Supplemental Digital Content is Available in the Text.
Keywords: enriched environment, recovery of function, rehabilitation, stroke, task-specific therapy
Abstract
Background and Purpose:
There is a need to translate promising basic research about environmental enrichment to clinical stroke settings. The aim of this study was to assess the effectiveness of enriched, task-specific therapy in individuals with chronic stroke.
Methods:
This is an exploratory study with a within-subject, repeated-measures design. The intervention was preceded by a baseline period to determine the stability of the outcome measures. Forty-one participants were enrolled at a mean of 36 months poststroke. The 3-week intervention combined physical therapy with social and cognitive stimulation inherent to environmental enrichment. The primary outcome was motor recovery measured by Modified Motor Assessment Scale (M-MAS). Secondary outcomes included balance, walking, distance walked in 6 minutes, grip strength, dexterity, and multiple dimensions of health. Assessments were made at baseline, immediately before and after the intervention, and at 3 and 6 months.
Results:
The baseline measures were stable. The 39 participants (95%) who completed the intervention had increases of 2.3 points in the M-MAS UAS and 5 points on the Berg Balance Scale (both P < 0.001; SRM >0.90), an improvement of comfortable and fast gait speed of 0.13 and 0.23 m/s, respectively. (P < 0.001; SRM = 0.88), an increased distance walked over 6 minutes (24.2 m; P < 0.001; SRM = 0.64), and significant improvements in multiple dimensions of health. The improvements were sustained at 6 months.
Discussion and Conclusions:
Enriched, task-specific therapy may provide durable benefits across a wide spectrum of motor deficits and impairments after stroke. Although the results must be interpreted cautiously, the findings have implications for enriching strategies in stroke rehabilitation.
Video Abstract available for more insights from the authors (see the Video, Supplemental Digital Content 1, available at: http://links.lww.com/JNPT/A304).
INTRODUCTION
The overall burden of stroke has increased across the globe and is the second commonest cause of death and a leading cause of adult disability worldwide.1 Many individuals with stroke face long-term consequences, which are usually complex and heterogeneous and can result in problems across multiple domains of functioning.2 The most common deficit after stroke is hemiparesis, which predisposes individuals to sedentary behaviors, seriously hampers postural control, and increases the risk of falls.3 Restoring impaired movement and associated functions is therefore a key goal in stroke rehabilitation.
Over the years, various approaches to physical rehabilitation for recovery of function and mobility after stroke have been developed.4 Many rehabilitation strategies used task-oriented and goal-directed training and include feedback, repetition, intensity, and specificity to regain lost functions.2,4 Such task- and context-specific training should target goals that are relevant for the needs of individuals with stroke.2 Many treatment methods are available to minimize functional disability, such as constraint-induced movement therapy, weight-supported treadmill training, cardiovascular training, and goal-directed physical exercise.2 High-intensity, high-dose, task-specific treatment strategies for stroke rehabilitation have also been developed.5 Nevertheless, individuals with stroke are increasingly left with persistent impairment,2 and many lack adequate stimulation, exercise, and socialization.6 The stroke rehabilitation field consequently faces a dual challenge: implementing new strategies to improve long-term outcome and tailoring treatment regimens to meet the needs of individuals with stroke.7
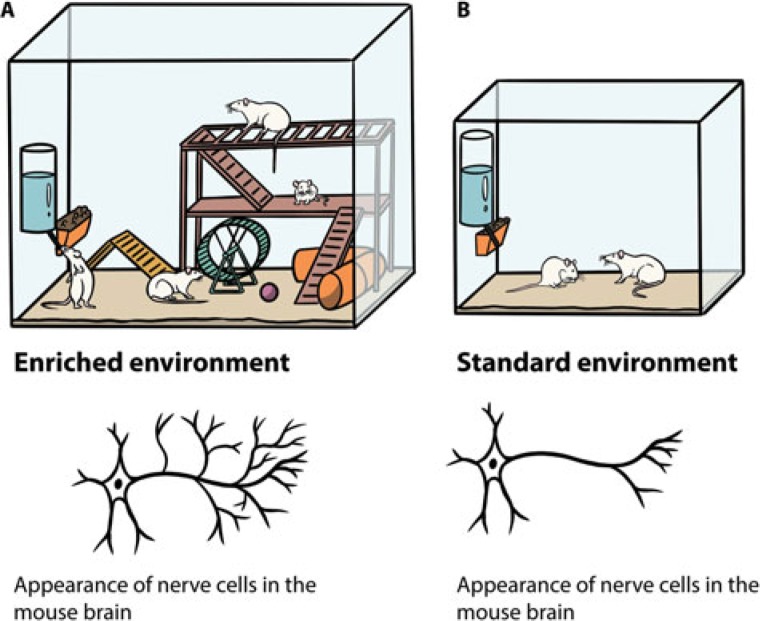
A growing amount of research suggests that the key to maximizing functional recovery after stroke is to combine a selection of components from different approaches.4,8,9 Combinational therapies have considerable potential to provide optimal gains in functional recovery after stroke by tapping into the multiple, complementary mechanisms that underlie neuroplasticity and repair.10 To further aid recovery from stroke, task-specific therapy could be combined with environmental enrichment (EE).10 Environmental enrichment that enhances motor, cognitive, sensory, and social stimulation is shown to increase neuroplasticity in rodents, as compared with standard housing (Figure 1A and B).8,10
Figure 1.
(A). A typical enriched environment condition composed of increased space and equipped with various objects that stimulate motor function by providing exercise, balancing or climbing activities (running wheel, igloos, tunnels, tube mazes, and ladders), and cognition (a variety of toys and objects to interact with and navigate in). The location and types of objects are changed regularly to maintain the concept of novelty and complexity in the environment, thereby offering multisensory stimulation (visual, acoustic, smell, touch, push, and sensory-motor challenges). Multiple animals are introduced to the stimulating environment simultaneously to facilitate social interaction (allogrooming, sniffing, and play-soliciting activities). (B). A standard housing condition that generally entails a cage with bedding and access to water and food.
A combination of different therapies is expected to have additive or even synergistic effects on neuroplasticity processes harnessed to aid rehabilitation after stroke.6,8,10,11 These findings support the idea that combinational therapies can aid recovery from stroke-related deficits.12 Despite the evidence that supports the potential of EE to enhance brain plasticity, it has largely remained a laboratory phenomenon, with little translation to clinical settings.13
Based on the fundamental principle of EE—that interventions should engage participants in concurrent physical, sensory, cognitive, and social activities or experiences—we designed an exploratory study of the EE paradigm in a clinical setting. Specifically, we investigated whether an intervention that combines high-dose and task-specific therapy with the sensory-motor, social, and cognitive stimulation inherent to EE could aid the recovery from stroke. The aim of the study was to assess the effectiveness of an enriched, task-specific therapy (ETT) program in enhancing functional motor performance as well as balance, gait, hand strength, and dexterity in individuals with residual hemiplegia in the chronic phase after stroke. We also investigated whether ETT improves confidence in task performance and health-related quality of life and reduces fatigue and depression.
