Abstract
The utilization of silver nanoparticles (AgNPs) in consumer products has significantly increased in recent years, primarily due to their antimicrobial properties. Increased use of AgNPs has raised ecological concerns. Once released into an aquatic environment, AgNPs may undergo oxidative dissolution leading to the generation of toxic Ag+. Therefore, it is critical to investigate the ecotoxicological potential of AgNPs and determine the physicochemical parameters that control their dissolution in aquatic environments. We have investigated the dissolution trends of aqueous colloidal AgNPs in five products, marketed as dietary supplements and surface sanitizers. The dissolution trends of AgNPs in studied products were compared to the dissolution trends of AgNPs in well-characterized laboratory-synthesized nanomaterials: citrate-coated AgNPs, polyvinylpyrrolidone-coated AgNPs, and branched polyethyleneimine-coated AgNPs. The characterization of the studied AgNPs included: particle size, anion content, metal content, silver speciation, and capping agent identification. There were small differences in the dissolved masses of Ag+ between products, but we did not observe any significant differences in the dissolution trends obtained for deionized water and tap water. The decrease of the dissolved mass of Ag+ in tap water could be due to the reaction between Ag+ and Cl−, forming AgCl and affecting their dissolution. We observed a rapid initial Ag+ release and particle size decrease for all AgNP suspensions due to the desorption of Ag+ from the nanoparticles surfaces. The observed differences in dissolution trends between AgNPs in products and laboratory-synthesized AgNPs could be caused by variances in capping agent, particle size, and total AgNP surface area in suspensions.
Keywords: Environmental fate of nanomaterials, pristine nanoparticles, laboratory-synthesized nanoparticles, aggregation, coating agent, surface area
Introduction
The utilization of engineered nanoparticles (ENPs) in consumer products has increased at a rapid pace over the last decade because of their unique properties and extensive variety of applications. Due to the plethora of products containing ENPs in the market, databases have been constructed to keep track of these products, such as Project on Emerging Nanomaterials (PEN), Nanotechnology Consumer Products Inventory (CPI), and Nanodatabase (Christian et al. 2008; Vance et al. 2015). Among these ENPs, silver nanoparticles (AgNPs) are the most widely used due to their antibacterial, antifungal, antiviral, and antimicrobial properties. These properties are directly related to the release of ionic silver from AgNPs (Foss Hansen et al. 2016).
Once released into an aquatic system, AgNPs undergo oxidative dissolution leading to silver cation (Ag+) generation (Froggett et al. 2014; Zhang et al. 2011). This oxidative dissolution mechanism can be triggered by oxidizing agents such as molecular oxygen and influenced by various environmental factors, including pH, capping agent, and chemical matrix. Dissolved Ag+ may have a severe effect on various organisms, including microbial communities, plants, invertebrates, and human cells (Benn et al. 2010). Toxicity of ionic silver, produced by dissolution of AgNPs, to aquatic environment results from the interaction of Ag+ ions with proteins and enzymes through specific cell-membrane functional groups like thiol (-SH) (Lowry et al. 2012). In addition to ionic silver release, the antibacterial properties of AgNPs have also been associated with direct interaction with the bacterial membrane, resulting in membrane damage from the production of reactive oxygen species (Siddhartha et al. 2007). Aggregation plays an important role in determining the toxicity of silver nanoparticles as dispersed silver nanoparticles provide enhanced bacterial effects over aggregated particles (Li and Lenhart 2012).
The dissolution of AgNPs from commercial products over the course of their intended utilization, while considering consumer pertinent conditions, is essential to further understand their environmental behavior and potential implications. Therefore, it is necessary to study the dissolution of AgNPs in consumer products (Benn and Westerhoff 2008; Echegoyen and Nerín 2013; Mackevica and Foss Hansen 2016; Pasricha et al. 2012). AgNPs are found in more than 430 consumer products (e.g. dietary supplements, textiles, food packaging, cleaning agents, surface sanitizers, medical instruments, etc.) (Artiaga et al. 2015; Mitrano et al. 2015; Rai et al. 2009). These consumer products have the potential to release AgNPs and Ag+ during different stages of their manufacturing process, product lifetime, and disposal (e.g. washing, ingestion, surface application, disposal on a solid waste landfill, etc.). Therefore, it is likely that these materials will be introduced into the environment (Mackevica and Foss Hansen 2016). The stability of released AgNPs and Ag+ will affect their mobility and reactivity when they reach waste water treatment plants (WWTPs) and the environment (Levard et al. 2012; Liu and Hurt 2010; Potter et al. 2019; Sheng et al. 2018).
Research regarding release and exposure of AgNPs have, thus far, primarily been focused on the understanding of the hazardous effects and mechanisms of toxicity related to laboratory-synthesized agents towards environmental microorganisms (Choi et al. 2008; Colombo et al. 2017; Grieger et al. 2012; Reidy et al. 2013). Although there are reports that address the silver release from various AgNP-containing consumer products, including paints (Kaegi et al. 2010), food containers (Huang et al. 2011; Mackevica et al. 2016), baby products (Quadros et al. 2013), textiles (Kulthong et al. 2010; Pasricha et al. 2012; Spielman-Sun et al. 2018), band-Aids for medical dressings (Rigo et al. 2012), medical supplies (catheters) (Joyce-Wöhrmann et al. 2000), and commercial toothbrushes (Mackevica et al. 2017), many products containing AgNPs have not been included in the Nanodatabase and have not been studied extensively (Vance et al. 2015). By the year 2016, only 76 publications reporting the release of ENPs from solid consumer products were available (Mackevica and Foss Hansen 2016) and only 34 of these studies showed AgNP release. However, the evaluations presented their results as total Ag release, rather than nanoparticle (NP) release, which may undermine the nanoparticle specific exposure and its associated effects. In addition, dissolution of AgNPs in aqueous colloidal consumer products has not been extensively investigated, since only products based on solid and textile matrices have been reported. So, the objective of this study was to investigate the dissolution trend of aqueous colloidal AgNPs in consumer products.
The characterization of pristine AgNPs has been extensively performed in various environmental matrices; but their characterization in consumer products and in colloidal suspensions has been particularly difficult for researchers. It is difficult to identify the actual NPs content in a number of consumer products from packing and labeling alone because of the lack of information regarding their synthesis process and their incorporation into complex and unknown matrices, as well as inconsistencies regarding their physicochemical characteristics reported by the vendors.
