Abstract
Objectives
The objective of this study was to assess and compare the performance of 68Ga-DOTA-conjugated-somatostatin-receptor-targeting-peptides (68Ga-DOTA-SST) PET/CT, octreoscan-SPECT/CT and 18F-FDG-PET/CT in the detection of tumor-induced osteomalacia (TIO).
Methods
Relevant studies reporting the performance 68Ga-DOTA-SST PET/CT, octreoscan-SPECT/CT and 18F-FDG-PET/CT in TIO were identified by searching PubMed, EMBASE, and Web of Science (last updated June 2019). Two authors independently extracted the numbers of true and false positives, and true and false negatives. The pooled estimates on a per-patient basis were calculated with 95% confidence interval (CI) obtained using a random-effects model.
Results
Fourteen studies comprising 346 patients were included in this meta-analysis. The meta-analysis provided the following results on a per-patient analysis. The pooled sensitivities of both 68Ga-DOTA-SST PET/CT (90%, 95% CI 82–95%) and octreoscan-SPECT/CT (83%, 95% CI 75–89%) were found to be significantly higher (P < 0.005) than that of 18F-FDG PET/CT (67%, 95% CI 53–80%). There was no statistically significant difference between the sensitivity of 68Ga-DOTA-SST PET/CT and octreoscan-SPECT/CT (P = 0.161). Owing to the low number of articles with true negative findings, the pooled specificities were not calculated. A total of 287 tumors were identified in 287 patients according to the data the included studies offered. The majority of the tumors were located in the lower extremities (59.6%, 171/287), followed by craniofacial regions (24.0%, 69/287), torso (9.4%%, 27/287), and upper extremities (6.9%, 20/287).
Conclusion
This meta-analysis demonstrates that somatostatin receptor-based imaging modalities outperformed 18F-FDG PET/CT in the detection of TIO, with 68Ga-DOTA-SST PET/CT performing slightly better than octreoscan-SPECT/CT.
Keywords: 18F-FDG, 68Ga-DOTA-SST, octreoscan, tumor-induced osteomalacia
Introduction
Tumor induced osteomalacia (TIO), also known as oncogenic osteomalacia, is an uncommon acquired paraneoplastic syndrome characterized by biochemical anomalies, such as hypophosphatemia and hyperphosphaturia, and manifested as long-term debilitating bone pain and muscle weakness [1]. The oncogenic osteomalacia is secondary to a phosphaturic mesenchymal tumor mixed connective tissue variant, which secretes fibroblast growth factor 23 (FGF23) and other phosphaturic proteins [2]. PMTMCTs are usually small, benign and slow-growing polymorphous neoplasms. The overproduction of FGF23 increases renal phosphate loss and decreases renal phosphate reabsorption, leading to hypophosphatemia and consequent osteomalacia [3]. The only curative therapy of the disease is complete resection of the responsible tumor or destruction via radiofrequency ablation [4,5]. PMTMCTs are rare neoplasms that approximately 53% of cases occurred within bones, 45% patients in soft tissue and 3% of patients in the skin [6]. Nevertheless, even with the recognition of TIO, the precise localization of the tumor can be challenging due to their small size and obscure anatomical situation [7]. In addition, the local symptoms directly caused by the slow-growing tumor are frequently overshadowed by the severe systematic symptoms of osteomalacia. These tumors prove to be difficult for conventional anatomy-based imaging techniques to detect.
Functional imaging modalities, such as PET and SPECT, have been utilized with various tracers to detect the culprit tumor inducing osteomalacia. The localization of TIO using 18F-FDG PET/CT have been described in several case reports and studies [8–11]. As these tumors are slowing growing and commonly small in size, the FDG uptake may be relatively low. TIOs are reported to overexpress somatostatin receptor, mainly subtype 2, allowing the use of somatostatin receptor imaging with PET or SPECT [12]. 68Ga-DOTA-SST PET/CT has higher resolution, better physical characteristic and higher affinity for somatostatin receptor 2, 5 compared to octreoscan SPECT/CT [13]. Therefore, it is speculated that 68Ga-DOTA-SST might have a higher sensitivity than octreoscan-SPECT/CT in detecting TIO [13]. However, evidence on the direct comparison of 68Ga-DOTA-SST PET/CT and octreoscan-SPECT/CT, as well as 18F-FDG PET/CT is limited. The aim of this meta-analysis is to assess and compare the efficacy of 68Ga-DOTA-SST PET/CT with octreoscan-SPECT/CT and 18F-FDG PET/CT in detecting TIO.
