Abstract
A global increase in the number of individuals that are either overweight or obese is leading to a higher incidence of type 2 diabetes (T2D). Behavioral interventions for the treatment of obesity have yet to deliver desired outcomes, thus introducing a pressing need for molecular- and cellular-based therapies. Excess energy from food is stored in the form of triglycerides in white adipose tissue (WAT), which expands during weight gain and can lead to insulin resistance and T2D. In contrast, brown adipose tissue (BAT) releases energy from metabolic substrates in the form of heat and secretes factors that can reverse metabolic disease by acting systemically. Therefore, the ability to increase BAT activity represents a promising approach to improve energy balance and metabolic homeostasis. Methods are now being developed to generate brown adipocytes from human induced pluripotent stem cells (hiPSCs), which would (1) provide an unlimited source of cellular material to study human brown adipogenesis, and (2) could be used to develop drug- and cell-based therapies for the treatment of metabolic complications associated with obesity. This article reviews basic BAT biology and details the current progress toward developing brown adipocytes from hiPSCs.
Keywords: adipogenesis, brown adipocytes, UCP1, metabolic syndrome, diabetes, mesoderm
Graphical abstract
The ability to reprogram adult somatic cells into human induced pluripotent stem cells (hiPSCs) represents a key discovery for cell-based therapies and has the potential to cure a wide range of diseases, including those associated with obesity. In this review, I will give an overview of brown adipogenesis and focus on the current state of methods that have been developed to generate thermogenically-active adipocytes from hiPSCs.
Introduction
Modern humans are exposed to an ever-increasing number of calories that are densely packed into high-energy yielding foods. Since the early 1960s and perhaps earlier, changes in diet coupled with a lifestyle that minimizes physical activity and maximizes sedentary behavior has led to a surge in the number of individuals that are either overweight or obese. According to the National Center for Health Statistics, in the United States 39.8% of adults were obese in 2015–2016, compared with just 13.4% surveyed between 1960 and 1962.1, 2 Such a statistic is not without consequence, as obesity is associated with an increased risk for a number of diseases including diabetes, stroke, heart disease, osteoarthritis, certain cancers, and depression.3 Regarding diabetes, in 2015 the U. S. Centers for Disease Control and Prevention estimated that the rate of diabetes and pre-diabetes among adults was 9.4% and 33.9%, respectively.4 Of those with diabetes, 87.5% were overweight or obese, making body weight the single largest risk factor.4 The number of individuals diagnosed with diabetes also increases with age, with those 65 and older having the highest rate (25.2%).4
As people get older, why do they find it harder to remain lean or lose weight? One reason may be loss of active brown adipose tissue (BAT), which declines progressively as people age, potentially contributing to a slower metabolism and increased weight gain.5, 6 Currently, our understanding of human BAT biology remains incomplete and there is a critical need to generate precursor cells to study brown adipogenesis and develop drug- and cell-based therapies. The ability to reprogram adult somatic cells into human induced pluripotent stem cells (hiPSCs) represents a key discovery for cell-based therapies and has the potential to cure a wide range of diseases, including those associated with obesity.7
In this review, I will give an overview of brown adipogenesis and focus on the current state of methods that have been developed to generate thermogenically-active adipocytes from hiPSCs.
The promise of brown adipose tissue as a therapeutic target tissue
Numerous types of adipose tissue are dispersed throughout the human body and serve important biological functions including protection from cold, structural support to organs, and importantly, the regulation of energy balance and metabolic homeostasis.8 White adipose tissue (WAT) stores energy that is utilized during periods of increased energy demand, such as high physical activity or fasting, when the body must rely on energy reserves. During chronic periods of overeating and limited physical activity, excess energy is stored as fats in WAT, and WAT accumulation in overweight individuals correlates with disease. Specifically, too much WAT in the deep abdominal region (known as visceral adipose) is highly correlated with the development of insulin resistance leading to type 2 diabetes (T2D).9
Attempts to address the public health risk of obesity have largely failed and researchers are now focusing on BAT, which is a promising therapeutic target tissue for treating human obesity and related metabolic disorders.10 During cold exposure, BAT regulates body temperature by generating heat through the release of energy stored in lipids and glucose. This process, known as non-shivering thermogenesis, results from sympathetic nervous system activation of BAT (reviewed in Ref. 11). BAT in humans was thought to primarily play a role in preventing hypothermia in newborns, which are susceptible to heat loss due to their high surface area to body mass ratio when compared to adults.11 However, in the past 10 years new imaging technologies have demonstrated that adult humans also possess active BAT, thus increasing our interest in targeting this tissue to treat obesity and its comorbidities.12–14 BAT activation by cold acclimation in humans improves glucose homeostasis and increases insulin sensitivity in patients with obesity and T2D.15–17 Additionally, BAT activity is inversely correlated with body mass index in humans5, 13 and exhibits anti-obesity effects in mice as well (reviewed in Ref. 19).
