Abstract
Hypophysitis is a heterogeneous condition that leads to inflammation of the sella and/or suprasellar region, potentially resulting in hormonal deficiencies and/or mass effects. A preponderance of hypophysitis subtypes have an underlying autoimmune aetiology. The overall incidence and prevalence of hypophysitis has dramatically increased over the past decade, mainly due to increased awareness of the condition in the medical community, improvements in imaging techniques, and a rise in the occurrence of certain forms of hypophysitis such as IgG4 hypophysitis (IgG4Hy) and immune checkpoint inhibitor induced hypophysitis (ICIHy). The clinical presentation varies from an asymptomatic condition to a fatal disease often as a result of electrolyte abnormalities due to glucocorticoid deficiency in the context of adrenal crisis from central adrenal insufficiency. Milder forms of hypophysitis are treated with replacement of deficient hormones while more acute presentations with mass effects require glucocorticoid therapy, immunosuppressive therapy or surgery. Timely diagnosis and interventions are keys to prevention of the lethal complications of this disease. In this review, we provide an update on the recent advances in the field of pituitary autoimmunity, with an emphasis on autoimmune hypophysitis and novel forms of hypophysitis such as anti-PIT1 hypophysitis, IgG4Hy and ICIHy.
Keywords: Pituitary, autoimmunity, hypophysitis, immune checkpoint inhibitors, immunotherapy, adrenal insufficiency
1. Introduction
Autoimmune endocrine disorders were initially described in animal models in the 1950s [1]. Since then, there has been a remarkable increase in the incidence and prevalence of autoimmune endocrinopathies in humans, which are known to affect various endocrine systems, including the pituitary gland. Hypophysitis is the general term used to describe any form of sellar and/or suprasellar inflammation that leads to structural changes in the hypothalamic-pituitary axis and varying degrees of anterior and/or posterior pituitary hormonal deficiencies [2]. Hypophysitis was initially thought to be primarily an autoimmune condition. However, recent definitions describe hypophysitis as a highly heterogeneous entity caused by various forms of autoimmune, inflammatory, infectious, and neoplastic disorders, with some having an unknown pathogenesis [2]. A majority of hypophysitis do have an autoimmune aetiology, while several forms of systemic diseases and medications are responsible for the remaining cases of hypophysitis [3, 4]. Clinically, the presentation of hypophysitis can range from an asymptomatic disease to a rapidly progressive disease potentially with fatal consequences [5].
Based on several reports, the estimated overall prevalence of hypophysitis has ranged from 0.2% to 0.88% [6–9]. As per Caturegli et al. (2005), the projected annual incidence of hypophysitis is 1 case per 9 million individuals [8]. However, this estimate was derived from a report on 5 biopsy-proven hypophysitis cases by Buxton and Robertson over 15 years from a single center in Nottingham, U. K. that provided care to a community of 3 million people [7]. Moreover, these cases were comprised of only lymphocytic or granulocytic forms of hypophysitis. Due to the rapid increase in the detection of several novel forms of hypophysitis over the past decade, the epidemiological landscape of hypophysitis has substantially transformed [10]. Since the reporting of the first magnetic resonance imaging (MRI)-proven hypophysitis case in late 1980s, the number of reports on hypophysitis have almost exponentially increased. By March 2013, the Johns Hopkins Hospital pathology department hypophysitis database identified a total of 857 patients described in the literature from 42 countries, with the highest prevalence being in Japan (27%) followed by the U.S.A. (19%) [11]. Between 2000 and 2010, the detection of two newly-described forms of hypophysitis has been responsible for the increase in hypophysitis reports, namely immune-checkpoint inhibitor (ICI) induced hypophysitis (ICIHy) and IgG4 hypophysitis (IgG4Hy) [11]. The number of hypophysitis cases is likely to increase in the upcoming years due to several factors: an increase in the knowledge and awareness of the condition among physicians, higher numbers of pituitary imaging and surgeries, and the rise in the number of certain forms of hypophysitis such as ICIHy. In addition, novel forms of hypophysitis called anti-pituitary-specific transcription factor-1 hypophysitis (anti-PIT1Hy) and isolated adrenocorticotropic hormone (ACTH) deficiency have been recently identified [12]. Several detailed reviews have been published on the topic of hypophysitis and pituitary autoimmunity over the past decades [2, 5, 6, 8, 10, 12–17]. Our review provides a more in-depth coverage of novel emerging pathophysiological concepts and recent updates in the field of pituitary autoimmunity, with an emphasis on autoimmune and drug-induced forms of hypophysitis.
2. Classification of hypophysitis
Historically, almost all cases of hypophysitis were deemed idiopathic as the underlying pathophysiologic mechanisms were not yet elucidated, although some cases were suspected to be due to autoimmune-mediated hypophyseal destruction. Due to a substantial increase in the number of hypophysitis cases being identified and studied over the past decades, more light has been shed on the pathogenesis of this disorder and several forms of hypophysitis have since been identified. Hypophysitis can be broadly classified into primary and secondary forms (Table 1) [15]. Primary hypophysitis (PHy) comprises of autoimmune forms of hypophysitis that have distinctive forms of immunological profiles and histopathological features, as well as idiopathic forms, in which the precise aetiology and pathogenesis is unknown [15]. Secondary hypophysitis (SHy) is caused by a variety of autoimmune, inflammatory, infectious, vascular and neoplastic conditions, or as an adverse effect of various medications [15]. Both PHy and SHy are rare disorders and are relatively uncommon in the general population. However, a reliable estimate for the incidence and prevalence of various forms of hypophysitis in the population is difficult to ascertain as several prior reports have not clearly distinguished the various forms of hypophysitis under their epidemiological description, and several subtle cases may have gone undiagnosed [15].
Table 1.
Causes of hypophysitis.
| A. Based on the aetiology: |
| I. Primary hypophysitis |
| 1. Lymphocytic hypophysitis (LHy) |
| 2. Granulomatous hypophysitis (GHy) |
| 3. IgG-4 hypophysitis (IgG4Hy) |
| 4. Xanthomatous hypophysitis (XHy) |
| 5. Necrotizing hypophysitis (NHy) |
| 6. Mixed forms (lymphogranulomatous, xanthogranulomatous hypophysitis) |
| II. Secondary hypophysitis |
| 1. Autoimmune endocrinopathies:a |
| • Autoimmune polyglandular syndromes |
| • Hashimoto’s thyroiditis |
| • Graves’ disease |
| • Type 1 diabetes mellitus |
| • Addison’s disease |
| 2. Systemic autoimmune diseases:a |
| • Sjogren’s syndrome |
| • Systemic lupus erythematosus |
| • Bechet’s disease |
| • Primary biliary cirrhosis |
| • Optic neuritis |
| • Atrophic gastritis |
| • Myocarditis |
| • Crohn’s disease |
| • Idiopathic thrombocytopenic pupura |
| • Autoimmune hepatitis |
| 3. Vasculitides: |
| • Temporal arteritis |
| • Takayasu arteritis |
| • Granulomatosis with polyangitis (Wegener’s granulomatosis) |
| • Microscopic polyangitis |
| • Cogan’s syndrome |
| 4. Inflammatory and proliferative disorders: |
| • Sarcoidosis |
| • IgG4 disease |
| • Langerhans cell histiocytosis |
| • Erdheim-Chester disease |
| • Tolosa-Hunt syndrome |
| • Rathke’s cleft cyst rupture |
| 5. Neoplastic lesions of the sellar and suprasellar regions: |
| • Germinoma |
| • Craniopharyngioma |
| • Pituitary adenoma |
| • Meningioma |
| • Glioma |
| • Chordoma |
| • Pituicytoma |
| • Pituitary adenoma |
| • Pituitary lymphoma |
| • Dermoid |
| • Epidermoid |
| 6. Drug-induced hypophysitis: |
| • Immune checkpoint inhibitor induced hypophysitis (ICIHy) |
| • Interferon alpha |
| • Ribavirin |
| • Ustekinumab |
| 7. Infections: |
| • Tuberculosis |
| • Syphilis |
| • Fungal infections (Nocardia, Aspergillus) |
| • Pituitary abscess |
| • Viruses (Coxsackie, Herpes simplex, Varicella zoster) |
| • Toxoplasma |
| 8. Paraneoplastic syndromes: |
| • Anti-PIT1 hypophysitis (Anti-PIT1Hy) - mostly secondary to thymomas |
| • Isolated ACTH deficiency - from large cell neuroendocrine carcinoma |
| B. Based on the anatomical site of involvement |
| I. Adenohypophysitis |
| II. Infundibuloneurohypophysitis |
| III. Panhypophysitis |
Hypophysitis associated with other autoimmune endocrinopathies and with other systemic autoimmune diseases are classified as ‘primary hypophysitis’ by some authors.
Autoimmune hypophysitis (AHy), as the name suggests, is a form of hypophysitis that results from autoimmunity-mediated destruction of the pituitary gland. AHy is the most common form of hypophysitis [15]. The first probable case of AHy was described by Simmonds in 1917 [18]. The first biopsy proven lymphocytic infiltration of pituitary gland was described by Goudie and Pinkerton in 1962 [19]. AHy includes most forms of hypophysitis under PHy category and those secondary to autoimmune polyendocrinopathies and systemic autoimmune diseases under SHy (Table 1). Identification of AHy cases has dramatically increased over the past few decades. For instance, from 1962 to 1981, only 16 AHy cases were reported, while from 1982 to 2001, 290 cases of AHy were described, and from 2002 to 2004, approximately 73 AHy cases were reported [8]. Hypophysitis can also be classified based on the anatomical site of pituitary gland involvement. Hypophysitis can affect only the anterior pituitary (adenohypophysitis), posterior pituitary (infundibuloneurohypophysitis), or the entire pituitary gland (panhypophysitis) (Table 1) [8, 15].
2.1. Primary hypophysitis
PHy is the most common form of hypophysitis, and in turn, the most common form of AHy. A vast majority of PHy cases are of lymphocytic hypophysitis (LHy) sub-type [15]. As of June 2016, there were 1,005 known cases of PHy, and analysis of this data has shown that the mean age of onset of PHy is 41±16 years, with a female predilection (female: male ratio: 2.5:1) [20]. The different forms of PHy are described in the following sections.
2.2.1. Lymphocytic hypophysitis
LHy is the most common form of hypophysitis and the most extensively studied sub-type of PHy/AHy in the literature. Previous reports have suggested that LHy constitutes about 71.8% of all cases of hypophysitis [6]. Over the years, studies from smaller cohorts have provided higher prevalence ranges of approximately 76% to 86% [21, 22]. Several prior articles have used the terms ‘LHy’ and ‘AHy’ interchangeably, which in turn confounds the precise estimation of incidence and prevalence of LHy per se [8, 15]. However, the 2013 worldwide data from 711 patients diagnosed with all forms of hypophysitis showed that biopsy-proven LHy was present in approximately 55% of the patients, and in up to 79% of patients with PHy [11]. Follow-up data on 674 histopathologically proven PHy patients from 2016 showed a somewhat lower LHy prevalence of 68% among PHy forms [20]. LHy can affect either anterior pituitary (lymphocytic adenohypophysitis; LADHy), posterior pituitary (lymphocytic infundibuloneurohypophysitis; LINHy), or the entire pituitary gland (lymphocytic panhypophysitis; LPAHy) [15]. LHy has been classically described to occur during pregnancy and the post-partum period. The first case of LHy was described by Goudie and Pinkerton in 1962 in a 22-year-old woman who succumbed to circulatory shock 14-months postpartum from supposed adrenal insufficiency (AI) [19]. An autopsy revealed a shrunken pituitary gland with lymphocytic infiltration of the adenohypophysis. LHy was previously thought to occur almost exclusively in women, as the first 20 or so reported patients with LHy were all women [15]. It is only from 1987 onwards that occurrence of LHy was also recognized in men [23].
Apart from varying forms of hormonal deficiencies, different forms of LHy demonstrate unique clinical characteristics. The LADHy sub-type is more common in women (about 75% to 80% of the cases; female:male ratio = 4:1), of which 30 to 70% of cases are associated with late pregnancy or the postpartum period, LPAHy seems to have a weak female preponderance (female:male ratio = 1.9:1), and LINHy does not demonstrate any gender predilection (female:male ratio = 1:1) [5, 11]. The mean age of presentation of LADHy is at a younger age for women (35 ± 13 years) as compared to men (49 ± 16 years). The mean age of presentation is 39 ± 20 years for LINHy and 43 ± 17 years for LPAHy in both men and women, and both of these sub-types do not show a clear association with pregnancy [11]. Up to 50% of LHy cases can be associated with other autoimmune endocrinopathies and systemic autoimmune diseases [15, 22].
There are several interesting features regarding the association between LHy/LADHy and pregnancy. While several autoimmune conditions improve during pregnancy, LHy paradoxically manifests during pregnancy [24]. This could be potentially explained due to the increase in pituitary antigens secondary to the pituitary hyperplasia that occurs during pregnancy, along with the change in the hypophyseal blood flow pattern in which the pituitary derives more blood supply from the systemic circulation than from the hypothalamic-hypophyseal portal system, thus increasing exposure to the immune system [24]. Molecular mimicry through co-expression of antigens such as enolase isoforms in both pituitary and placenta, which are known targets of certain pituitary antibodies, can also possibly explain the increased frequency of LHy during pregnancy [25]. LHy does not seem to adversely affect pregnancy or fetal outcomes in general [8]. Successful pregnancies have been achieved with a pre-existing diagnosis of LHy [8, 26]. Additionally, there have been reports where LHy has occurred in one pregnancy and resolved in a subsequent pregnancy [27, 28]. Another report also showed recurrent LHy in two consecutive pregnancies in a single patient [29]. There have been rare situations where pregnancy was associated with either LINHy or LPAHy [30–32].
The histopathology of LHy demonstrates the characteristic infiltration of lymphocytes into the pituitary gland, mainly in the interstitium, but partly in the pituitary acini, and these lymphocytes can sometimes even arrange themselves into lymphoid follicles with germinal centers [11, 15, 33]. The density of lymphocytes is higher in LHy when compared to other forms of PHy, and the lymphocytes can often be accompanied by plasma cells [33, 34]. The end result of LHy is pituitary fibrosis, which can often be severe [11]. The amount of lymphocyte infiltration usually inversely correlates with the amount of fibrosis [34]. Recently, two different patterns of immunological involvement have been identified with LHy, and both patterns demonstrate higher numbers of T lymphocytes than B lymphocytes [35]. The first pattern is an autoimmune process with T-helper cell 17 predominance without T regulatory cells, and with few B lymphocytes. The CD3 positive lymphocytes (T lymphocytes) also stain positive predominantly for CD4 in this pattern of LHy. In the second pattern, the lymphocytic infiltration is more organized forming germinal centers with relatively higher number of B lymphocytes as compared to the first pattern, and most CD3 positive cells stain positive for CD8. These data were obtained from two patients (one patient for each pattern) and further investigations are required to evaluate the frequency of occurrence of these histopathological and immunohistochemical patterns, as patients with autoimmune pattern of involvement could be candidates for immunosuppressive therapy. The reticulin network is generally maintained in hypophysitis, whereas it is disrupted in cases of pituitary adenomas [11].
