Abstract
Objective:
To evaluate the feasibility of Shortwave infrared (SWIR) otoscopy in a pediatric population and establish differences with visible otoscopy
Methods:
Pediatric patients 3 years of age and older seen in the otolaryngology clinic with an audiogram and tympanogram obtained within a week of the visit were recruited for video otoscopy using visible light otoscopy and SWIR otoscopy. Videos were rated by two otolaryngologists based on ability to identify the promontory, ability to identify the ossicular chain and presence or absence of middle ear fluid.
Results:
A total of 74 video recordings of ears were obtained in 20 patients. We obtained interpretable images in 63/74 (85.1%) ears. There was no statistical significance between ability to perform SWIR otoscopy versus white light video otoscopy as indicated by a p-value of 0.376.
There was high inter-rater agreement for identification of both the promontory and the ossicular chain with Kappa values of 0.81 and 0.92 respectively. There was statistical significance between SWIR otoscopy and visible otoscopy in the ability to image the promontory (p =0.012) and the ossicular chain (p=0.010). Increased contrast of middle ear fluid was seen in SWIR otoscopy when compared to visible otoscopy.
Conclusion:
SWIR otoscopy is feasible in a pediatric population and could offer some advantages over visible light otoscopy such as better visualization of the middle ear structures through the tympanic membrane and increased contrast for middle ear effusions.
Keywords: otoscope, infrared, middle ear effusion
Introduction
Otitis media is one of the most common reasons for pediatrician visits, antibiotic prescription, and surgery in the pediatric population1,2. The term otitis media encompasses a variety of conditions such as acute otitis media (AOM)3, otitis media with effusion (OME), and chronic suppurative otitis media (CSOM). These conditions are closely related and can overlap, but most importantly they are linked by presence of middle ear fluid or in the case of CSOM, fluid that drains out of the middle ear through a tympanic membrane perforation or a tympanostomy tube. While chronic suppurative otitis media is easily identifiable due to the presence of otorrhea; otoscopic diagnosis of otitis media with effusion and acute otitis media is not as straightforward4,5. The difficulty in identifying middle ear effusion (MEE) is largely responsible for both the overdiagnosis of cases of AOM and the frequent under-diagnosis of cases of OME4,6.
Visible light pneumatic otoscopy is considered the best currently available diagnostic tool for otitis media7,8. However, pneumatic otoscopy has various limitations. The speculum must create an adequate seal against the external auditory canal to obtain tympanic membrane movement, which is seldom possible with the standard disposable speculum. Also, sufficient training is required for effective pneumatic otoscopy, but there is frequently a gap in training for most clinicians9 . The implementation of pneumatic otoscopy by primary care physicians in their practice has not been optimal, leading to a lack of resident training and a perception that pneumatic otoscopy is difficult to master10,11. There is consensus that the diagnosis of MEE can be challenging and even experienced otolaryngologists require the use of adjuvant diagnostic methods such as the otologic microscope or tympanometry to better discern the presence of middle ear fluid in difficult cases12-14.
With these challenges in mind, we have recently described an otoscope sensitive to shortwave infrared (SWIR, 1-2 micrometer) wavelengths of light with the objective of improving middle ear disease diagnoses15. SWIR otoscopy provides two fundamental advantages over conventional visible light-based pneumatic otoscopy. First, a SWIR otoscope could help identify middle ear effusions based on the strong light absorption by middle ear fluid in the SWIR spectral region. Second, due to a longer wavelength, SWIR light can penetrate deeper through tissue, enabling a better view of middle ear anatomy behind the tympanic membrane.
The objective of this study is to present our preliminary findings evaluating the feasibility of using video rate SWIR imaging in a pediatric population with emphasis on identifying potential differences with visible light otoscopy.
Methods
The study was approved by the Institutional Review Board at Connecticut Children’s Medical Center and the Committee on the Use of Humans as Experimental Subjects at Massachusetts Institute of Technology.
Patients 3 years of age and older seen in the otolaryngology clinic with an audiogram and tympanogram obtained within a week of the visit were recruited for the study. Ears with a tympanic membrane perforation or history of cholesteatoma surgery were excluded from the study.
