Abstract
In this study, we aimed to investigate expression profile of long non‐coding RNA (lncRNA) SUMO1P3, and its role and molecular mechanisms in the progression of hepatocellular carcinoma (HCC).
The expression of SUMO1P3 in HCC tissues and cells was detected using quantitative real‐time polymerase chain reaction (qRT‐PCR). The chi‐squared test was used to estimate the relationship between SUMO1P3 levels and clinical characteristics of HCC cases. Cellular biological behaviours were investigated using MTT, transwell assays and wound healing assay. Bioinformatics and dual‐luciferase reporter assays were performed to identify potential target of SUMO1P3 in HCC. Additionally, protein analysis was carried out using Western blot.
The expression of SUMO1P3 was significantly higher in HCC tissues and cells than in non‐cancerous specimens and normal cells (P < .01). Moreover, its up‐regulation was closely correlated with lymph node metastasis (P = .027) and TNM stage (P = .019). SUMO1P3 knockdown inhibited the proliferation, migration and invasion of HCC cells. MiR‐320a was a potential target of SUMO1P3, and its expression was negatively regulated by SUMO1P3 in HCC SUMO1P3 could activate Wnt/β‐catenin pathway, which was mediated by miR‐320a.
Elevated expression of SUMO1P3 predicts malignant progression among HCC patients. SUMO1P3 enhances Wnt/β‐catenin pathway through sponging miR‐320a, thus contributing to aggressive progression of HCC.
Keywords: hepatocellular carcinoma, long non‐coding RNA, microRNAs, SUMO1P3, Wnt/β‐catenin pathway
1. INTRODUCTION
Hepatocellular carcinoma (HCC) is a frequently diagnosed fatal malignancy, posing a great threat to human health around the world, especially in China.1, 2 The morbidity of HCC is closely correlated with the infections of hepatitis B virus (HBV) and hepatitis C virus (HCV), smoking, alcohol abuse, etc3 In addition, cirrhosis, diabetes and obesity may also increase the risk of HCC.4 At present, therapeutic strategies for HCC mainly include liver transplantation, resection, local ablative therapies, adjuvant chemotherapy and immune treatment.5 Even though these treatments could improve the outcomes of HCC patients, long‐time prognosis of the disease is still unsatisfactory, due to high rates of recurrence and distant metastasis.6 Therefore, it is crucial to identify key factors that drive the tumorigenesis of HCC.
Long non‐coding RNAs (lncRNAs) are a class of endogenous RNAs with a length of more than 200 nucleotides.7 Although lncRNAs hold no or limited ability to code proteins, they may take part in various biological processes through regulating gene expression at transcriptional and post‐transcriptional levels.8 lncRNAs could interact with RNA and DNA through base pairing to form regulatory netweb with DNA, protein and RNA, thus playing important roles in physiological and pathological conditions.9, 10 In tumorigenesis, lncRNAs could exert promoting or repressing effects, or both.11 In HCC, expression patterns of lncRNA show close association with cell proliferation, apoptosis, invasion and metastasis, suggesting their possibilities to be employed as diagnostic and prognostic biomarkers, and therapeutic targets.12
Small ubiquitin‐like modifier (SUMO) 1 pseudogene 3 (SUMO1P3) is a pseudogene and belongs to a separate class of lncRNAs.13 The up‐regulation of lncRNA SUMO1P3 has been observed in several human cancers, such as breast cancer,13 gastric cancer 14 and bladder cancer.15 In HCC, the study performed by Zhou et al16 demonstrated that the knockdown of SUMO1P3 could inhibit cell proliferation, colony formation and invasion abilities. However, molecular mechanisms underlying the function of SUMO1P3 in HCC progression remained poorly understood.
In this study, we investigated expression patterns of lncRNA SUMO1P3 in HCC tissues and cells. Moreover, we estimated the association of SUMO1P3 expression with clinical characteristics of HCC patients. In addition, cell experiments were designed to explore the mechanisms of SUMO1P3 in HCC progression.
2. MATERIALS AND METHODS
2.1. Patients and tissue collection
The study was performed in Fujian Provincial Hospital, Shengli Clinical Medical College of Fujian Medical University. The patients who were newly diagnosed with HCC via pathological examinations in this hospital were included in our study. None of the patients had received any treatments, either surgery, radiotherapy, chemotherapy or immune therapy. The study was approved by the Ethics Committee of the hospital. Written informed consent was obtained from all patients or their families before surgery.
Hepatocellular carcinoma tissues and adjacent normal ones were collected in surgery. The tissues were immediately frozen in liquid nitrogen, and then stored at −80°C. Clinical characteristics of the patients were collected from their medical records. A total of 104 HCC patients including 60 males (57.7%) and 44 females (42.3%) were enrolled in our study, with an average age of 61.25 ± 10.36 years. Of the patients, 42 (40.4%) had smoking history, while 60 (57.7%) showed evidences for drinking. Additionally, 60 patients (57.7%) exhibited tumour size more than 3 cm, and lymph node metastasis was observed in 37 patients (35.6%). According to TNM (tumour node metastasis) staging, 59 (56.7%) patients were at stages I‐II, and 45 (43.3%) at stages III‐IV. Clinical information of the patients was summarized in Table 1.
Table 1.