METHODS
Study Design and Participants
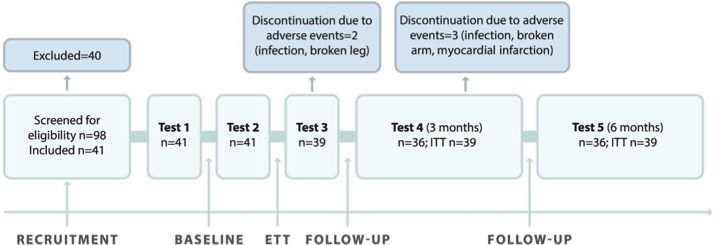
A within-subject, repeated-measures design with pretest-posttest evaluation was used to assess outcomes after ETT. The study enrolled Swedish or Norwegian community-dwelling individuals in the chronic phase after stroke who had applied to a Swedish agency that provides rehabilitation services in Spain. Individuals with slight to moderately severe hemiplegia after stroke were consecutively recruited from the wait list according to the eligibility criteria listed in Table 1. Figure 2 shows the enrollment of study participants, a flowchart of the study, and assessment time points.
Table 1. Eligibility Criteria.
| At least 6 mo and a maximum 10 y after the onset of stroke |
| Disability grades 2-4 on the Modified Rankin Scalea |
| Baseline motor deficit defined as less than a full score on the primary outcome measure (M-MAS UASb) |
| No other injury, illness, or addiction, making the individual unsuitable for participation, including exercise-induced epilepsy, assessed by the referring or prescribing physician |
| Cognitive and speech ability that enables instruction, intervention, and evaluation |
| Ability and willingness to travel to the place of evaluation |
| Able to perform sit-to-stand and stand-to-sit transfers independently or with assistance, without assistive technology such as mechanical lifts |
| Not having participated in a similar high-dose rehabilitation program (other than poststroke acute and subacute rehabilitation) within the previous 6 mo |
| Not scheduled for other treatment focused on intensive, high-dose training during the study period |
aAn ordinal disability rating scale, scored 0 to 6 (0 = no symptoms).
bModified Motor Assessment Scale developed at Uppsala University Hospital in 1999.
Figure 2.
Flowchart of the study design. ETT indicates enriched, task-specific therapy; ITT, intention-to-treat.
The participants underwent a baseline assessment (test 1), followed by a second baseline assessment 3 weeks later (test 2) to determine the stability of the outcome measures. Following the second baseline assessment, the participants underwent the 3-week intervention. Immediately after the intervention, the participants underwent the first postintervention assessment (test 3). To determine the retention of treatment effects, follow-up assessments were carried out 3 and 6 months after the intervention (tests 4 and 5). During the baseline period, the participants were not offered any new rehabilitation activities but were allowed to continue their regular treatment if engaged in any, for a maximum of 3 hours per week (eg, outpatient physiotherapy, occupational therapy, or speech and language therapy [SLT]). They were advised not to start any new therapies and were asked to record any participation in both therapist-led and self-managed rehabilitation activities during the baseline period. The physical therapist who conducted the assessments was independent and had no prior relation with the rehabilitation center.
Test 1 was done in Sweden, 3 weeks before the participants traveled to Spain. Depending on the participant's place of residence, test 1 was done at 2 Swedish hospitals, 1 in Gothenburg and 1 in Stockholm. Three weeks after test 1, participants flew to Spain on a charter flight; special assistance was offered to those who needed it. All participants were accommodated in handicap-friendly rehabilitation hotels, and most of the participants were accompanied by relatives or assistance personnel. Before the start of each ETT program, the study assessor went to the rehabilitation facility in Spain to conduct test 2, which was done upon the participants' arrival. The same assessor returned to Spain for the third assessment, done immediately after completion of the intervention. Three months after participants had returned to their home countries, a follow-up questionnaire was mailed to assess patient-reported outcome measures. The response frequency was maximized by telephone and postal reminders. The 6-month follow-up took place at the 2 Swedish assessment sites and included the whole assessment battery.
The study was approved by the Regional Ethical Review Board in Gothenburg, Sweden (reference number: 549-12) and was conducted in accordance with relevant ethical guidelines. All participants gave written informed consent and were told that they could withdraw from the study at any time. All participants chose to apply to the ETT program, which was paid for by the Swedish social insurance system (n = 16), the employer (n = 6), or was partly self-paid (n = 19).
Intervention
Enriched, Task-Specific Therapy
In this context, “enriched” refers to EE—an intervention to increase motor, sensory, cognitive, and social activity by providing a stimulating environment. “Task-specific” refers to repetitive functional training in everyday tasks, meaningful for the individual. The ETT program started immediately after the baseline period and took place at 2 rehabilitation facilities near Marbella and Malaga, Spain. The ETT was individually tailored, performed in groups of 4 to 9, and supervised by physical therapists. Depending on the level of impairments of the participants, as many as 3 physical therapists were sometimes required to supervise and assist the training. The ETT was characterized by large dosage of therapy. Rehabilitation activities were scheduled for 3 weeks, 5.5 hours on weekdays, and 3.5 hours on Saturdays. Everyone had Sundays off. The therapy was divided into 3 sessions of 1.5 to 2 hours each weekday, interspersed with social activities, such as scheduled coffee breaks and lunch.
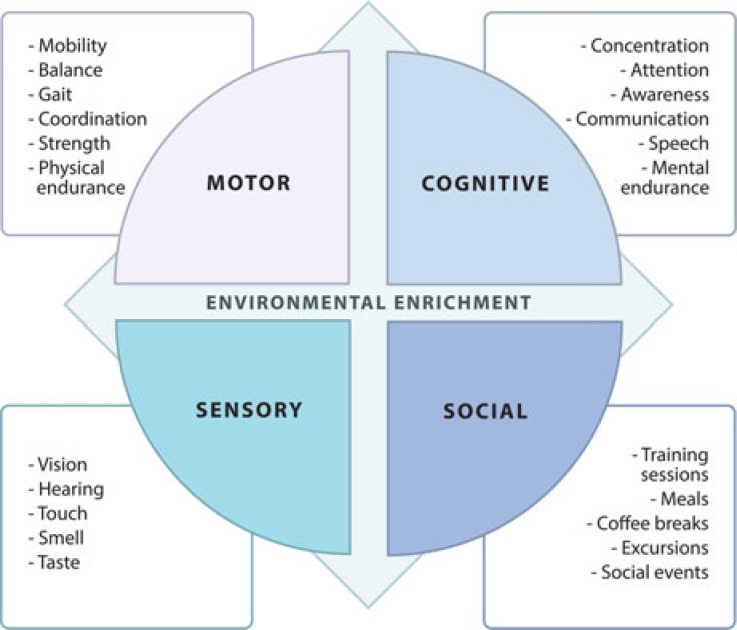
Participants with speech impairments (aphasia) received individualized treatment with a speech therapist for a maximum of 2 hours per day, included in the 5.5 (weekdays) or 3.5 hours (Saturdays). The physical therapy interventions were characterized by repetitive massed practice and based on noncompensatory strategies.5 The training consisted of 1 to 3 daily sessions of functional task training and 1 to 3 daily sessions of impairment-based training (for some examples, see the Figure, Supplemental Digital Content 2, http://links.lww.com/JNPT/A308). Beyond scheduled activities, participants were encouraged to physically engage in a challenging outdoor environment. The ETT also included enriching excursions with rehabilitation personnel, enabling goal-directed training in various environments. In addition, the participants interacted socially with each other and with accompanying family members at training sessions, meals, and social events after the scheduled activities. The various components acting in the clinical translation of the EE model in this study are presented in Figure 3. A detailed description of the ETT content is presented in the Figure, (see Supplemental Digital Content 2, http://links.lww.com/JNPT/A308).