Materials and methods
Search strategy
We performed a comprehensive literature search on PubMed, EMBASE and Web of Science. The search algorithm used was based on the combination of the following keywords: ‘tumor-induced osteomalacia OR oncogenic osteomalcia’; ‘18F-fluorodeoxyglucose OR FDG’; ‘68 Ga OR 68 gallium’; ‘99mTc-HYNIC-TOCOR 111In-pentetreotide OR octreotide scintigraphy OR somatostatin receptor scintigraphy’. We placed no restrictions on the language or date of publication. Reference lists of the retrieved articles and relevant review articles were also checked to identify studies that may have been missed by the initial database search.
Study selection
Studies were included based on the following criteria: (1) 68Ga-DOTA-SST or 18F-FDG PET/CT or Octreoscan-SPECT/CT were performed in patients suspicious of TIO; (2) studies that reported the diagnostic performance data; (3) clinical studies that included at least five patients. The exclusion criteria were as follows: (1) review articles, cases, editorials or letters, comments, conference proceedings, preclinical studies, animal studies; (2) patients with recurrent TIO; (3) duplicate data; (4) non-original articles.
Data extraction and quality assessment
Study and clinicopathological characteristics were extracted from the selected studies using a standardized form. The methodologic quality of included studies was evaluated using the Quality Assessment of Diagnostic Accuracy Studies-2 tool (QUADAS-2) [14]. The QUADAS-2 tool primarily assesses four domains: risk of bias in patient selection; index test; reference standard; and the timing of the reference test. Data extraction and quality assessment were independently performed by two independent reviewers; any disagreements were resolved by consensus.
Statistical analysis
The reference standard employed by the included studies was either pathological diagnosis or clinical diagnosis. In certain articles where there were no false-positives (FPs) and true-negatives (TNs), the ‘detection rate’ was used as ‘sensitivity’. From each included study, the number of true-positives, FPs, TNs and false-negatives were obtained on a per-patient basis if available. A random-effects model was used for statistical pooling of the data, taking into account the heterogeneity between studies. Heterogeneity among those eligible studies was assessed by the I2 test. Heterogeneity was considered low if I2 statistic was 25%, moderate if I2 statistic was 50% and high if I2 statistic was 75%. Since most of the studies did not apply a direct comparison among imaging methods, the Z test was used to find whether the pooled sensitivity was significantly different among imaging modalities [15–17]. For P value, the level of statistical significance was set to 0.05. All statistical analyses were performed using Meta-disc 1.4 software and SPSS version 21.
Results
Literature search and study characteristics
The process of selecting studies for the meta-analysis is shown in Fig. 1. A total of 370 articles were obtained through the initial search. After the removal of 192 duplicate articles and exclusion of 157 studies based on title and abstract review. There were 21 potentially eligible studies. Finally, 14 studies, comprising a total sample size of 346 patients with TIO met all inclusion criteria, and they were included in this meta-analysis. The summary of characteristics and statistics of the included studies are described in Tables 1 and 2. The imaging modalities used for TIO included 68Ga-DOTA-SST PET/CT, 18F-FDG PET/CT, 99mTc-HYNIC-TOC SPECT/CT and 111In-pentetreotide SPECT/CT.
Fig. 1.