The association of SUMO1P3 expression with clinical characteristics of HCC patients
| Characteristics | N (n = 104, %) | SUMO1P3 low expression (n = 46, %) | SUMO1P3 high expression (n = 58, %) | P‐values |
|---|---|---|---|---|
| Age (y) | ||||
| ≥60 | 64 (61.5) | 27 (48.7) | 37 (63.8) | .596 |
| <60 | 40 (38.5) | 19 (42.3) | 21 (36.2) | |
| Gender | ||||
| Male | 60 (57.7) | 26 (56.5) | 34 (58.6) | .850 |
| Female | 44 (42.3) | 20 (43.5) | 24 (42.4) | |
| Smoking | ||||
| Yes | 42 (40.4) | 18 (39.1) | 24 (41.4) | .816 |
| No | 62 (59.6) | 28 (60.9) | 34 (58.6) | |
| Drinking | ||||
| Yes | 60 (57.7) | 28 (60.9) | 32 (55.2) | .559 |
| No | 44 (42.3) | 18 (39.1) | 26 (44.8) | |
| Tumour size (cm) | ||||
| ≤3 | 60 (57.7) | 30 (65.2) | 30 (51.7) | .167 |
| >3 | 44 (42.3) | 16 (34.8) | 28 (48.3) | |
| Lymph node metastasis | ||||
| Yes | 37 (35.6) | 11 (23.9) | 26 (44.8) | .027 |
| No | 67 (64.4) | 35 (76.1) | 32 (22.2) | |
| TNM stage | ||||
| I‐II | 59 (56.7) | 32 (69.6) | 27 (46.6) | .019 |
| III‐IV | 45 (43.3) | 14 (30.4) | 31 (53.4) | |
2.2. Cell line and culture
In our study, in vitro experiments were carried out using human HCC cell line HepG2 (code: TCHu 72) and normal hepatocyte cell line THLE3 (code: GNHu40). Both of the two cell lines were bought from the Cell Bank of the Chinese Academic of Science (CBP600232; Shanghai, China). The cell lines were cultured using Roswell Park Memorial Institute 1640 (RPMI 1640) medium supplemented with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin. Cell mediums were maintained in an incubator containing 5% CO2 at 37°C.
2.3. RNA extraction and quantitative analysis
RNA template was extracted from collected tissue and cell samples using TRIzol reagent (Invitrogen, Thermo Fisher Scientific, Inc) according to the manufacturer's instruction. Then, RNA sample was used for the synthesis of the first‐strand cDNA, and the reaction was implemented using PrimeScript RT Reagent Kit (Takara). Quantitative analysis for the genes was carried out using quantitative real‐time polymerase chain reaction (qRT‐PCR) method. Specific primer sequences were as follows: GAPDH forward: 5′‐TGCACCACCAACTGCTTAGC‐3′; reverse: 5′‐GGCATGGACTGTGGTCATGAG‐3′; SUMO1P3 forward: 5′‐ACTGGGAATGGAGGAAGA‐3′; reverse: 5′‐TGAGAAAGGATTGAGGGAAAAG‐3′; U6 forward: 5′‐CTCGCTTCGGCAGCACA‐3′; reverse: 5′‐AACGCTTCACGAATTTGCGT‐3′; miR ‐320a forward: 5′‐GGGCTAAAAGCTGGGTTGA‐3′; reverse: 5′‐CAGTGCGTGTCGTGGAGT‐3′. GAPDH was employed as an internal control for mRNA detection, while U6 acted as a control for miRNA detection. 2−ΔΔCt equation was used to calculate relative expression of the genes. Each test was repeated three times.
2.4. Cell transfection
siRNA targeting SUMO1P3 (si‐SUMO1P3) and corresponding negative control (si‐NC) were designed and synthesized by HANBIO company. The recombinants were transfected to HCC cell line HepG2 using Lipofectamine® 2000 reagent (Invitrogen, Thermo Fisher Scientific, Inc) according to the instructions. Transfected cells were incubated at 37°C with 5% CO2 for 48 hours. Then, the cells were harvested, and qRT‐PCR method was used to investigate the expression of SUMO1P3 to estimate transfection efficacy.
2.5. Cell proliferation
Cell proliferation ability was estimated through MTT assay using MTT Cell Proliferation and Cytotoxicity Assay Kit (Sangon Biotech). In brief, cells were seeded to a 96‐well plate with a density of ×105 cells/mL. Then, the cells were incubated at 37°C with 5% CO2. At an interval of one day, 20 μL MTT was supplemented into cell medium and incubated for an additional 4 hours. Then, 150 μL DMSO was added and incubated at dark for 10 minutes to stop reaction. Subsequently, a microplate reader (TECAN) was used to detect the absorbance of the cell medium at 490 nm to estimate cell proliferation. Each test was carried out in triplicate.
2.6. Cell migration and invasion
The effects of SUMO1P3 expression on the motility of HCC cells were evaluated using Transwell assay (8.0 µm pore size, Costar). In migration analysis, 500 μL RPMI 1640 medium was added into the upper chamber, while the lower chamber was coated with 500 μL RPMI 1640 medium supplemented with 10% FBS. Two hundred micro litre cell suspension with a density of 5 × 104 cells/mL was seeded into the upper chamber, and then, the chamber was incubated at 37°C with 5% CO2. Forty eight hours later, the cells in the lower chamber were stained using crystal violet and counted under an inverted microscope (IX31; Olympus Corporation). For each sample, five random files were selected. For invasion analysis, Matrigel (Corning Glass Works) was added into the upper chamber, and the procedures were carried out in accordance with the Migration analysis. Each test had three repeats.