Several pituitary antibodies directed against various antigens have been described. Earlier reports have suggested growth hormone (GH) and alpha enolase as potential targets for pituitary antibodies [36, 37]. Moreover, certain enolase isoforms are co-expressed in the placenta and pituitary, which could potentially explain the increased incidence of LHy during pregnancy [25]. However, anti-alpha enolase antibody has also been found in 46% of patients with pituitary adenomas and 24% of patients with other pituitary conditions [38]. Other pituitary auto-antigens described in the literature include secretogranin 2, pituitary gland-specific factors 1a and 2, TPIT, PIT1, and chorionic somatomammotropin [11]. Anti-rabphilin 3A antibodies have been shown to be positive in cases of LINHy [31]. Anti-hypothalamic antibodies targeting corticotrophin-releasing hormone (CRH) secreting cells have also been identified [39]. In addition, antibodies directed against somatotroph, lactotroph, gonadotroph, and corticotroph cells causing selective hormonal deficiencies have been described [40–43]. However, the pathogenicity of these antibodies is not yet well-established. Due to the lack of high sensitivity and specificity, and lack of confirmation by independent investigators, utility of pituitary antibodies in clinical practice is of limited value [11]. Several HLA alleles are associated with LHy, including HLA DQ8, DR4, DR5, and DR53 [14, 44, 45]. Further research is required to establish a more definitive association between HLA and LHy pathogenesis.
2.2.2. Granulomatous hypophysitis
Granulomatous hypophysitis (GHy) is the second most common form of AHy, and accounts for approximately 20% of PHy cases [20, 46]. The first case of GHy was described in 1917, which may be the first description of AHy in the literature [18]. A systematic review of 82 cases of GHy has shown that GHy, similar to LHy, is a female predominant disease (female:male = 2.6:1), with a mean age at presentation being 44 ± 15 years [47]. However, unlike LHy, GHy does not seem to bear a strong association with pregnancy and peri-partum period [48]. GHy can also concomitantly occur with LHy, as some histological specimens have demonstrated lymphocytic infiltration into the pituitary gland [47]. The histopathology of GHy reveals numerous histiocytes, sometimes arranged in the form of granulomas, along with lymphocytes, multinucleated giant cells, and areas of fibrosis [47]. Several granulomatous conditions that cause SHy such as tuberculosis, sarcoidosis, vasculitides such as granulomatosis with polyangitis, Langerhan’s histiocytosis, syphilis, and ruptured Rathke’s cleft cyst, can mimic GHy, so these conditions causing SHy should be ruled out [46]. There has been an on-going debate on whether GHy represents a part of the clinical spectrum of the natural history of LHy. Development of GHy-like lesions (multinucleated giant cells) have been demonstrated several days after an initial lymphocyte-predominant pituitary infiltration in mouse models of AHy [49]. Moreover, GHy also has a strong female predilection and occurs about 8 years after the mean age of occurrence of LHy [47]. These observations would support the likelihood of GHy being a late histopathological manifestation of LHy. Further research is necessary to assess and delineate the pathophysiological relation between LHy and GHy.
2.2.3. IgG-4 hypophysitis
One of the novel emerging causes of hypophysitis is the IgG4Hy, also known as plasmacytic hypophysitis. IgG4Hy is usually a part of IgG4-related disease, a systemic disease involving multiple organs [50]. IgG4-related disease comprises of a variety of conditions, such as autoimmune pancreatitis, Mikulicz’s disease, Riedel’s thyroiditis, retroperitoneal fibrosis, inflammatory pseudotumor, and hypertrophic pachymeningitis, among others [2, 48]. However, IgG4Hy has also been reported to occur in isolation without involvement of other organs, and such isolated forms appear to be more common in females [13, 51, 52]. This form of hypophysitis was first suggested as a separate entity in 2004 [53], and the first histologically confirmed report was published in 2007 [54]. Since then, more than 100 cases have been identified, with more than half of the reports from Japan [13, 52]. Although reports have identified the prevalence of IgG4Hy at 4% from large cohorts [20], a more recent study showed that several cases previously designated as LHy or unspecified hypophysitis were in fact IgG4Hy based on histopathological examination, so the prevalence could actually be much higher [55]. However, the latter projection of disease prevalence was based on a much smaller study population.
Unlike other forms of PHy, IgG4Hy occurs more commonly in males (female:male = 1:3). However, the isolated forms of IgG4Hy without systemic involvement seems to be more common in females (female: male = 2:1) [55]. The overall mean age of onset is also higher (after 60 years) when compared to other forms of PHy, and the mean age of onset for females (56.4 years) is about 11 years younger than that of males (67.5 years) [13]. The presence of ≥10 IgG4 producing plasma cells per high power field was initially suggested as a requirement for the diagnosis of IgG4Hy [11, 56]. Currently, the more widely accepted diagnostic criteria is the one suggested by Leporati and colleagues [56], which consists of 5 diagnostic criteria for identification of IgG4Hy. A diagnosis of IgG4 can be established by the following combinations of criteria: 1) Biopsy-proven IgG4 disease of the pituitary gland alone (criterion 1); or 2) Positive imaging features of hypophysitis (criterion 2) with biopsy-proven IgG4 disease of other organs (criterion 3) in situations where no pituitary biopsy is available; or 3) Imaging evidence of hypophysitis (criterion 2) with elevated serum IgG4 antibody levels (>140 mg/dl) (criterion 4) and prompt response to glucocorticoid therapy (criterion 5) in cases where neither the biopsy samples of pituitary nor of other sites are available. Histopathology reveals mononuclear infiltration with ≥10 IgG4 positive plasma cells per high power field, storiform fibrosis (cartwheel pattern of fibrosis, with strands of collagen radiating from a central point), and rarely lymphoid follicles and eosinophils [57]. Establishing the diagnosis of IgG4Hy is crucial, since this form of hypophysitis is responsive to glucocorticoid therapy, which could be particularly beneficial in the inflammatory stage, and can also reduce circulating IgG4 levels [52, 58].
2.2.4. Xanthomatous hypophysitis
Initially described in 1998 in a series of 3 patients, xanthomatous hypophysitis (XHy) is a rare form of PHy/AHy that is characterized by the presence of foamy macrophages in the pituitary gland [59]. XHy accounts for approximately 3% of cases of PHy [20]. IHC demonstrates positivity for CD68 (macrophage marker), and lipid laden macrophages are evident on electron microscopy [59]. As with other forms of PHy, XHy is more common in women (female: male = 3:1) and the mean age of presentation is in the 4th decade [46]. Unlike LHy, XHy has no relation with pregnancy or post-partum period [48]. The disease mainly involves the anterior pituitary, central diabetes insipidus (DI) is seldom a manifestation of XHy, and visual symptoms are infrequent [60]. Cystic areas of liquefaction can occur within the pituitary and can often be visualized on MRI [46]. Secondary causes such as Erdheim-Chester disease can also give rise to xanthomatous or xanthogranulomatous lesions in the pituitary and must be suspected in supposed XHy cases with multi-organ involvement, especially if these lesions harbor the somatic BRAF V600E variant [61]. Conversely, DI is the most common endocrinopathy seen in patients with Erdeim-Chester disease, and can present as the first clinical manifestation. The pathogenesis of XHy is unclear. Several causes such as autoimmunity, infection, or regional endothelial dysfunction have been suggested, but the most plausible aetiology appears to be a local inflammatory response to a ruptured Rathke’s cleft cyst [48, 62].
2.2.5. Necrotizing and mixed forms of hypophysitis
Necrotizing hypophysitis (NHy) is the rarest form of PHy (0.6%) and very few cases have been reported in the literature [48, 63–65]. Females are more commonly affected than males (3:1) and the pathogenesis has not been elucidated [48]. Although the disease could be a separate entity altogether, it is more likely that NHy represent a more severe form of one of the other forms of hypophysitis. NHy can also occur with SHy, and has been reported in at least one case of ICIHy from the use of CTLA-4 inhibitor therapy [20]. Although the clinical and imaging features are similar to other forms of hypophysitis, NHy is more likely to present more acutely with mass effect symptoms (e.g., eye pain, visual defects) or even as aseptic meningitis [11, 65]. Histopathology reveals extensive areas of necrosis with infiltration of lymphocytes, plasmacytes, and a few eosinophils, and chronic inflammation can lead to fibrosis [48, 63]. Other conditions that can cause pituitary necrosis include Sheehan syndrome, macroadenomas, pituitary metastases, septic shock, and snake venom poisoning, and must be considered in the differential diagnoses [63].
Mixed forms of hypophysitis are observed in 4% of PHy cases [20]. Lymphogranulomatous hypophysitis can contain features of both LHy and GHy, but it is likely that this subset of hypophysitis histopathologically could represent a transition from an initial lymphocytic infiltrative pattern of LHy to a more granulomatous, chronic inflammatory pattern of GHy [47]. Xanthogranulomatous hypophysitis (XGHy) containing both foamy xanthoma cells and multinucleated giant cells along with cholesterol clefts and hemosiderin deposits has also been described in the literature [46, 66]. These lesions seem to share the pathogenesis of XHy, and appear to represent a disease spectrum, with XHy being the earlier manifestation followed by XGHy changes that occur later during the course of the disease [62].
2.3. Secondary hypophysitis
2.3.1. Immune checkpoint inhibitor induced hypophysitis
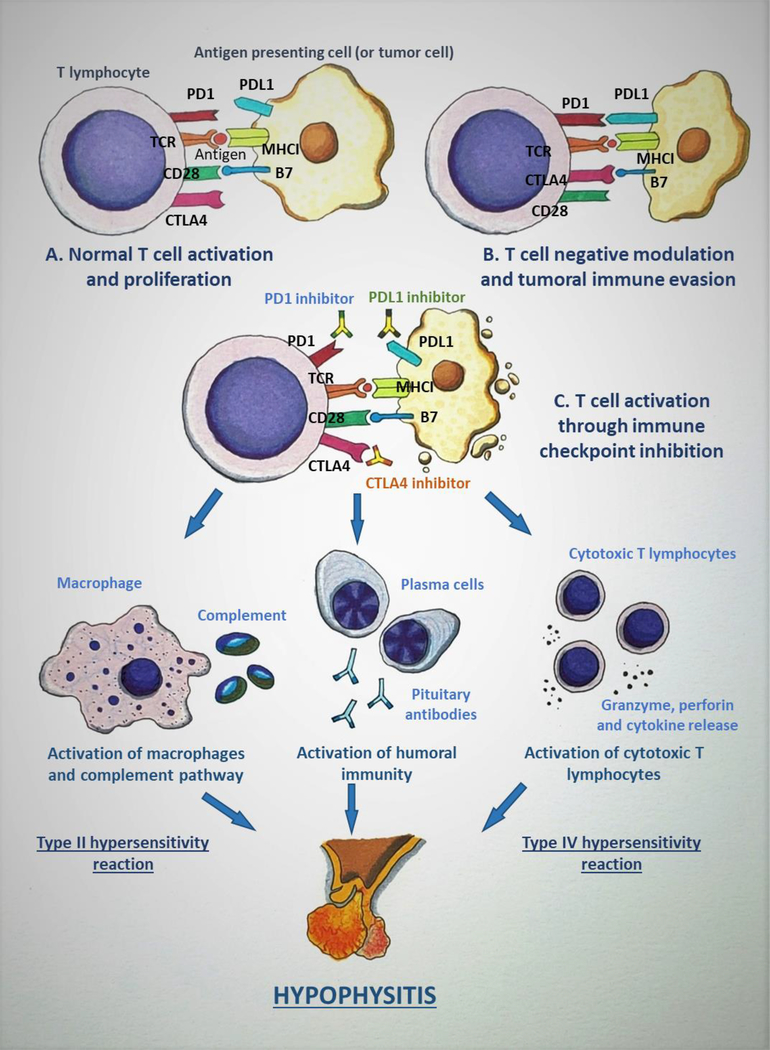
ICI therapy has revolutionized the field of oncology, and their treatment indications are being increasingly investigated in several forms of cancers. At least seven ICIs are currently approved by the U.S. Food and Drug Administration (ipilimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, cemiplimab, and avelumab), while other ICIs (such as tremelimumab) are being actively tested in clinical trials [67]. A diagrammatic representation of the mechanisms underlying ICIHy is shown in Figure 1.
Figure 1:
Mechanism of immune checkpoint inhibitor-induced hypophysitis (ICIH). A: The tumor antigen is presented to T lymphocyte (TLC) by antigen presenting cell (APC), which in this scenario can be a tumor cell, through the interaction between the T-cell receptor (TCR) and the major histocompatibility complex I (MHCI). Another simultaneous interaction occurs between CD28 on the TLC and B7 on the APC which leads to the completion of TLC activation. B: Cytotoxic T-lymphocyte antigen 4 (CTLA4) is a negative immunomodulator that is induced after the initial activation of TLC. CTLA4 binds to B7 with a higher affinity when compared to CD28, and causes inactivation of TLC. Programmed death 1 (PD1) is up-regulated in activated TLCs and, in response to the inflammatory state, various tissues express PD1 ligand 1 (PDL1) and the PD1/PDL1 interaction down-regulates TLC activation. These mechanisms act as immune checkpoints and are utilized by normal cells in the body to prevent immune-mediated destruction. But the tumor cells take advantage of these immunomodulatory pathways to evade the immune system. C: Immune checkpoint inhibitors (ICIs) are monoclonal antibodies that target the immune checkpoints (CTLA4, PD1/PDL1) and inhibit their action, leading to persistent TLC activation and proliferation. This up-regulation in TLC activity caused by ICIs leads to destruction of the tumor and, as an adverse effect, also causes ICIH and other immune-related adverse events. The binding of ICI to pituitary cells elicits various immune reactions: a type II hypersensitivity reaction leading to activation of macrophages and complement pathways, a type IV hypersensitivity reaction causing cytotoxic T lymphocyte activation resulting in the release of granzyme, perforin, and cytokines, and activation of humoral immunity pathway leading to the production of anti-pituitary antibodies, all of which cause the disruption of the pituitary gland. (Courtesy of Sriram Gubbi, NIDDK, NIH)
ICIs are monoclonal antibodies that bind to, and inhibit the action of, cytotoxic T-lymphocyte antigen 4 (CTLA4) and programmed cell death 1 (PD1) on the T lymphocytes, and the PD1 ligand 1 (PDL1) on the antigen presenting cells (Figure 1). These proteins are involved in T cell activity inhibition and T cell depletion, but their inhibition through ICI therapy reactivates the T cells and enhances detection of cancer cells, leading to immune-mediated tumor lysis [68, 69]. As with other forms of oncologic therapy, ICIs come with their own set of adverse effects, several of which are immune-related. ICI induced endocrinopathies (which includes ICIHy) comprise a major set of immune-related adverse events from this particular therapy [68]. As of March 2013, there were 101 cases with ICIHy described in the literature, which were comprised of data from 83 patients enrolled in clinical trials and from 18 case reports [11]. The report by Caturegli et al. from December 2016 was comprised of 128 cases with SHy from CTLA4 blockade [20]. Unlike LHy, ICIHy more commonly affects males (about 79%, female: male ratio: 1:4) and has an older age of onset (59±13 years) [20]. Based on the data from sizeable cohorts of patients treated with ipilimumab (CTLA4 inhibitor) for metastatic melanoma, the prevalence of ICIHy has been estimated to be 11% to 13.6% among patients receiving CTLA4 inhibitors [67, 70–73], but the prevalence of ICIHy is substantially lower (0.5%) with PD1/PDL1 inhibitors such as pembrolizumab and nivolumab [67, 74]. Also, the mean time to diagnosis of ICIHy after treatment initiation is earlier with CTLA4 inhibitors as compared to PD1/PDL1 inhibitors (9.3 weeks vs. 25.8 weeks, respectively) [67]. With combination therapy (CTLA4 plus PD1 inhibitors), the mean duration of onset is approximately 12.5 weeks [67].
