doses were observed to vary between 8.4 and 17.8 mSv, thus demonstrating the possibility of optimizing protocols in the country, which has been undertaken since the publication of INCAPS.318
Table 27.
Strategies for reducing radiation exposure
| 1 | Divulgate and apply appropriate exam indication criteria |
| 2 | Give preference to tracers that result in lower exposure to radiation. It is currently recommended to use technetium-99m (99mTc), which has a physical half-life of 6 hours, in comparison with thallium-201 (201Tl), whose half-life of 73 hours results in unfavorable dosimetry |
| 3 | Avoid protocols that inject both radioisotopes, 201Tl and 99mTc, in the same study (dual-isotope protocol), also considered an unfavorable dosimetry in combination |
| 4 | Avoid injecting any dose of 99mTc which is > 36 mCi or which results in an exposure dose of > 15 mSv for the complete exam |
| 5 | In the event that it is necessary to use 201Tl, avoid doses over 3.5 mCi |
| 6 | Attempt to decrease the number of patients who perform both stress and resting phases. In some cases, it is possible to answer the clinical question with the stress injection, provided that images are perfectly normal during this phase |
| 7 | Utilize more sensitive equipment which is able to detect lower injected doses of radiopharmaceutical, such as new gamma cameras with solid cadmium-zinc-telluride (CZT) detectors, as well as software which improves imaging quality, even at lower administered doses |
| 8 | Apply the dose adjustment table based on patient's size or body mass index (BMI) (Tables 28 and 29) |
| 9 | Avoid shine through. This phenomenon occurs in perfusion studies with 99mTc (one-day protocol, resting and stress phases) when the dose of the second injection is less than 3 times the first dose, and the residual activity in this step may interfere with interpretation of images corresponding to the second injection. This situation may result in a non-diagnostic study, making new investigations necessary and thus increasing the patient's total received dosage. |
Table 28.
Suggestions for dose limitation, adjusted to body mass index (BMI), using conventional gamma camera technology24
| One-day protocol | ||
| BMI | Dose 1 (mCi) | Dose 2 (mCi) |
| < 25 | 8 | 24 |
| 25 to 30 | 9 | 27 |
| 30 to 35 | 10 | 30* |
| > 35 | 12 | 36* |
| Two-day protocol | ||
| BMI | Dose 1 (mCi) | Dose 2 (mCi) |
| < 25 | 8 | 8 |
| 25 to 30 | 9 | 9 |
| 30 to 35 | 10 | 10 |
| > 35 | 12 | 12 |
Give preference to the two-day protocol.
Table 29.
Suggestions for dose limitation, adjusted to body mass index (BMI), using gamma cameras with cadmium-zinc-telluride technology24
| One-day protocol | ||
| BMI | Dose 1 (mCi) | Dose 2 (mCi) |
| < 25 | 4 | 12 |
| 25 to 30 | 4.5 | 13.5 |
| 30 to 35 | 5 | 15* |
| > 35 | 6 | 18* |
| Two-day protocol | ||
| BMI | Dose 1 (mCi) | Dose 2 (mCi) |
| < 25 | 4 | 4 |
| 25 to 30 | 4.5 | 4.5 |
| 30 to 35 | 5 | 5 |
| > 35 | 6 | 6 |
Give preference to the two-day protocol.
12.1. Reducing Radiation Using New Technologies, Image Quality, and Reliability of Findings
Given that exposure to high doses is a source of concern, considering the possibility of biological effects of ionizing radiation, which are known as deterministic (those which occur above certain limits of absorbed dose in a determined tissue, including skin erythema, loss of hair, and, possibly, direct cardiac toxicity) and stochastic (those whose radiation causes damage that may lead to malignancy which is generally long-term),319,320 there has been a demand for new technology to reduce doses of radiotracers, maintaining image quality and diagnostic accuracy. In this context, new cameras with CZT detectors have been launched in recent years. Differently from traditional Anger cameras, gamma radiation is directly converted into an electric pulse when it comes into contact with CZT detectors, increasing energy resolution and dispensing with photomultipliers, which makes the detectors much finer, lighter, and more sensible to photon detection. Protocols have suggested that it is possible to combine faster exams with lower dosimetry. Owing to high costs, they are still not widely used in Brazil, where there are few more than a dozen gamma cameras with this new technology , which has already begun to contribute relevant publications to the international scenario.321
One of the limitations, known as the “Achilles heel” of myocardial perfusion image interpretation is the attenuation which gamma rays undergo when they pass through tissues before reaching the detector. The problem lies in the fact that attenuation may simulate myocardial perfusion defects, and their recognition as cases of “false-positive” results greatly depends on the observer’s experience. These defects occur more frequently in the inferior wall in men (especially in patients with abdominal obesity), and they are described as diaphragmatic attenuation. In women, they are most commonly found in the anterior wall, due to attenuation caused by breast tissue. These imaging defects are more common in obese patients, for which reason they require higher injected doses. When the defects found are tenuous, when they occur in a similar way during resting and stress, and when they are accompanied by thickness in the walls of LV without abnormalities, the myocardial perfusion study may frequently be interpreted as normal, thus sparing the patient other investigations, both those that involve radiation and those that do not, and minimizing costs. Another way to lower the attenuation artifacts is to apply specific machines with other sources of radiation, such as an x-ray emitting tomograph, which calculates tissue attenuation factors and applies attenuation correction to the photons emitted by the radioactive tracer, reducing the effects of attenuation by means of software. This equipment, however, adds costs to the exam, and it is difficult in terms of financial viability. Even in the USA, which is the country with the highest volume of myocardial scintigraphy studies, it is estimated that only 20% of medical services routinely apply this method. Finally, another method, which is widely applied in practice to resolve apparent defects in uptake generated by attenuation of gamma photons when they penetrate tissue, is to acquire images in the prone and supine positions , especially during the stress phase, which significantly reduces the artifacts described. Some services have routinely implemented this practice, even considering the increased gamma camera utilization time with a new image acquisition series.
With respect to performing stress exam alone in order to reduce radiation by avoiding the resting dose, the following should be considered:
It is possible for the observing doctor to succeed in interpreting a study as “absence of ischemia,” based only on stress imaging, thus avoiding the resting injection. In order to do this, the observer must be confident that the image is perfectly normal and free of any perfusion defects, including “attenuation artifacts,” which are generally recognized by comparing stress and resting images. The alternative is described above, routinely applying attenuation correction, but this would involve increased costs.
Another situation, which is not frequent, but which may occur, is when the patient has homogenous tracer distribution in the LV and apparently normal perfusion, but with an observed transient increase or dilation in the same cavity during the stress phase, when compared to resting images (transient ischemic dilation). The following may, additionally, be observed: a) drop in LVEF following stress, compared to resting; and/or b) increased uptake in the RV (generally not visualized) during the phase corresponding to stress, in comparison with resting; these are markers of poor prognosis, even in the presence of perfusion which is apparently “normal” owing to homogenous distribution. In these situations, the patient is exceptionally identified as high-risk based on other findings which are not solely related to tracer distribution in the LV during stress. This analysis and these findings are only possible when comparing resting and stress images. In summary, the doctor interpreting the images should be sure that the stress exam is perfectly normal, in order to dispense with resting image acquisition. Whenever possible, nuclear cardiology should always be performed using exercise as a preferential form of stress instead of pharmacological tests, which serve as alternatives only in patients who are unable to exercise efficiently. In the presence of severe multivessel coronary disease (involving three arteries) and apparent relative homogeneous radiopharmaceutical distribution during stress, it is extremely rare not to observe other high-risk findings during stress testing, in addition to deterioration of LV function induced by stress itself (ischemic myocardial stunning).
In summary, in this section, various ways to reduce radiation exposure in patients undergoing nuclear cardiology exams have been covered. By means of relatively simple adjustments to protocols, injected doses may be optimized, guaranteeing image quality and, most of all, reliability of findings in an exam which is of great clinical importance to orient patient management.
13. Evaluation of Cardiac Sympathetic Activity by Scintigraphy with 123I-MIBG
13.1. Introduction
Autonomic cardiac innervation plays a fundamental role on cardiac performance by regulating myocardial blood flow, HR, and myocardial contractility. In several cardiac diseases, cardiac neuronal function is altered and frequently associated with worse evolution. Dysregulation of the autonomic cardiac nervous system increases the risk of potentially lethal arrhythmias and may be a marker of poor prognosis. Scintigraphy imaging of distribution and cardiac neuronal function facilitates the comprehension of the physiopathology of various diseases that affect the heart and may guide treatment, with consequent improvements to clinical results.322
The autonomic cardiac nervous system encompasses sympathetic and parasympathetic innervation, with its norepinephrine (NE) and acetylcholine neurotransmitters, respectively. These work in equilibrium, and they exert stimulating effects, via adrenergic receptors, and inhibitory effects, via muscarinic receptors, both of which are responsible for electrophysiological and hemodynamic adaptations in the cardiovascular system, in response to bodily demands.322
In this way, sympathetic stimuli are controlled by cerebral regulatory centers that integrate signals coming from other parts of the brain and receptors in the body. Efferent signals follow descending pathways in the spinal cord and make synapses with preganglionic fibers that emerge at levels T1 to L3. In this sequence, they establish synapses with the paravertebral stellate ganglion and innervate the RV, in addition to the anterior and lateral regions of the LV. In the heart, sympathetic nerves follow the coronary arteries in the subepicardium and then penetrate the myocardium.322,323
Parasympathetic fibers are scarce in number in comparison with sympathetic fibers, and they originate in the marrow, following the vagus nerve. They begin in the epicardium, crossing the atrioventricular groove and penetrating the myocardium. They are located in the subendocardium, predominantly innervating the atria, but they are less dense in the ventricles, with the exception of the inferior wall. They greatly control the function of sinoatrial and atrioventricular nodes.322
13.2. Cardiac Scintigraphy with 123I-MIBG
Metaiodobenzylguanidine (MIBG) is a molecule with a structure similar to that of NE, obtained by means of modifications to the molecular structure of guanethidine (a false neurotransmitter, also an NE analogue), which acts selectively on sympathetic nerves, without, however, being metabolized by monoamine oxidase or catechol-O-methyltransferase or exercising a stimulating effect, as NE does. For the objective of diagnosis, MIBG is labeled with iodine-123, forming the radiotracer MIBG-123I,322-325 demonstrating a good correlation between myocardial uptake of MIBG-123I and NE content in cardiac tissue.325 After the radiopharmaceutical is injected via intravenous administration, it spreads throughout synaptic space, and is taken up, concentrated, and stored in presynaptic nerve endings in a manner similar to that of NE. MIBG-123I is retained and localized in cardiac sympathetic nerve endings with scintigraphy images obtained by a conventional gamma camera.322-325
The radioisotope iodine-123 predominantly emits gamma photons with an energy of 159 keV and a physical half-life of 13.2 hours, making image acquisition easy and well tolerated. MIBG-123I is widely used in Europe and Japan, and it has recently been approved for cardiac use in the USA.326 In Brazil, this technique is available in some centers. Cardiac scintigraphy with MIBG-123I directly evaluates global and regional sympathetic function of the heart, including uptake, reuptake, storage, and NE release processes in presynaptic nerve endings.327
Intravenous injection of MIBG-123I is administered while resting, at least 30 minutes after oral administration of potassium iodide syrup or an iodine-containing solution, in order to block and protect the thyroid. Medications that may potentially interfere with catecholamine uptake, such as antidepressants, and some calcium channel blockers should be suspended for at least 24 hours before administration of the radiopharmaceutical. On the other hand, betablockers, angiotensin converting enzyme inhibitors (ACEI), and/or angiotensin receptor blockers (ARB) do not need to be discontinued.328 Approximately 15 minutes and 4 hours after administration of MIBG-123I, static images and tomography images (SPECT) of the thorax are obtained in anterior projection, with the patient in dorsal decubitus, with the left arm raised above the thorax. While tomography images are optional, they help evaluate myocardial sympathetic activity and compare with the perfusion study (see the subsequent topic “Evaluation of arrhythmias”). Global cardiac uptake of MIBG-123I is evaluated by static imaging of the thorax (Figure 52). The following 2 fundamental parameters are visually and semiquantitatively analyzed: the relation between cardiac and mediastinal uptake (heart to mediastinum ratio [HMR]) in early images (15 minutes after injection) and late imaging (4 hours after injection) and the myocardial washout rate (WR) of MIBG-123I.322-324,327-330
Figure 52.
Cardiac scintigraphy with MIBG-123I. Colored late imaging of the anterior thorax with the regions of interest (ROI) drawn in the mediastinal (M) and cardiac (C) areas, to calculate the heart to mediastinum ratio (HMR) of MIBG-123I uptake. This index is the measure of the ratio between the statistical count of the rate of average radiation per pixel in the ROI, drawn in the heart and mediastinum.
Normal HMR values vary from 1.9 to 2.8 with an approximate average of 2.2 ± 0.3 in late images, with results ≥ 1.6 considered predictive of lower risks.331 HMR reflects the density of receptors and, probably, depicts the integrity of presynaptic nerve endings and of the uptake 1 receptor. This elevated index indicates a predominant localization of the radiotracer in the myocardium, which is expected in normal hearts, to the extent that the finding of reduced HMR indicates lower myocardial uptake of MIBG-123I, translating as reduced density of cardiac adrenergic receptors. Late HMR combines information on neuronal function of uptake and the release of storage vesicles in cardiac nerve endings. Figures 53 and 54 show images of patients with normal and altered HMR, respectively.
Figure 53.
Cardiac scintigraphy with MIBG-123I showing normal radiopharmaceutical uptake in the cardiac area (arrow), which denotes preserved cardiac sympathetic activity. Black and white images of the anterior thorax, acquired approximately 15 minutes (early) and 4 hours (late) following intravenous injection of the radiopharmaceutical MIBG-123I.
Figure 54.
Abnormal cardiac scintigraphy with MIBG-123I of a patient with advanced heart failure and highly reduced radiopharmaceutical uptake in the cardiac area (arrow), which denotes cardiac sympathetic hyperactivity. Black and white images of the anterior thorax, acquired approximately 15 minutes (early) and 4 hours (late) following intravenous injection of the radiopharmaceutical MIBG-123I.
Myocardial washout rate (WR) of MIBG-123I is also an important measure of cardiac sympathetic innervation. WR is calculated as the difference in myocardial uptake between the early and late phases and is determined by the percentage of reduction in uptake between these steps, reflecting the amount of catecholamines released in the cardiac synaptic cleft. Cardiac sympathetic hyperactivity is associated with reduced retention of MIBG-123I in late images (reduced late HMR) and increased WR. Normal WR control values are 10% ± 9%.332 Higher values are predictive of worse prognosis,333 although there are several methods for determining this ratio in the literature. Intra- and inter-observer variability of these measurements is less than 5%.328
13.3. Clinical Applications of Cardiac Scintigraphy with MIBG-123I
Imaging studies of cardiac adrenergic activity may be useful in several clinical scenarios (Table 30), including: HF, ventricular arrhythmias (associated with HR and primary arrhythmias), ischemic heart disease, DM, patients undergoing cardiotoxic chemotherapy, pre- and post-cardiac transplant, Takotsubo syndrome, and cardiac involvement due to systemic conditions, as in Parkinson’s disease. Generally speaking, this exam has been established to have an effective capacity for risk stratification of patients with the previously described conditions, but the utility of this information in improving clinical results of patients has not yet been demonstrated.331
Table 30.
Established and potential indications for cardiac scintigraphy with MIBG-123I
| Clinical Scenarios | Established | Potential |
|---|---|---|
| HFrEF | • Risk stratification, regardless of other parameters; evaluation of progression of HF, arrhythmic events, and total cardiac mortality up to 2 years • Identification of a low-risk subgroup for cardiac events and mortality • Clinical follow-up of medical therapies indicated in the guidelines |
• Identification of patients most likely to benefit from CRT or LVAD • Guiding treatment of patients with LVAD: bridge to transplant, possible explant • Substitute marker for evaluating benefits of new medical therapies and devices |
| HFpEF | • Subanalyses of larger studies have shown a risk stratification similar to that seen in patients with HFrEF | • Identification of patients whose risks may be higher than clinically apparent |
| Arrhythmias associated with HF | • Risk stratification for lethal or potentially lethal ventricular arrhythmias for up to 2 years • Identification of patients with very low risks of lethal arrhythmic events for up to 2 years |
• Refining indication criteria for patients who will benefit from ICD • Helping identify patients who will no longer need ICD, at the end of battery life or device infection |
| Primary arrhythmic conditions | • Identification of patients with risks of worse outcomes, including arrhythmic events and total mortality | • Improving understanding of physiopathology of primary arrhythmic conditions • Guiding conduct for patients with primary arrhythmic conditions |
| Heart transplant | • Following post-transplant cardiac reinnervation after | • Identification of patients who are more likely to have complications following transplant, including transplant rejection and transplant by CAD |
| Ischemic heart disease | • Evaluation of area at risk in patients with acute coronary syndromes • Risk stratification in patients with hibernating myocardium |
• Guiding conduct for patients with acute coronary syndromes • Guiding conduct for patients following ischemic events • Ischemic memory |
| Diabetes mellitus | • Identification of cardiac autonomic abnormalities, including patients without extracardiac manifestations | • Identification of patients whose risks may be higher than clinically apparent, assisting in diagnosis and orienting appropriate treatment |
| Cardiotoxicity due to | • Identification and quantification of cardiac lesions in patients undergoing these treatments | • Guiding conduct of chemotherapy • Improving understanding of the physiopathology of toxicity due to drugs |
MIBG-123I: metaiodobenzylguanidine labeled with iodine-123; CAD: coronary artery disease; CRT: cardiac resynchronization therapy; HF: heart failure; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; ICD: implantable cardioverter defibrillator; LVAD: left ventricular assist device. Adapted from JCS Joint Working Group.339
13.3.1. Heart Failure
In this condition, altered cardiac adrenergic innervation is strongly correlated with mortality, and reduced cardiac uptake of MIBG-123I (late HMR) confers independent and additional long-term prognostic value to other established markers, such as LVEF and B-natriuretic peptide (BNP).334-336 Some studies have demonstrated that abnormalities in cardiac uptake of MIBG-123I may be predictive of increased risk of ventricular arrhythmia and sudden cardiac death,322,337 with attempts to standardize the procedure for the sake of routine clinical application.328-330 Cardiac scintigraphy with MIBG-123I has been approved for use in clinical practice in cardiology since 1992 in Japan,338 and it is considered a class I indication for evaluation of prognosis and severity of HF, with level of evidence B (Table 31).338 Studies with higher numbers of patients have recently been published in Europe and the USA. The AdreView Myocardial Imaging for Risk Evaluation in Heart Failure (ADMIRE-HF)336 multicenter, prospective, international study involving the use of MIBG-123I in HF independently validated the prognostic value of cardiac scintigraphy with MIBG-123I for the evaluation of patients with chronic HF.337 They included 961 patients with HF, NYHA HF functional class II-III, and LVEF ≤ 35%, undergoing cardiac scintigraphy with MIBG-123I, followed for an average of 17 months. Approximately 25% of patients (n = 237) had cardiac events, with approximately 70% of the first events being progression of chronic HF; 20% potentially lethal arrhythmic events (sustained VT > 30 seconds, heart arrest with cardiac arrest with resuscitation and appropriate ICD firing; and approximately 10% cardiac death; with 22% of patients presenting multiple events. Lower risks of the compound outcome were observed in patients with HMR ≥ 1.60 versus HMR < 1.60 (38%; hazard ratio: 0.40; p < 0.001) with a highly significant risk ratio for each individual component of the primary compound outcome evaluated. It is worth highlighting that total mortality over 2 years was around 5 times higher (16.1% versus 3.0%) in patients with late HMR < 1.60 compared to those with HMR ≥ 1.60, respectively.337
Table 31.
Recommendations for cardiac scintigraphy with MIBG-123I in accordance with the Japanese Circulation Society Guidelines339
| Indication | Class of recommendation | Level of evidence |
|---|---|---|
| Evaluation of severity and prognosis of patients with heart failure (HF) | I | B |
| Evaluation of the effects of HF treatment | IIa | C |
| Arrhythmogenic disease | IIb | C |
Considering this information, the FDA has approved MIBG-123I (AdreViewR), in 2013, for evaluation of cardiac sympathetic innervation in patients with New York Heart Association (NYHA) HF class II-III and LVEF < 35%.326
In addition to prognostic evaluation of HF,333-339 other applications which stand out include the following: evaluation of therapeutic response to medication;340,341 indication and evaluation of response to cardiac resynchronization therapy (CRT);342 indication for implant and explant of mechanical left ventricular assist device,343,344 and implantable cardioverter defibrillator (ICD); and evaluation of reinnervation following cardiac transplant.345
Late HMR of MIBG-123I in patients with severe chronic HF, in accordance with traditional classification criteria (LVEF, BNP, functional class), may help reclassify patients into a category of lower risk for events. Patients with late HMR ≥ 1.6 (even with very low LVEF and elevated BNP) have a low probability of severe cardiac events during a period of up to 2 years. This information may lead to changes in treatment.331 This marker may, also, help refine indication criteria for high-cost invasive therapies for HF, such as CRT342,346 and ICD implant.347,348 A Brazilian study carried out by Nishioka et al.342 has shown that HMR was the only independent predictor of therapy response. Patients with HMR < 1.36 had a lower chance of benefitting from CRT, suggesting that these patients with rather elevated cardiac sympathetic activity have terminal HF.342,346
The autonomic nervous system plays an important role in cardiac arrhythmias. Scintigraphy with MIBG-123I has the potential to select patients for ICD implant more accurately, in addition to identifying those at higher risks of sudden cardiac death, who would not be selected in accordance with current guidelines. Arora et al.,347 in a pilot study of 17 patients with advanced chronic HF and ICD, divided patients into groups according to the presence or absence of previous ICD firing. In cases with late HMR of MIBG-123I < 1.54, they observed a higher frequency of ICD firing and a positive predictive value of 71%, at the same time that increased HMR was observed to have a NPV of 83%.
The etiology of HF is frequently classified as ischemic (I) and non-ischemic (NI). Although the physiopathology and the initial lesion are different, investigation studies have suggested that, as the disease progresses, autonomic cardiac abnormalities are characteristic and common, regardless of etiology. Cardiac scintigraphy with MIBG-123I thus continues to be a strong prognostic marker. Wakabayashi et al.349 showed that, for both groups, late HMR was the strongest independent predictive factor for sudden cardiac death, although the cutoff points for HMR index values were different, namely 1.50 for ischemic cardiomyopathy and 2.02 for non-ischemic cardiomyopathy. For patients with LVEF < 40% and late HMR lower than the identified cutoff values, the rate of cardiac death was higher in the ischemic group (18.2% annually) than in the non-ischemic group (11.9% annually).