2.7. Wound healing assay
To test migration results from transwell assay, we conducted wound healing assay. HepG2 cells were seeded into 6‐well plates (4 × 105 cells/well), and 2% FBS‐supplemented medium was added to avoid cell proliferation before incubation at 37°C for 24 hours. si‐NC (NR) and si‐SUMO1P3 were transfected into HepG2 cells. Then, freshly changed 2% FBS‐supplemented medium was added after the medium was removed, and wounds were created with a sterile 200‐µL pipette tip in each well. Wound healing was monitored and photographed at 0, 24, 48 and 72 hours.
2.8. Dual‐luciferase reporter assay
Bioinformatics and dual‐luciferase reporter systems were used to confirm potential targeted genes of SUMO1P3 in HCC. Then, potential targeted miRNAs of SUMO1P3 were identified on starBase (http:// http://starbase.sysu.edu.cn/) and miRanda (http://www. microrna.org/microrna/home.do). The luciferase reporter plasmids containing SUMO1P3 wild type (wt) or SUMO1P3 mutant type (mut) were constructed, and cotransfected with miR ‐320a mimic or mimic NC into HCC cell line HepG2. Cell transfection was performed using Lipofectamine 3000 (Invitrogen, Thermo Fisher Scientific, Inc), and then, the cells were incubated at 37°C with 5% CO2. Forty eight hours later, luciferase activity of the cells was detected via Dual‐Luciferase Reporter Assay System (Promega Corporation). Renilla luciferase activity was normalized to firefly luciferase activity.
2.9. Western blot analysis
Protein expression was detected using Western blot analysis in our study. Protein samples were isolated from cell and tissue specimens using RIPA Lysis and Extraction Buffer (Thermo Scientific). Quantified analysis of protein sample was performed through BCA method, which was carried out using a BCA Protein Assay Kit (Thermo Scientific). Then, equal amount of protein samples was separated using 10% SDS‐PAGE analysis. Later, targeted proteins were transfected onto polyvinylidene fluoride membrane (0.45 µm pore size; EMD Millipore), and blocked employing 5% skimmed milk for 90 minutes at room temperature. Subsequently, the membranes were separated using specific primary antibodies, including anti‐β‐catenin antibody (1:5000, Abcam), anti‐C‐myc antibody (1:1000, Abcam), anti‐cyclin D1 antibody (1:10 000, Abcam) and anti‐GAPDH antibody (1:10 000, Abcam). GAPDH was employed as a loading control. Next, the membranes were incubated with secondary anti‐rabbit IgG antibody (1:2000, Abcam) at room temperature for 2 hours. Finally, band grey was detected by a ECL substrate reagent kit (GE Healthcare) on a Gel Doc XR imaging system (Bio‐Rad).
2.10. Statistical analysis
All data calculations were performed using SPSS 18.0 software (SPSS, Inc), and figures were plotted applying GraphPad Prism version 5.0 software (GraphPad). Continuous variables were shown as average ± standard deviation (SD), and their differences between two groups were analysed through Student's t test. Categorical data were recorded as case number, and their comparison between two groups was carried out using the chi‐squared test. All tests were two‐tailed, and P‐values <.05 meant the statistical significance of the results.
3. RESULTS
3.1. Up‐regulation of SUMO1P3 in HCC
qRT‐PCR was used to investigate the expression of SUMO1P3 mRNA in HCC tissues and cells. The results displayed in Figure 1 showed that the levels of SUMO1P3 were higher in HCC tissues than in adjacent normal ones (P < .01, Figure 1A). Moreover, compared to normal hepatic cells, the expression of SUMO1P3 was obviously enhanced in HCC cell line HepG2 (P < .01, Figure 1B).
Figure 1.
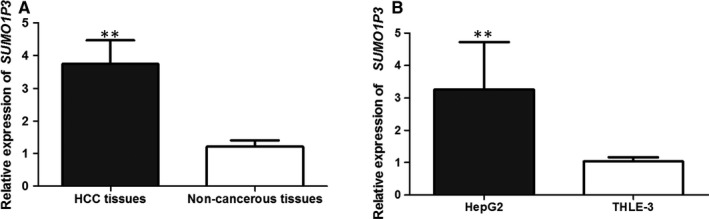
The expression of SUMO1P3 was significantly enhanced in HCC tissues (A) and cell line (B). **P < .01
3.2. Association of SUMO1P3 mRNA with clinical characteristics of HCC patients
According to the mean expression of SUMO1P3 mRNA in HCC tissues, the included HCC patients were divided into high (n = 58) and low (n = 46) expression groups. The chi‐squared test was applied to estimate the relationship between SUMO1P3 expression and clinical information of HCC patients. We found that the levels of SUMO1P3 mRNA were positively correlated with lymph node metastasis (P = .027) and TNM stage (P = .019). However, the expression of SUMO1P3 was not influenced by HCC patients' age, gender, smoking, drinking habits or tumour size (P > .05 for all) (Table 1).
3.3. Knockdown of SUMO1P3 suppressed HCC cell proliferation, migration and invasion
In order to investigate the function of SUMO1P3 in HCC progression, HepG2 cells were transfected by si‐SUMO1P3 vector to inhibit the expression of SUMO1P3. qRT‐PCR analysis suggested that the levels of SUMO1P3 were significantly decreased after the transfection with si‐SUMO1P3 (P < .001, Figure 2).