The exact pathophysiology of ICIHy has not yet been completely deciphered, but mice model experiments have demonstrated the expression of CTLA4 on the pituitary cells, and these mice develop complement deposition in the pituitary after CTLA4 antibody injection [75]. Interestingly, patients with ICIHy from CTLA4 treatment for melanoma develop CTLA4 antibodies [75]. Data on histopathological evaluation of the pituitary glands of patients with ICIHy is sparse because most, if not all, patients with ICIHy reported in the literature have not undergone pituitary biopsy or autopsy sampling [20]. However, histopathological evaluation of ICIHy in a few patients has revealed that the most likely underlying pathophysiologic process is a combination of type II and type IV hypersensitivity reactions [20]. The type II reaction is mediated through the binding of IgG antibody to target antigen on pituicytes (glial cells of the posterior pituitary), which in turn activates macrophages and complement pathways, whereas the type IV reaction is driven by T lymphocytes which cause granzyme and perforin-mediated cytotoxicity, cytokine release, and activation of the B lymphocytes and phagocytes [20]. In one case, there was evidence of emperipolesis (penetration of one living cell into another living cell) of lymphocytes into the pituitary acidophils, which is suggestive of an autoimmune process [20]. A case of histopathologically confirmed NHy induced by tremelimumab (CTLA4 inhibitor) has also been reported [20]. The above findings might also explain why CTLA4 inhibitors are more likely to cause ICIHy. Unlike CTLA4 inhibitors which are IgG1/IgG2 monoclonal antibodies, PD1/PDL1 inhibitors are IgG4 monoclonal antibodies and do not efficiently activate antibody-mediated cytotoxicity or the classic complement pathway [67]. Clinically, headaches from ICIHy are more common with CTLA4 inhibitors and combination therapy than with PD1 inhibitors [67]. More recent reports on patients developing ICIHy with PD1/PDL1 inhibitor therapy have shown a unique pattern of hypophysitis distinct from CTLA4 inhibitor mediated ICIHy. PD1/PDL1 inhibitor therapy often causes isolated ACTH deficiency, with hyponatraemia being a common presenting symptom, and MRI scans often do not reveal classic features of hypophysitis [76]. Patients with melanoma who develop hypophysitis from ipilimumab have a better overall survival irrespective of glucocorticoid therapy, when compared with those patient who do not develop hypophysitis [77].
2.3.2. Other forms of secondary hypophysitis
Several systemic autoimmune conditions are associated with hypophysitis. It is unclear as to where exactly in the classification the systemic autoimmune diseases fit. If classified based solely on the autoimmune aetiology, hypophysitis from any autoimmune disease would be classified as PHy. But if the underlying autoimmune disease is considered as a systemic disease, then the associated hypophysitis is classified as SHy. However, the classification of hypophysitis from autoimmune diseases is still a matter of debate. As these forms of hypophysitis are associated with autoimmune diseases, they are likely to be AHy. AHy has been associated with several autoimmune endocrine conditions such as Graves’ disease, Hashimoto’s thyroiditis, type 1 diabetes mellitus, or autoimmune polyglandular syndromes (APS) [19, 78–80]. Other autoimmune diseases such as systemic lupus erythematosus, Sjogren’s syndrome, Behcet’s syndrome, and systemic inflammatory diseases can also occur along with AHy [81–83]. Coeliac disease-associated haplotypes in Caucasian patients, particularly the ones linked to DQ8 and DQ2, have an increased association with hypophysitis, and anti-pituitary, anti-hypothalamus, and antinuclear antibodies were found in higher frequencies in patients with coeliac disease-associated haplotypes [84].
Vasculitic processes such as granulomatosis with polyangitis [85, 86], microscopic polyangitis [87], Takayasu arteriitis [88], and Cogan’s syndrome [89] have also been associated with AHy. Apart from ICI, other drugs are also known to cause AHy, including interferon alpha [90], interferon alpha/ribavirin combination therapy [91, 92], and ustekinumab [93]. Hypophysitis is also caused by several inflammatory conditions such as sarcoidosis, IgG4-related disease, and infectious diseases such as tuberculosis, syphilis, and fungal infections [15, 46, 61]. Proliferative disorders such as Langerhans cell histiocytosis, Erdheim Chester disease [94, 95], and neoplastic conditions such as germinoma, craniopharyngioma, and lymphoma can also give rise to hypophysitis [15, 46]. In particular, germinomas are highly immunogenic tumors and can cause peritumoral autoimmune reactions in the sellar and suprasellar region leading to an AHy-like picture [96].
2.3.3. Novel causes of secondary hypophysitis
Anti-PIT1Hy is a novel form of AHy that is characterized by selective deficiencies of GH, thyroid stimulating hormone (TSH), and prolactin [12]. PIT1 is a POU-domain transcription factor that is involved in the differentiation of somatotroph, thyrotroph, and lactotroph cells during organogenesis, and its mutations are known to cause congenital GH, TSH, and prolactin deficiencies [12, 97, 98]. Anti-PIT1Hy has been also referred to as ‘anti-PIT1 antibody syndrome’ as the condition is associated with circulating anti-PIT1 antibodies, which are specific markers for this condition [99]. But it is cytotoxic T lymphocytes (CTL) that are actually responsible for the development of the disease, because the circulating antibodies do not exhibit cytotoxic properties but rather serve as biomarkers for anti-PIT1Hy [100]. Recent experiments have demonstrated that PIT1 protein epitopes are presented by the MHC/HLA class I on the pituitary cells, which would further suggest a CTL-mediated destruction in this disorder [101]. Less than 10 cases have been described to date, with most of the reports in male patients [12]. The age of onset is usually after the 7th decade of life, although one case occurring at 44 years of age has been described [12]. Anti-PIT1Hy is a paraneoplastic manifestation, and the underlying primary tumor in most cases has been a thymoma; rarely, other underlying primary tumors such as bladder lymphoma have been reported [12]. Yamamoto et al. have suggested the following criteria for the diagnosis of anti-PIT1Hy: 1) Acquired specific GH, TSH, and prolactin deficiencies without impairment of other pituitary axes; 2) Presence of circulating anti-PIT1 antibodies or PIT1-reactive CTL; and 3) Underlying diagnosis of a thymoma or another malignant neoplasm [12]. A definitive diagnosis is established if criteria 1 and 2 are met, and the presence of criterion 1 alone suggests a probable diagnosis [12]. Anti-PIT1Hy can also concomitantly occur with other autoimmune endocrinopathies such as type 1 diabetes mellitus [12].
Another novel form of pituitary autoimmune condition is acquired, isolated ACTH-deficiency, which also is a paraneoplastic syndrome [12]. A recent case description reported a patient with an ectopic ACTH-expressing large cell neuroendocrine carcinoma who later developed isolated ACTH-deficiency [12]. Circulating anti-proopiomelanocortin (POMC) antibodies were detected and the peripheral blood lymphocytes were reactive to POMC protein, indicative of a CTL-mediated reaction. Similarly, isolated ACTH-deficiency has been reported in other malignancies such as gastric cancer and acute myeloid leukaemia [102, 103]. As isolated ACTH-deficiency has been associated with lymphocytic infiltration of the pituitary, it is likely that this condition could represent a novel form of AHy [104].
3. Clinical features of hypophysitis
Clinical presentation of hypophysitis has a wide spectrum: anywhere from being clinically asymptomatic, to having one or more features of hypopituitarism, to acute onset of mass effects including pituitary apoplexy and even death from circulatory collapse and adrenal crisis [5, 19]. All forms of hypophysitis can potentially manifest with one or more of the following four clinical features: 1) Mass effects such as headaches and visual symptoms; 2) Symptoms of deficiency of anterior pituitary hormones; 3) Central DI; and, 4) Hyperprolactinaemia [8, 11, 15]. Caturegli et al. collected data on PHy patients from 1917 to June 2016 and identified the following prevalence for the symptoms at presentation: headaches in 47%, low cortisol in 35%, polyuria and polydipsia in 35%, visual disturbance in 31%, low sex steroids in 20%, and low thyroxine in 16% of the patients [20]. In the same report, among the patients with SHy from CTLA-4 blockade, headache was prevalent in 60%, low cortisol in 72%, polydipsia and polyuria in 0.9%, visual disturbance in 3%, low sex steroids in 15%, and low thyroxine in 20% of the cohort [20].
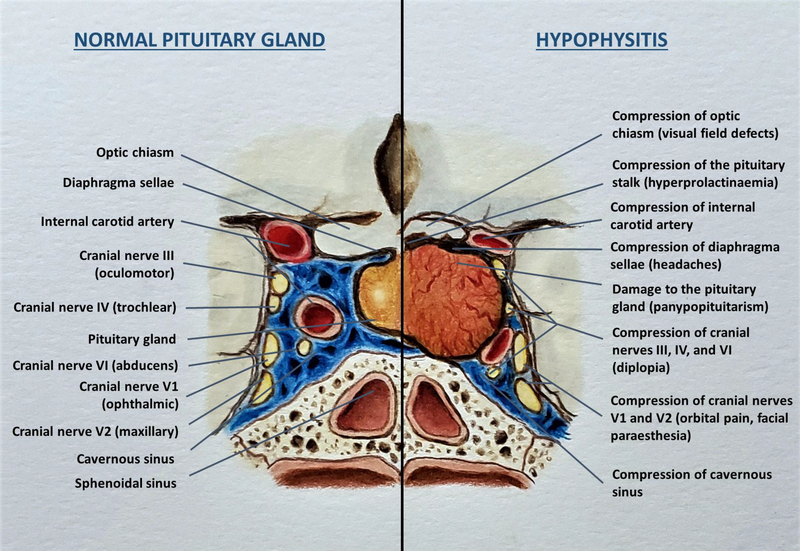
The most common presenting features of hypophysitis are the symptoms of mass effects, including headaches, and visual disturbances, especially in the acute or subacute phase [14, 105]. The anatomical disruption of the sellar region and the adjacent regions caused by hypophysitis is shown in Figure 2. Several studies have reported mass effects ranging from 24% to 83% of patients with PHy [9, 21, 105, 106]. Headaches occur in 57% to 83% and nausea, visual disturbances are observed in 50% to 70% of patients with acute AHy [21, 48]. Headache is the most common mass effect symptom, which is usually generalized and can often be severe [11]. Headaches occur in 47% to 68% of PHy patients and 60% to 61% of ICIHy patients [17, 20]. The likely cause is upward expansion of the inflamed, enlarged pituitary gland which impinges on the dura mater and optic chiasm, or lateral extension of the inflamed pituitary causing compression of the cavernous sinus [11, 107]. Headaches seem to be more severe in patients with PHy when compared to those with ICIHy, but occur more consistently in ICIHy patients [20]. The second common mass effect symptom is visual disturbance [11]. Visual symptoms can occur in up to 31% of PHy patients while they are extremely rare (approximately 3%) in ICIHy patients [20]. Similarly, visual symptoms are rare with XGHy [60]. Visual symptoms could manifest as visual field defects if the enlarged pituitary encroaches on the optic chiasm causing bitemporal quadrantopsia or hemianopsia, or pupillary defects or diplopia from compression of cranial nerves III (oculomotor), IV (trochlear), and VI (abducens), and rarely orbital pain or facial paraesthesia from compression of the branches of cranial nerve V (trigeminal nerve) [108–110]. Asthenia is another common symptom reported in up to 86% of PHy and 58% of ICIHy patients [17].
Figure 2:
Diagrammatic representation of normal anatomy of the sellar region and the adjacent neurovascular structures (left panel) and the mass effects of hypophysitis (right panel). (Courtesy of Sriram Gubbi, NIDDK, NIH)
Anterior pituitary hormone deficiencies leading to varying degrees of hypopituitarism is the next most commonly occurring feature of hypophysitis. The frequency of AHy-related anterior hypopituitarism reported in the literature ranges from 40% to 81% [9, 14, 21, 105, 106, 111]. A number of studies have provided varying frequencies of occurrence of different symptoms and hormonal deficiencies, including ACTH, GH, TSH, gonadotropins (follicle stimulating hormone; FSH, and luteinizing hormone; LH), and prolactin. The usual sequence of hormonal deficiencies seen with other causes of panhypopituitarism is loss of GH, followed by LH/FSH, TSH, and ACTH (mnemonic: Go Look For The Adenoma) [112]. In contrast, in hypophysitis, ACTH deficiency is the most frequently observed deficiency, followed by LH/FSH and TSH deficiencies, and then less frequently GH and prolactin deficiencies [14, 15]. Central AI has been reported in 20% to 75%, central hypogonadism in 15% to 60%, central hypothyroidism in 25% to 58%, GH deficiency in 5% to 41%, and prolactin deficiency in 13% to 25% of patients with various forms of PHy [8, 20, 21, 105, 111, 113, 114]. When compared with PHy, the frequency of ACTH, TSH, and LH/FSH deficiencies at diagnosis seem to be more profound in cases of SHy due to ICIHy [20]. Also unlike in PHy, where ACTH deficiency is more common, ACTH, TSH, and LH/FSH deficiencies occur at similar frequencies in ICIHy [20]. Based on the data compiled by Caturegli et al. on patients with SHy due to CTLA-4 blockade, secondary hypocortisolism was prevalent in 91%, secondary hypothyroidism in 84%, and secondary hypogonadism in 83% of the patients [20]. Hyponatraemia from isolated ACTH deficiency can be seen with ICIHy from PD1/PDL1 inhibitor therapy [76]. A variety of symptoms can manifest based on the type of hormonal deficiency, such as fatigue and muscle weakness (ACTH, TSH, FSH, LH, GH), orthostatic hypotension (ACTH), anorexia and nausea/vomiting (ACTH), hyponatraemia (ACTH, TSH), menstrual irregularities/amenorrhea (FSH, LH, TSH), erectile dysfunction (FSH, LH), hot flashes and vaginal dryness (FSH, LH) constipation and weight gain (TSH), weight loss (ACTH), and cold intolerance (TSH) [112]. Children with GH deficiency can present with short stature [48]. Prolactin deficiency can manifest as lactation failure in women during the post-partum period, which should particularly raise the concern for LHy [11]. Of all the manifestations of hypopituitarism, identifying the signs and symptoms of ACTH deficiency is crucial, as untreated AI can potentially be lethal [15, 48]. In addition, in cases of ICIHy, the symptoms of hypocortisolism (fatigue, tiredness, weight loss) can overlap with the symptoms of the underlying malignancy, which could potentially confound the physician’s assessment and lead to a missed diagnosis of AI [20].
Hyperprolactinaemia is prevalent in 18% to 48%, and central DI in 17% to 72% of patients with hypophysitis [9, 14, 21, 105, 106, 111, 114]. In PHy, central DI is seen in 39% and hyperprolactinaemia is seen in 37% of patients [20]. However, hyperprolactinaemia and central DI are uncommon in ICIHy, affecting 9% and 1% of patients, respectively [20]. The plausible explanations for hyperprolactinaemia with hypophysitis include disruption of the inhibitory hypothalamic dopaminergic signals due to pituitary stalk inflammation or compression, concomitant hypothalamic inflammation leading to decreased dopamine synthesis, or the presence of lactotroph-stimulating auto-antibodies causing prolactin synthesis and release [8, 48]. Hyperprolactinaemia can present as galactorrhea and amenorrhea in women, and as loss of libido and erectile dysfunction in men [115]. Central DI is more likely to occur in cases of infundibuloneurohypophysitis or panhypophysitis, and manifests as polyuria, polydipsia, nocturia and dehydration [112]. Central DI is rarely seen with XHy [60]. Simultaneous hypothalamic involvement could give rise to adipsic DI which can predispose the patient to the risk of severe hypernatremia [112, 116]. In patients with concomitant central AI, the central DI can be masked due to impaired free water clearance caused by glucocorticoid deficiency, and subsequent glucocorticoid replacement in central AI can unmask the central DI [112]. Sellar germinomas can mimic LINHy, and can also present with a triad of DI, hypopituitarism mainly as GH deficiency, and visual symptoms [48, 117].
