Abstract
Postoperative atrial fibrillation (POAF) following cardiac surgery remains a highly prevalent and costly condition that negatively impacts patient quality of life and survival. Numerous retrospective studies, meta-analysis and review papers have been reported identifying POAF risk based on patients’ risk factors and clinical biomarkers. In this narrative review, we report significant variations among selected pre- and peri-operative biomarkers used to predict POAF incidence in patients without a history of AF. POAF prediction based on BNP, NT-ProBNP, CRP, IL-6, Cr, and PAI-1 differs significantly among different studies, thereby limiting their clinical utility to predict POAF risk with high accuracy. Conversely, sVCAM-1, sCD40L, Gal-3 and aldosterone show promise for better POAF prediction. However, the current datasets for these selected biomarkers are not of sufficient size to validate the broad clinical application specifically for patients with no prior history of AF.
Keywords: Atrial fibrillation, Biomarkers, Coronary artery bypass graft, Postoperative atrial fibrillation
Coronary artery bypass grafting (CABG) is a well-established surgical procedure performed worldwide 1. However, post-CABG complications are prevalent and difficult to predict2 In particular, a sizeable cohort of patients undergoing cardiac surgery develop post-operative atrial fibrillation (POAF)3 It can be procedure-dependent, with an incidence of POAF of approximately 6–40% after CABG alone, 49% following CABG plus aortic valve replacements and 64% following CABG plus mitral valve replacements 4–6. Following CABG surgery, of the patients that develop POAF, 70% and 94% of patients developed POAF before the end of the fourth and the sixth postoperative days respectively, and it is linked to increased complications, financial burden, morbidity, and mortality rate 7,8.
Different formulas, algorithms, and other predictive methods have been reported to predict clinical outcome and recovery time based on family history, medical conditions, patient lifestyle and other patient co-morbidities9–16. Identifying the biomarkers that provide predictive power linked to POAF clinical outcome has greatly affected the field of medicine. Previous reports showed that biomarkers may be used as forecasters of a cardiac patients’ status post-cardiac surgery, and the applications are numerous 17–24 In this narrative review, we complied preoperative and perioperative biomarker data which have been shown to predict POAF incidence in patients undergoing either on-pump or off-pump CABG surgery with no prior history of AF. However, significant variation has been noted within biomarkers of interest, and thus the reported comprehensive data have added more questions regarding the clinical utility of these biomarkers to predict POAF incidence. Most importantly, many studies have reported postoperative biomarkers in their protocols as identifying patients at increased risk of POAF incidence, whereas those biomarkers were collected after cardiac surgery at day 1 and afterwards and this timeline overlaps with the typical onset of POAF. Finding appropriate biomarkers with predictive value and their concentrations preoperatively and intraoperatively will guide changes in cardiac surgery care specifically for those patients who would be at risk of POAF development and may also benefit from increased postsurgical monitoring, pre-emptive antiarrhythmic therapy, and/or additional surgical interventions (such as pulmonary vein isolation or surgical maze), and further developing personalized treatment strategies possibly including prophylactic interventions and closer monitoring to minimizing the risk of developing long-term AF.
Methods
Selection of Studies
We aimed to evaluate the studies by examining the quality of the previously reported preoperative and perioperative biomarkers data among patients with no prior AF history during either on-pump or off-pump CABG surgery. Articles were extracted using both PubMed and MEDLINE databases. The search strategy involved the MEsH keywords such as “biomarker”, “atrial fibrillation”, “Coronary Artery Bypass”, “creatinine” and the text keywords such as “postoperative atrial fibrillation”, “preoperative”, “perioperative / intraoperative”, “postoperative”, “biomarker”, “NT-proBNP”, “BNP”, “CRP”, “IL-6”, “inflammatory biomarkers”, “PAI-1”, “VCAM”, “aldosterone”, “sCD40L” and “Galectin-3”.
Data Extraction
Once an abstract’s general information had been identified, full articles were assessed and studied to ensure both inclusion and exclusion criteria. Studies not published as full-text articles, single case reports, opinion articles, and articles not written in English were excluded. No article was excluded based on preexisting antiarrhythmic drug therapy. Patients with history of atrial fibrillation and any prior heart surgical procedure were also excluded from the search. Both prospective and retrospective studies were included. Search was restricted to studies in adults (aged: 18+ years) with and without POAF incidence after cardiac surgery but none of the studies were excluded based on sex, race / ethnicity, BMI, obesity, diabetes mellitus and myocardial infarction condition. Finally, studies reported either preoperative biomarker data, perioperative biomarker data or both have been included. Only CABG procedures were considered. However, to increase the number of studies and patient population, both CABG and CABG+Valve were considered for PAI-1 and aldosterone. Finally, studies reported the postoperative data have also been included as long as they also showed preoperative data. Two members (M.S.K. and K.Y.) reviewed the published data in the articles and reached consensus.
Evaluation of Study Quality
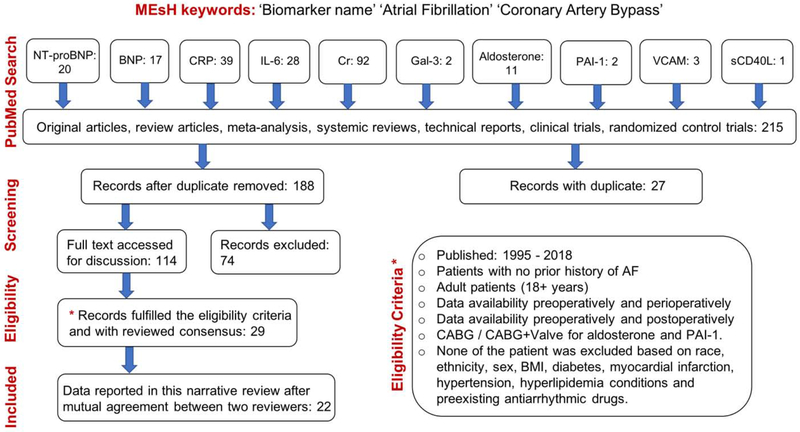
The following key points were required to ensure the study quality: clear identification of the study population, clear identification of procedure (CABG / CABG+Valve), clear identification of preoperative data and perioperative data, clear identification of standard units used for individual biomarker such as ‘mg/L’ for CRP, ‘pg/mL’ for IL-6, ‘μmol/L’ for Cr, ‘ng/mL’ for VCAM, ‘pg/ml’ for NT-proBNP and BNP, and ‘ng/mL’ for PAI-1 and sCD40L. Units were converted to standard units wherever required. Either standard variation (SD) or interquartile range (IQR) has been clearly identified for each biomarker data presented as preoperatively and perioperatively. Summary of the articles selection, data extraction and evaluation is shown in Fig. 1.
Fig. 1.
Flow diagram of selected studies included in this narrative review.
Results and Discussion
Quality of the individual biomarker has been accessed and compared for patients that develop POAF with patients that remained in sinus rhythm (SR). Variations in the biomarker-of-interest predicting POAF in patients with no previous AF episodes have been noted within the individual study and among different studies.