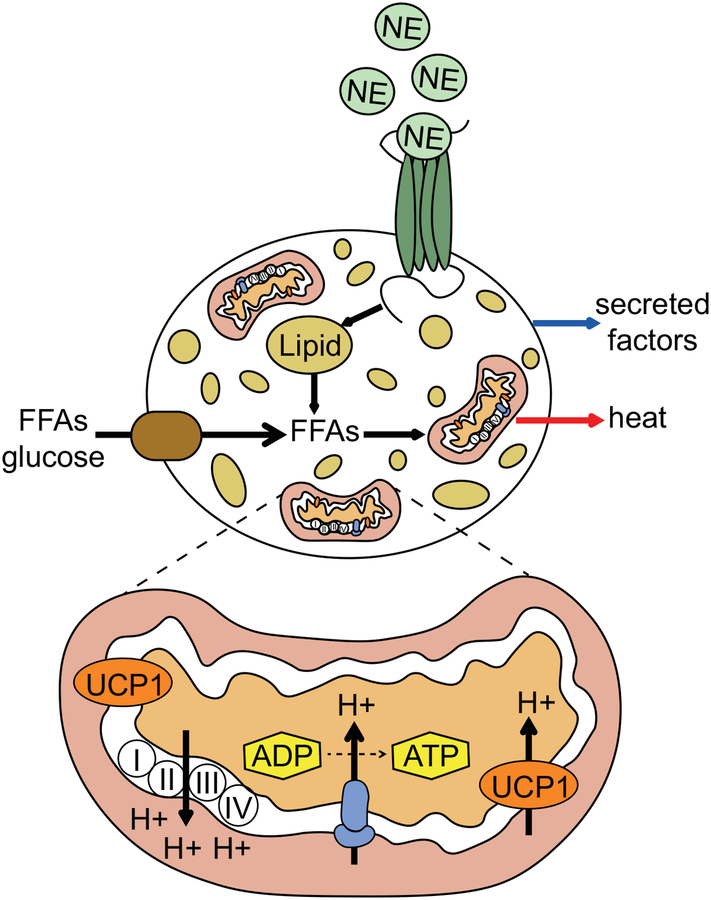
Brown adipocytes can be classified into two distinct subtypes. Classical brown adipocytes develop during the fetal period as a permanent tissue in the interscapular region in rodents and humans and are found mainly in the paravertebral, axillary and supraclavicular regions in adults.18–21 Functionally similar brown adipocytes, known as beige, can be induced predominantly in adult subcutaneous WAT (sWAT) in response to thermogenic activators of the sympathetic nervous system, such as cold and β-adrenergic receptor agonists.22, 23 Recruitment of beige adipocytes determines obesity resistance in specific strains of mice and rats, suggesting that this tissue, in addition to classical BAT, may also represent a therapeutic target for anti-obesity therapies.24–27 Both classical brown and beige adipocytes have multilocular lipid droplet morphology, high mitochondrial content and express a core set of brown fat-specific genes, such as uncoupling protein-1 (UCP1), which when activated increase thermogenesis and energy expenditure through proton leakage across the inner mitochondrial membrane (Fig. 1).28 In addition to UCP1-dependent thermogenesis, UCP1-independent mechanisms also exist in thermogenic adipocytes for heat generation, including creatine-driven substrate and SERCA2b-mediated calcium futile cycling.29, 30
Figure 1:
Activation of brown and beige adipocytes. During cold exposure norepinephrine (NE), secreted from sympathetic nerves, binds to β-adrenergic receptors (dark green) at the plasma membrane surface of brown and beige adipocytes. This increases cAMP and activates protein kinase A, triggering the breakdown of triglycerides within lipid droplets into free fatty acids (FFAs). Additionally, activated brown adipocytes increase uptake of FFAs and glucose from outside the cell. FFAs are transported into the mitochondria where they activate UCP1, which results in proton leak across the inner mitochondrial membrane and heat release. Activated brown adipocytes can also secrete factors that act locally or systemically to improve metabolic homeostasis.
Therapeutic advantages of hiPSC-derived brown and beige adipocytes
The derivation of brown and beige adipocytes from hiPSCs opens the door to study adipocyte development and can provide a source of cells for drug testing and cell-based transplantation therapies. However, a number of issues need to be addressed before adipocytes derived from hiPSCs can be used in the treatment of metabolic syndrome. These include the efficiency with which the adipocytes can be generated, proper functional and physiological properties, cell delivery into patients, and quality control and safety issues.31 Despite these challenges, much enthusiasm surrounds the successful use of hiPSC-derived brown and beige adipocytes for cellular-based therapies, which stems from a number of experiments showing that BAT transplantation can reverse metabolic disease in mice.32–34 One potential lesson from these mouse models is that secreted factors from the transplant may play a major role in reversing metabolic disease by acting in an endocrine manner to increase the activity of endogenous BAT.35 From a clinical standpoint, endogenous BAT represents a small fraction of adipose tissue in humans and is surgically difficult to obtain. BAT volume also decreases with increasing age and obesity and isolated adipogenic precursors have limited proliferation and differentiation potential with continued cell culture passaging.5, 6, 36, 37 Furthermore, while subcutaneous beige adipogenic precursors from obese or aged individuals are easier to procure than are precursors from BAT, they also lack significant expansion potential and ability to fully differentiate into beige adipocytes.38–41 Thus, in the patients that would benefit the most, the ability to isolate and expand primary autologous cells to generate material for therapeutic development is low. The recent development of human brown and beige adipocyte cellular models from hiPSCs brings forth new prospects for solving problems associated with surgically obtained adipogenic precursors, because adipogenic precursors derived from hiPSCs could be used to generate a virtually inexhaustible supply of cells for both research and clinical uses.42
Induction of adipogenic precursors into mature adipocytes
Adipocyte precursors, also known as preadipocytes, are derived from multipotent mesenchymal stem cells (MSCs) that are located within the stromal vascular cell compartment of adipose tissue.43 The first phase of adipocyte differentiation from MSCs to a committed preadipocyte and the extracellular cues that allow this to occur are poorly understood. However, studies have shown that bone morphogenetic proteins (BMP) 4 and BMP7 can induce commitment of MSCs to either a white or brown preadipocyte fate, respectively.44, 45 Terminal differentiation of committed preadipocytes into mature, lipid-containing adipocytes involves a cascade of transcriptional events mainly regulated by CCAAT-enhancer-binding proteins (CEBPA and CEBPB) and peroxisome proliferator-activated receptor gamma (PPARγ).46, 47 Ultimately, mature adipocytes acquire the necessary subcellular components for lipid and glucose transport, insulin responsiveness, and the secretion of factors that act to control whole body–mediated metabolic homeostasis.46 Brown and beige adipocytes have a specialized set of transcription factors that regulate their differentiation and function, and that distinguish them from white adipocytes, including the expression of PR domain zinc finger protein 16 (PRDM16), early B cell factor 2 (EBF2), and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) (reviewed in Ref. 47). These transcription factors coordinate with PPARγ to regulate brown and beige cell fate determination, and they are critical for increased expression of UCP1 and activation of the UCP1-mediated response to β-adrenergic receptor stimuli.
Differences between brown and beige adipocytes
Both brown and beige adipocytes are functionally similar and have the thermogenic potential to increase energy expenditure, yet there are a number of molecular markers that can be used to distinguish them. While the adipocyte specific function of many of these markers is unknown, their presence may be related to distinct developmental origins from which these cells have arisen. For example, brown preadipocytes isolated from interscapular BAT express a myogenic-specific gene expression signature that is absent from most beige preadipocytes isolated from sWAT.48 The myogenic signature likely results from the similar developmental origins (paraxial mesoderm) of BAT and skeletal muscle.49 In addition, brown adipocytes display higher expression of the transcription factor ZIC1, which unexpectedly functions as negative regulator of brown adipogenesis, and correlates negatively with increased BAT activity.50, 51 In mice, several markers show enriched expression in beige adipocytes compared to brown adipocytes, such as TMEM26, CD137, and TBX1, although no beige-specific function has been ascribed to these genes.52 Interestingly, certain BAT depots in adult humans show a marker profile similar to murine beige adipose tissue (positive for TMEM26, CD137, and TBX1).52, 53 Analysis of molecular markers from superficial human neck adipose tissue demonstrates a molecular pattern that resembles white and beige adipose tissue, while those from the deeper neck regions display a classical brown and beige pattern.54 To begin to understand whether this heterogeneity is due to a mixture of cell types, clonal analysis was performed on UCP1-positive supraclavicular BAT biopsies, which showed a profile more similar to murine beige adipocytes.55 However, the authors noted that their results may be biased due to the selection of highly proliferative cells during the clonal selection process. Together, these results suggest that human adult thermogenic adipose tissue likely displays a molecular profile more consistent with beige rather than classical brown adipose tissue, although whether this holds true for human fetal or neonatal BAT has not yet been described.
