There is considerable interest in syphilis preexposure (44.1%) and postexposure (60.1%) prophylaxis among gay, bisexual, and other men who have sex with men attending Toronto/Vancouver sexual health clinics.
Abstract
Background
We assessed the acceptability of doxycycline-based syphilis pre-exposure and post-exposure prophylaxis (PrEP/PEP) as well as human immunodeficiency virus (HIV) PrEP/PEP in gay, bisexual, and other men who have sex with men (gbMSM).
Methods
We recruited gbMSM from Toronto and Vancouver sexually transmitted infection (STI) clinics during routine visits from June 2018 to August 2018. We analyzed data using descriptive statistics and constructed multivariable logistic regression models for willingness to use syphilis and HIV PrEP and PEP respectively.
Results
Among 424 participants (56.4% Toronto, 43.6% Vancouver), median (interquartile range [IQR]) age was 31.0 years (26.0–39.0 years), 61.7% had completed postsecondary education and 54.4% were white. Median (IQR) number of male partners in the past 6 months was 6.0 (3.0–13.0), and 18.2% had 1 or more prior syphilis diagnosis. 60.1%/44.1% indicated willingness to use syphilis PEP/PrEP; 36.6% were unwilling to use either. Among HIV-negative participants, 74.0% and 75.2% were willing to use HIV PrEP and PEP, respectively. Most participants were familiar with antibiotic resistance (89.0%) and agreed that syphilis rates are rising in Canada (68.2%), but only 55.4% believed they were at risk for syphilis. Agreement with the latter statement was associated with willingness to use syphilis PrEP (adjusted odds ratio [aOR], 1.6; 95% confidence interval [95%CI], 1.0–2.5), as was previous/existing HIV PrEP use (aOR, 2.2; 95% CI, 1.1–4.3) and being “very concerned” about STI acquisition (aOR, 1.9; 95% CI, 1.0–3.4). Odds of being willing to use syphilis PEP were higher in Toronto versus Vancouver (aOR, 2.0; 95% CI, 1.2–3.4) and increased with the number of different STIs previously diagnosed (aOR, 1.4; 95% CI, 1.2,1.7).
Conclusions
There is considerable interest in syphilis PrEP/PEP in gbMSM attending Toronto/Vancouver STI clinics.
There are growing epidemics of bacterial sexually transmitted infections (STIs) in Canada, many of which disproportionately affect gay, bisexual, and other men who have sex with men (gbMSM). Syphilis has been on the rise since the 1990s, reaching a rate of 27.5 per 100,000 among Canadian men in 2017,1 and is associated with significant morbidity.2 Gonorrhea is the second most commonly reported STI in Canada,3 and the threat of untreatable infection is becoming a real possibility due to antimicrobial resistance.4 Though resistance to Chlamydia trachomatis remains rare, high reinfection rates have raised concern about treatment failures.5 Bacterial STIs are also known to increase human immunodeficiency virus (HIV) acquisition risk 3- to 4-fold,6 and syphilis may more commonly cause neurological complications in those already living with HIV.3
Meanwhile, biomedical advances in HIV prevention, including treatment as prevention, pre-exposure prophylaxis (PrEP) and post-exposure prophylaxis (PEP) may be changing sexual mixing and condom use patterns, inadvertently contributing to increasing bacterial STI rates.7 Thus, there is an urgent need for novel and effective STI prevention strategies that will be acceptable to gbMSM.
The success of HIV prevention technologies such as HIV PrEP has prompted researchers to consider an analogous approach to STI prevention. A small number of studies have already shown potential effectiveness of STI PrEP and PEP.8–10 In 2015, a pilot study of daily doxycycline as syphilis PrEP in HIV-positive gbMSM in Los Angeles demonstrated a decrease in bacterial STIs compared with a contingency management intervention.9,11 Another randomized trial assessed doxycycline as STI PEP versus no intervention in 232 HIV-negative gbMSM and found that PEP significantly reduced the incidence of a first STI overall (hazard ratio [HR], 0.53; 95% confidence interval [CI], 0.33–0.85), with similar results also seen for a first episode of chlamydia (HR, 0.30; 95% CI, 0.13–0.70) and syphilis (HR, 0.27; 95% CI, 0.07–0.98).10 However, these strategies are not without risk of side effects and may lead to antimicrobial resistance, leading many to express reservations about their appropriateness.11 Thus, it is important to assess current knowledge and opinions, as well as the acceptability of these emerging STI prevention techniques to predict potential uptake.
MATERIALS AND METHODS
Study Design and Objectives
The primary objective of this cross-sectional study was to determine the proportion of at-risk Toronto and Vancouver gbMSM who would be willing to use 2 hypothetical forms of STI prevention: doxycycline-based syphilis PrEP and PEP.
