Abstract
Background
Patients with heart failure with preserved ejection fraction (HFpEF) and chronic kidney disease (CKD) represent a high-risk phenotype. The Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Heart Failure with Preserved Ejection Fraction (RELAX) trial enrolled a high proportion of CKD participants, allowing investigation into differences in HFpEF by CKD status.
Methods and Results
Among 212 participants, we investigated the associations of CKD with biomarkers, cardiac structure, and exercise capacity, and identified predictors of change in estimated glomerular filtration rate (eGFR) over trial follow-up. CKD participants (eGFR ≤60 mL/min/1.73m2) were older, had more comorbidities, and had worse diastolic function. Lower eGFR was associated with higher levels of endothelin-1, NT-proBNP, aldosterone, uric acid, and biomarkers of fibrosis (P<0.05 for all). While lower eGFR was associated with worse peak VO2 after adjustment for demographics, clinical comorbidities, exercise modality, ejection fraction, and chronotropic index (β coefficient per 1-SD decrease in eGFR: −0.61, 95% CI: −1.01, −0.22, P=0.002), this association was attenuated after further adjustment for hemoglobin (β coefficient: −0.26, 95% CI: −0.68, 0.16, P=0.22). Hemoglobin mediated 35% of the association between eGFR and peak VO2. Sildenafil therapy was independently associated with worsening eGFR over the trial (β coefficient: −2.79, 95% CI: −5.34, −0.24, P=0.03).
Conclusion
Renal dysfunction in HFpEF is characterized by echocardiographic and biomarker profiles indicative of more advanced disease, and reduced hemoglobin is a strong mediator of the association between renal dysfunction and low exercise capacity. Sildenafil therapy was associated with worsening of renal function in RELAX.
Keywords: heart failure with preserved ejection fraction, chronic kidney disease, biomarkers, exercise capacity, trials, sildenafil
Heart failure with preserved ejection fraction (HFpEF) is a growing cardiovascular epidemic highlighted by exercise intolerance and accounts for nearly half of prevalent heart failure (HF) worldwide.1 While hospitalized HFpEF patients are burdened by similar long-term mortality rates to those with HF and reduced ejection fraction,2 there remain no pharmacologic or device therapies to alter the natural course of this disease. The current lack of therapeutic options may be in part due to the heterogeneity of the HFpEF syndrome. Efforts have thus been made to identify specific phenotypic groups of HFpEF, which may behave more uniformly in both disease progression and response to potential therapies.3 Patients with HFpEF and renal dysfunction comprise one such phenotype with a particularly poor prognosis.4
Both HFpEF and renal dysfunction are characterized by increased left ventricular (LV) mass, right ventricular dysfunction, and renal venous congestion.5 While the prognosis of patients with HFpEF and chronic kidney disease (CKD) is worse than HFpEF alone,4 the pathophysiologic mechanisms leading to such outcomes are not well defined and the influence of renal dysfunction in HFpEF on exercise capacity is unclear. As such, we aimed to (1) identify biomarkers of neurohormonal function, oxidative stress, and collagen metabolism that are associated with comorbid HFpEF and CKD, (2) understand the association between renal dysfunction and exercise capacity in HFpEF, and (3) identify independent predictors of worsening renal function during follow up of the Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Heart Failure with Preserved Ejection Fraction (RELAX) trial.
Material and Methods
Study Population
The RELAX trial was a National Heart, Lung, and Blood Institute (NHLBI)-sponsored, double-blind, parallel-group, randomized trial of sildenafil as compared with placebo among 216 participants with chronic HFpEF.6 Participants included in the trial were adults with LV ejection fraction (EF) ≥50%, New York Heart Association (NYHA) class II-IV symptoms and one of the following in the past 12 months: hospitalization for HF, intravenous diuretic or ultrafiltration, prescription for chronic loop diuretic and diastolic dysfunction on echocardiography, or elevated LV filling pressures on catheterization.7 In addition, evidence of markedly reduced (≤60% predicted) peak oxygen consumption (VO2) was required prior to trial enrollment. N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels >400 pg/mL were also required except in participants with high LV filling pressures on catheterization. Notably, participants with severe renal dysfunction, defined as estimated glomerular filtration rate (eGFR) <20 mL/min/1.73m2 were excluded.7 All participants enrolled in the RELAX trial provided written consent, informed consent, and the clinical trial protocol was approved by the institutional review board at all participating institutions.
Definition of CKD
We calculated eGFR using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation using core lab serum creatinine and cystatin-C measurements;8 eGFR by CKD-EPI was used for all analyses given improved precision and accuracy by this method.8 CKD was defined as eGFR ≤60 mL/min/1.73m2.
Biomarker Assessment
After enrollment, participants underwent baseline history and physical examinations and blood sampling for biomarkers. Biomarker assays were conducted in masse at a core laboratory (University of Vermont, Burlington, Vermont) and the following measures were obtained at baseline: neurohormonal function (NT-proBNP, aldosterone, and endothelin-1), oxidative stress (uric acid), myocardial injury (high-sensitivity troponin I [hs-TnI]), inflammation (C-reactive protein [CRP]), collagen metabolism (NT-procollagen III peptide, galectin-3, and carboxy-terminal telopeptide of collagen type I [CITP]), and renal function (creatinine and cystatin-C). Biomarker assays were also repeated at the final study visit (24 weeks from baseline) in enrolled participants.
Echocardiography, Cardiac Magnetic Resonance, Cardiopulmonary Exercise Testing, and Study Drug Protocols
Enrolled participants underwent six-minute walk distance (6MWD), core lab echocardiography (Mayo Clinic, Rochester, MN), core lab cardiac magnetic resonance imaging (cMRI; Duke University Medical Center, Durham, NC), electrocardiography, and core lab cardiopulmonary exercise testing (CPET; Massachusetts General Hospital, Boston, MA) at baseline. The protocols for echocardiography, cMRI, and CPET have been previously described.7 All measurements by echocardiography were completed at the core lab and were made according to American Society of Echocardiography guidelines.9, 10 Simpson’s rule was used for calculation of chamber volumes by cMRI. Aortic distensibility was calculated through the following equation: (aortic maximal cross-sectional area - aortic minimal cross-sectional area)/(aortic minimal cross-sectional area x pulse pressure).11 CPET protocol was designed specifically for HFpEF participants and utilized harmonized protocols for 2 exercise modalities: treadmill or cycle ergometer. Peak VO2 was determined by the highest 30-second median value of breath-by-breath VO2 measurements during the final minute of incremental exercise.7 Chronotropic index was defined as: (peak exercise heart rate [HR] – resting HR)/(age-predicted maximal HR – resting HR).12, 13 Age-predicted maximal HR was derived using the Astrand formula (220 – age).14
The study drug (sildenafil) was administered to a goal dose of 60 mg 3 times daily and repeat CPET was performed at both 12 and 24 weeks after therapy. Additionally, repeat 6MWD was performed at 12 and 24 weeks after therapy.