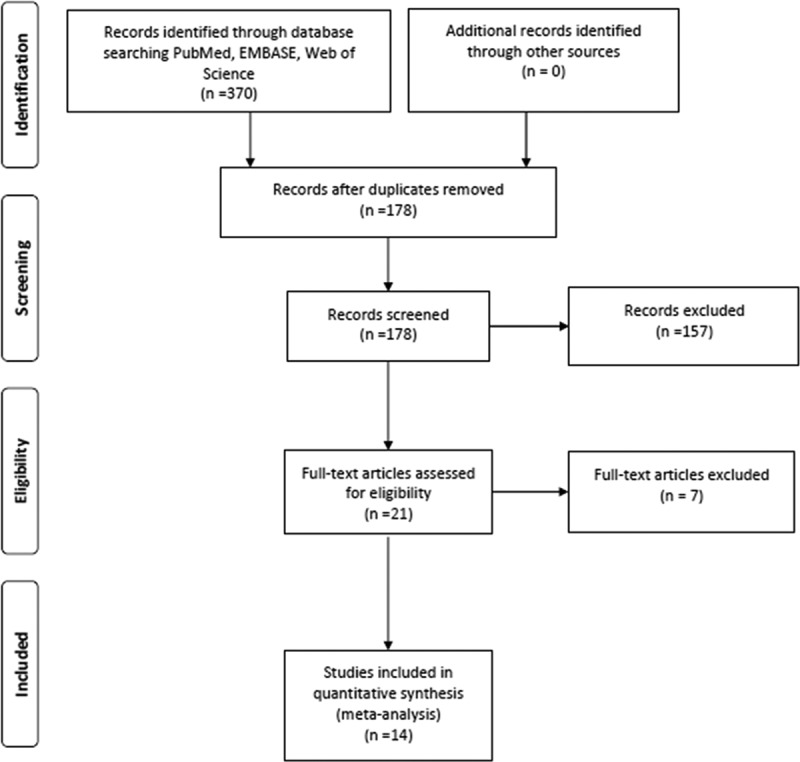
Flowchart diagram presenting the selection of eligible studies.
Table 1.
Characteristics of studies included in the meta-analysis
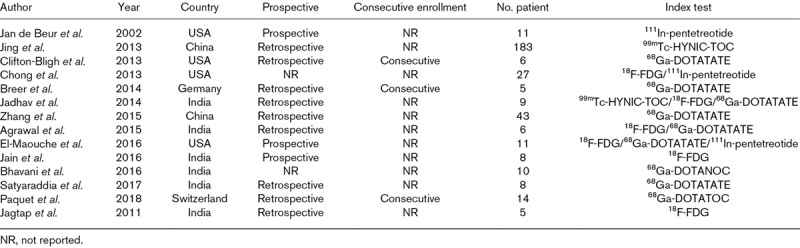
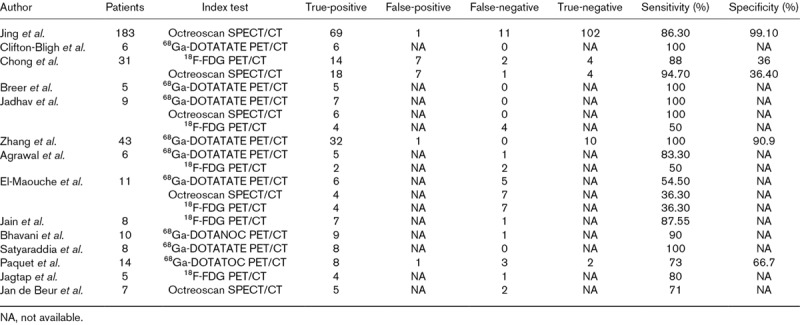
Table 2.
Summary of statistics of included articles
Quality assessment
Regarding the patient selection domain, 10 studies had a high risk of bias because they were retrospective and did not provide information about consecutive enrollment [18–27]. Regarding the index test domain, the included studies were all classified as unclear because they lacked information about whether they were interpreted without the knowledge of the reference standard. Even so, we believe that the index test interpretation was made without knowing the reference standard result because the surgical resection of the suspected TIO could only be performed when imaging techniques detect suspicious culprit tumor. Regarding the reference standard domain, in the same way, all studies showed a high risk of bias, as it was unclear whether reference standard interpretation was blinded to the index test results. Regarding the flow and timing domain, all studies had a high risk of bias, as the PET-reference standard interval was not provided. The applicability of the included studies was adequate and all classified as low.
