Abstract
Background
Meibomian gland dysfunction (MGD) is the major cause of evaporative dry eye disease, which is the more prevalent form of dry eye disease. Intense pulsed light (IPL) therapy, involving treatment of the skin near the eyelids, has emerged as a potential treatment for MGD.
Objectives
To evaluate the effectiveness and safety of intense pulsed light (IPL) for the management dry eye disease resulting from meibomian gland dysfunction (MGD).
Search methods
We searched CENTRAL, MEDLINE (Ovid), Embase Ovid and three trial registers for eligible clinical trials on 1 August 2019. There were no restrictions on publication status, date or language.
Selection criteria
We included randomised controlled trials (RCTs) studying the effectiveness or safety of IPL for treating MGD.
Data collection and analysis
Our outcomes of interest were the change from baseline in subjective dry eye symptoms, adverse events, changes to lipid layer thickness, tear break‐up time (TBUT), tear osmolarity, eyelid irregularity, eyelid telangiectasia, meibomian gland orifice plugging, meibomian gland dropout, corneal sodium fluorescein staining and conjunctival lissamine green staining.
Two review authors independently screened abstracts and full‐text articles, extracted data from eligible RCTs and judged the risk of bias using the Cochrane tool. We reached consensus on any disagreements by discussion. We summarised the overall certainty of the evidence using the GRADE Working Group approach.
Main results
We included three RCTs, one from New Zealand, one from Japan and one from China, published between 2015 and 2019. Together, these trials enrolled 114 adults (228 eyes). Two studies used a paired‐eye (inter‐eye comparison) design to evaluate the effects of a sham (control) IPL treatment relative to an actual IPL treatment. One study randomised individuals to either an IPL intervention combined with meibomian gland expression (MGX), or MGX alone (standard therapy). The study follow‐up periods ranged from 45 days to nine months. None of the trials were at low risk of bias in all seven domains. The first authors of two included studies were in receipt of funding from patents or the manufacturers of IPL devices. The funding sources and declaration of interests were not given in the report of the third included trial.
All three trials evaluated the effect of IPL on dry eye symptoms, quantified using the Standard Patient Evaluation of Eye Dryness (SPEED) questionnaire. Pooling data from two trials that used a paired‐eye design, the summary estimate for these studies indicated little to no reduction in dry eye symptoms with IPL relative to a sham intervention (mean difference (MD) –0.33 units, 95% confidence interval (CI) –2.56 to 1.89; I² = 0%; 2 studies, 144 eyes). The other study was not pooled as it had a unit‐of‐analysis error, but reported a reduction in symptoms in favour of IPL (MD –4.60, 95% CI –6.72 to –2.48; 84 eyes). The body of evidence for this outcome was of very low certainty, so we are uncertain about the effect of IPL on dry eye symptoms.
There were no relevant combinable data for any of the other secondary outcomes, thus the effect of IPL on clinical parameters relevant to dry eye disease are currently unclear.
For sodium fluorescein TBUT, two studies indicated that there may be an improvement in favour of IPL (MD 2.02 seconds, 95% CI 0.87 to 3.17; MD 2.40 seconds, 95% CI 2.27 to 2.53; 172 eyes total; low‐certainty evidence).
We are uncertain of the effect of IPL on non‐invasive tear break‐up time (MD 5.51 seconds, 95% CI 0.79 to 10.23; MD 3.20, 95% CI 3.09 to 3.31 seconds; two studies; 140 eyes total; very low‐certainty evidence).
For tear osmolarity, one study indicated that there may be an improvement in favour of IPL (MD –7.00 mOsmol/L, 95% –12.97 to –1.03; 56 eyes; low‐certainty evidence).
We are uncertain of the effect of IPL on meibomian gland orifice plugging (MD –1.20 clinical units, 95% CI –1.24 to –1.16; 84 eyes; very low‐certainty evidence).
We are uncertain of the effect of IPL on corneal sodium fluorescein staining. One study reported no evidence of a difference between the IPL and sham intervention arms at three months of follow‐up (P = 0.409), and a second study reported data favouring IPL (MD –1.00 units, 95% CI –1.07 to –0.93 units; 172 eyes in total; very low‐certainty evidence).
We considered the incidence of adverse events at the study endpoint, as a measure of safety. As most trials did not specifically report adverse events, the safety of IPL as a treatment for MGD could also not be determined with any certainty. Very low‐certainty results from individual studies suggest some adverse effects that may be experienced by participants, include mild pain and burning, and the potential for partially losing eyelashes (due to clinician error).
Authors' conclusions
This systematic review finds a scarcity of RCT evidence relating to the effectiveness and safety of IPL as a treatment for MGD. Whether IPL is of value for modifying the symptoms or signs of evaporative dry eye disease is currently uncertain. Due to a lack of comprehensive reporting of adverse events, the safety profile of IPL in this patient population is also unclear. The current limitations in the evidence base should be considered by clinicians using this intervention to treat MGD, and outlined to individuals potentially undergoing this procedure with the intent of treating dry eye disease.
The results of the 14 RCTs currently in progress will be of major importance for establishing a more definitive answer regarding the effectiveness and safety of IPL for treating MGD. We intend to update this review when results from these trials become available.
Plain language summary
Intense‐pulsed light (IPL) therapy for the treatment of meibomian gland dysfunction
Background: dry eye is an eye condition that can cause eye soreness or irritation and changes to vision. One of the main causes of dry eye is known as 'meibomian gland dysfunction' (MGD), which causes problems in the meibomian glands (glands located in the eyelids). These glands produce an oily substance (known as meibum). Meibum is important for keeping the tears and surface of the eye healthy. In MGD, the meibomian glands become blocked and the meibum is abnormal. Intense pulsed light (IPL) therapy is a light treatment applied to the skin near the bottom eyelids. IPL therapy has been suggested as a treatment for MGD.
Aim of the review: to summarise research on the use of IPL for treating MGD. We were interested in whether the treatment improved dry eye symptoms. We considered whether there were any side effects from IPL. We were also interested in several clinical tests, such as corneal sodium fluorescein staining (a test that uses orange dye (fluorescein) to detect damage to the surface of the eye). These tests give us information about whether the treatment improves the working of the meibomian glands.
Study characteristics: we searched for studies that had been published up to 1 August 2019. We identified three randomised controlled trials (RCTs; clinical studies where people are randomly put into one of two or more treatment groups) involving 114 adults (228 eyes) from three countries (New Zealand, Japan and China) that had been published between 2015 and 2019. The maximum time that people in the studies were followed up for after the treatment was nine months.
Key findings: because of very low‐quality evidence, we are unclear about the effect of IPL on dry eye symptoms. IPL may be helpful to improve some of the clinical signs of MGD (such as tear stability and tear composition ‐ both signs of how healthy the tears produced by the eye are). We are uncertain about the effect of IPL on meibomian gland blockage or corneal sodium fluorescein staining.
As most studies did not report side effects, we are uncertain about the safety of IPL as a treatment for MGD. Very low‐quality results from individual studies suggest there may be some side effects, including mild eye pain and burning, and partially losing eyelashes (due to mistakes when using the IPL device).
Quality of the evidence: the evidence for how effective and safe IPL is for treating MGD was of low or very low quality.
Conclusions: due to limited information in the clinical trials, we could not determine with certainty whether IPL treatment for MGD is effective or safe. The review findings indicate that more research is needed. It is important that eye care clinicians, and people considering having IPL as a dry eye treatment, are aware that there is limited high‐quality research to understand whether the procedure is effective or safe.
Summary of findings
Summary of findings for the main comparison. IPL (with/without MGX) compared to sham or no treatment (with/without MGX) for the treatment of meibomian gland dysfunction.
| IPL (± MGX) compared to sham or no treatment (± MGX) for the treatment of meibomian gland dysfunction | ||||||
|
Patient or population: people with meibomian gland dysfunction Setting: eye care clinic or hospital Intervention: IPL treatment (± MGX)a Comparison: sham (control) or no treatment (± MGX)a | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of eyes (studies) | Certainty of the evidence | Comments | |
| Assumed risk with sham treatment | Corresponding risk with IPL treatment | |||||
| Dry eye symptoms, measured using the SPEED score at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months) | 2 studies reported no evidence of a difference (Craig 2015: MD 0.00 SPEED units, 95% CI –3.67 to 3.67; Rong 2017: MD –0.53 SPEED units, 95% CI –3.33 to 2.27), and one study reported a reduction in dry eye symptoms, in favour of the IPL intervention (Arita 2019: MD –4.60, 95% CI –6.72 to –2.48). | 228 (3 studies) | ⊕⊝⊝⊝ Very lowb,c,d | Meta‐analysis was not performed due to substantial statistical heterogeneity. | ||
| Incidence of adverse events at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months) | Rong 2017 suggested there were some adverse effects, with 5 participants feeling mild pain and burning, and 1 participant experiencing an event that led to them partially missing their eyelashes "following mistakes from the doctors during treatment." However, no further details were provided. Arita 2019 reported that 3 participants in the MGX (control) group withdrew from the study because of pain experienced during the procedure. | — | ⊕⊝⊝⊝ Very lowc,e | Craig 2015 did not specifically report on adverse events. | ||
| Sodium fluorescein TBUT, measured in seconds, at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months) | Both studies reported an improvement in sodium fluorescein TBUT, in favour of the IPL intervention (Rong 2017: MD 2.02 seconds, 95% CI 0.87 to 3.17; 88 eyes; Arita 2019: MD 2.40 seconds, 95% CI 1.52 to 3.28, 84 eyes). | 172 (2 studies) | ⊕⊕⊝⊝ Lowb,c | Meta‐analysis was not performed due to a unit‐of‐analysis error in (Arita 2019). | ||
| NIBUT, measured in seconds, at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months) | Both studies reported a relative improvement in NIBUT, in favour of the IPL intervention (Craig 2015: MD 5.50 seconds, 95% CI 0.77 to 10.23; 56 eyes; Arita 2019: MD 3.20 seconds, 95% CI 3.09 to 3.31; 84 eyes). | 140 (2 studies) | ⊕⊝⊝⊝ Very lowb,f | Meta‐analysis was not performed due to a unit‐of‐analysis error in (Arita 2019). | ||
| Tear osmolarity, measured in mOsmol/L, at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months) | 1 study found a relative improvement in tear osmolarity, in favour of the IPL intervention (Craig 2015: MD –7.00 mOsmol/L, 95% CI –12.97 to –1.30). | 56 (1 study) | ⊕⊕⊝⊝ Lowg | — | ||
| Meibomian gland orifice plugging, at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months) | 1 study reported an inter‐group difference in extent of meibomian gland orifice plugging in favour of the IPL intervention (Arita 2019: MD –1.20 units, 95% CI –1.24 to –1.16, on a clinical scale from 0 to 3). | 84 (1 study) | ⊕⊝⊝⊝ Very lowb,h | This study used data from both eyes as independent samples (without appropriate within‐person correlation). | ||
| Corneal sodium fluorescein staining, measured using a validated clinical scale, at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months) | Rong 2017 reported no significant difference between intervention arms at 3 months of follow‐up (P = 0.409). Arita 2019 reported data favouring the IPL intervention arm (MD –1.00 units, 95% CI –1.07 to –0.93, on a scale from 0 to 9). | 172 (2 studies) | ⊕⊝⊝⊝ Very lowb,c,h | — | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IPL: intense pulsed light; MD: mean difference; MGX: meibomian gland expression; NIBUT: non‐invasive tear break‐up time; RR: risk ratio; SPEED: Standard Patient Evaluation of Eye Dryness; TBUT: tear break‐up time. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aTo be eligible for inclusion, MGX had to be applied in both the intervention and comparator arms. bDowngraded one level for risk of bias, due to absence of participant or outcome assessor masking in Arita 2019. cDowngraded one level for imprecision, as data were derived from studies of relatively small sample size and all studies had units of analysis errors. dDowngraded one level for inconsistency, as there was heterogeneity in the effect among trials with different units of analysis. eDowngraded two levels for risk of bias, due to lack of participant or outcome assessor masking in Arita 2019, and incomplete reporting of adverse outcomes in all studies. fDowngraded two levels for imprecision, as data were derived from two studies of small sample size, with wide confidence intervals and unit‐of‐analysis errors. gDowngraded two levels for imprecision, as data derived from one study with a unit‐of‐analysis error and a very small sample size. hDowngraded one level for inconsistency, as the two studies reported divergent effects.
Background
Description of the condition
Dry eye disease affects approximately 350 million individuals globally, with an estimated prevalence of 5% to 50% worldwide depending upon the geographic region (Craig 2017; Stapleton 2017). This complex, yet ubiquitous, condition adversely affects tear film integrity, which has sequelae for the health of the ocular surface and quality of life (Friedman 2010). The human tear film has multiple functions, ranging from ocular lubrication to imparting optical clarity. In dry eye disease, tear film homeostasis is disrupted, leading to compositional abnormalities, including tear hyperosmolarity. Two main subtypes of dry eye disease are recognised: evaporative and aqueous‐deficient, with potential overlap in their presentation. Meibomian gland dysfunction (MGD), leading to an abnormality in the tear lipid layer, is the leading cause of evaporative dry eye disease (Geerling 2017; Tomlinson 2011), and is the focus of this review. MGD is highly prevalent in Asian populations (Siak 2012). Other ocular (e.g. contact lens wear) and systemic factors (e.g. hormonal status and nutrition) may also contribute to the development of MGD (Galor 2014).
The meibomian glands are located in the upper and lower eyelids and secrete meibum, an oily substance that spreads to form the outermost layer of the tear film and aids in tear stabilisation (Knop 2011; Nichols 2011). MGD is characterised by changes to the quality and quantity of secreted meibum or obstruction of the meibomian glands, or both; these changes lead to compromised tear lipid and increased tear evaporation. An increase in the concentration of specific proinflammatory mediators has also been reported in the tear film of individuals with evaporative dry eye disease (Jackson 2016). Three clinical subforms of MGD are recognised: hypersecretory, hyposecretory and obstructive, with the latter the most common (Knop 2011). Animal models of obstructive MGD suggest that a key pathophysiological event is hyperkeratinisation of the meibomian gland ducts (Foulks 2003; Knop 2011). Foulks 2003 describe the process to involve the epithelial lining of the ducts undergoing hypertrophy, which reduces the lumen size, as well as shedding of epithelial cells into the meibum. Duct orifices are then recognised to become plugged by keratinised cells, with high levels of keratin present in expressed meibum. Back‐logged meibum secretions then lead to cystic dilation of the ducts and acini, followed by meibomian gland dropout as the acini atrophy from disuse in chronic cases. Meibomian gland dropout is considered to be a permanent pathological change, which can also be associated with age‐related, non‐obstructive acinar atrophy (Bron 2017).
A range of different treatment options currently exists for MGD (reviewed in Geerling 2011 and Nichols 2011, and more recently in Jones 2017). Some of these options include artificial tears (e.g. lubricating eye drops with lipid‐containing components), MGX (which forces meibum release from the glands), and warm compresses and thermal pulsation therapy (which both aim to liquify the meibum within the meibomian glands). Omega‐3 fatty acid supplementation may also be useful as a treatment for evaporative dry eye disease (Downie 2019; Epitropoulos 2016; Korb 2015). The presence of dry eye symptoms is associated with a relatively thin tear lipid layer (Blackie 2009). Broadly, these treatments aim to restore the stability of the tear film by improving lipid layer thickness or quality, or both. It has been shown that many of these treatments only impart temporary effects on patients' symptoms (Jiang 2016), and act as supportive therapies that do not necessarily target the key aetiological factor(s) driving the underlying MGD.
Description of the intervention
Intense pulsed light (IPL) therapy has traditionally been used to treat dermatological conditions, in particular the vascular skin lesions that occur in rosacea (Goldberg 2012; Wat 2014). IPL uses a high‐output flash lamp, to produce a broad wavelength, non‐coherent light, typically in the range of 500 nm to 1200 nm. Specific regions of the skin are exposed to the light output for brief flashes through an interfacing gel, with the intent of inducing coagulation of the superficial blood vessels. IPL is non‐ablative (i.e. does not remove the superficial layers of the skin) but induces photothermolysis, whereby the thermal damage is limited to haemoglobin and melanin in the skin to avoid non‐specific thermal injury to surrounding anatomical structures (Anderson 1983).
The first IPL device obtained regulatory approval from the Food and Drug Administration (FDA) in the US in 1995, for treating lower extremity telangiectasias. Over the past 20 years, there has been rapid development and proliferation of the technology, with application in multiple fields of medicine. The potential application of IPL for treating dry eye disease was a serendipitous clinical discovery, whereby it was recognised that individuals treated with IPL for rosacea, which has an association with MGD (Viso 2012), appeared to have a concurrent reduction in their dry eye symptoms (Toyos 2015). This discovery has led to the commercial development and promotion of IPL devices that are specific for dry eye treatment. Currently, the two main devices are the M22 Optima device (Lumenis Ltd, US) and the E>Eye device (E‐Swin, France). For the treatment of MGD, IPL is applied to multiple (typically five or six) locations across the face, under the inferior eyelids, starting nasally and finishing temporally. Typically, a course of treatment is recommended, involving three or four IPL sessions over approximately four months.
