Abstract
Background:
People living with human immunodeficiency virus (HIV+) have greater risk for sudden arrhythmic death than HIV-uninfected (HIV-) individuals. HIV-associated abnormal cardiac repolarization may contribute to this risk. We investigated whether HIV serostatus is associated with ventricular repolarization lability using QT variability index (QTVI), defined as a log measure of QT interval variance indexed to heart rate (HR) variance.
Methods:
We studied 1123 men (589 HIV+ and 534 HIV-) from the Multicenter AIDS Cohort Study (MACS), using the ZioXT® ambulatory electrocardiography (ECG) patch. Beat-to-beat analysis of up to 4 full days of ECG data per participant were performed using an automated algorithm [median analyzed duration (Q1-Q3): 78.3 (66.3–83.0) hours/person]. QTVI was modeled using linear mixed-effects models adjusted for demographics, cardiac risk factors, and HIV-related and inflammatory biomarkers.
Results:
Mean (SD) age was 60.1 (11.9) years among HIV- and 54.2 (11.2) years among HIV+ participants (p<0.001), 83% of whom had undetectable (<20 copies/mL) HIV-1 viral load (VL). Compared to HIV- men, HIV+ men had higher QTVI [adjusted difference of +0.077 (95% CI: +0.032 to +0.123)]. Magnitude of this association depended on the degree of viremia such that in HIV+ men with undetectable VL, adjusted QTVI was +0.064 (95% CI: +0.017 to +0.111) higher compared to HIV- men, while in HIV+ men with detectable VL adjusted QTVI was higher by +0.150 (95% CI: 0.072–0.228) compared to HIV- referents. Analysis of QTVI subcomponents showed HIV+ men had: (1) lower HR variability irrespective of VL status, and (2) higher QT variability if they had detectable—but not with undetectable—VL, when compared to HIV- men. Higher levels of C-reactive protein (CRP), interleukin-6 (IL-6), intercellular adhesion molecule (ICAM)-1, soluble tumor necrosis factor (TNF)-receptor 2, and soluble cluster of differentiation (CD)-163 (borderline), were associated with higher QTVI and partially attenuated the association with HIV serostatus.
Conclusions:
HIV+ men have greater beat-to-beat variability in QT interval (QTVI) compared to HIV- men, especially in the setting of HIV viremia and heightened inflammation. Among HIV+ men, higher QTVI suggests ventricular repolarization lability which can increase susceptibility to arrhythmias, while lower HR variability signals a component of autonomic dysfunction.
Keywords: QT variability, HIV, ventricular repolarization, inflammation, autonomic dysfunction, QTVI, ambulatory ECG
Background
The advent of highly active anti-retroviral therapy (HAART) has markedly increased the life expectancy of people living with human immunodeficiency virus (HIV+) and reduced mortality from acquired immunodeficiency syndrome (AIDS). However, cardiovascular disease (CVD) has emerged as the leading cause of non-AIDS mortality among HIV+ persons and the number of cardiac deaths continues to rise.1–3 A 4.5-fold higher than expected rate of sudden cardiac death (SCD), accounting for 86% of cardiac mortality, was reported among HIV+ patients attending a public clinic in San Fransisco.4 Yet, fundamental gaps in our understanding of mechanisms underlying increased risk of SCD in HIV+ persons and a dearth of validated biometric markers for arrhythmic risk persist.
Ventricular arrhythmias are a major cause of SCD and can be triggered by abnormal cardiac repolarization.5 There are multiple proposed mechanisms through which HIV infection could affect ventricular repolarization. Several antiretroviral drugs have been linked to repolarization abnormalities.6 Increased risk of subclinical coronary atherosclerosis, metabolic derangement, and a higher prevalence of traditional CVD risk factors among people living with HIV may place this population at increased risk.7 In addition, HIV Tat protein, the transactivator protein crucial for efficient replication of the virus, can inhibit potassium channels of repolarization,8, 9 and persistent inflammation can alter electrical properties and ventricular ionic currents, precipitating arrhythmias.10, 11 Finally, HIV is associated with dysfunction of the cardioprotective autonomic nervous system, resulting in sympathetic dominance which could affect repolarization and increase arrhythmic susceptibility.12
QT variability index (QTVI) is an electrocardiographic (ECG) marker of ventricular repolarization lability, defined as the log ratio of normalized beat-to-beat QT interval variance to normalized heart rate variance.13 QT dynamicity, of which QTVI is a marker, has been proposed as a biomarker of cardiovascular risk and in large cohorts with various forms of CVD, elevated QTVI was strongly predictive of SCD and ventricular arrhythmias.13–17 Explosive growth of lead-less mobile ECG affords an opportunity to acquire and study longer term, temporal fluctuations in the QT interval with the hope to improve our understanding of arrhythmic mechanisms and identification of at-risk individuals.18
We sought to compare QTVI between HIV+ and HIV- men, while assessing contributions of other clinical variables. To account for potential differences in day-to-day activities that can influence ventricular repolarization, we utilized an ambulatory, wearable ECG device.
Methods
The Multicenter AIDS Cohort Study (MACS) is an ongoing prospective cohort study of natural and treated histories of HIV-1 infection in men who have sex with men conducted in Baltimore/Washington DC, Chicago, Pittsburgh, and Los Angeles. Four periods of MACS enrollment occurred in 1984–1985, 1987–1991, 2001–2003 and 2010–2017. The cohort includes both HIV+ and HIV- men who attend semi-annual research visits for standardized interviews, physical examinations, and blood/urine collections for laboratory measurements. During visits, measured blood pressure, fasting serum glucose and lipid panels, body mass index (BMI), self-reported smoking status and use of prescribed medications and recreational drugs, including alcoholic drinks and opioids are recorded. Because of the sensitive nature of the data collected for this study, requests for datasets from qualified researchers trained in human subject confidentiality protocols will be reviewed as per the guidelines of the MACS WIHS Combined Cohort Study.19
From October 2016 to September 2017, all active MACS participants were invited to wear an ambulatory ECG device (ZioXT®, iRhythm Technologies, Inc., San Francisco, CA) which is a single-use, skin adhesive patch approved by the Food and Drug Administration for continuous ECG monitoring. ZioXT® records single-channel ECG (200Hz frequency) and concurrent 3-axis accelerometry (1.5Hz/axis; not FDA approved for accelerometry) data for up to 14 days. All participants signed informed consent forms and the study was approved by the Institutional Review Boards at all participating sites.
Medications were categorized by QT prolongation risk (known/possible vs. conditional/none) using the publicly available database at CredibleMeds.org, accessed April 8th 2019 (132 drugs excluding opioids and cocaine which we analyzed separately, see supplementary table 1).20 In HIV+ men, measures of HIV disease included plasma HIV-1 RNA (VL) concentrations (lower limit of detectability: 20 copies/mL), current and nadir CD4+ T-cell counts, medical record confirmation of prior AIDS-defining diseases, and ART medication type and duration. In a subset of individuals who had participated in another ancillary MACS study,21, 22 blood was analyzed for levels of inflammatory biomarkers, including acute phase reactants (fibrinogen, C-reactive protein (CRP), serum cytokines (interleukin-6 (IL-6)), soluble (s) immune receptors (sCD14; sCD163), intercellular adhesion molecule 1 (ICAM-1); soluble tumor necrosis factor-receptors 1 and 2 (sTNF-R1 and sTNF-R2)). Inflammatory biomarkers were assessed at a median of 5.6 years prior to ECG recording.
ECG data until 11:59 pm of the 3rd calendar day following initiation of recording for each participant were included (theoretical maximum of 4 full days) to reduce computational burden. A crude, manual inspection of each participant’s ECG tracing was performed to ensure reasonable signal quality, and segments with persistently low-quality were discarded. QT variability analysis was performed using a fully automated software program, which constructs a signal-averaged “template” beat for every 6-hours of ECG data and stretches or compresses each beat to maximize fit.13 Beats with any of the following criteria were rejected: non-sinus origin, inclusion of the P wave in the QT template time region, excessive signal noise, and the first beat following a non-sinus beat. ECG features were calculated and reported for every 5-minute interval. Intervals in which ≥10% of detected beats were automatically rejected were excluded.
For each acceptable interval, QT variability index was calculated using Formula 113:
Formula 1. Definition of QT variability index (QTVI)
Formula 1 was further deconstructed as Formula 2, to separate the effects of QT variability and HR variability.
Formula 2. QTVI, and therefore linear models of QTVI, can be deconstructed into distinct ‘heart rate portion’ and ‘QT portion’. QTVN and HRVN are normalized variances of the QT interval and HR respectively.