Functionally, one difference between brown and beige adipocytes is that beige adipocytes switch between brown- and white-like states and lose UCP1 expression upon withdrawal of either cold or β-adrenergic receptor stimuli.56 This appears to be due to several factors, including glucocorticoid receptor stimulation of Zfp423 expression, a known anti-beige adipocyte transcription factor, as well as an autophagy-mediated clearance pathway for mitochondria in beige cells that may be more limited in brown adipocytes.56, 57 Furthermore, in mice there exists heterogeneity in the function within the beige adipocyte lineage. A recent study has described a new type of beige adipocyte that is developmentally distinct in origin and function, and it can compensate in the absence of β-adrenergic signaling to provide energy homeostasis through enhanced glucose oxidation during cold adaptation.58 These cells are termed glycolytic beige fat, to distinguish them from cold-induced canonical beige fat that is normally stimulated through β-adrenergic signaling.
Embryonic origins of adipocytes
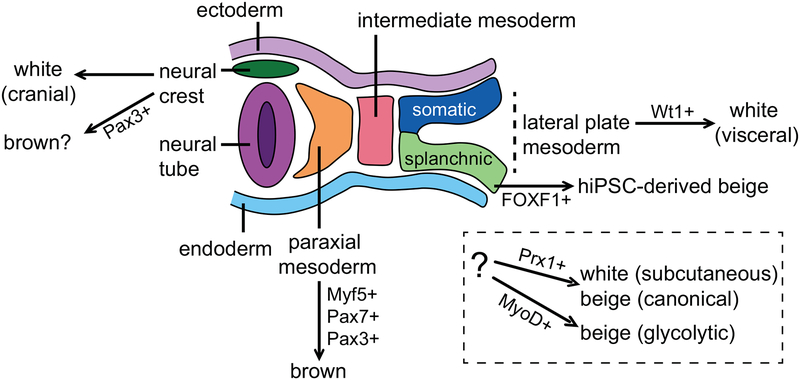
Much of the information regarding the developmental precursor lineages that give rise to adipocytes is derived from lineage tracing studies in mice. In humans, the early developmental steps leading to specific adipocyte populations are unclear, and human embryonic stem cells (hESCs) and hiPSCs will provide a unique opportunity to gain clarity into these early developmental events. Adipocytes, including other mesenchymal tissues (muscle, cartilage and bone) arise from the mesodermal layers that populate the space between the endoderm and ectoderm of the developing embryo (Fig. 2). Interscapular BAT, like skeletal muscle, derives from paraxial mesoderm precursors positive for Myf5, Pax7, and Pax3 (reviewed in Refs. 56,57). The closely related origin of BAT and skeletal muscle is also reflected in the expression of genes, such as PRDM16, that can be manipulated genetically to functionally toggle the cell-fate between muscle cells and brown adipocytes.59 While nearly all brown adipocytes found in interscapular BAT arise from the paraxial Myf5+/+ lineage, only half of the brown adipocytes found in cervical BAT are Myf5+/+ derived, while none of the brown adipocytes found in the peri-renal and peri-aortic BAT depots are derived from a Myf5+/+ mesodermal precursor.60
Figure 2:
Embryonic origins of adipocytes. Lineage tracing studies in mice demonstrate that white and brown adipocytes arise from different mesodermal layers during development, including the lateral plate (visceral white) and paraxial mesoderm (brown). In addition, some adipocytes are derived from the neural crest. The ultimate developmental origins of subcutaneous white and beige adipocytes are currently under investigation.
In the adult, white adipocytes arise from mural cells residing in the adipose vasculature, and it is generally accepted that they derive from a Myf5–/– precursor, potentially of lateral plate mesoderm origin.61, 62 Lineage tracing studies have demonstrated that a Wt1+/+ precursor population present within the lateral plate mesoderm generates a significant portion of all visceral WAT depots, whereas there is no Wt1+/+ precursor contribution to either BAT or sWAT.63 A number of studies have also demonstrated that at least some white adipocytes arise from a Myf5+/+ precursor pool, contradicting earlier hypotheses that only brown adipocytes arise from paraxial mesoderm.64, 65 Like white adipocytes, beige adipocytes also derive from progenitors that reside in the adipose tissue vasculature, however, it is currently not understood whether white and beige adipocytes arise from the same or distinct lineages of embryonic precursors.66 Lineage tracing with Myf5-Cre mice shows that beige adipocytes can arise from either Myf5+/+ or Myf5–/– lineages, depending on the specific location of the adipose depot.64 In the adult, subcutaneous white and beige adipocytes can both arise from a common Prx1+/+ precursor population found in the stromal vascular fraction, however, whether these precursors trace back to a single embryonic tissue of origin is unknown.67, 68 In addition, glycolytic beige fat, which can compensate to restore energy expenditure when β-adrenergic signaling is impaired or during chronic severe cold conditions, derives from a distinct Myod-derived beige precursor population found in the stromal vascular fraction.58 Finally, it should be mentioned that not all adipocytes arise from within the mesoderm, and some adipocytes, such as those found in the cranial regions, can be derived from the neural crest.69
Taken together as a whole, lineage-tracing data from mouse studies indicates a complex and heterogeneous embryonic origin for white, brown and beige adipocytes that resolves into more concrete patterns of embryonic inheritance depending on the future anatomical location of the mature adipose depot found in the adult. Though information regarding the developmental origins of adipocytes in mice is starting to become clearer, we are only beginning to understand if similar developmental patterns are seen in humans. As detailed below, the generation of distinct adipocyte lineages from hiPSCs is already beginning to contribute toward unraveling the developmental complexity of adipocyte origins. Conversely, understanding the origins of adipocytes during embryonic development in rodents will aid in identifying new markers and developmental pathways that can support the generation of patient-specific brown or beige adipocytes from hiPSCs.
Generation of adipocytes from hiPSCs
The main obstacle for generating adipocytes from hiPSCs has been the development of efficient methods for their differentiation. Information gleaned from developmental studies in mice and advanced tissue culture techniques, including new medium formulations and genetic engineering techniques have all been applied to overcome this obstacle. A number of earlier studies have demonstrated the generation of adipocytes from hESCs.70–72 Here, we will focus on the latest methods that have been developed over the past 10 years for the differentiation of hiPSCs into either white, brown or beige adipocytes (Table 1) and discuss the current limitations and potential improvements needed to advance this area of research.