13.3.2. Ventricular Arrhythmia
Sudden cardiac death continues to be one of the leading causes of death worldwide. Scarring and/or non-revascularized/ischemic myocardium provide important substrates for the occurrence of potentially lethal ventricular arrhythmias.350 Furthermore, the presence of clinical HF increases the risk of ventricular arrhythmia. The sympathetic nervous system is an important trigger of major arrhythmic events by means of global cardiac adrenergic hyperactivity and heterogeneity of regional myocardial sympathetic activity.322 Evaluation of the autonomic nervous system via myocardial scintigraphy (MS) with MIBG-123I may be useful in diverse clinical situations. The presence of denervated yet viable myocardium and the magnitude of denervation are potential markers of an individual’s susceptibility to triggering of severe arrhythmias. Several studies have demonstrated the ability of scintigraphy with MIBG-123I to identify patients at higher risks of developing spontaneous ventricular tachyarrhythmia, appropriate ICD shock,351,352 and sudden cardiac death.353-356
When analyzing the possibilities of scintigraphy imaging results, the finding of a mismatch between perfusion and myocardial innervation characterizes a scenario of higher risk for ventricular arrhythmia.350,351 The denervated regions respond to sympathetic stimuli differently than normal myocardium. This electrophysiological heterogeneity may serve as a substrate for VT and ventricular fibrillation (VF). In the same manner, tomography images (SPECT) of cardiac scintigraphy with MIBG-123I are useful for recognizing increased arrhythmogenicity. A prospective study of 50 patients with antecedents of myocardial infarction who underwent SPECT imaging with MIBG-123I and perfusion SPECT perfusion with Tetrofosmin-99mTc showed, via multivariate analysis, that a late MIBG-123I SPECT defect score of ≥ 37 was the only parameter capable of differentiating the group of patients who presented VT induced by electrophysiological testing, with a sensitivity of 77% and a specificity of 75%.357 There were no significant differences in late HMR and the mismatch scores obtained by subtraction of perfusion and sympathetic innervation between the groups with positive and negative induced VT.356
Moreover, the Prediction of Arrhythmic Events with Positron Emission Tomography (PAREPET) study evaluated quantification of denervated myocardium in 204 patients with ischemic heart disease (LVEF ≤ 35%), by means of PET imaging with 11C-meta-hydroxyephedrine (HED-11C), labeled with carbon-11. Perfusion and myocardial viability have also been characterized with 13N-ammonia and 18F-fluordeoxyglucose, respectively. The primary study objective was to observe the occurrence of sudden cardiac death, defined as arrhythmic death or ICD firing due to VF or VT > 240 beats/minute. After 4.1 years of follow-up, sudden cardiac death of 16.2% was registered. The quantification of infarction volume and LVEF were not factors predictive of sudden cardiac death. However, patients with higher volumes of denervated myocardium (33 ± 10% versus 26 ± 11% of the LV; p = 0.001) showed sudden arrhythmic death more frequently. The authors of this study concluded that, in ischemic cardiomyopathy, sympathetic denervation evaluated by HED-11C PET predicts sudden arrhythmic death regardless of LVEF and infarction volume. This information may improve identification of patients who will most likely benefit from ICD implant.358 One of the most peculiar aspects of the natural history of chronic Chagas cardiomyopathy is the occurrence of severe ventricular arrhythmia in individuals with preserved LV global systolic function which may evolve to sudden death during early phases of the disease.359,360 In 43 patients with chronic Chagas cardiopathy and LVEF ≥ 35%, the correlation between extent of sympathetic denervation, myocardial fibrosis, and severity of ventricular arrhythmias was investigated. Patients were divided into 3 groups, according to the presence of sustained VT, non-sustained VT, and the absence of VT on 24-hour Holter. Sympathetic denervation was evaluated via SPECT imaging with MIBG-123I and myocardial fibrosis via SPECT with 99mTc-sestamibi. The sums of perfusion scores (quantity of fibrosis) were similar in the 3 groups. The summed difference score between MIBG-123I and Sestamibi-99mTc, which evaluated the extension of denervated yet viable myocardium, was significantly larger in the group with sustained VT on Holter (score of 20.0 ± 8.0), when compared to the group without VT (2.0 ± 5.0; p < 0.0001) and NSVT (11.0 ± 8.0; p < 0.05). In conclusion, the occurrence of ventricular arrhythmias with different degrees of severity is quantitatively correlated with the extension of cardiac sympathetic denervation, but not with the extension of fibrosis, suggesting that myocardial sympathetic denervation plays a role in the generation of ventricular arrhythmia related to chronic Chagas cardiopathy.361
Sympathetic denervation may also occur in patients with stable angina in the absence of infarction. Cardiac scintigraphy with MIBG-123I may show inervation defects in these cases, in the absence of perfusion defects. Sympathetic nervous fibers are more susceptible to oxygen privation than cardiomyocytes and the occurrence of myocardial ischemia may thus lead to transient sympathetic denervation.362 The fact that recovery of inervation may be a slower process also makes it possible to use myocardial scintigraphy with MIBG-123I as a marker of ischemic memory in patients whose chest pain has resolved few hours or days prior.
Regional alterations of cardiac sympathetic activity may also be seen in primary arrhythmic conditions, in the absence of CAD,363 in Brugada syndrome,364 in hypertrophic cardiomyopathy,365,366 and in arrhythmogenic RV dysplasia.367 These findings in patients with primary arrhythmias support the potential use of cardiac scintigraphy with MIBG-123I for identifying patients at a risk of sudden cardiac death, who may benefit from ICD implant.
13.3.3. Cardiotoxicity Due to Chemotherapy
Over the past decades, there have been great advances in cancer diagnosis and treatment, offering oncology patients reduced mortality, increased survival, and better quality of life. On the other hand, progress in oncological treatment results in higher exposure to the cardiotoxic effects of chemotherapy. Screening for the occurrence of cardiotoxicity (CTX) is highly recommended before, during, and after the completion of chemotherapy. Several methods and diagnostic indexes have been suggested for the detection of CTX and therapeutic strategy planning. Although serial measure of LVEF by conventional echocardiogram is the most utilized strategy for monitoring myocardial damage, it does not appear to be sensitive enough to detect patients with risks of developing significant CTX in early phases of chemotherapy administration.323 The potential use of cardiac adrenergic imaging for monitoring the cardiotoxic effects of chemotherapy has been debated.368-370 There is evidence that reduced cardiac uptake of MIBG-123I precedes ejection fraction deterioration.371
A recent study has shown that, following 1 year of treatment with anthracyclines, late HMR was the strongest parameter of scintigraphy with MIBG-123I. This index correlates with conventional echocardiography variables and global indexes of radial and longitudinal strain, in addition to doses of galectin-3 in patients with breast cancer treated with anthracyclines. Altered late HMR was a predictor of abnormality in the global radial strain index on ECG.369
Cardiac scintigraphy with MIBG-123I was performed in 20 women with breast cancer and normal LVEF who had undergone treatment with anthracycline derivatives, associated and not associated with trastuzumab. It was observed that anthracycline use with trastuzumab promoted higher frequency and intensity of cardiac adrenergic hyperactivity.367 Carrió et al.371 identified abnormal MIBG-123I uptake in patients who used anthracyclines, where HMR of MIBG-123I decreased as the cumulative dose of this medication increased.371
The degree of cardiac uptake of MIBG-123I may thus be an early marker of CTX. However, multicenter studies with higher case numbers and standardized exam protocols comparing the evaluation of cardiac sympathetic activity with MIBG-123I before and after treatment need to be carried out in order to clarify these findings further.
13.3.4. Cardiac Autonomic Dysfunction in Diabetes Mellitus (DM)
In patients with DM, there is evidence of cardiac denervation in the absence of clinical manifestations.372 Diabetic autonomic neuropathy has been implied to be a cause of sudden cardiac death, with or without associated myocardial ischemia. Patients with DM and reduced HMR of MIBG-123I have an increased risk for clinical progression of HF.373 It is, however, not yet clear whether cardiac adrenergic imaging may improve clinical outcome in patients with diabetes.
13.3.5 Cardiac Transplant
Cardiac scintigraphy with MIBG-123I may be useful for evaluation of reinnervation in transplanted hearts. It identifies ventricular sympathetic reinnervation,345 which slowly develops from the cardiac base several months following surgery, and it is observed in 40% of patients 1 year following transplant.374 Even though the clinical implications and the mechanisms of cardiac reinnervation have yet to be completely made clear, restoration of cardiac sympathetic innervation probably increases physical capacity, due to improved HR and contractile function during exercise in patients with heart transplants.374 Evaluation of the process of cardiac reinnervation via scintigraphy with MIBG-123I seems to be useful for outpatient treatment of patients with heart transplants for the prescription of appropriate exercises, evaluation of the effect of physical training, and prediction of long-term survival.
13.3.6. Takotsubo Syndrome
Takotsubo syndrome, also known as neurogenic cardiomyopathy, stress-induced cardiomyopathy, or broken heart syndrome,375 is characterized by transient left ventricular dysfunction, electrocardiographic alterations similar to those present in AMI, and minimal alterations in cardiac enzymes in the absence of obstructive CAD. It was described in 1991 in Japan375 and denominated “Takotsubo” owing to the similarity of the morphological aspect which the LV assumes to a type of trap used to capture octopuses in Japan (round in the bottom and narrow in the upper part). It has recently been recognized as a new entity within the spectrum of acute coronary syndromes.376-378 Its real frequency is unknown, but it is estimated that it represents 1% to 2% of cases that present at the emergency room with acute coronary syndrome.377,379-381 It generally affects post-menopausal women (95% of cases occur in women between the ages of 60 and 80), and it is rarely (< 3%) seen in women under the age of 50 or in men. In up to 80% of cases, the syndrome is associated with previous events which produced strong physical or emotional stress, such as separations, financial loss, conflicts, loss of a loved one, illness of a loved one, severe disease, surgery, etc. In some cases, however, no preceding physical or emotional stress may be identified.
Several physiopathological mechanisms have been proposed as participants in generating the syndrome, such as occult atherosclerotic disease, multiple coronary spasms, endothelial dysfunction, and microvascular disease. Nevertheless, the most accepted hypothesis is an excess of sympathetic stimulation, with elevated circulating catecholamines causing dynamic obstruction of LV outflow and resulting in short periods of ischemia and ventricular “stunning.”377,379,380,382-384 In fact, excessive sympathetic activity with pronounced plasma elevation of catecholamines has been found in almost 75% of patients with Takotsubo syndrome.379
The reason why Takotsubo syndrome occurs much more frequently in women after menopause is unknown. Several explanations have been proposed, such as the influence of sexual hormones on the sympathetic neurohumoral axis379,385 and coronary vasoreactivity;379,386 higher vulnerability of women to myocardial stunning, mediated by the sympathetic system;379,387 and alterations in endothelial function following menopause, in response to reduced estrogen levels.388
Clinical presentation is characterized by intense, acute chest pain (similar to that of infarction), dyspnea, ischemic ST-segment alterations (ST-segment elevation and/or inversion of T waves and pathological Q waves), mild increase in cardiac enzymes, and segmental systolic dysfunction in the apex and middle third of the LV, with base hyperkinesis, in the absence of obstructive epicardial coronary disease.
The most accepted criteria for diagnosis are currently those proposed by the Mayo Clinic in 2008:389,390
Transient hypokinesis, akinesis, or dyskinesis in LV mid-segments, with or without apical involvement.
Regional abnormalities that extend beyond epicardial vascular distribution, often with a precipitating factor.
Absence of CAD or evidence of acute plaque rupture.
New ECG abnormalities (ST-segment elevation and/or T-wave inversion) or mild elevation in cardiac troponin (disproportional to the degree of LV dysfunction).
Absence of pheochromocytoma and myocarditis.
Evaluation via myocardial scintigraphy with MIBG-123I shows defects in the uptake of MIBG generally in the apex, with normal myocardial perfusion observed on perfusion scintigraphy with Sestamibi-99mTc (Figure 55). Semiquantitative analysis has also demonstrated reduced HMR and increased washout of MIBG-123I. Abnormalities on myocardial scintigraphy with MIBG-123I may be detected hours to days following ischemic injury.391 For this reason, alterations observed on myocardial scintigraphy with MIBG-123I suggest a physiopathological explanation for this syndrome.392,393 Prognosis of affected patients is generally favorable. In the vast majority of cases, the LV dysfunction is transient, and complete recovery commonly occurs in around 8 weeks. In rare cases, dysfunction may be accentuated, evolving to cardiogenic shock, ventricular arrhythmia, and death (< 1% intra-hospital mortality).394
Figure 55.
Patient with acute coronary syndrome (ACS). Normal myocardial perfusion scintigraphy (MPS) with Sestamibi-99mTc (top row). Myocardial scintigraphy (MS) with MIBG-123I (bottom row) demonstrates uptake defects in apical segments, suggestive of Takotsubo syndrome. Reduced tracer uptake is additionally observed in the inferior wall.
Personal source.
13.4. Final Considerations
The diagnostic and prognostic potential for evaluating the autonomic nervous system with nuclear cardiology is great. A growing amount of evidence has shown that cardiac scintigraphy with MIBG-123I may assist in selection of patients for more sophisticated HR treatments, such as CRT, as well as new medical approaches, and ICD implants for primary prevention. It is also a valuable tool for cardiovascular risk stratification (potentially lethal ventricular arrhythmias, progression of HF, and cardiac death). Due to the high sensitivity of autonomic nervous system fibers to ischemic injury and delayed recovery, myocardial scintigraphy with MIBG-123I is also useful as an ischemic memory marker or for the recognition of Takotsubo syndrome. Greater clinical experience with this method will, however, be necessary, with the aim of improving positive and negative predictive values, for the sake of greater differentiation of patients with low and high risks, thus contributing to more effective use of medical resources. Japan is the only country where the utility of this imaging technique has been characterized in guidelines. Data related to cost-effectiveness are still limited, and low availability in clinical practice make it difficult to use on a large scale.
14. New Applications of Nuclear Cardiology
14.1. Introduction
The applications of nuclear cardiology go beyond MPS for ischemic heart disease. Some of the indications which will be discussed are not relatively recent in the literature, but they are still little utilized within our context. In comparison to conventional investigation methods, new non-invasive methods of nuclear medicine in cardiology have the potential to improve early detection of affected myocardium, allowing for quantification of disease activity, orienting therapeutic interventions, and monitoring success of treatment.
14.2. Endocarditis
Early diagnosis of infectious endocarditis (IE) continues to be challenging. The pathology should, essentially, be suspected in the presence of fever of unknown origin, especially in association with laboratory signs of infection, anemia, microscopic hematuria, or manifestations of septic embolism. The modified Duke criteria, which are considered a reference, include clinical, microbiological, and echocardiography findings, resulting in a general sensitivity around 80%.395 Some limitations, however, stand out, especially in patients with prosthetic valves (PV) and cardiac implantable electronic devices (CIED),396 implying inadequate classification of up to 24% of patients with proven IE.395 Advanced imaging techniques for early, sensitive diagnosis of IE are, in fact, valuable tools in clinical practice. The combination of both evaluation of myocardial metabolism of glucose via PET/CT using a glucose analogue labeled with 18F, fluorodeoxyglucose (FDG) (FDG-18F - PET/CT), and modified Duke criteria resulted in increased sensitivity, without large alterations in specificity.397 Although FDG-18F - PET/CT is not reliable for evaluation of native valve endocarditis,398 it may accurately diagnose endocarditis in valve prostheses and its systemic complications.399 In recognition of its utility, FDG-18F - PET/CT was included in the European Society of Cardiology Guidelines, in 2015, as a diagnostic criterion (class of recommendation IIb) for IE in patients with valve prostheses.400 One option for further improving FDG-18F - PET/CT imaging is the incorporation of angio-CT (PET/angio-CT), resulting in sensitivity, specificity, positive predictive value, and negative predictive values of 91%, 91%, 93%, and 88%, respectively.401 As a more specific alternative to FDG-18F - PET/CT, guidelines on IE include scintigraphy using marked leukocytes with SPECT/CT imaging. SPECT/CT is the combination of nuclear medicine tomography imaging (SPECT) and anatomical imaging via CT, greatly increasing diagnostic accuracy. However, notwithstanding the proven value of this technique for detecting endocarditis402,403 (Figure 56), its widespread application is compromised due to limited sensitivity and the difficulty of locating inflammatory foci, but the very high specificity of scintigraphy with marked leukocytes for infection, when using SPECT/CT imaging, may be particularly useful in cases where diagnosis remains uncertain following echocardiography and FDG-18F - PET/CT, especially in patients who have undergone cardiac surgery over the past 2 months.404-407 As an additional possibility, the simultaneous combination of scintigraphy with marked leukocytes and MPS, acquired to improve localization of infectious points in relation to the valve plane defined by perfusion. Limitations to performing SPECT/CT with marked leukocytes are: the need for a specific structure with laminar flow, the manipulation of blood components, procedure duration, and inferior spatial resolution in relation to PET/CT.400
Figure 56.
Images from scintigraphy with labeledleukocytes with SPECT/CT demonstrate anomalous accumulation in the area of the percutaneously implanted aortic valve (arrows). Transesophageal echocardiogram was inconclusive, and blood culture was positive for Staphylococcus aureus. The patient was diagnosed with prosthetic valve infective endocarditis.
Personal source (courtesy of Dr. Alan Chambi).
Furthermore, new bacteria-specific tracers have become available, such as carbohydrates, which are metabolized exclusively by bacteria or antibodies directed against components of the bacterial cell membrane. For example, the protein component of the pilin structure of Enterococcus faecalis is being developed.408 This recent study has demonstrated the superior quality of images and another possibility for differentiating between infectious and inflammatory causes of endocarditis.
CIED have been increasingly used over recent years,400 with elevated rates of infection (1% to 3%), and they are associated with 1-year mortality over 10%.409 Doppler echocardiography is the first line imaging method for evaluation of suspected CIED infection, but its use is limited for investigating infection in extra-cardiac leads and device pockets. Both FDG-18F - PET/CT and SPECT/CT scintigraphy with marked leukocytes have demonstrated additional value for diagnosis of infections related to CIED or pacemaker. FDG-18F - PET/CT has been shown to be especially useful for diagnosing device pocket infections, but it is less reliable for diagnosing infections in the metallic device.410,411 The presence of a focal hotspot is considered the best criterion for infection,412 (Figures 57 and 58). It is worth noting that exam accuracy depends on patient preparation and post-implant interval, which is the case with applications involving FDG-18F. Mild FDG-18F uptake has been reported to be nonspecific in patients with CIED or pacemaker with no suspicion of acute-phase infection (≤ 2 months) following cardiac surgery.410 Moreover, attenuation correction artifacts due to metallic implants should be avoided by means of close evaluation of images without attenuation correction.
Figure 57.
Male patient, age 65, with pacemaker implant 4 months prior (removed due to subcutaneous pocket infection); new implantation with the distal end of the right chambers. He evolved with dyspnea and fever 20 days prior; blood culture was positive for S. aureus. FDG-18F - PET/CT study was positive for endocarditis in the implant site; maximum standard uptake value (SUV) = 8.1.
Source: INCOR, FMUSP, SP.
Figure 58.
Male patient, age 19, with biological prosthesis in the aorta and mitral annuloplasty for 45 days. He evolved with fever, bacteremia, and blood culture positive for S. Epidermidis. FDG-18F - PET/CT study was positive for infection in the aortic prosthesis; maximum standard uptake value (SUV) = 9.7.
Source: INCOR, FMUSP, SP.
Both FDG-18F - PET/CT and scintigraphy with marked leukocytes via SPECT/CT seem to be beneficial in diagnosing infections related to ventricular assist devices (VAD).413,414 FDG-18F - PET/CT is especially sensitive to infection in these devices. In a small retrospective study, sensitivity to VAD infection was 100%, and specificity was 80%. Furthermore, in 85% of cases, PET imaging had an impact on clinical management of patients.415
The role of FDG-18F - PET/CT in investigating extracardiac complications of infection was also studied. In a retrospective analysis of patients with suspected CIED infection, the performance of full body PET also identified septic embolism or infection disseminated into other sites in 28% of cases.416 These results were confirmed in a prospective study on known device endocarditis.417 In this cohort, FDG-18F - PET/CT found septic embolism in 10 patients (29%), including 7 cases of spondylodiscitis, 4 of which were not clinically visible and which resulted in significant modifications to therapy.
Guided myocardial biopsy may be another application of FDG-18F - PET/CT, as shown in other diseases.418 Furthermore, MR and PET/CT seem to be complementary in nature.419 Investigation of the incremental value of PET/MR, a new integrated imaging modality, may have great potential for diagnosing endocarditis.
14.3. Myocarditis
The most common causes of myocarditis are viral infections. Other causes include other types of infections, autoimmune disorders, or drug interactions. Clinical manifestations of myocarditis are highly variable, ranging from subclinical disease to sudden death. This spectrum also reflects the extent to which this histological disease’s severity, etiology, and stage of clinical presentation may vary. Inflammation of the myocardium may be focal or diffused, involving any of the cardiac chambers. Endomyocardial biopsy is currently the gold standard for diagnosis, but it has a low sensitivity (20-30%) and significant associated risk.420 MR is considered the imaging method of reference for non-invasive diagnosis of myocarditis, given that it allows for detection of several characteristics such as inflammatory hyperemia and edema, necrosis, and myocardial scarring, alterations in ventricular size and geometry, regional and global abnormalities in the movement of walls, and identification of pericardial effusion.421 MR criteria for diagnosis of myocarditis have been summarized in what are known as the Lake Louise Criteria.422 MR, however, has limitations that are particularly evident in chronic myocarditis, with low diagnostic precision (50% accuracy).423
Using FDG-18F - PET/CT, following adequate patient preparation with a carbohydrate-free diet, it is possible to visualize acute inflammation suggestive of active myocarditis. PET imaging may help distinguish active and chronic forms of the disease, following established working protocols.424,425 In a prospective study of 65 patients with suspected myocarditis, FDG-18F - PET was in agreement with MR findings.426 MR and FDG-18F - PET/CT seem to be complementary in nature.419 For this reason, cardiac PET/MR has potential as a diagnostic tool for myocarditis and a new field of research.427-429
14.4. Pericarditis
There are multiple causes of acute or chronic pericardial inflammation, including infections (viral, bacterial, or fungal), myocardial infarction, trauma, malign diseases (primary pericardial neoplasm, pericardial metastases, or paraneoplastic syndrome), autoimmune or inflammatory diseases, and metabolic disorders (uremia). Pericarditis may also be iatrogenic, as a collateral effect of medication. Radiotherapy or idiopathic causes are other possible origins. Although its etiology is variable, the pericardium’s response to different causes is not specific. Inflammation of pericardial layers and increased production of pericardial fluids are the most common, and they often manifest as chest pain. In the same manner, Doppler echocardiography stands out as a priority for diagnosis and therapeutic follow-up of pericarditis, externalizing findings such as pericardial effusion and thickness. Generally, CT and MR also allow for evaluation of pericardial effusion and thickness, allowing for better differentiation of pericardium and pericardial fluid.430
The use of FDG-18F - PET/CT in pericarditis is generally complementary, and it demonstrates the ability to detect inflammatory tissue, even in the absence of obvious anatomical changes.431,432 Non-infectious inflammatory pericarditis shows mild to moderate FDG-18F - PET uptake in the pericardium, with diffuse or focal uptake pattern. The literature is still scarce on the utility of FDG-18F - PET/CT for differential diagnosis of the underlying causes of this pathology. Some studies relate the possibility of differentiating infectious/inflammatory pericardial disease and neoplastic/metastatic disease, given that malignity, generally, presents intense metabolic activity.433 Constrictive or effusive pericarditis, an uncommon complication of chemotherapy, may also present pericardial uptake of FDG-18F, with mild intensity and wide distribution.431 Only a few case reports are available in the literature, and larger studies are still necessary to determine the accuracy of FDG-18F - PET/CT for pericarditis.
14.5. Cardiac Sarcoidosis
Sarcoidosis is a granulomatous disease whose etiology is unknown. It most commonly affects the lymphatic ganglia and the lungs, but it may involve any system of organs.434 The heart is frequently affected,435,436 and this represents one of the main causes of death due to this pathology in Japan and the USA.437 Due to its multifocal aspect and the irregular manner in which sarcoidosis affects the myocardium, the sensitivity of endomyocardial biopsy is extremely low (20% to 30%).438 In comparison with MR, the advantages of FDG-18F - PET/CT include the value of functional metabolic information, the detection of active inflammation, the potential for identifying cardiac and extracardiac involvement (Figure 59) of sarcoidosis, and the possibility of performing imaging in patients with CIED or renal insufficiency. In order to evaluate extracardiac involvement, it is important to perform full-body imaging.
Figure 59.
FDG-18F - PET/CT with suppression protocol for myocardial glucose uptake (diet), maximum-intensity-projection imaging (left) and coregistration with CT (right). Patient, age 88, with heart failure, reduced ejection fraction, and ventricular tachycardia. Endomyocardial biopsy was compatible with sarcoidosis. Images demonstrated abnormal tracer uptake in the right and left ventricles (arrows). Following immunosuppression, the patient showed clinical improvement and disappearance of abnormalities.
Personal source (courtesy of Dr. Evandro T. Mesquita).
Sarcoidosis normally manifests as an irregular focal uptake pattern. FDG-18F - PET/CT has demonstrated that it detects active cardiac and extracardiac forms reliably, with sensitivity between 81% and 89% and specificity between 78% and 82%, respectively.439,440 It is necessary to pay attention to the patient preparation required for image acquisition in these cases. It is essential for the patient’s diet to be low in carbohydrates and rich in fat the day before the exam and for the patient to be in fasting conditions in order to guarantee that there is no physiological uptake in the myocardium.
FDG-18F - PET/CT may often be combined with MPS synchronized with ECG (Figure 60), with the objective of ruling out CAD or even identifying resting perfusion defects suggestive of inflammation-induced tissue damage.441,442
Figure 60.
Patient with cardiac sarcoidosis. Myocardial perfusion scintigraphy (MPS) with MIBI-99mTc (left), showing evidence of accentuated persistent hypoperfusion in the anterior and anterolateral walls of the left ventricle; FDG-18F - PET imaging (right) for metabolic study shows that regions with apparent fibrosis were in fact inflammation due to sarcoidosis.
Source: INCOR, FMUSP, SP.
In addition to this, FDG-18F - PET/CT in combination with perfusion imaging has shown evidence of prognostic capability in patients with sarcoidosis,443 orienting myocardial biopsy,418 and demonstrating valor for predicting response and monitoring therapy.444
14.6. Cardiac Amyloidosis
The CA is a rare form of cardiomyopathy. Frequently subdiagnosed, it is characterized by extracellular deposition of fibrils, composed of varied serum protein subunits, which have low molecular weight. Although more than 30 different amyloid proteins have been described, the 2 that most frequently infiltrate the heart are: light chain immunoglobulin (AL) and transthyretin (TTR). The AL and TTR forms possess different clinical courses, prognoses, and distinct forms of treatment. In the AL form, fibrils are composed of light-chain immunoglobulins and produced by a population of plasma cell clones located in the bone marrow. In the TTR form, deposits are made up of anomalous monomers or dimers of the hepatic tetrameric protein whose origin may be related to genetic mutations of familial origin (mutated TTR or [mTTR]) or the wild type, formerly known as the senile type (sTTR). More than 100 known mutations are related to mTTR and to autosomal dominant inheritance, which may affect individuals in any age group, especially middle-aged men. The most common manifestation of CA is HF with preserved ejection fraction. In its final stage, it is present as restrictive cardiomyopathy, implying very poor prognosis. Definitive diagnosis requires amyloid deposits on endomyocardial biopsy or, in patients with suggestive cardiac findings, amyloid deposits on histological exams of other tissues (e.g., abdominal fat, rectum, or kidneys).445
Echocardiography is the initial non-invasive exam of choice for diagnosing CA, but its specificity is limited.446However, complementary sequence with MR, which has satisfactory sensitivity, may suggest a pattern of cardiomyopathy due to amyloid deposition, except in patients with moderate to severe kidney disease.
