Abstract
Background:
Diastolic dysfunction is a prevalent and therapeutically intractable feature of heart failure (HF). Increasing ventricular compliance can improve diastolic performance, but the viscoelastic forces that resist diastolic filling and become elevated in human HF are poorly defined. Having recently identified post-translationally detyrosinated microtubules as a source of viscoelasticity in cardiomyocytes, we sought to test whether microtubules contribute meaningful viscoelastic resistance to diastolic stretch in human myocardium.
Methods:
Experiments were conducted in isolated human cardiomyocytes and trabeculae. First, slow and rapid (diastolic) stretch was applied to intact cardiomyocytes from non-failing and HF hearts, and viscoelasticity was characterized following interventions targeting microtubules. Next, intact left-ventricular trabeculae from HF patient hearts were incubated with colchicine or vehicle and subject to pre- and post-treatment mechanical testing, which consisted of a staircase protocol and rapid stretches from slack length to increasing strains.
Results:
Viscoelasticity was increased during diastolic stretch of HF cardiomyocytes compared to non-failing counterparts. Reducing either microtubule density or detyrosination reduced myocyte stiffness, particularly at diastolic strain rates, indicating reduced viscous forces. In myocardial tissue, we found microtubule depolymerization reduced myocardial viscoelasticity, with an effect that decreased with increasing strain. Colchicine reduced viscoelasticity at strains below, but not above, 15%, with a two-fold reduction in energy dissipation upon microtubule depolymerization. Post-hoc sub-group analysis revealed that myocardium from patients with HF with reduced ejection fraction (HFrEF) were more fibrotic and elastic than myocardium from patients with HF with preserved ejection fraction (HFpEF), which were relatively more viscous. Colchicine reduced viscoelasticity in both HFpEF and HFrEF myocardium.
Conclusions:
Failing cardiomyocytes exhibit elevated viscosity, and reducing microtubule density or detyrosination lowers viscoelastic resistance to diastolic stretch in human myocytes and myocardium. In failing myocardium, microtubules elevate stiffness over the typical working range of strains and strain rates, but exhibited diminishing effects with increasing length, consistent with an increasing contribution of the extracellular matrix and/or myofilament proteins at larger excursions. These studies indicate that a stabilized microtubule network provides a viscous impediment to diastolic stretch, particularly in HF.
Keywords: Microtubule, viscoelasticity, mechanical stretch, cardiovascular disease, human myocardium, cytoskeletal dynamics, diastolic dysfunction
Introduction
Heart failure is pathologically diverse, but impaired left ventricular relaxation with elevated filling pressures is observed in the vast majority of patients with heart failure1. In patients with heart failure and reduced left ventricular ejection fraction (HFrEF), therapies aim to increase cardiac output by reducing afterload2 or by enhancing myocardial force production (inotropes)3, but these approaches are ineffective in patients with heart failure with preserved ejection fraction (HFpEF). An alternative method to improve cardiac performance is to enhance diastolic filling by reducing myocardial stiffness, which can increase end diastolic volume (EDV) and improve stroke volume via the Frank-Starling mechanism4. Previous studies have demonstrated that viscous forces can play a significant role resisting diastolic filling5–7 and are considerably increased in multiple etiologies of human heart disease, especially in patients with left ventricular (LV) hypertrophy6. Though ventricular filling would be improved by reducing myocardial viscoelasticity, the molecular determinants of elevated myocardial viscosity, particularly in human HF, are poorly understood.
Cardiomyocytes make up the bulk of the myocardium and consist of a dense cytoskeletal network that can be divided into two groups – the sarcomeric cytoskeleton made up of the contractile machinery, and the non-sarcomeric cytoskeleton composed primarily of microtubules and intermediate filaments. In pathological remodeling, the proliferation of microtubules and intermediate filaments and the post-translational detyrosination of microtubules increase myocyte viscoelasticity and physically impede contraction and relaxation of isolated myocytes8–11. Pathological remodeling is often accompanied by changes to myofilament proteins11 and the extracellular matrix (ECM)12 that will alter the myocyte-dependent contribution to myocardial stiffness13,14. As such, treatments to reduce the density or post-translational detyrosination of cardiac microtubules could serve to improve cardiac stiffness, but it remains to be demonstrated whether targeting cardiac microtubules can reduce viscous forces during diastolic strain of human myocardium.
The apparent contribution of the microtubule network to viscoelasticity has varied across studies, likely due to several factors reviewed recently15. While microtubules have been shown to contribute to the viscosity of cardiomyocytes in hypertrophied myocardium16,17 and microtubule stabilization is sufficient to increase myocyte viscosity16,18, the bulk of cardiac mechanical assays rely on cell permeabilization, myofilament isolation, or maintaining filaments in cold relaxation solution which compromises microtubule integrity19,20. Further, the contribution of microtubules to myocyte mechanics appears small in healthy animals16,17,21,22, increases with the severity of disease9,22,23, and is highly dependent on the rate of applied strain and the orientation of the mechanical test8,18. Thus, to properly assess the role of microtubule derived viscous forces, mechanical tests must faithfully replicate the physiological strain, strain-rate, and strain direction applied to intact myocytes and myocardium.
To date, therapeutic interventions targeting microtubules for the treatment of cardiovascular disease have focused on depolymerization of cardiac microtubules via administration of colchicine, which has been shown to improve myocardial performance in murine, feline, and canine models of HF21,24–29. Yet it is unclear if these benefits are conferred due to underlying changes in myocardial mechanics, and to our knowledge there has been no interrogation of the mechanical role of microtubules in human myocardium. Thus, the aim of this study was to characterize the contribution of cardiac microtubules to viscoelastic forces during diastolic stretch in primary human cardiomyocytes and myocardium. We demonstrate that microtubules contribute viscous resistance to stretch at diastolic strain-rates, that this contribution is regulated by microtubule detyrosination, and is greater in failing than non-failing myocytes. Using an analogous tissue-level assay, we demonstrate similar viscous forces attributable to microtubules in intact failing human myocardium, which can be reduced by microtubule depolymerization. Further, we identify that the microtubule contribution to viscoelasticity depends on the amplitude of diastolic stretch. Taken together, the results indicate that elevated viscosity in failing human myocytes is at least partly attributable to the microtubule network, and influences myocardial viscoelastic compliance during diastole.
Methods
Data Availability
The datasets generated or analyzed for the current study are available from the corresponding author upon reasonable request.
Procurement and characterization of human hearts
Procurement of human myocardial tissue was performed as described previously9, under protocols and ethical regulations approved by Institutional Review Boards at the University of Pennsylvania and the Gift-of-Life Donor Program (Pennsylvania, USA). Failing human hearts were procured at the time of orthotopic heart transplantation at the Hospital of the University of Pennsylvania after informed consent from all participants. Non-failing hearts were obtained at the time of organ donation from brain-dead organ donors. In all cases, hearts were arrested in situ using ice-cold cardioplegia solution and transported on wet ice. Whole hearts and dissected left ventricles were weighed to determine levels of hypertrophy by left ventricular mass index (LVMI – left ventricular mass/body surface area). Left ventricle ejection fraction (LVEF) was determined from echocardiography in subjects.
Classification standards: Failing hearts are etiologically defined by clinical diagnoses and are subdivided into heart failure with preserved ejection fraction (HFpEF: ejection fraction > 50%) and with reduced ejection fraction (HFrEF: ejection fraction < 35%), the former including cases of hypertrophic cardiomyopathy with diastolic dysfunction, the latter including cases of both hypertrophic and dilated cardiomyopathy with both diastolic and systolic dysfunction. Failing hearts with ischemic injury were excluded from this study.
Myocytes were isolated from 13 hearts (see method details below) for functional studies, including 7 non-failing donor hearts, and 6 explants characterized as HFrEF. Trabeculae were isolated from 10 different failing hearts of which 4 were HFpEF and 6 were HFrEF. All data collected from these subjects are included in this study. For patient statistics see Tables 1 and 2, and for available echocardiographic and hemodynamic data for each patient please see Supplemental Excel File.
Table 1: Patient Metrics for Isolated Cardiomyocyte Studies.
Metrics from human hearts used for intact myocyte mechanical assays. A total of 7 non-failing and 5 failing hearts were included in the study. Of these, 10 hearts were used in studies of freshly isolated cells (colchicine and parthenolide experiments) and 8 were used for myocyte culturing experiments (TTL overexpression). Five hearts were used for the comparison between freshly isolated and cultured cells (c.f. Supplemental Excel File). Data presented as mean +− 1SD, with unadjusted P-value for two-sample T-test comparing NF and HFrEF groups listed adjacent to means.
| Etiology | Non-Failing | HFrEF | Unadjusted P-Value |
|---|---|---|---|
| Gender | 3M, 4F | 3M, 2F | |
| Age | 58.4 ± 7.8 | 41.6 ± 15.9 | 0.035 |
| LVMI | 106.7 ± 21.3 | 129.6 ± 22.1 | 0.1 |
| LVEF | 65.8 ± 3.8 | 16.6 ± 5.3 | 2.20E-08 |
Table 2: Patient Metrics for Myocardial Studies.
Metrics from failing human hearts used for intact trabecular mechanical assays. A total of 10 failing hearts containing a mixture of HFpEF (LVEF > 55 %) and HFrEF (LVEF < 35%) hearts were used. Data presented as mean ± 1SD, with unadjusted P-value for two-sample T-test comparing HFrEF and HFpEF groups listed adjacent to means.
| CHF Etiology | HFrEF | HFpEF | Unadjusted P-Value |
|---|---|---|---|
| Gender | 4M, 2F | 1M, 3F | |
| Age | 50.0 ± 13.1 | 59.0 ± 14.8 | 0.393 |
| LVMI | 139.7 ± 40.7 | 144.4 ± 24.9 | 0.857 |
| LVEF | 14.1 ± 6.07 | 68.8 ± 5.5 | 1.18E-06 |
Human left ventricular myocyte isolation
Myocytes were disaggregated by isolation techniques described previously9, and detailed in the supplemental methods.
