Abstract
Retrospective analysis of 20 water systems from the USEPA’s Arsenic Demonstration Program revealed three patterns of arsenic levels at the tap, after arsenic treatment of the source well water. Following an initial destabilization period, Pattern A systems (6/20 with low iron/manganese in source water and plastic piping) had arsenic concentrations that did not change as water traveled to consumer taps (conservative contaminant behavior). Pattern B systems (8/20 with high iron/manganese in source water and iron piping) had consistently higher arsenic concentrations at consumer taps, above the arsenic content of incoming treated water, for months to more than a year after arsenic treatment (non-conservative behavior). Pattern C systems (6/20 with additional occasional arsenic treatment complications) experienced multiple arsenic spikes at consumer taps (non-conservative and unpredictable behavior). These field observations suggest that in some water distribution systems arsenic may linger long after it has been removed at its source.
Keywords: arsenic, iron, arsenic treatment, distribution system, non-conservative contaminant, accumulation, release
INTRODUCTION
Conservative versus non-conservative contaminants in the distribution system.
A drinking water contaminant is deemed conservative when its concentration entering the distribution system after water treatment equals its concentration exiting the system at points of water consumption, such as consumer taps. In this scenario pipe materials are inert, and the contaminant is unreactive (i.e., IN=OUT).
Chemical, physical and/or biological interactions between drinking water contaminants and plumbing materials challenge the notion of conservative contaminant behavior. A non-conservative contaminant accumulates within the distribution system through a variety of mechanisms such as sorption, precipitation or co-precipitation, and the result might be an apparent reduction in contaminant concentration at consumer taps (IN>OUT). This is because sorption and/or (co)precipitation reactions retain contaminants on the surface or within distribution system solids such as corrosion scales, biofilm, precipitates and sediment (e.g., Schock and Holm, 2003; Schock, 2005; USEPA 2006; Friedman et al., 2010; Hill et al., 2010; Makris et al., 2014; Friedman et al., 2016).
Once these equilibria are established, changes in water quality may reverse these reactions to allow for chemical re-equilibration. Physical and hydraulic disturbances may also destabilize distribution system solids and mobilize entrained contaminants. A contaminant may therefore be released back into the water through reverse mechanisms (e.g., desorption, dissolution) or other mechanisms (e.g., scale destabilization, generation from precursors), thereby resulting in higher concentration at the tap (IN<OUT) (e.g., Schock and Holm, 2003; Schock, 2005; USEPA 2006; Friedman et al., 2010; Hill et al., 2010; Makris et al., 2014; Friedman et al., 2016).
The first reported case of arsenic release from the distribution system (up to almost 5 mg/L) co-occurred with high iron, lead, and copper during red water complaints in a midwestern water utility (Rieber and Dostal, 2000). This and other notable cases of inorganic and radionuclide contaminant release, as summarized by Clement and Carlson (2004), Scanlan (2008), Lytle et al. (2010), and Friedman et al. (2016), prompted research on the subject in the last two decades.
Arsenic accumulation and release in distribution systems.
Arsenic (As) is a known human carcinogen with a maximum contaminant level (MCL) of 0.01 mg/L (or 10 μg/L) in drinking water in the United States (USEPA, 2003). Arsenic has been shown to carry over from the source water to the treatment plant and onto the scales of water pipes made from iron, copper, galvanized steel and even asbestos cement or plastic pipes containing internal buildup of iron coatings (Friedman et al., 2016; Lytle et al., 2010, 2004; Schock, 2005; Reiber and Dostal, 2000). Arsenic release has also been investigated at bench scale (e.g., Copeland et al., 2007; Friedman et al., 2016; Hammer 2018), pilot scale and modeling exercises (e.g., Burkhardt et al., 2017), collectively demonstrating the ability of arsenic to desorb from iron surfaces under a variety of experimental water quality conditions. These studies essentially proved the compelling case made by Bacso & Szalay (1978) and Schock (2005), that the abundant observations from the geochemistry literature on the interaction of arsenic with iron and manganese could be applied to drinking water distribution systems.
Early emphasis was justifiably placed on understanding the removal of arsenic from drinking water sources. For example, McNeill et al. (1995) measured arsenic at conventional treatment plant locations where changes in arsenic concentration or speciation were expected to occur. Regulatory compliance sampling for arsenic and most inorganic and radiological contaminants (except lead and copper) requires water sample collection at points of entry to the distribution system. To assess contaminant accumulation within distribution systems, alternative monitoring approaches can be used, such as tap water sampling, hydrant flushing solid/water sampling, and the examination of scales, sediments, and biofilms (USEPA, 2006). Work by Lytle et al. (2004; 2014), Schock et al. (2008), and Peng et al. (2012) is only a small representation of such efforts summarized by Friedman et al. (2016). Tap water sampling in particular is a direct measure of contaminant release at the point of water consumption. Yet tap water sampling is rarely conducted by water utilities except for studies specific to lead and copper contamination, or extensive investigations after visible water quality disturbances and consumer complaints.
USEPA Arsenic Demonstration Program.
Tap water sampling for arsenic and other inorganic contaminants was included in the USEPA Arsenic Demonstration Program (simply Arsenic Demo), which was conducted to assist small drinking water systems in complying with the then newly established arsenic MCL (USEPA, 2016). In three overlapping phases (Rounds 1, 2 and 2a), the Arsenic Demo evaluated full-scale arsenic removal technologies at 50 small water systems located in 26 states between 2001 and 2011 (Sorg, 2017). Key conclusions from the Arsenic Demo, including treatment technology effectiveness and costs, were summarized elsewhere (e.g., Sorg, 2007; Lytle et al, 2010; Wang and Chen, 2011; Sorg and Chen, 2012; Sorg et al., 2015; Sorg, 2017; Sorg et al, 2017a, 2017b; Chen et al, 2018). Final reports for each of the 50 systems were also made public through the Arsenic Demo website (USEPA, 2016).
Objectives.
The wealth of information in the EPA Arsenic Demo reports prompted a retrospective analysis of select water systems and water data, to identify possible patterns of arsenic release in distribution systems and potential contributing factors. Arsenic release was assessed by comparing temporal arsenic concentrations in consumer taps relative to distribution system entry points. In other words, this analysis aimed to understand whether arsenic behaved as a conservative contaminant in a given distribution system (i.e., IN=OUT), or as a non-conservative contaminant (i.e., IN≠OUT) during the Arsenic Demo. This unique analysis was performed for purely research purposes, since the data were never intended to be used for compliance with the arsenic MCL. Additional data on the elemental composition of hydrant flush solids were incorporated in the evaluation. This research did not aim to assess the efficacy of arsenic treatment in each system, as did the Arsenic Demo.
MATERIALS AND METHODS
Water systems selected from USEPA’s Arsenic Demo.
Water systems that met two criteria were included in this study. First, the water quality entering each distribution system originated from well water that was treated for arsenic without mixing/influence of other wells or other water sources that would complicate comparisons. Second, systems where 3 distribution sites were sampled at first draw (stagnation time ≥6 hours) were included. Out of 50 systems, 20 water systems in 15 states (Arsenic Demo Rounds 1, 2 and 2a) met these criteria (Table 1). The arsenic removal technologies at these 20 groundwater systems included adsorptive media, coagulation/filtration, iron removal, iron removal followed by adsorptive media, and oxidizing media followed by adsorptive media (Table 1). From the water quality information in the EPA reports, only data pertinent to the scope of this paper were extracted and analyzed.
Table 1.
Arsenic treatment technologies, number of water/solid samples collected, and plumbing material information in 20 select small water systems from the USEPA’s Arsenic Demonstration. Hydrant flush solids were occasionally collected. General plumbing material information (in the distribution system, service line and/or premises) was available in most EPA reports.
| System ID (State) |
As Removal Technology | Monthly Water Samples at Treatment Effluent and 3 Distribution Sites | Hydrant Flush Solids | General Plumbing Material Information |
|---|---|---|---|---|
| AL (TX) | Adsorptive Media | Before: 3–4 After: 12–14 |
0 | DS: Cast Fe SL: PVC & Galvanized Fe |
| BW (NH1) | Adsorptive Media | Before: 4 After: 17–26 |
0 | DS: PVC SL & Premises: PVC & Cu, Pb solder |
| BC (MI1) | Adsorptive Media | Before: 4 After: 18–24 |
Before: 2 After: 0 |
DS: Asbestos cement, ductile Fe, plastic |
| GF (NH2) | Adsorptive Media | Before: 4 After: 15–16 |
0 | DS: PVC SL & Premises: Cu |
| LD (SD) | Adsorptive Media | Before: 3 After: 12–16 |
0 | DS: Steel & PVC |
| DM (VT) | Adsorptive Media | Before: 4 After: 9–11 |
0 | DS: Pb, PVC, PE |
| FC (IN)* | Adsorptive Media | Before:1– 4 After: 12–16 |
0 | DS: Cu, galvanized Fe & PVC SL: galvanized Fe & PVC Premises: Mainly Cu |
| CM (MN1) | Coagulation/Filtration | Before: 4 After:12 −13 |
Before: 2 After: 2 |
DS: PVC Premises: PVC, Cu |
| LW (ND) | Coagulation/Filtration Engineering Modification | Before: 4 After: 6–9 |
Before: 2 After: 2 |
NA |
| PW (MI2) | Coagulation/Filtration | Before: 4 After: 11–22 |
0 | DS: ductile Fe, sand cast Fe SL: Cu |
| SF (OH) | Iron Removal & Adsorptive Media | Before: 4 After: 11–13 |
Before: 6 After: 0 |
DS: PVC, Cu, Fe SL: Cu, black PE Premises: PVC, Cu, polybutylene |
| ST (MN2) | Iron Removal & Adsorptive Media | Before: 4 After: 11–17 |
Before: 0 After: 3 |
DS: Cast Fe SL: Galvanized Fe, Cu, PVC |
| SV (CA)* | Oxidizing media & Adsorptive Media | Before: 3 After: 10–12 |
0 | SL & Premises: Cu, Galvanized Fe, PVC |
| WA (MA) | Oxidizing media & Adsorptive Media | Before: 4 After: 11–14 |
0 | DS, SL, & Premises: PVC |
| WL (UT) | Oxidizing media & Adsorptive Media | Before: 4 After: 11–13 |
0 | DS & SL: Galvanized Fe |
| WV (IL) | Iron Removal | Before: 6 After: 13–15 |
Before: 6 After: 0 |
DS: Cast Fe |
| CL (PA) | Iron Removal | Before: 4 After: 10–12 |
0 | NA |
| DV (WI) | Iron Removal | Before: 3–4 After: 13–18 |
0 | DS: Cu SL & Premises: Cu |
| SA (MN3) | Iron Removal | Before: 4 After: 13–18 |
Before: 0 After: 3 |
DS: Cast Fe & PVC |
| SC (MN4) | Iron Removal | Before: 3–4 After: 13 |
0 | DS: PVC |
School building, 3 school taps sampled instead of 3 homes; DS: Distribution system; SL: Service line PVC: Polyvinyl chloride; Pb: Lead, Cu: Copper, Fe: Iron, PE: Polyethylene; NA: Not Available in EPA report
Baseline water quality.