NT-ProBNP and BNP
In response to pressure and volume overload, the myocardium produces natriuretic peptides25. Myocyte stretch triggers the release of a precursor molecule (proBNP), which afterwards splits into B-type natriuretic peptide (BNP) and an N-terminal propeptide (NT-proBNP) 26. Preoperative blood levels for NT-proBNP in patients with cardiovascular disease, particularly those with heart failure (HF), have shown variations in comparison with patients with healthy hearts 25,26. Thus, preoperative measurements of NT-proBNP levels among CABG patients with no prior history of AF may be useful in predicting POAF risk27.
Schachner et al. conducted a large patient study (n=819) and found that patinets who had CABG surgery with preoperative NT-proBNP level >502 pg/mL were at higher risk of POAF (n=306) in comparison with those (<430 pg/mL) who remained in SR (n=513)21. Gasparovic et al. measured preoperative NT-ProBNP levels for 215 patients in SR undergoing CABG 20 and noted significantly lower concentration of preoperative NT-ProBNP in the subset of patients that maintained stable SR (273±347 pg/mL, n=160) in comparison to those that developed POAF (469±629 pg/mL, n=55; P<0.0001). Pretorius et al. reported the perioperative values for NT-proBNP with 253 patients28. NT-proBNP level was low in 73.5% of patients who remained in sinus after cardiac surgery, compared to those who developed POAF (182.0±30.6 pg/mL vs. 248.2±63.2 pg/mL, respectively, P=0.001) 28. Unlike Gasparovic et al., the blood samples collected in Pretorius et al. study were obtained immediately after separation from cardiopulmonary bypass (CPB) and processed utilizing the multiplex LINCOplex immunoassay. Thus, besides the patient risk factors and demographic information, the time required to process the collected preoperative samples is very critical in order for biomarkers to have practical utility to predict POAF. Cuthbertson et al., reported that the level of LV systolic function highly affects the preoperative NT-proBNP concentration in patients undergoing CABG surgery 29. They reported that patients (n=732) with normal LV systolic dysfunction had a median NT-proBNP level of 227 pg/mL (IQR 101–613). In comparison, those with mild systolic dysfunction had a median level of 468 pg/mL (IQR 159–1136), those with moderate dysfunction had median levels of 614 pg/mL (IQR 263–1356), and those with severe dysfunction had median levels of 818 pg/mL (IQR 565–9098).
BNP is a well-known predictive biomarker in both acute coronary syndrome and ambulatory HF patients and thus, has also been recognized as an indicator of cardiac function 30. In the setting of CABG surgery specifically, increased BNP is significantly associated with more recurrent unfavorable in-hospital cardiovascular events, and all-cause mortality after discharge 31. Other studies reported that preoperative BNP levels among CABG patients may be valuable in predicting postoperative HF hospitalization or death and decreased longer-term physical function 32,33. However, these reports did not elaborate on whether the patients had any pre-existing AF. Cuthbertson et al. reported a small study with 40 patients and had no prior record of AF 34. They found a high preoperative BNP level in 27.5% of patients who developed POAF, compared to those who remained in SR after CABG procedure (310 pg/mL (IQR 177–443) vs. 82 pg/mL (IQR 51–149), respectively, P=0.001) 34. In another study (n=187) conducted in patients with no AF history by Wazni et al., preoperative BNP concentration for CABG patients was measured to be 615 pg/mL (IQR 265–940) and 444 pg/mL (IQR 107–707) for patients with and without POAF respectively (P=0.005) 35. BNP levels reported in by Wazni et al., is about 2 times higher in comparison with the study reported by Cuthbertson et al. These differences could be due to patients’ clinical risk factors including age, left atrial diameter, left ventricular ejection fraction, chronic obstructive pulmonary disease, hypertension, and myocardial infarction as identified by Yamashita et al., in their meta-analysis study of POAF in cardiac surgery patients36.
C-Reactive Protein and lnterleukin-6
Both C-reactive protein (CRP) and interleukin-6 (IL-6) are inflammatory biomarkers and were claimed as independent POAF predictors among CABG patients. CRP is a plasma protein found in secretory cells (monocytes) and migrates to the injury site upon stimulation from stress or trauma 37. The risk of developing POAF in both CPB and off-pump CABG surgery is significantly higher in patients with high baseline CRP level (>3 mg/L)38. A large study (n=5491) including patients with no prior AF history reported by Aviles et al. revealed the possibility of utilizing the CRP level as a predictor for the presence of AF after off-pump CABG40. The median CRP value of 1.92 mg/L (IQR 0.97–3.41) was measured based on 897 subjects who developed POAF. It has been noted that subjects in higher quartiles of CRP had a more adverse event risk profile. In their report, the risk for AF was progressively higher with increasing CRP quartiles. For instance, the CRP level was higher among subjects with AF (median 2.41 mg/L, IQR 1.29–5.02 vs. 1.89 mg/L, IQR 0.95–3.37; P<0.001). Further, elevated levels for CRP, >1.5 mg/L) and IL-6, >2.2 pg/mL) were found to be a significant independent risk factor for the occurrence of POAF among patients with an increased waist circumference (>102 cm) 41.
IL-6 is a proinflammatory cytokine that plays an important role in the inflammatory cascade and is also associated with the higher risk of POAF 39. Pretorius et al. examined biomarkers in 253 patients that underwent on-pump CABG and 26% of patients developed POAF incidence 28. In their work, perioperative IL-6 level was almost twice in the POAF group than in those patients who did not develop POAF (380.6±151.1 pg/mL vs. 174.8±16.9 pg/mL). These perioperative samples were obtained immediately after separation from CPB and analyzed using multiplex LINCOplex biomarker immunoassays. In smaller study with 71 patients with no prior history of AF conducted by Canbaz et al.23, the preoperative level of CRP was found to be 23±17 mg/L in 18% of patients who developed POAF, compared to those who stayed in sinus after CABG procedure POAF (17±14 mg/L, P<0.05). The same study also reported the preoperative IL-6 level in POAF patients and was found to be 11 ±19 mg/L which was also slightly higher in comparison with those who did not develop any POAF (9±11 mg/L), P<.05 23. However, the data reported in their study was significantly higher in comparison with the study conducted by Ucar et al. with 49 patients22. The preoperative CRP concentration in Ucar et al. was measured to be 0.3±0.2 mg/L and 0.6±0.2 mg/L for patients without POAF and with POAF, P=0.03 respectively. The preoperative IL-6 concentration was measured to be 6.2±2.9 pg/mL and 7.4±3.6 pg/mL for patients without POAF and with POAF (p=0.24) respectively. They have also reported postoperative CRP and IL-6 levels before POAF onset (16.9±1.9 mg/L and 36.9±15.9 mg/L for no-POAF vs 22.4±4.1 pg/mL and 100.7±65.8 pg/mL for POAF). Cut-off points for postoperative first day IL-6, preoperative CRP, and postoperative first day CRP were 46.4 pg/mL with sensitivity of 92.9% and specificity of 80%, 0.46 mg/L with sensitivity of 71% and specificity of 75%, and 17.9 mg/L with sensitivity of 92.9% and specificity of 78%, respectively. A stepwise multivariate analysis conducted by Choi et al demonstrated that continuously elevated perioperative levels of CRP and IL-6 over the time immediately after surgery can also be used as a predictor for the possible development of POAF at day 1 21. In this study, among 315 CABG patients, 21% of patients developed POAF and their preoperative level of CRP was higher in comparison with those who remained in SR after surgery (0.66±1.27 vs. 0.47±1.14 mg/dL, P=0.25). On the other hand, a small study (n=19) conducted by Bruins et. al., reported a low preoperative CRP level of 0.23 mg/L (IQR 0.15–0.47) among CABG patients who developed POAF (37%) in comparison with 0.33 mg/L (IQR 0.15–0.47) in those who did not develop POAF 42. Another clinical study reported by Gasparovic et al. measured both the preoperative and postoperative CRP levels for 215 patients 20. They noted CRP values increased from 6±13 to 163±88 mg/L (P<.0001) in SR group and 6±16 to 163±104 mg/L (P<.0001) in the POAF group. All samples were analyzed using the Olympus AU 2700 clinical chemistry platform. However, their data did not show any significant difference in both cases (POAF vs. no-POAF).