Myocyte Mechanics
Either freshly isolated or cultured myocytes were diluted to a sparse density (~10x) into a large (22 × 50 mm) glass bottom petri dish coated with BSA to prevent myocyte adhesion with Normal Tyrodes solution. A small aliquot of Myotak™ was freshly thawed and a 1.5 μL droplet was placed in a dry area of the petri dish and allowed to polymerize (2–3 min). When the droplet had become “tacky” laser etched glass rods (Ion Optix LLC) were lowered into the solution several times to create a thin coating of Myotak which was allowed to dry for 90 seconds before the probes were submersed into the cell-containing bath. Myocytes were then attached to the glass holder by light contact and raised slightly above the coverslip before mechanical testing. The Myostretcher apparatus (Ion Optix LLC) was used to stretch myocytes by roughly 10% over either a 200 ms (diastolic) or 5 s (slow) interval while force and sarcomere length were recorded using the Ion Wizard software and MyoCam imaging system (live FFT). Each cell was subsequently imaged with transmitted light to obtain the cross-sectional area and convert forces into stresses. Following each protocol cells were removed by raising the cell holders out of solution several times, and an optical fiber was used to remove additional debris. If cells did not adhere well to the holders, probes were cleaned using a solution of Trypsin/EGTA, physically cleaned with an optical fiber, and recoated with Myotak.
Trabecula isolation and mechanics
Tissue sections from the left-ventricular free wall containing trabecular meshwork were excised and immediately transferred into oxygenated KHB containing 20 mM BDM and 10 mM taurine at 4°C. Sections were transferred into an isolation dish bubbled with 95/5% CO2 balanced oxygen. Trabeculae <1.5 mm and ideally < 1.0 mm diameter and roughly 8 mm in length were mounted to loops of platinum foil using No 7/0 silk suture (Medline) and transferred to the papillary muscle chamber (IonOptix LLC) containing KHB. Slack length (defined as suture - suture length) was determined by elongating the tissue until the onset of tension prior to washout of BDM. BDM was subsequently washed out while the tissue was maintained at slack length and the extracellular calcium concentration was increased from 0.25 mM to 1 mM. Tension was monitored periodically over the next 45 min and the tissue was held at slack length and stimulated at 0.5 Hz prior to the passive length tension protocol. Once resting tension levels were stable (~ 45 min in most samples) passive mechanics were assessed. Stimulation was paused during the passive length tension protocol and resumed afterward. The tissue was held for 90 min during the administration of either colchicine or vehicle perfusion. The solution was continuously perfused by pump and the solution bath was oxygenated by gentle bubbling with 95/5% CO2-balanced oxygen. Following drug treatment, the same mechanical protocol was performed. Mechanical tests were automated using the Ion Wizard Software (IonOptix LLC).
Drug treatment
Intact cardiomyocytes used acutely were maintained in NT for up to 6 h. Myocytes were treated with 10μM parthenolide (Fisher Scientific NC9013142 or Sigma P0667) or 10μM colchicine (Sigma) at room temperature in NT solution for 2 h prior to experimentation (as in9). Myocardial tissue was incubated in a perfusion bath of KHB containing 1 mM Ca2+.
NT Solution : NaCl, 140 mM; MgCl2, 0.5 mM; NaH2PO4, 0.33 mM; HEPES at pH 7.4, 5 mM; glucose, 5.5 mM; CaCl2, 1 mM; KCl, 5 mM; NaOH, pH to 7.4.
KHB: NaCl, 130 mM; NaH2CO3, 25 mM; Na-Pyruvate, 2 mM; KCl, 5.4 mM; NaH2PO4, 1.2 mM; MgSO4, 1.2 mM; Lactic Acid, 1.0 mM; glucose, 12.5 mM; CaCl2, 1 mM
Statistical Analyses
Statistical analysis and graphing were performed using Origin software (OriginLab, Northampton, MA). Statistical significance was determined by p<0.05, and the appropriate statistical test is denoted in the figure legend. For box plots, mean (line), standard deviation (whiskers) and 25–75 percentiles (box) are displayed. Line graphs are displayed as mean line ± standard error.
Results
Microtubules provide viscoelastic resistance to diastolic stretch
Strain rates in the human left ventricle vary during diastolic filling from 5–100% s−1,30,31 with the highest rates experienced during early diastolic filling. We designed our “diastolic stretch” assay to mimic early diastolic filling by applying a 10–12% strain on myocytes over 200 ms (~60% s−1), and conducted a second, slower stretch to better distinguish elastic from viscous forces. As can be seen from the average traces in Figure 1A, ventricular myocytes from non-failing donor hearts exhibited a significant and rapid stress relaxation that is attributable to viscoelastic forces following the diastolic stretch protocol, but which was greatly diminished during the slow stretch (Figure 1A, bottom). Stress relaxation is a characteristic of viscous forces (near instantaneous relaxation) and viscoelastic forces (gradual decay of stress). The sarcomere length change was the same upon slow or diastolic stretch (~1.94 to 2.16μm), and sarcomere length and cell length remained constant during the stress-relaxation phase (Figure 1A, middle) indicating that the stress relaxation is not attributable to cell slip or sarcomere relaxation.
Figure 1: Reducing microtubule density or detyrosination decreases human myocyte viscoelasticity.
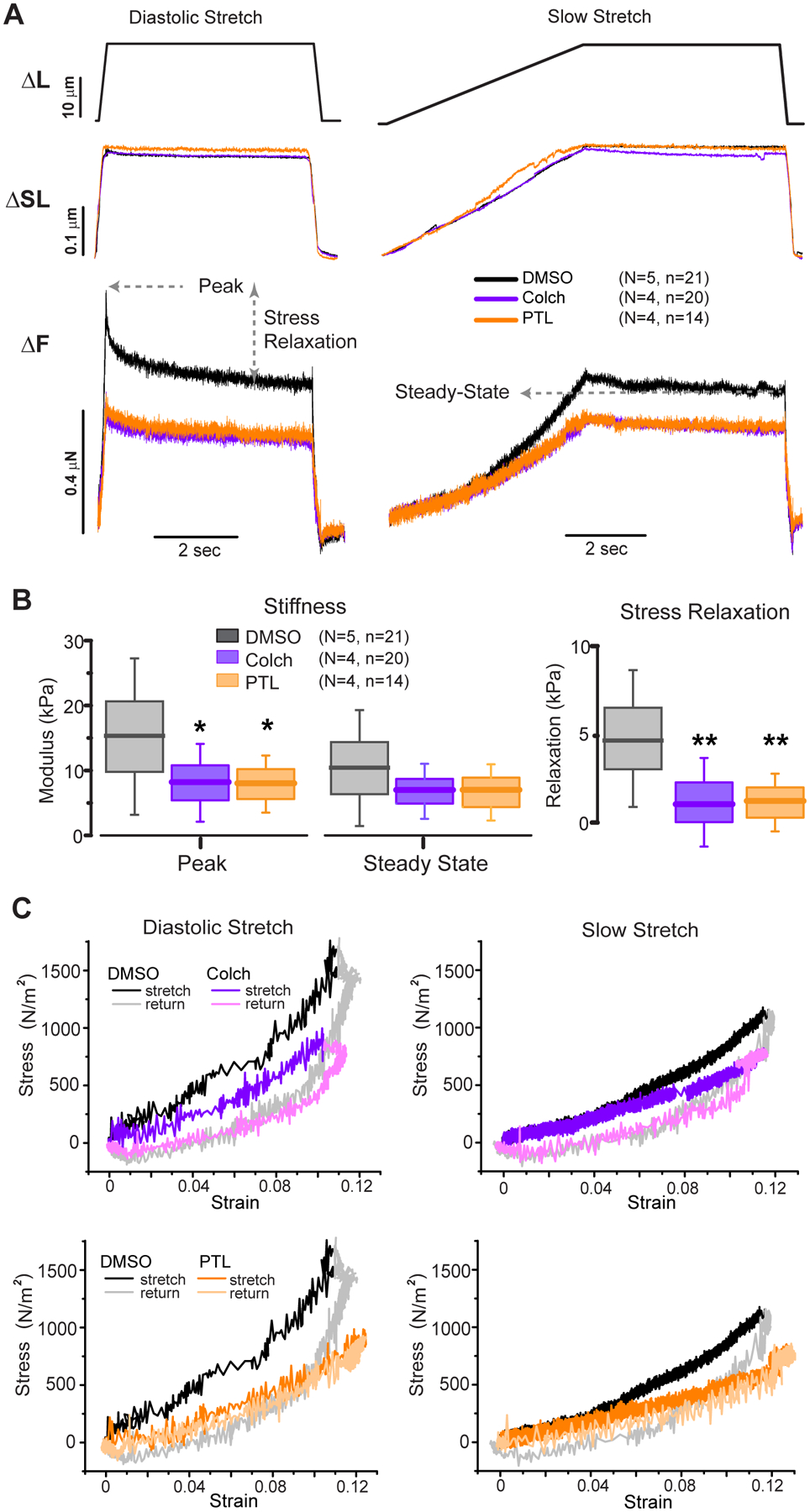
A, Average cellular responses to diastolic and slow stretching assay. Top: Stretching protocol showing either 200 ms or 5 s stretch followed by 5 s hold and 200 ms return to slack length. Middle: Corresponding sarcomere length change in response to stretch protocol. Bottom: force in response to stretch protocol. Peak, stress relaxation, and steady state forces are depicted. B, Box plots of peak (diastolic) and steady state (elastic) stiffness, and stress relaxation. Box plots represent mean (line), standard deviation (box) and 25–75th percentile (whisker). Significance *p<0.05, **p<0.01, one-way ANOVA with post-hoc Bonferroni comparison. C, Stress-strain plots showing hysteresis of cardiomyocytes treated with DMSO vehicle (black/gray - duplicated) or colchicine (purple – top) or parthenolide (orange – bottom) for diastolic stretch (left) or slow stretch (right). The reduction in slope indicates decreased stiffness at the respective stretching speed, while the loop area indicates the energy dissipated during the protocol.
