Abstract
Objectives
Clinical Practice Guidelines for Pancreatic Cancer were first published in 2006 by the Japan Pancreas Society, and they were revised in 2009, 2013, and 2016. In July 2019, the Clinical Practice Guidelines for Pancreatic Cancer 2019 were newly revised in Japanese.
Methods
For this version, we developed the new guidelines according to the Minds Manual for Guideline Development 2017, which includes the concepts of GRADE (Grading Recommendations Assessment, Development, and Evaluation), to enable a better understanding of the current guidelines.
Results
The guidelines show algorithms for the diagnosis, treatment, and chemotherapy of pancreatic cancer and address 7 subjects: diagnosis, surgical therapy, adjuvant therapy, radiation therapy, chemotherapy, stent therapy, and supportive and palliative medicine. They include 56 clinical questions and 84 statements. There are statements corresponding to clinical questions, evidence levels, recommendation strengths, and agreement rates.
Conclusions
These guidelines represent the most standard clinical and practical management guidelines at this time in Japan. This is the English synopsis of the Clinical Practice Guidelines for Pancreatic Cancer 2019 in Japanese and is an attempt to disseminate the Japanese guidelines worldwide for introducing the Japanese approach for clinical management of pancreatic cancer.
Key Words/Abbreviations: clinical guidelines; Japan Pancreas Society, Minds, GRADE system; pancreatic cancer; CT - computed tomography; ERCP - endoscopic retrograde cholangiopancreatography; EUS - endoscopic ultrasonography; JPS - Japan Pancreas Society; MDCT - multidetector-row CT; PET - positron emission tomography
Clinical Practice Guidelines for Pancreatic Cancer based on Evidence-Based Medicine 20061 were first published by the Japan Pancreas Society (JPS) and have been revised repeatedly: in July 2009,2,3 October 2013,4,5 and October 20166,7; a new revision has again been published in July 2019.8 For this latest version, we developed new guidelines according to the Minds Manual for Guideline Development 2017.9 In addition, the composition of the committee members changed for the revision, and more specialists from various fields were included to avoid biases in the recommendations. These guidelines represent the most standard guidelines for clinical and practical management of pancreatic cancer available at this time in Japan. This is the English synopsis of the Clinical Practice Guidelines for Pancreatic Cancer 2019 in Japanese, as an attempt to disseminate the Japanese guidelines worldwide, for introducing the Japanese approach for the clinical management of pancreatic cancer.
GENERAL OUTLINE OF THE REVISION PROCESS
The committee for Revision of the Clinical Guidelines for Pancreatic Cancer in the JPS consisted of Takuji Okusaka as chairman; Masafumi Nakamura as the vice-chairman; Masahiro Yoshida, Masayuki Kitano, Katsuhiko Uesaka, Yoshinori Ito, Junji Furuse, and Keiji Hanada as the chiefs of each of the groups; and 41 other specialists (medical doctors specialized in internal medicine, surgery, gastroenterology, medical oncology, radiology, endoscopy, psycho-oncology, nutrition, palliative and supportive medicine, a nurse specialized in cancer therapeutics, a cancer pharmacist, medical social workers, and a patient representative with pancreatic cancer) as committee members for the revision of the guidelines. In addition, 43 other specialists helped in the revision as assistants. The revision process with these committee members began in July 2017.
These new guidelines have the support of Prof Masahiro Yoshida, Mr Yosuke Hatakeyama, and Mr Sho Sasaki from Minds, under the special support program contracted by Minds and JPS. The committee members proposed the guidelines consisting of algorithms for the diagnosis (Fig. 1), treatment (Fig. 2), and chemotherapy (Fig. 3) of pancreatic cancer, and the general consensuses including those for uncontroversial “background questions” and well-established recommendations for diagnosis, surgical therapy, adjuvant therapy, radiotherapy, chemotherapy, stent therapy, and supportive and palliative therapy of pancreatic cancer, and the particular discussions consisting of “clinical questions (CQs)” and recommendations. A comprehensive search of the literature for the latest articles published after January 1990 (the year in which the literature search was performed for the first version of the guidelines) was performed for each CQ by a librarian (Mr Naohiko Yamaguchi). A total of 1014 articles were collected from 12,274 reports concerning pancreatic cancer that were listed on PubMed and Igaku Chuo Zasshi (ICHUSHI), a Japanese bibliographic database, from January 1990 to October 2017. The guidelines address particular discussions including the 6 subjects pertaining to the diagnosis of pancreatic cancer (15 CQs and 15 statements), treatment of resectable disease (13 CQs and 16 statements), treatment of borderline resectable disease (3 CQs and 5 statements), treatment of locally advanced disease (9 CQs and 17 statements), treatment of metastatic disease (6 CQs and 17 statements), and supportive and palliative medicine (10 CQs and 14 statements). The corresponding CQ numbers are inserted in the algorithms. There are statements corresponding to the CQs, with the evidence levels, recommendation strengths, and agreement rates.
FIGURE 1.
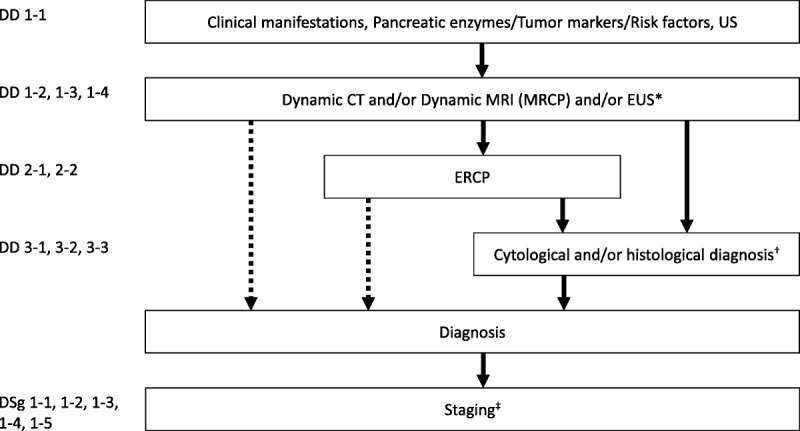
Algorithm for the diagnosis of pancreatic cancer. *It is desirable for endoscopic ultrasonography (EUS) to be performed at an institution where a high level of skill for EUS is available. †The diagnosis must be established by histopathology as much as possible. ‡Dynamic computed tomography (CT), dynamic magnetic resonance imaging (MRI), EUS, positron emission tomography (PET), and/or laparoscopic examination should be performed as needed. ERCP, endoscopic retrograde cholangiopancreatography; MRCP, magnetic resonance cholangiopancreatography.
FIGURE 2.
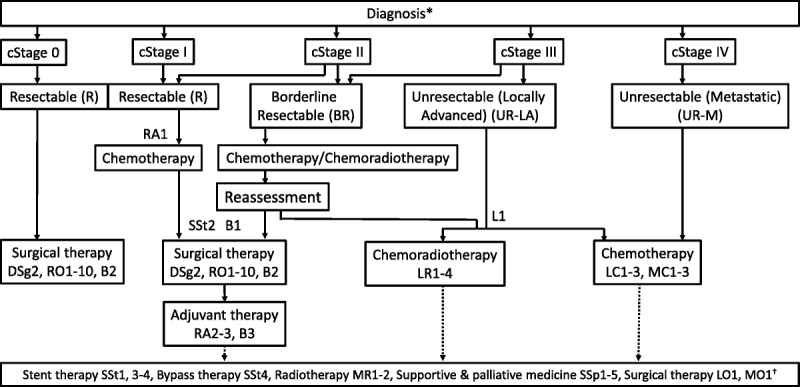
Algorithm for the treatment of pancreatic cancer. Cancer stage classification and resectability classification are based on the General Rules for the Study of Pancreatic Cancer, Seventh Edition, the JPS.10 *Supportive care for pain, digestion and absorption disorders, pancreatic diabetes, and anxiety is required even from the early stages after diagnosis in patients with pancreatic cancer. For further details, refer to the guidelines or the homepage of the Japanese Society for Palliative Medicine (http://www.jspm.ne.jp/guidelines/index.html). †Stent therapy, bypass therapy, radiotherapy, supportive and palliative medicine, and/or surgical therapy are recommended according to individual patients' conditions.