There are other reported studies which determined that the elevated CRP levels were independent risk factors for the incidence of POAF 40,43 However, based on aforementioned reports, a large standard deviation has been noted in both CRP and IL-6 levels and a significant variation has also been identified among different reports. Further, postoperative biomarker data presented in these and many other studies could be helpful from a research perspective but may not provide any predictive utility as in many cases this timeline overlaps the onset of POAF. More research with large patient cohert will likely be needed in CABG patients with no prior history of AF to determine conclusively if CRP and IL-6 can be utilized as an effective POAF risk predictors.
Serum Creatinine
With the increasing age of cardiac surgery patients and with increasing amounts of diabetes, hypertension, CHF and arteriosclerotic disease the amount of patients with decreased renal function is inevitably increasing 44 Patients with a mildly increased preoperative level of serum creatinine (Cr) have a higher risk of developing POAF risk in the ICU, and consequently, prolonged stay in hospital as a result from POAF 44 Previous studies have shown that preoperative renal failure with creatinine level >2.5 mg/dL (>221 μmol/L) has a significant impact on early and late AF in patients undergoing CABG surgery 45 Studies have shown that in-hospital mortality has been associated with high preoperative and postoperative Cr46. Alexandre et al. conducted a study with 137 patients undergoing CABG to evaluate the effectiveness of preoperative serum Cr level predicting POAF risk24. In their work, 24.8% patients developed POAF and the reported preoperative Cr level among POAF patients was 1.15±0.45 mg/dL (101.4±39.8 μmol/L) in comparison with those who did not develop POAF (0.96± 0.26 mg/dL (84.6±23.2 μmol/L), P<0.05). Another study conducted by Gasparovic et al. measured preoperative Cr level for 215 patients in SR undergoing CABG and found lower concentration in the subset of patients that maintained stable SR after cardiac surgery (1.16+ 0.28 mg/dL (103±25 μmol/L)) in comparison to those that went on to develop immediate POAF (1.29±0.41 mg/dL (114±36 μmol/L), P=0.12) 20. Thoren et al. reported one of the largest studies on the prediction of POAF in 7115 CABG patients based on Cr level and other factors such as male gender, NYHA class III/IV, current smoking, prior myocardial infarction and the absence of hyperlipidemia. Among 2270 (32%) patients that developed POAF condition, about 43% showed a much higher preoperative serum creatinine level of ≥1.69 mg/dL (150 μmol/L) in comparison of 3.2% of the total patient population. Further, from 4845 (68%) patients who were at sinus after cardiac surgery, only 129 patients (2.7%) showed high preoperative Cr level of 1.69 mg/dL. Based on preoperative cut-off value of creatinine ≥1.69 mg/dL (150 μmol/L), ROC curve for POAF prediction after CABG showed the AUC of 0.62 47. Mirhosseini et al. conducted cross-sectional study in 200 nondiabetic male patients undergoing elective off-pump CABG surgery. Patients with POAF (n=100) and without POAF (n=100) were included in the study 48. Their preoperative serum Cr (3 days before surgery) were 1.8±0.3 mg/dL (159.12±26.52 μmol/L ) and 1.0±0.2 mg/dL (88.4±17.68 μmol/L) in POAF and no-POAF groups. However, they did not observe the increased level in Cr on the day of surgery, and reported as 1.6±0.2 mg/dL (141.44±17.68 μmol/L) and 1.1±0.5 mg/dL (97.24±44.2 μmol/L), respectively. Radmehret al. conducted a study with 892 patients 49 and they indicated that 21.8% of patients with the mild increased level of Cr 1.3 – 2.2 mg/dL (114.92 – 194.48 μmol/L) were at the high POAF risk in comparison with those who did not develop POAF and maintained lower Cr level of 0.5 – 1.2 mg/dL (44.2 – 106.08 μmol/L), P=0.001. Although high serum Cr level has been shown to be a good candidate for prediction of POAF among patients with no prior AF history, further research is required in a large CABG patient cohort to study the significance of preoperative and perioperative Cr levels.
Plasminogen activator inhibitor-1
Plasminogen activator inhibitor-1 (PAI-1) is an acute-phase reactant and serves as the primary inhibitor of a tissue-type plasminogen activator (t-PA)50. The increased PAI-1 promotes fibrosis and reduces extracellular matrix turnover, which can alter the physiology of atrial substrate and possibly lead to high risk of POAF after cardiac surgery 51. In a study of 100 patients conducted by Fawzy et al., preoperative PAI-1 level was significantly higher in patients who developed POAF (28%) than those with no POAF (8.5 ng/mL, IQR 2.3–17.5 vs. 21 ng/ml_, IQR 12–34, P<0.01) 52. PAI-1 showed a sensitivity of 71.43% and a specificity of 70.83% at preoperative cut-off value of 15 ng/mL in predicting POAF. Pretorius et al. enrolled 253 adult patients with normal SR before on-pump CABG 28. In their study, in patients who developed POAF (26.5%), the level of preoperative PAI-1 was slightly higher in collected blood samples in comparison with patients who were stayed in SR (29.0±2.1 ng/mL vs. 24.3±0.9 ng/mL, P=0.036). The PAI-1 level among patients developed POAF is almost 3.2 times higher (29.0±2.1 vs. 8.5 ng/mL) in comparison with the study published by Fawzy et al.52. Based on these small patient cohort studies, it is not possible to determine the predictive power of PAI-1 levels to identify patients at highest risk for POAF. A large patient cohort study will add more power to determine the usefulness of PAI-1 in both preoperative and perioperative serum samples of those with no prior history of AF.