Statistical Analysis
Data for analysis were obtained through the NHLBI using the Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC). Continuous variables were compared using Student’s t test or Wilcoxon rank sum tests (depending on distribution) and categorical variables were compared using Pearson χ2 tests by CKD. For biomarker analyses, renal dysfunction was assessed by continuous values of eGFR. The relationships between all baseline levels of biomarkers and eGFR were tested for linear and/or non-linear trends by fitting a restricted cubic spline transformation using 3 knots.15 Depending on the overall relationship, linear regression models or general additive models assessed the association between levels of eGFR and biomarker levels. Generalized additive models were used for display purposes given the presence of non-linear relationships between eGFR and several biomarkers.
Multivariable linear regression models were used to evaluate the association between renal dysfunction (continuous values of eGFR and cystatin-C) and exercise capacity at baseline (peak VO2 and 6MWD). eGFR was also analyzed as a categorical variable (CKD vs. No CKD) for this analysis. The relationship between eGFR and peak VO2 was tested for non-linear trend by fitting a restricted cubic spline transformation using 3 knots. The first model adjusted for the following clinical variables based on clinical relevance: age, sex, exercise modality, atrial fibrillation, diabetes mellitus, EF, hypertension, loop diuretic use, jugular venous pressure, and chronotropic index. The second model further adjusted for hemoglobin level. Given the mechanistic relationship between renal dysfunction and anemia,16 we performed a formal mediation analysis to estimate the mediation effect of hemoglobin on the association between eGFR and peak VO2 using the mediation package in R (R Foundation for Statistical Computing). First, univariate regression models were performed to determine the independent associations between 1) eGFR (independent variable) and hemoglobin and 2) hemoglobin (independent variable) and peak VO2. Age- and sex- adjusted direct and indirect effects (i.e. mediation effect) were reported. 95% CIs were calculated using bootstrapping. Statistically significant mediation was determined if the indirect effect was significantly different from zero. We also evaluated for interaction between eGFR and hemoglobin level with regard to baseline peak VO2 using a linear regression model with an interaction term for hemoglobin.
We calculated the change in eGFR from baseline to the end of the study period (week 24). We used linear regression models adjusting for baseline eGFR to identify variables that were associated with change in eGFR over the course of the RELAX trial. Variables that were tested individually were (1) all biomarkers, (2) treatment arm (sildenafil vs. placebo) and (3) prespecified clinical variables: age, sex, diabetes mellitus, atrial fibrillation, EF, and BMI. Variables that predicted change in eGFR in these base models were then tested in multivariable models adjusting for baseline eGFR, age, sex, diabetes mellitus, atrial fibrillation, EF, and BMI. We also assessed for interaction between renal dysfunction and treatment group (sildenafil vs. placebo) with regard to change in peak VO2 at 24 weeks using linear regression models with interaction terms for metrics of renal dysfunction.
Statistical analyses were performed using R version 3.5.0 (R Foundation for Statistical Computing). This study was approved by the Institutional Review Board of Northwestern University.
Results
Study Population Characteristics
Among the RELAX cohort (n=216), there were 4 participants without serum creatinine or cystatin-C levels. The remaining 212 participants comprised the final study cohort, of which 114 participants (54%) had baseline CKD (eGFR ≤60 mL/min/1.73m2) and 27 participants (13%) had eGFR <30 mL/min/1.73m2. The baseline characteristics of the study population by eGFR category are displayed in Table 1. Relative to participants without CKD at baseline, participants with CKD were older, were more likely to have hypertension, diabetes mellitus, longer duration of HF, and to take diuretics (P<0.05 for all comparisons). A higher proportion of participants with CKD had NYHA functional class III symptoms compared with those with eGFR >60 mL/min/1.73m2 (68% vs 37%, P<0.001). CKD participants had significantly worse edema, higher jugular venous pressure, and lower diastolic blood pressure at baseline (Table 1).
Table 1.
Baseline Characteristics of RELAX Participants by Chronic Kidney Disease Status.
| Chronic Kidney Disease | |||
|---|---|---|---|
| Characteristic | No (n=98) | Yes (n= 114) | p-value |
| Age (years), mean (SD) | 65.2 (10.3) | 71.4 (9.4) | <0.001 |
| Female, n (%) | 45 (46) | 57 (50) | 0.65 |
| Race, n (%) | 0.83 | ||
| White | 89 (91) | 106 (93) | |
| Black | 6 (6) | 5 (4) | |
| Other | 3 (3) | 3 (3) | |
| Diabetes mellitus, n (%) | 34 (35) | 56 (49) | 0.03 |
