Abstract
Policy Points.
US maternal mortality rates (MMRs) display considerable racial disparities and exceed those of other developed countries. While worldwide MMRs have dropped sharply since the 1990s, the US MMR appears to be rising.
We provide strong evidence of the effectiveness of pregnancy‐related public health spending on improvements in maternal health.
Using longitudinal data from Florida counties, we found that spending on public health significantly reduced the MMR among black mothers and narrowed black‐white outcome disparities. Each 10% increase in pregnancy‐related public health expenditures was associated with a 13.5% decline in MMR among blacks and a 20.0% reduction in black‐white disparities.
Context
Maternal mortality rates in the United States exceed those of other developed countries. Moreover, these rates show considerable racial disparities, in which black mothers are at three to four times the risk compared with their white counterparts. With more than half of all maternal deaths deemed to be preventable, public health interventions have the potential to improve maternal health along with other pregnancy outcomes. This rigorous longitudinal study examines the impact of a package of pregnancy‐related public health programs on maternal mortality rates.
Methods
We analyzed administrative data on pregnancy‐related public health expenditures, maternal mortality rates, and sociodemographic factors from all 67 Florida counties between 2001 and 2014. Florida provides consistent counts of maternal deaths for the entire period of this analysis. We estimated both fixed‐effects ordinary least squares regressions (OLS) and generalized method of moments (GMM) models. GMM enabled us to identify the impact of public health expenditures on maternal mortality rates while also addressing both potential endogeneity and serial correlation problems. We also provide a series of robustness and falsification tests.
Findings
Overall, a 10% increase in targeted public health expenditures led to a weakly significant decline in overall maternal mortality rates of 3.9%. The estimated effect for white mothers was not statistically significant. However, we found statistically significant improvements for black mothers. Specifically, a 10% increase in pregnancy‐related public health spending led to a 13.5% decline in maternal mortality rates among black mothers and a 20.0% reduction in the black‐white maternal mortality gap.
Conclusions
Our analysis provides strong evidence of the effectiveness of public health programs in reducing maternal mortality rates and addressing racial disparities.
Keywords: Maternal mortality, public health, Florida, generalized method of moments
Maternal mortality rates (mmrs) in the united States not only exceed those of other developed countries, but they also exhibit considerable racial disparities.1, 2 In 2013, there were 12.7 deaths per 100,000 live births for white women and 43.5 for black women.3 The problem is significant enough to have been the subject of scrutiny even in the popular media. A 2017 investigative reporting series highlighted the alarming increase in “lost mothers” and concluded that “private tragedies point to a much bigger public health problem.”4 Later that same year, the near‐death experience of tennis star Serena Williams in childbirth led to further debate on the possible reasons for the racial disparities in both infant and maternal mortality in the United States.5
Moreover, the MMR in the United States seems to have risen by 49.2% between 1990 and 2013.6 In 2015, the United States—along with other countries such as Jamaica, North Korea, Serbia, and South Africa—was among the 13 countries in the entire world where MMRs were higher than in 1990.7 However, trends in the US MMR suffer from measurement issues. MacDorman and colleagues8 pointed out that in 2003, death certificates in the United States were revised to include a pregnancy check box in an effort to better capture maternal deaths. This led to a substantial rise in deaths attributed to maternal issues, which complicated studies of national time trends. Even though the pregnancy check box may have led to some overcounting,9 MMRs in the early 2000s were higher than previously reported, which suggests that maternal deaths had been a significant public health problem in the United States long before the revised measurements revealed it more clearly.8
Rising maternal mortality in the United States can be attributed to a number of factors. Clinicians have focused on the proximate medical factors contributing to the problem while mostly neglecting the social aspects that might also affect a mother's health before and after childbirth.2 Health services researchers, however, tend to consider other outcomes, such as maternal complications, that are far more common than maternal mortality.10 In general, there seems to be a decrease in the “classic” causes of maternal mortality, such as hemorrhage, whereas the number of deaths due to conditions like cardiovascular disease is on the rise.11 The increasing age of mothers and the higher prevalence of chronic health conditions such as hypertension, diabetes, and obesity can also complicate childbirth.12 The influence of these factors among low‐income and rural populations has grown over the last decade, which can explain some of the increases in racial disparities in maternal mortality.13 Leonard and colleagues14 examined trends in disparities over time and found that changes in the characteristics of pregnant women such as comorbidities did not explain why racial/ethnic differences in severe maternal morbidity persist over time. Beyond the impact of sociodemographic and obstetric characteristics as well as the comorbidities that are often associated with race, the strains of dealing with race‐based implicit bias, institutionalized racism, and segregation present yet another level of risk.15, 16
Although about 35% to 60% of all maternal deaths are estimated to be potentially preventable, this proportion varies by the specific cause of death and, in some cases, can be higher than 90%.17, 18, 19 Public health programs, with their explicit accommodation of racial, cultural, and ethnic differences, and a service focus that often extends from clinical to social needs, have the potential to mitigate some of the risk factors just discussed. They have been particularly useful in addressing disparities in infant health outcomes. For example, Moehling and Thomasson20 examined the introduction of the Sheppard‐Towner Act of 1921, which increased spending on public health education programs targeted at reducing infant and maternal mortality. They found that interventions such as nurse home visits and the establishment of health clinics were particularly effective among nonwhites in reducing state‐level infant mortality rates. Blacks do not appear to have been excluded from these programs and ended up benefiting more than whites did. This is because of the disproportional prevalence of the problem among nonwhites compared with whites, as well as the differences in health literacy and access to health care services. Similarly, Hoehn‐Velasco21 analyzed the provision of sanitation and child‐oriented health services by county health departments from 1908 to 1933 and found little to no reduction in white infant mortality rates. But for nonwhites in rural‐only counties and in counties with less wealth and access to health care, there were much greater declines in infant mortality.