Figure 3.
The various enriching components acting in the clinical translation of the EE model in this study.
Study Entry Characteristics and Outcome Measures
As screening measures, we used the Modified Rankin Scale (MRS) to assess the degree of disability or dependence in activities of daily living (ADLs) and the Montreal Cognitive Assessment14 to assess baseline cognitive performance. Treatment outcomes were assessed with multiple standardized measures. The primary outcome was functional motor performance, measured with the Modified Motor Assessment Scale (MAS) according to Uppsala University Hospital (M-MAS UAS), version 1999 (see Appendix A, Supplemental Digital Content 3, available at: http://links.lww.com/JNPT/A305), a modification of the original MAS.15 M-MAS UAS is a functional test designed to assess 8 motor components in individuals with stroke: supine to side lying, supine to sitting over side of bed, sitting, sitting to standing, walking, upper arm function, hand movements, and fine motor activities; the latter 3 components are assessed bilaterally. Each item is scored from 1 to 5; the maximum score of 55 indicates optimal motor function.16 In this study, we combined the first 5 components into the domain bed mobility and lower limb functional tasks and the last 3 components into the domain upper limb function. The M-MAS UAS total score and the domains BM-LL and upper limb function were used for statistical analyses. The M-MAS UAS is reliable and valid.16
Balance function was measured with the Berg Balance Scale (BBS).17 Measures of gait included walking ability and walking speed (comfortable and maximal), assessed with the 10-meter walk test,18 and distance walked in the 6-minute walk test.19 Gross manual dexterity was assessed with the Box and Blocks Test,20 and maximum isometric grip strength was assessed with a hydraulic hand dynamometer (JAMAR 5030J1, Sammons Preston Rolyan, USA.).21
The patient-reported outcome measures included perceived confidence in task performance, assessed with the Swedish modification of the Falls Efficacy Scale. Falls Efficacy Scale measures fear of falling and how that affects physical performance in personal and instrumental ADL.22 Life satisfaction was assessed with Life Satisfaction Checklist (LISAT-9).23 Effect of fatigue in ADL was measured with the Fatigue Impact Scale, which assesses physical, cognitive, and psychosocial function.24 Depression was measured with the Montgomery Åsberg Depression Rating Scale.25 Health-related quality of life was assessed with the EuroQol 5-dimensions questionnaire (EQ-5D 3L), which includes the dimensions mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, and an item measuring perceived overall health reported on a visual analogue scale (EQ-5D-VAS).26
Statistical Analyses
To rule out spontaneous improvement or an observer effect during the 3-week baseline period or improvement due to participants' regular treatment, the outcome was analyzed as the change from the first baseline assessment to the second baseline assessment. The outcome of ETT was analyzed as the change from the second baseline assessment to the assessment immediately after ETT. The outcomes at 3 and 6 months were analyzed as the change from the second baseline assessment to the 3- and 6-month follow-ups, respectively (Figure 2). At all testing points, QQ-plots and histograms as well as skewness and kurtosis were used to assess whether the outcome variables approximated a normal distribution. The data were not normally distributed, and therefore the Wilcoxon signed rank test and sign test were used. Treatment efficacy at 3 and 6 months was analyzed according to the intention-to-treat principle. Missing data at these time points were replaced with the last observation carried forward approach.
Demographics and baseline characteristics were summarized with descriptive statistics. Effect sizes were estimated with the Standardized Response Measure (mean post-ETT – mean pre-ETT)/SD (post-ETT – pre-ETT). Furthermore, to assess whether baseline characteristics (gender, age, time since stroke, stroke type, stroke localization, MRS, Montreal Cognitive Assessment, balance, and motor function at baseline) and dose-related aspects of rehabilitation activities (the amount of therapist-led and self-managed rehabilitation activities during the baseline period and the amount of physical therapy during the ETT program) might be useful in predicting functional benefit gained from the ETT program, univariate correlation and multiple stepwise regression analyses were used. The Mann-Whitney U test was used to assess the relation between dichotomous and continuous variables. Spearman test was used to assess the correlation between 2 continuous variables, and the Jonckheere-Terpstra test was used to assess the correlation between ordered categorical data (MRS), with the change of M-MAS UAS during ETT as the dependent variable. The variables significant at 0.10 level were included in a multiple stepwise linear regression analysis, which was corrected for heteroscedasticity. The residual plots were examined and found satisfactory. All tests of significance were 2-sided; P < 0.05 was considered statistically significant. Analyses were done with SPSS v 22.0 (IBM, Armonk, New York) and SAS software version 9.4 (SAS Institute, Cary, North Carolina). Values are reported as mean ± SD.
RESULTS
Between September 1, 2012, and July 31, 2015, 98 subjects were screened for eligibility; 41 eligible subjects with a mean age of 59.6 ±13.9 years agreed to participate in the study. We recruited participants who were scheduled for a 3-week rehabilitation program provided by the Swedish rehabilitation agency (Figure 2). The most frequent reasons for exclusion were inability (n = 18) or unwillingness (n = 17) to travel to the assessment location, need for assistive technology (such as mechanical lifts) during transfers (n = 15), less than 6 months or more than 10 years after stroke (n = 3), no hemiparesis (n = 2), and bilateral paresis (n = 1). Demographics and baseline characteristics of the participants are presented in Table 2.