| Hypertension, n (%) | 76 (78) | 103 (90) | 0.02 |
| Ischemic heart disease, n (%) | 37 (38) | 44 (39) | 0.99 |
| Duration of heart failure (years), mean (SD) | 2.1 (3.2) | 3.6 (4.7) | 0.01 |
| New York Heart Association Class, n (%) | <0.001 | ||
| II | 62 (63) | 37 (32) | |
| III | 36 (37) | 77 (68) | |
| Pacemaker, n (%) | 9 (9) | 25 (22) | 0.02 |
| Peripheral vascular disease, n (%) | 9 (9) | 21 (18) | 0.08 |
| Atrial fibrillation, n (%) | 48 (49) | 60 (53) | 0.70 |
| Hyperlipidemia, n (%) | 69 (70) | 87 (76) | 0.41 |
| Current smokers, n (%) | 14 (14) | 17 (15) | 0.94 |
| Chronic obstructive pulmonary disease, n (%) | 18 (18) | 24 (21) | 0.75 |
| Depression, n (%) | 36 (37) | 30 (26) | 0.14 |
| Medications | |||
| Beta blocker, n (%) | 70 (71) | 90 (79) | 0.27 |
| Angiotensin converting enzyme inhibitor, n (%) | 40 (41) | 57 (50) | 0.23 |
| Angiotensin receptor blocker, n (%) | 32 (33) | 25 (22) | 0.11 |
| Mineralocorticoid receptor antagonist, n (%) | 8 (8) | 13 (11) | 0.58 |
| Loop diuretic, n (%) | 65 (66) | 97 (85) | 0.002 |
| Thiazide diuretic, n (%) | 16 (16) | 23 (20) | 0.59 |
| Statin, n (%) | 61 (62) | 73 (64) | 0.79 |
| Physical Exam | |||
| Heart rate (bpm), mean (SD) | 71 (12) | 69 (11) | 0.16 |
| Systolic blood pressure (mmHg), mean (SD) | 129 (17) | 127 (18) | 0.51 |
| Diastolic blood pressure (mmHg), mean (SD) | 73 (10) | 68 (10) | 0.002 |
| Body mass index (kg/m2), mean (SD) | 34.1 (6.3) | 34.2 (7.6) | 0.87 |
| Jugular venous pressure, n (%) | 0.004 | ||
| <8 cm | 65 (68) | 47 (43) | |
| >8 cm | 31 (32) | 62 (57) | |
| Edema, n (%) | 0.02 | ||
| None | 49 (50) | 41 (36) | |
| Trace | 37 (38) | 42 (37) | |
| Moderate | 12 (12) | 21 (27) | |
| Laboratory | |||
| Hemoglobin (g/dL), mean (SD) | 13.5 (1.4) | 12.4 (1.4) | <0.001 |
| Sodium (mmol/L), mean (SD) | 139.3 (3.1) | 140.0 (3.3) | 0.12 |
| Potassium (mmol/L), mean (SD) | 4.3 (0.6) | 4.3 (0.5) | 0.55 |
| Blood urea nitrogen (mg/dL), median (IQR) | 17.5 (14.0 – 23.0) | 31.0 (24.0 – 40.0) | <0.001 |
| Creatinine (g/dL), median (IQR) | 0.84 (0.73 – 1.02) | 1.34 (1.13 – 1.70) | <0.001 |
| Cystatin-C (mg/L), median (IQR) | 1.04 (0.9 – 1.15) | 1.69 (1.47 −1.99) | <0.001 |
| Estimated glomerular filtration rate (mL/min/1.73m2), mean (SD) | 76.9 (13.3) | 39.8 (11.2) | <0.001 |
| NT-proBNP (pg/mL), median (IQR) | 465.7 (92.0 – 876.2) | 1248.5 (482.4 – 2107.0) | <0.001 |
| Aldosterone (pg/mL), median (IQR) | 167.9 (114.3–255.5) | 207.0 (123.3–317.5) | 0.03 |
| Endothelin-1 (pg/mL), median (IQR) | 2.16 (1.82–2.78) | 2.50 (2.12–3.36) | 0.001 |
| Troponin I (pg/mL), median (IQR) | 6.43 (3.64–14.09) | 11.45 (7.32–24.90) | <0.001 |
| CITP (ug/L), median (IQR) | 5.01 (4.02–6.13) | 8.05 (5.97–13.26) | <0.001 |
| Galectin-3 (ng/mL), median (IQR) | 12.2 (9.9–14.8) | 15.5 (12.8–20.9) | <0.001 |
| NT-procollagen III peptide (ug/L), median (IQR) | 7.06 (5.49–8.41) | 8.49 (6.42–11.03) | 0.001 |
| hs-CRP (mg/L), median (IQR) | 3.95 (1.65–8.27) | 3.58 (1.85–7.73) | 0.95 |
| Uric acid (mg/dL), median (IQR) | 6.3 (5.2–7.9) | 8.0 (6.7–9.5) | <0.001 |
CITP = carboxy-terminal telopeptide of collagen type I; hs-CRP = high sensitivity C-reactive protein; NT-proBNP = N-terminal pro-B-type natriuretic peptide.
CKD was defined as eGFR ≤60 mL/min/1.73m2.
Renal Dysfunction and Cardiac Structure and Function
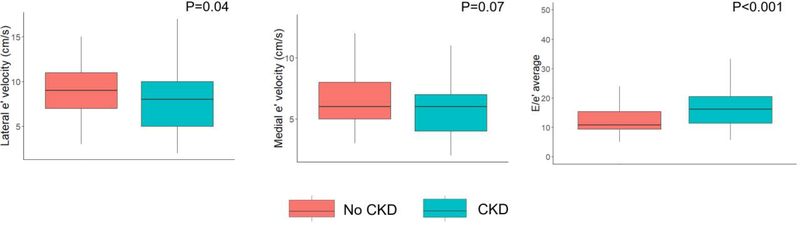
On echocardiographic analysis, lateral early diastolic (e’) tissue velocities were significantly lower among CKD participants and LV filling pressures were significantly higher among CKD participants after adjustment for age (Table 2; Figure 1). There was no difference in aortic distensibility or thickness by CKD status.
Table 2.
Baseline Cardiac Structure and Function in RELAX Participants by Chronic Kidney Disease Status.
| Chronic Kidney Disease | |||
|---|---|---|---|
| Variable | No (n=98) | Yes (n= 114) | p-value* |
| Left ventricular end diastolic volume index by cMRI (mL/m2), median (IQR) | 53.3 (45.8 – 63.5) | 60.6 (51.0 – 68.5) | 0.07 |
| Left ventricular end systolic volume index by cMRI (mL/m2), median (IQR) | 18.0 (14.1 – 25.3) | 19.5 (15.4 26.0) | 0.26 |
| Stroke volume index (mL/m2), median (IQR) | 36.7 (30.1 – 42.2) | 38.6 (30.2 – 44.3) | 0.49 |
| Ejection Fraction (%), mean (SD) | 60.8 (6.1) | 61.7 (7.3) | 0.22 |
| Left atrial volume index (mL/m2), median (IQR) | 40.7 (31.7 – 54.2) | 48.8 (39.3 – 59.6) | 0.63 |
| Medial e' (m/s), mean (SD) | 0.07 (0.02) | 0.06 (0.02) | 0.07 |
| Lateral e' (m/s), mean (SD) | 0.09 (0.03) | 0.08 (0.03) | 0.04 |
| E/e’ average, median (IQR) | 10.8 (9.4 – 15.4) | 16.3 (11.4 – 22.0) | <0.001 |
| Pulmonary artery systolic pressure (mmHg), mean (SD) | 50.5 (16.1) | 53.7 (15.5) | 0.20 |
| Aortic distensibility by cMRI (10−3 mmHg), median (IQR) | 1.15 (0.67 – 1.79) | 1.19 (0.69 – 1.74) | 0.14 |
| Aortic thickness by cMRI (mm), median (IQR) | 1.3 (1.1 – 1.4) | 1.3 (1.1 – 1.5) | 0.54 |
All indices of cardiac structure and function are derived from echocardiograms unless noted above. CKD was defined as eGFR ≤60 mL/min/1.73m2.