Soluble vascular endothelial cells adhesion molecule-1
Soluble vascular endothelial cells adhesion molecule-1 (sVCAM-1) gene expression is regulated by the transcription factor nuclear factor-kappa B (NF-κB), which may be activated by tumor nectoris factor-α (TNF-α) and reactive oxygen species53. The release of sVCAM-1 into the circulation has been associated with a number of cardiovascular disease processes including human POAF 54 Harling et al., conducted a study with 34 patients with no prior history of AF to investigate whether the recorded changes in serum sVCAM-1 follow the development of POAF 55. Preoperative sVCAM-1 level was significantly higher (>763.5 pg/mL) in 38.2% patients developing POAF when compared to no-POAF (587.7 pg/mL), P=0.022. However, no significant difference was observed in postoperative sVCAM-1 levels between the POAF and no-POAF groups (P=0.073 and 0.135 respectively) 55. ROC analysis revealed that at cut off of 763.5 ng/mL, sVCAM-1 had a sensitivity of 60.0% and specificity of 77.27%, with an overall diagnostic accuracy of 75.2% in predicting POAF. Thus, it would be beneficial to establish a new study to record sVCAM-1 level preoperatively and immediately after the cardiac surgery. In another small study conducted by Canbaz et al. with 71 patients, 13 patients (18.3%) developed POAF after on-pump CABG surgery23. The preoperative sVCAM-1 level was higher in POAF patients than those who remained in SR (902±320 ng/mL vs. 797±293 ng/mL, P<0.05). The presented data showed significant variations and did not reveal any details that whether the samples were collected immediately after the surgery. Based on these evidences, a large patient coherts study is highly recommended to validate the broad clinical applicability of sVCAM-1 as an independent predictor of POAF in CABG patients with no prior AF history.sCD40L sCD40L is a proinflammatory and prothrombotic molecule, belonging to the tumor necrosis factor (TNF) superfamily 56 Elevated sCD40L has been found in various cardiovascular conditions that are associated with AF 57. Indeed, evidence suggests that sCD40L reflects overall platelet activation, in particular, in patients with coronary atherosclerosis58. Increased levels of sCD40L would be associated with an increased risk of microthrombi formation and related ischemia after surgery 59. In those patients with pre-existing AF, sCD40L level surpassing 476 pg/mL had a nearly 5-fold higher likelihood of suffering a vascular event60. However, the association between an increased sCD40L concentration and the post-surgery AF development was not consistently demonstrated in case-control studies61. To find the sCD40L levels in patients with no prior record of AF and underwent off-pump CABG surgery, Antoniades et al. measured sCD40L level in 144 patients the day before the surgery62. Preoperative sCD40L levels were significantly higher in 29.9% of patients who developed POAF, compared to those who remained in SR (1.89 ng/mL with IQR 1.21–2.97 vs. 0.98 ng/mL with IQR 0.63–1.31). Nevertheless, this was the only study that reported sCD40L levels of patients undergoing off-pump CABG surgery with no prior record of AF. Thus, increased sCD40L level might be used as an independent predictor of POAF development after CABG. However, further work is needed to acquire preoperative and perioperative sCD40L levels in a large patient population and compare the outcome of two groups (patients with POAF vs. without POAF).
Aldosterone and Galectin-3
Aldosterone promotes myocardial inflammation and fibrosis, modulation of ionic currents and induces oxidative stress and therefore can create a substrate for the development of POAF 63. Similarly, Galectin-3 (Gal-3), a galactoside-binding lectin, has gained much attention as a novel biomarker of cardiac fibrosis and myocardium remodeling in HF 24. Chequel et al. presented evidence that renin–angiotensin–aldosterone system and Gal-3 could be of very useful predictive biomarkers of POAF and potentially interesting therapeutic target to prevent POAF occurrence 64. Alexandre et al. conducted a study with 137 patients (109 with CABG and 28 with CABG+AVR) in which POAF occurred in 34 (24.8%) patients24. Preoperative aldosterone levels were higher in the POAF group compared with the no-POAF group (183 pmol/L (IQR 138–300) vs 143 pmol/L (IQR 96.5–216.5) with P<.01) respectively. With a cut-off value of >155 pmol/L, aldosterone revealed 67.0% specificity and 79.4% sensitivity to predict POAF. In a same study, they further evaluated the usefulness of preoperative plasma Gal-3 levels among patients with no prior AF history and identified the risk of predicting POAF. In their finding, they concluded that Gal-3 levels were only available in a subgroup of 29 patients (14 with POAF, 15 with no-POAF). In the POAF group (48.3%), the median preoperative Gal-3 level was 14.5 ng/mL (IQR 9.2–15.6), whereas, in the no-POAF group, the median Gal-3 level was 7.4 ng/mL (IQR 5.5–8.3) with P<.002 24. Interestingly, in this subgroup, plasma aldosterone levels were also higher in the POAF group in comparison with the no-POAF group, 294.5 pmol/L (IQR 190.5–381) vs 96.0 pmol/L (66.5–120.0), P<.0001. Thus, carefully conducted studies involving a larger number of patients, excluding those with a history of AF, to confirm whether Gal-3 and aldosterone reflect the prediction of POAF are highly recommended. Further, the differences in methods utilized in analyzing the samples could cause intra-institutional variability. In addition to drawing the preoperative blood at different time intervals, underpower studies with small patient cohort could cause major differences in the results.
Conclusion
This review compiles available literature on selected preoperative and perioperative biomarkers that were used to evaluate patient POAF risk after either on-pump or off-pump CABG surgery. As shown in Table 1, studies summarized in this review were those that only included patients with no previous history of AF. However, a large standard deviation in dataset within the individual studies and a significant variation among different studies were seen in most of the investigated biomarkers, thereby limiting their clinical utility to predict POAF risk with the high accuracy. Preoperative cut-off values of selected biomarkers in patients that did not develop POAF condition after cardiac surgery are shown in Table 2. Antiarrhythmic therapy for all patients undergoing cardiac surgery has been proposed as a justifiable precaution given the risks associated with POAF. Various studies have investigated the use of b-blockers, amiodarone, or magnesium to prevent POAF 66, 67, but these approaches have not been adopted widely due to the risk of giving these drugs with known side effects to a particularly vulnerable population of cardiac surgery patients. POAF remains a substantially morbid, mortal, and socioeconomic problem, and it is critical that improved strategies with high accuracy be developed to identify patients at high risk in order to develop personalized treatment strategies possibly including prophylactic interventions, closer monitoring, and minimizing the risk of developing long-term AF. Rate control and rhythm control have been also studied to investigate POAF and may provide information to improve clinical decision making for this highly prevalent condition 68.
Table 1.