Quantitative synthesis
From the included study protocols, six used 68Ga-DOTA-SST PET/CT [21,23,24,28–30], two 18F-FDG PET/CT [26,31], two octreoscan-SPECT/CT [18,32], two 68Ga-DOTA-SST PET/CT, 18F-FDG PET/CT and octreoscan-SPECT/CT [20,33], one used 68Ga-DOTA-SST and 18F-FDG PET/CT [22], and one used 18F-FDG PET/CT and octreoscan-SPECT/CT [19] as index test examinations. For each study, the metrics of sensitivity and specificity were obtained. Due to the low number of articles providing TN and FP findings, pooled specificity was not calculated in this meta-analysis. The sensitivity values, with the corresponding 95% confidence intervals (CIs), were calculated for all included studies (Fig. 2). The pooled sensitivities of both 68Ga-DOTA-SST PET/CT (90%; 95% CI 82–95%) and octreoscan-SPECT/CT (83%; 95% CI 75–89%) were found to be significantly higher (P < 0.005) than that of 18F-FDG PET/CT (67%; 95% CI 53–80%). The pooled sensitivity of 68Ga-DOTA-SST PET/CT was higher than that of octreoscan-SPECT/CT, but the difference was not statistically significant (P = 0.161). The random-effects model was used because of moderate to substantial heterogeneity among the included studies (I2 = 66.9% for 68Ga-DOTA-SST PET/CT, 77.4% for octreoscan-SPECT/CT and 56.8% for 18F-FDG PET/CT). A total of 287 tumors were identified in 287 patients according to the data the included studies offered. We found that most of the tumors were located in the lower extremities (59.6%, 171/287), followed by craniofacial regions (24.0%, 69/287), torso (9.4%%, 27/287) and upper extremities (6.9%, n = 20/287).
Fig. 2.
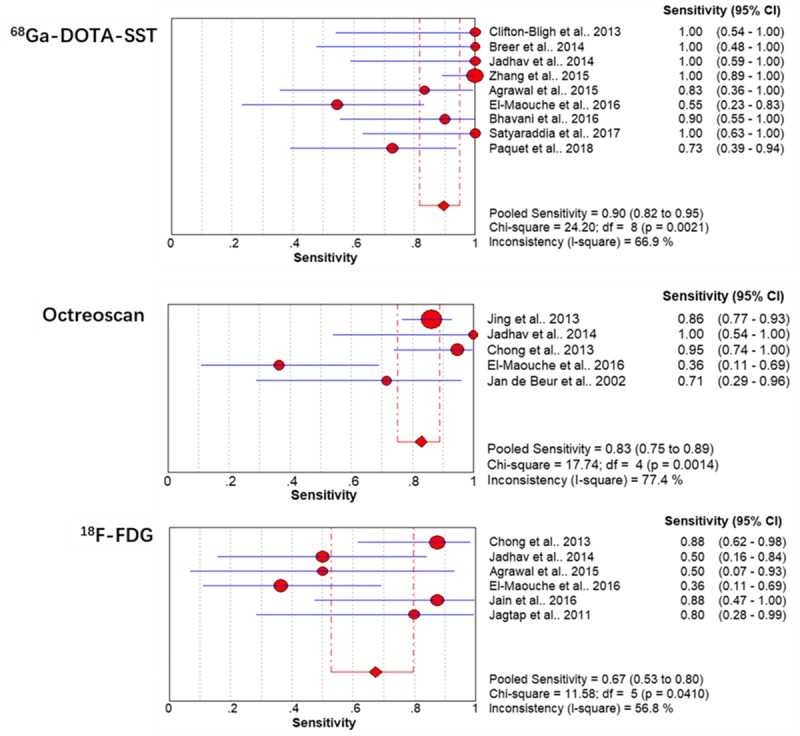
Forest plot of individual study results showing estimates of sensitivity and 95% CI. CI, confidence interval.
Discussion
Functional imaging methods have played a significant role in the detection of suspicious lesions of TIO. Several studies have used 68Ga-DOTA-SST PET/CT, octreoscan-SPECT/CT and 18F-FDG PET/CT in patients with TIO and reported favorable results. But only a few of them have compared these diagnostic techniques in the same group of patients. And these studies have limited power as they only enrolled small number of patients. This might be due to that TIO is a rare disease, for which large cohort studies are usually not feasible. Meta-analysis is regarded as an appropriate method to tackle this problem. Therefore, we have pooled reported data of the published studies in order to assess and compare the efficacy of 68Ga-DOTA-SST PET/CT, octreoscan-SPECT/CT and 18F-FDG PET/CT in detecting TIO.
As expected, results of quantitative indirect comparison of pooled estimates showed that 68Ga-DOTA-SST PET/CT exhibited the highest sensitivity in the detection of TIO, followed by octreoscan-SPECT/CT and 18F-FDG PET/CT. Both somatostatin receptor-based functional imaging modalities had significant higher sensitivity than 18F-FDG PET/CT. 18F-FDG is the most widely used radiotracer in nuclear medicine and also the first PET tracer reported in literature to be used in the detection of TIO [9]. The reported sensitivity of 18F-FDG PET/CT of published studies in detecting TIO varied greatly from 36.3 to 88%, with the pooled sensitivity being 67% and also the lowest among the three imaging modalities in this meta-analysis. This result might suggest the limitation in its utility. The relatively poor performance of 18F-FDG PET/CT might be mainly due to the benign nature and low metabolic activity of the mesenchymal tumors associated with TIO [31].