Adjusted for age.
cMRI = cardiac magnetic resonance imaging.
Figure 1. Box and whisker plots of various echocardiographic indices of diastolic function by chronic kidney disease status.
The thick black line represents the median value. The lower and upper limit of the boxes represent values of the 25th and 75th percentiles, respectively. The whiskers extend to 1.5 times the interquartile range. Shown are age-adjusted P-values. CKD = chronic kidney disease.
Renal Dysfunction and Biomarkers of Neurohormonal Function, Oxidative Stress, and Collagen Metabolism
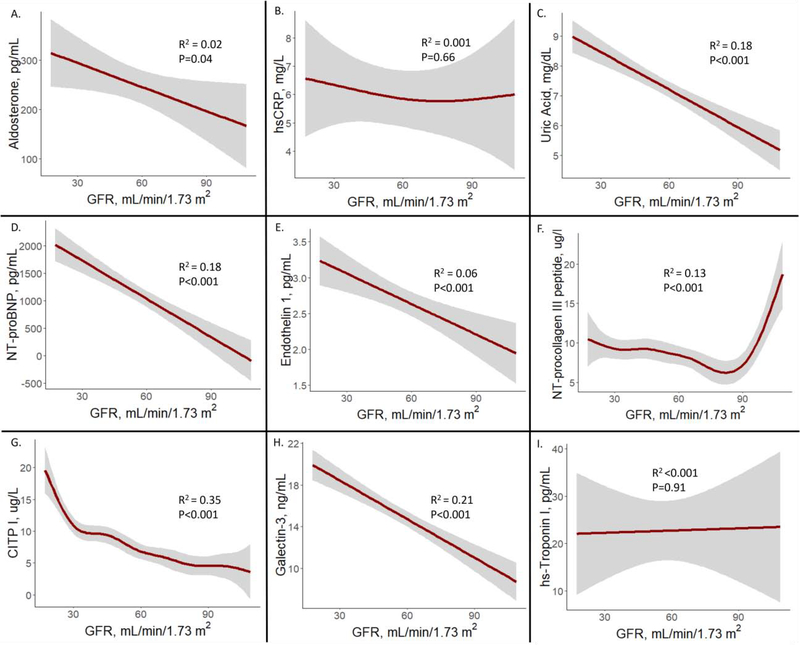
On raw analysis, history of CKD was significantly associated with higher levels of all biomarkers except for CRP (Table 1). The continuous associations between eGFR and biomarkers of neurohormonal function, oxidative stress, and collagen metabolism are displayed graphically in Figure 2. Lower eGFR was significantly associated with higher aldosterone (R2 = 0.02, P= 0.04; Figure 2A), uric acid (R2 = 0.18, P<0.001; Figure 2C), NT-proBNP (R2 = 0.18, P<0.001; Figure 2D), endothelin-1 (R2 = 0.06, P<0.001; Figure 2E), and galectin-3 (R2 = 0.21, P<0.001; Figure 2H) in a linear fashion. Lower eGFR was significantly associated with NT-procollagen III peptide levels (R2 = 0.13, P<0.001; Figure 2F) and CITP (R2 = 0.35, P<0.001; Figure 2G) in a non-linear fashion; the relationship between eGFR and NT-procollagen III peptide appeared U-shaped while and the relationship between eGFR and CITP appeared stronger at lower levels of eGFR (P for non-linearity <0.05). There was no significant association between eGFR and CRP or hs-TnI (Figure 2). Over the course of the 24-week trial, there were no other significant differences in changes in biomarkers between those with and without CKD (Supplemental Table 1).
Figure 2. Relationships between GFR and biomarkers of neurohormonal function, oxidative stress, and collagen metabolism.
Generalized additive models were used to display relationships between GFR and biomarkers. Shaded areas represent 95% CI. CITP = carboxy-terminal telopeptide of collagen type I; CRP = C-reactive protein; GFR = estimated glomerular filtration rate; hs=high sensitivity; NT-proBNP = N-terminal pro-B-type natriuretic peptide.
Renal Dysfunction and Baseline Exercise Capacity
Metrics of physical activity obtained at rest and peak exercise during CPET by CKD status are displayed in Table 3. With the exception of average diastolic blood pressure, which was lower among those with CKD compared with those without, resting parameters were similar between the two groups. Participants with CKD exercised for shorter duration and achieved lower peak HR and blood pressure parameters (P<0.05 for all comparisons; Table 3). CKD participants had lower chronotropic indices (0.44±0.28 vs. 0.58±0.28, P<0.001; reflecting worse chronotropic competence), achieved lower peak VO2 (11.2 ±2.9 mL/kg/min vs. 13.5±2.9 mL/kg/min, P<0.001), and had lower 6MWD (276.5±111.9m vs. 327.9 ±105.0m, P=0.001) compared with those without CKD. Upon exclusion of participants with pacemakers, CPET findings were consistent (Supplemental Table 2).
Table 3.
Baseline Cardiopulmonary Exercise Test Parameters in RELAX Participants by Chronic Kidney Disease Status.