Colchicine depolymerizes the majority of microtubules and parthenolide significantly reduces the level of microtubule detyrosination in myocytes10,32 following treatment for 90 min with 10 μM of either drug. Neither treatment altered myocyte resting sarcomere length (DMSO = 1.937 ± 0.016 μm; COLCH = 1.956 ± 0.014 μm; PTL = 1.949 ± 0.024 μm) nor peak sarcomere length upon 12% strain (~2.16 μm, Figure 1A middle). Reduction of either microtubule density (colchicine) or detyrosination (parthenolide) significantly reduced diastolic stiffness (peak stress) and stress relaxation, with a more modest, statistically insignificant reduction on elastic (steady state) stiffness (Figure 1B). Stress relaxation was reduced (Figure 1B, right) by similar amounts in response to colchicine or parthenolide treatment, indicating that detyrosination of microtubules is necessary for the substantial viscous forces observed during diastolic stretch.
Hysteresis loops (Figure 1C) are obtained from plotting stress vs strain from the average traces depicted in Figure 1A and provide a readout of myocyte stiffness (slope) at a given strain at each strain rate. The area within the loop represents the energy dissipated during each loading and unloading cycle. Viscous forces that typically depend linearly on loading rate are the primary source of energy dissipation, and thus hysteresis (loop area) is greater during diastolic stretch than slow stretch (Figure 1C). The greater slope of the relationship during diastolic stretch compared to slow stretch indicates that viscous forces are contributing to the stiffness at diastolic strain rates. Consistent with a reduction in viscous forces, both colchicine and parthenolide reduce the hysteresis during stretch, and consistent with changes in stiffness, both drugs reduce the slope of the curves at both slow and diastolic strain rates. Colchicine and parthenolide reduced diastolic stiffness to similar levels, and in both cases measurable stress relaxation remained, indicating that while microtubules contribute significantly to myocyte viscoelastic properties, there are other viscoelastic elements that give rise to rate-dependent mechanical properties, as previously demonstrated for titin and acto-myosin cross-bridges33,34.
Myocytes exhibit a pathological increase in diastolic stiffness
We next compared the diastolic compliance of myocytes isolated from 5 non-failing donors (NF) and 5 explanted hearts from patients with HF (Figure 2A). All HF patient hearts used for single myocyte assays exhibited both systolic and diastolic dysfunction (Table 1, Supplemental Excel File). HF hearts tended to be younger than NF hearts in this cohort, but no correlation between age and viscoelasticity was observed (Supplementary Figure 1).
Figure 2: Viscoelasticity is increased in failing human cardiomyocytes.
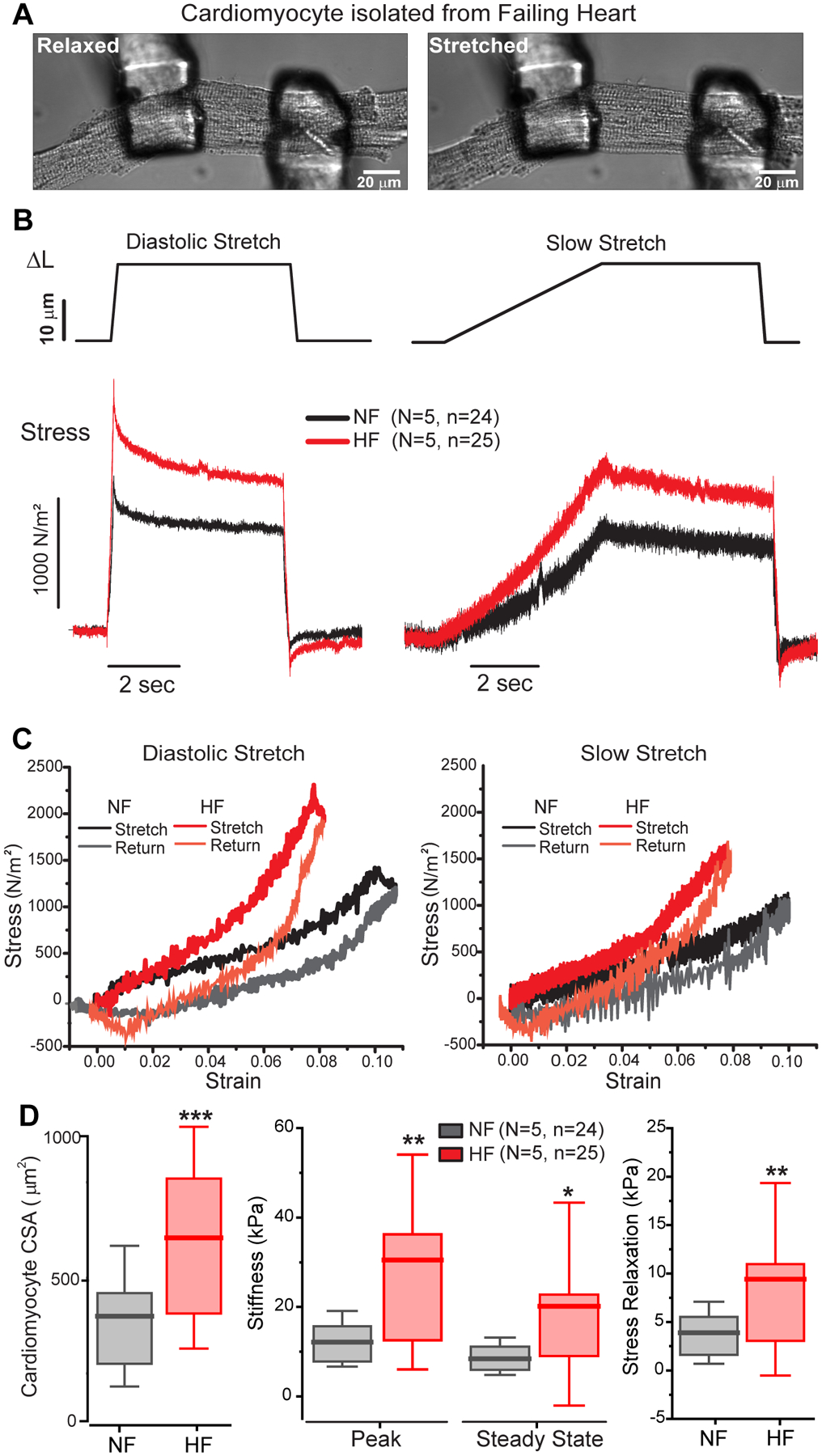
A, Transmitted light image of failing human myocyte before (relaxed) and during (stretched) diastolic stretching assay. B, Average stress-time plots comparing 20 um stretches at diastolic (200 ms - left) or slow (5 s - right) strain rates in NF (black) and HF (red) cardiomyocytes. C, Stress-strain plots for diastolic (left) and slow (right) stretches. Slope denotes effective stiffness at each rate, while hysteresis indicates energy dissipation. D, Summary of myocyte cross sectional area (left) and stiffness parameters in NF and HF myocytes (right). Statistical significance determined via two sample T-test, *p<0.05, **p<0.01, ***p<0.001.
Both viscous and elastic stresses were elevated in the failing myocytes (Figure 2B–D), which were twice as stiff as NF myocytes irrespective of strain rate. HF cardiomyocytes were also significantly larger than their NF counterparts (Figure 2D, left). Following the diastolic stretch protocol, greater stress relaxation was observed in the failing myocytes, consistent with previous observations that the microtubule network is proliferated and detyrosinated in human HF9,10. Stiffness (slope, Figure 2C) was greater in failing myocytes at both the diastolic and slow strain rates, and failing myocytes also exhibited a more pronounced non-linear behavior in stiffness as noted by the change in slope around 5% strain. At small strains and the slow rate, myocyte stiffness more closely matched the non-failing myocytes until the strain reached ~5%, while at diastolic strain rates the deviation was more apparent irrespective of strain amplitude. This is consistent with an increase in the viscous forces in failing myocytes at diastolic strain rates. Failing myocytes also exhibited increased hysteresis, particularly at diastolic strain rates. Overall, myocytes from failing hearts are larger, stiffer, more viscous, and dissipate more energy during diastolic stretch, consistent with reduced efficiency independent of systolic performance.
Genetic reduction of detyrosination reduces stiffness of failing cardiomyocytes
As an orthogonal approach, and in part due to potential off-target effects of pharmacological agents, we reduced microtubule detyrosination with a more specific genetic strategy, which can be achieved by adenoviral over-expression (OE) of the tyrosinating enzyme tubulin tyrosine ligase (TTL) in human cardiomyocytes9. We first compared data from freshly isolated cardiomyocytes to those transduced with control adenovirus (null) and cultured for 48hrs to test whether our culture conditions altered viscoelastic behavior. Myocytes cultured for 48h exhibited indistinguishable viscoelastic behavior from freshly isolated myocytes, regardless of whether they were from NF or HF hearts (Supplementary Figure 1), indicating that our short-term culture conditions did not alter myocyte viscoelasticity, which is consistent with our previous observations that tubulin detyrosination does not change with this duration of human cardiomyocyte culture9. As in freshly isolated myocytes (Figure 2), cultured HF myocytes exhibited increased viscoelasticity compared to NF controls (Figure 3A–C).
Figure 3: Overexpression of TTL is sufficient to reduce viscoelasticity in failing and non-failing human myocytes.