Because QT variance is significantly smaller than HR variance, QTVI is normally negative in healthy individuals but can become abnormally more positive when QT variability increases and/or HR variability decreases.23
As body posture affects the QT interval and QTVI through changes in autonomic nervous system tone,24 we utilized concurrent accelerometry data in a novel analytic approach to adjust for upright versus recumbent body positions. Accelerometer data was used to estimate each person’s real-time inclination of the chest wall from an estimated upright position. The initial posture classification was recumbent if chest inclination was greater than 45 degrees, and upright otherwise. Next, person-specific, mean-shift clustering was applied. For each cluster, a majority vote of initial classifications determined a second classification for the entire cluster. Finally, classifications were smoothed over time with a time point retaining its recumbent label only if it occurred in at least a 30-minute sequence of recumbent intervals. For each body position, we used the mean of each ECG feature for analysis (two data points per ECG feature per person: one corresponding to the average for all upright 5-minute intervals, and one recumbent counterpart).
Statistical Analysis
Continuous measurements are reported as mean (SD) or median (1st and 3rd quartiles) and categorical data are reported as counts (percentage). Demographic and clinical characteristics were compared using χ2 (categorical), Student’s t- or Wilcoxon rank sum (continuous) tests. For missing variables, latest recorded values from visits up to 3 years prior were substituted when available. All clinical covariates had <1% missing values, except for fasting triglycerides [130 (11.6%) missing], total and HDL cholesterols [24 (2.1%)], and serum creatinine [23 (2.0%)].
QTVI was modeled against HIV serostatus using linear mixed-effects models with participant-specific intercepts to account for correlation between each person’s upright and recumbent measurements. The models were progressively adjusted as follows:
Model A: Adjusted for body posture, age, race, enrollment center, and enrollment year (pre/post-2001).
Model B: Model A further adjusted for education <12 years, BMI, smoking status, recent opiate use (self-reported within the past 6 months; either recreational or prescribed), systolic blood pressure, use of medications to treat hypertension, diabetes, and/or high cholesterol, total and HDL cholesterol, serum creatinine (quartiles), triglycerides (log-transformed), and QT prolonging medication use.
Model C: Model B with HIV serostatus replaced with a categorical HIV status marker: (I) HIV-, (II) HIV+ with undetectable VL, and (III) HIV+ with detectable VL.
For intuitive comparison of progressive association between HIV serostatus/degree of viremia and QTVI, adjusted beta coefficients for markers of HIV status/VL were plotted. To ascertain independent associations between HR variability and QT variability with HIV serostatus, log10 (QTVN) and log10 (HRVN) were modeled separately with variables from models B and C.
Serum inflammatory biomarkers were analyzed as tertiles, each in separate models with covariates from Model B. Among HIV+ men, QTVI was modeled against HIV disease-specific factors in separate models adjusted for covariates from Model B (excluding HIV serostatus). HIV-specific factors include VL levels (undetectable: <20 copies/mL, low: 20–500 copies/mL, and high: >500 copies/mL), history of clinical AIDS, current and nadir CD4+ T-cell counts, years of HAART use, and current protease inhibitor use.
As a surrogate for electrical instability, high ventricular arrhythmia burden was defined as any episodes of non-sustained ventricular tachycardia (NSVT) >4 beats (182 individuals), and/or daily count of premature ventricular beats (PVC) in the highest quartile (89 men fit both criteria) per ZioXT®’s clinical report. Ventricular arrhythmia burden was compared across quartiles of mean QTVI among HIV+ men and also used in logistic regression models with quartiles of QTVI as the independent variable among all participants. The model was adjusted using the variables from Model B and exponentiated beta-coefficients reflecting odds ratios (OR) were reported.
QQ plots of residuals from each model were plotted to evaluate distributional assumptions. Scatter plots with loess smoothers between residuals, covariates, and fitted values were examined to assess linearity and equal variance. All models were fit using all cases with available covariates, implying a missing at random assumption. Multiple imputation by chained equations was performed to assess the sensitivity of the main results to this assumption.
Of 1783 MACS participants who presented for a MACS research visit during our study period 1331 (74.6%) agreed to participate. The most common reasons for non-participation were lack of interest, concern about discomfort, and concern about sweating/water contact. A total of 1243 unique recordings were obtained (50 devices not returned or malfunctioned, 32 for another ancillary study, and 6 corrupted/unanalyzable files). We excluded 49 participants with history of prior myocardial infarction, heart failure, or stroke, and 9 participants in persistent atrial fibrillation. To limit inclusion of poor-quality data, the following inclusion criteria were applied:
>3 hours of ECG data per body position (recumbent /upright)
QTVI calculable in >10% of 5-minute intervals for each body position
Of the remaining 1185 participants, 1123 (95%) met these criteria and were included in the analysis. Supplemental Table 2 compares the characteristics of the included and excluded participants. Compared to those included, men with study exclusion criteria had more cardiovascular risk factors. We also compared the characteristics of men who wore a ZioXT® to those who attended the visits and refused (n=452) or the ZioXT® was not returned/data were missing (n=50). Compared to the men with ZioXT® data, these men were less likely to be current smokers, use cholesterol or blood pressure medications or have diabetes (data not shown). There were no differences in age, race or HIV serostatus between the two groups.
Results
Characteristics of the Study Population
Baseline characteristics are presented in Table 1. Compared to HIV- men, HIV+ men [n=589 (52%)] were younger, less likely to be Caucasian, more likely to be current smokers and had lower BMI, higher serum triglycerides, and higher serum creatinine levels. Most measured inflammatory biomarker concentrations were higher in HIV+ compared with HIV− men. Most HIV+ men (82.5%) were virally suppressed and a minority had a history of AIDS (10.5%).
Table 1.
Cohort Characteristics
| HIV-uninfected (HIV-) (n=534) |
HIV-infected (HIV+) (n=589) |
P | |
|---|---|---|---|
| Age (years) | 60.1 (11.9) | 54.2 (11.2) | <0.001 |
| Race (%) | <0.001 | ||
| Caucasian | 384 (71.9) | 304 (51.6) | |
| Black | 103 (19.3) | 173 (29.4) | |
| Hispanic/other | 47 (8.8) | 112 (19.0) | |
| Body Mass Index (kg/m2) | 27.6 (5.3) | 26.6 (4.8) | 0.001 |
| Enrolled after 2001 (%) | 181 (33.9) | 365 (62.0) | <0.001 |
| ≥12 years of formal education (%) | 518 (97.0) | 542 (92.0) | <0.001 |
| Alcohol use >13 drinks per week (%) | 58 (11.0) | 45 (7.6) | 0.069 |
| Tobacco use (%) | 0.001 | ||
| Never smoker | 180 (34.0) | 180 (30.6) | |
| Former smoker | 256 (48.4) | 251 (42.6) | |
| Current smoker | 93 (17.6) | 158 (26.8) | |
| Opioid use (%) | 40 (7.5) | 50 (8.5) | 0.63 |
| On hypertension medications (%) | 210 (39.5) | 225 (38.2) | 0.71 |
| On diabetes medications (%) | 58 (10.9) | 66 (11.2) | 0.95 |
| On cholesterol lowering medications (%) | 205 (38.5) | 229 (38.9) | 0.95 |
| On QT prolonging medication (%) | 78 (14.6) | 90 (15.3) | 0.82 |
| Systolic blood pressure (mmHg) | 132.6 (15.3) | 128.7 (15.8) | <0.001 |
| Fasting glucose (mg/dL) | 93 [85, 102] | 93 [85, 103] | 0.60 |
| Total cholesterol (mg/dL) * | 181.7 (41.2) | 178.7 (40.4) | 0.22 |
| HDL cholesterol (mg/dL) * | 54.0 (14.1) | 50.0 (14.4) | <0.001 |
| Fasting triglycerides (mg/dL) * | 92 [68, 132] | 116 [79, 175] | <0.001 |
| Serum creatinine (mg/dL) * | 0.94 [0.86, 1.07] | 1.05 [0.91,1.20] | <0.001 |
| HIV Disease Factors | |||
| Detectable (>20 copies/mL) HIV RNA (%) | 103 (17.5) | ||
| Viral load (among detectable) | 165 [57, 1389] | ||
| CD4+ T-cell count (cells/mm3) | 698 [521, 906] | ||
| Nadir CD4+ T-cell count (cells/mm3) | 330 [212, 453] | ||
| On HAART (%) | 541 (91.9) | ||
| Duration of HAART (years) | 12.7 [5.2, 16.9] | ||
| On protease inhibitors (%) | 173 (29.4) | ||
| Duration of PI use (years) | 5.0 [0, 12.1] | ||
| History of Clinical AIDS (%) | 62 (10.5) | ||
| Serum inflammatory biomarkers (n= 219 HIV−/344 HIV+) | |||
| CRP (ug/mL) | 1.02 [0.55, 1.86] | 1.18 [0.63, 2.42] | 0.11 |
| Fibrinogen (mL/dL) | 336 [293, 377] | 318 [273, 356] | 0.001 |
| ICAM-1 (ng/mL) | 229 [196, 273] | 259 [210, 315] | <0.001 |
| IL-6 (pg/mL) | 1.29 [0.90, 2.03] | 1.47 [0.97, 2.18] | 0.13 |
| MCP1 (pg/mL) | 235 [183, 312] | 276 [216, 346] | <0.001 |
| sCD14 (ng/mL) | 1308 [1167, 1472] | 1589 [1390, 1818] | <0.001 |
| sCD163 (ng/mL) | 547 [445, 684] | 667 [519, 881] | <0.001 |
| sTNF-R1 (pg/mL) | 1142 [953, 1349] | 1174 [958, 1463] | 0.081 |
| sTNF-R2 (ng/mL) | 5875 [5021, 6847] | 6625 [5407, 7947] | <0.001 |
Categorical variables shown as count (%) and continuous variables as mean (SD) or median [Q1, Q3] if data were skewed.