Table 1:
Summary of methods used to generate adipocytes from hiPSCs
| Study | Technique | Mesoderm induction | Mesoderm markers | MSC phenotype | Adipocyte | Adipogenesis induction |
|---|---|---|---|---|---|---|
| Taura et al.73 | EB outgrowth | ES cell culture medium w/out FGF2 | Unknown | Unknown | Unknown | INS, IBMX, Dex, Indo, pioglitazone |
| Ahfeldt et al.37 | EB outgrowth, transgene | DMEM, 15% FBS | GSC/TBX3 | CD73, CD105 | White | INS, Dex, Rosi, PPARG transgene |
| Ahfeldt et al.37 | EB outgrowth, transgene | DMEM, 15% FBS | GSC/TBX3 | CD73, CD105 | Brown | INS, Dex, Rosi, PPARG + CEBPB transgenes |
| Nishio et al.76 | EB outgrowth | BPEL medium, BMP4, VEGFA, KITLG FLT3LG, IL-6, IGF-II | MYF5, PDFGRα, PAX7, PAX3 | Unknown | Brown | BMP7, VEGFA, KITLG FLT3LG, IL-6, IGF-2 |
| Mohsen-Kanson, et al.77 | EB outgrowth | DMEM/F12/20% KSR + retinoic acid | Unknown | CD73, CD105, CD90, CD146, PAX3 | White | INS, IBMX, Dex, T3, Rosi |
| Mohsen-Kanson, et al.77 | EB outgrowth | DMEM/F12/20% KSR | PAX3? | CD73, CD105, CD90, CD146, PAX3 | Brown | INS, IBMX, Dex, T3, Rosi |
| Hafner et al.80 | EB outgrowth | DMEM/F12/20% KSR | PAX3? | CD73, CD105, CD90 | Brown and Beige | INS, IBMX, Dex, T3, Rosi, SB431542, ascorbic acid, hydrocortisone, EGF |
| Guénantin et al.84 | Monolayer | STEMPro34 HSC medium + BMP4, Activin A | TBOX, MESP1 | PDGFRα, CD44, CD29 and LY6E | Beige | INS, IBMX, Dexa, Indo |
| Su et al.80 | Monolayer | STEMdiff™ Mesoderm Induction Medium | FOXF1, BAPX1, HOX11 | CD73, CD105, CD90, CD146, PDGFRβ, NG2 | Beige | INS, IBMX, Dex, Indo, T3, Rosi, SB431542, ascorbic acid, hydrocortisone, EGF |
EB – embryoid body, INS – insulin, Dex – dexamethasone, Indo – indomethacin, Rosi – Rosiglitazone, BPEL - Bovine Serum Albumin (BSA) Polyvinylalchohol Essential Lipids Medium, HSC – hematopoietic stem cell.
Generation of adipocytes from hiPSCs via embryoid body formation
With a potential goal for treatment of lypodystrophic patients, which lack adipose tissue and display severe insulin resistance, the Nakao group was the first to show that adipocyte-like cells could be generated from hiPSCs in 2009.73 This group took advantage of embryoid body (EB) formation, a 3D suspension culture technique routinely used to differentiate pluripotent stem cells toward the three germ lineages, which occurs due to cell adhesions and paracrine signaling within the EB microenvironment. After 12 days of suspension culture on non-adherent culture dishes in the presence of transient retinoic acid treatment (RA), the EBs were transferred to fibronectin and poly-L-ornithine coated culture plates where they formed outgrowths of adherent cells. Differentiation of outgrowths into adipocytes was then achieved using a cocktail of adipogenic factors commonly used to induce the differentiation of primary preadipocytes isolated from mouse or human adipose tissue (Table 1). Using this method, approximately 15% of the cells found in 50% of the EB colonies were positive for lipid-containing adipocytes as determined by Oil Red O staining. Compared to the undifferentiated hiPSCs, the hiPSC-derived adipocytes expressed markers of mature adipocytes including PPARγ2, CEBPA, fatty-acid-binding protein 4 (FABP4) and leptin (LEP). It is unclear whether these cells represented white, brown or beige adipocytes, as markers that distinguish between them were not analyzed. Importantly, they showed that multiple lines of hiPSCs were as efficient as hESCs in the generation of adipocytes, paving the way for potential adipocyte-based therapies from hiPSCs.
Transgenic induction of white and brown adipocytes from hiPSCs
In 2012, the Cowan group used EB formation and subsequent outgrowth on adherent culture plates to generate MSCs positive for CD73 and CD105 with the potential to differentiate into osteoblasts, chondrocytes and adipocytes.37 Adipogenesis was extremely low (3–10% of total cells), but when the MSCs were transduced with a doxycycline inducible PPARγ2 and then treated with an adipogenic cocktail, they found that 88% of the PPARγ2-programmed cells formed adipocytes. Morphologically, the adipocytes were unilocular and expressed CEBPA, FABP4 and hormone-sensitive lipase (HSL), consistent with formation of white adipocytes. Furthermore, the adipocytes could be maintained for an additional 4 weeks after removal of doxycycline, suggesting that a pulse of PPARγ2 expression could permanently switch the fate of the MSCs to the white adipocyte lineage. Microarray and lipid analysis showed a high concordance between the PPARγ2-programmed and primary adipose tissue-derived white adipocytes. Functionally, the programmed white adipocytes were able to secrete adiponectin (ADIPOQ) and LEP proteins and could breakdown triglycerides upon treatment with the β3-adrenergic receptor agonist isoproterenol. The white adipocytes were also able to respond to insulin, as determined by phosphorylation of AKT and glucose uptake. Lastly, they also developed insulin resistance in the presence of free fatty acids, suggesting that these programmed white adipocytes could be used to model human insulin resistance.
