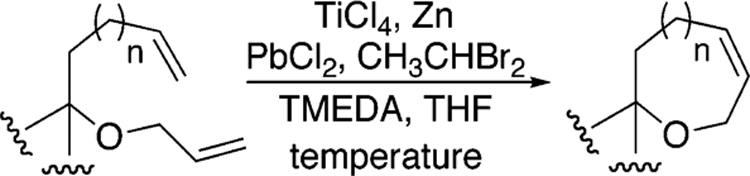
Because of their inherent reactivity, cyclic enol ethers serve as precursors to a number of interesting heterocyclic compounds and have garnered the attention of the chemical synthesis community. Among the variety of methods that have been reported for their synthesis, there has been considerable interest in the cyclization chemistry of olefinic esters via the two-step metathesis sequence outlined in Scheme 1.1,2
Scheme 1.
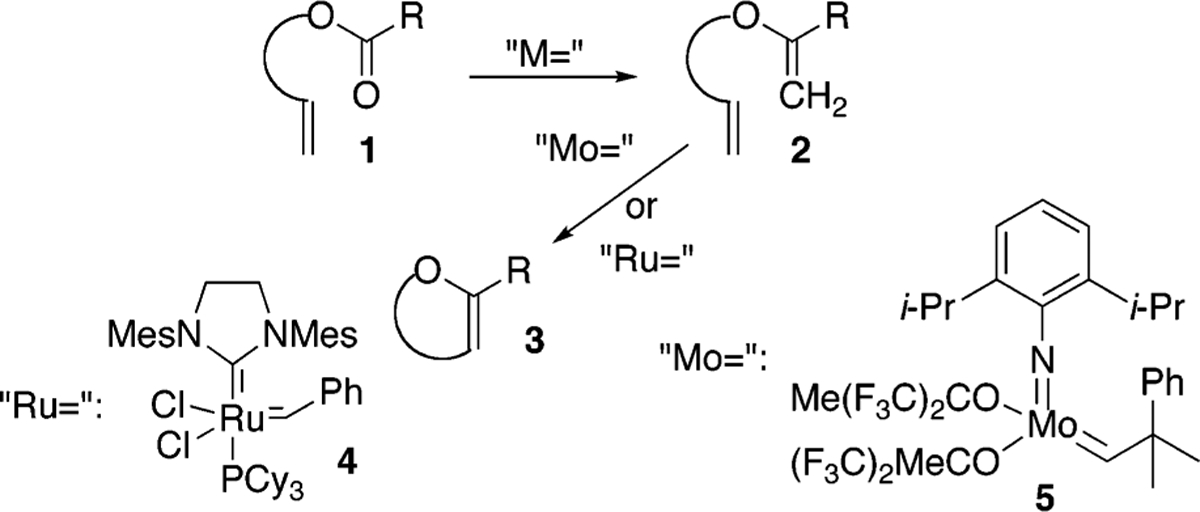
Clearly, a more efficient method of transforming 1 into 3 would bypass acyclic enol ether intermediates (e.g., 2). Along these lines, successes have been reported. In the mid 1980s, Grubbs, Stille, and Santarsiero successfully synthesized capnellene by utilizing strained olefinic esters (norbornenes) in ring-opening metathesis, carbonyl olefination reactions.3 Subsequently, Grubbs and Fu reported the use of a tungsten alkylidene to generate a cyclic enol ether directly from an acyclic olefinic ester.4 In 1996, Nicolaou and co-workers reported olefinic ester cyclization reactions using the Tebbe and Petasis reagents to generate fused ether compounds.5 While Nicolaou’s results were certainly impressive, the applicability of these reagents in olefinic ester cyclizations appears to be limited. A number of groups including ours have reported the reactions to be capricious and to lead, with some substrates, to a multitude of undesired side products.6
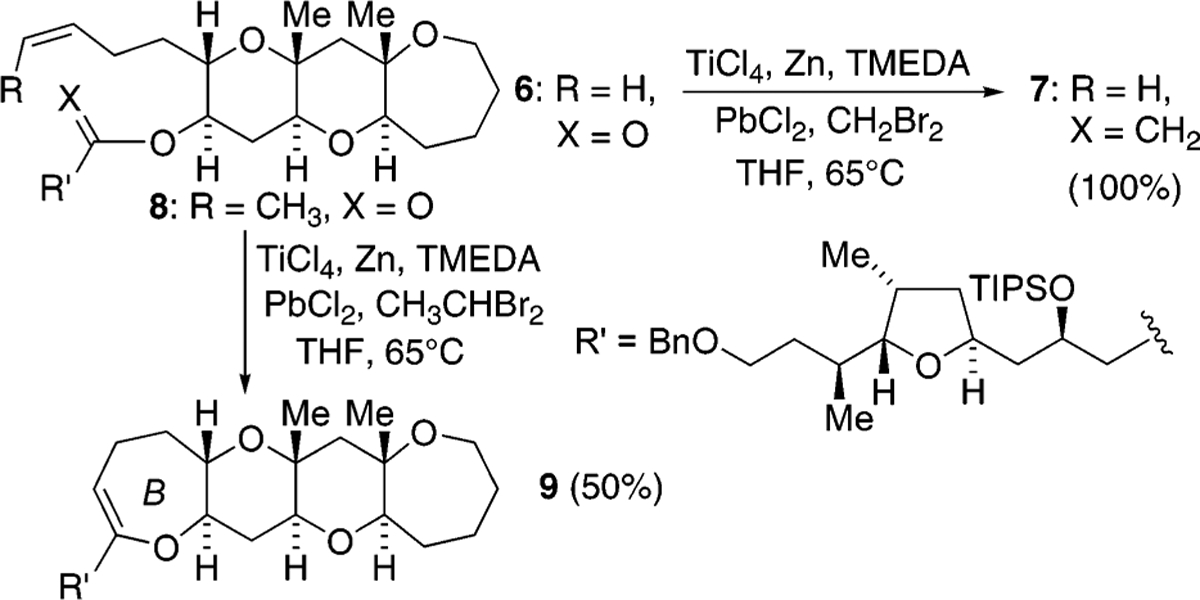
As a means of generating polycyclic ether natural products, we have also been interested in olefinic ester cyclizations and have employed the Takai–Utimoto reduced titanium reagent largely because of its in situ preparation, its increased reactivity relative to the Petasis reagent, and its diminished Lewis acidity relative to the Tebbe reagent.7,8 Although we previously described the use of this reagent to affect olefinic ester cyclizations, the reactions were limited to sterically hindered esters and relatively