Abstract
Background
High-grade gliomas are a type of malignant brain tumour. Optimal management often includes maximal surgical resection. 5-aminolevulinic acid hydrochloride (5-ALA) is an imaging agent that makes a high-grade glioma fluoresce under blue light, which can help guide the surgeon when removing the tumour. We conducted a health technology assessment of 5-ALA–guided surgical resection of high-grade gliomas, which included an evaluation of effectiveness, safety, the budget impact of publicly funding 5-ALA, and patient preferences and values.
Methods
We performed a systematic literature search of the clinical evidence to retrieve systematic reviews, and selected and reported results from one review that was recent, of high quality, and relevant to our research question. We complemented the identified systematic review with a literature search to identify randomized controlled trials published after the review. We reported the risk of bias of each included study and the quality of the body of evidence according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria. We also performed a systematic economic literature search to identify economic studies that compared 5-ALA–guided surgical resection of high-grade gliomas with standard surgical care or other intraoperative imaging modalities. We did not conduct a primary economic evaluation due to lack of high-quality published clinical evidence evaluating 5-ALA–guided surgical resection. From the perspective of the Ontario Ministry of Health, we analyzed the 5-year budget impact of publicly funding 5-ALA–guided surgical resection for adults with newly diagnosed, primary, high-grade gliomas for which resection is considered feasible. To contextualize the potential value of 5-ALA, we spoke with someone who had experience with high-grade glioma, 5-ALA–guided resection, and standard surgical treatment.
Results
We included one systematic review reporting on a single randomized controlled trial in the clinical evidence review. 5-ALA increased the proportion of patients achieving complete tumour resection compared with standard care (relative risk of incomplete resection 0.55, 95% confidence interval 0.42–0.71; GRADE: Low). Evidence was uncertain for an effect on overall survival with 5-ALA (hazard ratio for death 0.82, 95% confidence interval 0.62–1.07; GRADE: Low), but there may be an improvement in 6-month progression-free survival (GRADE: Very low). Adverse events between groups was insufficiently reported, but appeared similar between groups for overall and neurological adverse events, with an observed increase in neurological deficits 48 hours after surgery with 5-ALA (GRADE: Very low). The economic literature search identified five studies that met our inclusion criteria because they evaluated the cost-effectiveness of 5-ALA–guided surgical resection as compared with surgery with a standard operating microscope under white light (“white-light microscopy”). Most of these studies found 5-ALA–guided surgical resection was cost-effective compared to white-light microscopy for high-grade gliomas. However, all studies derived clinical model inputs of the comparative safety and effectiveness parameters of 5-ALA from limited and low-quality evidence. Public funding of 5-ALA–guided surgical resection in Ontario over the next 5 years would result in a budget impact of about $930,000 in year 1 to about $1,765,000 in year 5, yielding a total budget impact of about $7,500,000 over this period. The one participant we interviewed had experience with high-grade glioma, standard surgical treatment, and 5-ALA–guided resection. The participant felt that 5-ALA–guided resection resulted in accurate tumour removal and also found it reassuring that 5-ALA could help the surgeon better visualize the tumour.
Conclusions
5-ALA–guided surgical resection appears to improve the extent of resection of high-grade gliomas compared with surgery using standard white-light microscopy (GRADE: Low). The evidence suggests 5-ALA-guided resection may improve overall survival; however, we cannot exclude the possibility of no effect (Grade: Low). 5-ALA may improve 6-month progression-free survival, although the results are highly uncertain (GRADE: Very low). There is an uncertain impact on overall or neurological adverse events (GRADE: Very low). We did not identify any economic studies conducted from the perspective of the Ontario or Canadian public health care payer. Of the studies that met our inclusion criteria, most found 5-ALA–guided surgical resection was cost-effective compared to white-light microscopy for high-grade gliomas. However, clinical model inputs for the comparative effectiveness and safety of 5-ALA were based on limited and low-quality evidence. We estimate that publicly funding 5-ALA–guided surgical resection in Ontario over the next 5 years would result in a total 5-year budget impact of about $7,500,000. For people diagnosed with high-grade gliomas, 5-ALA is seen positively as a useful imaging tool for brain tumour resection.
OBJECTIVE
This health technology assessment evaluates the effectiveness and safety of 5-aminolevulinic acid hydrochloride (5-ALA)-guided surgical resection of high-grade gliomas in adults. It also evaluates the budget impact of publicly funding 5-ALA and the experiences, preferences, and values of a person with high-grade glioma.
BACKGROUND
Health Condition
Glioma is a general term used to define tumours arising from glial cells in the brain. Gliomas are the most common primary brain tumours in adults, accounting for over 25% of all primary brain tumours and 81% of malignant brain tumours.1
Symptoms of gliomas vary based on the subtype, as well as the location and size of the tumour. As a glioma grows, brain tissue is destroyed, and pressure is placed on adjacent tissue, which can result in changes in blood flow, damage to brain cells, and swelling of the brain. Common symptoms can include headaches, nausea, seizures, memory loss, visual changes, and/or changes in behaviour.2,3 Some people present with one-sided weakness, difficulty walking, or difficulties with speech.2
Initial assessment of a suspected glioma begins with contrast-enhanced magnetic resonance imaging (MRI) to evaluate the characteristics of the tumour.4 The definitive diagnosis of glioma and subsequent grading and classification are based on biopsy and histopathological assessment of the tumour. The World Health Organization classifies gliomas into Grades from I through IV based on the histological features of the tumour.5 Grade I gliomas are rare, slow-growing tumours typically seen in children and young adults that demonstrate no evidence of malignancy.6 Grade II gliomas are classified as benign, slow-growing tumours that most often progress to malignant tumours over several years.6,7 Grades III and IV gliomas are classified as “high-grade” tumours that are malignant, highly invasive, and infiltrative of surrounding brain tissue. Among these, the most common subtypes are glioblastoma multiforme (or glioblastoma, Grade IV), anaplastic astrocytoma (Grade III) and anaplastic oligodendroglioma (Grade III).1 Glioblastomas are the most common and most aggressive form of the disease, accounting for approximately 57% of all gliomas1 and 60% to 70% of all high-grade gliomas.8
Most high-grade gliomas are not linked to any specific risk factor and have no clear cause. Several factors that have been associated with an increased risk of glioma include previous exposure to ionizing radiation, a family history of brain tumours, and some rare hereditary diseases.7,9
Clinical Need and Target Population
Incidence and Prevalence
Gliomas are slightly more common in men than in women and occur more frequently in Caucasians compared with other ethnic groups.10
Although a rare tumour in comparison to other cancers, high-grade gliomas carry a significant burden of disease. The incidence of high-grade gliomas is approximately 3 to 5 per 100,000 people, with incidence increasing with age. The median age of onset for glioblastomas is 64 years, peaking between 75 and 85 years.10 Grade III gliomas occur in a younger population than glioblastoma, with a mean age of diagnosis in the fourth or fifth decade of life.11
Prognosis
Despite improvements in both the diagnosis and treatment of high-grade gliomas over time, these tumours are generally considered incurable. In the absence of treatment, the median survival for patients with glioblastoma is between 3 and 4 months.10 With maximal treatment, median survival is between 12 and 15 months for glioblastoma and between 2 and 3 years for Grade III gliomas.10,12 In Ontario, 5-year survival for glioblastoma, anaplastic astrocytoma, and anaplastic oligodendrogliomas are estimated at 4%, 18.2%, and 41.5%, respectively.13 Survival is strongly correlated with age, with substantially reduced survival in those over the age of 65 years.13
Current Treatment Options
Standard Intraoperative Imaging and Tumour Resection
Treatment for high-grade gliomas involves a combination of surgery, external beam radiotherapy and adjuvant chemotherapy. Treatment is not considered curative, but aims to improve patient symptoms, quality of life, and overall survival. Because of their aggressive ability to infiltrate brain tissue, nearly all high-grade gliomas are resistant to treatment and will recur, most often within 2 to 3 cm of the original tumour.14
Whenever possible, the first step in the clinical treatment pathway is surgical resection of the tumour. Resection allows for the pathological diagnosis of the tumour as well as the alleviation of patient symptoms resulting from the effects of the growing tumour mass on surrounding tissue. The extent of tumour resection has also been shown to be an important prognostic factor for high-grade gliomas. Numerous studies have found that when compared to subtotal tumour resection (a portion of the tumour still remains visible on postoperative contrast-enhanced MRI), gross total resection (no obvious tumour visible on postoperative contrast-enhanced MRI) improves both overall and progression-free survival among people with new glioblastoma tumours.15 Although the quality of this evidence remains moderate to low, both Canadian and international guidelines recommend removal of as much tumour as deemed safely possible.4,6,16–18 Evidence of improved survival with greater extent of tumour resection for patients with recurrent high-grade gliomas is available, but limited; however, surgical resection is recommended based on individual circumstances.14
Gross total surgical resection of high-grade gliomas remains challenging to the surgeon due to the infiltrative nature of these tumours and the inability to clearly visualize the tumour at its margins. Surgical resection must balance the goal of maximal tumour resection, while avoiding damage to regions of the brain associated with important neurological functions. Despite best practices, surgeons are estimated to achieve gross total resection in less than 40% of patients with new high-grade gliomas.19 Similarly, resection of recurrent tumours is limited by the difficulty of distinguishing tumour from reactive non-tumour, which occurs as a result of radiation treatment after the primary surgery.20
In Ontario, standard craniotomy and surgical resection rely on white-light microscopy (surgery with a standard operating microscope under white light) in combination with neuronavigation— an intraoperative, computer-assisted navigation system that helps visually guide surgical instruments within the skull based on preoperative MRI imaging data.21,22 Neuronavigation systems are primarily used to identify the site of craniotomy, tumour location, and critical neural structures. However, such systems can be limited by a progressive loss of accuracy during surgery due to “brain shift” (movement of brain tissue as a result of operative head positioning, swelling of the brain, and/or cerebral spinal fluid drainage).23 Brain structures are also altered by both surgical maneuvers and the removal of the tumour itself, further reducing the accuracy of neuronavigation.21 Imaging based on preoperative MRI is also limited when used for recurrent tumours, as it is difficult to distinguish tumour progression from treatment-related changes.20
Health Technology Under Review
5-aminolevulinic acid hydrochloride (5-ALA), is an imaging agent used to assist with intraoperative visualization of malignant gliomas.
5-ALA is a naturally occurring precursor of the heme biosynthesis pathway in human cells.24 When taken orally, 5-ALA selectively accumulates in high amounts within malignant glial cells, where it is converted into a fluorescent metabolite called protoporphyrin IX (PpIX).24 During surgery, PpIX fluorescence can be stimulated by blue light (400–410 nm wavelength), which is accomplished using special filters applied to a standard surgical microscope.25 The neurosurgeon can then visualize the tumour during surgery, with malignant glial cells appearing red or pink, and normal brain tissue appearing blue. It is proposed that this enhanced surgical visualization increases the extent of tumour resection and minimizes the removal of healthy brain tissue, resulting in an improved overall clinical course for patients with high-grade gliomas.
The suggested protocol for 5-ALA administration is an oral dose (20 mg per kilogram body weight) ingested 3 hours prior to anesthesia for surgical resection.25
Diagnostic Test Accuracy
The effectiveness of 5-ALA–guided surgical resection is dependent on its ability to accurately distinguish malignant gliomas from healthy brain tissue. The sensitivity and specificity of 5-ALA for high-grade glioma identification has been assessed in two meta-analyses.26,27 The pooled sensitivity of 5-ALA for high-grade gliomas ranged from 81% to 87% (individual study estimates ranging from 73%–95%) and the pooled specificity ranged from 89% to 90% (individual study estimates ranging from 71%–96%). While there was substantial statistical, clinical, and methodological heterogeneity between studies, there was consensus across these studies that 5-ALA provides greater surgical accuracy than would be observed with standard care alone. This was confirmed by two comparative studies included in the meta-analysis, which found the sensitivity and specificity of neuronavigation alone ranged from 58% to 66% and 57% to 68%, respectively, while the sensitivity and specificity with combined 5-ALA and neuronavigation was substantially higher, at 87% to 91% and 85% to 89%, respectively.27
Although the diagnostic test accuracy of 5-ALA appears promising, the impact of surgical resection guided by 5-ALA fluorescence on patient important clinical outcomes and harms is important to better understand the effectiveness of this intervention.
Safety/Harm
5-ALA is contraindicated in people with hypersensitivity to 5-ALA or porphyrins, acute or chronic types of porphyria, or pregnancy.25 Given a potential risk of phototoxic reactions, phototoxic drugs should not be administered during the perioperative period and exposure to sunlight should be reduced during the postoperative period.25
The ability of 5-ALA to accurately discriminate between cancerous and non-cancerous tissue is of vital importance. Since both false positive–fluorescence and false negative–fluorescence may occur during surgery, potentially resulting in less accurate tumour resection, adverse events associated with neurological outcomes will be assessed as primary outcomes of this review.
The safety of 5-ALA in relation to adverse effects and side effects directly associated with 5-ALA has not been assessed by Health Canada, but it was evaluated by the US Food and Drug Administration (FDA), which subsequently approved the use of 5-ALA in 2017.25 Adverse effects and side effects related to 5-ALA are considered secondary outcomes of interest and will be identified and reported in this health technology assessment.
Regulatory Information
5-ALA is currently not approved by Health Canada for the visualization of high-grade gliomas. According to the manufacturer, 5-ALA for this indication has not been reviewed by Health Canada and an application is currently being developed (Medexus Pharmaceuticals Inc., phone communication, April 4, 2019). However, the drug has been approved for use through Health Canada's Special Access Programme for neurosurgeons that have been formally trained in the use of 5-ALA for high-grade glioma resection. Since the summer of 2018, Health Canada has authorized 13 requests from six practitioners for 5-ALA use across Canada (Health Canada Special Access Programme, email communication, May 7, 2019). A total of 57 vials have been approved for use to date (Health Canada Special Access Programme, email communication, May 7, 2019).
5-ALA for high-grade glioma resection has been approved for use by the European Medicines Agency since 2007 and was subsequently approved for use in Asia and Australia. The US FDA approved 5-ALA as an optical imaging agent for patients with high-grade gliomas in June 2017.25
Ontario, Canadian, and International Context
5-ALA is not publicly funded in Ontario or any other province. Funding for this drug is currently provided through fundraising at some centres, and through hospital global budgets at others. The majority of the 13 requests for 5-ALA through Health Canada's Special Access Programme were from Ontario (Health Canada Special Access Programme, email communication, May 7, 2019).
According to industry consultations, a total of 13 neurosurgeons in Ontario (including international fellows) have received, or are signed up to receive, training in the use of 5-ALA for high-grade glioma resection (Medexus Pharmaceuticals Inc., phone communication, April 4, 2019).
Alternative Intraoperative Imaging Technologies
Several additional technologies are currently available and in use in Ontario to help guide surgical resection of high-grade gliomas. Based on expert opinion, these technologies are not considered standard care in Ontario as they are not widely used across the province (A. Mansouri, MD, phone communication, March 15, 2019; Z. Gelareh, MD, phone communication, April 2, 2019).
Intraoperative MRI involves the use of an MRI at selected times during the surgical procedure.28 The MRI uses a magnetic field and radio waves to create detailed images of the brain and the tumour during surgery.29 This allows surgeons to assess the extent of surgical tumour resection in real-time, and enables surgeons to tailor the resection, as required. Two types of intraoperative MRI devices are available: one that is portable and can be moved into the operating room during the procedure, and one that is not portable, requiring an adjacent diagnostic room.28,29 This technique is often limited by the size, portability, high cost of the device, and the need for extended surgical time.30
Similarly, intraoperative ultrasound devices are available to visualize the extent of tumour resection using ultrasonic wave pulses. An ultrasound probe is directed into the tissue of interest and allows for a three-dimensional representation of the brain.31 The primary disadvantages of intraoperative ultrasound is resolution and operator variability.31
International Guidelines and Funding Recommendations
In 2018, the National Institute for Health and Care Excellence (NICE) in the United Kingdom recommended in favour of the use of 5-ALA for surgical resection of high-grade gliomas, stating: “If a person has a radiologically enhancing suspected high-grade glioma and the multidisciplinary team thinks that surgical resection of all enhancing tumour is possible, offer 5-aminolevulinic acid (5-ALA)–guided resection as an adjunct to maximise resection at initial surgery.”16 In May 2019, the National Health Services stated it will release 5-ALA to all neurological centres in England.32
The Medical Services Advisory Committee in Australia made a recommendation against publicly funding 5-ALA in 2016, stating that they “did not support public funding of fluorescence-guided resection of high-grade glioma that are glioblastoma using oral 5-ALA,” based on the available evidence on clinical effectiveness, safety, and cost-effectiveness.33
Expert Consultation
We engaged with experts in the specialty areas of neurosurgery and neuro-oncology to help inform our understanding of aspects of the health technology and our methodologies and to contextualize the evidence.
CLINICAL EVIDENCE
Research Question
What are the effectiveness and safety of 5-aminolevulinic acid hydrochloride (5-ALA)-guided surgical resection of high-grade gliomas in adults compared with standard surgical care or other intraoperative imaging modalities?
Methods
Review Approach
Numerous recent systematic reviews and health technology assessments have been published evaluating the use of 5-ALA for tumour resection in people with high-grade gliomas. To avoid duplication of prior work and to build upon existing evidence, we systematically searched for, and identified, appropriate systematic reviews that matched our research question with the objective of selecting the best available systematic review. The systematic review selected for final inclusion was based on consideration of the following: alignment to our research question and population; the intervention used; comparators and outcomes; low risk of bias; recency; comprehensiveness of outcomes reported; and relevance of the review. We subsequently performed a systematic literature search for primary studies published after the literature search date of the chosen systematic review to update the body of evidence.
Clinical Literature Search
We performed a clinical literature search on April 10, 2019, using a methodological filter to retrieve systematic reviews, meta-analyses, and health technology assessments published from database inception until the search date. We used the Ovid interface in the following databases: MEDLINE, Embase, the Cochrane Database of Systematic Reviews, the Health Technology Assessment Database, and the National Health Service Economic Evaluation Database (NHS EED).
To update the chosen systematic review, we performed a clinical literature search on April 22, 2019, using our same search strategy with a methodological filter to retrieve randomized controlled trials published from January 1, 2017, until the search date. We used the Ovid interface in the following databases: MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, and NHS EED.
A medical librarian developed the search strategies using controlled vocabulary (e.g., Medical Subject Headings) and relevant keywords. Both final search strategies were peer-reviewed using the PRESS Checklist.34 For both searches, we created database auto-alerts in MEDLINE and Embase, and monitored them for the duration of the assessment period. We also performed a targeted grey literature search of health technology assessment agency websites and systematic review registries. See Appendix 1 for our literature search strategies, including all search terms.
Eligibility Criteria
Study Design—Systematic Reviews
Inclusion Criteria
English-language full-text publications
Studies published from database inception until April 10, 2019
Systematic reviews, meta-analyses, and health technology assessments that included a systematic review
-
Studies that matched our research question and population, intervention, comparator, and outcomes (see Participants, Interventions, Comparators, and Outcome Measures [PICO] below), including the following:
-
○
Systematic reviews with a broader scope than our review, if they included results for our specific question
-
○
Studies that provided information about literature search methods, including (at a minimum) information about the databases searched, search terms, and the search dates
Studies with prespecified eligibility criteria
Exclusion Criteria
Animal and in vitro studies
Nonsystematic reviews, abstracts, editorials, letters, case reports, and commentaries
Study Design—Primary Studies
Inclusion Criteria
English-language full-text publications
Study designs, as specified in the chosen systematic review
Studies evaluating the specific population, intervention, comparator and outcomes outlined for the review (see Participants, Interventions, Comparators, and Outcome Measures below)
Exclusion Criteria
Animal and in vitro studies
Nonsystematic reviews, abstracts, editorials, letters, case reports, and commentaries
Participants
Inclusion Criteria
Adults with suspected high-grade gliomas (defined as World Health Organization Grade III or Grade IV tumours identified on preoperative imaging)
Both new and recurrent tumours
Exclusion Criteria
Low-grade gliomas or studies of mixed high- and low-grade gliomas
Metastatic tumours
Interventions
Inclusion Criteria
5-ALA–guided surgical resection
Exclusion Criteria
5-ALA in combination with other imaging technologies that are not standard intraoperative imaging technologies (as specified in comparators below)
Comparators
-
Standard intraoperative imaging technologies, including the following:
-
○
White-light microscopy
-
○
Neuronavigation
-
○
-
Other intraoperative imaging technologies, including the following:
-
○
Intraoperative magnetic resonance imaging (MRI)
-
○
Intraoperative ultrasound
-
○
The only intraoperative health technologies evaluated were those currently adopted and in use in Ontario for surgical resection of high-grade gliomas, based on expert feedback.
Outcome Measures
Inclusion Criteria
Extent of tumour resection (as determined by postoperative MRI)
Overall survival
Progression-free survival
Quality of life
Neurological function
Adverse events
Exclusion Criteria
Diagnostic accuracy outcomes (sensitivity, specificity, positive predictive value, negative predictive value)
Literature Screening
A single reviewer conducted an initial screening of titles and abstracts using Covidence35 and then obtained the full texts of studies that appeared eligible for review, according to the inclusion criteria. A single reviewer then examined the full-text articles and selected studies eligible for inclusion.
Data Extraction
To identify the best systematic review for inclusion, we extracted relevant data on systematic review study characteristics and risk-of-bias items using a data form to collect information on the following: the review methods (e.g., eligibility criteria [i.e., population, intervention, comparator, outcomes], study types included, literature search information [e.g., date and databases searched]; number of studies; and Risk of Bias in Systematic Reviews [ROBIS] tool assessment items).
To answer the research question, we extracted relevant data on included primary studies (e.g., PICO), patient characteristics, summary estimates, risk of bias assessment items, and quality of the body of evidence assessment based on available data reported directly in the chosen systematic review.
Where further clarification was needed, we referenced and extracted data directly from the primary studies. We contacted systematic review study authors to provide clarification as needed.
Statistical Analysis
Since no primary studies were identified that were published after the selected systematic review, a de novo (novel) synthesis was not performed. All statistical analyses were reported as they were presented in the selected systematic review.
Outcomes were reported separately for each comparator under evaluation.
Critical Appraisal of Evidence
We assessed the risk of bias of systematic reviews using the ROBIS tool.37
In addition, we planned to assess the risk of bias of primary studies included in the selected systematic review directly by using the Cochrane Risk of Bias Tool37 for randomized controlled trials and the Risk of Bias Assessment tool for Non-randomized Studies (RoBANS).38 If the selected systematic review transparently reported on risk of bias of included studies, and used a validated tool, we reported the risk of bias as it was described in the review.
We planned to evaluate the quality of the body of evidence for each outcome according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Handbook.39 The body of evidence is assessed based on the following considerations: risk of bias, inconsistency, indirectness, imprecision, and publication bias. The overall rating reflects our certainty in the evidence. If the selected systematic review transparently evaluated and reported the quality of the body of evidence for each outcome according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Handbook, we reported the GRADE as assessed by the review authors.
Results
Clinical Literature Search for Systematic Reviews
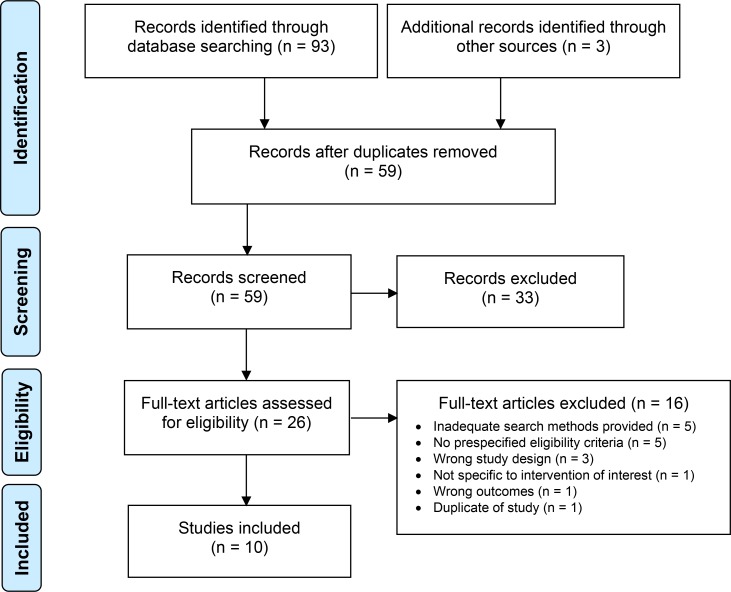
The clinical literature search for systematic reviews yielded 59 citations published from database inception until April 10, 2019, after removing duplicates. We identified 10 studies (nine published systematic reviews27,41–48 and one guideline with a systematic review16) that met our inclusion criteria. See Appendix 2 for a list of selected studies excluded after full-text review. Figure 1 presents the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the clinical literature search of systematic reviews.