Bhatia and colleagues22 reported that public health programs played a central role in reducing infant mortality in the United States in the early 20th century and explained that some of these structural interventions were designed in response to a growing awareness of racial/ethnic disparities in maternal and child mortality. Bekemeier and colleagues23 examined the black‐white mortality gap during a more recent time period, 1993 to 2005. They compiled county‐level information for a national sample from various data sources and identified 10 specific local health department service domains. After investigating which of these services were significantly associated with the racial disparity gap in all‐cause mortality rates across the United States, they concluded that maternal and child health activities were one of the two service domains significantly associated with drops in the black‐white mortality gap among 15‐ to 44‐year‐olds.
Empirical evidence on maternal mortality is much scarcer in the United States. Olds and colleagues24 conducted a randomized clinical trial of nurse home visits in a public system of obstetric and pediatric care in Memphis, Tennessee, between 1990 and 2011. The participants were recruited primarily among African American women and their first live‐born child living in disadvantaged urban neighborhoods. In addition to various pregnancy and child health outcomes through the first two years of life, Olds and colleagues also examined the effects on maternal mortality. They found statistically significant effects of nurse home visits on maternal mortality in some treatment groups but not others. The main reason was that even when individuals are observed over a two‐decade‐long period, maternal mortality is so seldom observed in a randomized‐controlled trial that it is difficult to draw meaningful conclusions.
As such, macro level studies at the state or county level can be much more informative. Typically, however, macro data on health outcomes, along with detailed information about public health activities, such as that used by Bekemeier and colleagues,23 is difficult to find. Studies trying to discern the effect of public health spending on particular health outcomes, including maternal and child health, have difficulty demonstrating its effectiveness because of the lack of uniform granular data regarding the specific use of funds. Mays and Smith25 and, more recently, Bernet and colleagues26 offer detailed discussions of these data limitations.
For this study, we created a panel data set of pregnancy‐related public health program expenditures for all Florida counties from 2001 through 2014, paired with MMRs as well as demographic and socioeconomic factors, to examine the effect of public health expenditures on MMRs. We specifically focused on maternal and infant health‐related spending and its role in mitigating maternal mortality and racial disparities. We used a uniquely detailed data set from the Florida Department of Health (FDOH), which allowed us to consistently disaggregate spending by use for all Florida counties during a 14‐year period. Our main contributions are threefold: (1) we concentrated on targeted spending by identifying expenditures on programs that could affect maternal health; (2) we estimated the impact of public health spending on maternal mortality; and (3) we examined whites and blacks separately, because public health spending may be particularly important to disadvantaged groups.
Data and Methods
Maternal Mortality Rates
For this study, we concentrated on a single state, Florida, which provides consistent counts of maternal deaths for the entire period of our analysis through its publicly accessible data portal, FLHealthCHARTS.com. MacDorman and colleagues8 suggest that MMR data ideally should be analyzed individually for each state in the United States because states have changed their measurement of MMRs at different times, making it difficult for researchers who want to examine national data. Our panel covers all 67 Florida counties for each year between 2001 and 2014. Since we utilize publicly available data aggregated at the county level, our study was exempt from approval by an institutional review board for research involving human subjects. Maternal deaths are all female deaths due to “complications during pregnancy, childbirth, or the period immediately following childbirth” (International Classification of Disease ICD‐10 codes A34, O00‐O95, O98‐O99). The period immediately following childbirth is defined as up to one year postpartum. MMR is expressed as the count of maternal deaths per 100,000 live births.
The count of maternal deaths we used is somewhat different from what Florida collects under its Pregnancy‐Associated Mortality Review (PAMR) registry. PAMR uses a broad set of criteria to identify maternal deaths—not only the ICD‐10 codes just listed but also a team review that relies on other information, including the pregnancy check box indicating that the decedent was pregnant or had a matching birth (or fetal death) record within the year prior to death. Therefore, FLHealthCHARTS.com likely underreports pregnancy‐related deaths compared with the PAMR registry figures.
When the US maternal mortality measures were modified in 2003, following the revision of the death certificates to include a pregnancy check box, the incident classifications employed in the Pregnancy Mortality Surveillance System were standardized.8 Florida adopted these standards by 2006, and all deaths between 1998 and 2005 were subsequently recoded in the PAMR registry using this system to allow for the proper evaluation of trends over time.27 In contrast, FLHealthCHARTS.com data have always reported the number of female deaths with a specific set of underlying ICD‐10 codes and have used the same counting method since 1999 (the introduction of 10th revision of ICD), well before the start of our analysis period.