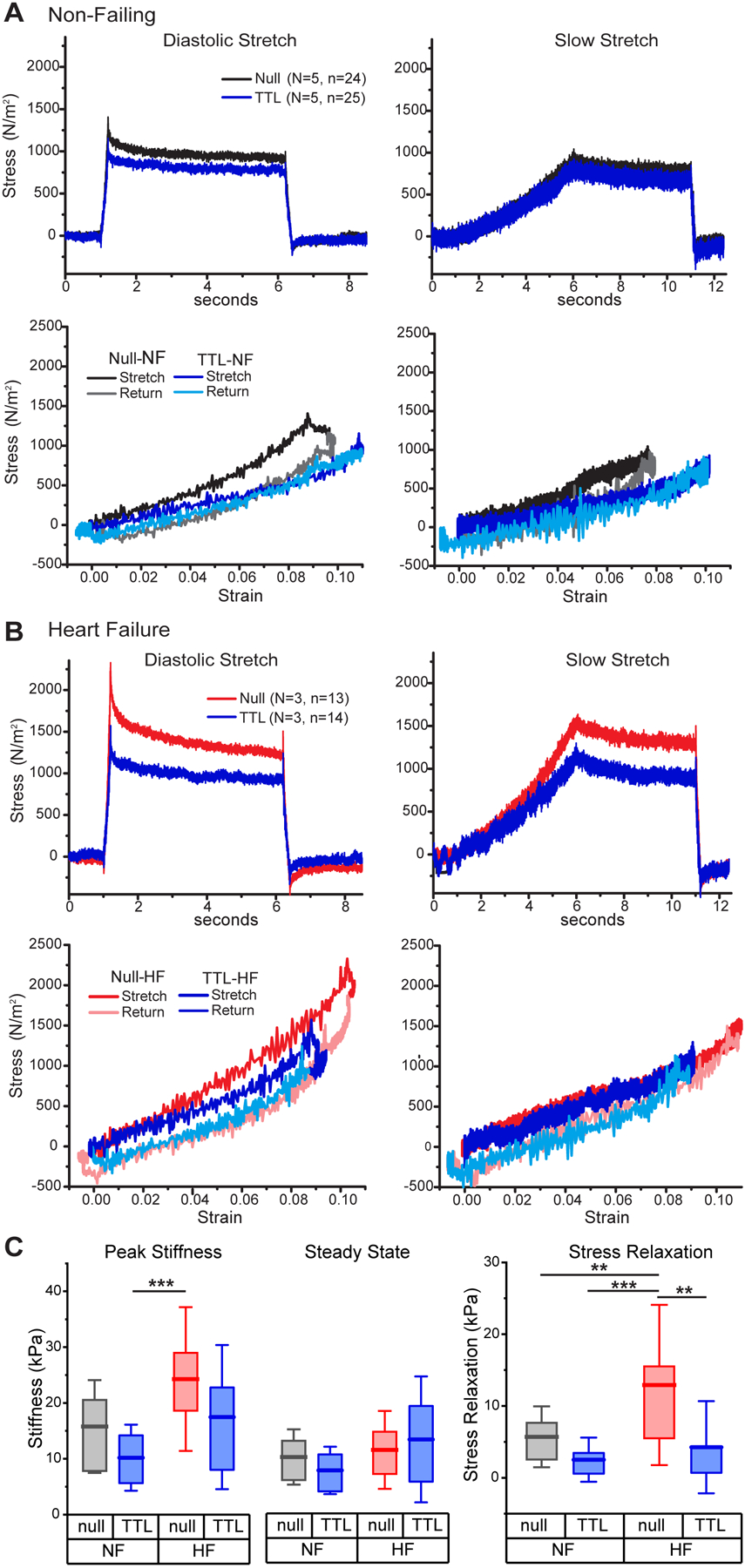
A-B, Average diastolic (left) and slow (right) stress-time (top) and stress-strain (bottom) plots in intact myocytes from NF and HF hearts following culture with either null-encoding adenovirus (Black, Red) or adenoviral overexpression (OE) of TTL (Blue). C, Diastolic and steady state stiffnesses (left) and stress relaxation (right) in NF and HF cardiomyocytes infected with null or TTL OE adenovirus. Statistical significance determined by two-way ANOVA with factors of etiology (NF or HF) and treatment (TTL OE vs. null). All significant interactions from this analysis are denoted in C, while significant factor effects are stated in the text, but not denoted on the figure for simplicity. Significance denoted as **p<0.01 and ***p<0.001
TTL OE in NF myocytes modestly reduced diastolic stiffness, stress relaxation, and hysteresis, with no observable effect on steady-state stiffness (Figure 3A). In failing myocytes, TTL OE more robustly reduced stress relaxation, diastolic stiffness and hysteresis, with no significant effect on elastic stiffness (Figure 3B). To assess the roles of etiology and treatment on these parameters, two-way ANOVA analysis was conducted, Figure 3C. HF increased peak stiffness (p=0.001) while TTL OE reduced it (p=0.008), but the reductions in peak stiffness with TTL OE did not reach significance within NF or HF groups. Steady state stiffness was more modestly increased in HF (p=0.04), but not affected by TTL OE. In the case of stress relaxation, HF significantly increased stress relaxation (p=0.003) while TTL OE robustly reduced it (p=0.0005). Within etiology, TTL OE significantly reduced stress relaxation only in the HF group. Together, this data indicates that reducing microtubule detyrosination reduces viscous stresses in failing myocytes at diastolic elongation rates, but has a minimal effect on elastic tension.
Microtubules provide viscous resistance to myocardial elongation
Having characterized the viscoelastic behavior of isolated cardiomyocytes, we next sought to interrogate the role of microtubules in intact myocardial tissue. Trabeculae were excised from the free-wall of failing human left ventricles and subjected to a stair-case loading and unloaded procedure (Figure 4A) to assess viscous energy dissipation, stress-relaxation and elasticity at different working lengths. Each trabecula was mechanically tested before (T0) and after 90 minutes (T90) of treatment with either colchicine or DMSO vehicle. Resting tissue sarcomere length was between 1.8–1.9 μm, and was not altered by colchicine treatment, as determined by analyzing identical regions of myocardium before and after treatment (Supplementary Figure 2). Analogous to single cell mechanical measurements, diastolic (peak) stress, viscoelasticity (stress relaxation), and elasticity (steady-state stress) were assessed at each lengthening step (~2.5% strain over 100 ms) at a 25% s−1 strain rate, covering strains from 0–17.5% over the staircase protocol.
Figure 4: Microtubules regulate myocardial stiffness and stress relaxation in a length-dependent fashion.
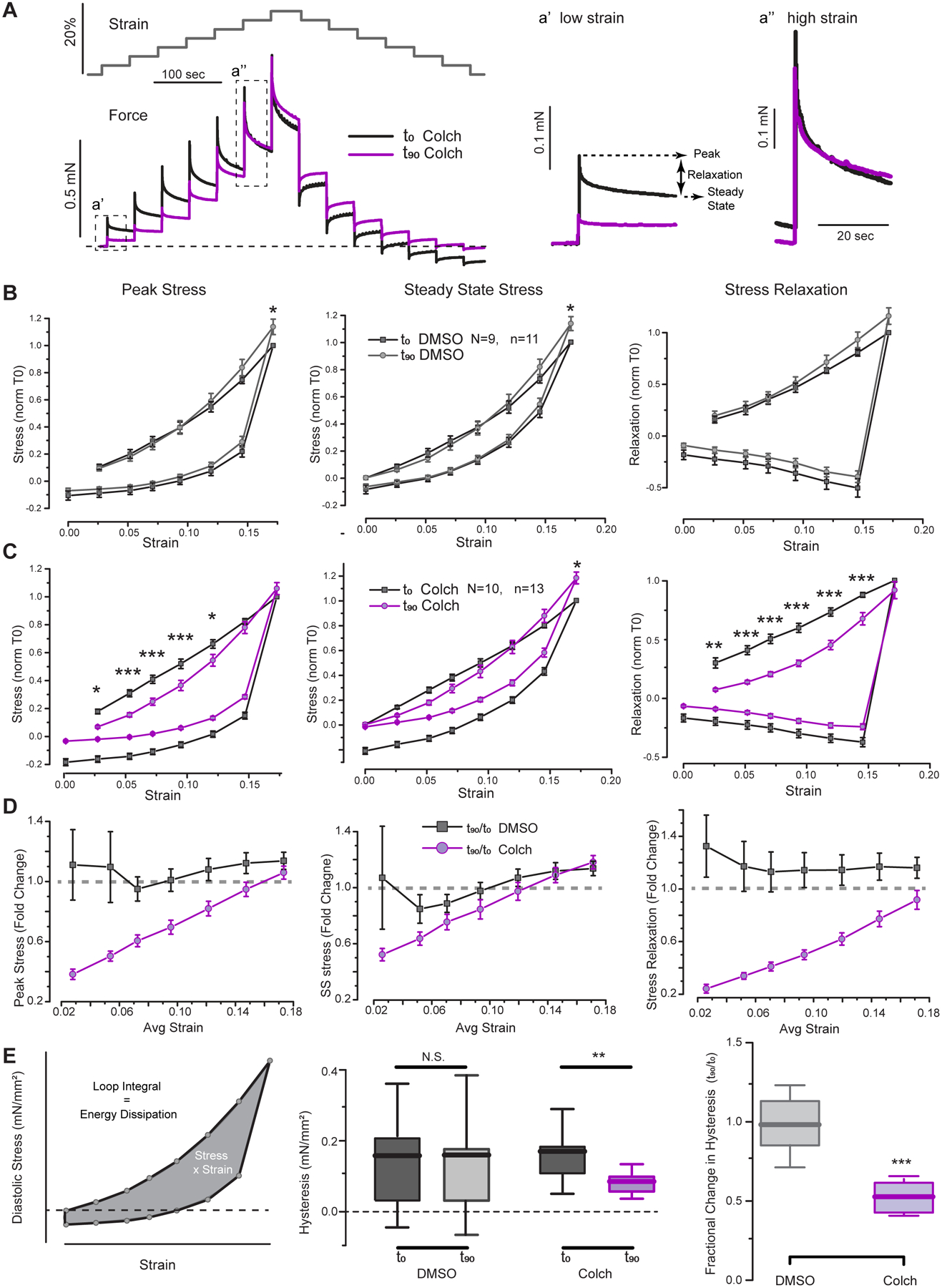
A, Experimental schematic: Gray trace shows summary of staircase protocol (stretch vs time). (Bottom) Representative trace of stress vs time for trabecula before (T0, black) and after (T90, purple) 90 min treatment with colchicine. Insets highlight stress relaxation behavior at small and large strains. B, Summary of mechanical parameters (peak stress, steady-state stress, and stress relaxation) before (black) and after 90 min treatment of trabecula with vehicle (gray). Measurements normalized within each trabecula to stiffness at maximal strain prior to treatment. Statistical significance determined via two-way, repeated measures ANOVA, *p<0.05, **p<0.01, ***p<0.001. C, Summary of mechanical parameters before (black) and after 90 min treatment of trabecula with colchicine (purple). D, Summary of fold change in each parameter after either vehicle (black) or colchicine (purple) treatment as a function of strain. E, Schematic representation for quantification of hysteresis (loop area) for diastolic stress-strain plots in staircase protocol. Middle panel shows raw values of energy dissipation, while right panel shows the within trabecula fractional change in hysteresis upon vehicle or colchicine treatment. Statistical significance determined via two-sample T-test.