Otoscopic evaluation was performed by two pediatric otolaryngologists using video otoscopy during the office visit. Consecutive videos were obtained for visible otoscopy and SWIR otoscopy. Videos for visible light otoscopy were obtained using a Macroview Welch Allyn (Skaneateles, New York) white-light otoscope adapted to a Thor Labs (Newton, New Jersey, USA) USB 2.0 CMOS 1280 x 1024 pixel, color camera and recorded in avi format. SWIR videos were recorded using a SWIR otoscope, which we have previously described in detail15. The system is composed of a fiber-coupled broadband light source, an otoscope head and ear speculum with a lens system to focus the reflected light onto an Indium Gallium Arsenide array detector. A filter holder in front of the sensor allows use of a variety of SWIR filters.
To determine differences between visible light otoscopy and SWIR otoscopy in the ability to identify the promontory and ossicular chain, videos were obtained with both modalities. All videos were de-identified and displayed on a 15 inch MacBook Pro for review. For each video, the otolaryngologist was asked to rate the quality of the video, ability to identify the promontory, ability to identify the ossicular chain and to determine the presence or absence of middle ear fluid. The SWIR and visible videos were not linked and videos of the same ear in both modalities were introduced at different time points.
Criteria to document absence of MEE were adequate movement of the ear drum during the office examination with pneumatic otoscopy and identifiable promontory and ossicles behind the tympanic membrane in visible and SWIR otoscopy. Criteria to document the presence of middle ear fluid included visible fluid in visible otoscopy and decreased light intensity from absorption of SWIR light by fluid in SWIR otoscopy. Results of the audiograms and tympanograms and ear examination were made available to the pediatric otolaryngologists at the time of the evaluation. Inter-rater agreement was performed using Cohen’s Kappa, and Chi square analysis was used for our nominal data using SPSS 22.0 (SPSS Inc. Chicago, Illinois). Differences were considered significant for a p value < 0.05. Contrast was quantified with ImageJ software by calculating the standard deviation in signal intensity divided by the mean signal intensity for a defined region of interest within the image. The region of interest encompassed areas both with and without suspected fluid accumulation. This calculation was carried out for four video frames and averaged.
Results
A total of 74 ear video recordings were obtained in 20 patients (see Table 1). Eleven videos were excluded from analysis due to poor image quality for reasons including patient movement, inadequate camera integration time, or presence of cerumen limiting view of the tympanic membrane. Of the excluded videos, 7 were from SWIR imaging and 4 were from visible light otoscopy. Images were deemed adequate for interpretation in 63/74 (85.1%) of videos examined. There was no statistical significance between ability to perform SWIR otoscopy versus white light video otoscopy as indicated by a p-value of 0.376.
Table 1.
Visible and SWIR videos collected
| Total Otoscopy Videos | 74 |
| Total Patients | 20 |
| Visible light otoscopy videos | 36 |
| SWIR otoscopy videos | 38 |
| Discarded Videos | 11 |
| Visible light otoscopy | 4 |
| SWIR otoscopy | 7 |
| Difference in successful image acquisition between visible and SWIR otoscopy | p=0.376 |
| Inter-rater Agreement | |
| Ability to see the Promontory | Kappa 0.81 |
| Ability to see Ossicular Chain | Kappa 0.92 |
| Difference between visible and SWIR otoscopy in the ability to see behind the tympanic membrane | |
| Ability to see Promontory | p=0.012 |
| Ability to see Ossicular Chain | p=0.010 |
| Cases of middle ear fluid present | 3* |
all cases were detected with both modalities
There was high inter-rater agreement between both examiners for identification of both the promontory and the ossicular chain with Kappa values of 0.81 and 0.92 respectively. In 8 cases, the ossicular chain was visible when using the SWIR otoscope compared to 1 case using the visible otoscope. There was improvement in the ability to see through the tympanic membrane using the SWIR otoscope. Showing statistical significance between SWIR otoscopy and visible otoscopy in the ability to image the promontory (p =0.012) and the ossicular chain (p=0.010) (see Figure 1).