Pregnancy‐Related Public Health Spending
Public health spending on pregnancy‐related programs is the key independent variable in our study. FDOH provides data on spending by county and program, and statewide accounting standards ensure the comparability of expenditures across all counties and years. We defined pregnancy‐related spending as the sum of expenditures on three specific services that can directly affect maternal mortality: (1) the Maternal Health and Improved Pregnancy Outcomes program provides pregnancy testing, nurse home visits to pregnant women, vitamin supplements, depression screening, educational material, and other services such as Spanish and Creole interpretation; (2) the Healthy Start program helps women, infants, and children up to age three with nutritional advice, psychosocial counseling, smoking cessation counseling, breast‐feeding training, interconception education, and home visits28; (3) and finally, Women, Infants, and Children is a federally funded nutrition program providing healthy foods, nutrition education, counseling, and other services. These programs provide nurse home visits, nutritional support, screening, and counseling both pre‐ and postpartum, all of which are expected to affect maternal outcomes. These same programs have also been shown to improve birth outcomes29 and reduce infant mortality.26 While other public health initiatives might improve maternal health, such as smoking cessation, we deliberately chose these three programs for two reasons. First, these programs directly targeted improvements in the health of infants and their mothers. Second, our data source allowed us to disaggregate expenditures that are spent on these three sets of services in each county and year.
Control Variables
We also accounted for a set of time‐varying county characteristics that could influence MMRs. We included demographic characteristics: the percentage of the population that is nonwhite, of Hispanic ethnicity, and of the child‐bearing ages 15 to 44 and ages 65 or older. We also added the unemployment rate, personal income per capita, percentage of births covered by Medicaid, and number of physicians and hospital beds per 100,000 people. These control variables were meant to capture demographic and socioeconomic factors, macroeconomic conditions, as well as access to health care services. The existing literature on maternal and child health outcomes informed our selection of control variables.30, 31, 32 Since we included county‐ and year‐specific fixed effects in all our models, as we later explain, variables that do not vary within‐county over time would not be meaningful to include. Some other factors that would be appropriate to include were, however, unavailable at the county level for each year and for all 67 counties of Florida. For example, the percentage of births covered by private health insurance plans could be an important determinant of maternal health, but such data were not available for all Florida counties and the years under study.
Empirical Methodology
Following the literature,31, 32 we first used ordinary least squares (OLS) with county and year fixed effects to estimate the following equation:
| (1) |
where MMRct is defined as the count of maternal deaths per 100,000 live births in county c and year t. We separately estimated the overall MMR, the black MMR, and the white MMR, as well as the black‐white MMR gap (defined as black MMR minus white MMR). The regressor Ln(PHExpct‐1), which is the focus of this study, represents the one‐year lagged pregnancy‐related real public health expenditures (as defined earlier) per 100,000 live births. We used the first lag to allow sufficient time to lapse between public spending and possible maternal death during pregnancy, birth, and postpartum. We also conducted robustness checks that utilized contemporaneous, second lag, and third lag of expenditures. The estimated coefficient for Ln(PHExpct‐1) can be interpreted as a semi‐elasticity, that is, a unit change in MMR that is associated with a 100% change in the public health spending rate. The vector X contains the first lags of time‐varying control variables described earlier. Year fixed effects (δt) accounted for annual statewide trends in the MMR. Time‐invariant regional differences in MMRs across Florida counties were accommodated through county‐specific fixed effects (γc), and εct is the error term.
OLS estimation fails to account for cyclical patterns: Higher public health expenditures can improve outcomes, but improved outcomes may lower spending. As a result, we modified the specification as
| (2) |
We included MMR ct‐1 to capture each county's previous outcomes. Introducing the lagged dependent variable leads to autocorrelation and inconsistent OLS estimates. In particular, if counties responded to worsening maternal and child health outcomes by increasing their pregnancy‐related spending, then the beneficial effect of public health efforts would be underestimated in a classic OLS model. In addition, public health spending may not be strictly exogenous. Concerns about both endogeneity and serial correlation can be addressed by using generalized method of moments (GMM) models.33 GMM allows us to specify public health expenditures and maternal mortality as endogenous and uses lagged differences and levels of the endogenous variables as instruments. Specifically, we estimated system GMM models using the first and the second lags of the two endogenous variables along with the year dummies as instruments.
Several identification assumptions underlie the GMM model. A critical assumption is that the utilized instruments are valid, that is, exogenously determined. Roodman34 described a Sargan/Hansen test of the overidentifying restrictions, which we implemented and reported in our tables. In an effort to avoid a “weak” set of instruments, one may be tempted to utilize a large number of lags as instruments. Including an excessive number of instruments in models with short time periods, however, can lead to a common pitfall with “implausibly good p‐values” close to 1.000.34 Hence, the choice of lags for instruments needs to be validated by the Hansen's J‐test for overidentifying restrictions. In addition to performing this test, we also experimented with various lags as instruments in a robustness check. Another set of diagnostic statistics we used, AR1 and AR2 tests, gauges the appropriateness of GMM models, as opposed to using OLS. AR1 (AR2) tests the null hypothesis of serial correlation of order one (two) in the residuals. If rejected, this test would indicate that serial correlation is a valid concern and that the OLS results are not consistent.