To make brown adipocytes, the Cowan group overexpressed PPARγ2, CEBPB and PRDM16, factors previously shown to induce primary-derived human and mouse fibroblasts into brown-like adipocytes.37, 74 After 21 days, the resulting adipocytes had a multilocular lipid droplet morphology, high mitochondrial content and strong cytoplasmic UCP1 staining, consistent with the formation of brown adipocytes. Microarray analysis of the iPSC-derived brown adipocytes showed that gene expression signatures clustered distinctly from both the programmed and primary adipose generated white adipocytes and expressed markers previously associated with BAT.75 Consistent with UCP1 expression and high mitochondrial activity, the programmed brown adipocytes displayed a higher maximal oxygen consumption rate (OCR) and increased proton leak-linked respiration compared to the programmed white adipocytes. Interestingly, they found that the PRDM16 transgene was expendable for brown adipocyte generation, possibly due to presence endogenous PRDM16 expression that was detected in the non-transduced MSCs. When the hiPSC-derived brown adipocytes were transplanted subcutaneously into Rag2–/– Il2γc–/– mice, they continued to express UCP1 after 4–6 weeks and were able to uptake 18F-fluordeoxyglucose (18F-FDG) as determined by positron-emission tomography computed tomography (PET/CT), a technology routinely used for BAT detection. These results demonstrate that MSCs generated from hiPSCs have a low adipogenic capacity and, in the absence of other unknown factors (described below and Refs. 72 and 73), require genetic enhancement with adipogenic regulatory genes for sufficient adipogenesis. This method also demonstrates that transgenic overexpression can be used to faithfully mimic white and brown adipocytes, which can be used to model human disease. However, due to integration of the transgenes, this hampers their ability to model the natural development of brown adipocytes and their use in cellular-based therapies.
Generation of integration-free brown adipocytes
In their quest to generate hematopoietic stem cells from hiPSCs, in 2012 the Saeki group inadvertently devised an integration-free method for brown adipocyte generation.76 Using the EB method and subsequent outgrowth on adherent culture plates in the presence of a cocktail of hematopoietic factors including BMP4 and BMP7 (Table 1), they observed a high level of multilocular lipid droplet formation in cells emanating from the EB colonies. BMP signaling was likely a key factor for brown adipocyte generation from hiPSCs using this protocol, as BMP7 null mice lack functional BAT and BMP7 can be used to induce brown adipocytes from MSCs and primary isolated preadipocytes.45 Further analysis of the differentiated EB outgrowths revealed that 95% of the adipocytes were positive for UCP1 and abundant in transverse cristae rich mitochondria. These cells showed proton-leak linked respiration and treatment with the β3-adrenergic receptor agonist isoproterenol increased expression of UCP1 and oxygen consumption rate, suggesting that the cells could be functionally activated by sympathetic neurons. Transplantation of the hiPSC-derived brown adipose into immunocompromised NOG mice resulted in reduced fasting lipid and glucose levels, and continued UCP1 expression, which could last for at least 3 weeks.
One question that arises from this study is, why would a hematopoietic stem cell cocktail of factors give rise to brown adipose? To answer this, the authors demonstrated that their hiPSC-derived brown adipocytes could serve as stroma for bone marrow-derived myeloid progenitors and that adipocytes within human bone marrow naturally express UCP1 and PRDM16. The presence of naturally occurring brown adipocytes was also verified using a group of young healthy volunteers, which displayed cold-stimulated 18F-FDG uptake in vertebral bone marrow as determined by PET/CT. These results suggest that brown adipocytes reside in bone marrow and may provide an important niche for hematopoiesis. From a developmental perspective, the authors demonstrated that the differentiating hiPSCs increase expression of paraxial mesoderm markers (MYF5, PDGFRα, PAX3, and PAX7), but not a marker of lateral plate mesoderm (VEGFR2), indicative of a progression toward classical brown adipocytes. However, there was no evidence presented that the differentiating EBs progressed through an adipogenic precursor or MSC intermediate and based upon the short duration of brown adipocyte formation from hiPSCs (only 12 days), it is more likely that the brown adipocytes were formed by direct induction from paraxial mesoderm. Therefore, whether this method could be used to enrich brown adipocyte precursor cells from other cells within the EB for transplantation-based therapies in humans is unknown. Nonetheless, this method represents the first integration-free generation of brown adipocytes and demonstrates that brown adipocytes in humans may also derive developmentally from the paraxial mesoderm as occurs in mice.
The role of PAX3 and the generation of adipogenic precursors from hiPSCs
For the above-mentioned studies, the generation of an expandable adipogenic precursor that gives rise to hiPSC-derived brown or white adipocytes had not been established. To address this, the Dani group performed a more detailed analysis of the cellular outgrowths emanating from Ebs.77 As in previous studies, they were able to generate adipocytes at a very low frequency (2%) from hiPSCs using the EB outgrowth method after treatment with an adipogenic cocktail of factors (Table 1). Analysis of the cellular outgrowths by flow cytometry identified precursor cells that expressed CD73, CD105, CD90, and CD146, akin to markers found on the surface of primary human adipose-derived stem cells (hADSCs).78 Similar to the Nakao group, transient treatment with RA during EB formation resulted in the development of white adipocytes from the cellular outgrowths. Conversely, they observed brown adipocytes after EBs were formed in the absence of RA and found that the hADSC-like precursors were enriched in PAX3, a marker previously lineage traced to brown, but not white or beige adipocytes.79 Additional analysis of human and mouse adipose tissue depots showed enrichment for PAX3 in BAT compared to WAT. These data support the notion that PAX3 may play a role in brown versus white adipocyte fate determination. When PAX3 was overexpressed in either hiPSC-derived hADSC-like precursors or primary hADSCs, PRDM16 expression increased, and this was associated with an increase in UCP1 expression after induction with an adipogenic cocktail of factors. Since the authors did not detect Myf5 expression during the differentiation of hiPSCs, the possibility exists that in addition to white adipocytes, brown adipocytes may also derive from PAX3+ neural crest (Fig. 2).69 However, since the authors did not conclusively lineage trace the origin of the precursors to PAX3+ neural crest, the possibility exists that Myf5 expression, indicative of paraxial mesoderm derivation, was simply not detected in these experiments. Overall, this study demonstrates that white or brown adipocytes generated from hiPSCs can arise from distinct adipogenic precursors, which display a marker phenotype overlapping with hADSCs. While the hADSC-like precursors in this study could be enriched through clonal analysis and subsequent passages, their differentiation still yielded poor adipogenesis, roughly 20 times lower than that observed for primary hADSCs. One question arising from this study is therefore, why does an enriched group of cells with similar surface marker expression to hADSCs (CD73, CD105, CD90, and CD146) display such a heterogeneous ability to form mature adipocytes?