Preoperative and Perioperative Biomarkers Collected From CABG Patients With No Prior History of AF
| Ref. | Biomarker Type | Total Patients | POAF Patients (%) | Surgery (CABG) | Preoperative |
Postoperative/Perioperative |
||
|---|---|---|---|---|---|---|---|---|
| Patients Without POAF | Patients With POAF | Patients Without POAF | Patients With POAF | |||||
| 27 | NT-proBNP | 819 | 37.4 | On-pump | <430 ng/mL | >502 pg/mL | NA | NA |
| 20 | NT-proBNP | 215 | 26 | On-pump | 273 ± 347 pg/mL | 469 ± 629 pg/mL | 3,110 ± 3,600 pg/mL | 4,625 ± 5,640 pg/mL |
| 65 | NT-proBNP | 253 | 26.5 | On-pump | NA | NA | 182.0 ± 30.6 pg/mL* | 248.2 ± 63.2 pg/mL* |
| 29 | NT-proBNP | 252 | 29.8 | Off-pump | 120-289 pg/mL | NA | 246 pg/mL (IQR105-747) | 388 pg/mL (IQR 150-1004) |
| 35 | BNP | 187 | 42.8 | NA | 444 pg/mL (IQR 107-707) | 615 pg/mL (IQR 265-940) | NA | NA |
| 34 | BNP | 40 | 27.5 | NA | 82 pg/mL (IQR 51-149) | 310 pg/mL (IQR 177-443) | 157 pg/mL (IQR 82-225) | 214 pg/mL (IQR 89-433) |
| 42 | CRP | 19 | 36.8 | On-pump | 0.33 mg/L (IQR 0.15-0.47) | 0.23 mg/L (IQR 0.15-0.47) | 65 mg/mL (IQR 50-75) | 51 mg/L (IQR 42-76) |
| 23 | CRP | 71 | 18.3 | On-pump | 17 ± 14 mg/L | 23 ± 17 mg/L | 45 ± 17 mg/L | 53 ± 17 mg/L |
| 21 | CRP | 315 | 20.9 | Off-pump | 0.47 ± 1.14 mg/dL | 0.66 ± 1.27 mg/ dL | NA | NA |
| 22 | CRP | 49 | 28.5 | On-pump | 0.3 ± 0.2 mg/L | 0.6 ± 0.2 mg/L | 16.9 ± 1.9 mg/L | 22.4 ± 4.1 mg/L |
| 20 | CRP | 215 | 25.6 | On-pump | 6 ± 13 mg/L | 6 ± 16 mg/L | 163 ± 188 mg/L | 163 ± 104 mg/L |
| 40 | CRP | 294 | 50† | On-pump | NA | >1.5 mg/mL† | NA | NA |
| 22 | IL-6 | 49 | 28.5 | On-pump | 6.2 ± 2.9 pg/mL | 7.4 ± 3.6 pg/mL | 36.9 ± 15.9 pg/mL | 100.7 ± 65.8 pg/mL |
| 23 | IL-6 | 71 | 18.3 | On-pump | 9 ± 11 pg/mL | 11 ± 19 pg/mL | 27 ± 37 pg/mL | 38 ± 36 pg/mL |
| 40 | IL-6 | 294 | 50† | On-pump | NA | >2.2 pg/mL† | NA | NA |
| 65 | IL-6 | 253 | 26.5 | On-pump | 3.3 ± 0.9 pg/mL | 2.7 ± 0.5 pg/mL | 174.8 ± 16.9 pg/mL* | 380.6 ± 151.1 pg/mL* |
| 24 | Creatinine | 137 | 24.8 | On-pump | 84.6 ± 23.2 μmol/L | 101.4 ± 39.8 μmol/L | NA | NA |
| 47 | Creatinine | 7115 | 32 | On/Off-pump | NA | ≥150 μmol/L | NA | NA |
| 49 | Creatinine | 892 | 27.8 | On-pump | 44.2-106 μmol/L | 114.9-194.5 μmol/L | NA | NA |
| 20 | Creatinine | 215 | 25.6 | On-pump | 103 ± 25 μmol/L | 114 ± 36 μmol/L | NA | NA |
| 52 | PAI-1‡ | 100 | 28 | On-pump | 8.5 ng/mL (IQR 2.3-17.5) | 21 ng/mL (IQR 12-34) | 12 ng/mL (IQR 6.7-23.0) | 43 ng/mL (IQR 29.5-54.5) |
| 65 | PAI-1 | 253 | 26.5 | On-pump | 24.3 ± 0.9 ng/mL | 29.0 ± 2.1 ng/mL | 14.6 ± 0.7 ng/mL* | 17.2 ± 1.2 ng/mL* |
| 55 | VCAM-1 | 34 | 38.2 | On-pump | < 587.7 ng/L | 765.5 ng/L | NA | NA |
| 28 | VCAM-1 | 253 | 26.5 | On-pump | NA | NA | 476.4 ± 12.4 ng/mL* | 514.5 ± 28.8 ng/mL* |
| 23 | VCAM-1 | 71 | 18.3 | On-pump | 797 ± 293 ng/mL | 902 ± 320 ng/mL | 924 ± 424 ng/mL | 1,021 ± 351 ng/mL |
| 24 | Aldosterone§ | 137 | 24.8 | On-pump | 143 pmol/L (IQR 96.5-216.5) | 183 pmol/L (IQR 138-300) | NA | NA |
| 24 | Galectin-3‖, | 29 | 48.3 | On-pump | 7.4 ng/mL (IQR 5.5-8.3) | 14.5 ng/mL (IQR 9.2-15.6) | NA | NA |
| 24 | Aldosterone‖, | 29 | 48.3 | On-pump | 96 pmol/L (IQR 66.5-120) | 294.5 pmol/L (IQR 190.5-381) | NA | NA |
| 62 | sCD40L | 144 | 29.9 | Off-pump | 0.98 ng/mL (IQR 0.63-1.31) | 1.89 ng/mL (IQR 1.21-2.97) | 1.97 ng/mL (IQR 1.21-3.22) | 5.62 ng/mL (IQR 3.93-5.94) |
Abbreviations: BNP, B-type natriuretic peptide; CABG, coronary artery bypass grafting; CRP, C-reactive protein; Il-6, interleukin-6; Gal-3, Galectin-3; IQR, interquartile range; NA, data not available; NT-proBNP, N-terminal pro B-type natriuretic peptide; PAI-1, plasminogen activator inhibitor-1; POAF, postoperative atrial fibrillation; sCD40L, soluble CD40 ligand; VCAM-1, vascular cell adhesion molecule-1.
Indicates data were recorded from blood samples collected immediately after separation from CPB.
Indicates nested case study: 147 patients among 2,214 were recruited who developed POAF. The presented data were compared with 147 patients as control (no POAF). Thus, 50% designated as POAF group with waist circumference of >102 cm.
Indicates CABG: 59 patients (20 with POAF), Valve (mitral/aortic/tricuspid): 38 patients (7 with POAF) and CABG + Valve patients: 3 (1 with POAF).
Indicates total patients: 137, CABG patients: 109 (27 with POAF), CABG + aortic valve replacement patients: 28 (7 with POAF).
Indicates a subgroup of 29 patients who were considered in to access the outcome for Gal-3 and aldosterone.
Table 2.
Preoperative cut-off value of selected biomarkers in patients that did not develop POAF condition after cardiac surgery
| REF. | Biomarker type | Preoperative Cut-off value |
|---|---|---|
| 29 | NT-proBNP | 204 (120–289) ng/L |
| 34 | BNP | 82 (51–149) pg/mL |
| 42 | CRP | 0.33 (0.15–0.47) mg/L |
| 22 | IL-6 | 6.2 (3.3–9.1) pg/mL |
| 49 | Creatinine | 44.2–106 μmol/L |
| 55 | sVCAM | 587.7 ng/mL |
| 24 | Gal-3 | 7.4 (5.5–8.3) ng/mL |
| 52 | PAI-1 | 8.5 (2.3–17.5) ng/mL |
| 24 | Aldosterone | 143 (96.5–216.5) pmol/L |
| 62 | sCD40L | 0.98 (0.63–1.31) |
Though numerous studies have been reported and claimed that NT-ProBNP, BNP, CRP, IL-6, creatinine, and PAI-1 could be utilized as POAF risk predictors, their preoperative results differed significantly. Conversely, VCAM-1, sCD40L, Gal-3 and aldosterone have shown promise for better POAF prediction. Unfortunately, the current dataset for these biomarkers are not of sufficient size to validate the broad clinical application of these biomarkers specifically for patients with no prior history of AF. It was also noted that recording these biomarkers postoperatively during POAF on day 2 and 3 do not provide predictive value as this timeline overlaps with the typical onset of POAF. Further, we suggest following steps for future clinical trials to test the validity of aldosterone, NT-proBNP, Cr, sVCAM, Gal-3, and sCD40L:
Multivariate and multicenter analyses are warranted to develop large CABG patient datasets (>1000).