DMSO-treated time control trabeculae showed no differences in peak stress, steady state stress, or stress relaxation over the working range of strains from T0 to T90, except for a slight increase in stress at 17.5% strain (Figure 4B). In contrast, colchicine-treated myocardium exhibited reduced peak stress and stress relaxation (Figure 4C). The largest fractional decrease was observed for stress relaxation (viscoelasticity, Figure 4C right panel), which was significantly reduced from strains of 2.5–15%, and exhibited a reduction in fold effect with increasing strain (Figure 4D). Reduced viscoelasticity underlies the reduction in peak stress observed up to 12.5% strain, which also shows a diminishing effect with increasing length (Figure 4D). The effect of colchicine was substantially less on elasticity, as steady-state stress was relatively insensitive to colchicine. Perhaps the most relevant strain for cardiac function is on the order of 10%, where colchicine reduced diastolic stress by ~30% and stress relaxation by ~50% compared to vehicle control. These findings support the idea that over the common working range of diastolic strain and strain rates, cardiac microtubules contribute to diastolic stiffness, but at larger excursions, where larger increases in stiffness are associated with titin and ECM extension35, the viscoelastic forces attributable to microtubules play a less significant role.
Colchicine reduces energy dissipation in failing myocardium
Analogous to the hysteresis curves obtained for single cells, the area was calculated within the loops generated by diastolic stress at each loading and unloading step against strain to measure the energy dissipated to viscosity for each trabecula during the staircase protocol (Figure 4E). Hysteresis can also be visualized by the trace in Figure 4A as the degree of negative force deflection below the resting level (dotted line) during the unloading phase of the staircase. There was no difference in hysteresis before and after DMSO treatment, but colchicine treatment significantly reduced hysteresis in failing myocardium by roughly 2-fold (Figure 4E). The reduced energy dissipation during length change implies an improvement in diastolic performance following colchicine treatment.
Colchicine reduces viscoelasticity during diastolic elongation in failing myocardium
The proper experiment to assess diastolic (peak) stiffness is not a staircase protocol, but a full stretch from slack length to a fixed length in a time period consistent with diastolic filling. While diastolic times and stroke volumes vary depending on etiology, 200 ms represents a typical early diastolic interval over which the net strain averages around 10 ± 5% depending on region and pathology30,31. Thus, we performed a series of stretches from slack length with strains increasing from ~2–12% over 200 ms (Figure 5A). Viscoelastic forces increased non-linearly with muscle strain, and no significant differences in stress relaxation or diastolic (peak) and elastic (steady state) stresses were observed after DMSO treatment (Figure 5B). Colchicine significantly reduced viscoelastic parameters at strains from 2–10%, and analogous to the staircase protocol, showed a diminishing effect with increasing length (Figure 5C).
Figure 5: The microtubule contribution to diastolic mechanics upon different step changes in length.
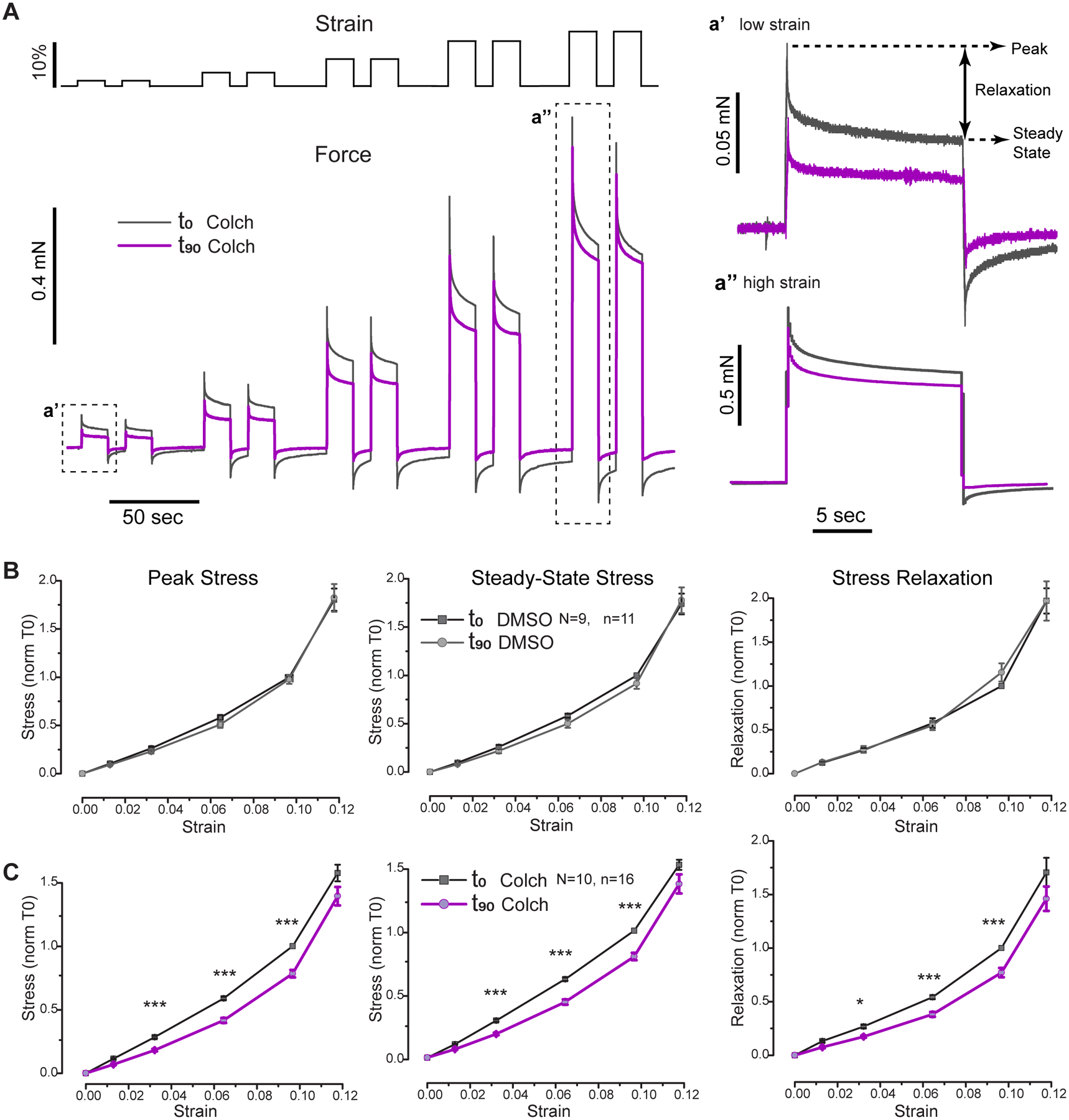
A, Overview of the diastolic stretching assay to assess diastolic mechanical parameters of intact human trabeculae at different strains. Representative trace shows the effect of 90 min colchicine treatment (T90, purple) in a trabecula relative to before treatment values (T0, black). Insets a’ and a” show differences in the effective behavior and fractional change at 2.5% and 12.5% strain, respectively. B-C, Average values of peak (diastolic) stress (left), steady state stress (center), and stress relaxation (right) before (black) and after 90 min of treatment with vehicle (gray, B) or colchicine (purple, C). Values are normalized within trabecula to pre-treatment value at 10% strain. Statistical significance determined via two-way, repeated measures ANOVA, *p<0.05, ***p<0.001.
HFpEF myocardium is less fibrotic and less elastic than HFrEF tissue
Mechanical data in Figures 4 and 5 is pooled from 24 trabeculae from 10 patients with non-ischemic HF, six with HFrEF and four with HFpEF. While diastolic dysfunction is common in both forms of HF, its increased significance in HFpEF motivated a post-hoc sub-group analysis. Histological analysis (Figure 6A) of the patient hearts used for mechanical testing revealed a similar degree of myocyte hypertrophy in HFrEF and HFpEF myocardium, as shown by a 2–3 fold increase in cardiomyocyte cross-sectional area compared to NF controls. Conversely, fibrosis was substantially increased only in HFrEF, but not HFpEF tissues.
Figure 6: Post-hoc analysis of HFpEF and HFrEF myocardium.