It has been reported that scintigraphy with intravenous administration of bisphosphonate radiotracers labeled with 99m-technetium (Pyrophosphate-99mTc is the most used in Brazil) localizes cardiac amyloid deposition. It is considered sensitive and highly specific for TTR CA, identifying the disease early at onset.447,448 One hypothesis for the binding of these bone markers to amyloid fibers is related to the higher quantity of calcium present in TTR protein, in relation to AL. In a recent multicenter study which included 1,217 patients with suspected CA, the combination of moderate to accentuated increase in myocardial uptake of the radiotracer and the absence of specific monoclonal protein in blood serum or urine, had specificity and positive predictive value of 100% for the TTR form of CA. However, scintigraphy with bisphosphonate radiotracers does not reliably detect other types of CA, and it cannot be used quantitatively for therapeutic monitoring.418
The intensity of the concentration of Pyrophosphate-99mTc in the cardiac area is correlated to the amyloid subtype. Degree of concentration is compared to bone uptake in the ribcage, considering the following: degree 3, greater uptake than the ribs; degree 2, equal to the intensity of concentration in the ribs; degree 1, lower concentration than in the ribs; and degree zero, no significant cardiac tracer concentration. Severely increased concentration (degrees 2 and 3) (Figure 61) is strongly associated with TTR CA, to the extent that some authors suggest dispensing cardiac biopsy in these situations. Less intense increased concentration (degree 1) and absence of increased concentration suggest the AL form, when there is clinical suspicion. Semiquantitative analysis of radiotracer uptake should also be performed (Figure 62).449
Figure 61.
Scintigraphy with Pyrophosphate-99mTc (left: planar imaging of the anterior thorax; right: axial cross section of the tomography image) demonstrated severe radiopharmaceutical uptake in the left ventricle (arrows) in a patient with confirmed transthyretin cardiac amyloidosis.
Personal source (courtesy of Dr. Rafael Willain Lopes).
Figure 62.
Quantification of uptake in planar imaging of anterior thorax scintigraphy with Pyrophosphate-99mTc. The image on the left represents a negative study, without uptake in the cardiac area. The image on the right represents a study positive for amyloidosis, with accentuated diffuse uptake in the left ventricle. For the purpose of quantification, circular regions of interest (ROI) have been drawn in both hemithoraxes, and the uptake ratio of the radiopharmaceutical between the ROI in the cardiac area (C) and the ROI in the contralateral (Cl) hemithorax. Results over 1.5 suggest TTR amyloidosis. The visual uptake score was equal to 1 in the image on the left and 3 in the image on the right.
Source: INCOR, FMUSP, SP.
A potential bone radiotracer for PET amyloid imaging is sodium fluoride labeled with 18F, or Sodium fluoride-18F. It has been described in single case series, whereas another study identified no increase in uptake of this tracer with TTR CA,450-452 indicating the need for further studies to investigate the potential value of PET/CT with Sodium fluoride-18F for CA.
A small amount of available data has demonstrated the limited application of FDG-18F - PET/CT for evaluation of CA.453,454 Up to the present moment, the most promising alternatives include other specific amyloid markers, such as the Pittsburgh B compound labeled with carbon-11 (PIB-11C),455,456 as well as other compounds labeled with 18F, such as Florbetapir457,458 and Florbetaben.459 All studies have reported promising results for diagnosis of CA, given that PIB-11C presents uptake in AC. It has additionally been demonstrated that PIB-11C has lower uptake in patients who have been treated with chemotherapy, in comparison with patients still undergoing treatment. Thus, PIB-11C - PET has the potential to be used for therapeutic monitoring of patients with light-chain CA as a marker of disease activity.460
14.7. Final Considerations
New applications of nuclear medicine in cardiology (Table 32) represent an important area which is little explored in our context. They have the capability to detect functional alterations in these pathologies, indicating whether a disease is or active or not and assisting in therapeutic monitoring. Cardiologists’ knowledge of these applications will be essential to their proper use and to the dissemination of these diagnostic methods.
Table 32.
Exam types and main scintigraphy findings of new applications of nuclear cardiology
| Pathology | Exam | Main findings |
|---|---|---|
| TTR CA (hereditary or wild) | Scintigraphy with 99mTc-pyrophosphate | Shows moderate to severe radiotracer uptake; high accuracy for detection of TTR form (PPV 100%); allows for early diagnosis; reflects extent of deposit; prognostic marker |
| Light chain CA | Scintigraphy with 99mTc-pyrophosphate | Absence of uptake or slight cardiac uptake |
| Sarcoidosis | FDG-18F - PET/CT | Cardiac hypermetabolism demonstrating active in-flammation |
| Myocardial perfusion scintigraphy | Persistent myocardial hy-poperfusion suggestive of tissue damage due to in-flammation | |
| Endocarditis | FDG-18F - PET/CT | Hypermetabolism in areas of infection |
| Scintigraphy with marked leukocytes | High uptake in areas of infection |
CA: cardiac amyloidosis; PPV: positive predictive value; TTR: transthyretin amyloidosis.
Table 21.
Percent probability of obstructive coronary artery disease, considering the presence of chest pain, sex, and age. Adapted from Diamond GA, Forrester JS and the Brazilian Cardiology Society's Third Guidelines on Exercise Testing117,118
| Age | Non-anginal chest pain | Atypical angina | Typical angina | |||
|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Women | Men | |
| 30-39 | 4 | 2 | 34 | 12 | 76 | 26 |
| 40-49 | 13 | 3 | 51 | 22 | 87 | 55 |
| 50-59 | 20 | 7 | 65 | 31 | 93 | 73 |
| 60-69 | 27 | 14 | 72 | 51 | 94 | 86 |
Acknowledgments
Special Acknowledgment: Juliano Cerci, Marcelo Vieira de Carvalho and Tales de Carvalho
Footnotes
To access the Addendum bibliography, go to: https://sbc-portal.s3.sa-east-1.amazonaws.com/diretrizes/Publicacoes/2020/Bibliografia-adendo/Bibliografia_ADENDO_INGLES_24-01-2020.pdf
Note: This guideline is for information purposes and should not replace the clinical judgment of a physician, who must ultimately determine the appropriate treatment for each patient.
References
- 1.Azambuja MIR, Foppa M, Maranhão MF, Achutti AC. Impacto econômico dos casos de doença cardiovascular grave no Brasil: uma estimativa baseada em dados secundários. Arq Bras Cardiol. 2008;91(3):163–171. doi: 10.1590/s0066-782x2008001500005. [DOI] [PubMed] [Google Scholar]
- 2.Teich V, Araujo DV. Estimativa de custo da síndrome coronariana aguda no Brasil. Rev Bras Cardiol. 2011;24(2):85–94. [Google Scholar]
- 3.Des Prez RD, Shaw LJ, Gillespie RL, Jaber WA, Noble GL, Soman P, et al. Cost-effectiveness of myocardial perfusion imaging: A summary of the currently available literature. J Nucl Cardiol. 2005;12(6):750–759. doi: 10.1016/j.nuclcard.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Shaw LJ, Hachamovitch R, Berman DS, Marwick TH, Lauer MS, Heller GV, et al. The economic consequences of available diagnostic and prognostic strategies for the evaluation of stable angina patients: An observational assessment of the value of precatheterization ischemia. J Am Coll Cardiol. 1999;33(3):661–669. doi: 10.1016/s0735-1097(98)00606-8. [DOI] [PubMed] [Google Scholar]
- 5.Volschan A. Challenges for Contemporary Medicine: Implementation of Evidence in Clinical Practice. Int J Cardiovasc Sci. 2016;29(4):250–252. [Google Scholar]
- 6.Underwood SR, Godman B, Salyani S, Ogle JR, Ell PJ. Economics of myocardial perfusion imaging in Europe-the EMPIRE Study. Eur Heart J. 1999;20(2):157–166. doi: 10.1053/euhj.1998.1196. [DOI] [PubMed] [Google Scholar]
- 7.Cerci JJ, Trindade E, Preto D, Cerci RJ, Lemos PA, Cesar LA, et al. Investigation route of the coronary patient in the public health system in Curitiba, Sao Paulo and in Incor - IMPACT study. Arq Bras Cardiol. 2014;103(3):192–200. doi: 10.5935/abc.20140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foy AJ, Dhruva SS, Peterson B, Mandrola JM, Morgan DJ, Redberg RF. Coronary computed tomography angiography vs functional stress testing for patients with suspected coronary artery disease: A systematic review and meta-analysis. JAMA Intern Med. 2017;177(11):1623–1631. doi: 10.1001/jamainternmed.2017.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stillman AE, Gatsonis C, Lima JA, Black WC, Cormack J, Gareen I, et al. Rationale and design of the Randomized Evaluation of patients with Stable angina Comparing Utilization of noninvasive Examinations (RESCUE) trial. Am Heart J. 2016 Sep;179:19–28. doi: 10.1016/j.ahj.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim AS, Johnston SC. Global variation in the relative burden of stroke and ischemic heart disease. Circulation. 2011;124(3):314–323. doi: 10.1161/CIRCULATIONAHA.111.018820. [DOI] [PubMed] [Google Scholar]
- 11.Hendel RC, Berman DS, Di Carli MF, Heidenreich PA, Henkin RE, Pellikka PA, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 Appropriate Use Criteria for Cardiac Radionuclide Imaging: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. J Am Coll Cardiol. 2009;53(23):2201–2229. doi: 10.1016/j.jacc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;126(25):e354–e471. doi: 10.1161/CIR.0b013e318277d6a0. [DOI] [PubMed] [Google Scholar]
- 13.Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher B, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;64(18):1929–1949. doi: 10.1016/j.jacc.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 14.Cesar LA, Ferreira JF, Armaganijan D, Gowdak LH, Mansur AP, Bodanese LC, et al. Diretriz de doença coronária estável. Arq Bras Cardiol. 2014;103(2) Suppl 2:1–59. doi: 10.5935/abc.2014s004. [DOI] [PubMed] [Google Scholar]
- 15.Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2014;35(37):2541–2619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 16.Kristensen SD, Knuuti J, Saraste A, Anker S, Bøtker HE, Hert SD, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA) Eur Heart J. 2014;35(35):2383–2431. doi: 10.1093/eurheartj/ehu282. [DOI] [PubMed] [Google Scholar]
- 17.Mastrocola LE, Lopes RW, Boccia D, Alves FB. Moreira MC, Montenegro ST, Paola AAV de. Livro Texto da Sociedade Brasileira de Cardiologia. Seção 8: Imagem não Invasiva: Ressonância Magnética, Tomografia Computadorizada e Medicina Nuclear. 2ªª ed. São Paulo: Manole; 2015. Cardiologia Nuclear: princípios; pp. 441– 452. Cap 1, Seção 8. [Google Scholar]
- 18.Meneghetti JC, Falcão AM, Buchpiguel CA, Mastrocola LE. Cardiologia Nuclear e pequisa de viabilidade miocárdica. In: Moreira MC, Montenegro ST, Paola AA, editors. Livro Texto da SBC. 2ªª ed. São Paulo: Manole; 2015. pp. 629–634. [Google Scholar]
- 19.Schinkel AF, Bax JJ, Delgado V, Poldermans D, Rahimtoola SH. Clinical relevance of hibernating myocardium in ischemic left ventricular dysfunction. Am J Med. 2010;123(11):978–986. doi: 10.1016/j.amjmed.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 20.Rahimtoola SH, Dilsizian V, Kramer CM, Marwick TH, Vanoverschelde JL. Chronic ischemic left ventricular dysfunction: from pathophysiology to imaging and its integration into clinical practice. JACC Cardiovasc Imaging. 2008;1(4):536–555. doi: 10.1016/j.jcmg.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soman P, Udelson JE, Bax JJ, Downey BC. Assessment of myocardial viability by nuclear imaging in coronary heart disease. Up to Date; 2017. [2018 June 10]. Available from: https://www.uptodate.com/contents/assessment-of-myocardial-viability-by-nuclear-imaging-in-coronary-heart-disease. [Google Scholar]
- 22.Dilsizian V. SPECT and PET myocardial perfusion imaging: Tracers and techniques. In: Dilsizian V, Narula J, Braunwald E, editors. Atlas of Nuclear Cardiology. 4th ed. New York: Springer; 2013. pp. 55–94. [Google Scholar]
- 23.Udelson JE, Dilsizian V, Bonow RO. Nuclear Cardiology: Technical aspects of image acquisition display and interpretation. In: Mann DL, Zipes DP, Libby P, Bonow RO, Braunwald E, editors. Braunwald's Heart Disease. A Textbook of Cardiovascular Medicine. 10th ed. Philadelphia: Elsevier; 2015. pp. 271–287. [Google Scholar]
- 24.INTERNATIONAL ATOMIC ENERGY AGENCY Nuclear Cardiology: Guidance on the Implementation of SPECT Myocardial Perfusion Imaging. IAEA Human Health Series. 2016;23(Rev. 1) [Google Scholar]
- 25.Medical Advisory Secretariat Single photon emission computed tomography for the diagnosis of coronary artery disease: an evidence-based analysis. Ont Health Technol Assess Ser. 2010;10(8):1–64. [PMC free article] [PubMed] [Google Scholar]
- 26.Nielsen AP, Morris KG, Murdock R, Bruno FP, Cobb FR. Linear relationship between the distribution of thallium-201 and blood flow in ischemic and nonischemic myocardium during exercise. Circulation. 1980;61(4):797–801. doi: 10.1161/01.cir.61.4.797. [DOI] [PubMed] [Google Scholar]
- 27.Krivokapich J, Smith GT, Huang SC, Hoffman EJ, Ratib O, Phelps ME, et al. 13N ammonia myocardial imaging at rest and with exercise in normal volunteers: quantification of absolute myocardial perfusion with dynamic positron emission tomography. Circulation. 1989;80(5):1328–1337. doi: 10.1161/01.cir.80.5.1328. [DOI] [PubMed] [Google Scholar]
- 28.Mastrocolla LE, Sousa AG, Smanio PE, Staico R, Pinto IF, Meneghelo RS, et al. Adenosine myocardial perfusion SPECT with Tc-99m-MIBI in patients with obstructive coronary artery disease: correlation between quantitative coronary angiography and intravascular ultrasound measurements. Arq Bras Cardiol. 2006;86(1):3–13. doi: 10.1590/s0066-782x2006000100002. [DOI] [PubMed] [Google Scholar]
- 29.Garcia EV. Quantitative nuclear cardiology: we are almost there! J Nucl Cardiol. 2012;19(3):424–437. doi: 10.1007/s12350-012-9551-8. [DOI] [PubMed] [Google Scholar]
- 30.Duvall WL, Croft LB, Ginsberg ES, Einstein AJ, Guma KA, George T, et al. Reduced isotope dose and imaging time with a high-efficiency CZT SPECT camera. J Nucl Cardiol. 2011;18(5):847–857. doi: 10.1007/s12350-011-9379-7. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Haim S, Kennedy J, Keidar Z. Novel cadmium zinc telluride devices for myocardial perfusion imaging-technological aspects and clinical applications. Semin Nucl Med. 2016;46(4):273–285. doi: 10.1053/j.semnuclmed.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Duvall WL, Hiensch RJ, Levine EJ, Croft LB, Henzlova MJ. The prognosis of a normal Tl-201 stress-only SPECT MPI study. J Nucl Cardiol. 2012;19(5):914–921. doi: 10.1007/s12350-012-9601-2. [DOI] [PubMed] [Google Scholar]
- 33.Ejlersen JA, May O, Mortensen J, Nielsen GL, Lauridsen JF, Allan J. Stress-only myocardial perfusion scintigraphy: a prospective study on the accuracy and observer agreement with quantitative coronary angiography as the gold standard. Nucl Med Commun. 2017;38(11):904–911. doi: 10.1097/MNM.0000000000000739. [DOI] [PubMed] [Google Scholar]
- 34.Tilkemeier PL, Bourque J, Doukky R, Sanghani R, Weinberg RL. ASNC imaging guidelines for nuclear cardiology procedures. Standardized reporting of nuclear cardiology procedures. J Nucl Cardiol. 2017;(24):2064–2118. doi: 10.1007/s12350-017-1057-y. [DOI] [PubMed] [Google Scholar]
- 35.Garcia EV, Galt JR, Faber TL, Chen J. Principles of nuclear cardiology imaging. In: Dilsizian V, Narula J, Braunwald E, editors. Atlas of Nuclear Cardiology. 4thth ed. New York: Springer; 2013. pp. 1–54. [Google Scholar]
- 36.Botvinick E. Assessment of cardiac function: first-pass, equilibrium blood pool, and gated myocardial SPECT. In: Dilsizian V, Narula J, Braunwald E, editors. Atlas of Nuclear Cardiology. 4thth ed. New York: Springer; 2013. pp. 195–245. [Google Scholar]
- 37.Germano G, Berman DS, editors. Clinical Gated Cardiac SPECT. Armonk (NY): Blackwell Futura; 2006. pp. 93–138. [Google Scholar]
- 38.Ficaro EP, Hansen CL, American Society of Nuclear Cardiology Imaging guidelines for nuclear cardiology procedures. [2018 Nov 10]. Internet. Available from: https://www.asnc.org/files/Stress%20Protocols%20and%20Tracers%202009.pdf.
- 39.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 40.Oliveira MA, Duarte PS, Gonzalez MM, Moises VA, Alonso G, Lima EV, et al. Biological factors and overestimation of left ventricular ejection fraction by gated SPECT. Arq Bras Cardiol. 2008;90(5):305–310. doi: 10.1590/s0066-782x2008000500005. [DOI] [PubMed] [Google Scholar]
- 41.Lomsky M, Johansson L, Gjertsson P, Björk J, Edenbrandt L. Normal limits for left ventricular ejection fraction and volumes determined by gated single photon emission computed tomography-a comparison between two quantification methods. Clin Physiol Funct Imaging. 2008;28(3):169–173. doi: 10.1111/j.1475-097X.2008.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cabrera-Rodríguez LO, Peix AT, Padrón KM, Chacón D, Carrillo R, Fernández Y, et al. Prognostic value of gated SPECT after reperfusion for acute myocardial infarction. MEDICC Rev. 2013;15(2):20–25. doi: 10.37757/MR2013V15.N2.5. [DOI] [PubMed] [Google Scholar]
- 43.Kwon DH, Hachamovitch R, Popovic ZB, Starling RC, Desai MY, Flamm SD, et al. Survival in patients with severe ischemic cardiomyopathy undergoing revascularization versus medical therapy: association with end-systolic volume and viability. Circulation. 2012;126(11 Suppl 1):S3–S8. doi: 10.1161/CIRCULATIONAHA.111.084434. [DOI] [PubMed] [Google Scholar]
- 44.Chalela WA, Moffa PJ, Meneghetti JC. Estresse cardiovascular. Princípios e aplicações clínicas. São Paulo: Roca; 2004. pp. 267, p.370. [Google Scholar]
- 45.Kim C, Kwok YS, Heagerty P, Redberg R. Pharmacologic stress testing for coronary disease diagnosis: A meta-analysis. Am Heart J. 2001;(142):934–944. doi: 10.1067/mhj.2001.119761. [DOI] [PubMed] [Google Scholar]
- 46.Leppo JA. Comparison of pharmacologic stress agents. Pt 2J Nucl Cardiol. 1996;3(6):S22–S26. doi: 10.1016/s1071-3581(96)90204-4. [DOI] [PubMed] [Google Scholar]
- 47.Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ. ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers. J Nucl Cardiol. 2016;23(3):606–639. doi: 10.1007/s12350-015-0387-x. [DOI] [PubMed] [Google Scholar]
- 48.Al Moudi M, Sun Z, Lenzo N. Diagnostic value of SPECT, PET and PET/CT in the diagnosis of coronary artery disease: A systematic review. Biomed Imaging Interv J. 2011;7(2):e9. doi: 10.2349/biij.7.2.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mastrocola LE, Saliba LA, Mastrocola F, Buchler RD. Avaliação pratica dos escores diagnósticos e prognósticos: implicações preditivas. In: Moreira MC, Montenegro ST, Paola AA, editors. Livro Texto da SOCIEDADE Brasileira de Cardiologia. 2ªª ed. São Paulo: Manole; 2015. pp. 441– 52. [Google Scholar]
- 50.Mark DB, Hlatky MA, Harrell FE Jr, Lee KL, Califf RM, Pryor DB. Exercise treadmill score for predicting prognosis in coronary artery disease. Ann Intern Med. 1987;106(6):793–800. doi: 10.7326/0003-4819-106-6-793. [DOI] [PubMed] [Google Scholar]
- 51.Mark DB, Shaw L, Harrell FE Jr, Hlatky MA, Lee KL, Bengtson JR, et al. Prognostic value of a treadmill exercise score in outpatients with suspected coronary artery disease. N Engl J Med. 1991;325(12):849–853. doi: 10.1056/NEJM199109193251204. [DOI] [PubMed] [Google Scholar]
- 52.Shaw LJ, Berman DS, Picard MH, Shaw LJ, Berman DS, Picard MH, et al. National Institutes of Health/National Heart, Lung, and Blood Institute-Sponsored ISCHEMIA Trial Investigators Comparative definitions for moderate-severe ischemia in stress nuclear, echocardiography, and magnetic resonance imaging. JACC Cardiovasc Imaging. 2014;7(6):593–604. doi: 10.1016/j.jcmg.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moroi M, Yamashina A, Tsukamoto K, Nishimura T, J-ACCESS Investigators Coronary revascularization does not decrease cardiac events in patients with stable ischemic heart disease but might do in those who showed moderate to severe ischemia. Int J Cardiol. 2012;158(2):246–252. doi: 10.1016/j.ijcard.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 54.Nudi F, Neri G, Schillaci O, Pinto A, Procaccini E, Vetere M, et al. Time to and risk of cardiac events after myocardial perfusion scintigraphy. J Cardiol. 2015;66(2):125–129. doi: 10.1016/j.jjcc.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 55.Nudi F, Pinto A, Procaccini E, Neri G, Vetere M, Tomai F, et al. A novel clinically relevant segmentation method and corresponding maximal ischemia score to risk-stratify patients undergoing myocardial perfusion scintigraphy. J Nucl Cardiol. 2014;21(4):807–818. doi: 10.1007/s12350-014-9877-5. [DOI] [PubMed] [Google Scholar]
- 56.Hachamovitch R, Berman DS, Shaw LJ, Kiat H, Cohen I, Cabico JA, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97(6):535–543. doi: 10.1161/01.cir.97.6.535. [DOI] [PubMed] [Google Scholar]
- 57.Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107(23):2900–2907. doi: 10.1161/01.CIR.0000072790.23090.41. [DOI] [PubMed] [Google Scholar]
- 58.Cremer P, Hachamovitch R, Tamarappoo B. Clinical decision making with myocardial perfusion imaging in patients with known or suspected coronary artery disease. Semin Nucl Med. 2014;44(4):320–329. doi: 10.1053/j.semnuclmed.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xie JX, Winchester DE, Phillips LM, Hachamovitch R, Berman DS, Blankstein R, et al. The elusive role of myocardial perfusion imaging in stable ischemic heart disease: Is ISCHEMIA the answer? J Nucl Cardiol. 2017;24(5):1610–1618. doi: 10.1007/s12350-017-0963-3. [DOI] [PubMed] [Google Scholar]
- 60.Meneghelo RS, Araújo CG, Stein R, Mastrocolla LE, Albuquerque PF, Serra SM, et al. Sociedade Brasileira de Cardiologia III Diretrizes da Sociedade Brasileira de Cardiologia sobre Teste Ergométrico. Arq Bras Cardiol. 2010;95(5) Suppl 1:1–26. doi: 10.1590/S0066-782X2010000800001. [DOI] [PubMed] [Google Scholar]
- 61.Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, et al. American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology. Council on Nutrition, Physical Activity and Metabolism. Council on Cardiovascular and Stroke Nursing. Council on Epidemiology and Prevention Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128(8):873–934. doi: 10.1161/CIR.0b013e31829b5b44. [DOI] [PubMed] [Google Scholar]
- 62.Feitosa GS, Nicolau JC, Chalela WA, Meneghetti JC, Ximenes AA, de Almeida CA, et al. I Diretriz da Sociedade Brasileira de Cardiologia sobre Cardiologia Nuclear. Arq Bras Cardiol. 2002;78(Suppl 3):1–42. [PubMed] [Google Scholar]
- 63.Mahmarian JJ, Shaw LJ, Filipchuk NG, Dakik HA, Iskander SS, Ruddy TD, INSPIRE Investigators et al. A multinational study to establish the value of early adenosine technetium-99m sestamibi myocardial perfusion imaging in identifying a low-risk group for early hospital discharge after acute myocardial infarction. J Am Coll Cardiol. 2006;48(12):2448–2457. doi: 10.1016/j.jacc.2006.07.069. [DOI] [PubMed] [Google Scholar]
- 64.Kim C, Kwok YS, Heagerty P, Redberg R. Pharmacologic stress testing for coronary disease diagnosis: A meta analysys. Am Heart J. 2001;142(6):934–944. doi: 10.1067/mhj.2001.119761. [DOI] [PubMed] [Google Scholar]
- 65.Nair SU, Ahlberg AW, Mathur S, Katten DM, Polk DM, Heller GV. The clinical value of single photon emission computed tomography myocardial perfusion imaging in cardiac risk stratification of very elderly patients (=80 years) with suspected coronary artery disease. J Nucl Cardiol. 2012;19(2):244–255. doi: 10.1007/s12350-011-9477-6. [DOI] [PubMed] [Google Scholar]
- 66.Cerci MS, Cerci JJ, Cerci RJ, Pereira Neto CC, Trindade E, Delbeke D, et al. Myocardial perfusion imaging is a strong predictor of death in women. JACC CVAS Imaging. 2011;4(8):880–888. doi: 10.1016/j.jcmg.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 67.Falcão A, Chalela W, Giorgi MC, Imada R, Soares J Jr, Do Val R, et al. Myocardial Blood flow assessment with 82 rubidium-PET imaging in patients with left bundle branch block. Clinics. 2015;70(11):726–732. doi: 10.6061/clinics/2015(11)02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fallahi B, Beiki D, Eftekhari M, Gilani KA, Fard-Esfahani A, Gholamrezanezhad A, et al. High accuracy of myocardial perfusion imaging in patients with left bundle branch block: comparison of four interpretation approaches. Hell J Nucl Med. 2009;12(2):132–137. [PubMed] [Google Scholar]
- 69.Bokhari S, Ficaro EP, McCallister BD Jr. Adenosine stress protocols for myocardial perfusion imaging. J Nucl Cardiol. 2007;14(3):415–416. doi: 10.1016/j.nuclcard.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 70.Center for Science in the Public Interest Caffeine content of food & drugs. 2014.Table containing the caffeine content of food and drugs. [2017 Dec 12]. Internet. Available from: http://www.cspinet.org/new/cafchart.htm.
