Abstract
The aim of this study was to evaluate the diagnostic performance of immunochromatographic tests (ICTs) for the detection of Mycoplasma pneumoniae. Medline/Pubmed, Embase, the Cochrane Library, and ISI Web of Science were searched through June 12, 2019 for relevant studies that used ICTs for the detection of M. pneumoniae infection with polymerase chain reaction (PCR) or microbial culturing as reference standards. Pooled diagnostic accuracy with 95% confidence interval (CI) was calculated using a bivariate random effects model. We also constructed summary receiver operating characteristic curves and calculated the area under the curve (AUC). Statistical heterogeneity was evaluated by χ2 test or Cochrane’s Q test. Thirteen studies including 2,235 samples were included in the meta-analysis. The pooled sensitivity and specificity for diagnosing M. pneumoniae infection were 0.70 (95% CI: 0.59–0.79) and 0.92 (95% CI: 0.87–0.95), respectively. The positive likelihood ratio (LR) was 8.94 (95% CI: 4.90–14.80), negative LR 0.33 (95% CI: 0.22–0.46), diagnostic odds ratio 29.20 (95% CI: 10.70–64.20), and AUC 0.904. In subgroup analysis, ICTs demonstrated similar pooled sensitivities and specificities in populations of children only and mixed populations (children + adults). Specimens obtained from oropharyngeal swabs exhibited a higher sensitivity and specificity than those of nasopharyngeal swab. Moreover, pooled estimates of sensitivity and accuracy for studies using PCR as a reference standard were higher than those using culture. The pooled sensitivity and specificity of Ribotest Mycoplasma®, the commercial kit most commonly used in the included studies, were 0.66 and 0.89, respectively. Overall, ICT is a rapid user-friendly method for diagnosing M. pneumoniae infection with moderate sensitivity, high specificity, and high accuracy. This suggests that ICT may be useful in the diagnostic workup of M. pneumoniae infection; however, additional studies are needed for evaluating the potential impact of ICT in clinical practice.
Introduction
Mycoplasma pneumoniae is an important cause of respiratory tract infection (RTI) in school-age children and young adults [1–4]. M. pneumoniae is responsible for approximately 10–40% of community-acquired pneumonia (CAP) cases [3, 5], rising to 70% in closed populations during epidemics [6–8]. M. pneumoniae infection is primarily known to present with a mild clinical course [6]; however, 3–4% of those are reported to develop into fulminant pneumonia with hypoxia [9, 10]. Extrapulmonary complications, primarily being central nervous system complications, may also occur in approximately 25% of M. pneumoniae-infected individuals [1, 11].
M. pneumoniae lacks a cell wall, and therefore, β-lactam antibiotics, which are active against most respiratory bacterial pathogens for RTI in children, are ineffective against M. pneumoniae [12]. A prompt and precise diagnosis of M. pneumoniae infection leads to the use of appropriate antibiotics. However, it is difficult to distinguish M. pneumoniae from other causative microorganisms of RTI early during the clinical course based on patient history, symptoms, physical examination, or a chest radiograph. Therefore, laboratory confirmation of the microorganism is crucial for planning the appropriate management [13–16].
While microbial culturing has been a gold standard for M. pneumoniae diagnosis, M. pneumoniae are fastidious and cultivation may require weeks for growth. Therefore, culturing is not routinely performed in clinical practice [17]. Serology is a more convenient and widely used method than culturing. A single high titer of M. pneumoniae-specific antibody is indicative of a recent infection; however, false-negative test results often occur early in the course of illness [17, 18]. An increase in the M. pneumoniae-specific IgG titer ≥ 4-fold during acute and convalescent phases of the clinical course also implies recent infection [19]; however, this is impractical in clinical practice as it requires 2–4 weeks of monitoring [17, 18]. ImmunoCard Mycoplasma (Meridian Bioscience, Cincinnati, OH, USA), a 10-min card-based enzyme-linked immunosorbent assay to detect M. pneumoniae IgM antibodies, has been developed and is commercially available [20, 21]. However, ImmunoCard Mycoplasma is an assay for IgM only and can exhibit false-positive results for an extended period of time after M. pneumoniae infection as M. pneumoniae IgM antibodies may persist for several months [21, 22]. Polymerase chain reaction (PCR) analysis is highly sensitive and currently used as a reference diagnostic method for M. pneumoniae detection; however, it requires complex and time-consuming sample pretreatment, skilled technical ability, and expensive equipment [23, 24].
Recently, several techniques for the rapid diagnosis of M. pneumoniae have been developed, including loop-mediated isothermal amplification (LAMP) [25, 26] and the immunochromatographic test (ICT) [27–30]. LAMP is a technique in which DNA is amplified under isothermal conditions within one hour [31, 32]. Although LAMP shows high sensitivity and specificity in the diagnosis of infectious diseases [33], it requires specific equipment for DNA amplification. In addition, an isolated room and a closed reaction system are recommended owing to unintended carryover contamination that may lead to false positives [34, 35]. Therefore, LAMP is not thought to be practical for use in primary care settings [36, 37].
The immunochromatographic test (ICT), often referred to as a lateral-flow assay, is a popular application of enzyme-immunoassays that utilize antigen and antibody properties as a sample passes along a membrane [38–41]. ICT has several positive qualities, including that it is simple and easy to perform, has a rapid assay time, exhibits long-term stability regardless of climate, is inexpensive, and is an instrument-free diagnostic test [42]. Moreover, results can be observed with the naked eye within 10–15 min [28, 29, 43, 44]. Recently, the diagnosis of CAP has been facilitated by the use of ICT-based urinary antigen tests for Streptococcus pneumoniae and Legionella pneumophila serogroup-1 [45, 46]. ICT targeting of M. pneumoniae antigen (e.g. ribosomal protein L7/L12) has also been developed and is commercially available [30, 36, 47–50]. However, the studies that have evaluated the performance characteristics of ICT for the detection of M. pneumoniae have not currently been systematically reviewed or integrated. Therefore, the aim of this systematic review and meta-analysis was to integrate and assess the evidence for the diagnostic accuracy of ICT for M. pneumoniae infection.
Materials and methods
This review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement (PRISMA) [51]. The protocol has been registered with Prospero: International prospective register of systematic reviews (registration number CRD42019140809).
Literature search
We searched on Medline/Pubmed, Embase, the Cochrane Library, and ISI Web of Science using the keywords Mycoplasma pneumoniae, immunochromatography and lateral flow assay. The search strategy included “Mycoplasma pneumoniae AND immunochromatography OR lateral flow assay” (S1 Search strategy). The search was executed on June 12, 2019. Additional studies were identified by examining the reference lists of the relevant articles. No language restrictions were applied. As the current study was based on a systematic review of previously published studies, institutional review board approval and patient consent were not necessary. This research received no specific grant from any funding.
Eligibility criteria
Studies were considered eligible if they assessed the accuracy of ICT for the diagnosis of M. pneumoniae infection and were detailed enough to allow the construction of a 2 × 2 table. We defined ICT as any assay identifying M. pneumoniae antigens in human respiratory specimens using ICT formats. Studies using PCR or microbial cultures as reference standards were eligible for inclusion in the current study. In vitro and in vivo animal studies were excluded. Editorials, letters to the editors, and conference abstracts were also excluded.
Study selection and data extraction
Two reviewers (SHY and JGY) independently screened the titles and abstracts for potential relevance and conducted full-text reviews of the selected publications. Any disagreements were resolved by a third reviewer (IKM) following a discussion with all three reviewers. Author names, country of origin for the study, publication year, study design, study period, age distribution of the study population (children were defined as ≤ 18 years of age), participant gender, index test assay, index test target, index test company, reference standard, type of specimen, sample size, and data regarding true positive, false positive, true negative, and false negative were extracted. If studies consisted of multiple groups, each group was treated as a single study. If there was insufficient information to construct the 2 × 2 table, we attempted to contact the corresponding authors by e-mail.