unhindered olefins.9,10 In contrast to these results, during our recent work targeting the generation of the B ring of gambieric acid A, we were surprised to find that the product distribution (acyclic vs cyclic enol ether) from the reaction of the reduced titanium alkylidene reagent was dependent upon the alkylidene reagent used (Scheme 2).11 That is, the titanium methylidene reagent that comes from the use of dibromomethane as the alkylidene source gave only acyclic enol ether while the corresponding ethylidene reagent from dibromoethane gave only cyclic enol ether. That substitution on the titanium alkylidene reagent could be used to direct reactivity was, to the best of our knowledge, unprecedented and in our opinion deserving of further study. Outlined here are our preliminary experiments aimed at uncovering the scope of this result.
Scheme 2.
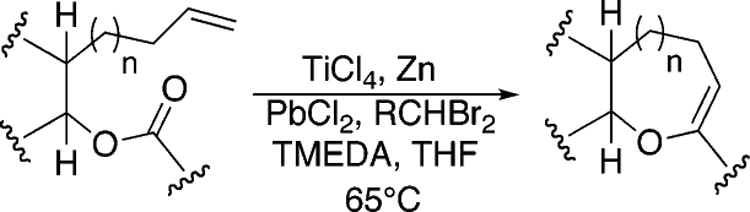
We initially chose to examine whether the reduced titanium ethylidene reagent could affect the cyclization of olefinic ester 10. We had previously examined the reaction of 10 with the corresponding titanium methylidene reagent and had found it to give a mixture of products (Table 1, entry 1).8 In a similar fashion to the results with 8, when we subjected 10 to the titanium ethylidene reagent, we isolated cyclic enol ether 12 as the only identifiable product in 75% yield (entry 2). We next explored the reaction on a more challenging substrate, olefinic ester 13, lacking a preformed cyclic template. When 13 was subjected to the titanium ethylidene reaction conditions, we isolated cyclic enol ether 15 as the only product in 70% yield (Table 1, entry 4). In contrast to this result, the corresponding methylidene reagent gave a 1:1 mixture of cyclic and acyclic enol ethers in 70% yield (entry 3). To get a better sense of the reaction scope, we also examined acyclic templates 16 and 18.2c Both substrates underwent successful cyclization to give dihydropyran 17 and oxepene 19, respectively (Table 1, entries 5 and 6).
Table 1.