| Chronic Kidney Disease | |||
|---|---|---|---|
| Variable | No (n=98) | Yes (n= 114) | p-value |
| Resting Variables | |||
| Heart rate (bpm), mean (SD) | 72 (13) | 68 (13) | 0.08 |
| Systolic blood pressure (mmHg), mean (SD) | 127.3 (22.5) | 125.2 (19.1) | 0.47 |
| Diastolic blood pressure (mmHg), mean (SD) | 73.6 (11.9) | 68.6 (9.7) | 0.001 |
| Pulse pressure (mmHg), mean (SD) | 53.7 (17.7) | 56.6 (17.0) | 0.23 |
| Oxygen saturation (%), mean (SD) | 97 (3) | 97 (3) | 0.49 |
| VO2 (mL/kg/min), mean (SD) | 3.1 (0.8) | 3.0 (0.7) | 0.29 |
| Borg score, mean (SD) | 0.6 (1.2) | 0.4 (1.0) | 0.17 |
| Oxygen pulse (mL/beat) | 4.4 (1.1) | 4.5 (1.2) | 0.65 |
| Exercise Variables | |||
| Exercise Mode | 0.52 | ||
| Treadmill, n (%) | 34 (35) | 46 (40) | |
| Bicycle, n (%) | 64 (65) | 68 (60) | |
| Exercise duration (min), mean (SD) | 10.8 (3.1) | 8.6 (2.8) | <0.001 |
| Peak heart rate (bpm), mean (SD) | 118 (24) | 103 (24) | <0.001 |
| Peak systolic blood pressure (mmHg), mean (SD) | 160.8 (32.6) | 146.3 (28.9) | 0.001 |
| Peak diastolic blood pressure (mmHg), mean (SD) | 76.5 (16.2) | 68.9 (12.7) | <0.001 |
| Peak oxygen saturation (%), mean (SD) | 96 (3) | 95 (4) | 0.23 |
| Respiratory exchange ratio | 1.10 (0.09) | 1.10 (0.12) | 0.79 |
| Peak VO2 (mL/kg/min), mean (SD) | 13.5 (2.9) | 11.2 (2.9) | <0.001 |
| Peak Borg score, mean (SD) | 7.2 (2.3) | 6.7 (2.3) | 0.16 |
| 6-minute walk distance (m), mean (SD) | 327.9 (105.0) | 276.5 (111.9) | 0.001 |
| Chronotropic index | 0.58 (0.28) | 0.44 (0.28) | <0.001 |
| Oxygen pulse (mL/beat), mean (SD) | 11.8 (3.1) | 11.1 (2.7) | 0.11 |
6MWD = 6-minute walk distance; VO2 = oxygen consumption. CKD was defined as eGFR ≤60 mL/min/1.73m2.
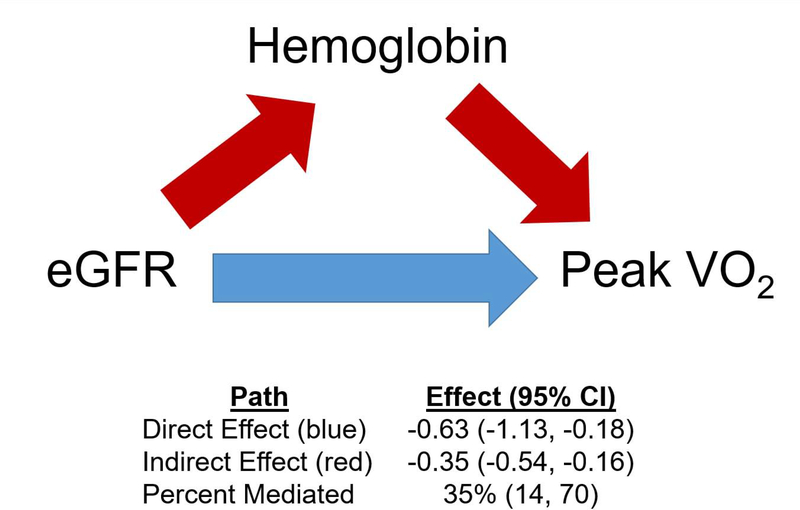
The associations of indices of renal dysfunction and baseline exercise capacity are shown in Table 4. Lower eGFR, higher cystatin-C, and CKD status were independently associated with lower baseline peak VO2 levels after adjustment for model 1 covariates. Of note, the relationship between eGFR and peak VO2 was linear (P for non-linearity = 0.43). The associations between eGFR, cystatin-C, and CKD status were all attenuated after further adjustment for hemoglobin level. Results of the mediation analysis are shown in Figure 3. Low hemoglobin was a significant mediator, explaining 35% (95% CI: 14%, 70%; P<0.001) of the association between reduced eGFR and lower peak VO2 (Figure 3). There was no statistically significant interaction between eGFR and hemoglobin level with respect to baseline peak VO2 (P-interaction = 0.62). While higher cystatin-C was independently associated with reduced 6MWD after adjustment for model 1 covariates, this association was attenuated after adjustment for hemoglobin level. There were no significant associations between continuous eGFR levels or CKD status and 6MWD after multivariable adjustment.
Table 4.
Association of Indices of Renal Function and Functional Capacity.
| eGFR (continuous) | Cystatin-C (continuous) | Chronic Kidney Disease | ||||
|---|---|---|---|---|---|---|
| Outcome Variable | β coefficient per 1-SD decrease (95% CI) | p-value | β coefficient per 1-SD increase (95% CI) | p-value | β coefficient (95% CI) | p-value |
| Peak Oxygen Consumption | ||||||
| Unadjusted | −1.22 (−1.61, −0.83) | <0.001 | −1.02 (−1.42, −0.62) | <0.001 | −2.28 (−3.08, −1.49) | <0.001 |
| Model 1* | −0.61 (−1.01, −0.22) | 0.002 | −0.68 (−1.06, −0.31) | <0.001 | −0.88 (−1.63, −0.13) | 0.02 |
| Model 2† | −0.26 (−0.68, 0.16) | 0.22 | −0.30 (−0.71, 0.12) | 0.16 | −0.38 (−1.12, 0.36) | 0.32 |
| 6-minute walk distance | ||||||
| Unadjusted | −27.8 (−42.5, −13.1) | <0.001 | −33.7 (−48.0, −19.4) | <0.001 | −51.4 (−80.9, −21.9) | <0.001 |
| Model 1* | −5.5 (−22.4, 11.5) | 0.52 | −14.9 (−31.1, 1.3) | 0.07 | −15.5 (−47.5, 16.5) | 0.34 |
| Model 2† | −7.3 (−11.5, 26.1) | 0.45 | −3.2 (−21.8, 15.4) | 0.73 | −1.6 (−34.7, 31.6) | 0.93 |
Adjusted for age, sex, exercise modality, left ventricular ejection fraction, atrial fibrillation, diabetes, hypertension, loop diuretic use, jugular venous pressure, and chronotropic index.
Adjusted for Model 1 variables plus hemoglobin.
eGFR = estimated glomerular filtration rate. CKD was defined as eGFR ≤60 mL/min/1.73
Figure 3. Mediation analysis of the association between eGFR, hemoglobin, and peak VO2.
The path model and mediation analysis describing mediation of the relationship between eGFR and peak VO2 by hemoglobin are displayed. Path effects are reported as the change in peak VO2 per 1-SD decrease in eGFR (95% CI). The total effect of the association between eGFR and peak VO2 was −0.98 (95% CI: −1.41,−0.57). eGFR = estimated glomerular filtration rate; peak VO2 = peak oxygen consumption.