Secondary objectives were: (i) to determine the proportion of at-risk gbMSM willing to use HIV PrEP and HIV PEP); (ii) to understand knowledge and opinions regarding emerging STI prevention strategies; and (iii) to identify characteristics associated with willingness to use these prevention strategies.
Participant Recruitment and Data Collection
We recruited consecutive, consenting gbMSM from community-based sexual health clinics in Toronto (1 site) and Vancouver (2 sites) during routine visits for sexual health services. Eligible participants had to be self-identified gbMSM of any age with sufficient English language proficiency. Data were collected from June to August of 2018 using a self-administered, anonymous questionnaire. The survey was preceded by a short letter of information, and participants who completed the survey were deemed to have provided implied consent. The questionnaire was administered via paper or electronic tablet, with a trained research assistant present to answer questions. Participants were compensated with a $10 CAD gift card.
Survey Instrument
The survey instrument was developed by team members with expertise in HIV and STI prevention and treatment. The questionnaire was pilot-tested for clarity and face validity in a sample of 34 eligible individuals recruited from an HIV treatment/prevention clinic at an academic hospital in Toronto. Adjustments were made based on this feedback, resulting in a final questionnaire with 54 items divided into 6 domains.
The first domain included questions regarding demographics and engagement in health services, while the second addressed HIV/STI status and testing history. Third, a sexual behavior domain incorporated the HIV Incidence Risk Index for men who have sex with men (HIRI-MSM), a validated tool for quantifying HIV risk in gbMSM.12 The fourth domain addressed knowledge and opinions related to HIV/STI risk and prevention strategies. The fifth section incorporated validated psychometric scales to screen for depression (Patient Health Questionnaire-2 score ≥4),13 problem alcohol use (Alcohol Use Disorders Identification Test-C score ≥4),14 and problem substance use (Drug Use Disorders Identification Test score ≥25)15; these cutpoints are associated with sensitivities and specificities of 0.62 and 0.95,13 0.86 and 0.72,14 and 0.90 and 0.88,15 respectively. We included these scales because prior literature has demonstrated mental health and substance use syndemics to be associated with an increased risk of HIV acquisition among gbMSM.16 The final domain included a Discrete Choice Experiment assessing participant preferences for sexual health services; results of this component will be reported separately.
Data Analysis
Characteristics of the study population were summarized using descriptive statistics. To achieve the primary objective, we calculated the proportion of participants responding “probably yes” or “definitely yes” to questionnaire items asking about their willingness to use hypothetical syphilis PrEP or syphilis PEP. The PrEP question was phrased as follows: “Syphilis PrEP would involve taking 1 antibiotic pill every day to prevent syphilis, and potentially chlamydia. It might cause side effects in some people such as mild stomach upset, and might increase the chance that future infections of all kinds could be harder to treat because of drug resistance (not just STIs). It would involve doing blood work and seeing a doctor every 3 months. If this strategy were approved in Canada and available to you, would you take it?” The question about PEP was identical, except for its definition: “Syphilis PEP would involve taking 2 antibiotic pills within 24 hours after sex.” We used a similar approach to assess willingness to use HIV PrEP and PEP.
To determine whether participant characteristics were associated with preferences for various STI prevention strategies, we built separate multivariable logistic regression models for willingness to use syphilis PrEP, syphilis PEP, HIV PrEP, and HIV PEP, using the total number of male sex partners within the last 6 months as the primary predictor variable. This choice of primary predictor variable was based on clinical judgment. All pertinent demographic, clinical, behavioral, opinion, and syndemic variables were considered for inclusion as covariates. The recruitment location variable was forced into all models to account for potential differences between Toronto and Vancouver participants. Variables were eligible for inclusion in the final model if they changed the primary predictor's beta-coefficient by at least 10% in adjusted versus unadjusted models and based on clinical judgment.17 To facilitate the interpretation of findings, we sought to include the same variables in the final PrEP and PEP models for each of syphilis or HIV. Finally, we used descriptive statistics to characterize knowledge and opinions regarding emerging STI prevention strategies. Missing data were excluded. Participants living with HIV were also excluded from both HIV models. All data analysis was conducted using R version 3.4.1.
Sample Size Considerations
The target sample size was 400 in total, based on the number needed to ascertain the proportion of gbMSM willing to use syphilis PrEP and/or PEP with reasonable precision, defined here as a 95% confidence interval of ±5%.18 Because an estimate for this proportion is lacking, we assumed P = 0.5, which generates the highest possible required sample size.