n= 478 HIV-/515 HIV+ for triglycerides, 524 HIV-/576 HIV+ for serum creatinine, and 522 HIV-/577 HIV+ for total and HDL cholesterol.
Unadjusted QT Variability in HIV+ vs. HIV- Participants
Unadjusted ambulatory ECG results are reported in Table 2. Markers of data quality did not differ by HIV serostatus. There was no significant difference in fraction of time spent in recumbency based on HIV serostatus. Average HR and the difference in HR between recumbent and upright postures were slightly higher in HIV+ men.
Table 2.
Unadjusted Ambulatory ECG results
| ECG parameters | HIV-uninfected (HIV-) |
HIV-infected (HIV+) |
P |
|---|---|---|---|
| Duration of data used (hours) | 78.3 [66.2, 83.1] | 78.5 [66.3, 83.1] | 0.81 |
| % of time in recumbency | 36.3 [30.6, 43.8] | 36.9 [30.4, 45.4] | 0.34 |
| % intervals with calculable HR | 99.7 [98.7, 100] | 99.8 [99.0, 100] | 0.12 |
| % intervals with calculable QTVI | 80.4 [65.2, 92.0] | 81.1 [67.7, 90.8] | 0.59 |
| Recumbent position | |||
| HR; bpm | 68.3 (9.7) | 71.4 (9.9) | <0.001 |
| QTVI | −1.47 (0.40) | −1.43 (0.43) | 0.11 |
| Log10(QTVN) | −1.22 (0.30) | −1.20 (0.29) | 0.17 |
| Log10(HRVN) | 0.25 (0.32) | 0.23 (0.36) | 0.42 |
| Upright position | |||
| HR; bpm | 81.9 (11.3) | 85.8 (11.4) | <0.001 |
| QTVI | −1.14 (0.38) | −1.12 (0.39) | 0.23 |
| Log10(QTVN) | −0.86 (0.25) | −0.82 (0.24) | 0.007 |
| Log10(HRVN) | 0.28 (0.30) | 0.29 (0.31) | 0.51 |
| Δ(Upright – Recumbent); ‘dipping’ | |||
| HR; bpm | 13.5 (6.4) | 14.4 (6.9) | 0.029 |
| QTVI | 0.33 (0.24) | 0.31 (0.25) | 0.39 |
| Log10(QTVN) | 0.36 (0.22) | 0.38 (0.21) | 0.21 |
| Log10(HRVN) | 0.04 (0.22) | 0.06 (0.22) | 0.030 |
Values shown as mean (SD) or median [Q1, Q3] if distribution was skewed.
To limit confounding by age and age-dependent factors, comparison of unadjusted QTVI was repeated in the largest participant subgroup with similar age distribution of HIV+ and HIV- men (age restricted to 55 to 70 years old) and the results were reported in Supplementary Table 3. In this age range, the difference in mean age was <1 year between the groups (61.5 years for HIV+ vs 62. 4 years for HIV-). Unadjusted QTVI were +0.10 (p=0.003) and +0.08 (p=0.015) higher in HIV+ compared to HIV- men in recumbent and standing body positions respectively. Age stratified unadjusted QTVI values are presented in Supplementary Figure 1 and showed a general tendency towards higher QTVI in HIV+ men, especially those with detectable VL.
Multivariable-Adjusted QT Variability in HIV+ vs. HIV- Participants
Multivariable-adjusted associations between QTVI, HIV serostatus and other covariates are reported in Table 3. After adjusting for age, race, center of study, time of enrollment (pre/post-2001), and upright posture, HIV+ men had significantly greater QTVI (+0.079, 95% CI: +0.034 to +0.124) than HIV- men. This association persisted with further adjustment for traditional CVD risk factors (+0.077, 95% CI: +0.032 to +0.123). The association between HIV and QTVI appeared more pronounced in participants with detectable VL than those with undetectable VL (p- interaction = 0.026). HIV+ men with undetectable VL had 0.065 higher adjusted QTVI (p=0.007) compared to HIV- men, while HIV+ men with viremia had 0.150 higher adjusted QTVI compared to HIV- men (p <0.001). A possible interaction between HIV serostatus and body posture with QTVI was not significant (p=0.87, data not shown).
Table 3.
Adjusted associations between HIV serostatus and QTVI
| QTVI | ||||||
|---|---|---|---|---|---|---|
| Model A*
(n= 1123) |
Model B*
(n= 983) |
P | Model C*
(n= 983) |
P | ||
|
Intercept w/ Centered Covariates** |
−1.55 (−1.61, −1.49) |
−1.57 (−1.69, −1.45) |
<0.001 | −1.57 (−1.69, −1.45) |
<0.001 | |
| HIV+ (vs. HIV-) | 0.079 (0.034,0.124) |
0.077 (0.032,0.123) |
<0.001 | HIV+ Undetectable† | 0.064 (0.017, 0.111) |
0.008 |
| HIV+ Detectable† | 0.150 (0.072, 0.228) |
<0.001 | ||||
| Upright Posture (vs. Recumbent) | 0.32 (0.31,0.33) | 0.32 (0.31, 0.34) | <0.001 | 0.32 (0.31, 0.34) | <0.001 | |
| Age, per 10 Years | 0.13 (0.10, 0.15) | 0.09 (0.07, 0.12) | <0.001 | 0.09 (0.06, 0.12) | <0.001 | |
| Black Race (vs White) | 0.10 (0.04, 0.16) | 0.08 (0.02,0.14) | 0.013 | 0.07 (0.01,0.13) | 0.020 | |
| BMI‡, per 10 units | 0.11 (0.07, 0.16) | <0.001 | 0.11 (0.07, 0.16) | <0.001 | ||
| Current Smoker (vs. Never) | 0.14 (0.08, 0.20) | <0.001 | 0.14 (0.08, 0.20) | <0.001 | ||
| Recent Opiate Use | 0.14 (0.06, 0.22) | <0.001 | 0.14 (0.06, 0.21) | <0.001 | ||
| Systolic BP, per 10 mmHg | 0.02 (0.01, 0.04) | 0.002 | 0.02 (0.01, 0.04) | 0.001 | ||
| Total Cholesterol, per 10 mg/dL | 0.00 (−0.01, 0.01) | 0.87 | 0.00 (−0.01, 0.01) | 0.94 | ||
| HDL Cholesterol, per 10 mg/dL | 0.01 (0.00, 0.03) | 0.13 | 0.01 (−0.01, 0.03) | 0.16 | ||
| Log Triglycerides, per 1 unit | 0.10 (0.05, 0.15) | <0.001 | 0.09 (0.04, 0.14) | <0.001 | ||
| Hypertension Medications | 0.08 (0.03, 0.13) | 0.001 | 0.08 (0.03, 0.13) | <0.001 | ||
| Diabetes Medications | 0.17 (0.10, 0.24) | <0.001 | 0.17 (0.10, 0.24) | <0.001 | ||
| Cholesterol Medications | 0.00 (−0.05, 0.05) | 0.88 | 0.00 (−0.05, 0.05) | 0.95 | ||
| QT prolonging Medications | 0.10 (0.04, 0.15) | 0.001 | 0.10 (0.04, 0.16) | 0.001 | ||
Beta-coefficients (95% confidence intervals (CI)) from mixed-effects linear regression models of QTVI are reported. Serum triglycerides were log transformed due to skewness. Quartiles of creatinine were used due to non-linear correlation with QTVI when visualized. Values are equal to expected difference in QTVI when other parameters are held constant. All models are also adjusted for Hispanic/other race (vs white), center of enrollment, time of enrollment (pre/post-2001), and model B and C are also adjusted for former tobacco use (vs. never), quartiles of serum creatinine, and education <12 years. Variables not reported were not statistically significant (P>0.05) except for center of enrollment (lower QTVI values were associated with the Chicago center).