Predictors of Worsening Renal Function
Overall, there was a modest decrease in eGFR among all RELAX participants over 24 weeks (median absolute change: −1.56 mL/min/1.73m2, interquartile range [IQR]: −6.62 – 2.24 mL/min/1.73m2]; median percentage change: 3%, IQR: −12% - +5%). After multivariable adjustment, diabetes mellitus (β coefficient: −3.80, 95% CI: −6.86, −0.74, P=0.02), atrial fibrillation (β coefficient: −3.18, 95% CI: −5.80, −0.55, P=0.02), sildenafil therapy (β coefficient: −2.79, 95% CI: −5.34, −0.24, P=0.03), and increased NT-proBNP (β coefficient per SD-increase: −1.93, 95% CI: −3.38, −0.48, P=0.009) were independently associated with worsening eGFR from baseline to 24 weeks. There were no other biomarkers that were significantly associated with change in eGFR (Supplemental Table 3).
Longitudinal Outcomes Among Participants With and Without CKD
There was no statistically significant interaction between renal function and sildenafil therapy with respect to change in peak VO2 at 24 weeks, which was the primary endpoint of the RELAX trial (eGFR P-interaction = 0.21; CKD status P-interaction = 0.12). While participants with CKD experienced numerically more deaths and hospitalizations during the RELAX trial compared with those without CKD, these differences were not significant (Supplemental Table 4). Additionally, the number of participants hospitalized for cardiovascular or renal causes in the 12 months preceding the RELAX trial was similar between the CKD and no CKD groups (Supplemental Table 4).
Discussion
In this analysis of renal dysfunction among participants with HFpEF, randomized to sildenafil vs. placebo in the RELAX trial, we found that compared to participants without CKD, those with CKD display more advanced cardiac functional derangements, higher levels of biomarkers reflecting neurohormonal activation, collagen metabolism/fibrosis, and oxidative stress compared to those without CKD. The association between worse eGFR and lower exercise capacity as measured by peak VO2 was strongly mediated by lower hemoglobin levels. Diabetes mellitus, atrial fibrillation, sildenafil therapy, and NT-proBNP were each independently associated with worsening renal function over the course of 24-week follow-up.
The RELAX trial enrolled a high proportion of CKD participants across a wide eGFR spectrum (≥20 mL/min/1.73 m2), allowing unique investigation into differences in HFpEF by CKD status. HFpEF with comorbid renal dysfunction represents a vulnerable cohort, driven by a bidirectional pathophysiology that ultimately portends worse clinical outcomes.3 HFpEF may instigate renal dysfunction via impaired renal arterial blood flow through 3 major mechanisms: RV dysfunction and subsequent renal venous congestion, an inability to augment cardiac output after systemic vasodilation due to chronotropic incompetence,12 and endothelial dysfunction.17 Chronotropic incompetence is especially common among patients with HFpEF-CKD. Indeed, in the RELAX cohort, we found that rates of pacemakers were higher and chronotropic indices were significantly worse among those with CKD. The lack of HR augmentation in the setting of systemic vasodilation (by pharmacotherapy or physical activity) reduces renal blood flow and promotes CKD.18 Conversely, CKD may promote HFpEF through systemic inflammation,19 deranged mineral metabolism including increased fibroblast growth factor 23 and imbalances in the calcium-phosphorous-parathyroid hormone axis,20 sympathetic overactivity/volume overload,21 and endothelial dysfunction.22 Such mechanisms may explain worse diastolic function by echocardiography and worse congestion metrics (i.e., edema and jugular venous pressure) among the CKD cohort in our study.
Our study findings provide insight into mechanisms that drive renal dysfunction and HFpEF to coexist. In RELAX, renal dysfunction was associated with higher levels of endothelin-1, a potent vasoconstrictor that is typically down-regulated by nitric oxide.23 Blockade of endothelin-1 is associated with reduction in LV mass in murine models of HFpEF via a mechanism independent of blood pressure reduction alone.24 Of note, circulating levels of low molecular weight (<30 kDa) biomarkers are determined largely by renal clearance. For such biomarkers, it is difficult to discern whether an elevation is biologically linked to cardiac pathophysiology or if an association with cardiovascular disease is confounded by CKD. Both the pulmonary and renal vascular beds produce and clear endothelin-1 in humans.25, 26 Whether the association between endothelin-1 levels and renal dysfunction noted here is influenced by reduced renal or pulmonary clearance or by increased renal production of endothelin-1 in kidney disease is unclear.
Of all the biomarkers, we noted the strongest relationships were noted between indices of renal dysfunction and certain markers of fibrosis (i.e. CITP, galectin-3). Our findings confirm the strong relationship between renal dysfunction and biomarkers of fibrosis in HFpEF.27 While antifibrotic therapies in HFpEF are currently limited, mineralocorticoid receptor antagonists have shown promise. However, risk of hyperkalemia may dissuade providers from utilizing mineralocorticoid receptor antagonists among those with renal dysfunction and HFpEF, a group which may most benefit from this therapy.28
While the impact of renal dysfunction in HFpEF on long-term clinical outcomes is well-established,4, 29, 30 the association between CKD and exercise capacity and mechanisms driving reduced exercise capacity in this cohort are less clear. Although the association between renal dysfunction and reduced peak VO2 was significant after controlling for several demographic and clinical variables in our analysis, this association was attenuated after further adjustment for hemoglobin level. Low hemoglobin has demonstrated a strong association with reduced exercise capacity in HFpEF,31 and our findings highlight that in the CKD population, the impact of low hemoglobin is particularly important. Indeed, we identified lower hemoglobin as a strong mediator, explaining 35% of the association between renal dysfunction and reduced exercise capacity. In HF, low hemoglobin levels may reflect true anemia or hemodilution in the setting of volume overload. As such, our findings provide evidence for anemia or increased plasma volume as mechanisms for reduced exercise capacity in the CKD-HFpEF cohort. Anemia in CKD is multifactorial, driven by deficiency in erythropoietin, shortened red blood cell survival, and nutritional deficiency (i.e. folate and vitamin B12).16 Impaired iron homeostasis, due to either true or functional iron deficiency is an increasingly recognized contributor to anemia in CKD.16 Although hemoglobin was not profoundly low in CKD participants in RELAX, hemoglobin levels may only be mildly reduced in the setting of substantial iron deficiency. Given hemoglobin is an important mediator of reduced exercise capacity in CKD, patients with comorbid HFpEF and CKD may particularly benefit from correction of anemia via intravenous iron therapy if truly iron deficient. Indeed, the FAIR-HFpEF ( NCT03074591) trial is currently underway, evaluating the effect of intravenous iron on exercise capacity in HFpEF. Conversely, hemoglobin is also marker of hemodilution and total body volume, and thus it is possible that lower exercise capacity in the CKD cohort is due to more profound volume overload. In our study, participants with CKD had higher rates of edema, elevated jugular venous pressure, diuretic use, and NYHA class III symptoms, suggesting increased volume overload in this subgroup. While decongestion in comorbid CKD-HFpEF is challenging due to diuretic resistance, our findings suggest concerted efforts to ameliorate volume overload may be of importance in this subgroup.