FIGURE 3.
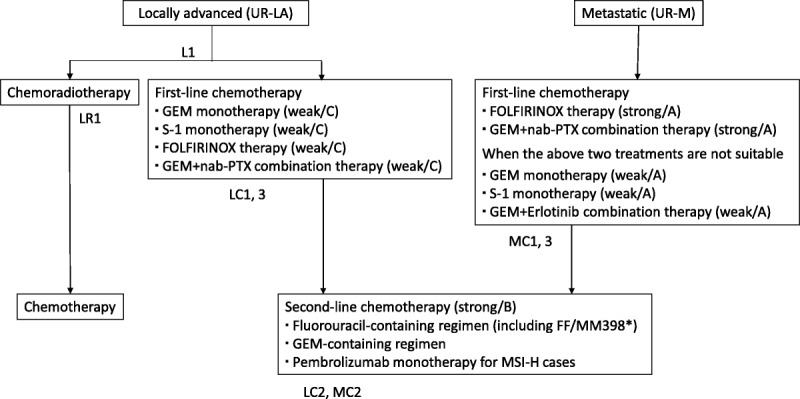
Algorithm for chemotherapy of pancreatic cancer. The recommendation strengths/evidence levels are given in parentheses. *MM-398 is not covered by health insurance in Japan. GEM indicates gemcitabine; nab-PTX, nab-paclitaxel; FF, fluorouracil + calcium folinate.
We used the Minds manual following the GRADE system approach. The overall quality of the body of evidence across gross studies for each important outcome was assessed. The evidence level was graded on a scale of A (strongest) to D (least strong). Each committee member specialized in the subject of each CQ prepared a draft of the statement, the evidence level, and the recommended strength. Committee members added some references from their own searches and performed meta-analyses independently as necessary. These were then reviewed, modified, and finalized by all committee members. The recommended strength was decided considering 4 factors: evidence level, balance of benefits and harms/burdens, patients' preferences, and cost benefits. Finally, the recommendation strengths were divided into 5 categories (1 = strong, recommend adoption on the approach, 2 = weak, propose adoption on the approach, 3 = weak, propose not to adopt the approach, 4 = strong, recommend against adoption of the approach, 5 = no recommendation) consensus of the attending committee members when at least 67% of the members attended. When the total agreement rate with recommendation of adopting or not adopting the approach was 50% or more and the total rate of agreement with the opposite view was less than 20%, the recommendation supported by 50% or more members was accepted by the committee. When the agreement rate with the recommendation of adopting or not adopting the approach was 70% or more, the strong recommendation was accepted by the committee members. If the voting results did not fulfill any of the aforementioned criteria, the committee members held discussions again, and a final voting was held. Nonfulfillment of the above criteria in the final voting led to the decision of “no recommendation.” Acceptability was determined by voting using an answer pad system by the committee members who attended.
To improve and confirm the validity of the guidelines, they were released in a draft form on the JPS website, inviting comments from the public. Simultaneously, they were reviewed by 2 external appraisal committees independently using AGREE Reporting Checklists: a group assigned by the JPS that consisted of surgeons (Koji Yamaguchi, Shuji Isaji, and Shoji Natsugoe), gastroenterologists (Hiroyuki Maguchi and Kazuma Fujimoto), an epidemiologist (Takeo Nakayama) and a patient representative with pancreatic cancer, and another group assigned by Minds, including specialists in guidelines methodology (Eiji Ishikawa, Hiroshi Okamoto, Hiroshi Koga, and Nobumasa Takagaki), independent of the revision committee members. Finally, taking into account the comments from the public and the external reviewers, the guidelines were reviewed and modified again by the revision committee members and finalized. These new Clinical Guidelines for Pancreatic Cancer 20198 follow the new General Rules for the Study of Pancreatic Cancer published by the JPS in July 2016.10
NOTES ON THE USE OF THE GUIDELINES
These guidelines represent the most standard guidelines for clinical and practical care of patients with pancreatic cancer available at this time. However, they should not be used inflexibly for the practical management of individual patients. The JPS is responsible for the statements in these guidelines. The JPS and the committee members are not liable for any consequences arising from any treatment, for which individual physicians involved in the treatment are responsible.
ALGORITHMS
The algorithms present the flow for diagnosis, treatment, and chemotherapy of pancreatic cancer. For detailed explanation of each CQ, please refer to the indicated box (Figs. 1-3).
I. Diagnosis
1. Diagnosis or D
A. Detection or D
DD1-1 Is ultrasonography recommended as a diagnostic method in subjects with suspected pancreatic cancer?
Statement:
Ultrasonography is recommended as a diagnostic tool in subjects with suspected pancreatic cancer.
Recommendation strength: weak; evidence level: C; agreement rates (N = 39): 1 = 10%, 2 = 77%, 3 = 10%, 4 = 0%, 5 = 3%*
(*N = number of voters; 1 = strong, recommend adoption of the approach; 2 = propose adoption of the approach; 3 = propose not to adopt the approach; 4 = strong, recommend against adoption of the approach; 5 = no recommendation)
DD1-2 Is contrast-enhanced computed tomography (CT) recommended as a diagnostic method in subjects with suspected pancreatic cancer?
Statement:
Contrast-enhanced CT (multidetector-row CT [MDCT] is desirable) is recommended as a diagnostic tool in subjects with suspected pancreatic cancer.
Recommendation strength: strong; evidence level: B; agreement rates (N = 40): 1 = 100%, 2 = 0%, 3 = 0%, 4 = 0%, 5 = 0%
DD1-3 Is abdominal magnetic resonance imaging (MRI) recommended as a diagnostic method in subjects with suspected pancreatic cancer?
Statement:
Abdominal MRI is recommended as a diagnostic tool in subjects with suspected pancreatic cancer.
Recommendation strength: weak; evidence level: C; agreement rates (N = 39): 1 = 10%, 2 = 90%, 3 = 0%, 4 = 0%, 5 = 0%
DD1-4 Is endoscopic ultrasonography (EUS) recommended as a diagnostic method in subjects with suspected pancreatic cancer?
Statement:
Endoscopic ultrasonography is recommended as a diagnostic tool in subjects with suspected pancreatic cancer, because it is more sensitive than other imaging modalities for the diagnosis of pancreatic cancer. However, the indication for EUS should be carefully determined, because it is a relatively invasive procedure.
Recommendation strength: weak; evidence level: C; agreement rates (N = 39): 1 = 3%, 2 = 97%, 3 = 0%, 4 = 0%, 5 = 0%
DD2-1 Is endoscopic retrograde cholangiopancreatography recommended as the next step in the diagnosis of pancreatic cancer?
Statement:
Endoscopic retrograde cholangiopancreatography is recommended for the diagnosis of pancreatic duct stenosis, which is difficult to differentiate from inflammatory lesions by other imaging modalities, or of pancreatic duct stenosis, which could be a manifestation of early pancreatic cancer.