Figure 2.
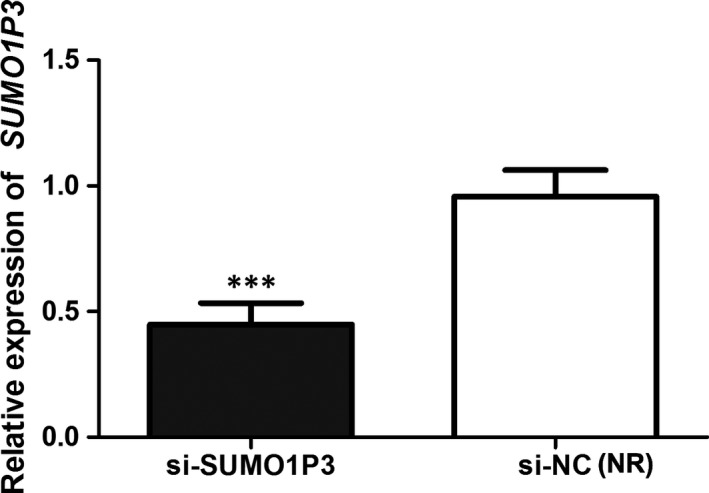
The transfection with si‐SUMO1P3 leads to obvious down‐regulation of SUMO1P3 in HepG2 cells. ***P < .001; NR: negative reference; si‐SUMO1P3: HepG2 cells transfected by si‐SUMO1P3 plasmid; si‐NC: HepG2 cells transfected by negative control plasmid, acted as NR
MTT and transwell assays were used to detect cell proliferation, migration and invasion of the transfected cells, separately. The results demonstrated that the transfection with si‐SUMO1P3 could significantly inhibit cell proliferation (P < .05, Figure 3A), migration (P < .05, Figure 3B) and invasion (P < .01, Figure 3C). Moreover, wound healing assay also verified results on migration (P < .05, Figure 3D). Knockdown of SUMO1P3 might inhibit the proliferation, migration and invasion abilities of HCC cells.
Figure 3.
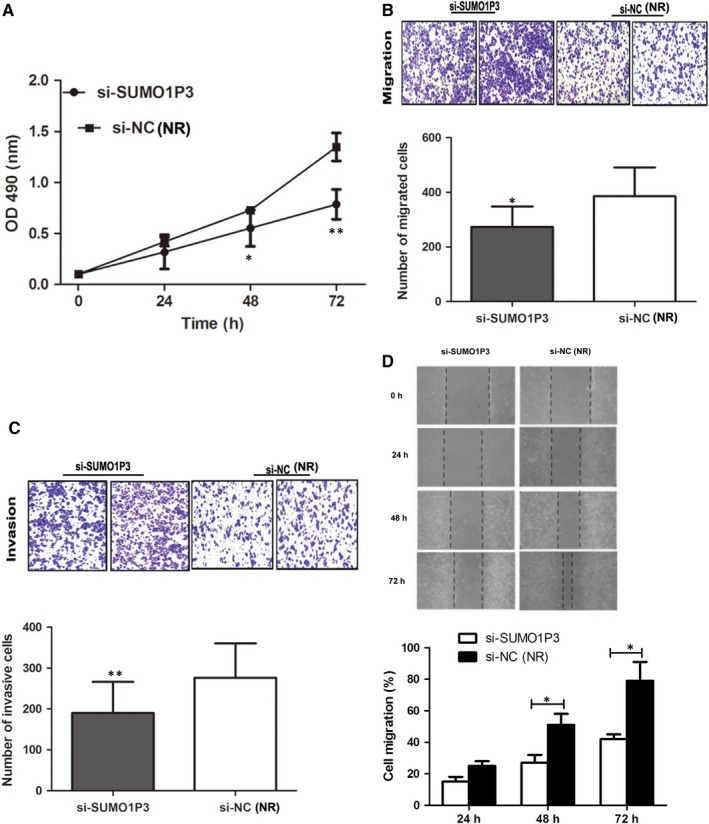
Knockdown of SUMO1P3 obviously suppresses HCC cell proliferation (A), migration (B, C) and invasion (D, E). *P < .05, **P < .01; NR: negative reference; si‐SUMO1P3: HepG2 cells transfected by si‐SUMO1P3 plasmid; si‐NC: HepG2 cells transfected by negative control plasmid, acted as NR
3.4. SUMO1P3 acted as sponge of miR‐320a in HCC
Bioinformatics analysis demonstrated that the 3′‐end of SUMO1P3 possessed complementary sequences of miR‐320a (Figure 4A). Thus, dual‐luciferase reporter assay was performed to verify whether miR‐320a was a potential target of SUMO1P3 in HCC. The results displayed in Figure 4B suggested that HCC cells cotransfected by SUMO1P3 wt and miR‐320a mimic exhibited obviously low luciferase activity than those cotransfected by SUMO1P3 wt and mimic NC (P < .01), while the cotransfection with miR‐320a mimic and SUMO1P3 mt did not significantly affect luciferase activity of the cells, compared to the control (P > .05). MiR‐320a might bind to SUMO1P3.
Figure 4.