Increasing the efficiency of adipocyte formation from hiPSCs
In a follow-up study, the Dani group was able to decipher important factors that might explain the low frequency associated with the generation of adipocytes from hiPSCs80. Previous studies have demonstrated that TGFβ activation of SMAD2/3 signaling plays an anti-adipogenic role and SMAD2/3 inhibition can result in the browning of WAT in mice.81, 82 Along these lines, the Dani group found that undifferentiated adipogenic precursors derived from hiPSCs expressed TGF-β–ligands that were sufficient to phosphorylate SMAD2/3 in the absence of exogenous ligands. Treatment of the precursors with an adipogenic cocktail (Table 1) in the presence of the TGF-β signaling inhibitor SB431542 resulted in a large increase in UCP1 expression and adipocyte formation. Additional treatment with ascorbic acid, hydrocortisone and EGF led to a further increase in adipocyte formation similar to what is observed with primary hADSCs after 30 days in culture. The resulting mature adipocytes were also responsive to β-adrenergic receptor stimuli and insulin signaling. Having successfully generated a highly efficient system for adipogenesis from hiPSCs, they were able to analyze the adipocyte subtypes present after differentiation. Analysis of markers for mature brown, beige and white adipocytes demonstrated that, in addition to UCP1, the brown adipogenic precursor method (RA–) generated adipocytes with a phenotype of both brown and beige markers, including expression of CD137 (beige) and ZIC1 (brown). Overall, these results suggest that adipogenic precursors derived from hiPSCs have an adipogenic capacity similar to adult hADSCs when differentiated in the presence of the appropriate factors. These studies also highlight the premise that distinct mechanisms (e.g., TGF-β) may control the differentiation of embryonic (and hiPSC-derived) versus adult adipocyte precursors, as has been previously observed in mice.83
Monolayer formation of adipocytes from hiPSCs
During EB formation, it is difficult to discern the specific signals that contribute to spontaneous formation of the three germ layers, including those that give rise to brown versus beige adipocytes. Additionally, this method results in heterogeneous formation of a large number of cell types of the developing embryo. In order to more accurately determine the developmental programs necessary for induction of a homogenous adipocyte population from hiPSCs, it will likely be necessary to move beyond the use of EBs. In 2017, the Vigouroux group described a monolayer culture method to generate hiPSC-derived beige adipocytes.84 Treatment of hiPSCs with BMP4 and Activin A in serum-free STEMPro34 hematopoietic stem cell medium was used to generate TBOX and MESP1 expressing mesoderm. Subsequent induction with an adipogenic cocktail (Table 1) was used to drive mesoderm precursors into lipid positive adipocytes by day 20 of the differentiation protocol. Marker analysis of differentiating mesodermal progenitors into mature adipocytes suggests that they transition through an adipogenic precursor intermediate positive for PDGFRα, CD44, CD29, and LY6E (human homolog of murine Sca1). LY6E expression has yet to be formally tested in hADSCs. The mature hiPSC-derived adipocytes expressed PPARγ2, CEBPA, insulin receptor (IR), and glucose transporter type 4 (GLUT4) proteins and responded to insulin through phosphorylation of the IR and its downstream target AKT. Analysis of overlapping brown and beige markers showed increased expression of UCP1, PCG1A and PRDM16. Differentiating adipocytes also increased expression of beige markers including CD137, CITED1, and TMEM26, but not MYF5 or ZIC1, markers specific to paraxial mesoderm and classical brown adipocytes, respectively. In response to 8Br-cAMP, adipocytes displayed increased mitochondrial content, shrinking lipid droplets and a 2.5-fold increase in oxygen consumption, however, protein-leak linked respiration and the presence of β-adrenergic receptors was not described. Subcutaneous injection of hiPSC-derived beige adipocytes in the interscapular region of athymic nude mice resulted in vascularized adipose tissue, which when stimulated with the β3-adrenergic receptor agonist isoproterenol, increased 18F-FDG uptake, mitochondrial content and expression of UCP1, as determined by staining with human specific antibodies. From a developmental perspective, the exact origin of these adipocytes is somewhat unclear. Mesp1 is expressed in paraxial mesoderm in mice, directly induces PDGFRα positive paraxial mesoderm and promotes skeletal myogenic differentiation, cardiac differentiation and hematopoiesis.85, 86 Moreover, CD29+, CD44+ myogenic precursors have also been described as originating from PDGFRα paraxial mesoderm.87 Further analysis with additional mesodermal markers will need to be performed using this method to determine the specific mesodermal origin that specifies these beige adipocytes.
Generation of beige adipocytes through an expandable iPSC-derived MSC intermediate population
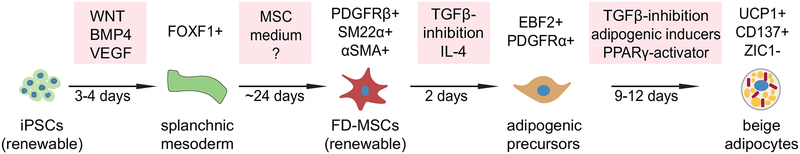
In 2018, our laboratory described the generation of human beige adipocytes from hiPSCs using a defined, 4-stage monolayer culture system.88 Our protocol includes the generation of mesoderm from hiPSCs followed by differentiation into a renewable source of MSCs, committed adipogenic precursors and finally beige adipocytes (Fig. 3). First, we identified a potential embryonic origin for beige adipocytes using a commercially available serum-free mesoderm induction medium (StemDiff™). We found that this medium is highly specific for generating FOXF1+ precursors that are common to the splanchnic layer within lateral plate mesoderm during embryonic development (Fig. 2). Splanchnic mesoderm is known to give rise to numerous tissues and cell types, including pericytes and vascular smooth muscle cells of the circulatory system (collectively termed mural cells), which are known precursors of beige adipocytes.59, 89, 90 Results with the commercial mesoderm induction medium could also be replicated in house with the WNT agonist CHIR99021, BMP4, and VEGFA when added to albumin polyvinylalcohol essential lipids (APEL) basal medium. After generation of FOXF1+ precursors, cells are continuously cultured in serum-free commercial MSC medium (MesenCult™-ACF) where they acquire a homogeneous marker phenotype indicative of mural cells (>98% purity). This includes surface expression of CD146, PDGFRβ, and NG2 and immunostaining for smooth muscle markers α-smooth muscle actin (αSMA) and smooth muscle protein-22-α (SM22α). The combination of factors contained within Mesencult-ACF™ medium are unknown (trade secret, StemCell Technologies, Inc.), but highly specific for the generation of beige adipocyte precursors as this medium could not be substituted with other serum-free defined commercial MSC media that were tested during the MSC differentiation stage of the protocol. Previous studies have shown that IL-4 can act directly on adipogenic precursors isolated from subcutaneous WAT and commit these precursors to a beige rather than white molecular phenotype.91, 92 We found that treatment of the FOXF1-derived MSCs (FD-MSCs) with a combination of IL-4 and the TGF-β inhibitor SB431542 was sufficient to push them into committed beige adipogenic precursors marked by expression of PDGFRα, EBF2, and PPARγ. Subsequent differentiation into mature adipocytes with the SB431542 containing adipogenic cocktail developed by the Dani group led to robust formation of beige adipocytes at a very high frequency (85–90%). These iPSC-derived beige adipocytes possess all of the major characteristics of primary beige adipocytes derived from the stromal vascular fraction of subcutaneous white adipose tissue. This includes robust UCP1 expression, expression of beige- (CD137, TMEM26, CITED1), but not brown-specific markers (ZIC1), and high proton leak–linked respiration. They also express β1- and β3-adrenergic receptors and increase fatty acid uptake and proton leak–linked respiration in response to the β3 adrenergic receptor agonist CL316,243. Furthermore, the beige adipocytes secrete anti-diabetic protein factors such as FGF21, IL6, NRG4 and ADIPOQ. Accordingly, conditioned medium from these cells increases insulin signaling and glucose uptake in cultured white adipocytes derived from T2D patients.