Other rare findings associated with hypophysitis include internal carotid artery occlusion, aseptic meningitis, and pituitary apoplexy [118–120]. Hypothalamic dysfunction from AHy manifesting as weight gain and temperature dysregulation are also reported [113, 121]. Hypercalcaemia is another uncommon feature observed in cases of AHy with AI [122, 123]. The proposed underlying mechanism is the increased efflux of calcium from bones along with increased tubular reabsorption and decreased renal excretion of calcium due to glucocorticoid deficiency [123]. In cases of SHy, features of other autoimmune endocrine disorders (Graves’ disease, Hashimoto thyroiditis, type 1 diabetes, APS), non-endocrine autoimmune conditions (systemic lupus erythematosus, rheumatoid arthritis, Sjogren’s syndrome, vasculitis, coeliac disease), or inflammatory conditions (sarcoidosis, IgG4 disease) can be evident on clinical presentation [14].
4. Diagnosis of hypophysitis
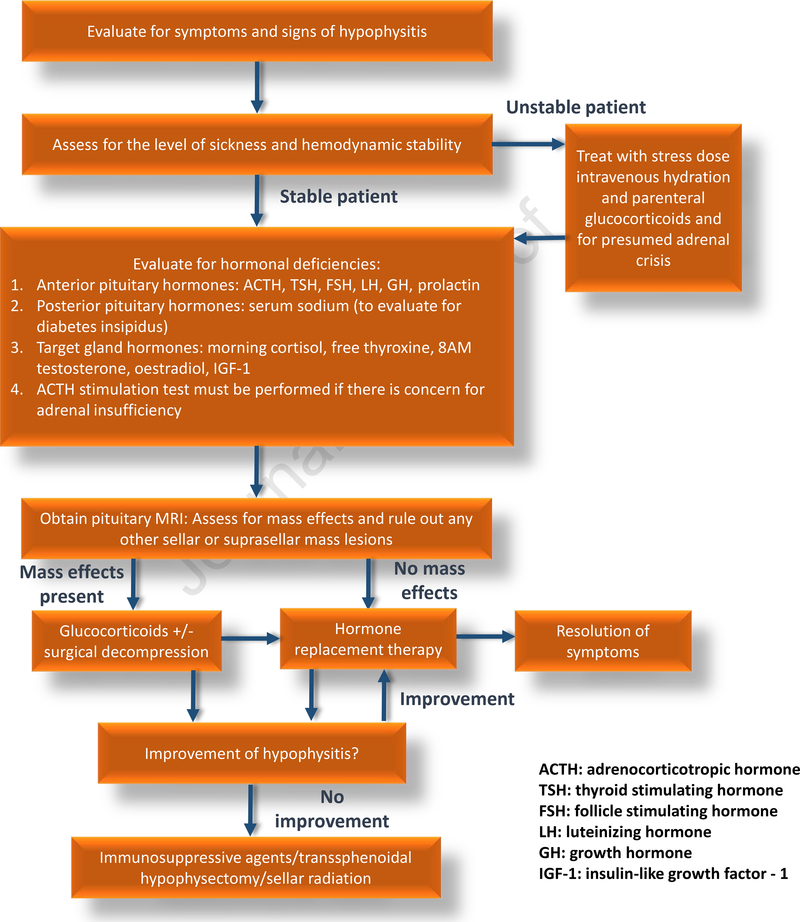
Making an accurate and timely diagnosis of hypophysitis continues to be a challenge to this day. Due to the relative rarity of the condition in the general population, clinically non-specific symptoms that could be erroneously attributed to other causes by physicians unaware of this condition, and the likelihood of late presentation with panhypopituitarism due to a clinically silent hypophysitis and fibrosis are all the factors that can explain a delayed or missed diagnosis [15]. The diagnostic approach towards hypophysitis can be broadly categorized into three groups: 1) Biochemical evaluation: 2) Imaging evaluation; and 3) Evaluation of secondary causes of AHy. A general algorithm for diagnosing and managing hypophysitis is shown in Figure 3.
Figure 3:
Diagnostic evaluation and treatment of hypophysitis.
Biochemical evaluation should comprise of measurement of pituitary hormones and the hormones of their target endocrine glands [112]. Pituitary hormones requiring evaluation include plasma ACTH, TSH, LH, FSH, GH, and prolactin. The target organ hormone measurements include plasma cortisol, free thyroxine, testosterone/oestradiol, and insulin-like growth factor – 1. Central adrenal insufficiency can be evaluated by measuring an 8 to 9 AM serum cortisol. A morning cortisol level of <3 mcg/dL is indicative of AI while a level of >15 mcg/dL likely excludes AI [112]. An ACTH stimulation test must be performed for AM cortisol values between 3 and 15 mcg/dL, and a value of <18.2 mcg/dL at 30 or 60 minutes after ACTH stimulation is suggestive of AI [112]. It is important to note that the ACTH stimulation test was not designed for evaluation of secondary AI [124]. Both the high- and low-dose ACTH stimulation test have similar diagnostic accuracy and are adequate to rule in, but not rule out, central AI [124]. In particular, ACTH stimulation tests can miss cases of secondary AI that have developed acutely. The gold standard test for evaluating central AI is the insulin tolerance test, which is rarely used given its inherent risks [124].
In secondary hypothyroidism, the free thyroxine is low and the TSH can be low, normal, or slightly elevated [112]. For males, along with LH and FSH, an AM serum testosterone (before 10 AM preferably after an overnight fast) should be measured, and a low AM serum testosterone along with low or normal gonadotropins indicates central hypogonadism [112]. In pre-menopausal women, normal menstruation suggests a normally functioning gonadotroph axis, and central hypogonadism can manifest with menstrual irregularities or amenorrhea. In cases of menstrual irregularities, LH, FSH and serum oestradiol must be measured, and pregnancy must be ruled out in patients with amenorrhea [112]. In post-menopausal women, absence of elevated LH, FSH is indicative of hypogonadotropic hypogonadism [112]. GH deficiency is difficult to diagnose with isolated GH or IGF-1 levels due to heterogeneous pulsatility in GH secretion and several confounding factors, including body weight and age, and up to 20% of adults with GH deficiency can have normal IGF-1 levels [112]. A diagnosis of GH deficiency can be firmly established only through GH stimulation testing [112]. However, with established deficiencies of three other pituitary hormones, GH deficiency testing is not necessary [112]. Prolactin levels must be measured to evaluate for prolactin deficiency and hyperprolactinaemia, both of which can occur with hypophysitis. In addition, hyperprolactinaemia can be associated with dysfunction of other pituitary axes, such as the gonadotroph and thyrotroph axes [112]. Central DI must be evaluated in patients with polyuria (defined as >50 ml/Kg/24 hours in adults) [116]. Serum sodium, and plasma and urine osmolality must be measured as initial laboratory tests. A serum sodium of >145 mmol/L, plasma osmolality of ≥295 mosm/Kg, and a urine osmolality of <300 mosm/Kg is indicative of DI [112, 116]. For indeterminate cases, especially in patients with urine osmolality >300 mosm/Kg and <800 mosm/Kg, further testing in the form of the indirect water deprivation test or hypertonic saline infusion test coupled with plasma copeptin measurement can be carried out if facilities are available for the respective tests [116, 125].
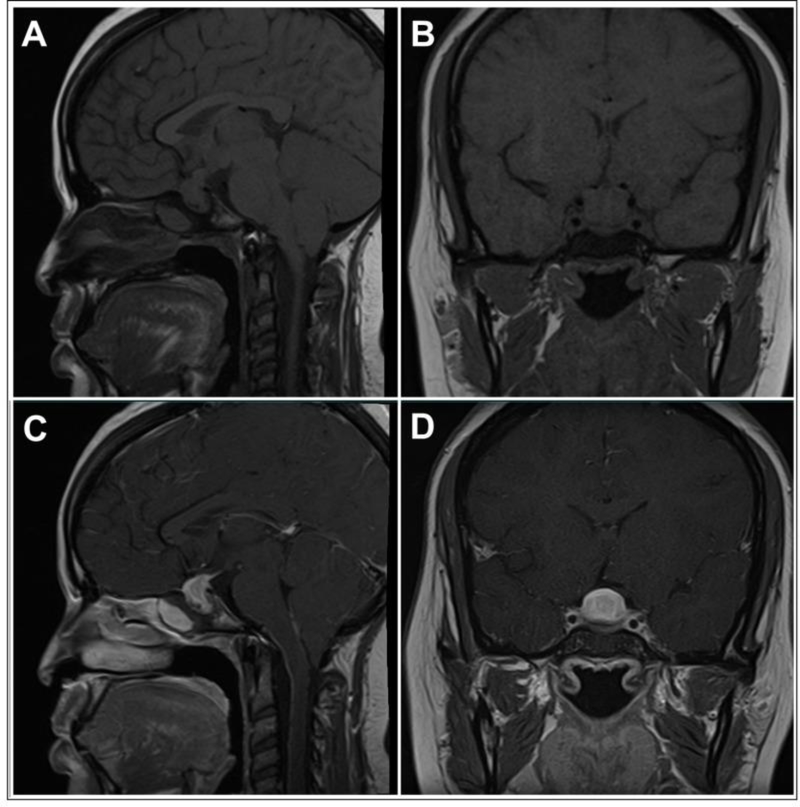
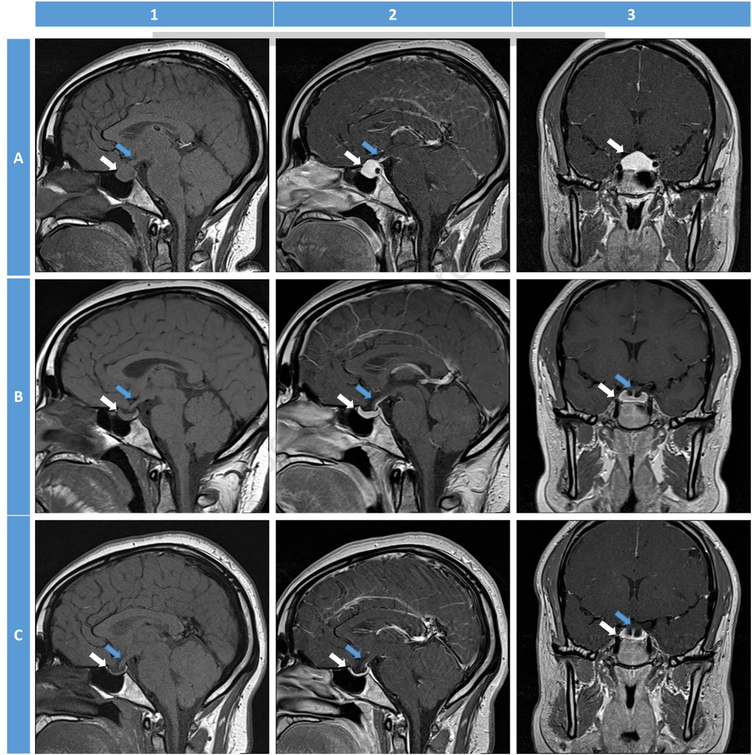
Currently, MRI is the best imaging modality for diagnosing hypophysitis. MRI abnormalities can be seen in up to 98% of PHy and 77% of ICIHy cases [20, 126]. Over the years, several MRI features have been noted to be associated with hypophysitis. The following are the common MRI findings and their typical frequency of occurrence in hypophysitis: pituitary enlargement in 23% to 93.2% of cases [21, 47, 105, 111, 127], homogeneous pituitary contrast enhancement in 23% to 91.7% of cases [21, 47, 105, 111, 113], pituitary stalk thickening (>4 mm antero-posterior diameter on sagittal section) in 33.8% to 96% of cases [11, 47, 105, 111, 113, 114], and loss of posterior pituitary bright spot on T1-weighted imaging in 18% to 70.8% [21, 105, 111]. Other frequently observed radiological signs include intrasellar plus suprasellar extension, seen in 50% to 92% of LHy/PHy patients [105, 111], and up to 100% of GHy patients in one report [113], and a parasellar T2 dark sign (seen in up to 50% of PHy patients) [105, 128]. Other rarer radiographic findings include parasellar extension, cystic changes, central necrosis/apoplexy, ‘figure of 8’ appearance, the presence of a ‘dural tail’ or a ‘meningeal tail’ (contrast-enhanced inflamed tissue along the dura mater), or an empty sella during late stages of the disease [11, 105, 129]. In XHy and XGHy, there can be mixed signal intensity on T1/T2 imaging due to cholesterol deposits [11, 48]. MRI can be normal in ICIHy from PD1/PDL1 inhibitor therapy [76]. In anti-PIT1Hy, the MRI can be normal, or can demonstrate an atrophic pituitary gland with heterogeneity [12]. Figures 4 and 5 illustrate some of the MRI features of hypophysitis.
Figure 4: Magnetic Resonance Imaging Findings in a Case of Primary Hypophysitis.
Panel A) T1-weighted image, sagittal section. Panel B) T1-weighted image, coronal section. Panel C) T1-weighted image post-gadolinium, sagittal section. Panel D) T1-weighted image post-gadolinium, coronal section. A homogeneous enlargement of the pituitary with thickening of the stalk can be seen. The mass shows intense and homogeneous enhancement post-gadolinium.
With permission from ref. 46
Prete A, Salvatori R. Hypophysitis. in: Feingold KR1, Anawalt B2, Boyce A3, Chrousos G4, Dungan K5, Grossman A6, Hershman JM7, Kaltsas G8, Koch C9, Kopp P10, Korbonits M11, McLachlan R12, Morley JE13, New M14, Perreault L15, Purnell J16, Rebar R17, Singer F18, Trence DL19, Vinik A20, Wilson DP21, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
2018 Aug 15.
Figure 5:
Magnetic resonance imaging (MRI) features (T1-weighted images) of hypophysitis. White arrows: pituitary gland; Blue arrows: pituitary stalk. 1: sagittal non-contrast T1-weighted image; 2: sagittal post-contrast; 3: coronal post-contrast. A: Initial MRI of acute hypophysitis in a lady presenting at 6-weeks post-partum with severe headaches, nausea, vomiting, and lactation failure without diabetes insipidus. MRI shows an enlarged pituitary gland along with thickened stalk. The post-contrast images demonstrate a homogeneous uptake within the pituitary gland. B: Follow-up MRI 2 months after initiation of glucocorticoid therapy (prednisone 40 mg per day for 2 months followed by 20 mg per day for 3 months, then gradual taper to 5 mg per day for 6 months, and then maintenance therapy with hydrocortisone for 6 months). There is evidence of reduction in the pituitary enlargement and stalk thickening. All symptoms resolved one month after initiation of therapy. C: Follow-up MRI 17 months after the initial MRI. There is resolution of all the features of acute hypophysitis. The patient continues to be on hormone replacement and is clinically doing well. (Courtesy of Joseph G. Verbalis, Georgetown University Medical Center, Washington, DC)
The main challenge in diagnosing hypophysitis on MRI lies in differentiating hypophysitis from a pituitary adenoma. A radiologic score was proposed by Gutenberg et al. to distinguish hypophysitis from a pituitary adenoma [130]. The following features favored a diagnosis of hypophysitis over an adenoma: 1) onset during late pregnancy and the early post-partum period; 2) relatively homogeneous uptake of gadolinium contrast and higher enhancement post-contrast; 3) absence of the posterior pituitary ‘bright spot’; and 4) pituitary stalk thickening. On the other hand, larger pituitary volume, asymmetric expansion of the pituitary, and sphenoid sinus mucosal thickening favors a diagnosis of pituitary adenoma. An aggregate of several of these findings provided a sensitivity and specificity of 92% and 99%, respectively, in identifying hypophysitis, and a correct diagnosis could be made in up to 97% of patients [130]. There are some exceptions to these criteria. A homogeneous intensity can be observed in macroadenomas that are secondarily inflamed, and cystic areas can occasionally develop in the pituitary, especially in XGHy, and can lead to heterogeneous enhancement [11, 15, 131]. A pre-contrast T1-weighted MRI of the pituitary normally reveals a posterior pituitary ‘bright spot’ which is thought to be caused by the phospholipid rich granules containing neurophysin-peptide complex that are stored in the posterior pituitary [132]. However, the presence of the ‘bright spot’ is subject to physiologic variations and can be absent in 8% to 25% of individuals, and in up to 29% among elderly subjects, and therefore cannot be used as a sole measure of hypophysitis and/or posterior pituitary damage [133, 134].