We reported these diagnostic test statistics for all the GMM models in our tables. Finally, in the GMM models, the standard error estimates are not necessarily robust to heteroskedasticity or serial correlation in the error terms.34 To address this, we computed and reported Windmeijer‐corrected cluster–robust standard errors that allow for the nonindependence of observations within each county. Further details on GMM, including other underlying assumptions of the two‐step system estimations, as well as the methodological advantages of GMM over OLS models, have been described elsewhere.26, 34, 35
Results
Figure 1 shows that the black MMR is higher than the white MMR in Florida and that both increased considerably over this period. While targeted public health expenditures increased somewhat after the Great Recession of 2007, there was a steady decline from 2011 to 2014. Table 1 provides descriptive statistics for all the variables, along with our data sources. From 2001 to 2014, the average MMR in Florida was 19.429 per 100,000 live births, with the MMR almost three‐times higher among black mothers than among white mothers (40.697 and 15.736, respectively). Spending on pregnancy‐related public health programs, described later, averaged $2.755 million per county per year (all amounts throughout this study are stated in constant 2014 dollars), or $105 million per 100,000 live births.
Figure 1.
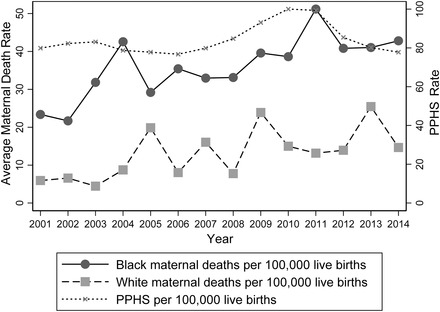
Maternal Mortality Rates and Pregnancy‐Related Public Health Spending (PPHS), Florida, 2001‐2014
Authors calculations using FDOH data; public health spending is in constant 2014 million dollars.
Table 1.
Descriptive Statistics for All Florida Counties, 2001‐2014 (N = 938 County‐Year Observations)
| Variable | Mean | Standard Deviation |
|---|---|---|
| Outcome measures a | ||
| Maternal mortality rate (per 100,000 live births) | 19.429 | 61.121 |
| Whites | 15.736 | 70.310 |
| Blacks | 40.697 | 215.901 |
| Pregnancy‐related public health spending b | ||
| Pregnancy‐related public health spendingb | 2,754,846 | 3,994,893 |
| Pregnancy‐related public health spending per 100,000 live birthsa , b | 105,000,000 | 57,100,000 |
| Control variables | ||
| Percentage of nonwhitesc | 18.19 | 9.69 |
| Percentage of Hispanic ethnicityc | 11.49 | 11.56 |
| Percentage of age 15‐44a | 38.28 | 6.36 |
| Percentage of age 65+a | 18.03 | 6.71 |
| Unemployment rated | 6.60 | 2.71 |
| Personal income per capitae | 36,008 | 11,795 |
| Percentage of births covered by Medicaida | 42.89 | 24.99 |
| Physicians per 100,000 peoplef | 141 | 108 |
| Hospital beds per 100,000 peopleg | 232 | 136 |
| Total live birthsa | 3,275 | 5,643 |
| Whites | 2,376 | 3,863 |
| Blacks | 730 | 1,599 |
Data from FloridaCHARTS.com Query System. FloridaCHARTS.com is provided by the Florida Department of Health, Division of Public Health Statistics & Performance Management.
Spending includes expenditures on the Maternal Health and Improved Pregnancy Outcomes, Healthy Start (Prenatal, Infants, and Interconception Woman), and the Women, Infants, and Children programs. Expressed in constant 2014 $. Data from the Florida Department of Health.
Data from the US Census Bureau.
Data from the US Department of Labor, Bureau of Labor Statistics.
Data from the US Department of Commerce, Bureau of Economic Analysis. Expressed in constant 2014 $.
Data from the Florida Department of Health, Division of Medical Quality Assurance.
Data from the Florida Agency for Health Care Administration (AHCA).
In Table 2, we present estimation results for both the OLS and GMM models, in which the dependent variable is the overall MMR in column 1. We then consider the white MMR, black MMR, and the black‐white MMR gap in Columns 2, 3, and 4, respectively. We report the estimated coefficients and corresponding robust standard errors (in parentheses) for all models. Model A, the OLS model with county and year fixed effects, yielded a barely statistically significant negative coefficient for health expenditures on the overall MMR, a positive but statistically insignificant effect for the white MMR, and a rather large and highly statistically significant negative coefficient for the black MMR.
Table 2.