An individual's suitability for IPL depends on their skin pigmentation level. The Fitzpatrick Skin Types classification (Fitzpatrick 1975), which provides a measure of the skin's tolerance to sunlight and its tendency to tan or burn, is commonly used to determine whether IPL may be an appropriate intervention option. There are six Fitzpatrick Skin Types, ranging from I (very fair skin, which always burns and never tans) through to VI (black skin, which tans easily). People with darker skin tones (types V to VI) are not good candidates for IPL, due to risks of inducing hypopigmentation and scarring. Moles or other pigmentation spots on the face should also be concealed. A range of other contraindications for IPL also exist, including certain autoimmune diseases, epilepsy, history of keloid scarring and the use of photosensitising medications. Eye shields, effective in attenuating transmission of the IPL wavelengths, must also be worn by the therapist and patient, to avoid potentially permanent eye injury.
How the intervention might work
The potential mechanism(s) of action of IPL in treating MGD remain(s) unclear. Several main theories exist, as follows:
Inducing thrombosis of telangiectatic blood vessels in the eyelids
Eyelid telangiectasia is a common sign in individuals with MGD (Schaumberg 2011). It has been suggested that IPL‐induced ablation of small vessels around the eyelid margins reduces local inflammation by decreasing the level of proinflammatory mediators reaching the eyelids and meibomian glands (Jiang 2016; Toyos 2015). A relatively hypoxic tissue environment has also been shown to be beneficial for meibomian gland function (Liu 2019).
Liquification of meibum
The temperature of the eyelids affects the physical properties of the meibum, which becomes increasingly more fluid with increasing temperature (Nagymihályi 2004). The temperature at which meibum changes from a semi‐solid to liquid state is known as the phase‐transition temperature. In individuals with MGD, the composition of lipids in the meibum is altered, resulting in a higher phase‐transition temperature compared with healthy meibomian gland secretions (Borchman 2011). Warming the eyelids, such as with warm compresses, is of value for promoting meibum warming and facilitating its expression. It has been suggested that IPL may warm the skin area adjacent to the meibomian glands, allowing for enhanced expression of blocked meibum (Dell 2017; Gupta 2016). However, this theory has been questioned by Craig 2015, who suggested that the effect of IPL on increasing skin temperature is modest and transient.
Reducing Demodex eyelid infestation
A potential contributor to the pathophysiology of MGD is the ectoparasite Demodex, which can reside in meibomian glands and consume meibum secretions (Liu 2010). Under physiological conditions, the number of Demodex mites is controlled to prevent so‐called 'infestation', which is a common feature in individuals with rosacea. Demodex infestation is typically accompanied by a heightened bacterial load (O'Reilly 2012), which can contribute to promoting a chronic pro‐inflammatory environment that adversely affects the eyelids, and subsequently the ocular surface.
It has been suggested that the exoskeleton of the Demodex mite may be vulnerable to IPL energy (Kirn 2002). Thus, IPL might contribute to treating MGD by reducing the Demodex load on the eyelids, to reduce the microbial load and thus reduce the ocular surface inflammation.
Promoting changes to meibomian gland architecture
One cohort study investigated the effects of IPL on the structure of dysfunctional meibomian glands. This study suggested that IPL could improve the microstructure and the macrostructure of the meibomian glands, as assessed using in vivo confocal microscopy (Yin 2018). These authors hypothesised that photomodulation of the glands stimulates cell activity and intracellular changes inside the glands, as well as decreasing inflammation surrounding them (Yin 2018).
Photomodulation
Photomodulation is a process whereby light induces intracellular changes at gene or protein (or both) levels. It has been suggested that IPL may stimulate mitochondria in the tarsal plate to increase adenosine triphosphate production, modify their output of reactive oxygen species and alter their transcription factors (Mejía 2019). These changes have been proposed to impart therapeutic effects on the meibomian gland acini.
Why it is important to do this review
IPL therapy has traditionally been used to treat dermatological conditions, in particular the vascular skin lesions that occur in rosacea. In recent years, this technology has been strongly marketed in multiple jurisdictions for the treatment of MGD. IPL is currently available in more than 50 countries globally, and is being offered by some eye care clinicians as a treatment for MGD, as an in‐office, multi‐visit course of clinical care. As an example of the rapid implementation of this technology into clinical practice, it is estimated that since 2014 more than 200 eye care practices have purchased IPL devices (about AUD $30,000 to AUD $s40,000) in Australia and New Zealand.
Despite this rapid clinical uptake, very few clinical trials have been conducted to evaluate IPL as a treatment for MGD; as such, there remains substantial clinical debate regarding whether IPL is efficacious and safe for treating MGD. The treatment has some risks, which include damage to the periocular skin (e.g. depigmentation, swelling, redness), hair or eyelash loss (or both), and permanent intraocular injury (e.g. iris transillumination). The treatment is also relatively costly for patients compared to conventional treatments for MGD (such as warm compresses and eyelid massage), often involving multiple visits that incur a charge of several hundred Australian dollars.
Recognising a need for evidence‐based guidance in relation to the use of IPL in eye care practice, primarily based upon safety concerns, the Canadian Agency for Drugs and Technologies in Health published a report, based upon a limited literature search, examining the clinical effectiveness of IPL for treating dry eye disease (Health Canada 2016). This report concluded that there was a paucity of high‐quality evidence to inform practice.
There is thus a strong clinical need for a systematic review to consider the current, best‐available research evidence relating to the effectiveness and safety of IPL as a treatment for MGD. Given the high prevalence of dry eye disease, we consider this topic to be of substantial relevance to clinicians, researchers and the wider community, and the findings will have substantial impact, at national and international levels. The undertaking of this review is also expected to identify important evidence gaps in the field, which will inform future research.
Objectives
To evaluate the effectiveness and safety of intense pulsed light (IPL) for the management dry eye disease resulting from meibomian gland dysfunction (MGD).
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs).
Types of participants
We included adults (i.e. aged 18 years or older) with MGD or evaporative dry eye disease, as defined by the study investigators.
Types of interventions
We included RCTs that compared IPL therapy applied to the facial area with the intent of treating MGD or evaporative dry eye disease, relative to standard therapy (e.g. warm compresses), placebo therapy (e.g. sham IPL) or no treatment. We excluded studies where participants were assigned with any other adjunctive treatments (e.g. MGX, artificial tears), unless this co‐intervention was administered in the same dose and frequency in the comparator group.
Types of outcome measures
Primary outcomes
Change from baseline in subjective dry eye symptoms (including dryness, foreign body sensation, burning, itching, sensitivity to light), as measured using a validated dry eye questionnaire (e.g. Standard Patient Evaluation of Eye Dryness (SPEED) or Ocular Surface Disease Index (OSDI)), at three months of follow‐up, with an acceptable follow‐up range of up to six months.
Secondary outcomes
Incidence of adverse events at the study endpoint was considered as the safety outcome.
We considered the following secondary outcomes, measured as the change from baseline at three months of follow‐up, with an acceptable follow‐up range of up to six months:
sodium fluorescein tear break‐up time (TBUT), measured in seconds;
non‐invasive tear break‐up time (NIBUT), measured in seconds;
tear osmolarity, measured in milliosmoles per litre;
lipid layer thickness, measured in nanometres, using tear film interferometry;
eyelid irregularity, measured using a validated slit lamp scale;
eyelid telangiectasia, measured using a validated slit lamp scale;
meibomian gland orifice plugging, measured using a validated slit lamp scale;
meibomian gland dropout (%), measured using meibography;
corneal sodium fluorescein staining, measured using a validated clinical scale;
conjunctival lissamine green staining, measured using a validated clinical scale.
Search methods for identification of studies
Electronic searches
We conducted systematic searches, without language or publication year restrictions, in the following databases:
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 7), which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched from inception to 1 August 2019; Appendix 1);
MEDLINE (Ovid) (search from 1946 to 1 August 2019; Appendix 2);
Embase Ovid (searched from 1980 to 1 August 2019; Appendix 3);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 1 August 2019; Appendix 4);
Australian and New Zealand Clinical Trials Registry (ANZCTR) (www.anzctr.org.au; searched 1 August 2019; Appendix 5);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 1 August 2019; Appendix 6).
We included studies regardless of their publication status.
Searching other resources
We undertook additional searching using the bibliographies of included RCTs to identify other potentially relevant studies. We did not handsearch conference abstracts for this review, as Cochrane Eyes and Vision routinely conducts handsearching for RCTs from major ophthalmology meetings and incorporates these results into the CENTRAL database.
Data collection and analysis
Selection of studies
Two review authors independently screened the titles and abstracts of the papers identified via the search strategies in Covidence. Based on the study inclusion and exclusion criteria, we classified the eligibility of each record as: yes (definitely include), no (definitely exclude), or maybe (eligibility unclear). For records where at least one review author categorised the eligibility to be yes or unclear, two review authors independently assessed the full‐text reports to classify each study as definitely include or definitely exclude. A third review author assisted with resolving any disagreements, if the two review authors were unable to reach consensus.
Data extraction and management
Two review authors independently extracted data, according to a standard data extraction form, for methodology, participants (including eligibility criteria), interventions and outcomes for each included study. For prespecified primary and secondary outcomes, we extracted all relevant quantitative data. When numeric data were not available, we presented the non‐numeric data. Any discrepancies in data extraction were resolved by discussion between the review authors. Data were exported into the Cochrane's statistical software, Review Manager 2014, by one review author and independently reviewed for accuracy by another review author.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias in the included studies according to the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 8; Higgins 2011a). We assessed the risk of bias in the following domains: selection bias (sequence generation and allocation concealment), performance and detection bias (masking (blinding) of participants, study personnel and outcome assessors), attrition bias (incomplete outcome data), reporting bias (selective outcome reporting) and other sources of bias. Risk of bias was graded as 'low risk', 'high risk' or 'unclear risk' for each included study. We contacted study authors when clarification was required. We used the information available within the full‐text when we were unable to contact, or failed to receive any response from, study authors after one month, or the study authors were unable to provide further information.
Measures of treatment effect
We undertook the data analyses according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 9; Deeks 2011). For continuous outcomes, we reported the mean difference (MD) between the control and intervention arms, with 95% confidence intervals (CIs).
Unit of analysis issues
The unit of analysis was the enrolled study eye of the participant. In two trials, the unit of analysis was the study eye, with individual eyes of participants randomised to either the control or intervention arm (Craig 2015; Rong 2017). However, the results presented in these papers did not appear to be derived from paired analyses, which would account for the correlation between eyes. This represents an analysis error, which limits our confidence in the reported inter‐group statistical differences.
Where the study included data for more than one eye per participant, we aimed to follow guidelines for clustering or paired‐eye design, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). There was one trial where this was the case (Arita 2019). Specifically, participants were randomly allocated to the intervention, but both eyes of each participant were included in the analysis as independent samples; this represents a 'unit‐of‐analysis' issue. As relevant information relating to the within‐person correlation was not provided in the study report and was not obtainable from the study authors, we were unable to include these data in the analyses.
Dealing with missing data
We attempted to contact study authors to clarify factors affecting the assessment of risk of bias or to obtain missing outcome data, or both. We used the information available within the full‐text whenever we were unable to contact, or failed to receive a response from, study authors after one month, or when the study authors could not provide further information. We did not impute data and relied on the data available within the study reports.
Assessment of heterogeneity
We examined clinical and methodological heterogeneity by examining the variability in the design, risk of bias, characteristics of participants, interventions and outcomes among included studies. We used the Chi² test and I² statistic to assess statistical heterogeneity among included studies. We interpreted an I² value greater than 60% to indicate substantial statistical heterogeneity.
Assessment of reporting biases
As there were fewer than 10 studies included in the meta‐analyses, we were unable to assess for potential publication biases or small‐study effects using a funnel plot. Selective outcome reporting was assessed as part of the risk of bias assessment for each included study.
Data synthesis
We undertook meta‐analyses for outcomes where the studies were considered similar (no heterogeneity) for their treatment, participants and intervention. We considered multiple potential sources of heterogeneity, including clinical (e.g. different aetiologies of dry eye disease), methodological (e.g. unit‐of‐analysis issues) and statistical (with a threshold of I² of 60% or less). We used a fixed‐effect model to combine the studies for analysis when there were fewer than three studies available. We presented a narrative summary of results when we did not undertake meta‐analyses due to substantial heterogeneity or insufficient reporting of data.
In the specific context of this review, where possible we pooled data from the studies in which the unit of analysis was the study eye (Craig 2015; Rong 2017), and have then separately reported the data from the study where both eyes of individuals were assigned to interventions and analysed as independent samples, resulting in a unit‐of‐analysis issue (Arita 2019).
Table 1 summarises the results of the analyses, using the approach described in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). The GRADE Working Group approach was adopted to grade the certainty of the evidence. Outcomes, measured between the intervention and control arms, include the change in each of: dry eye symptoms, TBUT, NITBUT, tear osmolarity, meibomian gland orifice plugging and corneal sodium fluorescein staining, as well as the incidence of adverse events with a probable link to the study intervention.
Subgroup analysis and investigation of heterogeneity
We were unable to conduct subgroup analyses due to the limited number of included studies. If there are more RCTs to evaluate in updates of this review, we will perform subgroup analyses to account for potential clinical differences in studies, such as: severity of disease, type of IPL technology and duration of treatment.
Sensitivity analysis
We did not perform a sensitivity analysis due to an insufficient number of included studies. For updates of this review, we will perform a sensitivity analysis to assess the impact of excluding studies with a high risk of bias, including lack of allocation concealment, lack of masking and a large proportion of participants lost to follow‐up (20% or more), industry funding, and unpublished studies when adequate data are available.
Results
Description of studies
Results of the search
The electronic searches yielded 221 records as of 1 August 2019 (Figure 1). After removal of duplicates, review authors independently screened 177 titles and abstracts for potential inclusion. We classified 37 reports as potentially eligible, and these articles proceeded to full‐text screening. We excluded 13 studies (see Characteristics of excluded studies table). The main reasons for exclusion were because the study was not a RCT (eight studies) or the study used an active (rather than inert) comparator (two studies).
1.
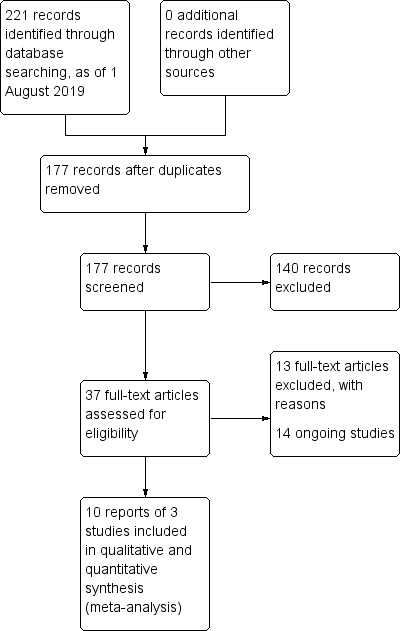
Study flow diagram.
We included 10 reports from three trials in the analyses (Arita 2019; Craig 2015; Rong 2017), and categorised 14 records as ongoing studies from clinical trial registries (see Characteristics of ongoing studies table). No studies were awaiting classification.
Included studies
A detailed description of the three trials included in this review is provided in the Characteristics of included studies table.
Studies
The included trials were conducted in Japan (Arita 2019), New Zealand (Craig 2015), and China (Rong 2017). All trials enrolled a relatively conservative number of participants, ranging from 28 to 44 people. Two studies used a paired‐eye (inter‐eye comparison) design to evaluate the effects of the control and intervention in the same participant (Craig 2015; Rong 2017). In these two studies, one eye randomly received IPL treatment and the fellow eye received the sham treatment. Arita 2019 randomised individuals to the control and intervention arms, and included data from both eyes in the analyses; this presented a unit‐of‐analysis issue.
The lead authors of two of the included studies made declarations of interest and were in receipt of funding from manufacturers of IPL devices (Arita 2019; Craig 2015). The lead author of Arita 2019 holds patents on IPL therapy, is a consultant for Kowa Company (Aichi, Japan) and Lumenis Japan (Tokyo, Japan) and has received financial support from TearScience (Morrisville, North Carolina, US). Craig 2015 declared France Medical, a manufacturer of IPL devices, as a funder of consumables in the trial, and the lead author declared the same company in their personal declarations of interest. The funding sources and declaration of interests were not given for Rong 2017.
Participants
The studies evaluated 228 eyes from 114 adults.
Overall, the mean age of participants across the three RCTs was approximately 50 years. In each of the three studies there were more women than men. Full details regarding the age and sex distribution of participants is provided in the Characteristics of included studies table.
Only one study explicitly reported the severity of dry eye disease (Craig 2015), which enrolled individuals with "mild to moderate clinical signs of MGD."
Interventions
All included trials adjusted the IPL light pulse intensity to the skin type of the participant according to the Fitzpatrick grading scale (Fitzpatrick 1975).
Arita 2019 administered IPL using the M22 system (Lumenis Inc., US), which was adjusted to the appropriate setting (ranging from 11 J/cm² to 14 J/cm²). Participants received about 13 light pulses (with slightly overlapping areas of application) from the left preauricular area, across the cheeks and nose, to the right preauricular area, with the treated area reaching up to the inferior boundary of the eye shields. The procedure was then repeated in a second pass. Participants in the intervention arm underwent eight IPL treatments at three‐week intervals.
All participants in both groups underwent a therapeutic MGX procedure on both the superior and inferior eyelids of each eye using an Arita Meibomian Gland Compressor (Katena, Japan) every three weeks. Eye drops containing 0.4% oxybuprocaine hydrochloride were administered prior to each procedure, to minimise pain.
After the eight MGX with or without IPL treatment sessions, all participants underwent three follow‐up examinations over the course of 11 weeks; each participant was involved in the study for 32 weeks in total.