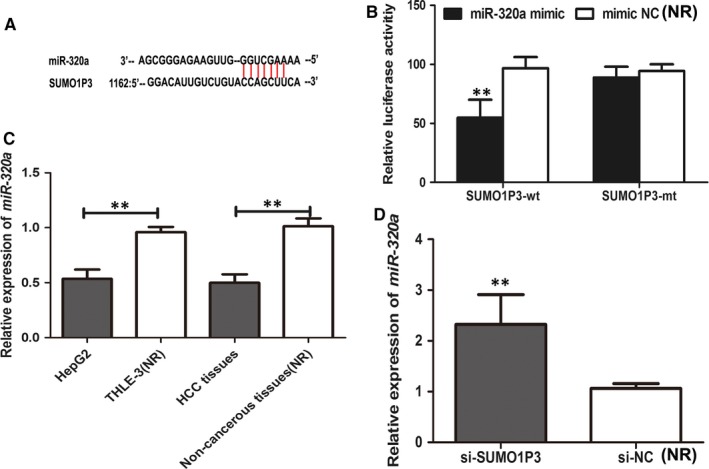
Bioinformatics analysis confirmed that the 3′‐end of SUMO1P3 had complementary sequences of miR‐320a (A). Dual‐luciferase reporter analysis was performed to ascertain the relationship between SUMO1P3 and miR‐320a. The presence of miR‐320a and SUMO1P3 decreased luciferase activity of HepG2 cells, but miR‐320a presence did not influence luciferase activity of HepG2 cells transfected by SUMO1P3‐mt (B). The expression of miR‐320a was decreased in HCC cells (HepG2) compared to normal hepatic cell line THLE‐3 (C), and the levels of miR‐320a were obviously lower in HCC tissues than in non‐malignant ones. But the knockdown of SUMO1P3 enhanced the expression of miR‐320a (D). **P < .01; NR: negative reference; si‐SUMO1P3: HepG2 cells transfected by si‐SUMO1P3 plasmid; si‐NC: HepG2 cells transfected by negative control plasmid, acted as NR
In addition, we examined the expression of miR‐320a in HCC tissues and HepG2 cells. The results demonstrated that the expression of miR‐320a was significantly decreased in HepG2 cells, compared to normal hepatic cells. Meanwhile, HCC tissues showed obviously reduced expression of miR‐320a in comparison with non‐cancerous ones (P < .01, Figure 4C). Moreover, the knockdown of SUMO1P3 could obviously enhance the expression of miR‐320a in HepG2 cells (P < .01, Figure 4D). SUMO1P3 could sponge the expression of miR‐320a.
3.5. SUMO1P3 suppressed Wnt/β‐catenin pathway in HCC
The study carried out by Lu et al17 reported that β‐catenin was a potential targeted gene of miR‐320a in HCC and that miR‐320a could regulate the activity of Wnt/β‐catenin pathway. Given the relationship between SUMO1P3 and miR‐320a, we investigated regulatory function of SUMO1P3 on Wnt/β‐catenin pathway in HCC. Western blot analysis suggested that the protein levels of β‐catenin, C‐myc and cyclin D1 were higher in HCC tissues than in non‐cancerous ones (P < .05 for all, Figure 5A). Moreover, the knockdown of SUMO1P3 could remarkably suppress the expression of β‐catenin, C‐myc and cyclin D1 proteins (P < .05 for all, Figure 5B), revealing the inactivation of Wnt/β‐catenin pathway.
Figure 5.
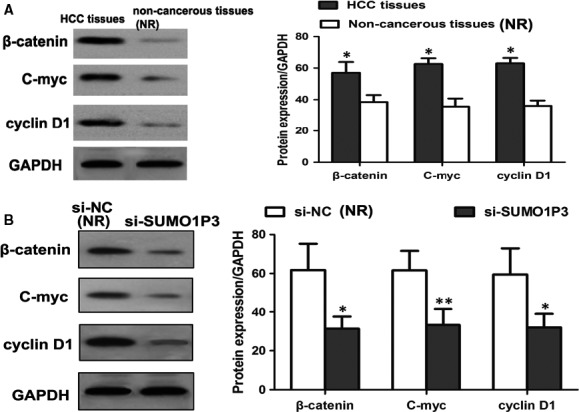
Compared to non‐cancerous ones, HCC tissues showed significantly increased expressions of β‐catenin, C‐myc and cyclin D1 proteins (A). The inhibition of SUMO1P3 in HepG2 cells resulted in the down‐regulation of β‐catenin, C‐myc and cyclin D1 proteins, revealing the inactivation of Wnt/β‐catenin pathway (B). *P < .05, **P < .01; NR: negative reference; si‐SUMO1P3: HepG2 cells transfected by si‐SUMO1P3 plasmid; si‐NC: HepG2 cells transfected by negative control plasmid, acted as NR
3.6. Oncogenic function of SUMO1P3 was mediated by miR‐320a
Additional experiments were designed to ascertain whether the function of SUMO1P3 in HCC progression was mediated by miR‐320a. HepG2 cells were cotransfected by si‐SUMO1P3 and miR‐320a inhibitor, and cells transfected by si‐SUMO1P3 vector were employed as controls. Western blot analysis suggested that compared to the controls, the cotransfection with si‐SUMO1P3 and miR‐320a inhibitor led to up‐regulated β‐catenin, C‐myc and cyclin D1 proteins (P < .05 for all; Figure 6).
Figure 6.