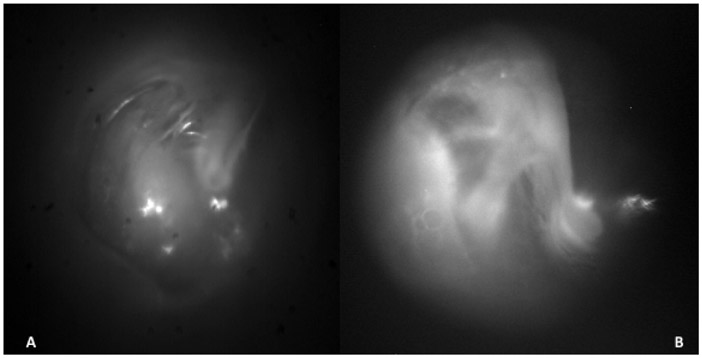
Figure 1.
Images in the SWIR of a right ear showing A) clear view of the promontory B) showing the tensor tympani and chorda tympani through the tympanic membrane.
The three patients with presence of middle ear effusion, confirmed by pneumatic otoscopy during the otolaryngology visit, were all identified using both visible and SWIR otoscopy. An improvement in contrast using SWIR otoscopy was observed in some areas where MEE was present in the middle ear (see Figures 2 and 3). The average contrast for visible otoscopy in the presence of middle ear effusion was 0.097 and for SWIR was 0.29.
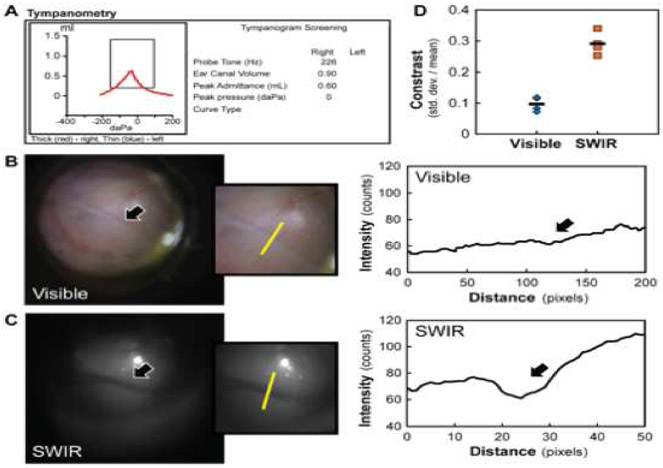
Figure 2.
Type A tympanogram of a right tympanic membrane and middle ear (A) with an air-fluid level detected using visible (B) and SWIR (C) otoscopy (indicated by a black arrow). The absorption of fluid is quantified by plotting the detected intensities across a region of interest containing fluid (yellow line). Contrast of the air-fluid level, defined as the standard deviation divided by the mean signal intensity for the region of interest (D), is more than double for SWIR imaging (orange squares, average of 4 images shown with a black line) than for visible imaging (blue diamonds, average of 4 images is shown with a black line).
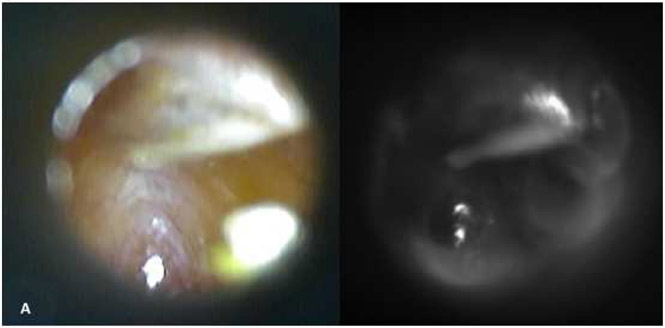
Figure 3.
Images of middle ear effusion with visible light otoscopy (A) and SWIR (B) otoscopy techniques. Notice complete “blackout effect “in the SWIR due to the presence of middle ear effusion.
In tympanic membranes with myringosclerosis, neither technique was able to see through areas of myringosclerosis . However, the SWIR otoscope was able to see through dried blood, dried secretions and thin dry areas of cerumen overlying the tympanic membrane (see Figure 4).
