Abstract
Purpose of review
The purpose of this review is to describe current concepts in the field of Less Invasive Surfactant Administration (LISA). The use of continuous positive airway pressure (CPAP) has become standard for the treatment of premature infants with respiratory problems throughout the world. However, if CPAP fails, technologies like LISA are needed that can combine surfactant delivery and spontaneous breathing with the support of noninvasive modes of ventilation.
Recent findings
LISA with thin catheters has been in use in Germany for more than 15 years. In the last 5 years, there was substantial interest in this method around the world. Randomized studies and recent metaanalyses indicate that the LISA technique helps to avoid mechanical ventilation especially in emerging respiratory distress syndrome (RDS). LISA is also associated with improved outcomes of preterm infants, specifically in the prevention of bronchopulmonary dysplasia (BPD) and intracranial hemorrhage (ICH). By now, a variety of different LISA catheters, devices and techniques have been described. However, most of the technologies are still connected with the unpleasant experience of laryngoscopy for the affected infants, so that the search for even less invasive techniques, for example, surfactant application by nebulization, goes on.
Summary
Maintenance of spontaneous breathing with support by the LISA technique holds big promise in the care of preterm infants. Patient comfort and lower complication rates are strong arguments to further investigate and promote the LISA approach. Open questions include exact indications for different patient groups, the usefulness of devices/catheters that have recently been built for the LISA technique and -- perhaps most urgently -- the issue of analgesia/sedation during the procedure. Studies on long-term outcome after LISA are under way.
Keywords: continuous positive airway pressure, less invasive surfactant administration, noninvasive ventilation
INTRODUCTION
Modified natural surfactants have been in clinical use for the treatment of respiratory distress syndrome (RDS) for about 30 years [1,2]. Replacement therapy with exogenous surfactant preparations derived from animal sources dramatically reduced RDS-related morbidity and mortality and became the most effective evidence-based therapy for RDS. In the first decade of replacement therapy, surfactant bolus administration during mechanical ventilation was the method of choice. In the 1990s, continuous positive airway pressure (CPAP) became increasingly popular especially in Scandinavia. In 1992, a ‘small catheter surfactant delivery method’ was described for the first time [3]. However, it was believed that at least a short interval of positive pressure ventilation was needed to move surfactant from the central airways to the lung periphery, so that the INSURE (Intubate SURfactant Extubate) method was developed [4]. However, with INSURE laryngoscopy, and intubation with a standard large diameter endotracheal tube are performed accompanied by analgesia and sedation. Following surfactant bolus administration, the infants are either ventilated with bag valve masks and/or connected to a mechanical ventilator delivering gas volumes with the help of positive pressure at least for a short interval. In that way, INSURE is different and more invasive than LISA.
When CPAP became more popular even for premature infants, it became evident that CPAP failure because of RDS, that is, surfactant deficiency, is common especially in the most immature infants [5]. This prompted the search for the LISA technique with the aim to effectively provide an adequate dose of surfactant while the infant is breathing spontaneously with the support of CPAP [6,7▪].
The ‘small diameter tube method’ (synonym for LISA) was rediscovered, about 10 years after its initial description [3] by Kribs et al.[6] in Cologne and from 2003 onwards, LISA was also used in Lübeck. The German Neonatal Network (GNN) facilitated the initial randomized trials (AMV and NINSAPP trials) [8,9] and additional observational studies on LISA [10] that underlined that LISA was also ready for routine use in neonatal ICUs (NICUs). The cohort of LISA-treated infants documented in the GNN has increased to more than 5000 patients by now [7▪]. More than 50% of all surfactant treatments are performed by LISA in Germany [7▪] and the international interest in this technique has steeply increased with studies reporting LISA results from, for example, Austria, Australia, Turkey, Spain, Iran and China [11–16]. Both the national German [17] and the recent European guidelines [18] for surfactant replacement therapy mention LISA as the method of choice for surfactant delivery in infants with RDS.
Box 1.
no caption available
EFFECTS OF LESS INVASIVE SURFACTANT ADMINISTRATION
LISA has been shown to reduce the need for mechanical ventilation in the randomized controlled AMV [8] and NINSAPP [9] trials. An observational study using a matched pairs design in more than 1000 infants demonstrated that this effect is robust in clinical practice also outside the specific setting of trials [10]. The effectiveness of LISA in preventing mechanical ventilation in the first 72 h strongly depends on gestational age [10]. Some of the most immature infants need intubation after the first days of life, mainly because of apnoea and/or muscular fatigue. However, the low intracranial hemorrhage (ICH) rate, for example, in the LISA group of the NINSAPP trial points [9] into the direction that postnatal circulatory adaptation under spontaneous breathing is of potential advantage also for the tiniest infants.