The overexpression of somatostatin receptor with a predominance of subtype 2 in these tumors made it possible for the utility of somatostatin receptor-based functional scans [12]. Somatostatin receptor-based imaging techniques include 99mTc-HYNIC-TOC SPECT/CT, and 111In-pentetreotide SPECT/CT and 68Ga-DOTA-SST PET/CT. To the best of our knowledge, only two studies with 20 subjects have performed a direct comparison between octreoscan-SPECT/CT and 68Ga-DOTA-TATE PET/CT in patients with TIO [20,33]. The reported sensitivities of octreoscan-SPECT/CT and 68Ga-DOTA-TATE PET/CT were 100% vs. 100% and 36.3% vs. 55%, respectively. However, the conclusions of these two studies are not solid due to the limited subjects enrolled. The strength of the current meta-analysis is the quantitative indirect comparison of the performance of octreoscan-SPECT/CT and 68Ga-DOTA-SST PET/CT involving a large sample size. There was no statistically significant difference between the sensitivity of 68Ga-DOTA-SST PET/CT and octreoscan-SPECT/CT (P = 0.161). Therefore, octreoscan-SPECT/CT is a valuable alternative to 68Ga-DOTA-SST PET/CT in clinic. Still, wherever available, 68Ga-DOTA-SST PET/CT should be preferred in light of the better physical characteristics and spatial resolution it offers than octreoscan-SPECT/CT.
The majority of the tumors were located in the lower extremities, followed by craniofacial regions, torso and upper extremities. The results are consistent with previously published studies [18,21,34]. More relevant researches are still needed to find out the underlying explanation for the location of tumors.
There are considerable limitations in this meta-analysis. Over half of the included studies are retrospective in nature, which might lead to overestimation of outcomes. Only one study performed a direct comparison among the three imaging modalities. Positive result publication bias is a major concern, because significant or favorable study results are easier to be published. Pooled specificity was not calculated in this meta-analysis, because most studies did not provide TN and FP findings. There are no other measures of diagnostic accuracy, including specificity, positive predictive value, negative predictive value and Diagnostic Odds Ratio, presented other than sensitivity data. Another major limitation is the lack of valid reference standard test and time and flow of imaging interpretation because all studies did not report whether reference standard was blinded to the index test and not specify the exact time interval between PET imaging and biopsy or surgery. There were heterogeneities in study design, patient selection, sample size, imaging acquisition among the included studies.
Conclusion
Tumor localization is a crucial step in the management of TIO. This meta-analysis demonstrates that somatostatin receptor-based imaging modalities outperformed 18F-FDG PET/CT in the detection of TIO, with 68Ga-DOTA-SST PET/CT performing slightly better than octreoscan-SPECT/CT.
Acknowledgements
This work is supported by National Natural Science Foundation of China (no. 81371588, no. 81101074).
Conception and design were done by W.C. Financial support by W.C. Collection and assembly of data was done by Y.J. and G.H. Writing was done by Y.J. and G.H.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Dr. Yuanyuan Jiang and Dr. Guozhu Hou contributed equally to the writing of this article.