We noted overall modest decrease in kidney function over the course of 24-week follow-up in RELAX. Sildenafil therapy was independently associated with worsening eGFR over follow-up. Our findings verify the concern that systemic vasodilation (via phosphodiesterase-5 inhibition) may be poorly tolerated in HFpEF patients as initially reported in the primary results of the RELAX trial.6 In a small HFpEF cohort with documented pulmonary hypertension, sildenafil was not associated with renal dysfunction, which may be explained by the drug’s pulmonary vasodilatory properties and lower rates of baseline CKD in this cohort.32 Sildenafil decreases contractility33 and increases mitochondrial and endoplasmic reticulum stress,34 which may explain its association with worsening renal function. Additionally, participants with comorbid atrial fibrillation and diabetes were noted to have worsening renal function over the course of the RELAX trial, indicating that HFpEF patients with these comorbidities may require close monitoring of kidney function.
Our study has limitations. As previously described, it is possible that some biomarkers obtained in the RELAX trial are cleared by the kidneys and are thus reflective of renal dysfunction as opposed to HFpEF itself. Certain biomarkers have been associated with CKD alone in the absence of HFpEF.35 However, certain biomarkers in our analysis, including endothelin-1, are cleared in part by mechanisms independent of the renal system. While the RELAX study sample size was relatively small, it represents a well-characterized cohort with comprehensive biomarker profiling, echocardiographic data, and CPET analysis. Given the clinical profile of participants within RELAX was unique due to the comprehensive diagnostic testing required prior to trial enrollment, our findings may not be applicable to other populations of HFpEF. Given multiple hypotheses tested in this analysis and relatively small effect sizes between eGFR and some biomarkers, our findings are subject to type I error. As CKD was associated with increased comorbidity burden, CKD-HFpEF may represent a more advanced disease state rather than a unique phenotype. However, given the relative underrepresentation of CKD patients in HFpEF trials and the poor outcomes associated with comorbid CKD-HFpEF, further characterization of this cohort is warranted. Indices of right ventricular size and function on echocardiogram and CO2 production on CPET were not available in the current dataset. Indices of proteinuria, which are associated with HF risk, were not measured. Data regarding changes in hemoglobin during follow-up and iron studies were not available to further understand the relationship between reduced eGFR, low hemoglobin and reduced peak VO2.
In this analysis of the RELAX trial, comorbid renal dysfunction was frequently noted in HFpEF participants, and represented a vulnerable, elderly cohort with high comorbidity burden and an echocardiographic profile of worse diastolic function. Renal dysfunction was significantly associated with biomarkers representative of neurohormonal dysfunction, oxidative stress, and fibrosis/collagen metabolism. Sildenafil therapy was independently predictive of worsening renal function during follow-up. Reduced hemoglobin is a strong mediator of the association between renal dysfunction and impaired exercise capacity. Further investigations to evaluate the mechanisms driving the association between reduced eGFR and low exercise capacity are required and could have relevance to therapeutic advances in the HFpEF-CKD cohort.
Supplementary Material
Acknowledgments
Funding
Research reported in this manuscript was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number U10 HL084904 and award numbers U10 HL110297, U10 HL110342, U10 HL110309, U10 HL110262, U10 HL110338, U10 HL110312, U10 HL110302, U10 HL110336, and U10 HL110337. Dr. Ravi Patel is supported by the NHLBI T32 postdoctoral training grant (T32HL069771). Dr. Ruth Dubin is supported by the NIH grants R03DK104013 and U01DK108809.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
Dr. Rupal Mehta has interest in Abbot Laboratories, AbbVie, Inc. and Teva Pharmaceuticals Industries Ltd.
Dr. Sanjiv J. Shah was supported by National Institutes of Health grants R01 HL105755, HL127028, R01 HL140731; and American Heart Association grants #16SFRN28780016 and #15CVGPSD27260148; and has received research grants from Actelion, AstraZeneca, Corvia, and Novartis; and consulting fees from Actelion, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Cardiora, Eisai, Ironwood, Merck, Novartis, Sanofi, Tenax, and United Therapeutics. All remaining authors have nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oktay AA, Rich JD and Shah SJ. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2013;10:401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, Yancy CW and Fonarow GC. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J Am Coll Cardiol. 2017;70:2476–2486. [DOI] [PubMed] [Google Scholar]
- 3.Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang CC and Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131:269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unger ED, Dubin RF, Deo R, Daruwalla V, Friedman JL, Medina C, Beussink L, Freed BH and Shah SJ. Association of chronic kidney disease with abnormal cardiac mechanics and adverse outcomes in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2016;18:103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gori M, Senni M, Gupta DK, Charytan DM, Kraigher-Krainer E, Pieske B, Claggett B, Shah AM, Santos AB, Zile MR, Voors AA, McMurray JJ, Packer M, Bransford T, Lefkowitz M, Solomon SD and Investigators P. Association between renal function and cardiovascular structure and function in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3442–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E and Trial R. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redfield MM, Borlaug BA, Lewis GD, Mohammed SF, Semigran MJ, Lewinter MM, Deswal A, Hernandez AF, Lee KL, Braunwald E and Heart Failure Clinical Research N. PhosphdiesteRasE-5 Inhibition to Improve CLinical Status and EXercise Capacity in Diastolic Heart Failure (RELAX) trial: rationale and design. Circ Heart Fail. 2012;5:653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS and Investigators C-E. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ, Chamber Quantification Writing G, American Society of Echocardiography’s G, Standards C and European Association of E. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. [DOI] [PubMed] [Google Scholar]
