Abstract
There is growing recognition that mucus and mucin biology have a considerable impact on respiratory health, and subsequent global morbidity and mortality. Mucins play a critical role in chronic lung disease, not only by providing a physical barrier and clearing pathogens, but also in immune homeostasis. The aim of this review is to familiarise the reader with the role of mucins in both lung health and disease, with particular focus on function in immunity, infection and inflammation. We will also discuss their receptors, termed glycan‐binding proteins, and how they provide an attractive prospect for therapeutic intervention.
Keywords: glycan‐binding protein, glycosylation, immunology, mucin, pulmonary
This review article explores the role of mucins in respiratory health and disease, with particular focus on their receptors, the glycan‐binding proteins.
Introduction
Mucus is one of the most ancient components of host defence. Its fundamental importance is emphasised by its conservation throughout the animal kingdom from snails to whales. The composition and function of mucus are much more complex than originally appreciated and are areas of growing interest and intrigue.
Mucus is a mixture of water, ions, glycoproteins, proteins and lipids.1 It plays a vital role in protecting the lungs from pathogens and toxins by forming the first line of innate defence in the respiratory tract. Mucins are major macromolecular components of mucus and are responsible for its chemical and physical properties.2 They are highly glycosylated proteins and are able to form a protective barrier as well as have a role in cell signalling, via interaction with their specific receptors, the glycan‐binding proteins (GBPs), on immune cells.
Although mucus and mucins are essential for airway defence, a number of diseases are associated with either abnormal mucus production, or alterations in mucin structure and glycosylation, which change the physical properties of the mucus barrier and the recognition of mucins by GBPs, thereby modulating the immune response. Mucin–GBP interactions protect the host by dampening and preventing excessive immune activation at the mucosal surface. Such homeostatic brakes have been co‐opted by pathogens and cancer cells for immune evasion and are increasingly recognised to play a key role in pathology.1
Further understanding of how mucins regulate the immune response in disease is a rapidly developing arena with the potential to identify novel therapeutic approaches.
Mucins
Mucins are predominantly produced by epithelial cells, on the luminal surface, and their type and quantity vary depending on location. To date, 21 mucins have been identified, of which at least eight have been found to be expressed in the respiratory tract (Table 1). Mucins are classified into two groups: secreted and membrane bound. In the lung, the most prevalent secreted mucins are MUC5AC and MUC5B making up the majority (90%) of the mucin content of sputum, with the membrane‐bound mucins (MUC1, MUC4 and MUC16) making up the remaining 10%.2
Table 1.
Mucins related to respiratory disease
| Mucin | Chromosome | No. of amino acid repeats | Location of expression in respiratory tract | Respiratory disease association |
|---|---|---|---|---|
| Secreted – gel‐forming | ||||
| MUC2 | 11p15.5 | 16 | Predominantly intestinal but a small amount is found in human lung | Asthma |
| MUC5AC | 11p15.5 | 8 | Upper respiratory tract predominant: trachea and bronchus | COPD, muco‐obstructive lung disease |
| MUC5B | 11p15.5 | 29 | Lower respiratory tract predominant: trachea and bronchus | IPF, RA‐ILD, |
| MUC19 | 12q12 | 7 | Trachea | Unknown |
| Secreted – non‐gel‐forming | ||||
| MUC7 | 4q13.3 | 23 | Saliva | Sjogren's, asthma |
| MUC8 | 12q24.3 | 41 | Maxillary sinus mucosa | Chronic rhinosinusitis, asthma and COPD |
| Membrane bound | ||||
| MUC1 | 1q21 | 20 | Upper and lower respiratory tract | Lung cancer, IPF, COPD |
| MUC4 | 3q29 | 16 | Upper and lower respiratory tract | Lung cancer, COPD, muco‐obstructive lung disease |
| MUC16 | 19p13.2 | 156 | Upper and lower respiratory tract | Lung cancer |
Mucin structure
Mucins are large glycoproteins which share common structural features consisting of a proline‐rich linear protein core and multiple O‐glycosylated carbohydrate side chains (glycans), producing a characteristic bottlebrush structure.3 The protein core comprises a variable number of tandem repeat (VNTR) domains rich in serine, threonine and proline (PTS) and is the primary site for O‐linked mucin‐type glycosylation.4 Glycans are covalently attached to the peptide backbone, predominantly by oxygen (O‐linked) via threonine and serine, and less commonly by nitrogen (N‐linked) via asparagine.5 N‐linked glycans are predominantly found outside the VNTR. In secreted polymeric mucins, the terminal regions are cysteine‐rich with little glycosylation and several conserved domains.6
Glycosylation, glycans and glycan‐binding proteins (GBPs)
Glycans are vital to a number of cellular processes including adhesion, motility, inflammation, immunity and infection.7 Mucin‐type O‐linked glycosylation is initiated in the Golgi with the addition of N‐acetylgalactosamine (GalNAc), by a family of GalNAc transferases, to threonines and serines to form the Tn antigen.8, 9 The O‐linked glycans are then built up sequentially by the addition of simple sugars [e.g. galactose (Gal) and N‐acetylglucosamine (GlcNAc)] in various ordered combinations to form a number of core structures (Figure 1a). The addition of Gal to Tn by T synthase (core 1 β3‐galactosyltransferase) encoded by the C1GALT1 gene forms the T antigen (core 1 structure). This enzyme requires the essential chaperone Cosmc for its correct folding in the ER.8 Further modification by adding GlcNAc to the T antigen forms the core 2 structure. The glycan side chains can be extended by accumulation of GlcNAc and Gal, depending on the tissue in which the cell resides. Such glycans can be further modified by processes such as fucosylation, sialylation and sulphation, which play a major role in the structure and function of the mucin.5, 7 It is clear that given such combinatorial variety, the glycans decorating any given mucin will vary in length, sequence and composition, which is key to their orchestration of the immune response to infection and their protection of the underlying epithelium against mechanical and chemical stress.10
Figure 1.

Glycan homeostasis in health and disease. (a) Schematic representation of the early stages of O‐linked mucin‐type glycosylation. (b) Schematic representation of aberrant glycosylation resulting in novel glyco‐epitopes and changes in O‐linked mucin glycosylation seen in disease (as indicated by the red arrows).