References
- 1.Carpenter TO. Oncogenic osteomalacia–a complex dance of factors. N Engl J Med. 2003; 348:1705–1708 [DOI] [PubMed] [Google Scholar]
- 2.Folpe AL, Fanburg-Smith JC, Billings SD, Bisceglia M, Bertoni F, Cho JY, et al. Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am J Surg Pathol. 2004; 28:1–30 [DOI] [PubMed] [Google Scholar]
- 3.Fukumoto S, Yamashita T. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003; 349:505–6; author reply 505 [PubMed] [Google Scholar]
- 4.Ward LM, Rauch F, White KE, Filler G, Matzinger MA, Letts M, et al. Resolution of severe, adolescent-onset hypophosphatemic rickets following resection of an FGF-23-producing tumour of the distal ulna. Bone. 2004; 34:905–911 [DOI] [PubMed] [Google Scholar]
- 5.Hesse E, Rosenthal H, Bastian L. Radiofrequency ablation of a tumor causing oncogenic osteomalacia. N Engl J Med. 2007; 357:422–424 [DOI] [PubMed] [Google Scholar]
- 6.Hautmann AH, Hautmann MG, Kölbl O, Herr W, Fleck M. Tumor-induced osteomalacia: an up-to-date review. Curr Rheumatol Rep. 2015; 17:512. [DOI] [PubMed] [Google Scholar]
- 7.Florenzano P, Gafni RI, Collins MT. Tumor-induced osteomalacia. Bone Rep. 2017; 7:90–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu W, Wang C, Ruan J, Chen F, Li N, Chen F. A case report of phosphaturic mesenchymal tumor-induced osteomalacia. Medicine (Baltimore). 2017; 96:e9470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dupond JL, Mahammedi H, Prié D, Collin F, Gil H, Blagosklonov O, et al. Oncogenic osteomalacia: diagnostic importance of fibroblast growth factor 23 and F-18 fluorodeoxyglucose PET/CT scan for the diagnosis and follow-up in one case. Bone. 2005; 36:375–378 [DOI] [PubMed] [Google Scholar]
- 10.Kaneuchi Y, Hakozaki M, Yamada H, Hasegawa O, Tajino T, Konno S. Missed causative tumors in diagnosing tumor-induced osteomalacia with (18)F-FDG PET/CT: a potential pitfall of standard-field imaging. Hell J Nucl Med. 2016; 19:46–48 [DOI] [PubMed] [Google Scholar]
- 11.Okamiya T, Takahashi K, Kamada H, Hirato J, Motoi T, Fukumoto S, Chikamatsu K. Oncogenic osteomalacia caused by an occult paranasal sinus tumor. Auris Nasus Larynx. 2015; 42:167–169 [DOI] [PubMed] [Google Scholar]
- 12.Seufert J, Ebert K, Müller J, Eulert J, Hendrich C, Werner E, et al. Octreotide therapy for tumor-induced osteomalacia. N Engl J Med. 2001; 345:1883–1888 [DOI] [PubMed] [Google Scholar]
- 13.Hofman MS, Lau WF, Hicks RJ. Somatostatin receptor imaging with 68ga DOTATATE PET/CT: clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiographics. 2015; 35:500–516 [DOI] [PubMed] [Google Scholar]
- 14.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. ; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011; 155:529–536 [DOI] [PubMed] [Google Scholar]
- 15.Lee YJ, Lee JM, Lee JS, Lee HY, Park BH, Kim YH, et al. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology. 2015; 275:97–109 [DOI] [PubMed] [Google Scholar]
- 16.Zhou J, Gou Z, Wu R, Yuan Y, Yu G, Zhao Y. Comparison of PSMA-PET/CT, choline-PET/CT, naf-PET/CT, MRI, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a systematic review and meta-analysis. Skeletal Radiol. 2019; 48:1915–1924 [DOI] [PubMed] [Google Scholar]
- 17.Basso Dias A, Zanon M, Altmayer S, Sartori Pacini G, Henz Concatto N, Watte G, et al. Fluorine 18-FDG PET/CT and diffusion-weighted MRI for malignant versus benign pulmonary lesions: a meta-analysis. Radiology. 2019; 290:525–534 [DOI] [PubMed] [Google Scholar]
- 18.Jing H, Li F, Zhuang H, Wang Z, Tian J, Xing X, et al. Effective detection of the tumors causing osteomalacia using [tc-99m]-HYNIC-octreotide (99mtc-HYNIC-TOC) whole body scan. Eur J Radiol. 2013; 82:2028–2034 [DOI] [PubMed] [Google Scholar]