Recommendation strength: weak; evidence level: C; agreement rates (N = 38): 1 = 8%, 2 = 92%, 3 = 0%, 4 = 0%, 5 = 0%
DD2-2 Is positron emission tomography (PET) recommended as the next step in the diagnosis of pancreatic cancer?
Statement:
Positron emission tomography is not recommended as the next step in the diagnosis of pancreatic cancer.
Recommendation strength: weak; evidence level: C; agreement rates (N = 39): 1 = 0%, 2 = 13%, 3 = 72%, 4 = 3%, 5 = 13%
DD3-1 Are cytology and histology recommended as definitive diagnostic procedures for pancreatic cancer?
Statement:
Cytological and histological examinations are recommended for the diagnosis of pancreatic cancer, as they have been shown to exhibit high sensitivity and specificity for the diagnosis of pancreatic cancer.
Recommendation strength: weak; evidence level: C; agreement rates (N = 39): 1 = 41%, 2 = 56%, 3 = 0%, 4 = 0%, 5 = 3%
DD3-2 In subjects presenting with a mass lesion in the pancreas, is EUS fine-needle aspiration recommended as a diagnostic procedure?
Statement:
Endoscopic ultrasonography fine-needle aspiration is recommended for the histopathological diagnosis of pancreatic masses, because it has been shown to exhibit a high sensitivity and specificity for the diagnosis and is useful for differentiating pancreatic cancer from other pancreatic masses.
Recommendation strength: weak; evidence level: B; agreement rates (N = 37): 1 = 19%, 2 = 78%, 3 = 0%, 4 = 0%, 5 = 3%
DD3-3 Is pancreatic fluid cytology via endoscopic retrograde cholangiopancreatography recommended in patients with abnormal pancreatic duct findings, but no mass lesions?
Statement:
Endoscopic retrograde cholangiopancreatography with pancreatic fluid cytology is recommended in patients with abnormal pancreatic duct findings, but no mass lesions. However, particular attention should be paid to the possible development of acute pancreatitis precipitated by endoscopic retrograde cholangiopancreatography.
Recommendation strength: weak; evidence level: D; agreement rates (N = 36): 1 = 6%, 2 = 92%, 3 = 0%, 4 = 0%, 5 = 3%
B. Staging or Sg
DSg1-1 Is contrast-enhanced MDCT recommended for the staging of pancreatic cancer and for assessment of its resectability?
Statement:
Contrast-enhanced CT (MDCT is desirable) is recommended for the staging of pancreatic cancer and for assessment of its resectability.
Recommendation strength: strong; evidence level: B; agreement rates (N = 35): 1 = 97%, 2 = 3%, 3 = 0%, 4 = 0%, 5 = 0%
DSg1-2 Is abdominal MRI recommended for the staging of pancreatic cancer and for assessment of its resectability?
Statement:
Performance of contrast-enhanced MRI for the staging pancreatic cancer and for assessment of its resectability.
Recommendation strength: weak; evidence level: C; agreement rates (N = 39): 1 = 3%, 2 = 90%, 3 = 3%, 4 = 0%, 5 = 5%
DSg1-3 Is EUS recommended for the staging of pancreatic cancer and for assessment of its resectability?
Statement:
When contrast-enhanced CT cannot definitively determine the disease stage/resectability, addition of EUS is recommended, because EUS is better than contrast-enhanced CT for diagnosing T-factor/vascular invasion.
Recommendation strength: weak; evidence level: C; agreement rates (N = 36): 1 = 6%, 2 = 86%, 3 = 3%, 4 = 0%, 5 = 6%
DSg1-4 Is PET recommended for the staging of pancreatic cancer?
Statement:
Positron emission tomography is recommended in patients with suspected distant metastasis, as PET is more specific than CT for the diagnosis of distant metastasis.
Recommendation strength: weak; evidence level: C; agreement rates (N = 37): 1 = 5%, 2 = 89%, 3 = 3%, 4 = 0%, 5 = 3%
DSg1-5 Is staging laparoscopy recommended for the staging of pancreatic cancer and for assessment of its resectability?
Statement:
When surgery is planned, but distant metastasis such as peritoneal dissemination cannot be ruled out, staging laparoscopy is recommended, because staging laparoscopy is useful for evaluating the presence/absence of hepatic micrometastases on the surface of the liver and peritoneal dissemination.
Recommendation strength: weak; evidence level: C; agreement rates (N = 37): 1 = 3%, 2 = 81%, 3 = 11%, 4 = 0%, 5 = 5%
DSg2 Is preoperative assessment of nutrition and body composition (muscle and fat mass) along with blood biochemical tests recommended in patients with pancreatic cancer?
Statement:
Assessment of the preoperative nutritional status and body composition is recommended, because these variables have been shown to contribute to prediction of the long-term prognosis and postoperative complications in patients undergoing surgery for pancreatic cancer.
Recommendation strength: weak; evidence level: C; agreement rates (N = 39): 1 = 5%, 2 = 92%, 3 = 0%, 4 = 0%, 5 = 3%
II. Treatment
1. Treatment of Resectable Disease or R
A. Operation or O
RO1 Is surgical treatment for pancreatic cancer recommended at a facility with a large volume of surgical cases?
Statement:
Surgical treatment for pancreatic cancer is recommended in a high-volume facility.
Recommendation strength: weak; evidence level: B; agreement rates (N = 39): 1 = 49%, 2 = 51%, 3 = 0%, 4 = 0%, 5 = 0%
RO2 Is surgical treatment recommended for pancreatic cancer patients with positive peritoneal lavage cytology?
Statement:
It is not clear whether surgical treatment is indicated for pancreatic cancer patients with positive peritoneal lavage cytology.
Recommendation strength: no recommendation; evidence level: D; agreement rates (N = 39): 1 = 3%, 2 = 5%, 3 = 5%, 4 = 3%, 5 = 85%
RO3 Is combined portal vein resection recommended in patients with pancreatic cancer?
Statement:
It is not clear whether combined portal vein resection for pancreatic cancer would improve the prognosis in patients with pancreatic cancer. When R0 resection is expected, combined portal vein resection is recommended.
Recommendation strength: weak; evidence level: D; agreement rates (N = 39): 1 = 31%, 2 = 69%, 3 = 0%, 4 = 0%, 5 = 0%
RO4 Is prophylactic extended lymph node and nerve plexus dissection recommended in patients with pancreatic cancer?
Statement:
Prophylactic extended lymph node and nerve plexus dissection is not recommended for patients with pancreatic cancer, because it has been shown to not contribute to improvement of the survival.
Recommendation strength: strong; evidence level: B; agreement rates (N = 39): 1 = 0%, 2 = 0%, 3 = 18%, 4 = 82%, 5 = 0%
RO5 Is laparoscopic pancreaticoduodenectomy recommended for patients with invasive ductal carcinoma who are candidates for pancreaticoduodenectomy?
Statement:
Laparoscopic pancreaticoduodenectomy has been reported to be associated with less intraoperative blood loss, a lower perioperative transfusion rate, a shorter length of hospital stay, and a longer recurrence-free survival as compared to open pancreaticoduodenectomy. However, these data have been obtained only from observational studies of operations performed at high-volume facilities for low-grade lesions, and it is still not clear whether the laparoscopic surgical approach is appropriate for patients with pancreatic cancer. Furthermore, it should be borne in mind that laparoscopic pancreaticoduodenectomy is not covered by health insurance in Japan. Based on the above, we conclude that while laparoscopic pancreaticoduodenectomy may be performed in clinical studies, this surgical approach is still not recommended in clinical practice in Japan.