Ethical Approval
The Research Ethics Boards of St. Michael's Hospital (REB 18-108) and The University of British Columbia (REB H18-01579) granted approval for the study at the Toronto and Vancouver locations, respectively.
RESULTS
Overall, 436 participants were recruited from community-based sexual health clinics in Toronto (n = 242) and Vancouver (n = 194). A refusal log was kept at the Toronto site only, showing that 20 additional individuals were approached but declined to participate; these individuals had similar age and gender identity compared with included participants (data not shown). Of the 436 men who completed the survey, 12 were subsequently excluded because they did not meet inclusion criteria (n = 9) or because primary outcome data were missing (n = 3), yielding a final sample of 424 participants.
Participants' median (interquartile range [IQR]) age was 31.0 years (26.0–39.0 years) (Table 1). Almost all participants self-identified as male (99.8%) and gay (87.9%), with only 1 identifying as transgender male. The majority were white (54.4%) or Asian (27.8%). Education level varied considerably between sites, with 67.2% and 16.1% of those recruited in Vancouver and Toronto reporting a high school diploma or less as their highest education, respectively. The median (IQR) number of male sex partners in the last 6 months was 6.0 (3.0, 13.0). Most (56.4%) participants reported ever having been diagnosed with 1 or more of chlamydia, gonorrhea or syphilis, and 28 (6.6%) participants reported living with HIV. Among HIV-negative participants, the mean HIRI-MSM score was 17.8, with 83.2% scoring of 10 or greater, the cutoff recommended by the scale's developers for identifying gbMSM at elevated HIV risk.12 Although only 9.0% and 5.9% screened positive for depression or problem drug use, respectively, 64.2% met screening criteria for problem alcohol use.
TABLE 1.
Characteristics of Participants Recruited at Community Sexual Health Clinics in Toronto and Vancouver, 2018
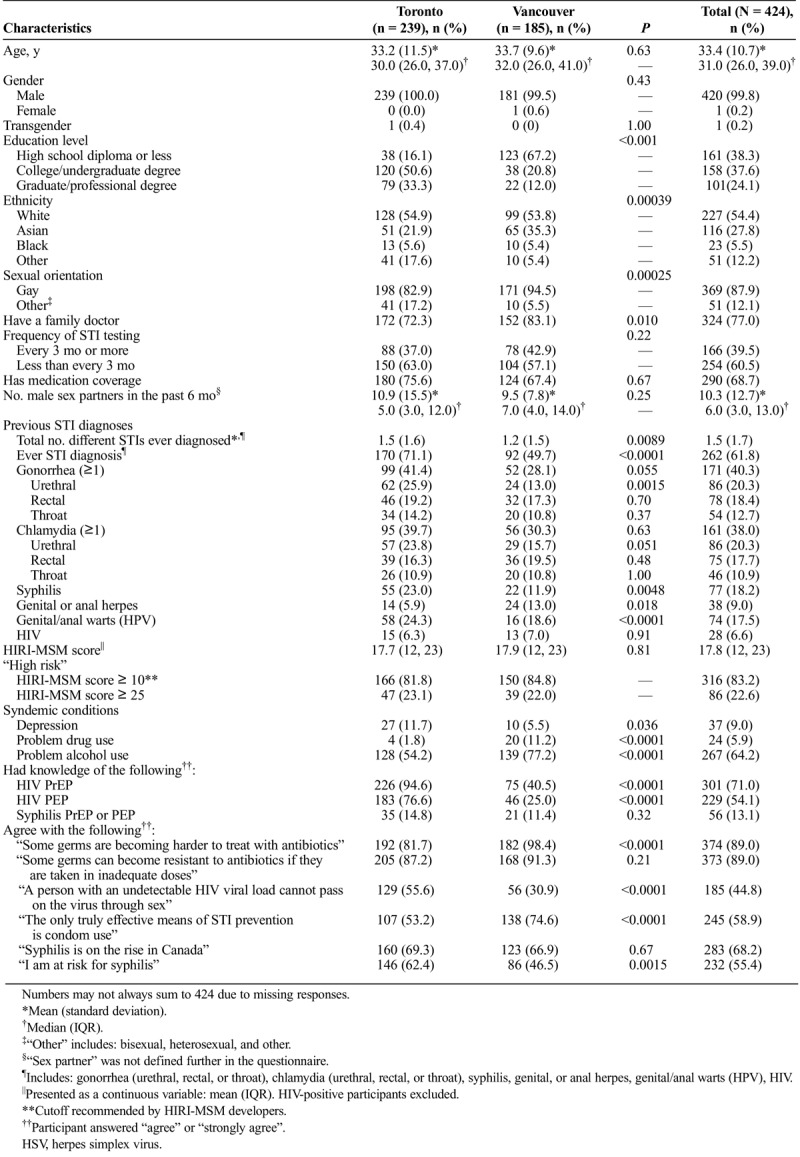
Willingness to Use Various Forms of STI Prevention
Although the majority of participants (60.1%) indicated willingness to use syphilis PEP in the future, only 44.1% of participants responded similarly for syphilis PrEP (Fig. 1). Moreover, 40.8% of participants were willing to use both syphilis prevention techniques, and 36.6% were unwilling to use either (kappa statistic = 0.56). Most participants were willing to use HIV PrEP and PEP (74.0% and 75.2% of the n = 396 HIV-negative participants that responded to each question respectively; Fig. 1).