These O‐glycans are recognised by the lectin family of GBPs that includes selectins, sialic acid‐binding immunoglobulin‐like lectins (siglecs) and galectins which are expressed on a range of immune cells and modulate the immune response.7
It is increasingly recognised that the ordered construction of glycan side chains may be dramatically modified during chronic lung diseases. In particular, the addition of sialylated, fucosylated or sulphated O‐glycans tends to be chain‐terminating, resulting in shorter glycans and leading to changes in the mucin physical properties and biochemical functions.1 In the tumor microenvironment, this has been shown to result in de novo glycan ligands for lectins on immune cells, often resulting in the activation of immunomodulatory pathways in these cells.11, 12 This is particularly relevant in cancers during which the glycocalyx of epithelial cells may transform to a structure made up of much simpler shorter glycans such as Tn or T that undergo sialylation to sialyl‐Tn and sialyl‐T, respectively9 (Figure 1b). At the same time, there is loss of epithelial cell polarity following an overexpression of the MUC1 cytoplasmic tail domain and inhibition of the Crumbs complex.13 Subsequently, mucins with repeated truncated glycans, no longer confined to the epithelial luminal surface, are expressed basally and can interact with receptors in the internal environment.
The mucociliary escalator
The composition of normally secreted mucins is > 80% carbohydrate,14 allowing them to effectively sequester water molecules essential for the formation of mucus and the mucociliary escalator (Figure 2). Considering the average adult inhales 7–8 L min−1 of bacteria/fungal/debris‐laden air, the lungs remain surprisingly free from infection. This is achieved by the unique structural properties of MUC5B and MUC5AC allowing effective mucociliary clearance (MCC). Understanding the mechanism underlying this is evolving, and the current model is dependent on the existence of a complex interplay between two different hydrogel polymer layers and different mucin subtypes.15 The two hydrogel layers are made up of secretory and membrane‐bound mucins, respectively. The periciliary layer (PCL) consists of membrane‐bound mucins (MUC1, 4 and 16), and sitting above the PCL is the second hydrogel layer comprising the secreted mucins (MUC5B and MUC5AC).16 These two hydrogel layers can interact directly with each other, sharing water molecules and ions. In health, the membrane‐bound mucin PCL gel exerts a higher osmotic pressure than the MUC5AC/MUC5B hydrogel that overlays it, ensuring the environment surrounding the cilia is adequately hydrated and facilitating efficient cilia beating. This allows for debris trapped by the secreted mucin layer to be transported out of the airway by the motion of the cilia with minimal resistance.16
Figure 2.
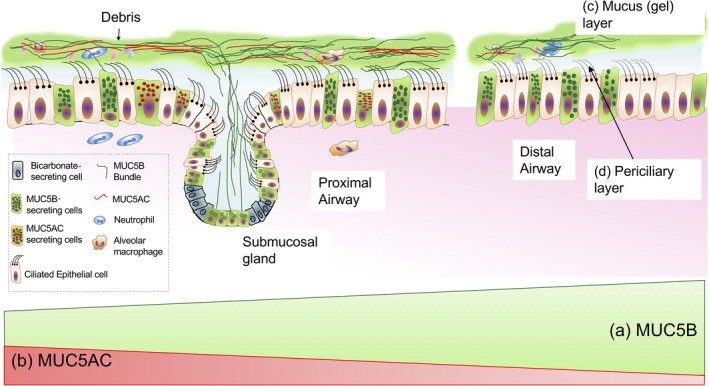
The mucociliary escalator. (a) MUC5B is the predominant mucin throughout the airway, and the majority is secreted distally. (b) MUC5AC is predominantly secreted in the proximal airway. (c) The mucus (gel) layer is made up of MUC5B, MUC5AC, water and ions. (d) The periciliary layer consists of the membrane‐bound mucins, water and ions.
Secreted airway mucins
In the normal lung, the secreted gel‐forming mucins, MUC5AC and MUC5B, are integral to the formation and function of mucus that lines airway epithelium, but their site‐specific expression is yet to be fully determined. Recent studies utilising RNA in situ hybridisation and immunohistochemistry in normal lung show that although MUC5B is constitutively secreted throughout the conducting airway except for the terminal bronchiole, the majority is produced in the distal airway by secretory epithelial cells.17 These cells together with submucosal glands are also responsible for MUC5B production in the proximal airway but to a lesser extent. In contrast, MUC5AC appears to be expressed predominantly in the proximal airway and is induced by a variety of triggers such as infection and cigarette smoke, as well as in allergic (type 2) inflammation, for example asthma.18 Cells either exclusively produce MUC5AC or MUC5B but not both, and MUC5B remains the predominant mucin throughout the airway.17
Secreted mucins are large glycoproteins that can have molecular weights in the region of 2–40 mDa. Cysteine‐rich von Willebrand factor‐like (vWF) D domains are found at either end, with D1, D2, D′, D3 found at the N terminus and D4 at the C‐terminal end6 (Figure 3). The mucins undergo post‐translational modification forming dimers in the ER, this occurs via disulphide bonds between the cysteine knot (CK domains) at their C‐termini.19 They are then transported to the Golgi where they are O‐glycosylated, further multimerised via disulphide bonding between D3 domains at their N termini and efficiently packed in a dehydrated and compact form into secretory vesicles. This process is dependent on noncovalent bonds formed by calcium ions between N‐terminal ends of dimers/polymers at acidic pH. When mucins are expelled from the secretory granule, the increased hydration and pH due to bicarbonate ions in the local vicinity result in the release of calcium ions holding the mucins in their compact conformation and their rapid volume expansion to form mucus.19
Figure 3.
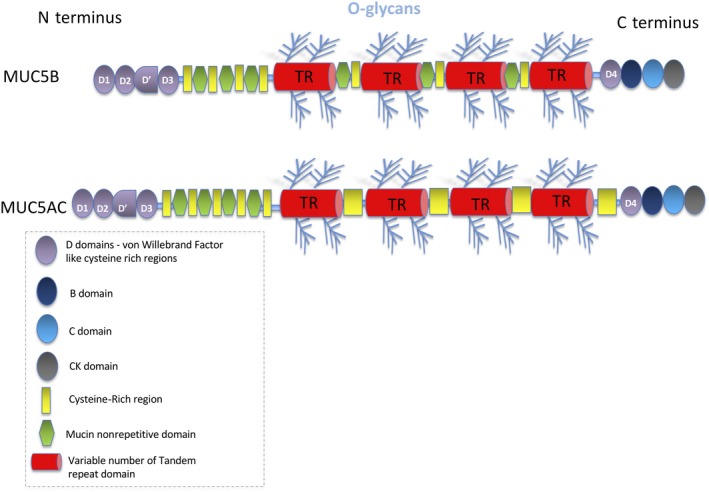
Structure of a secreted mucin. Schematic representation of a secreted mucin.