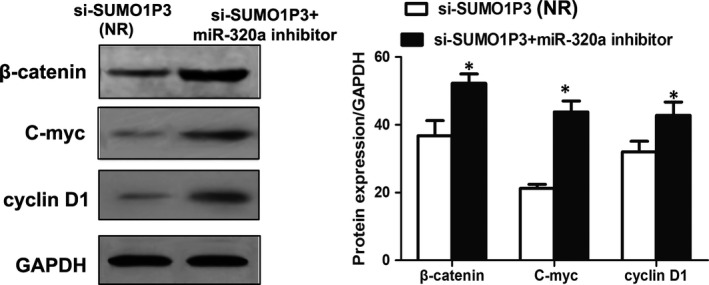
The cotransfection with si‐SUMO1P3 and miR‐320a inhibitor leads to the up‐regulation of β‐catenin, anti‐C‐myc and cyclin D1 proteins. *P < .05, **P < .01; NR: negative reference; si‐SUMO1P3: HepG2 cells transfected by si‐SUMO1P3 plasmid, which acted as NR; si‐SUMO1P3 + miR‐320a inhibitor: HepG2 cells cotransfected by si‐SUMO1P3 and miR‐320a inhibitor
In addition, MTT assay suggested that the presence of miR‐320a inhibitor significantly promoted HCC cell proliferation, and the migration and invasion ability were confirmed by transwell analysis (P < .05 for all; Figure 7). All data revealed that promoting function of SUMO1P3 in HCC progression was dependent on miR‐320a. MiR‐320a had the ability to reverse oncogenic function of SUMO1P3 in HCC.
Figure 7.
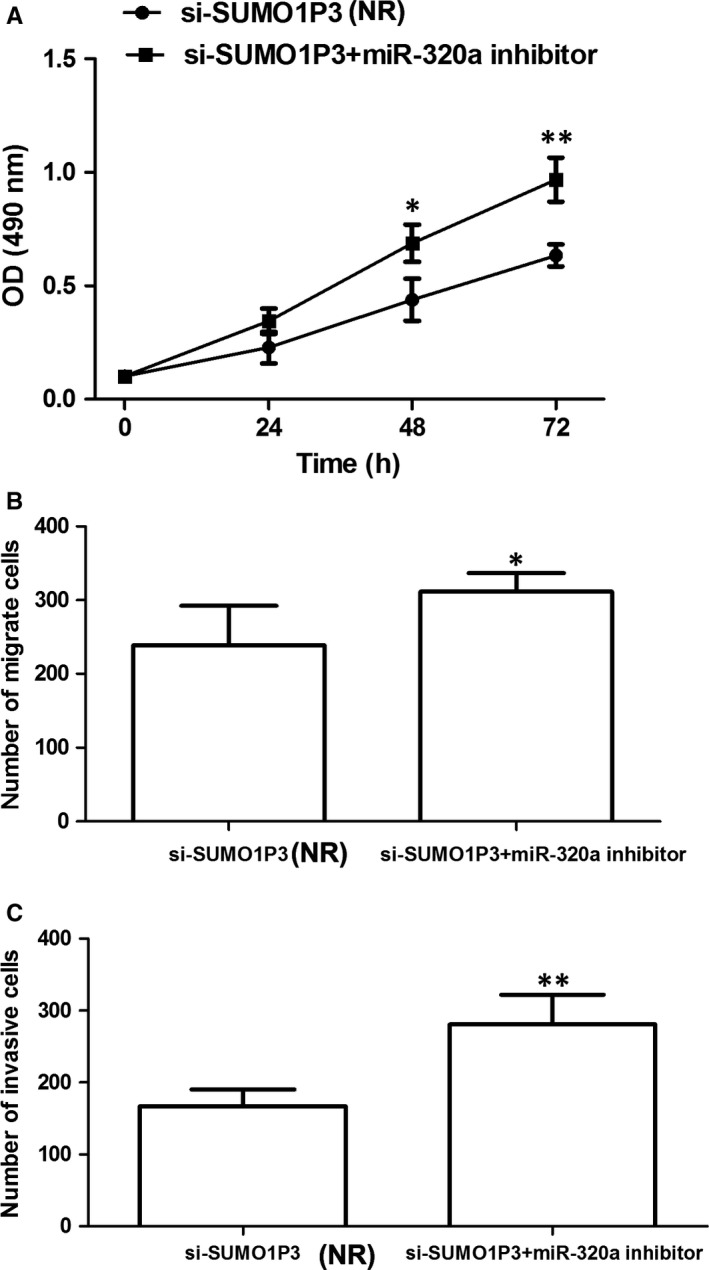
The presence of miR‐320a inhibitor significantly promoted HCC cell proliferation (A), migration (B) and invasion (C). *P < .05, **P < .01; NR: negative reference; si‐SUMO1P3: HepG2 cells transfected by si‐SUMO1P3 plasmid, which acted as NR; si‐SUMO1P3 + miR‐320a inhibitor: HepG2 cells cotransfected by si‐SUMO1P3 and miR‐320a inhibitor
4. DISCUSSION
The tumorigenesis of HCC is regulated by the interactions between genetic and epigenetic mutations.18 Gene silencing mediated by lncRNAs is a prevalent epigenetic change in tumorigenesis.19 In HCC, a variety of lncRNAs have been confirmed to be involved in cancer progression. For example, Zhu et al20 found that lncRNA NEAT1 interacting with miR‐384 contributed to HCC progression via promoting cell proliferation, migration and invasion. Wang et al21 demonstrated that lncRNA DGCR5 exerted suppressive function in HCC progression through miR‐346/KLF14 axis. lncRNAs are involved in various biological processes in HCC tumorigenesis, and may be employed as therapeutic targets, thus improving clinical outcomes of the patients. In this study, we investigated clinical significance and related molecular mechanisms of lncRNA SUMO1P3 in HCC progression. The results indicated that lncRNA SUMO1P3 might be an oncogene in HCC through enhancing cell proliferation, migration and invasion, and its up‐regulation showed close association with lymph node metastasis and TNM stage. SUMO1P3 could activate Wnt/β‐catenin pathway that is mediated by miR‐320a, thus contributing to malignant progression of HCC.