Figure 1.
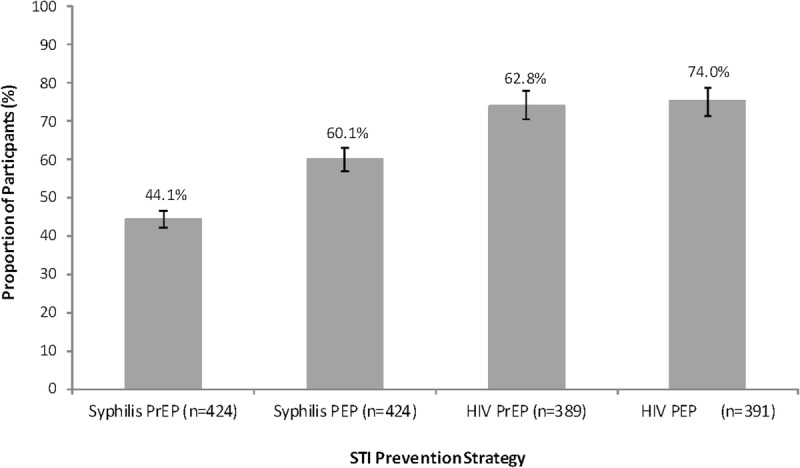
Willingness to use (% participants responding “probably yes” or “definitely yes”) various STI prevention strategies with associated 95% CIs.
Knowledge and Opinions Regarding Emerging STI Prevention Strategies
Few participants had ever heard of antibiotic-based prophylaxis prior to completing the questionnaire (13.1%). However, most were familiar with the concept of antimicrobial resistance, particularly that some bacteria are becoming harder to treat with antibiotics (89.0%) and can acquire resistance via antibiotic misuse (89.0%). The belief that condom use is “the only truly effective means of STI prevention” was common (58.9%) but not unanimous. In contrast, only 44.8% of participants had up-to-date knowledge about the “undetectable equals untransmittable” concept and agreed with the statement that “a person with an undetectable HIV viral load cannot pass on the virus through sex.” Most (68.2%) agreed that syphilis is on the rise in Canada and 55.4% of participants felt that they were at risk for syphilis. Although there were some differences in knowledge between Toronto and Vancouver, no clear pattern was discernible.
Characteristics Associated With Willingness to Use STI Prevention Strategies
Significant predictors for willingness to use syphilis and HIV PrEP/PEP varied (Tables 2 and 3). In multivariable analyses, willingness to use syphilis PrEP was significantly associated with being “very concerned” about STI acquisition (adjusted odds ratio [aOR], 1.9; 95% CI, 1.1–3.4), having ever used HIV PrEP (aOR, 2.2; 95% CI, 1.1–4.3), and although not statistically significant, it appeared to be associated with a higher number of different STIs previously diagnosed (aOR, 1.1; 95% CI, 0.98–1.3) and agreement with the statement “I am at risk for syphilis” (aOR, 1.6; 95% CI, 1.0–2.5). Interestingly, variables that are more traditionally related to syphilis risk, such as the total number of sex partners and a prior diagnosis of syphilis were not significantly associated with willingness to use syphilis PrEP in this analysis. In fact, the total number of sex partners, our primary predictor of interest, was not associated with interest in either syphilis PrEP or PEP. Willingness to use syphilis PEP was only significantly associated with a higher number of previously diagnosed STIs (aOR, 1.4; 95% CI, 1.2–1.7) and being in the Toronto rather than Vancouver sample (aOR, 2.0; 95% CI, 1.2–3.4).
TABLE 2.