Recommendation strength: weak; evidence level: D; agreement rates (N = 39): 1 = 0%, 2 = 0%, 3 = 72%, 4 = 18%, 5 = 10%
RO6 Is laparoscopic distal pancreatectomy recommended for patients with invasive ductal carcinoma who are candidates for distal pancreatectomy?
Statement:
Laparoscopic distal pancreatectomy may be beneficial. However, it should only be performed in patients without multiple organ invasion who do not require combined vascular resection, at facilities specializing in the treatment of pancreatic cancer.
Recommendation strength: weak; evidence level: D; agreement rates (N = 38): 1 = 0%, 2 = 79%, 3 = 11%, 4 = 3%, 5 = 8%
RO7 Is long-term regular surveillance recommended after surgical resection for pancreatic cancer?
Statement:
Continued regular long-term surveillance is recommended even in patients surviving for more than 5 years after resection of pancreatic cancer.
Recommendation strength: weak; evidence level: D; agreement rates (N = 39): 1 = 21%, 2 = 74%, 3 = 3%, 4 = 0%, 5 = 3%
RO8 Is perioperative nutrition therapy (enteral nutrition therapy) recommended after surgical resection for pancreatic cancer?
Statement:
Perioperative nutrition therapy (enteral nutrition therapy) is not recommended after surgical resection for pancreatic cancer.
Recommendation strength: weak; evidence level: C; agreement rates (N = 38): 1 = 0%, 2 = 16%, 3 = 55%, 4 = 0%, 5 = 29%
RO9 Is surgical treatment recommended for elderly patients with pancreatic cancer 80 years or older?
Statement:
Surgical treatment may be considered for elderly patients with pancreatic cancer 80 years or older if they wish to undergo the surgery and their general condition allows it. However, evidence for this recommendation is insufficient, and results of future studies are awaited.
Recommendation strength: weak; evidence level: D; agreement rates (N = 38): 1 = 3%, 2 = 92%, 3 = 0%, 4 = 0%, 5 = 5%
RO10 Is total pancreatectomy recommended to achieve R0 resection in patients with pancreatic cancer?
Statement:
Total pancreatectomy is recommended to achieve R0 resection in patients with pancreatic cancer.
Recommendation strength: weak; evidence level: C; agreement rates (N = 38): 1 = 24%, 2 = 76%, 3 = 0%, 4 = 0%, 5 = 0%
B. Adjuvant or A
RA1 Is neoadjuvant therapy recommended for patients with resectable pancreatic cancer?
Statement:
Combined gemcitabine + S-1 therapy is recommended as neoadjuvant therapy in patients with resectable pancreatic cancer.
Recommendation strength: weak; evidence level: C; agreement rates (N = 31): 1 = 0%, 2 = 90%, 3 = 0%, 4 = 0%, 5 = 10%
RA2 Is adjuvant chemoradiotherapy recommended for patients with pancreatic cancer?
Statement:
Adjuvant chemoradiotherapy is not recommended for patients with pancreatic cancer.
Recommendation strength: weak; evidence level: B; agreement rates (N = 41): 1 = 0%, 2 = 0%, 3 = 100%, 4 = 0%, 5 = 0%
RA3 Is adjuvant chemotherapy recommended in patients with pancreatic cancer?
Statement:
1. Adjuvant chemotherapy is recommended in patients with pancreatic cancer treated by macroscopic radical resection.
Recommendation strength: strong; evidence level: A; agreement rates (N = 38): 1 = 95%, 2 = 5%, 3 = 0%, 4 = 0%, 5 = 0%
2. S-1 monotherapy is recommended as an adjuvant chemotherapeutic agent in patients with pancreatic cancer.
Recommendation strength: strong; evidence level: A; agreement rates (N = 31): 1 = 87%, 2 = 13%, 3 = 0%, 4 = 0%, 5 = 0%
3. Gemcitabine hydrochloride monotherapy is recommended for patients with poor tolerance to S-1.
Recommendation strength: strong; evidence level: A; agreement rates (N = 36): 1 = 78%, 2 = 19%, 3 = 3%, 4 = 0%, 5 = 0%
4. Combined gemcitabine hydrochloride + capecitabine therapy (not covered by health insurance) and modified fluorouracil, leucovorin, irinotecan, and oxaliplatin (modified FOLFIRINOX) therapy (not covered by health insurance) are recommended for patients with pancreatic cancer, based on the results of phase III studies conducted outside Japan.
Combined gemcitabine hydrochloride + capecitabine therapy
Recommendation strength: weak; evidence level: A; agreement rates (N = 36): 1 = 0%, 2 = 69%, 3 = 8%, 4 = 0%, 5 = 22%
Modified FOLFIRINOX Therapy
Recommendation strength: weak; evidence level: A; agreement rates (N = 34): 1 = 0%, 2 = 74%, 3 = 3%, 4 = 3%, 5 = 21%
2. Treatment of Borderline Resectable Disease or B
B1 Is surgical treatment recommended for patients with borderline resectable pancreatic cancer?
Statement:
In patients with borderline resectable pancreatic cancer, it is recommended that a reassessment of the therapeutic efficacy be performed after neoadjuvant therapy, prior to surgery, in order to determine whether the cancer can be curatively resected.
Recommendation strength: weak; evidence level: C; agreement rates (N = 38): 1 = 8%, 2 = 89%, 3 = 0%, 4 = 0%, 5 = 3%
B2 Is combined arterial resection recommended in patients with pancreatic cancer?
Statement:
1. Distal pancreatectomy with celiac artery resection is recommended.
Recommendation strength: weak; evidence level: D; agreement rates (N = 38): 1 = 3%, 2 = 92%, 3 = 0%, 4 = 0%, 5 = 5%
2. Combined resection of the hepatic artery is recommended.
Recommendation strength: weak; evidence level: D; agreement rates (N = 39): 1 = 0%, 2 = 87%, 3 = 0%, 4 = 0%, 5 = 13%
3. Combined resection of the superior mesenteric artery is not recommended.
Recommendation strength: weak; evidence level: D; agreement rates (N = 39): 1 = 0%, 2 = 3%, 3 = 79%, 4 = 15%, 5 = 3%
B3 Is adjuvant chemotherapy recommended for patients with borderline resectable pancreatic cancer?
Statement:
Adjuvant chemotherapy is recommended for patients with borderline resectable pancreatic cancer.
Recommendation strength: weak; evidence level: C; agreement rates (N = 39): 1 = 8%, 2 = 92%, 3 = 0%, 4 = 0%, 5 = 0%
3. Treatment of Locally Advanced Disease or L
L1 What is the first-line treatment recommended for patients with locally advanced, unresectable pancreatic cancer?
Statement:
As first-line treatment for locally advanced, unresectable pancreatic cancer,
1. Chemoradiotherapy is recommended.
Recommendation strength: weak; evidence level: B; agreement rates (N = 39): 1 = 46%, 2 = 51%, 3 = 3%, 4 = 0%, 5 = 0%
2. Chemotherapy alone is recommended.
Recommendation strength: weak; evidence level: B; agreement rates (N = 39): 1 = 56%, 2 = 44%, 3 = 0%, 4 = 0%, 5 = 0%
A. Radiation or R
LR1 What is the chemoradiotherapy regimen recommended for patients with locally advanced, unresectable pancreatic cancer?
Statement:
1. For locally advanced, unresectable pancreatic cancer patients scheduled to receive chemoradiotherapy, concurrent use of fluoropyrimidine with radiotherapy is recommended.