Metaanalyses [19–21] point out that LISA is superior to CPAP alone or the INSURE technique both in terms of avoidance of bronchopulmonary dysplasia (BPD) and ICH [19]. However, the quality of some of the studies included in these metaanalyses was questionable, so that further confirmative randomized controlled studies (RCT) are still needed.
HOW TO PRACTICE LESS INVASIVE SURFACTANT ADMINISTRATION
Prenatal lung maturation with corticosteroids and good obstetrical care are very important factors for spontaneous breathing directly after birth, which is a prerequisite to make LISA work. Infants who are not crying/breathing are stabilized with a mask and distending pressure after delivery. Most centers use CPAP at 6--9 cmH2O after birth with a variety of different devices, whereas sustained inflations are not current practice. The use of high-flow nasal cannula (HFNC) is increasing in popularity, but in Germany, still uncommon in the delivery suite as CPAP seems to allow higher distending pressures. Giving regard to the low physiological saturation limits, adaptation to postnatal life is allowed with as little intervention as possible. Gentle stimulation, late cord clamping, caring for patency of the airways (often a lateral position is more adequate than keeping the baby in a strictly supine position), avoiding unnecessary suctioning, maintenance of body temperature and avoiding stress as much as possible are of utmost importance to establish effective spontaneous breathing. By now, the majority of infants below 1000 g in Germany receive caffeine for stimulation of breathing/apnoea prevention already in the delivery suite, although there is no clear evidence from RCTs supporting this approach. In this way, LISA is not simply a single technical procedure but rather a component of a complex care bundle supporting the individual capacity of a premature baby to adapt to extrauterine life (so called: minimal handling approach or ‘soft landing’).
If respiratory distress becomes evident shortly after birth, as indicated by increasing oxygen demand and/or tachypnoea, grunting and/or retractions, surfactant deficiency is likely and surfactant may be delivered by LISA already in the delivery room. The more stable, often also more mature, infants are transferred to the NICU under CPAP therapy and often receive surfactant only when certain thresholds in oxygen demand are reached (often a fraction of inspiratory oxygen (FiO2) greater than 0.30 or greater than 0.40 is used as indication limit, also considering the gestational age (see e.g. [18]).
For the most immature infants (e.g. <25 weeks of gestational age) some centers follow a (quasi-) prophylactic LISA approach and use LISA as early as at 20 min of life with the argument that in these infants with very delicate lung structures, surfactant treatment should be given as early as possible and most infants in this age group would receive surfactant anyhow in the hours to come. Clearly, RCTs are needed addressing the question of the superiority of such a ‘prophylactic’ approach compared with waitful watching and ‘rescue’ LISA treatment, if indicated.
In Germany, mainly thin (3.5--5.0 French) and soft catheters (e.g. gastric tubes, suction catheters, umbilical arterial or bladder catheters) are used for LISA and are mostly introduced into the larynx with the help of a laryngoscope and a Magill forceps (see also Fig. 1). Video laryngoscopy [22] holds some promise for well tolerated placement of the catheters in the trachea and also for teaching (see also Fig. 2). However, most neonatologists will tend to remove the laryngoscope for comfort reasons as soon as possible securing the adequate catheter position with the fingers on the lips/the nose of the babies. In the tiniest infants, the catheter is introduced only 1--2 cm beyond the vocal cords. Some gastric catheters have the disadvantage of side holes that are rather distant from the tip. Side holes/not inserting the catheter deep enough carries the risk of increased surfactant reflux, whereas too deep insertion of the catheter may result in unilateral surfactant deposition. The surfactant instillation is done in small boluses and only slowed down when bradycardia, apnoea or increased surfactant reflux is observed. Some air is often placed in the syringe ‘behind/on top of the surfactant’ to allow removal of the surfactant from the dead space in the syringe and the catheter. As silicone oil may bind to the surfactant lipids, it is preferable to use syringes that contain no lubricated rubber parts [23].
FIGURE 1.