Characteristics Associated With Willingness to Use Syphilis PrEP and Syphilis PEP
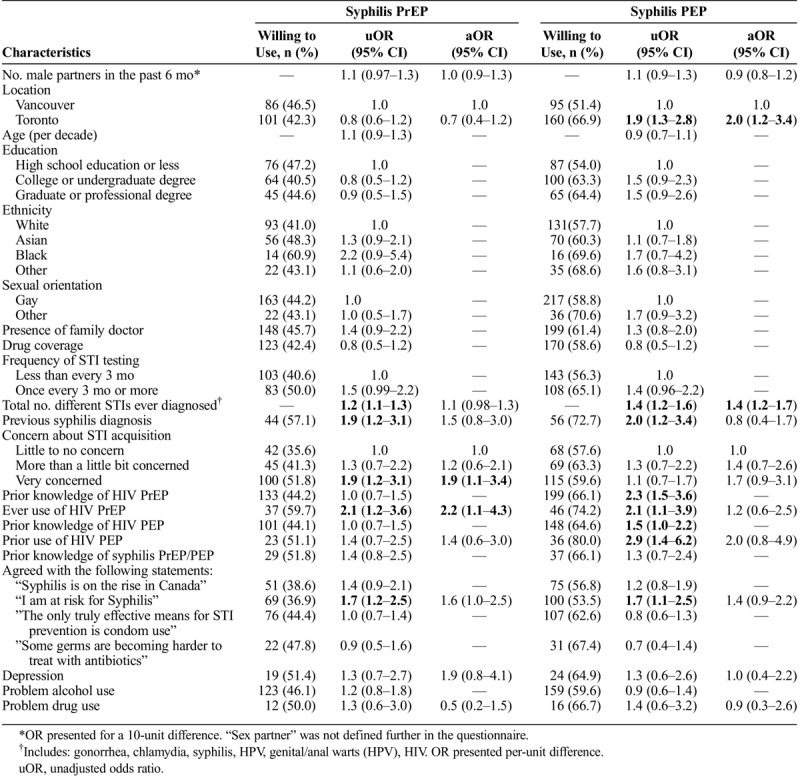
TABLE 3.
Characteristics Associated With Willingness to Use HIV PrEP and HIV PEP
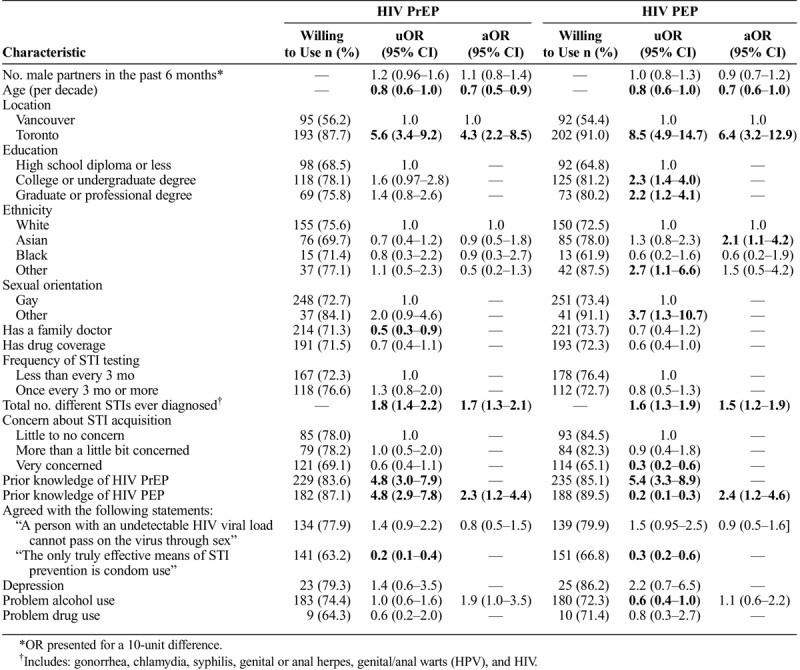
Several variables were significantly associated with willingness to use both HIV PrEP and PEP in multivariable models. Willingness decreased with age (PrEP aOR, 0.7; 95% CI, 0.5–0.9 and PEP aOR, 0.7; 95% CI, 0.6–1.0), increased with the number of different STIs previously diagnosed (PrEP aOR, 1.7; 95% CI, 1.3–2.1 and PEP aOR, 1.5; 95% CI, 1.2–1.9), was higher in Toronto than Vancouver (PrEP aOR, 4.3a; 95% CI, 2.2–8.5 and PEP aOR, 6.4; 95% CI, 3.2–12.9) and was higher in those with prior knowledge of HIV PEP (PrEP aOR, 2.3; 95% CI, 1.2–4.4 and PEP aOR, 2.4; 95% CI, 1.2–4.6). In addition, problem alcohol use appeared to be associated with willingness to use HIV PrEP but did not quite reach statistical significance (aOR, 1.9; 95% CI, 1.0–3.5), whereas Asian ethnicity (aOR = 2.1 compared to White participants; 95%CI = 1.1,4.2) was significantly associated with willingness to use HIV PEP.