- 71.Lette J, Tatum JL, Fraser S, Miller DD, Waters DD, Heller G, et al. Safety of dipyridamole testing in 73,806 patients: The multicenter dipyridamole safety study. J Nucl Cardiol. 1995;2(1):3–17. doi: 10.1016/s1071-3581(05)80003-0. [DOI] [PubMed] [Google Scholar]
- 72.Iskandrian AE, Bateman TM, Belardinelli L, Blackburn B, Cerqueira MD, Hendel RC, et al. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: Results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol. 2007;14(5):645–658. doi: 10.1016/j.nuclcard.2007.06.114. [DOI] [PubMed] [Google Scholar]
- 73.Geleijnse ML, Elhendy A, Fioretti PM, Roelandt JR. Dobutamine stress myocardial perfusion imaging. J Am Coll Cardiol. 2000;36(7):2017–2027. doi: 10.1016/s0735-1097(00)01012-3. [DOI] [PubMed] [Google Scholar]
- 74.Miller DD. Physiologic and pharmacologic stressors. In: Dilsizian V, Narula J, Braunwald E, editors. Atlas of Nuclear Cardiology. 4thth ed. New York: Springer; 2013. pp. 111–144. [Google Scholar]
- 75.Issa A, De Lorenzo A, Oliveira B, Pellini M, Lima R. Comparison between accelerated and conventional dobutamine stress protocols for myocardial perfusion scintigraphy. Int J Cardiovasc Imaging. 2012;28(7):1823–1828. doi: 10.1007/s10554-011-0002-4. [DOI] [PubMed] [Google Scholar]
- 76.Nkoulou R, Wolfrum M, Pazhenkottil AP, Fiechter M, Buechel RR, Gaemperli O, et al. Gated SPECT myocardial perfusion imaging with cadmium-zinc-telluride detectors allows real-time assessment of dobutamine-stress-induced wall motion abnormalities. J Nucl Cardiol. 2018 Jan 06; doi: 10.1007/s12350-018-1187-x. Epub ahead print. [DOI] [PubMed] [Google Scholar]
- 77.Vitola JV, Brambatti JC, Caligaris F, Lesse CR, Nogueira PR, Joaquim AI, et al. Exercise supplementation to dipyridamole prevents hypotension, improves electrocardiogram sensitivity and increases heart-to-liver ratio on Tc-99m sestamibi imaging. J Nucl Cardiol. 2001;8(6):652–659. doi: 10.1067/mnc.2001.117204. [DOI] [PubMed] [Google Scholar]
- 78.Iskandrian AE, Bateman TM, Belardinelli L, Blackburn B, Cerqueira MD, Hendel RC, et al. ADVANCE MPI Investigators Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol. 2007;14(5):645–658. doi: 10.1016/j.nuclcard.2007.06.114. [DOI] [PubMed] [Google Scholar]
- 79.Dilsizian V, Gewirtz H, Paivanas N, Kitsiou AN, Hage FG, Crone NE, et al. Serious and potentially life-threatening complications of cardiac stress testing: Physiological mechanisms and management strategies. J Nucl Cardiol. 2015;22(6):1198–1213. doi: 10.1007/s12350-015-0141-4. [DOI] [PubMed] [Google Scholar]
- 80.Gould KL, Lipscomb K. Effects of coronary stenoses on coronary flow reserve and resistance. Am J Cardiol. 1974;34(1):48–55. doi: 10.1016/0002-9149(74)90092-7. [DOI] [PubMed] [Google Scholar]
- 81.Johnson NP, Kirkeeide RL, Gould KL. same lesion, different artery, different FFR!? JACC Cardiovasc Imaging. 2018;(17):31154–31153. doi: 10.1016/j.jcmg.2017.11.029. [DOI] [PubMed] [Google Scholar]
- 82.Johnson NP, Gould LK. Fractional flow reserve returns to its origins. quantitative cardiac positron emission tomography. Circ Cardiovasc Imaging. 2016;9(9):e005435. doi: 10.1161/CIRCIMAGING.116.005435. [DOI] [PubMed] [Google Scholar]
- 83.Zimmermann FM, Ferrara A, Johnson NP, van Nunen LX, Escaned J, Albertsson P, et al. Deferral vs. performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial. Eur Heart J. 2015;36(45):3182–3188. doi: 10.1093/eurheartj/ehv452. [DOI] [PubMed] [Google Scholar]
- 84.van Nunen LX, Zimmermann FM, Tonino PA, Barbato E, Baumbach A, Engstrøm T, et al. FAME Study Investigators Fractional flow reserve versus angiography for guidance of PCI in patients with multivessel coronary artery disease (FAME): 5-year follow-up of a randomized controlled trial. Lancet. 2015;386(10006):1853–1860. doi: 10.1016/S0140-6736(15)00057-4. [DOI] [PubMed] [Google Scholar]
- 85.De Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino P, Piroth Z, et al. FAME 2 Trial Investigators Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med. 2014;371(13):1208–1217. doi: 10.1056/NEJMoa1408758. [DOI] [PubMed] [Google Scholar]
- 86.Johnson NP, Tóth GG, Lai D, Zhu H, Açar G, Agostoni P, et al. Prognostic value of fractional flow reserve: linking physiologic severity to clinical outcomes. J Am Coll Cardiol. 2014;64(16):1641–1654. doi: 10.1016/j.jacc.2014.07.973. [DOI] [PubMed] [Google Scholar]
- 87.Mustafa SJ, Morrison RR, Teng B, Pelleg A. Adenosine receptors and the heart: role in regulation of coronary blood flow and cardiac electrophysiology. Handb Exp Pharmacol. 2009;(193):161–188. doi: 10.1007/978-3-540-89615-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chan SY, Brunken RC, Czernin J, Porenta G, Kuhle W, Krivokapich J, et al. Comparison of maximal myocardial blood flow during adenosine infusion with that of intravenous dipyridamole in normal men. J Am Coll Cardiol. 1992;20(4):979–985. doi: 10.1016/0735-1097(92)90201-w. [DOI] [PubMed] [Google Scholar]
- 89.Chareonthaitawee P, Askew JW, Heller GV, Downey BC. Overview of stress radionuclide myocardial perfusion imaging. UpToDate; 2018. [2018 Dec 15]. Available from: https://www.uptodate.com/contents/overview-of-stress-radionuclide-myocardial-perfusion-imaging. [Google Scholar]
- 90.Lima RS, Watson DD, Goode AR, Siadaty MS, Ragosta M, Beller GA, et al. Incremental value of combined perfusion and function over perfusion alone by gated SPECT myocardial perfusion imaging for detection of severe three-vessel coronary artery disease. J Am Coll Cardiol. 2003;42(1):64–70. doi: 10.1016/s0735-1097(03)00562-x. [DOI] [PubMed] [Google Scholar]
- 91.Marwick TH, Shaw LJ, Lauer MS, Kesler K, Hachamovitch R, Heller GV, et al. The noninvasive prediction of cardiac mortality in men and women with known or suspected coronary artery disease. Economics of Noninvasive Diagnosis (END) Study Group. Am J Med. 1999;106(2):172–178. doi: 10.1016/s0002-9343(98)00388-x. [DOI] [PubMed] [Google Scholar]
- 92.Hachamovitch R, Berman DS, Shaw LJ, Kiat H, Cohen I, Cabico JA, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97(6):535–543. doi: 10.1161/01.cir.97.6.535. Errata em: Circulation. 1998;98(2):190. [DOI] [PubMed] [Google Scholar]
- 93.Brown KA. Prognostic value of thallium-201myocardial perfusion imaging. A diagnostic tool comes of age. Circulation. 1991;83(2):363–381. doi: 10.1161/01.cir.83.2.363. [DOI] [PubMed] [Google Scholar]
- 94.Bateman TM, O’Keefe JH Jr, Dong VM, Barnhart C, Ligon RW. Coronary angiographic rates after stress single-photon emission computed tomographic scintigraphy. J Nucl Cardiol. 1995;2(3):217–223. doi: 10.1016/s1071-3581(05)80058-3. [DOI] [PubMed] [Google Scholar]
- 95.Miller TD, Christian TF, Hodge DO, Mullan BP, Gibbons RJ. Prognostic value of exercise thallium-201 imaging performed within 2 years of coronary artery bypass graft surgery. J Am Coll Cardiol. 1998;31(4):848–854. doi: 10.1016/s0735-1097(98)00011-4. [DOI] [PubMed] [Google Scholar]
- 96.Eagle KA, Rihal CS, Mickel MC, Holmes DR, Foster ED, Gersh BJ. Cardiac risk of noncardiac surgery: influence of coronary disease and type of surgery in 3368 operations. CASS investigators and university of michigan heart care program. Coronary Artery Surgery Study. Circulation. 1997;96(6):1882–1887. doi: 10.1161/01.cir.96.6.1882. [DOI] [PubMed] [Google Scholar]
- 97.Udelson JE, Dilsizian V, Bonow RO. In: Nuclear Cardiology. Molecular imaging of the cardiovascular system. 10thth ed. Mann DL, Zipes DP, Libby P, Bonow RO, Braunwald's E, editors. Philadelphia: Elsevier; 2015. pp. 311–315. [Google Scholar]
- 98.Ottenhof MJ, Wai MC, Boiten HJ, Korbee RS, Valkema R, van Domburg RT, Schinkel AF. 12-Year outcome after normal myocardial perfusion SPECT in patients with known coronary artery disease. J Nucl Cardiol. 2013;20(5):748–754. doi: 10.1007/s12350-013-9713-3. [DOI] [PubMed] [Google Scholar]
- 99.Navare SM, Mather J, Shaw LJ, Fowler MS, Heller GV. Comparison of risk stratification with pharmacologic and exercise stress myocardial perfusion imaging: A meta-analysis. J Nucl Cardiol. 2004;11(5):551–556. doi: 10.1016/j.nuclcard.2004.06.128. [DOI] [PubMed] [Google Scholar]
- 100.Slim HB, Nair SU, Arora S, Heller GV. Does location matter? Prognostic value of single-photon emission computed tomography myocardial perfusion imaging by vascular territory. J Nucl Cardiol. 2012;19(3):458–464. doi: 10.1007/s12350-011-9486-5. [DOI] [PubMed] [Google Scholar]
- 101.Klocke FJ, Baird MG, Lorell BH, Bateman TM, Messer JV, Berman DS, et al. American College of Cardiology. American Heart Association. American Society for Nuclear Cardiology ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging-executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging) Circulation. 2003;108(11):1404–1418. doi: 10.1161/01.CIR.0000080946.42225.4D. [DOI] [PubMed] [Google Scholar]
- 102.Shaw LJ, Iskandrian AE. Prognostic value of stress gated SPECT in patients with known or suspected coronary artery disease. J Nucl Cardiol. 2004;11(2):171–185. doi: 10.1016/j.nuclcard.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 103.Shaw LJ, Berman DS, Blumenthal RS, Budoff MJ, Faber TL, Goraya T, et al. Clinical imaging for prevention: Directed strategies for improved detection of presymptomatic patients with undetected atherosclerosis-Part I: Clinical imaging for prevention. J Nucl Cardiol. 2008;15(1):e6–19. doi: 10.1016/j.nuclcard.2007.11.008. Errata em: J Nucl Cardiol. 2008;15(4):609. [DOI] [PubMed] [Google Scholar]
- 104.Ross R, Blair SN, Arena R, Church TS, Després JP, Franklin BA, et al. American Heart Association Physical Activity. Committee of the Council on Lifestyle and Cardiometabolic Health. Council on Clinical Cardiology. Council on Epidemiology and Prevention. Council on Cardiovascular and Stroke Nursing. Council on Functional Genomics and Translational Biology. Stroke Council Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134(24):e653–e699. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 105.Forman DE, Arena R, Boxer R, Dolansky MA, Eng JJ, Fleg JL, et al. American Heart Association Council on Clinical Cardiology. Council on Cardiovascular and Stroke Nursing. Council on Quality of Care and Outcomes Research. Stroke Council Prioritizing functional capacity as a principal end point for therapies oriented to older adults with CVAS disease: A scientific statement for healthcare professionals from the American Heart Association. Circulation. 2017;135:e894–e918. doi: 10.1161/CIR.0000000000000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, et al. American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology. Council on Nutrition, Physical Activity and Metabolism. Council on Cardiovascular and Stroke Nursing. Council on Epidemiology and Prevention Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128(8):873–934. doi: 10.1161/CIR.0b013e31829b5b44. [DOI] [PubMed] [Google Scholar]
- 107.Cremer P, Hachamovitch R, Tamarappoo B. Clinical decision making with myocardial perfusion imaging in patients with known or suspected coronary artery disease. Semin Nucl Med. 2014;44(4):320–329. doi: 10.1053/j.semnuclmed.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.ISCHEMIA Trial Research Group. Maron DJ, Hochman JS, O’Brien SM, Reynolds HR, Boden WE, et al. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial: Rationale and design. Am Heart J. 2018 Jul;201:124–135. doi: 10.1016/j.ahj.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moroi M, Yamashina A, Tsukamoto K, Nishimura T, J-ACCESS Investigators Coronary revascularization does not decrease cardiac events in patients with stable ischemic heart disease but might do in those who showed moderate to severe ischemia. Int J Cardiol. 2012;158(2):246–252. doi: 10.1016/j.ijcard.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 110.Hachamovitch R, Berman DS, Kiat H, Cohen I, Cabico JA, Friedman J, et al. Exercise myocardial perfusion SPECT in patients without known coronary artery disease: Incremental prognostic value and use in risk stratification. Circulation. 1996;93(5):905–914. doi: 10.1161/01.cir.93.5.905. [DOI] [PubMed] [Google Scholar]
- 111.Xie JX, Winchester DE, Phillips LM, Hachamovitch R, Berman DS, Blankstein R, et al. The elusive role of myocardial perfusion imaging in stable ischemic heart disease: Is ISCHEMIA the answer? J Nucl Cardiol. 2017;24(5):1610–1618. doi: 10.1007/s12350-017-0963-3. [DOI] [PubMed] [Google Scholar]
- 112.Gada H, Kirtane AJ, Kereiakes DJ, Bangalore S, Moses JW, Généreux P, et al. Meta-analysis of trials on mortality after percutaneous coronary intervention compared with medical therapy in patients with stable coronary heart disease and objective evidence of myocardial ischemia. Am J Cardiol. 2015;115(9):1194–1199. doi: 10.1016/j.amjcard.2015.01.556. [DOI] [PubMed] [Google Scholar]
- 113.De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, et al. FAME 2 Trial Investigators Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367(11):991–1001. doi: 10.1056/NEJMoa1205361. [DOI] [PubMed] [Google Scholar]
- 114.Petraco R, van de Hoef TP, Nijjer S, Sen S, van Lavieren MA, Foale RA, et al. Baseline instantaneous wave-free ratio as a pressure-only estimation of underlying coronary flow reserve: results of the JUSTIFY-CFR Study (Joined Coronary Pressure and Flow Analysis to Determine Diagnostic Characteristics of Basal and Hyperemic Indices of Functional Lesion Severity-Coronary Flow Reserve) Circ Cardiovasc Interv. 2014;7(4):492–502. doi: 10.1161/CIRCINTERVENTIONS.113.000926. [DOI] [PubMed] [Google Scholar]
- 115.Feher A, Sinusas AJ. Quantitative Assessment of Coronary Microvascular Function: Dynamic Single-Photon Emission Computed Tomography, Positron Emission Tomography, Ultrasound, Computed Tomography, and Magnetic Resonance Imaging. Circ Cardiovasc Imaging. 2017;10(8):e006427. doi: 10.1161/CIRCIMAGING.117.006427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Berman DS, Germano G. Berman DS, Germano G. Clinical Gated Cardiac SPECT. New York: Futura Publishing Company; 1999. Clinical Applications Of Nuclear Cardiology; pp. 3–10. [Google Scholar]
- 117.Meneghelo RS, Araújo CGS, Stein R, Mastrocolla LE, Albuquerque PF, Serra SM, et al. Sociedade Brasileira de Cardiologia III Diretrizes da Sociedade Brasileira de Cardiologia sobre Teste Ergométrico. Arq Bras Cardiol. 2010;95(5) Suppl 1:1–26. doi: 10.1590/S0066-782X2010000800001. [DOI] [PubMed] [Google Scholar]
- 118.Diamond GA, Forrester JS. Analysis probability as an aid in the clinical diagnosis of coronary artery disease. N Engl J Med. 1979;300(24):1350–1358. doi: 10.1056/NEJM197906143002402. [DOI] [PubMed] [Google Scholar]
- 119.Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, et al. American College of Cardiology. American Heart Association Task Force on practice guidelines (Committee on the Management of Patients With Chronic Stable Angina) ACC/AHA 2002 guideline update for the management of patients with chronic stable angina--summary article: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on the Management of Patients With Chronic Stable Angina) J Am Coll Cardiol. 2003;41(1):159–168. doi: 10.1016/s0735-1097(02)02848-6. [DOI] [PubMed] [Google Scholar]
- 120.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. ESC Scientific Document Group 2016 European Guidelines on cardiovascular disease prevention in clinical practice. The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 2016;37(29):2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nicolau JC, Chalela WA, Meneghetti JC, Ximenes AA, de Almeida CA, Vítola JV, et al. Primeira Diretriz da Sociedade Brasileira de Cardiologia sobre Cardiologia Nuclear. Arq Bras Cardiol. 2002;78(Suppl 3):1–42. [PubMed] [Google Scholar]
- 122.Gupta NC, Esterbrooks DJ, Hilleman DE, Mohiuddin SM. Comparison of adenosine and exercise thallium -201 single-photon emission computed tomography (SPECT) myocardial perfusion imaging. The GE SPECT Multicenter Adenosine Study Group. J Am Coll Cardiol. 1992;19(2):248–257. doi: 10.1016/0735-1097(92)90474-2. [DOI] [PubMed] [Google Scholar]
- 123.DePuey EG, Corbett JR, Friedman JD, Goldstein RA, Henzlova MJ, Hansen CL, et al. Imaging guidelines for nuclear cardiology procedures. J Nucl Cardiol. 2006;13(6):e21–171. [Google Scholar]
- 124.Thompson RC, Patil H, Thompson EC, Thomas GS, Al-Amoodi M, Kennedy KF, et al. Regadenoson pharmacologic stress for myocardial perfusion imaging: A three-way comparison between regadenoson administered at peak exercise, during walk recovery, or no-exercise. J Nucl Cardiol. 2013;20(2):214–221. doi: 10.1007/s12350-012-9660-4. [DOI] [PubMed] [Google Scholar]
- 125.Versteylen MO, Joosen IA, Shaw LJ, Narula J, Hofstra L. Comparison of Framingham, PROCAM, SCORE, and Diamond Forrester to predict coronary atherosclerosis and cardiovascular events. Journal of Nuclear Cardiology. 2011;18(5):904–911. doi: 10.1007/s12350-011-9425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Faludi AA, Izar MCO, Saraiva JFK, Chacra AP, Bianco HT, Afiune Neto A, et al. Atualização da Diretriz Brasileira de dislipidemias e prevenção da aterosclerose - 2017. Arq Bras Cardiol. 2017;109(2) Suppl 1:1–76. doi: 10.5935/abc.20170121. [DOI] [PubMed] [Google Scholar]
- 127.Cheng VY, Berman DS, Rozanski A, Dunning AM, Achenbach S, Al-Mallah M, et al. Performance of the traditional age, sex, and angina typicality- based approach for estimating pretest probability of angiographically significant CAD in patients undergoing CCTA: results from the multinational coronary CT angiography evaluation for clinical outcomes: an international multicenter registry (CONFIRM) Circulation. 2011;124(22):2423–2432. doi: 10.1161/CIRCULATIONAHA.111.039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB Sr, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25) Suppl 2:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. Errata em: Circulation. 2014;129(25 Suppl 2):S74-5. [DOI] [PubMed] [Google Scholar]
- 129.Chaitman B. Exercise stress testing. In: Bonow RO, Mann DL, Zipes DP, Libby P, Braunwald E, editors. Braunwald's Heart Disease. A Textbook of Cardiovascular Medicine. 9th. Philadelphia: Elsevier; 2012. pp. 179–179. [Google Scholar]
- 130.Udelson JE, Dilsizian V, Bonow RO. Nuclear cardiology: disease dection risk stratification and clinical devision making. In: Bonow RO, Mann DL, Zipes DP, Libby P, Braunwald E, editors. Braunwald's Heart Disease. A Textbook of Cardiovascular Medicine. 10thth ed. Philadelphia: Elsevier; 2015. pp. 297–310. [Google Scholar]
- 131.Weintraub WS, Madeira SW Jr., Bodenheimer MM, Seelaus PA, Katz RI, Feldman MS, et al. Critical analysis of the application of Bayes’ theorem to sequential testing in the noninvasive diagnosis of coronary artery disease. Am J Cardiol. 1984;54(1):43–49. doi: 10.1016/0002-9149(84)90301-1. [DOI] [PubMed] [Google Scholar]
- 132.Patterson RE, Eng C, Horowitz SF. Practical diagnosis of coronary artery disease: a Bayes’ theorem nomogram to correlate clinical data with noninvasive exercise tests. Am J Cardiol. 1984;53(1):252–256. doi: 10.1016/0002-9149(84)90722-7. [DOI] [PubMed] [Google Scholar]
- 133.Zou KH, O'Malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115(5):654–657. doi: 10.1161/CIRCULATIONAHA.105.594929. [DOI] [PubMed] [Google Scholar]
- 134.Beller GA, Zaret BL. Contributions of nuclear cardiology to diagnosis and prognosis of patients with coronary artery disease. Circulation. 2000;101(12):1465–1478. doi: 10.1161/01.cir.101.12.1465. [DOI] [PubMed] [Google Scholar]
- 135.Vanzetto G, Ormezzano O, Fagret D, Comet M, Denis B, Machecourt J. Long term additive prognostic value of thallium 201 myocardial perfusion imaging over clinical and exercise stress test in low to intermediate risk patients. Study in 1137 patients with 6-year follow up. Circulation. 1999;100(14):1521–1527. doi: 10.1161/01.cir.100.14.1521. [DOI] [PubMed] [Google Scholar]
- 136.Cerci MS, Cerci JJ, Cerci RJ, Pereira Neto CC, Trindade E, Delbeke D, et al. Myocardial perfusion imaging is a strong predictor of death in women. JACC Cardiovasc Imaging. 2011;4(8):880–888. doi: 10.1016/j.jcmg.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 137.Katsikis A, Theodorakos A, Papaioannou S, Kalkinis A, Kolovou G, Konstantinou K, et al. Adenosine stress myocardial perfusion imaging in octogenarians: Safety, tolerability, and long-term prognostic implications of hemodynamic response and SPECT-related variables. J Nucl Cardiol. 2019;26(1):250–262. doi: 10.1007/s12350-017-0893-0. [DOI] [PubMed] [Google Scholar]
- 138.Nappi C, Nicolai E, Daniele S, Acampa W, Gaudieri V, Assante R, et al. Long-term prognostic value of coronary artery calcium scanning, coronary computed tomographic angiography and stress myocardial perfusion imaging in patients with suspected coronary artery disease. J Nucl Cardiol. 2018;25(3):833–841. doi: 10.1007/s12350-016-0657-2. [DOI] [PubMed] [Google Scholar]
- 139.Duarte PS, Smanio PE, Oliveira CA, Martins LR, Mastrocolla LE, Pereira JC. Clinical significance of transient left ventricular dilation assessed during myocardial Tc-99m sestamibi scintigraphy. Arq Bras Cardiol. 2003;81(5):474–482. doi: 10.1590/s0066-782x2003001300004. [DOI] [PubMed] [Google Scholar]
- 140.Mazzanti M, Germano G, Kiat H, Kavanagh PB, Alexanderson E, Friedman JD. Identification of severe and extensive coronary artery disease by automatic measurement of transient ischemic dilation of the left ventricule in dual-isotope myocardial perfusion SPECT. J Am Coll Cardiol. 1996;27(7):1612–1620. doi: 10.1016/0735-1097(96)00052-6. [DOI] [PubMed] [Google Scholar]
- 141.Williams KA, Schneider CM. Increased stress right ventricular activity on dual isotope perfusion SPECT. J Am Coll Cardiol. 1999;34(2):420–427. doi: 10.1016/s0735-1097(99)00193-x. [DOI] [PubMed] [Google Scholar]
- 142.Ko KY, Wu YW, Liu CJ, Cheng MF, Yen RF, Tzen KY. Clinical significance of right ventricular activity on treadmill thallium-201 myocardial single-photon emission computerized tomography using cadmium-zinc-telluride cameras. Nucl Med Commun. 2016;37(6):650–657. doi: 10.1097/MNM.0000000000000494. [DOI] [PubMed] [Google Scholar]