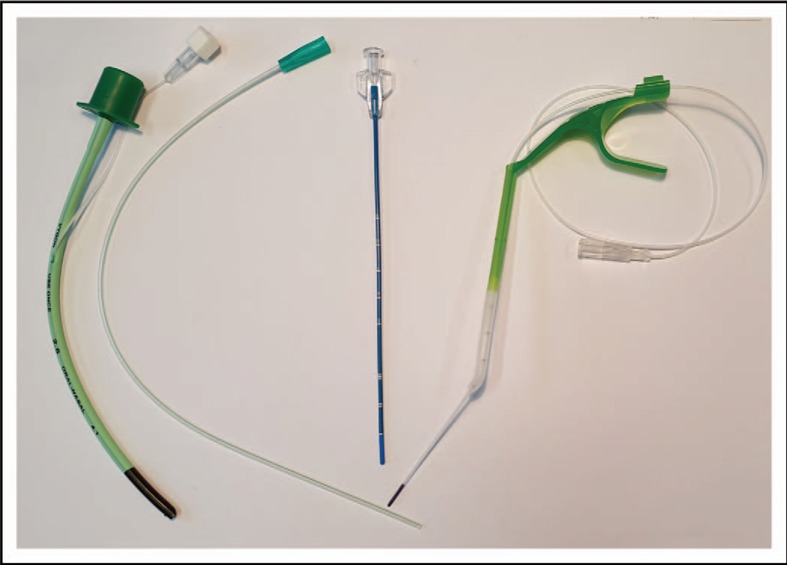
Devices for surfactant instillation by the standard and the less invasive surfactant administration method. From left to right: endotracheal tube size 2.5 (outer diameter 4.1 mm, see also Fig. 2a), soft suction catheter 5 French (outer diameter: 1.7 mm see also Fig. 2b), stiffer straight catheter (Lisacath) (outer diameter 1.7 mm) and special device (Neofact) with 3.5 French (outer diameter: 1.2 mm) catheter that is sliding out from the tip. Lisacath and Neofact have special ‘softer’ tips to avoid injury.
FIGURE 2.
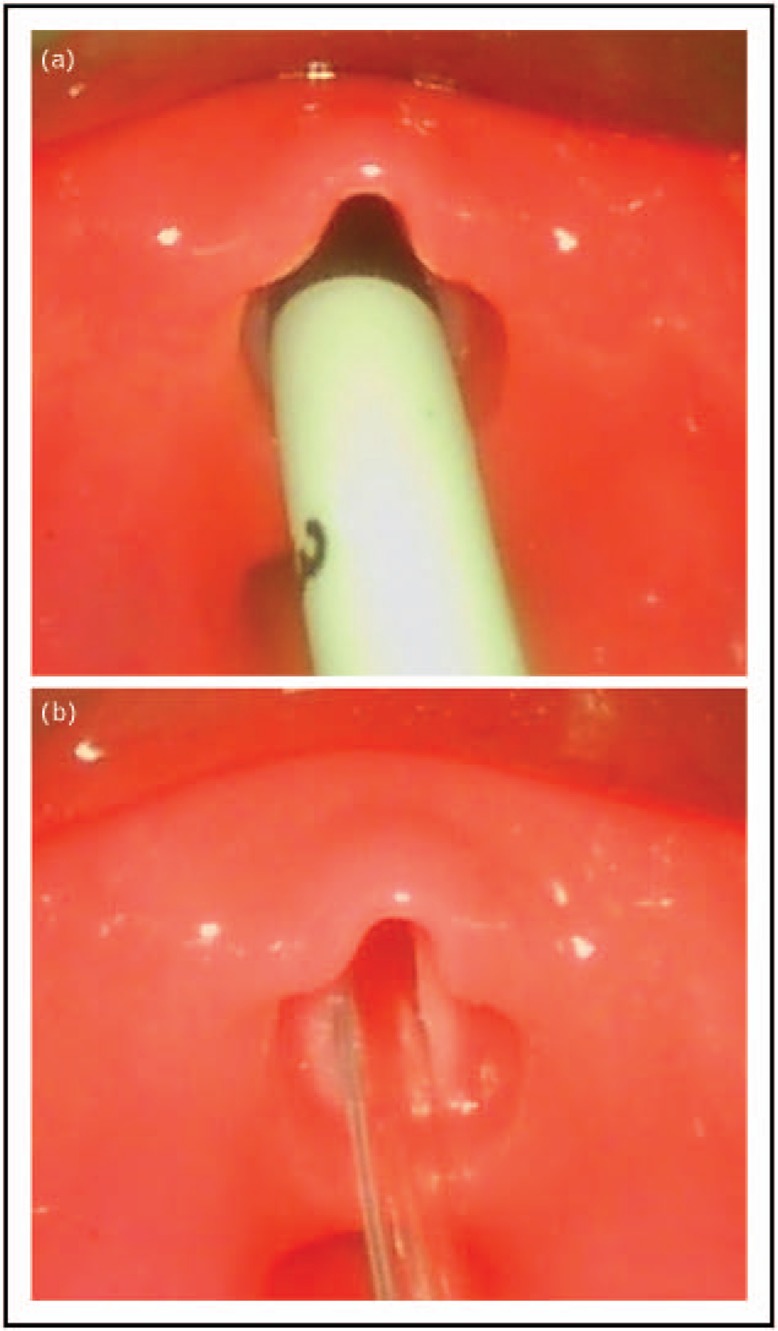
(a) 2.5 Portex endotracheal tube inserted into to the larynx. (b) 5 French Vygon suction catheter inserted into the larynx. Note that the endotracheal tube virtually occludes the laryngeal entrance (a), whereas the vocal cords and even a lumen can be seen above the small diameter suction tube (b) that is used for the LISA procedure. The pictures were taken with a video larnygoscope (C-MAC, Karl Storz, Tuttlingen, Germany) in a mannequin (PAUL, Sim Characters, Vienna, Austria) that simulates the conditions in a 1000 g, 27 +3 weeks premature baby with a body length of 35 cm. The larynx in the model goes back to an anatomically correct 3D printed larynx based on real-life MRI of a preterm baby with a corresponding gestational age. LISA, less invasive surfactant administration.