MUC5B is the predominant mucin in the mucus gel layer of the mucociliary escalator, and it forms a network to trap debris. The pore size is dependent on the level of concentration of this layer: the more concentrated it is, the smaller the pore size. Any pulmonary triggers that result in differing ratios of MUC5B and MUC5AC can result in excessively adherent mucus or reduced pore size of the mucus gel.20
In the trachea of pigs, long bundles of MUC5B have been seen, which can sweep along the airway and collect debris.21, 22 The submucosal glands located in the proximal airway may promote the formation of these long bundles. However, the majority of MUC5B is found in the distal airway which lacks submucosal glands, and the molecular organisation and mechanism of action of MUC5B in this location are under investigation.
This picture is further complicated by the observation that two glycoforms of MUC5B exist – a low‐charge form and a high‐charge form – which relate to the level of sialylation and sulphation of the attached O‐glycans. How these glycoforms affect the properties of the mucus gel is yet to be determined but the low‐charge form has been found in higher concentrations in respiratory diseases such as COPD.2, 23
The importance of MUC5B in MCC and immune homeostasis is further demonstrated by the Muc5b knockout mouse. These mice are prone to chronic bacterial infections, failure of inflammation resolution and reduced life expectancy.24
It is apparent that the secreted mucins display a range of properties geared towards host defence, providing not only a physical barrier, but are potentially able to broadly influence the immune response through specific interactions with GBPs, and in particular with lectins expressed on diverse immune cells. Future work aims to clarify the role that mucins may play in bridging the innate and adaptive arms of the immune system.
Membrane‐bound mucins
The predominant membrane‐bound mucins in the lung are MUC1, MUC4 and MUC16. These are membrane‐anchored glycoproteins expressed at the surface of respiratory epithelia, which span the cell membrane and do not form gels.25 MUC16 is the largest followed by MUC4 and then MUC1.25 However, MUC1 is the most extensively studied in the airway to date and will be the main membrane‐bound mucin discussed in this section. As work is done on the other mucins, it is possible that similarities will be identified.
The membrane‐bound mucins consist of various domains (Figure 4): the extracellular O‐glycosylated VNTR which protrudes at the cell surface presenting a glycoarray into the extracellular space and participates in a wide range of cell interactions and cellular signalling pathways26; the transmembrane domain and a cytoplasmic tail (CT) domain. Both are involved in activation of intracellular signal transduction pathways, control of inflammation, and regulation of cell differentiation and proliferation.27 The amino acid sequence and phosphorylation sites in these domains lead to a range of functions in cell signalling.28
Figure 4.

Structure of a membrane‐bound mucin. Schematic representation of a membrane‐bound mucin demonstrating the core structure and the domains unique to MUC4.
The extensive glycosylation of these mucins, not only serves as recognition sites for specific GBPs, but also regulates the biological properties of epithelial cells,28 whereby this hydrophilic environment is ideal for hydration and lubrication of the epithelia.29 The glycans extend further from the cell surface than most other extracellular receptors, which provides a barrier to the epithelium below, as well as a way for mucins to disrupt the adhesion of cells and pathogens.
MUC1 contains a single and MUC16 contains multiple sea urchin sperm protein enterokinase and agrin (SEA) domains. SEA domains are involved in protection against mechanical force and mucin degradation.27, 28 These are in the extracellular region of the mucin; they are able to self‐cleave and provide sites for glycosylation. The extracellular domain of MUC4 contains three epidermal growth factor (EGF)‐like domains that regulate signalling related to growth, motility and differentiation of the cell via interaction with EGF receptors.28 These domains contain conserved cysteine residues which may play a role in homodimerisation or oligomerisation of the mucin with itself as well as with other members of the mucin family.30 MUC4 also has the von Willebrand factor D‐like (vWF‐D) domain as well as two further unique domains, nidogen‐like (NIDO) and adhesion‐associated domain in MUC4 and other proteins (AMOP), which have been linked to cell adhesion, migration and angiogenesis.28 The various domains are listed in Table 2 (adapted from Corfield 20154).
Table 2.