This study aimed to investigate the dissolution trend of aqueous colloidal AgNPs in consumer products marketed as dietary supplements and surface sanitizers in deionized water and tap water. The obtained dissolution trends were compared to those of well-known laboratory-synthesized nanomaterials, where capping agents cover the pristine surface of NPs and prevent them from dissolution, aggregation, and reaction. The AgNPs capping agents are considered to influence the dissolution trend when exposed to deionized water, as a pristine medium in which the Ag+ may be dissolved, and tap water as the most common media with which the consumer products will interact during their application (Levard et al. 2011; Tejamaya et al. 2012). The laboratory-synthesized nanomaterials used in this study were citrate-coated AgNPs (AgNPs-Cit), polyvinylpyrrolidone-coated AgNPs (AgNPs-PVP), and branched polyethyleneimine-coated AgNPs (AgNPs-BPEI). Citrate, PVP, and BPEI represent some of the most common capping/stabilizing agents (Tolaymat et al. 2010) as they enable effective nanoparticle dispersion (El Badawy et al. 2012). We used these laboratory-synthesized AgNPs because they are representative of all the possible surface charge scenarios (positive, negative, and neutral), as well as all NP stabilization mechanisms (El Badawy et al. 2010). The choice of a capping agent for NP synthesis is crucial, as it can strongly influence their physicochemical properties (e.g. size, shape, and the interaction with solvent surroundings) (Raveendran et al. 2003). In addition, understanding the Ag+ dissolution trends of the consumer products in comparison with the laboratory-synthesized nanomaterials may help us better understand the behavior of these products in environmental systems.
Material and methods
Collection of AgNP consumer products
An online search of commercially-available consumer products that contain AgNPs was performed. The sources included peer-reviewed literature and The Project on Emerging Nanotechnologies Database (Vance et al. 2015). 22 colloidal AgNPs consumer products have been characterized in a previous publication (Rogers et al. 2018). We selected 5 consumer products from this group of 22 consumer products because of their high concentration of silver and because they represent a variety of particle sizes (10-20 nm) and matrices. These 5 products showed three different usage scenarios. Firstly, CP4, CP6, and CP16 are intended to be used as dietary supplements which undergo ingestion after mixing with water. Secondly, CP8 is advertised by the manufacturer as a surface sanitizer which will interact with tap water during cleaning or immediately after disposal. Thirdly, CP10 is intended to be used as a disinfectant for skin which will inevitably lead to tap water exposure during washing (Table S1). Observable product variations were noticeable in the color, turbidity, and odor of the consumer products. Table S1 contains the reported description of the utilized consumer products: name, color, nature of matrix, purpose of use.
Characterization of AgNPs suspension
The hydrodynamic diameter (HDD) and zeta potential (ζ) of the AgNPs were measured using Dynamic Light Scattering (DLS) in a Malvern Zetasizer Nano ZS (Malvern, UK). Matrix components, like oils, may interfere with DLS measurements (Instruments 2007). Despite this limitation, we believe the increasing or decreasing diameter trend over time is still accurate (Begum et al. 2017). HDD populations are identified using the DLS plot of volume (percent) vs. particle diameter (nm). Total silver concentration was measured by inductively coupled plasma-optical emission spectrometry (ICP-OES) (Thermo Scientific, Waltham, MA, USA) after acid digestion, following EPA SW846 methods 3015A. Transmission electron microscopy-energy dispersive X-ray (TEM-EDX) analysis was used to verify nanoparticles’ size and shape using a JEOL-1200 EX TEM (JEOL Inc.) and the EDX ( Oxford instrument, UK) was used to confirm the presence of Ag particles in the suspensions acquiring images at 300 kV. The average silver diameter (D) and size distribution were calculated for each nanoparticle sample by averaging particle counts of 100 to 1000 particles from TEM images using ImageJ software (ImageJ, U.S. National Institutes of Health, Bethesda, MD, USA). A Dionex ICS-5000 (Dionex, Sunnyvale, CA) was used to quantify inorganic anions in the dissolution media. A Liquid Chromatograph – Tandem Mass Spectrometry (LC-MS/MS) Agilent 1290 LC-6540 Q-TOF equipped with a Zorbax Eclipse Plus C-18 (Agilent Technologies, Santa Clara, CA) was used to search for organic compounds in the consumer products that could be potential capping agents. An X-ray Photoelectron Spectroscope (XPS) PHI Quantera II (Physical Electronics, Inc., Chanhassen, MN) was used to investigate the metal speciation within the reacted consumer products using an Al kα source at pass energies of 280 (broad range) and 6.5 (high resolution).
Silver nanoparticle dissolution batch tests
One set of AgNP suspensions were diluted to 50 mL in deionized water and another set of the same AgNPs were diluted to 50 mL in tap water to obtain a final concentration of 6 mg/L with continuous stirring (500 rpm). Our calculations result in 6 mg-Ag/L and not 6 mg-particle/L. The 6 mg/L was chosen to simulate approximately 20 drops of 600 mg/L of the suspension in 3.4 ounces of water which are representative values for the manufacture-suggested use. Sampling times were selected for 0 h, 4 h, 24 h, 168 h, and 720 h. By choosing these sampling times, we get information on two scenarios: 1) short term exposure that simulates addition to water before consumption and 2) long term stability in any aqueous media. While Wan et. al. has shown that dilution alone induced aggregation and subsequent sedimentation of the NPs (Wan et al. 2018), we used DLS to confirm that dilution caused no initial aggregates in our consumer products. The experiment was prepared in triplicates for each of the AgNPs in consumer products and laboratory-synthesized AgNPs sample. A positive control was prepared with AgNO3 at a final Ag+ concentration of 6 mg/L to determine sample loss and recovery. We used ultrafiltration to determine the form of the released silver (ionic vs nanoparticulate). For the ultrafiltration process, 6 mL of sample was transferred to a 10 kDa centrifuge unit (Amicon Ultra-15, 10 K, Millipore, Burlington, MA) and centrifuged at 4000 rpm (2147 g) for 20 mins. After determining initial Ag+ concentration (0 h), we normalized our results to this initial Ag+ mass to emphasize dissolution that occurs during the product usage instead of post-manufacture dissolution prior to product usage. The total silver concentrations of the retentate and filtrate were measured with ICP-OES. The tap water (water hardness 1.0-1.2 mg/L CaCO3, pH 7-8.5) was equilibrated with room temperature (22 °C) prior to the introduction of the silver nanoparticles.
Calculation of total AgNP surface area in suspensions
The number of particles (N) was calculated using the HDD value measured by DLS (Table 1). Assuming a spherical shape and uniform structure, N can be calculated by Eq. (1), where ρ is the density of silver (10.5 g/cm3), D is the HDD value, and W is the total mass of Ag (0.036 mg) after sample dilution (Liu et al. 2007). W is calculated using a 6 mL aliquot of 6 mg/L Ag. Despite these assumptions, the N values calculated of each suspension can still be compared to each other.
| (Eq. 1) |
After calculation of N, the total surface area (nm2) was determined by multiplying N times the surface area per particle (4πr2) to get total AgNP surface area (Table 1).