During routine monitoring prior to the installation of arsenic treatment, data had been collected at the 20 systems to understand the baseline quality of the source water. Illustrative data for important water quality parameters were compiled from the EPA reports, although emphasis was placed on arsenic, iron and manganese specifically.
Drinking water arsenic data.
After arsenic treatment was implemented, the treated well water (i.e., the treatment system effluent) was sampled weekly to evaluate the efficacy of a given arsenic removal technology. “Distribution sites” were also sampled as part of the Arsenic Demo, both before arsenic treatment (weekly for several months) and after arsenic treatment (monthly for the duration of the Arsenic Demo). Three “distribution sites”, meaning three homes or occasionally other building types like schools, were identified for each system. The selected sites were typically part of the water system’s Lead and Copper Rule (LCR) sampling pool and were sampled monthly by home-owners or plant operators during the Arsenic Demo according to LCR specifications. First-draw tap water was therefore collected in a 1-liter sample bottle after overnight stagnation of more than 6 hours (e-CFR, 2019). Total arsenic (and other metals of interest like iron and manganese) in the samples was analyzed by ICP-MS according to USEPA Method 200.8 (USEPA, 1994a) at Battelle Memorial Institute laboratories (Columbus, Ohio).
Temporal arsenic data in the treated water effluent, as well as in 3 distribution sites per system, were therefore available before and after arsenic treatment was installed. Water sampling before arsenic treatment at the 3 distribution sites spanned 3–4 months depending on system, corresponding to 3–4 individual samples per distribution site (one sample per month per distribution site for a period of 3–4 months) (Table 1). Water sampling after arsenic treatment spanned several months (e.g., 6–9 samples for each site of system LW) to more than a year (e.g., 15–16 samples for each site of system GF) (Table 1).
Drinking water arsenic data analysis.
Arsenic concentrations at the treatment system effluent (assumed to reflect water entering the distribution system, i.e., IN) versus the 3 distribution system taps (i.e., OUT) were compared, to identify patterns of arsenic release for each distribution system. Only those weekly treatment system effluent data that closely matched the monthly distribution site sampling dates were included (ideally collected on the same day, with some exceptions of several days apart). Graphs of arsenic concentration at the different sampling locations versus time were then created for each system, and summary arsenic statistics were calculated. This basic exploratory data analysis allowed the categorization of the 20 systems into general patterns of arsenic release. Due to the high temporal variability of data in some systems, effort was placed in basic exploratory analysis, thereby focusing on practical significance of this assessment rather than statistical significance. With that in mind, the percent of tap water samples exceeding the health-based arsenic threshold of 10 μg/L was also calculated. A preliminary presentation by Triantafyllidou et al. (2016) was improved and expanded for this paper.
Supplemental plumbing material information.
General plumbing material information was extracted from the EPA reports. This information was reported by plant operators as part of the system background information and was not specific to the distribution system sampling sites. Despite reporting variations and uncertainties, the plumbing material information distinguished between distribution system mains, service lines, and premise plumbing (Table 1), and could somewhat aid in data interpretation.
Supplemental hydrant flush solid data.
Hydrant flush solids were analyzed from some participating utilities in a previous parallel study to assess their elemental composition. Overall, 28 hydrant flush samples were collected from 7 participating systems before and/or after arsenic treatment (Table 1). The remaining 13 systems either did not conduct routine hydrant flushing or did not participate in the sampling. Although these limited samples were not intended to represent each system spatially or temporally, they are indicative of potential sources of arsenic that can be released back into the distribution systems.
Hydrant-flushed water samples (5-L container) were collected by utility personnel from the flowing water stream during routine hydrant flushing (see Figure 1a). Upon arrival to the laboratory, hydrant-flushed water samples were gravity-settled, concentrated by centrifugation, and the solids were subsequently air dried and stored in a desiccator, as detailed in Lytle et al. (2010). The resulting solid samples (≥1 g) were acid-digested according to USEPA Method 3050B (USEPA, 1996), followed by ICP-MS analysis for metal quantification according to USEPA Method 200.8 (1994a).
Figure 1.
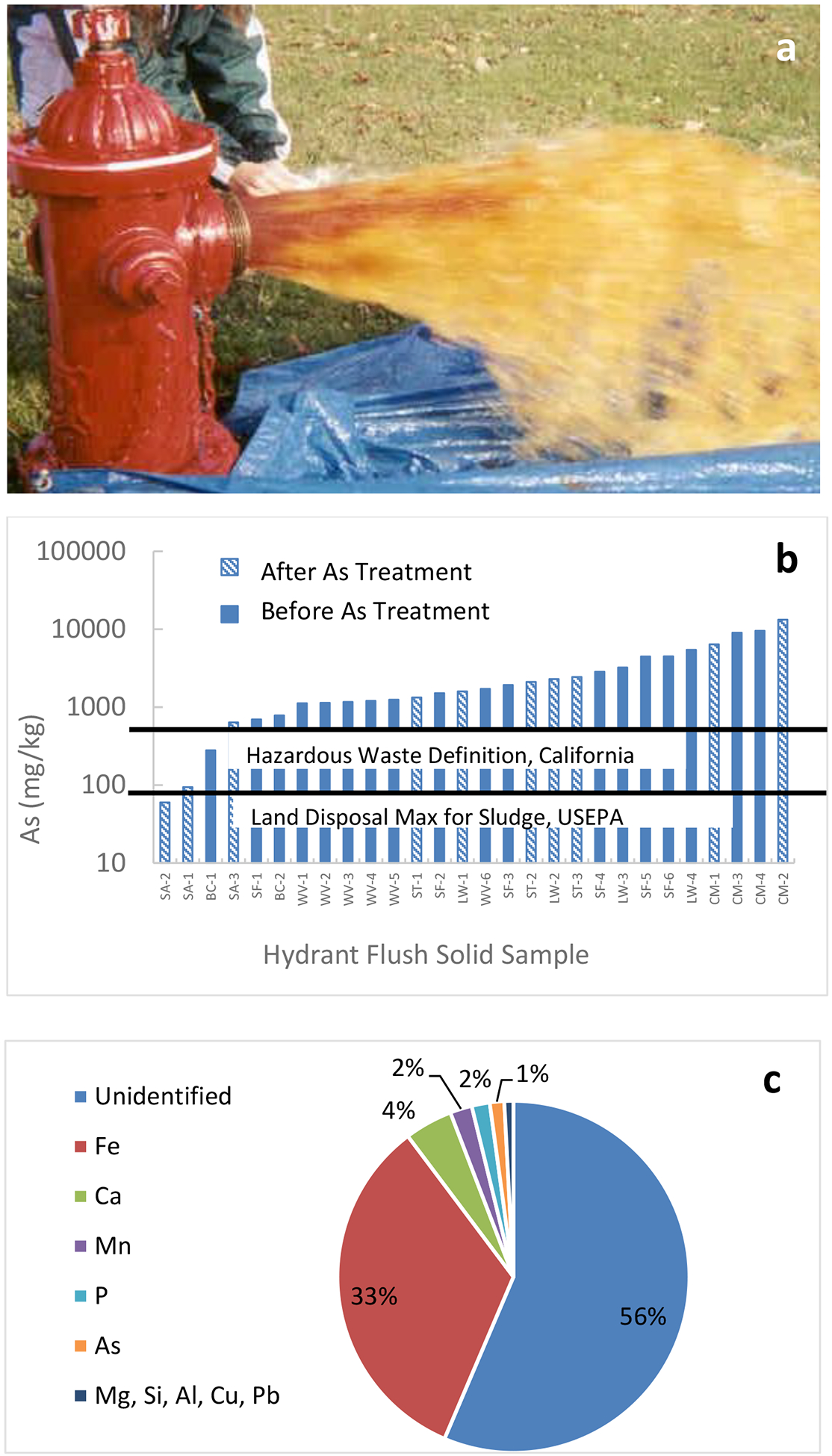
Hydrant flush solid samples were collected (a) from 7/20 studied systems. The arsenic content of the 28 hydrant solid samples (b) made up to 1% by weight of the solids’ elemental composition (c, corresponding to highest sample CM-2).
RESULTS AND DISCUSSION
Arsenic in hydrant flush solids.
Hydrant flush samples likely contain loose superficial contaminant deposits that can be released back into the water due to hydraulic disturbances (Friedman et al, 2010; Lytle et al., 2010). As such, they offer one indication of the presence and propensity of contaminants to be released back into the water from the various reversible sinks that exist in distribution systems. Twenty eight samples were collected overall from 7/20 systems, with 5/7 systems having before or after-treatment samples but not both. The results were therefore grouped together in order of increasing arsenic content (Figure 1b).