Recommendation strength: weak; evidence level: C; agreement rates (N = 38): 1 = 53%, 2 = 47%, 3 = 0%, 4 = 0%, 5 = 0%
2. For patients with locally advanced, unresectable pancreatic cancer patients scheduled to receive chemoradiotherapy, concurrent use of gemcitabine hydrochloride with radiotherapy is recommended.
Recommendation strength: weak; evidence level: C; agreement rates (N = 40): 1 = 33%, 2 = 65%, 3 = 3%, 4 = 0%, 5 = 0%
LR2 Is elective nodal irradiation for regional lymph nodes recommended in radiotherapy for locally advanced, unresectable pancreatic cancer patients?
Statement:
In patients with locally advanced, unresectable pancreatic cancer scheduled to receive radiotherapy, elective nodal irradiation for the para-aortic lymph nodes is not recommended.
Recommendation strength: weak; evidence level: D; agreement rates (N = 31): 1 = 0%, 2 = 0%, 3 = 100%, 4 = 0%, 5 = 0%
LR3 Is induction chemotherapy recommended prior to chemoradiotherapy for patients with locally advanced, unresectable pancreatic cancer?
Statement:
Induction chemotherapy is not recommended prior to chemoradiotherapy in patients with locally advanced, unresectable pancreatic cancer.
Recommendation strength: weak; evidence level: C; agreement rates (N = 37): 1 = 0%, 2 = 14%, 3 = 76%, 4 = 3%, 5 = 8%
LR4 Are radiotherapy and chemoradiotherapy recommended for locally advanced, unresectable pancreatic cancer patients with local symptoms such as pain?
Statement:
Radiotherapy or chemoradiotherapy is recommended for patients with locally advanced, unresectable pancreatic cancer with local symptoms such as pain.
Recommendation strength: weak; evidence level: C; agreement rates (N = 39): 1 = 0%, 2 = 95%, 3 = 0%, 4 = 0%, 5 = 5%
B. Chemotherapy or C
LC1 What is the first-line chemotherapy recommended for patients with locally advanced, unresectable pancreatic cancer?
Statement:
1. Gemcitabine hydrochloride monotherapy is recommended.
Recommendation strength: weak; evidence level: C; agreement rates (N = 36): 1 = 14%, 2 = 83%, 3 = 3%, 4 = 0%, 5 = 0%
2. S-1 monotherapy is recommended.
Recommendation strength: weak; evidence level: C; agreement rates (N = 32): 1 = 6%, 2 = 94%, 3 = 0%, 4 = 0%, 5 = 0%
3. Combined fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) therapy is recommended.
Recommendation strength: weak; evidence level: C; agreement rates (N = 35): 1 = 11%, 2 = 89%, 3 = 0%, 4 = 0%, 5 = 0%
4. Combined gemcitabine hydrochloride + nab-paclitaxel therapy is recommended.
Recommendation strength: weak; evidence level: C; agreement rates (N = 34): 1 = 21%, 2 = 79%, 3 = 0%, 4 = 0%, 5 = 0%
LC2 (MC2) Is second-line chemotherapy recommended for patients with unresectable pancreatic cancer?
Statement:
1. Second-line chemotherapy is recommended for unresectable pancreatic cancer patients who are refractory to first-line therapy.
Recommendation strength: strong; evidence level: B; agreement rates (N = 34): 1 = 74%, 2 = 26%, 3 = 0%, 4 = 0%, 5 = 0%
2. Use of a fluorouracil-containing regimen (including fluorouracil + calcium folinate + MM-398; MM-398 is not covered by health insurance) is recommended after a gemcitabine hydrochloride–containing regimen.
Recommendation strength: weak; evidence level: C; agreement rates (N = 33): 1 = 24%, 2 = 73%, 3 = 0%, 4 = 0%, 5 = 3%
3. Use of a gemcitabine hydrochloride–containing regimen is recommended after a fluorouracil-containing regimen.
Recommendation strength: weak; evidence level: C; agreement rates (N = 33): 1 = 27%, 2 = 73%, 3 = 0%, 4 = 0%, 5 = 0%
4. Pembrolizumab monotherapy (still not approved for health insurance coverage at the time of voting in October 2018) is recommended for microsatellite instability-high cases.
Recommendation strength: weak; evidence level: C; agreement rates (N = 33): 1 = 0%, 2 = 70%, 3 = 0%, 4 = 0%, 5 = 30%
LC3 (MC3) Is continuation of chemotherapy until disease progression becomes obvious recommended in patients with unresectable pancreatic cancer?
Statement:
Continuation of chemotherapy is recommended until the onset of an adverse event that would make further continuation of chemotherapy difficult or disease progression becomes obvious in patients with unresectable pancreatic cancer.
Recommendation strength: weak; evidence level: D; agreement rates (N = 34): 1 = 44%, 2 = 47%, 3 = 0%, 4 = 0%, 5 = 9%
C. Operation or O
LO1 Is resection of the primary lesion after multidisciplinary treatment recommended in patients with locally advanced cancer who were judged as being unsuitable candidates for resection at the first examination?
Statement:
Resection of the primary lesion after multidisciplinary treatment is one of the treatment options for patients with locally advanced pancreatic cancer in whom the disease was judged as being unresectable at the first examination, because good survival or recurrence-free survival may be obtained in patients in whom the treatment is successful and the cancer becomes resectable.
Recommendation strength: weak; evidence level: C; agreement rates (N = 36): 1 = 6%, 2 = 86%, 3 = 6%, 4 = 0%, 5 = 3%
4. Treatment of Locally Metastatic Disease or M
A. Chemotherapy or C
MC1 What is the first-line chemotherapy is recommended for pancreatic cancer patients with distant metastasis?
Statement:
As first-line chemotherapy for pancreatic cancer with distant metastasis,
1. FOLFIRINOX therapy is recommended.
Recommendation strength: strong; evidence level: A; agreement rates (N = 34): 1 = 85%, 2 = 15%, 3 = 0%, 4 = 0%, 5 = 0%
2. Combined gemcitabine hydrochloride + nab-paclitaxel therapy is recommended.
Recommendation strength: strong; evidence level: A; agreement rates (N = 34): 1 = 94%, 2 = 6%, 3 = 0%, 4 = 0%, 5 = 0%
For patients in whom the above treatments are inappropriate due to the general condition, age, or other reasons,
3. Gemcitabine hydrochloride monotherapy is recommended.
Recommendation strength: weak; evidence level: A; agreement rates (N = 34): 1 = 35%, 2 = 65%, 3 = 0%, 4 = 0%, 5 = 0%
4. S-1 monotherapy is recommended.
Recommendation strength: weak; evidence level: A; agreement rates (N = 34): 1 = 26%, 2 = 74%, 3 = 0%, 4 = 0%, 5 = 0%
5. Combined gemcitabine hydrochloride + erlotinib hydrochloride therapy is recommended.
Recommendation strength: weak; evidence level: A; agreement rates (N = 34): 1 = 0%, 2 = 76%, 3 = 15%, 4 = 0%, 5 = 9%
MC2 (LC2) Is second-line chemotherapy recommended for patients with unresectable pancreatic cancer?
Statement:
1. Second-line chemotherapy is recommended for unresectable pancreatic cancer patients who are refractory to first-line therapy.
Recommendation strength: strong; evidence level: B; agreement rates (N = 34): 1 = 74%, 2 = 26%, 3 = 0%, 4 = 0%, 5 = 0%
2. Use of a fluorouracil-containing regimen (including fluorouracil + calcium folinate + MM-398; MM-398 is not covered by health insurance) is recommended after a gemcitabine hydrochloride–containing regimen.