Surfactant has unique spreading properties once it is instilled in the large airways. From animal experiments, it is known that infusing surfactant at too slow rates results in less even distribution compared with the bolus approach [24]. The surfactant instillation with LISA can usually be completed in less than 60 s. Rarely, 2 min or more are needed, when intermittent bradycardia/apnoea occurs and the injection has to be slowed down. There are no data comparing different speeds of surfactant delivery with LISA.
Mainly animal-derived modified natural surfactants have been studied for LISA. The AMV study [8] compared three different types of surfactant but was underpowered for valid comparisons in terms of LISA effectiveness between different surfactants. The AMV-trial [8] used a phospholipid dose of 100 mg/kg, the NINSAPP trial [9] a whole vial of 120 mg porcine surfactant per infant and a retrospective study a dose of 200 mg/kg [11]. In daily practice, often whole vials of surfactant are used, especially when treatment is done in the delivery room and an exact weight is not available. The pragmatic way to use a whole vial of, for example, 120 mg surfactant in a baby with an estimated weight of 800 g will result in a phospholipid dose of at least 150 mg/kg. There is hope that such a dose results in higher surfactant pool size than the 100 mg/kg used, for example, in the AMV study, where the rest of the vial content was discarded. Most studies report the need for retreatment in 20--30% of LISA-treated infants. Theoretically, a higher initial surfactant dose should supply a higher surfactant pool and hopefully thereby reduce the need for (stressful) retreatment. Again, clearly RCTs are needed here as well, before definitive dose recommendations can be made.
Given special experience, LISA can be successfully applied in infants as immature as 22 weeks [25]. Personally, we do not favour the use of LISA in infants above 32 weeks. There is no evidence for a benefit of LISA in this group so far and the risk for BPD is low in this group [13]. More mature infants often struggle, and therefore, need sedation/analgesia to tolerate the procedure. On the other hand, medication interferes with the spontaneous breathing that is crucial to make LISA work.
CATHETER TYPES
A variety of catheter types and instruments are used for the LISA procedure (see Table 1 and Fig. 1). The placement of a soft catheter with the help of the Magill forceps is the method that most centers in Germany use. The catheter is often introduced via the mouth and has already been mounted into the forceps before the start of the procedure. Alternatively, the soft flexible catheter is introduced via the nose (while a mononasal CPAP is in place in the other nostril) and then taken up with the forceps similar to the technique of nasal intubation. Soft catheter introduction without a forceps has been described as the so-called Take Care procedure [15]. Not all neonatologists are familiar with nasal intubation and the use of a Magill forceps. Oral intubation is much more common in other parts of the world, so that direct laryngoscopy and the use of a stiff vascular catheter was described by Peter Dargaville as the so called Hobart method (MIST, minimal invasive surfactant therapy) in 2011 [27].
Table 1.
Published methods to combine continuous positive airway pressure, spontaneous breathing and surfactant administration
| Name | Device type | Procedure/instruments | Reference |
| Cologne method | Flexible suction catheter | Laryngoscope + Magill forceps | Kribs et al. [6] |
| SONSURE | Flexible nasogastric tube | Laryngoscope + Magill forceps | Aguar et al. [26] |
| Take Care method | Flexible nasogastric tube | Laryngoscope, no forceps | Kanmaz et al. [15] |
| Hobart method | Semi-rigid vascular catheter Device name: for example, Lisacath | Laryngoscope, no forceps | Dargaville et al. [27] |
| QuickSF | Soft catheter Device name: Neofact | Laryngoscope + intrapharyngeal guidance device | Maiwald et al. [28] |
| INSURE | Endotracheal tube | Laryngoscope | Verder et al. [3] |
| Laryngeal Mask method | Special device placed in hypopharynx | No Laryngoscope, no forceps | Roberts et al. [29▪] |
| Aerosol method | No catheter Nebuliser with, for example, mask/prongues | No Laryngoscope, no forceps | Pillow et al. [30] |
| Pharyngeal Surfactant | Flexible short tube and syringe Injection into the pharynx | No Laryngoscope, no forceps | Kattwinkel et al. [31] |
For review see [32▪▪].
By now, companies have developed straight [33▪] (Lisacath) or catheters with an angulated tip (Surfcath) that are easy to use via the oral route. These catheters are relatively stiff, so that no extra devices are needed to introduce the tip through the vocal cords. Special introducers [28] (Neocath) allowing to slide out a soft thin catheter from the tip after the device has been placed in front of the larynx and devices to guide the catheter during video laryngoscopy have recently reached the market.