Continuous covariates were centered to their respective means to improve intercept interpretability. Intercept with centered covariate can be interpreted as expected QTVI for a participant with average continuous biomarkers and reference values in categorical biomarkers (aka. average healthy participant).
Detectable: HIV+ with viral load measured at ≥20 HIV RNA copies/mL. Undetectable: HIV+ with viral load measured at <20 HIV RNA copies/mL. Both are compared to HIV-.
BMI: Body Mass Index
Results of separate models for the QT component and the HR component of QTVI (Formula 2) are reported in Table 4. HIV+ men had lower HR variability irrespective of their VL status (−0.042, p=0.020 for undetectable VL vs HIV-; −0.074, p=0.015 for detectable VL vs HIV-). However, QTVN was significantly higher among HIV+ men with detectable VL when compared to HIV- referents (+0.077, p=0.011) but not statistically different among HIV+ men with undetectable VL vs. HIV- men (+0.022, p=0.23).
Table 4.
Adjusted association of HIV with each QTVI component (Formula 2)
| Log10 (QTVN) | |||||
|---|---|---|---|---|---|
| Model B | P | Model C | P | ||
| HIV+ * | 0.030 (−0.004, 0.065) |
0.086 | Undetectable *,† | 0.022 (−0.014, 0.057) |
0.23 |
| Detectable *,† | 0.077 (0.018, 0.135) |
0.011 | |||
| Log10 (HRVN) | |||||
| Model B | P | Model C | P | ||
| HIV+ * | −0.047 (−0.082, −0.013) |
0.008 | Undetectable *,† | −0.042 (−0.078, −0.007) |
0.020 |
| Detectable *,† | −0.074 (−0.133, −0.015) |
0.015 | |||
All beta-coefficients are vs HIV- as reference.
Detectable: HIV+ with viral load measured at ≥20 HIV RNA copies/mL. Undetectable: HIV+ with viral load <20 HIV RNA copies/mL.
Association between HIV specific factors and QTVI demonstrated a progressive trend towards higher adjusted QTVI with higher degrees of viremia (Table 5), but no association with current or nadir CD4 counts. A clinical AIDS diagnosis was associated with higher QTVI as well.
Table 5.
Adjusted associations between HIV specific factors and QTVI
| HIV-Related Factors (N* = 510) | Beta-coefficient (95% CI) |
P |
|---|---|---|
| Viral load | ||
| Undetectable (<20 copies/mL); N = 417 (82%) | Ref | Ref |
| Low (20–500 copies/mL); N = 60 (12%) | 0.04 (−0.06,0.14) | 0.40 |
| High (>500 copies/mL); N = 33 (7%) | 0.12 (0.00,0.24) | 0.057 |
| Current CD4+ T-Cell Count (cells/mL) | ||
| >400; N = 450 (89%) | Ref | Ref |
| ≤400; N = 60 (11%) | 0.05 (−0.05,0.14) | 0.32 |
| Nadir CD4+ T-Cell Count (cells/mL) | ||
| >400; N = 179 (35%) | Ref | Ref |
| ≤400; N = 326 (65%) | −0.06 (−0.13, 0.00) | 0.067 |
| Clinical history of AIDS; N = 53 (10%) | 0.12 (0.02, 0.22) | 0.024 |
| Duration of HAART use (per 10 years) | 0.00 (−0.06, 0.05) | 0.90 |
| Current Protease Inhibitor Use | 0.05 (−0.02, 0.12) | 0.13 |
Beta-coefficients (95% confidence intervals (CI)) for associations with QTVI reported from mixed-effects linear regression models (similar to model B and C except for HIV serostatus) conducted among HIV+ subgroup.
Of 589 HIV+ men, 79 were missing at least one clinical variable used for adjustment and were excluded.
Associations between inflammatory biomarker levels and QTVI are shown in Table 6. Compared with the lowest tertile concentrations, the highest tertile concentrations of CRP, ICAM-1, and sTNF-R2, and both middle and highest tertile concentrations of IL-6 were associated with greater QTVI. The highest tertile of sCD163 showed nominally higher QTVI as well. Inclusion of each inflammatory marker in the multivariable model slightly attenuated the association of HIV+ serostatus with QTVI (4–13% smaller beta-coefficient).
Table 6.
QTVI differences associated with inflammatory marker concentrations (n= 219 HIV−/ 344 HIV+) and the residual association for HIV
| 2nd Tertile (vs. 1st) |
P | 3rd Tertile (vs. 1st) |
P | HIV+ (vs. HIV-) |
|
|---|---|---|---|---|---|
| Model B | 0.089 (0.022,0.157) |
||||
|
Model B +
CRP (μg/mL) |
0.050 (−0.021,0.122) |
0.17 | 0.092 (0.016,0.168) |
0.018 | 0.081 (0.013,0.148) |
|
Model B +
ICAM-1 (ng/mL) |
0.014 (−0.059,0.088) |
0.70 | 0.075 (0.001, 0.149) |
0.049 | 0.079 (0.010, 0.147) |
|
Model B +
IL-6 (pg/mL) |
0.113 (0.041, 0.186) |
0.002 | 0.135 (0.059, 0.211) |
<0.001 | 0.084 (0.016,0.151) |
| Model B + sCD163 (ng/mL) | 0.029 (−0.045, 0.103) |
0.44 | 0.074 (−0.006, 0.154) |
0.070 | 0.081 (0.011, 0.150) |
|
Model B +
sTNF- R2 (ng/mL) |
0.049 (−0.025, 0.122) |
0.20 | 0.083 (0.006, 0.161) |
0.035 | 0.085 (0.017, 0.153) |
Each row corresponds to a separate mixed-effects model of QTVI with adjustments from Model B and the tertiles of the inflammatory marker of interest as the independent variable. Fibrinogen, MCP1, sCD14, and sTNF-R1 were also modeled but had P-values >0.10 and are not reported.
QTVI vs Ventricular Arrhythmias
Among HIV+ individuals, those in the highest quartile of QTVI had the most arrhythmia burden (any NSVT and/or highest quartile of PVCs/day; see Table 7). In all men, highest quartile of QTVI was associated with high arrhythmia burden (OR: 2.88; p<0.001). This association was attenuated but remained significant after adjustments (OR: 1.93; p=0.006).
Table 7.
Prevalence of ventricular arrhythmias by QTVI quartile among HIV+ men
| Mean QTVI Quartiles (HIV+ only) * | |||||
|---|---|---|---|---|---|
| 1st
(lowest) |
2nd | 3rd | 4th
(Highest) |
||
| Number (%) of Participants with High Arrhythmia Burden | 25 (17%) | 45 (31%) | 52 (35%) | 58 (40%) | |
| Mean QTVI Quartiles (All participants) | |||||
| Odds Ratio** (P-value) for High Arrhythmia Burden | Unadjusted | Ref | 1.96(<0.001) | 2.51 (<0.001) | 2.88 (<0.001) |
| Adjusted | Ref | 1.58 (0.037) | 1.93 (0.003) |
1.93 (0.006) |
|
Unadjusted prevalence of high arrhythmia burden (any NSVT or highest quartile of PVCs/day) stratified by QTVI quartiles among HIV+ participants (n= 589).
Exponentiated beta-coefficients of logistic regression with high arrhythmia burden as the dependent variable. Unadjusted model includes quartiles of QTVI as independent variable. Adjusted model adds the covariates from Model B as independent variables. Among other clinical covariates, age (OR: 1.75 per 10 years, p<0.001) was associated with higher likelihood of arrhythmia burden. Other variables were not statistically significant. Participants with missing covariates are excluded from adjusted models (unadjusted n = 1123, adjusted n= 983).
Sensitivity analyses
Multiple imputation (10 models) of missing covariates did not affect our findings. Similarly, alternate filtering criteria of 0 and 6 hours/body position (instead of 3 hours/body position), and >0% and >25% calculable QTVI (instead of >10% calculable QTVI) did not significantly alter our results. Additional adjustment for fasting glucose level, self-reported binge drinking (>13 drinks/week), cocaine use, use of beta-blockers, calcium channel blockers, ACE inhibitors/ARBs, and diuretics in the past 6 months did not affect the results and were not associated with statistically significant changes in QTVI (not shown). Among HIV+ men, no associations between non-nucleoside reverse transcriptase inhibitors and integrase inhibitor subclasses of antiretroviral therapy and QTVI were found (not shown). Analysis of all HIV medication subclasses were repeated without adjustment for QT prolonging medications to avoid overlap by some drugs but the findings were not affected.