Figure 3:
Generation of beige adipocytes through multiple well-defined cellular stages. hiPSCs can be differentiated into beige adipocytes by simulating multiple differentiation stages in cell culture as they occur naturally during mammalian development. Figure adopted and modified from Ref. 91.
Our method is distinguished from prior methods by producing beige adipocytes through the differentiation of iPSCs toward a well-defined and renewable MSC population (>15 passages). Furthermore, this method can also be used to generate beige adipocytes from urine cell derived, integration-free iPSCs. This permits beige adipocytes to be generated easily from large cohorts of human patients in a non-invasive manner. In addition, since these beige adipocytes can be generated using media that is produced commercially under high-quality controlled conditions, reproducibility is predicted to be high across numerous laboratories. Overall, this method offers an accurate model to study beige adipocyte development and regulation as it occurs naturally in mammals and can be used to generate sufficient material for testing cell-based therapies that can target metabolic syndrome in humans.
Next steps toward generation and characterization of thermogenic adipocytes from hiPSCs
Methods developed for the differentiation of hiPSCs into adipocytes are quite diverse and one of the primary concerns has been the efficiency associated with generation of functional cells. In particular, to acquire functional adipocytes from hiPSCs at a high frequency for therapeutic purposes, it will be necessary to obtain an expandable and committed adipogenic precursor. This would serve several purposes. First, once derived, these precursors should possess a certain level of expansion potential, reducing the time required to generate mature adipocytes on a routine basis. Second, direct comparison of the adipogenic potential of hiPSC-derived adipogenic precursors with primary precursors isolated from human BAT and WAT would provide a standard for producing better adipocytes. Third, this would allow for direct testing of adipogenic precursors in a transplantation setting, and finally, enrichment at the level of the adipogenic precursor would allow removal of non-adipocyte lineages or undifferentiated hiPSCs from the culture, which could result in unknown and perhaps unwanted cell types after transplantation.
An important aspect for the use of hiPSC-derived brown or beige adipocytes in a clinical setting is the length of time the cells can be maintained in a therapeutically beneficial state. The innervation of sympathetic nerves and/or vascularization of hiPSC-derived brown or beige adipocyte transplants may aid in prolonging their activation and overall effectiveness. With the technology developed thus far, cellular interactions between the hiPSC-derived adipocytes and other cell types found within the adipose tissue niche have yet to be modeled sufficiently.
Another important feature of hiPSC-derived brown and beige adipocytes may be potential cellular functions beyond increased thermogenesis, including the secretion of anti-diabetic factors that can act locally and systemically (reviewed in Ref. 89). This characteristic of naturally occurring adipocytes may represent one of the most important considerations when determining the clinical relevance of hiPSC-derived adipocytes. A number of BAT-secreted factors, including NRG4 and FGF21 have been shown to attenuate hepatic steatosis, increase fatty acid β-oxidation, promote WAT browning and stimulate sympathetic outflow to adipose tissues (reviewed in Ref. 90). To take advantage of these factors, assays need to be developed to test the secretion potential of hiPSC-derived adipocytes, as well as their ability to increase insulin sensitivity, glucose uptake and fatty acid oxidation in other cell types affected in T2D, such as white adipocytes, hepatocytes and muscle cells.
In summary, considerable progress has been made toward the development of hiPSC-derived adipocytes that appear to functionally mirror many of the attributes associated with tissue-derived brown and beige adipocytes. The development of new and efficient methods for adipocyte derivation from hiPSCs, as well as a deeper characterization of their functional properties will be necessary to realize their ultimate potential in developing safe and effective cellular therapies for obesity related disorders.
Acknowledgements
This work was supported by NIH COBRE award P20GM121301 (A. Brown, L. Liaw, and C.J. Rosen). Shameem Fakory, Julieta Martino, and Daniel Nguyen kindly provided assistance with illustrations and editing.
Footnotes
Competing interests
The author declares no competing interests.
References
- 1.Hales CM, C. M, Fryar CD, Ogden CL. 2017, October Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS Data Brief. No. 288. [PubMed] [Google Scholar]
- 2.Ogden CL, C. M 2010, June Prevalence of Overweight, Obesity, and Extreme Obesity Among Adults: United States, Trends 1960–1962 Through 2007–2008. National Center for Health Statistics. [Google Scholar]
- 3.Pi-Sunyer X 2009. The medical risks of obesity. Postgrad Med. 121: 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Dept of Health and Human Services. Centers for Disease Control and Prevention. 2017. National Diabetes Statistics Report.