Figure 1: PRISMA Flow Diagram—Clinical Search Strategy for Systematic Reviews.
Source: Adapted from Moher et al, 2009.48
Characteristics of Identified Reviews
Systematic Reviews
Ten systematic reviews initially met our eligibility criteria.16,27,41–48 The reviews were published between 2014 and 2018, and all included selection criteria that captured studies evaluating the use of 5-ALA for tumour resection among individuals with high-grade glioma. While all 10 systematic reviews had a broader scope than our review, they included studies meeting our specific selection criteria. After careful analysis of all 10 reviews, only one was chosen for our review. Details about the reviews’ characteristics and designs are provided in Appendix 3, and the ROBIS risk of bias assessment is provided in Appendix 4.
Of the nine reviews excluded, one was omitted because it did not report results quantitatively,42 and three others were excluded because they did not clearly subgroup or report results specific to our PICO limiting to: high-grade gliomas,46 5-ALA,47 or comparative studies.43 Three additional reviews were excluded due to high risk of bias, with one or more of the following characteristics: unclear study selection criteria; no risk of bias assessment for individual studies; limited synthesis of data or inappropriate synthesis of data.27,44,46 The remaining three systematic reviews published met our study selection criteria and had a low risk of bias.16,41,45 However, the review by Barone et al41 had an outdated literature search and was therefore not selected.
The remaining two reviews45 were published in 2018 and evaluated high-grade and low-grade glioma populations—a population broader than our inclusion criteria, but included subgroup or sensitivity analyses for high-grade gliomas only. Both reviews included a validated risk of bias assessment as well as an assessment of the quality of the body of evidence for each outcome using GRADE. Both reviews limited their inclusion to randomized controlled trials and identified the same study for inclusion. However, we ultimately selected the Cochrane review by Jenkinson et al45 for our analysis because it included a search of recurrent high-grade gliomas and provided more detailed information regarding included study designs, outcomes, and risk of bias assessment. Inclusion criteria characteristics for the selected systematic review are provided in Table 1.
Table 1:
Inclusion Criteria for Selected Cochrane Systematic Review (Jenkinson et al)45
| Author, Year Literature Search End Date | Population | Intervention | Comparators | Outcomes | Study Types Included |
|---|---|---|---|---|---|
| Jenkinson et al, 201845 June 2017 |
|
Fluorescence-guided surgery (including 5-ALA)a |
|
|
RCTs |
Abbreviations: 5-ALA, 5-aminolevulinic acid hydrochloride; MRI, magnetic resonance imaging; RCT, randomized controlled trials.
The systematic review evaluated all interventions and comparators to one another.
Clinical Literature Search for Primary Studies
The clinical literature search for primary studies was based on the study design (limiting to randomized controlled trials) and end date (June 2017) of the literature search used by the Cochrane review by Jenkinson et al.45 We started our literature search from January 1, 2017, which allowed us to identify randomized controlled trials that may have been published prior to June 2017, but were added to the database after the search was performed by Jenkinson et al.
The literature search yielded 32 citations published between January 1, 2017, and April 22, 2019, after removing duplicates. We identified no studies that met our inclusion criteria. Figure 2 presents the PRISMA flow diagram for the clinical literature search of primary studies.
Figure 2: PRISMA Flow Diagram—Clinical Search Strategy for Randomized Controlled Trials.
Source: Adapted from Moher et al, 2009.48
Primary Studies
No additional randomized controlled trials were identified from the search for primary studies published after the selected systematic review by Jenkinson et al.45
Results from Selected Systematic Review
Since no additional randomized controlled trials were identified, a de novo synthesis was not required. We report results as they are presented in the Jenkinson et al45 systematic review because they transparently evaluated risk of bias using the Cochrane Risk of Bias tool and assessed the quality of the body of evidence using GRADE.
The systematic review by Jenkinson et al45 identified one randomized controlled trial (with results published in four separate publications) that met their study selection criteria for the comparison of 5-ALA–guided resection to resection guided by standard or other intraoperative imaging devices. The included randomized controlled trial was published by Stummer et al19 in 2006, and included 322 adult patients with newly diagnosed and untreated malignant gliomas that were eligible for complete resection due to tumour location. The included study compared 5-ALA–guided resection to conventional surgery with white-light microscopy, and both arms of the study could include neuronavigation.45
No studies meeting the selection criteria by Jenkinson et al45 were identified that evaluated the comparison between 5-ALA and intraoperative MRI or intraoperative ultrasound. In addition, no studies were identified that evaluated the use of 5-ALA in people with recurrent gliomas.
Risk of Primary Study Bias Assessment From Selected Systematic Review
Jenkinson et al45 evaluated the risk of bias of the included randomized controlled trial using the Cochrane Risk of Bias tool.37 The authors assessed the included randomized controlled trial by Stummer et al as having a low risk of bias for random sequence generation, allocation concealment, and blinding of neuropathology and neuroradiology outcome assessors. The authors judged a high risk of bias for all outcomes because there was no blinding of study participants, surgeons, and those involved in the treatment of participants. The study was further judged by the review authors as having a high risk of bias due to incomplete reporting of outcome data; particularly for the exclusion of 47 of 322 randomized participants from both the intention to treat and per protocol analyses due to violations of MRI inclusion criteria or not meeting histological criteria for the study after surgery was completed.45 Issues with selective outcome reporting for adverse events, progression-free survival, and survival were also noted by Jenkinson et al,45 in addition to potential bias related to industry involvement.
Extent of Tumour Resection
Jenkinson et al45 reported an increase in complete resection of tumour (residual tumour defined as a volume greater than 0.175 cm3, as measured by postoperative MRI) with 5-ALA–guided resection compared with the control group, increasing from 35% to 65%, respectively. This corresponded to a relative risk of incomplete resection of 0.55 (95% confidence interval [CI] 0.42–0.71; Table 2), in favour of 5-ALA-guided resection. The GRADE for this body of evidence was assessed by the review authors as low. The evidence was downgraded two levels due to multiple issues related to the risk of bias assessment, as discussed above.
Table 2:
Extent of Tumour Resection With 5-ALA in Comparison to Standard Care
| Complete Tumour Resection | ||||
|---|---|---|---|---|
| Systematic Review Author, Year | Number of Included Studies | 5-ALA (%, n/N) | Standard Care (%, n/N) | RR Incomplete Resection (95% CI) |
| Jenkinson et al, 201845 | 1 | 65% (90/139) | 36% (47/131) | 0.55 (0.42–0.71) |
Abbreviations: 5-ALA, 5-aminolevulinic acid hydrochloride; CI, confidence interval; RR, relative risk.
Overall Survival
Jenkinson et al45 reported no clear difference in overall survival with 5-ALA–guided resection when compared with standard care (hazard ratio for death 0.82, 95% CI 0.62–1.07; Table 3). The review authors assessed the GRADE for this body of evidence as low due to several limitations related to risk of bias.
Table 3:
Overall Survival With 5-ALA in Comparison to Standard Care
| Overall Survival | ||||
|---|---|---|---|---|
| Systematic Review Author, Year | Number of Included Studies | 5-ALA Median (Months) (95% CI) | Standard Care Median (Months) (95% CI) | Hazard Ratio (95% CI) |
| Jenkinson et al, 201845 | 1 | 15.2 (12.9-17.5) | 13.5 (12.0-14.7) | 0.82 (0.62-1.07) |
Abbreviations: 5-ALA, 5-aminolevulinic acid hydrochloride; CI, confidence interval.
Progression-Free Survival
The systematic review by Jenkinson et al45 identified one randomized controlled trial19 that evaluated the outcome of progression-free survival (with progression defined by the included study as a new tumour lesion or an increase in residual tumour volume of greater than 25%). The review authors stated that the format of results prespecified for this outcome (hazard ratios and their confidences intervals) were not available and could not be calculated from the information reported in the randomized controlled trial. The review did report a small improvement in the median progression-free survival with 5-ALA relative to standard care (Table 4), although statistical analysis was not performed. The review authors assessed the GRADE for this body of evidence as very low because the outcome was not adequately reported by the included trial.
Table 4:
Progression-Free Survival With 5-ALA in Comparison to Standard Care
| Progression-Free Survival | ||||
|---|---|---|---|---|
| Systematic Review Author, Year | Number of Included Studies | 5-ALA Median (Months) (95% CI) | Standard Care Median (Months) (95% CI) | Hazard Ratio (95% CI) |
| Jenkinson et al, 201845 | 1 | 5.1 (3.4-6.0) | 3.6 (3.2-4.4) | Not available and could not be calculated |
Abbreviations: 5-ALA, 5-aminolevulinic acid hydrochloride; CI, confidence interval.
Since Jenkinson et al stated that outcomes for progression-free survival did not meet their prespecified format for reporting, we extracted data for this outcome directly from Stummer et al19 to capture their reported primary outcome of interest. Stummer et al reported an improved 6-month progression-free survival for patients assigned 5-ALA in comparison to the white-light microscopy group (41% [95% CI 32.8–49.2] vs. 21.1% [95% CI 14.0-28.2], respectively; P= 0.003).19
Adverse Events
Jenkinson et al45 found limited and inconsistent reporting of adverse events in the trial assessing 5-ALA. Since no denominators were provided for each result, the review authors could not calculate the relative risk and associated confidence intervals surrounding the estimates. No information regarding the timing of events or the number of individuals with more than one event were available. Overall, they identified no major differences between the two groups for overall or neurological adverse events (Table 5). The authors reported greater deterioration in the National Institutes of Health (NIH) Stroke Scale compared with baseline values at 48 hours for 5-ALA-guided resection (26.2%) compared with control (14.5%), with no major differences in this measure at subsequent follow-up. Since no statistical analyses were reported, the variation between groups is unclear. Although not stated as statistically significant by review authors, NIH Stroke Scale scores were higher in the 5-ALA group until the 6-week follow-up, with no observed difference at 3 months. The GRADE for this body of evidence was assessed as very low by the review authors45 because adverse events were inadequately and inconsistently reported in the trial.
Table 5:
Adverse Events with 5-ALA in Comparison to Standard Care
| Systematic Review Author, Year | Number of Included Studies | Adverse Outcome | 5-ALA (%) | Standard Care (%) |
|---|---|---|---|---|
| Jenkinson et al, 201845 | 1 | Overall adverse events | 58.7 | 57.8 |
| Neurological adverse events | 42.8 | 44.5 | ||
| Grades III and IVa | 7 | 5.2 | ||
| Significant neurological adverse eventsb | 12.4 | 11.6 | ||
| Deterioration in NIH Stroke Scale compared to baseline—48 hours | 26.2 | 14.5 | ||
| Deterioration in NIH Stroke Scale compared to baseline—7 days | 20.5 | 10.7 | ||
| Deterioration in NIH Stroke Scale compared to baseline—6 weeks | 17.1 | 11.3 | ||
| Deterioration in NIH Stroke Scale compared to baseline—3 months | 19.6 | 18.6 |
Abbreviations: 5-ALA, 5-aminolevulinic acid hydrochloride; NIH, National Institutes of Health.
According to the Common Toxicity Criteria (data obtained from the primary Stummer et al article for further clarification).50
Defined as aphasia, convulsions, grand mal seizure, hemiparesis, intracranial hypertension and stupor (data obtained from the primary Stummer et al50 article for further clarification)
Quality of Life
Jenkinson et al45 did not find any studies evaluating the outcome of quality of life associated with 5-ALA compared with standard care or other comparators.
Discussion
Maximal safe surgical resection is the standard treatment for individuals with high-grade gliomas. We systematically selected and reported on the best available systematic review with a low risk of bias, which evaluated the use of 5-ALA–guided surgical resection of glioma compared with standard surgical imaging or other intraoperative imaging modalities.
The systematic review we selected identified a single randomized controlled trial comparing 5-ALA–guided surgical resection with surgical resection using standard white-light microscopy. 5-ALA–guided surgery resulted in greater complete tumour resection when compared with standard white-light microscopy, although this was based on low-quality evidence. There was a potential improvement in overall survival; however, the confidence interval included the possibility of no difference in overall survival. Progression-free survival was not reported by the included randomized controlled trial in the format prespecified by the selected systematic review, and therefore was not reported by that review. However, we noted that the included randomized controlled trial found an increase in 6-month progression-free survival among individuals in the 5-ALA cohort relative to control, based on very low–quality evidence. No data on the impact of 5-ALA on quality of life were identified.
The primary goal of maximal surgical glioma resection guided by 5-ALA is to improve the survival of individuals with this disease. While the extent of resection has been suggested to have an important role in improving prognosis in high-grade gliomas,15 and is the outcome most directly associated with the impact of 5-ALA, it remains a surrogate outcome for patient survival. The impact of 5-ALA on overall survival was not clear, and the randomized controlled trial by Stummer et al19 was not designed or powered to detect a difference in this outcome. Progression-free survival is often used as a reasonable surrogate for overall survival; however, this measure is limited by assessor subjectivity, timing of assessment, and debate over the relationship between progression-free-survival and overall survival in glioblastoma.51,52 Additionally, the shorter time-to-event benefit of progression-free survival is arguably less justified in this context given the short overall survival period of individuals with high-grade gliomas. However, differences in progression-free survival and overall survival may be more difficult to demonstrate relative to complete resection in this study as there was no standardization of treatment provided after surgical resection of glioma, or after subsequent tumour recurrence, which may have a greater impact on survival than 5-ALA–guided surgical resection.
The observed benefit of increased tumour resection must be balanced with the potential risk of impact on neurological function. No differences in overall adverse events and serious neurological adverse events were observed between groups. Although there was a trend of increased neurological deficits based on the NIH Stroke Scale in the 5-ALA group in the early postoperative period (48 hours, 7 days, and 6 weeks), these differences were not observed between groups at 3 months. Data on adverse events and neurological outcomes were extremely limited, and therefore substantial uncertainty remains. The aim of 5-ALA–guided surgery is to maximize the extent of resection; however, this tool will not necessarily determine whether maximal resection will cause a neurological deficit. Neurosurgeon clinical judgment and additional techniques to understand critical neural structures are still required with the use of this technology to help minimize the impact of related adverse neurological deficits.
Strengths and Limitations
This clinical evidence review avoided duplication of prior work and leveraged knowledge of existing systematic reviews by selecting and reporting on the one highest quality review with a low risk of bias that answered our specific research question. Because we relied on results from one systematic review, it is possible that relevant studies or data were missed or not reported. There may also be potential variations in the interpretation of the evidence by review authors. Additionally, the chosen review was limited to randomized controlled trials, and we were therefore not able to capture published observational studies. Despite these potential limitations, we remain confident in the results presented. All other identified systematic reviews and health technology assessments included the same single randomized controlled trial reported,16,27,42,44,46–48 and we performed an update of the literature to capture any additional randomized controlled trials that have since been published. Further, conclusions of other systematic reviews with similar selection criteria were in line with our conclusions, with an emphasis on results from the randomized controlled trial by Stummer et al,19 regardless of the study designs included.16,46 The systematic review performed by the National Institute for Health and Care Excellence (NICE) had the same final GRADE quality of evidence assessment rating for each outcome reported in the Cochrane review.
Only results for the clinical utility and safety of the technology were evaluated as outcomes of our clinical review. A large proportion of the evidence on 5-ALA reports on diagnostic test accuracy, which are surrogate outcomes (indirect outcomes) for patient-important outcomes. The ability of the intervention to appropriately distinguish between “true tumour cells” and healthy brain tissue is reflected in outcomes related to clinical effectiveness, adverse events, and neurological function.
Generalizability to Ontario
Several issues related to the generalizability of results to Ontario need to be considered. As reported by Jenkinson et al,45 the included randomized controlled trial selected patients who were younger and had higher performance status than would be expected in a general population. The randomized controlled trial further excluded any patients for whom tumour location did not enable complete resection, and therefore would likely observe greater success rates and fewer adverse events related to tumours within eloquent regions of the brain. Additionally, most patients included in the randomized controlled trial had glioblastoma (88% in each group). While this is reflective of the general population with high-grade glioma in Ontario, it remains unclear if results of 5-ALA-guided surgical resection of Grade III gliomas would be as robust as those with Grade IV gliomas. The majority of studies evaluating the uptake of 5-ALA in high-grade glioma cells have focused on glioblastoma; however, some studies have suggested similar uptake and diagnostic accuracy among Grade III gliomas, while others have shown lower accuracy among this patient population.24,46
Similarly, no studies were identified that evaluated the use of 5-ALA compared with standard care for people with recurrent gliomas. Diagnostic accuracy studies in recurrent gliomas suggest lower sensitivity and specificity of 5-ALA relative to new tumours, and therefore the impact on clinical outcomes could vary as well.
Further, treatment protocols used in the included randomized controlled trial are based on data from 2006 and may differ from current standard treatment practice. In particular, the study was conducted before postoperative temozolomide chemotherapy became standard care for high-grade glioma treatment in 2005,4 and therefore may not reflect survival outcomes that would be observed for patients today.
Lastly, while the level of training of surgeons involved in the included randomized controlled trial was not reported by Jenkinson et al,45 they stated that centres were reported as being highly specialized. It is therefore possible that a learning curve could be observed with the use of 5-ALA-surgical resection and may be dependent on surgeon experience.
Conclusions
Based on results from a single randomized controlled trial, 5-ALA-guided surgical resection appears to improve the extent of resection of high-grade gliomas when compared with surgery using standard white-light microscopy (GRADE: Low). The evidence suggests 5-ALA-guided resection may improve overall survival; however, we cannot exclude the possibility of no effect (GRADE: Low). 5-ALA-guided resection may improve 6-month progression-free survival, although results are highly uncertain (GRADE: Very low). There is an uncertain impact on overall or neurological adverse events (GRADE: Very low).
ECONOMIC EVIDENCE
Research Question
What is the cost-effectiveness of 5-aminolevulinic acid hydrochloride (5-ALA)–guided surgical resection of high-grade gliomas in adults compared with standard surgical care or other intraoperative imaging modalities?
Methods
Economic Literature Search
We performed an economic literature search on April 11, 2019, to retrieve studies published from database inception until the search date. To retrieve relevant studies, we developed a search using the clinical search strategy with an economic and costing filter applied. We used the Ovid interface in the following databases: MEDLINE, Embase, the Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials, the Health Technology Assessment Database, and the National Health Service Economic Evaluation Database (NHS EED).
We created database auto-alerts in MEDLINE and Embase, and monitored them for the duration of the assessment period. We also performed a targeted grey literature search of health technology assessment agency websites, systematic review registries, and the Tufts Cost-Effectiveness Analysis Registry. See the Clinical Literature Search section, above, for further details on methods used. See Appendix 1 for our literature search strategies, including all search terms.
Eligibility Criteria
Studies
Inclusion Criteria
English-language full-text publications
Studies published from database inception until search date
Studies in adults with high-grade (World Health Organization [WHO] Grade III and Grade IV)53 gliomas (e.g., anaplastic astrocytoma, anaplastic oligodendroglioma, and glioblastoma or glioblastoma multiforme)
Studies comparing 5-ALA–guided surgical resection to standard surgical care, including surgery using standard white-light operating microscope (“white-light microscopy”), neuronavigation, intraoperative ultrasound, intraoperative magnetic resonance imaging (MRI), or other intraoperative imaging technologies
Cost-utility analyses, cost-effectiveness analysis, cost-benefit analyses, and cost minimization analyses
Exclusion Criteria
Narrative reviews, editorials, case reports, commentaries, or conference abstracts
Systematic reviews
Cost of illness studies, feasibility and implementation studies
Population
Adults with high-grade (WHO Grade III and Grade IV)53 gliomas (e.g., anaplastic astrocytoma, anaplastic oligodendroglioma, and glioblastoma or glioblastoma multiforme)
Interventions and Comparators
5-ALA–guided surgical resection
Surgery with a standard operating microscope under white light (“white-light microscopy”) only or together with any intraoperative technologies (e.g., neuronavigation, intraoperative ultrasound, intraoperative magnetic resonance imaging)
Outcome Measures
Costs, health outcomes (e.g., quality-adjusted life-years)
Incremental costs, incremental effectiveness
Incremental cost-effectiveness ratios
Literature Screening
A single reviewer conducted an initial screening of titles and abstracts using Covidence and then obtained the full texts of studies that appeared eligible for review according to the inclusion criteria. A single reviewer then examined the full-text articles and selected studies eligible for inclusion.
Data Extraction
We extracted relevant data on study characteristics and outcomes to collect information about the following:
Source (e.g., citation information, study type)
Methods (e.g., study design, analytic technique, perspective, time horizon, population, intervention[s], comparator[s])
Outcomes (e.g., health outcomes, costs, incremental cost-effectiveness ratios)
Study Applicability and Limitations
We determined the usefulness of each identified study for decision-making by applying a modified quality appraisal checklist for economic evaluations originally developed by the National Institute for Health and Care Excellence (NICE) in the United Kingdom to inform the development of NICE's clinical guidelines.53 We modified the wording of the questions to remove references to guidelines and to make it specific to Ontario. Next, we separated the checklist into two sections. In the first section, we assessed the applicability of each study to the research question (directly, partially, or not applicable). In the second section, we assessed the limitations (minor, potentially serious, or very serious) of the studies that we found to be directly applicable.
Results
Economic Literature Search
The economic literature search yielded 50 citations published from database inception until April 11, 2019, after removing duplicates. We identified five studies that met our inclusion criteria. Figure 3 presents the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the economic literature search.
Figure 3: PRISMA Flow Diagram—Economic Search Strategy.
Source: Adapted from Moher et al, 2009.48
Overview of Included Economic Studies
We identified five cost-utility analyses studies16,55–58 that evaluated 5-ALA–guided surgical resection for high-grade gliomas. The economic review results are summarized in Table 6.