Most participants (98%) also had a resting 12-lead ECG as part of their MACS visit. The prevalence of ventricular conduction abnormality (QRS >120msec) was 6% and did not differ by HIV serostatus. Presence of ventricular conduction abnormality was associated with significantly higher adjusted QTVI (+0.29, p<0.001 for left bundle block/nonspecific interventricular conduction delay, n=18; +0.16, p=0.001 for right bundle block, n=49; vs. no abnormality). Exclusion of participants with prolonged QRS did not affect our results significantly. Within our cohort 382 men underwent a research coronary CT angiography study between 2015 and 2017. We found no association between coronary artery disease (presence of >50% stenosis) and QTVI in the adjusted model and inclusion of coronary artery disease in the model did not attenuate the association between HIV and QTVI. There were 621 men with echocardiography obtained ~1 year after participation in our study. Prevalence of diastolic dysfunction25 was 7% and did not differ by HIV serostatus. Diastolic dysfunction was associated with higher adjusted QTVI (+0.11, p=0.034); however, adjustment for diastolic dysfunction in our models did not affect the association between HIV and QTVI.
Discussion
We investigated QT variability in a well-characterized cohort of HIV+ and HIV- men with comparable clinical and behavioral features using a wearable ambulatory ECG device. To our knowledge, this is the longest duration of ECG monitoring in any investigation of QT variability with close to 80 hours/participant and approximately 90,000 hours of total ECG data analyzed beat-by-beat. While QT-interval prolongation is reported among HIV+ individuals,26, 27 no study has characterized QTVI or other markers of temporal QT dynamicity in this population. Our study is the first to investigate and report greater repolarization lability and autonomic dysfunction, which could contribute to increased susceptibility to SCD among HIV+ compared to HIV- men.
Effect of HIV Serostatus and Viremia on Repolarization Lability
QTVI was 0.077 higher among HIV+ compared to HIV- men, independent of traditional CVD risk factors. Our findings add to what is already known about QT variability. More positive QTVI has been associated with the presence of ischemic heart disease,28, 29 hypertension,30, 31 left ventricular hypertrophy,32 and systolic dysfunction.17, 33 QTVI in diabetics has been correlated with the extent of cardiovascular autonomic dysfunction.34
In a heart failure cohort, higher QTVI correlated with higher New York Heart Association (NYHA) class.35 In the same cohort, QTVI in the top quartile was associated with an adjusted hazard ratio of 2.1 for cardiac death.15 Similarly, the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II, which included patients with history of myocardial infarction and ejection fraction <30%, found an almost 2-fold increased risk of ventricular tachycardia and fibrillation (VT/VF) among those with QTVI in the top quartile compared to the other participants.36 Similar findings were reported in a cohort with subclinical cardiomyopathy.33 While a universally accepted threshold separating healthy and unhealthy QTVI is not well-established, these studies demonstrate a strong association between higher QTVI and adverse cardiac outcomes and its relevance as a potential risk metric. Our sensitivity analysis further demonstrated that conduction disorders (which are strongly linked to risk of SCD)37 were associated with higher QTVI. As our algorithm masks the QRS interval during template-matching,13 this finding serves to illustrate QTVI’s sensitivity to electrophysiological abnormalities and that the effects of such conduction abnormalities permeate the repolarization phase. We also used ZioXT®’s pre-established utility in detecting NSVT and PVCs and found higher burden of ventricular arrhythmias in participants with highest QTVI even after adjustments. These results are consistent with elevated QTVI being a potential risk marker for ventricular arrhythmias.38 In fact, there are reports of beat-to-beat QT variability predicting torsades de pointes with higher sensitivity than QTc interval prolongation.39
Our findings demonstrate the independent association of HIV viral load to higher QTVI. We found that men with HIV and detectable VL had +0.150 higher adjusted QTVI compared to HIV- men—a larger effect size than any other CVD risk factor except for diabetes. Progressive association between presence and magnitude of HIV viremia (Figure 1) and higher QTVI could suggest possible viremia dependent loss of repolarization reserve leading to susceptibility to ventricular arrhythmias and SCD in people living with HIV. Our results are of particular interest in light of laboratory studies reporting inhibition of myocardial slow (Iks) and rapid (Ikr) rectifier potassium channels by the Tat protein encoded in the HIV genome.8, 9 As these channels are crucial for ventricular repolarization, direct disruption of myocardial repolarization by viral components could be plausible.
Figure 1. Progressive association between HIV serostatus/viral load and QTVI.
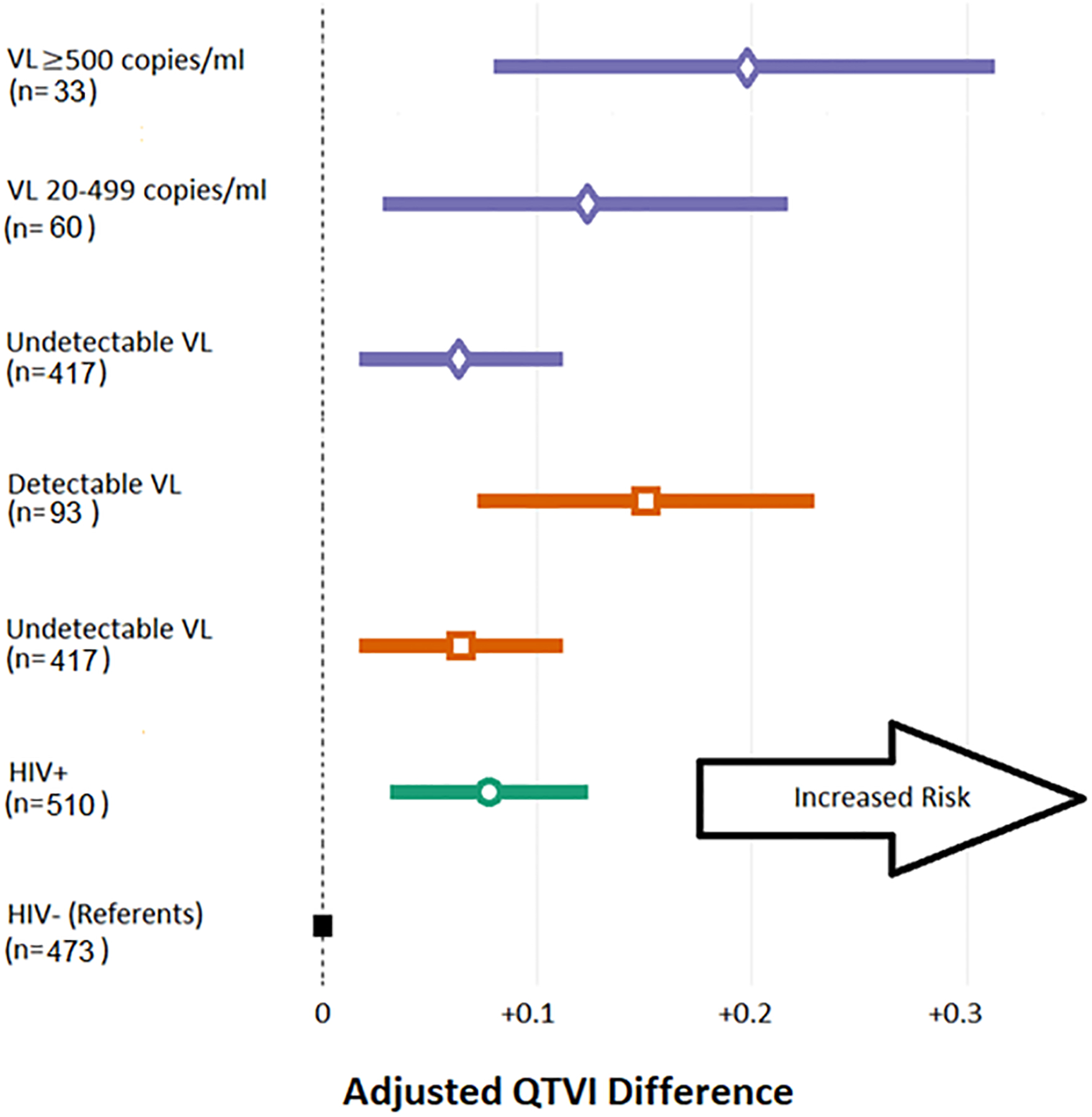
Beta-coefficients and 95% confidence intervals from models adjusted for demographic, socioeconomic, and cardiovascular risk factors when comparing HIV+ subgroups based on viral load (VL) burden vs HIV- as reference (each color denotes a separate model).