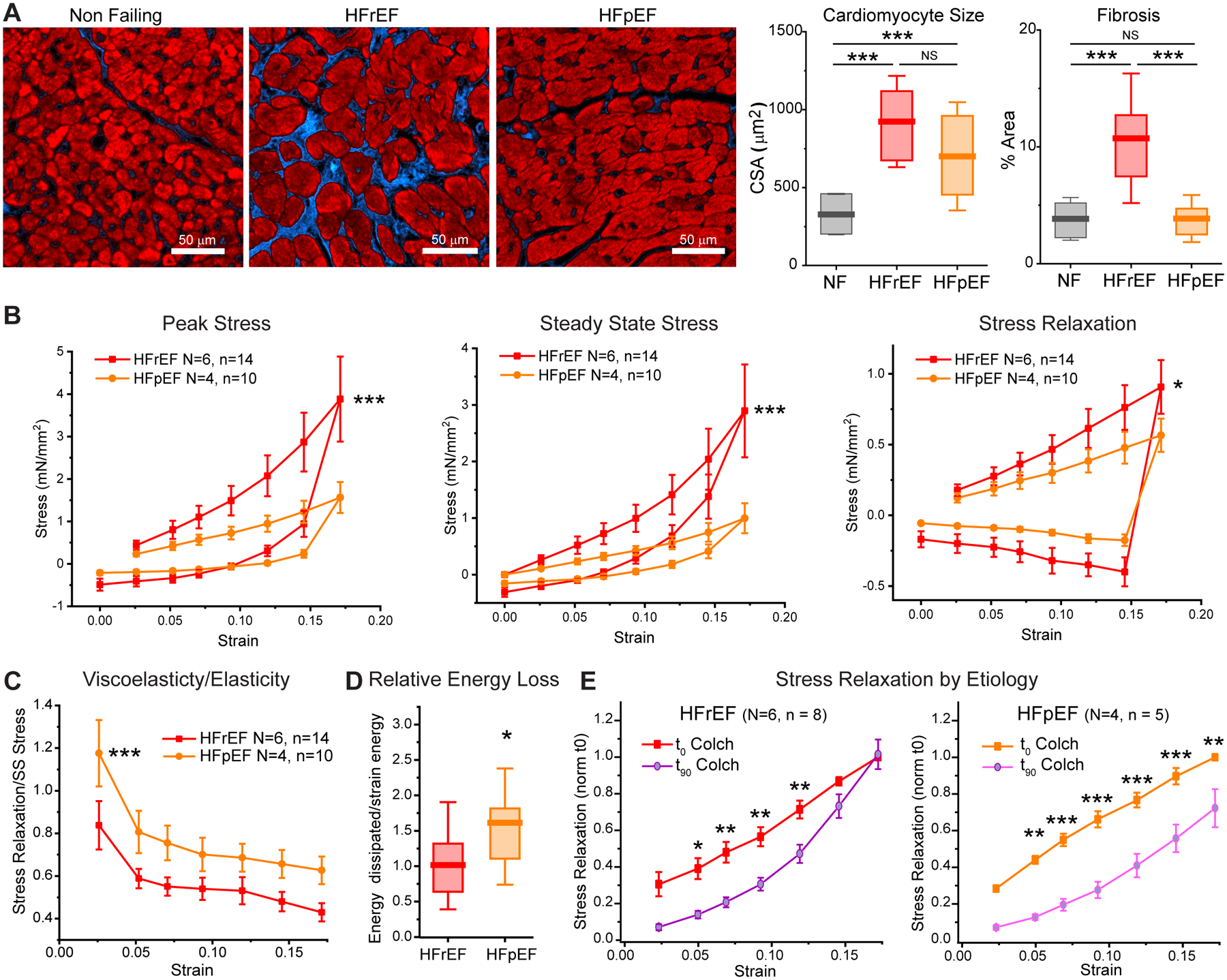
A, Histological analysis of NF vs. HFrEF and HFpEF myocardium from hearts used for mechanical testing. Left: representative transverse sections of LV free wall (left) with Masson’s Trichrome staining (myocardium in red, fibrosis in blue). Right: quantification of cardiomyocyte cross sectional area and fibrosis. For each heart, n>100 cell were analyzed from 3 independent areas. N = 3, 5, and 4 hearts analyzed for NF, HFrEF, and HFpEF respectively. Statistical significance determined via one-way ANOVA with post-hoc Bonferroni comparison, ***p<0.001. B, Comparison of viscoelastic properties between HFrEF and HFpEF trabecula prior to any treatment. C, The relative proportion of viscoelastic to elastic stress (Stress Relaxation/Steady State Stress) as a function of strain. For B and C, asterisks denote significant effect of etiology as determined via two-way ANOVA (with factors of etiology and strain). *p<0.05, ***p,0.001. D, Relative energy loss (energy dissipated/energy stored) was calculated from the ratio of energy dissipated (hysteresis loop) to elastic strain energy. Two-sample t-test, *p<0.05. E, Effect of colchicine on stress relaxation during ascending staircase of HFrEF and HFpEF trabecula. Statistical significance determined via two-way, repeated measures ANOVA, *p<0.05, **p<0.01, ***p<0.001.
Consistent with more fibrotic myocardium, diastolic and elastic stiffness (as assessed by the slope of the stress-strain relationship) were significantly elevated in HFrEF (n=14) compared to HFpEF (n=10) trabeculae (Figure 6B): elastic stiffness was ~3 fold higher in HFrEF than in HFpEF, whereas increases in viscoelasticity (stress relaxation) with HFrEF were less pronounced. Figure 6C displays the tissue viscoelasticity relative to elasticity as a function of strain in HFrEF and HFpEF myocardium. Regardless of etiology, the myocardium is more viscoelastic at low strains, and becomes increasingly elastic with increasing length, consistent with an increasing contribution of extracellular matrix. HFpEF myocardium is also relatively more viscoelastic compared to HFrEF tissue. Energy dissipation normalized to elastic strain energy (energy lost per energy stored) was increased in HFpEF myocardium, indicating greater relative energy dissipation due to viscosity in the HFpEF myocardium studied here (Figure 6D).
Consistent with pooled HF data (Figure 4), colchicine reduced diastolic stress with a modest or insignificant effect on steady state stress in both HFrEF (n=8) and HFpEF trabecula (n=5) (Supplementary Figure 3). Colchicine produced a greater reduction in stress relaxation in HFpEF than HFrEF myocardium, particularly at higher strains. Consistent with HFpEF tissues being less fibrotic and relatively more viscous, colchicine reduced stress relaxation even at 17.5% strain, whereas in HFrEF myocardial viscoelasticity was insensitive to colchicine at strains beyond 12.5% (Figure 6E). Thus, the diminishing effect of colchicine at longer lengths in HFrEF tissue may be attributable to increased fibrosis (Figure 6A) that contributes more to mechanical properties at longer lengths35.
Discussion
Microtubules and diastolic viscoelasticity
The above results indicate that the microtubule network regulates the compliance of human cardiomyocytes and myocardium. The microtubule contribution to stiffness depends on the rate of loading, indicating that it is viscous in nature, and viscous forces are significant at rates of lengthening consistent with diastolic filling in the intact heart. HF myocytes exhibit increased viscoelastic forces compared to NF myocytes, consistent with the proliferation of stable, detyrosinated microtubules in several etiologies of human HF9,36,37. Consequently, HF myocytes exhibit reduced viscoelasticity when microtubules are mechanically uncoupled in the myocyte by reducing detyrosination. A similar viscoelastic role of microtubules is observed in failing myocardium, where the effect of microtubules is observed at physiological strains and decreases with increasing length. At larger excursions, non-linear stiffening of myocardium, likely by other elements such as ECM or titin, begins to mask the contribution of microtubules.
Translation of single cell parameters to intact myocardium
Reductions in microtubules or microtubule detyrosination leads to an acceleration of both myocyte shortening and relaxation8,9,22,23, which can be explained by reduced viscosity, but not solely by a change in elasticity. Our work here is consistent with these observations, whereby a viscoelastic contribution of microtubules at the cardiomyocyte level translates to the myocardium upon diastolic strain. Because the effect of viscosity will be largest under low loads (such as upon unloaded shortening)38, increased myocardial viscosity is most likely to affect the cardiac cycle in settings of lower distending pressure and high internal strain rates, such as during the internal elastic recoil that occurs upon isovolumic relaxation and rapid stretch during early diastolic filling4. Inhibiting the speed of relaxation or filling can elevate diastolic pressures or reduce end-diastolic volume, hallmarks of diastolic dysfunction39. Thus, elevated myocardial viscosity may impair diastolic function, independent of a change to systolic function or afterload.
Molecular underpinnings of viscous forces
Previous experiments in intact and skinned cardiac muscle and myofibril preparations have demonstrated that cardiomyocytes exhibit non-linear, rate-dependent mechanical properties that depend largely on the giant muscle protein titin, which is responsible for the majority of passive stiffness33,40. Yet we observe, particularly at modest strains, that as much as half of diastolic stiffness is sensitive to microtubule manipulations. While at first this may seem counterintuitive, we believe it is largely in agreement with these previous findings. Differences in experimental conditions aside, an important consideration is that the stiffness of series elements do not add, and thus the total stiffness of a system will be less than the sum of individual element’s contributions. In the case of the myocardium, a dense network of highly cross-linked sarcomeric, cytoskeletal, and extracellular proteins create a complicated viscoelastic meshwork. Cross-linkers such as plectins, microtubule associated proteins, and molecular motors provide interconnectivity between microtubules, intermediate filaments, and myofilaments in the myocyte15. Under stress, the rearrangement of these cross-linkers gives rise to increased viscosity. Previous visualization of detyrosinated microtubules buckling at sarcomeric periodicities is evidence for a detyrosination-dependent linkage between the microtubule network and sarcomeres10, and thus their mechanical properties are likely to be linked. As further support of interconnectivity, in the absence of the intermediate filament desmin, reducing microtubule detyrosination has no further effect on myocyte viscoelasticity10. We observe here that manipulations of the microtubule network decrease the viscoelastic stiffness of myocytes and myocardium in the presence of the diastolic tension provided by sarcomeres. This work cannot distinguish whether microtubule modifications reduce viscoelasticity through downstream interactions that influence cross-bridge activation or as independent mechanical components. Additional work is needed to determine mechanisms of cross-linking, how it changes in disease, and to test the possibility that microtubules may also regulate biochemical reactions that impact stiffness, such as post-translational modifications of known contributors to myofilament stiffness. The characterization of these molecular sources of viscosity may inform the design of new therapeutic approaches to improve diastolic function.
Microtubule-based therapies
Microtubule stabilizers (taxanes) are effective chemotherapeutic agents but are associated with cardiomyopathy, conduction abnormalities, and impaired contractility, particularly when combined with anthracyclines41,42. While cardiac complications associated with chemotherapy are likely multi-factorial, in-vivo imaging of non-failing patient hearts before and after a chemotherapeutic regiment of taxane treatment indicates compromised diastolic function with preserved systolic parameters43, consistent with microtubule-based viscoelastic changes primarily altering diastolic function.
In contrast, low-dose colchicine, a microtubule destabilizer, is associated with reduced risk of overall cardiovascular events44. However, establishing efficacy for modifying the course of coronary artery disease or HF requires further investigation, and the benefits to patients likely arise largely from colchicine’s anti-inflammatory effects, since the tolerable doses of colchicine in patients are below the threshold required for the robust depolymerization of cardiac microtubules that has been observed in animal models24,25,28,29,45. Thus, the unfavorable benefit:adverse effect ratio of colchicine may limit its potential as a treatment for HF, and derivatives with improved pharmacological profiles or more granular approaches should be explored.