Recommendation strength: weak; evidence level: C; agreement rates (N = 33): 1 = 24%, 2 = 73%, 3 = 0%, 4 = 0%, 5 = 3%
3. Use of a gemcitabine hydrochloride-containing regimen is recommended after a fluorouracil-containing regimen.
Recommendation strength: weak; evidence level: C; agreement rates (N = 33): 1 = 27%, 2 = 73%, 3 = 0%, 4 = 0%, 5 = 0%
4. Pembrolizumab monotherapy (still not approved for health insurance coverage at the time of voting in October 2018) is recommended for microsatellite instability-high cases.
Recommendation strength: weak; evidence level: C; agreement rates (N = 33): 1 = 0%, 2 = 70%, 3 = 0%, 4 = 0%, 5 = 30%
MC3 (LC3) Is continuation of chemotherapy until disease progression becomes obvious recommended in unresectable pancreatic cancer?
Statement:
Continuation of chemotherapy is recommended until the onset of an adverse event that would make further continuation of chemotherapy difficult or disease progression becomes obvious in patients with unresectable pancreatic cancer.
Recommendation strength: weak; evidence level: D; agreement rates (N = 34): 1 = 44%, 2 = 47%, 3 = 0%, 4 = 0%, 5 = 9%
B. Radiation or R
MR1 Is radiotherapy recommended for painful bone metastases in patients with pancreatic cancer?
Statement:
Radiotherapy is recommended for painful bone metastases in patients with pancreatic cancer.
Recommendation strength: strong; evidence level: B; agreement rates (N = 38): 1 = 79%, 2 = 21%, 3 = 0%, 4 = 0%, 5 = 0%
MR2 Is radiotherapy recommended for pancreatic cancer patients with postoperative metastatic/recurrent lesions?
Statement:
1. Radiotherapy is recommended for local recurrence and regional lymph node metastases.
Recommendation strength: weak; evidence level: D; agreement rates (N = 39): 1 = 0%, 2 = 95%, 3 = 3%, 4 = 0%, 5 = 3%
2. Radiotherapy is recommended for lung metastases.
Recommendation strength: weak; evidence level: D; agreement rates (N = 39): 1 = 0%, 2 = 85%, 3 = 0%, 4 = 0%, 5 = 15%
3. Radiotherapy is not recommended for liver metastases.
Recommendation strength: weak; evidence level: D; agreement rates (N = 39): 1 = 0%, 2 = 3%, 3 = 85%, 4 = 10%, 5 = 3%
C. Operation or O
MO1 Is surgical resection recommended for pancreatic cancer patients with postoperative metastatic/recurrent lesions?
Statement:
1. Surgical resection of the remnant pancreas is recommended.
Recommendation strength: weak, evidence level: D, agreement rates (N = 36): 1 = 0%, 2 = 92%, 3 = 3%, 4 = 0%, 5 = 6%
2. Surgical resection of lung metastases is recommended after carefully confirming the indication for surgical resection.
Recommendation strength: weak, evidence level: D, agreement rates (N = 37): 1 = 0%, 2 = 97%, 3 = 0%, 4 = 0%, 5 = 3%
3. Surgical resection is not recommended for other metastases (eg, liver metastases).
Recommendation strength: weak, evidence level: D, agreement rates (N = 39): 1 = 0%, 2 = 5%, 3 = 74%, 4 = 10%, 5 = 10%
5. Supportive and Palliative Medicine or S
A. Stenting or St
SSt1 Which of the following is the optimal biliary drainage approach in patients with unresectable pancreatic cancer: percutaneous transhepatic, endoscopic transpapillary, or endoscopic transmural biliary drainage?
Statement:
Endoscopic transpapillary biliary drainage is recommended as the first-line approach for biliary drainage in patients with unresectable pancreatic cancer.
Recommendation strength: weak; evidence level: B; agreement rates (N = 37): 1 = 19%, 2 = 81%, 3 = 0%, 4 = 0%, 5 = 0%
SSt2 Which of the following types of stent is recommended for preoperative pancreatic cancer patients with obstructive jaundice: plastic or metallic stent?
Statement:
1. Use of a metallic stent is recommended.
Recommendation strength: weak; evidence level: C; agreement rates (N = 37): 1 = 5%, 2 = 92%, 3 = 0%, 4 = 0%, 5 = 3%
-
2. When the waiting period for surgery is short, use of a plastic stent is recommended.
Recommendation strength: weak; evidence level: C; agreement rates (N = 37): 1 = 8%, 2 = 92%, 3 = 0%, 4 = 0%, 5 = 0%
SSt3-1 Which of the following types of stent is recommended for unresectable pancreatic cancer patients with obstructive jaundice: plastic or metallic stent?
Statement:
Use of a metallic stent is recommended.
Recommendation strength: strong; evidence level: A; agreement rates (N = 39): 1 = 85%, 2 = 13%, 3 = 0%, 4 = 0%, 5 = 3%
SSt3-2 Which of the following types of stent is recommended for unresectable pancreatic cancer patients with obstructive jaundice: covered or uncovered metallic stent?
Statement:
Use of a covered metallic stent is recommended.
Recommendation strength: weak; evidence level: B; agreement rates (N = 35): 1 = 11%, 2 = 89%, 3 = 0%, 4 = 0%, 5 = 0%
SSt4 Is gastrointestinal stenting recommended over surgical gastrojejunostomy in unresectable pancreatic cancer patients with gastrointestinal obstruction?
Statement:
It is not clear whether gastrointestinal stenting is superior to surgical gastrojejunostomy, and either may be selected according to the patient's condition and wishes and the circumstances at the facility.
Recommendation strength: no recommendation; evidence level: B; agreement rates (N = 35): 1 = 0%, 2 = 23%, 3 = 0%, 4 = 0%, 5 = 77%
B. Supportive and Palliative Medicine or Sp
SSp1 Are interventions directed at reducing the psychological distress recommended for patients with pancreatic cancer and their families?
Statement:
Provision of systematic support by a multidisciplinary team consisting of multiple experts, such as a palliative care team, is recommended even from the early stages of treatment for patients with advanced pancreatic cancer and their families.
Recommendation strength: weak; evidence level: C; agreement rates (N = 35): 1 = 14%, 2 = 86%, 3 = 0%, 4 = 0%, 5 = 0%
SSp2 Are non-opioid analgesics, opioid analgesics, adjuvant analgesics, and nerve blocks recommended for pancreatic cancer patients with upper abdominal/back pain?
Statement:
1. Pain treatment with a nonopioid/opioid analgesic(s) is recommended.
Recommendation strength: strong; evidence level: B; agreement rates (N = 35): 1 = 89%, 2 = 11%, 3 = 0%, 4 = 0%, 5 = 0%
2. When sufficient pain relief is not obtained despite increase of the dose of the opioid analgesic(s) within an acceptable range, concurrent use of adjuvant analgesics is recommended.
Recommendation strength: weak; evidence level: C; agreement rates (N = 35): 1 = 9%, 2 = 89%, 3 = 0%, 4 = 0%, 5 = 3%
3. Celiac nerve block may be performed as one of the treatment options.
Recommendation strength: weak; evidence level: A; agreement rates (N = 35): 1 = 0%, 2 = 94%, 3 = 0%, 4 = 0%, 5 = 6%
SSp3 Is exercise therapy recommended after surgery in patients with pancreatic cancer?
Statement:
Exercise therapy is recommended after surgery in patients with pancreatic cancer.