- 143.Hendel RC, Berman DS, Di Carli MF, Heidenreich PA, Henkin RE, Pellikka PA, et al. ACCF/ASNC/ACR/AHA/ASE/ SCCT/ SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine: endorsed by the American College of Emergency Physicians. Circulation. 2009;119(22):e561–e587. doi: 10.1161/CIRCULATIONAHA.109.192519. [DOI] [PubMed] [Google Scholar]
- 144.Wolk MJ, Bailey SR, Doherty JU, Douglas PS, Hendel RC, Kramer CM, et al. ACCF/AHA/ASE/ASNC/HFSA /HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;63(4):380–406. doi: 10.1016/j.jacc.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 145.Cherry SR, Sorenson JA, Phelps ME. Physics in nuclear medicine. 3rdrd ed. Philadelphia: Saunders/Elsevier; 2012. Tracer kinetic modeling; pp. 379–406. Chap 21. [Google Scholar]
- 146.Patton JA. Delbeke D, Vitola JV. Nuclear cardiology & correlative imaging. A teaching file. New York: Springer-Verlag; 2004. Physics principles and instrumentation in nuclear cardiology; pp. 24–26. [Google Scholar]
- 147.Garcia EV, Galt R, Faber TL, Chen J. Principles of nuclear cardiology imaging. In: Dilsizian V, Narula J, editors. Atlas of nuclear cardiology. 3rdrd ed. Switzerland: Springer; 2009. pp. 1–53. [Google Scholar]
- 148.Ruzsics B, Lee H, Zwerner PL, Gebregziabher M, Costello P, Schoepf UJ. Dual-energy CT of the heart for diagnosing coronary artery stenosis and myocardial ischemia-initial experience. Eur Radiol. 2008;18(11):2414–2424. doi: 10.1007/s00330-008-1022-x. [DOI] [PubMed] [Google Scholar]
- 149.Ficaro EP, Lee BC, Kritzman JN, Corbett JR. Corridor DM: The Michigan method for quantitative nuclear cardiology. J Nucl Cardiol. 2007;14(1):455–465. doi: 10.1016/j.nuclcard.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 150.Germano G, Kavanagh PB, Slomka PJ, Van Kriekinge SD, Pollard G, Berman DS. Quantitation in gated perfusion SPECT imaging: The Cedars-Sinai approach. J Nucl Cardiol. 2007;14(4):433–454. doi: 10.1016/j.nuclcard.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 151.Garcia EV, Faber TL, Cooke CD, Folks RD, Chen J, Santana C. The increasing role of quantification in nuclear cardiology: the Emory approach. J Nucl Cardiol. 2007;14(4):420–432. doi: 10.1016/j.nuclcard.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 152.Mastrocola F, Mastrocola LE. Lapa Santos EC, Figuinha FC, Lima AG, Henares BB, Mastrocola F. Manual de Cardiologia Cardiopapers. Rio de Janeiro: Atheneu; 2013. Cintilografia do Miocárdio; pp. 317–330. [Google Scholar]
- 153.Pitts SR, Niska RW, Xu J, Burt CW. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. Natl Health Stat Report. 2008;6(7):1–38. [PubMed] [Google Scholar]
- 154.American Heart Association Exercise, Cardiac Rehabilitation. Prevention Committee of the Council on Clinical Cardiology. Council on Cardiovascular Nursing. Interdisciplinary Council on Quality of Care and Outcomes Research. Amsterdam EA, Kirk JD, Bluemke DA, Diercks D, Farkouh ME, Garvey JL. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation. 2010;122(17):1756–1776. doi: 10.1161/CIR.0b013e3181ec61df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(25):e344–e426. doi: 10.1161/CIR.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 156.ESC Scientific Document Group. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2016;37(3):267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 157.Working Group on Cardiovascular Pharmacotherapy. Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio AL, et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J. 2017;38(3):143–153. doi: 10.1093/eurheartj/ehw149. [DOI] [PubMed] [Google Scholar]
- 158.Pasupathy S, Tavella R, Beltrame JF. Myocardial Infarction With Nonobstructive Coronary Arteries (MINOCA) The Past, Present, and Future Management. Circulation. 2017;135(16):1490–1493. doi: 10.1161/CIRCULATIONAHA.117.027666. [DOI] [PubMed] [Google Scholar]
- 159.Beltrame JF. Assessing patients with myocardial infarction and nonobstructed coronary arteries (MINOCA) J Intern Med. 2013;273(2):182–185. doi: 10.1111/j.1365-2796.2012.02591.x. [DOI] [PubMed] [Google Scholar]
- 160.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368(21):2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 161.Crea F, Libby P. Acute coronary syndromes: The way forward from mechanisms to precision treatment. Circulation. 2017;136(12):1155–1166. doi: 10.1161/CIRCULATIONAHA.117.029870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39(2):119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 163.O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, et al. American College of Cardiology Foundation. American Heart Association Task Force on Practice Guidelines. American College of Emergency Physicians. Society for Cardiovascular Angiography and Interventions 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the American College of Emergency Physicians and Society for Cardiovascular Angiography and Interventions. Circulation. 2013;127(4):e362–e425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 164.Amsterdam EA, Kirk JD, Diercks DB, Lewis WR, Turnipseed SD. Immediate exercise testing to evaluate low-risk patients presenting to the emergency department with chest pain. J Am Coll Cardiol. 2002;40(2):251–256. doi: 10.1016/s0735-1097(02)01968-x. [DOI] [PubMed] [Google Scholar]
- 165.Meneghelo RS, Araújo CG, Stein R, Mastrocolla LE, Albuquerque PF, Serra SM, et al. Sociedade Brasileira de Cardiologia III Diretrizes da Sociedade Brasileira de Cardiologia sobre teste ergométrico. Arq Bras Cardiol. 2010;95(5) Suppl 1:1–26. doi: 10.1590/S0066-782X2010000800001. [DOI] [PubMed] [Google Scholar]
- 166.Peels CH, Visser CA, Kupper AJ, Visser FC, Roos JP. Usefulness of two-dimensional echocardiography for immediate detection of myocardial ischemia in the emergency room. Am J Cardiol. 1990;65(11):687–691. doi: 10.1016/0002-9149(90)90143-o. [DOI] [PubMed] [Google Scholar]
- 167.Sabia P, Abbott RD, Afrookteh A, Keller MW, Touchstone DA, Kaul S. Importance of two-dimensional echocardiographic assessment of left ventricular systolic function in patients presenting to the emergency room with cardiac-related symptoms. Circulation. 1991;84(4):1615–1624. doi: 10.1161/01.cir.84.4.1615. [DOI] [PubMed] [Google Scholar]
- 168.Sabia P, Afrookteh A, Touchstone DA, Keller MW, Esquivel L, Kaul S. Value of regional wall motion abnormality in the emergency room diagnosis of acute myocardial infarction: a prospective study using two-dimensional echocardiography. Circulation. 1991;84(3) Suppl:I85–I92. [PubMed] [Google Scholar]
- 169.Meyersohn NM, Szilveszter B, Staziaki PV, Scholtz JE, Takx RAP, Hoffmann U, et al. Coronary CT angiography in the emergency department utilizing second and third generation dual source CT. J Cardiovasc Comput Tomogr. 2017;11(4):249–257. doi: 10.1016/j.jcct.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tesche C, Caruso D, De Cecco CN, Shuler DC, Rames JD, Albrecht MH, et al. Coronary computed tomography angiography-derived plaque quantification in patients with acute coronary syndrome. Am J Cardiol. 2017;119(5):712–718. doi: 10.1016/j.amjcard.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 171.Dedic A, Nieman K, Hoffmann U, Ferencik M. Is there still a role for cardiac CT in the emergency department in the era of highly sensitive troponins? Minerva Cardioangiol. 2017;65(3):214–224. doi: 10.23736/S0026-4725.16.04291-2. [DOI] [PubMed] [Google Scholar]
- 172.Ghoshhajra BB, Takx RA, Staziaki PV, Vadvala H, Kim P, Neilan TG, et al. MGH Emergency Cardiac CTA Program Contributors. Clinical implementation of an emergency department coronary computed tomographic angiography protocol for triage of patients with suspected acute coronary syndrome. Eur Radiol. 2017;27(7):2784–2793. doi: 10.1007/s00330-016-4562-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hoffmann U, Truong QA, Schoenfeld DA, Chou ET, Woodard PK, Nagurney JT, et al. ROMICAT-II Investigators Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367(4):299–308. doi: 10.1056/NEJMoa1201161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.de la Mora Cervantes R, Dennie C. Triple rule-out cardiac computed tomography: is it finally a reality? Minerva Cardioangiol. 2017;65(3):225–234. doi: 10.23736/S0026-4725.16.04272-9. [DOI] [PubMed] [Google Scholar]
- 175.Udelson JE, Beshansky JR, Ballin DS, Feldman JA, Griffith JL, Handler J, et al. Myocardial perfusion imaging for evaluation and triage of patients with suspected acute cardiac ischemia: a randomized controlled trial. Send to JAMA. 2002;288(21):2693–2700. doi: 10.1001/jama.288.21.2693. [DOI] [PubMed] [Google Scholar]
- 176.Kontos MC, Schmidt KL, McCue M, Rossiter LF, Jurgensen M, Nicholson CS, et al. A comprehensive strategy for the evaluation and triage of the chest pain patient: a cost comparison study. J Nucl Cardiol. 2003;10(3):284–290. doi: 10.1016/s1071-3581(03)00361-1. [DOI] [PubMed] [Google Scholar]
- 177.Radensky PW, Hilton TC, Fulmer H, McLaughlin BA, Stowers SA. Potential cost effectiveness of initial myocardial perfusion imaging for assessment of emergency department patients with chest pain. Am J Cardiol. 1997;79(5):595–599. doi: 10.1016/s0002-9149(96)00822-3. [DOI] [PubMed] [Google Scholar]
- 178.Stowers SA, Eisenstein EL, Th Wackers FJ, Berman DS, Blackshear JL, Jones AD Jr, et al. An economic analysis of an aggressive diagnostic strategy with single photon emission computed tomography myocardial perfusion imaging and early exercise stress testing in emergency department patients who present with chest pain but nondiagnostic electrocardiograms: results from a randomized trial. Ann Emerg Med. 2000;35(1):17–25. doi: 10.1016/S0196-0644(00)70100-4. [DOI] [PubMed] [Google Scholar]
- 179.Wackers FJ, Brown KA, Heller GV, Kontos MC, Tatum JL, Udelson JE, et al. American Society of Nuclear Cardiology position statement on radionuclide imaging in patients with suspected acute ischemic syndromes in the emergency department or chest pain center. J Nucl Cardiol. 2002;9(2):246–250. doi: 10.1067/mnc.2002.122630. [DOI] [PubMed] [Google Scholar]
- 180.Schaeffer MW, Brennan TD, Hughes JA, Gibler WB, Gerson MC. Resting radionuclide myocardial perfusion imaging in a chest pain center including an overnight delayed image acquisition protocol. J Nucl Med Technol. 2007;35(4):242–245. doi: 10.2967/jnmt.107.042796. [DOI] [PubMed] [Google Scholar]
- 181.Delaney MC, Neth M, Thomas JJ. Chest pain triage: Current trends in the emergency departments in the United States. J Nucl Cardiol. 2017;24(6):2004–2011. doi: 10.1007/s12350-016-0578-0. [DOI] [PubMed] [Google Scholar]
- 182.Nabi F, Chang SM, Xu J, Gigliotti E, Mahmarian JJ. Assessing risk in acute chest pain: The value of stress myocardial perfusion imaging in patients admitted through the emergency department. J Nucl Cardiol. 2012;19(2):233–243. doi: 10.1007/s12350-011-9484-7. [DOI] [PubMed] [Google Scholar]
- 183.Cremer PC, Khalaf S, Agarwal S, Mayer-Sabik E, Ellis SG, Menon V, et al. Myocardial perfusion imaging in emergency department patients with negative cardiac biomarkers: yield for detecting ischemia, short-term events, and impact of downstream revascularization on mortality. Circ Cardiovasc Imaging. 2014;7(6):912–919. doi: 10.1161/CIRCIMAGING.114.002401. [DOI] [PubMed] [Google Scholar]
- 184.Better N, Karthikeyan G, Vitola J, Fatima A, Peix A, Novak MD, et al. Performance of rest myocardial perfusion imaging in the management of acute chest pain in the emergency room in developing nations (PREMIER trial) J Nucl Cardiol. 2012;19(6):1146–1153. doi: 10.1007/s12350-012-9622-x. [DOI] [PubMed] [Google Scholar]
- 185.Jain D, Thompson B, Wackers FJ, Zaret BL. Relevance of increased lung thallium uptake on stress imaging in patients with unstable angina and non-Q wave myocardial infarction: results of the Thrombolysis in Myocardial Infarction (TIMI)-IIIB Study. J Am Coll Cardiol. 1997;30(2):421–429. doi: 10.1016/s0735-1097(97)00164-2. [DOI] [PubMed] [Google Scholar]
- 186.Kapetanopoulos A, Heller GV, Selker HP, Ruthazer R, Beshansky JR, Feldman JA, et al. Acute resting myocardial perfusion imaging in patients with diabetes mellitus: results from the Emergency Room Assessment of Sestamibi for Evaluation of Chest Pain (ERASE Chest Pain) trial. J Nucl Cardiol. 2004;11(5):570–577. doi: 10.1016/j.nuclcard.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 187.Klocke FJ, Baird MG, Lorell BH, Bateman TM, Messer JV, Berman DS, et al. American College of Cardiology. American Heart Association. American Society for Nuclear Cardiology. American College of Cardiology. American Heart Association Task Force on Practice Guidelines. American Society for Nuclear Cardiology ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging) J Am Coll Cardiol. 2003;42(7):1318–1333. doi: 10.1016/j.jacc.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 188.Hendel RC, Berman DS, Di Carli MF, Heidenreich PA, Henkin RE, Pellikka PA, et al. American College of Cardiology Foundation Appropriate Use Criteria Task Force. American Society of Nuclear Cardiology. American College of Radiology. American Heart Association. American Society of Echocardiology. Society of Cardiovascular Computed Tomography. Society for Cardiovascular Magnetic Resonance. Society of Nuclear Medicine ACCF/ASNC/ACR/AHA/ASE /SCCT/SCMR/SNM 2009. Appropriate Use Criteria for Cardiac Radionuclide Imaging: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. J Am Coll Cardiol. 2009;53(23):2201–2229. doi: 10.1016/j.jacc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 189.Osborne AD, Moore B, Ross MA, Pitts SR. The feasibility of Rubidium-82 positron emission tomography stress testing in low-risk chest pain protocol patients. Crit Pathw Cardiol. 2011;10(1):41–43. doi: 10.1097/HPC.0b013e31820d6a2e. [DOI] [PubMed] [Google Scholar]
- 190.Klocke FJ, Baird MG, Lorell BH, Bateman TM, Messer JV, Berman DS, et al. American College of Cardiology. American Heart Association. American Society for Nuclear Cardiology ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging) J Am Coll Cardiol. 2003;42(7):1318–1333. doi: 10.1016/j.jacc.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 191.Chalela WA, Camargo EE, Marin-Neto JA, Meneghetti JC, Barroso AA, Ximenes AA, et al. Sociedade Brasileira de Cardiologia Atualização da Diretriz da Sociedade Brasileira de Cardiologia sobre Cardiologia Nuclear. Arq Bras Cardiol. 2006;86(Suppl 1):1–61. [PubMed] [Google Scholar]
- 192.Hachamovitch R, Berman DS, Shaw LJ, Kiat H, Cohen I, Cabico JA. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97(6):535–543. doi: 10.1161/01.cir.97.6.535. Erratum in Circulation. 1998;98(2):190. [DOI] [PubMed] [Google Scholar]
- 193.Klocke FJ, Baird MG, Lorell BH, Bateman TM, Messer JV, Berman DS, et al. American College of Cardiology. American Heart Association. American Society for Nuclear Cardiology ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging-executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging) Circulation. 2003;108(11):1404–1418. doi: 10.1161/01.CIR.0000080946.42225.4D. [DOI] [PubMed] [Google Scholar]
- 194.Marwick TH, Shaw LJ, Lauer MS, Kesler K, Hachamovitch R, Heller GV, et al. The noninvasive prediction of cardiac mortality in men and women with known or suspected coronary artery disease. Economics of Noninvasive Diagnosis (END) Study Group. Am J Med. 1999;106(2):172–178. doi: 10.1016/s0002-9343(98)00388-x. [DOI] [PubMed] [Google Scholar]
- 195.Mastrocola F, Mastrocola LE. Lapa Santos EC, Figuinha FC, Lima AG, Henares BB, Mastrocola F. Manual de cardiologia Cardiopapers. São Paulo: Atheneu; 2013. Cintilografia do miocárdio; pp. 317–330. [Google Scholar]
- 196.Udelson JE, Dilsizian V, Bonow RO. Nuclear cardiology: appropriate use criteria. In: Bonow RO, Mann DL, Zipes DP, Libby P, Bonow R, Braunwald E, editors. Braunwald's heart disease: a textbook of cardiovascular medicine. 10th ed. Philadelphia: Elsevier; 2018. pp. 316–319. [Google Scholar]
- 197.Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, Dorbala S, et al. ASNC imaging guidelines/ SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol. 2016;23(5):1187–1226. doi: 10.1007/s12350-016-0522-3. [DOI] [PubMed] [Google Scholar]
- 198.Gould KL, Schelbert HR, Phelps ME, Hoffman EJ. Noninvasive assessment of coronary stenoses with myocardial perfusion imaging during pharmacologic coronary vasodilatation. V. Detection of 47 percent diameter coronary stenosis with intravenous nitrogen-13 ammonia and emission-computed tomography in intact dogs. Am J Cardiol. 1979;43(2):200–208. doi: 10.1016/s0002-9149(79)80005-3. [DOI] [PubMed] [Google Scholar]
- 199.Schelbert HR, Henze E, Phelps ME, Kuhl DE. Assessment of regional myocardial ischemia by positron-emission computed tomography. Pt 2Am Heart J. 1982;103(4):588–597. doi: 10.1016/0002-8703(82)90462-8. [DOI] [PubMed] [Google Scholar]
- 200.Schelbert HR, Wisenberg G, Phelps ME, Gould KL, Henze E, Hoffman EJ, et al. Noninvasive assessment of coronary stenoses by myocardial imaging during pharmacologic coronary vasodilation. VI. Detection of coronary artery disease in human beings with intravenous N-13 ammonia and positron computed tomography. Am J Cardiol. 1982;49(5):1197–1207. doi: 10.1016/0002-9149(82)90045-5. [DOI] [PubMed] [Google Scholar]
- 201.Bateman TM, Heller GV, McGhie AI, Friedman JD, Case JA, Bryngelson JR, et al. Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET: comparison with ECG-gated Tc-99m sestamibi SPECT. J Nucl Cardiol. 2006;13(1):24–33. doi: 10.1016/j.nuclcard.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 202.Sampson UK, Dorbala S, Limaye A, Kwong R, Di Carli MF. Diagnostic accuracy of rubidium-82 myocardial perfusion imaging with hybrid positron emission tomography/computed tomography in the detection of coronary artery disease. J Am Coll Cardiol. 2007;49(10):1052–1058. doi: 10.1016/j.jacc.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 203.Ziadi MC, Dekemp RA, Williams KA, Guo A, Chow BJ, Renaud JM, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol. 2011;58(7):740–748. doi: 10.1016/j.jacc.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 204.Beanlands RS, Nichol G, Huszti E, Humen D, Racine N, Freeman M, et al. F-18-fluorodeoxyglucose positron emission tomography imaging-assisted management of patients with severe left ventricular dysfunction and suspected coronary disease: a randomized, controlled trial (PARR-2) J Am Coll Cardiol. 2007;50(20):2002–2012. doi: 10.1016/j.jacc.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 205.Ghosh N, Rimoldi OE, Beanlands RS, Camici PG. Assessment of myocardial ischaemia and viability: role of positron emission tomography. Eur Heart J. 2010;31(24):2984–2995. doi: 10.1093/eurheartj/ehq361. [DOI] [PubMed] [Google Scholar]
- 206.Marwick TH, MacIntyre WJ, Lafont A, Nemec JJ, Salcedo EE. Metabolic responses of hibernating and infarcted myocardium to revascularization. A follow-up study of regional perfusion, function, and metabolism. Circulation. 1992;85(4):1347–1353. doi: 10.1161/01.cir.85.4.1347. [DOI] [PubMed] [Google Scholar]
- 207.Schinkel AF, Bax JJ, Poldermans D, Elhendy A, Ferrari R, Rahimtoola SH. Hibernating myocardium: diagnosis and patient outcomes. Curr Probl Cardiol. 2007;32(7):375–410. doi: 10.1016/j.cpcardiol.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 208.Allman KC, Wieland DM, Muzik O, Degrado TR, Wolfe ER Jr, Schwaiger M. Carbon-11 hydroxyephedrine with positron emission tomography for serial assessment of cardiac adrenergic neuronal function after acute myocardial infarction in humans. J Am Coll Cardiol. 1993;22(2):368–375. doi: 10.1016/0735-1097(93)90039-4. [DOI] [PubMed] [Google Scholar]
- 209.Hartmann F, Ziegler S, Nekolla S, Hadamitzky M, Seyfarth M, Richardt G, et al. Regional patterns of myocardial sympathetic denervation in dilated cardiomyopathy: an analysis using carbon-11 hydroxyephedrine and positron emission tomography. Heart. 1999;81(3):262–270. doi: 10.1136/hrt.81.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mc Ardle BA, Leung E, Ohira H, Cocker MS, deKemp RA, DaSilva J, et al. The role of F(18)-fluorodeoxyglucose positron emission tomography in guiding diagnosis and management in patients with known or suspected cardiac sarcoidosis. J Nucl Cardiol. 2013;20(2):297–306. doi: 10.1007/s12350-012-9668-9. [DOI] [PubMed] [Google Scholar]
- 211.Tahara N, Imaizumi T, Virmani R, Narula J. Clinical feasibility of molecular imaging of plaque inflammation in atherosclerosis. J Nucl Med. 2009;50(3):331–334. doi: 10.2967/jnumed.108.060376. [DOI] [PubMed] [Google Scholar]
- 212.Yamagishi H, Shirai N, Takagi M, Yoshiyama M, Akioka K, Takeuchi K, et al. Identification of cardiac sarcoidosis with (13)N-NH(3)/(18)F-FDG PET. J Nucl Med. 2003;44(7):1030–1036. [PubMed] [Google Scholar]
- 213.Padilha BG, Sabino D, Giorgi MC, Soares J Jr, Izaki M, Meneghetti JC. Case Report: Multivessel Coronary Disease Assessment with SPECT 99mTc-Sestamibi and Rubidium-82 PET/CT. Arq Bras Cardiol. 2017;108(1):87–90. doi: 10.5935/abc.20160198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Senthamizhchelvan S, Bravo PE, Lodge MA, Merrill J, Bengel FM, Sgouros G. Radiation dosimetry of 82Rb in humans under pharmacologic stress. J Nucl Med. 2011;52(3):485–491. doi: 10.2967/jnumed.110.083477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chow BJ, Beanlands RS, Lee A, DaSilva JN, deKemp RA, Alkahtani A, et al. Treadmill exercise produces larger perfusion defects than dipyridamole stress N-13 ammonia positron emission tomography. J Am Coll Cardiol. 2006;47(2):411–416. doi: 10.1016/j.jacc.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 216.Huisman MC, Higuchi T, Reder S, Nekolla SG, Poethko T, Wester HJ, et al. Initial characterization of an 18F-labeled myocardial perfusion tracer. J Nucl Med. 2008;49(4):630–636. doi: 10.2967/jnumed.107.044727. [DOI] [PubMed] [Google Scholar]
- 217.Maddahi J. Properties of an ideal PET perfusion tracer: new PET tracer cases and data. J Nucl Cardiol. 2012;19(Suppl 1):S30–S37. doi: 10.1007/s12350-011-9491-8. [DOI] [PubMed] [Google Scholar]
- 218.Nekolla SG, Reder S, Saraste A, Higuchi T, Dzewas G, Preissel A, et al. Evaluation of the novel myocardial perfusion positron-emission tomography tracer 18F-BMS-747158-02: comparison to 13N-ammonia and validation with microspheres in a pig model. Circulation. 2009;119(17):2333–2342. doi: 10.1161/CIRCULATIONAHA.108.797761. [DOI] [PubMed] [Google Scholar]