Subcomponents of QTVI vs. HIV Serostatus
Since QTVI is defined as QT variability (QTVN from Formula 2) indexed to HR variability (HRVN from Formula 2), changes to either component affect its value. The MADIT II study found elevations in the QT-only portion of QTVI (QTVN) were more strongly associated with incidence of VT/VF compared to elevated QTVI itself. On the other hand, QTVN did not predict mortality within the defibrillator arm of that study, whereas high QTVI remained associated with ‘defibrillator-resistant’ cardiac death in that group. Similarly, in a study of QTVI among people with heart failure, higher QTVI driven by lower HR variability (HRVN), but not higher QTVN, strongly predicted non-sudden cardiac death with a three-fold greater risk for the highest quartile of QTVI vs the other 3 quartiles, but did not predict SCD.17 These findings imply that QTVI is a sensitive, composite marker encompassing both arrhythmia risk through increased QTVN, and degeneration of overall health (pump failure, autonomic impairment, etc.) through lower HRVN. Comparable results were reported in studies of QTVI in cohorts of patients with familial dysautonomia, spinal injury, and obesity (increased QTVI through lower HRVN) as well as cohorts with hypertrophic cardiomyopathy and long QT syndrome (increased QTVI through higher QTVN).23
Interestingly, we found HIV infection to be independently associated with both greater QT variability, as well as lower HR variability in HIV+ men with detectable VL, resulting in an amplified effect on QTVI. However, HIV+ men with undetectable VL only had lower HR variability contributing to higher QTVI, compared to HIV men. Reduced HR variability in HIV+ individuals has been reported previously and is believed to be caused by impaired autonomic function and sympatho-vagal imbalance.12 However, our report of HIV-associated effects on both QTVN and HRVN among viremic HIV+ individuals is a novel finding that implies potential virus-associated effects on both repolarization lability and autonomic dysfunction, which may contribute to a unique dual mechanism for increased susceptibility to SCD among people living with uncontrolled HIV. Recently, there have been reports that heart failure patients living with HIV are at particularly high risk of SCD, especially in the setting of viremia and that this increased risk was not explained by ejection fraction.40, 41 Our findings could point to a potential mechanism for this increased risk through more unstable repolarization (QT portion of QTVI) and decreased overall health (HR portion of QTVI).
Role of Inflammation in Labile Repolarization
HIV infection is associated with increased systemic inflammation and we previously reported an association between concentrations of inflammatory biomarkers and the QT interval.27 Here, we found novel associations between increased concentrations of inflammatory markers, namely, CRP, IL-6, ICAM-1, sTNF-R2, and sCD163 (borderline significance) and higher QTVI in our exploratory analysis. CD163 is a macrophage specific scavenger receptor which is shed upon activation and becomes solubilized (sCD163). sCD163 has been found to correspond to viral activity in HIV infection42 and is linked to development of atherosclerotic disease in HIV.43 CD163-stained macrophages are integral to the cardiac conduction system and contribute to electrical conduction through connexin-43 gap junctions with neighboring cardiomyocytes as well.44 Whether monocytes are involved in repolarization remains unknown. A recent study among aging HIV+ women also reports an association between sCD163 and myocardial fibrosis which could be a substrate for arrhythmias.45 Our data cannot distinguish between systemic or cardiac sources of sCD163, but further investigation into the effects of macrophage activation on ventricular repolarization may be warranted. TNF-α and IL-6 are also reported to inhibit the same potassium channels affected by the HIV-Tat protein (human Ether-à-go-go-Related Gene aka hERG coded Ikr related channels) and to increase the risk of arrhythmias in people living with inflammatory diseases.46 Inhibition of IL-6 in rheumatoid arthritis has been found to counteract QTc prolongation caused by this inflammatory disease while lowering CRP concentrations simultanously.30 Our findings are consistent with an association between greater cardiac repolarization lability and higher systemic inflammation. The observed attenuation of the association between HIV serostatus and higher QTVI by inflammation further suggests inflammation could be a partial mediator of the observed greater repolarization lability in HIV+ men. However, most of the HIV-attributable effect was independent of measured inflammation.
Body Posture vs QTVI
Our study demonstrates feasibility of controlling for real-time body posture in QT variability analysis using ambulatory ECG. QTVI was significantly higher in the upright position. Effect of posture on the QT interval and QTVI is a previously documented24 interaction that could act as a strong confounder if not taken into account. This confounding effect has long been recognized, but to our knowledge no suitable solution existed and previous studies of diurnal changes in the QT interval used self-reported sleep diaries or stratification by time of day.35, 47, 48 We incorporated accelerometry data recorded concurrently with the ECG data to account for postural effects which is a novel approach in studies of QT dynamicity.
Strengths and Limitations
The strengths of our investigation include a large sample size derived from a well-characterized cohort with similar risk behaviors among the groups of interest. Previous studies of QTVI used 5-minute ECG tracings from hospital settings or up to 24-hours of Holter monitoring, and our study uses the longest duration of ECG data to date. We also used a fully automated, template-matching algorithm to analyze ECG data to improve consistency and eliminate human error as recommended by current guidelines for QT dynamicity studies.49 Finally, our use of objective, participant-specific accelerometry to determine posture improves accuracy and accounts for individual differences in sleep pattern/duration.
There are limitations to our study. The MACS includes only men, and it is unknown if the results differ among HIV+ women. While the QTVI calculation is designed to compensate for fluctuations in autonomic tone, we cannot rule out the possibility that autonomic state variations may influence measured QTVI (e.g. through catecholamine effects on the myocardial substrate). MACS is an observational study so residual confounding cannot be excluded and our ability to investigate etiologic mechanisms for increased QTVI among HIV+ men is limited. Inflammatory markers were only available for approximately half the participants and from samples drawn a median of 5.6 years prior to our study. However, previous studies in MACS have suggested these markers are relatively stable.50 The analysis of ventricular arrhythmias was limited by the fact that QTVI was not measured during intervals with frequent ventricular ectopy. Lastly, ZioXT® patch records a single-channel ECG at 200Hz. Inability to choose among different ECG channels meant the T-wave was less prominent for some participants resulting in fewer intervals with calculable QTVI. Also, a previous study has suggested that sampling frequencies below 500Hz could inflate measured QT variability;49 However, such additional noise would be expected to affect our participant groups similarly and would be expected to favor the null hypothesis.
Conclusion
Analysis of a large, well-characterized cohort reveals higher repolarization lability among HIV+ compared to HIV- men, due to lower HR variability, as well as higher QT variability limited to those with persistent viral replication. Progressively greater QTVI with higher viral loads could be the result of direct viral effects on electrophysiologic properties. Increased systemic inflammation was also associated with higher QTVI and inclusion of inflammatory markers partially attenuated the QTVI difference attributable to HIV. Our findings suggest increased ventricular repolarization lability and dysfunction of the cardioprotective autonomic nervous system among men living with HIV. Particularly among HIV+ men who were not virally suppressed in whom there was elevated systemic inflammation, increased QTVI could signal higher susceptibility to ventricular dysrhythmias, autonomic dysfunction and poor general health and warrants further investigation as a viable risk stratification biomarker.
Supplementary Material
Clinical Perspective.
What is new?
In this largest study of QT variability to date, HIV infection and viral load were progressively associated with higher QT variability index (QTVI), a marker of QT interval dynamicity reflecting ventricular repolarization lability and increased arrhythmia risk.
Elevated levels of inflammatory markers were associated with higher QTVI and partially explained the relationship between HIV and abnormal repolarization.
Clinical implications
Men living with HIV exhibit lower heart rate variability (HRV), regardless of viral load.
Viremia among men living with HIV is associated with higher QT variability.
Abnormal HRV reflecting autonomic dysfunction and abnormal QT variability suggesting ventricular repolarization lability may explain mechanisms for increased sudden cardiac death among people living with HIV.
Acknowledgments
The authors acknowledge the valuable contributions of the other MACS investigators, staff and participants.
Sources of Funding
Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS). MACS (Principal Investigators): Johns Hopkins University Bloomberg School of Public Health (Joseph Margolick, Todd Brown), U01-AI35042; Northwestern University (Steven Wolinsky), U01-AI35039; University of California, Los Angeles (Roger Detels, Otoniel Martinez-Maza), U01-AI35040; University of Pittsburgh (Charles Rinaldo, Jeremy Martinson), U01-AI35041; the Center for Analysis and Management of MACS, Johns Hopkins University Bloomberg School of Public Health (Lisa Jacobson, Gypsyamber D’Souza), UM1-AI35043. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute of Mental Health (NIMH). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute on Deafness and Communication Disorders (NIDCD). Some of the biomarker data were derived from RO1 HL095129 (Post). MACS data collection is also supported by UL1-TR001079 (JHU ICTR) from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Dr. Ashikaga receives research funding from The Fondation Leducq Transatlantic Network of Excellence (16CVD02). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH), Johns Hopkins ICTR, or NCATS. The MACS website is located at http://aidscohortstudy.org/.