Table 6:
Summary Results of Economic Literature Review
| Author, Year, Country of Publication | Analytic Technique, Study Design, Perspective, Time Horizon, Discount Rate, Currency | Population | Intervention(s) and Comparator(s) | Results | ||
|---|---|---|---|---|---|---|
| Health Outcomes | Costs | Cost-Effectiveness | ||||
| NICE, 2018, United Kingdom16 |
Type of analysis: Cost-utility analysis Study design: Partitioned survival analysis approach Perspective: NHS and PSS Time horizon: 5 years Discount rate: 3.5% (health outcomes and costs) Currency and cost year: £ (GBP), 2016 |
Total N/Age/Male (%): NR Other: Adults with WHO Grade IV glioma KPS > 70 No previous surgical treatments for tumour Suitable for surgery Tumour not located in midline, basal ganglia, cerebellum, or brain stem |
Intervention: 5-ALA Comparator: White-light |
Mean per person: 5-ALA: 1.2903 QALYs White-light: 1.1504 QALYs Mean difference: 5-ALA vs. white-light: 0.14 QALYs |
Mean per person: 5-ALA: £3,131.00 GBP White-light: £1,874.00 GBP Mean difference: 5-ALA vs. white-light £1,257.00 GBP |
Reference case: 5-ALA vs. white-light: £8,991.00 GBP/QALY gained Deterministic sensitivity analysis: Results remained robust Probabilistic sensitivity analysis: Probability of 5-ALA being cost-effective is 84% at a willingness to pay of £20,000.00 GBP/QALY |
| MSAC, 2016, Australia33 |
Type of analysis: Cost-utility analysis Cost-effectiveness analysis (LY, DFS) Study design: Markov model Perspective: Australian health care system Time horizon: 5 years Discount rate: 5% (health outcomes and costs) Currency and cost year: AUD, NR |
Total N/Age/Male (%): NR Other: Adults with WHO Grade IV glioma that are GBM |
Intervention: 5-ALA Comparator: White-light |
Mean per person: 5-ALA: redacted White-light: redacted Mean difference: 5-ALA vs. white-light: redacted |
Mean per person: 5-ALA: redacted White-light: redacted Mean difference: 5-ALA vs. white-light: redacted |
Reference case: 5-ALA vs. white-light: $56,836.00 AUD/QALY gained $41,233.00 AUD/LY gained $53,613.00 AUD/DFS gained Deterministic sensitivity analysis: NR Probabilistic sensitivity analysis: NR |
| Elijamel et al, 2016, NR57 |
Type of analysis: Cost-utility analysis Cost-effectiveness analysis (GTR) Study design: Meta-analyses techniques Perspective/time horizon/discount rate: NR Currency and cost year: USD, NR |
Total N: 919 study participants (across 15 included studies) Age/Male (%): NR Other: Adults with diagnosed high-grade glioma (WHO Grade III/IV) |
Interventions: 5-ALA Fluorescein iUS iMRI Comparator: White-light |
Mean per person: 5-ALA: NR White-light: NR Mean difference: 5-ALA vs. white-light: 0.11 QALY |
Mean per person: 5-ALA: $1,407.00 USD White-light: NR Mean difference: 5-ALA vs. white-light $1,784.00 USD |
Reference case: 5-ALA vs. white-light: $16,218.18 USD/QALY gained Deterministic sensitivity analysis: Not conducted Probabilistic sensitivity analysis: Not conducted |
| Esteves et al, 2015, Portugal55 |
Type of analysis: Cost-utility analysis Cost-effectiveness analysis (PFLY, LY) Study design: Markov model Perspective: Portuguese National Health Services Time horizon: 7.9 years Discount rate: 5% (health outcomes and costs) Currency and cost year: € (EUR), 2012 |
Total N/Age/Male(%): NR Other: Adults with newly diagnosed high-grade gliomas (WHO Grade III/IV) |
Intervention: 5-ALA Comparator: White-light |
Mean per person: 5-ALA: 1.36 QALYs 1.90 LYs 1.10 PFLYs White-light: 1.20 QALYs 1.68 LYs 0.93 PFLYs Mean difference: 5-ALA vs. white-light: 0.16 QALY 0.22 LY 0.17 PFLY |
Mean per person: 5-ALA: €22,516.61 EUR White-light: €21,028.64 EUR Mean difference: 5-ALA vs. white-light: €1,487.97 EUR |
Reference case: 5-ALA vs. white-light: €9,097.47 EUR/QALY gained €6,675.52 EUR/LY gained €8,780.84 EUR/PFLY gained Deterministic sensitivity analysis: Results remained robust ICERs remained < €14,000.00 EUR/QALY gained in all variations tested Probabilistic sensitivity analysis: ICERs ranged between €8,282.90 EUR/QALY gained and €21,000 EUR/QALY gained in 95% of cases |
| Slof et al, 2015, Spain56 |
Type of analysis: Cost-utility analysis Cost-effectiveness analysis (CR) Study design/Time horizon: NR Perspective: Spanish health care system Discount rate: 0% Currency and cost year: € (EUR), 2012 |
Total N: 5-ALA: 131 (8 Grade III; 123 Grade IV) White-light: 120 (13 Grade III; 105 Grade IV) Age/Male(%): NR Other: Adults with high-grade gliomas (WHO Grade III/IV) Based on VISIONA60 study database |
Intervention: 5-ALA Comparator: White-light |
Mean per person: 5-ALA: NR White-light: NR Mean difference: 5-ALA vs. white-light: 0.11 QALY |
Mean per person: 5-ALA: NR White-light: NR Mean difference: 5-ALA vs. white-light: €1,010.00 EUR |
Reference case: 5-ALA vs. white-light: €9,021.00 EUR/QALY gained €4,550.00 EUR/additional CR achieved Deterministic sensitivity analysis: Results remained robust Probabilistic sensitivity analysis: Not conducted |
Abbreviations: 5-ALA, 5-aminolevulinic acid hydrochloride; AUD, Australian Dollar; CR, complete resection; DFS, disease-free survival (year gained free of progression); GBM, glioblastoma multiforme; GTR, gross total resection; ICERs, incremental cost-effectiveness ratios; iMRI, intraoperative magnetic resonance imaging; iUS, intraoperative ultrasound; KPS, Karnosfsky performance status; LY, life-year; MSAC, Medical Services Advisory Committee; N, number; NHS, National Health Services; NICE, The National Institute for Health and Care Excellence; NR, not reported; PFLYs, progression-free life-year; PSS, Personal Social Services; QALY, quality-adjusted life-year; WHO, World Health Organization.
No studies were identified that evaluated the comparison between 5-ALA–guided surgical resection and the use of alternative intraoperative technologies, including neuronavigation, intraoperative ultrasound, or intraoperative MRI. All studies that met our inclusion criteria compared 5-ALA–guided surgical resection with surgery using standard white-light operating microscope (“white-light microscopy”) only.
Four studies (Esteves et al,55 Slof et al,56 NICE health technology assessment [HTA],16 and Medical Services Advisory Committee [MSAC] HTA33) were conducted from the perspective of the public health payer in Portugal, Spain, the United Kingdom, and Australia, and one study (Eljamel et al)57 did not report the study perspective.
The majority of studies16,55–57 found that 5-ALA–guided surgical resection was cost-effective as compared to white-light microscopy, and reported incremental cost-effectiveness ratios (ICERs).16 The remaining study was an HTA submission to the MSAC in Australia made by Specialised Therapeutics Australia Pty Ltd (“the applicant”) requesting Medicare Benefits Schedule listing (i.e., public funding) for fluorescence-guided resection of high-grade glioma that are glioblastoma multiforme using oral 5-ALA.33 In the applicant's economic model provided to MSAC, 5-ALA–guided surgical resection compared with white-light microscopy had an ICER of $53,613 AUD per quality-adjusted life-year (QALY) gained. The MSAC recommended against public funding of 5-ALA, citing the following considerations in its funding decision: lack of unbiased evidence supporting comparative safety and effectiveness of 5-ALA; lack of evidence demonstrating improvements in overall survival; and the high unit cost of 5-ALA without justification (at $3,990 AUD [cost year not reported] per vial33).
Applicability and Limitations of the Included Studies
Appendix 5 (Tables A4 and A5) provides the results of the quality appraisal checklist for economic evaluations applied to the included studies. Four studies (Esteves et al,55 Slof et al,56 NICE HTA,16 and MSAC HTA33) were deemed only partially applicable to our research question, as they had key attributes (e.g., study perspective) of the cost-effectiveness analysis that differ from the Ontario setting. In addition, three (Esteves et al,55 NICE HTA,16 and MSAC HTA33) of the four studies had focused their target population on Grade IV gliomas only. While the fourth study (Slof et al)56 included all malignant gliomas, Grade III gliomas were largely underrepresented. The remaining study (Eljamel et al)57 was deemed not applicable to our research question because a number of key attributes (e.g., perspective, discount rate) of its cost-effectiveness analysis were not clearly stated. Moreover, its reference case results were produced using meta-analysis techniques, rather than decision-analysis modelling. For these reasons, there was limited information to determine whether the health care system studied is sufficiently similar to Ontario.
We assessed the limitations of the included studies and found that four studies (Esteves et al,55 Slof et al,56 NICE HTA,16 and MSAC HTA33) had potentially serious limitations and one study (Eljamel et al57) had very serious limitations. Most studies (Esteves et al,55 Slof et al,56 Eljamel et al,57 and NICE HTA16) shared the key assumption that 5-ALA–guided surgical resection would take place at the initial surgery only, at one vial per person, regardless of body weight. Moreover, most studies also did not account for the cost of the fluorescence module in their reference case. Only two studies (Slof et al56 and NICE HTA16,56) explored the impact of module costs on the cost-effectiveness of 5-ALA–guided surgical resection in scenario analyses. Both studies found that reference case results remained robust when assuming the highest price of module at €45,000 EUR (reported in 2012) over an 8-year depreciation period at a minimum of 20 procedures per year56 or at £47,392 GBP (reported in 2016) over an 8-year depreciation period at a minimum of five procedures per year.16 The remaining study (Eljamel et al57) did not explicitly report its model parameter input values for costs, or the methods used for identifying the prices of relevant resources.
Lastly, except for the NICE HTA,16 other studies did not explore all uncertain parameter values that would be appropriate in a sensitivity analysis (e.g., unit cost of 5-ALA, proportion of patients requiring an additional vial, and cost of module), and had either declared author affiliation or financial support from the manufacturer or distributor of 5-ALA, or did not indicate whether or not there was a potential conflict of interest.
Discussion
Four studies (Esteves et al,55 Slof et al,56 Eljamel et al,57 and NICE HTA16) found 5-ALA–guided surgical resection as compared with white-light microscopy was cost-effective at ICERs that fell below the cost per QALY gained for which interventions are considered cost-effective by NICE (i.e., £20,000 GBP per QALY gained). Of these four studies, one was a NICE HTA,16 which recommended public funding of the 5-ALA as an adjunct to maximize resection at the initial surgery for newly diagnosed, primary high-grade gliomas.16 The remaining study33 was an MSAC HTA, and in contrast, did not support public funding for 5-ALA–guided surgical resection for high-grade gliomas that are glioblastoma or glioblastoma multiforme (e.g., Grade IV gliomas). Although, it should be noted that the ICER associated with 5-ALA–guided surgical resection was marginally above $50,000 AUD per QALY gained, the threshold for which MSAC considers an intervention cost-effective.58
In summary, there was inconsistency between the ICER reported in the MSAC HTA ($53,613 AUD per QALY gained) as compared to ICERs reported in the other studies (all below €20,000 EUR per QALY gained). This variability may be explained by the different model parameter input values used for two primary drivers of cost-effectiveness of 5-ALA: the proportion of patients requiring two vials of 5-ALA (based on the recommended dose per kilogram body weight, at one vial per 75 kg59) and the unit cost per vial. The model evaluated by MSAC assumed that 50% of patients would require a second vial of 5-ALA at a unit cost of $3,990 AUD (cost year not reported) per vial, whereas other studies considered one vial per patient at the unit cost of £1,016.44 GBP (reported in 2016) per vial in the NICE HTA,16 €1,000 EUR (reported in 2012) per vial in Slof et al,56 and €980 EUR (reported in 2012) per vial Esteves et al.55 As such, if the MSAC HTA58 used similar cost parameter inputs as the other studies, it is likely that the ICERs would be more consistent across all studies.
Strengths and Limitations
Most studies had developed an economic model that accordingly modelled the natural history of high-grade gliomas. Of these models, two55,58 had additionally modelled the clinical treatment pathway for high-grade gliomas that is considered appropriate to Canadian clinical practice guidelines18,60 and the clinical practice in Ontario (A. Mansouri, MD, phone communication, May 1, 2019). As such, the models across studies are structurally robust and may be generalizable to the Ontario setting.
However, there were important limitations associated with the five studies that met our inclusion criteria.
First, across all studies, the clinical model inputs of the comparative safety and effectiveness parameters of 5-ALA were derived from low- or very low–quality evidence, according to GRADE (Grading of Recommendations, Assessment, Development and Evaluation) criteria.16,45 For instance, the randomized, open-label trial by Stummer et al19 published in 2006 was the primary source from which all16,55,57,58 but one study (Slof et al)56 derived their clinical model inputs. Outcomes based on the Stummer et al19 trial were downgraded to low- or very low–quality evidence by both systematic reviews by Cochrane45 and NICE16 for reasons related to risk of bias, imprecision of estimates, and inadequate reporting of data. No additional randomized controlled trials evaluating 5-ALA–guided surgical resection were identified in our clinical literature search for systematic reviews or in our clinical literature search for primary studies. As such, this 2006 study (Stummer et al)19 remains the only randomized controlled trial to date on 5-ALA–guided surgical resection. Slof et al,56 the only included study that did not derive clinical parameter values from the Stummer et al19 trial for its model input, relied on a single retrospective observational trial60 instead.
Second, while 5-ALA is indicated for use as an adjunct to visualize tumours during surgery in people with suspected high-grade glioma (WHO Grade III or IV)53 based on preoperative imaging,61 all studies derived clinical model inputs from trials that had an underrepresentation of Grade III gliomas. It is therefore not known whether, or to what extent, the reference case results of these studies are applicable to Grade III gliomas.
Third, most studies55,56,65 also did not account for the cost of the fluorescence module in their reference case.
Lastly, none of the studies evaluated the effect of 5-ALA–guided surgical resection on extent of tumour resection for recurrent gliomas. For instance, the models in both the NICE16 and MSAC33 HTAs assumed that 5-ALA would only be used at the initial surgery, and that the subsequent surgery for recurrent tumours would use white-light microscopy.16
Conclusions
We did not identify any studies conducted from the perspective of the Ontario or Canadian public health care payer. Of the studies that met our inclusion criteria, most found 5-ALA–guided surgical resection was cost-effective compared to white-light microscopy for high-grade gliomas. However, clinical model inputs for the comparative effectiveness and safety of 5-ALA were based on limited and low-quality evidence (according to GRADE criteria); namely a single randomized, open-label trial19 that was downgraded by recent systematic reviews by Cochrane and NICE.16,45 As such, until there is further research and development of high-quality evidence of 5-ALA–guided surgical outcomes, future cost-effectiveness analyses will most likely also be limited by similar clinical parameter uncertainties.
PRIMARY ECONOMIC EVALUATION
To our knowledge, there is no high-quality published evidence16,45 evaluating 5-aminolevulinic acid hydrochloride (5-ALA)–guided surgical resection as compared with the standard care for newly diagnosed or recurrent high-grade gliomas.
As such, any de novo (novel) economic model evaluating the cost-effectiveness of 5-ALA– guided surgical resection as compared with the standard surgical resection of high-grade gliomas would likely be limited by similar parameter uncertainties as previous models. These models have demonstrated structural robustness and appropriately align with Canadian clinical practice guidelines18,60 and clinical practice of high-grade gliomas in Ontario (A. Mansouri, MD, phone communication, May 1, 2019). We therefore anticipated that conducting a primary economic evaluation would produce similar results, and would not differ substantially in the model structure or parameter assumptions in the absence of new evidence. For instance, the de novo economic model developed in the recent National Institute for Health and Care Excellence health technology assessment16 came to largely the same conclusions as the previous economic evaluations. For these reasons, we did not conduct a primary economic evaluation.
BUDGET IMPACT ANALYSIS
Research Question
What is the 5-year budget impact for the Ontario Ministry of Health of publicly funding 5-aminolevulinic acid hydrochloride (5-ALA)–guided surgical resection for adults with high-grade gliomas?
Methods
Analytic Framework
We estimated the budget impact of publicly funding 5-ALA–guided surgical resection using the cost difference between two scenarios: (1) current clinical practice of using white-light microscopy and neuronavigation, with or without intraoperative ultrasound (the current scenario) and (2) anticipated clinical practice with public funding for 5-ALA as an adjunct to white-light microscopy and neuronavigation, with or without intraoperative ultrasound (the new scenario). Figure 4 presents the budget impact model schematic.
Figure 4: Schematic Model of Budget Impact.
We conducted a reference case analysis and sensitivity analyses. Our reference case analysis represents the analysis with the most likely set of input parameters and model assumptions. Our sensitivity analyses explored how the results are affected by varying input parameters and model assumptions.
Key Assumptions
Use of 5-ALA as an adjunct to standard care in Ontario (i.e., white-light microscopy in combination with neuronavigation) minimally disrupts the flow of tumour resection and clinical pathway of individuals with high-grade gliomas in Ontario, given that use of 5-ALA does not require any major changes to the standard surgical procedure66
If publicly funded, 5-ALA–guided surgical resection will be performed at neuro-oncology surgical sites in the first 5 years of uptake (A. Mansouri, MD, email and phone communications, March to May 2019; Z. Gelareh, MD, email and phone communications, April to May 2019)
-
To perform 5-ALA–guided surgical resection, sites must meet the requirements that are currently consistent with the US Food and Drug Administration and the European Medicines Agency drug approval packages for 5-ALA.59,61 These requirements are as follows:
-
○
Standard neurosurgical microscope must have the appropriate modifications (e.g., fluorescence modules Zeiss BLUE 400 or LEICA FL400) with the recommended wavelength to visualize the 5-ALA–induced fluorescence during surgery
-
○
Operating neurosurgeon must complete, as part of the manufacturer's risk management plan,59,61 certified training on the safe and effective use of 5-ALA
-
○
There is no additional cost associated with the training and certification of neurosurgeons in the safe and effective use of 5-ALA for the visualization of high-grade gliomas; this educational training program developed by Professor Walter Stummer, will continue to be offered by the manufacturer over the next 5 years (Medexus Pharmaceuticals Inc., phone communications, April 4, 2019)
The level of resource utilization of the current mix of standard care (i.e., white-light microscopy combined with neuronavigation alone or with intraoperative ultrasound) in surgery for high-grade gliomas will remain unchanged when combined with the adjunct of 5-ALA (A. Mansouri, MD, email and phone communications, March to May 2019)
-
Of our target population, 30% will require an additional vial of 5-ALA, based on the recommended dose per body weight, at one vial per 75 kg.59 This estimate was approximated by taking into account:
-
○
Mean age-adjusted body weight was 89.8 kg (197.9 lb) and 77.4 kg (170.6 lb) for men and women, respectively, in the United States in 2015 to 201664
-
○
Based on historical trends, the average body weight of adults are generally lower in Canada than in the United States65
-
○
Assuming that average body weight of adults with high-grade gliomas are similar to that of the general population
-
○
Incidence of newly diagnosed, primary high-grade gliomas will remain relatively stable over the next 5 years (as is consistent with incidence trends of newly diagnosed, primary malignant brain tumours in Ontario over the previous 5 years, between 2013 and 2017; Appendix 6, Table A6)
5-ALA–guided surgical resection should be offered, as appropriate, as an adjunct to help maximize resection of newly diagnosed primary high-grade gliomas at the initial surgery. Standard care white-light microscopy in combination with neuronavigation, either alone or together with intraoperative ultrasound, would continue as is current clinical practice for recurrent tumours. This is consistent with National Institute for Health and Care Excellence guideline on primary brain tumours and brain metastases in adults16
Target Population
The target population of our budget impact analysis are adults (18 years of age or older) with newly diagnosed, primary high-grade (World Health Organization [WHO] Grade III and Grade IV)53 gliomas (e.g., anaplastic astrocytoma, anaplastic oligodendroglioma, and glioblastoma or glioblastoma multiforme) for which resection is feasible.53 Given that the extent of safe resection has been shown to be a prognostic variable, most high-grade gliomas are managed with the standard treatment of removing as much of the tumour as possible (“maximal safe resection”).69–71 However, to achieve the right balance of minimizing morbidity, extending survival, and maximizing quality of life, the extent of safe tumour resection is reliant on several key clinical factors, including patient age, tumour location (e.g., noneloquent vs. eloquent location of brain), and patient performance status (e.g., Karnofsky Performance Status). As such, the choice of surgical approach (e.g., maximal safe resection, partial section, open biopsy, or stereotactic biopsy) should ultimately be individualized to each patient and subject to the clinical judgment of the physician.69–71
Using the reported annual incidence of primary brain tumours in Ontario and estimates from literature,1 we estimated that each year, there are roughly 730 newly diagnosed, primary high-grade gliomas for which resection is feasible (Appendix 6, Table A7).
To provide a more precise estimate of our target population, we used data from the Discharge Abstract Database (DAD), the national health database that captures administrative, clinical, and demographic information on hospital discharges.69 Through DAD, we identified all inpatient discharges with a valid health care card number in Ontario aged 18 years or older with International Classification of Diseases for Oncology (ICD-10) diagnosis codes for malignant neoplasm of the brain, specific to the anatomic locations of gliomas. We then filtered the results by the Canadian Classification of Health Interventions (CCI) intervention code for excision of the brain using craniotomy. Using this methodology, we estimated that each year, there are roughly 800 newly diagnosed, primary high-grade gliomas for which tumour resection is feasible (Table 7). We were not able to identify the histology type of these results, as there is no diagnosis code associated with gliomas. As such, our estimate can be considered on the higher end.
Table 7:
Estimated Annual Incidence of Newly Diagnosed, Primary High-Grade Gliomas in Ontario for Which Maximal Safe Resection is Feasible
| ICD-10-CA and CCI | Averagea | Source | |
|---|---|---|---|
| A | Newly diagnosed, primary malignant brain tumours in adults (≥ 18 years of age) (ICD-10-CA codes C71.0, C71.2, C71.3, C71.4, C71.5, C71.6, C71.8, C71.9) | 1,590 | DADb |
| B | “A” filtered by CCI intervention codes 1.AN.87.SZ.c for partial excision of the brain using craniotomyc | 785 | DADd |
Source: IntelliHealth Ontario.
Abbreviations: CCI, Canadian Classification of Health Interventions; DAD, Discharge Abstract Database; ICD-10, International Classification of Disease for Oncology.
Estimates from calculations are rounded to the nearest five.
Average of annual number of cases ≥ 18 years of age, assigned ICD-10-CA codes C71.0 (cerebrum, except lobes and ventricles), C71.1 (frontal lobe), C71.2 (temporal lobe), C71.3 (parietal lobe), C71.4 (occipital lobe), C71.5 (cerebral), C71.6 (cerebellum), C71.7 (brain stem), C71.8 (lesion of brain), C71.9 (optic nerve) from 2013 to 2017.
CCI intervention code 1.AN.87.SZ.^^ includes: 1.AN.87.SZ.AG, 1.AN.87.SZ.AZ, and 1.AN.87.SZ.GX.
Average of annual number of cases ≥ 18 years of age, assigned ICD-10-CA codes C71.0, C71.2, C71.3, C71.4, C71.5, C71.6, C71.8, and C71.9, filtered by CCI code 1.AN.87.^^ from 2013 to 2017.
Current Intervention Mix
At the time of writing this review, there were six neuro-oncology surgical sites in Ontario, defined as sites with established multidisciplinary health care teams with specialization and clinical practice focus on the surgical management of brain tumours (A. Mansouri, MD, email and phone communications, March to May 2019). Based on information captured in DAD, the large majority (around 75%) of surgical resections of malignant brain tumours in the province were performed at these sites, with the remainder performed at general neurosurgical sites (see Appendix 6, Table A6). Table 8 lists the hospitals that performed surgical resections of high-grade gliomas in the province and their corresponding average surgical volumes of malignant brain tumours per year, from 2013 to 2017.
Table 8:
Ontario Hospitals Performing Surgical Resection of Adult High-Grade Gliomas and Their Corresponding Average Surgical Volumes of Malignant Brain Tumours Per Year, 2013 to 2017a
| Hospital | Average Volume/Yearb | Proportion |
|---|---|---|
| Neuro-oncology Surgical Sites | 590c | |
| The Ottawa Hospital | 105 | 75% (590/785) |
| St. Michael's Hospital | 117 | |
| Toronto Western Hospital | 87 | |
| Sunnybrook Health Sciences Centre | 83 | |
| London Health Sciences Centre | 70 | |
| Hamilton Health Sciences Centre | 128 | |
| General Neurosurgical Sites | 195c | |
| Kington General Hospital | 36 | 25% (195/785) |
| Trillium Health Partners | 89 | |
| Health Sciences North | 29 | |
| Thunder Bay Regional Hospital | 7 | |
| Windsor Regional Hospital | 32 | |
| Total | 785c | 100% |
Source: IntelliHealth Ontario.
Report generated from IntelliHealth Ontario, using ICD-10-CA codes for malignant neoplasm of the brain, specific to the anatomic locations of gliomas (C71.0 to C71.9), filtered by the Canadian Classification of Health Interventions (CCI) intervention codes for excision of the brain using craniotomy (1.AN.87.SZ.^^), and by the age group 18 years of age or older; results are reported in annual hospital average of number of inpatient discharges with valid health care card number in Ontario for the calendar years 2013 to 2017.
May not be exact due to rounding.
Estimates are rounded to the nearest five.
In Ontario, the standard surgical care of high-grade gliomas consists of surgery with a standard operating microscope under white light (“white-light microscopy”) in combination with neuronavigation, a computer-assisted technology that superimposes the position of surgical instruments onto a three-dimensional model of the patient brain created from preoperative imaging (A. Mansouri, MD, email and phone communication, March to May 2019; Z. Gelareh, MD, email and phone communication, April to May 2019). Several additional technologies, such as intraoperative magnetic resonance imaging (MRI) and intraoperative ultrasound, are also sometimes used to guide the surgical resection of high-grade gliomas. However, the use of these resources is not uniform across the province (A. Mansouri, MD, email and phone communication, March to May 2019; Z. Gelareh, MD, email and phone communication, April to May 2019).
For instance, in Ontario, intraoperative MRI is rarely used, as it is considered expensive, disruptive to the flow of surgery, and may extend surgical time by up to approximately one hour.73 Similarly, based on information captured by the Ontario Case Costing Initiative (OCCI), intraoperative ultrasound is used, on average, for only about 5% of all surgical resections of malignant brain tumours each year (Appendix 6, Table A8). Its heterogenous use across the province can be attributed to the high sensitivity of this technology to the differences between individual operators.31,74
Lastly, while the first 5-ALA–guided surgical resection was first performed in Ontario in fall of 2018,72 it is still currently being accessed through the Health Canada Special Access Programme (Health Canada Special Access Programme, phone communication, April 7, 2019), and provided through various funding sources, such as hospital foundations,72 fundraising support,73 or hospital global budget (A. Mansouri, MD, email and phone communication, March to May 2019). As such, we did not consider 5-ALA as part of the current mix of standard surgical care and intraoperative technologies in our budget impact analysis.
