Abstract
BACKGROUND AND PURPOSE:
Graph theory uses structural similarity to analyze cortical structural connectivity. We used a voxel-based definition of cortical covariance networks to quantify and assess the relationship of network characteristics to cognition in a cohort of patients with relapsing-remitting MS with and without cognitive impairment.
MATERIALS AND METHODS:
We compared subject-specific structural gray matter network properties of 18 healthy controls, 25 patients with MS with cognitive impairment, and 55 patients with MS without cognitive impairment. Network parameters were compared, and predictive value for cognition was assessed, adjusting for confounders (sex, education, gray matter volume, network size and degree, and T1 and T2 lesion load). Backward stepwise multivariable regression quantified predictive factors for 5 neurocognitive domain test scores.
RESULTS:
Greater path length (r = –0.28, P < .0057) and lower normalized path length (r = 0.36, P < .0004) demonstrated a correlation with average cognition when comparing healthy controls with patients with MS. Similarly, MS with cognitive impairment demonstrated a correlation between lower normalized path length (r = 0.40, P < .001) and reduced average cognition. Increased normalized path length was associated with better performance for processing (P < .001), learning (P < .001), and executive domain function (P = .0235), while reduced path length was associated with better executive (P = .0031) and visual domains. Normalized path length improved prediction for processing (R2 = 43.6%, G2 = 20.9; P < .0001) and learning (R2 = 40.4%, G2 = 26.1; P < .0001) over a null model comprising confounders. Similarly, higher normalized path length improved prediction of average z scores (G2 = 21.3; P < .0001) and, combined with WM volume, explained 52% of average cognition variance.
CONCLUSIONS:
Patients with MS and cognitive impairment demonstrate more random network features and reduced global efficiency, impacting multiple cognitive domains. A model of normalized path length with normal-appearing white matter volume improved average cognitive z score prediction, explaining 52% of variance.
MS is a chronic inflammatory disease of the central nervous system characterized by WM and GM axonal loss and demyelination and associated with whole-brain atrophy.1 The most commonly described manifestation of MS is physical disability, but cognitive impairment is common and underrecognized.2 Historically, WM lesions were considered the primary contributing factor to impairment; however, WM damage only partly accounts for functional status, and recent studies have shown that structural GM measures are crucial contributors to disease manifestation.3,4 A recently introduced method of GM analysis differs from traditional voxel- and surfaced-based methods by taking advantage of graph theory applications and MR imaging to obtain parameters that reflect the structural connectivity of the cortex.5
By means of a graph theoretical approach, sets of nodes describe spatial regions of gray matter, either obtained from an atlas6 or defined by cubes of voxels,5 and the edges describe the structural similarity between 2 nodes. Networks defined in this way have been found to exhibit small world properties.7 Small world networks are more efficient systems, characterized by higher clustering and similar characteristic path length compared with randomly created networks of the same size and degree of distribution.8 In comparison, “regular” networks show both higher clustering and higher characteristic path length than random networks.8 The graph theoretical approach supports the axonal tension hypothesis, which theorizes that interconnected areas of the brain are structurally similar due to mutual tension between axons.9 Studies have shown that structural networks become disorganized in patients with Alzheimer disease,10 schizophrenia,11 and diabetes.12 However, studies applying this approach to patients with MS have shown contradictory results.6,13 Rimkus et al13 showed that patients with MS have a more random network topology than healthy controls (HC), whereas Tewarie et al6 showed that patients with MS have a more regular network topology. This inconsistency may be due to different methods of extracting structural networks and a higher percentage of patients with secondary-progressive MS included in the latter study. Patients with secondary-progressive MS have been shown to undergo different cortical atrophy patterns than patients with relapsing-remitting MS (RRMS),14 potentially altering the structural gray matter network topology and affecting whole-group analysis. Thus, although graph theoretical approaches have shown promise in other disease processes, methodologic inconsistencies in previous MS studies have prevented meaningful comparison of results.6,12,15
In this study, we used a voxel-based definition of the GM structural covariance networks to determine network characteristics in a cohort of patients with RRMS with (CI) and without cognitive impairment (CP). Using the same method as Rimkus et al,13 we sought to determine the association between network parameters and CI. Consistent with a more small world network in normal health and degeneration to a more random network in disease, we hypothesized a higher degree and density in HC compared with patients with MS and a higher degree, density, and normalized (λ) and reduced path length in MS-CP versus MS-CI. We expand on prior reported findings by quantifying the added contribution to a model of CI of network parameters over traditional determinants such as white matter lesion load.
MATERIALS AND METHODS
Patient Cohort
Patients with RRMS were prospectively recruited for this ethics board–approved study from tertiary referral MS clinics and were age- and sex-matched with HC. MS diagnosis was established using the revised McDonald (2017) criteria by a senior MS neurologist (20 years’ experience).16 Participants’ clinical histories, including age, sex, education level, and disease duration, were recorded. Exclusion criteria were drug/alcohol abuse, relapse or corticosteroid use within the past 3 months, premorbid psychiatric history, head injury (including loss of consciousness), and concurrent morbidity (cerebrovascular disease and MR imaging/gadolinium contraindications, including impaired renal function). Eighty patients with RRMS (55 CP, 25 CI) and 18 healthy controls were recruited.