DISCUSSION
This multicity study of at-risk Canadian gbMSM found that most participants (60.1%) were willing to use doxycycline-based PEP to prevent syphilis acquisition after a high-risk exposure, demonstrating fairly high acceptability of this STI prevention strategy within the population of interest. Willingness to use doxycycline-based PrEP was lower, at 44.1%. Because the definitions of doxycycline PEP and PrEP provided to participants only differed in dosing frequency (“1 antibiotic pill every day” versus “2 antibiotic pills within 24 hours after sex”), this discrepancy is likely explained by a preference for taking fewer pills. Other studies are consistent with this trend, with 52.7% of 2095 gay male survey participants in Australia willing to use syphilis PrEP,19 compared with 84% of 1301 gbMSM survey respondents in the United States willing to try syphilis PEP in the future.20
In our study, willingness appeared to be associated both with participants' subjective assessments regarding the chance of acquiring STIs, such as feeling at risk for syphilis and being concerned about acquiring it, and with traditional epidemiologic risk factors, such as the total number of different STIs previously diagnosed. Of interest, however, the magnitude of association appeared to be stronger for the subjective than the objective assessments of risk; in fact, neither the total number of sex partners in the past 6 months nor a prior history of syphilis was associated with willingness to use syphilis PrEP or PEP in multivariable models. Given that many prior studies have shown that patients commonly underestimate their own health risks,21,22 these findings underscore the need for interventions to aid individuals in accurately understanding these probabilities.
It is also noteworthy that prior use of HIV PrEP was the variable most strongly associated with willingness to use syphilis PrEP in our multivariable model. This finding is encouraging, given the high incidence of STIs in HIV PrEP users,23 and ongoing concern about the potential for HIV PrEP to contribute to these epidemics. Members of our team are currently conducting an exploratory trial of combined syphilis and HIV PrEP using both daily doxycycline and tenofovir disoproxil fumarate/emtricitabine, with final results expected in summer/fall 2020.24
Participants' willingness to use HIV PrEP and PEP were considerably higher than for syphilis PrEP and PEP, at 74.0% and 75.2% respectively. These figures are similar to that of a prior study in Vancouver, which estimated interest in HIV PrEP to be 81% among MSM,25 and suggest an increase in acceptability among at-risk MSM in Toronto, which has been previously estimated at 66.1%,26 54.9%,27 and 56.5%.28 This increase is not surprising, given the efforts to promote PrEP use in recent years. For instance, both Toronto and Vancouver have active community-based organizations that vocally support broader HIV PrEP and PEP rollout. Further, partial public funding for PrEP medication has been in place in Ontario since September 2017, whereas universal PrEP access for those meeting high-risk criteria has been available in British Columbia since January 2018. Work from our team has shown a significant positive association between the availability of public funding and population-level PrEP uptake.29 Together, these factors may suggest an increasingly favorable environment for the potential use of syphilis PrEP and PEP within the target population. Indeed, being a safe and affordable drug used in the treatment of many common infections, doxycycline is already publicly covered in both Ontario and British Columbia.
Interestingly, although 89% of participants were aware of the concept of antimicrobial resistance, this knowledge was not associated with doxycycline PrEP and PEP acceptability. However, the potential for long-term doxycycline use to induce resistance in both normal host flora and in STI pathogens remains a major concern among clinical and public health stakeholders. Indeed, just months after the positive results of the IPERGAY substudy on doxycycline-based PEP were presented, Public Health England and the British Association for Sexual Health and HIV released a joint policy statement warning that the potential benefit of doxycycline PEP for STIs would be outweighed by the risk of antimicrobial resistance.30
To date, neither of the published studies on doxycycline-based STI PrEP or PEP have formally evaluated the emergence of tetracycline class resistance in host normal flora, and the limited available data from Neisseria gonorrhoeae and C. trachomatis isolates in the doxycycline PEP trial has suggested no increase in resistance or resistance genes in the treated group. Data on these outcomes from planned and ongoing trials are thus eagerly anticipated.