The current mix of standard surgical care and intraoperative technologies for high-grade gliomas in Ontario is summarized in Table 9.
Table 9:
Distribution of the Current Mix of Standard Care and Intraoperative Technologies in the Surgical Management of High-Grade Gliomas in Ontario
| Technology | Distribution | Source |
|---|---|---|
| White-light microscopy | 100% | A. Mansouri, MD, email and phone conversation, March to May 2019; Z. Gelareh, MD, email and phone communication, April to May 2019 |
| Neuronavigation | 100% | A. Mansouri, MD, email and phone conversation, March to May 2019; Z. Gelareh, MD, email and phone communication, April to May 2019 |
| iUS | 5% | OCCIa |
| iMRI | 0% | Key assumptionb |
| 5-ALA–guided surgical resection | 0% | Key assumptionc |
Abbreviations: 5-ALA, 5-aminolevulinic acid hydrochloride; iMRI, intraoperative magnetic resonance imaging; iUS, intraoperative ultrasound; OCCI, Ontario Case Costing Initiative.
Average proportion of iUS use derived from report generated from OCCI, using Canadian Classification of Health Interventions (CCI) intervention codes for excision of the brain using craniotomy (1.AN.87.SZ.^^), and filtered by the age group 18 years of age or older for fiscal years 2014/15 to 2016/17. For further details, see Appendix 6, Table A8.
This is a simplifying assumption after considering that the current use of this intraoperative technology in the surgical resection of high-grade gliomas is rare, not captured by Ontario health databases, and considered prohibitively expensive, disruptive to the flow of surgery, and may extend surgical time by up to 57 minutes.73
This is a simplifying assumption after considering that 5-ALA is currently being accessed through Health Canada Special Access Programme (Health Canada Special Access Programme, phone communication, April 7, 2019), and provided through various funding sources, such as hospital foundations,72 fundraiser support,73 or hospital global budget (A. Mansouri, MD, email and phone communication, March to May 2019).
Uptake of the New Intervention and New Intervention Mix
We expect that the current mix of standard care (i.e., white-light microscopy) and intraoperative technologies in the surgical management of high-grade gliomas would remain unchanged in Ontario when combined with the adjunct of 5-ALA (A. Mansouri, MD, email and phone communication, March to May 2019; Z. Gelareh, MD, email and phone communication, April to May 2019).
To determine the uptake rate of 5-ALA–guided surgical resection for high-grade (WHO Grade III and Grade IV)53 gliomas in Ontario over the next 5 years, we took into account the following factors:
Diffusion of 5-ALA is currently taking place at three neuro-oncology surgical sites in Ontario. These sites have met the infrastructure (i.e., fluorescence module) and physician training requirements to perform 5-ALA–guided surgical resections
-
Likely readiness of other sites to meet these two requirements is high, given the following details:
-
○
All neuro-oncology surgical sites in Ontario either have the most current models of surgical microscopes (e.g., ZEISS KINEVO 900 or LEICA M530) with built-in and fully integrated Zeiss BLUE 400 or Leica FL400, or have earlier models of the above surgical microscopes (e.g., Zeiss OMNI PENTERO 800, Zeiss OMNI PENTERO 900, LEICA M720) that are compatible and can be upgraded with the necessary fluorescence modules (A. Mansouri, MD, email and phone communication, March to May 2019; Z. Gelareh, MD, email and phone communication, April to May 2019)
-
○
Past training courses appear to be relatively accessible, as they have been delivered at multiple locations across Ontario (Medexus Pharmaceuticals Inc., phone communication, April 4, 2019)
-
○
Taking these factors into consideration, we assumed the three neuro-oncology surgical sites that have currently obtained special access to 5-ALA through Health Canada Special Access Programme would be ready to completely and quickly adopt 5-ALA–guided surgical resection (A. Mansouri, MD, email and phone communication, March to May 2019; Z. Gelareh, MD, email and phone communication, April to May 2019).
Table 10 lists the estimated annual volumes of standard surgical treatment and 5-ALA–guided resection for the first 5 years. On average, the three neuro-oncology surgical sites with special access to 5-ALA account for roughly 40% of the annual volume of surgical resections of high-grade gliomas across the province. We therefore estimated that if publicly funded, the uptake rate for 5-ALA in year 1 would be 40%. We assumed that in the subsequent years, the remaining neuro-oncology surgical sites will adopt 5-ALA–guided surgical resection starting with half of all surgeries before fully adopting the use of 5-ALA as an adjunct to standard care in the following year. By this assumption, all six neuro-oncology surgical sites in Ontario are assumed to completely adopt 5-ALA by year 3, at the uptake rate of 75% of annual surgical resections of high-grade gliomas in the province (Appendix 6, Table A9).
Table 10:
Annual Volumes of Standard Surgical Treatment and 5-ALA–Guided Surgical Resection, Year 1 Through Year 5a
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | ||
|---|---|---|---|---|---|---|
| Current Scenario | 785 | 785 | 785 | 785 | 785 | |
| Distribution of current mix of standard care | White-light microscopy + neuronavigation | 745 | 745 | 745 | 745 | 745 |
| White-light microscopy + neuronavigation + iUS | 40 | 40 | 40 | 40 | 40 | |
| New Scenario | 785 | 785 | 785 | 785 | 785 | |
| Distribution of 5-ALA combined with standard care | 5-ALA–guided resection | 315 | 430 | 590 | 590 | 590 |
| Uptake rate | 40% | 55% | 75% | 75% | 75% | |
| White-light microscopy+ neuronavigation | 430 | 315 | 155 | 155 | 155 | |
| White-light microscopy + neuronavigation + iUS | 40 | 40 | 40 | 40 | 40 |
Source: IntelliHealth Ontario.
Abbreviation: 5-ALA, 5-aminolevulinic acid hydrochloride; iUS, intraoperative ultrasound.
Estimates are rounded to the nearest five.
Resources and Costs
Our budget impact analysis accounted for cost of the unit price of 5-ALA, costs of the procurement of fluorescence module (e.g., Zeiss BLUE 400 or Leica FL400), insured physician service fees and hospital costs associated with white-light microscopy of malignant brain tumours with neuronavigation, and the additional hospital cost of intraoperative ultrasound use during surgery, as appropriate.
The listing price of 5-ALA in Ontario is $2,265 per vial (Medexus Pharmaceuticals Inc., email communication, April 15, 2019). Cost of the fluorescence module, estimated at around $71,810 was the median cost from the price range reported in Slof et al,56 adjusted to the Canadian dollar in 2019, using power purchasing parities from the Organisation for Economic Co-operation and Development74 and consumer price index for Heath and Personal Care from Statistics Canada75 (Table 11). The cost of the module was included in our budget impact analysis as the additional cost per procedure, using the formula adapted from Slof et al56: (purchase price of module) ÷ (useful life of module × procedures per year). The useful life of module was assumed to be 8 years at around 95 procedures per year, to account for the total annual average volumes of malignant brain surgeries at neuro-oncology surgical sites that currently do not have the florescence modules in place (Appendix 6, Table A10). We accounted for the cost of the fluorescence module component only, regardless of whether a hospital procures a surgical microscope with the fluorescence module functionality built-in, or if the fluorescence module is acquired separately to upgrade an existing surgical microscope.
Table 11:
Additional Costs Associated With 5-ALA–Guided Surgical Resection
| Resource | Name of Technology | Cost |
|---|---|---|
| 5-ALA (1.5 g vial) | Gliolan | $2,265a |
| Fluorescence module | Zeiss BLUE 400 | $71,810b |
| Leica FL400 |
Abbreviations: 5-ALA, 5-aminolevulinic acid hydrochloride; CPI, consumer price index; OECD, Organisation for Economic Co-operation and Development; PPP, power purchasing parities.
Medexus Pharmaceuticals Inc., email communications, April 15, 2019.
€37,500 EUR, median of cost range (€30,000–€45,000 EUR) of adding the fluorescence module to older microscopes reported in Slof et al,56 adjusted to the Canadian dollar in 2019 using PPP from the OECD74 and CPI for Heath and Personal Care from Statistics Canada75; converted price was rounded to the nearest $5.
The cost of insured physician services ($3,131.48) included the professional fees of the neurosurgeon and anaesthesiologist associated with craniotomy and excision of brain tumours located in the cerebral hemispheres (“supratentorial tumours”) with use of an operating microscope and neuronavigation (“intracranial stereotaxis”; Table 12). While gliomas can be found in the infratentorial region of the brain as well, the majority (61.2%) are located in the supratentorium (frontal, temporal, parietal, and occipital lobes combined).1 As such, for simplicity, we deferred all intervention volumes to the insured physician services for surgical procedure of the supratentorial region of the brain.
Table 12:
Per-Patient Surgical Procedure Costs of Resection for Supratentorial High-Grade Gliomas (Tumours Located in the Cerebral Hemispheres)a
| OHIP Code | Component | Component Breakdown | Cost |
|---|---|---|---|
| N103 | Craniotomy plus excision, supratentorial | Surgeon | $1,562.90 |
| Anesthesiologist | $795.53b | ||
| E901 | With operating microscope | Surgeon | $234.65 |
| N123 | Stereotaxis, intracranial (to include ventriculography) | Surgeon | $538.40 |
| Total cost | $3,131.48 |
Abbreviation: OHIP, Ontario Health Insurance Plan.
The majority of high-grade gliomas are located in the cerebral hemispheres, as opposed to the back of the brain. As such, for simplicity, we assumed insured physician services for the surgical procedure of supratentorial tumours only. Counterpart OHIP codes for the procedure done in the infratentorial part of the brain are: N151, E901, and N123.
Calculated based on 15 basic units and 38 time units of anesthesiologists’ unit fee of $15.04.
Hospital costs of $15,120 per procedure were derived from the OCCI and include all direct costs (e.g., operating room, nursing, inpatient hospital stay, etc.) and indirect costs (e.g., administration, finance, human resources, hospital operations, etc.) associated with the surgical procedure, using the aforementioned CCI intervention codes, filtered for the age group 18 years of age or older (Table 13). Lastly, the additional hospital cost of intraoperative ultrasound estimated at around $185 per use during surgery were similarly derived from the OCCI, identified via the appropriate functional centre cost associated with this technology (Appendix 6, Table A11). Note that the previously described hospital cost associated with the procedure was adjusted to exclude for the additional cost of intraoperative ultrasound to avoid double counting.
Table 13:
Per-Patient Hospital Cost Associated With White-Light Microscopy and Neuronavigation With and Without Intraoperative Ultrasound for the Surgical Resection of Malignant Brain Tumours (Weighted Average of Reported Costs), Fiscal Years 2014/15 to 2016/17ab
| Procedure | Costc |
|---|---|
| White-light microscopy and neuronavigation only | $15,120 ($15,305d – $185e) |
| White-light microscopy, neuronavigation, and intraoperative iUS | $15,305d |
Source: Ontario Case Costing Initiative.
Abbreviations: CCI, Canadian Classification of Health Interventions; iUS, intraoperative ultrasound; OCCI, Ontario Case Costing Initiative.
Includes both direct and indirect costs associated with 1.AN.87.SZ.^^.
Report generated from OCCI, using CCI intervention codes for excision of the brain using craniotomy (1.AN.87.SZ.^^), filtered by the age group 18 years of age or older for fiscal years 2014/15 through 2016/17.
Estimates from calculations are rounded to the nearest five.
See Appendix 6, Table A12.
See Appendix 6, Table A11.
Based on the information presented in Tables 11, 12, and 13, the total average per-person cost is around $21,480 for 5-ALA–guided surgical resection and $18,435 for standard surgical care. The estimated total per-person costs in the new scenario are about $3,000 higher than in the current scenario (Table 14).
Table 14:
Total Average Per-Person Costs, associated with surgical resectiona
| Estimated Average Costs | Current Scenario | New Scenario | Source |
|---|---|---|---|
| 5-ALA (optical imaging agent) | NA | $2,265 | Medexus Pharmaceuticals Inc. |
| Fluorescence module | NA | $100b | Slof et al56 |
| Insured physician services | $3,130 | $3,130 | OHIP SoB76 |
| Hospital costs (white-light microscopy and neuronavigation) | $15,120 | $15,120 | OCCI |
| Hospital costs (iUS) | $185 | $185 | OCCI |
| Totalc | $18,435 | $21,480 |
Abbreviations: 5-ALA, 5-aminolevulinic acid hydrochloride; NA, not applicable; OCCI, Ontario Case Costing Initiative; OHIP SoB, Ontario Health Insurance Plan Schedule of Benefits; iUS, intraoperative ultrasound.
Estimates rounded to the nearest $5.
Additional costs calculated as: (purchase price of module) ÷ (useful life of module × procedures per year), where purchase price = $71,810; useful life of module = 8 years; and procedures per year = 95.
Total per-person costs without iUS is $18,250 and $21,295 under current scenario and new scenario, respectively.
Internal Validation
The secondary health economist conducted a formal internal validation. This process included checking for errors and ensuring the accuracy of parameter inputs and equations in the budget impact analysis.
Analysis
Reference Case
We conducted a reference case analysis that examined the budget impact as the difference in total costs between the current and new scenarios. We estimated the cost of the current scenario using insured physician services and hospital costs associated with the standard surgical treatment for high-grade gliomas in Ontario. We estimated the cost of the new scenario by combining the additional costs associated with 5-ALA–guided surgical resection with the cost of standard surgical treatment, and accounting for an uptake rate of the technology over 5 years.
Sensitivity Analysis
We explored the impact to our reference case results by varying our cost parameters and key assumptions in nine scenarios:
Scenarios 1 and 2 assumed a unit cost of 5-ALA at 50% ($1,132.50/vial) and 30% ($679.50/vial) of the reference case, respectively (reference case: $2,265/vial)
Scenarios 3 and 4 assumed that 0% and 50% of patients required an additional vial of 5-ALA, respectively (reference case: 30%)
Scenario 5 assumed an uptake rate of 20% in year 1, increasing by 10% per year, from year 2 to year 5 (reference case: 40% in year 1, 55% in year 2, 75% in years 3 through 5)
Scenario 6 excluded the cost of the fluorescence module
Scenarios 7, 8, and 9 accounted for three potential volumes per year of recurrent tumours for 5-ALA–guided surgical resection (at 220, 105, and 60 cases, respectively) based on model parameter input used in Esteves et al55 and DAD administrative data
Results
Reference Case
The results of publicly funding 5-ALA–guided surgical resection for adults with high-grade gliomas are summarized in Table 15. Adopting 5-ALA–guided surgical resection at an uptake rate of 40% in year 1, 55% in year 2, and 75% in years 3 to 5 would lead to additional costs of about $930,000 in year 1 to $1,765,000 in year 5. We estimated a total budget impact of $7,500,000 over the next 5 years.
Table 15:
Budget Impact Analysis Results
| Budget Impact, $ab | |||||||
|---|---|---|---|---|---|---|---|
| Scenario | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total | |
| Current Scenario | 14,333,650 | 14,333,650 | 14,333,650 | 14,333,650 | 14,333,650 | 71,668,250 | |
| Standard care | White-light + neuronavigation | 13,596,250 | 13,596,250 | 13,596,250 | 13,596,250 | 13,596,250 | 67,981,250 |
| White-light + neuronavigation + iUS | 737,400 | 737,400 | 737,400 | 737,400 | 737,400 | 3,687,000 | |
| Future Scenario | 15,261,170 | 15,613,785 | 16,098,905 | 16,098,905 | 16,098,905 | 79,171,670 | |
| 5-ALA + Standard care | 5-ALA–guided surgical resection | 6,676,268 | 9,127,635 | 12,532,755 | 12,532,755 | 12,532,755 | 53,402,168 |
| Uptake rate | 40% | 55% | 75% | 75% | 75% | ||
| Standard care | 8,584,900 | 6,486,150 | 3,566,150 | 3,566,150 | 3,566,150 | 25,769,500 | |
| Budget Impact | 927,520 | 1,280,135 | 1,765,255 | 1,765,255 | 1,765,255 | 7,503,420 | |
Abbreviation: 5-ALA, 5-aminolevulinic acid hydrochloride; iUS, intraoperative ultrasound.
All costs are reported in 2019 Canadian dollars.
Estimates from calculations are rounded to the nearest $5.
Sensitivity Analysis
Table 16 details the results of our sensitivity analysis. Overall, the unit cost of 5-ALA was the main driver of the budget impact. When 50% (Scenario 1) and 30% (Scenario 2) of the unit cost is assumed (at $1,132.50/vial and $679.50/vial respectively), the 5-year reference case budget impact is reduced by just under $4,000,000 and just over $5,000,000, respectively. In comparison, the rest of the scenarios impacted the reference case budget impact between around $100,000 (Scenario 6) to $2,800,000 (Scenario 5).
Table 16:
Budget Impact Sensitivity Analysis Results
| Budget Impact, $ab | ||||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total | |
| Reference Case | ||||||
| Current | 14,333,650 | 14,333,650 | 14,333,650 | 14,333,650 | 14,333,650 | 71,668,250 |
| Future | 15,261,170 | 15,613,785 | 16,098,905 | 16,098,905 | 16,098,905 | 79,171,670 |
| Budget Impact | 927,520 | 1,280,135 | 1,765,255 | 1,765,255 | 1,765,255 | 7,503,420 |
| Scenario 1: Unit Cost of 5-ALA at 50% of Reference Case ($1,132.50/vial) | ||||||
| Current | 14,333,650 | 14,333,650 | 14,333,650 | 14,333,650 | 14,333,650 | 71,668,250 |
| Future | 14,797,410 | 14,980,720 | 15,230,280 | 15,230,280 | 15,230,280 | 75,468,970 |
| Budget Impact | 463,760 | 647,070 | 896,630 | 896,630 | 896,630 | 3,800,720 |
| Scenario 2: Unit Cost of 5-ALA at 30% of Reference Case ($679.50/vial) | ||||||
| Current | 14,333,650 | 14,333,650 | 14,333,650 | 14,333,650 | 14,333,650 | 71,668,250 |
| Future | 14,611,905 | 14,727,490 | 14,882,825 | 14,882,825 | 14,882,825 | 73,987,870 |
| Budget Impact | 278,255.00 | 393,840.00 | 549,175 | 549,175 | 549,175 | 2,319,620 |
| Scenario 3: Proportion of Patients Requiring an Additional Vial of 5-ALA Assumed at 0% | ||||||
| Current | 14,333,650 | 14,333,650 | 14,333,650 | 14,333,650 | 14,333,650 | 71,668,250 |
| Future | 15,047,125 | 15,321,600 | 15,698,000 | 15,698,000 | 15,698,000 | 77,462,725 |
| Budget Impact | 713,475 | 987,950 | 1,364,350 | 1,364,350 | 1,364,350 | 5,794,475 |
| Scenario 4: Proportion of Patients Requiring an Additional Vial of 5-ALA Assumed at 50% | ||||||
| Current | 14,333,650 | 14,333,650 | 14,333,650 | 14,333,650 | 14,333,650 | 71,668,250 |
| Future | 15,403,865 | 15,808,575 | 16,366,175 | 16,366,175 | 16,366,175 | 80,310,965 |
| Budget Impact | 1,070,215 | 1,474,925 | 2,032,525 | 2,032,525 | 2,032,525 | 8,642,715 |
| Scenario 5: Uptake Rate Assumed 20% in Year 1, Increasing by 10% Per Year, From Year 2 to Year 5 | ||||||
| Current | 14,333,650 | 14,333,650 | 14,333,650 | 14,333,650 | 14,333,650 | 71,668,250 |
| Future | 14,790,050 | 15,039,610 | 15,289,170 | 15,524,730 | 15,745,565 | 76,389,125 |
| Budget Impact | 456,400 | 705,960 | 955,520 | 1,191,080 | 1,411,915 | 4,720,875 |
| Scenario 6: Excluded Costs of Fluorescence Module | ||||||
| Current | 14,333,650 | 14,333,650 | 14,333,650 | 14,333,650 | 14,333,650 | 71,668,250 |
| Future | 15,261,170 | 15,599,785 | 16,070,905 | 16,070,905 | 16,070,905 | 79,073,670 |
| Budget Impact | 927,520 | 1,266,135 | 1,737,255 | 1,737,255 | 1,737,255 | 7,405,420 |
| Scenario 7: Assumed 220b Additional Surgical Volumes of High-Grade Gliomas Per Year to Account for Recurrent Tumours | ||||||
| Current | 18,348,650 | 18,348,650 | 18,348,650 | 18,348,650 | 18,348,650 | 91,743,250 |
| Future | 19,526,450 | 19,996,850 | 20,599,750 | 20,599,750 | 20,599,750 | 101,322,550 |
| Budget Impact | 1,177,800 | 1,648,200 | 2,251,100 | 2,251,100 | 2,251,100 | 9,579,300 |
| Scenario 8: Assumed 105b Additional Surgical Volumes of High-Grade Gliomas Per Year to Account for Recurrent Tumours | ||||||
| Current | 16,249,900 | 16,249,900 | 16,249,900 | 16,249,90 | 16,249,900 | 81,249,500 |
| Future | 17,295,200 | 17,706,705 | 18,250,715 | 18,250,715 | 18,250,715 | 89,754,050 |
| Budget Impact | 1,045,300 | 1,456,805 | 2,000,815 | 2,000,815 | 2,000,815 | 8,504,550 |
| Scenario 9: Assumed 60b Additional Surgical Volumes of High-Grade Gliomas Per Year to Account for Recurrent Tumours | ||||||
| Current | 15,428,650 | 15,428,650 | 15,428,650 | 15,428,650 | 15,428,650 | 77,143,250 |
| Future | 16,429,780 | 16,811,845 | 17,326,410 | 17,326,410 | 17,326,410 | 85,220,855 |
| Budget Impact | 1,001,130 | 1,383,195 | 1,897,760 | 1,897,760.00 | 1,897,760 | 8,077,605 |
Abbreviations: 5-ALA, 5-aminolevulinic acid hydrochloride.
All costs are reported in 2019 Canadian dollars.
Estimates from calculations are rounded to the nearest five.
Discussion
We conducted a budget impact analysis to explore adopting 5-ALA–guided surgical resection for adults with newly diagnosed, primary high-grade gliomas. Assuming an uptake rate of 40% in year 1, 55% in year 2, and 75% in years 3 to 5, we estimated the total budget impact would be about $7,500,000 over this period (from about $930,000 in year 1 to about $1,765,000 in year 5). This finding accounts for the complete uptake of 5-ALA–guided surgical resection in all established neuro-oncology surgical sites in Ontario.
Our sensitivity analyses explored changes in the budget impact estimates by varying the unit price of 5-ALA, the proportion of target population requiring an additional vial, and the uptake rate. In addition, we also explored scenarios that excluded the cost of the fluorescence module and assumed that 5-ALA would be used to guide maximal safe resections in recurrent tumours as well.
Overall, the unit cost of 5-ALA was the main driver of budget impact. At 50% (Scenario 1) and 30% (Scenario 2) of the 5-ALA unit price, the reference case budget impact can be reduced by around $4,000,000 and $5,000,000, respectively. By comparison, the remaining scenarios impacted the reference case results by less than $3,000,000. For instance, excluding the cost of the fluorescence module (Scenario 6) had the least effect on the reference case result, reducing the budget impact by less than $100,000 over the analyses period. Due to its minimal effect on budget impact, we did not explore a range in the cost of the fluorescence module in our scenario analyses.
Strengths and Limitations
We based our annual volume and the majority of cost estimates on hospitalization data from an Ontario health administrative database (i.e., DAD) and the current Ontario Health Insurance Plan Schedule of Benefits for Physician Services under the Health Insurance Act. We identified relevant data using the appropriate ICD-10-CA and CCI intervention codes for surgical treatment of malignant brain tumours. Our methodology and estimates were further informed and validated by expert consultations. The unit cost of 5-ALA was based on its listing price in Ontario, provided by Medexus Pharmaceuticals Inc. The single cost estimate informed by secondary and non-Canadian sources was the cost of the fluorescence module, which demonstrated only a minimal effect on the overall budget impact. Our estimates can therefore be considered updated, specific to Ontario, and inclusive of all important and relevant costs, having accounted for both direct (e.g., insured physician services, operating room) and indirect costs (e.g., hospital operations).