Patients with no prior history of atrial fibrillation must be enrolled prospectively.
Patients must be consented regardless of race, ethnicity, sex, BMI, diabetes, COPD, hypertension, hyperlipidemia, PVD, PAD, myocardial infarction, PCI, TIA, CAD.
For each patient, preoperative antiarrhythmic drug and LVEF must be reported.
Patient blood samples must be collected preoperatively (24 h and 6 h before surgery) and perioperatively (during surgery before bypass).
Same technique (ELISA, flow cytometry, etc.) must be utilized to process and analyze the patient samples.
Pre- and peri-operative data must be reported in SI units for consistency.
At the end of each study, positive / negative predictive value for all the biomarkers must be highlighted.
Patients must be classified as ‘POAF’ for those who will develop atrial fibrillation within six days after cardiac surgery and ‘no-POAF’ for those who will remain at sinus rhythm after cardiac surgery.
A regression model must be developed to study the outcome of these biomarkers individually and collectively.
In addition, more comprehensive models will need to be developed that could create risk scores based on the compilation of clinical characteristics and, perhaps, an array of biologic markers.
Acknowledgment:
Research reported in this publication was supported by National Heart, Lung, and Blood Institute of the National Institutes of Health, NIH under award: R01HL128752 (DJD), a research grant from the Nora Eccles Treadwell Foundation (DJD) and American Heart Association, AHA under award: 9POST34450115 (MSK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH/AHA.
Abbreviations and Acronyms
- AF
Atrial fibrillation
- AVR
Aortic valve replacement
- BMI
Body mass index
- BNP
B-type natriuretic peptide
- CABG
Coronary artery bypass grafting
- CAD
Coronary artery disease
- COPD
Chronic obstructive pulmonary disease
- CPB
Cardiopulmonary bypass
- CVD
Cardiovascular disease
- Cr
Creatinine
- CRP
C-reactive protein
- DM
Diabetes mellitus
- EF
Ejection fraction
- Gal-3
Galectin-3
- HF
Heart failure
- ICU
Intensive care unit
- IL-6
Interleukin-6
- IQR
Interquartile range
- LVEF
Left ventricular ejection fraction
- MI
Myocardial infarction
- NA
Not available
- NT-proBNP
N-terminal pro b-type natriuretic peptide
- PAI-1
Plasminogen activator inhibitor-1
- PC
Prospective cohort
- POAF
Postoperative atrial fibrillation
- PVD
Peripheral vascular disease
- RC
Retrospective cohort
- SD
Standard deviation
- sVCAM
Soluble vascular endothelial cells adhesion molecule-1
- TNF
Tumor necrosis factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Authors declare no conflict of interest.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics-2011 update: A report from the American Heart Association. Circulation. 2011;123(4):e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ho CH, Chen YC, Chu CC, Wang JJ, Liao KM. Postoperative complications after coronary artery bypass grafting in patients with chronic obstructive pulmonary disease. Medicine (Baltimore). 2016;95(8):e2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillinov AM, Bagiella E, Moskowitz AJ, et al. Rate Control versus Rhythm Control for Atrial Fibrillation after Cardiac Surgery. N Engl J Med. 2016;374(20):1911–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathew JP, Fontes ML, Tudor IC, et al. A Multicenter Risk Index for Atrial Fibrillation After Cardiac Surgery. JAMA. 2004;291(14):1720. [DOI] [PubMed] [Google Scholar]
- 5.Villareal RP, Hariharan R, Liu BC, et al. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol. 2004;43(5):742–748. [DOI] [PubMed] [Google Scholar]
- 6.Creswell LL, Schuessler RB, Rosenbloom M, Cox JL. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993;56(3):539–549. [DOI] [PubMed] [Google Scholar]
- 7.Alqahtani A Atrial fibrillation post cardiac surgery trends toward management. Hear Views. 2010;11 (2):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Brien B, Burrage PS, Ngai JY, et al. Society of Cardiovascular Anesthesiologists/European Association of Cardiothoracic Anesthetists Practice Advisory for the Management of Perioperative Atrial Fibrillation in Patients Undergoing Cardiac Surgery. J. Cardiothorac. Vase. Anesth 2019; 33:12–26. [DOI] [PubMed] [Google Scholar]
- 9.Yin L, Ling X, Zhang Y, et al. CHADS2 and CHA2DS2-VASc Scoring Systems for Predicting Atrial Fibrillation following Cardiac Valve Surgery. PLoS One. 2015;10(4):e0123858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chua S-K, Shyu K-G, Lu M-J, et al. Renal dysfunction and the risk of postoperative atrial fibrillation after cardiac surgery: role beyond the CHA 2 DS 2 - VASc score. Europace. 2015; 17(9): 1363–1370. [DOI] [PubMed] [Google Scholar]
- 11.Gu J, Andreasen JJ, Melgaard J, et al. Preoperative Electrocardiogram Score for Predicting New-Onset Postoperative Atrial Fibrillation in Patients Undergoing Cardiac Surgery. J Cardiothorac Vase Anesth. 2016;31(1):69–76. [DOI] [PubMed] [Google Scholar]
- 12.Scarabelli CC, Faggian G. Postoperative Atrial Fibrillation is Associated with Higher Euroscore, but not with Hospital Mortality after Coronary Artery Bypass Graft Surgery. J Clin Exp Cardiolog. 2013;61(10):002. [Google Scholar]
- 13.Emren V, Aldemir M, Duygu H, et al. Usefulness of HATCH score as a predictor of atrial fibrillation after coronary artery bypass graft. Kardiol Pol. 2016;74(8):749–753. [DOI] [PubMed] [Google Scholar]
- 14.Perrier S, Meyer N, Hoang Minh T, et al. Predictors of Atrial Fibrillation After Coronary Artery Bypass Grafting: A Bayesian Analysis. Ann Thorac Surg. 2017;103(1):92–97. [DOI] [PubMed] [Google Scholar]
- 15.Bessissow A, Khan J, Devereaux PJ, Alvarez-Garcia J, Alonso-Coello P. Postoperative atrial fibrillation in non-cardiac and cardiac surgery: An overview. J Thromb Haemost. 2015;13(1):S304–S312. [DOI] [PubMed] [Google Scholar]
- 16.Borde D, Gandhe U, Hargave N, Mathew M, Joshi S, Pandey K. Prediction of postoperative atrial fibrillation after coronary artery bypass grafting surgery: Is CHA 2 DS 2 -VASc score useful? Ann Card Anaesth. 2014;17(3):182–187. [DOI] [PubMed] [Google Scholar]