Overall, the arsenic content of 28 hydrant flush solids from 7 systems (see Table 1) varied widely, from 60 mg As/kg solid to 13,250 mg As/kg solid (or 0.006% to 1.32% As by weight) (Figure 1b). To put this range in perspective, 27/28 hydrant flush solids contained higher arsenic than the 75 mg/kg permissible ceiling concentration for the land application of sewage sludge, as set by the EPA (USEPA, 1994b). In addition, 25/28 hydrant flush solids contained higher arsenic than the 500 mg/kg total threshold limit concentration for hazardous waste, as set by the State of California (California Water Board, 1986). The arsenic content of these solids is comparable to prior observations of 107–9,936 mg As/kg solid in 30 hydrant flush solids from 15 utilities in 3 US states (Lytle et al. 2004), and comparable (albeit typically higher) to the 926–1,394 mg As/kg solid in 6 hydrant flush solids from 1 US utility as retained on nets (Friedman et al., 2016)
The major component in most solid samples was iron (ranging from 103,140 to 555,210 mg/kg) (see illustrative example of CM in Figure 1, bottom), presumably from iron pipes in the distribution system and/or from the inherent iron content of the well water for these systems (see Table 2). The iron content of the solids is important because arsenic release is often accompanied by elevated iron concentrations since arsenic has a high affinity for iron. In addition to iron and arsenic, the solid samples contained calcium, manganese, phosphorus, magnesium, silicon, aluminum, copper and lead (see illustrative example in Figure 1c). This method does not detect carbon, oxygen and hydrogen, therefore a significant fraction of the solids was not identifiable (Figure 1c).
Table 2.
Baseline quality of the source well water in 20 select small water systems from the USEPA’s Arsenic Demonstration. These illustrative results reflect a specific sampling date prior to the Arsenic Demo, as listed below.
| AL | BW | BC | GF | LD | DM | FC | CM | LW | PW | SF | ST | SV | WA | WL | WV | CL | DV | SA | SC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Date (MM/YY) | 2/05 | 4/04 | 7/03 | 9/04 | 12/06 | 9/04 | 9/06 | 7/03 | 7/03 | 8/04 | 8/04 | 8/04 | 10/04 | 9/04 | 11/06 | 12/06 | 10/06 | 9/04 | 8/04 | 8/04 |
| pH (units) | NA | 6.8 | 7.3 | 6.9 | 7.3 | 7.9 | 7.5 | 7.4 | 7.2 | 6.9 | NA | 7.7 | 7.5 | 8.6 | 7.5 | 7 | 8 | 7.5 | 7.3 | 7.1 |
| Alkalinity (mg/L CaCO3) | 379 | 54 | 235 | 85 | 162 | 137 | 337 | 304 | 344 | 141 | 319 | 424 | 82 | 65 | 137 | 681 | 147 | 384 | 302 | 363 |
| Hardness (mg/L CaCO3) | NA | 92.7 | 83.2 | 25 | 163 | 156 | 255 | 228 | 513 | 252 | 381 | 246 | 40 | 53 | 108 | 481 | 154 | 346 | 752 | 360 |
| Turbidity (NTU) | 2 | NA | NA | 0.2 | 0.7 | 0.4 | 5.8 | NA | NA | 2.3 | 23 | 7 | 0.9 | 0.1 | 2.6 | 35 | 1.5 | 20 | 7.1 | 30 |
| TOC (mg/L) | NA | <0.5 | <0.5 | <0.7 | <1** | <0.7 | 1.6 | <1.0 | <1.0 | 2.5 | <1.0 | 7.2 | 1 | <0.7 | <1 | 9.0 | <1 | 1.8 | 2 | 3.9 |
| Chloride (mg/L) | 110 | 41 | 51 | 1.2 | 1.0 | 51 | 2.0 | 190 | 82 | 130 | 14 | 7.2 | 2.1 | 7.6 | 23.0 | 7.0 | 14.0 | <1.0 | 34 | <1.0 |
| Sulfate (mg/L) | 1 | 12 | 74 | 5.8 | 2 | 20 | 2 | 120 | 390 | 1 | 27 | <5.0 | 17 | 18 | 6 | <1 | 21 | <1.0 | 410 | <5.0 |
| Silica (mg/L SiO2) | 16.7 | 19.7 | 8.1 | 25.7 | 15 | 12.3 | 13.9 | 27.3 | 29.4 | 11.1 | 19.4 | 26.6 | 14.5 | 10.7 | 13.3 | 20.1 | 12.7 | 14.3 | 29.7 | 25 |
| As tot. (μg/L) | 34.5 | 39.2 | 14.2 | 32.7 | 23.9 | 30 | 26.9 | 38.7 | 147 | 13.4 | 24.6 | 41.7 | 36.7 | 37.7 | 15.4 | 28.3 | 28.4 | 20.1 | 13.9 | 25.3 |
| As sol. (μg/L) | NA | 44.1 | 12 | 33.1 | 23 | 30.1 | 18.1 | 34.6 | 126 | 13.2 | 24.3 | 32.9 | 36.6 | 38 | 13.6 | 21.2 | 28 | 20.5 | 12.6 | 20.7 |
| As part. (μg/L) | NA | <0.1 | 2.2 | <0.1 | 0.9 | <0.1 | 8.8 | 4.2 | 20.3 | 0.2 | 0.3 | 8.8 | 0.1 | <0.1 | 1.8 | 7.1 | 0.4 | <0.1 | 1.3 | 4.6 |
| As (III) (μg/L)* | NA | 0.5 | 11.2 | 0.8 | 0.5 | 1.5 | 12.6 | 34.8 | 121 | 11.1 | 24.7 | 31.9 | 31.9 | 33.4 | 6.0 | 17.4 | 25.8 | 19.1 | 5.1 | 13.6 |
| As (V) (μg/L)* | NA | 43.6 | 0.8 | 32.3 | 22.5 | 28.6 | 5.5 | <0.1 | 5.3 | 2.1 | <0.1 | 1 | 4.7 | 4.6 | 7.6 | 3.8 | 2.2 | 1.4 | 7.5 | 7.1 |
| Fe tot. (μg/L) | 317 | <25 | 127 | <25 | <25 | <25 | 1547 | 546 | 1325 | 466 | 1615 | 1344 | 125 | <25 | 332 | 2659 | 157 | 1499 | 854 | 3078 |
| Fe sol. (μg/L) | NA | <25 | 118 | <25 | <25 | <25 | 855 | 540 | 1316 | 465 | 1635 | 1359 | <25 | <25 | 129 | 2350 | 151 | 1400 | 844 | 3149 |
| Mn tot. (μg/L) | 55.4 | 2.1 | 13 | 13.5 | 2.8 | 5.1 | 53.5 | 128 | 675 | 32.4 | 18.5 | 27 | 5.6 | 10.3 | 180 | 18.9 | 61 | 20.2 | 327 | 150 |
| Mn sol. (μg/L) | NA | 1.5 | 15 | 2.8 | 0.8 | 4.2 | 53.1 | 130 | 665 | 32.6 | 18.8 | 28 | 5.5 | 9.6 | 165 | 18.4 | 66.3 | 18.3 | 331 | 154 |
| Ca tot. (mg/L) | 12 | 28.3 | 20.6 | 7 | 49.9 | 28 | 57.9 | 60.6 | 148 | 56 | 89 | 56 | 11.2 | 18 | 35.6 | 103 | 50 | 71.4 | 173 | 87 |
| Mg tot. (mg/L) | 3.2 | 5.3 | 7.7 | 2 | 9.3 | 21 | 26.7 | 18.5 | 35 | 27 | 39 | 26 | 2.9 | 2 | 4.7 | 54 | 7.1 | 40.7 | 78 | 35 |
NA: Not analyzed.
The sum of As(III) and As(V) equals As sol.
Sample analyzed outside of holding time
Overall, this analysis illustrates the persistent effect of the prior untreated water quality on superficial pipe arsenic composition and possibly highlights the propensity of the studied distribution systems to switch from accumulation sinks to eventual sources of arsenic and other inorganic contaminants.
Baseline arsenic and other water data.
All systems exceeded the arsenic MCL of 10 μg/L during “baseline” well water sampling before the installation of arsenic treatment (Table 2). Total arsenic ranged from 13 μg/L in PW to 147 μg/L in LW, and the form of arsenic was dominantly soluble (>67% in all systems). Except for LW, arsenic levels ranged between 13 μg/L and 42 μg/L. Arsenic speciation of well water for dissolved versus particulate arsenic and As(III) versus As(V) was conducted according to Sorg et al. (2014), to obtain important baseline information for the selection of a suitable treatment strategy (see Table 1). However, only total arsenic, total iron and total manganese were measured in water sampled at distribution system sites. The following discussion will therefore only focus on total concentrations.
Twelve systems had source waters that contained iron levels greater than the secondary MCL (SMCL) of 0.3 mg/L (300 μg/L) and 8 systems had source waters that contained manganese greater than the SMCL of 0.05 mg/L (50 μg/L). However, in 5 systems (BW, DM, GF, LD, WA) iron in the well water was not detected (<25 μg/L) and manganese was low (2.1–13.5 μg/L) (Table 2). The amount of iron in the source water was important in identifying appropriate arsenic treatment for the Arsenic Demo, but also important in the subsequent analysis herein. This is because arsenic release is often accompanied by elevated iron concentrations and naturally-formed iron precipitates have a sorption capacity to remove arsenic from water.
Patterns of arsenic release at distribution system sites.
Three patterns of arsenic release at the tap were identified among the 20 water systems:
Pattern A. Mostly conservative behavior in the distribution system: Arsenic concentration did not change during the travel of water through pipes, except for brief destabilization period(s)
Pattern B. Non-conservative behavior in the distribution system: Arsenic concentration increased during the travel of water through pipes (continuous slow release at sampled taps)
Pattern C. Non-conservative behavior in the distribution system: Arsenic concentration increased during the travel of water through pipes (spikes and unpredictable variability at sampled taps)
Two illustrative examples from each pattern are presented in detail (Figures 2, 3, and 4). Arsenic in water that entered the distribution system “after treatment” (i.e., IN) was compared to arsenic in water exiting the distribution system at three “distribution sites” (reflecting three consumer taps in the context of this paper, i.e., OUT) Summary statistics and pattern classification for all 20 systems are presented in Table 3.