Table 2. Demographic Variables and Baseline Characteristics of 41 Study Participants at Test 1a.
| Characteristics | |
| Women | 8 (20%) |
| Men | 33 (80%) |
| Mean age, SD, y; median [minimum; maximum] | 59.6 (13.9); 64 [22; 84] |
| Nationality: Swedish/Norwegian | 40 (98%)/1 (2%) |
| Stroke demographics | |
| Right hemispheric stroke | 18 (44%) |
| Left hemispheric stroke | 23 (56%) |
| Infarct | 36 (89%) |
| Hemorrhage | 3 (9%) |
| First-time stroke | 38 (93%) |
| Recurrent stroke | 3 (7%) |
| Months since stroke onset, median (SD) median [minimum; maximum] | 35.5 (29.5) 26 [6; 130] |
| Hemiplegia of dominant side | 23 (56%) |
| Modified Rankin Scaleb | |
| Mean grade, mean (SD); median [minimum; maximum] | 3.4 (0.7); 3 [2; 4] |
| Grade 2 | 5 (12%) |
| Grade 3 | 16 (39%) |
| Grade 4 | 20 (49%) |
| MOCAc, mean (SD); median [minimum; maximum] (n = 25) | 22.6 (5.3); 24 [6; 28] |
| M-MAS UAS, mean (SD); median [minimum; maximum] | 37.8 (9.0); 36 [20; 55] |
| BBS, mean (SD); median [minimum; maximum] | 36.1 (18.8); 44 [3; 56] |
Abbreviations: BBS, Berg Balance Scale; M-MAS UAS, Modified Motor Assessment Scale (Uppsala University Hospital); MOCA, Montreal Cognitive Assessment.
aMean (SD)/median [minimum; maximum] is given for continuous variables and n (%) for categorical variables.
bAn ordinal disability rating scale scored 0 to 6. 2—Slight disability. Able to look after own affairs without assistance but unable to carry out all previous activities. 3—Moderate disability. Requires some help but able to walk unassisted. 4—Moderately severe disability. Unable to attend to own bodily needs without assistance and unable to walk unassisted.
cExcluding participants with severe aphasia who were unable to undertake the test.
All participants completed the 3-week baseline period. During the baseline period, 26 of the 41 participants (63%) reported having received a mean of 5.6 ± 3.8 hours of therapist-led rehabilitation activities including 3.8 ± 2.0 hours of physiotherapy (N = 19), 3.2 ± 1.0 hours of occupational therapy (N = 6), 2.8 ± 1.7 hours of SLT (N = 9) and 2.6 ± 1.4 hours of other treatment modalities such as acupuncture, rhythm therapy, and so forth (N = 10). Twenty-four participants reported engagement in self-managed rehabilitation activities (such as gait training, home exercise programs, and stationary bike training) amounting to a mean of 7.7 ± 4.2 hours.
All participants completed the second baseline assessment (test 2) and started the 3-week ETT program. Two participants had adverse events and discontinued the program; one had an infection that started before initiation of ETT, and the second broke a leg during ETT activities (Figure 2). Two participants deviated from the protocol; one did receive extended SLT (1.5 hours per day) at his own request, and one became ill during about a week but did resume the ETT as soon as he got better. During the ETT program, the whole study cohort received a mean of 75.3 ± 19.2 hours of physical therapy. Participants with aphasia (n = 18) received a mean of 55.8 ± 5.3 hours of physical therapy and 17.6 ± 19.2 hours of SLT. Participants without speech impairment (n = 20) received a mean of 93 ± 0 hours of physical therapy. Demographics, the amount of individualized therapy provided to each person, and data from each individual assessment are provided in Appendix B (see Supplemental Digital Content 4, available at: http://links.lww.com/JNPT/A306).
After the post-ETT assessment, 3 participants had adverse events and discontinued follow-up: one had an infection that started during ETT, the second had a broken arm, and the third had a myocardial infarction between tests 3 and 4 (Figure 2). None of the adverse events were related to baseline activities. In all 3 cases, the last observation carried forward approach was used in the analyses at 3 and 6 months.
The only significant change after the baseline period was a decrease in health-related quality of life, measured with the EQ-5D usual activity dimension (mean change: 0.23 ± 0.62, P = 0.029). The results of the within-subject analyses after completion of the ETT program are summarized in Table 3. Immediately after the intervention, significant improvements were shown in functional motor performance (2.3-point increase in the M-MAS UAS, P < 0.001). Significant gains were also observed in balance and gait, as shown by a 5.0-point improvement on the BBS (P < 0.001) (Figure 3), by increases in mean comfortable gait speed (from 0.56 to 0.69 m/s, P < 0.001) and mean fast gait speed (from 0.78 to 1.01 m/s, P = 0.001), and by an increase in distance walked over 6 minutes (26.3 m, P < 0.001). The Bocks and blocks test of manual dexterity showed changes from 11.8 to 13.1 blocks/minute (P = 0.028), but no significant change in grip strength was seen (10.9 to 12.1 N, P = 0.11). Enriched, task-specific therapy also increased participants' confidence in task performance as measured by Falls Efficacy Scale and improved the perception of life satisfaction as measured by LISAT. The level of depression and fatigue was also significantly improved after treatment completion, as was patient-reported mobility, anxiety/depression, and the overall health status according to EQ-5D. The effect sizes are given in Table 3.
Table 3. Changes in Outcome Measures Between ETT-Pretest (Test 2) and ETT-Posttest (Test 3) and Between ETT-Pretest (Test 2) and 6-Month Follow-up (Test 5)a.
| N | ETT-Pretest (Test 2) Mean (SD), Median [Minimum; Maximum] | ETT-Posttest (Test 3) Mean (SD), Median [Minimum; Maximum] | Change ETT-Pretest to ETT-Posttest Mean (SD), Median [Minimum; Maximum] | P | SRM | 6 mo (Test 5) Mean (SD), Median [Minimum; Maximum] | Change ETT-Pretest to 6 mo Mean (SD), Median [Minimum; Maximum] | P | SRM | |