However, due to limited evidence, we relied on a few simplifying assumptions. Firstly, we did not identify information on the average body weight of individuals with malignant brain tumours. As such, we assumed that it would be similar to that of the general population. We then used the reported average body weight and trends of the adult general population in the United States and Canada to approximate this estimate. Secondly, we did not account for the use of 5-ALA for recurrent tumours in our reference case, as this clinical parameter has not been sufficiently studied. However, should 5-ALA–guided surgical resection become the recommended clinical practice in the province, the budget impact would increase by no greater than around $2,000,000 when accounting for the upper estimate (i.e., 220/year) of additional surgical volumes due to recurrent tumours. Thirdly, our target population excluded children since 5-ALA has been largely studied for use in adult high-grade gliomas, and that its safety and efficacy has not yet been tested in individuals less than 18 years of age.62,80,81 In addition, high-grade gliomas are considered rare in children. According to the Central Brain Tumour Registry of the United States, between 2008 and 2012, only about 7% of all reported brain tumours occurred in children (≤ 19 years of age), of which just under 15% were high-grade gliomas (11.7% malignant gliomas, not otherwise specified; 2.9% glioblastomas).82 As such, our reference case budget impact would not be significantly affected even when accounting for the potential future use of 5-ALA in visualizing tumours to guide maximal safe resection in children.
Lastly, we did not account for medical costs associated with managing adverse effects of 5-ALA–guided surgical resection. As previously discussed in the Clinical Evidence section, the Cochrane systematic review by Jenkinson et al45 did not identify major differences in overall or neurological adverse events between the 5-ALA group and the control group from the randomized control trial by Stummer.19 However, a greater deterioration in the National Institute of Health stroke scale from baseline values was reported in the 5-ALA (26.2%) compared with control (14.5%) at 48 hours. No statistical analyses were performed or reported. However, in subsequent follow-ups, no major differences were observed between the two groups.
Conclusions
Based on the findings of our budget impact analysis, we estimated that publicly funding 5-ALA– guided surgical resection for adults with high-grade gliomas would be associated with additional costs of about $930,00 in year 1 to about $1,765,000 in year 5. We estimated that the total budget impact would be about $7,500,000 over this 5-year period, accounting for the complete uptake of 5-ALA–guided surgical resection by all established neuro-oncology surgical sites in Ontario.
PATIENT PREFERENCES AND VALUES
Objective
The objective of this analysis was to explore the underlying preferences, values, needs, and priorities of those who have lived experience with high-grade glioma.
Background
Exploring patient preferences and values provides a unique source of information about people's experiences of a health condition and the health technologies or interventions used to manage or treat the health condition. It includes the impact of the condition and its treatment on the person with the health condition, their family and other caregivers, and the person's personal environment. Engagement also provides insights into how a health condition is managed by the province's health system.
Information shared from lived experience can also identify gaps or limitations in published research (e.g., outcomes important to those with lived experience that are not reflected in the literature).83–85 Additionally, lived experience can provide information and perspectives on the ethical and social values implications of health technologies or interventions.
Because the needs, preferences, priorities, and values of those with lived experience in Ontario are not often adequately explored in published literature, we speak directly with people who live with a given health condition, including those with experience with the intervention we are exploring.
Methods
Engagement Plan
The engagement plan for this health technology assessment focused on consultation to examine the experiences of people with high-grade glioma and those of their families and other caregivers. Due to the frail condition of the patients, we spoke with only one participant for this assessment. We engaged the participant via a telephone interview and a follow-up was done through email.
We used a qualitative interview, as this method of engagement allowed us to explore the meaning of central themes in the experiences of the participant with high-grade glioma.83 The sensitive nature of exploring people's experiences of a health condition and their quality of life are other factors that support our choice of an interview methodology.
Participant Outreach
We used an approach called purposive sampling,87–90 which involves actively reaching out to people with direct experience of the health condition and health technology or intervention being reviewed. We reached out clinical experts who provided support for this assessment, and to an online, international support group to spread the word about this engagement activity and to contact people who have had experience with high-grade glioma and/or 5-aminolevulinic acid hydrochloride (5-ALA) and their caregivers and family members.
Inclusion Criteria
We sought to speak with people who have been actively managing their condition after being diagnosed with high-grade glioma and have received 5-ALA, as well as their family members and caregivers.
Exclusion Criteria
We did not set exclusion criteria.
Participants
For this project, we spoke with one person with high-grade glioma living outside of Canada. The person with whom we spoke had direct experience with standard surgical treatment and 5-ALA– guided resection for their brain tumour.
Approach
At the beginning of the interview, we explained the role of our organization, the purpose of the health technology assessment, the risks of participation, and how the participant's personal health information would be protected. We also gave this information to the participant in a printed letter of information (Appendix 7). We obtained the participant's verbal consent before starting the interview. With the participant's consent, we audio-recorded and then transcribed the interview.
The interview lasted approximately 30 minutes. The interview was loosely structured and consisted of a series of open-ended questions. Questions were based on a list developed by the Health Technology Assessment International Interest Group on Patient and Citizen Involvement in Health Technology Assessment.88 Questions focused on the impact of high-grade glioma on the person's quality of life, their experiences with treatments to manage or treat the condition, their experiences with 5-ALA–guided resection, and their perceptions of the benefits or limitations of 5-ALA in glioma resection. See Appendix 8 for our interview guide.
Data Extraction and Analysis
We used a modified version of a grounded-theory methodology to analyze the interview transcript. The grounded-theory approach allowed us to organize information on the experiences of the participant. This method consists of a process of obtaining, documenting, and analyzing responses while simultaneously collecting, analyzing, and comparing information.89,90 We used the qualitative data analysis software program NVivo91 to identify and interpret patterns in the interview data. The patterns we identified allowed us to highlight the impact of high-grade glioma and 5-ALA on the patient we interviewed.
Results
Diagnosis of Glioma
Although the physical and emotional impact of high-grade glioma can be considerable in some people, the participant did not experience any obvious symptoms at first:
It was a lucky find, as it had no symptoms … [I] went for my yearly medical [exam] and [my ears were not symmetrical. My] doctor said to get [an] MRI to check it … There was a mass almost 3 cm [long] on the left side [of my head] … [The biopsy] came back as a Grade II glioma.
Impact
The participant reported that the news of the diagnosis left family members and friends in shock. The poor prognosis associated with gliomas also contributed to the shock, although the patient reported that not much changed in their day-to-day life:
[The diagnosis] of [the] brain tumour, for most people, was quite shocking. Like some other cancers that have a good chance of healing, I think with brain tumours—especially glioma—chances are slim to none. So that changes something in your everyday life, but not much changed for me.
Treatment: Standard Surgical Resection
The participant had experience with both the current standard surgical treatment for glioma resection and 5-ALA–guided resection for a brain tumour that had progressed. They were initially diagnosed with Grade II glioma:
First, I had a … [stereotactic] biopsy. I am lucky that the tumour is on top, so [surgeons] didn't have to go through the brain [for the biopsy], and it came back as Grade II glioma.
With Grade II glioma, the participant was not eligible to receive 5-ALA–guided resection for their first surgery, and they underwent standard surgical resection. The participant reported that while the surgeon removed everything they could see, they had to leave part of the tumour behind to avoid damaging their swallowing reflex:
Since [it was] near my speech centre, they suggested [I stay] awake [during] surgery. They got everything they could see, [but] there was a one small corner [where the neurosurgeon] said … “I cannot cut that, as it will inhibit [your] swallowing reflex.”
Diagnosis of High-Grade Glioma
With regular follow-up scans, the participant reported that they found another mass in the same spot, but that it seemed to have progressed to Grade III or IV glioma:
I went on to [get an MRI] every 6 months … right after [an] MRI, the technician said there was a less than 1 cm mass [in] the same spot. From what I understand … the colour on the MRI was different colour [that was] much brighter, so they expected it to be Grade III or IV.
After confirming that the glioma had progressed to Grade III or IV, the participant reported needing to undergo a second surgery. However, the participant now qualified for 5-ALA–guided resection. The surgeon recommending using 5-ALA, but the participant was able to make the final decision about whether or not to use it:
After the PET scan and MRI, I had a brief meeting with [the neurosurgeon] where he … basically [said]: “it looks like the metabolism of the cell has gone up, either Grade III or IV” … My plan was to have a surgery and not wait too long; I think I had 10 days between diagnosis and surgery. Then he said … “this time, we can [use] 5-ALA.” It was my decision and his suggestion. You have good chance of getting more, if not all, of [the] tumour, just because it is better and easier to see what is tumour and … [what are] healthy brain cells. Of course, the decision is always the patient's.
Treatment: 5-ALA–Guided Resection
The participant reported going to the hospital the day before surgery for necessary testing. On the day of the surgery, the participant described drinking the 5-ALA before surgery, and that it tasted bitter:
[The nurse] said to “chuck it” as it's not very tasty … I [didn't] really do it; [it] was a bit too much … [But] it doesn't taste very good. It's a bit bitter … It's not making you throw up; it's just not tasty.
The participant also stated that the 5-ALA liquid created a strange sensation on their teeth:
On the teeth, it gave a bit of a weird sensation like … if you go to the dentist sometimes as a child [and] get the fluoride … it's like a gel, so for a few minutes, [there was no] taste; [just] a bit sticky in the mouth. [I] didn't get nauseous or anything; [it was] just a bit strange.
The participant reported that the main benefit of 5-ALA was that it gave assurance to both the neurosurgeon and the patient that most of the tumour is removed. The participant felt that the 5-ALA–guided procedure may provide more accurate results, and that it helped provide a visual to see how much of their tumour was removed:
Everything that was coloured [as] 5-ALA was removed plus [a] 1–2 cm margin. My sense is that the surgery went better, [and] the result was better—more accurate, because of the 5-ALA—just because it's a visual thing. It's not just plotting tumour on the MRI or under the microscope; it is also for us who are not doctors to [visually] recognize [the] tumour.
The participant reported that one of the main risks discussed with them was the potential sensitivity to light that can occur after taking 5-ALA. The participant also stated that it was advised by the staff to not look directly at their phone for long periods.
Discussion
The participant we interviewed had direct experience with high-grade glioma, and both standard surgical resection and 5-ALA–guided resection. They reported that they had no apparent symptoms, but that a yearly checkup led to their glioma diagnosis.
The participant identified an unpleasant, bitter taste to the 5-ALA, and the need to protect against potential light sensitivity following surgery with 5-ALA. The participant was able to directly compare standard surgical resection with 5-ALA–guided resection, and felt the 5-ALA– guided resection resulted in more accurate resection, and that it felt more reassuring to see how much tumour was removed through visual confirmation with 5-ALA. The participant resided outside of Canada; however, no barriers to receiving 5-ALA (including cost) were identified.
Overall, the participant reported a positive experience with 5-ALA–guided resection and they felt it produced better results for accuracy, maximal tumour removal, and in the emotional reassurance and satisfaction of seeing how much of the tumour was removed.
Because we only interviewed one patient, we recognize that the experiences reported here may not reflect those of other patients with high grade gliomas and/or other patients who have had 5-ALA–guided surgery. Our participant outreach was conducted through an international, online support group and through the clinical experts contributing to this health technology assessment. A diagnosis of high-grade glioma can be physically and/or emotionally demanding, and as a result, patients may not have been available to participate.
Conclusions
Although we were able to interview only one participant for this assessment, the participant had the unique perspective and opportunity to comment on both standard surgical resection and 5-ALA–guided resection. The patient reported that 5-ALA had a bitter taste and resulted in a strange sensation on the teeth. Overall, the participant expressed greater satisfaction with the 5-ALA–guided resection compared with standard surgical resection.
CONCLUSIONS OF THE HEALTH TECHNOLOGY ASSESSMENT
5-aminolevulinic acid hydrochloride (5-ALA)–guided surgical resection of high-grade gliomas appears to improve the extent of tumour resection relative to standard care (GRADE: Low). The evidence suggests 5-ALA may improve overall survival; however, we cannot exclude the possibility of no effect (GRADE: Low). 5-ALA–guided resection may improve 6-month progression-free survival, although results remain highly uncertain (GRADE: Very low). The impact on adverse events was also very uncertain (GRADE: Very low). We did not identify any economic studies conducted from the perspective of the Ontario or Canadian public health care payer. Of the studies that met our inclusion criteria, most found 5-ALA–guided surgical resection was cost-effective compared to white-light microscopy for high-grade gliomas. However, clinical model inputs for the comparative effectiveness and safety of 5-ALA were based on limited and low-quality evidence. We estimate that publicly funding 5-ALA–guided surgical resection in Ontario over the next 5 years would result in a budget impact of about $930,000 in year 1 to about $1,765,000 in year 5, yielding a total 5-year budget impact of about $7,500,000. The one participant we interviewed had experience with high-grade glioma, standard surgical treatment, and 5-ALA–guided resection. The participant perceived 5-ALA–guided resection to result in accurate tumour removal and found it reassuring that 5-ALA could help the surgeon better visualize the tumour.
Acknowledgments
This report was developed by a multidisciplinary team from the Quality business unit at Ontario Health. The clinical epidemiologist was Milica Jokic, the health economics associate was Jennifer Guo, the secondary health economist was Olga Gajic-Veljanoski, the patient and public partnership analysts were Ammara Shafique and David Wells, and the medical librarian was Melissa Walter.
The medical editor was Ansely Wong. Others involved in the development and production of this report were Merissa Mohamed, Claude Soulodre, Kara Cowan, Elisabeth Smitko, Kathryn Schwarz, Sarah McDowell, Vivian Ng, Andrée Mitchell, Amy Lang, Nancy Sikich, and Irfan Dhalla.
We would like to thank the following individuals and organization for lending their expertise to the development of this report:
Dr. Alireza Mansouri, Toronto Western Hospital, University Health Network
Dr. John Sinclair, University of Ottawa
Dr. Gelareh Zadeh, Toronto Western Hospital, University Health Network
Medexus Pharmaceuticals Inc.
We also thank our lived experience participant who generously gave their time to share their story with us for this report.
The statements, conclusions, and views expressed in this report do not necessarily represent the views of those we consulted.
ABBREVIATIONS
- 5-ALA
5-Aminolevulinic acid hydrochloride
- CCI
Canadian Classification of Health Interventions
- CI
Confidence interval
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- ICER
Incremental cost-effectiveness ratio
- MRI
Magnetic resonance imaging
- NICE
National Institutes for Health and Care Excellence
- OCCI
Ontario Case Costing Initiative
- QALY
Quality-adjusted life-year
- WHO
World Health Organization
GLOSSARY
- Adverse event
An adverse event is an unexpected medical problem that happens during treatment for a health condition. Adverse events may be caused by something other than the treatment.
- Budget impact analysis
A budget impact analysis estimates the financial impact of adopting a new health care intervention on the current budget (i.e., the affordability of the new intervention). It is based on predictions of how changes in the intervention mix will impact the level of health care spending for a specific population. Budget impact analyses are typically conducted for a short-term period (e.g., 5 years). The budget impact, sometimes referred to as the net budget impact, is the estimated cost difference between the current scenario (i.e., the anticipated amount of spending for a specific population without using the new intervention) and the new scenario (i.e., the anticipated amount of spending for a specific population following the introduction of the new intervention).
- Cost-effective
A health care intervention is considered cost-effective when it provides additional benefits, compared with relevant alternatives, at an additional cost that is acceptable to a decision-maker based on the maximum willingness-to-pay value.
- Cost-effectiveness analysis
Used broadly, “cost-effectiveness analysis” may refer to an economic evaluation used to compare the benefits of two or more health care interventions with their costs. It may encompass several types of analysis (e.g., cost-effectiveness analysis, cost–utility analysis). Used more specifically, “cost-effectiveness analysis” may refer to a type of economic evaluation in which the main outcome measure is the incremental cost per natural unit of health (e.g., life-year, symptom-free day) gained.
- Cost–utility analysis
A cost–utility analysis is a type of economic evaluation used to compare the benefits of two or more health care interventions with their costs. The benefits are measured using quality-adjusted life-years, which capture both the quality and quantity of life. In a cost– utility analysis, the main outcome measure is the incremental cost per quality-adjusted life-year gained.
- Deterministic sensitivity analysis
Deterministic sensitivity analysis is an approach used to explore uncertainty in the results of an economic evaluation by varying parameter values to observe the potential impact on the cost-effectiveness of the health care intervention of interest. One-way sensitivity analysis accounts for uncertainty in parameter values one at a time, whereas multiway sensitivity analysis accounts for uncertainty in a combination of parameter values simultaneously.
- Discounting
Discounting is a method used in economic evaluations to adjust for the differential timing of the costs incurred and the benefits generated by a health care intervention over time. Discounting reflects the concept of positive time preference, whereby future costs and benefits are reduced to reflect their present value. The health technology assessments conducted by Ontario Health (Quality) use an annual discount rate of 1.5% for both future costs and future benefits.
- Health state
A health state is a particular status of health (e.g., sick, well, dead). A health state is associated with some amount of benefit and may be associated with specific costs. Benefit is captured through individual or societal preferences for the time spent in each health state and is expressed in quality-adjusted weights called utility values. In a Markov model, a finite number of mutually exclusive health states are used to represent discrete states of health.
- Incremental cost
The incremental cost is the additional cost, typically per person, of a health care intervention versus a comparator.
- Incremental cost-effectiveness ratio (ICER)
The incremental cost-effectiveness ratio (ICER) is a summary measure that indicates, for a given health care intervention, how much more a health care consumer must pay to get an additional unit of benefit relative to an alternative intervention. It is obtained by dividing the incremental cost by the incremental effectiveness. Incremental cost-effectiveness ratios are typically presented as the cost per life-year gained or the cost per quality-adjusted life-year gained.
- Markov model
A Markov model is a type of decision-analytic model used in economic evaluations to estimate the costs and health outcomes (e.g., quality-adjusted life-years gained) associated with using a particular health care intervention. Markov models are useful for clinical problems that involve events of interest that may recur over time (e.g., stroke). A Markov model consists of mutually exclusive, exhaustive health states. Patients remain in a given health state for a certain period of time before moving to another health state based on transition probabilities. The health states and events modelled may be associated with specific costs and health outcomes.
- Multiway sensitivity analysis
A multiway sensitivity analysis is used to explore uncertainty in the results of an economic evaluation. It is done by varying a combination of model input (i.e., parameter) values simultaneously between plausible extremes to observe the potential impact on the cost-effectiveness of the health care intervention of interest.
- One-way sensitivity analysis
A one-way sensitivity analysis is used to explore uncertainty in the results of an economic evaluation. It is done by varying one model input (i.e., a parameter) at a time between its minimum and maximum values to observe the potential impact on the cost-effectiveness of the health care intervention of interest.
- Quality-adjusted life-year (QALY)
The quality-adjusted life-year (QALY) is a generic health outcome measure commonly used in cost–utility analyses to reflect the quantity and quality of life-years lived. The life-years lived are adjusted for quality of life using individual or societal preferences (i.e., utility values) for being in a particular health state. One year of perfect health is represented by one quality-adjusted life-year.
- Reference case
The reference case is a preferred set of methods and principles that provide the guidelines for economic evaluations. Its purpose is to standardize the approach of conducting and reporting economic evaluations, so that results can be compared across studies.
- Sensitivity analysis
Every economic evaluation contains some degree of uncertainty, and results can vary depending on the values taken by key parameters and the assumptions made. Sensitivity analysis allows these factors to be varied and shows the impact of these variations on the results of the evaluation. There are various types of sensitivity analysis, including deterministic, probabilistic, and scenario.
- Time horizon
In economic evaluations, the time horizon is the time frame over which costs and benefits are examined and calculated. The relevant time horizon is chosen based on the nature of the disease and health care intervention being assessed, as well as the purpose of the analysis. For instance, a lifetime horizon would be chosen to capture the long-term health and cost consequences over a patient's lifetime.
- Utility
A utility is a value that represents a person's preference for various health states. Typically, utility values are anchored at 0 (death) and 1 (perfect health). In some scoring systems, a negative utility value indicates a state of health valued as being worse than death. Utility values can be aggregated over time to derive quality-adjusted life-years, a common outcome measure in economic evaluations.
- Willingness-to-pay value
A willingness-to-pay value is the monetary value a health care consumer is willing to pay for added health benefits. When conducting a cost–utility analysis, the willingness-to-pay value represents the cost a consumer is willing to pay for an additional quality-adjusted life-year. If the incremental cost-effectiveness ratio is less than the willingness-to-pay value, the health care intervention of interest is considered cost-effective. If the incremental cost-effectiveness ratio is more than the willingness-to-pay value, the intervention is considered not to be cost-effective.