- 17.Turagam MK, Mirza M, Werner PH, et al. Circulating biomarkers predictive of postoperative atrial fibrillation. Cardiol Rev. 2016;24(2):76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reckman YJ, Creemers EE. Circulating circles predict postoperative atrial fibrillation. J Am Heart Assoc. 2018;7(2):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Preeshagul I, Gharbaran R, Jeong KH, et al. Potential biomarkers for predicting outcomes in CABG cardiothoracic surgeries. J Cardiothorac Surg. 2013;8(176):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gasparovic H, Burcar I, Kopjar T, et al. NT-pro-BNP, but not C-reactive protein, is predictive of atrial fibrillation in patients undergoing coronary artery bypass surgery. Eur J Cardio-thoracic Surg. 2010;37(1):100–105. [DOI] [PubMed] [Google Scholar]
- 21.Choi YS, Shim JK, Hong SW, Kim DH, Kim JC, Kwak YL. Risk factors of atrial fibrillation following off-pump coronary artery bypass graft surgery: predictive value of C-reactive protein and transfusion requirement. Eur J Cardio-thoracic Surg. 2009;36(5):838–843. [DOI] [PubMed] [Google Scholar]
- 22.Ucar HI, Tok M, Atalar E, et al. Predictive significance of plasma levels of interleukin-6 and high-sensitivity C-reactive protein in atrial fibrillation after coronary artery bypass surgery. Heart Surg Forum. 2007;10(2):131–135. [DOI] [PubMed] [Google Scholar]
- 23.Canbaz S, Erbas H, Huseyin S, Duran E. The role of inflammation in atrial fibrillation following open heart surgery. J Int Med Res. 2008;36(5):1070–1076. [DOI] [PubMed] [Google Scholar]
- 24.Alexandre J, Saloux E, Chequel M, et al. Preoperative plasma aldosterone and the risk of atrial fibrillation after coronary artery bypass surgery: A prospective cohort study. J Hypertens. 2016;34(12):2449–2457. [DOI] [PubMed] [Google Scholar]
- 25.De Lemos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet. 2003;362(9380):316–322. [DOI] [PubMed] [Google Scholar]
- 26.Weber M, Hamm C. Role of B-type natriuretic peptide (BNP) and NT-PROBNP in clinical routine. Heart. 2006;92(6):843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schachner T, Wiedemann D, Fetz H, Laufer G, Kocher A, Bonaros N. Influence of preoperative serum N-terminal pro-brain type natriuretic peptide on the postoperative outcome and survival rates of coronary artery bypass patients. Clinics (Sao Paulo). 2010;65(12):1239–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pretorius M, Donahue BS, Yu C, Greelish JP, Roden DM, Brown NJ. Plasminogen activator inhibitor-1 as a predictor of postoperative atrial fibrillation after cardiopulmonary bypass. Circulation. 2007;116(11):1–7. [DOI] [PubMed] [Google Scholar]
- 29.Cuthbertson BH, Croal BL, Rae D, et al. N-terminal pro-B-type natriuretic peptide levels and early outcome after cardiac surgery: A prospective cohort study. Br J Anaesth. 2009;103(5):647–653. [DOI] [PubMed] [Google Scholar]
- 30.Doust JA, Pietrzak E, Dobson A, Glasziou P. How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. BMJ. 2005;330(7492):625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox AA, Muehlschlegel JD, Body SC, et al. Comparison of the Utility of Preoperative versus Postoperative B-type Natriuretic Peptide for Predicting Hospital Length of Stay and Mortality after Primary Coronary Artery Bypass Grafting. Anesthesiology. 2010;112(4):842–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen T-H, Lin C-L, Shih JJ-M, et al. Plasma B-type natriuretic peptide in predicting outcomes of elective coronary artery bypass surgery. Kaohsiung J Med Sci. 2013;29(5):254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox AA, Nascimben L, Body SC, et al. Increased Perioperative B-type Natriuretic Peptide Associates with Heart Failure Hospitalization or Heart Failure Death after Coronary Artery Bypass Graft Surgery. Anesthesiology. 2013;119(2):284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuthbertson BH, Card G, Croal BL, McNeilly J, Hillis GS. The utility of B-type natriuretic peptide in predicting postoperative cardiac events and mortality in patients undergoing major emergency non-cardiac surgery. Anaesthesia. 2007. ;62(9):875–881. [DOI] [PubMed] [Google Scholar]
- 35.Wazni OM, Martin DO, Marrouche NF, et al. Plasma B-Type Natriuretic Peptide Levels Predict Postoperative Atrial Fibrillation in Patients Undergoing Cardiac Surgery. Circulation. 2004;110(2):124–127. [DOI] [PubMed] [Google Scholar]
- 36.Yamashita K, Selzman C, Ranjan R, Hu N, Dosdall D. Clinical Risk Factors for Post-operative Atrial Fibrillation among Patients after Cardiac Surgery. Thorac Cardiovasc Surg. 2019;67(2):107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mogues T, Etzerodt M, Hall C, Engelich G, Graversen JH, Hartshorn KL. Tetranectin binds to the kringle 1–4 form of angiostatin and modifies its functional activity. J Biomed Biotechnol. 2004;2004(2):73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lo B, Fijnheer R, Nierich AP, Bruins P, Kalkman CJ. C-Reactive Protein is a Risk Indicator for Atrial Fibrillation After Myocardial Revascularization. Ann Thorac Surg. 2005;79(5):1530–1535. [DOI] [PubMed] [Google Scholar]
- 39.Gaudino M, Andreotti F, Zamparelli R, et al. The -174G/C Interleukin-6 Polymorphism Influences Postoperative Interleukin-6 Levels and Postoperative Atrial Fibrillation. Is Atrial Fibrillation an Inflammatory Complication? Circulation. 2003;108(10):195–199. [DOI] [PubMed] [Google Scholar]
- 40.Aviles RJ, Martin DO, Apperson-Hansen C, et al. Inflammation as a Risk Factor for Atrial Fibrillation. Circulation. 2003;108(24):3006–3010. [DOI] [PubMed] [Google Scholar]
- 41.Girerd N, Pibarot P, Fournier D, et al. Middle-aged men with increased waist circumference and elevated C-reactive protein level are at higher risk for postoperative atrial fibrillation following coronary artery bypass grafting surgery. Eur Heart J. 2009;30(10):1270–1278. [DOI] [PubMed] [Google Scholar]
- 42.Bruins P, Te Velthuis H, Yazdanbakhsh AP, et al. Activation of the complement system during and after cardiopulmonary bypass surgery: Postsurgery activation involves c-reactive protein and is associated with postoperative arrhythmia. Circulation. 1997;96(10):3542–3548. [DOI] [PubMed] [Google Scholar]