|---|---|---|---|---|---|---|---|---|---|---|
| Motor outcome measures | ||||||||||
| M-MAS UAS (0–55) | 39 | 38.4 (8.9) | 40.7 (8.2) | 2.3 (1.8) | <0.001b | 1.28 | 40.8 (7.6) | 2.4 (2.6) | <0.001b | 0.92 |
| 38 [22; 55] | 40 [25; 55] | 2.0 [−1; 7] | 41 [24; 55] | 2.0 [−1; 10] | ||||||
| M-MAS UAS BM-LL (0–25) | 39 | 19.9 (5.4) 22 [8; 25] | 21.2 (4.6) 23 [11; 25] | 1.3 (1.6) 1 [0; 6] | <0.001b | 0.81 | 21.4 (4.13) 23 [9; 25] | 1.46 (2.21) 1 [−2; 9] | <0.001b | 0.66 |
| M-MAS UAS UL (0-30) | 39 | 18.5 (4.6) 17 [13; 30] | 19.5 (5.0) 17 [14; 30] | 0.97 (1.2) 1 [−1; 4] | <0.001 | 0.80 | 19.5 (4.6) 18 [15; 30] | 0.92 (1.2) 1 [−1; 3] | <0.001b | 0.76 |
| BBS (0-56) | 39 | 37.3 (18.3) 43 [3; 56] | 42.3 (16.1) 49 [4; 56] | 5.0 (4.0) 4.0 [−1; 13] | <0.001b | 1.25 | 43.1 (15.2) 49 [−1; 13] | 5.7 (5.6) 5.0 [0; 20] | <0.001b | 1.01 |
| 6MWT, m | 36 | 212.2 (164.0) 160 [0; 555] | 238.5 (165.8) 195 [27; 555] | 26.3 (27.8) 20 [-5; 135] | <0.001b | 0.94 | 234.3 (163.8) 173 [32; 585] | 22.1 (34.8) 17.5 [−75; 115] | <0.001b | 0.64 |
| 10MWT, m/s | ||||||||||
| Comfortable speed | 35 | 0.56 (0.38) 0.48 [0.06; 1.33] | 0.69 (0.42) 0.68 [0.06; 1.47] | 0.13 (0.12) 0.10 [−0.07; 0.44] | <0.001b | 1.01 | 0.68 (0.43) 0.53 [0.09; 1.49] | 0.12 (0.15) 0.10 [−0.18; 0.58] | <0.001b | 0.80 |
| Fast speed | 32 | 0.78 (0.52) 0.73 [0.07; 1.61] | 1.01 (0.67) 0.91 [0.11; 2.50] | 0.23 (0.33) 0.21 [−0.76; 1.31] | <0.001b | 0.70 | 0.93 (0.63) 0.78 [0.12; 2.44] | 0.15 (0.26) 0.10 [−0.31;0.90] | 0.001b | 0.58 |
| BBT, n/min | 17 | 11.8 (14.3) 7 [0; 46] | 13.1 (13.7) 0 [0; 44] | 1.35 (2.21) 1 [−2; 6] | 0.028b | 0.61 | 11.5 (14.9) 0 [0; 45] | −0.3 (3.60) 0 [−12; 4] | 0.82b | −0.08 |
| JAMAR, Newton | ||||||||||
| Maximum grip force (affected hand) | 39 | 10.9 (13.6) 7 [0; 59] | 12.1 (14.7) 9 [0; 63] | 1.2 (6.0) 0 [−16; 30] | 0.11b | 0.20 | 8.6 (10.5) 5 [0; 52] | −2.4 (10.4) 0 [57; 10] | 0.33b | −0.23 |
| PROM:s | ||||||||||
| FES total score (0–130) | 39 | 66.3 (39.3) 59 [8; 130] | 77.8 (36.1) 81 [9; 130] | 11.5 (19.9) 8 [−20; 61] | 0.002 | 0.58 | 77.0 (37.1) 81 [12; 130] | 10.7 (19.3) 11 [−29; 51] | 0.003b | 0.55 |
| Instrumental ADL (0-60) | 39 | 23.6 (19.2) 16 [0; 16] | 28.6 (19.7) 32 [0; 60] | 4.9 (10.0) 2 [−13; 26] | 0.011b | 0.49 | 28.1 (20.0) 26 [0; 60] | 4.4 (10.1) 2 [−21; 29] | 0.012b | 0.44 |
| Personal ADL (0-60) | 39 | 37.5 (19.2) 40 [3; 60] | 42.2 (16.3) 47 [8; 60] | 4.7 (10.5) 2 [−14; 32] | 0.011b | 0.46 | 42.2 (16.6) 47 [6; 60] | 4.8 (10.5) 3 [−12; 31] | 0.016b | 0.46 |
| LISAT (9-54) | 36 | 29.5 (7.0) 28.5 [12; 47] | 32.4 (6.9) 32 [13; 47] | 2.9 (4.1) 2 [−5; 15] | <0.001b | 0.71 | 32.0 (7.0) 32 [20; 54] | 2.4 (5.2) 1.5 [−8; 15] | 0.016b | 0.46 |
| FIS | ||||||||||
| Cognitive (0-80) | 38 | 22.8 (12.4) 26 [0; 48] | 20.1 (12.4) 21 [0; 41] | −2.7 (5.0) −1 [−18; 6] | 0.001b | −0.54 | 18.5 (12.0) 19 [0; 45] | −4.3 (7.7) −1.5 [−26; 13] | 0.001b | -0.56 |
| Social (0-40) | 39 | 20.5 (12.5) 22 [0; 47] | 18.3 (12.0) 17 [0; 43] | −2.3 (5.6) −1 [−18; 10] | 0.017b | −0.41 | 17.3 (12.7) 18 [0; 44] | −3.2 (9.5) −2 [−26; 25] | 0.016b | -0.34 |
| Physical (0-40) | 38 | 26.0 (13.7) 29 [0; 51] | 23.6 (13.4) 23 [0; 49] | −2.5 (6.0) −1 [−17; 10] | 0.016b | −0.42 | 20.4 (14.1) 19 [0; 48] | −5.6 (10.5) −4 [−41; 11] | 0.002b | -0.53 |
| MADRS (0-54) | 39 | 12.0 (6.9) 12.5 [0; 27] | 9.1 (6.5) 8 [0; 28] | −2.9 (4.6) −4 [−12; 10] | 0.001b | −0.63 | 8.6 (5.9) 9 [0; 20] | −3.4 (5.1) −2 [−19; 5] | <0.001b | -0.67 |
| EQ5D-VAS 0-100 | 38 | 58.8 (21.2) 55 [18; 99] | 63.2 (17.4) 61.5 [28; 100] | 4.4 (14.1) 6 [−40; 30] | 0.034b | 0.31 | 63.9 (18.8) 64 [20; 100] | 5.1 (14.7) 4.5 [−30; 30] | 0.032b | 0.35 |
| EQ5D | n | n (%) Change ETT-Pretest–ETT Posttest | P | SRM | (%) Change ETT-Pretest–6 mo | P | SRM | |||
| Mobility (0-2) | 39 | Improved | 10 (25.6%) | 0.012c | −0.48 | Improved | 6 (15.4%) | 0.29c | −0.23 | |
| Deteriorated | 1 (2.5%) | Deteriorated | 2 (5.1%) | |||||||
| Ties | 28 (71.8%) | Ties | 31 (79.5%) | |||||||
| Self-care (0-2) | 39 | Improved | 2 (5.1%) | 1.0c | 0.07 | Improved | 4 (10.3%) | 0.75c | 0.10 | |
| Deteriorated | 3 (7.7%) | Deteriorated | 6 (15.4%) | |||||||
| Ties | 34 (87.2%) | Ties | 29 (74.4%) | |||||||
| Usual activities (0-2) | 39 | Improved | 11 (28.2%) | 0.210c | −0.28 | Improved | 13 (33.3%) | 0.007c | −0.50 | |
| Deteriorated | 5 (12.8%) | Deteriorated | 2 (5.1%) | |||||||
| Ties | 23 (58.9%) | Ties | 24 (61.5%) | |||||||
| Pain/discomfort (0-2) | 39 | Improved | 7 (17.9%) | 0.34c | −0.20 | Improved | 7 (17.9%) | 0.77c | −0.09 | |
| Deteriorated | 3 (7.8%) | Deteriorated | 5 (12.8%) | |||||||
| Ties | 29 (74.3%) | Ties | 27 (69.2%) | |||||||
| Anxiety/depression (0-2) | 39 | Improved | 11 (28.2%) | 0.022c | −0.43 | Improved | 8 (20.5%) | 0.39c | −0.19 | |
| Deteriorated | 2 (5.1%) | Deteriorated | 4 (10.3%) | |||||||
| Ties | 26 (66.7%) | Ties | 27 (69.2%) | |||||||
Abbreviations: ADL, activities of daily living; BBS, Berg Balance Scale; BBT, The Box and Block Test; BM, bed mobility; EQ5D, European Quality of Life five-dimension questionnaire; EQ5D-VAS, visual analog scale of perceived overall health; FES, Falls Efficacy Scale; FIS, Fatigue Impact Scale; LISAT, Life Satisfaction Checklist; LL, lower limb functional tasks; MADRS, Montgomery Åsberg Depression Rating Scale; M-MAS UAS, Modified Motor Assessment Scale (Uppsala University Hospital); PROM, Patient Reported Outcome Measure; 6MWT, 6-minute walk test; SRM, Standardized Response Measure; 10MWT, 10-meter walk test; Ties, no change; UL, upper limb function.
aThe changes from ETT-pretest to ETT-posttest/6 months post-ETT were analyzed with the Wilcoxon signed-rank test and sign test.
bWilcoxon signed-rank test.
cSign test.