Rong 2017 administered IPL treatment by delivering light pulses of 14 J/cm² to 16 J/cm² to the upper and lower eyelids using the M22 system (Lumenis Inc., US). The treatment eye received IPL to the skin areas around both the upper and lower eyelids, with monthly applications over a three‐month period. The light pulses were applied to six treatment areas of the skin, while the eyes were protected by goggles. The control eye received a sham IPL therapy, using the same device, with an energy of 0 J/cm². MGX was performed immediately after IPL treatment using an Arita tarsal gland massager, in both the control (sham) and IPL treatment arms; all participants also received polyethylene glycol (lubricant) eye drops (three times daily) and local ice‐pack treatment for five minutes after the IPL intervention (to reduce skin heat or redness, or both) as co‐interventions.
Craig 2015 used lower energy pulses, of 9 J/cm² to 13 J/cm², applied to the lower eyelid of the intervention eye using an E>Eye IPL system (E‐Swin, France). IPL was administered to the skin area immediately below the lower eyelid during three separate treatment sessions every two weeks, on study days 1, 15 and 45, as per manufacturer recommendations. Treatment was applied to four areas below the eyelid while the eyes were protected by opaque goggles. The control eye received pulses from the same IPL device with a light‐blocking filter at the tip, as a sham IPL treatment. There were no co‐interventions.
Outcomes
Only one study clearly specified primary and secondary outcomes (Rong 2017). Although the three included studies included similar outcomes, the investigators did not consistently follow the same procedures and reported measurements at different time points.
All three included studies measured subjective dry eye symptoms and quantified best‐corrected visual acuity. All measured subjective dry eye symptoms using the Standard Patient Evaluation of Eye Dryness (SPEED) questionnaire. This is a validated questionnaire that gives a score from 0 to 28 depending on the frequency and severity of the following symptoms: dryness, grittiness, scratchiness, irritation, burning, watering, soreness, and eye fatigue (Ngo 2013). Arita 2019 was the only study to quantify lipid layer thickness, degree of eyelid irregularity, extent of eyelid telangiectasia and degree of meibomian gland orifice plugging, and to report quantitative data relating to meibography (quantified using the Meiboscore).
Craig 2015 and Rong 2017 measured corneal staining using fluorescein, while Arita 2019 quantified the extent of combined corneal and conjunctival fluorescein staining. Two trials measured tear stability using NIBUT (Arita 2019; Craig 2015). Arita 2019 and Rong 2017 used the more traditional measure of TBUT, quantified involving the instillation of sodium fluorescein. Craig 2015 evaluated several other outcomes, including conjunctival staining, lipid layer grade, tear meniscus height, tear osmolarity and tear evaporation rate. Arita 2019 also reported data relating to meibum grade and Schirmer test score. Rong 2017 evaluated the meibomian gland yielding secretion score (MGYSS). Only Rong 2017 explicitly reported adverse events.
Excluded studies
Following full‐text evaluation, we excluded 13 studies from the review. These trials are listed in the Characteristics of excluded studies table, with the primary reason for exclusion. Overall, the two main reasons were due to a non‐RCT study design (eight studies) and use of an ineligible comparator (two studies).
Studies awaiting classification
There were no studies awaiting classification.
Ongoing studies
We identified 14 ongoing studies (see Characteristics of ongoing studies table).
Risk of bias in included studies
The risk of bias assessment for included trials is summarised in Figure 2 and Figure 3. Information on the risk of bias judgements for individual studies is also provided in the Characteristics of included studies table.
2.
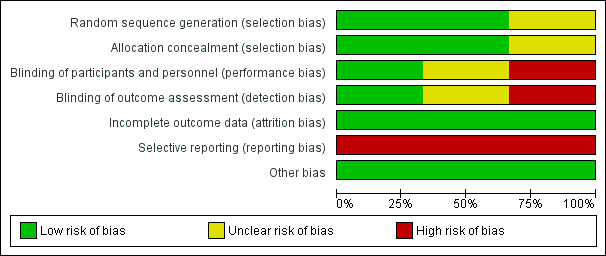
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Craig 2015 and Arita 2019 were at low risk of bias for both allocation concealment and sequence generation, while Rong 2017 was at unclear risk in both domains. Both Craig 2015 and Arita 2019 used a computer‐generated randomisation sequence to derive the treatment allocation, and email communication with the lead authors of both studies confirmed that the allocation to intervention was concealed from study investigators randomising participants to the interventions. Rong 2017 was described as "randomised" but the authors did not report how the randomisation list was generated or how the treatment allocation was administered.
Blinding
For Craig 2015, the risk of bias domains for 'blinding of participants and personnel' and 'blinding of outcome assessors' were low, as they clearly reported procedures for masking. The risk of bias in both of these domains was unclear in Rong 2017, which was reported as a "double‐blind" study but with no further details relating to how this was achieved. Arita 2019 provided no information in relation to masking. We assumed that, in the absence of reporting, personnel and outcome assessors were not masked, which corresponds to a high potential risk of bias in these domains.
Incomplete outcome data
All three included studies were at low risk of attrition bias. The participant follow‐up rates for Arita 2019 and Rong 2017 were more than 80%, with relatively equal follow‐up in the two study groups and the reasons for dropout not linked to adverse events. The Craig 2015 study had 100% participant follow‐up.
Selective reporting
All trials were at high risk of reporting bias. For Rong 2017, there was retrospective registration of the study on a clinical trial registry. For both Arita 2019 and Craig 2015, some outcomes reported in the published report were not listed in the clinical trial registry entry, and not all items listed on the trial registry were described in the publication.
Other potential sources of bias
All three included studies were at low risk of other bias, as no other potential sources of bias were identified.
Effects of interventions
See: Table 1
Table 1 summarises the effect of the intervention (IPL) compared with the control (sham IPL), for the prespecified outcomes.
Primary outcome
Dry eye symptoms
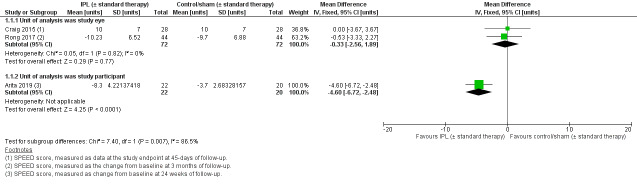
All three trials used the SPEED questionnaire to evaluate dry eye symptoms. Craig 2015, who compared IPL versus sham, reported dry eye symptom scores at the study endpoint (i.e. 45 days of follow‐up). Rong 2017, who compared IPL plus MGX versus sham plus MGX reported the change from baseline in dry eye symptoms at three months of follow‐up. We pooled data from these trials, in which the unit of analysis was the study eye (Craig 2015; Rong 2017). The summary estimate for these two studies indicated little to no reduction in dry eye symptoms with IPL relative to sham (MD –0.33 SPEED units, 95% CI –2.56 to 1.89; 2 studies, 144 eyes; Analysis 1.1; Figure 4). The results presented in these papers did not appear to be derived from paired analyses, which account for the correlation between eyes. Therefore, we would expect the CIs for this result to be wider had the correct, paired analysis been applied. The level of statistical heterogeneity was negligible (I² = 0%).
1.1. Analysis.
Comparison 1 Intense pulsed light (IPL) (with/without standard therapy) versus sham (with/without standard therapy), Outcome 1 Subjective dry eye symptoms, as measured using a validated dry eye questionnaire at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months).
4.
Forest plot of comparison: 1 Intense pulsed light (IPL) (with/without standard therapy) versus sham (with/without standard therapy), outcome: 1.1 Subjective dry eye symptoms, as measured using a validated dry eye questionnaire at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months) [units].
Arita 2019 assigned both eyes of participants to the same intervention and included them in the analysis as separate samples. This study reported SPEED scores at 24 and 32 weeks after treatment onset. The MD between intervention arms (IPL plus MGX versus MGX alone) favoured the IPL plus MGX intervention arm, with a MD of –4.60 (95% CI –6.72 to –2.48 SPEED units) at 24 weeks, and similar results at 32 weeks.
We used the GRADE approach to judge the certainty of the body of evidence for this outcome, and downgraded the findings by three levels to very low, for risk of bias (one level, due to lack of participant or outcome assessor masking in Arita 2019), imprecision (one level, as data derived from studies of small sample size with units of analysis errors in all three trials) and inconsistency (as there was heterogeneity in the effect estimate among trials with different units of analysis).
Secondary outcomes
Adverse effects
None of the trials comprehensively reported adverse events. The publication by Rong 2017 suggested some adverse effects were experienced by the participants, with five participants feeling mild pain and burning, and one participant experiencing an event that led to them partially losing their eyelashes "following mistakes from the doctors during treatment." These authors also indicated that none of the participants experienced inflammation, retinal damage, ocular surface, or injury to the posterior eye. Craig 2015 did not provide any details about adverse events. Arita 2019 reported that three participants in the MGX (control) group withdrew from the study because of pain experienced during the procedure.
The certainty of the body of the evidence for this outcome, assessed using the GRADE approach, was downgraded by three levels to very low, for risk of bias (two levels, due to lack of participant or outcome assessor masking in Arita 2019 and incomplete reporting of adverse outcomes in all studies) and imprecision (one level, as data derived from three studies of small sample size with unit of analyses errors).
Traditional measurement of TBUT involves the instillation of fluorescein into the eye (Mengher 1985), while NIBUT is a less‐invasive (non‐dye) method (Cho 1995). Both methods are considered to provide measures of tear film stability, but are not interchangeable (Wolffsohn 2017). Arita 2019 and Rong 2017 assessed tear stability using TBUT with sodium fluorescein, and Arita 2019 and Craig 2015 used a NIBUT; the two types of tear stability measures are considered as separate outcomes in this review.
Sodium fluorescein tear break‐up time
As summarised in Analysis 1.2, Rong 2017 measured the change from baseline in TBUT, using sodium fluorescein, and found a significant inter‐group difference at three months of follow‐up favouring the IPL intervention arm (MD 2.02 seconds, 95% CI 0.87 to 3.17; 88 eyes). Arita 2019, which considered data from the individual eyes of participants as independent samples, without statistical adjustment for within‐person correlation, reported a similar change for this outcome at 24 weeks of follow‐up, favouring the IPL group (MD 2.40 seconds, 95% CI 2.27 to 2.53 seconds; 84 eyes). Due to the unit‐of‐analysis errors in both of these studies, we would expect the CIs to be wider had the correct paired analysis been used.
1.2. Analysis.
Comparison 1 Intense pulsed light (IPL) (with/without standard therapy) versus sham (with/without standard therapy), Outcome 2 Sodium fluorescein tear break‐up time at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months).
The certainty of the body of evidence for this outcome, assessed using the GRADE approach, was downgraded by two levels to low, for risk of bias (one level, due to lack of participant or outcome assessor masking in Arita 2019), and imprecision (one level, as data derived from two studies of small sample size with unit of analysis errors).
Non‐invasive tear break‐up time
NIBUT was reported as study endpoint values (at 45 days of follow‐up) in Craig 2015, and as the change from baseline at 24 weeks of follow‐up in Arita 2019 (Analysis 1.3). A meta‐analysis was not possible for this outcome, owing to the unit‐of‐analysis issue in Arita 2019. Both studies reported significant inter‐group differences in tear NIBUT. In Craig 2015, NIBUT improved with IPL treatment, relative to a sham intervention (MD 5.51 seconds, 95% CI 0.79 to 10.23). Arita 2019 also reported data favouring the IPL intervention arm (MD 3.20 seconds, 95% CI 3.09 to 3.31). Owing to the unit‐of‐analysis errors in both of these studies, we would expect the CIs to be wider had the correct paired analysis been used.
1.3. Analysis.
Comparison 1 Intense pulsed light (IPL) (with/without standard therapy) versus sham (with/without standard therapy), Outcome 3 Non‐invasive tear break‐up time (NIBUT) at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months).
The certainty of the body of evidence for this outcome, assessed using the GRADE approach, was downgraded by three levels to very low, for risk of bias (one level, due to lack of participant or outcome assessor masking in Arita 2019) and imprecision (two levels, as data derived from two studies of small sample size with wide CIs and unit‐of‐analysis errors).
Tear osmolarity
One study quantified tear osmolarity (Craig 2015). These authors reported endpoint values at 45 days of follow‐up (available from their 'supplementary material' table), with a significant inter‐group difference at this time point favouring the IPL arm (MD –7.00 mOsmol/L, 95% CI –12.97 to –1.03; Analysis 1.4). Due to the unit‐of‐analysis error, we would expect that this estimate is more precise than if the correct, paired analysis had been applied.
1.4. Analysis.
Comparison 1 Intense pulsed light (IPL) (with/without standard therapy) versus sham (with/without standard therapy), Outcome 4 Tear osmolarity at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months).
We used the GRADE classification to judge the certainty of the body of evidence for this outcome, and downgraded the findings by two levels to low, due to imprecision (two levels, as data derived from one study of very small sample size with a unit‐of‐analysis error).
Lipid layer thickness
Craig 2015 qualitatively measured lipid layer grade (LLG) using tear film interferometry (Tearscope Plus; Keeler, UK), and reported a higher (improved) LLG at day 45 in the IPL‐treated eye, relative to the sham‐treated eye (P = 0.002). Rong 2017 did not consider this outcome.
Arita 2019 quantified tear lipid layer thickness using the LipiView (TearScience, US) interferometry device. The authors of this study reported a relative increase in lipid layer thickness at 24 weeks of follow‐up in favour of the IPL intervention arm (MD 19.50 nm, 95% CI 13.19 to 25.82; Analysis 1.5); although, use of data from both eyes as independent samples (without appropriate adjustment for within‐person correlation) should be noted.
1.5. Analysis.
Comparison 1 Intense pulsed light (IPL) (with/without standard therapy) versus sham (with/without standard therapy), Outcome 5 Lipid layer thickness at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months).
Slit lamp biomicroscopy signs: eyelid irregularity, eyelid telangiectasia, eyelid thickening and meibomian gland orifice plugging
Although both Craig 2015 and Rong 2017 undertook slit lamp examinations as part of the clinical trial protocol, neither study considered these specific outcomes.
We prespecified four clinical biomicroscopic signs relating to eyelid parameters as outcome measures, namely eyelid irregularity, eyelid telangiectasia, eyelid thickening and meibomian gland orifice plugging. None of the included studies reported on eyelid thickening. Only Arita 2019, which considered outcomes in 84 eyes (42 participants), reported these specific outcome measures.
There were no significant inter‐group differences for the extent of eyelid irregularity at 24 weeks of follow‐up (Analysis 1.6).
1.6. Analysis.
Comparison 1 Intense pulsed light (IPL) (with/without standard therapy) versus sham (with/without standard therapy), Outcome 6 Eyelid irregularity at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months).
At 24 weeks of follow‐up, these authors reported a relative improvement favouring the IPL treatment arm for both eyelid telangiectasia, termed 'vascularity' in the study (MD –1.30 units, 95% CI –1.50 to –1.10 on a clinical scale from 0 to 3; Analysis 1.7), and meibomian gland plugging (MD –1.20 units, 95% CI –1.24 to –1.16 on a clinical scale from 0 to 3; Analysis 1.8). The certainty of the body of evidence for the extent of meibomian gland orifice plugging was very low, downgraded one level for risk of bias (due to an absence of participant or outcome assessor masking in this study) and by two levels for imprecision (as data derived from one study of very small sample size, with a unit of analysis error).
1.7. Analysis.
Comparison 1 Intense pulsed light (IPL) (with/without standard therapy) versus sham (with/without standard therapy), Outcome 7 Eyelid telangiectasia at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months).
1.8. Analysis.
Comparison 1 Intense pulsed light (IPL) (with/without standard therapy) versus sham (with/without standard therapy), Outcome 8 Meibomian gland orifice plugging at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months).
Meibomian gland dropout
Based upon the clinical trial registry entry (ACTRN12614000162617), Craig 2015 undertook meibography, however the publication reported no data. Through personal communication with the corresponding author of this study, it was revealed that the extent of meibomian gland dropout did not significantly change over the course of the study in either treatment group. Rong 2017 evaluated meibography using a four‐step 'meibomian gland score' (MGS) relating to the extent of missing tarsal glands, graded from 0 to 3 for each of the upper and lower eyelids. These authors reported that the MGS showed no significant change from baseline at the end of the treatment period in either intervention arm, but did not provide quantitative data.
Arita 2019 used the Meiboscore (Arita 2008), graded from 0 to 3, and reported an inter‐group difference favouring the IPL intervention arm (MD –0.30, 95% CI –0.33 to –0.27; Analysis 1.9), notwithstanding the unit‐of‐analysis issue (as previously discussed).
1.9. Analysis.
Comparison 1 Intense pulsed light (IPL) (with/without standard therapy) versus sham (with/without standard therapy), Outcome 9 Meibomian gland dropout at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months).
Corneal sodium fluorescein staining
Craig 2015 and Rong 2017 reported including corneal sodium fluorescein staining as an outcome measure, although there were insufficient data provided for a meta‐analysis. Rong 2017 reported no significant difference between arms at three months of follow‐up (P = 0.409). Craig 2015 did not report data relating to this outcome measure.
Arita 2019 reported data favouring the IPL intervention arm relating to combined corneal and conjunctival fluorescein staining at 24 weeks of follow‐up (MD –1.00 units, 95% CI –1.07 to –0.93 on a scale from 0 to 9; Analysis 1.10).
1.10. Analysis.
Comparison 1 Intense pulsed light (IPL) (with/without standard therapy) versus sham (with/without standard therapy), Outcome 10 Corneal sodium fluorescein staining at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months).
The certainty of the body of evidence for this outcome was very low, downgraded one level for risk of bias (due to an absence of participant or outcome assessor masking in Arita 2019), one level for imprecision (as data derived from studies of small sample size with units of analysis errors) and one level for inconsistency (as the two studies reported divergent effects).