Neuropsychological Assessment
All participants were assessed for cognitive impairment using the minimal assessment of cognitive function in MS, assessing 5 cognitive domains with 7 tests. These domains include learning and memory (California Verbal Leaning Test-II, Brief Visuospatial Test-revised); processing speed and working memory (Paced Auditory Serial Addition, Symbol Digit Modalities Test); executive function (Delis-Kaplan Executive Function System); verbal fluency (Controlled Oral Work Association Test); and visuospatial perception/spatial processing (Judgment of Line Orientation test). Age- and sex-adjusted normative data were used to convert raw test scores to z scores.17 Z scores less than −1.5 for a single test defined impairment, and patients impaired on ≥2 tests were considered impaired for MS group dichotomization, whereas an average z score was also calculated for each patient.
Image Acquisition
MR imaging was performed on a 3T MR imaging system (Magnetom Prisma; Siemens, Erlangen, Germany) with a 20-channel phased array coil. The acquisitions included sagittal volumetric T1 (TR/TE/flip angle, 2300 ms/2.26 ms/9°; number of averages, 1; FOV, 256 mm; section thickness, 1 mm; matrix size, 256 × 256 mm); T2 sampling perfection with application-optimized contrasts by using different flip angle evolution (SPACE sequence; Siemens) (TR/TE, 3200/408 ms; FOV, 230 mm; section thickness, 0.9 mm; matrix, 230 × 230 mm); T2 FLAIR (TR/TE, 500/387 ms; FOV, 230 mm; section thickness, 0.9 mm; matrix, 230 × 230 mm); and phase-sensitive inversion recovery (TR/TE, 2900/9.5 ms; FOV, 220 mm; section thickness, 2 mm; matrix, 220 × 176).
Segmentation and Lesion Measurement
T2 FLAIR images were coregistered to structural T1-weighted images by Statistical Parametric Mapping software, Version 12 (SPM 12; http://www.fil.ion.ucl.ac.uk/spm/software/spm12). A neuroradiologist (17 years of experience) used the threshold function in Analyze (Version 12.0; AnalyzeDirect, Overland Park, Kansas) to derive the WM T2-hyperintense and T1-hypointense lesion tracings on T1 and T2 images. Lesion volumes were filled using the SPM SLF Toolbox (https://github.com/NIC-VICOROB/SLF) to remove segmentation errors.18 Lesion-filled T1 images were automatically segmented into cortical GM, WM, and CSF volumes using the SPM12 segmentation tool (https://neuroimage.usc.edu/brainstorm/Tutorials/SegCAT12) with the minimum probability of cortical GM set to 70% to correct for GM/WM partial volume effects. The segmentations were inspected visually, and no scan was excluded. GM volumes were realigned with the standard space Montreal Neurological Institute T1 template and resliced into 2 × 2 × 2 mm3 isotropic voxels. Deep GM structure volumes (ie, basal ganglia and thalamus) were segmented using the FMRIB Integrated Registration and Segmentation Tool, Version 5 (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FIRST).
Gray Matter Network Construction
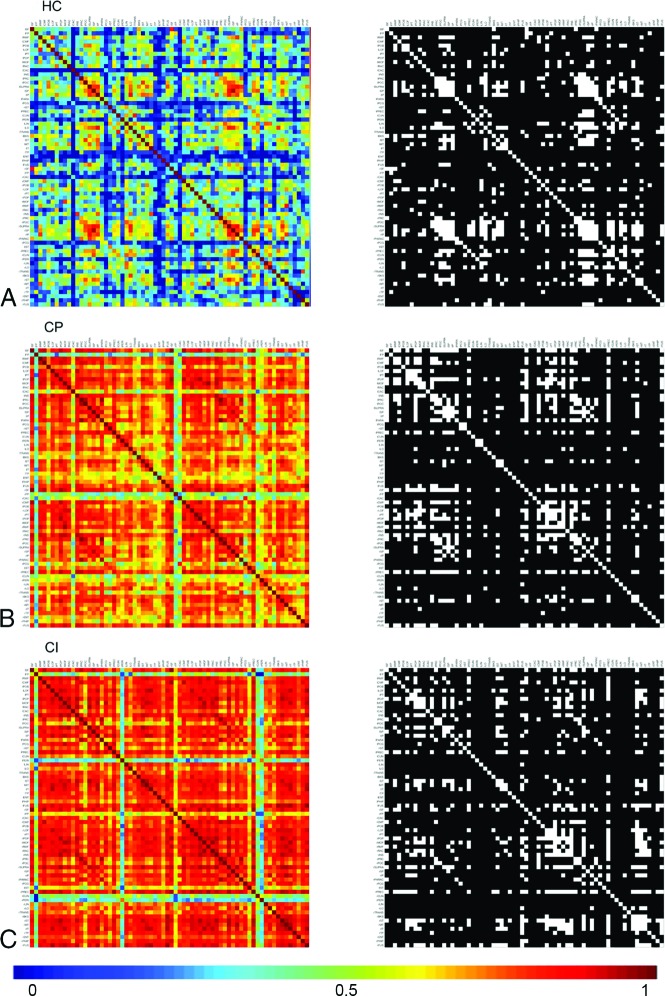
Network construction was performed using a previously published process available on-line at https://github.com/bettytijms/Single_Subject_Gray_Matter_Networks.6 Briefly, realigned and resliced GM segmentations were divided into 3 × 3 × 3 voxel cubes representing the nodes in the structural network. Two nodes were connected by an edge when their Pearson correlation coefficient exceeded a certain threshold. To correct for cortical curvature, we rotated cubes by multiples of 45° and reflected them over all axes to find the maximum correlation coefficient between each pairing (Figure, left column matrices). The similarity matrices were then binarized using a subject-specific threshold based on permutation testing to ensure a similar chance of including 5% spurious correlations for each patient (Figure, right column matrices).13 The resulting unweighted, undirected networks were used to calculate the following network parameters: size, degree, connectivity density, betweenness centrality, characteristic clustering coefficient (C), and characteristic path length (L). Network measures were calculated using the Brain Connectivity Toolbox (https://www.nitrc.org/projects/bct/). Definitions for the network parameters are the following19—size: total number of nodes present in the network; degree: average number of edges connected to each node divided by the total number of nodes; density: number of edges present in the network divided by the number of possible edges; betweenness centrality: number of shortest paths between any 2 pairs of nodes that pass through a given node; C: how many of the nodes are connected to a given node, which are also connected to one another; and L: average of smallest number of edges connecting all possible pairs of nodes.