Respiratory mucins and their domains (adapted from Corfield 20154)
| Domain | Domain function | Mucin | Mucin type |
|---|---|---|---|
| PTS – tandem repeat | Site for O‐linked glycosylation | All mucins | Secreted and membrane bound |
| Signal sequence at N terminus | Mediates secretion or membrane delivery | All mucins | Secreted and membrane bound |
| Cysteine rich and CYS domains | Enable mucin–mucin interactions | MUC5AC, MUC5B | Secreted |
| Cysteine knot | Dimerisation | MUC5AC, MUC5B | Secreted |
| Von Willebrand Factor D3 | Mediates oligomerisation | MUC5AC, MUC5B | Secreted |
| Von Willebrand Factor D4 | Contains GDPH autocatalytic cleavage site | MUC4, MUC5AC | Secreted and membrane bound |
| Cytoplasmic tail | Contains phosphorylation sites involved in signalling | MUC1, MUC4, MUC16 | Membrane bound |
| SEA | Contains autocatalytic proteolytic cleavage site | MUC1, MUC16 | Membrane bound |
| EGF | Mediate interactions between mucin subunits and ERBB receptors | MUC4 | Membrane bound |
| Transmembrane | Membrane‐spanning sequence typical for membrane proteins | MUC1, MUC4, MUC16, MUC20 | Membrane bound |
Membrane‐bound mucins are cleaved post‐translationally into two subunits but remain associated throughout intracellular processing and insertion into the membrane by noncovalent bonds.27 They are also able to shed their extracellular domain via cleavage sites in their penultimate and/or last SEA domains and release soluble forms into the external environment. It is thought this process is triggered by phosphorylation events in the intracellular CT domain. The link between binding and shedding of the extracellular domain and activation of the intracellular domain is not proven; however, it has been postulated that the glycosylated extracellular domain senses the external environment and can feedback via the intracellular domain to stimulate essential mucosal maintenance and repair pathways.27
As well as being produced in the lung, MUC1 is also expressed in the mammary gland, female reproductive system, gastrointestinal tract and at lower levels by some immune cells. Levels increase dramatically during pregnancy and lactation, and a soluble form has been detected in breastmilk, peripheral blood, urine and the supernatant of cultures from MUC1‐positive cancer cell lines.1, 29 Shedding can be stimulated by proteases such as neutrophil elastase (NE). Shed MUC1 may form a gel, act as a decoy for pathogens, contribute to mucus obstruction, shield cancerous cells from the immune response or bind to and activate lectins on other cells.31
The CT domain of MUC1 contains several potential sites for kinase‐mediated phosphorylation and cell signalling. Six of the seven tyrosine residues are conserved across species suggesting functionality. The MUC1 CT domain can associate with various growth factor receptors including fibroblast growth factor receptor 3 (FGFR‐3),32 platelet‐derived growth factor receptor (PDGFR), epidermal growth factor receptor (EGFR) and other ErbB family members.33 Binding of ligands to these receptors increases their association with MUC1, stimulates phosphorylation of the MUC1 CT domain and results in intracellular signalling. The majority of such growth factor receptors would be expressed basally and would normally be separated from MUC1 by epithelial tight junctions, so that they would only be able to associate following damage to epithelial integrity or a loss of apical–basal polarity as is seen in cancer cells. MUC1 is not only able to influence cellular growth but also the local stroma and angiogenesis by increasing the levels of neuropilin‐1 (NRP1), a coreceptor of vascular endothelial growth factor (VEGF) and its ligand VEGF.34 It is clear that these events have potential roles, not only in the physiology of normal epithelial homeostasis and repair, but also in malignant cell growth and progression,35 and as such are being targeted in clinical trials.36
Mucins and their interacting glycan‐binding proteins
Both membrane‐bound and secreted mucins are able to present glycan ligands for recognition by GBPs on immune cells. It is believed that GBPs recognise such mucin ligands via motifs that encompass both discrete O‐linked glycan structures and elements of their underlying scaffolds.
The GBPs are a large family that includes the lectins, selectins, siglecs and galectins, and we discuss each of these in turn in this review.
Selectins
The selectins, consisting of E‐, L‐ and P‐selectin, are a family of single‐pass transmembrane cell adhesion proteins with an outer carbohydrate recognition domain that are widely expressed on endothelium, leucocytes and platelets. The selectins play a key role in leucocyte trafficking out of the circulation and into the tissues. However, the selectins can also bind O‐glycans expressed on mucins in luminal organs including the lung.37 The minimum structure for selectin recognition is a tetrasaccharide sialyl Lewis X (SLex/CD15s) or its isomer Sialyl Lewis A (SLeA/CD19), containing sialic acid, fucose, Gal and GlcNAc. SLeA is recognised as the circulating tumor marker CA19.9, which may be found in the O‐linked glycans on MUC1, MUC5AC and MUC16.38 Invasion of tumor cells coupled with a loss of epithelial cell polarity is thought to underlie basal release of the normally luminally expressed form into the circulation. This derangement of mucin expression of selectin ligands results in abnormal cell adhesion, implicated in the haematogenous spread of tumor metastasis and cancer‐associated thrombosis, increased angiogenesis and an altered immune recognition of and response to the mucin expressing cells.29 The reader is referred to an excellent review of this subject.38
Another scenario that results in selectin ligand expression on airway mucins is during inflammation. In one striking example, pyocyanin from Pseudomonas aeuroginosa (PA) upregulates SLex on mucins expressed by the bronchial epithelium to allow the bacterium to adhere and invade.39 However, although both cancer and inflammation are associated with abnormal mucin glycosylation, what is much less clear is whether there are physiological selectin ligands expressed on mucins in the healthy lung that play a role in normal lung homeostasis.
Siglecs
The second GBP lectin family are the siglecs that are differentially expressed on restricted subsets of immune cells. There are 14 different mammalian siglecs but the identification of ligands for each individual family member is difficult because of their broad and overlapping affinities.40 Once again the physiological ligands for these GBPs are largely unknown but are thought to include sialoglycans on mucins. The siglecs are immunomodulatory receptors and ligand engagement usually results in reduced inflammation though immune inhibitory sequences (ITIMs) on cytoplasmic tails of most siglecs7 to mediate immune cell death, inhibit immune mediator release and/or enhance anti‐inflammatory mediator release. For example, antibodies or synthetic ligands are able to induce apoptosis of eosinophils, neutrophils and depletion of B cells through Siglec‐8, Siglec‐9 or Siglec‐2 (CD22), respectively. Although in general siglecs are inhibitory, there are certain siglecs, Siglec‐4 and Siglec‐14 to Siglec‐16, that do not have an ITIM or ITIM like motif, but instead signal through the association of DNAX activation protein (DAP)12.41 These are called activating siglecs as they promote immune activation through p38 MAPK and AKT signalling pathways. In some cases, activating and inhibitory siglecs are found on the same cell and thereby balance the immune response.
Probably the most important siglecs to be considered in chronic lung disease are Siglec‐8 on eosinophils, mast cells and basophils, and Siglec‐9 on neutrophils, monocytes and some T cells. Siglec‐8 and Siglec‐9 have been shown to play a role in the resolution of ongoing inflammation in asthma and COPD, respectively, with polymorphisms of SIGLEC8 and SIGLEC9 associated with disease severity.40, 42 Circulating levels of soluble Siglec‐9 have been proposed to impair disease resolution in COPD patients by ‘mopping‐up’ ligands for Siglec‐9 that would otherwise tone down inflammatory responses of alveolar neutrophils. Pathogens, specifically group B streptococcus (GBS) and PA, as well as tumor cells have developed mechanisms of sialoglycan overexpression to escape immune surveillance in an MHC class I‐independent fashion, through Siglec‐9 engagement.43, 44, 45 These observations have led to the suggestion that siglec‐directed therapies, particularly involving Siglec‐8 and Siglec‐9, may be appropriate for inflammatory, autoimmune, allergic and infectious diseases.46 However, much is still left to understand the role of endogenous airway sialoglycans in promoting siglec‐mediated immune regulation and the role of mucins in these events.