WHAT MAKES LESS INVASIVE SURFACTANT ADMINISTRATION DIFFERENT?
LISA is different from other modes of surfactant delivery as it allows the infant to keep on breathing and to use the physiological function of the larynx without (nearly complete) obstruction by a larger diameter endotracheal tube. Figure 2a demonstrates how the glottis is nearly completely obstructed by a size 2.5 endotracheal tube (inner diameter 2.5 mm, outer diameter 4.1 mm) in a mannequin airway simulating a 1000 g baby, whereas a small diameter catheter (e.g. 5 French, external diameter: 1.7 mm) leaves most of the airway open allowing flow of gas through the vocal cords with the catheter in place (Fig. 2b). A 5-French tube occludes a cross sectional area of approximately 1.8 mm2. A standard endotracheal size 2.5 tube has a total cross sectional area of 13.2 mm2. It must be emphasized that only 4.9 mm2 of this area is are available for respiration. The rest (8.3 mm2) is occluded by the wall of the endotracheal tube [34].
Videos demonstrate that such small catheters allow movements of the vocal cords (e.g. Lancet TV: https://www.youtube.com/watch?v=IYf92NN1kV0).
Recently, the use of a 2.0 endotracheal tube (the smallest available size) has been described for LISA [35] but still this corresponds to an outer diameter of 8 French (2.7 mm).
UNANSWERED QUESTIONS
In addition to the technical issues, the dose question and the matter of exact indications/treatment thresholds for different groups of patients, there is an ongoing debate whether analgesia and sedation during the LISA procedure is necessary, or may actually endanger the success of the method.
Analgesia and sedation during the less invasive surfactant administration procedure
It has to be stated clearly that the use of LISA does not exclude analgesia and/or sedation per se. Stress and pain in the neonatal period may have long-term negative effects and should be avoided whenever possible. Currently, in Germany, in infants less than 26 weeks, most centres will do the first LISA attempt without analgesia [25]. If the infant struggles, analgesia and sedation is used for the second attempt. Nonpharmacological methods of analgesia like positioning, holding, (’facilitated tucking’) and/or sucrose solutions are often used. In more mature and vigorous infants, it seems wise to use analgesia and sedation right away. So far, there is no ideal combination of drugs that would allow analgesia and sedation with a rapid onset, a short duration, no suppression of spontaneous breathing and a favourable overall short and long-term safety profile. This is reflected by the fact that a variety of drugs have been studied for the purpose of analgesia/sedation during INSURE or LISA; fentanyl, ketamine and propofol were the most frequently used medications. First studies indicate that these drugs may help to reduce pain scores, but on the other hand, interfere with spontaneous breathing and increase the number of infants that need respiratory support/mechanical ventilation during LISA [36▪▪]. Thus, practice patterns of using these drugs vary widely between countries and NICUs [37].
Short-term side effects
Under the condition that LISA is performed by neonatologists experienced in airway management, there are few acute side effects. Failure to insert the catheter through the vocal cords at first attempt, significant surfactant reflux, acute desaturations, bradycardia and/or need for positive pressure ventilation during LISA were observed in less than 10% [8] to more than 30% [13] of LISA/MIST manipulations. Short apnoea, hypoxia and bradycardia can often be handled by slowing down the injection speed/interrupting the surfactant administration or, if needed, by a couple of manual breaths via a CPAP device that in our experience should stay in place during the LISA procedure. Studies with continuous monitoring of saturation and regional (e.g. also cerebral) saturation by near-infrared spectroscopy (NIRS) seem to indicate that the laryngoscopy is more often the source of side effects than the surfactant instillation itself [38]. The only potential adverse effect observed so far was a slight increase in the rate of focal intestinal perforation (FIP) in a subset of infants born at 23 and 24 weeks of gestation receiving LISA [39]. This finding may be related to the distension of the fragile intestinal wall in consequence the positive end-expiratory pressure applied during noninvasive ventilation, but clearly this has to be followed in more detail.
Long-term outcome
In individual studies [8,9] and metaanalyses [19,21] LISA reduces the incidence of ICH and BPD, and thus, bears the potential to improve also the long-term outcome. Small retrospective follow-up studies on LISA infants suggested favourable neurocognitive outcome compared with historical controls [40–43]. Unpublished data from the 5-year follow-up of LISA infants in the GNN cohort suggest better lung function (FEV1) and better neuro-outcome/intellectual properties (WPPSI score) in infants who received surfactant via LISA compared with infants who received surfactant via the standard route. Follow-up results from the randomized studies will soon become available.