Figure.
Pearson correlations (edges) between all pairs of GM regions (nodes) for a group of subjects. Here the rows/columns denote the nodes, and the warmer colors represent the greater edge weights/connectivity between the nodes. The colored matrices (left column) here show the weighted undirected network, where the edges are associated with the strength of the connection and are undirected (ie, if node j is connected to node k, then node k is also connected to node j), resulting in a symmetric connectivity matrix. The black-white matrices are binary undirected networks, where edges are either 0 or 1, indicating the absence or presence of a connection, and they have no directionality. Nodal correlations are proportional to longer path length. MS-CI shows a warmer matrix than MS-CP or HC.
Normalized clustering coefficient (γ) and normalized path length (λ) were calculated by dividing C and L respectively by C and L, obtained from averaging over 20 randomized networks of the same size and degree distribution.13 The small world coefficient of the network was then calculated by dividing γ/λ. Small world networks have a characteristic clustering coefficient much larger than random networks and a characteristic path length approximately equal to those of random networks. Thus, a small world network can be seen when γ/λ > 1.20
Size, degree, and density are included as potential confounders for more complex network parameters. Characteristic clustering coefficient and path length are included as important descriptors of graph topology and graph connectivity. γ, λ, and small world coefficient are included as quantifiers for randomness and small worldness. The betweenness coefficient is included to determine regional hubs in future analyses.
Statistical Analysis
Demographic factors were compared between all patients with MS and HC or between patients with MS-CI and MS-CP using the Wilcoxon rank sum test for continuous variables and the Fisher exact test or χ2 test for categoric variables as appropriate. Using the ANCOVA with the Fisher least significant difference test correction, we compared the network parameters between groups, accounting for confounding factors including sex, education, global GM volume, NAWM, and factors of size, degree, and density. Confounding factors included sex, education, global gray matter volume, network size and degree, and T1 and T2 lesion loads. To normalize distributions for T1 and T2 lesion loads, we used natural log transformation. Similarly, the associations between each of 9 network parameters and each of 6 neurocognitive domain test scores were tested in patients with MS only. To control for the multiple comparisons, we applied the Benjamini-Hochberg adaptive linear step-up method,21 and the adjusted P value < .05 was considered statistically significant. Partial correlation coefficients were calculated after accounting for confounding factors to determine the correlation of each network parameter to average cognition by calculating an average z score for each study participant. Using the backward stepwise selection procedure in the multivariable linear regression analysis, we would search for the most significant predictive factors for each of 5 neurocognitive domain test scores in MS. R2 was estimated as the goodness-of-fit for each of the models. The higher the R2 value, the better was the model fit. To evaluate significant influence from network predictors associated to neurocognitive domain scores, we performed the G2 likelihood ratio test. The G2 likelihood ratio statistic is the difference between −2L (log likelihood) of the fitted model (ie, the model includes both significant network predictors and demographic variables) and the reference null model (ie, the model includes only confounding factors of sex, education, global GM volume, and T1 and T2 lesion loads). The P value was obtained from the G2 likelihood ratio χ2 test, and P < .05 was considered statistically significant. Multicollinearity was tested using the variance inflation factor. All analyses were conducted by SAS, Version 9.4 for Windows (SAS Institute, Cary, North Carolina).