Non-standard Abbreviations and Acronyms:
- HAART
Highly active anti-retroviral therapy
- HRV
Heart rate variability
- HRVN
Normalized heart rate variance
- MACS
Multicenter AIDS Cohort Stud
- QTVI
QT variability index
- QTVN
Normalized QT variance
Footnotes
Disclosures
FJP is on the speaker’s bureau for Gilead Sciences, Janssen Pharmaceuticals, Merck & Co, Inc. and Bristol‐Myers Squibb. MJB has received grants from General Electric Company. TTB has served as a consultant to Gilead Sciences, Merck, Theratechnologies and EMD-Serono.
References
- 1.Farahani M, Mulinder H, Farahani A, Marlink R. Prevalence and distribution of non-AIDS causes of death among HIV-infected individuals receiving antiretroviral therapy: a systematic review and meta-analysis. Int J STD AIDS. 2017;28:636–650. doi: 10.1177/0956462416632428 [DOI] [PubMed] [Google Scholar]
- 2.Freiberg MS, So-Armah K. HIV and cardiovascular disease: we need a mechanism, and we need a plan. J Am Heart Assoc. 2016;4:e003411. doi: 10.1161/JAHA.116.003411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marin B, Thiebaut R, Bucher HC, Rondeau V, Costagliola D, Dorrucci M, Hamouda O, Prins M, Walker S, Porter K, et al. Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. AIDS. 2009;23:1743–1753. doi: 10.1097/QAD.0b013e32832e9b78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tseng ZH, Secemsky EA, Dowdy D, Vittinghoff E, Moyers B, Wong JK, Havlir DV, Hsue PY. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012;59:1891–1896. doi: 10.1016/j.jacc.2012.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–1482. doi: 10.1056/NEJMra000650 [DOI] [PubMed] [Google Scholar]
- 6.Thienemann F, Sliwa K, Rockstroh JK. HIV and the heart: the impact of antiretroviral therapy: a global perspective. Eur Heart J. 2013;34:3538–3546. doi: 10.1093/eurheartj/eht388 [DOI] [PubMed] [Google Scholar]
- 7.Feinstein MJ, Hsue PY, Benjamin LA, Bloomfield GS, Currier JS, Freiberg MS, Grinspoon SK, Levin J, Longenecker CT, Post WS. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: a scientific statement from the American Heart Association. Circulation. 2019;140:e98–e124. doi: 10.1161/CIR.0000000000000695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai YL, Liu HB, Sun B, Zhang Y, Li Q, Hu CW, Zhu JX, Gong DM, Teng X, Zhang Q, et al. HIV Tat protein inhibits hERG K+ channels: a potential mechanism of HIV infection induced LQTs. J Mol Cell Cardiol. 2011;51:876–880. doi: 10.1016/j.yjmcc.2011.07.017 [DOI] [PubMed] [Google Scholar]
- 9.Es-Salah-Lamoureux Z, Jouni M, Malak OA, Belbachir N, Al Sayed ZR, Gandon-Renard M, Lamirault G, Gauthier C, Baro I, Charpentier F, et al. HIV-Tat induces a decrease in IKr and IKs via reduction in phosphatidylinositol-(4,5)-bisphosphate availability. J Mol Cell Cardiol. 2016;99:1–13. doi: 10.1016/j.yjmcc.2016.08.022 [DOI] [PubMed] [Google Scholar]
- 10.Brouillette J, Cyr S, Fiset C. Mechanisms of arrhythmia and sudden cardiac death in patients with HIV infection. Can J Cardiol. 2019;35:310–319. doi: 10.1016/j.cjca.2018.12.015 [DOI] [PubMed] [Google Scholar]
- 11.Nou E, Lo J, Grinspoon SK. Inflammation, immune activation, and cardiovascular disease in HIV. AIDS. 2016;30:1495–1509. doi: 10.1097/QAD.0000000000001109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McIntosh RC, Lobo JD, Hurwitz BE. Current assessment of heart rate variability and QTc interval length in HIV/AIDS. Curr Opin HIV AIDS. 2017;12:528–533. doi: 10.1097/COH.0000000000000408 [DOI] [PubMed] [Google Scholar]
- 13.Berger RD, Kasper EK, Baughman KL, Marban E, Calkins H, Tomaselli GF. Beat-to-beat QT interval variability: novel evidence for repolarization lability in ischemic and nonischemic dilated cardiomyopathy. Circulation. 1997;96:1557–1565. doi: 10.1161/01.CIR.96.5.1557 [DOI] [PubMed] [Google Scholar]
- 14.Bilchick K, Viitasalo M, Oikarinen L, Fetics B, Tomaselli G, Swan H, Laitinen PJ, Vaananen H, Kontula K, Berger RD. Temporal repolarization lability differences among genotyped patients with the long QT syndrome. Am J Cardiol. 2004;94:1312–1316. doi: 10.1016/j.amjcard.2004.07.123 [DOI] [PubMed] [Google Scholar]
- 15.Dobson CP, La Rovere MT, Pinna GD, Goldstein R, Olsen C, Bernardinangeli M, Veniani M, Midi P, Tavazzi L, Haigney M, et al. QT variability index on 24-hour Holter independently predicts mortality in patients with heart failure: analysis of Gruppo Italiano per lo Studio della Sopravvivenza nell’Insufficienza Cardiaca (GISSI-HF) trial. Heart Rhythm. 2011;8:1237–1242. doi: 10.1016/j.hrthm.2011.03.055 [DOI] [PubMed] [Google Scholar]
- 16.Nayyar S, Hasan MA, Roberts-Thomson KC, Sullivan T, Baumert M. Effect of loss of heart rate variability on T-Wave heterogeneity and QT variability in heart failure patients: implications in ventricular arrhythmogenesis. Cardiovasc Eng Technol. 2017;8:219–228. doi: 10.1007/s13239-017-0299-9 [DOI] [PubMed] [Google Scholar]
- 17.Tereshchenko LG, Cygankiewicz I, McNitt S, Vazquez R, Bayes-Genis A, Han L, Sur S, Couderc JP, Berger RD, de Luna AB, et al. Predictive value of beat-to-beat QT variability index across the continuum of left ventricular dysfunction: competing risks of noncardiac or cardiovascular death and sudden or nonsudden cardiac death. Circ Arrhythm Electrophysiol. 2012;5:719–727. doi: 10.1161/CIRCEP.112.970541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giudicessi JR, Noseworthy PA, Ackerman MJ. The QT interval. Circulation. 2019;139:2711–2713. doi: 10.1161/CIRCULATIONAHA.119.039598 [DOI] [PubMed] [Google Scholar]
- 19.Data Analysis and Coordination Center for MACS/WIHS. Investigator How-To’s. https://mwccs.org/investigator-how-tos/. Accessed November 1, 2019. [Google Scholar]
- 20.Woosley RL, Heise CW, Romero KA. QT drugs list. https://www.crediblemeds.org. Accessed April 8, 2019 AZCERT, Inc. 1822 Innovation Park Dr., Oro Valley, AZ: 85 `755. [Google Scholar]
- 21.Bahrami H, Budoff M, Haberlen SA, Rezaeian P, Ketlogetswe K, Tracy R, Palella F, Witt MD, McConnell MV, Kingsley L, et al. Inflammatory markers associated with subclinical coronary artery disease: the Multicenter AIDS Cohort Study. J Am Heart Assoc. 2016;5:e003371. doi: 10.1161/JAHA.116.003371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKibben RA, Margolick JB, Grinspoon S, Li X, Palella FJ Jr., Kingsley LA, Witt MD, George RT, Jacobson LP, Budoff M, et al. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis. 2015;211:1219–1228. doi: 10.1093/infdis/jiu594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tereshchenko LG, Berger RD. Towards a better understanding of QT interval variability. Ther Adv Drug Saf. 2011;2:245–251. doi: 10.1177/2042098611421209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeragani VK, Pohl R, Jampala VC, Balon R, Kay J, Igel G. Effect of posture and isoproterenol on beat-to-beat heart rate and QT variability. Neuropsychobiology. 2000;41:113–123. doi: 10.1159/000026642 [DOI] [PubMed] [Google Scholar]
- 25.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 26.Reinsch N, Buhr C, Krings P, Kaelsch H, Neuhaus K, Wieneke H, Erbel R, Neumann T, German Heart Failure Network. Prevalence and risk factors of prolonged QTc interval in HIV-infected patients: results of the HIV-HEART study. HIV Clin Trials. 2009;10:261–268. doi: 10.1310/hct1004-261 [DOI] [PubMed] [Google Scholar]