The role of HLA testing and pituitary antibody measurement is currently of uncertain value and further research is required to establish the role of pituitary antibodies and HLA testing in diagnosing AHy [14]. Immunoglobulin levels must be measured if IgG4Hy is suspected [10]. Pituitary biopsy is considered as the gold standard for establishing the diagnosis of AHy [14]. However, the procedure is invasive, and because biochemical testing and MRI can detect most cases of AHy, pituitary biopsy is not routinely performed. Pituitary biopsy should be undertaken only in select cases where the diagnosis is uncertain and if the results of the biopsy are likely to change the course of management [46]. The procedure must be performed in well-equipped centers by an experienced neurosurgeon [46].
The differential diagnosis of hypophysitis are listed in Table 1, and includes several other inflammatory, infectious, and neoplastic causes [15]. These secondary causes must be appropriately evaluated (e.g., Quantiferon testing, serum angiotensin converting enzyme levels, and ANCA antibodies in cases of suspected GHy to rule out tuberculosis, sarcoidosis, and vasculitis; whole body bone scan, skeletal survey and biopsy in cases of Erdheim-Chester disease or Langerhan’s histiocytosis; serum alpha fetoprotein and beta-HCG in cases of sellar/suprasellar germinomas) [10]. Congenital disorders with abnormal pituitary development due to mutations in genes such as PIT1 or PROP1 can give rise to varying degrees of panhypopituitarism and present usually during infancy or early childhood, and these conditions must be on the list of differential diagnoses when evaluating for hypophysitis in the paediatric age-group [98, 135]. Some of the physiologic pituitary changes that can mimic hypophysitis include pituitary hypertrophy in children and during puberty, and pregnancy. Other causes such as Sheehan’s syndrome and isolated thyrotroph hyperplasia from severe, long-standing primary hypothyroidism can also mimic hypophysitis on imaging studies [14, 46].
5. Treatment of hypophysitis
There is no clear consensus on the optimal strategy for treatment of hypophysitis because of the rarity of the disease, heterogeneity in clinical presentation and natural history of the disease, and lack of clinical trials comparing the efficacy of different treatment modalities [48]. The therapeutic strategies employed for hypophysitis addresses one or both of the following issues: 1) Treatment of hormonal abnormalities; and 2) Alleviation of neurological and mass effects.
Hormonal abnormality pattern is identified based on biochemical testing, and those hormones that are deficient should be replaced. AI is treated with hydrocortisone 15 to 20 mg that is typically body-surface area based, and administered in two or more divided doses, with the highest dose to be taken in the morning after waking up [112]. All patients with AI should be educated about AI and its symptoms and dosing for physical stresses, and should be provided with a medical alert card/necklace/bracelet and supplies of injectable hydrocortisone along with instructions about its handling [112]. Central hypothyroidism is treated with levothyroxine (average dose of 1.6 mcg/Kg/day) and the biochemical follow-up should be via measurement of free thyroxine levels, with a goal level of mid to upper half of the reference range [112]. As secondary AI and hypothyroidism are more common in patients with AHy, treatment of AI should precede the treatment of central hypothyroidism in order to avoid the theoretical risk for thyroid hormone-induced adrenal crisis [112]. Testosterone replacement is necessary for male patients with central hypogonadism in order to improve bone mineral density, libido, sexual function, maintenance of muscle mass, strength, and prevention of anaemia [112]. Several testosterone formulations are available and choice of the formulation depends on the patient’s preference, regional availability, and cost [112]. In premenopausal women with central hypogonadism, hormone replacement therapy should be provided (combined oestrogen – progestin formulations for women with intact uterus and oestrogen-only formulations for those who have undergone hysterectomy) [112]. GH replacement could be considered in patients with proven GH deficiency, with a starting dose of 0.2 – 0.4 mg/day in patients <60 years of age and 0.1–0.2 mg/day in patients >60 years of age [112]. Central DI can be treated with desmopressin, but in some instances, partial DI might not bother the patient much and no treatment may be necessary [112]. Caution must be implemented in treating patients with adipsic DI, with careful adjustments of fluid intake and desmopressin based on urine output. Symptomatic hyperprolactinaemia is treated with dopamine agonists (cabergoline, bromocriptine) and the dosage adjusted based on serum prolactin levels [115].
Immunosuppressive therapy is utilized for patients with rapid onset of neurological symptoms and mass effects due to pituitary enlargement. Immunosuppressive drugs have demonstrated efficacy in reducing pituitary swelling, and with restoring pituitary function [16]. Glucocorticoids are the first-line immunosuppressive agents for the treatment of AHy. Although hormonal replacement alone is sufficient in a majority of cases with AHy, glucocorticoid treatment is the mainstay of therapy in some forms of hyophysitis such as IgG4Hy [46]. Various forms of glucocorticoids (prednisone, methylprednisone, or dexamethasone) have been utilized in the treatment of hypophysitis, either as monotherapy or in combination with other immunosuppressive agents [10, 16, 72, 109, 114, 136]. Glucocorticoid therapy is less effective in GHy and XHy [60]. In some studies, two-thirds of the patients experienced improvement/reversal in PHy (65.5% to 66.7%) after two years of average follow-up [136, 137], while another study showed up to 75% improvement in PHy after a median follow-up of 8 months after initiation of immunosuppressive therapy [105]. In another report, partial recovery occurred in 23% and complete recovery occurred in 4% of PHy patients after 6 months of glucocorticoid or combination therapy [21]. In contrast to PHy, ICIHy appears to be more refractory to glucocorticoid therapy, and studies have demonstrated the lack of improvement or recovery of hormonal function of pituitary function even after 12 months of glucocorticoid treatment [70, 72, 138]. Regardless, glucocorticoid therapy is recommended in ICIHy based on the severity. For grade 1 (asymptomatic) or grade 2 (mildly symptomatic) ICIHy, hormone replacement can be provided and there is no need for discontinuation of ICI therapy [139]. For grade 3 (moderately symptomatic) ICIHy, ICI therapy must be delayed and re-initiation of ICI must be considered based on clinical response to hormonal replacement/glucocorticoid treatment. For grade 4 (severe or life-threatening) ICIHy, ICI therapy must be discontinued and high dose glucocorticoid therapy must be administered [139]. There is concern of reduced survival among patients who receive glucocorticoids for ICIHy based on a study in a melanoma cohort receiving ipilimumab [77]. But even those patients who received glucocorticoids for ICIHy had a better overall survival compared to patients who did not develop ICIHy. Nevertheless, high dose glucocorticoids are not recommended for ICIHy except for acute presentations such as symptomatic mass effects [77].
For glucocorticoid treatment of hypophysitis in general, after an initial high dose treatment (1mg/kg/day of prednisone or its equivalent), glucocorticoids are tapered over several weeks to months, and the duration of treatment is guided by the clinical response [11]. Other immunosuppressive agents such as azathioprine, methotrexate, and cyclosporine A can be used in patients who relapse or do not respond to glucocorticoid therapy, and these modalities have been shown to be effective in both PHy and SHy [109, 140–142]. Rituximab can be of potential benefit, especially among those patients with IgG4Hy or those with a biopsy-proven B-lymphocyte predominant, steroid-refractory hypophysitis [142–144].
Surgery should be reserved for cases of uncertain diagnosis, rapid progression of neurological symptoms, and large space-occupying lesions that are accessible through transsphenoidal approach [136]. An advantage of surgery is that it allows for conclusive histopathological diagnosis and can definitively reduce mass-effects from AHy [48]. Moreover, surgery could be a better option for certain forms of PHy, such as GHy, where it has shown better resolution of symptoms, lesser need for hormone replacement, and lower rates of recurrence when compared to glucocorticoid treatment [47]. In cases that are refractory to both medical and surgical modalities, stereotactic radiotherapy has been shown to be effective [145, 146].
The salient features of each major form of hypophysitis is summarized in Table 2. The data on the natural course of hypophysitis is highly variable, mainly due to the rarity of the disease and a small patient population in most studies. The disease can be self-limiting and spontaneous remissions can occur [46]. Anterior pituitary function can spontaneously improve in up to 33% of patients, although the rate of recovery is highly variable [14, 105, 114]. Glucocorticoid treatment has also shown somewhat similar rates in improvement in anterior pituitary function (12% to 41%) [105, 114, 136]. Cortisol and gonadotroph axes tend to improve more frequently with therapy, while DI rarely recovers is more often refractory. DI has been shown to be a negative prognostic factor in the response of AHy to glucocorticoid therapy [105, 147]. Approximately 71% to 72% of patients require long-term hormone replacement therapy [14, 105]. Initial improvements in mass effects and radiologic features with glucocorticoid therapy are observed in over 75% of patients, with different studies showing response rates of 40% to 89%, and the pituitary lesion size can remain stable in up to 31% of patients [16, 114, 136, 148]. However, relapse/recurrence rates are not uncommon following glucocorticoid therapy, and have been observed in 38% to 46% of patients, with disease progression in 3% [114, 136]. Data on the influence of the dose and duration of glucocorticoids on the long-term prognosis of AHy is insufficient [46]. Also, prognostic data on some forms of AHy such as anti-PIT1Hy or isolated ACTH-deficiency is lacking due to the extreme rarity of these conditions. Surgery can result in rapid improvements in mass effects and neurological symptoms, and radiologic shrinkage in the size of the lesion can be observed in up to 68% of patients [136]. But even after surgery, the pituitary mass lesion can remain unchanged (approximately 21%) or progress (approximately 3%) [136]. The disease can be fatal in 6% to 7% of patients, mainly due to adrenal crisis [11, 14]. Long-term inflammation in hypophysitis can cause fibrosis and shrinkage of the pituitary, which in turn can lead to the development of an empty sella [15, 46].
Table 2.
Salient features of common forms of hypophysitis.
| 1. Lymphocytic hypophysitis |
| • Most common form of hypophysitis (68% of PHy). |
| • Common in females (female:male = 4:1 for LADHy, 1.9:1 for LINHy, and 1:1 for LPHy). |
| • Mean age of presentation: females: 35 years, men: 49 years for LAHy. For LINHy, 39 years and for LPHy, 43 years in both sexes. |
| • Association with pregnancy and post-partum period. |
| • Histopathology: lymphocytic infiltration in the interstitium and in the pituitary acini. Lymphoid follicles with germinal centers can be observed. |
| 2. Granulomatous hypophysitis |
| • Second most common form (20% of PHy). |
| • Common in females (female:male = 2.6:1). |
| • Mean age of presentation: 44 years. |
| • Histopathology: arranged in the form of granulomas, along with lymphocytes, multinucleated giant cells, and areas of fibrosis. |
| • Might represent a later stage in the course of natural history of lymphocytic hypophysitis. |
| 3. IgG4 hypophysitis |
| • Prevalence of 4% among PHy (although some cohorts suggest as high as 41%). |
| • Often part of a systemic disease known as IgG4 disease. |
| • More common in males in cases of systemic involvement (female:male = 3:1), but isolated hypophysitis is more common in females (female:male = 2:1) |
| • Mean age of presentation is earlier in females (females: 56.4 years; males: 67.5 years). |
| • More common in eastern countries such as Japan. |
| • Histopathology: mononuclear infiltration with ≥10 IgG4 positive plasma cells per high power field, storiform fibrosis, rarely lymphoid follicles and eosinophils. |
| • Highly responsive to glucocorticoid therapy. |
| • Diagnosis can be established based on the following criteria: 1. Histopathological evidence on pituitary biopsy, 2. Hypophysitis on MRI, 3. Biopsy-proven systemic involvement, 4. Serum IgG4 levels >140 mg/dl, and 5. Excellent response to glucocorticoid therapy. Criterion 1 alone, or criteria 2 + 3, or criteria 2 + 4 + 5 establish the diagnosis. |
| 4. Xanthomatous hypophysitis |
| • Prevalence of about 3% among PHy. |
| • More common in females (female:male = 3:1). |
| • Commonly presents in the 4th decade. |
| • Usually causes adenohypophysitis. Central DI is uncommon. |
| • Histopathology: infiltration with CD68 positive foamy macrophages. Xanthogranulomatous sub-type can contain cholesterol clefts and hemosiderin deposits. |
| • MRI can show cystic areas of liquefaction. |
| • Visual symptoms are uncommon in this type of hypophysitis. |
| • Precise aetiology unclear: likely an inflammatory reaction to a ruptured Rathke’s cleft cyst. |
| 5. Necrotizing hypophysitis: |
| • Rarest histopathological form (0.6% of PHy). |
| • Can also occur in association with CTLA4 inhibitor therapy. |
| • Common in females (female:male = 3:1). |
| • More likely to present with acute onset of symptoms with mass effects. |
| • Histopathology: extensive areas of necrosis with infiltration of lymphocytes, plasmacytes, and a few eosinophils. Fibrosis can result from chronic inflammation. |
| 6. Immune checkpoint therapy induced hypophysitis: |
| • Most common form of secondary hypophysitis. |
| • Common in males (female:male = 1:4) |
| • Mean age of presentation: 59 ± 13 years |
| • Aetiology: combination of a type II and type IV hypersensitivity reaction, along with humoral immune response. |
| • More common with CTLA4 inhibitors (13.6%) as compared to PD1/PDL1 inhibitors (0.5%). |
| • Headaches are more common but visual disturbances are less on presentation when compared to PHy. |
| • Central DI is uncommon, and occurs in about 1% of patients. |
| • Isolated ACTH deficiency with hyponatraemia and a normal pituitary MRI is more common with PD1/PDL1 inhibitor therapy. |
| • Relatively refractory to glucocorticoid treatment. |
PHy: primary hypophysitis; LADHy: lymphocytic adenohypophysitis; LINHy: lymphocytic infundibuloneurohypophysitis; LPAHy: lymphocytic panhypophysitis; DI: diabetes insipidus; ACTH: adrenocorticotrophic hormone; MRI: magnetic resonance imaging.