RESULTS
Group Demographics
Eighteen HC (mean age, 48.7 ± 7.2 years; 72% women) and 80 patients with MS (mean age, 51.8 ± 8.6 years; 68% women) with a mean education of 15 ± 2.4 years, disease duration of 15.8 ± 8.9 years, and mean Expanded Disability Status Scale score of 4.3 ± 2.3) were included. There was no difference in the frequency of disease-modifying drug use between groups (P = .80).
CI was present in 25/80 (31%) of patients. Increased T1 and T2 lesion loads and reduced global GM volume and lower average and individual domain z scores were present in MS-CI versus MS-CP. A greater proportion of women composed the MS-CP cohort (75% versus 52%). Compared with HC, patients with MS demonstrated an average of 2 fewer years of education and reduced global GM volume and z scores for processing, learning, and average cognition (Table 1). Size and degree were higher in HC versus patients with MS (Table 2), but only degree remained significantly different after correcting for other confounders. Therefore, degree was added as an additional confounder when comparing the other network parameters between HCs and the MS cohort. Size, density, and degree were not significantly different between MS-CP and MS-CI.
Table 1:
Demographic and clinical characteristics of healthy controls and patients with MS
| Characteristics | HC | MS |
||
|---|---|---|---|---|
| Total Sample | MS-CP | MS-CI | ||
| No. | 18 | 80 | 55 | 25 |
| Female sex (No.) (%) | 13 (72%) | 54 (68%) | 41 (75%) | 13 (52%)a |
| Age (mean) (SD) (yr) | 48.7 (7.2) | 51.8 (8.6) | 52.7 (8.8) | 49.7 (7.9) |
| Education (mean) (SD) (yr) | 17.0 (2.9) | 15.0 (2.4)b | 15.2 (2.2) | 14.6 (2.7) |
| Disease duration (mean) (SD) (yr) | NA | 15.8 (8.9) | 15.7 (8.4) | 15.9 (9.9) |
| EDSS (mean) (SD) | NA | 4.3 (2.3) | 4.1 (2.4) | 4.6 (2.2) |
| T1 lesion load (mean) (SD) | NA | 4.0 (5.6) | 2.8 (3.3) | 6.7 (8.3)c |
| T2 lesion load (mean) (SD) | NA | 15.8 (15.8) | 11.8 (11.2) | 24.6 (20.5)c |
| Global gray matter volume (mean) (SD) | 875.9 (48.9) | 748.5 (69.6)d | 761.2 (65.9) | 720.6 (70.8)a |
| NAWM volume (mean) (SD) | 616.2 (39.9) | 510.9 (101.3)d | 540.9 (56.3) | 444.7 (141.5)e |
| Neurocognitive domain score (mean) (SD) | ||||
| Processing | −0.17 (0.74) | −0.90 (0.99)e | −0.43 (0.68) | −2.01 (0.65)f |
| Learning | 0.12 (0.73) | −0.80 (1.29)e | −0.093 (0.76) | −2.35 (0.74)f |
| Executive functioning | 0.50 (0.75) | −0.062 (1.12) | 0.30 (0.80) | −0.85 (1.32)f |
| Visual | 0.98 (0.20) | 0.55 (0.91) | 0.79 (0.86) | 0.010 (0.80)f |
| Language | −0.70 (0.84) | −0.86 (1.05) | −0.56 (0.84) | −1.53 (1.17)f |
| Average z score | 0.15 (0.41) | −0.42 (0.80)e | −0.003 (0.48) | −1.34 (0.54)f |
Note:—NA indicates not applicable; EDSS, Expanded Disability Status Scale.
Adjusted P < .05 (comparison within MS groups is based on cognitive impairment status).
Adjusted P < .05 (comparison of total sample of MS patients with healthy control).
Adjusted P < .01 (comparison within MS groups is based on cognitive impairment status).
Adjusted P < .001 (comparison of total sample of MS patients with healthy control).
Adjusted P < .01 (comparison of total sample of MS patients with healthy control).
Adjusted P < .001 (comparison within MS groups is based on cognitive impairment status).
Table 2:
Values of global gray matter network properties between HC and patients with MS and within the MS sample based on cognitive statusa
| Network Properties (Mean) (SD) | HC | MS |
||
|---|---|---|---|---|
| Total Sample | MS-CP | MS-CI | ||
| No. | 18 | 80 | 55 | 25 |
| Size | 6950.17 (700.80) | 6677.81 (589.84) | 6607.64 (575.60) | 6832.20 (603.02) |
| Degree | 1234.59 (129.73) | 1064.42 (124.89)b | 1080.08 (113.24) | 1029.97 (143.86) |
| Density (%) | 17.77 (0.77) | 15.97 (1.59) | 16.37 (1.31) | 15.10 (1.82) |
| Clustering | 0.439 (0.013) | 0.396 (0.034) | 0.405 (0.027) | 0.377 (0.040) |
| Betweenness | 5998.11 (609.99) | 5816.27 (534.55) | 5741.29 (513.53) | 5981.22 (553.17) |
| Path length | 1.863 (0.009) | 1.871 (0.014)b | 1.869 (0.012) | 1.875 (0.016) |
| γ | 1.086 (0.003) | 1.081 (0.005) | 1.081 (0.004) | 1.079 (0.006) |
| λ | 1.021 (0.003) | 1.015 (0.004)b | 1.016 (0.003) | 1.012 (0.004)c |
| Small world | 1.064 (0.003) | 1.065 (0.004) | 1.064 (0.004) | 1.067 (0.005) |
HC versus MS group was statistically compared taking into account sex, education, and global gray matter volume; and the MS-CP versus MS-CI groups were compared with 2 additional confounding factors of T1 and T2 lesion loads (log). Data are corrected for multiple comparisons.