Strengths of this study include our recruitment of participants from popular urban sexual health clinics, whose clientele likely reflect the sexually active gbMSM population to which future STI prevention efforts will be targeted, and our use of validated scales to assess HIV risk and syndemic conditions. Limitations include the potential for recall bias, particularly regarding questions about behaviors 6 months before participation (although the direction of such bias is unclear). The low number of HIV-positive participants precluded subgroup analyses by HIV status. In addition, data on willingness to use syphilis PrEP/PEP may not be an accurate indicator of future behavior. Further, because we did not specify the efficacy of syphilis PrEP/PEP in the wording of our questions, results may have been biased toward increased willingness if participants assumed these interventions would confer complete protection. Also, our descriptions of syphilis PrEP/PEP assumed quarterly bloodwork/clinic visits, mimicking the schedules being used in clinical trials and the recommended STI screening frequency for sexually active gbMSM according to Canadian guidelines, and thus findings may not reflect willingness to use these interventions under a different follow-up schedule. In addition, questionnaire items assessing awareness of antimicrobial resistance also did not distinguish between different organisms and their varying levels of resistance. Lastly, the demographic profile of the Vancouver and Toronto participants differed for several variables, including education, ethnicity, syndemic conditions, having a family doctor and previous STI diagnoses. Although the reasons for these differences are unclear since recruitment sites service similar populations, 1 potential reason could be participants in Vancouver were recruited only during daytime hours, while Toronto sites included evening hours as well.
To our knowledge, this is the first study to assess the acceptability of syphilis PrEP and PEP in Canada. Overall, we observed that a substantial proportion of at-risk gbMSM in Toronto and Vancouver would be willing to use it if available and efficacious. Although preliminary studies have indicated the effectiveness of syphilis chemoprophylaxis, larger clinical trials are underway to substantiate these results and are eagerly awaited.
Footnotes
Conflicts of Interest and Source of Funding: DHST reports grants from Canadian Institutes of Health Research and Ontario HIV Treatment Network during the conduct of the study and grants from Gilead Sciences Inc. and Viiv Healthcare outside the submitted work. D.H.S.T. is also a site investigator of a clinical trial sponsored by Glaxo Smith Kline outside the submitted work. M.H. reports honoraria for speaking engagements and advisory boards from Gilead and Merck, and honoraria for advisory boards from Viiv, outside the submitted work and paid to his institution. All remaining authors have nothing to disclose.
REFERENCES
- 1.Public Health Agency of Canada. Reported cases from 1991 to 2017 in Canada—Notifiable diseases on-line. Available at: https://diseases.canada.ca/notifiable/charts?c=yl. Accessed December 4, 2019.
- 2.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012; 380:2197–2223. [DOI] [PubMed] [Google Scholar]
- 3.Public Halth Agency of Canada. Report on sexually transmitted infections in Canada: 2013–2014. Available at: https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/diseases-conditions/report-sexually-transmitted-infections-canada-2013-14/report-sexually-transmitted-infections-canada-2013-14-eng.pdf. Accessed December 13, 2018.
- 4.Hottes TS, Lester RT, Hoang LM, et al. Cephalosporin and azithromycin susceptibility in Neisseria gonorrhoeae isolates by site of infection, British Columbia, 2006 to 2011. Sex Transm Dis 2013; 40:46–51. [DOI] [PubMed] [Google Scholar]
- 5.Kong FY, Hocking JS. Treatment challenges for urogenital and anorectal Chlamydia trachomatis. BMC Infect Dis 2015; 15:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen MS. Sexually transmitted diseases enhance HIV transmission: No longer a hypothesis. Lancet 1998; 351(suppl 3):5–7. [DOI] [PubMed] [Google Scholar]
- 7.Freeborn K, Portillo CJ. Does pre-exposure prophylaxis for HIV prevention in men who have sex with men change risk behaviour? A systematic review. J Clin Nurs 2018; 27(17–18):3254–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaul R, Kimani J, Nagelkerke NJ, et al. Monthly antibiotic chemoprophylaxis and incidence of sexually transmitted infections and HIV-1 infection in Kenyan sex workers: A randomized controlled trial. JAMA 2004; 291:2555–2562. [DOI] [PubMed] [Google Scholar]
- 9.Bolan RK, Beymer MR, Weiss RE, et al. Doxycycline prophylaxis to reduce incident syphilis among HIV-infected men who have sex with men who continue to engage in high-risk sex: A randomized, controlled pilot study. Sex Transm Dis 2015; 42:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molina JM, Charreau I, Chidiac C, et al. Post-exposure prophylaxis with doxycycline to prevent sexually transmitted infections in men who have sex with men: An open-label randomised substudy of the ANRS IPERGAY trial. Lancet Infect Dis 2018; 18:308–317. [DOI] [PubMed] [Google Scholar]
- 11.Golden MR, Handsfield HH. Pre-exposure prophylaxis to prevent bacterial sexually transmitted infections in men who have sex with men. Sex Transm Dis 2015; 42:104–106. [DOI] [PubMed] [Google Scholar]
- 12.Smith DK, Pals SL, Herbst JH, et al. Development of a clinical screening index predictive of incident HIV infection among men who have sex with men in the United States. J Acquir Immune Defic Syndr 2012; 60:421–427. [DOI] [PubMed] [Google Scholar]
- 13.Kroenke K, Spitzer RL, Williams JB. The patient health questionnaire-2: Validity of a two-item depression screener. Med Care 2003; 41:1284–1292. [DOI] [PubMed] [Google Scholar]
- 14.Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—II. Addiction 1993; 88:791–804. [DOI] [PubMed] [Google Scholar]
- 15.Berman AH, Bergman H, Palmstierna T, et al. The drug use disorders identification test manual. Available at: https://paihdelinkki.fi/sites/default/files/duditmanual.pdf. Accessed December 13, 2018.