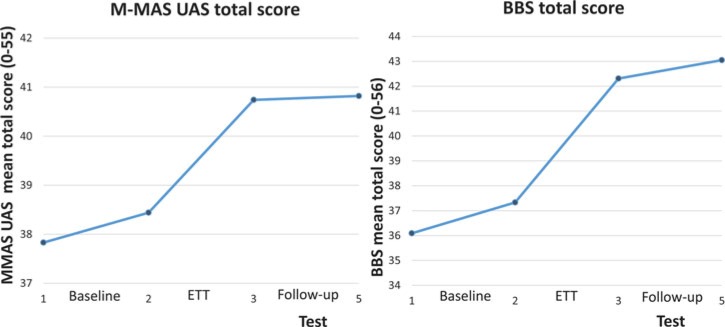
Outcomes at 3- and 6-month follow-up were similar to those observed immediately following the intervention. Therefore, only the outcomes at 6 months are presented (Table 3). These results demonstrate sustained improvement in functional motor performance measured by M-MAS UAS and continued improvement in balance relative to participants' second baseline performance on the BBS (5.7 points) (Figure 4). The significant improvements in comfortable and fast gait speed were sustained, as were those in the distance walked over 6 minutes, perceived confidence in task performance, life satisfaction, fatigue, and depression. Gains in patient-reported mobility and overall health status measured by EQ-5D were also sustained. The 6-month follow-up also revealed improved performance in usual activities as measured by the EQ-5D (P < 0.007).
Figure 4.
Line charts of the total mean scores on the Berg Balance Scale (BBS) and the Modified Motor Assessment Scale (MAS) according to Uppsala University Hospital at tests 1, 2, 3, and 5. BBS indicates Berg Balance Scale; ETT, enriched, task-specific therapy; M-MAS, Modified Motor Assessment Scale.
The analyses investigating whether any baseline characteristics and dose-related aspects of rehabilitation activities could predict the functional gains from the ETT program showed that the correlation between balance at baseline as measured by BBS and the outcome of M-MAS UAS was statistically significant (rs = −0.42, P = 0.0071). There were also significant correlations between baseline motor function as measured by M-MAS UAS and the M-MAS UAS outcome (rs = −0.35, P = 0.029), between baseline disability as measured by MRS and the M-MAS UAS outcome (rs = 0.37, P = 0.022), and between hours of therapist-led rehabilitation activities during baseline (rs = 0.46, P = 0.0084) and the M-MAS UAS outcome. Multiple stepwise regression analysis including these 4 variables revealed no significant and independent correlation with larger gains in motor function at ETT completion as measured by M-MAS UAS. Analysis of the impact of baseline characteristics on the outcome detected no characteristics that influenced outcome for stroke localization, stroke type, gender, age, time since stroke, cognition, hours of self-managed training during baseline, and amount of physical therapy during the ETT program.
DISCUSSION
This study shows that an ETT program emphasizing task-oriented therapy was associated with reduced disability and improved health status even many years after stroke. Participants with chronic stroke who took part in the ETT program demonstrated sustained improvements in functional motor performance as measured by M-MAS UAS. The M-MAS UAS was selected as a primary measure since it is recommended as a standardized and clinically relevant overall measure to quantify the progress of individuals, or the total population of a rehabilitation unit.27 The minimal detectable change (MDC) and the minimal clinically important difference of the scale, however, remain to be established.
Enriched, task-specific therapy was associated with significant gains in many of the secondary outcome measures, including balance and gait. Improvements were sustained at 6 months. In a study of individuals in the chronic phase after stroke, designed to identify the sensitivity of common outcome measures, the reported MDC of the BBS was 4.66.28 This is encouraging for this study, demonstrating a 5-point mean increase (13%) in the BBS postintervention and a 5.7-point mean increase (15%) at 6 months. Furthermore, at the time of intervention completion, the mean increases in comfortable and fast gait speed were 0.13 and 0.23 m/s, respectively. In a recent study by Lewek and Sykes,29 it is suggested that the MDC for gait speed in populations with chronic stroke should be differentiated on the basis of the individual's baseline gait speed. In the aforementioned study,29 the participants with chronic stroke (n = 76) were stratified according to a speed-based classification system into a low: less than 0.4 m/s, moderate: 0.4 to 0.8 m/s, and high functioning groups: greater than 0.8 m/s. According to this classification system, our study participants who were able to perform the 10-meter walk test (n = 35) with a mean baseline comfortable gait speed of 0.56 ± 0.38 m/s would be classified as a moderate functioning group. Lewek and Sykes29 found that the MDC values for comfortable gait speed increased with increasing gait speed in their study population (low: 0.10 m/s; moderate: 0.15 m/s; and high: 0.18 m/s). Similar findings were observed for fast gait speed.29 According to their findings in the moderate functioning group (n = 29), the gains in comfortable gait speed among our study participants nearly reached the level of MDC for comfortable gait speed (0.13 m/s vs 0.15 m/s) and exceeded the MDC for fast gait speed (0.23 vs 0.17 m/s).
Gains in gait speed in this study were sustained at 6 months and fast gait speed reached the level that was previously established as a substantial meaningful change (0.14 m/s).30 The poststroke gait speed is an important determinant of long-distance walking capacity,31 suggesting that gains in gait speed after ETT may be related to the increased distance walked during 6 minutes (26.3 m). The improvements in gait in this study may be considered notable, as a systematic review of gait training after stroke32 showed that poststroke gait speed (comfortable speed in 7–9 studies) increased by 0.07 m/s and the distance in the 6-minute walk test increased by 26.06 m. In the LEAPS trial,33 gait speed in the late locomotor training group improved by 0.11 m/s at 1 year after stroke.