Adjusted P < .05 (comparison of total sample of MS patients with healthy control).
Adjusted P < .01 (comparison within MS group based on cognitive impairment status).
Patients with MS demonstrated increased path length (P = .03) and reduced λ (P = .02) compared with HC. λ (P = .006) was reduced in MS-CI versus MS-CP (Table 2). Lower λ (r = 0.36, P < .0004) and greater path length (r = –0.28, P < .0057) demonstrated a correlation with average cognition when comparing HC with patients with MS. Similarly, MS-CI demonstrated a correlation between reduced λ (r = 0.40, P < .001) and lower average cognition.
Association between Network Parameters and Cognitive Domains
The On-line Table demonstrates the association in patients with MS between global GM network properties and the 5 cognitive domains. Network parameters positively associated with average cognition were density (P = .002), degree (P = .02), clustering (P = .001), and λ (P < .001), while path length (P = .04) was inversely associated. Increased λ was associated with better performance for processing (P < .001), learning (P < .001), and executive domain function (P = .0235), while reduced path length was associated with better executive (P = .0031) and visual domains. Higher clustering was seen in association with executive (P = .0004) and visual (P = .0007) domains. Increased degree and density were seen with better executive and visual (P = .0269 and P = .0008) domains, respectively, whereas size was increased with better executive functioning (P = .0315). Betweenness, γ, and small world parameters were not associated with any domains.
Predictors of Cognitive Impairment
Network parameters were significantly associated with models of cognitive impairment in each domain. The predictive models of cognitive performance that included the network parameters demonstrated a better model fit than the null model in the processing, learning, and visual domains and for average cognition. λ improved prediction for processing (R2 = 43.6%, G2 = 20.9; P < .0001) and learning (R2 = 40.4%, G2 = 26.1; P < .0001). Density improved prediction of impairment in the visual domain (R2 = 27.5%, G2 = 9.2; P = .0024). While degree was significantly associated with executive and language domains, the parameter did not improve the predictive model significantly over the null model. A higher λ improved prediction of the average z score, (G2 = 21.3; P < .0001) and, together with WM volume, explained 52% of average cognition model variance (Table 3).
Table 3:
Predictors of neurocognitive domain scores in total groups of patients with MSa
| Standardized Coefficients β |
Comparing with the Reference Modelb (G2) (P Value) | ||
|---|---|---|---|
| β (95% CI) | Significance | ||
| Processing (R2 = 43.6%) | 20.9 (<.0001) | ||
| NAWM volume | 0.298 (0.047–0.616) | .0229 | |
| λ | 0.548 (0.236–0.895) | .0011 | |
| Learning (R2 = 40.4%) | 26.1 (<.0001) | ||
| Education | 0.205 (0.022–0.388) | .0283 | |
| T1 lesion loads (log) | −0.300 (−0.581 to −0.019) | .0369 | |
| λ | 0.622 (0.332–0.913) | <.0001 | |
| Executive functioning (R2 = 41.0%) | 4.4 (.1108) | ||
| Education | 0.354 (0.170–0.539) | .0003 | |
| NAWM volume | 0.274 (0.020–0.527) | .0348 | |
| Degree | 0.313 (0.095–0.531) | .0055 | |
| Visual (R2 = 27.5%) | 9.2 (.0024) | ||
| Sex (M/F) | 0.293 (0.056–0.530) | .0160 | |
| T2 lesion loads (log) | 0.367 (0.204–0.714) | .0383 | |
| Density (%) | 0.523 (0.236–0.809) | .0005 | |
| Language (R2 = 35.8%) | 7.9 (.0953) | ||
| EDSS (log) | −0.376 (−0.648 to −0.104) | .0074 | |
| Disease duration (log) | 0.224 (0.007–0.441) | .0433 | |
| NAWM volume | 0.318 (0.037–0.600) | .0274 | |
| Degree | −0.254 (−0.486 to −0.021) | .0330 | |
| Average score (R2 = 56.2%) | 21.3 (<.0001) | ||
| Education | 0.305 (0.139–0.471) | .0005 | |
| T1 lesion loads (log) | −0.261 (−0.515 to −0.008) | .0434 | |
| NAWM volume | 0.343 (0.109–0.576) | .0046 | |
| λ | 0.404 (0.12–0.696) | .0073 | |
Backward stepwise elimination multivariable regression was conducted after accounting for sex, education, global gray matter volume, and T1 and T2 lesion loads (log). The Table shows the best regression model for each dependent variable (neurocognitive domain scores).