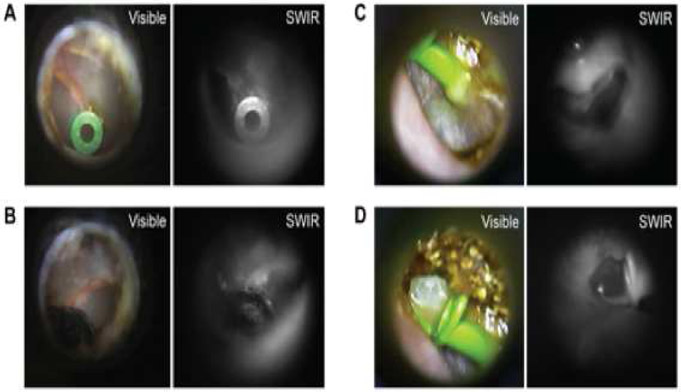
Figure 4.
Images of tympanostomy tubes using both visible and SWIR otoscopy. An ear with dry blood blocking the inferior aspect of the tympanic membrane is imaged using visible (A) and SWIR otoscopy. (B) A tympanostomy tube is seen through the dry blood in the SWIR image but is obscured in the visible image. ( C ) A tympanostomy tube with otorrhea is also imaged with visible and SWIR otoscopy. SWIR images show the ability to see through dry secretions and cerumen which enables visualization of the tympanostomy tube flange (C). Fluid draining from the tympanostomy tube (D) can also be seen with greater contrast using SWIR otoscopy compared to visible otoscopy. Notice increased fluid contrast on the tip of the tympanostomy tube on the SWIR image and the ability to see through dry secretions and cerumen allowing to see the flange of the T tube.
Discussion
Improving primary care provider’s ability to diagnose OM has an evident public health benefit16. AOM is often over-diagnosed due to the difficulty of confirming presence of middle ear fluid, resulting in unnecessary prescription of antibiotics thus facilitating antibiotic resistance. Meanwhile, OME is often under-diagnosed due to the asymptomatic nature of this condition and the difficulty in diagnosing MEE with traditional otoscopy17,18. OME is the most common reason for conductive hearing loss in the pediatric population and has been associated with behavioral and learning difficulties19. Recent research on cases of reversible conductive hearing loss has even identified changes that occur in the neurological pathways that persist long after the conductive hearing loss has resolved20.
Identification of MEE using visible light otoscopy and pneumatic otoscopy has been a long standing challenge for clinicians. Despite the tympanic membrane being semi-translucent there is significant scattering and absorption of light, limiting the view of middle ear structures15. The current diagnostic instrument of choice, pneumatic otoscopy, uses movement of the eardrum following air insufflation as a surrogate for seeing the middle ear fluid. This technique has some difficulties such as achieving an adequate seal in the ear canal and interpreting the actual movement of the tympanic membrane upon insufflation. These challenges can in part account for the low adoption rate among practitioners11. In the United States, the prevalence of pneumatic otoscopy in clinical practice is only between 7% and 33% for primary care physicians21,22.
To compensate for the limitations of pneumatic otoscopy, other diagnostic tools such as tympanometry and acoustic reflectometry have emerged to assist clinicians with the diagnosis of MEE. Current AAO-HNS guidelines for management of OME recommend use of tympanometry to assist with the diagnosis of middle ear effusion when pneumatic otoscopy examination is not conclusive7.
Tympanometry has comparable sensitivity to pneumatic otoscopy with 90- 94% sensitivity for the diagnosis of MEE; however its specificity is lower, between 50-75%8. In practice, the use of tympanometry in primary care settings is low when compared to standard otoscopy despite its clinical relevance3,14. Barriers to the utilization of tympanometry include the need for additional training, increased time during the visit, and the cost of the device. However, studies show that primary care providers find the use of tympanometry easier than pneumatic otoscopy14.
Another tool to help identify the presence of MEE is acoustic reflectometry. This technique measures how much sound is reflected off the tympanic membrane. However, despite there being an economical consumer version, adoption among physicians has been low23,24.