Galectins
A third class of GBP lectins are the galectins that are expressed by many immune cells including activated macrophages, dendritic cells, and B and T cells. They bind galactose‐terminated oligosaccharides on mucins, to direct immune cell maturation, survival and activation.7 Galectins have also been implicated in tumor cell adhesion and progression, immunity, inflammation and wound healing. Galectin (Gal)‐1 demonstrates both pro‐ and anti‐inflammatory features that are not fully understood and has been shown to interact with MUC16.28 Gal‐1 agonists are under development as anti‐inflammatories but there are no clinical studies at the moment in chronic lung disease. The most well‐described galectin in relation to mucins is Gal‐3 that can interact with MUC1, MUC4 and MUC16 to alter cell surface polarisation, enhance tumor cell homotypic aggregation and increase growth factor signalling pathways.28, 47 The observation that the Gal‐3 knockout mouse is resistant to bleomycin‐induced lung fibrosis,48 and Gal‐3 is highly expressed in fibrotic tissue, suggested Gal‐3 as a potential therapeutic target in fibrotic lung disease. Although the precise mechanism by which Gal‐3 promotes fibrosis is ill‐defined, it has been shown to promote TGF‐β1‐mediated signalling in fibroblasts in vitro. Pulmonary fibrosis is associated with aberrant forms of MUC‐1; for example, the truncated MUC1‐ST (see below), which has fewer glycosylated branches and may expose ligand binding sites for Gal‐3, or via steric effects, makes interaction more accessible. Subsequently, this may result in increased transduction of profibrotic signalling pathways hitherto concealed. Two Gal‐3 inhibitors have been developed with this in mind: the modified disaccharide TD139a (Galecto Biotech) and a modified naturally occurring large galactose‐containing carbohydrate polymer GR‐MD‐02 (Galectin Therapeutics). Neither drug is absorbed orally and must be given intravenously (GR‐MD‐02) or inhaled (TD139), and both have a similar protective effect in a mouse model of lung fibrosis.49 A phase II study is currently being planned to evaluate the efficacy and safety of TD139 by dry powder inhalation over 52 weeks in patients with idiopathic pulmonary fibrosis (IPF).
In summary, the lectin group of GBPs recognise glycoproteins and their scaffolds to initiate and regulate ongoing inflammatory responses and are essential for homeostasis. However, tumor cells9, 11 and pathogens50, 51 are able to alter their glycans to engage lectins and so downregulate and evade the immune response.
Disease associations
Mucins in pulmonary fibrosis
Secreted and membrane‐bound mucins are both implicated in the development of fibrotic lung diseases. IPF is a rapidly progressive interstitial lung disease (ILD) which radiologically appears as honeycomb cysts (HC). A similar disease occurs in rheumatoid arthritis termed rheumatoid arthritis‐associated ILD (RA‐ILD).
The single greatest risk factor, genetic or otherwise, for the development of IPF and RA‐ILD is a gain of function, single nucleotide polymorphism (SNP) in the promoter region of the MUC5B gene (promoter variant rs35705950).52, 53 The wild‐type guanine (G) is switched for the thymidine (T) nucleotide (risk allele). This variant has a dose‐dependent effect, with those individuals heterozygous for the risk allele (GT) 4.5 times more likely to develop the disease, increasing to twenty times more likely if homozygous for the risk allele (TT). This SNP rs35705950 results in increased production of structurally normal MUC5B with an identical amino acid sequence to the wild‐type G version. It is associated with the development of HC. Recent data suggest that in IPF, MUC5B is not restricted to the conducting airways but co‐expressed with surfactant protein C in type 2 alveolar (AT2) cells and epithelial cells lining the HC.54, 55 Despite the propensity to develop IPF or RA‐ILD with this SNP, it appears to be somewhat protective with life expectancy extending beyond the usual 3–5 years, although a recent paper has challenged this association, suggesting that MUC5B actually decreases survival.56 The mechanism by which excess MUC5B results in IPF is unknown but current speculation involves impairment of the physical barrier resulting in adherent mucus that is difficult to expectorate and susceptible to colonisation by virulent bacterial strains. The finding that IPF patients have loss of heterogeneity of bacterial flora supports this theory.57 ER stress due to increased production and subsequent misfolding of MUC5B in distal epithelial cells may result in an impaired response to injury and development of fibrosis.
Krebs von den Lungen‐6 (KL‐6), an aberrantly glycosylated form of MUC1 which either carries the sialylated T antigen (MUC1‐ST) or a longer sialylated core 2 structure, or a mixture of both, shows increased levels of expression on airway epithelial cells in IPF.58 Injured AT2 cells release KL‐6, and measuring serum KL‐6 levels forms the basis of a biomarker used in Japan to assess disease progression and treatment response.59 The MUC1 intracellular cytoplasmic tail has also been shown to be activated in type 2 epithelial cells and fibroblasts both in animal models of pulmonary fibrosis and in the human disease. Phosphorylation of the cytoplasmic tail results in formation of a MUC1/beta‐catenin nuclear complex that promotes epithelial to mesenchymal and fibroblast to myofibroblast transition.60
We speculate that nintedanib, a novel antifibrotic and tyrosine kinase inhibitor, may achieve its therapeutic effect by reducing aberrant growth factor signalling by blocking the CT domain of MUC1 that may in part be enhanced by overexpression of MUC1‐ST in fibrotic lung.