Figure 2.
Total arsenic concentration in tap water of Pattern A systems GF (a) and LD (b) before/after the installation of arsenic treatment (at time 0). “After treatment” samples reflect water entering the distribution system. “Distribution site” samples reflect water exiting the distribution system at three consumer taps. System GF (a) experienced adsorptive media breakthrough after t=270 days.
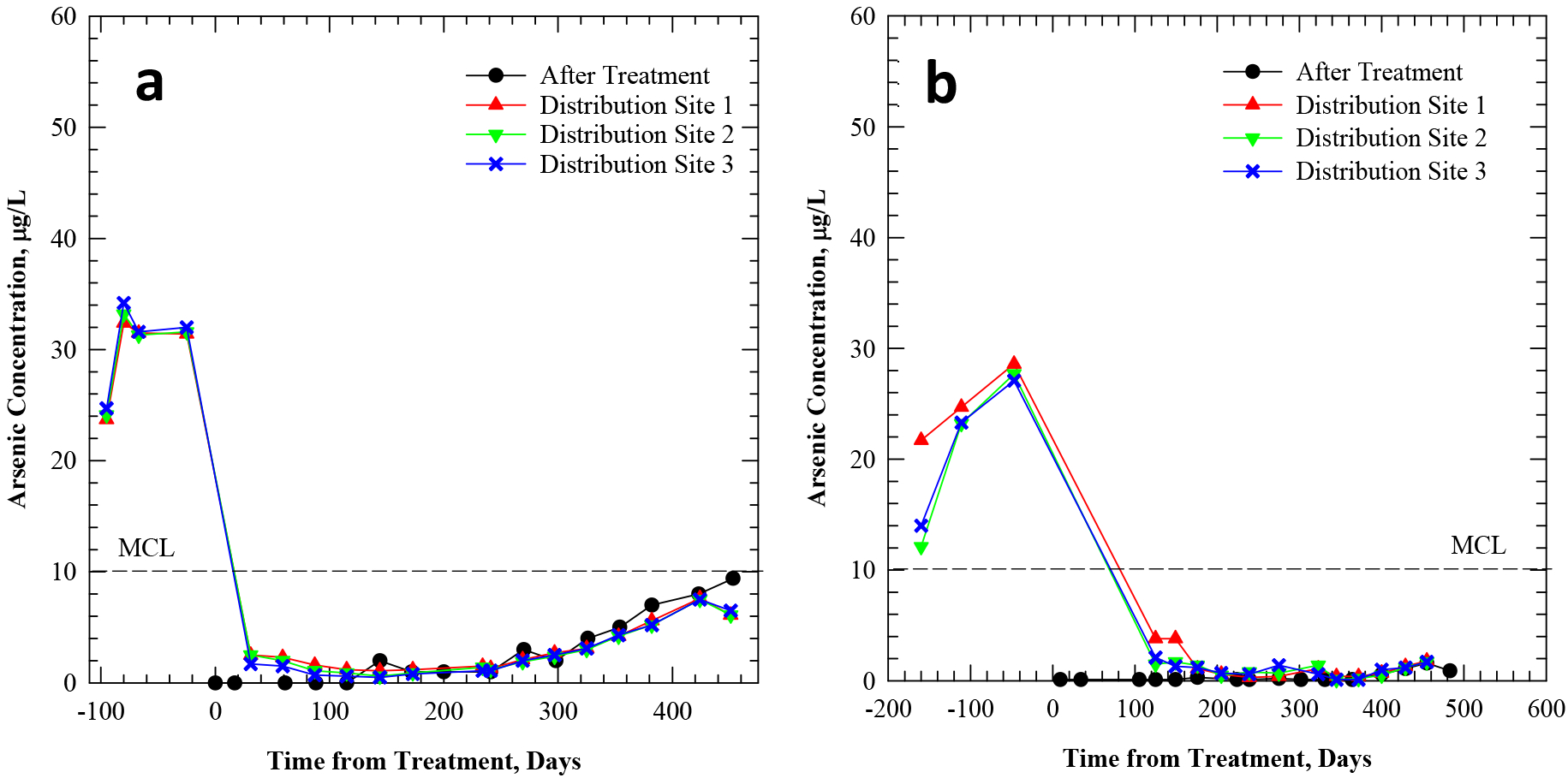
Figure 3.
Total arsenic concentration (a, b) and total iron concentration (c, d) in tap water of Pattern B systems LW and WV, before/after the installation of arsenic treatment. “After treatment” samples reflect water entering the distribution system. “Distribution site” samples reflect water exiting the distribution system at three consumer taps.
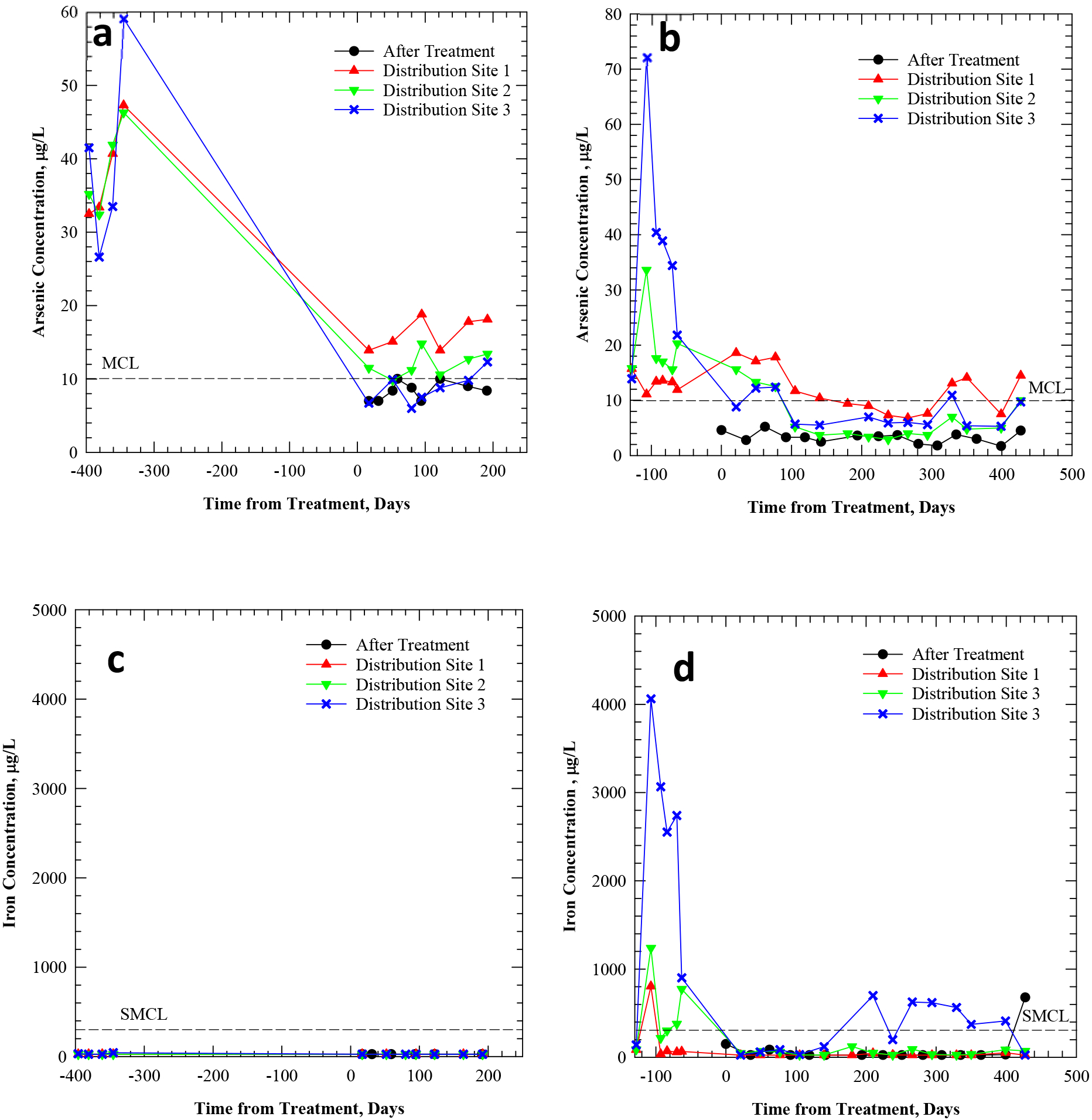
Figure 4.