- 10.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA and Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–33. [DOI] [PubMed] [Google Scholar]
- 11.Hundley WG, Kitzman DW, Morgan TM, Hamilton CA, Darty SN, Stewart KP, Herrington DM, Link KM and Little WC. Cardiac cycle-dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001;38:796–802. [DOI] [PubMed] [Google Scholar]
- 12.Klein DA, Katz DH, Beussink-Nelson L, Sanchez CL, Strzelczyk TA and Shah SJ. Association of Chronic Kidney Disease With Chronotropic Incompetence in Heart Failure With Preserved Ejection Fraction. Am J Cardiol. 2015;116:1093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindman BR, Davila-Roman VG, Mann DL, McNulty S, Semigran MJ, Lewis GD, de las Fuentes L, Joseph SM, Vader J, Hernandez AF and Redfield MM. Cardiovascular phenotype in HFpEF patients with or without diabetes: a RELAX trial ancillary study. J Am Coll Cardiol. 2014;64:541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Astrand PO. Physical performance as a function of age. JAMA. 1968;205:729–33. [DOI] [PubMed] [Google Scholar]
- 15.Durrleman S and Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–61. [DOI] [PubMed] [Google Scholar]
- 16.Babitt JL and Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012;23:1631–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Dijk CG, Oosterhuis NR, Xu YJ, Brandt M, Paulus WJ, van Heerebeek L, Duncker DJ, Verhaar MC, Fontoura D, Lourenco AP, Leite-Moreira AF, Falcao-Pires I, Joles JA and Cheng C. Distinct Endothelial Cell Responses in the Heart and Kidney Microvasculature Characterize the Progression of Heart Failure With Preserved Ejection Fraction in the Obese ZSF1 Rat With Cardiorenal Metabolic Syndrome. Circ Heart Fail. 2016;9:e002760. [DOI] [PubMed] [Google Scholar]
- 18.Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA and Borlaug BA. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol. 2012;59:442–51. [DOI] [PubMed] [Google Scholar]
- 19.Ter Maaten JM, Damman K, Verhaar MC, Paulus WJ, Duncker DJ, Cheng C, van Heerebeek L, Hillege HL, Lam CS, Navis G and Voors AA. Connecting heart failure with preserved ejection fraction and renal dysfunction: the role of endothelial dysfunction and inflammation. Eur J Heart Fail. 2016;18:588–98. [DOI] [PubMed] [Google Scholar]
- 20.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro OM, Kusek JW, Keane MG and Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neumann J, Ligtenberg G, Klein II, Koomans HA and Blankestijn PJ. Sympathetic hyperactivity in chronic kidney disease: pathogenesis, clinical relevance, and treatment. Kidney Int. 2004;65:1568–76. [DOI] [PubMed] [Google Scholar]
- 22.Dubin RF, Guajardo I, Ayer A, Mills C, Donovan C, Beussink L, Scherzer R, Ganz P and Shah SJ. Associations of Macro- and Microvascular Endothelial Dysfunction With Subclinical Ventricular Dysfunction in End-Stage Renal Disease. Hypertension. 2016;68:913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marasciulo FL, Montagnani M and Potenza MA. Endothelin-1: the yin and yang on vascular function. Curr Med Chem. 2006;13:1655–65. [DOI] [PubMed] [Google Scholar]
- 24.Valero-Munoz M, Li S, Wilson RM, Boldbaatar B, Iglarz M and Sam F. Dual Endothelin-A/Endothelin-B Receptor Blockade and Cardiac Remodeling in Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhaun N and Webb DJ. Endothelins in cardiovascular biology and therapeutics. Nat Rev Cardiol. 2019. [DOI] [PubMed] [Google Scholar]
- 26.Neuhofer W and Pittrow D. Endothelin receptor selectivity in chronic kidney disease: rationale and review of recent evidence. Eur J Clin Invest. 2009;39 Suppl 2:50–67. [DOI] [PubMed] [Google Scholar]
- 27.AbouEzzeddine OF, Haines P, Stevens S, Nativi-Nicolau J, Felker GM, Borlaug BA, Chen HH, Tracy RP, Braunwald E and Redfield MM. Galectin-3 in heart failure with preserved ejection fraction. A RELAX trial substudy (Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Diastolic Heart Failure). JACC Heart Fail. 2015;3:245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beldhuis IE, Myhre PL, Claggett B, Damman K, Fang JC, Lewis EF, O’Meara E, Pitt B, Shah SJ, Voors AA, Pfeffer MA, Solomon SD and Desai AS. Efficacy and Safety of Spironolactone in Patients With HFpEF and Chronic Kidney Disease. JACC Heart Fail. 2019;7:25–32. [DOI] [PubMed] [Google Scholar]
- 29.Damman K, Valente MA, Voors AA, O’Connor CM, van Veldhuisen DJ and Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. 2014;35:455–69. [DOI] [PubMed] [Google Scholar]
- 30.Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, Granger CB, Michelson EL, Ostergren J, Cornel JH, de Zeeuw D, Pocock S, van Veldhuisen DJ, Candesartan in Heart Failure: Assessment of Reduction in M and Morbidity I. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671–8. [DOI] [PubMed] [Google Scholar]
- 31.Mohammed SF, Borlaug BA, McNulty S, Lewis GD, Lin G, Zakeri R, Semigran MJ, LeWinter M, Hernandez AF, Braunwald E and Redfield MM. Resting ventricular-vascular function and exercise capacity in heart failure with preserved ejection fraction: a RELAX trial ancillary study. Circ Heart Fail. 2014;7:580–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu LC, Hummel YM, van der Meer P, Berger RM, Damman K, van Veldhuisen DJ, Voors AA and Hoendermis ES. Effects of sildenafil on cardiac structure and function, cardiopulmonary exercise testing and health-related quality of life measures in heart failure patients with preserved ejection fraction and pulmonary hypertension. Eur J Heart Fail. 2017;19:116–125. [DOI] [PubMed] [Google Scholar]
- 33.Borlaug BA, Lewis GD, McNulty SE, Semigran MJ, LeWinter M, Chen H, Lin G, Deswal A, Margulies KB and Redfield MM. Effects of sildenafil on ventricular and vascular function in heart failure with preserved ejection fraction. Circ Heart Fail. 2015;8:533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H, Anstrom K, Ilkayeva O, Muehlbauer MJ, Bain JR, McNulty S, Newgard CB, Kraus WE, Hernandez A, Felker GM, Redfield M and Shah SH. Sildenafil Treatment in Heart Failure With Preserved Ejection Fraction: Targeted Metabolomic Profiling in the RELAX Trial. JAMA Cardiol. 2017;2:896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rebholz CM, Harman JL, Grams ME, Correa A, Shimbo D, Coresh J and Young BA. Association between Endothelin-1 Levels and Kidney Disease among Blacks. J Am Soc Nephrol. 2017;28:3337–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.