Table 1.
Particle size diameter measured by DLS and TEM, number of nanoparticles, and silver concentration claimed, measured
| Sample name | PDI | Particle size, HDD | TEM Particle Diameter | Number of NPs* (1012) | Surface area per particle (nm2) | Total surface area (1012) (nm2) | Claimed Ag (mg/L) | Measured Ag (mg/L) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Size 1 (Prime) | Size 2 (Aggregates) | P-1 (nm) | P-2 (nm) | |||||||||
| HDD (nm) | Vol % | HDD (nm) | Vol % | |||||||||
| AgNP-PVP | 0.36 | 15.0 | 99.2 | 32.7 | 0.80 | 13.8 | - | 1.94 | 323 | 626.9 | - | - |
| AgNP-Cit | 0.42 | 12.6 | 100 | - | - | 11.2 | - | 3.27 | 549 | 1797 | - | - |
| AgNP-BPEI | 0.39 | 11.5 | 98.5 | 39.9 | 1.50 | 9.23 | - | 4.31 | 743 | 3199 | - | - |
| CP4 | 0.50 | 9.10 | 99.6 | 34.2 | 0.40 | 4.50 | 20-40 | 4.92 | 950 | 4674 | 500 | 466 |
| CP6 | 0.50 | 11.0 | 100 | - | - | 3.60 | 15-40 | 6.69 | 1098 | 7345 | 200 | 183 |
| CP8 | 0.37 | 14.4 | 97.8 | 621 | 2.20 | 5.20 | 15-30 | 2.17 | 435 | 944.1 | NR | 8.31 |
| CP10 | 0.76 | 13.8 | 93.0 | 5470 | 7.00 | 5.10 | 20 | 2.47 | 461 | 1139 | 240 | 146 |
| CP16 | 0.63 | 13.2 | 100 | - | - | 4.60 | 15-40 | 2.81 | 501 | 1410 | 20 | 22.9 |
PDI: Polydispersity, HDD: Hydrodynamic Diameter, P-1: Particle one (small range), P-2: Particle two (large size), and NR: Not Recorded.
Number of NPs calculated from Eq. 1.
Synthesis and purification of nanomaterials
The nanomaterials synthesis procedure and basic physical properties are described by El Badawy et al. (2010).
Statistical analysis
A paired-samples t-test was conducted to determine the variations in dissolution data due to the tap water where the statistical significance was calculated using the dissolution data of AgNPs in deionized water and in tap water and a p-value < 0.01 were considered as significant.
Results and discussion
Total Silver Determination
The five consumer products investigated were advertised by the manufacturer to contain AgNPs and their concentrations were listed on the labels for all products except for CP8 (Table 1). The total silver concentration ranged from 8.31 mg/L to 466 mg/L. Some of the measured concentrations were fairly close to the labeled Ag values while others were either above or below the claimed values. For example, CP10 contained ~60% of the claimed amount of silver while the other four products were within 10% of their claimed concentrations (Rogers et al. 2018). CP16 had a total silver value of 22.9 mg/L, neighboring the 21.3 mg/L reported by Reed et al. (Reed et al. 2014). While Wasukan et al. (Wasukan et al. 2015) found differences of 5 orders of magnitude between measured and reported total silver concentrations in personal care nanoproducts.
Particle size determination
The AgNP HDD in the examined consumer products ranged between 9.1-14.4 (± 0.23) nm. Spherical AgNPs were synthesized in the size range between 11.5-15 (± 0.97) nm. The laboratory-synthesized particles were less polydisperse than the consumer products with polydispersity (PDI) values < 0.5. The consumer products, except CP8, had PDI values of ≥ 0.5, indicating a high degree of particle size heterogeneity. This suspension polydispersity may be due to dissolution during aging of consumer products in their packaging (Reidy et al. 2013).
The TEM micrographs for the laboratory-synthesized nanoparticles showed particles with diameter between 9.23-13.8 nm (Table 1, Fig. 1). However; for consumer products, the TEM measurements generally showed two particle size ranges. The first range contained a greater number of small particles with diameter between 3.6-5.2 nm and the second range contained a smaller number of larger particles between 20-40 nm. The particles within the smaller diameter range seemed to be primarily spherical and showed less degree of aggregation than the bigger particles. Even though the particles appear to be of lower density, the existence of Ag was confirmed by EDX analysis (Fig. S1). The reasons for the differences found in particle sizes in these colloidal silver products might be related to their different composition, formulation, preparation, and production methods. The larger particle size values may be due to particle aggregation (Rogers et al. 2018). Also, these variations could be due to potential environmental factors like storage conditions, aging in their packaging, or exposure to light (Korshed et al. 2018).
Fig. 1.
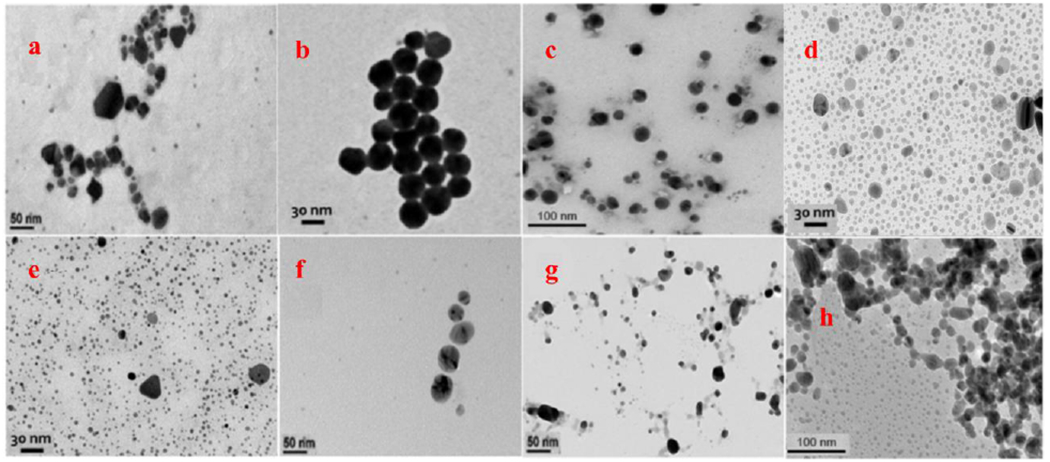
Representative TEM micrographs for AgNPs a) PVP-capped, b) Citrate-capped, c) BPEI-capped and products d) CP4, e) CP6, f) CP8, g) CP10 and h) CP16.