- 43.Marott SCW, Nordestgaard BG, Zacho J, et al. Does elevated C-reactive protein increase atrial fibrillation risk? A mendelian randomization of 47,000 individuals from the general population. J Am Coll Cardiol. 2010;56(10):789–795. [DOI] [PubMed] [Google Scholar]
- 44.Jyrala A, Weiss RE, Jeffries RA, Kay GL. Effect of mild renal dysfunction (s-crea 1.2-2.2 mg/dl) on presentation characteristics and short- and long-term outcomes of on-pump cardiac surgery patients. Interact Cardiovasc Thorac Surg. 2010;10(5):777–782. [DOI] [PubMed] [Google Scholar]
- 45.Filsoufi F, Rahmanian PB, Castillo JG, Chikwe J, Carpentier A, Adams DH. Early and late outcomes of cardiac surgery in patients with moderate to severe preoperative renal dysfunction without dialysis. Interact Cardiovasc Thorac Surg. 2008;7(1):90–95. [DOI] [PubMed] [Google Scholar]
- 46.Tolpin DA, Collard CD, Lee V-V, et al. Subclinical changes in serum creatinine and mortality after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2012;143(3):682–688.e1. [DOI] [PubMed] [Google Scholar]
- 47.Thoren E, Hellgren L, Jideus L, Stahle E. Prediction of postoperative atrial fibrillation in a large coronary artery bypass grafting cohort. Interact Cardiovasc Thorac Surg. 2012;14(5):588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mirhosseini SJ, Ali-Hassan-Sayegh S, Karimi-Bondarabadi AA, Mozayan MR. Can preoperative serum level of creatinine predict new-onset atrial fibrillation in non-diabetic male patients undergoing open heart surgery? A retrograde view. Acta Med Iran. 2013;51(12):861–863. [PubMed] [Google Scholar]
- 49.Radmehr H, Forouzannia SK, Bakhshandeh AR, Sanatkar M. Relation between preoperative mild increased in serum creatinine level and early outcomes after coronary artery bypass grafting. Acta Med Iran. 2011;49(2):89–92. [PubMed] [Google Scholar]
- 50.Rerolle J-P, Hertig A, Nguyen G, Sraer J-D, Rondeau EP. Plasminogen activator inhibitor type 1 is a potential target in renal fibrogenesis. Kidney Int. 2000;58(5):1841–1850. [DOI] [PubMed] [Google Scholar]
- 51.Kaikita K, Fogo AB, Ma L, Schoenhard JA, Brown NJ, Vaughan DE. Plasminogen activator inhibitor-1 deficiency prevents hypertension and vascular fibrosis in response to long-term nitric oxide synthase inhibition. Circulation. 2001. ;104(7):839–844. [DOI] [PubMed] [Google Scholar]
- 52.Fawzy S, Abdel Aleem M, El Badawy M, Mohamed I. Plasminogen activator inhibitor-1 as a predictor after cardiopulmonary bypass for postoperative atrial fibrillation. Res Opin Anesth Intensive Care. 2018;5(1):27–34. [Google Scholar]
- 53.Marui N, Offermann MK, Swerlick R, et al. Vascular cell adhesion molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells. J Clin Invest. 1993;92(4):1866–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verdejo H, Roldan J, Garcia L, et al. Systemic vascular cell adhesion molecule-1 predicts the occurrence of post-operative atrial fibrillation. Int J Cardiol. 2011;150(3):270–276. [DOI] [PubMed] [Google Scholar]
- 55.Harling L, Lambert J, Ashrafian H, Darzi A, Gooderham NJ, Athanasiou T. Preoperative serum VCAM-1 as a biomarker of atrial fibrillation after coronary artery bypass grafting. J Cardiothorac Surg. 2017;12(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kroczek RA, Henn V, Slupsky JR, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391 (6667):591–594. [DOI] [PubMed] [Google Scholar]
- 57.Pamukcu B, Lip GYH, Snezhitskiy V, Shantsila E. The CD40-CD40L system in cardiovascular disease. Ann Med. 2011;43(5):331–340. [DOI] [PubMed] [Google Scholar]
- 58.Tousoulis D, Antoniades C, Nikolopoulou A, et al. Interaction between cytokines and sCD40L in patients with stable and unstable coronary syndromes. Eur J Clin Invest. 2007;37(8):623–628. [DOI] [PubMed] [Google Scholar]
- 59.Choudhury A, Freestone B, Patel J, Lip GYH. Relationship of Soluble CD40 Ligand to Vascular Endothelial Growth Factor, Angiopoietins, and Tissue Factor in Atrial Fibrillation. Chest. 2007;132(6):1913–1919. [DOI] [PubMed] [Google Scholar]
- 60.Ferro D, Loffredo L, Polimeni L, et al. Soluble CD40 ligand predicts ischemic stroke and myocardial infarction in patients with nonvalvular atrial fibrillation. Arterioscler Thromb Vasc Biol. 2007;27(12):2763–2768. [DOI] [PubMed] [Google Scholar]
- 61.Choudhury A, Chung I, Panja N, Patel J, Lip GYH. Soluble CD40 Ligand, Platelet Surface CD40 Ligand, and Total Platelet CD40 Ligand in Atrial Fibrillation: Relationship to Soluble P-Selectin, Stroke Risk Factors, and Risk Factor Intervention. Chest. 2008;134(3):574–581. [DOI] [PubMed] [Google Scholar]
- 62.Antoniades C, Van-Assche T, Shirodaria C, et al. Preoperative sCD40L levels predict risk of atrial fibrillation after off-pump coronary artery bypass graft surgery. Circulation. 2009;120(11 ):170–176. [DOI] [PubMed] [Google Scholar]
- 63.Brown NJ. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat Rev Nephrol. 2013;9(8):459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chequel M, Ollitrault P, Saloux E, et al. Preoperative Plasma Aldosterone Levels and Postoperative Atrial Fibrillation Occurrence Following Cardiac Surgery: A Review of Literature and Design of the ALDO-POAF Study (ALDOsterone for Prediction of Post-Operative Atrial Fibrillation). Curr Clin Pharmacol. 2016;11 (3):150–158. [DOI] [PubMed] [Google Scholar]
- 65.Pretorius M, Donahue BS, Yu C, Greelish JP, Roden DM, Brown NJ. Plasminogen activator inhibitor-1 as a predictor of postoperative atrial fibrillation after cardiopulmonary bypass. Circulation. 2007;116(11):1–7. [DOI] [PubMed] [Google Scholar]
- 66.Yao X, Gersh BJ, Holmes DR Jr, Melduni RM,et al. Association of Surgical Left Atrial Appendage Occlusion With Subsequent Stroke and Mortality Among Patients Undergoing Cardiac Surgery. JAMA. 2018;319(20):2116–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith H, Yeung C, Gowing S, Sadek M, et al. A review and analysis of strategies for prediction, prevention and management of post-operative atrial fibrillation after non-cardiac thoracic surgery. J Thorac Dis. 2018;10(Suppl 32):S3799–S808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gillinov AM, Bagiella E, Moskowitz AJ, et al. Rate Control versus Rhythm Control for Atrial Fibrillation after Cardiac Surgery. N. Engl. J. Med. 2016; 374:1911–21. [DOI] [PMC free article] [PubMed] [Google Scholar]