Expression levels of lncRNA SUMO1P3 were significantly higher in HCC tissues and cells than in non‐cancerous specimens. Moreover, its up‐regulation predicted positive lymph node metastasis and advanced TNM stages. The overexpression of SUMO1P3 might contribute to malignant progression of HCC. In subsequent cell experiments, we found that the knockdown of SUMO1P3 obviously suppressed the proliferation, migration and invasion abilities of HCC cells. The conclusion was consistent with that from published article. Zhou et al16 reported that HCC patients with high expression of SUMO1P3 were more likely entering advanced TNM stages, while its inhibition might suppress cell proliferation and motility, and promote cell apoptosis. All data revealed that SUMO1P3 acted as an oncogene in HCC.
Despite the lack of protein‐coding ability, lncRNAs could regulate gene expression at both transcriptional and post‐transcriptional levels.8 At post‐transcriptional level, interaction between lncRNAs and microRNAs (miRNAs) is an important pathway to regulate gene expression.22 In our study, we found that the 3′ end of lncRNA SUMO1P3 had complementary sequence of miR‐320a. Moreover, subsequent dual‐luciferase reporter assay demonstrated that miR‐320a was a potential target of SUMO1P3. SUMO1P3 could negatively regulate the expression of miR‐320a in HCC. The up‐regulation of SUMO1P3 resulted in the down‐regulation of miR‐320a, which could promote HCC cell proliferation and invasion, thus contributing to malignant growth and metastasis.23, 24, 25 Our results were in line with those from published articles. The interaction between SUMO1P3 and miR‐320a was also confirmed in breast cancer.13 SUMO1P3 interacting with miR‐320a played an important role in HCC progression.
Growing evidences have demonstrated that SUMO1P3 promotes tumorigenesis through regulating epithelial‐mesenchymal transition (EMT) signalling pathway and angiogenesis.26, 27 Molecular mechanisms of SUMO1P3 in HCC progression were also explored in our study. The study carried out by Lu et al17 suggested that miR‐320a could suppress HCC progression based on its inhibition on Wnt/β‐catenin pathway. β‐Catenin was a direct target of miR‐320a in HCC. In the current study, we found that the knockdown of SUMO1P3 led to the inactivation of Wnt/β‐catenin signalling pathway. Moreover, regulatory function of SUMO1P3 on Wnt/β‐catenin pathway was depended on miR‐320a. The absence of miR‐320a could reverse anti‐tumour action caused by the knockdown of SUMO1P3. Taken together, SUMO1P3 contributed to malignant progression of HCC through activating Wnt/β‐catenin pathway via negatively regulating miR‐320a. However, the study carried out by Xie et al25 demonstrated that miR‐320a played anti‐tumour action in HCC through directly targeting c‐Myc. In addition, HMGB1 was confirmed to be a targeted gene of miR‐320a in HCC.23 All evidences proved that SUMO1P3 might influence multiple signalling pathways through its targeted genes in HCC. However, due to limited study period, regulatory netweb of SUMO1P3 in HCC was not completely explored in our study. Besides, the sample size was relatively small. Clinical significance of SUMO1P3 in HCC requires further investigations with extended sample size. Additionally, experiments in our study only proved that the expression levels of SUMO1P3 could influence the oncogenicity of HCC cells, and whether the overexpression of SUMO1P3 could endow normal hepatic cells with oncogenic ability remained unclear. Lastly, the absence of animal experiments might limit statistical power of our results. Therefore, further researches are in urgent need to address the above issues.
In conclusion, lncRNA SUMO1P3 is up‐regulated in HCC specimens and positively correlated with metastasis and tumour stage. SUMO1P3 has the ability to promote the proliferation, invasion and migration of HCC cells. In HCC, SUMO1P3 could activate miR‐320a–mediated Wnt/β‐catenin signalling pathway, thus contributing to aggressive progression of the disease.
CONFLICT OF INTEREST
None.
AUTHORS' CONTRIBUTIONS
SW and SC conceived and designed the experiments, analysed the data and wrote the paper. NL and JY performed the experiments. All authors read and approved the final manuscript.
Wu S, Chen S, Lin N, Yang J. Long non‐coding RNA SUMO1P3 promotes hepatocellular carcinoma progression through activating Wnt/β‐catenin signalling pathway by targeting miR‐320a . J Cell Mol Med. 2020;24:3108–3116. 10.1111/jcmm.14977
Songsong Wu and Sheng Chen are co‐first authors.
DATA AVAILABILITY STATEMENT
All data generated or analysed during this study are included in this article.