Quality assessment
The validity of the included studies was assessed using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool [52]. QUADAS-2 evaluates the risk of bias and the applicability of diagnostic accuracy studies, which consists of four key domains (patient selection, index test, reference standard, and flow and timing). Each domain was assessed in terms of risk of bias and the first three domains with respect to concerns regarding applicability.
Statistical analysis
Summary estimates of sensitivity and specificity along with 95% confidence intervals were calculated based on a bivariate random effects model [53]. From the pooled estimates, we derived the diagnostic odds ratio (DOR), positive LR, negative LR, and 95% CI [54].
Summary receiver operating characteristic (SROC) curves and the area under the curve (AUC) obtained from the fitted bivariate random effects model were used to summarize the overall test performance. SROC curves were plotted with the confidence region and prediction region. Heterogeneity of sensitivity and specificity was assessed by visual inspection of forest plots and by χ2 test analysis (p < 0.05 indicated significant heterogeneity). If heterogeneity between studies existed, a bivariate random effects model was adopted [55–57]. A fixed value (0.5) was added as a continuity correction to all cells for studies with zero values.
We planned subgroup analyses prior to starting the evaluations as heterogeneity among the studies was expected. The following variables were expected to be possible sources of heterogeneity: age of the population (children, adult, mixed), type of index test and type of specimen, reference standard used, and blinding procedures. Potential publication bias was visually assessed using a funnel plot [58, 59]. The statistical significance of publication bias was tested using the Egger’s test [60]. R package, version 3.6.0 (The R Foundation for Statistical Computing, Vienna, Austria), was used for all statistical analyses.
Results
Characteristics of the included studies
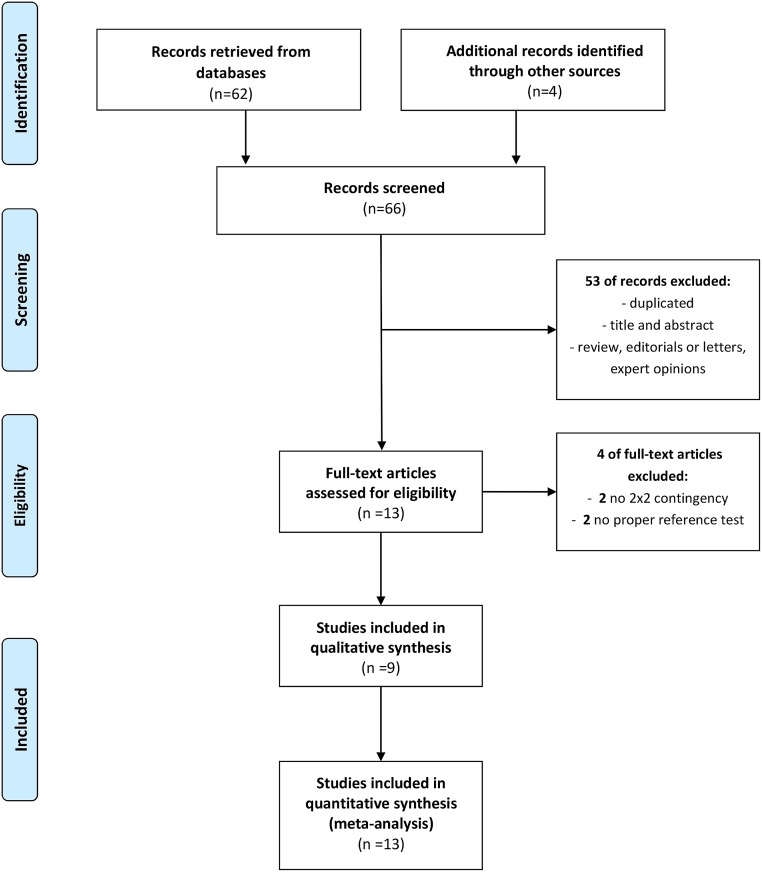
Totally, 66 articles were retrieved. After the removal of duplicate articles and exclusion of studies based on titles and abstracts, 13 articles remained for full text review. Four of the 13 studies were excluded as two studies did not provide sufficient data for generation of a 2 × 2 contingency table and the other two studies did not use PCR or microbial cultures as reference standards. Of the nine studies not excluded, several evaluated more than one reference method [28] and different patient groups [48], brand of index test [36], and type of specimen [49]. Each dataset from these studies was considered separately. Therefore, 13 studies (11 in English, 1 in Korean, 1 Japanese) [28–30, 36, 47–50, 61] were ultimately included for quantitative data synthesis and meta-analysis (Fig 1).
Fig 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart of the selection process used for eligible studies.
The data sets were extracted from the 13 articles and consisted of 2,235 samples. Descriptive characteristics of the studies are summarized in Table 1. All the studies were conducted in Asia (2 in China [29, 61], 10 in Japan [28, 30, 36, 47–49], and 1 in Korea [50]). Seven studies (53.8%) included both adults and children [28, 36, 47, 48] while the remaining six studies (46.2%) included only children [29, 30, 49, 50, 61]. No studies specifically evaluated adult populations (≥ 18 years of age). Among the ICTs used in the studies included in our meta-analysis, Ribotest Mycoplasma® (Asahi Kasei Pharma Co., Tokyo, Japan) was the most frequently assessed with eight (57.6%) of the studies evaluating this ICT [30, 36, 47–50]. PCR was used as the reference standard in 11 (84.6%) of the studies [28–30, 36, 47–49, 61] and microbial culturing was used in two (15.4%) of the studies [28, 50]. No studies described the duration of symptoms prior to testing. The specific age ranges of enrolled patients and gender proportions are summarized in S1 Table.
Table 1. Characteristics of the studies included in the meta-analysis.
| Year, Author | Country | Study periods | Age | Specimen | Patients | Index test assay | Index test target | Company | Reference standard | MP confirmed/non-MP confirmed (n) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2015, Li | China | Feb 2014 to Aug 2014 | Children* | OP swab + sputum | pneumonia + suspected MP infection | Colloidal gold-based IC assay | MP membrane protein P1 | In-house ICT | PCR | 78/224 |
| 2015, Miyashita | Japan | Nov 2013 to Oct 2014 | children + adult | NP swab | CAP | Ribotest Mycoplasma® | MP L7/L12 ribosomal protein | Asahi Kasei Pharma, Tokyo, Japan | PCR | 8/110 |
| 2015, Yamazaki | Japan | Sep 2012 to Mar 2013 | children | NP swab | pneumonia or bronchitis | Ribotest Mycoplasma® | MP L7/L12 ribosomal protein | Asahi Kasei Pharma, Tokyo, Japan | PCR | 85/127 |
| 2016, Miyashita-1 | Japan | May 2015 to Aug 2015 | children + adult | NP swab | RTI | Ribotest Mycoplasma® | MP L7/L12 ribosomal protein | Asahi Kasei Pharma, Tokyo, Japan | PCR | 46/355 |
| 2016, Miyashita-2 | Japan | May 2015 to Aug 2015 | children + adult | NP swab | CAP | Ribotest Mycoplasma® | MP L7/L12 ribosomal protein | Asahi Kasei Pharma, Tokyo, Japan | PCR | 8/60 |
| 2016, Sano-1 | Japan | − | children + adult | pharyngeal swab§ | RTI | Mycoplasma RP-L7/L12 ICT | MP L7/L12 ribosomal protein | In-house ICT | PCR | 33/143 |
| 2016, Sano-2 | Japan | − | children + adult | pharyngeal swab§ | RTI | Mycoplasma RP-L7/L12 ICT | MP L7/L12 ribosomal protein | In-house ICT | culture | 35/141 |
| 2017, Kakuya-1 | Japan | Dec 2015 to Aug 2016 | children | NP swab | community-acquired lower RTI | Ribotest Mycoplasma® | MP L7/L12 ribosomal protein | Asahi Kasei Pharma, Tokyo, Japan | PCR | 15/43 |
| 2017, Kakuya-2 | Japan | Dec 2015 to Aug 2016 | children | OP swab | community-acquired lower RTI | Ribotest Mycoplasma® | MP L7/L12 ribosomal protein | Asahi Kasei Pharma, Tokyo, Japan | PCR | 15/43 |
| 2017, Song | China | Dec 2016 to Jan 2017 | children | OP swab | pneumonia | SWCNT/CGIC strip | MP membrane protein P1 | In-house ICT | PCR | 97/40 |
| 2018, Namkoong-1 | Japan | Dec 2015 to Dec 2016 | children + adult | OP swab | clinically suspected MP infection | SAI system¶ | MP antigen | Mizuho Medy, Saga, Japan or Fujifilm, Kanagawa, Japan | PCR | 73/84 |
| 2018, Namkoong-2 | Japan | Dec 2015 to Dec 2016 | children + adult | OP swab | clinically suspected MP infection | Ribotest Mycoplasma® | MP L7/L12 ribosomal protein | Asahi Kasei Pharma, Tokyo, Japan | PCR | 73/84 |
| 2019, Yang | Korea | Aug 2010 to Aug 2018 | children | NP aspirates | lower RTI | Ribotest Mycoplasma® | MP L7/L12 ribosomal protein | Asahi Kasei Pharma, Tokyo, Japan | culture | 119/96 |
CAP, community acquired pneumonia; MP, Mycoplasma pneumoniae; PCR, polymerase chain reaction; RTI, respiratory tract infection; SWCNT/CGIC, single-walled carbon nanotubes coupled with the colloidal gold-monoclonal antibody immunochromatographic strips; ICT, immunochromatographic test; NP, naso-pharyngeal; OP, oropharyngeal; SAI, silver amplification immunochromatography
−: Not given.