Conjunctival lissamine green staining
The methods section of Craig 2015 reported that conjunctival staining with lissamine green was assessed; however, no data were reported. Neither Arita 2019 nor Rong 2017 considered this outcome measure.
Discussion
Summary of main results
The objective of this systematic review was to examine the effectiveness and safety of IPL therapy for treating dry eye disease due to MGD. The main results, and judgements regarding the certainty of the body of evidence, are provided in the Table 1.
We identified three eligible RCTs, which collectively evaluated outcomes in 114 participants with evaporative dry eye disease or MGD, as defined by the study authors. The study follow‐up periods were 45 days (Craig 2015), three months (Rong 2017), and 32 weeks (Arita 2019). Two trials were paired‐eye trials, whereby IPL was applied to the 'treatment' eye and a "sham" treatment was applied to the 'control' eye (Craig 2015; Rong 2017). However, the results presented in both these studies did not appear to be derived from paired analyses, which would account for the correlation between eyes. This represents an analysis error, which limits our confidence in the reported inter‐group statistical differences.
In Rong 2017, a single physical MGX was also applied to both the control and treatment eyes. Arita 2019 randomised individuals to either an IPL intervention combined with MGX, or MGX alone. Arita 2019 included data from both eyes as independent samples, constituting a unit‐of‐analysis error.
Craig 2015 was at low risk of bias in domains relating to selection bias, performance bias, detection bias and attrition bias. However, this study was at high risk of bias for selective outcome reporting. This trial also received funding support from the medical company (E>Eye, France) that manufactured the IPL device studied in the trial. There were unclear risks of bias in the majority of domains in the Rong 2017 trial; the risk of bias was low for attrition bias and other bias for this study. The study by Arita 2019 was at high risk of bias in domains relating to masking of study participants and outcome assessors, and selective outcome reporting.
All three trials provided data relevant to the primary outcome, change in subjective dry eye symptoms, quantified using the SPEED questionnaire. The SPEED questionnaire is considered an appropriate subjective measure for evaluating evaporative dry eye symptoms (Finis 2014), and suitable for use as an outcome measure in dry eye clinical trials (Wolffsohn 2017). Given dry eye disease is a symptomatic condition, changes in this parameter are considered of major clinical relevance. We performed a meta‐analysis, pooling data from the two paired‐eye trials (Craig 2015; Rong 2017). The summary estimate indicated little to no reduction in dry eye symptoms with IPL relative to sham (MD –0.33 SPEED units, 95% CI –2.56 to 1.89; 2 studies, 144 eyes; Analysis 1.1; Figure 4). The level of statistical heterogeneity was negligible (I² = 0%).
Arita 2019 reported a reduction in dry eye symptoms in favour of the IPL intervention, with the acknowledged limitation of the unit‐of‐analysis issue relating to including both eyes as independent samples.
The certainty of the evidence for this outcome relating to dry eye symptoms was very low, owing to the risk of bias in the trials, imprecision and inconsistency.
There were two outcomes relevant to tear film stability, that is, sodium fluorescein TBUT and NIBUT. Although a pooled data analysis could not be performed, individual studies reported relative improvements in tear stability with the IPL intervention, relative to the control for both outcomes. The certainty of the evidence for tear stability outcomes was low for sodium fluorescein TBUT and very low for NIBUT, owing to the risk of bias in the included trials and imprecision.
There was limited ability to draw conclusions in relation to all other secondary effectiveness outcomes, as data derived from one study, Arita 2019, which had a unit‐of‐analysis error (as previously outlined). The certainty of the evidence for all other secondary effectiveness outcomes was low or very low.
In terms of potential adverse events, Rong 2017 reported several adverse events, although it was unclear which intervention group these occurred in. These authors reported that five participants felt mild pain and burning after the IPL intervention, and one participant experienced an event that led to them partially missing their eyelashes "following mistakes from the doctors during treatment." Craig 2015 and Arita 2019 did not specifically report adverse events. Arita 2019 reported that three participants in the MGX (control) group withdrew from the study because of pain experienced during the procedure. Due to a lack of comprehensive reporting of adverse events, there was low certainty of the safety profile of IPL in this patient population.
Overall, this systematic review identified a paucity of evidence relating to the effectiveness or safety of IPL for the treatment of MGD.
Overall completeness and applicability of evidence
This systematic review found only three RCTs evaluating the effectiveness or safety of IPL for treating MGD. The three trials included in this review considered the use of IPL, as a treatment for MGD, over treatment durations ranging from 45 days to 32 weeks. In addition to the limitation of few participants in each trial, several factors limited our ability to synthesise the available evidence, and our ability to draw more definitive conclusions surrounding the effectiveness and safety of IPL for treating MGD, in particular:
Type of intense pulsed light device
The trials used different IPL devices. Craig 2015 used the E>Eye (E‐Swin) and Arita 2019 and Rong 2017 used the M22 (Lumenis) system. These devices inherently use different wavelengths and intensities of light. Although some of these details are proprietary, the M22 system is known to apply light pulses of 11 J/cm² to 16 J/cm², while the E>Eye devices delivers light pulses of 9.8 J/cm² to 13 J/cm². Given the availability of only three studies, we were unable to conduct a sub‐group analysis to compare any potential device‐related differences in outcomes. Thus, it is currently not known whether different devices may yield differential therapeutic or adverse effects (or both). In addition, it has not been comprehensively investigated whether single (Rong 2017) or multiple (Arita 2019) adjunctive in‐office MGX(s) yield more substantial clinical effects.
Intense pulsed light protocol and skin types
The number and time spacing of IPL treatments, as well as the post‐intervention follow‐up time point(s), may also impact study outcomes, although this could not be determined from the available data. Arita 2019 performed the examinations prior to administration of the interventions. Craig 2015 conducted the follow‐up examination directly after administering the intervention, whereas it was undertaken one day later in Rong 2017. It is unclear whether any changes reported by Craig 2015 reflect short‐term clinical improvement (potentially due to the immediate benefit of heat generated by the IPL device) rather than long‐term physiological changes to the meibomian glands.
All trials reported adjusting the IPL pulse intensity to the skin type of the study participants, ranked using the Fitzpatrick grading scale (from I to VI) (Fitzpatrick 1975). This procedure acknowledges that the risk of adverse events, in particular skin hypopigmentation, are significantly higher in individuals with darker skin tones when higher‐intensity light pulses are applied (Gupta 2016). However, none of the studies provided a clear explanation regarding the protocol used to determine the intensity of treatment for the skin type of each participant. The Arita 2019 and Rong 2017 studies were ambiguous in terms of the procedures used to allocate participants to a particular IPL intensity, reporting only that it was adjusted to the Fitzpatrick skin type. Craig 2015 reported that the protocol followed the procedure recommended by the device manufacturer, and provided details in relation to the pulse intensity each skin type received.
None of the studies reported data for each Fitzpatrick skin type. Rather, they pooled overall findings across the full cohort of participants. Therefore, it is not possible to determine whether there are differential effects relating to the effectiveness and safety of IPL in individuals with different skin tones.
Study populations
The three single‐centre studies in this review considered potentially different patient populations, although specific information about ethnicity was not provided. The Craig 2015 study was undertaken in New Zealand, and recruited individuals with "mild to moderate clinical signs of MGD," but without clear definition of the clinical criteria that were adopted or whether a threshold dry eye symptom score was required. The Rong 2017 trial, undertaken in China, presumably involved Asian participants, who were enrolled on the basis of a SPEED score of at least six units, and a Meibomian Gland Yielding Secretion Score of 12 or less. The trial by Arita 2019 was performed in Japan and evaluated "the skin type of most Japanese individuals… classified as Fitzpatrick type 3," and acknowledged that the findings reported in the study may not be representative of results in individuals of other ethnicity or skin type.
The Craig 2015 and Rong 2017 studies enrolled participants of a similar age (mean age about 45 years), whereas participants were generally older in the Arita 2019 trial (mean age about 61 years). To date, there have been no RCTs evaluating the effectiveness or safety of IPL in children and thus the effectiveness and safety of this intervention in this population is unknown.
The data presented in the included studies is thus insufficient for assessing whether MGD populations of different ethnicity, age, and disease severity might have differential responses to IPL.
Inability to synthesise data
We were unable to conduct meta‐analyses for most of the prespecified outcomes, which significantly impacted our ability to draw definitive conclusions regarding the effectiveness or safety of IPL treating MGD. This is of concern given that in 2016, Health Canada released a warning in relation to the use of IPL devices, due to the potential risk of skin burns (Health Canada 2016). There is thus a need to ensure the safety of this therapy before its diffuse implementation in clinical practice.
Trial design and the control (sham) intervention
Two trials adopted a paired‐eye design, whereby one eye of a participant received the IPL intervention and the fellow eye received a "sham" intervention (Craig 2015; Rong 2017). Several potential limitations to this trial design, which may confound the reported findings, include:
the potential for sympathetic ocular improvement, whereby performing a treatment in one eye can yield clinical improvement in the fellow eye. This phenomenon may affect the ability to detect an inter‐eye, and thus inter‐intervention, difference;
the challenge of ensuring that participants are not unmasked to the intervention. For example, IPL involves the generation of substantial heat on the surface of the skin, which would be present with the active intervention but absent from the sham intervention; this differential may inadvertently unmask participants. Neither trial assessed the extent of successful masking by asking participants to guess the per‐eye treatment allocation;
the assessment of ocular comfort on a 'per eye' basis is not validated, and may be challenging, thus limiting the capacity to detect changes in dry eye symptom scores. It is possible that participants may have found it challenging to individually distinguish ocular comfort changes in each eye, and rather reported an overall change to both eyes.
In addition, the results presented in these papers did not appear to be derived from paired analyses, which would account for the correlation between eyes. This represents an analysis error, which limits our confidence in the reported inter‐group statistical differences.
Quality of the evidence
For all effectiveness outcomes where quantitative data were available, we judged the certainty of the evidence to be very low (symptoms, NIBUT, corneal sodium fluorescein staining, extent of meibomian gland orifice plugging and adverse events) or low (sodium fluorescein TBUT and tear osmolarity) using the GRADE approach. The main reasons for downgrading the certainty of the findings were due to risks of bias (e.g. the absence of participant or outcome assessor masking in Arita 2019), imprecision (as data derived from a limited number of studies of modest sample size) and inconsistency (due to heterogeneity in effects).
There is currently a paucity of data relating to the safety of IPL for treating MGD.
Potential biases in the review process
We used the standard methodological procedures recommended in theCochrane Handbook for Systematic Reviews of Interventions to minimise any potential source of bias during the review process (Higgins 2011b).
The review protocol was prospectively registered (Downie 2018, CRD42018099359), and as such all outcome measures were specified in advance of undertaking the review. An a priori search strategy was developed that was comprehensive and did not exclude grey or non‐English literature, minimising selection bias. Two review authors independently oversaw each stage of the review process.
We acknowledge the potential limitations of including paired‐eye studies that did not account for contralateral eye effects, and that these studies did not appear to undertake paired statistical analyses. Given the limited high‐quality data available, we opted to include these studies in the review and to report their findings, notwithstanding the potential limitations.
Agreements and disagreements with other studies or reviews
This is, to our knowledge, the first systematic review to evaluate the effectiveness and safety of IPL for treating MGD.
In the Tear Film and Ocular surface Society (TFOS) International Dry Eye WorkShop II (DEWS II), involving a comprehensive narrative synthesis of current modalities for treating and managing dry eye disease (Jones 2017), the authors described results from three publications (Craig 2015; Gupta 2016; Vegunta 2016). The studies by Gupta 2016 and Vegunta 2016 were excluded from the present review, as they are not RCTs.
In 2018, the Canadian Agency for Drugs and Technologies in Health published a 'Rapid Response Report: Summary with Critical Appraisal' (Rennick 2018). This review involved a limited literature search of three electronic databases, and applied no methodological restrictions. This review, which included four studies, noted that most studies lacked suitable control populations for comparing the effectiveness and safety of IPL. Consistent with the present review, Rennick 2018 also noted that there is no consistent protocol for performing IPL to manage MGD, and the number of treatments required to impart therapeutic benefit remains unclear.
Authors' conclusions
Implications for practice.
Based upon our consideration of the current, best‐available clinical trial evidence, we find a dearth of high‐quality evidence relating to the effectiveness or safety of IPL for treating MGD.
Whether IPL is of value for modifying the symptoms or signs of evaporative dry eye disease is currently uncertain. Due to a lack of comprehensive reporting of adverse events, the safety profile of IPL in this patient population is also unclear. These factors should be considered by clinicians using this intervention and clearly outlined to patients potentially undergoing this procedure, to ensure an appropriate level of informed consent.
All of the studies included in this review also excluded individuals with certain skin types (V and VI on the Fitzpatrick scale), due to the potential increased risk of adverse effects when IPL is applied to darker skin types. As a therapy, IPL is thus only applicable to individuals with skin types I to IV (Fitzpatrick scale). As this is a known caveat of the applicability of this technology more generally, this factor did not contribute to downgrading the certainty of the body of evidence.
The relative effectiveness of IPL relative to other established treatments for MGD (e.g. thermal pulsation therapy) is also still not currently known; head‐to‐head trials are required to ascertain how IPL compares, both in terms of effectiveness and safety, to other modalities.
The results of the 14 RCTs currently in progress will be of importance for establishing a more definitive answer regarding the effectiveness and safety of IPL for treating MGD (see Characteristics of ongoing studies table).
Implications for research.
This is the first systematic review to appraise and synthesise RCT evidence relating to the effectiveness and safety of IPL for the treatment of MGD. While there have been several open‐label and non‐randomised studies of IPL that suggest that there may be a potential benefit in dry eye populations (e.g. Albietz 2018; Gupta 2016), we only identified three relevant RCTs that collectively considered a total of 114 participants. Two of these trials used a pair‐eye design which, as discussed, has several limitations that may confound the reported findings (Craig 2015; Rong 2017). The other trial randomised individual participants to the intervention groups, but performed the data analysis using both eyes as independent samples, without statistical adjustment for within‐person correlation (Arita 2019).
There is thus a need for suitably powered, robust clinical trials to further evaluate the effectiveness and safety of IPL as a treatment for MGD. Such trials should be: of adequately powered, randomise individuals (rather than eyes) to each intervention and analyse the per‐eye data using statistically robust methods, and clearly define the patient population (including factors such as ethnicity and MGD severity using a standard classification). In view of the ties between the manufacturers or sponsors in at least two of the included trials, it would be preferable for there to be greater independence in the design, conduct and reporting of future trials in this field. Some of these issues may be addressed by the 14 trials of IPL for MGD that are currently ongoing.
As emphasised in other systematic reviews in this field (e.g. Downie 2019), there is a need to develop a 'core outcome set' for dry eye clinical trials (Saldanha 2018) to improve the consistency of outcome reporting. This will enhance the ability to synthesise data in meta‐analyses, and thus draw more certain conclusions about the relative effectiveness and safety of dry eye interventions.
The trial by Craig 2015 noted a cumulative effect from IPL treatment over 45 days; however, due to the short duration of the included studies (up to three months in Rong 2017 and six months of active intervention in Arita 2019), we were unable to explore this possibility. Longer study durations are thus required in order to determine the optimal intervention period. The timing of the clinical evaluation (including symptoms and signs), relative to the administration of the intervention should also be carefully considered. For example, Arita 2019 performed the clinical assessment prior to the intervention and Craig 2015 assessed clinical outcomes immediately post‐IPL. Rong 2017 performed the postintervention assessment one day after the intervention, and in a separate follow‐up paper reported continuous improvement in meibomian gland secretion function and tear break‐up time six months following treatment (Rong 2018). Separating the intervention from the assessment minimises the risk of only capturing short‐term clinical changes induced by the heating effect of IPL, as opposed to any potential longer‐term effects of the intervention.
Due to the risk of adverse events being higher in people with darker skin tones, it would have been useful for the studies to separate the participants into subgroups to investigate whether skin type and the associated light intensities affected the effectiveness or safety (or both) outcomes. For example, if relatively higher intensities for lighter skin types had a significant effect on outcomes compared to individuals necessarily treated with lower intensity light pulses. Adverse event reporting should also be stratified by Fitzpatrick scores.
There is also a need to more clearly establish the relative effectiveness and safety of: the different types of commercially available IPL devices, IPL relative to other MGD therapies and combining IPL with other forms of MGD management approaches.
The mechanism of action for IPL treatment in MGD is also not yet established, and thus is an additional area requiring further research.
Acknowledgements
We acknowledge Anne Gad (research assistant) for her contribution to the conception and development of the protocol of this review. We thank Dayton Ming Jun Lee for translating the Rong 2017 paper from Chinese into English. We thank Robin Featherstone (Cochrane Editorial and Methods Department Information Specialist) for her assistance with constructing the search strategies.
The Cochrane Editorial and Methods Department managed the editorial process for this review. We thank Helen Wakeford (Managing Editor), Nuala Livingstone (Associate Editor) and Kerry Dwan (Statistical Editor) from the Editorial and Methods Department for their feedback.
We acknowledge the peer reviewers Luis F Mejia, Anat Galor, Katrina Schmid and William Ngo and consumer referees David McDonald, Abdul Shakoor and Christian Fau who provided helpful comments on this review.
We thank Cochrane Eyes and Vision, particularly Co‐ordinating Editor Richard Wormald, for acting as sign‐off editor. We also acknowledge Peter Tugwell, Senior Editor of the Cochrane Muscular, Oral, Skin, and Sensory (MOSS) Network for acting as sign‐off editor. We also thank Anne Lawson for copy editing this review.