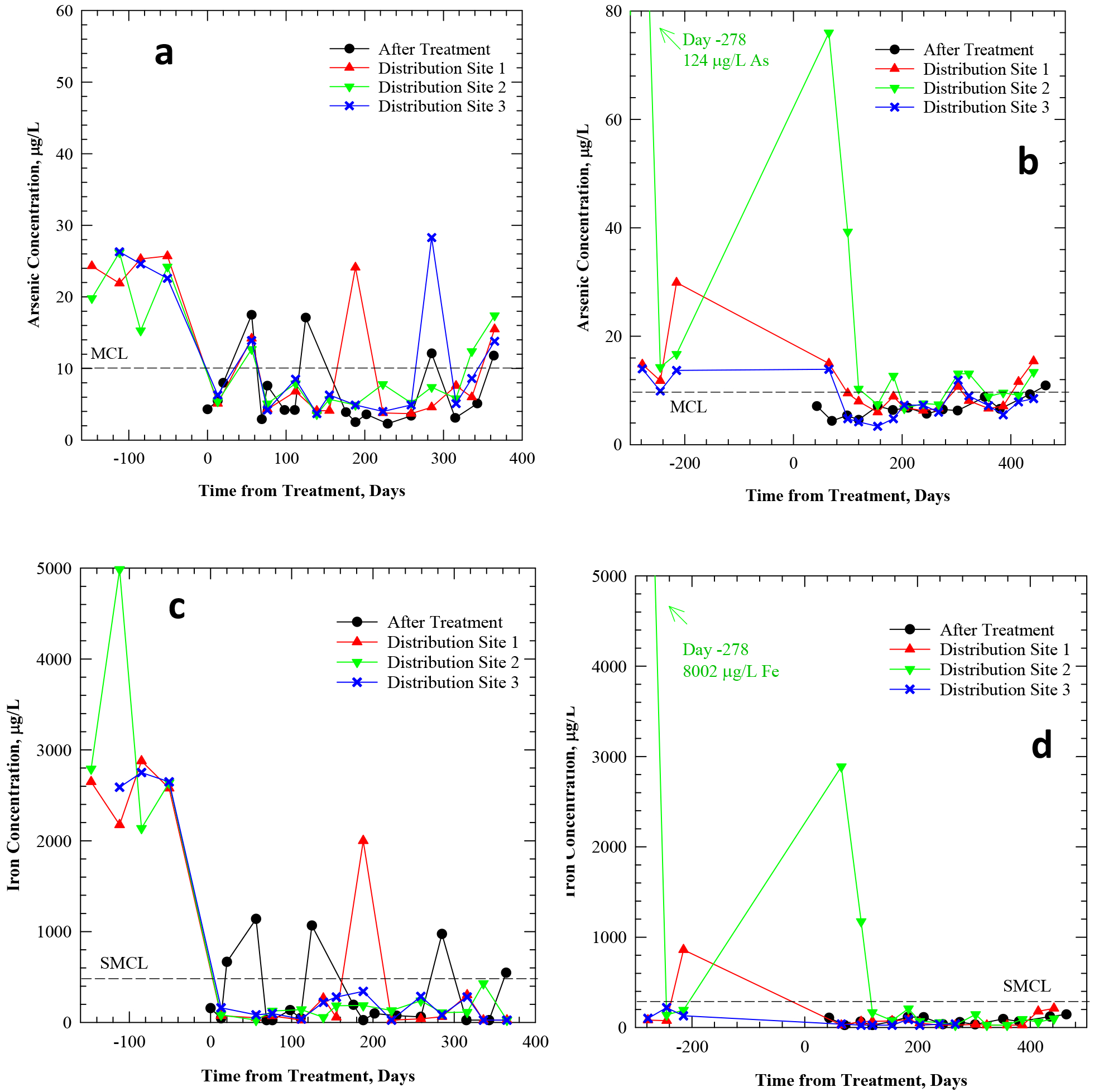
Total arsenic concentration (a, b) and total iron concentration (c, d) in tap water of Pattern C systems SC and SA, before/after the installation of arsenic treatment. “After treatment” samples reflect water entering the distribution system. “Distribution site” samples reflect water exiting the distribution system at three consumer taps.
Table 3.
Summary statistics for arsenic in first-draw water samples and categorization of systems based on the pattern of arsenic release.
| System |
Arsenic Before Treatment AVG ± STDEV, μg/L |
Arsenic After Treatment AVG ± STDEV, μg/L |
Arsenic Pattern | |||||
|---|---|---|---|---|---|---|---|---|
| # samples over 10.0 μg/L, % | # samples over 10.0 μg/L, % | |||||||
| DS1 | DS2 | DS3 | Treatment Effluent | DS1 | DS2 | DS3 | ||
| Adsorptive Media | ||||||||
| AL | 47±31 | 31±2 | 36±10 | 1±1 | 3±4 (2±1)* | 2±1 | 2±1 | A |
| 100% | 100% | 100% | 0% | 8% (0%)* | 0% | 0% | ||
| BW | 46±7 | 44±4 | 45±5 | 14±12 | 14±10 | 13±9 | 15±9 | A |
| 100% | 100% | 100% | 54% | 53% | 61% | 56% | ||
| BC | 12±1 | 9±1 | 11±1 | 4±3 | 6±2 | 6±2 | 6±3 | A |
| 100% | 0% | 75% | 4% | 6% | 6% | 11% | . | |
| GF | 30±4 | 30±4 | 31±4 | 3±3 | 3±2 | 3±2 | 3±2 | A |
| 100% | 100% | 100% | 0% | 0% | 0% | 0% | ||
| LD | 25±3 | 21±8 | 21±7 | 0.2±0.1 | 1±1 | 1±1 | 1±1 | A |
| 100% | 100% | 100% | 0% | 0% | 0% | 0% | ||
| DM | 29±2 | 34±5 | 39±8 | 1±0.5 | 9±10* | 3±2 | 5±3 | B |
| 100% | 100% | 100% | 0% | 33% | 0% | 11% | ||
| FC | 14±16 (6±1)* | 22±NA | 24±NA | 4±1 | 5±3 | 5±2 | 5±2 | C |
| 25% (0)* | NA | NA | 0% | 8% | 0% | 0% | ||
| Coagulation/Filtration | ||||||||
| CM | 33±8 | 37±11 | 41±12 | 10±4 | 51±136 (12±3)* | 13±2 | 11±3 | B |
| 100 | 100 | 100 | 39% | 75% (73%)* | 83% | 67% | ||
| LW | 39±7 | 39±6 | 40±14 | 8±1 | 16±2 | 12±2 | 9±2 | B |
| 100% | 100% | 100% | 0% | 100% | 86% | 14% | ||
| PW | 16±1 | 17±5 | 17±1 | 8±4 | 7±1 | 8±3 | 8±2 | C |
| 100% | 100% | 100% | 18% | 0% | 33% | 25% | ||
| Iron Removal & Adsorptive Media | ||||||||
| SF | 43±19 | 17±8 | 12±2 | 0.2±0.1 | 2±1 | 0.5±0.3 | 2±0.5 | B |
| 100% | 100% | 75% | 0% | 0% | 0% | 0% | ||
| ST | 29±2 | 35±3 | 29±1 | 2±2 | 6±2 | 5±2 | 8±4 | B |
| 100% | 100% | 100% | 0% | 0% | 0% | 8% | ||
| Oxidizing media & Adsorptive Media | ||||||||
| SV | 34±17 | 31±11 | 26±13 | 0.1±0.0 | 1±0.4 | 1±0.6 | 2±2 | B |
| 100% | 100% | 100% | 0% | 0% | 0% | 0% | ||
| WA | 35±4 | 36±4 | 36±3 | 10±15 | 11±16 | 11±17 | 12±17 | A |
| 100% | 100% | 100% | 36% | 36% | 36% | 36% | ||
| WL | 12±2 | 12±1 | 11±1 | 0.5±0.2 | 4±2 | 2±2 | 4±1 | B |
| 100% | 100% | 100% | 0% | 0% | 0% | 0% | ||
| Iron Removal | ||||||||
| WV | 13±2 | 20±7 | 40±20 | 3±1 | 12±4 | 7±4 | 8±3 | B |
| 100% | 100% | 100% | 0% | 60% | 20% | 20% | ||
| CL | 11±7 | 8±5 | 13±6 | 4±5 | 3±2 | 5±5 | 7±6 | C |
| 25% | 25% | 75% | 17% | 0% | 9% | 10% | ||
| DV | 13±3 | 16±2 | 16±1 | 8±5 | 10±6 | 8±6 | 10±6 | C |
| 67% | 100% | 100% | 28% | 39% | 23% | 23% | ||
| SA | 16±10 | 55±52 | 12±2 | 7±2 | 9±3 | 17±19 | 7±3 | C |
| 75% | 100% | 50% | 7% | 29% | 50% | 14% | ||
| SC | 24±2 | 21±5 | 25±2 | 6±4 | 8±6 | 8±4 | 9±7 | C |
| 100% | 100% | 100% | 21% | 23% | 23% | 23% | ||
AVG Average; STDEV Standard Deviation; NA: Not Applicable; DS1, DS2, DS3: Three distribution system sites sampled (i.e., homes or occasionally other buildings like schools);
single irregular spike that skews distribution removed from calculation in parenthesis
Pattern A (6 systems) was mostly characterized by similar arsenic concentrations in the treatment system effluent (i.e., IN) and in “distribution sites” (i.e., OUT). The exception was a relatively short period after treatment start-up (several months), when arsenic in the tap water as sampled in distribution sites 1, 2 and 3 was greater than in the respective treatment system effluent. This constituted an adjustment period (or lag phase), for the benefits of arsenic removal at the well to be fully realized at the sampled taps.
Pattern A was reflected by the arsenic trends in GF (Figure 2a). The lag phase in this system lasted for the initial 100 days of arsenic treatment (or 10,000 bed volumes [BV] of adsorptive media throughput). During this period, arsenic levels at the distribution system taps (~2±1 μg/L) were greater than those in the treatment system effluent (0.1±0.0 μg/L). After 100 days, the arsenic levels at the distribution system taps were nearly identical to those entering the distribution system. This trend even extended to a period when arsenic started breaking through the adsorptive media system (between 270 and 450 days). Overall, during 450 days of the Arsenic Demo, average arsenic levels at the three distribution system sites (3±2 μg/L) were practically identical to those in the treatment effluent (3±3 μg/L) (Table 3). The baseline source well water had undetectable iron of <25 μg/L and low manganese of 14 μg/L (Table 2). The sampled tap water contained <25 μg/L of iron and <3 μg/L of manganese before and during the Arsenic Demo, indicating little or no release from the distribution system (iron and manganese data were not plotted here due to their low concentrations). This distribution system was made from PVC, whereas buildings contained copper pipe (Table 1).
LD was another Pattern A system, where the lag phase lasted for about 170 days of treatment (or 45,000 BV [Figure 2b]). After that, arsenic concentrations in the 3 sampled taps essentially mirrored those of the treatment effluent. Baseline source well water in this system also had undetectable iron (Table 2). The distribution system does not appear to have released any iron or manganese before and during the Arsenic Demo (consistently <25 μg/L iron and low manganese of 3 μg/L at sampled tap water, not plotted here). The distribution system was constructed of steel and PVC, whereas no information was available regarding building plumbing materials (Table 1).