APPENDICES
Appendix 1: Literature Search Strategies
Clinical Evidence Search—Systematic Reviews
Search date: April 10, 2019
Databases searched: Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, CRD Health Technology Assessment Database, and NHS Economic Evaluation Database
Database: EBM Reviews – Cochrane Database of Systematic Reviews <2005 to March 27, 2019>, EBM Reviews – Health Technology Assessment <4th Quarter 2016>, EBM Reviews –NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2019 Week 14>, Ovid MEDLINE(R) ALL <1946 to April 09, 2019>
Search strategy:
-
1
∗Brain Neoplasms/ (98247)
-
2
((brain or cerebral or intracerebral or intracranial or intra cranial) adj5 (cancer∗ or tumor∗ or tumour∗ or malignan∗ or neoplas∗ or carcinoma∗)).ti. (56921)
-
3
exp Glioma/ (194864)
-
4
(glioma∗ or astrocytoma∗ or xanthoastrocytoma∗ or glioblastoma∗ or GBM∗ or gliosarcoma∗ or oligodendroglioma∗ or oligoastrocytoma∗ or astroblastoma∗ or ganglioglioma∗ or ependymoma∗).ti,ab,kf. (210785)
-
5
((astrocytic or oligodendroglial or ependymal or glial) adj5 (cancer∗ or tumor∗ or tumour∗ or malignan∗ or neoplas∗ or carcinoma∗)).ti,ab,kf. (14417)
-
6
or/1-5 (319482)
-
7
Aminolevulinic Acid/ (13309)
-
8
(5ALA or 5 ALA or aminol?evulin∗ or amino l?evulin∗ or gliolan∗ or gleolan∗ or levulan∗).ti,ab,kf. (18257)
-
9
7 or 8 (22146)
-
10
6 and 9 (1620)
-
11
Fluorescence/ (156251)
-
12
Microscopy, Fluorescence/ (125150)
-
13
Fluorescent Dyes/ (113879)
-
14
(fluorescen∗ or fluorescing).ti,ab,kf. (927840)
-
15
or/11-14 (1039459)
-
16
Surgical Procedures, Operative/ (481477)
-
17
Brain Neoplasms/dg (11286)
-
18
Brain Neoplasms/su (22135)
-
19
Neurosurgery/ (68175)
-
20
Neurosurgical Procedures/ (78684)
-
21
Cytoreduction Surgical Procedures/ (14647)
-
22
Margins of Excision/ (8128)
-
23
Microsurgery/ (52649)
-
24
Surgery, Computer-Assisted/ (22836)
-
25
Monitoring, Intraoperative/ (19266)
-
26
Intraoperative Period/ (50126)
-
27
(surg∗ or neurosurg∗ or microsurg∗ or ablat∗ or biops∗ or cytoreduc∗ or cyto reduc∗ or debulk∗ or de bulk∗ or resect∗ or excis∗ or operat∗ or interoperat∗ or intraoperat∗ or perioperat∗).ti,ab,kf. (6923191)
-
28
Craniotomy/ (40067)
-
29
(craniotom∗ or craniectom∗).ti,ab,kf. (38016)
-
30
or/16-29 (7043782)
-
31
6 and 15 and 30 (2858)
-
32
10 or 31 (3431)
-
33
(Systematic Reviews or Meta Analysis).pt. (99337)
-
34
Systematic Review/ or Systematic Reviews as Topic/ or Meta-Analysis/ or exp Meta-Analysis as Topic/ or exp Technology Assessment, Biomedical/ (516735)
-
35
((systematic∗ or methodologic∗) adj3 (review∗ or overview∗)).ti,ab,kf. (350514)
-
36
(meta analy∗ or metaanaly∗ or met analy∗ or metanaly∗ or meta review∗ or metareview∗ or health technolog∗ assess∗ or HTA or HTAs or (technolog∗ adj (assessment∗ or overview∗ or appraisal∗))).ti,ab,kf. (358587)
-
37
(evidence adj (review∗ or overview∗ or synthes#s)).ti,ab,kf. (13495)
-
38
(review of reviews or overview of reviews).ti,ab,kf. (1249)
-
39
umbrella review∗.ti,ab,kf. (457)
-
40
GRADE Approach/ (83)
-
41
((pool∗ adj3 analy∗) or published studies or published literature or hand search∗ or handsearch∗ or manual search∗ or ((database∗ or systematic∗) adj2 search∗) or reference list∗ or bibliograph∗ or relevant journals or data synthes∗ or data extraction∗ or data abstraction∗).ti,ab,kf. (390799)
-
42
(medline or pubmed or medlars or embase or cinahl or web of science or ovid or ebsco∗ or scopus).ab. (400374)
-
43
cochrane.ti,ab,kf. (168662)
-
44
(meta regress∗ or metaregress∗).ti,ab,kf. (16225)
-
45
(((integrative or collaborative or quantitative) adj3 (review∗ or overview∗ or synthes∗)) or (research adj3 overview∗)).ti,ab,kf. (22452)
-
46
(cochrane or (health adj2 technology assessment) or evidence report or systematic review∗).jw. (60210)
-
47
((comparative adj3 (efficacy or effectiveness)) or relative effectiveness or ((indirect or indirect treatment or mixed-treatment) adj comparison∗)).ti,ab,kf. (39748)
-
48
or/33-47 (1073515)
-
49
32 and 48 (95)
-
50
exp Animals/ not Humans/ (15935384)
-
51
49 not 50 (59)
-
52
Case Reports/ or Comment.pt. or Editorial.pt. or (Letter not (Letter and Randomized Controlled Trial)).pt. or Congresses.pt. (5077690)
-
53
51 not 52 (59)
-
54
limit 53 to english language [Limit not valid in CDSR; records were retained] (58)
-
55
54 use medall,cleed (36)
-
56
limit 32 to english language [Limit not valid in CDSR; records were retained] (3292)
-
57
56 use coch,clhta (2)
-
58
55 or 57 (38)
-
59
∗brain tumor/ (120140)
-
60
∗brain cancer/ (89459)
-
61
((brain or cerebral or intracerebral or intracranial or intra cranial) adj5 (cancer∗ or tumor∗ or tumour∗ or malignan∗ or neoplas∗ or carcinoma∗)).ti. (56921)
-
62
exp glioma/ (194864)
-
63
(glioma∗ or astrocytoma∗ or xanthoastrocytoma∗ or glioblastoma∗ or GBM∗ or gliosarcoma∗ or oligodendroglioma∗ or oligoastrocytoma∗ or astroblastoma∗ or ganglioglioma∗ or ependymoma∗).tw,kw. (212544)
-
64
((astrocytic or oligodendroglial or ependymal or glial) adj5 (cancer∗ or tumor∗ or tumour∗ or malignan∗ or neoplas∗ or carcinoma∗)).tw,kw. (14523)
-
65
or/59-64 (326887)
-
66
aminolevulinic acid/ (13309)
-
67
(5ALA or 5 ALA or aminol?evulin∗ or amino l?evulin∗ or gliolan∗ or gleolan∗ or levulan∗.tw,kw,dv,du. (21668)
-
68
66 or 67 (22835)
-
69
65 and 68 (1642)
-
70
Fluorescence/ (156251)
-
71
fluorescence imaging/ (26500)
-
72
fluorescence microscopy/ (139727)
-
73
(fluorescen∗ or fluorescing).tw,kw,dv,du. (945157)
-
74
or/70-73 (1039699)
-
75
surgery/ (506746)
-
76
surgical technology/ (1851)
-
77
surgical technique/ (332974)
-
78
brain tumor/su (27742)
-
79
brain cancer/su (18838)
-
80
neurosurgery/ (68175)
-
81
brain surgery/ (10928)
-
82
cancer surgery/ (217861)
-
83
cytoreductive surgery/ (14647)
-
84
radical resection/ (4631)
-
85
surgical margin/ (8128)
-
86
microsurgery/ (52649)
-
87
image guided surgery/ (15861)
-
88
computer assisted surgery/ (24644)
-
89
intraoperative monitoring/ (19294)
-
90
intraoperative period/ (50126)
-
91
(surg∗ or neurosurg∗ or microsurg∗ or ablat∗ or biops∗ or cytoreduc∗ or cyto reduc∗ or debulk∗ or de bulk∗ or resect∗ or excis∗ or operat∗ or interoperat∗ or intraoperat∗ or perioperat∗).tw,kw. (6918974)
-
92
craniotomy/ (40067)
-
93
(craniotom∗ or craniectom∗).tw,kw. (38680)
-
94
or/75-93 (7165601)
-
95
65 and 74 and 94 (2940)
-
96
fluorescence guided surgery/ (96)
-
97
65 and 96 (40)
-
98
69 or 95 or 97 (3503)
-
99
Systematic review/ or “systematic review (topic)”/ or exp Meta Analysis/ or “Meta Analysis (Topic)”/ or Biomedical Technology Assessment/ (510175)
-
100
(meta analy∗ or metaanaly∗ or health technolog∗ assess∗ or systematic review∗).hw. (504595)
-
101
((systematic∗ or methodologic∗) adj3 (review∗ or overview∗)).tw,kw. (361951)
-
102
(meta analy∗ or metaanaly∗ or met analy∗ or metanaly∗ or meta review∗ or metareview∗ or health technolog∗ assess∗ or HTA or HTAs or (technolog∗ adj (assessment∗ or overview∗ or appraisal∗))).tw,kw. (385371)
-
103
(evidence adj (review∗ or overview∗ or synthes#s)).tw,kw. (13872)
-
104
(review of reviews or overview of reviews).tw,kw. (1436)
-
105
umbrella review∗.tw,kw. (495)
-
106
((pool∗ adj3 analy∗) or published studies or published literature or hand search∗ or handsearch∗ or manual search∗ or ((database∗ or systematic∗) adj2 search∗) or reference list∗ or bibliograph∗ or relevant journals or data synthes∗ or data extraction∗ or data abstraction∗).tw,kw. (416037)
-
107
(medline or pubmed or medlars or embase or cinahl or web of science or ovid or ebsco∗ or scopus).ab. (400374)
-
108
cochrane.tw,kw. (172275)
-
109
(meta regress∗ or metaregress∗).tw,kw. (17126)
-
110
(((integrative or collaborative or quantitative) adj3 (review∗ or overview∗ or synthes∗)) or (research adj3 overview∗)).tw,kw. (23312)
-
111
(cochrane or (health adj2 technology assessment) or evidence report or systematic review∗).jw. (60210)
-
112
((comparative adj3 (efficacy or effectiveness)) or relative effectiveness or ((indirect or indirect treatment or mixed-treatment) adj comparison∗)).tw,kw. (41367)
-
113
or/99-112 (1100184)
-
114
98 and 113 (104)
-
115
(exp animal/ or nonhuman/) not exp human/ (10227090)
-
116
114 not 115 (103)
-
117
Case Report/ or Comment/ or Editorial/ or (letter.pt. not (letter.pt. and randomized controlled trial/)) or conference abstract.pt. (10310476)
-
118
116 not 117 (98)
-
119
limit 118 to english language [Limit not valid in CDSR; records were retained] (96)
-
120
119 use emez (55)
-
121
58 or 120 (93)
-
122
121 use medall (36)
-
123
121 use coch (2)
-
124
121 use clhta (0)
-
125
121 use cleed (0)
-
126
121 use emez (55)
-
127
remove duplicates from 121 (57)
Clinical Evidence Search—Primary Studies
Search date: April 22, 2019
Databases searched: Ovid MEDLINE, Embase, Cochrane Central Register of Controlled Trials, and NHS Economic Evaluation Database
Database: EBM Reviews – Cochrane Central Register of Controlled Trials <March 2019>, EBM Reviews – NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2019 Week 16>, Ovid MEDLINE(R) ALL <1946 to April 19, 2019>
Search strategy:
-
1
∗Brain Neoplasms/ (98464)
-
2
((brain or cerebral or intracerebral or intracranial or intra cranial) adj5 (cancer∗ or tumor∗ or tumour∗ or malignan∗ or neoplas∗ or carcinoma∗)).ti. (58139)
-
3
exp Glioma/ (196476)
-
4
(glioma∗ or astrocytoma∗ or xanthoastrocytoma∗ or glioblastoma∗ or GBM∗ or gliosarcoma∗ or oligodendroglioma∗ or oligoastrocytoma∗ or astroblastoma∗ or ganglioglioma∗ or ependymoma∗).ti,ab,kf. (214439)
-
5
((astrocytic or oligodendroglial or ependymal or glial) adj5 (cancer∗ or tumor∗ or tumour∗ or malignan∗ or neoplas∗ or carcinoma∗)).ti,ab,kf. (14519)
-
6
or/1-5 (324279)
-
7
Aminolevulinic Acid/ (13747)
-
8
(5ALA or 5 ALA or aminol?evulin∗ or amino l?evulin∗ or gliolan∗ or gleolan∗ or levulan∗).ti,ab,kf. (19033)
-
9
7 or 8 (23010)
-
10
6 and 9 (1664)
-
11
Fluorescence/ (157126)
-
12
Microscopy, Fluorescence/ (125579)
-
13
Fluorescent Dyes/ (114406)
-
14
(fluorescen∗ or fluorescing).ti,ab,kf. (934022)
-
15
or/11-14 (1046060)
-
16
Surgical Procedures, Operative/ (487233)
-
17
Brain Neoplasms/dg (11311)
-
18
Brain Neoplasms/su (22174)
-
19
Neurosurgery/ (68390)
-
20
Neurosurgical Procedures/ (79271)
-
21
Cytoreduction Surgical Procedures/ (15021)
-
22
Margins of Excision/ (8463)
-
23
Microsurgery/ (53258)
-
24
Surgery, Computer-Assisted/ (23493)
-
25
Monitoring, Intraoperative/ (20781)
-
26
Intraoperative Period/ (51427)
-
27
(surg∗ or neurosurg∗ or microsurg∗ or ablat∗ or biops∗ or cytoreduc∗ or cyto reduc∗ or debulk∗ or de bulk∗ or resect∗ or excis∗ or operat∗ or interoperat∗ or intraoperat∗ or perioperat∗).ti,ab,kf. (7193317)
-
28
Craniotomy/ (40545)
-
29
(craniotom∗ or craniectom∗).ti,ab,kf. (39278)
-
30
or/16-29 (7315974)
-
31
6 and 15 and 30 (2910)
-
32
10 or 31 (3497)
-
33
Clinical Trials as Topic/ (290760)
-
34
controlled clinical trials as topic/ (14136)
-
35
exp Randomized Controlled Trials as Topic/ (291239)
-
36
controlled clinical trial.pt. (183869)
-
37
randomized controlled trial.pt. (948648)
-
38
Pragmatic Clinical Trial.pt. (2025)
-
39
Random Allocation/ (197245)
-
40
Single-Blind Method/ (78496)
-
41
Double-Blind Method/ (403688)
-
42
Placebos/ (319374)
-
43
trial.ti. (731719)
-
44
(random∗ or sham or placebo∗ or RCT∗1).ti,ab,kf. (3662479)
-
45
((singl∗ or doubl∗) adj (blind∗ or dumm∗ or mask∗)).ti,ab,kf. (620931)
-
46
((tripl∗ or trebl∗) adj (blind∗ or dumm∗ or mask∗)).ti,ab,kf. (3227)
-
47
or/33-46 (4659869)
-
48
exp Animals/ not Humans/ (15933708)
-
49
47 not 48 (3516030)
-
50
32 and 49 (161)
-
51
Case Reports/ or Comment.pt. or Editorial.pt. or (Letter not (Letter and Randomized Controlled Trial)).pt. or Congresses.pt. (5090930)
-
52
50 not 51 (159)
-
53
limit 52 to english language (146)
-
54
limit 53 to yr=“2017 –Current” (45)
-
55
54 use medall,cleed (19)
-
56
limit 32 to english language (3333)
-
57
limit 56 to yr=“2017 –Current” (801)
-
58
57 use cctr (9)
-
59
55 or 58 (28)
-
60
∗brain tumor/ (120340)
-
61
∗brain cancer/ (89617)
-
62
((brain or cerebral or intracerebral or intracranial or intra cranial) adj5 (cancer∗ or tumor∗ or tumour∗ or malignan∗ or neoplas∗ or carcinoma∗)).ti. (58139)
-
63
exp glioma/ (196476)
-
64
(glioma∗ or astrocytoma∗ or xanthoastrocytoma∗ or glioblastoma∗ or GBM∗ or gliosarcoma∗ or oligodendroglioma∗ or oligoastrocytoma∗ or astroblastoma∗ or ganglioglioma∗ or ependymoma∗).tw,kw. (216272)
-
65
((astrocytic or oligodendroglial or ependymal or glial) adj5 (cancer∗ or tumor∗ or tumour∗ or malignan∗ or neoplas∗ or carcinoma∗)).tw,kw. (14608)
-
66
or/60-65 (331729)
-
67
aminolevulinic acid/ (13747)
-
68
(5ALA or 5 ALA or aminol?evulin∗ or amino l?evulin∗ or gliolan∗ or gleolan∗ or levulan∗).tw,kw,dv,du. (22440)
-
69
67 or 68 (23684)
-
70
66 and 69 (1684)
-
71
Fluorescence/ (157126)
-
72
fluorescence imaging/ (26878)
-
73
fluorescence microscopy/ (140156)
-
74
(fluorescen∗ or fluorescing).tw,kw,dv,du. (951790)
-
75
or/71-74 (1046721)
-
76
surgery/ (511581)
-
77
surgical technology/ (1854)
-
78
surgical technique/ (333603)
-
79
brain tumor/su (27781)
-
80
brain cancer/su (18862)
-
81
neurosurgery/ (68390)
-
82
brain surgery/ (10955)
-
83
cancer surgery/ (221228)
-
84
cytoreductive surgery/ (14990)
-
85
radical resection/ (4688)
-
86
surgical margin/ (8424)
-
87
microsurgery/ (53258)
-
88
image guided surgery/ (16497)
-
89
computer assisted surgery/ (25301)
-
90
intraoperative monitoring/ (20809)
-
91
intraoperative period/ (51427)
-
92
(surg∗ or neurosurg∗ or microsurg∗ or ablat∗ or biops∗ or cytoreduc∗ or cyto reduc∗ or debulk∗ or de bulk∗ or resect∗ or excis∗ or operat∗ or interoperat∗ or intraoperat∗ or perioperat∗).tw,kw. (7194261)
-
93
craniotomy/ (40545)
-
94
(craniotom∗ or craniectom∗).tw,kw. (39995)
-
95
or/76-94 (7443135)
-
96
66 and 75 and 95 (2990)
-
97
fluorescence guided surgery/ (96)
-
98
66 and 97 (40)
-
99
70 or 96 or 98 (3566)
-
100
“clinical trial (topic)”/ (100214)
-
101
“controlled clinical trial (topic)”/ (9952)
-
102
“randomized controlled trial (topic)”/ (158124)
-
103
randomization/ (180388)
-
104
Single Blind Procedure/ (34649)
-
105
Double Blind Procedure/ (156326)
-
106
placebo/ (318462)
-
107
trial.ti. (731719)
-
108
(random∗ or sham or placebo∗ or RCT∗1).tw,kw. (3719670)
-
109
((singl∗ or doubl∗) adj (blind∗ or dumm∗ or mask∗)).tw,kw. (644680)
-
110
((tripl∗ or trebl∗) adj (blind∗ or dumm∗ or mask∗)).tw,kw. (3246)
-
111
or/100-110 (4278701)
-
112
(exp animal/ or nonhuman/) not exp human/ (10245323)
-
113
111 not 112 (3817406)
-
114
99 and 113 (228)
-
115
Case Report/ or Comment/ or Editorial/ or (letter.pt. not (letter.pt. and randomized controlled trial/)) or conference abstract.pt. (10367620)
-
116
114 not 115 (186)
-
117
limit 116 to english language (173)
-
118
limit 117 to yr=“2017 –Current” (47)
-
119
118 use emez (24)
-
120
59 or 119 (52)
-
121
120 use medall (19)
-
122
120 use cctr (9)
-
123
120 use cleed (0)
-
124
120 use emez (24)
-
125
remove duplicates from 120 (34)
Economic Evidence Search
Search date: April 11, 2019
Databases searched: Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, CRD Health Technology Assessment Database, and NHS Economic Evaluation Database
Database: EBM Reviews – Cochrane Central Register of Controlled Trials <March 2019>, EBM Reviews – Cochrane Database of Systematic Reviews <2005 to April 10, 2019>, EBM Reviews –Health Technology Assessment <4th Quarter 2016>, EBM Reviews – NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2019 Week 14>, Ovid MEDLINE(R) ALL <1946 to April 10, 2019>
Search strategy:
-
1
∗Brain Neoplasms/ (98268)
-
2
((brain or cerebral or intracerebral or intracranial or intra cranial) adj5 (cancer∗ or tumor∗ or tumour∗ or malignan∗ or neoplas∗ or carcinoma∗)).ti. (58009)
-
3
exp Glioma/ (195903)
-
4
(glioma∗ or astrocytoma∗ or xanthoastrocytoma∗ or glioblastoma∗ or GBM∗ or gliosarcoma∗ or oligodendroglioma∗ or oligoastrocytoma∗ or astroblastoma∗ or ganglioglioma∗ or ependymoma∗).ti,ab,kf. (213745)
-
5
((astrocytic or oligodendroglial or ependymal or glial) adj5 (cancer∗ or tumor∗ or tumour∗ or malignan∗ or neoplas∗ or carcinoma∗)).ti,ab,kf. (14489)
-
6
or/1-5 (323363)
-
7
Aminolevulinic Acid/ (13718)
-
8
(5ALA or 5 ALA or aminol?evulin∗ or amino l?evulin∗ or gliolan∗ or gleolan∗ or levulan∗).ti,ab,kf. (19017)
-
9
7 or 8 (22981)
-
10
6 and 9 (1661)
-
11
Fluorescence/ (156475)
-
12
Microscopy, Fluorescence/ (125254)
-
13
Fluorescent Dyes/ (114061)
-
14
(fluorescen∗ or fluorescing).ti,ab,kf. (931234)
-
15
or/11-14 (1043060)
-
16
Surgical Procedures, Operative/ (482449)
-
17
Brain Neoplasms/dg (11290)
-
18
Brain Neoplasms/su (22136)
-
19
Neurosurgery/ (68251)
-
20
Neurosurgical Procedures/ (79117)
-
21
Cytoreduction Surgical Procedures/ (14680)
-
22
Margins of Excision/ (8172)
-
23
Microsurgery/ (53134)
-
24
Surgery, Computer-Assisted/ (23527)
-
25
Monitoring, Intraoperative/ (20775)
-
26
Intraoperative Period/ (51331)
-
27
(surg∗ or neurosurg∗ or microsurg∗ or ablat∗ or biops∗ or cytoreduc∗ or cyto reduc∗ or debulk∗ or de bulk∗ or resect∗ or excis∗ or operat∗ or interoperat∗ or intraoperat∗ or perioperat∗).ti,ab,kf. (7173655)
-
28
Craniotomy/ (40447)
-
29
(craniotom∗ or craniectom∗).ti,ab,kf. (39155)
-
30
or/16-29 (7295233)
-
31
6 and 15 and 30 (2900)
-
32
10 or 31 (3484)
-
33
economics/ (251355)
-
34
economics, medical/ or economics, pharmaceutical/ or exp economics, hospital/ or economics, nursing/ or economics, dental/ (812102)
-
35
economics.fs. (417679)
-
36
(econom∗ or price or prices or pricing or priced or discount∗ or expenditure∗ or budget∗ or pharmacoeconomic∗ or pharmaco-economic∗).ti,ab,kf. (861226)
-
37
exp “costs and cost analysis”/ (569244)
-
38
(cost or costs or costing or costly).ti. (257561)
-
39
cost effective∗.ti,ab,kf. (315945)
-
40
(cost∗ adj2 (util∗ or efficacy∗ or benefit∗ or minimi∗ or analy∗ or saving∗ or estimate∗ or allocation or control or sharing or instrument∗ or technolog∗)).ab,kf. (207425)
-
41
models, economic/ (12410)
-
42
markov chains/ or monte carlo method/ (78412)
-
43
(decision adj1 (tree∗ or analy∗ or model∗)).ti,ab,kf. (40843)
-
44
(markov or markow or monte carlo).ti,ab,kf. (125168)
-
45
quality-adjusted life years/ (38524)
-
46
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).ti,ab,kf. (70726)
-
47
((adjusted adj1 (quality or life)) or (willing∗ adj2 pay) or sensitivity analys∗s).ti,ab,kf. (114778)
-
48
or/33-47 (2483925)
-
49
32 and 48 (103)
-
50
Case Reports/ or Comment.pt. or Editorial.pt. or (Letter not (Letter and Randomized Controlled Trial)).pt. or Congresses.pt. (5080536)
-
51
49 not 50 (100)
-
52
exp Animals/ not Humans/ (15935908)
-
53
51 not 52 (59)
-
54
limit 53 to english language [Limit not valid in CDSR; records were retained] (55)
-
55
54 use medall,coch,cctr,clhta (31)
-
56
limit 32 to english language [Limit not valid in CDSR; records were retained] (3321)
-
57
56 use cleed (0)
-
58
55 or 57 (31)
-
59
∗brain tumor/ (120161)
-
60
∗brain cancer/ (89480)
-
61
((brain or cerebral or intracerebral or intracranial or intra cranial) adj5 (cancer∗ or tumor∗ or tumour∗ or malignan∗ or neoplas∗ or carcinoma∗)).ti. (58009)
-
62
exp glioma/ (195903)
-
63
(glioma∗ or astrocytoma∗ or xanthoastrocytoma∗ or glioblastoma∗ or GBM∗ or gliosarcoma∗ or oligodendroglioma∗ or oligoastrocytoma∗ or astroblastoma∗ or ganglioglioma∗ or ependymoma∗).tw,kw. (215628)
-
64
((astrocytic or oligodendroglial or ependymal or glial) adj5 (cancer∗ or tumor∗ or tumour∗ or malignan∗ or neoplas∗ or carcinoma∗)).tw,kw. (14595)
-
65
or/59-64 (330869)
-
66
aminolevulinic acid/ (13718)
-
67
(5ALA or 5 ALA or aminol?evulin∗ or amino l?evulin∗ or gliolan∗ or gleolan∗ or levulan∗).tw,kw,dv,du. (22436)
-
68
66 or 67 (23678)
-
69
65 and 68 (1683)
-
70
Fluorescence/ (156475)
-
71
fluorescence imaging/ (26546)
-
72
fluorescence microscopy/ (139831)
-
73
(fluorescen∗ or fluorescing).tw,kw,dv,du. (949087)
-
74
or/70-73 (1043749)
-
75
surgery/ (506756)
-
76
surgical technology/ (1851)
-
77
surgical technique/ (332975)
-
78
brain tumor/su (27743)
-
79
brain cancer/su (18839)
-
80
neurosurgery/ (68251)
-
81
brain surgery/ (10928)
-
82
cancer surgery/ (217861)
-
83
cytoreductive surgery/ (14649)
-
84
radical resection/ (4631)
-
85
surgical margin/ (8133)
-
86
microsurgery/ (53134)
-
87
image guided surgery/ (16552)
-
88
computer assisted surgery/ (25335)
-
89
intraoperative monitoring/ (20803)
-
90
intraoperative period/ (51331)
-
91
(surg∗ or neurosurg∗ or microsurg∗ or ablat∗ or biops∗ or cytoreduc∗ or cyto reduc∗ or debulk∗ or de bulk∗ or resect∗ or excis∗ or operat∗ or interoperat∗ or intraoperat∗ or perioperat∗).tw,kw. (7179978)
-
92
craniotomy/ (40447)
-
93
(craniotom∗ or craniectom∗).tw,kw. (39903)
-
94
or/75-93 (7427407)
-
95
65 and 74 and 94 (2985)
-
96
fluorescence guided surgery/ (96)
-
97
65 and 96 (40)
-
98
69 or 95 or 97 (3559)
-
99
Economics/ (251355)
-
100
Health Economics/ or Pharmacoeconomics/ or Drug Cost/ or Drug Formulary/ (126795)
-
101
Economic Aspect/ or exp Economic Evaluation/ (445673)
-
102
(econom∗ or price or prices or pricing or priced or discount∗ or expenditure∗ or budget∗ or pharmacoeconomic∗ or pharmaco-economic∗).tw,kw. (886697)
-
103
exp “Cost”/ (569244)
-
104
(cost or costs or costing or costly).ti. (257561)
-
105
cost effective∗.tw,kw. (328133)
-
106
(cost∗ adj2 (util∗ or efficac∗ or benefit∗ or minimi∗ or analy∗ or saving∗ or estimate∗ or allocation or control or sharing or instrument∗ or technolog∗)).ab,kw. (218263)
-
107
Monte Carlo Method/ (62556)
-
108
(decision adj1 (tree∗ or analy∗ or model∗)).tw,kw. (44640)
-
109
(markov or markow or monte carlo).tw,kw. (130219)
-
110
Quality-Adjusted Life Years/ (38524)
-
111
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw,kw. (74549)
-
112
((adjusted adj1 (quality or life)) or (willing∗ adj2 pay) or sensitivity analys∗s).tw,kw. (135345)
-
113
or/99-112 (2127538)
-
114
98 and 113 (91)
-
115
Case Report/ or Comment/ or Editorial/ or (letter.pt. not (letter.pt. and randomized controlled trial/)) or conference abstract.pt. (10332230)
-
116
114 not 115 (76)
-
117
(exp animal/ or nonhuman/) not exp human/ (10227620)
-
118
116 not 117 (75)
-
119
limit 118 to english language [Limit not valid in CDSR; records were retained] (71)
-
120
119 use emez (40)
-
121
58 or 120 (71)
-
122
121 use medall (29)
-
123
121 use coch (1)
-
124
121 use cctr (1)
-
125
121 use clhta (0)
-
126
121 use cleed (0)
-
127
121 use emez (40)
-
128
remove duplicates from 121 (50)
Grey Literature Search
Performed: April 9–11, 2019
Websites searched:
HTA Database Canadian Repository, Alberta Health Technologies Decision Process reviews, Canadian Agency for Drugs and Technologies in Health (CADTH), Institut national d'excellence en santé et en services sociaux (INESSS), Institute of Health Economics (IHE), McGill University Health Centre Health Technology Assessment Unit, National Institute for Health and Care Excellence (NICE), Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Centers, Australian Government Medical Services Advisory Committee, Centers for Medicare & Medicaid Services Technology Assessments, Institute for Clinical and Economic Review, Ireland Health Information and Quality Authority Health Technology Assessments, Washington State Health Care Authority Health Technology Reviews, PROSPERO, EUnetHTA, Tuft's Cost-Effectiveness Analysis Registry
Keywords used: glioma, gliomas, glioblastoma, glioblastomas, 5ALA, 5 ALA, aminolevulinic, aminolaevulinic, fluorescence, fluorescent
Results (included in PRISMA): 3
Ongoing HTAs (PROSPERO/EUnetHTA): 4
Appendix 2: Selected Excluded Studies—Clinical Evidence
For transparency, we provide a list of studies that readers might have expected to see but that did not meet the inclusion criteria, along with the primary reason for exclusion.