6. Summary: Challenges and Future directions
A substantial wealth of knowledge has acclimated over the past decade on the pathophysiology, diagnostic approach, and treatment strategies for management of hypophysitis and pituitary autoimmune conditions. However, many areas in this disease need further refinement. The precise classification of hypophysitis is still a matter of debate. Some publications include all the autoimmune forms of hypophysitis such as those associated with vasculitis or APS under PHy [46], while others have classified hypophysitis from systemic autoimmune disease under SHy [2, 15]. On the other hand, some forms of PHy such as IgG4Hy (which have multi-organ involvement) are placed under both PHy and SHy [2, 15, 48]. Therefore, further research into molecular and histopathological demarcation between the various forms of hypophysitis is necessary for a more precise classification of the condition. Additionally, future investigations on the role of autoantibodies and HLA phenotypes in the pathogenesis of AHy could provide more insights into the relationship between the pituitary gland and the immune system. Other potential areas of further investigations include the histopathological and immunohistochemical phenotyping of hypophysitis and the role of immunosuppressive and immunomodulatory therapies in affecting the long-term prognosis of hypophysitis. Autoimmune patterns of hypophysitis can lack T regulator cells, which is a pattern also seen with other autoimmune conditions such as rheumatoid arthritis and multiple sclerosis [35]. Therefore, those patients who have an autoimmune pattern of pituitary involvement might benefit from immunosuppressive therapy. Furthermore, those patients with B-lymphocyte predominant AHy can be potentially treated with rituximab-based regimen [143, 144]. Large scale prospective studies on ICIHy are currently lacking and there is no strong data on the impact of discontinuing a potentially life-saving treatment such as ICI in order to tackle a manageable adverse effect of ICIHy [138]. Therefore, a multi-disciplinary team approach comprising of endocrinologists, oncologists, neuroradiologists, and pathologists is necessary for shared decision-making in order to formulate individualized treatment. As more data on hypophysitis and pituitary autoimmunity gather in the coming years, several of these questions can potentially be answered in the near future.
Practice Points.
Hypophysitis is a heterogeneous condition that leads to inflammation of the sella and/or suprasellar region, potentially resulting in hormonal deficiencies and/or mass effects
A preponderance of hypophysitis subtypes have an underlying autoimmune aetiology
Coeliac disease-associated haplotypes in Caucasian patients, particularly the ones linked to DQ8 and DQ2, have an increased association with hypophysitis
The overall incidence and prevalence of hypophysitis has dramatically increased over the past decade, with a rise in the occurrence of certain forms of hypophysitis such as IgG4 hypophysitis (IgG4Hy) and immune checkpoint inhibitor induced hypophysitis (ICIHy)
The clinical presentation varies from an asymptomatic condition to a fatal disease often as a result of electrolyte abnormalities due to glucocorticoid deficiency in the context of adrenal crisis from central adrenal insufficiency
Milder forms of hypophysitis are treated with replacement of deficient hormones while more acute presentations with mass effects require glucocorticoid therapy, immunosuppressive therapy or surgery
Headaches from ICIHy are more common with CTLA4 inhibitors and combination therapy than with PD1 inhibitors
PD1/PDL1 inhibitor therapy often causes isolated ACTH deficiency, with hyponatraemia being a common presenting symptom, and MRI scans often do not reveal classic features of hypophysitis
Patients with melanoma who develop hypophysitis from ipilimumab have a better overall survival irrespective of glucocorticoid therapy, when compared with those patient who do not develop hypophysitis
MRI is the best imaging modality for diagnosing hypophysitis and MRI abnormalities can be seen in up to 98% of PHy and 77% of ICIHy cases
Glucocorticoids are the first-line immunosuppressive agents for the treatment of AHy
For grade 1 (asymptomatic) or grade 2 (mildly symptomatic) ICIHy, hormone replacement can be provided and there is no need for discontinuation of ICI therapy
For grade 3 (moderately symptomatic) ICIHy, ICI therapy must be delayed and re-initiation of ICI must be considered based on clinical response to hormonal replacement/glucocorticoid treatment
For grade 4 (severe or life-threatening) ICIHy, ICI therapy must be discontinued and high dose glucocorticoid therapy must be administered
For glucocorticoid treatment of hypophysitis in general, after an initial high dose treatment (1mg/kg/day of prednisone or its equivalent), glucocorticoids are tapered over several weeks to months, and the duration of treatment is guided by the clinical response
Surgery should be reserved for cases of uncertain diagnosis, rapid progression of neurological symptoms, and large space-occupying lesions that are accessible through transsphenoidal approach
71% to 72% of patients require long-term hormone replacement therapy
Research Agenda.
Elucidate the role of pituitary antibodies and HLA testing in diagnosing AHy
Establish a more definitive association between HLA and LHy pathogenesis
Better understand molecular mimicry through co-expression of antigens such as enolase isoforms in both pituitary and placenta, which are known targets of certain pituitary antibodies
Decipher the pathogenesis of IgG4Hy, also known as plasmacytic hypophysitis, which can be part of IgG4-related disease, a systemic disease involving multiple organs
Acknowledgments
Funding information: This work was funded in part by the Intramural Research Program of the National Institutes of Health (NIH).
Declaration of Competing Interest
This research was supported in part by the Intramural Research Program of the NIDDK and Eunice Kennedy Shriver NICHD, National Institutes of Health, Bethesda, MD.
List of useful abbreviations
- PHy
Primary hypophysitis
- SHy
Secondary hypophysitis
- AHy
Autoimmune hypophysitis
- LHy
Lymphocytic hypophysitis
- GHy
Granulomatous hypophysitis
- IgG4Hy
IgG-4 hypophysitis
- XHy
Xanthomatous hypophysitis
- NHy
Necrotizing hypophysitis
- ICIHy
Immune-checkpoint inhibitor (ICI) induced hypophysitis
- Anti-PIT1Hy
Anti-pituitary-specific transcription factor-1 (Anti-PIT1) hypophysitis
- LAHy
Lymphocytic adenohypophysitis
- LINHy
Lymphocytic infundibuloneurohypophysitis
- LPHy
Lymphocytic panhypophysitis
Footnotes
Disclosure statement: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Colover J, Glynn L. Experimental iso-immune adrenalitis. Immunology. 1958;1(2):172. [PMC free article] [PubMed] [Google Scholar]
- 2.*.Wehbeh L, Alreddawi S, Salvatori R. Hypophysitis in the era of immune checkpoint inhibitors and immunoglobulin G4-related disease. Expert review of endocrinology & metabolism. 2019;14(3):167–78. [DOI] [PubMed] [Google Scholar]
- 3.*.Ferrari SM, Fallahi P, Elia G, Ragusa F, Ruffilli I, Patrizio A, Galdiero MR, Baldini E, Ulisse S, Marone G, Antonelli A. Autoimmune endocrine dysfunctions associated with cancer immunotherapies. International journal of molecular sciences. 2019;20(10):2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koch CA, Antonelli A. Immunoendocrinology: When (neuro) endocrinology and immunology meet. Reviews in Endocrine and Metabolic Disorders. 2018;19(4):277–82. [DOI] [PubMed] [Google Scholar]
- 5.Falorni A, Minarelli V, Bartoloni E, Alunno A, Gerli R. Diagnosis and classification of autoimmune hypophysitis. Autoimmunity Reviews. 2014;13(4–5):412–6. [DOI] [PubMed] [Google Scholar]
- 6.Bellastella G, Maiorino MI, Bizzarro A, Giugliano D, Esposito K, Bellastella A, De Bellis A. Revisitation of autoimmune hypophysitis: knowledge and uncertainties on pathophysiological and clinical aspects. Pituitary. 2016;19(6):625–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buxton N, Robertson I. Lymphocytic and granulocytic hypophysitis: a single centre experience. British journal of neurosurgery. 2001;15(3):242–6. [DOI] [PubMed] [Google Scholar]
- 8.*.Caturegli P, Newschaffer C, Olivi A, Pomper MG, Burger PC, Rose NR. Autoimmune Hypophysitis. Endocrine Reviews. 2005;26(5):599–614. [DOI] [PubMed] [Google Scholar]
- 9.Chiloiro S, Tartaglione T, Angelini F, Bianchi A, Arena V, Giampietro A, Mormando M, Sciandra M, Laino ME, De Marinis L. An overview of diagnosis of primary autoimmune hypophysitis in a prospective single-center experience. Neuroendocrinology. 2017;104(3):280–90. [DOI] [PubMed] [Google Scholar]
- 10.*.Joshi MN, Whitelaw BC, Carroll PV. Mechanisms in endocrinology: hypophysitis: diagnosis and treatment. European journal of endocrinology. 2018;179(3):R151–R63. [DOI] [PubMed] [Google Scholar]
- 11.Caturegli P, Lupi I, Gutenberg A. Autoimmune hypophysitis The Autoimmune Diseases. 5th Edition ed: Elsevier; 2014. p. 633–46. [Google Scholar]
- 12.*.Yamamoto M, Iguchi G, Bando H, Kanie K, Hidaka-Takeno R, Fukuoka H, Takahashi Y. Autoimmune pituitary disease: New concepts with clinical implications. Endocrine reviews. 2019. [DOI] [PubMed] [Google Scholar]
- 13.Shikuma J, Kan K, Ito R, Hara K, Sakai H, Miwa T, Kanazawa A, Odawara M. Critical review of IgG4-related hypophysitis. Pituitary. 2017;20(2):282–91. [DOI] [PubMed] [Google Scholar]
- 14.Caturegli P, Lupi I, Landek-Salgado M, Kimura H, Rose NR. Pituitary autoimmunity: 30 years later. Autoimmunity reviews. 2008;7(8):631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gubbi S, Hannah-Shmouni F, Stratakis CA, Koch CA. Primary hypophysitis and other autoimmune disorders of the sellar and suprasellar regions. Reviews in Endocrine and Metabolic Disorders. 2018;19(4):335–47. [DOI] [PubMed] [Google Scholar]
- 16.Lupi I, Manetti L, Raffaelli V, Lombardi M, Cosottini M, Iannelli A, Basolo F, Proietti A, Bogazzi F, Caturegli P. Diagnosis and treatment of autoimmune hypophysitis: a short review. Journal of endocrinological investigation. 2011;34(8):e245–e52. [DOI] [PubMed] [Google Scholar]
- 17.*.Chiloiro S, Capoluongo ED, Tartaglione T, Giampietro A, Bianchi A, Giustina A, Pontecorvi A, De Marinis L. The Changing Clinical Spectrum of Hypophysitis. Trends in Endocrinology & Metabolism. 2019. [DOI] [PubMed] [Google Scholar]
- 18.Simmonds M Über das Vorkommen von Riesenzellen in der Hypophyse. Virchows Archiv für pathologische Anatomie und Physiologie und für klinische Medizin. 1917;223(3):281–90. [Google Scholar]
- 19.Goudie R, Pinkerton P. Anterior hypophysitis and Hashimoto’s disease in a young woman. The Journal of pathology and bacteriology. 1962;83(2):584–5. [PubMed] [Google Scholar]
- 20.Caturegli P, Di Dalmazi G, Lombardi M, Grosso F, Larman HB, Larman T, Taverna G, Cosottini M, Lupi I. Hypophysitis secondary to cytotoxic T-lymphocyte–associated protein 4 blockade: insights into pathogenesis from an autopsy series. The American journal of pathology. 2016;186(12):3225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angelousi A, Cohen C, Sosa S, Danilowicz K, Papanastasiou L, Tsoli M, Pal A, Piaditis G, Grossman A, Kaltsas G. Clinical, endocrine and imaging characteristics of patients with primary hypophysitis. Hormone and Metabolic Research. 2018;50(04):296–302. [DOI] [PubMed] [Google Scholar]
- 22.Imber BS, Lee HS, Kunwar S, Blevins LS, Aghi MK. Hypophysitis: a single-center case series. Pituitary. 2015;18(5):630–41. [DOI] [PubMed] [Google Scholar]
- 23.GUAY AT, AGNELLO V, TRONIC BC, GRESHAM DG, FREIDBERG SR. Lymphocytic hypophysitis in a man. The Journal of Clinical Endocrinology & Metabolism. 1987;64(3):631–4. [DOI] [PubMed] [Google Scholar]
- 24.Laway BA, Mir SA. Pregnancy and pituitary disorders: challenges in diagnosis and management. Indian journal of endocrinology and metabolism. 2013;17(6):996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’dwyer D, Clifton V, Hall A, Smith R, Robinson P, Crock P. Pituitary Autoantibodies in Lymphocytic Hypophysitis Target Both γ-and a-Enolase–A Link with Pregnancy? Archives of physiology and biochemistry. 2002;110(1–2):94–8. [DOI] [PubMed] [Google Scholar]
- 26.Tsur A, Leibowitz G, Samueloff A, Gross DJ. Successful pregnancy in a patient with preexisting lymphocytic hypophysitis. Acta obstetricia et gynecologica Scandinavica. 1996;75(8):772–4. [DOI] [PubMed] [Google Scholar]
- 27.Gagneja H, Arafah B, Taylor HC, editors. Histologically proven lymphocytic hypophysitis: spontaneous resolution and subsequent pregnancy Mayo Clinic Proceedings; 1999: Elsevier. [DOI] [PubMed] [Google Scholar]
- 28.Hayes F, McKenna T. The occurrence of lymphocytic hypophysitis in a first but not subsequent pregnancy. The Journal of Clinical Endocrinology & Metabolism. 1996;81(8):3131–2. [DOI] [PubMed] [Google Scholar]
- 29.Chandan JS, Gittoes N, Toogood A, Karavitaki N. Recurrent lymphocytic hypophysitis during two pregnancies: a very rare care. Society for Endocrinology BES. 2017;2017. [Google Scholar]
- 30.Davies EC, Jakobiec FA, Stagner AM, Rizzo JF. An atypical case of lymphocytic panhypophysitis in a pregnant woman. Journal of Neuro-Ophthalmology. 2016;36(3):313–6. [DOI] [PubMed] [Google Scholar]
- 31.Sakurai K, Yamashita R, Niituma S, Iwama S, Sugimura Y, Arihara Z, Takahashi K. Usefulness of anti-rabphilin-3A antibodies for diagnosing central diabetes insipidus in the third trimester of pregnancy. Endocrine journal. 2017;64(6):645–50. [DOI] [PubMed] [Google Scholar]
- 32.Panicker HK, Janicic N, Nguyen D, Verbalis J. Presumed infundibuloneurohypophysitis: Unusual presentation in a postpartum patient. American journal of neuroradiology. 2005;26(2):357–9. [PMC free article] [PubMed] [Google Scholar]
- 33.Fehn M, Sommer C, Lüdecke DK, Plöckinger U, Saeger W. Lymphocytic hypophysitis: light and electron microscopic findings and correlation to clinical appearance. Endocrine pathology. 1998;9(1):71–8. [DOI] [PubMed] [Google Scholar]