Typically, DLS diameter values are larger than TEM diameter values for the same suspension (Cascio et al. 2015; Cascio et al. 2014). The reason for this difference is that DLS is susceptible to change in capping agent and matrix (Cascio et al. 2014). Oil-containing matrices may interfere with the accuracy of DLS measurements but the change in HDD over time can still indicate a trend (Begum et al. 2017).
Dissolution of AgNPs in laboratory-synthesized and consumer products
Silver release into deionized water
The dissolution trends were considered for when AgNPs are exposed to tap water, the most common media with which the consumer products will interact during their application and usage scenarios, and deionized water. An ultrafiltration unit was used to separate the ionic and nano fractions of the released silver. Although we assumed that the silver passing the membrane was ionic, it is possible for extremely small AgNPs (< 1 nm) to pass through the membrane.
Batch dissolution experiments revealed that the dissolved Ag+ mass increased over time until reaching a plateau at 168 h in all AgNP samples, except AgNPs-Cit which continued to increase until 720 h (Fig. 2). CP8 exhibited no Ag+ dissolution, possibly due to compounds from its oily matrix interfering with or replacing its manufactured capping agent (Rahimi-Nasrabadi et al. 2014). CP4, CP6, and AgNPs-BPEI showed the highest overall dissolution of 0.006, 0.0073, and 0.0054 mg Ag, respectively, compared to the rest of the AgNPs (Fig. 2). For AgNPs-BPEI, CP6, CP10, and CP16 the total dissolved mass of silver reached its maximum level within the first 24 h and thereafter continued at a slower rate until reaching a plateau at 168 h. The total dissolved mass of AgNPs-PVP, AgNPs-Cit, and CP4 showed the highest release rate within the first 4 h and continued to release Ag+ thereafter until 168 h sampling point. At 168 h the AgNPs-PVP and CP4 reached a plateau, while AgNPs-Cit continued increasing (Fig. 2).
Fig. 2.
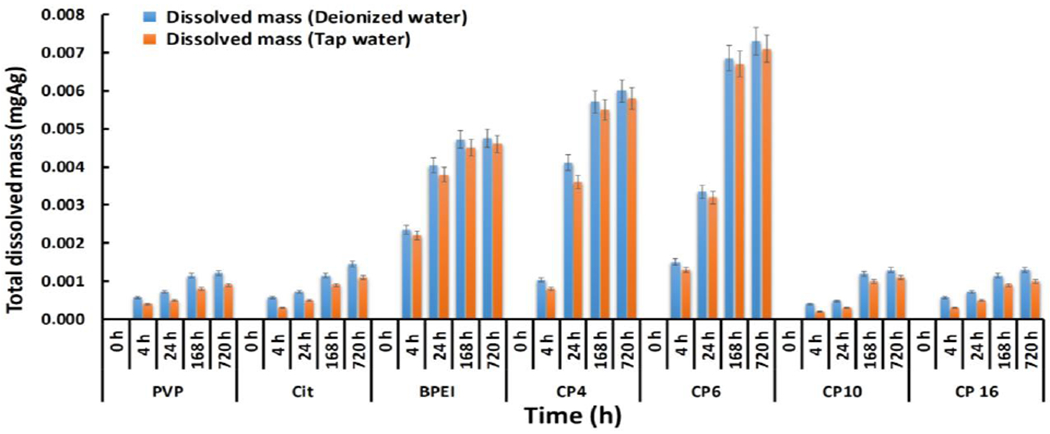
Total dissolved mass of silver (mgAg) found in lab-synthesized nanomaterials and in consumer products in the batch dissolution experiments with deionized water and tap water
It has been hypothesized that the dissolution trend of AgNPs is influenced by the stabilization mechanism and the characteristics of the capping agent (El Badawy et al. 2012). The stabilization mechanisms of coated AgNPs include electrostatic (AgNPs-Cit), steric (AgNPs-PVP) and electrosteric (AgNPs-BPEI). However; AgNPs-BPEI showed the highest amount of dissolution among these laboratory-synthesized AgNPs. In order to better understand the reason behind this phenomenon, and considering that nanoparticle suspensions were synthesized to contain similar sizes and shapes, we calculated the theoretical total AgNP surface area in suspensions (Table 1, Fig. 3) (Liu et al. 2007). Even though the Eq. 1 assumes spherical shape and uniform size, the use of the average HDD leads to comparable results between suspensions. According to our calculations, BPEI contained a higher total AgNP surface area compared to PVP and citrate (Fig. 3) due to AgNPs-BPEI having a slightly smaller diameter and approximately half the average particle volume. Between two solutions with the same concentration of Ag, the solution with more particles will have significantly higher total surface area and therefore higher dissolution is expected.
Fig. 3.
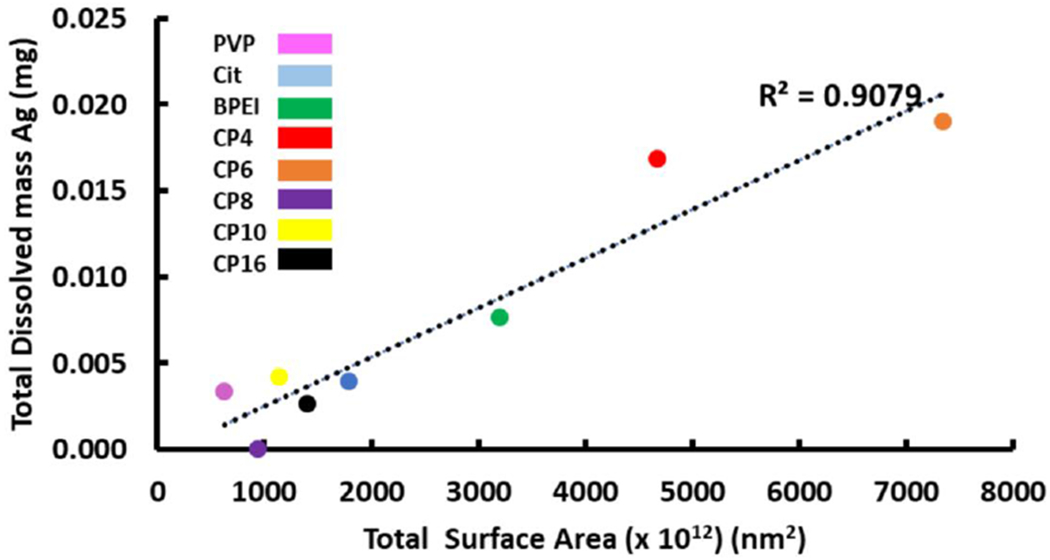
Total dissolved mass of silver (mgAg) after 720 hours vs. total surface area of AgNPs in lab-synthesized nanoparticles and in consumer products
Particle size fluctuations in deionized water
Particle size has a direct impact on silver nanoparticle dissolution rates (Li et al. 2010). The existence of aggregates will impact the dissolution trend due to a decrease in surface area. Hence, to study the correlation between particle size and aggregation with dissolution, a size measurement was performed on fresh AgNPs prior to exposure and again after 720 h.