Reference model included only confounding factors of sex, education, global gray matter volume, and T1 and T2 lesion loads (log). The G2 likelihood ratio test was used to compare the best regression model with the reference model. P < .05 was considered statistically significant.
DISCUSSION
We demonstrate a reduction in λ between HC and patients with MS-CP and MS-CI after correcting for confounding factors, including global GM, sex and education, and lesion load in the MS subgroup. Reduced λ was directly correlated with average cognition and associated with impaired performance in processing, learning, and executive domains. λ improved prediction for performance within the processing domain and accounted for 43.6% of the variance. λ also accounted for 40% of the variance within the learning domain. Path length was increased in patients with MS compared with HC and correlated with reduced executive and visual domain functioning. No associations with cognition were found for betweenness, γ, or small world variables.
Graph theory or network neuroscience is used to represent the matrix of global brain organization and structural or functional connectivity by providing a mathematic framework to model the pair-wise communications between elements of a network. This, in turn, gives insight into how cognitive function is linked to neuronal network structure. Loss of white matter structural integrity and network integration is posited as a significant determinant of cognitive impairment in the “disconnection hypothesis”; however, white matter lesions are characterized by varying degrees of axonal and myelin loss, despite similar macroscopic appearances on MR imaging.22 By controlling for multiple confounding factors, including both T1 and T2 white matter lesion load, our results indicate that network parameters explain variance in cognition beyond that of conventional structural parameters. Normalized path length or λ together with normal-appearing white matter volume added to a model of average z score explained 52% of variance. Rimkus et al13 demonstrated that a lower λ value was associated with worse average cognition, processing, and executive functioning, while Dicks et al23 showed an association between λ reduction and executive functioning.
We extend these findings by showing that λ was also associated with the learning domain and improved predictive models, accounting for a high degree of variance for processing and learning. Reduction in λ is indicative of a more random network and mirrors prior findings in MS, mild cognitive impairment, and Alzheimer disease.13,23,24 Our results reinforce the link between reduced white matter integrity and impairment, particularly in executive functioning and processing speed.25 fMRI studies suggest that executive functioning is dependent on a bilateral brain network that requires efficient pathways connecting the dorsolateral prefrontal, anterior cingulate, and parietal cortices. Similarly, macrostructural white matter abnormality is linked to impairment in processing speed in neurologically healthy elderly subjects from a population-based random sample.26
A prior study showed that a short path length is a characteristic feature of the normal human cortex7 and corresponds to a high global efficiency. Progressive white matter disease burden in MS causes impairment in global efficiency, characterized by node disconnection and an increase in path length, similar to our findings.24,27 Reduced global efficiency is also consistent with prior fMRI studies demonstrating lower functional network integration in patients with MS.28,29 The relationship between white matter damage and global efficiency may support the axonal tension hypothesis, which purports that axonal tension between interrelated cortical areas induces gyration and influences the degree of compactness of neural circuits within the brain.9 The codependence of path length and white matter disease extent may also explain why path length did not appear in predictive modeling of cognitive domains. Our findings are disparate from those of Rimkus et al,13 who observed that a higher path length value could be a consequence of loss of density of connections and reported a shift with cognitive impairment from increased-to-reduced path length after correcting for density. We explored the association between degree and density and all network parameters including path length but revealed a confounding effect only for degree, not density. Degree depends on the number and quality of connections between nodes and thus provides more information than standard structural parameters. In contrast, GM atrophy decreases the volume available for creating nodes, directly affecting graph size and explaining why only degree remained significant after correcting for GM volume.
Prior studies have reported both increased and reduced path lengths with cognitive impairment in MS, mild cognitive impairment, Alzheimer disease, and patients with diabetic retinopathy.6,12,13,23,27,30 Multiple potential explanations exist for this apparent disparity. There is a paucity of data characterizing changes in network parameters with disease duration or disease subtype, especially in MS. Study differences may therefore reflect cohort differences in disease subtype and duration between the 2 studies or indicate a nonlinear path length response with disease. Further clarification of the effect of MS disease subtype and longitudinal network changes is needed. Tewarie et al6 showed a heterogeneous pattern of spatial reconfiguration of interregional cortical thickness in RRMS. While the global structural covariance was unaffected compared with HC, the association between functional connectivity and covariation in cortical thickness showed both higher and lower functional connectivity, dependent on the magnetoencephalography frequency band. Differences were attributed to differential cortical layer responses to frequency and differences in local and global characteristics of the neuronal population. Similarly, functional and diffusion tensor MR imaging studies have demonstrated both increased and reduced cortical activations and connectivity, respectively, in patients with MS-CI compared with HC, thought to reflect network reorganization and cortical plasticity, a known hallmark of early disease.15,21
Limitations of the study include the use of a 3 × 3 voxel size to represent individual nodes. It is uncertain what the optimum cube size is for adequate representation of cortical folding, convolution, and thickness. However, the cube size used in this study is consistent with prior publications. Reproducibility of node selection was not tested but is previously reported in HC.31 While we studied global network parameters and cognition, previous studies have shown correlations between regional (rather than global) parameters and cognitive outcomes for attention and executive functioning domains,15 average cognition, and information processing.6 A regional analysis would be useful to validate prior studies and better define the topographic relationship between graph parameters and cognitive domains. The cross-sectional design of this study cannot address temporal changes in network parameters or evaluate the impact of progressive cognitive or motor deterioration on these parameters. A better understanding of the impact of structural and clinical changes on network parameters is important but can only be addressed with longitudinal studies. Pathologic correlates of measured network parameters are unknown, and further study will be needed to ascertain the utility of these measures as biomarkers of disease assessment and prognostication. However, monitoring of graph parameters may potentially provide the clinician with insight into the structural or functional integrity of the connectome. Used in this way, the parameters could serve as surrogates for monitoring the efficacy of disease control and to detect early changes that may signify a decline in network efficiency that preempts cognitive impairment. The biologic processes underlying the graph theoretical approach are not yet fully understood.5 Further research is needed to confirm that structural similarity is a suitable biomarker of physical connectedness.