* Children and adults were defined as younger and older than 18 years of age, respectively.
§ Authors did not provide details regarding the source of the swabs (nasopharyngeal or oropharyngeal).
¶ The SAI system consists of a Quick Chaser® Auto Myco (Mizuho Medy, Saga, Japan) or FUJI DRI-CHEM IMMUNO AG
Cartridge Myco (Fujifilm, Kanagawa, Japan) combined with an analyzer Quick Chaser Immuno Reader (Mizuho Medy,
Saga, Japan) or FUJI DRI-CHEM IMMUNO AG1 (Fujifilm, Kanagawa, Japan).
Quality assessment
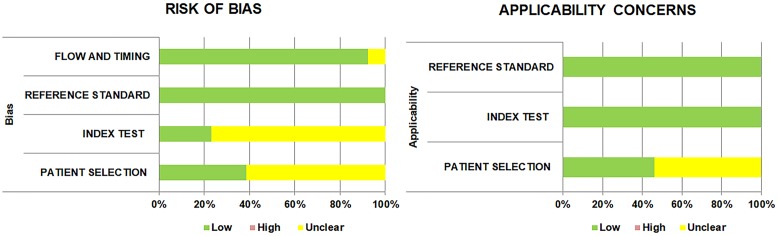
Results of QUADAS-2 assessment for evaluating the quality of the studies are shown in Fig 2 and S2 Table. With respect to the risk of bias, in the patient selection domain, 61.5% of the studies were considered “unclear” risk of bias as they failed to specify the methods used for enrollment of the patients, whether consecutive or random. The remaining studies were classified as “low” risk of bias. In the index test domain, most of the studies (76.9%) were classified as “unclear” risk of bias since the authors did not report the index test, did not clarify whether the ICT results were identified without knowledge of the results of the reference standard [62, 63]. However, if index test results were interpreted with dedicated ICT readers, the studies were judged to be at low risk of bias. In the reference standard domain, all studies were at “low” risk of bias because PCR and culturing were regarded as being objective methods, regardless of whether they were interpreted without knowledge of the index test results. In the flow and timing domain, most studies (12/13, 92.3%) were at “low” risk of bias. Applicability was of low concern for all the studies in the index and reference standard domain. The patient selection domain was assessed to be an “unclear” concern for seven of the studies (53.8%) as they enrolled only lower RTI patients.
Fig 2. Quality assessment of the diagnostic accuracy studies-2 (QUADAS-2).
Overall accuracy of IC
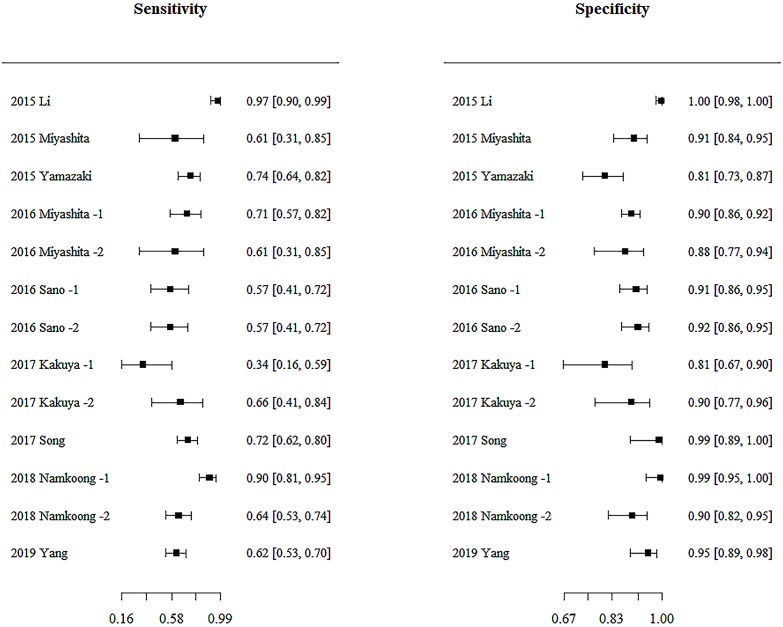
The sensitivity and specificity of each study included in the analysis are shown in the form of a forest plot in Fig 3. Significant heterogeneity between studies was noted in terms of sensitivity (χ2 = 63.75; p < 0.0001) and specificity (χ2 = 60.62; p < 0.0001). Taking into account the statistical heterogeneity, a meta-analysis was performed using a bivariate random effects model. Funnel plot asymmetry (p = 0.0001 from Egger’s test) revealed the existence of publication bias among the included studies (S1 Fig).
Fig 3. Coupled forest plots of the sensitivity and specificity of immunochromatographic tests for diagnosing Mycoplasma pneumoniae infection.
The studies are indicated by year and author name. The numbers are pooled estimates with 95% confidence interval (CI) in brackets. Horizontal lines indicate 95% CIs.
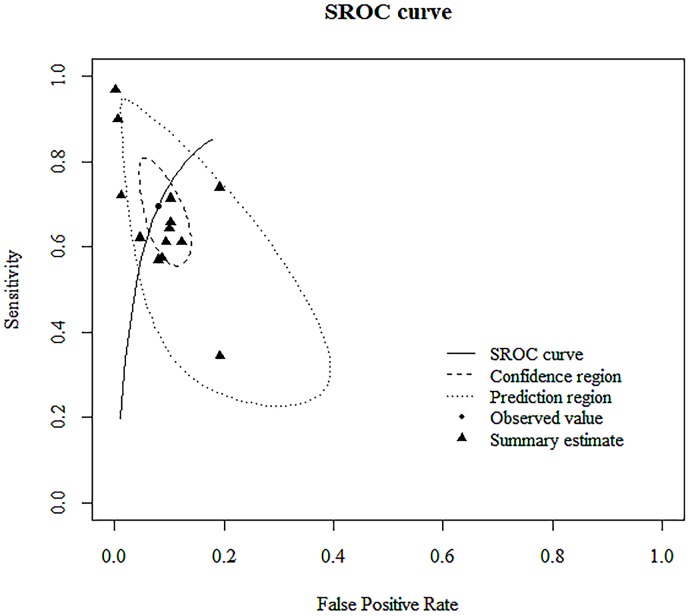
The overall sensitivity of the studies included in the analysis was estimated from the bivariate random effects model to be 0.70 (95% CI; 0.59–0.79). Similarly, the overall specificity was estimated to be 0.92 (95% CI; 0.87–0.95). DOR, as shown in Table 2, was 29.20 (95% CI; 10.70–64.20). The AUC for the SROC was 0.904 (Fig 4).