- 34.*.Gutenberg A, Buslei R, Fahlbusch R, Buchfelder M, Brück W. Immunopathology of primary hypophysitis: implications for pathogenesis. The American journal of surgical pathology. 2005;29(3):329–38. [DOI] [PubMed] [Google Scholar]
- 35.Mirocha S, Elagin R, Salamat S, Jaume J. T regulatory cells distinguish two types of primary hypophysitis. Clinical & Experimental Immunology. 2009;155(3):403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’dwyer DT, Smith AI, Matthew ML, Andronicos NM, Ranson M, Robinson PJ, Crock PA. Identification of the 49-kDa autoantigen associated with lymphocytic hypophysitis as α-enolase. The Journal of Clinical Endocrinology & Metabolism. 2002;87(2):752–7. [DOI] [PubMed] [Google Scholar]
- 37.Takao T, Nanamiya W, Matsumoto R, Asaba K, Okabayashi T, Hashimoto K. Antipituitary antibodies in patients with lymphocytic hypophysitis. Hormone Research in Paediatrics. 2001;55(6):288–92. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka S, Tatsumi K-i, TAKANO T, MURAKAMI Y, TAKAO T, YAMAKITA N, TAHARA S, TERAMOTO A, HASHIMOTO K, KATO Y. Anti-alpha-enolase antibodies in pituitary disease. Endocrine journal. 2003;50(6):697–702. [DOI] [PubMed] [Google Scholar]
- 39.De Bellis A, Sinisi A, Pane E, Dello Iacovo A, Bellastella G, Di Scala G, Falorni A, Giavoli C, Gasco V, Giordano R. Involvement of hypothalamus autoimmunity in patients with autoimmune hypopituitarism: role of antibodies to hypothalamic cells. The Journal of Clinical Endocrinology & Metabolism. 2012;97(10):3684–90. [DOI] [PubMed] [Google Scholar]
- 40.Andrioli M, Giraldi FP, Cavagnini F. Isolated corticotrophin deficiency. Pituitary. 2006;9(4):289–95. [DOI] [PubMed] [Google Scholar]
- 41.De Bellis A, Bellastella G, Maiorino MI, Aitella E, Lucci E, Cozzolino D, Bellastella A, Bizzarro A, Giugliano D, Esposito K. Longitudinal behavior of autoimmune GH deficiency: from childhood to transition age. European journal of endocrinology. 2016;174(3):381–7. [DOI] [PubMed] [Google Scholar]
- 42.Iwama S, Welt CK, Romero CJ, Radovick S, Caturegli P. Isolated prolactin deficiency associated with serum autoantibodies against prolactin-secreting cells. The Journal of Clinical Endocrinology & Metabolism. 2013;98(10):3920–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cocco C, Brancia C, D’Amato F, Noli B. Pituitary gonadotropins and autoimmunity. Molecular and cellular endocrinology. 2014;385(1–2):97–104. [DOI] [PubMed] [Google Scholar]
- 44.Bellastella A, Bizzarro A, Coronella C, Bellastella G, Sinisi AA, De Bellis A. Lymphocytic hypophysitis: a rare or underestimated disease? European Journal of Endocrinology. 2003;149(5):363–76. [DOI] [PubMed] [Google Scholar]
- 45.Heaney AP, Sumerel B, Rajalingam R, Bergsneider M, Yong WH, Liau LM. HLA markers DQ8 and DR53 are associated with lymphocytic hypophysitis and may aid in differential diagnosis. The Journal of Clinical Endocrinology & Metabolism. 2015;100(11):4092–7. [DOI] [PubMed] [Google Scholar]
- 46.*.Prete A, Salvatori R. Hypophysitis In: in: Feingold KR1 AB, Boyce A3, Chrousos G4, Dungan K5, Grossman A6, Hershman JM7, Kaltsas G8, Koch C9, Kopp P10, Korbonits M11, McLachlan R12, Morley JE13, New M14, Perreault L15, Purnell J16, Rebar R17, Singer F18, Trence DL19, Vinik A20, Wilson DP21, editors., editor Endotext [Internet] South Dartmouth (MA): : MDText.com, Inc.; 2000-. https://www.ncbi.nlm.nih.gov/books/NBK519842/; 2018 Aug 15. [Google Scholar]
- 47.Hunn BH, Martin WG, Simpson S, Mclean CA. Idiopathic granulomatous hypophysitis: a systematic review of 82 cases in the literature. Pituitary. 2014;17(4):357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guaraldi F, Giordano R, Grottoli S, Ghizzoni L, Arvat E, Ghigo E. Pituitary autoimmunity. Endocrine Immunology. 48: Karger Publishers; 2017. p. 48–68. [DOI] [PubMed] [Google Scholar]
- 49.Tzou S-C, Lupi I, Landek M, Gutenberg A, Tzou Y-M, Kimura H, Pinna G, Rose NR, Caturegli P. Autoimmune hypophysitis of SJL mice: clinical insights from a new animal model. Endocrinology. 2008;149(7):3461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, Okamoto A, Egawa N, Nakajima H. A new clinicopathological entity of IgG4-related autoimmune disease. Journal of gastroenterology. 2003;38(10):982–4. [DOI] [PubMed] [Google Scholar]
- 51.Bando H, Iguchi G, Fukuoka H, Taniguchi M, Yamamoto M, Matsumoto R, Suda K, Nishizawa H, Takahashi M, Kohmura E. The prevalence of IgG4-related hypophysitis in 170 consecutive patients with hypopituitarism and/or central diabetes insipidus and review of the literature. European journal of endocrinology. 2014;170(2):161–72. [DOI] [PubMed] [Google Scholar]
- 52.Yuen KC, Moloney KJ, Mercado JU, Rostad S, McCullough BJ, Litvack ZN, Delashaw JB, Mayberg MR. A case series of atypical features of patients with biopsy-proven isolated IgG4-related hypophysitis and normal serum IgG4 levels. Pituitary. 2018;21(3):238–46. [DOI] [PubMed] [Google Scholar]
- 53.van der Vliet HJ, Perenboom RM. Multiple pseudotumors in IgG4-associated multifocal systemic fibrosis. Annals of internal medicine. 2004;141(11):896. [DOI] [PubMed] [Google Scholar]
- 54.Wong S, Lam WY, Wong WK, Lee KC. Hypophysitis presented as inflammatory pseudotumor in immunoglobulin G4-related systemic disease. Human pathology. 2007;38(11):1720–3. [DOI] [PubMed] [Google Scholar]
- 55.Bernreuther C, Illies C, Flitsch J, Buchfelder M, Buslei R, Glatzel M, Saeger W. IgG4 related hypophysitis is highly prevalent among cases of histologically confirmed hypophysitis. Brain Pathology. 2017;27(6):839–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leporati P, Landek-Salgado MA, Lupi I, Chiovato L, Caturegli P. IgG4-related hypophysitis: a new addition to the hypophysitis spectrum. The Journal of Clinical Endocrinology & Metabolism. 2011;96(7):1971–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uccella S, Amaglio C, Brouland J-P, Bianconi E, Ippolito S, Messerer M, Rouiller N, Tanda ML, Sessa F, La Rosa S. Disease heterogeneity in IgG4-related hypophysitis: report of two histopathologically proven cases and review of the literature. Virchows Archiv. 2019:1–9. [DOI] [PubMed] [Google Scholar]
- 58.Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. The Lancet. 2015;385(9976):1460–71. [DOI] [PubMed] [Google Scholar]
- 59.Folkerth RD, Price DL Jr, Schwartz M, Black PM, De Girolami U. Xanthomatous hypophysitis. The American journal of surgical pathology. 1998;22(6):736–41. [DOI] [PubMed] [Google Scholar]
- 60.Gutenberg A, Hans V, Puchner MJ, reutzer Br ck Jr, Caturegli P, Buchfelder M. Primary hypophysitis: clinical-pathological correlations. European Journal of Endocrinology. 2006;155(1):101–7. [DOI] [PubMed] [Google Scholar]
- 61.Yuen KC, Popovic V, Trainer PJ. New Causes of Hypophysitis. Best Practice & Research Clinical Endocrinology & Metabolism. 2019. [DOI] [PubMed] [Google Scholar]
- 62.Kleinschmidt DeMasters B, Lillehei KO, Hankinson TC. Review of xanthomatous lesions of the sella. Brain Pathology. 2017;27(3):377–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gutenberg A, Caturegli P, Metz I, Martinez R, Mohr A, Brück W, Rohde V. Necrotizing infundibulo-hypophysitis: an entity too rare to be true? Pituitary. 2012;15(2):202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahmed SR, Aiello DP, Page R, Hopper K, Towfighi J, Santen RJ. Necrotizing infundibulo-hypophysitis: a unique syndrome of diabetes insipidus and hypopituitarism. The Journal of Clinical Endocrinology & Metabolism. 1993;76(6):1499–504. [DOI] [PubMed] [Google Scholar]
- 65.Ćaćić M, Marinković J, ruljac I, Perić B, Čerina V, Stipić D, Pažanin L, Pećina HI, Vrkljan M. Ischemic Pituitary Apoplexy, Hypopituitarism and Diabetes Insipidus: a Triad Unique to Necrotizing Hypophysitis. Acta Clinica Croatica. 2018;57(4):768–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tashiro T, Sano T, Xu B, Wakatsuki S, Kagawa N, Nishioka H, Yamada S, Kovacs K. Spectrum of different types of hypophysitis: a clinicopathologic study of hypophysitis in 31 cases. Endocrine pathology. 2002;13(3):183–95. [DOI] [PubMed] [Google Scholar]
- 67.Faje A, Reynolds K, Zubiri L, Lawrence D, Cohen J, Sullivan R, Nachtigall LB, Tritos N. Hypophysitis secondary to nivolumab and pembrolizumab is a clinical entity distinct from ipilimumab-associated hypophysitis. European journal of endocrinology. 2019;1(aop). [DOI] [PubMed] [Google Scholar]
- 68.Byun DJ, Wolchok JD, Rosenberg LM, Girotra M. Cancer immunotherapy—immune checkpoint blockade and associated endocrinopathies. Nature Reviews Endocrinology. 2017;13(4):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer. 2012;12(4):252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Albarel F, Gaudy C, Castinetti F, Carré T, Morange I, Conte-Devolx B, Grob J-J, Brue T. Long-term follow-up of ipilimumab-induced hypophysitis, a common adverse event of the anti-CTLA-4 antibody in melanoma. European Journal of Endocrinology. 2015;172(2):195–204. [DOI] [PubMed] [Google Scholar]
- 71.Faje AT, Sullivan R, Lawrence D, Tritos NA, Fadden R, Klibanski A, Nachtigall L. Ipilimumab-induced hypophysitis: a detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. The Journal of Clinical Endocrinology & Metabolism. 2014;99(11):4078–85. [DOI] [PubMed] [Google Scholar]
- 72.Min L, Hodi FS, Giobbie-Hurder A, Ott PA, Luke JJ, Donahue H, Davis M, Carroll RS, Kaiser UB. Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: a retrospective cohort study. Clinical Cancer Research. 2015;21(4):749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wei KZ, Baxter M, Casasola R. Hypophysitis induced by immune checkpoint inhibitors in a Scottish melanoma population. Melanoma Management. 2019;6(1):MMT13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, Tolaney SM. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA oncology. 2018;4(2):173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Science translational medicine. 2014;6(230):230ra45–ra45. [DOI] [PubMed] [Google Scholar]
- 76.Lupi I, Brancatella A, Cosottini M, Viola N, Lanzolla G, Sgrò D, Di Dalmazi G, Latrofa F, Caturegli P, Marcocci C. Clinical heterogeneity of hypophysitis secondary to PD-1/PD-L1 blockade: insights from four cases. Endocrinology, diabetes & metabolism case reports. 2019;2019(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Faje AT, Lawrence D, Flaherty K, Freedman C, Fadden R, Rubin K, Cohen J, Sullivan RJ. High dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer. 2018;124(18):3706–14. [DOI] [PubMed] [Google Scholar]
- 78.Bayram F, eleştimur F, Özt rk F, Selcuklu A, Patiroğlu T, Beyhan Z. Lymphocytic hypophysitis in a patient with Graves’ disease. Journal of endocrinological investigation. 1998;21(3):193–7. [DOI] [PubMed] [Google Scholar]
- 79.Hussain S, Shahriar HM, Drake W, editors. Lymphocytic hypophysitis in a pregnant patient with type 1 diabetes. Society for Endocrinology Endocrine Update 2018; 2018: BioScientifica. [Google Scholar]
- 80.Bando H, Iguchi G, Yamamoto M, Hidaka-Takeno R, Takahashi Y. Anti-PIT-1 antibody syndrome; a novel clinical entity leading to hypopituitarism. Pediatric endocrinology reviews: PER. 2015;12(3):290–6. [PubMed] [Google Scholar]
- 81.Katano H, Umemura A, Kamiya K, Kanai H, Yamada K. Visual disturbance by lymphocytic hypophysitis in a non-pregnant woman with systemic lupus erythematosus. Lupus. 1998;7(8):554–6. [DOI] [PubMed] [Google Scholar]
- 82.Ashraf V, Bhasi R, Kumar RP, Girija A. Primary Sjögren’s syndrome manifesting as multiple cranial neuropathies: MRI findings. Annals of Indian Academy of Neurology. 2009;12(2):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tao T, Zhang Z, Li H. Lymphocytic hypophysitis associated with Behcet’s disease: A case report and review of the literature. Neuroendocrinology Letters. 2018;39(1). [PubMed] [Google Scholar]
- 84.Chiloiro S, Capoluongo ED, Tartaglione T, Bianchi A, Giampietro A, Angelini F, Arena V, Pontecorvi A, De Marinis L. Human leucocyte antigens coeliac haplotypes and primary autoimmune hypophysitis in caucasian patients. Clinical endocrinology. 2018;88(5):692–9. [DOI] [PubMed] [Google Scholar]
- 85.McIntyre EA, Perros P. Fatal inflammatory hypophysitis. Pituitary. 2007;10(1):107–11. [DOI] [PubMed] [Google Scholar]
- 86.Chu JN, Goglin S, Patzek S, Brondfield S. Granulomatosis with Polyangiitis Presenting as Hypophysitis. The American journal of medicine. 2019;132(1):e21–e2. [DOI] [PubMed] [Google Scholar]
- 87.Funauchi M, Nozaki Y, Hashimoto K, Suk Yoo B, Ohno M, Kinoshita K, Kanamaru A. Microscopic polyangitis as a possible cause of diabetes insipidus. Clinical rheumatology. 2002;21(6):540-. [DOI] [PubMed] [Google Scholar]
- 88.Toth M, Szabo P, Racz K, Szende B, Balogh I, Czirjak S, Slowik F, Glaz E. Granulomatous hypophysitis associated with Takayasu’s disease. Clinical endocrinology. 1996;45(4):499–503. [DOI] [PubMed] [Google Scholar]
- 89.KANATANI M, NAKAMURA R, KUROKAWA K, TAODA M, NEMOTO Y, KAMAKURA K, KUGAI N, NAGATA N, TAKATANI O, TSUCHIYA K. Hypopituitarism associated with Cogan’s syndrome; high-dose glucocorticoid therapy reverses pituitary swelling. Japanese journal of medicine. 1991;30(2):164–9. [DOI] [PubMed] [Google Scholar]
- 90.Chan W, Cockram C. Panhypopituitarism in association with interferon-alpha treatment. Singapore Med J. 2004;45(2):93–4. [PubMed] [Google Scholar]
- 91.Ridruejo E, Flor A, Mando OG. Central hypothyroidism and hypophysitis during treatment of chronic hepatitis C with pegylated interferon alpha and ribavirin. European journal of gastroenterology & hepatology. 2006;18(6):693–4. [DOI] [PubMed] [Google Scholar]
- 92.Tebben P, Atkinson J, Scheithauer B, Erickson D. Granulomatous adenohypophysitis after interferon and ribavirin therapy. Endocrine Practice. 2007;13(2):169–75. [DOI] [PubMed] [Google Scholar]