CONCLUSIONS
Patients with MS with CI demonstrate more random network features and reduced global efficiency impacting multiple cognitive domains. A model of λ with normal-appearing white matter volume improved average cognitive z score prediction, explaining 52% of variance.
ABBREVIATIONS:
- C
characteristic clustering coefficient
- CI
cognitive impairment
- CP
Cognitively preserved
- γ
normalized clustering coefficient
- HC
healthy controls
- L
characteristic path length
- λ
normalized path length
- NAWM
normal-appearing white matter
- RRMS
relapsing-remitting MS
Footnotes
Biostatistician: Dr Liying Zhang
Dr Aviv was supported by a Physicians Services Incorporated Foundation grant No. 16-40.
Disclosures: Liesly Lee—UNRELATED: Consultancy: Roche Canada Advisory Board, Genzyme Canada Advisory Board; Payment for Lectures Including Service on Speakers Bureaus: Genzyme Canada evening dinner lecture. Richard I. Aviv—RELATED: Grant: Physician Services Incorporated Foundation.* Sarah Morrow—UNRELATED: Board Membership: Consortium of Multiple Sclerosis Centers, Americas Committee for Treatment and Research in Multiple Sclerosis; Consultancy: Biogen, Celgene, EMD Serono, Genzyme, Novartis, Roche, Teva Neuroscience; Grants/Grants Pending: AbbVie, Biogen, EMD Serono, Genzyme, Novartis, Roche*; Payment for Lectures Including Service on Speakers Bureaus: Biogen, Celgene, EMD Serono, Genzyme, Novartis, Roche. *Money paid to the institution.
References
- 1.Kipp M, van der Valk P, Amor S. Pathology of multiple sclerosis. CNS Neurol Disord Drug Targets 2012;11:506–17 10.2174/187152712801661248 [DOI] [PubMed] [Google Scholar]
- 2.Sbardella E, Petsas N, Tona F, et al. . Assessing the correlation between grey and white matter damage with motor and cognitive impairment in multiple sclerosis patients. PLoS One 2013;8:e63250 10.1371/journal.pone.0063250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geurts JJ, Barkhof F. Grey matter pathology in multiple sclerosis. Lancet Neurol 2008;7:841–51 10.1016/S1474-4422(08)70191-1 [DOI] [PubMed] [Google Scholar]
- 4.Bo L, Geurts JJ, van der Valk P, et al. . Lack of correlation between cortical demyelination and white matter pathologic changes in multiple sclerosis. Arch Neurol 2007;64:76–80 10.1001/archneur.64.1.76 [DOI] [PubMed] [Google Scholar]
- 5.Tijms BM, Series P, Willshaw DJ, et al. . Similarity-based extraction of individual networks from gray matter MRI scans. Cereb Cortex 2012;22:1530–41 10.1093/cercor/bhr221 [DOI] [PubMed] [Google Scholar]
- 6.Tewarie P, Steenwijk MD, Tijms BM, et al. . Disruption of structural and functional networks in long-standing multiple sclerosis. Hum Brain Mapp 2014;35:5946–61 10.1002/hbm.22596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He Y, Chen ZJ, Evans AC. Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb Cortex 2007;17:2407–19 10.1093/cercor/bhl149 [DOI] [PubMed] [Google Scholar]
- 8.Watts DJ, Strogatz SH. Collective dynamics of ‘small-world’ networks. Nature 1998;393:440–42 10.1038/30918 [DOI] [PubMed] [Google Scholar]
- 9.Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 1997;385:313–18 10.1038/385313a0 [DOI] [PubMed] [Google Scholar]
- 10.He Y, Chen Z, Gong G, et al. . Neuronal networks in Alzheimer’s disease. Neuroscientist 2009;15:333–50 10.1177/1073858409334423 [DOI] [PubMed] [Google Scholar]
- 11.Tijms BM, Sprooten E, Job D, et al. . Grey matter networks in people at increased familial risk for schizophrenia. Schizophr Res 2015;168:1–8 10.1016/j.schres.2015.08.025 [DOI] [PubMed] [Google Scholar]
- 12.van Duinkerken E, Ijzerman RG, Klein M, et al. . Disrupted subject-specific gray matter network properties and cognitive dysfunction in type 1 diabetes patients with and without proliferative retinopathy. Hum Brain Mapp 2016;37:1194–208 10.1002/hbm.23096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rimkus CM, Schoonheim MM, Steenwijk MD, et al. . Gray matter networks and cognitive impairment in multiple sclerosis. Mult Scler 2018;25:382–91 10.1177/1352458517751650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pagani E, Rocca MA, Gallo A, et al. . Regional brain atrophy evolves differently in patients with multiple sclerosis according to clinical phenotype. AJNR Am J Neuroradiol 2005;26:341–46 [PMC free article] [PubMed] [Google Scholar]