Recommendation strength: weak; evidence level: C; agreement rates (N = 35): 1 = 3%, 2 = 97%, 3 = 0%, 4 = 0%, 5 = 0%
SSp4 Is advance care planning recommended for patients with advanced pancreatic cancer?
Statement:
Advance care planning is recommended for patients with advanced pancreatic cancer.
Recommendation strength: weak; evidence level: C; agreement rates (N = 36): 1 = 3%, 2 = 97%, 3 = 0%, 4 = 0%, 5 = 0%
SSp5 Are pregabalin and duloxetine recommended for peripheral neuropathy caused by FOLFIRINOX therapy or gemcitabine hydrochloride + nab-paclitaxel combination therapy in patients with pancreatic cancer?
Statement:
1. Use of duloxetine is recommended for peripheral neuropathy associated with FOLFIRINOX therapy or combined gemcitabine hydrochloride + nab-paclitaxel therapy in patients with pancreatic cancer.
Recommendation strength: weak; evidence level: C; agreement rates (N = 34): 1 = 0%, 2 = 76%, 3 = 6%, 4 = 0%, 5 = 18%
2. Use of pregabalin may be considered for peripheral neuropathy associated with FOLFIRINOX therapy or combined gemcitabine hydrochloride + nab-paclitaxel therapy in patients with pancreatic cancer.
Recommendation strength: weak; evidence level: D; agreement rates (N = 34): 1 = 0%, 2 = 62%, 3 = 6%, 4 = 0%, 5 = 32%
COMMITTEE MEMBERS
Structure of the Committee for Revision of the Clinical Guidelines for Pancreatic Cancer of the Japan Pancreas Society:
Chairman: Takuji Okusaka (National Cancer Center Hospital)
Vice chairman: Masafumi Nakamura (Kyushu University)
Guidelines Methodology
Masahiro Yoshida (International University of Health and Welfare)
Kohei Nakata (Kyushu University)
Diagnosis
Masayuki Kitano (Wakayama Medical University)
Takao Itoi (Tokyo Medical University)
Ken Kamata (Kindai University)
Manabu Kawai (Wakayama Medical University)
Atsushi Kanno (Tohoku University)
Masahiro Serikawa (Hiroshima University)
Makoto Takaoka (Kansai Medical University)
Kyoichi Takaori (Kyoto University)
Yukiko Takayama (Tokyo Women's Medical University)
Keiji Hanada (JA Onomichi General Hospital)
Surgical Therapy
Masafumi Nakamura (Kyushu University)
Itaru Endo (Yokohama City University)
Takao Ohtsuka (Kyushu University)
Masato Ozaka (Cancer Institute Hospital of the Japanese Foundation for Cancer Research)
Shunsuke Onoe (Nagoya University)
Manabu Kawai (Wakayama Medical University)
Sohei Satoi (Kansai Medical University)
Tsutomu Fujii (University of Toyama)
Ippei Matsumoto (Kindai University)
Fuyuhiko Motoi (Tohoku University)
Adjuvant Therapy
Katsuhiko Uesaka (Shizuoka Cancer Center)
Hidetoshi Eguchi (Osaka University)
Atsushi Kanno (Tohoku University)
Toshio Nakagohri (Tokai University)
Satoaki Nakamura (Kansai Medical University)
Akira Fukutomi (Shizuoka Cancer Center)
Radiotherapy
Yoshinori Ito (Showa University)
Tatsuya Ioka (Osaka International Cancer Institute)
Takayuki Ohguri (University of Occupational and Environmental Health)
Keiko Shibuya (Osaka City University)
Masanori Someya (Sapporo Medical University)
Satoaki Nakamura (Kansai Medical University)
Akira Fukutomi (Shizuoka Cancer Center)
Chemotherapy
Junji Furuse (Kyorin University)
Tatsuya Ioka (Osaka International Cancer Institute)
Takao Ohtsuka (Kyushu University)
Takuji Okusaka (National Cancer Center Hospital)
Masato Ozaka (Cancer Institute Hospital of the Japanese Foundation for Cancer Research)
Sohei Satoi (Kansai Medical University)
Akira Fukutomi (Shizuoka Cancer Center)
Nobumasa Mizuno (Aichi Cancer Center Hospital)
Stent Therapy
Keiji Hanada (JA Onomichi General Hospital)
Hiroyuki Isayama (Juntendo University)
Takao Itoi (Tokyo Medical University)
Hironari Kato (Okayama University)
Yasunari Sakamoto (National Cancer Center Hospital)
Yousuke Nakai (The University of Tokyo)
Supportive and Palliative Medicine
Takuji Okusaka (National Cancer Center Hospital)
Asao Ogawa (National Cancer Center Hospital East)
Hatoe Sakamoto (National Cancer Center Hospital East)
Yoichi Shimizu (National Cancer Center Hospital)
Kenichi Suzuki (Hoshi University)
Yusuke Tando (Hirosaki University)
Tetsuya Tsuji (Keio University)
Maiko Fujimori (National Cancer Center)
Masanori Mori (Seirei Mikatahara General Hospital)
Chikao Yasuda (Cancer Institute Hospital of the Japanese Foundation for Cancer Research)
Kinomi Yomiya (Saitama Cancer Center)
Patient Representative
Yoshiyuki Majima (PanCAN Japan)
Advisers from Department of EBM and Guidelines Japan Council for Quality Health Care (Minds)
Sho Sasaki (Japan Council for Quality Health Care)
Yosuke Hatakeyama (Japan Council for Quality Health Care)
Librarian who was responsible for the comprehensive literature search
Naohiko Yamaguchi (Seirei Sakura Citizen Hospital)
Secretariat
Yasunari Sakamoto (National Cancer Center Hospital)
Keiko Kondo (National Cancer Center Hospital)
Specialists for the Revision as Assistants
Mariko Asai (Teikyo Heisei University)
Tsukasa Ikeura (Kansai Meidical University)
Yasutaka Ishii (Hiroshima University)
Rei Umezawa (Tohoku University)
Yu Uneno (Seirei Mikatahara General Hospital)
Masanori Enokido (National Cancer Center Hospital East)
Shunsuke Omoto (Kindai University)
Yoshiro Okajima (Jichi Medical University)
Ken-ichi Okada (Wakayama Medical University)
Keiko Kamei (Kindai University)
Masahiro Kitami (Tohoku University)
Kiyoshi Kume (Tohoku University)
Yu Sawada (Yokohama City University)
Makoto Shinoto (Kyushu University)
Kazuto Shibuya (University of Toyama)
Teiichi Sugiura (Shizuoka Cancer Center)
Atsushi Sofuni (Tokyo Medical University)
Shotaro Takahashi (Yamaguchi University)
Michinori Takahashi (Sendai Medical Center)
Naminatsu Takahara (The University of Tokyo)
Junko Tahara (Tokyo Women's University)
Takashi Tamura (Wakayama Medical University)
Takayoshi Tsuchiya (Tokyo Medical University)
Nnbuhiro Tsuchiya (Yokohama City University)
Tomofumi Tsuboi (Hiroshima University)
Yoshito Tomimaru (Toyonaka Municipal Hospital)
Kohei Nakata (Kyushu University)
Kyoko Nakanishi (Saitama Cancer Center)
Tatsuo Hata (Tohoku University)
Shin Hamada (Tohoku University)
Yuji Higuchi (Taiyo Hills Hospital)
Masakazu Hori (Sapporo Medical University)
Kazuyuki Matsumoto (Okayama University)
Eri Mikami (Hirosaki University)
Shuichi Mitsunaga (National Cancer Center Hospital)
Tomoyuki Minami (JA Onomichi General Hospital)
Yasuhiro Yabushita (Yokohama City University)
Yasunobu Yamashita (Wakayama Medical University)
Tomohisa Yamamoto (Kansai Medical University)
Takashi Yokokawa (Cancer Institute Hospital of the Japanese Foundation for Cancer Research)
Yukihiro Yokoyama (Nagoya University)
Isaku Yoshioka (University of Toyama)
External Appraisal Committee Assigned by the JPS
Koji Yamaguchi (Clinic of Fukuoka Government Building, Hamanomachi Hospital)
Hiroyuki Maguchi (Teine Keijinkai Hospital, Kameda Medical Center)
Shuji Isaji (Mie University Hospital)
Kazuma Fujimoto (Saga University)
Shoji Natugoe (Kagoshima University)
Takeo Nakayama (Kyoto University)
Anonymous (Patient Representative)
External Appraisal Committee Assigned by Department of EBM and Guidelines Japan Council for Quality Health Care (Minds)
Eiji Ishikawa (Mie University)
Hiroshi Okamoto (Kurashiki Central Hospital)
Hiroshi Koga (Shinshu University)
Nobumasa Takagaki (Senshukai Hospital)
Hoshi Murai (Japan Council for Quality Health Care)
Footnotes
The funding of the guidelines was supported by the Japan Pancreas Society (President, Kazuichi Okazaki).