Estimation Results for Maternal Mortality Rates
| Overall | Whites | Blacks | Blacks‐Whites | |
|---|---|---|---|---|
| (1) | (2) | (3) | (4) | |
| Model A: Fixed‐effects OLS regression (N = 871) | ||||
| Ln(Pregnancy‐related public health spending per 100,000 live births)t‐1 | ‒6.688* | 1.805 | −57.118*** | ‒58.923*** |
| (3.713) | (3.29) | (19.390) | (20.342) | |
| Model B: Two‐step system GMM estimation (N = 871) | ||||
| Ln(Pregnancy‐related public health spending per 100,000 live births)t‐1 | ‒7.645* | ‒1.121 | ‒55.046** | ‒53.656*** |
| (3.946) | (4.43) | (26.108) | (20.600) | |
| AR1 test: p‐value | 0.005 | 0.054 | 0.013 | 0.006 |
| AR2 test: p‐value | 0.219 | 0.909 | 0.281 | 0.419 |
| Hansen test: p‐value | 0.948 | 0.996 | 0.941 | 0.982 |
| Model C: Two‐step system GMM weighted estimation (N = 871) | ||||
| Ln(Pregnancy‐related public health spending per 100,000 live births)t‐1 | ‒10.433* | ‒3.419 | ‒40.021** | ‒53.133*** |
| (6.272) | (8.189) | (19.665) | (15.596) | |
| AR1 test: p‐value | 0.013 | 0.076 | 0.025 | 0.009 |
| AR2 test: p‐value | 0.279 | 0.712 | 0.094 | 0.380 |
| Hansen test: p‐value | 0.966 | 0.982 | 0.934 | 0.894 |
| Model D: Two‐step system GMM estimation using the second lag of spending (N = 804) | ||||
| Ln(Pregnancy‐related public health spending per 100,000 live births)t‐2 | ‒10.553* | ‒3.221 | ‒69.792*** | ‒67.748** |
| (6.190) | (4.70) | (26.029) | (27.683) | |
| AR1 test: p‐value | 0.006 | 0.055 | 0.011 | 0.006 |
| AR2 test: p‐value | 0.405 | 0.560 | 0.132 | 0.179 |
| Hansen test: p‐value | 0.910 | 0.879 | 0.641 | 0.803 |
The dependent variable is the maternal mortality rate per 100,000 live births. All models include year and county fixed‐effects, a constant term, and the control variables listed in Table 1. Models B, C, and D also include lagged dependent variable as a regressor. Robust (and Windmeijer‐corrected in Models B, C, and D) standard errors are in parentheses.
*, **, ***; Significance at the 10%, 5%, and 1% level, respectively.
In Model B of Table 2, the GMM estimates suggest that a 100% increase in targeted public health expenditures has a weakly statistically significant effect of 7.645 decline in the overall MMR. If we consider a more reasonable 10% increase in targeted public health expenditures, it would lead to a 0.765 decline in the MMR, an improvement of 3.9%. Even this relatively small effect is larger than the 2.07% decrease in the infant mortality rate in response to a 10% increase in targeted spending reported by Bernet and colleagues.26 Although the estimate for white mothers in Model B turns negative, it still is not statistically significant. Among black mothers, each 10% increase in pregnancy‐related public health spending (per 100,000 live births) led to a statistically significant 5.505 decline in the MMR, holding everything else constant. Based on the mean black MMR reported in Table 1, this translates into an improvement of 13.5%. Most strikingly, a 10% increase in targeted spending led to a 5.366 reduction in the black‐white MMR difference, which is a decline of 20.0%, considering that the average gap in our sample is 26.8.
Under each column in Model B of Table 2, we provide p‐values for AR1, AR2, and Sargan/Hansen tests that validate the choice of GMM and the instruments used. Specifically, the Sargan/Hansen test statistics for overidentifying restrictions never reject the null hypothesis that the instruments are jointly valid. While the null hypothesis of no serial correlation of order one in the residuals (AR1 test) is rejected, the null hypothesis of no serial correlation of order two in the residuals (AR2 test) is never rejected. This suggests that serial correlation in this context is a valid concern, and thus the OLS results are not consistent. Nevertheless, the OLS and GMM estimates in the two top panels of Table 2 are rather similar.
In Model C, we present GMM regressions weighted by the number of live births in each county. Weighted regressions are more efficient only if the form of heteroskedasticity is chosen correctly. Bearing this caveat in mind, a comparison of estimates in Models A and B with those in C reveals that the OLS estimate for overall MMR may be biased downward and that the OLS estimate for the black MMR may be biased upward. Our overall conclusions, however, remain the same. In Model D, we re‐estimated all our models using the second lag of expenditures instead of the first one. This exercise yielded estimates larger than those of Model B and showed larger differences than those of the OLS estimates, suggesting that public health expenditures from two years prior exert a larger influence on maternal mortality. This may be true if earlier interventions are likely to matter more.
In the following, we concentrate on Model B results mainly because they are more conservative estimates compared with those in Model D. We also tried replacing the first lag of the expenditures with contemporaneous expenditures and then with the third lag of expenditures. As expected, contemporaneous spending yielded similar but relatively smaller estimates than those in Model B, and the third lag of expenditures did not have any statistically significant effect on MMRs.
Finally, in Table 3, we present additional evidence that the observed effects are not spurious. Panel I in this table repeats the baseline results from Table 2 (Model B) for comparison purposes. In Panel II, we display the estimated effect of total public health expenditures on MMRs. We found that overall public health spending does not yield any statistically significant effects on MMRs, which is consistent with the existing findings.26 This is not surprising, as aggregate public health expenditures fail to distinguish between spending items specifically devoted to maternal health versus other outcomes. Next, as falsification tests, we replaced pregnancy‐related spending with other relatively large public health expenditures, first those on immunization (Panel III) and then on tobacco control programs (Panel IV). As expected, neither of these two expenditures yielded any statistically significant effects on MMRs.