Previous findings indicating that the degree of functional mobility and independence in ADL affect health-related quality of life34 suggest that improvements in those domains may have contributed to participants' improvements in multiple secondary outcome measures. The participants' average degree of depression according to Montgomery Åsberg Depression Rating Scale severity gradation was mild (7–19) at baseline but approached the cutoff Montgomery Åsberg Depression Rating Scale score for “no depression” (0-6)35 at 6 months. The significant improvement in the EQ-5D activity dimension at 6 months may reflect continued recovery after completion of treatment. Only minor or no hand-specific gains were demonstrated in our study, consistent with previous findings,36 showing the impact of repetitive task training on lower but not upper limb functions. The severe upper limb motor impairment in some participants in this study, including total paralysis of the muscles of the hand, may explain the small improvements in hand function. Most intervention studies have focused on mildly to moderately impaired individuals with stroke. By including individuals with more severe hemiplegia, we had a more representative sample of the large number of individuals with significant impairments, for whom rehabilitation approaches that yield enduring improvement are urgently needed.
This study has 4 main strengths: a low withdrawal rate; high compliance with therapy; the inclusion of individuals with stroke who differed in age, gender, stroke severity, and degree of deficit; and the measurement of a broad range of functions and health-related factors. The ability of the individuals to tolerate the dose and duration of the therapy sessions was not a limiting factor, as the intervention was individually tailored and shifted between physical, social, and environmental content. The broken leg during ETT activities was an accident and not related to any lack of patient safety.
Limitations
Inherent limitations of our methods must be considered. Since we recruited participants who had applied to the ETT program, we used a within-subject, repeated-measures design. Thus, there was no comparison group to account for effects of participating in a rehabilitation program of any type. However, the baseline period did control for the passage of time and made it possible to separate out the effects of spontaneous recovery or observer effect during this time. During the baseline period, the participants were allowed to continue their regular treatment (eg, physiotherapeutic training or occupational or speech and language therapy) but were advised not to start any new therapy during the study. In general, treatment is seldom offered in the chronic phase after the stroke.37 In this study, more than half of the study participants had received therapist-led rehabilitation, and more or less as many had been engaged in self-managed rehabilitation during the baseline period. Since the participants themselves took the initiative to apply to the ETT program, they may represent a population of individuals with stroke who actively seek possibilities to enhance their recovery. The rather high frequency of rehabilitation activities reported during the baseline period is indicative of this characteristic. The results at test 2 demonstrated that the 3-week baseline period did not produce treatment effect or spontaneous recovery or observer effect. The univariate correlation analysis revealed that the amount of therapist-led rehabilitation during the baseline period was significantly and positively correlated with the functional gains at completion of the ETT program, yet having no significant or independent impact, when included in the multiple stepwise regression analysis. Adding the fact that the participants were repeatedly assessed, we cannot rule out that these factors might somehow have influenced or primed the response to the ETT.
The within-subject design was chosen because its advantage in indicating the potential effects of EE in a clinical population of individuals with stroke outweighed its limitations. Our method of recruitment was not ideal, as individuals applying to rehabilitation services may be highly motivated. Another potential limitation is the lack of blinding of the outcome assessor. Since the assessor had to travel to Spain to conduct the assessment before the intervention started, blinding was not feasible. Because of the large geographical spread of participants, it was not logistically possible to have them travel to the Swedish assessment site after completing the ETT program. Therefore, the assessor returned to Spain to conduct the assessment immediately after the intervention. This procedure minimized the withdrawal rate and enabled assessment directly after completion of the intervention, with no time delay. Possible introduction of systematic bias was reduced by using an independent assessor who was not otherwise involved in the ETT program and by using valid and reliable outcomes measures.
Although the ETT was mainly group-based, the content was individually tailored and targeted participants' individual goals. As many as three therapists were sometimes required, depending on the severity of the impairments. The choice and amount of physical training differed among participants depending on their individual needs. But the fact that part of the ETT program was devoted to SLT for almost half the study cohort did not affect the outcome in terms of motor function. Future research should explore the cost-benefits of multimodal poststroke therapies and also investigate whether the combination of therapeutic modalities and enriched physical and social surroundings renders an enhanced overall effect, which is substantially greater than the therapeutic components in isolation. In accordance with previous findings,33,38 the participants were responsive to an exercise intervention despite being in the chronic phase after stroke. This possibility contradicts the notion that individuals with stroke reach a recovery plateau after stroke39 and is supported by research suggesting that combining modalities has synergistic effects on stroke recovery.8
To the best of our knowledge, this is the first attempt to translate an EE paradigm incorporating high-dose, task-specific therapy to a clinical stroke setting. A pleasant and stimulating climate as well as the social environment may have affected the psychological well-being of participants. As suggested in the literature, the combination of different ETT components most likely had additive or even synergistic effects on neuroplasticity processes harnessed to aid recovery from stroke-related impairments. To give a decisive answer about the efficacy of an ETT program, future studies should incorporate blinded assessments, larger groups, and long-term between-group comparisons. Moreover, the mechanisms that drive recovery from impairments and disabilities late after stroke need to be better understood. Thus, it remains to be investigated whether recovery is underpinned by neuroplasticity processes, behavioral compensation, or a combination of both.40 To investigate this further within the context of the ETT program, this study protocol includes kinematic motion analysis and blood-based biomarker studies that remain to be completed.
CONCLUSIONS
A therapy program that combines the physical, sensory, and social stimulation inherent in EE may provide durable benefits across the wide spectrum of motor deficits and impairments, even years after stroke. Although it is necessary to be cautious in our interpretation of the results of this exploratory study, we encourage controlled replication with blinded assessment as the findings have implications for enriching strategies in stroke rehabilitation that may provide guidance for future research.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the staff at the rehabilitation facilities in Spain, the physical therapist responsible for the outcome assessment, and Aldina Pivodic, Nils-Gunnar Pehrsson, and Jan Kowalski for statistical guidance.
Footnotes
ClinicalTrials.gov identifier: NCT02889939
The authors thank the following funding agencies for supporting the study: the Aina Wallström's and Mary-Ann Sjöblom's Foundation, Peter Eriksson Foundation, the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (725241), Promobilia foundation, The Swedish Stroke Association, Rune and Ulla Almlöv's foundation, P-O Ahl & J-B Wennerström foundation, Handlaren Hjalmar Svensson's Foundation, Brain Athletics, the Swedish Medical Research Council, and the Foundation for Rehabilitation and Medical Science.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jnpt.org).
The authors declare no conflict of interest.