- 93.Ramos-Leví AM, Gargallo M, Serrano-Somavilla A, Sampedro-Núñez MA, Fraga J, Marazuela M. Hypophysitis following treatment with Ustekinumab: Radiological and pathological Findings. Frontiers in endocrinology. 2018;9:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marinelli JP, Peters PA, Vaglio A, Van Gompel JJ, Lane JI, Carlson ML. Skull Base Manifestations of Erdheim-Chester Disease: A Case Series and Systematic Review. Neurosurgery. 2019. [DOI] [PubMed] [Google Scholar]
- 95.Imashuku S, Kudo N, Kaneda S, Kuroda H, Shiwa T, Hiraiwa T, Inagaki A, Morimoto A. Treatment of patients with hypothalamic-pituitary lesions as adult-onset Langerhans cell histiocytosis. International journal of hematology. 2011;94(6):556–60. [DOI] [PubMed] [Google Scholar]
- 96.Pal R, Rai A, Vaiphei K, Gangadhar P, Gupta P, Mukherjee KK, Singh P, Ray N, Bhansali A, Dutta P. Intracranial Germinoma Masquerading as Secondary Granulomatous Hypophysitis: A Case Report and Review of Literature. Neuroendocrinology. 2019. [DOI] [PubMed] [Google Scholar]
- 97.Li S, Crenshaw EB, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990;347(6293):528. [DOI] [PubMed] [Google Scholar]
- 98.Pfaffle R, Blankenstin O, Wüller S, Kentrup H. Combined pituirary hormone deficency: role of Pit 1 and Prop-1. Acta Pædiatrica. 1999;88:33–41. [DOI] [PubMed] [Google Scholar]
- 99.Yamamoto M, Iguchi G, Takeno R, Okimura Y, Sano T, Takahashi M, Nishizawa H, Handayaningshi AE, Fukuoka H, Tobita M. Adult combined GH, prolactin, and TSH deficiency associated with circulating PIT-1 antibody in humans. The Journal of clinical investigation. 2011;121(1):113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bando H, Iguchi G, Fukuoka H, Yamamoto M, Hidaka-Takeno R, Okimura Y, Matsumoto R, Suda K, Nishizawa H, Takahashi M. Involvement of PIT-1-reactive cytotoxic T lymphocytes in anti-PIT-1 antibody syndrome. The Journal of Clinical Endocrinology & Metabolism. 2014;99(9):E1744–E9. [DOI] [PubMed] [Google Scholar]
- 101.Kanie K, Bando H, Iguchi G, Muguruma K, Matsumoto R, Hidaka-Takeno R, Okimura Y, Yamamoto M, Fujita Y, Fukuoka H. Pathogenesis of anti-PIT-1 antibody syndrome: PIT-1 presentation by HLA class I on anterior pituitary cells. Journal of the Endocrine Society. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kamiya Y, Murakami M. Type 2 diabetes mellitus accompanied by isolated adrenocorticotropic hormone deficiency and gastric cancer. Internal Medicine. 2009;48(12):1031–5. [DOI] [PubMed] [Google Scholar]
- 103.Yamaguchi H, Nakamura H, Mamiya Y, Yamamoto Y, Tajika K, Sugihara H, Gomi S, Inokuchi K, Hasegawa S, Shibazaki T. Acute lymphoblastic leukemia with isolated adrenocorticotropic hormone deficiency. Internal medicine. 1997;36(11):819–21. [DOI] [PubMed] [Google Scholar]
- 104.Kubo S-i, Kitamura O, Orihara Y, Tsuda R, Hirose W, Nakasono I. Isolated adrenocorticotropic hormone deficiency: an autopsy case of adrenal crisis: a case report. The American journal of forensic medicine and pathology. 1997;18(2):202–5. [DOI] [PubMed] [Google Scholar]
- 105.Khare S, Jagtap VS, Budyal SR, Kasaliwal R, Kakade HR, Bukan A, Sankhe S, Lila AR, Bandgar T, Menon PS. Primary (autoimmune) hypophysitis: a single centre experience. Pituitary. 2015;18(1):16–22. [DOI] [PubMed] [Google Scholar]
- 106.Thodou E, Asa SL, Kontogeorgos G, Kovacs K, Horvath E, Ezzat S. Clinical case seminar: lymphocytic hypophysitis: clinicopathological findings. The Journal of Clinical Endocrinology & Metabolism. 1995;80(8):2302–11. [DOI] [PubMed] [Google Scholar]
- 107.Nussbaum CE, Shige-Hisa O, Jacobs LS. Lymphocytic hypophysitis with involvement of the cavernous sinus and hypothalamus. Neurosurgery. 1991;28(3):440–4. [DOI] [PubMed] [Google Scholar]
- 108.Pekic S, Bogosavljevic V, Peker S, Doknic M, Miljic D, Stojanovic M, Skender-Gazibara M, Gacic EM, Popovic V, Petakov M. Lymphocytic hypophysitis successfully treated with stereotactic radiosurgery: case report and review of the literature. Journal of Neurological Surgery Part A: Central European Neurosurgery. 2018;79(01):077–85. [DOI] [PubMed] [Google Scholar]
- 109.Tubridy N, Saunders D, Thom M, Asa S, Powell M, Plant G, Howard R. Infundibulohypophysitis in a man presenting with diabetes insipidus and cavernous sinus involvement. Journal of Neurology, Neurosurgery & Psychiatry. 2001;71(6):798–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Supler ML, Mickle JP. Lymphocytic hypophysitis: report of a case in a man with cavernous sinus involvement. Surgical neurology. 1992;37(6):472–6. [DOI] [PubMed] [Google Scholar]
- 111.Zhu Q, Qian K, Jia G, Lv G, Wang J, Zhong L, Yu S. Clinical Features, Magnetic Resonance Imaging, and Treatment Experience of 20 Patients with Lymphocytic Hypophysitis in a Single Center. World neurosurgery. 2019. [DOI] [PubMed] [Google Scholar]
- 112.Fleseriu M, Hashim IA, Karavitaki N, Melmed S, Murad MH, Salvatori R, Samuels MH. Hormonal replacement in hypopituitarism in adults: an endocrine society clinical practice guideline. The Journal of Clinical Endocrinology & Metabolism. 2016;101(11):3888–921. [DOI] [PubMed] [Google Scholar]
- 113.Honegger J, Schlaffer S, Menzel C, Droste M, Werner S, Elbelt U, Strasburger C, Störmann S, Küppers A, Streetz-van der Werf C. Diagnosis of primary hypophysitis in Germany. The Journal of Clinical Endocrinology & Metabolism. 2015;100(10):3841–9. [DOI] [PubMed] [Google Scholar]
- 114.Wang S, Wang L, Yao Y, Feng F, Yang H, Liang Z, Deng K, You H, Sun J, Xing B. Primary lymphocytic hypophysitis: Clinical characteristics and treatment of 50 cases in a single centre in China over 18 years. Clinical endocrinology. 2017;87(2):177–84. [DOI] [PubMed] [Google Scholar]
- 115.Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, Wass JA. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. The Journal of Clinical Endocrinology & Metabolism. 2011;96(2):273–88. [DOI] [PubMed] [Google Scholar]
- 116.Gubbi S, Hannah-Shmouni F, Koch CA, Verbalis JG. Diagnostic Testing for Diabetes Insipidus In: In: Feingold KRAB, Boyce A, et al. , editors., editor. Endotext [Internet]: MDText.com, Inc South Dartmouth (MA) Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537591/; 2019 Feb 10.. [Google Scholar]
- 117.Gutenberg A, Bell JJ, Lupi I, Tzou S-C, Landek-Salgado MA, Kimura H, Su J, Karaviti LP, Salvatori R, Caturegli P. Pituitary and systemic autoimmunity in a case of intrasellar germinoma. Pituitary. 2011;14(4):388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Husain Q, Zouzias A, Kanumuri VV, Eloy JA, Liu JK. Idiopathic granulomatous hypophysitis presenting as pituitary apoplexy. Journal of Clinical Neuroscience. 2014;21(3):510–2. [DOI] [PubMed] [Google Scholar]
- 119.IKEDA J-i, KURATSU J-i, MIURA M, KAI Y, USHIO Y. Lymphocytic adenohypophysitis accompanying occlusion of bilateral internal carotid arteries. Neurologia medico-chirurgica. 1990;30(5):346–9. [DOI] [PubMed] [Google Scholar]
- 120.Katoh N, Machida K, Satoh S, Yahikozawa H, Ikeda S-i. A clinically diagnosed lymphocytic hypophysitis presenting as recurrent meningitis. Rinsho shinkeigaku= Clinical neurology. 2007;47(7):419–22. [PubMed] [Google Scholar]
- 121.Jain A, DhAnwAl DK. A rare case of autoimmune hypophysitis presenting as temperature dysregulation. Journal of Clinical and Diagnostic Research: JCDR. 2015;9(2):OD09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Richtsmeier AJ, Henry RA, Bloodworth J, Ehrlich EN. Lymphoid hypophysitis with selective adrenocorticotropic hormone deficiency. Archives of Internal Medicine. 1980;140(9):1243–5. [PubMed] [Google Scholar]
- 123.Vasikaran S, Tallis G, Braund W. Secondary hypoadrenalism presenting with hypercalcaemia. Clinical endocrinology. 1994;41(2):261–4. [DOI] [PubMed] [Google Scholar]
- 124.Ospina NS, Al Nofal A, Bancos I, Javed A, Benkhadra K, Kapoor E, Lteif AN, Natt N, Murad MH. ACTH stimulation tests for the diagnosis of adrenal insufficiency: systematic review and meta-analysis. The Journal of Clinical Endocrinology & Metabolism. 2016;101(2):427–34. [DOI] [PubMed] [Google Scholar]
- 125.Christ-Crain M, Bichet DG, Fenske WK, Goldman MB, Rittig S, Verbalis JG, Verkman AS. Diabetes insipidus. Nature Reviews Disease Primers. 2019;5(1):1–20. [DOI] [PubMed] [Google Scholar]
- 126.Araujo P, Coelho M, Arruda M, Gadelha M, Neto L. Ipilimumab-induced hypophysitis: review of the literature. Journal of endocrinological investigation. 2015;38(11):1159–66. [DOI] [PubMed] [Google Scholar]
- 127.Tartaglione T, Chiloiro S, Laino ME, Giampietro A, Gaudino S, Zoli A, Bianchi A, Pontecorvi A, Colosimo C, De Marinis L. Neuro-radiological features can predict hypopituitarism in primary autoimmune hypophysitis. Pituitary. 2018;21(4):414–24. [DOI] [PubMed] [Google Scholar]
- 128.Nakata Y, Sato N, Masumoto T, Mori H, Akai H, Nobusawa H, Adachi Y, Oba H, Ohtomo K. Parasellar T2 dark sign on MR imaging in patients with lymphocytic hypophysitis. American Journal of Neuroradiology. 2010;31(10):1944–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ahmadi J, Meyers GS, Segall HD, Sharma OP, Hinton DR. Lymphocytic adenohypophysitis: contrast-enhanced MR imaging in five cases. Radiology. 1995;195(1):30–4. [DOI] [PubMed] [Google Scholar]
- 130.Gutenberg A, Larsen J, Lupi I, Rohde V, Caturegli P. A radiologic score to distinguish autoimmune hypophysitis from nonsecreting pituitary adenoma preoperatively. American Journal of Neuroradiology. 2009;30(9):1766–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Flanagan D, Ibrahim A, Ellison D, Armitage M, Gawne-Cain M, Lees P. Inflammatory hypophysitis–the spectrum of disease. Acta neurochirurgica. 2002;144(1):47–56. [DOI] [PubMed] [Google Scholar]
- 132.Holder CA, Elster AD. Magnetization transfer imaging of the pituitary: further insights into the nature of the posterior “bright spot”. Journal of computer assisted tomography. 1997;21(2):171–4. [DOI] [PubMed] [Google Scholar]
- 133.Brooks BS, El Gammal T, Allison JD, Hoffman WH. Frequency and variation of the posterior pituitary bright signal on MR images. American Journal of Roentgenology. 1989;153(5):1033–8. [DOI] [PubMed] [Google Scholar]
- 134.Terano T, Seya A, Tamura Y, Yoshida S, Hirayama T. Characteristics of the pituitary gland in elderly subjects from magnetic resonance images: relationship to pituitary hormone secretion. Clinical endocrinology. 1996;45(3):273–9. [DOI] [PubMed] [Google Scholar]
- 135.Böttner A, Keller E, Kratzsch Jr, Stobbe H, Weigel JF, Keller A, Hirsch W, Kiess W, Blum WF, Pfäffle RW. PROP1 mutations cause progressive deterioration of anterior pituitary function including adrenal insufficiency: a longitudinal analysis. The Journal of Clinical Endocrinology & Metabolism. 2004;89(10):5256–65. [DOI] [PubMed] [Google Scholar]
- 136.*.Honegger J, Buchfelder M, Schlaffer S, Droste M, Werner S, Strasburger C, Störmann S, Schopohl J, Kacheva S, Deutschbein T. Treatment of primary hypophysitis in Germany. The Journal of Clinical Endocrinology & Metabolism. 2015;100(9):3460–9. [DOI] [PubMed] [Google Scholar]
- 137.*.Chiloiro S, Tartaglione T, Capoluongo ED, Angelini F, Arena V, Giampietro A, Bianchi A, Zoli A, Pontecorvi A, Colosimo C. Hypophysitis outcome and factors predicting responsiveness to glucocorticoid therapy: a prospective and double-arm study. The Journal of Clinical Endocrinology & Metabolism. 2018;103(10):3877–89. [DOI] [PubMed] [Google Scholar]
- 138.Albarel F, Castinetti F, Brue T. MANAGEMENT OF ENDOCRINE DISEASE: Immune check point inhibitors-induced hypophysitis. European journal of endocrinology. 2019;181(3):R107–R18. [DOI] [PubMed] [Google Scholar]
- 139.Joshi M, Whitelaw B, Palomar M, Wu Y, Carroll P. Immune checkpoint inhibitor related hypophysitis and endocrine dysfunction: clinical review. Clinical Endocrinology. 2016;85(3):331–9. [DOI] [PubMed] [Google Scholar]
- 140.Papanastasiou L, Pappa T, Tsiavos V, Tseniklidi E, Androulakis I, Kontogeorgos G, Piaditis G. Azathioprine as an alternative treatment in primary hypophysitis. Pituitary. 2011;14(1):16–22. [DOI] [PubMed] [Google Scholar]
- 141.Ward L, Paquette J, Seidman E, Huot Cl, Alvarez F, Crock P, Delvin E, Kämpe O, Deal C. Severe autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy in an adolescent girl with a novel AIRE mutation: response to immunosuppressive therapy. The Journal of Clinical Endocrinology & Metabolism. 1999;84(3):844–52. [DOI] [PubMed] [Google Scholar]
- 142.Schreckinger M, Francis T, Rajah G, Jagannathan J, Guthikonda M, Mittal S. Novel strategy to treat a case of recurrent lymphocytic hypophysitis using rituximab: Case report. Journal of neurosurgery. 2012;116(6):1318–23. [DOI] [PubMed] [Google Scholar]
- 143.Bullock DR, Miller BS, Clark HB, Hobday PM. Rituximab treatment for isolated IgG4-related hypophysitis in a teenage female. Endocrinology, diabetes & metabolism case reports. 2018;2018(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lupi I, Urbani C, Manetti L, Cosottini M, Brancatella A, Cappellani D, Bogazzi F, editors. IgG4-related neuroinfundibulo-hypophysitis treated by rituximab and corticosteroids. 20th European Congress of Endocrinology; 2018: BioScientifica. [Google Scholar]
- 145.Ray DK, Yen CP, Vance ML, Laws ER, Lopes B, Sheehan JP. Gamma Knife surgery for lymphocytic hypophysitis: Case report. Journal of neurosurgery. 2010;112(1):118–21. [DOI] [PubMed] [Google Scholar]
- 146.Selch MT, DeSalles AA, Kelly DF, Frighetto L, Vinters HV, Cabatan-Awang C, Wallace RE, Solberg TD. Stereotactic radiotherapy for the treatment of lymphocytic hypophysitis: report of two cases. Journal of neurosurgery. 2003;99(3):591–6. [DOI] [PubMed] [Google Scholar]
- 147.Chiloiro S, De Marinis L. Diabetes insipidus is an unfavorable prognostic factor for response to glucocorticoids in patients with autoimmune hypophysitis. European journal of endocrinology. 2018;178(2):L1–L. [DOI] [PubMed] [Google Scholar]
- 148.Kristof RA, Van Roost D, Klingmüller D, Springer W, Schramm J. Lymphocytic hypophysitis: non-invasive diagnosis and treatment by high dose methylprednisolone pulse therapy? Journal of Neurology, Neurosurgery & Psychiatry. 1999;67(3):398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]