Appendices
Appendix 1. CENTRAL search strategy
1.[Intense Pulsed Light Therapy] explode all trees 2.[Phototherapy] explode all trees 3.(intense near/3 puls*):ti,ab,kw 4.(puls* near/2 light):ti,ab,kw 5.(light near/3 therapy):ti,ab,kw 6.IPL:ti,ab,kw 7.#1 or #2 or #3 or #4 or #5 or #6 8.[Dry Eye Syndromes] explode all trees 9.[Tears] explode all trees 10.[Meibomian Glands] explode all trees 11.[Eyelids] explode all trees 12.[Blepharitis] explode all trees 13.[Keratoconjunctivitis] explode all tree 14.Meibomian:ti,ab,kw 15.(dry NEXT eye*):ti,ab,kw 16.((eye NEXT lid*) or eyelid*):ti,ab,kw 17.Tear NEXT film:ti,ab,kw 18.(tear NEXT stabil*):ti,ab,kw 19.(tear NEXT instab*):ti,ab,kw 20.(“evaporative dry” NEXT eye*):ti,ab,kw 21.meibum:ti,ab,kw 22.lipid*:ti,ab,kw 23.“eye dryness”:ti,ab,kw 24.MGD:ti,ab,kw 25.#8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 26.#7 and #25
Appendix 2. MEDLINE search strategy
1.randomized controlled trial.pt. 2.controlled clinical trial.pt. 3.(randomised OR randomized).ab,ti. 4.placebo.ab. 5.drug therapy.fs. 6.randomly.ab. 7.trial.ab. 8.groups.ab. 9.1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 10.exp animals/ not humans.sh. 11.9 not 10 12.exp Intense Pulsed Light Therapy/ 13.(intense adj3 puls*).tw. 14.(light adj3 therapy).tw. 15.(puls* adj2 light).tw. 16."IPL".tw. 17.or/ 12‐16 18.exp Dry Eye Syndromes/ 19.exp eyelids/ or conjunctiva/ or eyelashes/ or meibomian glands/ 20.exp Blepharitis/ 21.exp Tears/ 22.exp Keratoconjunctivitis/ 23.meibomian.tw. 24.dry eye*.tw. 25.(eyelid* or eye lid*).tw. 26.tear film.tw. 27.tear stabil*.tw. 28.tear instab*.tw. 29.evaporative dry eye*.tw. 30.meibum.tw. 31.lipid*.tw. 32.eye dryness.tw. 33.MGD.tw. 34.or/ 18‐33 35.11 and 17 and 34
Appendix 3. EMBASE search strategy
1.crossover‐procedure/ 2.double‐blind procedure/ 3.randomized controlled trial/ 4.single‐blind procedure/ 5.random*.mp. 6.(crossover* OR cross over*).mp. 7.placebo*.mp. 8.(doubl* adj blind*).mp. 9.(singl* adj blind*).mp. 10.allocat*.mp. 11.1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 12.exp intense pulsed light therapy/ 13.(intense adj3 puls*).ti,ab,kw. 14.(light adj3 therapy).ti,ab,kw. 15.(puls* adj2 light).ti,ab,kw. 16.IPL.ti,ab,kw. 17.12 or 13 or 14 or 15 or 16 18.exp keratoconjunctivitis/ 19.meibomian.mp. or exp meibomian gland/ 20.dry eye.mp. or exp dry eye/ 21.(eye lid* or eyelid*).mp. or exp eyelid/ 22.conjunctiva*.mp. or exp conjunctiva/ 23.eyelash*.mp. or exp eyelash/ 24.tear*.mp. or exp lacrimal fluid/ 25.exp tear film/ 26.meibomian gland*.ti,ab,kw. 27.dry eye*.ti,ab,kw. 28.tear film.ti,ab,kw. 29.tear stabil*.ti,ab,kw. 30.tear instab*.ti,ab,kw. 31.evaporative dry eye*.ti,ab,kw. 32.meibum.ti,ab,kw. 33.lipid.ti,ab,kw. 34.eye dryness.ti,ab,kw 35.MGD.ti,ab,kw. 36.18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 37.11 and 17 and 36
Appendix 4. ICTRP search strategy
Condition = dry eye OR tear OR evaporative Intervention = intense OR pulsed light OR light therapy
Appendix 5. ANZCTR search strategy
("intense pulse* light" OR "IPL" OR "light therapy") AND ("meibomian gland" OR "dry eye" OR "tear")
Appendix 6. Clincaltrials.gov search strategy
Condition = dry eye OR evaporative Intervention = intense OR pulsed light OR light therapy
Data and analyses
Comparison 1. Intense pulsed light (IPL) (with/without standard therapy) versus sham (with/without standard therapy).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Subjective dry eye symptoms, as measured using a validated dry eye questionnaire at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Unit of analysis was study eye | 2 | 144 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐2.56, 1.89] |
| 1.2 Unit of analysis was study participant | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐4.60 [‐6.72, ‐2.48] |
| 2 Sodium fluorescein tear break‐up time at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Unit of analysis was study eye | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Unit of analysis was study participant | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Non‐invasive tear break‐up time (NIBUT) at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Unit of analysis was the study eye | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Unit of analysis was the study participant | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Tear osmolarity at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Lipid layer thickness at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Eyelid irregularity at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Eyelid telangiectasia at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Meibomian gland orifice plugging at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Meibomian gland dropout at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Corneal sodium fluorescein staining at 3 months of follow‐up (with an acceptable follow‐up range ≤ 6 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arita 2019.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group, where both eyes from an individual participant were considered as independent samples for the statistical analysis (representing a unit of analysis issue). Exclusions after randomisation (if Yes, provide relevant details from the paper): 3 participants in the MGX (control) group subsequently withdrew from the study because of pain during the procedure. Percentage of participant follow‐up (include details for all intervention groups): MGX group: 20/23 participants (87% follow‐up); IPL‐MGX group: 22/22 participants (100% follow‐up) Study duration (of intervention): quote: "Each patient underwent a series of eight treatment sessions at 3‐week intervals. After the eight treatment sessions, each patient underwent three follow‐up examinations over the course of 11 weeks." Was a sample size calculation reported (Yes/No): yes |
|
| Participants |
Baseline characteristics IPL + MGX group
MGX only group
Overall
Inclusion criteria:
Exclusion criteria: none reported. Significant pretreatment baseline differences? No significant inter‐group differences at baseline. Severity of dry eye: reported as "refractory MGD;" dry eye severity not explicitly reported. |
|
| Interventions |
IPL + MGX group
MGX only group
|
|
| Outcomes |
(As reported in the paper) Primary and secondary outcomes: not explicitly stated Measurements included:
|
|
| Identification |
Funding sources: no specific grant from funding agencies in the public, commercial or not‐for‐profit sectors. Declarations of interest: RA holds patents on the non‐contact meibography technique described in this manuscript (Japanese patent registration no. 5281846; US patent publication no. 2011–0273550A1; European patent publication no. 2189108A1), is a consultant for Kowa Company (Aichi, Japan) and Lumenis Japan (Tokyo, Japan), and has received financial support from TearScience (Morrisville, North Carolina, US). The other authors declared no potential conflict of interest. Country: Japan Setting: Itoh Clinic Comments: Publication status: published study Journal of publication: Ocular Surface Language: English Trial registration number: UMIN000022747. Contacting study investigators: 1 review author (LED) contacted the corresponding author August 2019 to confirm that data presented in the paper represented the inclusion of data from both eyes, as independent samples, without adjustment for within‐person correlation. LED contacted the corresponding author in November 2019 to obtain further information about the random sequence generation and allocation concealment methods, which informed the risk of bias assessment. LED asked for relevant information relating to the within‐person correlation. However, the authors advised that they would not be able to provide this information or the data to facilitate its calculation. Date study conducted: May 2016 to August 2017 Corresponding author's name: Reiko Arita Institution: Itoh Clinic, Saitama, Japan Email: ritoh@za2.so‐net.ne.jp Address: Department of Ophthalmology, Itoh Clinic, 26‐11 Minami‐Nakano, Minumaku, Saitama, Saitama, 337‐0042, Japan |
|
| Notes | Adverse events: not explicitly reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Refractory MGD patients were randomly assigned to receive either IPL with MGX (IPL‐MGX) or MGX alone as a control." Judgement comment: email correspondence with Dr Arita (5 November 2019) confirmed that the randomisation code was generated using a computer‐generated list. |
| Allocation concealment (selection bias) | Low risk | Quote: "Refractory MGD patients were randomly assigned to receive either IPL with MGX (IPL‐MGX) or MGX alone as a control." Judgement comment: email correspondence with Dr Arita (5 November 2019) confirmed that the allocation was concealed by means of a computer‐based system for participant randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: open label or no information on masking. We assume that in the absence of reporting, participants and personnel were not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: open label or no information on masking. We assume that in the absence of reporting, outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Ninety eyes of 45 patients were enrolled in the study. Three patients in the MGX (control) group subsequently withdrew from the study because of pain during the procedure, leaving a total of 20 patients in the MGX group and 22 patients in the IPL‐MGX group." Judgement comment: missing data < 20% (i.e. > 80% participant follow‐up) and relatively equal follow‐up in both groups and no obvious reason why loss to follow‐up should be related to outcome. |
| Selective reporting (reporting bias) | High risk | Judgement comment: mismatches between clinical trial registry entry for outcome measures, and how data were reported in the paper. For example, corneal and conjunctival fluorescein staining score and NIBUT are reported in the paper but not listed in the clinical trial registry. The primary outcome measure listed on the clinical trial registry (meibum grade quality) is reported in the paper, but not specified as the primary outcome measure. |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias. |
Craig 2015.
| Methods |
Study design: randomised controlled trial Study grouping: intra‐person (between eye) comparative trial Exclusions after randomisation? (If Yes, provide relevant details from the paper): none (follow‐up data available for all 28 enrolled participants) Percentage of participant follow‐up (include details from all intervention groups): 100% Study duration (of intervention): 45 days Was a sample size calculation reported? (Yes/No): no (although a sample size calculation was available on the clinical trial registry entry) |
|
| Participants |
Baseline characteristics IPL group
Sham (control) group
Overall
Inclusion criteria:
Exclusion criteria:
Significant pretreatment baseline differences? No. Quote: "At baseline, there was no significant difference between the treated and control eyes in any outcome variable (p > 0.05 in all cases)." Severity of dry eye: mild‐to‐moderate MGD |
|
| Interventions |
IPL group
Sham (control) group
|
|
| Outcomes |
(As reported in the paper) Primary and secondary outcomes: not explicitly stated. Measurements included: best spectacle corrected visual acuity (logMAR), bulbar conjunctival injection graded on a VAS; NIBUT; fluorescein and lissamine green corneal and conjunctival staining; assignment of the tear LLG through tear film interferometry (Tearscope Plus, Keeler, UK), TMH, tear osmolality (TearLab Osmolarity System; TearLab, San Diego, California, US), TER (VapoMeter; Delfin, Kuopio, Finland), patient symptoms (measured with SPEED validated questionnaire and perceived severity of dry eye symptoms using a VAS anchored at each end with 'No symptoms' and 'Constant symptoms' as descriptors), at baseline (day 1), 15 and 45. |
|
| Identification |
Funding sources: supported by a summer studentship grant from the New Zealand Association of Optometrists (YHC) and consumables funding from France Medical. Declarations of interest: JP Craig, France Medical (F); YH Chen, none; PRK Turnbull, none Country: New Zealand Setting: eye clinic Comments: Publication status: published study Journal of publication: Investigative Ophthalmology and Visual Science Language: English Trial registration number: ACTRN12614000162617 Contacting study investigators: 1 review author (LED) contacted the trial corresponding author (A/Prof Craig) in September 2018 to clarify the method of allocation concealment. LED contacted Associate Professor Craig in January 2020 to clarify the quantitative data reported for the NIBUT outcome, as numeric values were inconsistent between the abstract and main text. The abstract values were confirmed to be correct. Date study conducted: not reported Corresponding author's name: Jennifer Craig Institution: University of Auckland, New Zealand Email: jp.craig@auckland.ac.nz Address: Department of Ophthalmology, University of Auckland, Private Bag 92019, Auckland 1142, New Zealand |
|
| Notes |
Adverse events: not reported Comments on statistical analysis: this study was an intra‐person (between eye) comparative trial. Although it appears from the text in the paper that a paired analysis was performed (to account for the correlation between eyes), the results presented in the paper appear not to be from a paired analysis. This represents a statistical analysis error, which limits our confidence in the reported inter‐eye differences. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "One eye was selected for treatment according to a computer‐generated randomization program, with the other eye assigned to serve as a mock‐treated control." Judgement comment: computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Low risk | Judgement comment: not reported how allocation was administered. Contacted trial author (Craig) and clarified that the allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double masked"; "participant masking was employed with a white‐blocking filter applied over the tip of the IPL probe during application to the nontreated eye only." Judgement comment: clearly stated that participants were masked; there were no associated personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The researcher collecting the clinical data was masked as to which eye was treated, and participant masking was employed with a white‐blocking filter applied over the tip of the IPL probe during application to the non‐treated eye only." Judgement comment: clearly stated that the outcome assessor was masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The full cohort of 28 enrolled participants completed measurements across all three appointments and were included in the analysis." Judgement comment: complete follow‐up reported. |
| Selective reporting (reporting bias) | High risk | Judgement comment: all outcomes in the clinical trial registry (ACTRN12614000162617) were reported. However, selective outcome reporting was suspected as the following additional outcomes (not listed in the clinical trial registry entry) were also reported in the methods section of the paper: TER, TMH, tear osmolarity, SPEED symptom questionnaire, and lissamine green corneal and conjunctival staining. In the results, findings for meibography, and fluorescein and lissamine green staining were also not provided in the paper. |
| Other bias | Low risk | Judgement comment: no other apparent significant sources of bias. |
Rong 2017.
| Methods |
Study design: randomised controlled trial Study grouping: intra‐person (between eye) comparative trial Exclusions after randomisation? (If Yes, provide relevant details from the paper): 2 exclusions, but the paper did not state the time point that this occurred. Percentage of participant follow‐up (include details from all intervention groups): 95.7%; quote: "Two patients quit the study due to reasons not related to the study, and were not included in the analysis." Study duration (of intervention): not reported Was a sample size calculation reported? (Yes/No): no |
|
| Participants |
Baseline characteristics IPL group
Sham (control) group
Overall
Inclusion criteria:
Exclusion criteria:
Significant pretreatment baseline differences? None Severity of dry eye: not reported |
|
| Interventions |
IPL group
Sham (control) group
|
|
| Outcomes |
Primary outcome: MGYSS evaluated using the MGE‐1000 to assess tarsal excretion function. Each tarsal gland scored using the standard schema: the secreted liquid fat is: clear = 3 points, sticky white or light yellow fat = 2 points, concentrated toothpaste‐like fat = 1 point, no excretion = 0 points. Each squeeze with the MGE device can assess 5 connecting tarsal gland openings. In total, 15 glands were assessed and the MGYSS of upper and lower eyelids gives a combined score of 0–45. Secondary outcomes: tear film BUT, SPEED dry eye symptoms questionnaire (score 0–28), corneal fluorescein dye (scored using a 12‐point system), meibomian gland score (meibography‐based), safety evaluation (checking for eyelid burns, blisters, missing eyelashes or brow and skin pigmentation, Snellen vision chart BCVA, non‐contact intraocular pressure, slit lamp examination and OCT). |
|
| Identification |
Funding sources: none reported Declarations of interest: none reported Country: China Setting: eye hospital Comments: Publication status: published study Journal of publication: various Language: Chinese Trial registration number: ChiCRT‐INR‐16010256 Contacting study investigators: 1 review author (LED) contacted the corresponding author in August 2019 to clarify whether the study population in this study was the same as the Rong 2018 study, which was confirmed to be the case. LED contacted the corresponding author in November 2019 to obtain further information about the random sequence generation and allocation concealment methods, to inform the risk of bias assessment, but received no response. Date study conducted: not reported Corresponding author's name: Xiaoming Yan Institution: Peking University First Hospital Email: yanxiaoming7908@163.com Address: 8 Xishiku Street, Xicheng District, Beijing, China |
|
| Notes |
Additional treatments (in both intervention arms)
Adverse events: 5 participants had mild pain and burning, and 1 participant experienced an event that led to them partially missing their eyelashes "following mistakes from the doctors during treatment." These authors indicated that 0 participants experienced inflammation, retinal damage, ocular surface injury or injury to the posterior eye. Comments on statistical analysis: this study was an intra‐person (between eye) comparative trial. However, the results presented in the paper appeared not to be from a paired analysis. This represents a statistical analysis error, which limits our confidence in the reported inter‐eye differences. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Trial was described as "randomised" but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation administration not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: stated as "double‐blind" but no indication of who was masked. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: stated as "double‐blind" but no indication of who was masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: missing data < 20% (i.e. > 80% participant follow‐up) and equal follow‐up in both groups and no obvious reason why loss to follow‐up should be related to outcome. |
| Selective reporting (reporting bias) | High risk | Judgement comment: retrospective registration of trial. |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias. |
BCVA: best‐corrected visual acuity; BUT: break‐up time; CFS: corneal fluorescein staining; IPL: intense pulsed light; LLT: lipid layer thickness; MGD: meibomian gland dysfunction; MGX: meibomian gland expression; MGYSS: Meibomian Glands Yielding Secretion Score; NIBUT: non‐invasive break‐up time; OCT: optical coherence tomography; SD: standard deviation; SPEED: Standard Patient Evaluation of Eye Dryness; TER: tear evaporation rate; TMH: tear meniscus height; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ChiCTR‐ONC‐17010867 | Ineligible study design (not an RCT). |
| ChiCTR‐ONN‐17013864 | Ineligible study design (not an RCT). |
| ChiCTR‐OON‐15007125 | Ineligible study design (not a RCT). |
| ChiCTR1800014847 | Ineligible study design (not an RCT). |
| ChiCTR1900020576 | Ineligible comparator – active comparator (broad band light as the comparator). |
| Li 2019 | Ineligible comparator – active comparator (2 IPL methods compared). |
| NCT01917539 | Study withdrawn. |
| NCT02066051 | Ineligible study design (not an RCT). |
| NCT02621593 | Ineligible study design (not an RCT). |
| NCT02992535 | Ineligible study design (not an RCT). |
| NCT03658811 | Ineligible study design (not an RCT). |
| NCT03788486 | Ineligible intervention (blue light‐emitting diode rather than IPL). |
| Zhang 2019 | Ineligible patient population (participants had ocular Demodex infestation rather than meibomian gland dysfunction). |
IPL: intense pulsed light; RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12616000667415.