- 15.Fleischer V, Groger A, Koirala N, et al. . Increased structural white and grey matter network connectivity compensates for functional decline in early multiple sclerosis. Mult Scler 2017;23:432–41 10.1177/1352458516651503 [DOI] [PubMed] [Google Scholar]
- 16.Thompson AJ, Banwell BL, Barkhof F, et al. . Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018;17:162–73 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 17.Walker LA, Marino D, Berard JA, et al. . Canadian Normative Data for Minimal Assessment of Cognitive Function in Multiple Sclerosis. Can J Neurol Sci 2017;44:547–55 10.1017/cjn.2017.199 [DOI] [PubMed] [Google Scholar]
- 18.Valverde S, Oliver A, Llado X. A white matter lesion-filling approach to improve brain tissue volume measurements. Neuroimage Clin 2014;6:86–92 10.1016/j.nicl.2014.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 2010;52:1059–69 10.1016/j.neuroimage.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 20.Humphries MD, Gurney K. Network ‘small-world-ness’: a quantitative method for determining canonical network equivalence. PLoS One 2008;3:e0002051 10.1371/journal.pone.0002051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staffen W, Mair A, Zauner H, et al. . Cognitive function and fMRI in patients with multiple sclerosis: evidence for compensatory cortical activation during an attention task. Brain 2002;125:1275–82 10.1093/brain/awf125 [DOI] [PubMed] [Google Scholar]
- 22.Hogan AM, Vargha-Khadem F, Saunders DE, et al. . Impact of frontal white matter lesions on performance monitoring: ERP evidence for cortical disconnection. Brain 2006;129:2177–88 10.1093/brain/awl160 [DOI] [PubMed] [Google Scholar]
- 23.Dicks E, Tijms BM, Ten Kate M, et al. . Gray matter network measures are associated with cognitive decline in mild cognitive impairment. Neurobiol Aging 2018;61:198–206 10.1016/j.neurobiolaging.2017.09.029 [DOI] [PubMed] [Google Scholar]
- 24.Tijms BM, Moller C, Vrenken H, et al. . Single-subject grey matter graphs in Alzheimer’s disease. PLoS One 2013;8:e58921 10.1371/journal.pone.0058921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prins ND, van Dijk EJ, den Heijer T, et al. . Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain 2005;128:2034–41 10.1093/brain/awh553 [DOI] [PubMed] [Google Scholar]
- 26.Ylikoski R, Ylikoski A, Erkinjuntti T, et al. . White matter changes in healthy elderly persons correlate with attention and speed of mental processing. Arch Neurol 1993;50:818–24 10.1001/archneur.1993.00540080029009 [DOI] [PubMed] [Google Scholar]
- 27.He Y, Dagher A, Chen Z, et al. . Impaired small-world efficiency in structural cortical networks in multiple sclerosis associated with white matter lesion load. Brain 2009;132:3366–79 10.1093/brain/awp089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gamboa OL, Tagliazucchi E, von Wegner F, et al. . Working memory performance of early patients with MS correlates inversely with modularity increases in resting state functional connectivity networks. Neuroimage 2014;94:385–95 10.1016/j.neuroimage.2013.12.008 [DOI] [PubMed] [Google Scholar]
- 29.Louapre C, Perlbarg V, Garcia-Lorenzo D, et al. . Brain networks disconnection in early multiple sclerosis cognitive deficits: an anatomofunctional study. Hum Brain Mapp 2014;35:4706–17 10.1002/hbm.22505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo CY, Wang PN, Chou KH, et al. . Diffusion tensor tractography reveals abnormal topological organization in structural cortical networks in Alzheimer’s disease. J Neurosci 2010;30:16876–85 10.1523/JNEUROSCI.4136-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tijms BM, Wink AM, de Haan W, et al. . Alzheimer’s disease: connecting findings from graph theoretical studies of brain networks. Neurobiol Aging 2013;34:2023–36 10.1016/j.neurobiolaging.2013.02.020 [DOI] [PubMed] [Google Scholar]