CONCLUSION
LISA is part of the strategy of a minimal handling approach supporting the concept of spontaneous breathing. LISA reduces the need for mechanical ventilation, and is therefore, in increasing use in NICUs around the world [44–50,51▪]. Short-term (ICH and BPD) and first long-term follow-up data on neurocognitive and pulmonary function are encouraging. The search for better strategies allowing to deliver surfactant in an even gentler way goes on. In this context, especially surfactant nebulization may become one of the options on the horizon.
Acknowledgements
We are grateful to Philipp Jung, MD for help with the video laryngoscopy (Fig. 2).
Financial support and sponsorship
Most of the clinical data on LISA in Germany were obtained with the help of the German Neonatal Network (GNN; www.vlbw.de) that is sponsored by the German Ministry for Education and Research (BMBF-grant-No: 01ER0805 and 01ER1501). This manuscript is in part based on the results of an international meeting on Less Invasive Surfactant Application (LISA) that took part in Lübeck, Germany, on 31 May 2018. This workshop was sponsored by the German Research Foundation (DFG): Grant: DFG-He 2072-3.
Patient consent/Ethics: Written consent from the parents for the participation in the German Neonatal Network was obtained prior to enrollment. Approval by the local institutional review board for research in human subjects of the University of Lübeck (file number 08-022) and by the local ethic committees of all participating centres has been given. We are grateful for the support of the families and of the colleagues contributing to this network.
Conflicts of interest
E.H., C.H. and W.G. have received study support, honoraria for presentations and travel support from Chiesi Farmaceutici, a surfactant producer. E.H. and C.H. served as advisors for Draeger Medical, a company producing incubators, monitors and ventilators.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.More K, Sakhuja P, Shah PS. Minimally invasive surfactant administration in preterm infants: a meta-narrative review. JAMA Pediatr 2014; 168:901–908. [DOI] [PubMed] [Google Scholar]
- 2.Herting E. Less Invasive Surfactant Administration (LISA) - ways to deliver surfactant in spontaneously breathing infants. Early Hum Dev 2013; 89:875–880. [DOI] [PubMed] [Google Scholar]
- 3.Verder H, Agertoft L, Albertsen P, et al. Surfactant treatment of newborn infants with respiratory distress syndrome primarily treated with nasal continuous positive air pressure. A pilot study. Ugeskr Laeger 1992; 154:2136–2139. [PubMed] [Google Scholar]
- 4.Verder H, Robertson B, Greisen G, et al. Surfactant therapy and nasal continuous positive airway pressure for newborns with respiratory distress syndrome. Danish-Swedish Multicenter Study Group. N Engl J Med 1994; 331:1051–1055. [DOI] [PubMed] [Google Scholar]
- 5.Dargaville PA, Gerber A, Johansson S, et al. Australian and New Zealand Neonatal Network. Incidence and outcome of CPAP failure in preterm infants. Pediatrics 2016; 138: pii: e20153985. [DOI] [PubMed] [Google Scholar]
- 6.Kribs A, Pillekamp F, Hunseler C, et al. Early administration of surfactant in spontaneous breathing with nCPAP: feasibility and outcome in extremely premature infants (postmenstrual age ≤27 weeks). Paediatr Anaesth 2007; 17:364–369. [DOI] [PubMed] [Google Scholar]
- 7▪.Herting E, Härtel C, Göpel W. Less invasive surfactant administration (LISA): chances and limitations. Arch Dis Child Fetal Neonatal Ed 2019; 104:F655–F659. [DOI] [PMC free article] [PubMed] [Google Scholar]; Recent review on current trends with LISA.