Table 2. Summary estimates of the diagnostic accuracy of immunochromatographic tests used to diagnose Mycoplasma pneumoniae.
| References (year and author) |
Sensitivity (95% CI) | Specificity (95% CI) | +LR (95% CI) | −LR (95% CI) | DOR (95% CI) |
|---|---|---|---|---|---|
| 2015 Li | 0.97 (0.90–0.99) | 1.00 (0.98–1.00) | 435.76 (27.33–6947.27) | 0.03 (0.01–0.11) | 13739.40 (652.36–289,367.43) |
| 2015 Miyashita | 0.61 (0.31–0.85) | 0.91 (0.84–0.95) | 6.46 (2.97–14.04) | 0.43 (0.19–0.98) | 15.04 (3.41–66.29) |
| 2015 Yamazaki | 0.74 (0.64–0.82) | 0.81 (0.73–0.87) | 3.86 (2.64–5.63) | 0.32 (0.22–0.47) | 11.92 (6.21–22.88) |
| 2016 Miyashita -1 | 0.71 (0.57–0.82) | 0.90 (0.86–0.92) | 6.95 (4.87–9.93) | 0.32 (0.20–0.50) | 21.72 (10.59–44.57) |
| 2016 Miyashita -2 | 0.61 (0.31–0.85) | 0.88 (0.77–0.94) | 4.97 (2.13–11.62) | 0.44 (0.19–1.01) | 11.21 (2.40–52.43) |
| 2016 Sano -1 | 0.57 (0.41–0.72) | 0.91 (0.86–0.95) | 6.61 (3.61–12.09) | 0.47 (0.32–0.69) | 14.15 (5.79–34.57) |
| 2016 Sano -2 | 0.57 (0.41–0.72) | 0.92 (0.86–0.95) | 7.03 (3.77–13.11) | 0.47 (0.32–0.68) | 15.01 (6.14–36.68) |
| 2017 Kakuya -1 | 0.34 (0.16–0.59) | 0.81 (0.67–0.90) | 1.78 (0.72–4.41) | 0.81 (0.55–1.19) | 2.19 (0.61–7.83) |
| 2017 Kakuya -2 | 0.66 (0.41–0.84) | 0.90 (0.77–0.96) | 6.42 (2.50–16.50) | 0.38 (0.19–0.76) | 16.76 (4.05–69.30) |
| 2017 Song | 0.72 (0.62–0.80) | 0.99 (0.89–1.00) | 58.99 (3.74–929.82) | 0.28 (0.21–0.39) | 207.65 (12.33–3,495.88) |
| 2018 Namkoong -1 | 0.90 (0.81–0.95) | 0.99 (0.95–1.00) | 152.77 (9.62–2,425.12) | 0.10 (0.05–0.20) | 1498.47 (84.06–26,712.36) |
| 2018 Namkoong -2 | 0.64 (0.53–0.74) | 0.90 (0.82–0.95) | 6.42 (3.32–12.42) | 0.40 (0.29–0.54) | 16.13 (6.87–37.87) |
| 2019 Yang | 0.62 (0.53–0.70) | 0.95 (0.89–0.98) | 13.38 (5.37–33.35) | 0.40 (0.31–0.50) | 33.66 (12.19–92.92) |
| Summary estimates | 0.70 (0.59–0.79) | 0.92 (0.87–0.95) | 8.94 (4.90–14.80) | 0.33 (0.22–0.46) | 29.20 (10.70–64.20) |
CI, confidence interval; +LR, positive likelihood ratio, −LR, negative likelihood ratio, DOR, diagnostic odds ratio
Fig 4. Summary receiver operating characteristic (SROC) curves of the diagnostic accuracy of immunochromatographic tests (ICTs) for Mycoplasma pneumoniae infection.
Summary points of the sensitivity and specificity, SROC curve, 95% confidence region, and 95% prediction region are shown. The area under the curve of the SROC curve for ICT was 0.904.
Subgroup analysis
According to our covariate significance test using a bivariate random effects model, the index test assay was the only significant heterogeneity factor (S3 Table). However, because subgroup analysis may be valuable based on the clinical characteristics, we conducted subgroup analysis to identify each potential source of heterogeneity. The summary estimates for the different subgroups are presented in Table 3.
Table 3. Subgroup analyses: Summary estimates using a bivariate random effects model.
| Variables | Sensitivity | Specificity | +LR | −LR | DOR | AUC§ |
|---|---|---|---|---|---|---|
| Population | ||||||
| Children* (n = 6) | 0.72 (0.49–0.87) | 0.94 (0.80–0.98) | 15.40 (2.93–45.20) | 0.32 (0.13–0.59) | 70.80 (5.19–285.00) | 0.911 |
| Mixed (children + adult) (n = 7) | 0.68 (0.56–0.78) | 0.91 (0.88–0.92) | 7.12 (5.48–8.98) | 0.36 (0.24–0.49) | 20.80 (11.60–34.50) | 0.906 |
| Type of specimen | ||||||
| Nasopharyngeal swab (n = 5) | 0.64 (0.48–0.77) | 0.87 (0.82–0.91) | 4.92 (3.24–7.03) | 0.42 (0.26–0.60) | 12.50 (5.57–24.50) | 0.866 |
| Oropharyngeal swab (n = 4) | 0.74 (0.58–0.86) | 0.96 (0.84–0.99) | 21.50 (3.98–64.80) | 0.29 (0.15–0.47) | 98.60 (8.84–371.00) | 0.907 |
| Reference standard | ||||||
| PCR (n = 11) | 0.72 (0.59–0.82) | 0.92 (0.86–0.95) | 8.88 (4.52–15.10) | 0.31 (0.20–0.46) | 31.80 (10.50–73.20) | 0.908 |
| Culture (n = 2) | 0.61 (0.52–0.69) | 0.94 (0.89–0.97) | 10.20 (5.13–18.30) | 0.43 (0.34–0.52) | 24.70 (10.70–46.90) | 0.763 |
| Index test assay | ||||||
| Ribotest Mycoplasma® (n = 8) | 0.66 (0.60–0.71) | 0.89 (0.85–0.92) | 6.00 (4.55–7.88) | 0.39 (0.33–0.45) | 15.70 (11.00–21.40) | 0.786 |
| Others (n = 5) | 0.79 (0.55–0.92) | 0.98 (0.91–1.00) | 49.20 (6.61–157.00) | 0.23 (0.08–0.47) | 378.00 (14.40–1750.00) | 0.962 |
Numbers are pooled estimates with 95% confidence intervals (CI) in parentheses. Horizontal lines indicate 95% CIs.
+LR, positive likelihood ratio,
−LR, negative likelihood ratio, DOR, diagnostic odds ratio; AUC, area under the summary receiver operating characteristic curve.
*Children and adults were defined as younger and older than 18 years of age, respectively.
§ The area under the summary receiver operating characteristic curve was obtained from a fitted bivariate random effects model and used to summarize overall test performance.