All AgNPs in consumer products, except CP8, demonstrated a decrease in particle size over the duration of the experiment. Among them, CP4 and CP6 showed the highest decrease in HDD, from 9.1 nm and 11 nm at 0 h to 4.9 nm and 6.5 nm after 720 h, respectively (Table 2). On the contrary, dissolution of CP8 in deionized water resulted in no significant change in particle sizes within 720 h. CP8 contains oils in its matrix that could decrease the accuracy of DLS measurements. While this may affect the accuracy of the HDD value for CP8, we are confident that the HDD change of CP8 over 720 h (14.45 to 14.55 nm) indicates no diameter change (Table 2) and therefore no dissolution. The size measurements of these particles were consistent with the total dissolved silver. Similarly, the laboratory-synthesized AgNPs displayed a decrease in HDD over the duration of the experiment. The dissolution trend of AgNPs in AgNPS-BPEI, AgNPs-Cit, and AgNPs-PVP led to 35%, 25%, and 27% HDD decrease over 720 h, respectively. The observed decrease in HDD was also consistent with the dissolution trends measured.
Table 2.
Particle size over duration of experiment (DLS).
| Zero hour | 720 hours | |||||||
|---|---|---|---|---|---|---|---|---|
| Size 1 (Prime) | Size 2 (Aggregates) | Size 1 (Prime) | Size 2 (Aggregates) | |||||
| HDD (nm) | Vol % | HDD (nm) | Vol % | HDD (nm) | Vol % | HDD (nm) | Vol % | |
| PVP- DI | 15.00 | 97.2 | 32.69 | 2.8 | 8.50 | 93.5 | 38.23 | 6.5 |
| PVP-Tap | 15.03 | 97.1 | 36.65 | 2.8 | 9.52 | 93.5 | 46.62 | 6.5 |
| Cit- DI | 12.60 | 100 | - | - | 7.00 | 94.0 | 45.60 | 6.0 |
| Cit-Tap | 12.53 | 100 | - | - | 7.74 | 94.0 | 45.15 | 6.0 |
| BPEI- DI | 11.50 | 96.0 | 39.85 | 3.0 | 6.50 | 91.0 | 62.50 | 11.5 |
| BPEI-Tap | 11.51 | 96.4 | 50.65 | 3.5 | 7.63 | 88.0 | 65.91 | 12.0 |
| CP4- DI | 9.10 | 95.0 | 34.23 | 5.0 | 4.90 | 89.5 | 70.65 | 10.5 |
| CP4-Tap | 10.15 | 94.9 | 34.18 | 5.1 | 5.81 | 87.6 | 58.35 | 12.3 |
| CP6- DI | 11.00 | 100 | - | - | 6.50 | 88.0 | 689 | 12.0 |
| CP6-Tap | 11.00 | 100 | - | - | 7.59 | 87.0 | 673 | 13.0 |
| CP8- DI | 14.45 | 98.0 | 621 | 2.0 | 14.55 | 96.8 | 655 | 2.00 |
| CP8-Tap | 14.32 | 97.9 | 627 | 2.1 | 14.99 | 96.0 | 668 | 4.00 |
| CP10- DI | 13.84 | 85.0 | 5470 | 15.0 | 7.50 | 78.5 | 5214 | 21.5 |
| CP10-Tap | 13.59 | 84.7 | 5475 | 15.3 | 8.70 | 78.5 | 5214 | 21.4 |
| CP16- DI | 13.25 | 100 | - | - | 7.09 | 93.5 | 40.21 | 6.50 |
| CP16-Tap | 13.52 | 100 | - | - | 6.86 | 94.0 | 41.65 | 6.00 |
DI: Deionized water, HDD: Hydrodynamic Diameter.
Nanoparticle aggregation was simultaneously observed over the duration of the experiment. Size measurements (Table 2) showed an increase in the % of aggregation for all suspensions of AgNPs except for CP8 over the duration of the study. The % aggregation for CP4, CP6, CP10, and CP16 increased from 5%, 0%, 15%, and 0% at zero h to 10.5%, 12%, 21.5%, and 6.5%, respectively, after 720 h. Furthermore, laboratory-synthesized AgNPs showed a similar increase in the % of aggregation ranging from 2.8% to 11.5% after 720 h (Table 2). The decrease observed in particle size measurements agrees with the release of ionic silver in solution (Figs. 2 & 4).
Fig. 4.
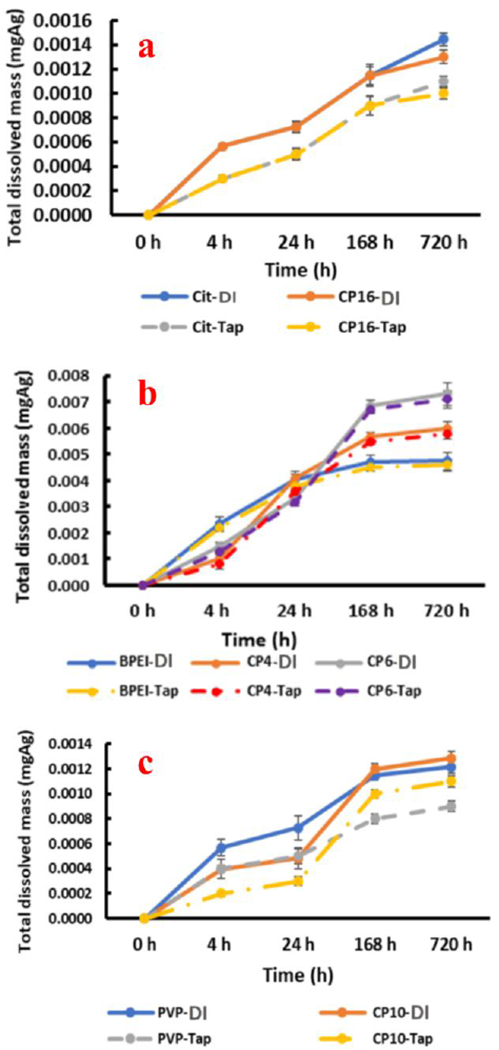
Total dissolved mass of silver (mgAg) in the dissolution experiments with deionized water and tap water for (a) Citrate-batch and CP16, (b) BPEI-batch, CP4 and CP6, (c) PVP-batch and CP10
Comparison of dissolution trends
The properties and behavior of AgNP in consumer products were compared with those of laboratory-synthesized AgNPs in two different ways: comparison of dissolution trends and conclusive identification of capping agents by LC-MS/MS. The dissolution trends of some AgNP consumer products were statistically similar to laboratory-synthesized AgNPs: AgNPs-Cit and CP16 (Fig. 4a), AgNPs-PVP and CP10 (Fig. 4c), and AgNPs-BPEI and CP4 (Fig. 4b). Linear regression was carried out to assess the relationship between dissolved mass of laboratory-synthesized AgNPs and consumer products containing AgNPs, where R2 values may assist in the identification of the capping agents or stabilization mechanisms in silver consumer products based on their similarity to the laboratory-synthesized AgNPs. The R2 values between AgNPs-Cit and CP16, AgNPs-PVP and CP10, and AgNPs-BPEI and CP4 were 0.9963, 0.9415, and 0.9251 in deionized water, and 0.9985, 0.9000, and 0.9114 in tap water, respectively (Fig. S2 a&b).