Table 3.
Falsification Tests Using Two‐Step System GMM Estimation (N = 871)
| Overall | Whites | Blacks | Blacks‐Whites | |
|---|---|---|---|---|
| (1) | (2) | (3) | (4) | |
| Panel I: Baseline results from Table 2, Model B using pregnancy‐related public health spending | ||||
| Ln(Pregnancy‐related public health spending per 100,000 live births)t–1 | ‒7.645* | ‒1.121 | ‒55.046** | ‒53.656*** |
| (3.946) | (4.437) | (26.108) | (20.600) | |
| AR1 test: p‐value | 0.005 | 0.054 | 0.013 | 0.006 |
| AR2 test: p‐value | 0.219 | 0.909 | 0.281 | 0.419 |
| Hansen test: p‐value | 0.948 | 0.996 | 0.941 | 0.982 |
| Panel II: Using total public health spending | ||||
| Ln(Total public health spending per 100,000 live births)t‐1 | ‒3.146 | ‒12.197 | 31.067 | 82.759 |
| (20.727) | (14.691) | (68.750) | (88.480) | |
| AR1 test: p‐value | 0.005 | 0.051 | 0.009 | 0.005 |
| AR2 test: p‐value | 0.138 | 0.416 | 0.168 | 0.254 |
| Hansen test: p‐value | 0.999 | 0.999 | 0.667 | 0.965 |
| Panel III: Using immunization‐related public health spending | ||||
| Ln(Immunization‐related public health spending per 100,000 live births)t‐1 | ‒15.848 | ‒14.972 | ‒7.406 | 16.221 |
| (12.638) | (12.283) | (67.832) | (62.563) | |
| AR1 test: p‐value | 0.006 | 0.054 | 0.009 | 0.004 |
| AR2 test: p‐value | 0.398 | 0.261 | 0.168 | 0.237 |
| Hansen test: p‐value | 0.990 | 0.982 | 0.888 | 0.932 |
| Panel IV: Using tobacco‐related public health spending | ||||
| Ln(Tobacco‐related public health spending per 100,000 live births)t‐1 | ‒0.716 | ‒0.963 | ‒2.613 | ‒1.270 |
| (1.013) | (1.645) | (2.369) | (2.761) | |
| AR1 test: p‐value | 0.007 | 0.056 | 0.009 | 0.004 |
| AR2 test: p‐value | 0.615 | 0.807 | 0.131 | 0.228 |
| Hansen test: p‐value | 0.706 | 0.731 | 0.929 | 0.994 |
The dependent variable is the maternal mortality rate per 100,000 live births. All models include year and county fixed‐effects, a constant term, and the control variables listed in Table 1. All models include lagged dependent variable as a regressor. Robust and Windmeijer‐corrected standard errors are in parentheses.
*, **, ***; Significance at the 10%, 5%, and 1% level, respectively.
In addition to these results, we conducted other robustness checks, in which we estimated the GMM models using only one lag and then the first three lags of the endogenous variables (along with year dummies) as instruments. These exercises support our preference for Model B presented in Table 2 based on specification test results. All those results not reported here are available from the authors upon request.
Conclusions
Maternal mortality has been the focus of increased attention in the past decade partly because of the poor performance of the United States relative to that of other developed nations and partly because of disparities that place black mothers at three to four times the risk of white mothers.11 A recent CDC report determined that about three in five pregnancy‐related deaths in the United States could be preventable.19 Yet very few studies have looked at how specific public health efforts could reduce MMRs, typically because of the lack of granular data that allow researchers to identify service‐specific public health expenditures. As we demonstrated in this study, total public health expenditures do not always improve specific health outcomes such as maternal deaths. In that sense, one of the main contributions of this study is the longitudinal analysis of how a targeted public health package of pregnancy‐related programs can influence MMRs. If increases in maternal mortality in the United States can be attributed to an increase in chronic conditions like hypertension and diabetes, or societal factors such as bias or racism, all of which increase the risks associated with childbirth, effective solutions may not lie in improving hospital care alone but rather in improving access to early and regular interventions through publicly funded programs.
Our results show that spending on pregnancy‐related public health programs leads to statistically significant reductions in black maternal mortality and the black‐white maternal mortality gap in Florida. Specifically, a 10% increase in program spending led to a 13.5% decline in the MMR among black mothers and a 20.0% reduction in black‐white disparities. This is after accounting for a variety of potential confounders such as income, unemployment, and access to care that are typically associated with race. These results are consistent with earlier reports of a higher proportion of preventable maternal deaths among black women compared with white women.17 They also are consistent with the existing literature, which finds beneficial effects of public health efforts on racial disparities.20, 21, 23, 26
This study has several limitations. First, we focused on a single state. Although we considered well‐established public health services, replicating our analysis for another state may prove somewhat difficult, since the specific mix of public health programs may include different service combinations. The reason is that each state tailors its public health programs according to its particular needs. Nonetheless, Florida is demographically diverse and the third most populous state in the United States, which does enhance the generalizability of our findings. Second, we examined only one outcome, maternal mortality, which is a relatively rare event. Investigations of other maternal health outcomes, such as severe maternal morbidity along with MMRs, would have allowed us to gain a more complete perspective. Third, while we control for some structural factors such as poverty and access to health care, we do not explicitly account for negative experiences of bias that potentially contribute to existing health disparities. Our results suggest that public health programs can overcome some racial inequities but the mechanisms through which this occurs are beyond the scope of our analysis. Future studies need to further examine how directing public health resources to nonwhite communities can address such inequities. Finally, our observations were at the county and year levels, which may miss variations at finer geographical levels and time intervals. This really is a current data limitation, but surveillance is one of the core public health functions, and observations at finer levels in the future could provide further insights.