Table A1:
Selected Excluded Studies—Clinical Evidence
| Citation | Primary Reason for Exclusion |
|---|---|
| Eljamel S. 5-ALA fluorescence image guided resection of glioblastoma multiforme: a meta-analysis of the literature. Int J Mol Sci. 2015;16(5):10443–56. | No search dates provided |
| Barbosa BJ, Mariano ED, Batista CM, Marie SK, Teixeira MJ, Pereira CU, Tatagiba MS, Lepski GA. Intraoperative assistive technologies and extent of resection in gliomasurgery: a systematic review of prospective controlled studies. Neurosurg Rev. 2015;38(2):217–26; discussion 226–7. | No search dates provided |
| Eljamel SM, Mahboob OS. The effectiveness and cost-effectiveness of intraoperative imaging in high-grade glioma resection; a comparative review of intraoperative ALA, fluorescein, ultrasound and MRI. Photodiagnosis Photodyn Ther. 2016;16:35–43. | No search dates provided |
| Suero Molina E, Schipmann S, Stummer W. Maximizing safe resections: the roles of 5-aminolevulinic acid and intraoperative MR imaging in glioma surgery-review of the literature. Neurosurg Rev. 2019;42(2):197–208. | No population or outcomes prespecified |
Appendix 3: Summary of Identified Systematic Reviews and Health Technology Assessments Meeting Study Selection Criteria
Table A2:
Characteristics of Systematic Reviews Considered for Inclusion
| Author, Year Literature Search End Date | Population | Intervention | Comparator(s) Relevant to our Review | Outcomes | Study Types Included |
| Coburger et al, 201942 March 2018 |
|
|
NS |
|
Not clear |
| Jenkinson et al, 201845 June 2017 |
|
Fluorescence-guided surgery (including 5-ALA)a |
|
|
RCTs |
| Haider et al, 201844 June 2018 |
|
5-ALA | NS | Unclear | |
| NICE, 201816 May 2017 |
|
5-ALAa |
|
|
RCTs |
| Senders et al, 201747 March 2016 |
|
Fluorescent agents | NS |
|
Articles in clinical and preclinical setting |
| Mansouri et al, 201646 February 2015 |
|
5-ALA as adjunctive tool | Conventional surgery (specific interventions not prespecified) |
|
Excluded: case reports, case series, < 3 patients |
| Ferraro et al, 201643 May 2014 |
|
5-ALA | NS |
|
Retrospective, prospective, and clinical trials |
| Barone et al, 201441 March 2013 |
|
Fluorescence-guided surgery (including 5-ALA)a |
|
|
RCTs |
| Su et al, 201448 September 2013 |
|
Fluorescence-guided resection (including 5-ALA) | NS (but studies required a control) |
|
RCTs, quasi-RCTs, and studies with a control |
| Zhao et al, 201327 October 2012 |
|
5-ALA | Conventional neuronavigation-guided resection |
|
Prospective studies, level 1 and 2 evidence |
Abbreviations: 5-ALA, 5-aminolevulinic acid hydrochloride; CNS, central nervous system; iMRI, intraoperative magnetic resonance imaging; iUS, intraoperative ultrasound; NICE, National Institute for Health and Care Excellence; NS, not specified; QOL, quality of life; RCT, randomized controlled trial.
Systematic review compared all interventions to each other.
Reported on in results, but not specified in methods.
Appendix 4: Critical Appraisal of Clinical Evidence
Table A3:
Risk of Biasa Among Systematic Reviews (ROBIS Tool)
| Phase 2 | Phase 3 | ||||
|---|---|---|---|---|---|
| Author, Year | Study Eligibility Criteria | Identification and Selection of Studies | Data Collection and Study Appraisal | Synthesis and Findings | Risk of Bias in the Review |
| Coburger et al, 201942 | High | High | High | High | High |
| Jenkinson et al, 201845 | Low | Low | Low | Low | Low |
| Haider et al, 201844 | High | High | High | High | High |
| NICE, 201816 | Low | Low | High | Low | Low |
| Senders et al, 201747 | High | Low | High | High | High |
| Mansouri et al, 201646 | Low | Low | High | High | High |
| Ferraro et al, 201643 | High | High | High | High | High |
| Barone et al, 201441 | Low | Low | Low | Low | Low |
| Su et al, 201448 | Low | High | High | High | High |
| Zhao et al, 201327 | Low | Low | Unclear | High | High |
Abbreviation: ROBIS, Risk of Bias in Systematic Reviews.
Possible risk of bias levels: low, high, unclear.
Appendix 5: Results of Applicability and Limitation Checklists for Studies Included in the Economic Literature Review
Table A4:
Assessment of the Applicability of Studies Evaluating the Cost-Effectiveness of 5-ALA–Guided Resection
| Author, Year, Country of Publication | Is the study population similar to the question? | Are the interventions similar to the question? | Is the health care system studied sufficiently similar to Ontario? | Were the perspectives clearly stated? If yes, what were they? | Are all direct effects included? Are all other effects included where they are material? | Are all future costs and outcomes discounted? If yes, at what rate? | Is the value of health effects expressed in terms of quality-adjusted life-years? | Are costs and outcomes from other sectors fully and appropriately measured and valued? | Overall Judgmenta |
|---|---|---|---|---|---|---|---|---|---|
| NICE, 2018, United Kingdom16 | Partially | Yes | Partially | Yes; NHS and PSS | Partially | Yes; 3.5% for both future costs and outcomes | Yes | Partially | Partially applicable |
| MSAC, 2016, Australia33 | Partially | Yes | Partially | Yes; Australian health care system | Partially | Yes; 5% for both future costs and outcomes | Yes | Partially | Partially applicable |
| Elijamel et al, 2016, NR57 | Yes | Yes | Unclear | No | Partially | Unclear | Yes | Partially | Not applicable |
| Esteves et al, 2015, Portugal55 | Yes | Yes | Partially | Yes; Portuguese National Health Services | Partially | Yes; 5% for both future costs and outcomes | Yes | Partially | Partially applicable |
| Slof et al, 2015, Spain56 | Partially | Yes | Partially | Yes; Spanish health care system | Partially | No; no discounting | Yes | Partially | Partially applicable |
Abbreviations: MSAC, Medical Services Advisory Committee; NHS, National Health Service; NICE, National Institute for Health and Care Excellence; NR, not reported; PFS, progression free survival; PSS, Personal Social Services.
Note: Response options for all items were “yes,” “partially,” “no,” “unclear,” and “NA” (not applicable).
Overall judgment may be “directly applicable,” “partially applicable,” or “not applicable.”
Table A5:
Assessment of the Limitations of Studies Evaluating the Cost-Effectiveness of 5-ALA–Guided Surgical Resection
| Author, Year, Country of Publication | Does the model structure adequately reflect the nature of the health condition under evaluation? | Is the time horizon sufficiently long to reflect all important differences in costs and outcomes? | Are all important and relevant health outcomes included? | Are the clinical inputsa obtained from the best available sources? | Do the clinical inputsa match the estimates contained in the clinical sources? | Are all important and relevant (direct) costs included in the analysis? | Are the estimates of resource use obtained from the best available sources? | Are the unit costs of resources obtained from the best available sources? | Is an appropriate incremental analysis presented, or can it be calculated from the reported data? | Are all important and uncertain parameters subjected to appropriate sensitivity analysis? | Is there a potential conflict of interest? | Overall Judgmentb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NICE, 2018, United Kingdom16 | Partiallyc | Yes | Partially | Unclear | Yes | Partially | Yes | Yes | Yes | Yes | No | Potentially serious limitations |
| MSAC, 2016, Australia33 | Partiallyc | Yes | Partially | Unclear | Yes | Partiallyd | Yes | Partiallye | Yes | Nof | Not applicable | Potentially serious limitations |
| Elijamel et al, 2016, NR57 | Unclear | Unclear | Partially | Unclear | Yes | Unclear | Yes | Unclear | Yes | Nof | Unclear | Very serious limitations |
| Esteves et al, 2015, Portugal55 | Partiallyc | Yes | Partially | Unclear | Yes | Partiallyd | Yes | Yes | Yes | Nof | Yesg | Potentially serious limitations |
| Slof et al, 2015, Spain56 | Partiallyc | Unclear | Partially | Noh | Yes | Partiallyd | Yes | Yes | Yes | Partiallyf | Yesi | Potentially serious limitations |
Abbreviations: 5-ALA, 5-aminolevulinic acid hydrochloride; HTA, health technology assessment; MSAC, Medical Services Advisory Committee; NICE, National Institute for Health and Care Excellence.
Note: Response options for all items were “yes,” “partially,” “no,” “unclear,” and “NA” (not applicable).
Clinical inputs include relative treatment effects, natural history, and utilities.
Overall judgment may be “very serious limitations,” “potentially serious limitations,” or “minor limitations.”
Model accounts for 5-ALA at initial surgery only.
Reference case did not account for at least one of the following: cost of additional vial of 5-ALA required per patient >75 kg body weight, cost of fluorescence module, and/or cost of additional surgical resections accounting for recurrent tumours.
Unit cost of 5-ALA used in MSAC HTA, at $3,990 AUD (cost year not reported), was markedly higher as compared with previously reported prices.
Sensitivity analysis did not explore how results are affected by varying input parameters and/or assumptions for the unit cost of 5-ALA and/or cost of fluorescence module.
Support for this study was provided by medac GmbH. Dr. Esteves, M., Alves, and Dr. Castel-Branco received a grant from medac GmbH in relation to this study. Dr. Stummer received lecturing fees from medac.
Clinical inputs were derived from VISIONA observational trial.60
This study was financed by Laboratorios Gebro Pharma.
Appendix 6: Additional Tables—Budget Impact Analysis
Table A6:
Annual Surgical Volumes of Newly Diagnosed, Primary Malignant Brain Tumours in Ontario, 2013 to 2017a
| Year | ||||||||
|---|---|---|---|---|---|---|---|---|
| 2017 | 2016 | 2015 | 2014 | 2013 | Average, by Hospital | Average, by Typeb | Proportion, by Type | |
| Neuro-oncology Surgical Sites | ||||||||
| The Ottawa Hospital | 93 | 97 | 124 | 111 | 97 | 105 | 590c | 75% |
| St. Michael's Hospital | 123 | 136 | 104 | 95 | 128 | 117 | (590/785) | |
| Toronto Western Hospital | 82 | 83 | 92 | 97 | 83 | 87 | ||
| Sunnybrook Health Sciences Centre | 101 | 89 | 70 | 77 | 79 | 83 | ||
| London Health Sciences Centre | 73 | 66 | 67 | 79 | 67 | 70 | ||
| Hamilton Health Sciences Centre | 138 | 123 | 122 | 137 | 121 | 128 | ||
| General Neurosurgical Sites | ||||||||
| Kingston General Hospital | 24 | 49 | 41 | 33 | 34 | 36 | 190c | 25% |
| Trillium Health Partners | 100 | 90 | 102 | 70 | 82 | 89 | (195/785) | |
| Health Sciences North | 21 | 20 | 38 | 27 | 40 | 29 | ||
| Thunder Bay Regional Hospital | 5 | 9 | 7 | 8 | 7 | 7 | ||
| Windsor Regional Hospital | 44 | 33 | 34 | 20 | 31 | 32 | ||
| Total | 804 | 795 | 801 | 754 | 769 | 785 | 785 | 100% |
Source: IntelliHealth Ontario.
Report generated from IntelliHealth Ontario, using ICD-10-CA codes for malignant neoplasm of the brain, specific to the anatomic locations of gliomas (C71.0 to C71.9), filtered by the CCI intervention codes for excision of the brain using craniotomy (1.AN.87.SZ.^^), and by the age group 18 years of age or older; results are reported in annual hospital average of number of inpatient discharges with valid health care card number in Ontario for the calendar years 2013 to 2017.
May not be exact due to rounding.
Estimates are rounded to the nearest five.
Table A7:
Estimated Annual Incidence of Newly Diagnosed, Primary High-Grade Gliomas in Ontario for Which Surgical Resection is Feasible—Based on 2013 Reported Incidence (Ontario Cancer Profile 2018)
| Description | Data/Calculation | Source |
|---|---|---|
| Annual incidence of newly diagnosed, primary brain tumoursa | 1,975 | Ontario Cancer Profile 2018 |
| Percentage of newly diagnosed, primary brain tumours that are malignant | 53.5% | Ostrom et al, 20181 |
| Percentage of newly diagnosed, primary malignant brain tumours that are gliomas | 81% | Ostrom et al, 20181 |
| Percentage of newly diagnosed, primary high-grade gliomas for which maximal safe resection is feasible | 85.3% | Ostrom et al, 20181 |
| Estimate of annual incidence of primary malignant brain tumours in Ontario | 1,057 (1,975 × 0.535) | Calculationb |
| Estimate of annual incidence of newly diagnosed, primary high-grade gliomas | 856 (1,057 × 0.81) | Calculationb |
| Estimate of resectable high-grade gliomas | 730 (856 × 0.853) | Calculationb |
Ontario Cancer Profile 2018.
Estimates from calculations are rounded to the nearest whole number.
Table A8:
Average Annual Proportion of Malignant Brain Tumour Surgeries (≥ 18 Years of Age) Using Intraoperative Ultrasound, Fiscal Years 2014/15 to 2016/17a
| Fiscal Year | Cases With iUS (n) | Total Cases (n) | Proportion |
|---|---|---|---|
| 2014/15 | 25 | 472 | 5% (25/472) |
| 2015/16 | 33 | 375 | 9% (33/375) |
| 2016/17 | 20 | 587 | 3% (20/587) |
| Average proportion | 5%bc | ||
| Average number of cases (n) | 45bc |
Source: OCCI.
Abbreviations: CCI, Canadian Classification of Health Interventions; iUS, intraoperative ultrasound; OCCI, Ontario Case Costing Initiative.
Report generated from OCCI, using the CCI intervention codes for excision of the brain using craniotomy (1.AN.87.SZ.^^), filtered by the age group 18 years of age or older for fiscal years 2014/15 to 2016/17. Results are in number of cases with the appropriate functional centre cost associated with iUS.
Estimates rounded to the nearest five.
May not be exact due to rounding.
Table A9:
Estimated Uptake Rate and Corresponding Volumes of 5-ALA–Guided Surgical Resection at Neuro-oncology Surgical Sites in Future Scenario of Budget Impact Analysisa
| Name of Hospital | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
|---|---|---|---|---|---|
| The Ottawa Hospitalb | 104 | 104 | 104 | 104 | 104 |
| St. Michael's Hospitalb | 117 | 117 | 117 | 117 | 117 |
| Toronto Western Hospitalb | 87 | 87 | 87 | 87 | 87 |
| Sunnybrook Health Sciences Centrec | 0 | 42 | 83 | 83 | 83 |
| London Health Sciences Centrec | 0 | 35 | 70 | 70 | 70 |
| Hamilton Health Sciences Centrec | 0 | 65 | 128 | 128 | 128 |
| Total 5-ALAde | 310 | 450 | 590 | 590 | 590 |
| Uptake ratede | 40% (310/785) | 55% (450/785) | 75% (590/785) | 75% (590/785) | 75% (590/785) |
| Incremental uptake rate/yearde | 40% | 15% | 15% | 0% | 0% |
Source: IntelliHealth Ontario.
Abbreviations: 5-ALA, 5-aminolevulinic acid hydrochloride; ICD, International Classification of Disease; SAP, Special Access Programme.
Uptake rate and corresponding volumes calculated from report generated from IntelliHealth Ontario, using ICD-10-CA codes for malignant neoplasm of the brain, specific to the anatomic locations of gliomas (C71.0 to C71.9), filtered by the CCI intervention codes for excision of the brain using craniotomy (1.AN.87.SZ.^^), and by the age group 18 years of age or older.
Assumed that neuro-oncology surgical sites that have currently obtained special access to 5-ALA through the Health Canada SAP would be ready to completely adopt 5-ALA–guided surgical resection in year 1.
Assumed that the remaining neuro-oncology surgical sites will adopt 5-ALA–guided surgical resection for 50% of their volumes in year 2, before fully adopting the use of 5-ALA by year 3.
Estimates are rounded to the nearest five.
May not be exact due to rounding.
Table A10:
Mean Additional Cost of Fluorescence Module Per Procedure
| Neuro-oncology Surgical Sites | Average Annual Volumea | Purchase Price | Useful Life (Years) | Average Additional Cost of Module Per Procedure, by Site | Overall Average Additional Cost of Module Per Procedure |
|---|---|---|---|---|---|
| Sunnybrook Health Sciences Centre | 83 | $107.89 | |||
| London Health Sciences Centre | 70 | $71,810.00 | 8 | $127.50 | $100.00b |
| Hamilton Health Sciences Centre | 128 | $70.02 |
Source: OCCI.
Abbreviation: OCCI, Ontario Case Costing Initiative.
See Table A6.
Estimates rounded to nearest $5.
Table A11:
Per-Patient Hospital Cost Associated With iUS for the Surgical Resection of Malignant Brain Tumours (Weighted Average of Reported Costs), Fiscal Years 2014/15 to 2016/17ab
| Fiscal Year | Age Group | Cases (n) | Average Cost | Weight |
|---|---|---|---|---|
| 2014/15 | 18+ | 25 | $190.14c | 0.32 (25 ÷ [25 + 33 + 20]) |
| 18-69 | 18 | $174.00 | ||
| 70+ | 7 | $232.00 | ||
| 2015/16 | 18+ | 33 | $207.49c | 0.42 (33 ÷ [25 + 33 + 20]) |
| 18-69 | 27 | $202.00 | ||
| 70+ | 6 | $230.00 | ||
| 2016/17 | 18+ | 20 | $153.08c | 0.26 (20 ÷ [25 + 33 + 20]) |
| 18-69 | 20 | $153.08 | ||
| 70+ | FOId | FOId | ||
| Total, n | 18+ | 78 | ||
| Cost, weighted averagee | $185.00 |
Source: OCCI.
Abbreviations: CCI, Canadian Classification of Health Interventions; FOI, Freedom of Information; iUS, intraoperative ultrasound; OCCI, Ontario Case Costing Initiative.
Includes both direct and indirect costs associated with 1.AN.87.SZ.^^.
Appropriate functional centre cost associated with iUS derived from report generated from OCCI using the CCI intervention codes for excision of the brain using craniotomy (1.AN.87.SZ.^^), filtered by the age group 18 years of age or older for fiscal years 2014/15 to 2016/17.
Weighted average of costs from age groups 18–69 years and 70+ years.
OCCI supresses information associated with 5 or less cases in order to comply with Freedom of Information (FOI) directives.
Estimates rounded to the nearest $5.
Table A12:
Per-Patient Hospital Cost Associated With White-Light Microscopy, Neuronavigation, and iUS for the Surgical Resection of Malignant Brain Tumours (Weighted Average of Reported Costs), Fiscal Years 2014/15 to 2016/17ab
| Fiscal Year | Age Group | Cases (n) | Average Cost | Weight |
|---|---|---|---|---|
| 2014/15 | 18+ | 472 | $15, 409c | 0.33 |
| 18-69 | 387 | $14,926 | (472 ÷ [472 + 375 + 578]) | |
| 70+ | 85 | $17,608 | ||
| 2015/16 | 18+ | 375 | $15,161c | 0.26 |
| 18-69 | 261 | $14,413 | (375 ÷ [472 + 375 + 578]) | |
| 70+ | 114 | $16,875 | ||
| 2016/17 | 18+ | 578 | $15,344c | 0.41 |
| 18-69 | 492 | $15,273 | (578 ÷ [472 + 375 + 578]) | |
| 70+ | 95 | $15,709 | ||
| Total, n | 18+ | 1,434 | ||
| Cost, weighted averaged | $15,305 |
Source: OCCI.
Abbreviations: CCI, Canadian Classification of Health Interventions; iUS, intraoperative ultrasound; OCCI, Ontario Case Costing Initiative.
Includes both direct and indirect costs associated with 1.AN.87.SZ.^^.
Report generated from OCCI, using the CCI intervention codes for excision of the brain using craniotomy (1.AN.87.SZ.^^), filtered by the age group 18 years of age or older for fiscal years 2014/15 to 2016/17.
Weighted average of costs from age groups 18–69 years and 70+ years.
Estimates rounded to the nearest $5.
Appendix 7: Letter of Informationa
LETTER OF INFORMATION
Health Quality Ontario is conducting a review of 5-ALA for people who have been diagnosed with high-grade glioma. The purpose is to understand whether this test should be publicly funded in Ontario.
An important part of this review involves gathering perspectives of patients and caregivers with experience with 5-ALA or other current testing for removal of brain tumor for people who have been diagnosed with high-grade glioma. They could have had the gene expression profiling test, recently or in the past or could be considering it in the future.
WHAT DO YOU NEED FROM ME
✓ Willingness to share your story
✓ 30 minutes of your time for a phone or videoconference
✓ Permission to audio- (not video-) record the interview
WHAT YOUR PARTICIPATION INVOLVES
If you agree to share your experiences, you will be asked to have an interview with Health Quality Ontario staff. The interview will last about 30 minutes. It will be held over the telephone or videoconference. With your permission, the interview will be audio-taped. The interviewer will ask you questions about your or your loved one's condition and your perspectives about treatment options in Ontario.
Participation is voluntary. You may refuse to participate, refuse to answer any questions or withdraw before or at any point during your interview. Withdrawal will in no way affect the care you receive.
CONFIDENTIALITY
All information you share will be kept confidential and your privacy will be protected except as required by law. The results of this review will be published, however no identifying information will be released or published. Any records containing information from your interview will be stored securely until project completion. After the project completion, the records will be destroyed.
RISKS TO PARTICIPATION
There are no known physical risks to participating. Some participants may experience discomfort or anxiety after speaking about their experience.
IF YOU ARE INTERESTED, PLEASE CONTACT US BEFORE MAY 31, 2019:
Health Quality Ontario is now the Quality business unit at Ontario Health.
Appendix 8: Interview Guide
Introduction
Health Quality Ontariob is a provincial advisor to the Ontario Ministry of Health. We do a few things for the ministry, but one of our roles is to conduct health technology assessments. These assessments look at new technologies and health services for the consideration of public funding. If any of the following questions cause you emotional distress or make you uncomfortable, please let me know and you can choose to not answer the question or say as little as you like. Do you have any questions for me?
History of the condition (high-grade glioma)
Experience with the condition
Health Quality Ontario is now the Quality business unit at Ontario Health.
Lived Experience of High-Grade Glioma
What is your day-to-day routine?
What has been the impact and effect on your quality of life?
Did you see any loss of independence?
Did the condition have an impact on your loved ones/caregivers, work, and/or friends?