LC-MS/MS results
LC-MS/MS analysis confirmed the presence of citrate anion in CP16 (Figs. S3&4). While it is possible that citrate was added post-synthesis as a preservative or flavoring agent, CP16 does not list any additives on the label and therefore these LC-MS/MS results suggest that the AgNPs in CP16 are likely citrate-capped. No capping agent was identified in any of the other consumer products although this does not rule out any potential candidates. Due to the low concentration of capping agents in purified AgNP solutions, the capping agents in these consumer products are likely below the detection limit of this instrument. While it is possible that these AgNPs are uncapped, it is not probable that manufacturers would leave them uncapped and susceptible to reaction prior to consumer purchase and use.
Variations due to tap water
The dissolution trend of the AgNPs in tap water was similar to that of deionized water (Fig. 2). According student t-test, AgNPs-PVP, AgNPs-Cit, CP10, and CP16 demonstrated a statistically significant decrease in dissolved Ag mass in tap water (M = 0.0007; SD = 0.00005; N = 20) compared to deionized water (M = 0.0005; SD = 0.00004; N = 20). The null hypothesis was rejected, t(38) = 2.02; p < 0.01, at all sampling points . CP4, at 4 h and 24 h, displayed a statistically significant difference in dissolved Ag+ mass between deionized water (M = 0.0005; SD = 0.00073; N = 2) and tap water (M = 0.0004; SD = 0.00057; N = 2). The null hypothesis was rejected, t(3) = 3.18; p < 0.01, at 4 h and 24 h but not at 168 h and 720 h. This may be related to the various characteristics of the capping agents utilized which may affect the interactions with the background media leading to changes in dissolution trends (Benn et al. 2010).
The difference in dissolved Ag+ in tap water compared to deionized water might relate to the chloride ions (Table S3) detected in tap water at levels of ~22.5 mg/L. Chloride can form AgCl(s)0 shells around AgNPs, potentially reducing the available AgNP surface area and dramatically changing the surface characteristics which may directly inhibit dissolution and lead to particle aggregation and precipitation (Choi et al. 2008; Gitipour et al. 2017; Li et al. 2010). XPS analysis confirmed the presence of AgCl in CP6 after 720 h in tap water (Fig. S5).
Size measurements of AgNPs at 0 h and at 720 h for all AgNPs displayed similar trends in tap water and deionized water (Table 2). In tap water, they showed a significant decrease in HDD due to dissolution of AgNPs (up to 30%). Among the consumer products, CP6 and CP4 displayed the largest decrease (30%) in HDD in tap water over 720 h. On the contrary, CP8 displayed no significant change in particle size in tap water. The size stability of CP8 was expected and corresponds to low ionic silver release. The overall dissolution of AgNPS-BPEI, AgNPs-Cit, and AgNPs-PVP in tap water resulted in 25%, 16%, and 19% HDD decrease over 720 h, respectively. This decrease in HDD is consistent with the dissolution trends measured. The increase in the percentage of aggregation of the AgNPs in tap water displayed a similar trend to that observed in deionized water (Table 2).
Conclusions
To expand our understanding on the fundamental mechanisms of dissolution of AgNPs in consumer products, the dissolution behavior of AgNPs in various consumer products was compared to laboratory-synthesized AgNPs under various conditions. We designed an experimental system where the capping agent and the total AgNP surface area in suspensions were the primary contributors to the dissolution trends by controlling the initial particle size and dissolution media. Even though studied nanoparticles were chosen to be in the same size range, small differences in their diameter lead to large differences in particle volume, number of particles, and their surface area. When the mass of silver is constant, a higher initial number of AgNPs leads to a higher overall available surface area and to a higher dissolution rate.
The dissolution results obtained for CP16, the product that did not have any other additives, suggest that the particles in these products would behave identically to their laboratory-synthesized (AgNPs-Cit) counterparts. The particle sizes at zero-hour and at one-month for AgNPs-Cit and CP16 in the dissolution experiments were ~13 nm and ~7.5 nm for deionized water and tap water, respectively, and both types of AgNP suspensions exhibited the same aggregation volume (~6%) with aggregation particle size ~43 nm after 720 h. Moreover, the linear regression (R2) between the dissolved mass of AgNPs-Cit and CP16, in deionized water and in tap water, were 0.9963 and 0.9985, respectively, while LC-MS/MS analysis confirmed the presence of the citrate anion in CP16.
On the contrary, when there are other additives (i.e., CP8), AgNPs behave unpredictably. CP8 reportedly contains a complex matrix of many different components, such as plant extracts and essential oils. Many of these components are used as reducing/capping agents during the preparation of AgNPs in consumer products using green synthesis techniques. The oily matrix of CP8 possibly replaces the capping agent and covers the NPs, hindering their dissolution in water.
While we did not detect differences in overall dissolution trends of AgNPs between deionized and tap water, there were differences in Ag+ dissolved masses. The decrease in Ag+ release in tap water compared to deionized water could be due to reaction between the ionic Ag and the chloride anion to form AgCl.
All AgNP suspensions (consumer products and laboratory-synthesized) were shown to have the potential to persist as nanoparticles in deionized water and tap water for up to one month. This persistence of AgNPs could lead to longer lifetime or increased transport in the environment. Finally, the results from this study suggest that future studies on the dissolution of AgNPs should consider the differences between pristine laboratory-synthesized particles and consumer products because AgNP consumer products with complex matrices tend to behave unpredictably.
Supplementary Material
Acknowledgements
This research was supported in part by a PhD research grant from the Egyptian Ministry of Higher Education and Scientific Research (Grant No. 1582014) by providing stipend to Mr. Radwan and by appointments in the Research Participation Program at the Office of Research and Development (ORD), EPA administered by the Oak Ridge Institute for Science and Education (92431601). This manuscript was subjected to EPA internal reviews and quality assurance approval. The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency.