| Trial name or title | Evaluation of intense pulsed light therapy for dry eye relief |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Exclusions after randomisation? Not reported Percentage of participant follow‐up: not applicable Study duration (of intervention): 105 days Was a sample size calculation reported? (Yes/No): no |
| Participants |
Baseline characteristics IPL group
Control group
Overall
Inclusion criteria: symptomatic dry eye caused by MGD; aged ≥ 18 years; both genders. Exclusion criteria: contraindications to light therapy, e.g. clinical skin treatments within last 2 months; implants beneath the lower eyelid area, tattoos, semi‐permanent make‐up or pigmented lesions in the treatment area; contact lens wearers must refrain from wearing contacts within 1 week of commencing the study, and during the study; individuals taking prescribed photosensitising medications such as doxycycline within 3 months of study commencement Significant pretreatment baseline differences? Not reported Severity of dry eye: not reported |
| Interventions |
IPL
Control
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Starting date | Anticipated date of first participant recruitment: 24 May 2016 No further update as at 15 July 2019 |
| Contact information | Associate Professor Jennifer P Craig Department of Ophthalmology, The University of Auckland, Private Bag 92019, Auckland 1142, New Zealand Email: jp.craig@auckland.ac.nz Telephone: +6499238173 |
| Notes | None |
ChiCTR‐1800014787.
| Trial name or title | A prospective, multi‐center, randomized, and controlled clinical trial to evaluate the effectiveness and safety of intense pulsed light and laser system (M22) in dry eye patients caused by meibomian gland dysfunction (MGD) compared to basic physical therapy |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Percentage of participant follow‐up (include details from all intervention groups): not applicable Study duration (of intervention): not reported Was a sample size calculation reported? (Yes/No): no Exclusions after randomisation: not reported |
| Participants |
Baseline characteristics IPL group
Control (warm compress) group
Overall
Inclusion criteria: aged ≥ 18 years; Fitzpatrick skin type 1–4; SPEED score ≥ 6; Meibomian gland function score ≤ 12; TBUT ≤ 10 seconds*; corneal fluorescein staining score ≥ 1; must sign an informed consent form, willing to comply with the treatment and follow‐up schedule, and participate voluntarily in this study. *Note: if TBUT ≤ 5 seconds, do not considerate criteria of corneal fluorescein staining score ≥ 1. Exclusion criteria: pregnancy and nursing; contact lens wearer; acute ocular inflammation or infection; obvious scar or keratinisation on the eyelid; received eye surgery or eyelid surgery within 6 months prior to enrolment; neuroparalysis occurred in the treatment area within 6 months before enrolment; tear plug is being used; precancerous lesions in the treatment area, skin cancer or pigmentation; received LASIK surgery within 6 months prior to enrolment; treated area has diseases that may be stimulated by light waves of 560–1200 nm, such as herpes simplex types 1 and 2, systemic lupus erythematosus and porphyria; taking photosensitisers, such as isotretinoin, tetracycline or St John's wort; eye drops for dry eye within 48 hours before enrolment (except for artificial tears); history of head and neck radiotherapy within 1 year before enrolment, or radiotherapy within 8 weeks after intensive pulsed light therapy is expected; chemotherapy history within the first 8 weeks of enrolment, or chemotherapy within 8 weeks after IPL therapy is expected; history of migraine or epilepsy; face IPL treatment was performed within 1 year before enrolment; excessive exposure in the first 4 weeks before enrolment; other conditions judged by the researcher as unsuitable for this clinical trial. Significant pretreatment baseline differences? Not reported Severity of dry eye: not reported |
| Interventions |
IPL
Control
|
| Outcomes |
Primary outcome: tear BUT Secondary outcomes: meibomian gland assessment; SPEED questionnaire; corneal fluorescein staining; standard vision; intraocular pressure; observation of the palpebral margin and anterior segment. |
| Starting date | Study execution dates listed as: 9 October 2017 to 28 February 2019 |
| Contact information | Xiaoming Yan 8 Xishiku Street, Xicheng District, Beijing, China Email: 13501297605@163.com Telephone: +86 13502197605 |
| Notes | None |
ChiCTR‐INR‐16009781.
| Trial name or title | The clinical application and significance of ocular surface function and tear lipid layer examination |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Exclusions after randomisation? Not reported Percentage of participant follow‐up: not applicable Study duration (of intervention): not reported Was a sample size calculation reported: not reported |
| Participants |
Baseline characteristics Standard therapy group
IPL group
LipiFlow
Overall
Inclusion criteria: aged 18–80 years; willing to follow study protocol; diagnosed with MGD; SPEED score ≥ 6; meibomian gland secretion score ≤ 12 for 15 glands of the lower lid; complete informed consent. Exclusion criteria: pregnant or breastfeeding woman; SPEED score ≥ 15; meibomian gland dropout area of any lower lid ≥ 50%; any co‐existing ocular conditions that could interfere with dry eye (e.g. use of systemic antihistamines, anti‐inflammatory drugs or corticosteroids); any co‐existing ocular conditions that could interfere with treatment (e.g. Fitzpatrick skin type VI). Significant pretreatment baseline differences? Not reported Severity of dry eye: not reported |
| Interventions |
Standard therapy
IPL
LipiFlow
|
| Outcomes |
Primary outcomes:
Secondary outcome:
(Time points not reported in clinical trial registry) |
| Starting date | Not yet recruiting (as of 18 September 2019) |
| Contact information | Study leader: Billian Ke 100 Haining Road, Hongkou District, Shanghai, China Email: kebilian@126.com Telephone: +86 13386259873 |
| Notes | None |
ChiCTR‐IOR‐17013767.
| Trial name or title | The treatment of intense pulsed light for meibomian gland dysfunction reduced dry eye |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Exclusions after randomisation? Not applicable Percentage of participant follow‐up: not applicable Study duration (of intervention): not reported Was a sample size calculation reported: no |
| Participants |
Baseline characteristics IPL + warm massage group
Warm massage (standard therapy) group
IPL only
Overall
Inclusion criteria: aged > 18 years; Fitzpatrick grade 1–4; SPEED rating > 6; MGD rating ≤ 12; TBUT ≤ 10 seconds; corneal fluorescein staining score ≥ 1, if TBUT ≤ 5 seconds corneal fluorescein staining score can be ignored. Exclusion criteria: lactating or pregnant; contact lens wearers; infection of ocular surface; scar or keratinisation of lids; ocular or eyelid surgery in 6 months; neural paralyses of face in 6 months; lacrimal duct plugs; LASIK surgery in 6 months. Significant pretreatment baseline differences? Not applicable Severity of dry eye: not reported |
| Interventions |
IPL + warm massage
Standard therapy
IPL
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Starting date | Recruiting status: recruiting (as at 18 September 2019) |
| Contact information | Study leader: Wei Chen The Eye Hospital of Wenzhou Medical University, 270 Xueyuan Road West, Wenzhou, Zhejiang, China Email: chenweimd@hotmail.com Telephone: +86 13757728118 |
| Notes | None |
ChiCTR1800014775.
| Trial name or title | Comparative study of the effects of intense pulse light and traditional massage on the subcutaneous nerve plexus and dendritic cells in the cornea of MGD patients |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Exclusions after randomisation? Not applicable Percentage of participant follow‐up: not applicable Study duration (of intervention): not reported Was a sample size calculation reported: no |
| Participants |
Baseline characteristics Hot compress + massage (standard therapy) group
IPL group
Overall
Inclusion criteria: adults; diagnosed with MGD (> stage 1, according to the 2011 International Workshop on MGD; had not conducted eyelid hygiene or undergone any alternative treatments for ≥ 3 months. Diagnostic criteria: symptoms of ocular discomfort, such as eye irritation that limited activities; clinical signs: meibum quality grade ≥ 4 or MGX ≥ 1 Exclusion criteria: previous ocular surgery or trauma (excluding chalazion section); blepharal dysraphism; history of blepharal and periorbital skin disease in 1 month; acute inflammation Significant pretreatment baseline differences? Not applicable Severity of dry eye: not reported |
| Interventions |
Standard therapy
IPL
|
| Outcomes |
Primary outcomes:
Secondary outcome:
|
| Starting date | Recruiting status: recruiting (as of 18 September 2019) |
| Contact information | Study leader: Jun Cheng Shandong Eye Institute, Qingdao Eye Hospital, 5 Yanerdao Road, Qingdao, Shandong, China Email: alice.567@163.com Telephone: +86 18653280868 |
| Notes | None |
ChiCTR1800019782.
| Trial name or title | The effect of Intense pulsed light on moderate meibomian gland dysfunction |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Exclusions after randomisation? Not applicable Percentage of participant follow‐up: not applicable Study duration (of intervention): not reported Was a sample size calculation reported: no |
| Participants |
Baseline characteristics IPL + MGX group
MGX (standard therapy) group
Overall
Inclusion criteria: aged ≥ 18 years; SPEED score ≥ 6 points; eyelid margin obtuse or hypertrophy or new blood vessels; meibomian gland orifices obstruction, uplift or lipid suppository form; meibum quality score of a single eyelid 5–10 points; Schimer test > 5 mm Exclusion criteria: eye infection, surgery or trauma in past 6 months; eyelid closure insufficiency, entropion or ectropion, etc.; Fitzpatrick skin category 5–6; treatment before 4 weeks with a tan or tanning, tender skin treatment or have been too sensitive or allergic symptoms; pretreatment area is skin cancer or pigmentary lesions; in past month have dry eye physical therapy or point with anti‐inflammatory drugs; systemic immune‐related diseases, such as Sjogren's syndrome, Stevens‐Johnson syndrome, rheumatism, etc.; nerve lesions, such as trigeminal nerve tumours, surgery or trauma, virus damage, etc.; people with metabolic diseases such as diabetes mellitus, hypothyroidism and thyroid hyperfunction, etc. Significant pretreatment baseline differences? Not applicable Severity of dry eye: not reported |
| Interventions |
IPL + MGX
Standard therapy only
|
| Outcomes |
Primary outcomes:
|
| Starting date | Recruiting status: recruiting (as of 18 September 2019) |
| Contact information | Study leader: Zeng Qingyan Hankou Aier Eye Hospital, 328 Machang Road, Jianghan District, Wuhan, Hubei, China Email: zengqingyan1972@163.com Telephone: +86 13971009610 |
| Notes | None |
ChiCTR1900021273.
| Trial name or title | Clinic results of intraductal meibomian gland probing combined intense pulsed light in treating patients with refractory obstructive meibomian gland dysfunction: a randomized controlled trial |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Exclusions after randomisation? Not applicable Percentage of participant follow‐up: not applicable Study duration (of intervention): not reported Was a sample size calculation reported: not reported |
| Participants |
Baseline characteristics IPL + intraductal probing group
Intraductal probing group
IPL
Overall
Inclusion criteria: aged > 18 years; Fitzpatrick skin type 1–4; SPEED questionnaire ≥ 6; meibum grade ≤ 24 and more than half of the 15 evaluated meibomian gland orifices in each eyelid were obstructed and had no lipid secretion with extrusion; TBUT ≤ 5 seconds; Schirmer test > 5 seconds; Meibo‐Scan (OCULUS) showed the atrophy area of meibomian gland in both upper and lower eyelids < 1/3 of the total area; did not have symptom relief with conservative treatment (eyelid warming, massage and artificial tears) for ≥ 1 year before study treatment; sign informed consent Exclusion criteria: history of corneal contact lens, mite blepharitis, acute eye inflammation or infection and apparent eyelid margin scar as well as patients using lacrimal plug or receiving LASIK Significant pretreatment baseline differences? Not applicable Severity of dry eye: not specifically reported, although intends to recruit people with "refractory obstructive meibomian gland dysfunction." |
| Interventions |
IPL + intraductal probing
Standard therapy only
IPL only
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Starting date | Recruiting status: recruiting (as at 18 September 2019) |
| Contact information | Study leader: Jin Xiuming Eye Center, Affiliated Second Hospital, School of Medicine, Zhejiang University, 88 Jiefang Road, Shangcheng District, Hangzhou, China Email: lzyjxm@zju.edu.cn Telephone: +86 571 87783897 |
| Notes | None |
NCT02958514.
| Trial name or title | Effectiveness: comparison of two kinds of treatment in treating dry eye caused by meibomian gland dysfunction |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Exclusions after randomisation? Not applicable Percentage of participant follow‐up: not applicable Study duration (of intervention): 12 weeks with 3 cycles of IPL of 4 weeks' interval and artificial tears 4 times daily Was a sample size calculation reported: no |
| Participants |
Baseline characteristics IPL group
Topical antibiotics (standard therapy) group
Overall
Inclusion criteria: people with dry eye syndrome and diagnosed as MGD Exclusion criteria: infection or inflammatory disease; ocular surgical history within last 3 months; Sjogren's syndrome Significant pretreatment baseline differences? Not applicable Severity of dry eye: not reported |
| Interventions |
IPL
Topical antibiotic
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Starting date | First posted: 8 November 2016; no further updates. |
| Contact information | Responsible party: Hong Qi Peking University Third Hospital, 49 Huayuan N Road, Haidian District, Beijing, China |
| Notes | Open‐label trial |
NCT03089580.
| Trial name or title | Intense pulsed light study for dry eye disease |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Exclusions after randomisation? Not specified Percentage of participant follow‐up: not applicable Study duration (of intervention): 4 months (for primary outcome), 7 months (for secondary outcome) Was a sample size calculation reported? (Yes/No): no |
| Participants |
Baseline characteristics IPL group
Sham (control) group
Overall
Inclusion criteria: willing and able to provide informed consent; diagnosed with evaporative dry eye disease with symptoms for ≥ 6 months; able and willing to comply with follow‐up visits, telephone calls and IPL treatments; agree to using an effective method of birth control during course of study; agree to continue current dry eye treatments during course of study; Fitzpatrick skin scale of 1 (very fair) to 4 (olive) as determined by investigator Exclusion criteria: Fitzpatrick scale 5 and 6 as determined by investigator; neurotrophic keratitis; ectropion, trauma or any other lid abnormalities; previous diagnosis of Stevens‐Johnson syndrome or GVHD; ocular burn, active ocular infection or active ocular inflammation; currently pregnant or trying to become pregnant in next 5 months; systemic conditions or currently taking medications which makes light therapy contraindicated (the use of doxycycline is allowed); tattoos in the treatment area; people who have had IPL therapy, LipiFlow or Meibothermoflo within past 6 months; contact lens wear > 1 time/week or history of refractive surgery; glaucoma drop use; ophthalmic steroid use within past 30 days; punctal plugs if instilled within 30 days of the start of the study; obvious asymmetry between the 2 eyes deemed significant by the investigators (such as punctal plugs or cautery in only 1 eye, etc); history of trabeculectomy or tube surgery; uncontrolled ocular or systemic disease; ocular or eyelid surgery within the last 6 months; any condition which leads the investigator to believe that the person cannot comply with the study requirements or the person may be placed at risk with participation, or both. Significant pretreatment baseline differences? Not reported Severity of dry eye: not reported |
| Interventions |
IPL
Control
|
| Outcomes |
Primary outcome:
Secondary outcome measure:
|
| Starting date | 27 March 2017 |
| Contact information | Sarah Wood University of Michigan Kellogg Eye Center, University of Michigan, Ann Arbor, Michigan, US, 48105 Email: not reported Telephone: not reported |
| Notes | None |
NCT03194698.
| Trial name or title | Efficacy of IPL treatment of dry eye and ocular rosacea |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Exclusions after randomisation? (If Yes, provide relevant details from the paper): not reported Percentage of participant follow‐up (include details from all intervention groups): not reported Study duration (of intervention): 4 months Was a sample size calculation reported? (Yes/No): no |
| Participants |
Baseline characteristics IPL group
Control group
Overall
Inclusion criteria: dry eye of moderate severity with ocular rosacea diagnosed by ophthalmologist; dry eye symptoms must be alleviated with topical anaesthetic; must have ≥ 50% meibomian glands viable on meibography; contact lenses and refractive surgery are permitted; aged 18–100 years; either gender Exclusion criteria: healthy volunteers; contraindications of severe ocular surface disease or inability to be safely treated with IPL; GVHD, Stevens‐Johnson syndrome, active allergic conjunctivitis or other conjunctivitis, alkali burn history; new treatments for dry eye in past 6 months Significant pretreatment baseline differences? Not reported Severity of dry eye: moderate |
| Interventions |
IPL
Control
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Starting date | 17 August 2017 |
| Contact information | Joanne F Shen Department of Ophthalmology – Arizona, Mayo Clinic, Scottsdale, Arizona, US, 85259 Email: not reported Telephone: not reported |
| Notes | None |
NCT03265652.