Olefinic Ester Cyclizations
 | ||||
|---|---|---|---|---|
| entry | starting material/acyclic enol ether | RCHBr2 | cyclic enol ether | yield (cyclic:acyclic) |
| 1 | 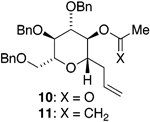 |
CH2Br2 | 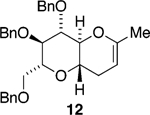 |
80% (5:3) |
| 2 | 10 | CH3CHBr2 | 12 | 75% (>95:5) |
| 3a | 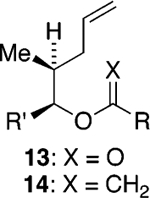 |
CH2Br2 | 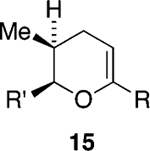 |
70% (1:1) |
| 4 | 13 | CH3CHBr2 | 15 | 70% (>95:5) |
| 5 | 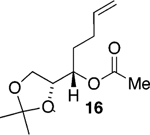 |
CH3CHBr2 | 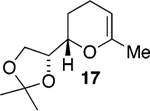 |
78% (>95:5) |
| 6 | 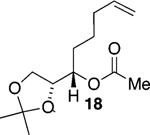 |
CH3CHBr2 | 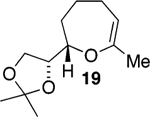 |
82% (>95:5) |
R = CH2CH2CH2CH(OMe)2, R′ = CH2CH2CH2OTBDPS
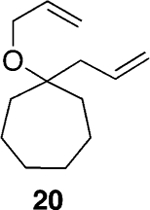
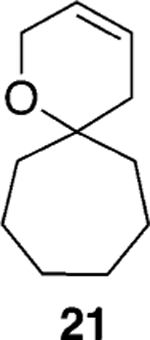
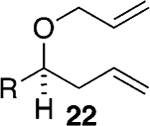
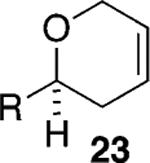
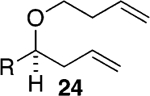
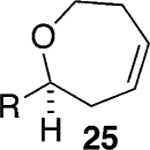
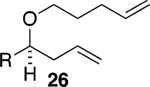
Assuming titanium alkylidenes to be involved in the olefinic ester chemistry outlined above, we became intrigued by the possibility that the titanium reagent might also induce diene ring-closing metathesis (RCM) cyclizations. With the exception of Nicolaou’s enol ether–olefin RCM chemistry,5 to the best of our knowledge, there are no reports of unstrained dienes undergoing RCM reactions using titanium alkylidenes.12 In light of this, we were pleasantly surprised to isolate spirocyclic allyl ether 2113 in quantitative yield when diene 20 was subjected to the titanium ethylidene reagent (Table 2, entry 1). Impressively, this transformation even proceeded at room temperature. We were also pleased to be able to generate dihydropyran 23,14 oxepene 25,15 and oxocene 2716 from the reactions of the titanium ethylidene reagent with the acyclic allyl ethers 22, 24, and 26, respectively (Table 2, entries 2–4).
Table 2.
Reduced Titanium-Mediated Diene RCM
R = CH2CH2Ph
In summary, this communication has described the unique and unprecedented reactivity of an in-situ-generated reduced titanium ethylidene reagent. Although optimization of both olefinic ester and diene RCM cyclization reactions is clearly required, for example, we have not explored the effect of stoichiometry or additives nor have we focused on the scale-up of the reaction (the largest scale carried out thus far in our hands has been 0.5 g), we believe that these studies represent a significant breakthrough. The reduced titanium reagent described here is relatively inexpensive, it is generated in situ, and it is tolerant of a wide variety of functionality.1c We intend to continue to study the scope of its reactivity.
Acknowledgment.
We are grateful to the National Institutes of Health, General Medical Sciences (GM56677) for support of this work. We would like to thank the support staff at the University of Utah and especially Dr. Charles Mayne (NMR) and Dr. Jim Muller (mass spectrometry) for help in obtaining data. We would also like to thank Professor Gary Keck and Mr. Lars Heumann for graciously providing the homoallyl alcohol precursor to 22, 24, and 26.