In some systems of Pattern A, arsenic concentrations in tap water exceeded the MCL post-treatment. For example, 53–61% of distribution site samples (i.e., 9/17 samples at site DS1 to 11/18 at DS2) were above 10 μg/L As in BW, 6–11% of distribution site samples (i.e., 1/18 at DS1 & DS2 to 2/18 in DS3) were above 10 μg/L in BC, and 36% were above 10 μg/L in WA (i.e., 4/11 at each of DS1, DS2 & DS3) (Table 3). These occasionally increased arsenic levels were still consistent with the increased arsenic content of the incoming treated water, possibly reflecting adsorptive media breakthrough (due to multiple adsorption runs in BW and WA or initial absence of pre-chlorination in BC). These systems were therefore also classified under Pattern A (Table 3). An occasional single and inexplicable arsenic spike (>10 μg/L) could still be observed in tap water for some Pattern A systems (e.g., As=17 μg/L, Fe <25 μg/L, Mn=17 μg/L at DS1 on Day 22 for AL, not plotted here).
Pattern B (8 systems) was characterized by higher arsenic concentrations in distribution system taps (i.e., OUT) relative to the treatment system effluent (i.e., IN) throughout the Arsenic Demo duration, implying release of arsenic from the distribution system. In this category, at least two of the three sampled sites from each system had consistently higher arsenic in tap water than the treated water, above and beyond the occasional single spike observed in some Pattern A systems.
In the case of Pattern B system LW, the treated water entering the distribution system (i.e., IN) contained an average of 8±1 μg/L arsenic during the Arsenic Demo. Water sampled at the three Distribution Sites (i.e., OUT) averaged 16±2, 12±2 and 9±2 μg/L respectively, over the same period (Figure 3a, Table 3). These observations indicate continual slow release of arsenic from the distribution system materials during an extended period after system engineering modifications. The specific reasons for the arsenic concentration differences among the sampled sites are not possible to identify, mostly because detailed plumbing material investigations were not part of the initial Arsenic Demo scope (see Table 1). However, the differing concentrations demonstrate the variable and complex nature of arsenic release among locations of the same community receiving water from the same treated source. Out of the 20 systems, this system had the highest arsenic content (147 μg/L) in source water, concurrent with high iron (1,325 μg/L) and manganese levels (675 μg/L) (Table 2). It is possible that a significant amount of arsenic in treated water prior to the engineering modifications had accumulated on distribution system solids and that some arsenic was subsequently released back into the water. Notably, iron concentrations at the distribution system entry point and at the 3 distribution system sites were low (mostly below the detection limit of 25 μg/L) (Figure 3.c).
WV was another Pattern B system, which demonstrated a similar characteristic continual slow arsenic release in water sampled from building taps. The sampled sites experienced a lag-phase in the beginning of the Arsenic Demo (Figure 3b), similar but more excessive (i.e., >10 μg/L) than that observed in Pattern A systems. However, unlike Pattern A, arsenic levels at these distribution sites (i.e., OUT) never dropped to levels observed in the treatment system effluent (i.e., IN). Specifically, arsenic concentrations at the three distribution sites averaged 12±4, 7±4 and 8±3 μg/L, over the 430-day Arsenic Demo. In contrast, arsenic after treatment averaged 3±1 μg/L (Table 3). These observations illustrate the relatively consistent and continual release of arsenic from distribution pipes and other plumbing materials for more than a year after start-up of arsenic treatment. Except for Distribution Site 3, iron concentrations at the distribution system entry point and at the other two distribution sites were typically low (Figure 3.d). Source water prior to arsenic treatment contained about 28 μg/L As, concurrent with more than 2,500 μg/L iron but low manganese of 19 μg/L (Table 1). The six hydrant flush solid samples from this system had appreciable arsenic (see Figure 1b) and iron content, and the distribution system contained cast iron.
Levels of arsenic in the sampling locations at these two Pattern B systems (LW and WV) remained mostly above the MCL after arsenic treatment (e.g., 86% to 100% of the time for DS1 and DS2 in LW) despite being below the MCL at the entry point to the distribution system (regulatory compliance location). This observation, however, was not consistent across the systems that followed Pattern B (Table 3). Overall, levels of arsenic at the sampled sites of Pattern B remained either above the MCL after treatment (LW, WV, CM) or dropped below the MCL after treatment (DM, SF, ST, SV, WL) (Table 3).
An example from the latter group of Pattern B is SF (not plotted but summarized in Table 3), where the concentrations of arsenic overall were low. This may be due to plastic pipe materials in this system (Table 1) that may have served as modest reversible contaminant accumulation sinks, although some accumulation was still possible. Work by Lytle et al. (2010) analyzed a scraped solid sample from a PVC distribution pipe from this system and found it had accumulated 1,230 mg As/kg and 144,200 mg Fe/kg. This illustrates that even plastic pipes serve as sinks for contaminant accumulation and potential release, consistent with Li et al. (2018), Salehi et al. (2017), Liu et al., (2017), Friedman et al. (2016) and Cerrato et al. (2006). The 6 additional hydrant flush solid samples reported here (see Table 1) contained 700 mg As/Kg to 4,470 mg As/Kg (or 0.07% to 0.45% As by weight) (Figure 1b), co-occurring with 103,140 to 353,350 mg Fe /Kg (or 10.3% to 35.3% Fe by weight). This system had about 25 μg/L of total As in untreated well water, concurrent with about 1600 μg/L Fe and low Mn (Table 2).
In addition to the continual slow arsenic release pattern, distribution sites of Pattern B also occasionally experienced isolated arsenic spikes after treatment was installed, which was indicative of random particulate arsenic release episodes. For example, DS1 in CM (not plotted here but summarized in Table 3) experienced a visible red water event as reported by the homeowner, that coincided with a 484 μg/L arsenic spike captured during a sampling event (Day 20 after the start-up of arsenic treatment). The sample also contained 13,903 μg/L Fe and 1,290 μg/L Mn, suggesting release of arsenic-containing iron particles from the distribution system. Thus, in addition to the rather consistent slow background release of As, additional release due to isolated but severe spikes was possible for systems in Pattern B. Although samples were not filtered for dissolved versus particulate arsenic determination, the very high concentrations of arsenic, co-occurring with very high iron and manganese suggest particulate release in that instance. Prior to arsenic treatment, CM had 39 μg/L As, 546 μg/L Fe and 128 μg/L Mn at the source well water (Table 2), and buildings had copper plumbing or PVC (Table 1).
Pattern C (6 systems) was characterized by several arsenic spikes in the system effluent (i.e., IN) and/or at the 3 sampled taps (i.e., OUT), more than the single irregular spike occasionally observed in systems that fell under Patterns A or B. Arsenic fluctuations were occasionally above the arsenic MCL and did not necessarily stabilize post-treatment. Arsenic fluctuations in the system effluent reflected insufficient removal of arsenic at the treatment plant during intermittent operational problems associated with chlorine and/or FeCl3 addition or particulate breakthrough from filters, as explained in the respective EPA reports.
For example, SC experienced arsenic spikes >10 μg/L at the plant effluent on several sampling dates (Figure 4a), co-occurring with elevated iron (Figure 4c), which were sometimes reflected at some or all sampled building taps (e.g., on Days 56, 285 and 364). These spikes were possibly attributable to particulate breakthrough from pressure filters. The spikes at the plant effluent did not always correspond to fluctuations at sampled taps (e.g., on Day 125), due to sampling lag times and dilution effects from plant to tap. Distribution Site 1 experienced 24 μg/L arsenic on Day 188, at a sampled tap that was not frequently used. Although additional information is not available on water use patterns at the sampled taps during the Arsenic Demo, this indicates that water use may also play an important role in the variability of arsenic concentrations from tap to tap. Distribution Site 3 also experienced a spike of 28.3 μg/L arsenic on Day 285 (Figure 4a), which was not concurrent with increased iron (Figure 4c).
Pattern C system SA also experienced irregular arsenic spikes (Figure 4b) in the sampling locations. Distribution Site 2, in particular, experienced arsenic spikes before arsenic treatment at the well (Time=−278 days, As=124 μg/L) and after treatment (e.g., Time=65 days, As=76 μg/L; Time=100 days, As=39 μg/L; Time=184 days, As=12.7 μg/L). This sampling tap was reportedly located in the older part of town and initially had higher arsenic and iron levels due to a history of periodic release of particulates (tubercles) from the distribution system. The distribution system materials included cast Fe and PVC for this system, while no plumbing material information was available for premises. The history of particulate release in Distribution Site 2 may be attributable to a cast iron main, or possibly galvanized iron piping within the building. Although the continual slow arsenic release of Pattern B was also observed here, the several additional irregular arsenic spikes increased variability (e.g., see Figure 4 and Table 3) and distinguished Pattern C from Pattern B.
SUMMARY
The arsenic content of opportunistic hydrant flush solid samples highlighted the propensity of distribution systems to switch from arsenic accumulation sinks to sources, consistent with prior observations. More importantly, analysis of unique datasets from the EPA’s Arsenic Demonstration Program revealed three patterns of arsenic release at consumer taps, after arsenic treatment of the source well water (Figure 5). These temporal trends (three patterns of arsenic release during several months to more than a year depending on system) and spatial trends (three patterns of arsenic release among 20 distribution systems, and occasional differences between 3 sampled building taps within each system), as supplemented with other inorganic data (iron and manganese) and some general information on pipe materials, allow some speculation on plausible underlying arsenic release mechanisms from distribution system solids back into the water (Figure 5). Commonalities within each pattern also suggest that factors contributing to the behavior of arsenic conceivably were the source water arsenic and iron levels, the resulting type of arsenic treatment, and the distribution system pipe materials.
Figure 5.