- 219.Yu M, Guaraldi MT, Mistry M, Kagan M, McDonald JL, Drew K, et al. BMS-747158-02: a novel PET myocardial perfusion imaging agent. J Nucl Cardiol. 2007;14(6):789–798. doi: 10.1016/j.nuclcard.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 220.Ziadi MC, Beanlands RS. The clinical utility of assessing myocardial blood flow using positron emission tomography. J Nucl Cardiol. 2010;17(4):571–581. doi: 10.1007/s12350-010-9258-7. [DOI] [PubMed] [Google Scholar]
- 221.Mc Ardle BA, Dowsley TF, deKemp RA, Wells GA, Beanlands RS. Does rubidium-82 PET have superior accuracy to SPECT perfusion imaging for the diagnosis of obstructive coronary disease? A systematic review and meta-analysis. J Am Coll Cardiol. 2012;60(18):1828–1837. doi: 10.1016/j.jacc.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 222.Parker MW, Iskandar A, Limone B, Perugini A, Kim H, Jones C, et al. Diagnostic accuracy of cardiac positron emission tomography versus single photon emission computed tomography for coronary artery disease: a bivariate meta-analysis. Circ Cardiovasc Imaging. 2012;5(6):700–707. doi: 10.1161/CIRCIMAGING.112.978270. [DOI] [PubMed] [Google Scholar]
- 223.Stewart RE, Schwaiger M, Molina E, Popma J, Gacioch GM, Kalus M, et al. Comparison of rubidium-82 positron emission tomography and thallium-201 SPECT imaging for detection of coronary artery disease. Am J Cardiol. 1991;67(16):1303–1310. doi: 10.1016/0002-9149(91)90456-u. [DOI] [PubMed] [Google Scholar]
- 224.Parkash R, de Kemp RA, Ruddy TD, Kitsikis A, Hart R, Beauchesne L, et al. Potential utility of rubidium 82 PET quantification in patients with 3-vessel coronary artery disease. J Nucl Cardiol. 2004;11(4):440–449. doi: 10.1016/j.nuclcard.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 225.Ziadi MC, Dekemp RA, Williams K, Guo A, Renaud JM, Chow BJ, et al. Does quantification of myocardial flow reserve using rubidium-82 positron emission tomography facilitate detection of multivessel coronary artery disease? J Nucl Cardiol. 2012;19(4):670–680. doi: 10.1007/s12350-011-9506-5. [DOI] [PubMed] [Google Scholar]
- 226.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’ t Veer M, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 227.Johnson NP, Gould KL. Integrating noninvasive absolute flow, coronary flow reserve, and ischemic thresholds into a comprehensive map of physiological severity. JACC Cardiovasc Imaging. 2012;5(4):430–440. doi: 10.1016/j.jcmg.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 228.Johnson NP, Kirkeeide RL, Gould KL. Is discordance of coronary flow reserve and fractional flow reserve due to methodology or clinically relevant coronary pathophysiology? JACC Cardiovasc Imaging. 2012;5(2):193–202. doi: 10.1016/j.jcmg.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 229.Dorbala S, Di Carli MF, Beanlands RS, Merhige ME, Williams BA, Veledar E, et al. Prognostic value of stress myocardial perfusion positron emission tomography: results from a multicenter observational registry. J Am Coll Cardiol. 2013;61(2):176–184. doi: 10.1016/j.jacc.2012.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Adenaw N, Salerno M. PET/MRI: current state of the art and future potential for cardiovascular applications. J Nucl Cardiol. 2013;20(6):976–989. doi: 10.1007/s12350-013-9780-5. [DOI] [PubMed] [Google Scholar]
- 231.Heller GV, Calnon D, Dorbala S. Recent advances in cardiac PET and PET/CT myocardial perfusion imaging. J Nucl Cardiol. 2009;16(6):962–969. doi: 10.1007/s12350-009-9142-5. [DOI] [PubMed] [Google Scholar]
- 232.Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Hainer J, et al. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015;131(1):19–27. doi: 10.1161/CIRCULATIONAHA.114.011939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Task Force Members. Montalescot G, Sechtem U, Achenbach S, Andreotti F, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 234.Vítola JV. A need to reduce premature CV mortality in the developing world: How could appropriate use of non-invasive imaging help? J Nucl Cardiol. 2019;26(3):975–985. doi: 10.1007/s12350-018-01526-6. [DOI] [PubMed] [Google Scholar]
- 235.Vítola JV, Wanderley MR Jr, Cerci RJ, Pereira Neto CC, Kormann O, Neto OF, et al. Outcome of patients with high-risk Duke treadmill score and normal myocardial perfusion imaging on spect. J Nucl Cardiol. 2016;23(6):1291–1300. doi: 10.1007/s12350-015-0156-x. [DOI] [PubMed] [Google Scholar]
- 236.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2006;113(1):30–37. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 237.Shaw LJ, Giambrone AE, Blaha MJ, Knapper JT, Berman DS, Bellam N, et al. Long-Term Prognosis After Coronary Artery Calcification Testing in Asymptomatic Patients: A Cohort Study. Ann Intern Med. 2015;163(1):14–21. doi: 10.7326/M14-0612. [DOI] [PubMed] [Google Scholar]
- 238.Kelkar AA, Schultz WM, Khosa F, Schulman-Marcus J, O’Hartaigh BW, Gransar H, et al. Long-Term prognosis after coronary artery calcium scoring among low-intermediate risk women and men. Circ Cardiovasc Imaging. 2016;9(4):e003742. doi: 10.1161/CIRCIMAGING.115.003742. [DOI] [PubMed] [Google Scholar]
- 239.Chang SM, Nabi F, Xu J, Pratt CM, Mahmarian AC, Frias ME, et al. Value of CACS compared with ETT and myocardial perfusion imaging for predicting long-term cardiac outcome in asymptomatic and symptomatic patients at low risk for coronary disease: clinical implications in a multimodality imaging world. JACC Cardiovasc Imaging. 2015;8(2):134–144. doi: 10.1016/j.jcmg.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 240.Cho I, Chang HJ, Ó Hartaigh B, Shin S, Sung JM, Lin FY, et al. Incremental prognostic utility of coronary CT angiography for asymptomatic patients based upon extent and severity of coronary artery calcium: results from the Coronary CT Angiography EvaluatioN For Clinical Outcomes InteRnational Multicenter (CONFIRM) study. Eur Heart J. 2015;36(8):501–508. doi: 10.1093/eurheartj/ehu358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Rana JS, Rozanski A, Berman DS. Combination of myocardial perfusion imaging and coronary artery calcium scanning: potential synergies for improving risk assessment in subjects with suspected coronary artery disease. Curr Atheroscler Rep. 2011;13(5):381–389. doi: 10.1007/s11883-011-0192-1. [DOI] [PubMed] [Google Scholar]
- 242.Cerci RJ, Zapparoli M, Cunha C, Cerci JJ, Kormann OJ, Stier AL Jr, et al. P26 Coronary calcium score ability to predict ischemia in patients from a developing nation. Moderated Posters Session- Ischaemia Detection. Eur Heart J- Cardiovasc Imaging. 2017;18(1):i7–i9. [Google Scholar]
- 243.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, COURAGE Trial Research Group Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356(15):1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 244.BARI 2D Study Group. Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360(24):2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107(23):2900–2907. doi: 10.1161/01.CIR.0000072790.23090.41. [DOI] [PubMed] [Google Scholar]
- 246.Stone GW, Hochman JS, Williams DO, Boden WE, Ferguson TB Jr, Harrington RA, et al. Medical Therapy With Versus Without Revascularization in Stable Patients With Moderate and Severe Ischemia: The Case for Community Equipoise. J Am Coll Cardiol. 2016;67(1):81–99. doi: 10.1016/j.jacc.2015.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, PROMISE Investigators et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372(14):1291–1300. doi: 10.1056/NEJMoa1415516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Karthikeyan G, Guzic SB, Jug B, Devasenapathy N, Alexanderson E, Vitola J, et al. Functional compared to anatomical imaging in the initial evaluation of patients with suspected coronary artery disease: An international, multi-center, randomized controlled trial (IAEA-SPECT/CTA study) J Nucl Cardiol. 2017;24(2):507–517. doi: 10.1007/s12350-016-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Hulten EA, Carbonaro S, Petrillo SP, Mitchell JD, Villines TC. Prognostic value of cardiac computed tomography angiography: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;57(10):1237–1247. doi: 10.1016/j.jacc.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 250.Taqueti VR, Shaw LJ, Cook NR, Murthy VL, Shah NR, Foster CR, et al. Excess cardiovascular risk in women relative to men referred for coronary angiography is associated with severely impaired coronary flow reserve, not obstructive disease. Circulation. 2017;135(6):566–577. doi: 10.1161/CIRCULATIONAHA.116.023266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Bittencourt MS, Hulten E, Ghoshhajra B, O'Leary D, Christman MP, Montana P, et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging. 2014;7(2):282–291. doi: 10.1161/CIRCIMAGING.113.001047. [DOI] [PubMed] [Google Scholar]
- 252.Cerci RJ, Zanin H, Zapparoli M, Zier SS, Cunha C, Cerci JJ, et al. P122 clinical management, safety and cost impact of performing coronary computed tomography angiography in patients with mild ischemia by SPECT-MPI. Poster session I, European Heart Journal - Cardiovascular Imaging. 2017;18(1):i20–i44. [Google Scholar]
- 253.Bittencourt MS, Hulten EA, Murthy VL, Cheezum M, Rochitte CE, Di Carli MF, et al. Clinical outcomes after evaluation of stable chest pain by coronary computed tomographic angiography versus usual care: A meta-analysis. Circ Cardiovasc Imaging. 2016;9(4):e004419. doi: 10.1161/CIRCIMAGING.115.004419. [DOI] [PubMed] [Google Scholar]
- 254.National Institute for Health and Care Excellence . Clinical Guideline(CG95) London: 2016. Chest pain of recent onset: assessment and diagnosis of recent onset. [PubMed] [Google Scholar]
- 255.Dewey FE, Kapoor JR, Williams RS, Lipinski MJ, Ashley EA, Hadley D, et al. Ventricular arrhythmias during clinical treadmill testing and prognosis. Arch Intern Med. 2008;168(2):225–234. doi: 10.1001/archinte.168.2.225. [DOI] [PubMed] [Google Scholar]
- 256.Al-Mallah MH, Qureshi W, Lin FY, Achenbach S, Berman DS, Budoff MJ, et al. Does coronary CT angiography improve risk stratification over coronary calcium scoring in symptomatic patients with suspected coronary artery disease? Results from the prospective multicenter international CONFIRM registry. Eur Heart J Cardiovasc Imaging. 2014;15(3):267–274. doi: 10.1093/ehjci/jet148. [DOI] [PubMed] [Google Scholar]
- 257.Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ, et al. CONFIRM Investigators Age and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (CoronaryCT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol. 2011;58(8):849–860. doi: 10.1016/j.jacc.2011.02.074. [DOI] [PubMed] [Google Scholar]
- 258.Pepine CJ, Ferdinand KC, Shaw LJ, Light-McGroary KA, Shah RU, Gulati M, et al. ACC CVD in Women Committee Emergence of nonobstructive coronary artery disease: a woman's problem and need for change in definition on angiography. J Am Coll Cardiol. 2015;66(17):1918–1933. doi: 10.1016/j.jacc.2015.08.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Taqueti VR, Shaw LJ, Cook NR, Murthy VL, Shah NR, Foster CR, et al. Excess cardiovascular risk in women relative to men referred for coronary angiography is associated with severely impaired coronary flow reserve, not obstructive disease. Circulation. 2017;135(6):566–577. doi: 10.1161/CIRCULATIONAHA.116.023266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Taqueti VR, Dorbala S, Wolinsky D, Abbott B, Heller GV, Bateman TM, et al. Myocardial perfusion imaging in women for the evaluation os stable ischemic heart disease. State-of-the-evidence and clinical recommendations. J Nucl Cardiol. 2017;24(4):1402–1426. doi: 10.1007/s12350-017-0926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Jamiel A, Ebid M, Ahmed AM, Ahmed D, Al-Mallah MH. The role of myocardial viability in contemporary cardiac practice. Heart Fail Rev. 2017;22(4):401–413. doi: 10.1007/s10741-017-9626-3. [DOI] [PubMed] [Google Scholar]
- 262.Udelson JE, Dilsizian V, Bonow RO. Nuclear cardiology: Assement of myocardial cellular metabolism and physiology. In: Bonow RO, Mann DL, Zipes DP, Libby P, Braunwald E, editors. Braunwald's Heart Disease. A textbook of cardiovascular medicine. 10th. Philadelphia: Elsevier; 2015. pp. 292–295. [Google Scholar]
- 263.Rahimtoola SH, Dilsizian V, Kramer CM, Marwick TH, Vanoverschelde JL. Chronic ischemic left ventricular dysfunction: from pathophysiology to imaging and its integration into clinical practice. JACC Cardiovasc Imaging. 2008;1(4):536–555. doi: 10.1016/j.jcmg.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Topol EJ, Weiss JL, Guzman PA, Dorsey-Lima S, Blanck TJ, Humphrey LS, et al. Immediate improvement of dysfunctional myocardial segments after coronary revascularization: detection by intraoperative transesophageal echocardiography. J Am Coll Cardiol. 1984;4(6):1123–1134. doi: 10.1016/s0735-1097(84)80131-x. [DOI] [PubMed] [Google Scholar]
- 265.Jennings RB, Murry CE, Reimer KA. Energy metabolism in preconditioned and control myocardium: effect of total ischemia. J Mol Cell Cardiol. 1991;23(12):1449–1458. doi: 10.1016/0022-2828(91)90190-w. [DOI] [PubMed] [Google Scholar]
- 266.Hendel RC, Berman DS, Di Carli MF, Heidenreich PA, Henkin RE, Pellikka PA, et al. ACCF/ASNC/ACR/AHA/ASE/ SCCT/ SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine: endorsed by the American College of Emergency Physicians. Circulation. 2009;119(22):e561–e587. doi: 10.1161/CIRCULATIONAHA.109.192519. [DOI] [PubMed] [Google Scholar]
- 267.Mastrocola LE, Lopes RW, Boccia D, Alves FB. Cardiologia Nuclear: Princípios. In: Moreira MC, Montenegro ST, Paola AA, editors. Livro texto da Sociedade Brasileira de Cardiologia. 2ªª ed. São Paulo: Manole; 2015. pp. 750–758. [Google Scholar]
- 268.Klocke FJ, Baird MG, Bateman TM, Berman DS, Carabello BA, Cerqueira MD, et al. American College of Cardiology. American Heart Association. American Society for Nuclear Cardiology ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging - executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (ACC/AHA/ASNC Committee to revise the 1995 guidelines for the clinical use of cardiac radionuclide imaging) Circulation. 2003;108(11):1404–1418. doi: 10.1161/01.CIR.0000080946.42225.4D. [DOI] [PubMed] [Google Scholar]
- 269.Wang W, Li X, Tian C, Zhao S, Hacker M, Zhang X. Cardiac death in patients with left ventricular aneurysm, remodeling and myocardial viability by gated 99mTc-MIBI SPECT and gated 18F-FDG PET. Int J Cardiovasc Imaging. 2018;34(3):485–493. doi: 10.1007/s10554-017-1234-8. [DOI] [PubMed] [Google Scholar]
- 270.Kusunose K, Obuchowski NA, Gillinov M, Popovic ZB, Flamm SD, Griffin BP, et al. Predictors of Mortality in Patients With Severe Ischemic Cardiomyopathy Undergoing Surgical Mitral Valve Intervention. J Am Heart Assoc. 2017;6(11):1–9. doi: 10.1161/JAHA.117.007163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Bax JJ, Delgado V. Myocardial viability as integral part of the diagnostic and therapeutic approach to ischemic heart failure. J J Nucl Cardiol. 2015;22(2):229–245. doi: 10.1007/s12350-015-0096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Gheorghiade M, Sopko G, De LL, Velazquez EJ, Parker JD, Binkley PF, et al. Navigating the crossroads of coronary artery disease and heart failure. Circulation. 2006;114(11):1202–1213. doi: 10.1161/CIRCULATIONAHA.106.623199. [DOI] [PubMed] [Google Scholar]
- 273.Redwood SR, Ferrari R, Marber MS. Myocardial hibernation and stunning: from physiological principles to clinical practice. Heart. 1998;80(3):218–222. doi: 10.1136/hrt.80.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Bito V, van der Velden J, Claus P, Dommke C, Van Lommel A, Mortelmans L, et al. Reduced force generating capacity in myocytes from chronically ischemic, hibernating myocardium. Circ Res. 2007;100(2):229–237. doi: 10.1161/01.RES.0000257829.07721.57. [DOI] [PubMed] [Google Scholar]
- 275.Shabana A, El-Menyar A. Myocardial viability: What we knew and what is new. Cardiol Res Pract. 2012;2012:607486–607486. doi: 10.1155/2012/607486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Kaandorp TA, Lamb HJ, van der Wall EE, de Roos A, Bax JJ. Cardiovascular MR to access myocardial viability in chronic ischaemic LV dysfunction. Heart. 2005;91:1359–1365. doi: 10.1136/hrt.2003.025353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Bax JJ, Wijns W, Cornel JH, Visser FC, Boersma E, Fioretti PM. Accuracy of currently available techniques for prediction of functional recovery after revascularization in patients with left ventricular dysfunction due to chronic coronary artery disease: comparison of pooled data. J Am Coll Cardiol. 1997;30(6):1451–1460. doi: 10.1016/s0735-1097(97)00352-5. [DOI] [PubMed] [Google Scholar]
- 278.Dilszian V, Rocco TP, Freedman NM, Leon MB, Bonow RO. Enhanced detection but viable myocardium by the reinjection of thallium after stress: redistribution imaging. N Engl J Med. 1990;323(3):141–146. doi: 10.1056/NEJM199007193230301. [DOI] [PubMed] [Google Scholar]
- 279.Levine MG, McGill CC, Ahlberg AW, White MP, Giri S, Shareef B, et al. Functional assessment with electrocardiographic gated single-photon emission computed tomography improves the ability of technetium-99m sestamibi myocardial perfusion imaging to predict myocardial viability in patients undergoing revascularization. Am J Cardiol. 1999;83(1):1–5. doi: 10.1016/s0002-9149(98)00772-3. [DOI] [PubMed] [Google Scholar]
- 280.Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ. ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols and tracers. J Nucl Cardiol. 2016;23(3):640–642. doi: 10.1007/s12350-015-0387-x. [DOI] [PubMed] [Google Scholar]
- 281.Yang MF, Keng F, He ZX. Nitrate-augmented myocardial perfusion imaging for assessment of myocardial viability: recent advances. Nucl Med Commun. 2009;30(6):415–419. doi: 10.1097/MNM.0b013e32832b2ed1. [DOI] [PubMed] [Google Scholar]
- 282.Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, Dorbala S, et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol. 2016;23(5):1187–1226. doi: 10.1007/s12350-016-0522-3. [DOI] [PubMed] [Google Scholar]
- 283.Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, Gropler R, et al. ASNC imaging guidelines for nuclear cardiology procedures PET myocardial perfusion and metabolism clinical imaging. J Nucl Cardiol. 2009 Jun;16:651–651. [Google Scholar]
- 284.Hedgire SS, Osborne M, Verdini DJ, Ghoshhajra BB. Updates on stress imaging testing and myocardial viability with advanced imaging modalities. Curr Treat Options Cardiovasc Med. 2017;19(4):26–26. doi: 10.1007/s11936-017-0525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Beanlands RS, Nichol G, Huszti E, Humen D, Racine N, Freeman M, et al. F-18-fluorodeoxyglucose positron emission tomography imaging-assisted management of patients with severe left ventricular dysfunction and suspected coronary disease: a randomized, controlled trial (PARR-2) J Am Coll Cardiol. 2007;50:2002–2012. doi: 10.1016/j.jacc.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 286.Di Carli MF, Hachamovitch R, Berman DS. The art and science of predicting postrevascularization improvement in left ventricular (LV) function in patients with severely depressed LV function. JACC. 2002;40(10):1744–1747. doi: 10.1016/s0735-1097(02)02498-1. [DOI] [PubMed] [Google Scholar]
- 287.Allman KC, Shaw LJ, Hachamovitch R, Udelson JE. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol. 2002;39(7):1151–1158. doi: 10.1016/s0735-1097(02)01726-6. [DOI] [PubMed] [Google Scholar]
- 288.Bonow RO, Maurer G, Lee KL, Holly TA, Binkley PF, Desvigne-Nickens P, et al. STICH Trial Investigators Myocardial viability and survival in ischemic left ventricular diysfunction. N Engl J Med. 2011;364(17):1617–1625. doi: 10.1056/NEJMoa1100358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Patel H, Mazur W, Williams KA Sr, Kalra DK. Myocardial viability-State of the art: Is it still relevant and how to best assess it with imaging? Trends Cardiovasc Med. 2018;28(1):24–37. doi: 10.1016/j.tcm.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 290.Bhat A, Gan GC, Tan TC, Hsu C, Denniss AR. Myocardial Viability: From Proof of Concept to Clinical Practice. Cardiol Res Pract. 2016;2016:1020818–1020818. doi: 10.1155/2016/1020818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Gewirtz H, Dilsizian V. Myocardial Viability: Survival mechanisms and molecular imaging targets in acute and chronic ischemia. Circ Res. 2017;120(7):1197–1212. doi: 10.1161/CIRCRESAHA.116.307898. [DOI] [PubMed] [Google Scholar]
- 292.Klocke FJ, Baird MG, Lorell BH, Bateman TM, Messer JV, Berman DS, et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging-Executive Summary. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging) J Am Coll Cardiol. 2003;42(7):1318–1333. doi: 10.1016/j.jacc.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 293.Slomka PJ, Nishina H, Berman DS, Kang X, Friedman JD, Hayes SW, et al. Automatic quantification of myocardial perfusion stress-rest change: a new measure of ischemia. J Nucl Med. 2004;45(2):183–191. [PubMed] [Google Scholar]
- 294.Bocher M, Blevis IM, Tsukerman L, Shrem Y, Kovalski G, Volokh L. A fast cardiac gamma camera with dynamic SPECT capabilities: Design, system validation and future potential. Eur J Nucl Med Mol Imaging. 2010;37(10):1887–1902. doi: 10.1007/s00259-010-1488-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Buechel RR, Herzog BA, Husmann L, Burger IA, Pazhenkottil AP, Treyer V, et al. Ultrafast nuclear myocardial perfusion imaging on a new gamma camera with semiconductor detector technique: first clinical validation. Eur J Nucl Med Mol Imaging. 2010;37(4):773–778. doi: 10.1007/s00259-009-1375-7. Errata em: Eur J Nucl Med Mol Imaging. 2011;38(6):1172. [DOI] [PubMed] [Google Scholar]
- 296.Garcia EV, Faber TL, Esteves FP. Cardiac dedicated ultrafast SPECT cameras: New designs and clinical implications. J Nucl Med. 2011;52(2):210–217. doi: 10.2967/jnumed.110.081323. [DOI] [PubMed] [Google Scholar]