- 19.Chong WH, Andreopoulou P, Chen CC, Reynolds J, Guthrie L, Kelly M, et al. Tumor localization and biochemical response to cure in tumor-induced osteomalacia. J Bone Miner Res. 2013; 28:1386–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jadhav S, Kasaliwal R, Lele V, Rangarajan V, Chandra P, Shah H, et al. Functional imaging in primary tumour-induced osteomalacia: relative performance of FDG PET/CT vs somatostatin receptor-based functional scans: a series of nine patients. Clin Endocrinol (Oxf). 2014; 81:31–37 [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Zhu Z, Zhong D, Dang Y, Xing H, Du Y, et al. 68ga DOTATATE PET/CT is an accurate imaging modality in the detection of culprit tumors causing osteomalacia. Clin Nucl Med. 2015; 40:642–646 [DOI] [PubMed] [Google Scholar]
- 22.Agrawal K, Bhadada S, Mittal BR, Shukla J, Sood A, Bhattacharya A, Bhansali A. Comparison of 18F-FDG and 68ga DOTATATE PET/CT in localization of tumor causing oncogenic osteomalacia. Clin Nucl Med. 2015; 40:e6–e10 [DOI] [PubMed] [Google Scholar]
- 23.Bhavani N, Reena Asirvatham A, Kallur K, Menon AS, Pavithran PV, Nair V, et al. Utility of gallium-68 DOTANOC PET/CT in the localization of tumour-induced osteomalacia. Clin Endocrinol (Oxf). 2016; 84:134–140 [DOI] [PubMed] [Google Scholar]
- 24.Satyaraddi A, Cherian KE, Shetty S, Kapoor N, Jebasingh FK, Cherian VM, et al. Musculoskeletal oncogenic osteomalacia-an experience from a single centre in south india. J Orthop. 2017; 14:184–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M, Zhou L, Xue HD, Jiang Y, Zhong DR, Zhu ZH. [Clinical and radiologic characteristics of craniomaxillofacial primary tumor induced osteomalacia: a retrospective analysis]. Zhonghua Kou Qiang Yi Xue Za Zhi. 2016; 51:341–345 [DOI] [PubMed] [Google Scholar]
- 26.Jagtap VS, Sarathi V, Lila AR, Malhotra G, Sankhe SS, Bandgar T, et al. Tumor-induced osteomalacia: a single center experience. Endocr Pract. 2011; 17:177–184 [DOI] [PubMed] [Google Scholar]
- 27.Jiang Y, Xia WB, Xing XP, Silva BC, Li M, Wang O, et al. Tumor-induced osteomalacia: an important cause of adult-onset hypophosphatemic osteomalacia in China: report of 39 cases and review of the literature. J Bone Miner Res. 2012; 27:1967–1975 [DOI] [PubMed] [Google Scholar]
- 28.Clifton-Bligh RJ, Hofman MS, Duncan E, Sim IeW, Darnell D, Clarkson A, et al. Improving diagnosis of tumor-induced osteomalacia with gallium-68 DOTATATE PET/CT. J Clin Endocrinol Metab. 2013; 98:687–694 [DOI] [PubMed] [Google Scholar]
- 29.Breer S, Brunkhorst T, Beil FT, Peldschus K, Heiland M, Klutmann S, et al. 68ga DOTA-TATE PET/CT allows tumor localization in patients with tumor-induced osteomalacia but negative 111in-octreotide SPECT/CT. Bone. 2014; 64:222–227 [DOI] [PubMed] [Google Scholar]
- 30.Paquet M, Gauthé M, Zhang Yin J, Nataf V, Bélissant O, Orcel P, et al. Diagnostic performance and impact on patient management of 68ga-DOTA-TOC PET/CT for detecting osteomalacia-associated tumours. Eur J Nucl Med Mol Imaging. 2018; 45:1710–1720 [DOI] [PubMed] [Google Scholar]
- 31.Jain AS, Shelley S, Muthukrishnan I, Kalal S, Amalachandran J, Chandran S. Diagnostic importance of contrast enhanced (18)F-fluorodeoxyglucose positron emission computed tomography in patients with tumor induced osteomalacia: our experience. Indian J Nucl Med. 2016; 31:14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jan de Beur SM, Streeten EA, Civelek AC, McCarthy EF, Uribe L, Marx SJ, et al. Localisation of mesenchymal tumours by somatostatin receptor imaging. Lancet. 2002; 359:761–763 [DOI] [PubMed] [Google Scholar]
- 33.El-Maouche D, Sadowski SM, Papadakis GZ, Guthrie L, Cottle-Delisle C, Merkel R, et al. 68ga-DOTATATE for tumor localization in tumor-induced osteomalacia. J Clin Endocrinol Metab. 2016; 101:3575–3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang S, Wang L, Wang T, Xing HQ, Huo L, Li F. [Value of 68ga-DOTA-TATE positron emission tomography/computed tomography in the localization of culprit tumors causing osteomalacia with negative 99mtc-HYNIC-TOC single photo emission computed tomography]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2018; 40:757–764 [DOI] [PubMed] [Google Scholar]