Funding information
This research was funded and conducted by the National Risk Management Research Laboratory of U.S. Environmental Protection Agency (EPA), Cincinnati, Ohio under the CSS program.
Footnotes
Additional figures and details for Materials and Method and Results and Discussion are presented.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Artiaga G, Ramos K, Ramos L, Camara C, Gomez-Gomez M (2015) Migration and characterisation of nanosilver from food containers by AF(4)-ICP-MS Food chemistry 166:76–85 doi: 10.1016/j.foodchem.2014.05.139 [DOI] [PubMed] [Google Scholar]
- Begum F, Jahan SA, Mollah M, Rahman M, Susan M (2017) Stability and Aggregation Kinetics of Silver Nanoparticles in Water in Oil Microemulsions of Cetyltrimethylammonium Bromide and Triton X-100 Journal of Scientific Research 9:431–447 [Google Scholar]
- Benn T, Cavanagh B, Hristovski K, Posner JD, Westerhoff P (2010) The release of nanosilver from consumer products used in the home Journal of environmental quality 39:1875–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benn TM, Westerhoff P (2008) Nanoparticle silver released into water from commercially available sock fabrics Environmental science & technology 42:4133–4139 [DOI] [PubMed] [Google Scholar]
- Cascio C, Geiss O, Franchini F, Ojea-Jimenez I, Rossi F, Gilliland D, Calzolai L (2015) Detection, quantification and derivation of number size distribution of silver nanoparticles in antimicrobial consumer products Journal of Analytical Atomic Spectrometry 30:1255–1265 [Google Scholar]
- Cascio C, Gilliland D, Rossi F, Calzolai L, Contado C (2014) Critical experimental evaluation of key methods to detect, size and quantify nanoparticulate silver Analytical chemistry 86:12143–12151 doi: 10.1021/ac503307r [DOI] [PubMed] [Google Scholar]
- Choi O, Deng KK, Kim N-J, Ross L Jr, Surampalli RY, Hu Z (2008) The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth Water research 42:3066–3074 [DOI] [PubMed] [Google Scholar]
- Christian P, Von der Kammer F, Baalousha M, Hofmann T (2008) Nanoparticles: structure, properties, preparation and behaviour in environmental media Ecotoxicology 17:326–343 [DOI] [PubMed] [Google Scholar]
- Colombo A, Saibene M, Moschini E, Bonfanti P, Collini M, Kasemets K, Mantecca P (2017) Teratogenic hazard of BPEI-coated silver nanoparticles to Xenopus laevis Nanotoxicology 11:405–418 doi: 10.1080/17435390.2017.1309703 [DOI] [PubMed] [Google Scholar]
- Echegoyen Y, Nerín C (2013) Nanoparticle release from nano-silver antimicrobial food containers Food and Chemical Toxicology 62:16–22 [DOI] [PubMed] [Google Scholar]
- El Badawy AM, Luxton TP, Silva RG, Scheckel KG, Suidan MT, Tolaymat TM (2010) Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions Environmental science & technology 44:1260–1266 doi: 10.1021/es902240k [DOI] [PubMed] [Google Scholar]
- El Badawy AM, Scheckel KG, Suidan M, Tolaymat T (2012) The impact of stabilization mechanism on the aggregation kinetics of silver nanoparticles Science of the total environment 429:325–331 [DOI] [PubMed] [Google Scholar]
- Foss Hansen S, Heggelund LR, Revilla Besora P, Mackevica A, Boldrin A, Baun A (2016) Nanoproducts - what is actually available to European consumers? Environmental Science: Nano 3:169–180 doi: 10.1039/C5EN00182J [DOI] [Google Scholar]
- Froggett SJ, Clancy SF, Boverhof DR, Canady RA (2014) A review and perspective of existing research on the release of nanomaterials from solid nanocomposites Particle and fibre toxicology 11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitipour A, Al-Abed SR, Thiel SW, Scheckel KG, Tolaymat T (2017) Nanosilver as a disinfectant in dental unit waterlines: assessment of the physicochemical transformations of the AgNPs Chemosphere 173:245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieger KD, Linkov I, Hansen SF, Baun A (2012) Environmental risk analysis for nanomaterials: review and evaluation of frameworks Nanotoxicology 6:196–212 doi: 10.3109/17435390.2011.569095 [DOI] [PubMed] [Google Scholar]
- Huang Y, Chen S, Bing X, Gao C, Wang T, Yuan B (2011) Nanosilver Migrated into Food-Simulating Solutions from Commercially Available Food Fresh Containers Packaging Technology and Science 24:291–297 doi: 10.1002/pts.938 [DOI] [Google Scholar]
- Instruments M (2007) Zetasizer nano user manual Malvern Instruments Ltd, Worcestershire WR14 1XZ, UK [Google Scholar]
- Joyce-Wöhrmann RM, Hentschel T, Münstedt H (2000) Thermoplastic Silver-Filled Polyurethanes for Antimicrobial Catheters Advanced Engineering Materials 2:380–386 [Google Scholar]
- Kaegi R et al. (2010) Release of silver nanoparticles from outdoor facades Environmental pollution 158:2900–2905 doi: 10.1016/j.envpol.2010.06.009 [DOI] [PubMed] [Google Scholar]
- Korshed P, Li L, Wang T (2018) Effect of Storage Conditions on the Long-Term Stability of Bactericidal Effects for Laser Generated Silver Nanoparticles Nanomaterials 8:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulthong K, Srisung S, Boonpavanitchakul K, Kangwansupamonkon W, Maniratanachote R (2010) Determination of silver nanoparticle release from antibacterial fabrics into artificial sweat Particle and fibre toxicology 7:8 doi: 10.1186/1743-8977-7-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levard C, Hotze EM, Lowry GV, Brown GE Jr (2012) Environmental transformations of silver nanoparticles: impact on stability and toxicity Environmental science & technology 46:6900–6914 [DOI] [PubMed] [Google Scholar]
- Levard C, Reinsch BC, Michel FM, Oumahi C, Lowry GV, Brown GE Jr (2011) Sulfidation processes of PVP-coated silver nanoparticles in aqueous solution: impact on dissolution rate Environmental science & technology 45:5260–5266 [DOI] [PubMed] [Google Scholar]
- Li X, Lenhart JJ (2012) Aggregation and dissolution of silver nanoparticles in natural surface water Environmental science & technology 46:5378–5386 [DOI] [PubMed] [Google Scholar]
- Li X, Lenhart JJ, Walker HW (2010) Dissolution-accompanied aggregation kinetics of silver nanoparticles Langmuir 26:16690–16698 [DOI] [PubMed] [Google Scholar]