- 297.Duvall WL, Croft LB, Godiwala T, Ginsberg E, George T, Henzlova MJ. Reduced isotope dose with rapid SPECT MPI imaging: initial experience with a CZT camera. J Nucl Cardiol. 2010;17(6):1009–1014. doi: 10.1007/s12350-010-9215-5. [DOI] [PubMed] [Google Scholar]
- 298.Gimelli A, Bottai M, Genovesi D, Giorgetti A, Di Martino F, Marzullo P. High diagnostic accuracy of low-dose gated-SPECT with solid-state ultrafast detectors: preliminary clinical results. Eur J Nucl Med Mol Imaging. 2012;39(1):83–90. doi: 10.1007/s00259-011-1918-6. [DOI] [PubMed] [Google Scholar]
- 299.Hindorf C, Oddstig J, Hedeer F, Hansson MJ, Jögi J, Engblom H. Importance of correct patient positioning in myocardial perfusion SPECT when using a CZT camera. J Nucl Cardiol. 2014;21(4):695–702. doi: 10.1007/s12350-014-9897-1. [DOI] [PubMed] [Google Scholar]
- 300.Oldan JD, Shaw LK, Hofmann P, Phelan M, Nelson J, Pagnanelli R, et al. Prognostic value of the cadmium-zinc-telluride camera: A comparison with a conventional (anger) camera. J Nucl Cardiol. 2015;23(6):1280–1287. doi: 10.1007/s12350-015-0181-9. [DOI] [PubMed] [Google Scholar]
- 301.Lima R, Peclat T, Soares T, Ferreira C, Souza AC, Camargo G. Comparison of the prognostic value of myocardial perfusion imaging using a CZT-SPECT camera with a conventional anger camera. J Nucl Cardiol. 2016;24(1):245–251. doi: 10.1007/s12350-016-0618-9. [DOI] [PubMed] [Google Scholar]
- 302.Slomka PJ, Patton JA, Berman DS, Germano G. Advances in technical aspects of myocardial perfusion SPECT imaging. J Nucl Cardiol. 2009;16(2):255–276. doi: 10.1007/s12350-009-9052-6. [DOI] [PubMed] [Google Scholar]
- 303.Hudson HM, Larkin RS. Accelerated image reconstruction using ordered subsets of projection data. IEEE Trans Med Imaging. 1994;13(4):601–609. doi: 10.1109/42.363108. [DOI] [PubMed] [Google Scholar]
- 304.DePuey E, Gadiraju R, Clark J, Thompson L, Anstett F, Shwartz S. Ordered subset expectation maximization and wide beam reconstruction (WBR) ''half-time'' gated myocardial perfusion SPECT functional imaging: A comparison to ''full-time'' filtered back projection. J Nucl Cardiol. 2008;15(4):547–563. doi: 10.1016/j.nuclcard.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 305.Lima R, DeLorenzo A, Camargo G, Oliveira G, Reis T, Peclat T, et al. Prognostic value of myocardium perfusion imaging with a new reconstruction algorithm. J Nucl Cardiol. 2014;21(1):149–157. doi: 10.1007/s12350-013-9824-x. [DOI] [PubMed] [Google Scholar]
- 306.Falcão A, Chalela W, Giorgi MC, Imada R, Soares Jr J, Do Val R, et al. Myocardial blood flow assessment with 82rubidium-PET imaging in patients with left bundle branch block. Clinics. 2015;70(11):726–732. doi: 10.6061/clinics/2015(11)02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, et al. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol. 2009;54(2):150–156. doi: 10.1016/j.jacc.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 308.Ziadi MC, Dekemp RA, Williams KA, Guo A, Chow BJ, Renaud JM, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol. 2011;58(7):740–748. doi: 10.1016/j.jacc.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 309.Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124(20):2215–2224. doi: 10.1161/CIRCULATIONAHA.111.050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Majmudar MD, Murthy VL, Shah RV, Kolli S, Mousavi N, Foster CR, et al. Quantification of coronary flow reserve in patients with ischaemic and non-ischaemic cardiomyopathy and its association with clinical outcomes. Eur Heart J Cardiovasc Imaging. 2015;16(8):900–909. doi: 10.1093/ehjci/jev012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Cecchi F, Olivotto I, Gistri R, Lorenzoni R, Chiriatti G, Camici PG. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med. 2003;349(11):1027–1035. doi: 10.1056/NEJMoa025050. [DOI] [PubMed] [Google Scholar]
- 312.McArdle BA, Davies RA, Chen L, Small GR, Ruddy TD, Dwivedi G, et al. Prognostic value of rubidium-82 positron emission tomography in patients after heart transplant. Circ Cardiovasc Imaging. 2014;7(6):930–937. doi: 10.1161/CIRCIMAGING.114.002184. [DOI] [PubMed] [Google Scholar]
- 313.Ito Y, Katoh C, Noriyasu K, Kuge Y, Furuyama H, Morita K, et al. Estimation of myocardial blood flow and myocardial flow reserve by 99m-technetium sestamibi imaging: comparison with the results of 15 H2O PET. Eur J Nucl Med Mol Imaging. 2003;30(2):281–287. doi: 10.1007/s00259-002-1031-y. [DOI] [PubMed] [Google Scholar]
- 314.Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM. Long-term prognostic value of 13N-ammonia myocardial perfusion PET: added value of coronary flow reserve. J Am Coll Cardiol. 2009;54(2):150–156. doi: 10.1016/j.jacc.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 315.Wells RG, Timmins R, Klein R, Lockwood J, Marvin B, deKemp RA, et al. Dynamic SPECT measurement of absolute myocardial blood flow in a porcine model. J Nucl Med. 2014;55(10):1685–1691. doi: 10.2967/jnumed.114.139782. [DOI] [PubMed] [Google Scholar]
- 316.Ben Bouallègue F, Roubille F, Lattuca B, Cung TT, Macia JC, Gervasoni R, et al. SPECT myocardial perfusion reserve in patients with multivessel coronary disease: correlation with angiographic findings and invasive fractional flow reserve measurements. J Nucl Med Off Publ Soc Nucl Med. 2015;56(11):1712–1717. doi: 10.2967/jnumed.114.143164. [DOI] [PubMed] [Google Scholar]
- 317.Einstein AJ, Pascual TN, Mercuri M, Karthikeyan G, Vitola JV, Mahmarian JJ, et al. Incaps Investigators group Current worldwide nuclear cardiology practices and radiation exposure: Results from the 65 country IAEA nuclear cardiology protocols. Eur Heart J. 2015;36(26):1689–1696. doi: 10.1093/eurheartj/ehv117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Vitola JV, Mut F, Alexánderson E, Pascual TN, Mercuri M, Karthikeyan G, INCAPS Investigators et al. Opportunities for improvement on current nuclear cardiology practices and radiation exposure in Latin America: Findings from the 65-country IAEA Nuclear Cardiology Protocols Cross-sectional Study (INCAPS) J Nucl Cardiol. 2017;24(3):851–859. doi: 10.1007/s12350-016-0433-3. [DOI] [PubMed] [Google Scholar]
- 319.Mastrocola LE, Alves FB, Lopes RW. Exames de imagem e risco da exposição às radiações. conceitos fundamentais. Rev DERC. 2012;18(2):44–45. [Google Scholar]
- 320.Albuquerque AS, Mastrocola LE. Radiação e exames diagnósticos: qual o risco real ? Rev Soc Cardiol Estado de São Paulo. 2017;27(2):82–87. [Google Scholar]
- 321.Lima R, Peclat T, Soares T, Ferreira C, Souza AC, Camargo G. Comparison of the prognostic value of myocardial perfusion Imaging using a CZT-SPECT camera with a conventional anger camera. J Nucl Cardiol. 2017;24(1):245–251. doi: 10.1007/s12350-016-0618-9. [DOI] [PubMed] [Google Scholar]
- 322.Chirumamilla A, Travin MI. Cardiac applications of 123I-mIBG imaging. Semin Nucl Med. 2011;41(5):374–387. doi: 10.1053/j.semnuclmed.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 323.Carrió I. Cardiac neurotransmission imaging. J Nucl Med. 2001;42(7):1062–1076. [PubMed] [Google Scholar]
- 324.Petretta M, Pellegrino T, Cuocolo A. Cardiac neuronal imaging with 123I-meta-iodobenzylguanidine in heart failure: implications of endpoint selection and quantitative analysis on clinical decisions. Eur J Nucl Med Mol Imaging. 2014;41(9):1663–1665. doi: 10.1007/s00259-014-2827-2. [DOI] [PubMed] [Google Scholar]
- 325.Gaudino M, Giordano A, Santarelli P, Alessandrini F, Nori SL, Trani C, et al. Immunohistochemical-scintigraphic correlation of sympathetic cardiac innervation in postischemic left ventricular aneurysms. J Nucl Cardiol. 2002;9(6):601–607. doi: 10.1067/mnc.2002.126681. [DOI] [PubMed] [Google Scholar]
- 326.Stiles S. FDA Approves Heart Sympathetic Activity Imaging Agent for HF Evaluation. [2013 Feb 10]. Emedicine.medscape.com Available from: https://www.medscape.com/viewarticle/791544.
- 327.Angelidis G, Giamouzis G, Karagiannis G, Butler J, Tsougos L, Valotassiou V, et al. SPECT and PET in ischemic heart failure. Heart Fail Rev. 2017;22(2):243–261. doi: 10.1007/s10741-017-9594-7. [DOI] [PubMed] [Google Scholar]
- 328.Flotats A, Carrió I, Agostini D, Le Guludec D, Marcassa C, Schaffers M, et al. Proposal for standardization of 123I-metaiodobenzylguanidine (MIBG) cardiac sympathetic imaging by the EANM Cardiovascular Committee and the European Council of Nuclear Cardiology. Eur J Nucl Med Mol Imaging. 2010;37(9):1802–1812. doi: 10.1007/s00259-010-1491-4. [DOI] [PubMed] [Google Scholar]
- 329.Rocha ET, Alves WEFM, Verschure DO, Verbene HJ. Uso do 123I-MIBG cardíaco na rotina clínica: a necessidade de padronizar. Int J Cardiovasc Sci. 2017;30(6):533–541. [Google Scholar]
- 330.Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ. ASNC imaging guidelines for SPECT nuclear cardiology procedures: stress, protocols, and tracers. J Nucl Cardiol. 2016;23(3):606–639. doi: 10.1007/s12350-015-0387-x. [DOI] [PubMed] [Google Scholar]
- 331.Travin MI. Current clinical applications and next steps for cardiac innervation imaging. Curr Cardiol Rep. 2017;19(1):1–1. doi: 10.1007/s11886-017-0817-2. [DOI] [PubMed] [Google Scholar]
- 332.Morozumi T, Kusuoka H, Fukuchi K, Tani A, Uehara T, Matsuda S, et al. Myocardial iodine-123-metaiodobenzylguanidine images and autonomic nerve activity in normal subjects. J Nucl Med. 1997;38(1):49–52. [PubMed] [Google Scholar]
- 333.Tamaki S, Yamada T, Okuyama Y, Morita T, Sanada S, Tsukamoto Y, et al. Cardiac iodine-123 metaiodobenzylguanidine imaging predicts sudden cardiac death independently of left ventricular ejection fraction in patients with chronic heart failure and left ventricular systolic dysfunction: results from a comparative study with signal-averaged electrocardiogram, heart rate variability, and QT dispersion. J Am Coll Cardiol. 2009;53(5):426–435. doi: 10.1016/j.jacc.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 334.Nakata T, Nakajima K, Yamashina S, Yamada T, Momose M, Kasama S, et al. A pooled analysis of multicenter cohort studies of (123)I-mIBG imaging of sympathetic innervation for assessment of long-term prognosis in heart failure. JACC Cardiovasc Imaging. 2013;6(7):772–784. doi: 10.1016/j.jcmg.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 335.Verberne HJ, Brewster LM, Somsen GA, van Eck-Smit BL. Prognostic value of myocardial 123I-metaiodobenzylguanidine (MIBG) parameters in patients with heart failure: a systematic review. Eur Heart J. 2008;29(9):1147–1159. doi: 10.1093/eurheartj/ehn113. [DOI] [PubMed] [Google Scholar]
- 336.Agostini D, Verberne HJ, Burchert W, Knuuti J, Povinec P, Sambuceti G, et al. I-123-mIBG myocardial imaging for assessment of risk for a major cardiac event in heart failure patients: insights from a retrospective European multicenter study. Eur J Nucl Med Mol Imaging. 2008;35(3):535–546. doi: 10.1007/s00259-007-0639-3. [DOI] [PubMed] [Google Scholar]
- 337.Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, et al. Myocardial iodine-123 metaiodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView myocardial imaging for risk evaluation in heart failure) study. J Am Coll Cardiol. 2010;55(20):2212–2221. doi: 10.1016/j.jacc.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 338.Nakajima K, Nakata T. Cardiac 123I-MIBG Imaging for Clinical Decision Making: 22-Year Experience in Japan. J Nucl Med. 2015;56(Suppl 4):11S–119. doi: 10.2967/jnumed.114.142794. [DOI] [PubMed] [Google Scholar]
- 339.JCS Joint Working Group Guidelines for Clinical Use of Cardiac Nuclear Medicine (JCS 2010): digest version. Circ J. 2012;76(3):761–767. doi: 10.1253/circj.cj-88-0019. [DOI] [PubMed] [Google Scholar]
- 340.Toyama T, Aihara Y, Iwasaki T, Hasegawa A, Suzuki T, Nagai R, et al. Cardiac sympathetic activity estimated by 123I-MIBG myocardial imaging in patients with dilated cardiomyopathy after beta-blocker or angiotensin-converting enzyme inhibitor therapy. J Nucl Med. 1999;40(2):217–223. [PubMed] [Google Scholar]
- 341.Matsuo Y, Kasama S, Toyama T, Funada R, Takama N, Koitabashi N, et al. Comparative effects of long-acting and short-acting loop diuretics on cardiac sympathetic nerve activity in patients with chronic heart failure. Open Heart. 2016;3(1):e000276. doi: 10.1136/openhrt-2015-000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Nishioka SA, Martinelli Filho M, Brandão SC, Giorgi MC, Vieira ML, Costa R, et al. Cardiac sympathetic activity pre and post resynchronization therapy evaluated by 123I-MIBG myocardial scintigraphy. J Nucl Cardiol. 2007;14(6):852–859. doi: 10.1016/j.nuclcard.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 343.Ambardekar AV, Buttrick PM. Reverse remodeling with left ventricular assist devices: a review of clinical, cellular, and mechanistic effects. Circ Heart Fail. 2011;4(2):224–233. doi: 10.1161/CIRCHEARTFAILURE.110.959684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Drakos SG, Athanasoulis T, Malliaras KG, Terrovitis JV, Diakos N, Koudoumas G, et al. Myocardial sympathetic innervation and long-term left ventricular mechanical unloading. J Am Coll Cardiol Img. 2010;3(1):64–70. doi: 10.1016/j.jcmg.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 345.Buendia-Fuentes F, Almenar L, Ruiz C, Vercher JL, Sánchez-Lázaro I, Martínez-Dolz L, et al. Sympathetic reinnervation 1 year after heart transplantation, assessed using iodine-123 metaiodobenzylguanidine. Transplant Proc. 2011;43(6):2247–2248. doi: 10.1016/j.transproceed.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 346.Brandão SC, Giorgi MC, Nishioka SA, Meneghetti JC. Papel da medicina nuclear na terapia de ressincronização cardíaca. Rev bras ecocardiogr imagem cardiovasc. 2011;24(4):62–72. [Google Scholar]
- 347.Arora R, Ferrick KJ, Nakata T, Kaplan RC, Rozengarten M, Latif F, et al. I-123 MIBG imaging and heart rate variability analysis to predict the need for an implantable cardioverter defibrillator. J Nucl Cardiol. 2003;10(2):121–131. doi: 10.1067/mnc.2003.2. [DOI] [PubMed] [Google Scholar]
- 348.Verschure DO, de Groot JR, Mirzaei S, Gheysens O, Nakajima K, van Eck-Smit BLF. Cardiac 123I-mIBG scintigraphy is associated with freedom of appropriate ICD therapy in stable chronic heart failure patients. Int J Cardiol. 2017 Dec;248:403–408. doi: 10.1016/j.ijcard.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 349.Wakabayashi T, Nakata T, Hashimoto A, Yuda S, Tsuchihashi K, Travin MI, et al. Assessment of underlying etiology and cardiac sympathetic innervation to identify patients at high risk of cardiac death. J Nucl Med. 2001;42(12):1757–1767. [PubMed] [Google Scholar]
- 350.Bello D, Fieno DS, Kim RJ, Pereles FS, Passman R, Song G, et al. Infarct morphology identifies patients with substrate for sustained ventricular tachycardia. J Am Coll Cardiol. 2005;45(7):1104–1108. doi: 10.1016/j.jacc.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 351.Zipes DP. Heart-brain interactions in cardiac arrhythmias: Role of the autonomic nervous system. Clev Clin J Med. 2008;75(Suppl 2):S94–S96. doi: 10.3949/ccjm.75.suppl_2.s94. [DOI] [PubMed] [Google Scholar]
- 352.Boogers MJ, Borleffs CJ, Henneman MM, van Bommel RJ, van Ramshorst J, Boersma E, et al. Cardiac sympathetic denervation assessed with 123-iodine metaiodobenzylguanidine imaging predicts ventricular arrhythmias in implantable cardioverter-defibrillator patients. J Am Coll Cardiol. 2010;55:2769–2777. doi: 10.1016/j.jacc.2009.12.066. [DOI] [PubMed] [Google Scholar]
- 353.Tamaki S, Yamada T, Okuyama Y, Morita T, Sanada S, Tsukamoto Y, et al. Cardiac iodine-123 metaiodobenzylguanidine imaging predicts sudden cardiac death independently of left ventricular ejection fraction in patients with chronic heart failure and left ventricular systolic dysfunction: results from a comparative study with signal-averaged electrocardiogram, heart rate variability, and QT dispersion. J Am Coll Cardiol. 2009;53(5):426–435. doi: 10.1016/j.jacc.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 354.Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, et al. ADMIRE-HF Investigators Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure: Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol. 2010;55(20):2212–2221. doi: 10.1016/j.jacc.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 355.Kuramoto Y, Yamada T, Tamaki S, Okuyama Y, Morita T, Furukawa Y, et al. Usefulness of cardiac iodine-123 meta-iodobenzylguanidine imaging to improve prognostic power of Seattle heart failure model in patients with chronic heart failure. Am J Cardiol. 2011;107(8):1185–1190. doi: 10.1016/j.amjcard.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 356.Silva MI, Ferreira MJ, Moreira AP. Utilização da cintilografia com iodo-123-metaiodobenzilguanidina na estratificação do risco de morte súbita an insuficiência cardíaca. Rev Port Cardiol. 2013;32(6):509–516. [Google Scholar]
- 357.Bax JJ, Kraft O, Buxton AE, Fjeld JG, Parízek P, Agostini D, et al. 123 I-mIBG scintigraphy to predict inducibility of ventricular arrhythmias on cardiac electrophysiology testing: a prospective multicenter pilot study. Circ Cardiovasc Imaging. 2008;1(2):131–140. doi: 10.1161/CIRCIMAGING.108.782433. [DOI] [PubMed] [Google Scholar]
- 358.Fallavollita JA, Heavey BM, Luisi AJ Jr, Michalek SM, Baldwa S, Mashtare TL Jr, et al. Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. J Am Coll Cardiol. 2014;63(2):141–149. doi: 10.1016/j.jacc.2013.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Rassi A Jr, Rassi SG, Rassi A. Sudden death in Chagas’ disease. Arq Bras Cardiol. 2001;76(1):75–96. doi: 10.1590/s0066-782x2001000100008. [DOI] [PubMed] [Google Scholar]
- 360.Rassi A Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375(9723):1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 361.Gadioli LP, Miranda CH, Pintya AO, de Figueiredo AB, Schmidt A, Maciel BC, et al. The severity of ventricular arrhythmia correlates with the extent of myocardial sympathetic denervation, but not with myocardial fibrosis extent in chronic Chagas cardiomyopathy: Chagas disease, denervation and arrhythmia. J Nucl Cardiol. 2018;25(1):75–83. doi: 10.1007/s12350-016-0556-6. [DOI] [PubMed] [Google Scholar]
- 362.Zipes DP. Influence of myocardial ischemia and infarction on autonomic innervation of heart. Circulation. 1990;82(4):1095–1105. doi: 10.1161/01.cir.82.4.1095. [DOI] [PubMed] [Google Scholar]
- 363.Mitrani RD, Klein LS, Miles WM, Hackett FK, Burt RW, Wellman HN, et al. Regional cardiac sympathetic denervation in patients with ventricular tachycardia in the absence of coronary artery disease. J Am Coll Cardiol. 1993;22(5):1344–1353. doi: 10.1016/0735-1097(93)90541-8. [DOI] [PubMed] [Google Scholar]
- 364.Wichter T, Matheja P, Eckardt L, Kies P, Schäfers K, Schulze-Bahr E, et al. Cardiac autonomic dysfunction in Brugada syndrome. Circulation. 2002;105(6):702–706. doi: 10.1161/hc0602.103677. [DOI] [PubMed] [Google Scholar]
- 365.Taki J, Nakajima K, Bunko H, Simizu M, Muramori A, Hisada K. Whole-body distribution of iodine 123 metaiodobenzylguanidine in hypertrophic cardiomyopathy: significance of its washout from the heart. Eur J Nucl Med. 1990;17(5):264–268. doi: 10.1007/BF00812368. [DOI] [PubMed] [Google Scholar]
- 366.Verschure DO, van Eck-Smit BL, Somsen GA, Verberne HJ. Cardiac sympathetic activity in hypertrophic cardiomyopathy and Tako-tsubo cardiomyopathy. Clin Transl Imaging. 2015;3(5):379–385. doi: 10.1007/s40336-015-0133-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Takahashi N, Ishida Y, Maeno M, Hirose Y, Kawano S, Fukuoka S, et al. Noninvasive identification of left ventricular involvements in arrhythmogenic right ventricular dysplasia: comparison of 123I-MIBG, 201TlCl, magnetic resonance imaging and ultrafast computed tomography. Ann Nucl Med. 1997;11(3):233–241. doi: 10.1007/BF03164769. [DOI] [PubMed] [Google Scholar]
- 368.Simoni LJ, Brandão SC. New Imaging Methods for Detection of Drug-Induced Cardiotoxicity in Cancer Patients. Curr Cardiovasc Imaging Rep. 2017;10(6):18–18. [Google Scholar]
- 369.Guimarães SL, Brandão SC, Andrade LR, Maia RJ, Markman Filho B. Cardiac sympathetic hyperactivity after chemotherapy: early sign of cardiotoxicity? Arq Bras Cardiol. 2015;105(3):228–234. doi: 10.5935/abc.20150075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Bulten BF, Verberne HJ, Bellersen L, Oyen WJ, Sabaté-Llobera A, Mavinkurve-Groothuis AM, et al. Relationship of promising methods in the detection of anthracycline-induced cardiotoxicity in breast cancer patients. Cancer Chemother Pharmacol. 2015;76(5):957–967. doi: 10.1007/s00280-015-2874-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Carrió I, Cowie MR, Yamazaki J, Udelson J, Camici PG. Cardiac sympathetic imaging with mIBG in heart failure. J Am Coll Cardiol Cardiovasc Imaging. 2010;3(1):92–100. doi: 10.1016/j.jcmg.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 372.Stevens MJ, Raffel DM, Allman KC, Dayanikli F, Ficaro E, Sandford T, et al. Cardiac sympathetic dysinnervation in diabetes: implications for enhanced cardiovascular risk. Circulation. 1998;98(10):961–968. doi: 10.1161/01.cir.98.10.961. [DOI] [PubMed] [Google Scholar]
- 373.Fadi G. Hage, Ami E. Iskandrian. Cardiac Autonomic Denervation in Diabetes Mellitus. Circ Cardiovasc Imaging. 2011 Mar;4:79–81. doi: 10.1161/CIRCIMAGING.111.963967. [DOI] [PubMed] [Google Scholar]
- 374.Bengel FM, Ueberfuhr P, Schiepel N, Nekolla SG, Reichart B, Schwaiger M. Effect of sympathetic reinnervation on cardiac performance after heart transplantation. N Engl J Med. 2001;345(10):731–738. doi: 10.1056/NEJMoa010519. [DOI] [PubMed] [Google Scholar]
- 375.Bybee KA, Kara T, Prasad A, Lerman A, Barsness GW, Wright RS, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004;141(11):858–865. doi: 10.7326/0003-4819-141-11-200412070-00010. [DOI] [PubMed] [Google Scholar]
- 376.Sharkey SW, Lesser JR, Zenovich AG, Maron MS, Lindberg J, Longe TF, et al. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005;111(4):472–479. doi: 10.1161/01.CIR.0000153801.51470.EB. [DOI] [PubMed] [Google Scholar]
- 377.Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006;27(13):1523–1529. doi: 10.1093/eurheartj/ehl032. [DOI] [PubMed] [Google Scholar]