- 16.Stall R, Mills TC, Williamson J, et al. Association of co-occurring psychosocial health problems and increased vulnerability to HIV/AIDS among urban men who have sex with men. Am J Public Health 2003; 93:939–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol 1993; 138:923–936. [DOI] [PubMed] [Google Scholar]
- 18.Daniel W. Biostatistics: A Foundation for Analysis in the Health Sciences. 7th ed New York, NY: John Wiley & Sons, 1999. [Google Scholar]
- 19.Wilson DP, Prestage GP, Gray RT, et al. Chemoprophylaxis is likely to be acceptable and could mitigate syphilis epidemics among populations of gay men. Sex Transm Dis 2011; 38:573–579. [DOI] [PubMed] [Google Scholar]
- 20.Spinelli MA, Scott H, Vittinghoff E, et al. High interest in doxycycline for sexually transmitted infection post-exposure prophylaxis (doxy-PEP) in a multi-city survey of men having sex with men (MSM) using a social-networking app. Presented at: Open Forum Infectious Diseases [S673]; 2018. [Google Scholar]
- 21.Clifton S, Mercer CH, Sonnenberg P, et al. STI risk perception in the British population and how it relates to sexual behaviour and STI healthcare use: Findings from a cross-sectional survey (Natsal-3). EClinicalMedicine 2018; 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallagher T, Link L, Ramos M, et al. Self-perception of HIV risk and candidacy for pre-exposure prophylaxis among men who have sex with men testing for HIV at commercial sex venues in New York City. Available at: https://www.theaidsinstitute.org/sites/default/files/attachments/HIV Risk, Self Perceptions, MSM, PrEP Candidacy_JLGBT Health_2014.pdf. Accessed June 5, 2019. [DOI] [PubMed]
- 23.Kojima N, Davey DJ, Klausner JD. Pre-exposure prophylaxis for HIV infection and new sexually transmitted infections among men who have sex with men. AIDS 2016; 30:2251–2252. [DOI] [PubMed] [Google Scholar]
- 24.Grant JS, Stafylis C, Celum C, et al. Doxycycline prophylaxis for bacterial sexually transmitted infections. Clin Infect Dis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lachowsky N, Lawson Tattersall T, Sereda P, et al. Community awareness of, use of and attitudes towards HIV pre-exposure prophylaxis (PrEP) among men who have sex with men in Vancouver, Canada: Preparing health promotion for a publicly funded PrEP program. Sex Health 2019; 16:180–186. [DOI] [PubMed] [Google Scholar]
- 26.Leonardi M, Lee E, Tan DH. Awareness of, usage of and willingness to use HIV pre-exposure prophylaxis among men in downtown Toronto, Canada. Int J STD AIDS 2011; 22:738–741. [DOI] [PubMed] [Google Scholar]
- 27.Kesler MA, Kaul R, Myers T, et al. Perceived HIV risk, actual sexual HIV risk and willingness to take pre-exposure prophylaxis among men who have sex with men in Toronto, Canada. AIDS Care 2016; 28:1378–1385. [DOI] [PubMed] [Google Scholar]
- 28.Rana J, Wilton J, Fowler S, et al. Trends in the awareness, acceptability, and usage of HIV pre-exposure prophylaxis among at-risk men who have sex with men in Toronto. Can J Public Health 2018; 109:342–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan D, Dashwood T, Wilton J, et al. PrEP uptake in Ontario remains far below guideline recommendations despite favourable policy changes. Presented at: 28th Annual Canadian Conference on HIV/AIDS Research Poster Presentation [EPHP107]. Saskatoon, 2019. [Google Scholar]
- 30.British Association for Sexual Health and HIV. Position statement on doxycycline as post-exposure prophylaxis for sexually transmitted infections. Available at: https://www.bashhguidelines.org/media/1156/doxy_pep_statement_v5_phe_bashh.pdf. Accessed June 5, 2019.