ICT showed similar pooled sensitivity and specificity in populations of children and mixed populations (children + adults). The specimens obtained from oropharyngeal swabs showed a higher sensitivity and specificity than those from nasopharyngeal swabs. In addition, the pooled estimates of sensitivity and accuracy for studies using PCR reference standards were higher than those using microbial culturing (Table 3).
Among the 13 studies included in our current analysis, eight of the studies consisting of 1,287 samples used the Ribotest Mycoplasma® brand of ICT [30, 36, 47–50]. The pooled sensitivity, specificity, positive LR, negative LR, and DOR of Ribotest Mycoplasma® for M. pneumoniae infection were 0.66 (95% CI, 0.60–0.71), 0.89 (95% CI, 0.85–0.92), 6.00 (95% CI, 4.55–7.88), 0.39 (95% CI, 0.33–0.45) and 15.70 (95% CI, 11.00–21.40), respectively (S4 Table). The overall accuracy of Ribotest Mycoplasma® was 0.786 (S2 Fig). As only one study provided information regarding blinding prior to testing, we were unable to conduct subgroup analysis.
Discussion
The current systematic review and meta-analysis is the first to establish an overview of the diagnostic accuracy of ICT for M. pneumoniae infection. ICT showed high specificity (0.92), with modest sensitivity (0.70) for the diagnosis of M. pneumoniae infection. The SROC AUC was 0.904, which indicates that ICT was highly accurate in the diagnosis of M. pneumoniae infection. This means that if a test result was positive, it was unlikely to be a false-positive result [64]. Therefore, physicians can confidently make a diagnosis of M. pneumoniae infection for a patient with respiratory symptoms and a positive ICT result and can then start proper antibiotic treatment to control the infection. Unfortunately, negative ICT results cannot be used to definitely rule out M. pneumoniae infection [64]. Therefore, the diagnosis should be confirmed using other laboratory methods if the test result can influence management decisions of patients.
Regardless, the easy-to-perform, rapid, accurate diagnosis of M. pneumoniae infection using ICT has the potential to decrease disease burden by the early prevention of outbreaks in closed populations, such as schools, colleges, and nursing home [5, 65]. ICT may also be a useful test during epidemic outbreaks, even in environments such as private hospitals that may not have specialized laboratories or emergency rooms required for making quick diagnoses. In addition, M. pneumoniae infection can cause significant morbidity, and even mortality, in patients of extreme age [5]. Prompt diagnosis and treatment of M. pneumoniae infection in these patients may be specifically beneficial.
Moreover, the prevalence of macrolide-resistant M. pneumoniae (MRMP) has recently increased worldwide, reaching prevalence rates up to 80–90%, especially in Asian countries [66–70]. MRMP is associated with severe clinical course (e.g., longer durations of fever, cough, and hospital stays) and more extrapulmonary complications [69, 71]. As macrolides are dramatically less effective against MRMP than against macrolide-sensitive M. pneumoniae, alternative antibiotic treatment including tetracyclines or fluoroquinolones is warranted in severe cases [12, 70–73]. Although ICT is not able to identify whether a particular strain of M. pneumoniae is resistant to macrolide or not, until additional genetic testing for MRMP strains become available, rapid M. pneumoniae diagnosis can provide a clinical basis for the use of alternative antibiotics when no clinical improvement is observed with the use of macrolides as the first line of antibiotics [74].
As for the target age group, our study demonstrated that the diagnostic accuracy of ICT for M. pneumoniae infection was similar between the groups containing both adults and children and the groups containing only children. This finding suggests that ICT may be used regardless of patient age. Nevertheless, clinical studies evaluating ICT focusing on adult populations and studies that compare the accuracy of ICT between children and adults are still warranted.
The only significant heterogeneity factor in our covariate significance analysis was the index test used. The majority of index tests used in the studies analyzed was the commercially available Ribotest Mycoplasma® kit. Pooled estimates of the sensitivities, specificities, and AUC for Ribotest Mycoplasma® was lower than that of other index tests. The other commercial ICT kits used included the FUJI DRI-CHEM IMMUNO AG Cartridge Myco (Fujifilm, Kanagawa, Japan) and the Quick Chaser® Auto Myco M. pneumoniae antigen detection kit (Mizuho Medy, Saga, Japan), which uses silver amplification. These have been shown to exhibit high sensitivity and specificity (0.904 and 1.0, respectively), even surpassing Ribotest Mycoplasma® (0.644 and 0.905, respectively) in head-to-head comparisons [36]. Test results derived from commercial kits would be more concern for clinicians in the practical use, but there remains a lack of research evaluating the head-to-head performance of ICTs across commercial brands.
The best sampling site for detecting M. pneumoniae, whether OP swabs or NP swabs, remains controversial [75–77]. In our meta-analysis, higher sensitivity and specificity were found for OP swabs. However, our findings were limited since head-to-head comparisons were not performed in most of the studies included in our analyses. Only one study reported that using OP swab specimens for ICT analysis showed higher accuracy in detecting lower RTIs caused by of M. pneumoniae than that of NP swab specimens when the samples were concomitantly obtained [49]. The authors suspected the reasons for their findings was due to varied M. pneumoniae density in the NP swab specimens, which were collected in a blinded fashion, and the larger OP swab tip, which was able to reach deeper into the airway resulting in a specimen higher load of M. pneumoniae. In addition, it has been reported that a higher copy number of M. pneumoniae is found in the alveoli than on the epithelium of the upper respiratory tract [78, 79].
Microbial culturing and PCR are currently the most commonly used reference standards for M. pneumoniae diagnosis. M. pneumoniae culturing has specific short-comings, including being less sensitive, difficult to perform, and requiring longer than PCR to obtain results [17, 80–84]. In our current analysis, the overall sensitivity and accuracy of ICT using PCR as the reference standard were higher than of ICT using microbial culturing, but the overall specificity was similar. Accordingly, we suggest that PCR is a more useful reference standard for ICT because of the moderate pooled sensitivity of ICT.
It is noteworthy that our current study had several limitations. First, there were no studies included that assessed the difference in ICT performance between macrolide-sensitive and macrolide-resistant M. pneumoniae strains. In addition, industrial sponsorship, inclusion/exclusion of comorbid conditions, duration of clinical symptoms prior to testing, time lapse before specimen processing, and blinded assessment of ICT were so rarely reported that we were not able to evaluate their effects.
Conclusions
ICT is a rapid and easy-to-use detection method with moderate sensitivity, high specificity, and high accuracy in diagnosing M. pneumoniae infection, regardless of patient age. This suggests that ICT is a useful test during the diagnostic workup of RTIs. Major practical advantages of ICT are its user-friendly format and short time requirements (usually ≤ 20 minutes). ICT could function as the point-of-care in clinical practice, instead of serology, PCR and microbial culturing, especially in resource-limited settings. If physicians are aware of the limitations of ICT, such as false negative results, they could make educated decisions in using ICT to implement appropriate antibiotics stewardship and infection control as well as to help make decision regarding the use of other diagnostic modalities. However, additional studies regarding the potential impact of ICT in clinical practice are necessary. These include the cost-effectiveness of routine ICT use and whether ICT may allow for decreases in additional diagnostic tests and result in reducing the excessive use of macrolides.
Supporting information
(DOC)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(EPS)
Summary points of the sensitivity and specificity, summary receiver operating characteristic (SROC) curve, 95% confidence region, and 95% prediction region are shown. The area under the curve of the SROC for the immunochromatographic tests (ICTs) was 0.786.