SWIR otoscopy could provide an adjuvant method for identification of MEE. SWIR light, due to the longer wavelength, results in deeper tissue penetration and decreased light scattering, allowing a better view of the promontory and ossicular chain in the absence of middle ear fluid. As we approach the diagnostic challenges of otitis media it is essential to recognize that it is as important that a diagnostic instrument be able to determine absence of middle ear fluid as it is to be able to identify its presence. SWIR otoscopy allows for both by having better penetration through the tympanic membrane making it easier to identify the promontory and ossicular chain in the absence of MEE (Figure 1). If MEE is present in the middle ear, SWIR otoscopy provides greater contrast through a “blackout effect” in the areas where effusion is present. Furthermore, using SWIR otoscopy does not add time or complexity to a diagnosis, and requires no additional training of medical practitioners.
Aside from improved visibility through the tympanic membrane we have also shown other benefits of SWIR otoscopy such as the ability to see through opaque media as illustrated in the case of dry blood covering a tympanostomy tube (see Figure 4). We acknowledge this study has some limitations such as excluding patients under the age of 3 and patients with other middle ear pathologies such as cholesteatomas. We decided against including patients younger than three years old due to the need for a separate examination with the SWIR and the white light otoscope for comparison. Another reason for exclusion of younger patients was that the SWIR otoscope is a research prototype with an optical path optimized for a 4mm speculum. Another limitation was that videos were reviewed at a different time point from the clinic visit and pneumatic otoscopy examination. We also recognize that the study was conducted in a small number of patients with only 3 ears having middle ear effusion.
Despite this, we believe that compared to the subtle visual change observed by visible otoscopy in the presence of MEE, SWIR endogenous contrast and deeper tissue penetration could provide a useful adjunctive tool to assist in the determination of the presence or absence of MEE. We show in this preliminary study the feasibility of SWIR otoscopy in a pediatric population and identify clinically relevant differences from visible light pneumatic otoscopy.
Conclusion
Pediatric otoscopy for diagnosis of otitis media is challenging even in the hands of an experienced pediatric otolaryngologist. We have demonstrated that SWIR otoscopy is feasible in a pediatric population. Furthermore, we show that expanding otoscopy into the SWIR can offer some advantages over traditional visible otoscopy, such as better visualization of the middle ear structures and increased contrast of middle ear effusion.
Supplementary Material
Acknowledgments
Funding Sources:
NIH through the Laser Biomedical Research Center, Grant 9-P41-EB015871-26A1 (to M.G.B.) and the Massachusetts Institute of Technology SPARK program through the University of Connecticut
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Dr Bawendi, Dr Bruns, Dr Valdez and Ms. Carr have filed intellectual Property for the Shortwave Infrared Otoscope
Financial Disclosure: Nothing to Declare
References
- 1.Rovers MM, Black N, Browning GG, Maw R, Zielhuis GA, Haggard MP. Grommets in otitis media with effusion: an individual patient data meta-analysis. Arch Dis Child 2005; 90:480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rovers MM, Glasziou P, Appelman CL et al. Antibiotics for acute otitis media: a meta-analysis with individual patient data. Lancet 2006; 368:1429–1435. [DOI] [PubMed] [Google Scholar]
- 3.Lieberthal AS, Carroll AE, Chonmaitree T et al. The diagnosis and management of acute otitis media. Pediatrics 2013; 131:e964–999. [DOI] [PubMed] [Google Scholar]
- 4.Pichichero ME, Poole MD. Assessing diagnostic accuracy and tympanocentesis skills in the management of otitis media. Arch Pediatr Adolesc Med 2001; 155:1137–1142. [DOI] [PubMed] [Google Scholar]