We used one such approach here with parthenolide, a naturally occurring sesquiterpene lactone known for its anti-inflammatory characteristics as a remedy for arthritis and migraine. Parthenolide inhibits the tubulin carboxypeptidase to reduce microtubule detyrosination46, but may also be limited for the treatment of HF as it can alter E-C coupling in human myocytes9 and has additional off-target effects including inhibition of histone deacetylase 147, inhibition of NFkB activity48, and others15. Thus, a need exists for safer, more specific therapies to target cardiac microtubule detyrosination. The recent identification of the tubulin carboxypeptidase complex, VASH/SVBP,49,50 provides a promising new target for the development of novel HF therapeutics.
Limitations and Future Directions
Due to experimental limitations, stretches beyond 12% elongation were not attempted in intact single myocytes. With tissue preparations demonstrating a length-dependent effect on the microtubule contribution to viscoelasticity, it would be of further interest to attempt larger stretches in single myocytes to identify whether the diminishing effect of microtubules arises from mechanical elements from within the myocyte (i.e. titin) or depends on extracellular constituents. Cardiac myofilaments exhibit non-linear elastic behavior with robust stiffening as strains exceed 20%, but we observe that the diminishing effect of microtubules in failing myocardium begins to occur before 10% strain, consistent with an additional source of tissue stiffness such as extracellular matrix35. Tissue is well known to be a non-linear material, and the determination of cellular and extracellular sources of non-linearity in healthy and failing tissue is of considerable interest.
Because specifically targeting detyrosination demonstrates similar decreases in viscoelasticity as gross microtubule depolymerization with colchicine, the development of specific inhibitors of the detyrosinating enzyme complex VASH/SVBP would provide a refined approach to reducing myocardial viscoelasticity. Alternatively, since long-term modifications to the microtubule network are likely to have multiple consequences due to their roles in mRNA delivery, protein turnover, and maintenance of T-tubules and sarcomeres15, additional work to understand molecular determinants of myocardial viscosity may unlock new, more specific targets to restore myocardial mechanics in HF.
While post-hoc analysis of pre-treatment myocardial mechanics (Figure 6B–D) was performed on 14 HFrEF and 10 HFpEF trabecula, post-hoc assessment of within-etiology colchicine effects (Supplemental Figure 3, Figure 6E) was restricted to a smaller sample (n=8 and n=5, respectively), as numerous trabeculae were used for DMSO time controls. Thus, future study is needed to robustly assess differential effects of microtubule-targeting agents in different etiologies of HF, which should include different sub-groups within the HFpEF population as well as ischemic HF, which was not assessed here. We speculate that increased fibrosis and elasticity in ischemic myocardium may limit the effect of a microtubule destabilizing agent, but this remains to be tested.
In sum, this study provides the first evidence that targeting microtubules reduces viscous forces during diastolic stretch of failing human myocardium, improving myocardial compliance. This work motivates the development of therapies to target microtubule detyrosination specifically, or cardiac viscoelasticity more broadly.
Supplementary Material
Clinical Perspective:
What Is New?
We interrogated the role of the microtubule network in the diastolic mechanics of human cardiomyocytes and myocardium.
We found that stable, detyrosinated microtubules contribute viscous forces during diastolic stretch that increase cardiomyocyte stiffness, particularly in patients with heart failure.
Depolymerizing microtubules reduced myocardial stiffness over the range of strains and strain rates associated with early rapid filling in tissue from patients with diastolic dysfunction.
What Are The Clinical Implications?
This work pinpoints detyrosinated microtubules as a therapeutic target to potentially reduce myocardial stiffness and improve diastolic mechanics in heart failure.
Post-hoc analysis of myocardial samples suggests that a microtubule-based therapy may confer greater benefit in conditions with limited myocardial fibrosis, including heart failure with preserved ejection fraction (HFpEF).
Acknowledgements
The authors acknowledge the assistance of the Gift-of-Life Donor Program, Philadelphia, PA who helped provide nonfailing heart tissue from unused donor hearts for this research.
Sources of Funding
This work was supported by funding from NHLBI R01-HL133080 to B.L.P., NIAMS grant 5T32AR053461 to M.A.C., American Heart Association Fellowship 19POST34400012 to C.Y.C, Sanofi iAwards to B.L.P. and K.B.M., and the Gund Family Fund to K.B.M.
Non-standard abbreviations and acronyms:
- BDM
butanedione monoxime
- ECM
extracellular matrix
- EDV
end diastolic volume
- KHB
Krebs–Henseleit buffer
- LV
left ventricle
- LVMI
left-ventricular mass index
- LVEF
left-ventricular ejection fraction
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- NF
non-failing
- NT
normal tyrode’s solution
- PTL
parthenolide
- TTL
tubulin tyrosine ligase
Footnotes
Conflict of Interest Disclosures
Significant financial interests: Research Grant, Sanofi-Aventis, U.S., LLC – sponsored research >$50,000. Invention disclosure/patent, inventor: composition and methods for improving heart function and treating heart failure. US patent application No.15/959,181 USA 2018.
References
- (1).Little WC, Applegate RJ. Congestive Heart Failure: Systolic and Diastolic Function. J. Cardiothorac. Vasc. Anesth 1993; 7: 2–5. [DOI] [PubMed] [Google Scholar]
- (2).Machaj F, Dembowska E, Rosik J, Szostak B, Mazurek-Mochol M, Pawlik A. New Therapies for the Treatment of Heart Failure: A Summary of Recent Accomplishments. Ther. Clin. Risk Manag 2019; 15: 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Psotka MA, Gottlieb SS, Francis GS, Allen LA, Teerlink JR, Adams KF, Rosano GMC, Lancellotti P. Cardiac Calcitropes, Myotropes, and Mitotropes. J. Am. Coll. Cardiol 2019; 73: 2345–2353. [DOI] [PubMed] [Google Scholar]
- (4).Kass DA, Bronzwaer JGF, Paulus WJ. What Mechanisms Underlie Diastolic Dysfunction in Heart Failure? Circ. Res 2004; 94: 1533–1542. [DOI] [PubMed] [Google Scholar]
- (5).Rankin JS, Arentzen CE, McHale PA, Ling D, Anderson RW. Viscoelastic Properties of the Diastolic Left Ventricle in the Conscious Dog. Circ. Res 1977; 41: 37–45. [DOI] [PubMed] [Google Scholar]
- (6).Hess OM, Grimm J, Krayenbuehl HP. Diastolic Simple Elastic and Viscoelastic Properties of the Left Ventricle in Man. Circulation. 1979; 59: 1178–1187. [DOI] [PubMed] [Google Scholar]
- (7).Fraites TJ, Saeki A, Kass DA. Effect of Altering Filling Pattern on Diastolic Pressure-Volume Curve. Circulation. 1997; 96: 4408–4414. [DOI] [PubMed] [Google Scholar]
- (8).Caporizzo MA, Chen CY, Salomon AK, Margulies KB, Prosser BL. Microtubules Provide a Viscoelastic Resistance to Myocyte Motion. Biophys. J 2018; 115: 1796–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Chen CY, Caporizzo MA, Bedi K, Vite A, Bogush AI, Robison P, Heffler JG, Salomon AK, Kelly NA, Babu A, et al. Suppression of Detyrosinated Microtubules Improves Cardiomyocyte Function in Human Heart Failure. Nat. Med 2018; 24: 1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Robison P, Caporizzo MA, Ahmadzadeh H, Bogush AI, Chen CY, Margulies KB, Shenoy VB, Prosser BL. Detyrosinated Microtubules Buckle and Bear Load in Contracting Cardiomyocytes. Science. 2016; 352: aaf0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Bottillo I, D’Angelantonio D, Caputo V, Paiardini A, Lipari M, De Bernardo C, Giannarelli D, Pizzuti A, Majore S, Castori M, et al. Molecular Analysis of Sarcomeric and Non-Sarcomeric Genes in Patients with Hypertrophic Cardiomyopathy. Gene. 2016; 577: 227–235. [DOI] [PubMed] [Google Scholar]
- (12).Frangogiannis NG. The Extracellular Matrix in Ischemic and Nonischemic Heart Failure. Circ. Res 2019; 125: 117–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Vélez-Rendón D, Pursell ER, Shieh J, Valdez-Jasso D. Relative Contributions of Matrix and Myocytes to Biaxial Mechanics of the Right Ventricle in Pulmonary Arterial Hypertension. J. Biomech. Eng 2019; 141: 091011. [DOI] [PubMed] [Google Scholar]
- (14).Sewanan LR, Schwan J, Kluger J, Park J, Jacoby DL, Qyang Y, Campbell SG. Extracellular Matrix From Hypertrophic Myocardium Provokes Impaired Twitch Dynamics in Healthy Cardiomyocytes. JACC Basic Transl. Sci 2019; 4: 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Caporizzo MA, Chen CY, Prosser BL. Cardiac Microtubules in Health and Heart Disease. Exp. Biol. Med 2019; 0:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Tagawa H, Wang N, Narishige T, Ingber DE, Zile MR, Cooper G. Cytoskeletal Mechanics in Pressure-Overload Cardiac Hypertrophy. Circ. Res 1997; 80: 281–289. [DOI] [PubMed] [Google Scholar]