- 8.Göpel W, Kribs A, Ziegler A, et al. German Neonatal Network. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open-label, randomised, controlled trial. Lancet 2011; 378:1627–1634. [DOI] [PubMed] [Google Scholar]
- 9.Kribs A, Roll C, Göpel W, et al. NINSAPP Trial Investigators. Nonintubated surfactant application vs conventional therapy in extremely preterm infants: a randomized clinical trial. JAMA Pediatr 2015; 169:723–730. [DOI] [PubMed] [Google Scholar]
- 10.Göpel W, Kribs A, Härtel C, et al. German Neonatal Network (GNN). Less invasive surfactant administration is associated with improved pulmonary outcomes in spontaneously breathing preterm infants. Acta Paediatr 2015; 104:241–246. [DOI] [PubMed] [Google Scholar]
- 11.Klebermass-Schrehof K, Wald M, Schwindt J, et al. Less invasive surfactant administration in extremely preterm infants: impact on mortality and morbidity. Neonatology 2013; 103:252–258. [DOI] [PubMed] [Google Scholar]
- 12.Dargaville PA, Aiyappan A, De Paoli AG, et al. Minimally-invasive surfactant therapy in preterm infants on continuous positive airway pressure. Arch Dis Child Fetal Neonatal Ed 2013; 98:F122–F126. [DOI] [PubMed] [Google Scholar]
- 13.Dargaville PA, Ali SKM, Jackson HD, et al. Impact of minimally invasive surfactant therapy in preterm infants at 29-32 weeks gestation. Neonatology 2018; 113:7–14. [DOI] [PubMed] [Google Scholar]
- 14.Bao Y, Zhang G, Wu M, et al. A pilot study of less invasive surfactant administration in very preterm infants in a Chinese tertiary center. BMC Pediatr 2015; 15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanmaz HG, Erdeve O, Canpolat FE, et al. Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial. Pediatrics 2013; 131:e502–e509. [DOI] [PubMed] [Google Scholar]
- 16.Berneau P, Nguyen Phuc Thu T, Pladys P, et al. Impact of surfactant administration through a thin catheter in the delivery room: A quality control chart analysis coupled with a propensity score matched cohort study in preterm infants. PLoS One 2018; 13:e0208252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gortner L, Schuller SS, Herting E. Review demonstrates that less invasive surfactant administration in preterm neonates leads to fewer complications. Acta Paediatr 2018; 107:736–743. [DOI] [PubMed] [Google Scholar]
- 18.Sweet DG, Carnielli V, Greisen G, et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome - 2016 Update. Neonatology 2017; 111:107–125. [DOI] [PubMed] [Google Scholar]
- 19.Isayama T, Iwami H, McDonald S, Beyene J. Association of noninvasive ventilation strategies with mortality and bronchopulmonary dysplasia among preterm infants: a systematic review and meta-analysis. JAMA 2016; 316:611–624. [DOI] [PubMed] [Google Scholar]
- 20.Rigo V, Lefebvre C, Broux I. Surfactant instillation in spontaneously breathing preterm infants: a systematic review and meta-analysis. Eur J Pediatr 2016; 175:1933–1942. [DOI] [PubMed] [Google Scholar]
- 21.Aldana-Aguirre JC, Pinto M, Featherstone RM, et al. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 2017; 102:F17–F23. [DOI] [PubMed] [Google Scholar]
- 22.O'Shea JE, Loganathan P, Thio M, et al. Analysis of unsuccessful intubations in neonates using videolaryngoscopy recordings. Arch Dis Child Fetal Neonatal Ed 2018; 103:F408–F412. [DOI] [PubMed] [Google Scholar]
- 23.Herting E, Stichtenoth G, Robertson B. Syringes with rubber-coated plungers and inactivation of surfactant. Lancet 2003; 361:311–313. [DOI] [PubMed] [Google Scholar]
- 24.Segerer H, van Gelder W, Angenent FW, et al. Pulmonary distribution and efficacy of exogenous surfactant in lung-lavaged rabbits are influenced by the instillation technique. Pediatr Res 1993; 34:490–494. [DOI] [PubMed] [Google Scholar]
- 25.Mehler K, Oberthuer A, Keller T, et al. Survival among infants born at 22 or 23 weeks’ gestation following active prenatal and postnatal care. JAMA Pediatr 2016; 170:671–677. [DOI] [PubMed] [Google Scholar]
- 26.Aguar M, Cernada M, Brugada M, et al. Minimally invasive surfactant therapy with a gastric tube is as effective as the intubation, surfactant, and extubation technique in preterm babies. Acta Paediatr 2014; 103:e229–e233. [DOI] [PubMed] [Google Scholar]
- 27.Dargaville PA, Aiyappan A, Cornelius A, et al. Preliminary evaluation of a new technique of minimally invasive surfactant therapy. Arch Dis Child Fetal Neonatal Ed 2011; 96:F243–F248. [DOI] [PubMed] [Google Scholar]
- 28.Maiwald CA, Neuberger P, Vochem M, et al. Quick SF: a new technique in surfactant administration. Neonatology 2017; 111:211–213. [DOI] [PubMed] [Google Scholar]
- 29▪.Roberts KD, Brown R, Lampland AL, et al. Laryngeal mask airway for surfactant administration in neonates: a randomized, controlled trial. J Pediatr 2018; 193:40–46. [DOI] [PubMed] [Google Scholar]; Study demonstrating that the laryngeal mask airway may become an alternative for surfactant treatment especially in more mature infants.
- 30.Pillow JJ, Minocchieri S. Innovation in surfactant therapy II: surfactant administration by aerosolization. Neonatology 2012; 101:337–344. [DOI] [PubMed] [Google Scholar]
- 31.Kattwinkel J, Robinson M, Bloom BT, et al. Technique for intrapartum administration of surfactant without requirement for an endotracheal tube. J Perinatol 2004; 24:360–365. [DOI] [PubMed] [Google Scholar]
- 32▪▪.Vento M, Bohlin K, Herting E, et al. Surfactant administration via thin catheter: a practical guide. Neonatology 2019; 116:211–226. [DOI] [PubMed] [Google Scholar]; Most comprehensive review available on LISA addressing all the practical aspects of LISA treatment.