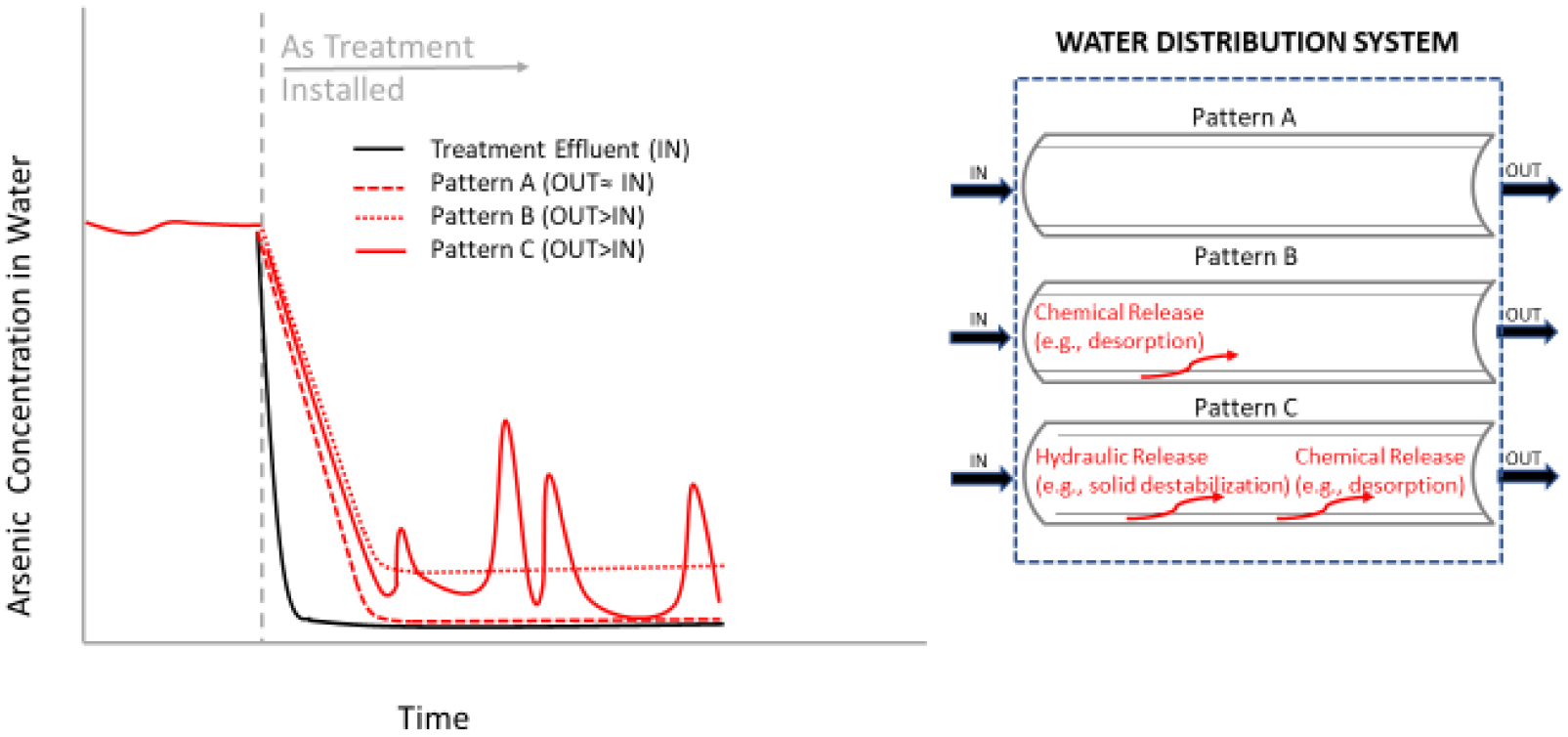
Illustrative and simplified arsenic profiles (not to scale), summarizing arsenic release under Pattern A, B, and C, accompanied by plausible underlying release-controlling mechanisms. The worst-case of Pattern C (hydraulic release with some additional chemical release) is depicted.
Pattern A (30% of the water systems) was characterized by an initial short phase of “non-conservative” contaminant behavior (IN<OUT), possibly indicating some initial redissolution and/or resuspension of arsenic that had previously accumulated in the distribution system. This phase was followed by a longer period of chemically idealized “conservative” behavior, where arsenic concentration entering the distribution system did not change as water travelled through the mains and other pipes (IN≈OUT). In Pattern A systems, arsenic levels at the well before treatment were 14 μg/L to 37 μg/L (Table 2). Because iron and manganese levels at the well before treatment were low (Table 2), adsorptive media were employed for arsenic removal in these cases (see Table 1). The low source water iron concentration reduced the amount of iron that could accumulate in the distribution system. Coupled with the prevalence of PVC or plastic as distribution system materials (see Table 1), the measured arsenic levels at consumer taps followed a rather conservative pattern overall.
Pattern B (40% of systems) was characterized by non-conservative arsenic release (IN<OUT) throughout the Arsenic Demo. Whether the observed continual arsenic release was temporally mostly minor (<10 μg/L) or major (>10 μg/L), at least 2/3 of the distribution sites following Pattern B contained higher arsenic in tap water than the treated well water. Because the arsenic release trend was steady and consistent, it was suggestive of a desorption arsenic release control mechanism from distribution system solids back to the water. In Pattern B systems, arsenic levels at the well before treatment were 15 μg/L to 147 μg/L (Table 2). With two exceptions, systems of this Pattern had iron and manganese levels at the well before treatment that were typically much greater than their respective SMCLs (Table 2), and employed iron removal or coagulation/filtration (see Table 1). Systems that fell under this category had a variety of plumbing materials listed, from plastic pipes (e.g., PVC, black PE, polybutylene) to copper, cast iron or galvanized iron (see Table 1). It is speculated that the specific locations sampled were receiving water from cast iron mains and/or galvanized premise pipes, which are more likely to accumulate arsenic onto iron surfaces.
Pattern C (30% of systems) was indicative of non-conservative arsenic release throughout the Arsenic Demo, which was more complex/unpredictable than the continual slow release of Pattern B. Pattern C possibly reflected a complex combination of intermittent arsenic release mechanisms in addition to arsenic desorption. This may have included particle breakthrough from the treatment system or particle destabilization from distribution system solids. Overall, the initial elevated arsenic levels at Pattern C wells (ranging 13–27 μg/L) concurrent with elevated iron levels >300 μg/L (see Table 2), the presence of iron within distribution system plumbing materials, the reported arsenic treatment operational problems (e.g., backwash failures, temporary chlorine addition problems, temporary ferric chloride feed problems), and the reported history of particulate arsenic release in some building taps, are believed to have exacerbated arsenic spikes in systems of this Pattern even after arsenic treatment at the well. Apart from two exceptions, Pattern C systems employed iron removal technologies (Table 1), where backwash cycles are particularly important. If backwash cycles do not take place frequently, then longer filter runs result in iron and arsenic particle breakthrough from the treatment system.
As with any retrospective field study of this extent, certain limitations were unavoidable. The inadvertently different timing of water sampling at the treatment effluent versus consumer taps, in combination with the lag time for water to move through each distribution system, means that arsenic levels could not be monitored in the exact same volume of water as it moved from plant to tap. Dissolved versus particulate arsenic fractions were not distinguished, which limited the ability to verify certain underlying arsenic release mechanisms. The 1-L first-draw sample volume at 3 sampled building taps reflected water that previously traveled through a given distribution system and stagnated for ≥6 hours within approximately the last 20 feet of building piping, assuming an internal piping ID of 1/2 inch. The influence of this short plumbing section to the sampled stagnant water quality is important, but such specific plumbing material information was not available. The analysis was limited to the duration of the As Demo in each system, for which data were available. Any subsequent water treatment or other changes could potentially alter the release of arsenic in the studied systems.
Limitations notwithstanding, the unique information in this extensive dataset did allow for valuable general conclusions. The three arsenic release patterns at consumer taps were of increasing complexity. They ranged from mostly conservative behavior in “simpler” systems (low iron/manganese in source well water and plastic pipe materials in the distribution system) to non-conservative release in more “complex” systems (high iron/manganese in source well water, iron pipe materials, and occasional arsenic treatment complications). This study suggests that in some complex systems arsenic and possibly other legacy inorganic contaminants may linger long after they have been removed at their source, as has already been demonstrated for lead (e.g., up to 4 years after lead service line replacement in Schock et al., 2014). Managing expectations after removing/treating inorganic contaminant sources is advisable, based on these field observations.
Distribution systems are dynamic in space (miles of diverse pipe materials with complex configurations under differing hydraulic patterns), and dynamic in time (possibly decades or more than a century of service to adapt to chemical and hydraulic changes). Tap water sampling at points of water consumption gives invaluable information on the spatial and temporal behavior of legacy contaminants as they move through distribution systems. More importantly, it offers a more complete picture of the water quality customers may conceivably be consuming, in addition to sampling at distribution system entry points.
ACKNOWLEDGMENTS
The authors acknowledge water utilities that participated in the Arsenic Demo, as well as operators and homeowners who collected water samples. Several Battelle staff members oversaw the collection and analysis of water and fire hydrant samples. The authors also acknowledge Katherine Webster (Pegasus Technical Services, Inc at the time of this study) for extracting data from the EPA reports. Andrea Porter (EPA Region 5), Regan Murray and Jonathan Burkhardt (both EPA ORD), as well as Alessandro Ossola (Macquarie University) provided comments to this manuscript. Michael R. Schock (EPA ORD) was especially helpful with comments to the manuscript. This work has been subjected to USEPA peer and administrative review and has been approved for publication. Any opinions expressed in this paper are those of the authors and do not necessarily reflect the official positions and policies of the USEPA.