Mucins in asthma and COPD
Postmortem studies from patients with fatal asthma revealed death occurred from airflow obstruction because of extensive mucus plugging and bronchoconstriction. Mucus from patients with acute asthma has an increased proportion of MUC5AC and the low‐charge MUC5B glycoform. It is thought that MUC5AC together with smooth muscle contractility may be responsible for the airway hyperreactivity, as well as producing a hyperconcentrated mucus that is thicker, adherent and liable to form plaques and plugs.61
Muco‐obstructive plugs in COPD also consist of the low‐charge glycoform of MUC5B as well as MUC5AC. This hyperconcentrated mucus raft is more adherent, suppresses the cilia motion and arrests MCC. This results in repeated infections with mucus viscosity impairing the action of antimicrobial proteins secreted by the airway epithelial cell. These airways have elevated levels of NE, which correlates with disease severity and drives the development of bronchiectasis as NE‐induced mucus is more adherent.62
Mucins in cystic fibrosis (CF) and non‐CF bronchiectasis
Normally, during short‐lived infections, increased mucus production is beneficial in dealing with the increased pathogen load, with cough acting as an important rescue mechanism to clear this excess mucus. The defects in CF mucus have led to a greater understanding of the protective role of mucus and MCC in airway infection. Thick mucus is the hallmark of CF and results from impaired mucus hydration.14 The CF transmembrane conductance regulator (CFTR) in goblet cells releases bicarbonate which increases pH and hence results in Ca2+ precipitating out and allowing mucins to be secreted into the lumen. In CF patients, defective bicarbonate release results in slower mucin unfolding and ultimately dehydrated and hyperconcentrated mucus. This occurs from birth and results in colonisation by oral anaerobes and eventually more virulent Gram‐negative species. It is this vicious cycle of persistent infection and inflammation that results in disease and loss of lung function. Non‐CF bronchiectasis likely results from a similar interplay between environmental stressors and inherent host defence defects resulting in bronchiectasis as the final common pathway.
Mucins in infection and inflammation
The most common pathogens found in chronic respiratory diseases are Pseudomonas aeuroginosa (PA), Staphylococcus aureus and Haemophillus influenza and are associated with increased mucin production and mucus hypersecretion.1 These pathogens can activate MAPK pathways, which are important in transmitting extracellular signals from the cell surface to the nucleus to upregulate mucin genes.1
MUC1 is a receptor for PA where it has an anti‐inflammatory role; PA binds to the extracellular domain of MUC1 and triggers shedding of the extracellular domain which in turn induces phosphorylation of the CT domain to suppress Toll‐like receptor 5 (TLR) signalling.63 Furthermore, Muc1 knockout mice show increased inflammation than the wild type, indicating an anti‐inflammatory role for MUC1 during airway infection.63
Mucins in epithelial cancers
Overexpression and aberrant glycosylation of mucins have been a great focus of attention in epithelial cancers, such as lung, breast, ovarian and pancreas.4 The most frequently occurring cancer‐associated changes in glycosylation are increased sialylation which generates a terminal structure that cannot be extended, frequently involving Tn and T antigens and their sialylated counterparts – sialyl‐Tn and sialyl‐T antigens.4 These modified structures provide an enormous range of potential ligands for interaction with other receptors at the cell surface, which allows them to control the local microenvironment allowing tumors to grow, metastasise and invade, thereby evading the normal immune response.
Of the various mucins, MUC1 is predominantly found in lung adenocarcinoma and correlates with disease progression.64 The Muc1 knockout mouse model has helped in understanding the critical role played by MUC1 in cancer pathobiology.65 During cancer development, epithelial cells lose their polarity resulting in reposition of MUC1 over the entire epithelial cell surface membrane, rather than just the apical surface. This means it can play a different role in cell–cell or cell–extracellular matrix interactions, not only by interacting with growth factor receptors expressed on the basal domain, but also with the intracellular adhesion molecule‐1 (ICAM‐1) via the protein component of its VNTR domains to facilitate tumor cell metastasis.28 MUC1‐ST can interact with siglecs expressed on leucocytes to affect downstream signalling pathways, which further amplify the permissive tumor microenvironment including T‐cell suppression and enhanced tumor cell growth.11 Interestingly, Muc1 knockout mice display slower tumor progression and fewer metastases in multiple tumor models.65, 66
MUC4 can modulate cell apoptosis, regulate cell–cell adhesion and serve as a tumor marker or target for cancer therapy, most of which occurs through modulation of Erb family member signalling. Much work has been done looking at MUC4 overexpression in pancreatic cancer, where the NIDO domain is thought to play a key role in metastases.67 Through its extracellular EGF‐like domain, MUC4 interacts with the receptor tyrosine kinase, ErbB2, and controls ErbB2 and ErbB3 tyrosine phosphorylation. Overexpression of MUC4 is also seen in lung cancer, particularly in squamous cell carcinoma.64
The gel‐forming mucins are also involved in cancer. In lung adenocarcinomas, MUC5B and MUC5AC are strongly expressed, and MUC5AC specifically is associated with poorer survival where there is an associated KRAS mutation.68 In gastric cancers, MUC5AC expression is reduced, compared to the high expression of MUC5AC which usually characterises the healthy epithelial surface of the gastric tract, and this expression has been shown to be inversely associated with tumor stage including the extent of invasion and metastatic potential.69 MUC6 expression is also reduced in gastric cancers and is an independent predictor for malignant progression.70 MUC2 is the major structural component of colonic mucosa and has been associated with colorectal cancer, where its reduced expression results in increased inflammation and promotes IL‐6‐induced epithelial to mesenchymal transition.71
The CA125 antigen is a well‐recognised marker for ovarian cancer and corresponds to the cleaved extracellular TR region of MUC16. Mutations of MUC16 have also been associated with air pollution‐related lung cancer.72
Understanding the mechanisms leading to these biochemical and molecular changes of mucin structure in epithelial cell cancers may provide novel candidate biomarkers as well as potential therapeutic targets.
Conclusion
In summary, mucins are complex glycoproteins with a multitude of both positive and negative effects in lung host defence. Although in mucus they form an essential physical barrier, their functions extend far beyond this with emerging roles in cell signalling, inflammation and cancer. The aberrant mucin glycosylation seen in tumor cells opens an expansive avenue of potential therapeutic targets including their receptors, the GBPs. Work has already begun on overcoming the immune checkpoint blockade by targeting siglecs and galectins in cancer. Beyond cancer, further understanding of mucin biology is critical in the search for novel treatments in a host of chronic lung diseases.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by Breathing Matters and undertaken at UCLH/UCL who received a proportion of funding from the Department of Health's NIHR Biomedical Research Centres funding scheme. DT is supported by the Medical Research Council (grant number MR/R002800/1).
Contributor Information
Emma Denneny, Email: e.denneny@ucl.ac.uk.
Joanna Porter, Email: Joanna.porter@ucl.ac.uk.