Footnotes
Supporting Information Available: General experimental procedure for the cyclization reaction and spectroscopic data for all new cyclic compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).(a) Deiters A; Martin SF Chem. Rev 2004, 104, 2199. [DOI] [PubMed] [Google Scholar]; (b) Brown RCD; Satcharoen V Heterocycles 2006, 70, 705. [Google Scholar]; (c) Hartley RC; McKiernan GJJ Chem. Soc., Perkin Trans 1 2002, 2763. [Google Scholar]
- (2).(a) Fujimura O; Fu GC; Grubbs RH J. Org. Chem 1994, 59, 4029. [Google Scholar]; (b) Clark JS; Kettle JG Tetrahedron Lett 1997, 38, 123. [Google Scholar]; (c) Clark JS; Kettle JG Tetrahedron Lett 1997, 38, 127. [Google Scholar]; (d) Postema MH D. J. Org. Chem 1999, 64, 1770. [DOI] [PubMed] [Google Scholar]; (e) Clark JS; Hamelin O Angew. Chem., Int. Ed 2000, 39, 372. [DOI] [PubMed] [Google Scholar]; (f) Chatterjee AK; Morgan JP; Scholl M; Grubbs RH J. Am. Chem. Soc 2000, 122, 3783. [Google Scholar]; (g) Postema MHD; Calimente D; Liu L; Behrmann TJ Org. Chem 2000, 65, 6061. [DOI] [PubMed] [Google Scholar]; (h) Rainier JD; Cox JM; Allwein SP Tetrahedron Lett 2001, 42, 179. [Google Scholar]; (i) Liu L; Postema MH D. J. Am. Chem. Soc 2001, 123, 8602. [DOI] [PubMed] [Google Scholar]
- (3).(a) Stille JR; Grubbs RH J. Am. Chem. Soc 1986, 108, 855. [Google Scholar]; (b) Stille JR; Santarsiero BD; Grubbs RH J. Org. Chem 1990, 55, 843. [Google Scholar]
- (4).Fu GC; Grubbs RH J. Am. Chem. Soc 1993, 115, 3800. [Google Scholar]
- (5).(a) Nicolaou KC; Postema MHD; Claiborne CF J. Am. Chem. Soc 1996, 118, 1565. [Google Scholar]; (b) Nicolaou KC; Postema MHD; Yue EW; Nadin AJ Am. Chem. Soc 1996, 118, 10335. [Google Scholar]
- (6).See refs 8 and 10b and:; (a) Oishi T; Uehara H; Nagumo Y; Shoji M; Le Brazidec J-Y; Kosaka M; Hirama M. Chem. Commun 2001, 381. [Google Scholar]; (b) Uehara H; Oishi T; Inoue M; Shoji M; Nagumo Y; Kosaka M; Le Brazidec J-Y; Hirama M Tetrahedron 2002, 58, 6493. [Google Scholar]; (c) Kadota I; Kadowaki C; Park C-H; Takamura H; Sato K; Chan PWH; Thorand S; Yamamoto Y Tetrahedron 2002, 58, 1799. [Google Scholar]
- (7).Takai K; Kakiuchi T; Kataoka Y; Utimoto KJ Org. Chem 1994, 59, 2668. [Google Scholar]
- (8).Allwein SP; Cox JM; Howard BE; Johnson HWB; Rainier JD Tetrahedron 2002, 58, 1997. [Google Scholar]
- (9).Related dithiane ester cyclization reactions have been reported by Takeda and Hirama. See ref 6 and Takeda T Bull. Chem. Soc. Jpn 2005, 78, 195. [Google Scholar]
- (10).(a) We have demonstrated that the cyclic products came from an olefin metathesis, carbonyl olefination mechanistic sequence. See: Rainier JD; Allwein SP; Cox JM J. Org. Chem 2001, 66, 1380. [DOI] [PubMed] [Google Scholar]; (b) Majumder U; Rainier JD Tetrahedron Lett 2005, 46, 7209. [Google Scholar]
- (11).Roberts SW; Rainier JD Org. Lett 2007, 9, 2227. [DOI] [PubMed] [Google Scholar]
- (12).We are aware of a single example of the use of norbornene–olefin ROM/RCM using the Tebbe reagent. See: Halterman RL Ramsey TM J. Organomet. Chem 1997, 547, 41. [Google Scholar]
- (13).Maier ME; Bugl M Synlett 1998, 1390. [Google Scholar]
- (14).Schmidt BJ Org. Chem 2004, 69, 7672. [DOI] [PubMed] [Google Scholar]
- (15).Castaneda A; Kucera DJ; Overman LE J. Org. Chem 1989, 54, 5695. [Google Scholar]
- (16).Blumenkopf TA; Bratz M; Castaneda A; Look GC; Overman LE; Rodriguez D; Thompson AS J. Am. Chem. Soc 1990, 112, 4386. [Google Scholar]