- 33▪.Fabbri L, Klebermass-Schrehof K, Aguar M, et al. Five-country manikin study found that neonatologists preferred using the LISAcath rather than the Angiocath for less invasive surfactant administration. Acta Paediatr 2018; 107:780–783. [DOI] [PMC free article] [PubMed] [Google Scholar]; First results published with a special catheter to deliver surfactant under spontaneous breathing.
- 34.Göpel W, Kribs A, Herting E. LISA for prevention of BPD. In: Bronchopulmonary dysplasia. Bhandari (ed.) Springer 2016, pp: 315–324. [Google Scholar]
- 35.Gengaimuthu K. Minimally invasive surfactant therapy using a 2.0 mm uncuffed endotracheal tube as the conduit: an easily adaptable technique. Cureus 2019; 11:e5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36▪▪.Dekker J, Lopriore E, van Zanten HA, et al. Sedation during minimal invasive surfactant therapy: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2018-315015 doi: 10.1136. [DOI] [PubMed] [Google Scholar]; Study underlining the dilemma of analgesia and the LISA procedure. With analgesia, the pain scores were lower, and also more infants needed respiratory support.
- 37.Borenstein LL, Synnes A, Grunau RE, et al. Canadian Neonatal Network Investigators. Narcotics and sedative use in preterm neonates. J Pediatr 2017; 180:92.e1–98.e1. [DOI] [PubMed] [Google Scholar]
- 38.Hanke K, Rausch TK, Paul P, et al. The effect of less invasive surfactant administration on cerebral oxygenation in preterm infants. Acta Paediatr 2019; 109:291–299. [DOI] [PubMed] [Google Scholar]
- 39.Härtel C, Paul P, Hanke K, et al. Less invasive surfactant administration and complications of preterm birth. Sci Rep 2018; 8:8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porath M, Korp L, Wendrich D, et al. Surfactant in spontaneous breathing with nCPAP: neurodevelopmental outcome at early school age of infants ≤27 weeks. Acta Paediatr 2011; 100:352–359. [DOI] [PubMed] [Google Scholar]
- 41.Teig N, Weitkämper A, Rothermel J, et al. Observational study on less invasive surfactant administration (LISA) in preterm infants <29 weeks - short and long-term outcomes. Z Geburtshilfe Neonatol 2015; 219:266–273. [DOI] [PubMed] [Google Scholar]
- 42.Ramos-Navarro C, Sánchez-Luna M, Zeballos-Sarrato S, et al. Three-year perinatal outcomes of less invasive beractant administration in preterm infants with respiratory distress syndrome. J Matern Fetal Neonatal Med 2018; 1–171. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 43.Márquez Isidro E, Sánchez Luna M, Ramos-Navarro C. Long-term outcomes of preterm infants treated with less invasive surfactant technique (LISA). J Matern Fetal Neonatal Med 2019; 12:1–6. [DOI] [PubMed] [Google Scholar]
- 44.Kurepa D, Perveen S, Lipener Y, Kakkilaya V. The use of less invasive surfactant administration (LISA) in the United States with review of the literature. J Perinatol 2019; 39:426–432. [DOI] [PubMed] [Google Scholar]
- 45.Fernandez C, Boix H, Camba F, et al. Less Invasive Surfactant Administration in Spain: a survey regarding its practice, the target population, and premedication use. Am J Perinatol 2019; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 46.Heiring C, Jonsson B, Andersson S, Björklund LJ. Survey shows large differences between the Nordic countries in the use of less invasive surfactant administration. Acta Paediatr 2017; 106:382–386. [DOI] [PubMed] [Google Scholar]
- 47.Klotz D, Porcaro U, Fleck T, Fuchs H. European perspective on less invasive surfactant administration-a survey. Eur J Pediatr 2017; 176:147–154. [DOI] [PubMed] [Google Scholar]
- 48.Beltempo M, Isayama T, Vento M, et al. on behalf of the International Network for Evaluating Outcomes of Neonates. Respiratory management of extremely preterm infants: an international survey. Neonatology 2018; 114:28–36. [DOI] [PubMed] [Google Scholar]
- 49.Bhayat S, Kaur A, Premadeva I, et al. Survey of less invasive surfactant administration in England, slow adoption and variable practice. Acta Paediatr 2019; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 50.Jena SR, Bains HS, Pandita A, et al. Surfactant therapy in premature babies: SurE or InSurE. Pediatr Pulmonol 2019; 54:1747–1752. [DOI] [PubMed] [Google Scholar]
- 51▪.Guthrie SO, Cummings JJ. Not sure? How should we give exogenous surfactant to newborns with RDS. Pediatr Pulmonol 2019; doi: 10.1002/ppul.24543. [DOI] [PubMed] [Google Scholar]; Editorial describing the current discussion around LISA or INSURE