REFERENCES
- Bacso J & Szalay A (1978). Accumulation of arsenic, lead and other heavy elements in the iron-manganese oxide-hydroxide precipitation in the pipelines of city waterworks. The Science of the Total Environment, 9, 271–276. doi: 10.1016/0048-9697(78)90017–7 [DOI] [Google Scholar]
- Burkhardt JB, Szabo J, Klosterman S, Hall J, & Murray R (2017). Modeling fate and transport of arsenic in a chlorinated distribution system. Environmental Modelling & Software, 93, 322–331. doi: 10.1016/j.envsoft.2017.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- California Regional Water Quality Control Board, Central Valley Region. (1986). The designated level methodology for waste classification and cleanup level determination. Accessed 5/31/2018 at https://www.waterboards.ca.gov/rwqcb5/plans_policies/guidance/dlm.pdf
- Cerrato JM, Reyes LP, Alvarado CN, & Dietrich AM (2006). Effect of PVC and iron materials on Mn(II) deposition in drinking water distribution systems. Water Research, 40, 2720–6. doi: 10.1016/j.watres.2006.04.035 [DOI] [PubMed] [Google Scholar]
- Chen ASC, Wang L, Lytle DA, & Sorg TJ (2018). Arsenic/iron removal from groundwater with elevated ammonia and natural organic matter. JAWWA, 110, E3–E17. doi: 10.5942/jawwa.2018.110.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement R, & Carlson G (2004). Contaminant accumulation in the distribution system: case histories AWWA Inorganic Contaminants Conference, Reno, Nevada. [Google Scholar]
- Copeland RC, Lytle DA, & Dionysiou DD (2007). Desorption of arsenic from drinking water distribution system solids. Environmental Monitoring and Assessment. 127, 523–535. doi: 10.1007/s10661-006-9299-1 [DOI] [PubMed] [Google Scholar]
- e-CFR (Electronic Code of Federal Regulations), 2019. National primary drinking water regulations. Title 40 part 141 subpart i control of lead and copper. Accessed at http://www.ecfr.gov/cgi-bin/text-idx?tpl=/ecfrbrowse/Title40/40cfr141_main_02.tpl [Google Scholar]
- Friedman MJ, Hill AS, Reiber SH, Valentine RL, & Korshin GV (2010). Assessment of inorganics accumulation in drinking water system scales and sediments. Water Research Foundation Project #3118. [Google Scholar]
- Friedman M, Hill A, Booth S, Hallett M, McNeill L, McLean J, Stevens D, Sorensen D, Hammer T, Kent W, De Haan M, MacArthur K, & Mitchell K (2016). Metals accumulation and release within the distribution system: evaluation and mitigation. Water Research Foundation PDF report 4509. [Google Scholar]
- Hammer TW (2018). Desorption of trace inorganic contaminants from solids in drinking water distribution systems. Master’s thesis, Utah State University. [Google Scholar]
- Hill A, Friedman M, Reiber S, & Korshin GV (2010). Behavior of trace inorganic contaminants in drinking water distribution system. JAWWA, 102, 107–118. doi: 10.1002/j.1551-8833.2010.tb10153.x [DOI] [Google Scholar]
- Li G, Ding Y, Xu H, Jin J, & Shi B (2018). Characterization and release profile of (Mn, Al)-bearing deposits in drinking water distribution systems. Chemosphere 197, 73–80. doi: 10.1016/j.chemosphere.2018.01.027 [DOI] [PubMed] [Google Scholar]
- Liu G, Tao Y, Zhang Y, Lut M, Knibbe WJ, van der Wielen P, Liu W, Medema G, & van der Meer W (2017). Hotspots for selected metal elements and microbes accumulation and the corresponding water quality deterioration potential in an unchlorinated drinking water distribution system. Water Research, 124, 435–445. doi: 10.1016/j.watres.2017.08.002 [DOI] [PubMed] [Google Scholar]
- Lytle DA, Sorg TJ, & Frietch C (2004). Accumulation of arsenic in drinking water distribution systems. Environmental Science and Technology, 38, 5365–72. doi: 10.1021/es049850v [DOI] [PubMed] [Google Scholar]
- Lytle DA, Sorg TJ, Muhlen C, & Wang L, (2010). Particulate arsenic release in a drinking water distribution system. JAWWA, 102, 87. doi: 10.1002/j.1551-8833.2010.tb10075.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytle DA, Sorg T, Wang L, & Chen A (2014). The accumulation of radioactive contaminants in drinking water distribution systems. Water Research, 50, 396–407. [DOI] [PubMed] [Google Scholar]
- Makris KC, Andra SS, & Botsaris G (2014). Pipe scales and biofilms in drinking-water distribution systems: undermining finished water quality. Critical Reviews in Environmental Science and Technology, 44, 1477–1523. doi: 10.1080/10643389.2013.790746 [DOI] [Google Scholar]
- McNeill LS, & Edwards M (1995) Soluble arsenic removal at water treatment plants. JAWWA, 87, 105–113. doi: 10.1002/j.1551-8833.1995.tb06346.x [DOI] [Google Scholar]
- Peng CY, Hill AS, Friedman MJ, Valentine RL, Larson GS, Romero AMY, Reiber SH, & Korshin GV (2012). Occurrence of trace inorganic contaminants in drinking water distribution systems. JAWWA, 104, E181–E193. doi: 10.5942/jawwa.2012.104.0042 [DOI] [Google Scholar]
- Reiber S, & Dostal G (2000). Well Water Disinfection Sparks Surprises. Opflow, 26:3:1 [Google Scholar]
- Salehi M, Li X, Whelton AJ (2017). Metal accumulation in representative plastic drinking water plumbing systems. JAWWA, 109, E479–E493. doi: 10.5942/jawwa.2017.109.0117 [DOI] [Google Scholar]
- Scanlan LP (2008). Metals surprise: precautions for treatment plant operators. AWWA Inorganic Contaminants Workshop, January 28 [Google Scholar]
- Schock M, Cantor A, Triantafyllidou S, & DeSantis M (2014). Importance of pipe deposits to lead and copper rule compliance. JAWWA, 106, E336–E349. doi: 10.5942/jawwa.2014.106.0064 [DOI] [Google Scholar]
- Schock MR, Hyland RN, & Welch MM (2008). Occurrence of contaminant accumulation in lead pipe scales from domestic drinking-water distribution systems. Environmental Science and Technology, 42, 4285–91. doi: 10.1021/es702488v [DOI] [PubMed] [Google Scholar]
- Schock MR (2005). Distribution systems as reservoirs and reactors for inorganic contaminants Chapter 6, MacPhee MJ (ed.), distribution system water quality challenges in the 21st century - a strategic guide. AWWA Research Foundation, Denver, CO, 105–140. [Google Scholar]
- Schock MR, & Holm TR (2003). Are we monitoring in the right places for inorganics and radionuclides? Journal of The New England Water Works Association, 117, 102 –116. [Google Scholar]
- Sorg T (2017). The USEPA arsenic demonstration program: status of the treatment systems demonstrated. AWWA International Symposium on Inorganics, Detroit, MI, March 21 – 22. [Google Scholar]
- Sorg T (2007). Arsenic: USEPA demonstration program and latest research results. Workshop on Inorganic Contaminant Issues, Cincinnati, OH, August 21–23. [Google Scholar]
- Sorg T, Wang L, & Chen A (2015). The costs of small drinking water systems removing arsenic from groundwater. Journal of Water Supply: Research and Technology - AQUA, 64, 219–234. doi: 10.2166/aqua.2014.044 [DOI] [Google Scholar]
- Sorg T, Chen A, & Wang L (2014). Arsenic species in drinking water wells in the USA with high arsenic concentrations. Water Research 48, 156–169. doi: 10.1016/j.watres.2013.09.016 [DOI] [PubMed] [Google Scholar]
- Sorg TJ, & Chen ASC (2012). Using a solid oxidizing media to enhance arsenic (AS[III]) removal at a very small system. AWWA Ohio Section Newsletter, 38–43. [Google Scholar]
- Sorg TJ, Chen ASC, Wang L, & Kolisz R (2017a). Regenerating an arsenic removal iron‐based adsorptive media system, part 1: the regeneration process. JAWWA, 109:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg TJ, Kolisz R, Chen ASC, & Wang L (2017b). Regenerating an arsenic removal iron‐based adsorptive media system, part 2: performance and cost. JAWWA 109, E122–E128. doi: 10.5942/jawwa.2017.109.0046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafyllidou S, Lytle D, Sorg T, & Muhlen C (2016). Release of accumulated arsenic from distribution pipes into tap water after arsenic treatment of source water AWWA Water Quality and Technology Conference, Indianapolis, November 13–17. [Google Scholar]
- USEPA (2016). Arsenic treatment technology demonstrations. Accessed on 10/20/2018 at https://www.epa.gov/water-research/arsenic-treatment-technology-demonstrations
- USEPA (2006). Inorganic contaminant accumulation in potable water distribution systems Total coliform rule issue paper. Office of Water, Office of Ground Water and Drinking Water, Washington, DC: Prepared by HDR Engineering, Inc. Accessed on 10/24/2018 at https://pdfs.semanticscholar.org/a867/2acddc0b092ecb325770934cb6734cb91ce3.pdf [Google Scholar]
- USEPA (2003). 40 CFR Part 141. National Primary Drinking Water Regulations [Google Scholar]
- USEPA (1996). Method 3050B. EPA 660 13–75–009 Acid digestion of sediments, sludges and soils, revision 2. Washington, D.C. [Google Scholar]
- USEPA (1994a). Method 200.8: determination of trace elements in waters and wastes by inductively coupled plasma - mass spectrometry, revision 5.4 Environmental Monitoring Systems Laboratory, Office of Research and Development: Cincinnati, OH. [Google Scholar]
- USEPA (1994b). Land application of sewage sludge: a guide for land appliers on the requirements of the federal standards for the use or disposal of sewage sludge, 40 CFR Part 503. Office of Enforcement Environmental and Compliance Assurance, DC 20460. EPA/831-B-93–002b, accessed 5/31/2018 at https://www3.epa.gov/npdes/pubs/sludge.pdf
- Wang L, & Chen ASC (2011). Costs of arsenic removal technologies for small systems. US Environmental Protection Agency technology demonstration program. EPA/600/R-11/090, https://nepis.epa.gov/Exe/ZyPDF.cgi/P100CAXP.PDF?Dockey=P100CAXP.PDF