T.O. has received consultancy services for Taiho Pharmaceutical Co, Ltd; Daiichi Sankyo Co, Ltd; Dainippon Sumitomo Pharma Co, Ltd; Bristol-Myers K.K.; AstraZeneca K.K.; and Zeria Pharmaceutical Co, Ltd; grants from Kowa Company, LTD, Pfizer Japan Inc; Yakult Honsha Co, Ltd; Bayer Yakuhin, Ltd; Ono Pharmaceutical Co, Ltd; Kyowa Hakko Kirin Co, Ltd; Eli Lilly Japan K.K.; AstraZeneca K.K.; Chugai Pharmaceutical Co, Ltd; Eisai Co, Ltd;Ltd; Novartis Pharma K.K.; Bristol-Myers K.K.; AstraZeneca K.K.; Taiho Pharmaceutical Co, Ltd; Dainippon Sumitomo Pharma Co, Ltd; MSD K.K.; and Baxter, and honoraria from EA Pharma Co, Ltd; MSD K.K.; Shire, AstraZeneca K.K.; AbbVie Inc; Eisai Co, Ltd; Ono Pharmaceutical Co, Ltd; Yakult Honsha Co, Ltd; Shire, Daiichi Sankyo Co, Ltd; Taiho Pharmaceutical Co, Ltd; Takeda Pharmaceutical Co, Ltd; Chugai Pharmaceutical Co, Ltd; Teijin Pharma Ltd; Eli Lilly Japan K.K.; Nobelpharma Co, Ltd; Novartis Pharma K.K.; Bayer Yakuhin, Ltd; Pfizer Japan Inc; FUJIFILM RI Pharma Co, Ltd; Bristol-Myers K.K.; Mundipharma K.K.; Nihon Servier Co, Ltd; Nippon Shinyaku Co, Ltd; and Celgene, K.K.; and payment for manuscript preparation from Eisai Co, Ltd; Takeda Pharmaceutical Co, Ltd; Lilly Japan K.K.; and Nobelpharma Co, Ltd. M.N. has received grants from Taiho, Takeda, Kaken, Daiichisankyo, Medtronic, Bostonscientific, Tsumura, Shionogi, EA Pharma, Eli Lilly Japan, Asahikasei-pharma, and Chugai, and payment for lectures from Novartis, Yakult, Daiichisankyo, Terumo, Chugai, Johnson & Johnson, Taisho-pharma, Medicon, MSD, Asahikasei-pharma, Ono, Takeda, Kaken, Medtronic, Miyarisan, Otsuka, Taiho, Tsumura, Pfizer, Sumitomo Dainippon Pharma, Nihon Pharmaceutical, Sanofi, Teijin Pharma, Mylan, Fuji Pharma, and Bristol-Myers Squibb. M.K. has received grants from EA Pharma Co Ltd; Takeda Pharaceutical Co Ltd; Olympus Co Ltd; and EA Pharma Co Ltd. J.F. has received grants from MSD, Yakult Honsha, Taiho Pharmaceutical, Chugai Pharma, Daiichi Sankyo, Eli Lilly Japan, Mochida Pharmaceutical, Ono Pharmaceutical, Sumitomo Dainippon, and Taiho Pharmaceutical; honorarium from Taiho Pharmaceutical, Yakult Honsha, Shionogi, Eli Lilly Japan, Chugai Pharma, Mochida Pharmaceutical, Nihon Servier, Sawai Pharmaceutical, Daiichi Sankyo, and MSD; and payment for lectures from Eisai, Bayer Yakuhin, Ono Pharmaceutical, Novartis, Teijin pharma, EA Pharma, Takeda, Sanofi, Fujifilm, Toyama Chemical, Nobel pharma, Pfizer, Sumitomo Dainippon, Merck Serono, Shire, and Kyowa Hakko Kirin. K.H. has received honorarium from Gadelius Medical. The other authors declare no conflict of interest.
REFERENCES
- 1.Japan Pancreas Society Evidence-Based Medicine Clinical Practice Guidelines for Pancreatic Cancer 2006. Tokyo, Japan: Kanahara & Co, Ltd; 2006. [Google Scholar]
- 2.Japan Pancreas Society Evidence-Based Medicine Clinical Practice Guidelines for Pancreatic Cancer 2009. Tokyo, Japan: Kanahara & Co, Ltd; 2009. [Google Scholar]
- 3.Yamaguchi K, Tanaka M, Committee for Revision of Clinical Guidelines for Pancreatic Cancer of Japan Pancreas Society EBM-based clinical guidelines for pancreatic cancer 2009 from the Japan Pancreas Society: a synopsis. Jpn J Clin Oncol. 2011;41:836–840. [DOI] [PubMed] [Google Scholar]
- 4.Japan Pancreas Society Evidence-Based Medicine Clinical Practice Guidelines for Pancreatic Cancer 2013. Tokyo, Japan: Kanahara & Co, Ltd; 2013. [Google Scholar]
- 5.Yamaguchi K, Okusaka T, Shimizu K, et al. EBM-based clinical guidelines for pancreatic cancer (2013) issued by the Japan Pancreas Society: a synopsis. Jpn J Clin Oncol. 2014;44:883–888. [DOI] [PubMed] [Google Scholar]
- 6.Japan Pancreas Society Clinical Practice Guidelines for Pancreatic Cancer 2016. Tokyo, Japan: Kanahara & Co, Ltd; 2016. [Google Scholar]
- 7.Yamaguchi K, Okusaka T, Shimizu K, et al. Clinical practice guidelines for pancreatic cancer 2016 from the Japan Pancreas Society: a synopsis. Pancreas. 2017;46:595–604. [DOI] [PubMed] [Google Scholar]
- 8.Japan Pancreas Society Clinical Practice Guidelines for Pancreatic Cancer 2019. Tokyo, Japan: Kanahara & Co, Ltd; 2019. [Google Scholar]
- 9.Medical Information Network Distribution Service (Minds) Minds Manual for Guideline Development 2017. December 27, 2017. Available at: https://minds.jcqhc.or.jp/s/guidance_2017_0_h. Accessed December 27, 2017.
- 10.Japan Pancreas Society General Rules for the Study of Pancreatic Cancer. 7th ed. Tokyo, Japan: Kanehara & Co, Ltd; 2016. [Google Scholar]