| Trial name or title | IPL and MGX versus MGX alone in the treatment of dry eye disease secondary to MGD |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Exclusions after randomisation? (If Yes, provide relevant details from the paper): not applicable Percentage of participant follow‐up (include details from all intervention groups): not applicable Study duration (of intervention): 10 weeks Was a sample size calculation reported? (Yes/No): no |
| Participants |
Baseline characteristics IPL group
Control group
Overall
Inclusion criteria: able to read, understand and sign an informed consent form; aged ≥ 18 years; Fitzpatrick skin type I–IV; SPEED questionnaire ≥ 10; OSDI questionnaire ≥ 23; in both eyes, ≥ 5 non‐atrophied meibomian glands on the lower eyelid; in both eyes, tear BUT ≤ 7 seconds; in both eyes, MGA (total MGS for 15 glands of the lower eyelid) ≤ 12. Exclusion criteria: contact lens wear within the month prior to screening; unwilling to discontinue use of contact lenses for duration of study; ocular surgery or eyelid surgery within 6 months prior to screening; neuro‐paralysis in the planned treatment area within 6 months prior to screening; other uncontrolled eye disorders affecting the ocular surface, for example active allergies; current use of punctal plugs; precancerous lesions, skin cancer or pigmented lesions in the planned treatment area; uncontrolled infections or uncontrolled immunosuppressive diseases; ocular infections within 6 months prior to screening; history of cold sores or rashes in perioral area or in the planned treatment area that could be stimulated by light at a wavelength 560–1200 nm (e.g. Herpes simplex 1 or 2, systemic lupus erythematosus, porphyria); use of photosensitive medication or herbs that may cause sensitivity to 560–1200 nm light exposure, such as isotretinoin, tetracycline, doxycycline, or St John's wort within 3 months prior to screening; overexposure to sun within 4 weeks prior to screening, in the judgement of the investigator; administration of prescription eye drops for dry eye within 7 days prior to screening, excluding artificial tears and glaucoma drops; radiotherapy to the head or neck within 12 months prior to screening, or planned radiotherapy within 8 weeks after completion of all IPL treatments; treatment with chemotherapeutic agent within 8 weeks prior to screening, or planned chemotherapy within 8 weeks after completion of all IPL treatments; new topical treatments within the area to be treated, or oral therapies within 3 months prior to screening, except non‐prescription paracetamol‐based analgesics (such as Extra Strength Tylenol®) for pain management after study treatment, new oral omega 3 fatty acid supplements and topical artificial tears; change in dosage of any systemic medication within 3 months prior to screening; anticipated relocation or extensive travel outside of the local study area preventing compliance with follow‐up over the study period; legally blind in either or both eyes; history of migraines, seizures or epilepsy; IPL treatment within 12 months prior to screening; LipiFlow treatment, or any other thermal treatment of the eyelids, within 6 months prior to screening; expression of the meibomian glands within 6 months prior to screening; any condition revealed during the eligibility screening process whereby the investigator deems the person inappropriate for the study; women below the age of menopause (50 years of age). Significant pretreatment baseline differences? Not reported Severity of dry eye: not reported |
| Interventions |
IPL
Control
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Other outcomes:
|
| Starting date | 30 March 2018 |
| Contact information | David Zadok Shmu'el Bait St 12, Jerusalem, 9103102, Israel Email: not reported Telephone: not reported |
| Notes | None |
NCT03396913.
| Trial name or title | Effectiveness of intense pulsed light for improving dry eye syndrome |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Exclusions after randomisation? (If Yes, provide relevant details from the paper): not applicable Percentage of participant follow‐up (include details from all intervention groups): not applicable Study duration (of intervention): 10 weeks Was a sample size calculation reported? (Yes/No): no |
| Participants |
Baseline characteristics IPL group
Control group
Overall
Inclusion criteria: able to read, understand and sign an informed consent form; aged 22̵–85 years; able and willing to comply with the treatment/follow‐up schedule and requirements; in the study eye, TBUT ≤ 7 seconds; in the study eye, MGS ≤ 12; in the study eye, ≥ 5 non‐atrophied meibomian glands in the lower eyelid; symptoms self‐assessed using the OSDI questionnaire ≥ 23 Exclusion criteria: Fitzpatrick skin type V or VI; contact lens wear within month prior to screening; unwilling to discontinue use of contact lenses for duration of study; ocular surgery or eyelid surgery, within 6 months prior to screening; neuro‐paralysis in the planned treatment area, within 6 months prior to screening; other uncontrolled eye disorders affecting the ocular surface, e.g. active allergies; current use of punctal plugs; precancerous lesions, skin cancer or pigmented lesions in the planned treatment area; uncontrolled infections or uncontrolled immunosuppressive diseases; ocular infections, within 6 months prior to screening; history of cold sores or rashes in the perioral area or in the planned treatment area that could be stimulated by light at a wavelength of 560–1200 nm, including: Herpes simplex 1 or 2, systemic lupus erythematosus, and porphyria; within 3 months prior to screening, use of photosensitive medication or herbs that may cause sensitivity to 560–1200 nm light exposure, including: isotretinoin, tetracycline, doxycycline, and St John's wort; overexposure to sun, within 4 weeks prior to screening; use of prescription eye drops for dry eye, within 7 days prior to screening, excluding artificial tears and glaucoma drops; radiotherapy to the head or neck, within 12 months prior to screening; planned radiotherapy, within 8 weeks after the last treatment session; treatment with chemotherapeutic agent, within 8 weeks prior to screening; planned chemotherapy, within 8 weeks after the last treatment session; new topical treatments within the area to be treated, or oral therapies, within 3 months prior to screening except non‐prescription paracetamol‐based analgesics for pain management, new oral omega 3 fatty acid supplements and topical artificial tears; change in dosage of any systemic medication, within 3 months prior to screening; anticipated relocation or extensive travel outside of the local study area preventing compliance with follow‐up over the study period; legally blind in either eye; history of migraines, seizures or epilepsy; facial IPL treatment, within 12 months prior to screening; any thermal treatment of the eyelids, including LipiFlow, within 6 months prior to screening; expression of the meibomian glands, within 6 months prior to screening; In either eye, moderate‐to‐severe (Grade 3–4) inflammation of the conjunctiva, including: allergic, vernal or giant papillary conjunctivitis; in either eye, severe (Grade 4) inflammation of the eyelid, including: blepharochalasis, staphylococcal blepharitis or seborrhoeic blepharitis; ocular surface abnormality that may compromise corneal integrity in either eye (e.g. prior chemical burn, recurrent corneal erosion, corneal epithelial defect, Grade 3 corneal fluorescein staining or map dot fingerprint dystrophy); eyelid abnormalities that affect lid function in either eye, including: entropion, ectropion, tumour, oedema, blepharospasm, lagophthalmos, severe trichiasis and severe ptosis; any systemic condition that may cause dry eye disease, including: Stevens‐Johnson syndrome, vitamin A deficiency, rheumatoid arthritis, Wegener's granulomatosis, sarcoidosis, leukaemia, Riley‐Day syndrome, systemic lupus erythematosus and Sjögren's syndrome; unwilling or unable to abstain from the use of medications known to cause eye dryness (e.g. isotretinoin, antihistamines) throughout the study duration. People must discontinue these medications for ≥ 1 month prior to the baseline visit. Any condition revealed whereby the investigator deems the person inappropriate for this study. Significant pretreatment baseline differences? Not reported Severity of dry eye: not reported |
| Interventions |
IPL
Control
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Other outcomes:
|
| Starting date | 10 January 2018 |
| Contact information | Rolando Toyos Toyos Clinic, Nashville, Tennessee, US, 37215 Email: Contact Dillon O'Brien, dobrien@toyosclinic.com Telephone: Contact Dillon O'Brien, 615‐327‐4015 |
| Notes | None |
NCT03518398.
| Trial name or title | Effectiveness and safety of intense pulsed light in patients with meibomian gland dysfunction |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Exclusions after randomisation? Not applicable Percentage of participant follow‐up: not applicable Study duration (of intervention): 45 days Was a sample size calculation reported: not reported |
| Participants |
Baseline characteristics IPL group
Sham IPL group
Overall
Inclusion criteria: able to read, understand and sign an informed consent form; aged 18–80 years; Fitzpatrick skin type 1–5; able and willing to comply with the treatment/follow‐up schedule and requirements; presence of meibomian gland on each lower eyelid's meibography; current diagnosis of stage1–4 of MGD in both eyes, according to the International Workshop on Meibomian Gland Dysfunction: Report of the Subcommittee on Management and Treatment of Meibomian Gland Dysfunction. Exclusion criteria: contact lens wearer within past month and throughout the study; recent ocular surgery or eyelid surgery within past 6 months; neuro‐paralysis in the planned treatment area within past 6 months; current use of punctual plugs; precancerous lesions, skin cancer or pigmented lesions in the planned treatment area; uncontrolled infections or uncontrolled immunosuppressive diseases; undergone refractive surgery within past 6 months; diseases in the planned treatment area that could be stimulated by light at 560–1200 nm (e.g. Herpes simplex 1 and 2, systemic lupus erythematosus, porphyria); use of photosensitive medications or herbs that may cause sensitivity to 560–1200 nm light exposure, such as isotretinoin, tetracycline or St John's wort; pregnancy and lactation; radiotherapy to the head or neck within past year, or planned radiotherapy throughout study period; treatment with chemotherapeutic agent within past 8 weeks, or planned chemotherapy throughout study period; any condition revealed during the eligibility screening process whereby the physician deems the person inappropriate for this study; declared legally blind in 1 eye; IPL treatment within past 12 months; LipiFlow treatment, or any equivalent treatments, within past 12 months; Any anti‐glaucomatous eye drop uses within past 3 months and throughout study period. Significant pretreatment baseline differences? Not applicable Severity of dry eye: not reported |
| Interventions |
IPL
Sham IPL
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Starting date | 3 July 2018 and completed 2 April 2019 |
| Contact information | Principal Investigator: Yonrawee Piyacomn Department of Ophthalmology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand, 10330 |
| Notes | None |
NCT03950115.
| Trial name or title | Effects and prognostic factors of intensive pulse light treatment for meibomian gland dysfunction |
| Methods |
Study design: randomised controlled trial Study grouping: cross‐over Exclusions after randomisation? Not applicable Percentage of participant follow‐up: not applicable Study duration (of intervention): 8 weeks Was a sample size calculation reported: not reported |
| Participants |
Baseline characteristics IPL + MGX group
IPL + MGX (active comparator) group
Overall
Inclusion criteria: clinical diagnosis of MGD Exclusion criteria: medical conditions in which IPL is contraindicated (pregnancy, breastfeeding, lupus and any major uncontrolled health problem); contact lens wearer; previous ocular surgery; previous thermal treatment for dry eye disease (e.g. LipiFlow) Significant pretreatment baseline differences? Not reported Severity of dry eye: not reported |
| Interventions |
IPL + MGX
IPL + MGX (active comparator)
|
| Outcomes |
Primary outcomes:
|
| Starting date | Start date: 18 April 2019 |
| Contact information | Responsible party: Prof Tae‐Young Chung Samsung Medical Center, Seoul, Republic of Korea |
| Notes | None |
BUT: break‐up time; GVHD: graft‐versus‐host disease; IL: interleukin; IPL: intense pulsed light; LLT: lipid layer thickness; MGA: meibomian gland area; MGD: meibomian gland dysfunction; MGP: meibomian gland probing; MGS: Meibomian Gland Score; MGX: meibomian gland expression; MGYLSS: Meibomian Glands Yielding Secretion Score; OSDI: Ocular Surface Disease Index; RNA: ribonucleic acid; SANDE: Symptom Assessment in Dry Eye; SPEED: Standard Patient Evaluation of Eye Dryness; TBUT: tear film break‐up time; TGF: transforming growth factor; TMH: tear meniscus height.
Differences between protocol and review
The review protocol was prospectively registered in Downie 2018. There were no differences between the registered protocol and how the review was performed.
Contributions of authors
LED conceived the systematic review concept.
The full systematic review protocol was developed by SNC, VGA, AM, AM‐XL, CL JAO, KSN and LED, with input into the statistical methods from LB and input into the search strategies from ACZ.
The study screening, risk of bias assessment and data extractions were performed by SNC, ACZ, VGA, AM, AM‐XL, CL, JAO, KSN and LED.
All authors contributed to the drafting and revisions of the systematic review.
All authors approved the final version of the manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This project was supported by the UK National Institute for Health Research, through Cochrane Infrastructure funding. The views and opinions expressed are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
Declarations of interest
SC: none.
ACZ: none.
VA: none.
AM: none.
CL: none.
JO: none.
KN: none.
LB: none.
LED: has received research funding for dry eye clinical trials, not related to IPL therapy, from Allergan Pty Ltd, Alcon Pty Ltd and Azura Ophthalmics Pty Ltd, and has collaborated and co‐published with one of the authors of a study included in this review (Jennifer Craig), for non‐IPL studies.
New
References
References to studies included in this review
Arita 2019 {published data only}
- Arita R, Fukuoka S, Morishige N. Therapeutic efficacy of intense pulsed light in patients with refractory meibomian gland dysfunction. Ocular Surface 2019;17(1):104‐10. [DOI: 10.1016/j.jtos.2018.11.004] [DOI] [PubMed] [Google Scholar]
Craig 2015 {published data only}
- ACTRN12614000162617. Effect of intense pulsed light technology in treating meibomian gland dysfunction (MGD). anzctr.org.au/Trial/Registration/TrialReview.aspx?id=365741 (first received 4 February 2014).
- Craig J, Turnbull P, Chen A. Placebo‐controlled trial of intense pulsed light (IPL) therapy for meibomian gland dysfunction (MGD). Clinical and Experimental Ophthalmology 2014;1:66. [DOI: 10.1111/ceo.12449] [DOI] [Google Scholar]
- Craig JP, Chen YH, Trunbull PR. Cumulative effect of intense pulsed light (IPL) therapy for meibomian gland dysfunction (MGD) confirmed in prospective, double‐masked, placebo controlled trial. Investigative Ophthalmology and Visual Science 2015;56(7):6194. [DOI] [PubMed] [Google Scholar]
- Craig JP, Chen YH, Turnbull PR. Prospective trial of intense pulsed light for the treatment of meibomian gland dysfunction. Investigative Ophthalmology and Visual Science 2015;56(3):1965‐70. [DOI: 10.1167/iovs.14-15764] [DOI] [PubMed] [Google Scholar]
Rong 2017 {published data only}
- ChiCTR‐INR‐16010256. Therapeutic effect of intense pulsed light therapy on meibomian gland dysfunction. chictr.org.cn/showprojen.aspx?proj=17444 (first received 26 December 2016).
- Liu R, Rong B, Tu P, Tang Y, Song W, Toyos R, et al. Analysis of cytokine levels in tears and clinical correlations after intense pulsed light treating meibomian gland dysfunction. American Journal of Ophthalmology 2017;183:81‐90. [DOI: 10.1016/j.ajo.2017.08.021] [DOI] [PubMed] [Google Scholar]
- Rong B, Tang Y, Liu R, Tu P, Qiao J, Song W, et al. Long‐term effects of intense pulsed light combined with meibomian gland expression in the treatment of meibomian gland dysfunction. Photomedicine and Laser Surgery 2018;36(10):562‐7. [DOI] [PubMed] [Google Scholar]
- Rong B, Tang Y, Tu P, Liu R, Qiao J, Song W, et al. Intense pulsed light applied directly on eyelids combined with meibomian gland expression to treat meibomian gland dysfunction. Photomedicine and Laser Surgery 2018;36(6):326‐32. [DOI: 10.1089/pho.2017.4402] [DOI] [PubMed] [Google Scholar]
- Rong B, Tu P, Tang Y, Liu RX, Song WJ, Yan XM. Evaluation of short‐term effect of intense pulsed light combined with meibomian gland expression in the treatment of meibomian gland dysfunction. Chung‐Hua Yen Ko Tsa Chih [Chinese Journal of Ophthalmology] 2017;53(9):675‐81. [DOI: 10.3760/cma.j.issn.0412-4081.2017.09.008] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ChiCTR1800014847 {published data only}
- ChiCTR1800014847. Evaluation of therapeutic effect and exploration of mechanism of intense pulsed light combined with meibomian gland massage in the treatment of meibomian gland dysfunction. chictr.org.cn/showproj.aspx?proj=24566 (first received 9 February 2018).
ChiCTR1900020576 {published data only}
- ChiCTR1900020576. The safety and efficacy of broadband light (BBL) compared with narrow spectrum intense pulsed light for the treatment of meibomian gland dysfunction (MGD). chictr.org.cn/showproj.aspx?proj=34518 (first received January 2019).
ChiCTR‐ONC‐17010867 {published data only}
- ChiCTR‐ONC‐17010867. Intense pulsed light treatment for dry eye disease due to meibomian gland function. chictr.org.cn/showprojen.aspx?proj=18418 (first received 14 March 2017).
ChiCTR‐ONN‐17013864 {published data only}
- ChiCTR‐ONN‐17013864. The effect of optimized pulsed light technology assisted therapy for moderate and severe blepharitis associated keratoconjunctivitis. chictr.org.cn/showproj.aspx?proj=23850 (first received 12 December 2017).
ChiCTR‐OON‐15007125 {published data only}
- ChiCTR‐OON‐15007125. The effect of orbital intense pulsed light treatment on ocular surface. chictr.org.cn/showproj.aspx?proj=11993 (first received 23 September 2015).