Other states, including Ohio, Massachusetts,36 and Illinois,37 have been exploring their maternal mortality surveillance system data in an effort to inform public health practice. Recently, Main and colleagues38 analyzed another very large state with a diverse population, California, and the efforts of the California Department of Public Health to investigate and reduce maternal deaths. They explained that California's Maternal Quality Care Collaborative, a statewide public‐private partnership established in 2006, helped the state achieve a “large‐scale and sustained improvement in maternity outcomes.” Even though the national MMR rose in the 2010s, California managed to reduce its rate nearly in half, bringing it down to a level comparable to the rates in Western European countries. Main and colleagues38 identified the key steps in this process and credited various maternal health improvement activities that could provide a road map for other public health departments across the country.
Our findings here certainly echo the California experience, with one major difference. Main and colleagues showed that while MMRs fell drastically for both black and white mothers in California between 1999 and 2013, the black‐white maternal mortality gap remained the same. The FDOH has declared that one of the state's priorities is to “improve access to health care for women to improve preconception and interconception health, specifically women who face significant barriers to better health.”39 Our findings suggest that Florida's public health programs may be on target if we specifically consider maternal mortality and the racial disparities therein.
Funding/Support
None.
Acknowledgments: We thank the Florida Department of Health, Office of the Deputy Secretary for County Health Systems and the Division of Public Health Statistics & Performance Management for their support and assistance in providing the Florida public health administrative data. We also thank two anonymous referees for their thoughtful and constructive feedback on earlier versions of this article.
Conflict of Interest Disclosures: All authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest. No conflicts were reported.
References
- 1. Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy‐related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130(2):366‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mann S, Hollier LM, McKay K, Brown H. What we can do about maternal mortality—and how to do it quickly. N Engl J Med. 2018;379(18):1689‐1691. [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention (CDC) . Pregnancy mortality surveillance system, 2018. Atlanta, GA: CDC; 2018. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. Accessed July 19, 2018. [Google Scholar]
- 4. Martin N, Cillekens E, Freitas A. Lost mothers. ProPublica. July 17, 2017. https://www.propublica.org/article/lost-mothers-maternal-health-died-childbirth-pregnancy. Accessed July 19, 2018. [Google Scholar]
- 5. Villarosa L. Why America's black mothers and babies are in a life‐or‐death crisis. New York Times April 11, 2018. https://www.nytimes.com/2018/04/11/magazine/black-mothers-babies-death-maternal-mortality.html. Accessed July 19, 2018.
- 6. Kassebaum NJ, Bertozzi‐Villa A, Coggeshall MS, et al. Global, regional, and national levels and causes of maternal mortality during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9947):980‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization (WHO) . Trends in maternal mortality: 1990 to 2015: estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. Geneva, Switzerland: WHO; 2015. [Google Scholar]
- 8. MacDorman MF, Declercq E, Cabral H, Morton C. Recent increases in the US maternal mortality rate: disentangling trends from measurement issues. Obstet Gynecol. 2018;131(5):934‐935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. MacDorman MF, Declercq E. The failure of United States maternal mortality reporting and its impact on women's lives. Birth. 2018;45(2):105‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lorch SA, Srinivas SK, Ahlberg C, Small DS. The impact of obstetric unit closures on maternal and infant pregnancy outcomes. Health Serv Res. 2013;48(2, pt. 1):455‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Race, ethnicity, and nativity differentials in pregnancy‐related mortality in the United States: 1993–2006. Obstet Gynecol. 2012;120(2):261‐268. [DOI] [PubMed] [Google Scholar]
- 12. Neggers YH. Trends in maternal mortality in the United States. Reprod Toxicol. 2016;64:72‐76. [DOI] [PubMed] [Google Scholar]
- 13. Admon LK, Winkelman TN, Moniz MH, Davis MM, Heisler M, Dalton VK. Disparities in chronic conditions among women hospitalized for delivery in the United States, 2005–2014. Obstet Gynecol. 2017;130(6):1319‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leonard SA, Main EK, Scott KA, Profit J, Carmichael SL. Racial and ethnic disparities in severe maternal morbidity prevalence and trends. Ann Epidemiol. 2019;33:30‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Howell, EA. Reducing disparities in severe maternal morbidity and mortality. Clin Obstet Gynecol. 2018;61(2):387‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Howell EA, Brown H, Brumley J, et al. Reduction of peripartum racial and ethnic disparities: a conceptual framework and maternal safety consensus bundle. J Obstet Gynecol Neonatal Nurs. 2018;47(3):275‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berg CJ, Harper MA, Atkinson SM, et al. Preventability of pregnancy‐related deaths: results of a state‐wide review. Obstet Gynecol. 2005;106(6):1228‐1234. [DOI] [PubMed] [Google Scholar]