- 27.Wu KC, Zhang L, Haberlen SA, Ashikaga H, Brown TT, Budoff MJ, D’Souza G, Kingsley LA, Palella FJ, Margolick JB, et al. Predictors of electrocardiographic QT interval prolongation in men with HIV. Heart. 2019;105:559–565. doi: 10.1136/heartjnl-2018-313667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Li P, Wang X, Karmakar C, Liu C, Liu C. Short-term QT interval variability in patients with coronary artery disease and congestive heart failure: a comparison with healthy control subjects. Med Biol Eng Comput. 2019;57:389–400. doi: 10.1007/s11517-018-1870-8 [DOI] [PubMed] [Google Scholar]
- 29.Murabayashi T, Fetics B, Kass D, Nevo E, Gramatikov B, Berger RD. Beat-to-beat QT interval variability associated with acute myocardial ischemia. J Electrocardiol. 2002;35:19–25. doi: 10.1054/jelc.2002.30250 [DOI] [PubMed] [Google Scholar]
- 30.Baumert M, Schlaich MP, Nalivaiko E, Lambert E, Sari CI, Kaye DM, Elser MD, Sanders P, Lambert G. Relation between QT interval variability and cardiac sympathetic activity in hypertension. Am J Physiol Heart Circ Physiol. 2011;300:H1412–1417. doi: 10.1152/ajpheart.01184.2010 [DOI] [PubMed] [Google Scholar]
- 31.Myredal A, Gao S, Friberg P, Jensen G, Larsson L, Johansson M. Increased myocardial repolarization lability and reduced cardiac baroreflex sensitivity in individuals with high-normal blood pressure. J Hypertens. 2005;23:1751–1756. doi: 10.1097/01.hjh.0000179762.93291.94 [DOI] [PubMed] [Google Scholar]
- 32.Piccirillo G, Germano G, Quaglione R, Nocco M, Lintas F, Lionetti M, Moise A, Ragazzo M, Marigliano V, Cacciafesta M. QT-interval variability and autonomic control in hypertensive subjects with left ventricular hypertrophy. Clin Sci (Lond). 2002;102:363–371. doi: 10.1042/cs1020363 [DOI] [PubMed] [Google Scholar]
- 33.Piccirillo G, Magri D, Matera S, Magnanti M, Torrini A, Pasquazzi E, Schifano E, Velitti S, Marigliano V, Quaglione R, et al. QT variability strongly predicts sudden cardiac death in asymptomatic subjects with mild or moderate left ventricular systolic dysfunction: a prospective study. Eur Heart J. 2007;28:1344–1350. doi: 10.1093/eurheartj/ehl367 [DOI] [PubMed] [Google Scholar]
- 34.Khandoker AH, Imam MH, Couderc JP, Palaniswami M, Jelinek HF. QT variability index changes with severity of cardiovascular autonomic neuropathy. IEEE Trans Inf Technol Biomed. 2012;16:900–906. doi: 10.1109/TITB.2012.2205010 [DOI] [PubMed] [Google Scholar]
- 35.Dobson CP, La Rovere MT, Olsen C, Berardinangeli M, Veniani M, Midi P, Tavazzi L, Haigney M, GISSI-HF Investigators. 24-hour QT variability in heart failure. J Electrocardiol. 2009;42:500–504. doi: 10.1016/j.jelectrocard.2009.06.021 [DOI] [PubMed] [Google Scholar]
- 36.Haigney MC, Zareba W, Gentlesk PJ, Goldstein RE, Illovsky M, McNitt S, Andrews ML, Moss AJ, Multicenter Automatic Defibrillator Implantation Trial II investigators. QT interval variability and spontaneous ventricular tachycardia or fibrillation in the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II patients. J Am Coll Cardiol. 2004;44:1481–1487. doi: 10.1016/j.jacc.2004.06.063 [DOI] [PubMed] [Google Scholar]
- 37.Sondergaard MM, Nielsen JB, Mortensen RN, Gislason G, Kober L, Lippert F, Graff C, Haunso S, Svendsen JH, Kragholm KH, et al. Associations between common ECG abnormalities and out-of-hospital cardiac arrest. Open Heart. 2019;6:e000905. doi: 10.1136/openhrt-2018-000905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dobson CP, Kim A, Haigney M. QT variability index. Prog Cardiovasc Dis. 2013;56:186–194. doi: 10.1016/j.pcad.2013.07.004 [DOI] [PubMed] [Google Scholar]
- 39.Hinterseer M, Thomsen MB, Beckmann BM, Pfeufer A, Schimpf R, Wichmann HE, Steinbeck G, Vos MA, Kaab S. Beat-to-beat variability of QT intervals is increased in patients with drug-induced long-QT syndrome: a case control pilot study. Eur Heart J. 2008;29:185–190. doi: 10.1093/eurheartj/ehm586 [DOI] [PubMed] [Google Scholar]
- 40.Alvi RM, Neilan AM, Tariq N, Hassan MO, Awadalla M, Zhang L, Afshar M, Rokicki A, Mulligan CP, Triant VA, et al. The risk for sudden cardiac death among patients living with heart failure and Human Immunodeficiency Virus. JACC Heart Fail. 2019;7:759–767. doi: 10.1016/j.jchf.2019.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tseng ZH. Presumed sudden cardiac deaths among persons with HIV and heart failure. JACC Heart Fail. 2019;7:768–770. doi: 10.1016/j.jchf.2019.06.007 [DOI] [PubMed] [Google Scholar]
- 42.Burdo TH, Lentz MR, Autissier P, Krishnan A, Halpern E, Letendre S, Rosenberg ES, Ellis RJ, Williams KC. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis. 2011;204:154–163. doi: 10.1093/infdis/jir214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanna DB, Lin J, Post WS, Hodis HN, Xue X, Anastos K, Cohen MH, Gange SJ, Haberlen SA, Heath SL, et al. Association of macrophage inflammation biomarkers with progression of subclinical carotid artery atherosclerosis in HIV-infected women and men. J Infect Dis. 2017;215:1352–1361. doi: 10.1093/infdis/jix082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hulsmans M, Clauss S, Xiao L, Aguirre AD, King KR, Hanley A, Hucker WJ, Wulfers EM, Seemann G, Courties G, et al. Macrophages facilitate electrical conduction in the heart. Cell. 2017;169:510–522 e520. doi: 10.1016/j.cell.2017.03.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zanni MV, Awadalla M, Toribio M, Robinson J, Stone LA, Cagliero D, Rokicki A, Mulligan CP, Ho JE, Neilan AM, et al. Immune correlates of diffuse myocardial fibrosis and diastolic dysfunction among aging women with Human Immunodeficiency Virus [published online ahead of print May 17, 2019]. J Infect Dis. doi: 10.1093/infdis/jiz184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lazzerini PE, Capecchi PL, El-Sherif N, Laghi-Pasini F, Boutjdir M. Emerging arrhythmic risk of autoimmune and inflammatory cardiac channelopathies. J Am Heart Assoc. 2018;7:e010595. doi: 10.1161/JAHA.118.010595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Molnar J, Zhang F, Weiss J, Ehlert FA, Rosenthal JE. Diurnal pattern of QTc interval: how long is prolonged? Possible relation to circadian triggers of cardiovascular events. J Am Coll Cardiol. 1996;27:76–83. doi: 10.1016/0735-1097(95)00426-2 [DOI] [PubMed] [Google Scholar]
- 48.Morganroth J, Brozovich FV, McDonald JT, Jacobs RA. Variability of the QT measurement in healthy men, with implications for selection of an abnormal QT value to predict drug toxicity and proarrhythmia. Am J Cardiol. 1991;67:774–776. doi: 10.1016/0002-9149(91)90541-r [DOI] [PubMed] [Google Scholar]
- 49.Baumert M, Porta A, Vos MA, Malik M, Couderc JP, Laguna P, Piccirillo G, Smith GL, Tereshchenko LG, Volders PG. QT interval variability in body surface ECG: measurement, physiological basis, and clinical value: position statement and consensus guidance endorsed by the European Heart Rhythm Association jointly with the ESC Working Group on Cardiac Cellular Electrophysiology. Europace. 2016;18:925–944. doi: 10.1093/europace/euv405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wada NI, Jacobson LP, Margolick JB, Breen EC, Macatangay B, Penugonda S, Martinez-Maza O, Bream JH. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS. 2015;29:463–471. doi: 10.1097/QAD.0000000000000545 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