- Liu J, Hurt RH (2010) Ion release kinetics and particle persistence in aqueous nano-silver colloids Environmental science & technology 44:2169–2175 [DOI] [PubMed] [Google Scholar]
- Liu X, Atwater M, Wang J, Huo Q (2007) Extinction coefficient of gold nanoparticles with different sizes and different capping ligands Colloids and Surfaces B: Biointerfaces 58:3–7 [DOI] [PubMed] [Google Scholar]
- Lowry GV, Gregory KB, Apte SC, Lead JR (2012) Transformations of nanomaterials in the environment. ACS Publications, [DOI] [PubMed] [Google Scholar]
- Mackevica A, Foss Hansen S (2016) Release of nanomaterials from solid nanocomposites and consumer exposure assessment–a forward-looking review Nanotoxicology 10:641–653 [DOI] [PubMed] [Google Scholar]
- Mackevica A, Olsson ME, Hansen SF (2016) Silver nanoparticle release from commercially available plastic food containers into food simulants Journal of Nanoparticle Research 18:5 doi: 10.1007/s11051-015-3313-x [DOI] [Google Scholar]
- Mackevica A, Olsson ME, Hansen SF (2017) The release of silver nanoparticles from commercial toothbrushes Journal of hazardous materials 322:270–275 doi: 10.1016/j.jhazmat.2016.03.067 [DOI] [PubMed] [Google Scholar]
- Mitrano DM, Motellier S, Clavaguera S, Nowack B (2015) Review of nanomaterial aging and transformations through the life cycle of nano-enhanced products Environment international 77:132–147 [DOI] [PubMed] [Google Scholar]
- Pasricha A, Jangra SL, Singh N, Dilbaghi N, Sood K, Arora K, Pasricha R (2012) Comparative study of leaching of silver nanoparticles from fabric and effective effluent treatment Journal of environmental sciences 24:852–859 [DOI] [PubMed] [Google Scholar]
- Potter PM, Navratilova J, Rogers KR, Al-Abed SR (2019) Transformation of silver nanoparticle consumer products during simulated usage and disposal Environmental Science: Nano [PMC free article] [PubMed] [Google Scholar]
- Quadros ME, Pierson Rt, Tulve NS, Willis R, Rogers K, Thomas TA, Marr LC (2013) Release of silver from nanotechnology-based consumer products for children Environmental science & technology 47:8894–8901 doi: 10.1021/es4015844 [DOI] [PubMed] [Google Scholar]
- Rahimi-Nasrabadi M, Pourmortazavi SM, Shandiz SAS, Ahmadi F, Batooli H (2014) Green synthesis of silver nanoparticles using Eucalyptus leucoxylon leaves extract and evaluating the antioxidant activities of extract Natural product research 28:1964–1969 [DOI] [PubMed] [Google Scholar]
- Rai MK, Asthana P, Singh SK, Jaiswal VS, Jaiswal U (2009) The encapsulation technology in fruit plants--a review Biotechnology advances 27:671–679 doi: 10.1016/j.biotechadv.2009.04.025 [DOI] [PubMed] [Google Scholar]
- Raveendran P, Fu J, Wallen SL (2003) Completely “green” synthesis and stabilization of metal nanoparticles Journal of the American Chemical Society 125:13940–13941 doi: 10.1021/ja029267j [DOI] [PubMed] [Google Scholar]
- Reed RB, Faust JJ, Yang Y, Doudrick K, Capco DG, Hristovski K, Westerhoff P (2014) Characterization of nanomaterials in metal colloid-containing dietary supplement drinks and assessment of their potential interactions after ingestion Acs Sustainable Chemistry & Engineering 2:1616–1624 [Google Scholar]
- Reidy B, Haase A, Luch A, Dawson KA, Lynch I (2013) Mechanisms of silver nanoparticle release, transformation and toxicity: a critical review of current knowledge and recommendations for future studies and applications Materials 6:2295–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigo C, Roman M, Munivrana I, Vindigni V, Azzena B, Barbante C, Cairns WR (2012) Characterization and evaluation of silver release from four different dressings used in burns care Burns : journal of the International Society for Burn Injuries 38:1131–1142 doi: 10.1016/j.burns.2012.06.013 [DOI] [PubMed] [Google Scholar]
- Rogers KR et al. (2018) Characterization of engineered nanoparticles in commercially available spray disinfectant products advertised to contain colloidal silver Science of The Total Environment 619–620:1375–1384 doi: 10.1016/j.scitotenv.2017.11.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Z, Van Nostrand JD, Zhou J, Liu Y (2018) Contradictory effects of silver nanoparticles on activated sludge wastewater treatment Journal of hazardous materials 341:448–456 [DOI] [PubMed] [Google Scholar]
- Siddhartha S, Tanmay B, Arnab R, Gajendra S, Ramachandrarao P, Debabrata D (2007) Characterization of enhanced antibacterial effects of novel silver nanoparticles Nanotechnology 18:225103. [DOI] [PubMed] [Google Scholar]
- Spielman-Sun E, Zaikova T, Dankovich T, Yun J, Ryan M, Hutchison JE, Lowry GV (2018) Effect of silver concentration and chemical transformations on release and antibacterial efficacy in silver-containing textiles NanoImpact 11:51–57 [Google Scholar]
- Tejamaya M, Römer I, Merrifield RC, Lead JR (2012) Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media Environmental science & technology 46:7011–7017 [DOI] [PubMed] [Google Scholar]
- Tolaymat TM, El Badawy AM, Genaidy A, Scheckel KG, Luxton TP, Suidan M (2010) An evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applications: a systematic review and critical appraisal of peer-reviewed scientific papers Science of the total environment 408:999–1006 [DOI] [PubMed] [Google Scholar]
- Vance ME, Kuiken T, Vejerano EP, McGinnis SP, Hochella MF Jr., Rejeski D, Hull MS (2015) Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory Beilstein journal of nanotechnology 6:1769–1780 doi: 10.3762/bjnano.6.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Kim Y, Mulvihill MJ, Tokunaga TK (2018) Dilution destabilizes engineered ligand-coated nanoparticles in aqueous suspensions Environmental toxicology and chemistry 37:1301–1308 [DOI] [PubMed] [Google Scholar]
- Wasukan N, Srisung S, Kulthong K, Boonrungsiman S, Maniratanachote R (2015) Determination of silver in personal care nanoproducts and effects on dermal exposure Journal of Nanoparticle Research 17:425 [Google Scholar]
- Zhang W, Yao Y, Sullivan N, Chen Y (2011) Modeling the primary size effects of citrate-coated silver nanoparticles on their ion release kinetics Environmental science & technology 45:4422–4428 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