REFERENCES
- 1. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941‐1953. [DOI] [PubMed] [Google Scholar]
- 2. Fan JH, Wang JB, Jiang Y, et al. Attributable causes of liver cancer mortality and incidence in china. Asian Pac J Cancer Prev. 2013;14:7251‐7256. [DOI] [PubMed] [Google Scholar]
- 3. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301‐1314. [DOI] [PubMed] [Google Scholar]
- 4. Said A, Ghufran A. Epidemic of non‐alcoholic fatty liver disease and hepatocellular carcinoma. World J Clin Oncol. 2017;8:429‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rich NE, Yopp AC, Singal AG. Medical management of hepatocellular carcinoma. J Oncol Practi. 2017;13:356‐364. [DOI] [PubMed] [Google Scholar]
- 6. Medavaram S, Zhang Y. Emerging therapies in advanced hepatocellular carcinoma. Exp Hematol Oncol. 2018;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beermann J, Piccoli MT, Viereck J, Thum T. Non‐coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol Rev. 2016;96:1297‐1325. [DOI] [PubMed] [Google Scholar]
- 8. Melissari MT, Grote P. Roles for long non‐coding RNAs in physiology and disease. Pflugers Arch. 2016;468:945‐958. [DOI] [PubMed] [Google Scholar]
- 9. Schmitz SU, Grote P, Herrmann BG. Mechanisms of long noncoding RNA function in development and disease. Cell Mol Life Sci. 2016;73:2491‐2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rafiee A, Riazi‐Rad F, Havaskary M, Nuri F. Long noncoding RNAs: regulation, function and cancer. Biotechnol Genet Eng Rev. 2018;34:153‐180. [DOI] [PubMed] [Google Scholar]
- 11. Fang Y, Fullwood MJ. Roles, functions, and mechanisms of long non‐coding RNAs in cancer. Genomics Proteomics Bioinformatics. 2016;14:42‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qiu L, Tang Q, Li G, Chen K. Long non‐coding RNAs as biomarkers and therapeutic targets: Recent insights into hepatocellular carcinoma. Life Sci. 2017;191:273‐282. [DOI] [PubMed] [Google Scholar]
- 13. Liu J, Song Z, Feng C, et al. The long non‐coding RNA SUMO1P3 facilitates breast cancer progression by negatively regulating miR‐320a. Am J Transl Res. 2017;9:5594‐5602. [PMC free article] [PubMed] [Google Scholar]
- 14. Mei D, Song H, Wang K, et al. Up‐regulation of SUMO1 pseudogene 3 (SUMO1P3) in gastric cancer and its clinical association. Med Oncol. 2013;30:709. [DOI] [PubMed] [Google Scholar]
- 15. Zhan Y, Liu Y, Wang C, et al. Increased expression of SUMO1P3 predicts poor prognosis and promotes tumor growth and metastasis in bladder cancer. Oncotarget. 2016;7:16038‐16048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou Y, He P, Xie X, Sun C. Knockdown of SUMO1P3 represses tumor growth and invasion and enhances radiosensitivity in hepatocellular carcinoma. Mol Cell Biochem. 2019;450(1‐2):125‐134. [DOI] [PubMed] [Google Scholar]
- 17. Lu C, Liao Z, Cai M, Zhang G. MicroRNA‐320a downregulation mediates human liver cancer cell proliferation through the Wnt/beta‐catenin signaling pathway. Oncol Lett. 2017;13:573‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee S‐M, Kim‐Ha J, Choi W‐Y, et al. Interplay of genetic and epigenetic alterations in hepatocellular carcinoma. Epigenomics. 2016;8:993‐1005. [DOI] [PubMed] [Google Scholar]
- 19. Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: a new paradigm. Can Res. 2017;77:3965‐3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhu L, Yang N, Li C, Liu G, Pan W, Li X. Long noncoding RNA NEAT1 promotes cell proliferation, migration, and invasion in hepatocellular carcinoma through interacting with miR‐384. J Cell Biochem. 2019;120(2):1997‐2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang YG, Liu J, Shi M, Chen FX. LncRNA DGCR5 represses the development of hepatocellular carcinoma by targeting the miR‐346/KLF14 axis. J Cell Physiol. 2018;234:572‐580. [DOI] [PubMed] [Google Scholar]
- 22. Dykes IM, Emanueli C. Transcriptional and Post‐transcriptional Gene Regulation by Long Non‐coding RNA. Genomics Proteomics Bioinformatics. 2017;15:177‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lv G, Wu M, Wang M, et al. miR‐320a regulates high mobility group box 1 expression and inhibits invasion and metastasis in hepatocellular carcinoma. Liver Int. 2017;37:1354‐1364. [DOI] [PubMed] [Google Scholar]
- 24. Zhang Z, Li X, Sun W, et al. Loss of exosomal miR‐320a from cancer‐associated fibroblasts contributes to HCC proliferation and metastasis. Cancer Lett. 2017;397:33‐42. [DOI] [PubMed] [Google Scholar]
- 25. Xie F, Yuan Y, Xie L, et al. miRNA‐320a inhibits tumor proliferation and invasion by targeting c‐Myc in human hepatocellular carcinoma. Onco Targets Ther. 2017;10:885‐894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tian C, Jin Y, Shi S. Long non‐coding RNA SUMO1P3 may promote cell proliferation, migration, and invasion of pancreatic cancer via EMT signaling pathway. Oncol Lett. 2018;16:6109‐6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang LM, Wang P, Liu XM, Zhang YJ. LncRNA SUMO1P3 drives colon cancer growth, metastasis and angiogenesis. Am J Transl Res. 2017;9:5461‐5472. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this article.