References
- 1. Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev 2006; 86: 245–278. [DOI] [PubMed] [Google Scholar]
- 2. Ridley C, Thornton DJ. Mucins: the frontline defence of the lung. Biochem Soc Trans 2018; 46: 1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schnaar RL. Glycobiology simplified: diverse roles of glycan recognition in inflammation. J Leukoc Biol 2016; 99: 825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corfield AP. Mucins: a biologically relevant glycan barrier in mucosal protection. Biochim Biophys Acta 2015; 1850: 236–252. [DOI] [PubMed] [Google Scholar]
- 5. Jensen PH, Kolarich D, Packer NH. Mucin‐type O‐glycosylation ‐ putting the pieces together. FEBS J 2010; 277: 81–94. [DOI] [PubMed] [Google Scholar]
- 6. Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol 2008; 70: 459–486. [DOI] [PubMed] [Google Scholar]
- 7. Schnaar RL. Glycans and glycan‐binding proteins in immune regulation: a concise introduction to glycobiology for the allergist. J Allergy Clin Immunol 2015; 135: 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burchell JM, Beatson R, Graham R et al O‐linked mucin‐type glycosylation in breast cancer. Biochem Soc Trans 2018; 46: 779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer 2015; 15: 540–555. [DOI] [PubMed] [Google Scholar]
- 10. Zhang X‐L. Roles of glycans and glycopeptides in immune system and immune‐related diseases. Curr Med Chem 2006; 13: 1141–1147. [DOI] [PubMed] [Google Scholar]
- 11. Beatson R, Tajadura‐Ortega V, Achkova D et al The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec‐9. Nat Immunol 2016; 17: 1273–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rodríguez E, Schetters STT, Van Kooyk Y. The tumour glyco‐code as a novel immune checkpoint for immunotherapy. Nat Rev Immunol 2018; 18: 204–211. [DOI] [PubMed] [Google Scholar]
- 13. Alam M, Bouillez A, Tagde A et al MUC1‐C represses the Crumbs complex polarity factor CRB3 and downregulates the Hippo pathway. Mol Cancer Res 2016; 14: 1266–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hansson GC. Mucus and mucins in diseases of the intestinal and respiratory tracts. J Intern Med 2019; 285: 479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boucher RC. Muco‐obstructive lung diseases. N Engl J Med 2019; 380: 1941–1953. [DOI] [PubMed] [Google Scholar]
- 16. Button B, Cai LH, Ehre C et al A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 2012; 337: 937–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Okuda K, Chen G, Subramani DB et al Localization of secretory mucins MUC5AC and MUC5B in normal/healthy human airways. Am J Respir Crit Care Med 2019; 199: 715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Evans CM, Raclawska DS, Ttofali F et al The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nat Commun 2015; 6: 6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ridley C, Kirkham S, Williamson SJ et al Biosynthesis of the polymeric gel‐forming mucin MUC5B. Am J Physiol Cell Mol Physiol 2016; 310: L993–L1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Henderson AG, Ehre C, Button B et al Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J Clin Invest 2014; 124: 3047–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ermund A, Meiss LN, Rodriguez‐Pineiro AM et al The normal trachea is cleaned by MUC5B mucin bundles from the submucosal glands coated with the MUC5AC mucin. Biochem Biophys Res Commun 2017; 492: 331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fischer AJ, Pino‐Argumedo MI, Hilkin BM et al Mucus strands from submucosal glands initiate mucociliary transport of large particles. JCI Insight 2019; 4: e124863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kirkham S, Kolsum U, Rousseau K et al MUC5B is the major mucin in the gel phase of sputum in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008; 178: 1033–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roy MG, Livraghi‐Butrico A, Fletcher AA et al Muc5b is required for airway defence. Nature 2014; 505: 412–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bafna S, Kaur S, Batra SK. Membrane‐bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene 2010; 29: 2893–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lillehoj EP, Kato K, Lu W et al Cellular and molecular biology of airway mucins. Int Rev Cell Mol Biol 2013; 303: 139–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Van Putten JPM, Strijbis K. Transmembrane mucins: signaling receptors at the intersection of inflammation and cancer. J Innate Immun 2017; 9: 281–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jonckheere N, Skrypek N, Frénois F et al Membrane‐bound mucin modular domains: from structure to function. Biochimie 2013; 95: 1077–1086. [DOI] [PubMed] [Google Scholar]
- 29. Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol 2007; 70: 431–457. [DOI] [PubMed] [Google Scholar]
- 30. Chaturvedi P, Singh AP, Batra SK. Structure, evolution, and biology of the MUC4 mucin. FASEB J 2008; 22: 966–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lindén SK, Sheng YH, Every AL et al MUC1 limits Helicobacter pylori infection both by steric hindrance and by acting as a releasable decoy. PLoS Pathog 2009; 5: e1000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ren J, Raina D, Chen W et al MUC1 oncoprotein functions in activation of fibroblast growth factor receptor signaling. Mol Cancer Res 2006; 4: 873–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lallemand D, Manent J, Couvelard A et al Merlin regulates transmembrane receptor accumulation and signaling at the plasma membrane in primary mouse Schwann cells and in human schwannomas. Oncogene 2009; 28: 854–865. [DOI] [PubMed] [Google Scholar]
- 34. Zhou R, Curry JM, Das Roy L et al A novel association of neuropilin‐1 and MUC1 in pancreatic ductal adenocarcinoma: role in induction of VEGF signaling and angiogenesis. Oncogene 2016; 35: 5608–5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carson DD. The cytoplasmic tail of MUC1: a very busy place. Sci Signal 2008; 1: pe35. [DOI] [PubMed] [Google Scholar]
- 36. A phase I/II trial of the MUC1 inhibitor, GO‐203‐2C in patients with relapsed or refractory acute myeloid leukemia. clinicaltrials.gov; 2014; 2014–2021. https://clinicaltrials.gov/ct2/show/NCT02204085. Accessed June 7, 2019. [Google Scholar]