- 5.Verhoeff M, van der Veen EL, Rovers MM, Sanders EA, Schilder AG. Chronic suppurative otitis media: a review. Int J Pediatr Otorhinolaryngol 2006; 70:1–12. [DOI] [PubMed] [Google Scholar]
- 6.Rosenfeld RM. Diagnostic certainty for acute otitis media. Int J Pediatr Otorhinolaryngol 2002; 64:89–95. [DOI] [PubMed] [Google Scholar]
- 7.Rosenfeld RM, Shin JJ, Schwartz SR et al. Clinical Practice Guideline: Otitis Media with Effusion (Update). Otolaryngol Head Neck Surg 2016; 154:S1–S41. [DOI] [PubMed] [Google Scholar]
- 8.Takata GS, Chan LS, Morphew T, Mangione-Smith R, Morton SC, Shekelle P. Evidence assessment of the accuracy of methods of diagnosing middle ear effusion in children with otitis media with effusion. Pediatrics 2003; 112:1379–1387. [DOI] [PubMed] [Google Scholar]
- 9.M. innes Ahser CCG. Infections of the Upper Respiratory Tract In: Lynn M Taussig M, and Louis I. Landau, ed. Pediatric Respiratory Medicine. Philadelphia PA: Mosby Elsevier, 2008. [Google Scholar]
- 10.MacClements JE, Parchman M, Passmore C. Otitis media in children: use of diagnostic tools by family practice residents. Fam Med 2002; 34:598–603. [PubMed] [Google Scholar]
- 11.Ouedraogo E, Labrecque M, Cote L, Charbonneau K, Legare F. Use and teaching of pneumatic otoscopy in a family medicine residency program. Can Fam Physician 2013; 59:972–979. [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers DJ, Boseley ME, Adams MT, Makowski RL, Hohman MH. Prospective comparison of handheld pneumatic otoscopy, binocular microscopy, and tympanometry in identifying middle ear effusions in children. Int J Pediatr Otorhinolaryngol 2010; 74:1140–1143. [DOI] [PubMed] [Google Scholar]
- 13.Lee DH. How to improve the accuracy of diagnosing otitis media with effusion in a pediatric population. Int J Pediatr Otorhinolaryngol 2010; 74:151–153. [DOI] [PubMed] [Google Scholar]
- 14.Abbott P, Rosenkranz S, Hu W, Gunasekera H, Reath J. The effect and acceptability of tympanometry and pneumatic otoscopy in general practitioner diagnosis and management of childhood ear disease. BMC Fam Pract 2014; 15:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carr JA, Valdez TA, Bruns OT, Bawendi MG. Using the shortwave infrared to image middle ear pathologies. Proc Natl Acad Sci U S A 2016; 113:9989–9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schilder AG, Chonmaitree T, Cripps AW et al. Otitis media. Nat Rev Dis Primers 2016; 2:16063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchanan CM, Pothier DD. Recognition of paediatric otopathology by General Practitioners. Int J Pediatr Otorhinolaryngol 2008; 72:669–673. [DOI] [PubMed] [Google Scholar]
- 18.Legros JM, Hitoto H, Garnier F, Dagorne C, Parot-Schinkel E, Fanello S. Clinical qualitative evaluation of the diagnosis of acute otitis media in general practice. Int J Pediatr Otorhinolaryngol 2008; 72:23–30. [DOI] [PubMed] [Google Scholar]
- 19.Bennett KE, Haggard MP, Silva PA, Stewart IA. Behaviour and developmental effects of otitis media with effusion into the teens. Arch Dis Child 2001; 85:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarkson C, Antunes FM, Rubio ME. Conductive Hearing Loss Has Long-Lasting Structural and Molecular Effects on Presynaptic and Postsynaptic Structures of Auditory Nerve Synapses in the Cochlear Nucleus. J Neurosci 2016; 36:10214–10227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lannon C, Peterson LE, Goudie A. Quality measures for the care of children with otitis media with effusion. Pediatrics 2011; 127:e1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forrest CB, Fiks AG, Bailey LC et al. Improving adherence to otitis media guidelines with clinical decision support and physician feedback. Pediatrics 2013; 131:e1071–1081. [DOI] [PubMed] [Google Scholar]
- 23.Combs JT, Combs MK. Acoustic reflectometry: spectral analysis and the conductive hearing loss of otitis media. Pediatr Infect Dis J 1996; 15:683–686. [DOI] [PubMed] [Google Scholar]
- 24.Muderris T, Yazici A, Bercin S, Yalciner G, Sevil E, Kiris M. Consumer acoustic reflectometry: accuracy in diagnosis of otitis media with effusion in children. Int J Pediatr Otorhinolaryngol 2013; 77:1771–1774. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.