Brain Tumour Resection With the Use of 5-ALA
What was the process for receiving 5-ALA?
What were the side effects?
What were the benefits?
What were the limitations and barriers?
Were there issues related to cost, access, knowledge of the health care system, etc.?
Did it meet your needs for treatment?
In the conversation between you and the physician, were you involved in making decisions about your care? If not, would you have preferred to be part of that decision making?
Brain Tumour Resection Using Standard Surgical Treatment
What was the process for standard surgical treatment?
How long did you need to wait to receive it?
What were the side effects?
What were the benefits?
What were the limitations and barriers?
Were there issues related to cost, access, knowledge of the health care system, etc.?
Did it meet your needs for treatment?
In the conversation between you and the physician, were you involved in making decisions about your care? If not, would you have preferred to be part of that decision making?
Lived Experience After Receiving 5-ALA and/or Receiving Standard Surgical Treatment
What is your day-to-day routine?
What has been the impact and effect on your quality of life?
Did you see any loss of independence?
Did it have an impact on your loved/caregivers, work, and/or friends?
Do you feel more comfortable with it now compared to before?
Author contributions
This report was developed by a multidisciplinary team from the Quality business unit at Ontario Health. The clinical epidemiologist was Milica Jokic, the health economics associate was Jennifer Guo, the secondary health economist was Olga Gajic-Veljanoski, the patient and public partnership analysts were Ammara Shafique and David Wells, and the medical librarian was Melissa Walter.
KEY MESSAGES
What Is This Health Technology Assessment About?
High-grade gliomas are a type of fast-growing brain tumour that can invade nearby brain tissue. Treatment of high-grade gliomas involves surgery to remove as much of the tumour as safely as possible to help people feel better and live longer. Removing the brain tumour can be challenging for surgeons because it is difficult to see the difference between the tumour and healthy brain tissue. Surgical tumour removal is also challenging because surgeons need to avoid areas of the brain that control function, such as speech, senses, or movement.
5-aminolevulinic acid hydrochloride (5-ALA) is a drug that is used to help surgeons see the tumour during surgery and guide its removal. After 5-ALA is administered, areas within the tumour glow pink or red, and healthy brain tissue appears blue when exposed to a special blue light during surgery.
This health technology assessment looked at how safe and effective 5-ALA–guided surgical resection is for people with high-grade gliomas. It also looked at the budget impact of publicly funding 5-ALA and the experiences, preferences, and values of an adult who had experience with high-grade glioma, standard surgical treatment, and 5-ALA–guided resection.
What Did This Health Technology Assessment Find?
People who receive 5-ALA–guided surgery appear to have complete resection of their tumour more often than people who receive standard surgical care. Based on low- to very low–quality evidence, 5-ALA– guided surgical resection of high-grade gliomas may improve 6-month progression-free survival, and may improve overall survival; however, we cannot exclude the possibility of no effect on overall survival. There is uncertainty regarding the impact of 5-ALA on adverse events after surgical resection.
We estimate that publicly funding 5-ALA–guided surgical resection in Ontario over the next 5 years would result in a budget impact of about $930,000 in year 1 to about $1,765,000 in year 5, yielding a total 5-year budget impact of about $7,500,000.
A participant with high-grade glioma reported a positive experience with 5-ALA and felt more satisfied with the 5-ALA–guided resection compared with standard surgical treatment.
Contributor Information
Ontario Health (Quality):
Milica Jokic, Jennifer Guo, Olga Gajic-Veljanoski, Ammara Shafique, David Wells, and Melissa Walter
About Us
This health technology assessment was produced by the Quality business unit at Ontario Health, the government agency that when fully established will be responsible for ensuring all Ontarians receive high-quality health care where and when they need it.
For more information about Ontario Health, visit ontariohealth.ca.
REFERENCES
- (1). Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol. 2018;20(suppl_4):iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2). American Brain Tumor Association. Glioma [Internet]. 2019. [cited 2019 March 2019]. Available from: https://www.abta.org/tumor_types/glioma/
- (3). John Hopkins Medicine. Gliomas [Internet]: The Johns Hopkins University; 2019. Available from: https://www.hopkinsmedicine.org/healthlibrary/conditions/nervous_system_disorders/gliomas_134,22 [Google Scholar]
- (4). Mason WP, Maestro RD, Eisenstat D, Forsyth P, Fulton D, Laperriere N, et al. Canadian recommendations for the treatment of glioblastoma multiforme. Curr Oncol. 2007;14(3):110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5). Gupta A, Dwivedi T. A simplified overview of World Health Organization classification update of central nervous system tumors. J Neurosci Rural Pract 2016;8(4):629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6). Stupp R, Brada M, van den Bent MJ, Tonn JC, Pentheroudakis G, Esmo Guidelines Working Group. High-grade glioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii93–101. [DOI] [PubMed] [Google Scholar]
- (7). American Brain Tumor Association. Glioblastoma and malignant astrocytoma [Internet]. 2017. [cited 2019 Mar 2019]. Available from: https://2knaef3o0jpz4ff42k23tr6l-wpengine.netdna-ssl.com/wp-content/uploads/2018/03/glioblastoma-brochure.pdf
- (8). Manrique-Guzmán S, Herrada-Pineda T, Revilla-Pacheco F. Surgical management of glioblastoma. 2017. In: Glioblastoma [Internet]. Brisbane (AU): Codon Publications; Available from: https://www.ncbi.nlm.nih.gov/books/NBK469999/ [PubMed] [Google Scholar]
- (9). Braganza M, Kitahara C, Berrington de González A. Ionizing radiation and the risk of brain and central nervous system tumors: a systematic review. Neuro Oncol. 2012;14(11):1316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10). Tamimi A, Juweid M. Epidemiology and outcome of glioblastoma. In: De Vleeschouwer S, editor. Glioblastoma. Brisbane (AU): Codon Publications; 2017. [PubMed] [Google Scholar]
- (11). Canadian Cancer Society. Brain and spinal cord tumours [Internet]. Toronto (ON): Canadian Cancer Society; c2019. Available from: http://www.cancer.ca/en/cancer-information/cancer-type/brain-spinal/brain-and-spinal-tumours/?region=on [Google Scholar]
- (12). Canadian Cancer Society's Advisory Committee on Cancer Statistics. Canadian cancer statistics [Internet]. Toronto (ON): Canadian Cancer Society; 2016. Available from: http://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2016-EN.pdf?la=en [Google Scholar]
- (13). Yuan Y, Shi Q, Li M, Nagamuthu C, Andres E, Davis FG. Canadian brain cancer survival rates by tumour type and region: 1992-2008. Can J Public Health. 2016;107(1):e37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14). Easaw JC, Mason WP, Perry J, Laperriere N, Eisenstat DD, Del Maestro R, et al. Canadian recommendations for the treatment of recurrent or progressive glioblastoma multiforme. Curr Oncol. 2011;18(3):e126-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15). Brown TJ, Brennan MC, Li M, Church EW, Brandmeir NJ, Rakszawski KL, et al. Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol. 2016;2(11):1460–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16). National Institute for Health and Care Excellence. Brain tumours (primary) and brain metastases in adults. Evidence reviews for the investigation, management and follow-up of glioma. Evidence Report A. London (UK): The Institute; 2018. [PubMed] [Google Scholar]
- (17). Alberta Health Services. Glioblastoma. Clinical practice guideline [Internet]. Edmonton (AB): Alberta Health Services; 2012. [cited 2019 Feb]. Available from: www.albertahealthservices.ca [Google Scholar]
- (18). Perry J, Brown J, Ang L-C, Das S, Laperriere N, Mason W, et al. An endorsement of the 2017 European Association for NeuroOncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Toronto (ON): Cancer Care Ontario; 2018. [Google Scholar]
- (19). Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401. [DOI] [PubMed] [Google Scholar]
- (20). Chohan MO, Berger MS. 5-Aminolevulinic acid fluorescence guided surgery for recurrent high-grade gliomas. J Neurooncol. 2019;141(3):517–22. [DOI] [PubMed] [Google Scholar]
- (21). Orringer DA, Golby A, Jolesz F. Neuronavigation in the surgical management of brain tumors: current and future trends. Expert Rev Med Devices. 2012;9(5):491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22). Thomas NWD, Sinclair J. Image-guided neurosurgery: history and current clinical applications. J Med Imaging Radiat Sci. 2015;46(3):331–42. [DOI] [PubMed] [Google Scholar]
- (23). Gerard IJ, Kersten-Oertel M, Petrecca K, Sirhan D, Hall JA, Collins DL. Brain shift in neuronavigation of brain tumors: a review. Med Image Anal. 2017;35:403–20. [DOI] [PubMed] [Google Scholar]
- (24). Halani SH, Adamson DC. Clinical utility of 5-aminolevulinic acid HCl to better visualize and more completely remove gliomas. Onco Targets Ther. 2016;9:5629–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25). US Food and Drug Administration. Drug approval package: Gleolan (aminolevulinic acid hydrochloride) [Internet]. Silver Spring (MD): The Administration; 2018. [cited 2019 March]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208630Orig1s000TOC.cfm [Google Scholar]
- (26). Eljamel S. 5-ALA fluorescence image guided resection of glioblastoma multiforme: A meta-analysis of the literature. Int J Mol Sci. 2015;16(5):10443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27). Zhao S, Wu J, Wang C, Liu H, Dong X, Shi C, et al. Intraoperative fluorescence-guided resection of high-grade malignant gliomas using 5-aminolevulinic acid-induced porphyrins: a systematic review and meta-analysis of prospective studies [Internet]: PLoS ONE; 2013. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0063682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28). Garzon-Muvdi T, Kut C, Li X, Chaichana KL. Intraoperative imaging techniques for glioma surgery. Future Oncol. 2017;13(19):1731–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29). Mayo Clinic. Intraoperative magnetic resonance imaging (iMRI) [Internet]. 2019. [Available from: https://www.mayoclinic.org/tests-procedures/intraoperative-magnetic-resonance-imaging/about/pac-20394451
- (30). Liang D, Schulder M. The role of intraoperative magnetic resonance imaging in glioma surgery. Surg Neurol Int. 2012;3(Suppl 4):S320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31). Pino MA, Imperato A, Musca I, Maugeri R, Giammalva GR, Costantino G, et al. New hope in brain glioma surgery: the role of intraoperative ultrasound. A review. Brain sci. 2018;8(11):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32). The Telegraph. More people to be given dye to highlight brain tumours to surgeons say NICE [Internet]. 2019. May 12 [cited 2019 July]. Available from: https://www.telegraph.co.uk/news/2019/05/12/brain-cancer-pink-drink-campaigned-tessa-jowell-rolled-nhs-memory/
- (33). Medical Services Advisory Committee. Fluorescence guided resection of high grade (grade IV) glioma that are glioblastoma multiforme using Gliolan (aminolevulinic acid): application no. 1395 [Internet]. Canberra (AU): Commonwealth of Australia; 2016 November. [cited 2019 Jan]. Available from: http://www.msac.gov.au/internet/msac/publishing.nsf/Content/1395-public [Google Scholar]
- (34). McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. [DOI] [PubMed] [Google Scholar]
- (35). Covidence systematic review software. Veritas Health Innovation. Melbourne (Australia). Available at: https://www.covidence.org/home.
- (36). Whiting P, Savović J, Higgins JP, Caldwell DM, Reeves BC, Shea B, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37). Higgins J, Altman D, Sterne J. Assessing risk of bias in included studies. In: Higgins JPT, Green S. (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Internet]. The Cochrane Collaboration, 2011. [updated 2011 Mar; cited 2017 Jan 19]. Available from www.handbook.cochrane.org. [Google Scholar]
- (38). Kim SY, Park JE, Lee YJ, Seo HJ, Sheen SS, Hahn S, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013;66(4):408–14. [DOI] [PubMed] [Google Scholar]
- (39). Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook [Internet]. Hamilton (ON): GRADE Working Group; 2013. [cited 2017 Dec]. Available from http://gdt.guidelinedevelopment.org/app/handbook/handbook.html [Internet]. [Google Scholar]
- (40). Barone DG, Lawrie TA, Hart MG. Image guided surgery for the resection of brain tumours. Cochrane Database Syst Rev. 2014;1:CD009685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41). Coburger J, Wirtz CR. Fluorescence guided surgery by 5-ALA and intraoperative MRI in high grade glioma: a systematic review. J Neurooncol. 2019;141(3):533–46. [DOI] [PubMed] [Google Scholar]
- (42). Ferraro N, Barbarite E, Albert TR, Berchmans E, Shah AH, Bregy A, et al. The role of 5-aminolevulinic acid in brain tumor surgery: a systematic review. Neurosurg Rev. 2016;39(4):545–55. [DOI] [PubMed] [Google Scholar]
- (43). Haider SA, Lim S, Kalkanis SN, Lee IY. The impact of 5-aminolevulinic acid on extent of resection in newly diagnosed high grade gliomas: a systematic review and single institutional experience. J Neurooncol. 2019;141(3):507–15. [DOI] [PubMed] [Google Scholar]
- (44). Jenkinson MD, Barone DG, Bryant A, Vale L, Bulbeck H, Lawrie TA, et al. Intraoperative imaging technology to maximise extent of resection for glioma. Cochrane Database Syst Rev. 2018;1:CD012788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45). Mansouri A, Mansouri S, Hachem LD, Klironomos G, Vogelbaum MA, Bernstein M, et al. The role of 5-aminolevulinic acid in enhancing surgery for high-grade glioma, its current boundaries, and future perspectives: a systematic review. Cancer. 2016;122(16):2469–78. [DOI] [PubMed] [Google Scholar]
- (46). Senders JT, Muskens IS, Schnoor R, Karhade AV, Cote DJ, Smith TR, et al. Agents for fluorescence-guided glioma surgery: a systematic review of preclinical and clinical results. Acta Neurochir (Wien). 2017;159(1):151–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47). Su X, Huang QF, Chen HL, Chen J. Fluorescence-guided resection of high-grade gliomas: a systematic review and meta-analysis. Photodiagnosis Photodyn Ther. 2014;11(4):451–8. [DOI] [PubMed] [Google Scholar]
- (48). Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(6):e1000097. [PMC free article] [PubMed] [Google Scholar]
- (49). Stummer W, Tonn JC, Mehdorn HM, Nestler U, Franz K, Goetz C, et al. Counterbalancing risks and gains from extended resections in malignant glioma surgery: a supplemental analysis from the randomized 5-aminolevulinic acid glioma resection study. Clinical article. J Neurosurg. 2011;114(3):613–23. [DOI] [PubMed] [Google Scholar]
- (50). Alexander BM, Galanis E, Yung WK, Ballman KV, Boyett JM, Cloughesy TF, et al. Brain Malignancy Steering Committee clinical trials planning workshop: report from the Targeted Therapies Working Group. Neuro Oncol. 2015;17(2):180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51). Han K, Ren M, Wick W, Abrey L, Das A, Jin J, et al. Progression-free survival as a surrogate endpoint for overall survival in glioblastoma: a literature-based meta-analysis from 91 trials. Neuro Oncol. 2014;16(5):696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52). Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–20. [DOI] [PubMed] [Google Scholar]
- (53). National Institute for Health and Care Excellence. Process and methods guides. Appendix I: Quality appraisal checklist–economic evaluations [Internet]. London: The Institute; 2012. [cited 2016 Jan]. Available from: https://www.nice.org.uk/process/pmg4/chapter/appendix-i-quality-appraisal-checklist-economic-evaluations [Google Scholar]
- (54). Esteves S, Alves M, Castel-Branco M, Stummer W. A pilot cost-effectiveness analysis of treatments in newly diagnosed high-grade gliomas: the example of 5-aminolevulinic acid compared with white-light surgery. Neurosurgery. 2015;76(5):552–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55). Slof J, Diez Valle R, Galvan J. Cost-effectiveness of 5-aminolevulinic acid-induced fluorescence in malignant glioma surgery. Neurologia. 2015;30(3):163–8. [DOI] [PubMed] [Google Scholar]
- (56). Eljamel MS, Mahboob SO. The effectiveness and cost-effectiveness of intraoperative imaging in high-grade glioma resection; a comparative review of intraoperative ALA, fluorescein, ultrasound and MRI. Photodiagnosis Photodyn Ther. 2016;16:35–43. [DOI] [PubMed] [Google Scholar]
- (57). Diez Valle R, Slof J, Galvan J, Arza C, Romariz C, Vidal C. Observational, retrospective study of the effectiveness of 5-aminolevulinic acid in malignant glioma surgery in Spain (The VISIONA study). Neurologia. 2014;29(3):131–8. [DOI] [PubMed] [Google Scholar]
- (58). Taylor C, Jan S. Economic evaluation of medicines. Aust Prescr. 2017;40(2):76–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59). European Medicines Agency. Annex I: summary of product characteristics. 2007. In: Gliolan: EPAR – product information [Internet]. Amsterdam European Medicines Agency; Available from: https://www.ema.europa.eu/en/documents/product-information/gliolan-epar-product-information_en.pdf [Google Scholar]
- (60). Alberta Health Services. Glioblastoma. Clinical practice guideline [Internet]. Edmonton (AB): Alberta Health Services; 2012. [cited 2019 Feb]. Available from: www.albertahealthservices.ca [Google Scholar]
- (61). IDT Biologika GmbH. 1.14.1.3 Draft labeling text: Gleolan [package insert]. Washington, D.C.: US Food and Drug Administration; June 2017. [Google Scholar]
- (62). Applicant submitted protocol for fluorescence guided resection of high grade (grade IV) glioma that are glioblastoma multiforme (GBM) using oral aminolevulinic acid hydrochloride (ALA): application 1395. Canberra (AU) Medical Services Advisory Committee; February 2016. [Google Scholar]
- (63). Hadjipanayis CG, Widhalm G, Stummer W. What is the surgical benefit of utilizing 5-aminolevulinic acid for fluorescence-guided surgery of malignant gliomas? Neurosurgery. 2015;77(5):663–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64). Fryar CD, Kruszon-Moran D, Gu Q, Ogden CL. Mean body weight, height, waist circumference, and body mass index among adults: United States, 1999-2000 through 2015-2016. National health statistics reports. 2018(122):1–16. [PubMed] [Google Scholar]
- (65). Shields M, Carroll MD, Ogden CL. Adult obesity prevalence in Canada and the United States: NCHS Data Brief No. 56 [Internet]. Washington, D.C.: US Department of Health and Human Services; March 2011. Available from: https://www.cdc.gov/nchs/products/databriefs/db56.htm [PubMed] [Google Scholar]
- (66). Noiphithak R, Veerasarn K. Clinical predictors for survival and treatment outcome of high-grade glioma in Prasat Neurological Institute. Asian J Neurosurg. 2017;12(1):28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67). Venur VA, Peereboom DM, Ahluwalia MS. Current medical treatment of glioblastoma. Cancer Treat Res. 2015;163:103–15. [DOI] [PubMed] [Google Scholar]
- (68). Young RM, Jamshidi A, Davis G, Sherman JH. Current trends in the surgical management and treatment of adult glioblastoma. Annals of translational medicine. 2015;3(9):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69). Juurlink D, Preyra C, Croxford R, Chong A, Austin P, Tu J, et al. Canadian Institute for Health Information Discharge Abstract Database: a validation study [Internet]. Toronto (ON): Institute for Clinical Evaluative Sciences; June 2016. Available from: https://www.ices.on.ca/Publications/Atlases-and-Reports/2006/Canadian-Institute-for-Health-Information [Google Scholar]
- (70). Ahmadi R, Campos B, Haux D, Rieke J, Beigel B, Unterberg A. Assessing perioperative complications associated with use of intraoperative magnetic resonance imaging during glioma surgery – a single centre experience with 516 cases. Br J Neurosurg. 2016;30(4):397–400. [DOI] [PubMed] [Google Scholar]
- (71). Sastry R, Bi WL, Pieper S, Frisken S, Kapur T, Wells W, 3rd, et al. Applications of ultrasound in the resection of brain tumors. J Neuroimaging. 2017;27(1):5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72). Ottawa Regional Cancer Foundation [Internet]. Ottawa (ON): The Foundation Press release, Ottawa Regional Cancer Foundation funds first-in-Canada approach to brain cancer surgery; 2018. Sep 17 [cited 2019 Jan]. Available from: https://www.newswire.ca/news-releases/ottawa-regional-cancer-foundation-funds-first-in-canada-approach-to-brain-cancer-surgery-693508481.html [Google Scholar]
- (73). St. Michael's Hospital Foundation. New breakthrough [Internet]. Toronto (ON): St. Michael's Hospital Foundation; Available from: http://www.stmichaelsfoundation.com/news/articles/new-breakthrough.html [Google Scholar]
- (74). OECD. Purchasing power parities (PPP) [Internet]. Paris: Organisation for Economic Co-operation and Development; c2019. Available from: https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm [Google Scholar]
- (75). Statistics Canada. Table:18-10-0004-01. Consumer Price Index, monthly, not seasonally adjusted [Internet]. Ottawa (ON): Statistics Canada; 2019. [cited 2019 Apr 30]. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/cv.action?pid=1810000401#timeframe [Google Scholar]
- (76). Ministry of Health and Long-Term Care. Schedule of benefits: physician services under the Health Insurance Act [Internet]. Toronto (ON): Queen's Printer for Ontario; 2015. [cited 2019 Apr]. Available from: http://www.health.gov.on.ca/en/pro/programs/ohip/sob/physserv/sob_master20160401.pdf [Google Scholar]
- (77). European Medicines Agency. Gliolan; INN: 5-aminolevulinic acid hydrochloride [Internet]. Amsterdam: Eiropean Medicines Agency; 2007. Available from: https://www.ema.europa.eu/en/documents/scientific-discussion/gliolan-epar-scientific-discussion_en.pdf [Google Scholar]
- (78). US Food and Drug Administration. FDA briefing document: medical imaging drugs advisory committee meeting, May 10, 2017 [Internet]. Washington, D.C.: The Administration; 2017. Available from: https://www.fda.gov/media/104856/download [Google Scholar]
- (79). Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro Oncol. 2015;17 Suppl 4:iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80). Barham L. Public and patient involvement at the UK National Institute for Health and Clinical Excellence. The patient. 2011;4(1):1–10. [DOI] [PubMed] [Google Scholar]
- (81). Messina J, Grainger DL. A pilot study to identify areas for further improvements in patient and public involvement in health technology assessments for medicines. The patient. 2012;5(3):199–211. [DOI] [PubMed] [Google Scholar]
- (82). Ontario Health Technology Advisory Committee Public Engagement Subcommittee. Public engagement for health technology assessment at Health Quality Ontario—final report from the Ontario Health Technology Advisory Committee Public Engagement Subcommittee [Internet]. Toronto (ON): Queen's Printer for Ontario; 2015. Apr [cited 2018 Apr 30]. Available from: http://www.hqontario.ca/Portals/0/documents/evidence/special-reports/report-subcommittee-20150407-en.pdf [Google Scholar]
- (83). Kvale S. Interviews: an introduction to qualitative research interviewing. Thousand Oaks (CA): Sage; 1996. [Google Scholar]
- (84). Kuzel AJ. Sampling in qualitative inquiry. In: Miller WL, Crabtree BF, editors. Doing qualitative research. Thousand Oaks (CA): Sage; 1999. p. 33–45. [Google Scholar]
- (85). Morse J. Emerging from the data: cognitive processes of analysis in qualitative research. In: Morse J, editor. Critical issues in qualitative research methods. Thousand Oaks (CA): Sage; 1994. p. 23–41. [Google Scholar]
- (86). Patton MQ. Qualitative research and evaluation methods. 3rd ed. Thousand Oaks (CA): Sage; 2002. [Google Scholar]
- (87). Strauss AL, Corbin JM. Basics of qualitative research: techniques and procedures of developing a grounded theory. 2nd ed. Thousand Oaks (CA): Sage; 1998. [Google Scholar]
- (88). Health Technology Assessment International. Introduction to health technology assessment [Internet]. Edmonton (AB): Health Technology Assessment International; 2015. [cited 2018 Apr 30]. Available from: http://www.htai.org/fileadmin/HTAi_Files/ISG/PatientInvolvement/v2_files/Resource/PCISG-Resource-Intro_to_HTA__KFacey__Jun13.pdf [Google Scholar]
- (89). Strauss AL, Corbin JM. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol. 1990;13(1):3–21. [Google Scholar]
- (90). Strauss AL, Corbin JM. Grounded theory methodology: an overview. In: Denzin NK, Lincoln YS, editors. Handbook of qualitative research. Thousand Oaks (CA): Sage; 1994. p. 273–85. [Google Scholar]
- (91). NVivo qualitative data analysis software. QSR International. Doncaster, Victoria (Australia). Available at: https://www.qsrinternational.com/nvivo/home.