- (17).Harris TS, Baicu CF, Conrad CH Koide M, Buckley JM, Barnes M, Cooper G, Zile MR. Constitutive Properties of Hypertrophied Myocardium: Cellular Contribution to Changes in Myocardial Stiffness. Am. J. Physiol.-Heart Circ. Physiol 2002; 282: H2173–H2182. [DOI] [PubMed] [Google Scholar]
- (18).Nishimura S, Nagai S, Katoh M, Yamashita H, Saeki Y, Okada J, Hisada T, Nagai R, Sugiura S. Microtubules Modulate the Stiffness of Cardiomyocytes Against Shear Stress. Circ. Res 2006; 98: 81–87. [DOI] [PubMed] [Google Scholar]
- (19).Drechsel DN, Kirschner MW. The Minimum GTP Cap Required to Stabilize Microtubules. Curr. Biol 1994; 4: 1053–1061. [DOI] [PubMed] [Google Scholar]
- (20).Delphin C, Bouvier D, Seggio M, Couriol E, Saoudi Y, Denarier E, Bosc C, Valiron O, Bisbal M, Arnal I, et al. MAP6-F Is a Temperature Sensor That Directly Binds to and Protects Microtubules from Cold-Induced Depolymerization. J. Biol. Chem 2012; 287: 35127–35138.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Yamamoto S, Tsutsui H, Takahashi M, Ishibashi Y, Tagawa H, Imanaka-Yoshida K, Saeki Y, Takeshita A. Role of Microtubules in the Viscoelastic Properties of Isolated Cardiac Muscle. J. Mol. Cell. Cardiol 1998; 30: 1841–1853. [DOI] [PubMed] [Google Scholar]
- (22).Cooper G Cytoskeletal Networks and the Regulation of Cardiac Contractility: Microtubules, Hypertrophy, and Cardiac Dysfunction. Am. J. Physiol.-Heart Circ. Physiol 2006; 291: H1003–H1014. [DOI] [PubMed] [Google Scholar]
- (23).Tsutsui H, Ishihara K, Cooper G. Cytoskeletal Role in the Contractile Dysfunction of Hypertrophied Myocardium. Science. 1993; 260: 682–687. [DOI] [PubMed] [Google Scholar]
- (24).Prins KW, Tian L, Wu D, Thenappan T, Metzger JM, Archer SL. Colchicine Depolymerizes Microtubules, Increases Junctophilin‐2, and Improves Right Ventricular Function in Experimental Pulmonary Arterial Hypertension. J. Am. Heart Assoc 2017; 6: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Scopacasa BS, Teixeira VPA, Franchini KG. Colchicine Attenuates Left Ventricular Hypertrophy but Preserves Cardiac Function of Aortic-Constricted Rats. J. Appl. Physiol 2003; 94: 1627–1633. [DOI] [PubMed] [Google Scholar]
- (26).Zhang C, Chen B, Guo A, Zhu Y, Miller JD, Gao S, Yuan C, Kutschke W, Zimmerman K, Weiss RM, et al. Microtubule-Mediated Defects in Junctophilin-2 Trafficking Contribute to Myocyte Transverse-Tubule Remodeling and Ca 2+ Handling Dysfunction in Heart Failure. Circulation. 2014; 129: 1742–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Zile MR, Koide M, Sato H, Ishiguro Y, Conrad CH, Buckley JM, Morgan JP, Cooper G. Role of Microtubules in the Contractile Dysfunction of Hypertrophied Myocardium. J. Am. Coll. Cardiol 1999; 33: 250–260. [DOI] [PubMed] [Google Scholar]
- (28).Koide M, Hamawaki M, Narishige T, Sato H, Nemoto S, DeFreyte G, Zile MR, Cooper G, Carabello BA. Microtubule Depolymerization Normalizes In Vivo Myocardial Contractile Function in Dogs With Pressure-Overload Left Ventricular Hypertrophy. Circulation. 2000; 102: 1045–1052. [DOI] [PubMed] [Google Scholar]
- (29).Fassett JT, Hu X, Xu X, Lu Z, Zhang P, Chen Y, Bache RJ. AMPK Attenuates Microtubule Proliferation in Cardiac Hypertrophy. Am. J. Physiol.-Heart Circ. Physiol 2013; 304: H749–H758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Sharif H, Ting S, Forsythe L, McGregor G, Banerjee P, O’Leary D, Ditor D, George K, Zehnder D, Oxborough D. Layer-Specific Systolic and Diastolic Strain in Hypertensive Patients with and without Mild Diastolic Dysfunction. Echo Res. Pract 2018; 5: 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Urheim S, Edvardsen T, Torp H, Angelsen B, Smiseth OA. Myocardial Strain by Doppler Echocardiography: Validation of a New Method to Quantify Regional Myocardial Function. Circulation. 2000; 102: 1158–1164. [DOI] [PubMed] [Google Scholar]
- (32).Kerr JP, Robison P, Shi G, Bogush AI, Kempema AM, Hexum JK, Becerra N, Harki DA, Martin SS, Raiteri R, et al. Detyrosinated Microtubules Modulate Mechanotransduction in Heart and Skeletal Muscle. Nat. Commun 2015; 6: 8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Linke WA, Ivemeyer M, Labeit S, Hinssen H, Rüegg JC, Gautel M. Actin-Titin Interaction in Cardiac Myofibrils: Probing a Physiological Role. Biophys. J 1997; 73: 905–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Donaldson C, Palmer BM, Zile M, Maughan DW, Ikonomidis JS, Granzier H, Meyer M, VanBuren P, LeWinter MM, Myosin Cross-Bridge Dynamics in Patients With Hypertension and Concentric Left Ventricular Remodeling. Circ. Heart Fail 2012; 5: 803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Linke WA, Fernandez JM. Cardiac Titin: Molecular Basis of Elasticity and Cellular Contribution to Elastic and Viscous Stiffness Components in Myocardium In Mechanics of Elastic Biomolecules; Linke WA, Granzier H, Kellermayer MSZ. Eds.; Springer; Netherlands: Dordrecht, 2003; pp 483–497. [DOI] [PubMed] [Google Scholar]
- (36).Dorsch LM, Schuldt M, dos Remedios CG, Schinkel AFL, de Jong PL, Michels M, Kuster DWD, Brundel BJJM, van der Velden J Protein Quality Control Activation and Microtubule Remodeling in Hypertrophic Cardiomyopathy. Cells. 2019; 8: 741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Zile MR, Green GR, Schuyler GT, Aurigemma GP, Miller DC, Cooper G. Cardiocyte Cytoskeleton in Patients with Left Ventricular Pressure Overload Hypertrophy. J. Am. Coll. Cardiol 2001; 37: 1080–1084. [DOI] [PubMed] [Google Scholar]
- (38).de Tombe PP, ter Keurs HE. An Internal Viscous Element Limits Unloaded Velocity of Sarcomere Shortening in Rat Myocardium. J. Physiol 1992; 454: 619–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Zile MR, Brutsaert DL. New Concepts in Diastolic Dysfunction and Diastolic Heart Failure: Part I: Diagnosis, Prognosis, and Measurements of Diastolic Function. Circulation. 2002; 105: 1387–1393. [DOI] [PubMed] [Google Scholar]
- (40).Granzier HL, Irving TC. Passive Tension in Cardiac Muscle: Contribution of Collagen, Titin, Microtubules, and Intermediate Filaments. Biophys. J 1995; 68: 1027–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Gehl J, Boesgaard M, Paaske T, Jensen BV, Dombernowsky P. Combined Doxorubicin and Paclitaxel in Advanced Breast Cancer: Effective and Cardiotoxic. Ann. Oncol 1996; 7: 687–693. [DOI] [PubMed] [Google Scholar]
- (42).Gianni L, Munzone E, Capri G, Fulfaro F, Tarenzi E, Villani F, Spreafico C, Laffranchi A, Caraceni A, Martini C. Paclitaxel by 3-Hour Infusion in Combination with Bolus Doxorubicin in Women with Untreated Metastatic Breast Cancer: High Antitumor Efficacy and Cardiac Effects in a Dose-Finding and Sequence-Finding Study. J. Clin. Oncol 1995; 13: 2688–2699. [DOI] [PubMed] [Google Scholar]
- (43).Altin C, Sade LE, Demirtas S, Karacaglar E, Kanyilmaz S, Simsek V, Ayhan A, Muderrisoglu H. Effects of Paclitaxel and Carboplatin Combination on Mechanical Myocardial and Microvascular Functions: A Transthoracic Doppler Echocardiography and Two-Dimensional Strain Imaging Study. Echocardiography. 2015; 32: 238–247. [DOI] [PubMed] [Google Scholar]
- (44).Slobodnick A, Shah B, Krasnokutsky S, Pillinger MH. Update on Colchicine, 2017. Rheumatology. 2018; 57: i4–i11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Tsutsui H, Ishibashi Y, Takahashi M, Namba T, Tagawa H, Imanaka-Yoshida K, Takeshita A. Chronic Colchicine Administration Attenuates Cardiac Hypertrophy in Spontaneously Hypertensive Rats. J. Mol. Cell. Cardiol 1999; 31: 1203–1213. [DOI] [PubMed] [Google Scholar]
- (46).Fonrose X, Ausseil F, Soleilhac E, Masson V, David B, Pouny I, Cintrat J-C, Rousseau B, Barette C, Massiot G, et al. Parthenolide Inhibits Tubulin Carboxypeptidase Activity. Cancer Res 2007; 67: 3371–3378. [DOI] [PubMed] [Google Scholar]
- (47).Gopal YNV, Arora TS, Van Dyke MW. Parthenolide Specifically Depletes Histone Deacetylase 1 Protein and Induces Cell Death through Ataxia Telangiectasia Mutated. Chem. Biol 2007; 14: 813–823. [DOI] [PubMed] [Google Scholar]
- (48).Hehner SP, Hofmann TG, Dröge W, Schmitz ML. The Antiinflammatory Sesquiterpene Lactone Parthenolide Inhibits NF-KB by Targeting the IκB Kinase Complex. J. Immunol 1999; 163: 5617. [PubMed] [Google Scholar]
- (49).Nieuwenhuis J, Adamopoulos A, Bleijerveld OB, Mazouzi A, Stickel E, Celie P, Altelaar M, Knipscheer P, Perrakis A, Blomen VA, et al. Vasohibins Encode Tubulin Detyrosinating Activity. Science. 2017; 358: 1453–1456. [DOI] [PubMed] [Google Scholar]
- (50).Aillaud C, Bosc C, Peris L, Bosson A, Heemeryck P, Van Dijk J, Le Friec J, Boulan B, Vossier F, Sanman LE, et al. Vasohibins/SVBP Are Tubulin Carboxypeptidases (TCPs) That Regulate Neuron Differentiation. Science. 2017; 358: 1448–1453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated or analyzed for the current study are available from the corresponding author upon reasonable request.