(EPS)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17: 697–728, table of contents. 10.1128/CMR.17.4.697-728.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson TP, Balish MF, Waites KB. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiology Reviews. 2008;32: 956–973. 10.1111/j.1574-6976.2008.00129.x [DOI] [PubMed] [Google Scholar]
- 3.Miyashita N, Fukano H, Mouri K, Fukuda M, Yoshida K, Kobashi Y, et al. Community-acquired pneumonia in Japan: a prospective ambulatory and hospitalized patient study. Journal of Medical Microbiology. 2005;54: 395–400. 10.1099/jmm.0.45920-0 [DOI] [PubMed] [Google Scholar]
- 4.MIYASHITA N, KAWAI Y, AKAIKE H, OUCHI K, HAYASHI T, KURIHARA T, et al. Influence of age on the clinical differentiation of atypical pneumonia in adults. Respirology. 2012;17: 1073–1079. [DOI] [PubMed] [Google Scholar]
- 5.Bajantri B, Venkatram S, Diaz-Fuentes G. Mycoplasma pneumoniae: A Potentially Severe Infection. Journal of clinical medicine research. 2018;10: 535–544. 10.14740/jocmr3421w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobs E, Ehrhardt I, Dumke R. New insights in the outbreak pattern of Mycoplasma pneumoniae. Int J Med Microbiol. 2015;305: 705–708. 10.1016/j.ijmm.2015.08.021 [DOI] [PubMed] [Google Scholar]
- 7.Loens K, Goossens H, Ieven M. Acute respiratory infection due to Mycoplasma pneumoniae: current status of diagnostic methods. Eur J Clin Microbiol Infect Dis. 2010;29: 1055–1069. 10.1007/s10096-010-0975-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waites KB, Xiao L, Liu Y, Balish MF, Atkinson TP. Mycoplasma pneumoniae from the Respiratory Tract and Beyond. Clinical microbiology reviews. 2017;30: 747–809. 10.1128/CMR.00114-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izumikawa K, Izumikawa K, Takazono T, Kosai K, Morinaga Y, Nakamura S, et al. Clinical features, risk factors and treatment of fulminant Mycoplasma pneumoniae pneumonia: A review of the Japanese literature. Journal of Infection and Chemotherapy. 2014;20: 181–185. 10.1016/j.jiac.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 10.Miyashita N, Obase Y, Ouchi K, Kawasaki K, Kawai Y, Kobashi Y, et al. Clinical features of severe Mycoplasma pneumoniae pneumonia in adults admitted to an intensive care unit. Journal of Medical Microbiology. 2007;56: 1625–1629. 10.1099/jmm.0.47119-0 [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Mei S, Zhou Y, Huang M, Dong G, Chen Z. Cytokines as the good predictors of refractory Mycoplasma pneumoniae pneumonia in school-aged children. Sci Rep. 2016;6: 37037 10.1038/srep37037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyashita N, Akaike H, Teranishi H, Ouchi K, Okimoto N. Macrolide-resistant Mycoplasma pneumoniae pneumonia in adolescents and adults: clinical findings, drug susceptibility, and therapeutic efficacy. Antimicrobial agents and chemotherapy. 2013;57: 5181–5185. 10.1128/AAC.00737-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Principi N, Esposito S. Macrolide-resistant Mycoplasma pneumoniae: its role in respiratory infection. J Antimicrob Chemother. 2013;68: 506–511. 10.1093/jac/dks457 [DOI] [PubMed] [Google Scholar]
- 14.Cunha BA. The atypical pneumonias: clinical diagnosis and importance. Clin Microbiol Infect. 2006;12 Suppl 3: 12–24. 10.1111/j.1469-0691.2006.01393.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niederman MS. In the Clinic: Community-Acquired Pneumonia. Ann Intern Med. 2015;163: Itc1–17. 10.7326/aitc201510060 [DOI] [PubMed] [Google Scholar]
- 16.Prina E, Ranzani OT, Torres A. Community-acquired pneumonia. Lancet. 2015;386: 1097–1108. 10.1016/S0140-6736(15)60733-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.She RC, Thurber A, Hymas WC, Stevenson J, Langer J, Litwin CM, et al. Limited utility of culture for Mycoplasma pneumoniae and Chlamydophila pneumoniae for diagnosis of respiratory tract infections. J Clin Microbiol. 2010;48: 3380–3382. 10.1128/JCM.00321-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kishaba T. Community-Acquired Pneumonia Caused by Mycoplasma pneumoniae: How Physical and Radiological Examination Contribute to Successful Diagnosis. Frontiers in Medicine. 2016;3 10.3389/fmed.2016.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S-C, Youn Y-S, Rhim J-W, Kang J-H, Lee K-Y. Early Serologic Diagnosis of Mycoplasma pneumoniae Pneumonia: An Observational Study on Changes in Titers of Specific-IgM Antibodies and Cold Agglutinins. Medicine. 2016;95: e3605–e3605. 10.1097/MD.0000000000003605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexander TS, Gray LD, Kraft JA, Leland DS, Nikaido MT, Willis DH. Performance of Meridian ImmunoCard Mycoplasma test in a multicenter clinical trial. J Clin Microbiol. 1996;34: 1180–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matas L, Dominguez J, De Ory F, Garcia N, Gali N, Cardona PJ, et al. Evaluation of Meridian ImmunoCard Mycoplasma test for the detection of Mycoplasma pneumoniae-specific IgM in paediatric patients. Scand J Infect Dis. 1998;30: 289–293. 10.1080/00365549850160954 [DOI] [PubMed] [Google Scholar]
- 22.Miyashita N, Kawai Y, Yamaguchi T, Ouchi K, Oka M. Clinical potential of diagnostic methods for the rapid diagnosis of Mycoplasma pneumoniae pneumonia in adults. Eur J Clin Microbiol Infect Dis. 2011;30: 439–446. 10.1007/s10096-010-1107-8 [DOI] [PubMed] [Google Scholar]
- 23.Busson L, Van den Wijngaert S, Dahma H, Decolvenaer M, Di Cesare L, Martin A, et al. Evaluation of 10 serological assays for diagnosing Mycoplasma pneumoniae infection. Diagn Microbiol Infect Dis. 2013;76: 133–137. 10.1016/j.diagmicrobio.2013.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitt BH, Sloan LM, Patel R. Real-time PCR detection of Mycoplasma pneumoniae in respiratory specimens. Diagn Microbiol Infect Dis. 2013;77: 202–205. 10.1016/j.diagmicrobio.2013.07.016 [DOI] [PubMed] [Google Scholar]
- 25.Gotoh K, Nishimura N, Ohshima Y, Arakawa Y, Hosono H, Yamamoto Y, et al. Detection of Mycoplasma pneumoniae by loop-mediated isothermal amplification (LAMP) assay and serology in pediatric community-acquired pneumonia. Journal of Infection and Chemotherapy. 2012;18: 662–667. 10.1007/s10156-012-0388-5 [DOI] [PubMed] [Google Scholar]
- 26.Aizawa Y, Oishi T, Tsukano S, Taguchi T, Saitoh A. Clinical utility of loop-mediated isothermal amplification for rapid diagnosis of Mycoplasma pneumoniae in children. J Med Microbiol. 2014;63: 248–251. 10.1099/jmm.0.068288-0 [DOI] [PubMed] [Google Scholar]
- 27.Luer CA, Wong K-P. Conformational stability of ribosomal protein L7/L12: effects of pH, temperature, and guanidinium chloride. Biochemistry. 1980;19: 176–183. 10.1021/bi00542a027 [DOI] [PubMed] [Google Scholar]
- 28.Sano G, Itagaki T, Ishiwada N, Matsubara K, Iwata S, Nakamori Y, et al. Characterization and evaluation of a novel immunochromatographic assay for pharyngeal Mycoplasma pneumoniae ribosomal protein L7/L12 antigens. Journal of Medical Microbiology. 2016;65: 1105–1110. 10.1099/jmm.0.000336 [DOI] [PubMed] [Google Scholar]