- 378.Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352(6):539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 379.Akashi YJ, Nakazawa K, Sakakibara M, Miyake F, Musha H, Sasaka K. 123I-MIBG myocardial scintigraphy in patients with "takotsubo" cardiomyopathy. J Nucl Med. 2004;45(7):1121–1127. [PubMed] [Google Scholar]
- 380.Matsuoka K, Okubo S, Fujii E, Uchida F, Kasai A, Aoki T, et al. Evaluation of the arrhythmogenicity of stress-induced 'takotsubo cardiomyopathy' from the time course of the 12-lead surface electrocardiogram. Am J Cardiol. 2003;92(2):230–233. doi: 10.1016/s0002-9149(03)00547-2. [DOI] [PubMed] [Google Scholar]
- 381.Ito K, Sugihara H, Katoh S, Azuma A, Nakagawa M. Assessment of Takotsubo (ampulla) cardiomyopathy using 99mTc-tetrofosmin myocardial SPECT-comparison with acute coronary syndrome. Ann Nucl Med. 2003;17(2):115–122. doi: 10.1007/BF02988449. [DOI] [PubMed] [Google Scholar]
- 382.Stöllberger C, Finsterer J, Schneider B. Takotsubo-like left ventricular dysfunction: clinical presentation, instrumental findings, additional cardiac and non-cardiac diseases and potential pathomechanisms. Minerva Cardioangiol. 2005;53(2):139–145. [PubMed] [Google Scholar]
- 383.Desmet WJ, Adriaenssens BF, Dens JA. Apical ballooning of the left ventricle: first series in white patients. Heart. 2003;89(9):1027–1031. doi: 10.1136/heart.89.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Akashi YJ, Goldstein DS, Bárbaro G, Ueyama T. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation. 2008;118(25):2754–2762. doi: 10.1161/CIRCULATIONAHA.108.767012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Hinojosa-Laborde C, Chapa I, Lange D, Haywood JR. Gender differences in sympathetic nervous system regulation. Clin Exp Pharmacol Physiol. 1999;26(2):122–126. doi: 10.1046/j.1440-1681.1999.02995.x. [DOI] [PubMed] [Google Scholar]
- 386.Sader MA, Celermajer DS. Endothelial function, vascular reactivity and gender differences in the cardiovascular system. Cardiovasc Res. 2002;53(3):597–604. doi: 10.1016/s0008-6363(01)00473-4. [DOI] [PubMed] [Google Scholar]
- 387.Lambert G, Naredi S, Eden E, Rydenhag B, Friberg P. Monoamine metabolism and sympathetic nervous activation following subarachnoid haemorrhage: influence of gender and hydrocephalus. Brain Res Bull. 2002;58(1):77–82. doi: 10.1016/s0361-9230(02)00762-1. [DOI] [PubMed] [Google Scholar]
- 388.Taddei S, Virdis A, Ghiadoni L, Mattei P, Sudano I, Bernini G, et al. Menopause is associated with endothelial dysfunction in women. Hypertension. 1996;28(4):576–582. doi: 10.1161/01.hyp.28.4.576. [DOI] [PubMed] [Google Scholar]
- 389.Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Takotsuboor stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155(3):408–417. doi: 10.1016/j.ahj.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 390.Akashi YJ, Goldstein DS, Barbaro G, Ueyama T. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation. 2008;118(25):2754–2762. doi: 10.1161/CIRCULATIONAHA.108.767012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Inobe Y, Kugiyama K, Miyagi H, Ohgushi M, Tomiguchi S, Takahashi M, et al. Long-lasting abnormalities in cardiac sympathetic nervous system in patients with coronary spastic angina: quantitative analysis with iodine 123 metaiodobenzylguanidine myocardial scintigraphy. Am. Heart J. 1997;134(1):112–118. doi: 10.1016/s0002-8703(97)70114-5. [DOI] [PubMed] [Google Scholar]
- 392.Villarroel AH, Vitola JV, Stier AL Jr, Dippe T Jr, Cunha C. Takotsubo or stress cardiomyopathy: role of nuclear cardiology using 123I-MIBG. Expert Rev Cardiovasc Ther. 2009;7(7):847–852. doi: 10.1586/erc.09.50. [DOI] [PubMed] [Google Scholar]
- 393.Burgdorf C, von Hof K, Schunkert H, Kurowski V. Regional alterations in myocardial sympathetic innervation in patients with transient left-ventricular apical ballooning (Takotsubo cardiomyopathy) J Nucl Cardiol. 2008;15(1):65–72. doi: 10.1016/j.nuclcard.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 394.Pilgrim TM, Wyss TR. Takotsubo cardiomyopathy or transient left ventricular apical ballooning syndrome: a systematic review. Int J Cardiol. 2008;124(3):283–292. doi: 10.1016/j.ijcard.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 395.Habib G, Derumeaux G, Avierinos JF, Casalta JP, Jamal F, Volot F, et al. Value and limitations of the Duke criteria for the diagnosis of infective endocarditis. J Am Coll Cardiol. 1999;33(7):2023–2029. doi: 10.1016/s0735-1097(99)00116-3. [DOI] [PubMed] [Google Scholar]
- 396.Thuny F, Grisoli D, Collart F, Habib G, Raoult D. Management of infective endocarditis: challenges and perspectives. Lancet. 2012;379(9819):965–975. doi: 10.1016/S0140-6736(11)60755-1. [DOI] [PubMed] [Google Scholar]
- 397.Saby L, Laas O, Habib G, Cammilleri S, Mancini J, Tessonnier L, et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol. 2013;61(23):2374–2382. doi: 10.1016/j.jacc.2013.01.092. [DOI] [PubMed] [Google Scholar]
- 398.Granados U, Fuster D, Pericas JM, Llopis JL, Ninot S, Quintana E, et al. Diagnostic accuracy of 18F-FDG PET/CT in infective endocarditis and implantable cardiac electronic device infection: a cross-sectional study. J Nucl Med. 2016;57(11):1726–1732. doi: 10.2967/jnumed.116.173690. [DOI] [PubMed] [Google Scholar]
- 399.Lancellotti P, Habib G, Oury C, Nchimi A. Positron emission tomography/computed tomography imaging in device infective endocarditis: ready for prime time. Circulation. 2015;132(12):1076–1080. doi: 10.1161/CIRCULATIONAHA.115.018521. [DOI] [PubMed] [Google Scholar]
- 400.Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, et al. 2015 ESC Guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM) Eur Heart J. 2015;36(44):3075–3128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 401.Pizzi MN, Roque A, Fernández-Hidalgo N, Cuéllar-Calabria H, Ferreira-González I, Gonzàlez-Alujas MT, et al. Improving the diagnosis of infective endocarditis in prosthetic valves and intracardiac devices with 18f-fluordeoxyglucose positron emission tomography/computed tomography angiography: initial results at an infective endocarditis referral center. Circulation. 2015;132(12):1113–1126. doi: 10.1161/CIRCULATIONAHA.115.015316. [DOI] [PubMed] [Google Scholar]
- 402.Cerqueira MD, Jacobson AF. Indium-111 leukocyte scintigraphic detection of myocardial abscess formation in patients with endocarditis. J Nucl Med. 1989;30(5):703–706. [PubMed] [Google Scholar]
- 403.Rouzet F, Chequer R, Benali K, Lepage L, Ghodbane W, Duval X, et al. Respective performance of 18F-FDG PET and radiolabeled leukocyte scintigraphy for the diagnosis of prosthetic valve endocarditis. J Nucl Med. 2014;55(12):1980–1985. doi: 10.2967/jnumed.114.141895. [DOI] [PubMed] [Google Scholar]
- 404.Bonfiglioli R, Nanni C, Morigi JJ, Graziosi M, Trapani F, Bartoletti M, et al. (1)(8)F-FDG PET/CT diagnosis of unexpected extracardiac septic embolisms in patients with suspected cardiac endocarditis. Eur J Nucl Med Mol Imaging. 2013;40(8):1190–1196. doi: 10.1007/s00259-013-2426-7. [DOI] [PubMed] [Google Scholar]
- 405.Sarrazin JF, Philippon F, Tessier M, Guimond J, Molin F, Champagne J, et al. Usefulness of fluorine-18 positron emission tomography/computed tomography for identification of cardiovascular implantable electronic device infections. J Am Coll Cardiol. 2012;59(18):1616–1625. doi: 10.1016/j.jacc.2011.11.059. [DOI] [PubMed] [Google Scholar]
- 406.Adjtoutah D, Azhari A, Larabi Y, Dorigo E, Merlin C, Marcaggi X, et al. Advantages of 18F-fluorodeoxyglucose positron emission tomography combined with computed tomography in detecting post cardiac surgery infections. J Saudi Heart Assoc. 2014;26(1):57–61. doi: 10.1016/j.jsha.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Thuny F, Lass O, Saby L, Tessonnier L, Cammilleri S, Casalta JP, et al. Reply: positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis. J Am Coll Cardiol. 2014;63(2):187–189. doi: 10.1016/j.jacc.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 408.Pinkston KL, Gao P, Singh KV, Azhdarinia A, Murray BE, Sevick-Muraca EM, et al. Antibody guided molecular imaging of infective endocarditis. Methods Mol Biol. 2017 Jan;1535:229–241. doi: 10.1007/978-1-4939-6673-8_15. [DOI] [PubMed] [Google Scholar]
- 409.Tarakji KG, Chan EJ, Cantillon DJ, Doonan AL, Hu T, Schmitt S, et al. Cardiac implantable electronic device infections: presentation, management, and patient outcomes. Heart Rhythm. 2010;7(8):1043–1047. doi: 10.1016/j.hrthm.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 410.Sarrazin JF, Philippon F, Tessier M, Guimond J, Molin F, Champagne J, et al. Usefulness of fluorine-18 positron emission tomography/computed tomography for identification of cardiovascular implantable electronic device infections. J Am Coll Cardiol. 2012;59(18):1616–1625. doi: 10.1016/j.jacc.2011.11.059. [DOI] [PubMed] [Google Scholar]
- 411.Bensimhon L, Lavergne T, Hugonnet F, Mainardi JL, Latremouille C, Maunoury C, et al. Whole body [(18) F] fluorodeoxyglucose positron emission tomography imaging for the diagnosis of pacemaker or implantable cardioverter defibrillator infection: a preliminary prospective study. Clin Microbiol Infect. 2011;17(6):836–844. doi: 10.1111/j.1469-0691.2010.03312.x. [DOI] [PubMed] [Google Scholar]
- 412.Yeh CL, Liou JY, Chen SW, Chen YK. Infective endocarditis detected by 18F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in a patient with occult infection. Kaohsiung J Med Sci. 2011;27(11):528–531. doi: 10.1016/j.kjms.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 413.Kim J, Feller ED, Chen W, Dilsizian V. FDG PET/CT imaging for LVAD associated infections. JACC Cardiovasc Imaging. 2014;7(8):839–842. doi: 10.1016/j.jcmg.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 414.Litzler PY, Manrique A, Etienne M, Salles A, Edet-Sanson A, Vera P, et al. Leukocyte SPECT/CT for detecting infection of left-ventricular-assist devices: preliminary results. J Nucl Med. 2010;51(7):1044–1048. doi: 10.2967/jnumed.109.070664. [DOI] [PubMed] [Google Scholar]
- 415.Dell’Aquila AM, Mastrobuoni S, Alles S, Wenning C, Henryk W, Schneider SR, et al. Contributory role of fluorine 18-fluorodeoxyglucose positron emission tomography/computed tomography in the diagnosis and clinical management of infections in patients supported with a continuous-flow left ventricular assist device. Ann Thorac Surg. 2016;101(1):87–94. doi: 10.1016/j.athoracsur.2015.06.066. [DOI] [PubMed] [Google Scholar]
- 416.Tlili G, Amraoui S, Mesguich C, Rivière A, Bordachar P, Hindié E, et al. High performances of (18)F-fluorodeoxyglucose PET-CT in cardiac implantable device infections: a study of 40 patients. J Nucl Cardiol. 2015;22(4):787–798. doi: 10.1007/s12350-015-0067-x. [DOI] [PubMed] [Google Scholar]
- 417.Amraoui S, Tlili G, Sohal M, Berte B, Hindié E, Ritter P, et al. Contribution of PET imaging to the diagnosis of septic embolism in patients with pacing lead endocarditis. JACC Cardiovasc Imaging. 2016;9(3):283–290. doi: 10.1016/j.jcmg.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 418.Simonen P, Lehtonen J, Kandolin R, Schildt J, Marjasuo S, Miettinen H, et al. F-18-fluorodeoxyglucose positron emission tomography-guided sampling of mediastinal lymph nodes in the diagnosis of cardiac sarcoidosis. Am J Cardiol. 2015;116(10):1581–1585. doi: 10.1016/j.amjcard.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 419.Piriou N, Sassier J, Pallardy A, Serfaty JM, Trochu JN. Utility of cardiac FDG-PET imaging coupled to magnetic resonance for the management of an acute myocarditis with non-informative endomyocardial biopsy. Eur Heart J Cardiovasc Imaging. 2015;16(5):574–574. doi: 10.1093/ehjci/jeu319. [DOI] [PubMed] [Google Scholar]
- 420.Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. Eur Heart J. 2007;28(24):3076–3093. doi: 10.1093/eurheartj/ehm456. [DOI] [PubMed] [Google Scholar]
- 421.Friedrich MG, Marcotte F. Cardiac magnetic resonance assessment of myocarditis. Circ Cardiovasc Imaging. 2013;6(5):833–839. doi: 10.1161/CIRCIMAGING.113.000416. [DOI] [PubMed] [Google Scholar]
- 422.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, et al. International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53(17):1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Lurz P, Eitel I, Adam J, Steiner J, Grothoff M, Desch S, et al. Diagnostic performance of CMR imaging compared with EMB in patients with suspected myocarditis. JACC Cardiovasc Imaging. 2012;5(5):513–524. doi: 10.1016/j.jcmg.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 424.Ozawa K, Funabashi N, Daimon M, Takaoka H, Takano H, Uehara M, et al. Determination of optimum periods between onset of suspected acute myocarditis and (1)(8)F-fluorodeoxyglucose positron emission tomography in the diagnosis of inflammatory left ventricular myocardium. Int J Cardiol. 2013;169(3):196–200. doi: 10.1016/j.ijcard.2013.08.098. [DOI] [PubMed] [Google Scholar]
- 425.von Olshausen G, Hyafil F, Langwieser N, Laugwitz KL, Schwaiger M2, Ibrahim T. Detection of acute inflammatory myocarditis in Epstein Barr virus infection using hybrid 18F-fluoro-deoxyglucose-positron emission tomography/magnetic resonance imaging. Circulation. 2014;130(11):925–926. doi: 10.1161/CIRCULATIONAHA.114.011000. [DOI] [PubMed] [Google Scholar]
- 426.Nensa F, Kloth J, Tezgah E, Poeppel TD, Heusch P, Goebel J, et al. Feasibility of FDG-PET in myocarditis: comparison to CMR using integrated PET/MRI. J Nucl Cardiol. 2018;25(3):785–794. doi: 10.1007/s12350-016-0616-y. [DOI] [PubMed] [Google Scholar]
- 427.Takano H, Nakagawa K, Ishio N, Daimon M, Daimon M, Kobayashi Y, et al. Active myocarditis in a patient with chronic active Epstein-Barr virus infection. Int J Cardiol. 2008;130(1):e11–e13. doi: 10.1016/j.ijcard.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 428.Nensa F, Poeppel TD, Beiderwellen K, Schelhorn J, Mahabadi AA, Erbel R, et al. Hybrid PET/MR imaging of the heart: feasibility and initial results. Radiology. 2013;268(2):366–373. doi: 10.1148/radiol.13130231. [DOI] [PubMed] [Google Scholar]
- 429.Nensa F, Poeppel TD, Krings P, Schlosser T. Multiparametric assessment of myocarditis using simultaneous positron emission tomography/magnetic resonance imaging. Eur Heart J. 2014;35(32):2173–2173. doi: 10.1093/eurheartj/ehu086. [DOI] [PubMed] [Google Scholar]
- 430.Alter P, Figiel JH, Rupp TP, Bachmann GF, Maisch B, Rominger MB. MR, CT, and PET imaging in pericardial disease. Heart Fail Rev. 2013;18(3):289–306. doi: 10.1007/s10741-012-9309-z. [DOI] [PubMed] [Google Scholar]
- 431.Losik SB, Studentsova Y, Margouleff D. Chemotherapy-induced pericarditis on F-18 FDG positron emission tomography scan. Clin Nucl Med. 2003;28(11):913–915. doi: 10.1097/01.rlu.0000093087.97892.62. [DOI] [PubMed] [Google Scholar]
- 432.Salomäki SP, Hohenthal U, Kemppainen J, Pirilä L, Saraste A. Visualization of pericarditis by fluorodeoxyglucose PET. Eur Heart J Cardiovasc Imaging. 2014;15(3):291–291. doi: 10.1093/ehjci/jet179. [DOI] [PubMed] [Google Scholar]
- 433.Shao D, Wang SX, Liang CH, Gao Q. Differentiation of malignant from benign heart and pericardial lesions using positron emission tomography and computed tomography. J Nucl Cardiol. 2011;18(4):668–677. doi: 10.1007/s12350-011-9398-4. [DOI] [PubMed] [Google Scholar]
- 434.Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H Jr, Bresnitz EA, et al. Case Control Etiologic Study of Sarcoidosis (ACCESS) research group Clinical characteristics of patients in a case control study of sarcoidosis. Pt 1Am J Respir Crit Care Med. 2001;164(10):1885–1889. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 435.Mehta D, Lubitz SA, Frankel Z, Wisnivesky JP, Einstein AJ, Goldman M, et al. Cardiac involvement in patients with sarcoidosis: diagnostic and prognostic value of outpatient testing. Chest. 2008;133(6):1426–1435. doi: 10.1378/chest.07-2784. [DOI] [PubMed] [Google Scholar]
- 436.Patel MR, Cawley PJ, Heitner JF, Klem I, Parker MA, Jaroudi WA, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120(20):1969–1977. doi: 10.1161/CIRCULATIONAHA.109.851352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Gideon NM, Mannino DM. Sarcoidosis mortality in the United States 1979-1991: an analysis of multiple-cause mortality data. Am J Med. 1996;100(4):423–427. doi: 10.1016/S0002-9343(97)89518-6. [DOI] [PubMed] [Google Scholar]
- 438.Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. Eur Heart J. 2007;28(24):3076–3093. doi: 10.1093/eurheartj/ehm456. [DOI] [PubMed] [Google Scholar]
- 439.Youssef G, Leung E, Mylonas I, Nery P, Williams K, Wisenberg G, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metanalysis including the Ontario experience. J Nucl Med. 2012;53(2):241–248. doi: 10.2967/jnumed.111.090662. [DOI] [PubMed] [Google Scholar]
- 440.Tang R, Wang JT, Wang L, Le K, Huang Y, Hickey AJ, et al. Impact of patient preparation on the diagnostic performance of 18F-FDG PET in cardiac sarcoidosis: a systematic review and meta-analysis. Clin Nucl Med. 2016;41(7):e327–e339. doi: 10.1097/RLU.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 441.McArdle B, Dowsley TF, Cocker MS, Ohira H, deKemp RA, DaSilva J, et al. Cardiac PET: metabolic and functional imaging of the myocardium. Semin Nucl Med. 2013;43(6):434–448. doi: 10.1053/j.semnuclmed.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 442.Schatka I, Bengel FM. Advanced imaging of cardiac sarcoidosis. J Nucl Med. 2014;55(1):99–106. doi: 10.2967/jnumed.112.115121. [DOI] [PubMed] [Google Scholar]
- 443.Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VL, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63(4):329–336. doi: 10.1016/j.jacc.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Hulten E, Aslam S, Osborne M, Abbasi S, Bittencourt MS, Blankstein R. Cardiac sarcoidosis-state of the art review. Cardiovasc Diagn Ther. 2016;6(1):50–63. doi: 10.3978/j.issn.2223-3652.2015.12.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Bhogal S, Ladia V, Sitwala P, Cook E, Bajaj K, Ramu V, et al. Cardiac amyloidosis: An updated review with emphasis on diagnosis and future directions. Curr Probl Cardiol. 2018;43(1):10–34. doi: 10.1016/j.cpcardiol.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 446.Carroll JD, Gaasch WH, McAdam KP. Amyloid cardiomyopathy: characterization by a distinctive voltage/mass relation. Am J Cardiol. 1982;49(1):9–13. doi: 10.1016/0002-9149(82)90270-3. [DOI] [PubMed] [Google Scholar]
- 447.Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133(24):2404–2412. doi: 10.1161/CIRCULATIONAHA.116.021612. [DOI] [PubMed] [Google Scholar]
- 448.Glaudemans AW, van Rheenen RW, van den Berg MP, Noordzij W, Koole M, Blokzijl H, et al. Bone scintigraphy with (99m) technetium-hydroxymethylene diphosphonate allows early diagnosis of cardiac involvement in patients with transthyretin derived systemic amyloidosis. Amyloid. 2014;21(1):35–44. doi: 10.3109/13506129.2013.871250. [DOI] [PubMed] [Google Scholar]
- 449.Bokhari S, Castaño A, Pozniakoff T, Deslisle S, Latif F, Maurer MS. 99mTc-PYP Scintigraphy for Cardiac Amyloidosis Circ Cardiovasc. Imaging. 2013;6(2):195–201. doi: 10.1161/CIRCIMAGING.112.000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Van Der Gucht A, Galat A, Rosso J, Guellich A, Garot J, Bodez D, et al. [18F]-NaF PET/CT imaging in cardiac amyloidosis. J Nucl Cardiol. 2016;23(4):846–849. doi: 10.1007/s12350-015-0287-0. [DOI] [PubMed] [Google Scholar]
- 451.Gagliardi C, Tabacchi E, Bonfiglioli R, Diodato S, Nanni C, Guidalotti P, et al. Does the etiology of cardiac amyloidosis determine the myocardial uptake of [18F]-NaF PET/CT? J Nucl Cardiol. 2017;24(2):746–749. doi: 10.1007/s12350-016-0457-8. [DOI] [PubMed] [Google Scholar]
- 452.Trivieri MG, Dweck MR, Abgral R, Robson PM, Karakatsanis NA, Lala A, et al. 18F-sodium fluoride PET/MR for the assessment of cardiac amyloidosis. J Am Coll Cardiol. 2016;68(24):2712–2714. doi: 10.1016/j.jacc.2016.09.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Kung J, Zhuang H, Yu JQ, Duarte PS, Alavi A. Intense fluorodeoxyglucose activity in pulmonary amyloid lesions on positron emission tomography. Clin Nucl Med. 2003;28(12):975–976. doi: 10.1097/01.rlu.0000099807.66221.64. [DOI] [PubMed] [Google Scholar]
- 454.Mekinian A, Jaccard A, Soussan M, Launay D, Berthier S, Federici L, et al. Centre de Référence des Amyloses immunoglobulinémiques et autres maladies liées aux dépots des immunoglobulines monoclonales 18F-FDG PET/CT in patients with amyloid light-chain amyloidosis: case series and literature review. Amyloid. 2012;19(2):94–98. doi: 10.3109/13506129.2012.682833. [DOI] [PubMed] [Google Scholar]
- 455.Lee SP, Lee ES, Choi H, Im HJ, Koh Y, Lee MH, et al. 11C-Pittsburgh B PET imaging in cardiac amyloidosis. JACC Cardiovasc Imaging. 2015;8(1):50–59. doi: 10.1016/j.jcmg.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 456.Pilebro B, Arvidsson S, Lindqvist P, Sundström T, Westermark P, Antoni G, et al. Positron emission tomography (PET) utilizing Pittsburgh compound B (PIB) for detection of amyloid heart deposits in hereditary transthyretin amyloidosis (ATTR) J Nucl Cardiol. 2018;25(1):240–248. doi: 10.1007/s12350-016-0638-5. [DOI] [PubMed] [Google Scholar]
- 457.Dorbala S, Vangala D, Semer J, Strader C, Bruyere JR Jr, Di Carli MF, et al. Imaging cardiac amyloidosis: a pilot study using 18F-florbetapir positron emission tomography. Eur J Nucl Med Mol Imaging. 2014;41(9):1652–1662. doi: 10.1007/s00259-014-2787-6. [DOI] [PubMed] [Google Scholar]
- 458.Osborne DR, Acuff SN, Stuckey A, Wall JS. A routine PET/CT protocol with streamlined calculations for assessing cardiac amyloidosis using (18)F-florbetapir. Front Cardiovasc Med. 2015 May;2:23–23. doi: 10.3389/fcvm.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Law WP, Wang WY, Moore PT, Mollee PN, Ng AC. Cardiac amyloid imaging with 18F-florbetaben PET: a pilot study. J Nucl Med. 2016;57(11):1733–1739. doi: 10.2967/jnumed.115.169870. [DOI] [PubMed] [Google Scholar]
- 460.Lee SP, Lee ES, Choi H, Im HJ, Koh Y, Lee MH, et al. 11C-Pittsburgh B PET imaging in cardiac amyloidosis. JACC Cardiovasc Imaging. 2015;8(1):50–59. doi: 10.1016/j.jcmg.2014.09.018. [DOI] [PubMed] [Google Scholar]