REFERENCES
- 1.Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383(9913):245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011;377(9778):1693–1702. [DOI] [PubMed] [Google Scholar]
- 3.Weerdesteyn V, de Niet M, van Duijnhoven HJ, Geurts AC. Falls in individuals with stroke. J Rehabil Res Dev. 2008;45(8):1195–1213. [PubMed] [Google Scholar]
- 4.Pollock A, Baer G, Campbell P, et al. Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Database Syst Rev. 2014;(4):CD001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296(17):2095–2104. [DOI] [PubMed] [Google Scholar]
- 6.Venna VR, Xu Y, Doran SJ, Patrizz A, McCullough LD. Social interaction plays a critical role in neurogenesis and recovery after stroke. Transl Psychiatry. 2014;4:e351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer S, Verheyden G, Brinkmann N, et al. Functional and motor outcome 5 years after stroke is equivalent to outcome at 2 months: follow-up of the collaborative evaluation of rehabilitation in stroke across Europe. Stroke. 2015;46(6):1613–1619. [DOI] [PubMed] [Google Scholar]
- 8.Corbett D, Jeffers M, Nguemeni C, Gomez-Smith M, Livingston-Thomas J. Lost in translation: rethinking approaches to stroke recovery. Prog Brain Res. 2015;218:413–434. [DOI] [PubMed] [Google Scholar]
- 9.Mala H, Rasmussen CP. The effect of combined therapies on recovery after acquired brain injury: systematic review of preclinical studies combining enriched environment, exercise, or task-specific training with other therapies. Restor Neurol Neurosci. 2017;35(1):25–64. [DOI] [PubMed] [Google Scholar]
- 10.Corbett D, Nguemeni C, Gomez-Smith M. How can you mend a broken brain? Neurorestorative approaches to stroke recovery. Cerebrovasc Dis. 2014;38(4):233–239. [DOI] [PubMed] [Google Scholar]
- 11.Livingston-Thomas J, Nelson P, Karthikeyan S, et al. Exercise and environmental enrichment as enablers of task-specific neuroplasticity and stroke recovery. Neurotherapeutics. 2016;13(2):395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janssen H, Ada L, Bernhardt J, et al. An enriched environment increases activity in stroke patients undergoing rehabilitation in a mixed rehabilitation unit: a pilot non-randomized controlled trial. Disabil Rehabil. 2014;36(3):255–262. [DOI] [PubMed] [Google Scholar]
- 13.McDonald MW, Hayward KS, Rosbergen ICM, Jeffers MS, Corbett D. Is environmental enrichment ready for clinical application in human post-stroke rehabilitation? Front Behav Neurosci. 2018;12:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. [DOI] [PubMed] [Google Scholar]
- 15.Carr J, Shepherd R. Modified Motor Assessment Scale. Phys Ther. 1989;69(9):780. [DOI] [PubMed] [Google Scholar]
- 16.Barkelius K, Johansson A, Korm K, Lindmark B. Reliabilitet-och validitetsprövning av Motor assessment Scale enligt Uppsala Akademiska sjukhus-95. Nordisk fysioterapi. 1997;1:121. [Google Scholar]
- 17.Berg K, Wood-Dauphinee S, Williams JI. The Balance Scale: reliability assessment with elderly residents and patients with an acute stroke. Scand J Rehabil Med. 1995;27(1):27–36. [PubMed] [Google Scholar]
- 18.Bohannon RW. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing. 1997;26(1):15–19. [DOI] [PubMed] [Google Scholar]
- 19.Butland RJ, Pang J, Gross ER, Woodcock AA, Geddes DM. Two-, six-, and 12-minute walking tests in respiratory disease. Br Med J (Clin Res Ed). 1982;284(6329):1607–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther. 1985;39(6):386–391. [DOI] [PubMed] [Google Scholar]
- 21.Bohannon RW, Schaubert KL. Test-retest reliability of grip-strength measures obtained over a 12-week interval from community-dwelling elders. J Hand Ther. 2005;18(4):426–427, quiz 428. [DOI] [PubMed] [Google Scholar]
- 22.Hellstrom K, Lindmark B, Fugl-Meyer A. The Falls-Efficacy Scale, Swedish version: does it reflect clinically meaningful changes after stroke? Disabil Rehabil. 2002;24(9):471–481. [DOI] [PubMed] [Google Scholar]
- 23.Fugl-Meyer AR, Melin R, Fugl-Meyer KS. Life satisfaction in 18- to 64-year-old Swedes: in relation to gender, age, partner and immigrant status. J Rehabil Med. 2002;34(5):239–246. [DOI] [PubMed] [Google Scholar]
- 24.Flensner G, Ek AC, Soderhamn O. Reliability and validity of the Swedish version of the Fatigue Impact Scale (FIS). Scand J Occup Ther. 2005;12(4):170–180. [DOI] [PubMed] [Google Scholar]
- 25.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. [DOI] [PubMed] [Google Scholar]
- 26.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–343. [DOI] [PubMed] [Google Scholar]
- 27.Loewen SC, Anderson BA. Reliability of the Modified Motor Assessment Scale and the Barthel Index. Phys Ther. 1988;68(7):1077–1081. [DOI] [PubMed] [Google Scholar]
- 28.Hiengkaew V, Jitaree K, Chaiyawat P. Minimal detectable changes of the Berg Balance Scale, Fugl-Meyer Assessment Scale, Timed “Up & Go” Test, gait speeds, and 2-minute walk test in individuals with chronic stroke with different degrees of ankle plantarflexor tone. Arch Phys Med Rehabil. 2012;93(7):1201–1208. [DOI] [PubMed] [Google Scholar]
- 29.Lewek MD, Sykes R., III Minimal detectable change for gait speed depends on baseline speed in individuals with chronic stroke. J Neurol Phys Ther. 2019;43(2):122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–749. [DOI] [PubMed] [Google Scholar]
- 31.Awad LN, Reisman DS, Wright TR, Roos MA, Binder-Macleod SA. Maximum walking speed is a key determinant of long distance walking function after stroke. Top Stroke Rehabil. 2014;21(6):502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.States RA, Pappas E, Salem Y. Overground physical therapy gait training for chronic stroke patients with mobility deficits. Cochrane Database Syst Rev. 2009;(3):CD006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duncan PW, Sullivan KJ, Behrman AL, et al. Body-weight-supported treadmill rehabilitation after stroke. N Engl J Med. 2011;364(21):2026–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langhammer B, Stanghelle JK, Lindmark B. Exercise and health-related quality of life during the first year following acute stroke. A randomized controlled trial. Brain Inj. 2008;22(2):135–145. [DOI] [PubMed] [Google Scholar]
- 35.Herrmann N, Black SE, Lawrence J, Szekely C, Szalai JP. The Sunnybrook Stroke Study: a prospective study of depressive symptoms and functional outcome. Stroke. 1998;29(3):618–624. [DOI] [PubMed] [Google Scholar]
- 36.French B, Thomas L, Leathley M, et al. Does repetitive task training improve functional activity after stroke? A Cochrane systematic review and meta-analysis. J Rehabil Med. 2010;42(1):9–14. [DOI] [PubMed] [Google Scholar]
- 37.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bunketorp-Kall L, Lundgren-Nilsson A, Samuelsson H, et al. Long-term improvements after multimodal rehabilitation in late phase after stroke: a randomized controlled trial. Stroke. 2017;48(7):1916–1924. [DOI] [PubMed] [Google Scholar]
- 39.Ferrarello F, Baccini M, Rinaldi LA, et al. Efficacy of physiotherapy interventions late after stroke: a meta-analysis. J Neurol Neurosurg Psychiatry. 2011;82(2):136–143. [DOI] [PubMed] [Google Scholar]
- 40.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10(12):861–872. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.