- 29.Li W, Liu Y, Zhao Y, Tao R, Li Y, Shang S. Rapid diagnosis of Mycoplasma pneumoniae in children with pneumonia by an immuno-chromatographic antigen assay. Sci Rep. 2015;5: 15539 10.1038/srep15539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamazaki T, Kuroki H, Itagaki T, Iwata S, Tateda K. [Evaluation of a Rapid Antigen Detection Kit Targeting L7/L12 Ribosomal Protein for Mycoplasma pneumoniae]. Kansenshogaku Zasshi. 2015;89: 394–399. 10.11150/kansenshogakuzasshi.89.394 [DOI] [PubMed] [Google Scholar]
- 31.Mori Y, Kanda H, Notomi T. Loop-mediated isothermal amplification (LAMP): recent progress in research and development. J Infect Chemother. 2013;19: 404–411. 10.1007/s10156-013-0590-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thirapanmethee K, Pothisamutyothin K, Nathisuwan S, Chomnawang MT, Wiwat C. Loop-mediated isothermal amplification assay targeting the blaCTX-M9 gene for detection of extended spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Microbiol Immunol. 2014;58: 655–665. 10.1111/1348-0421.12205 [DOI] [PubMed] [Google Scholar]
- 33.Mori Y, Notomi T. Loop-mediated isothermal amplification (LAMP): a rapid, accurate, and cost-effective diagnostic method for infectious diseases. J Infect Chemother. 2009;15: 62–69. 10.1007/s10156-009-0669-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng M-H, Zhong L-Y, Kamolnetr O, Limpanont Y, Lv Z-Y. Detection of helminths by loop-mediated isothermal amplification assay: a review of updated technology and future outlook. Infectious Diseases of Poverty. 2019;8: 20 10.1186/s40249-019-0530-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karthik K, Rathore R, Thomas P, Arun TR, Viswas KN, Dhama K, et al. New closed tube loop mediated isothermal amplification assay for prevention of product cross-contamination. MethodsX. 2014;1: 137–143. 10.1016/j.mex.2014.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Namkoong H, Yamazaki M, Ishizaki M, Endo I, Harada N, Aramaki M, et al. Clinical Evaluation of the Immunochromatographic System Using Silver Amplification for the Rapid Detection of Mycoplasma pneumoniae. Scientific Reports. 2018;8 10.1038/s41598-018-19734-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai Z-H, Dai Y-Y, Huang L-Y, Zhang W-S, Guo X-G. Diagnosis of mycoplasma pneumoniae by loop-mediated isothermal amplification: systematic review and meta-analysis. BMC Infectious Diseases. 2019;19: 173 10.1186/s12879-019-3799-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lou SC, Patel C, Ching S, Gordon J. One-step competitive immunochromatographic assay for semiquantitative determination of lipoprotein(a) in plasma. Clin Chem. 1993;39: 619–624 [PubMed] [Google Scholar]
- 39.Shim WB, Yang ZY, Kim JY, Choi JG, Je JH, Kang SJ, et al. Immunochromatography using colloidal gold-antibody probe for the detection of atrazine in water samples. J Agric Food Chem. 2006;54: 9728–9734. 10.1021/jf0620057 [DOI] [PubMed] [Google Scholar]
- 40.Shim WB, Yang ZY, Kim JS, Kim JY, Kang SJ, Woo GJ, et al. Development of immunochromatography strip-test using nanocolloidal gold-antibody probe for the rapid detection of aflatoxin B1 in grain and feed samples. J Microbiol Biotechnol. 2007;17: 1629–1637 [PubMed] [Google Scholar]
- 41.Goryacheva IY. Chapter 2—Rapid Tests Progress Through the Years In: Goryacheva IY, editor. Comprehensive Analytical Chemistry: Elsevier; 2016. pp. 5–32. [Google Scholar]
- 42.Cho YJ, Lee DH, Kim DO, Min WK, Bong KT, Lee GG, et al. Production of a monoclonal antibody against ochratoxin A and its application to immunochromatographic assay. J Agric Food Chem. 2005;53: 8447–8451. 10.1021/jf051681q [DOI] [PubMed] [Google Scholar]
- 43.Dzantiev BB, Byzova NA, Urusov AE, Zherdev AV. Immunochromatographic methods in food analysis. TrAC Trends in Analytical Chemistry. 2014;55: 81–93. 10.1016/j.trac.2013.11.007 [DOI] [Google Scholar]
- 44.Zhao Y, Zhang G, Liu Q, Teng M, Yang J, Wang J. Development of a Lateral Flow Colloidal Gold Immunoassay Strip for the Rapid Detection of Enrofloxacin Residues. Journal of Agricultural and Food Chemistry. 2008;56: 12138–12142. 10.1021/jf802648z [DOI] [PubMed] [Google Scholar]
- 45.Postma DF, van Werkhoven CH, Huijts SM, Bolkenbaas M, Oosterheert JJ, Bonten MJ. New trends in the prevention and management of community-acquired pneumonia. Neth J Med. 2012;70: 337–348 [PubMed] [Google Scholar]
- 46.Couturier MR, Graf EH, Griffin AT. Urine antigen tests for the diagnosis of respiratory infections: legionellosis, histoplasmosis, pneumococcal pneumonia. Clin Lab Med. 2014;34: 219–236. 10.1016/j.cll.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 47.Miyashita N, Kawai Y, Tanaka T, Akaike H, Teranishi H, Wakabayashi T, et al. Diagnostic sensitivity of a rapid antigen test for the detection of Mycoplasma pneumoniae: Comparison with real-time PCR. J Infect Chemother. 2015;21: 473–475. 10.1016/j.jiac.2015.02.007 [DOI] [PubMed] [Google Scholar]
- 48.Miyashita N, Kawai Y, Kato T, Tanaka T, Akaike H, Teranishi H, et al. Rapid diagnostic method for the identification of Mycoplasma pneumoniae respiratory tract infection. J Infect Chemother. 2016;22: 327–330. 10.1016/j.jiac.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 49.Kakuya F, Kinebuchi T, Okubo H, Matsuo K. Comparison of Oropharyngeal and Nasopharyngeal Swab Specimens for the Detection of Mycoplasma pneumoniae in Children with Lower Respiratory Tract Infection. J Pediatr. 2017;189: 218–221. 10.1016/j.jpeds.2017.06.038 [DOI] [PubMed] [Google Scholar]
- 50.Yang SI, Han MS, Kim SJ, Lee SY, Choi EH. Evaluation of a Rapid Diagnostic Antigen Test Kit Ribotest Mycoplasma® for the Detection of Mycoplasma pneumoniae. Pediatr Infect Vaccine. 2019;26: 81–88 [Google Scholar]
- 51.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62: 1006–1012. 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 52.Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Annals of Internal Medicine. 2011;155: 529–536. 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 53.Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58: 982–990. 10.1016/j.jclinepi.2005.02.022 [DOI] [PubMed] [Google Scholar]
- 54.Zwinderman AH, Bossuyt PM. We should not pool diagnostic likelihood ratios in systematic reviews. Stat Med. 2008;27: 687–697. 10.1002/sim.2992 [DOI] [PubMed] [Google Scholar]
- 55.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327: 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21: 1539–1558. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 57.McGrath TA, McInnes MDF, Korevaar DA, Bossuyt PMM. Meta-Analyses of Diagnostic Accuracy in Imaging Journals: Analysis of Pooling Techniques and Their Effect on Summary Estimates of Diagnostic Accuracy. Radiology. 2016;281: 78–85. 10.1148/radiol.2016152229 [DOI] [PubMed] [Google Scholar]
- 58.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54: 1046–1055. 10.1016/s0895-4356(01)00377-8 [DOI] [PubMed] [Google Scholar]
- 59.Song F, Khan KS, Dinnes J, Sutton AJ. Asymmetric funnel plots and publication bias in meta-analyses of diagnostic accuracy. Int J Epidemiol. 2002;31: 88–95. 10.1093/ije/31.1.88 [DOI] [PubMed] [Google Scholar]