- 18. Geller SE, Koch AR, Martin NJ, Rosenberg D, Bigger HR. Assessing preventability of maternal mortality in Illinois: 2002–2012. Am J Obstet Gynecol. 2014;211(6):698.e1. [DOI] [PubMed] [Google Scholar]
- 19. Petersen EE, Davis NL, Goodman D, et al. Vital signs: pregnancy‐related deaths, United States, 2011–2015, and strategies for prevention, 13 states, 2013–2017. Morbidity Mortality Weekly Rep. 2019;68(18):423‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moehling CM, Thomasson MA. Saving babies: the impact of public education programs on infant mortality. Demography. 2014;51(2):367‐386. [DOI] [PubMed] [Google Scholar]
- 21. Hoehn‐Velasco L. Explaining declines in US rural mortality, 1910–1933: the role of county health departments. Explorations in Economic History. 2018;70:42‐72. [Google Scholar]
- 22. Bhatia A, Krieger N, Subramanian SV. Learning from history about reducing infant mortality: contrasting the centrality of structural interventions to early 20th‐century successes in the United States to their neglect in current global initiatives. Milbank Q. 2019;97(1):285‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bekemeier B, Grembowski D, Yang Y, Herting JR. Are local public health department services related to racial disparities in mortality? SAGE Open. 2014;4(1):2158244014527989. [Google Scholar]
- 24. Olds DL, Kitzman H, Knudtson MD, Anson E, Smith JA, Cole R. Effect of home visiting by nurses on maternal and child mortality: results of a 2‐decade follow‐up of a randomized clinical trial. JAMA Pediatr. 2014;168(9):800‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mays GP, Smith SA. Geographic variation in public health spending: correlates and consequences. Health Serv Res. 2009;44(5, pt. 2):1796‐1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bernet PM, Gumus G, Vishwasrao S. Effectiveness of public health spending on infant mortality in Florida, 2001–2014. Soc Sci Med. 2018;211:31‐38. [DOI] [PubMed] [Google Scholar]
- 27. Burch D, Noell D, Hill WC, Delke I. Pregnancy‐associated mortality review: the Florida experience. Semin Perinatol. 2012;36(1):31‐36. [DOI] [PubMed] [Google Scholar]
- 28. Florida Department of Health (FDOH) . Healthy Start Standards and Guidelines . Chap. 16: Performance based contracts and memoranda of agreement. Tallahassee, FL: October 18, 2007. http://www.floridahealth.gov/programs-and-services/childrens-health/healthy-start/_documents/new-hssg-chapter-16.pdf. Accessed January 5, 2017.
- 29. Hoynes H, Page M, Stevens AH. Can targeted transfers improve birth outcomes? Evidence from the introduction of the WIC program. J Public Economics. 2011;95(7‐8):813‐827. [Google Scholar]
- 30. Erwin PC, Greene SB, Mays GP, Ricketts TC, Davis MV. The association of changes in local health department resources with changes in state‐level health outcomes. Am J Public Health. 2011;101(4):609‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mays GP, Smith SA. Evidence links increases in public health spending to declines in preventable deaths. Health Aff. 2011;30(8):1585‐1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bekemeier B, Yang Y, Dunbar MD, Pantazis A, Grembowski DE. Targeted health department expenditures benefit birth outcomes at the county level. Am J Prev Med. 2014;46(6):569‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arellano M, Bond S. Some tests of specification for panel data: Monte Carlo evidence and an application to employment equations. Rev Economic Studies. 1991;58(2):277‐297. [Google Scholar]
- 34. Roodman D. How to do xtabond2: an introduction to difference and system GMM in Stata. Stata J. 2009;9(1):86‐136. [Google Scholar]
- 35. Mishra P, Newhouse D. Does health aid matter? J Health Economics. 2009;28(4):855‐872. [DOI] [PubMed] [Google Scholar]
- 36. Conrey EJ, Manning SE, Shellhaas C, et al. Severe maternal morbidity, a tale of 2 states using data for action—Ohio and Massachusetts. Maternal Child Health J. 2019;23(8):989‐995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kilpatrick SJ, Prentice P, Jones RL, Geller S. Reducing maternal deaths through state maternal mortality review. J Womens Health. 2012;21(9):905‐909. [DOI] [PubMed] [Google Scholar]
- 38. Main EK, Markow C, Gould J. Addressing maternal mortality and morbidity in California through public‐private partnerships. Health Aff. 2018; 37(9):1484‐1493. [DOI] [PubMed] [Google Scholar]
- 39. Florida Department of Health (FDOH) . Maternal and Child Health Services Title V Block Grant, Florida, FY2018 Application/FY2016 Annual Report. September 18, 2007. http://www.floridahealth.gov/programs-and-services/womens-health/pregnancy/_documents/mch-block-grant-fy2018.pdf. Accessed November 30, 2018.