Li 2019 {published data only}
- Li D, Lin S, Cheng B. Intense pulsed light treatment for meibomian gland dysfunction in skin types III/IV. Photobiomodulation Photomedicine and Laser Surgery 2019;37(2):70‐6. [10.1089/photob.2018.4509] [DOI] [PubMed] [Google Scholar]
NCT01917539 {unpublished data only}
- NCT01917539. Efficacy of pulsed light therapy for meibomian gland dysfunction and dry eye syndrome. clinicaltrials.gov/show/nct01917539 (first received 6 August 2013).
NCT02066051 {published data only}
- NCT02066051. IPL and meibomian gland expression to treat ocular rosacea ocular GVHD. clinicalTrials.gov/show/NCT02066051 (first received 25 November 2015).
NCT02621593 {published data only}
- NCT02621593. Feasibility of IPL for reducing dry eye symptoms caused by MGD. clinicaltrials.gov/show/NCT02621593 (first received 3 December 2015).
NCT02992535 {published data only}
- NCT02992535. Effect of intense pulse light (IPL) treatment on tear film osmolarity. clinicaltrials.gov/show/NCT02992535 (first received 14 December 2016).
NCT03658811 {published data only}
- NCT0365881. Intense pulse light treatment with meibomian gland expression of the upper eyelids in dry eye disease. clinicaltrials.gov/ct2/show/NCT0365881 (first received 5 September 2018).
NCT03788486 {published data only}
- NCT03788486. Efficacy and safety of meibomian gland dysfunction and dry eye with an led blue treatment device. clinicaltrials.gov/ct2/show/NCT03788486 (first received 27 December 2018).
Zhang 2019 {published data only}
- Zhang X, Song N, Gong Lan. Therapeutic effect of intense pulsed light on ocular demodicosis. Current Eye Research 2019;44(3):250‐6. [DOI: 10.1080/02713683.2018.1536217] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12616000667415 {published data only}
- ACTRN12616000667415. Evaluation of intense pulsed light therapy for dry eye relief. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=370710 (first received 16 May 2016).
ChiCTR1800014775 {published data only}
- ChiCTR1800014775. Comparative study of the effects of intense pulse light and traditional massage on the subcutaneous nerve plexus and dendritic cells in the cornea of MGD patients. chictr.org.cn/showprojen.aspx?proj=25223 (first received 4 February 2018).
ChiCTR‐1800014787 {published data only}
- ChiCTR1800014787. A prospective, multi‐center, randomized, and controlled clinical trial to evaluate the effectiveness and safety of intense pulsed light and laser system (M22) in dry eye patients caused by meibomian gland dysfunction (MGD) compared to basic physical therapy. 2018. chictr.org.cn/showprojen.aspx?proj=25123 (first received 5 February 2018).
ChiCTR1800019782 {published data only}
- ChiCTR1800019782. The effect of Intense pulsed light on moderate meibomian gland dysfunction. chictr.org.cn/showproj.aspx?proj=33393 (first received 28 November 2018).
ChiCTR1900021273 {published data only}
- ChiCTR1900021273. Clinic results of intraductal meibomian gland probing combined intense pulsed light in treating patients with refractory obstructive meibomian gland dysfunction: a randomized controlled trial. chictr.org.cn/showproj.aspx?proj=34935 (first received 9 February 2019). [DOI] [PMC free article] [PubMed]
ChiCTR‐INR‐16009781 {published data only}
- ChiCTR‐INR‐16009781. The clinical application and significance of ocular surface function and tear lipid layer examination. chictr.org.cn/showproj.aspx?proj=16643 (first received 8 November 2016).
ChiCTR‐IOR‐17013767 {published data only}
- ChiCTR‐IOR‐17013767. The treatment of intense pulsed light for meibomian gland dysfunction reduced dry eye. chictr.org.cn/showproj.aspx?proj=23707 (first received 8 December 2017).
NCT02958514 {published data only}
- NCT02958514. Efficacy comparison of two kinds of treatment in treating dry eye caused by meibomian gland dysfunction. clinicalTrials.gov/show/NCT02958514 (first received 8 November 2016).
NCT03089580 {published data only}
- NCT03089580. Intense pulsed light study for dry eye disease. clinicaltrials.gov/ct2/show/NCT03089580 (first received 1 March 2017).
NCT03194698 {published data only}
- NCT03194698. Efficacy of IPL treatment of dry eye and ocular rosacea. clinicaltrials.gov/ct2/show/NCT03194698 (first received 21 June 2017).
NCT03265652 {published data only}
- NCT03265652. IPL and MGX versus MGX alone in the treatment of dry eye disease secondary to MGD. clinicaltrials.gov/ct2/show/NCT03265652 (first received 29 August 2017).
NCT03396913 {published data only}
- NCT03396913. Effectiveness of intense pulsed light for improving dry eye syndrome. clinicaltrials.gov/ct2/show/NCT03396913 (first received 11 January 2011).
NCT03518398 {published data only}
- NCT03518398. Effectiveness and safety of intense pulsed light in patients with meibomian gland dysfunction. clinicaltrials.gov/show/NCT03518398 (first received 8 May 2018).
NCT03950115 {published data only}
- NCT03950115. Effects and prognostic factors of intensive pulse light treatment for meibomian gland dysfunction. clinicaltrials.gov/show/NCT03950115 (first received 15 May 2019).
Additional references
Albietz 2018
- Albietz JM, Schmid KL. Intense pulsed light treatment and meibomian gland expression for moderate to advanced meibomian gland dysfunction. Clinical and Experimental Optometry 2018;101(1):23‐33. [DOI] [PubMed] [Google Scholar]
Anderson 1983
- Anderson RR, Parrish RR. Selective photothermolysis: precise microsurgery by selective absorption of pulse radiation. Science 1983;220:524‐7. [DOI] [PubMed] [Google Scholar]
Arita 2008
- Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age‐related changes of the meibomian glands in a normal population. Ophthalmology 2008;115(5):911‐5. [DOI] [PubMed] [Google Scholar]
Blackie 2009
- Blackie CA, Solomon JD, Scaffidi RC, Greiner JV, Lemp MA, Korb DR. The relationship between dry eye symptoms and lipid layer thickness. Cornea 2009;28:789‐94. [DOI] [PubMed] [Google Scholar]
Borchman 2011
- Borchman D, Foulks GN, Yappert MC, Bell J, Wells E, Neravetla S, et al. Human meibum lipid conformation and thermodynamic changes with meibomian‐gland dysfunction. Investigative Ophthalmology & Visual Science 2011;52(6):3805‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bron 2017
- Bron AJ, Paiva C, Chauhan SK, Bonini S, Gabison EE, Jain S, et al. TFOS DEWS II pathophysiology report. Ocular Surface 2017;15:438‐510. [DOI: 10.1016/j.jtos.2017.05.011] [DOI] [PubMed] [Google Scholar]
Cho 1995
- Cho P, Douthwaite W. The relation between invasive and noninvasive tear break‐up time. Optometry Visual Science 1995;72:17‐22. [DOI] [PubMed] [Google Scholar]
Craig 2017
- Craig JP, Nichols KK, Akpek EK, Caffrey B, Dua HS, Joo CK, et al. TFOS DEWS II definition and classification report. Ocular Surface 2017;15:276‐83. [DOI: ] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Dell 2017
- Dell SJ. Intense pulsed light for evaporative dry eye disease. Clin Ophthalmol 2017;11:1167‐73.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Downie 2019
- Downie LE, Ng S, Lindsley K, Akpek E. Omega‐3 and omega‐6 polyunsaturated fatty acids for dry eye disease. Cochrane Database of Systematic Reviews 2019, Issue 12. [DOI: 10.1002/14651858.CD011016.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Epitropoulos 2016
- Epitropoulos AT, Donnenfeld ED, Shah ZA, Holland EJ, Gross M, Faulkner WJ, et al. Effect of oral re‐esterified omega‐3 nutritional supplementation on dry eyes. Cornea 2016;35(9):1185‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Finis 2014
- Finis D, Pischel N. Konig C, Hayajneh J, Borrelli M, Schrader S, et al. Comparison of the OSDI and SPEED questionnaires for the evaluation of dry eye disease in clinical routine. Ophthalmologe 2014;111:1050‐6. [DOI] [PubMed] [Google Scholar]
Fitzpatrick 1975
- Fitzpatrick TB. Sun and skin [Soleil et peau]. Journal de Médecine Esthétique 1975;2:33‐4. [Google Scholar]
Foulks 2003
- Foulks GN, Bron AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocular Surface 2003;1(3):107‐26. [DOI] [PubMed] [Google Scholar]
Friedman 2010
- Friedman NJ. Impact of dry eye disease and treatment on quality of life. Current Opinion in Ophthalmology 2010;21(4):310‐6. [DOI] [PubMed] [Google Scholar]
Galor 2014
- Galor A. MGD: definition versus dry eye disease, risk factors. Current Ophthalmology Reports 2014;2(2):58‐64. [Google Scholar]
Geerling 2011
- Geerling G, Tauber J, Baudouin C, Goto E, Matsumoto Y, O'Brien T, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Investigative Ophthalmology Visual Science 2011;52:2050‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Geerling 2017
- Geerling G, Baudouin C, Aragona P, Rolando M, Boboridis K, Benitez‐del‐Castillo J, et al. Emerging strategies for the diagnosis and treatment of meibomian gland dysfunction: proceedings of the OCEAN group meeting. Ocular Surface 2017;15(2):179‐92. [DOI] [PubMed] [Google Scholar]
Goldberg 2012
- Goldberg DJ. Current trends in intense pulsed light. Journal of Clinical and Aesthetic Dermatology 2012;5(6):45‐53. [PMC free article] [PubMed] [Google Scholar]
Gupta 2016
- Gupta PK, Vora GK, Matossian C, Kim M, Stinnett S. Outcomes of intense pulsed light therapy for treatment of evaporative dry eye disease. Canadian Journal of Ophthalmology 2016;51(4):249‐53. [DOI] [PubMed] [Google Scholar]
Health Canada 2016
- Health Canada. Risk of thermal harm from therapeutic medical lasers, intense pulsed light and light emitting diodes. www.canada.ca/en/health‐canada/services/drugs‐health‐products/medical‐devices/activities/announcements/notice‐risk‐thermal‐harm‐therapeutic‐medical‐lasers‐intense‐pulsed‐light‐emitting‐diodes.html (accessed 16 June 2019).
Higgins 2011a
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jackson 2016
- Jackson DC, Zeng W, Wong CY, Mifsud E, Williamson NA, Ang C‐S, et al. Tear interferon‐gamma as a biomarker for evaporative dry eye disease. Investigative Ophthalmology and Visual Science 2016;57(11):4824‐30. [DOI] [PubMed] [Google Scholar]
Jiang 2016
- Jiang X, Lv H, Song H, Zhang M, Liu Y, Hu X, et al. Evaluation of the safety and effectiveness of intense pulsed light in the treatment of meibomian gland dysfunction. Journal of Ophthalmology 2016;2016:1910694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2017
- Jones L, Downie L, Korb D, Benitez‐del‐Castillo J, Dana R, Deng S, et al. TFOS DEWS II management and therapy report. Ocular Surface 2017;15(3):575‐628. [DOI: 10.1016/j.jtos.2017.05.006] [DOI] [PubMed] [Google Scholar]
Kirn 2002
- Kirn T. Intense pulsed light eradicates Demodex mites. Skin Allergy News 2002;33(1):37. [Google Scholar]
Knop 2011
- Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Investigative Ophthalmology and Visual Science 2011;52(4):1938‐78. [DOI: 10.1167/iovs.10-6997c] [DOI] [PMC free article] [PubMed] [Google Scholar]
Korb 2015
- Korb DR, Blackie CA, Finnemore VM, Douglass T. Effect of using a combination of lid wipes, eye drops, and omega‐3 supplements on meibomian gland functionality in patients with lipid deficient/evaporative dry eye. Cornea 2015;34(4):407‐12. [DOI] [PubMed] [Google Scholar]
Liu 2010
- Liu J, Sheha H, Tseng SC. Pathogenic role of Demodex mites in blepharitis. Current Opinion Allergy Clinical Immunology 2010;10:505‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2019
- Liu Y, Chen D, Chen X, Kam WR, Hatton MP, Sullivan DA. Hypoxia: a breath of fresh air for the meibomian gland. Ocular Surface 2019;17(2):310‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mejía 2019
- Mejía LF, Gil JC, Jaramillo M. Intense pulsed light therapy: a promising complementary treatment for dry eye disease. Archivos de la Sociedad Española de Oftalmología 2019;94(7):331‐6. [DOI] [PubMed] [Google Scholar]
Mengher 1985
- Mengher LS, Bron AJ, Tonge SR, Gilbert DJ. Effect of fluorescein instillation on the pre‐corneal tear film stability. Current Eye Research 1985;4(1):9‐12. [DOI] [PubMed] [Google Scholar]
Nagymihályi 2004
- Nagymihályi A, Dikstein S, Tiffany J. The influence of eyelid temperature on the delivery of meibomian oil. Experimental Eye Research 2004;78(3):367‐70. [DOI] [PubMed] [Google Scholar]
Ngo 2013
- Ngo W, Situ P, Keir N, Korb D, Blackie C, Simpson T. Psychometric properties and validation of the Standard Patient Evaluation of Eye Dryness questionnaire. Cornea 2013;32(9):1204‐10.. [DOI] [PubMed] [Google Scholar]
Nichols 2011
- Nichols KK, Foulks GN, Bron AJ, Glasgow BJ, Dogru M, Tsubota K, et al. The international workshop on meibomian gland dysfunction: executive summary. Investigative Ophthalmology and Visual Science 2011;52(4):1922‐9. [DOI: 10.1167/iovs.10-6997a] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Reilly 2012
- O'Reilly N, Menezes N, Kavanagh K. Positive correlation between serum immunoreactivity to Demodex‐associated Bacillus proteins and erythematotelangiectatic rosacea. British Journal of Dermatology 2012;167(5):1032‐6. [DOI] [PubMed] [Google Scholar]
Rennick 2018
- Rennick S, Adcock L. Intense Pulsed Light Therapy for Meibomian Gland Dysfunction: a Review of Clinical Effectiveness and Guidelines. Ottawa (Canada): Canadian Agency for Drugs and Technologies in Health, 2018. [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manage (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rong 2018
- Rong B, Tang Y, Liu R, Tu P, Qiao J, Song W, et al. Long‐term effects of intense pulsed light combined with meibomian gland expression in the treatment of meibomian gland dysfunction. Photomedicine and Laser Surgery 2018;36(10):562‐7. [DOI] [PubMed] [Google Scholar]
Saldanha 2018
- Saldanha IJ, Petris R, Han G, Dickersin K, Akpek E. Research questions and outcomes prioritized by patients with dry eye. JAMA Ophthalmology 2018;136(10):1170‐9. [DOI: 10.1001/jamaophthalmol.2018.3352] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schaumberg 2011
- Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for MGD. Investigative Ophthalmology and Visual Science 2011;52(4):1994‐2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siak 2012
- Siak JJ, Tong L, Wong WL, Cajucom‐Uy H, Rosman M, Saw SM, et al. Prevalence and risk factors of meibomian gland dysfunction: the Singapore Malay eye study. Cornea 2012;31(11):1223‐8. [DOI] [PubMed] [Google Scholar]
Stapleton 2017
- Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. TFOS DEWS II epidemiology report. Ocular Surface 2017;15(3):334‐65. [DOI: 10.1016/j.jtos.2017.05.003] [DOI] [PubMed] [Google Scholar]
Tomlinson 2011
- Tomlinson A, Bron AJ, Korb DR, Amano S, Paugh JR, Pearce EI, et al. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Investigative Ophthalmology and Visual Science 2011;52(4):2006‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Toyos 2015
- Toyos R, McGill W, Briscoe D. Intense pulsed light treatment for dry eye disease due to meibomian gland dysfunction: a 3‐year retrospective study. Photomedicine and Laser Surgery 2015;33(1):41‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vegunta 2016
- Vegunta S, Patel D, Shen JF. Combination therapy of intense pulsed light therapy and meibomian gland expression (IPL/MGX) can improve dry eye symptoms and meibomian gland function in patients with refractory dry eye: a retrospective analysis. Cornea 2016;35(3):22. [DOI] [PubMed] [Google Scholar]
Viso 2012
- Viso E, Rodríguez‐Ares MD, Oubiña B, Gude F. Prevalence of asymptomatic and symptomatic meibomian gland dysfunction in the general population of Spain. Investigative Ophthalmology and Visual Science 2012;53(6):2601‐6. [DOI] [PubMed] [Google Scholar]
Wat 2014
- Wat H, Wu D, Rao J, Goldman M. Application of intense pulsed light in the treatment of dermatologic disease: a systematic review. Dermatological Surgery 2014;40(4):359‐77. [DOI] [PubMed] [Google Scholar]
Wolffsohn 2017
- Wolffsohn J, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, et al. TFOS DEWS II diagnostic methodology report. Ocular Surface 2017;15(3):539‐74. [DOI] [PubMed] [Google Scholar]
Yin 2018
- Yin Y, Liu N, Gong L, Song N. Changes in the meibomian gland after exposure to intense pulsed light in meibomian gland dysfunction (MGD) patients. Current Eye Research 2018;43:308‐13. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Downie 2018
- Downie LE, Ahmdzai V, Cote S, Li C, Li A, Maleken A, et al. Intense pulsed light (IPL) therapy for the treatment of meibomian gland dysfunction: a systematic review. www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018099359 2018. [DOI] [PMC free article] [PubMed]