- 60.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315: 629–634. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song M, Zhang Y, Li S, Zhang C, Tao M, Tang Y, et al. A sensitive and rapid immunoassay for Mycoplasma pneumoniae in children with pneumonia based on single-walled carbon nanotubes. Sci Rep. 2017;7: 16442 10.1038/s41598-017-16652-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whiting PF, Rutjes AWS, Westwood ME, Mallett S. A systematic review classifies sources of bias and variation in diagnostic test accuracy studies. Journal of Clinical Epidemiology. 2013;66: 1093–1104. 10.1016/j.jclinepi.2013.05.014 [DOI] [PubMed] [Google Scholar]
- 63.Chartrand C, Tremblay N, Renaud C, Papenburg J. Diagnostic Accuracy of Rapid Antigen Detection Tests for Respiratory Syncytial Virus Infection: Systematic Review and Meta-analysis. J Journal of Clinical Microbiology 2015;53: 3738–3749. 10.1128/JCM.01816-15% [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trevethan R. Sensitivity, Specificity, and Predictive Values: Foundations, Pliabilities, and Pitfalls in Research and Practice. Frontiers in public health. 2017;5: 307–307. 10.3389/fpubh.2017.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diaz MH, Benitez AJ, Winchell JM. Investigations of Mycoplasma pneumoniae infections in the United States: trends in molecular typing and macrolide resistance from 2006 to 2013. J Clin Microbiol. 2015;53: 124–130. 10.1128/JCM.02597-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kawai Y, Miyashita N, Kubo M, Akaike H, Kato A, Nishizawa Y, et al. Nationwide surveillance of macrolide-resistant Mycoplasma pneumoniae infection in pediatric patients. Antimicrob Agents Chemother. 2013;57: 4046–4049. 10.1128/AAC.00663-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cao B, Zhao CJ, Yin YD, Zhao F, Song SF, Bai L, et al. High prevalence of macrolide resistance in Mycoplasma pneumoniae isolates from adult and adolescent patients with respiratory tract infection in China. Clin Infect Dis. 2010;51: 189–194. 10.1086/653535 [DOI] [PubMed] [Google Scholar]
- 68.Zhou Z, Li X, Chen X, Luo F, Pan C, Zheng X, et al. Macrolide-resistant Mycoplasma pneumoniae in adults in Zhejiang, China. Antimicrob Agents Chemother. 2015;59: 1048–1051. 10.1128/AAC.04308-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hong KB, Choi EH, Lee HJ, Lee SY, Cho EY, Choi JH, et al. Macrolide resistance of Mycoplasma pneumoniae, South Korea, 2000–2011. Emerg Infect Dis. 2013;19: 1281–1284. 10.3201/eid1908.121455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang H-J, Song DJ, Shim JY. Mechanism of resistance acquisition and treatment of macrolide-resistant Mycoplasma pneumoniae pneumonia in children. Korean J Pediatr. 2017;60: 167–174. 10.3345/kjp.2017.60.6.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pereyre S, Goret J, Bébéar C. Mycoplasma pneumoniae: Current Knowledge on Macrolide Resistance and Treatment. Frontiers in microbiology. 2016;7: 974–974. 10.3389/fmicb.2016.00974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kawai Y, Miyashita N, Kubo M, Akaike H, Kato A, Nishizawa Y, et al. Therapeutic efficacy of macrolides, minocycline, and tosufloxacin against macrolide-resistant Mycoplasma pneumoniae pneumonia in pediatric patients. Antimicrob Agents Chemother. 2013;57: 2252–2258. 10.1128/AAC.00048-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ishiguro N, Koseki N, Kaiho M, Ariga T, Kikuta H, Togashi T, et al. Therapeutic efficacy of azithromycin, clarithromycin, minocycline and tosufloxacin against macrolide-resistant and macrolide-sensitive Mycoplasma pneumoniae pneumonia in pediatric patients. PLOS ONE. 2017;12: e0173635 10.1371/journal.pone.0173635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang T-I, Chang T-H, Lu C-Y, Chen J-M, Lee P-I, Huang L-M, et al. Mycoplasma pneumoniae in pediatric patients: Do macrolide-resistance and/or delayed treatment matter? Journal of Microbiology, Immunology and Infection. 2019;52: 329–335. 10.1016/j.jmii.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 75.Gnarpe J, Lundback A, Gnarpe H, Sundelof B. Comparison of nasopharyngeal and throat swabs for the detection of Chlamydia pneumoniae and Mycoplasma pneumoniae by polymerase chain reaction. Scand J Infect Dis Suppl. 1997;104: 11–12 [PubMed] [Google Scholar]
- 76.Dorigo-Zetsma JW, Verkooyen RP, van Helden HP, van der Nat H, van den Bosch JM. Molecular detection of Mycoplasma pneumoniae in adults with community-acquired pneumonia requiring hospitalization. J Clin Microbiol. 2001;39: 1184–1186. 10.1128/JCM.39.3.1184-1186.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Michelow IC, Olsen K, Lozano J, Duffy LB, McCracken GH, Hardy RD. Diagnostic utility and clinical significance of naso- and oropharyngeal samples used in a PCR assay to diagnose Mycoplasma pneumoniae infection in children with community-acquired pneumonia. J Clin Microbiol. 2004;42: 3339–3341. 10.1128/JCM.42.7.3339-3341.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang L, Yang S, Yan X, Liu T, Feng Z, Li G. Comparing the yield of oropharyngeal swabs and sputum for detection of 11 common pathogens in hospitalized children with lower respiratory tract infection. Virology Journal. 2019;16: 84 10.1186/s12985-019-1177-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Collier AM, Clyde WA Jr. Appearance of Mycoplasma pneumoniae in lungs of experimentally infected hamsters and sputum from patients with natural disease. Am Rev Respir Dis. 1974;110: 765–773. 10.1164/arrd.1974.110.6P1.765 [DOI] [PubMed] [Google Scholar]
- 80.Nilsson AC, Björkman P, Persson K. Polymerase chain reaction is superior to serology for the diagnosis of acute Mycoplasma pneumoniae infection and reveals a high rate of persistent infection. BMC Microbiology. 2008;8: 93 10.1186/1471-2180-8-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Daxboeck F, Krause R, Wenisch C. Laboratory diagnosis of Mycoplasma pneumoniae infection. Clinical Microbiology and Infection. 2003;9: 263–273. 10.1046/j.1469-0691.2003.00590.x [DOI] [PubMed] [Google Scholar]
- 82.Morozumi M, Ito A, Murayama SY, Hasegawa K, Kobayashi R, Iwata S, et al. Assessment of real-time PCR for diagnosis of Mycoplasma pneumoniae pneumonia in pediatric patients. Canadian Journal of Microbiology. 2006;52: 125–129. 10.1139/w05-118 [DOI] [PubMed] [Google Scholar]
- 83.van Kuppeveld FJ, Johansson KE, Galama JM, Kissing J, Bolske G, Hjelm E, et al. 16S rRNA based polymerase chain reaction compared with culture and serological methods for diagnosis of Mycoplasma pneumoniae infection. Eur J Clin Microbiol Infect Dis. 1994;13: 401–405. 10.1007/bf01971997 [DOI] [PubMed] [Google Scholar]
- 84.Waring AL, Halse TA, Csiza CK, Carlyn CJ, Arruda Musser K, Limberger RJ. Development of a genomics-based PCR assay for detection of Mycoplasma pneumoniae in a large outbreak in New York State. J Clin Microbiol. 2001;39: 1385–1390. 10.1128/JCM.39.4.1385-1390.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(EPS)
Summary points of the sensitivity and specificity, summary receiver operating characteristic (SROC) curve, 95% confidence region, and 95% prediction region are shown. The area under the curve of the SROC for the immunochromatographic tests (ICTs) was 0.786.
(EPS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.