- 37. Kappelmayer J, Nagy B. The interaction of selectins and PSGL‐1 as a key component in thrombus formation and cancer progression. Biomed Res Int 2017; 2017: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Trinchera M, Aronica A, Dall'Olio F. Selectin ligands sialyl‐lewis a and sialyl‐lewis x in gastrointestinal cancers. Biology 2017; 6: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jeffries JL, Jia J, Choi W et al Pseudomonas aeruginosa pyocyanin modulates mucin glycosylation with sialyl‐Lewis x to increase binding to airway epithelial cells. Mucosal Immunol 2016; 9: 1039–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yu H, Gonzalez‐Gil A, Wei Y et al Siglec‐8 and Siglec‐9 binding specificities and endogenous airway ligand distributions and properties. Glycobiology 2017; 27: 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. MacAuley MS, Crocker PR, Paulson JC. Siglec‐mediated regulation of immune cell function in disease. Nat Rev Immunol 2014; 14: 653–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zeng Z, Li M, Wang M et al Increased expression of Siglec‐9 in chronic obstructive pulmonary disease. Sci Rep 2017; 7: 10116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Carlin AF, Uchiyama S, Chang YC et al Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec‐9 and dampen the innate immune response. Blood 2009; 113: 3333–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Carlin AF, Chang YC, Areschoug T et al Group B Streptococcus suppression of phagocyte functions by protein‐mediated engagement of human Siglec‐5. J Exp Med 2009; 206: 1691–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Khatua B, Bhattacharya K, Mandal C. Sialoglycoproteins adsorbed by Pseudomonas aeruginosa facilitate their survival by impeding neutrophil extracellular trap through siglec‐9. J Leukoc Biol 2012; 91: 641–655. [DOI] [PubMed] [Google Scholar]
- 46. Lübbers J, Rodríguez E, van Kooyk Y. Modulation of immune tolerance via siglec‐sialic acid interactions. Front Immunol 2018; 9: 2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Piyush T, Chacko AR, Sindrewicz P et al Interaction of galectin‐3 with MUC1 on cell surface promotes EGFR dimerization and activation in human epithelial cancer cells. Cell Death Differ 2017; 24: 1931–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. MacKinnon AC, Gibbons MA, Farnworth SL et al Regulation of transforming growth factor‐β1‐driven lung fibrosis by galectin‐3. Am J Respir Crit Care Med 2012; 185: 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hirani N, Mackinnon A, Nicol L et al TD139, A novel inhaled galectin‐3 inhibitor for the treatment of idiopathic pulmonary fibrosis (IPF). Results from the first in (IPF) patients study. Am J Respir Crit Care Med 2017; 195: A7560. [Google Scholar]
- 50. McAuley JL, Corcilius L, Tan HX et al The cell surface mucin MUC1 limits the severity of influenza A virus infection. Mucosal Immunol 2017; 10: 1581–1593. [DOI] [PubMed] [Google Scholar]
- 51. Han J, Perez JT, Chen C et al Genome‐wide CRISPR/Cas9 screen identifies host factors essential for influenza virus replication. Cell Rep 2018; 23: 596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Juge P‐A, Lee JS, Ebstein E et al MUC5B promoter variant and rheumatoid arthritis with interstitial lung disease. N Engl J Med 2018; 379: 2209–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Seibold MA, Wise AL, Speer MC et al A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med 2011; 364: 1503–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Seibold MA, Smith RW, Urbanek C et al The idiopathic pulmonary fibrosis honeycomb cyst contains a mucocilary pseudostratified epithelium. PLoS One 2013; 8: e58658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hancock LA, Hennessy CE, Solomon GM et al Muc5b overexpression causes mucociliary dysfunction and enhances lung fibrosis in mice. Nat Commun 2018; 9: 5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dudbridge F, Allen RJ, Sheehan NA et al Adjustment for index event bias in genome‐wide association studies of subsequent events. Nat Commun 2019; 10: 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Molyneaux PL, Cox MJ, Willis‐Owen SAG et al The role of bacteria in the pathogenesis and progression of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2014; 190: 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ohyabu N, Hinou H, Matsushita T et al An essential epitope of anti‐MUC1 monoclonal antibody KL‐6 revealed by focused glycopeptide library. J Am Chem Soc 2009; 131: 17102–17109. [DOI] [PubMed] [Google Scholar]
- 59. Bergantini L, Bargagli E, Cameli P et al Serial KL‐6 analysis in patients with idiopathic pulmonary fibrosis treated with nintedanib. Respir Investig 2019; 57: 290–29. [DOI] [PubMed] [Google Scholar]
- 60. Milara J, Ballester B, Montero P et al MUC1 intracellular bioactivation mediates lung fibrosis. Thorax 2019; 75: 132–142. [DOI] [PubMed] [Google Scholar]
- 61. Welsh KG, Rousseau K, Fisher G et al MUC5AC and a glycosylated variant of MUC5B alter mucin composition in children with acute asthma. Chest 2017; 152: 771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kesimer M, Ford AA, Ceppe A et al Airway mucin concentration as a marker of chronic bronchitis. N Engl J Med 2017; 377: 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kim KC. Role of epithelial mucins during airway infection. Pulm Pharmacol Ther 2012; 25: 415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lakshmanan I, Ponnusamy MP, Macha MA et al Mucins in lung cancer: diagnostic, prognostic, and therapeutic implications. J Thorac Oncol 2015; 10: 19–27. [DOI] [PubMed] [Google Scholar]
- 65. Joshi S, Kumar S, Bafna S et al Genetically engineered mucin mouse models for inflammation and cancer. Cancer Metastasis Rev 2015; 34: 593–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xu X, Chen W, Leng S et al Muc1 knockout potentiates murine lung carcinogenesis involving an epiregulin‐mediated EGFR activation feedback loop. Carcinogenesis 2017; 38: 604–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kumar S, Cruz E, Joshi S et al Genetic variants of mucins: Unexplored conundrum. Carcinogenesis 2017; 38: 671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bauer AK, Umer M, Richardson VL et al Requirement for MUC5AC in KRAS‐dependent lung carcinogenesis. JCI Insight 2018; 3: e120941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Krishn SR, Ganguly K, Kaur S et al Ramifications of secreted mucin MUC5AC in malignant journey: a holistic view. Carcinogenesis 2018; 39: 633–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Boltin D, Niv Y. Mucins in gastric cancer ‐ an update. J Gastrointest Dig Syst 2013; 03: 15519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Li C, Zuo D, Yin L et al Prognostic value of MUC2 expression in colorectal cancer: a systematic review and meta‐analysis. Gastroenterol Res Pract 2018; 2018: 6986870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kanwal M, Ding X‐J, Song X et al MUC16 overexpression induced by gene mutations promotes lung cancer cell growth and invasion. Oncotarget 2018; 9: 12226–12239. [DOI] [PMC free article] [PubMed] [Google Scholar]