- 5.Saito M, Okamatsu-Ogura Y, Matsushita M, et al. 2009. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 58: 1526–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoneshiro T, Aita S, Matsushita M, et al. 2011. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (Silver Spring). 19: 1755–1760. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, Tanabe K, Ohnuki M, et al. 2007. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 131: 861–872. [DOI] [PubMed] [Google Scholar]
- 8.Rosen ED & Spiegelman BM. 2014. What we talk about when we talk about fat. Cell. 156: 20–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freedland ES 2004. Role of a critical visceral adipose tissue threshold (CVATT) in metabolic syndrome: implications for controlling dietary carbohydrates: a review. Nutr Metab (Lond). 1: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saito M 2013. Brown adipose tissue as a therapeutic target for human obesity. Obes Res Clin Pract. 7: e432–438. [DOI] [PubMed] [Google Scholar]
- 11.Lean ME 1989. Brown adipose tissue in humans. Proc Nutr Soc. 48: 243–256. [DOI] [PubMed] [Google Scholar]
- 12.Cypess AM, Lehman S, Williams G, et al. 2009. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 360: 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. 2009. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 360: 1500–1508. [DOI] [PubMed] [Google Scholar]
- 14.Virtanen KA, Lidell ME, Orava J, et al. 2009. Functional brown adipose tissue in healthy adults. N Engl J Med. 360: 1518–1525. [DOI] [PubMed] [Google Scholar]
- 15.Chondronikola M, Volpi E, Borsheim E, et al. 2014. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 63: 4089–4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanssen MJ, van der Lans AA, Brans B, et al. 2016. Short-term Cold Acclimation Recruits Brown Adipose Tissue in Obese Humans. Diabetes. 65: 1179–1189. [DOI] [PubMed] [Google Scholar]
- 17.Hanssen MJ, Hoeks J, Brans B, et al. 2015. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nature medicine. 21: 863–865. [DOI] [PubMed] [Google Scholar]
- 18.Napolitano L & Fawcett D. 1958. The fine structure of brown adipose tissue in the newborn mouse and rat. J Biophys Biochem Cytol. 4: 685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heaton JM 1972. The distribution of brown adipose tissue in the human. J Anat. 112: 35–39. [PMC free article] [PubMed] [Google Scholar]
- 20.Sacks H & Symonds ME. 2013. Anatomical locations of human brown adipose tissue: functional relevance and implications in obesity and type 2 diabetes. Diabetes. 62: 1783–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leitner BP, Huang S, Brychta RJ, et al. 2017. Mapping of human brown adipose tissue in lean and obese young men. Proceedings of the National Academy of Sciences of the United States of America. 114: 8649–8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cannon B & Nedergaard J. 2004. Brown adipose tissue: function and physiological significance. Physiological reviews. 84: 277–359. [DOI] [PubMed] [Google Scholar]
- 23.Wu J, Cohen P & Spiegelman BM. 2013. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 27: 234–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kopecky J, Clarke G, Enerback S, et al. 1995. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest. 96: 2914–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins S, Daniel KW, Petro AE, et al. 1997. Strain-specific response to beta 3-adrenergic receptor agonist treatment of diet-induced obesity in mice. Endocrinology. 138: 405–413. [DOI] [PubMed] [Google Scholar]
- 26.Ghorbani M & Himms-Hagen J. 1997. Appearance of brown adipocytes in white adipose tissue during CL 316,243-induced reversal of obesity and diabetes in Zucker fa/fa rats. Int J Obes Relat Metab Disord. 21: 465–475. [DOI] [PubMed] [Google Scholar]
- 27.Guerra C, Koza RA, Yamashita H, et al. 1998. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest. 102: 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harms M & Seale P. 2013. Brown and beige fat: development, function and therapeutic potential. Nature medicine. 19: 1252–1263. [DOI] [PubMed] [Google Scholar]
- 29.Kazak L, Chouchani ET, Jedrychowski MP, et al. 2015. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell. 163: 643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikeda K, Kang Q, Yoneshiro T, et al. 2017. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nature medicine. 23: 1454–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh VK, Kalsan M, Kumar N, et al. 2015. Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front Cell Dev Biol. 3: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanford KI, Middelbeek RJ, Townsend KL, et al. 2013. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 123: 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X, Zheng Z, Zhu X, et al. 2013. Brown adipose tissue transplantation improves whole-body energy metabolism. Cell Res. 23: 851–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gunawardana SC & Piston DW. 2012. Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes. 61: 674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villarroya F & Giralt M. 2015. The Beneficial Effects of Brown Fat Transplantation: Further Evidence of an Endocrine Role of Brown Adipose Tissue. Endocrinology. 156: 2368–2370. [DOI] [PubMed] [Google Scholar]
- 36.Frontini A & Cinti S. 2010. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell metabolism. 11: 253–256. [DOI] [PubMed] [Google Scholar]
- 37.Ahfeldt T, Schinzel RT, Lee YK, et al. 2012. Programming human pluripotent stem cells into white and brown adipocytes. Nat Cell Biol. 14: 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berry DC, Jiang Y, Arpke RW, et al. 2017. Cellular Aging Contributes to Failure of Cold-Induced Beige Adipocyte Formation in Old Mice and Humans. Cell metabolism. 25: 481. [DOI] [PubMed] [Google Scholar]
- 39.Carey AL, Vorlander C, Reddy-Luthmoodoo M, et al. 2014. Reduced UCP-1 content in in vitro differentiated beige/brite adipocytes derived from preadipocytes of human subcutaneous white adipose tissues in obesity. PLoS One. 9: e91997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel P & Abate N. 2013. Role of subcutaneous adipose tissue in the pathogenesis of insulin resistance. J Obes. 2013: 489187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chung KJ, Chatzigeorgiou A, Economopoulou M, et al. 2017. A self-sustained loop of inflammation-driven inhibition of beige adipogenesis in obesity. Nat Immunol. 18: 654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carobbio S, Rosen B & Vidal-Puig A. 2013. Adipogenesis: new insights into brown adipose tissue differentiation. J Mol Endocrinol. 51: T75–85. [DOI] [PubMed] [Google Scholar]
- 43.Tang QQ & Lane MD. 2012. Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem. 81: 715–736. [DOI] [PubMed] [Google Scholar]
- 44.Bowers RR & Lane MD. 2007. A role for bone morphogenetic protein-4 in adipocyte development. Cell Cycle. 6: 385–389. [DOI] [PubMed] [Google Scholar]
- 45.Tseng YH, Kokkotou E, Schulz TJ, et al. 2008. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 454: 1000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosen ED & MacDougald OA. 2006. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 7: 885–896. [DOI] [PubMed] [Google Scholar]
- 47.Christodoulides C, Lagathu C, Sethi JK, et al. 2009. Adipogenesis and WNT signalling. Trends Endocrinol Metab. 20: 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Timmons JA, Wennmalm K, Larsson O, et al. 2007. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proceedings of the National Academy of Sciences of the United States of America. 104: 4401–4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atit R, Sgaier SK, Mohamed OA, et al. 2006. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Developmental biology. 296: 164–176. [DOI] [PubMed] [Google Scholar]
- 50.Nascimento EBM, Sparks LM, Divoux A, et al. 2018. Genetic Markers of Brown Adipose Tissue Identity and In Vitro Brown Adipose Tissue Activity in Humans. Obesity (Silver Spring). 26: 135–140. [DOI] [PubMed] [Google Scholar]
- 51.Zhang H, Huang Y, Lee HJ, et al. 2015. Zic1 negatively regulates brown adipogenesis in C3H10T1/2 cells. Science Bulletin. 60: 1033–1035. [Google Scholar]
- 52.Wu J, Bostrom P, Sparks LM, et al. 2012. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 150: 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jespersen NZ, Larsen TJ, Peijs L, et al. 2013. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell metabolism. 17: 798–805. [DOI] [PubMed] [Google Scholar]
- 54.Cypess AM, White AP, Vernochet C, et al. 2013. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nature medicine. 19: 635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shinoda K, Luijten IH, Hasegawa Y, et al. 2015. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nature medicine. 21: 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Altshuler-Keylin S, Shinoda K, Hasegawa Y, et al. 2016. Beige Adipocyte Maintenance Is Regulated by Autophagy-Induced Mitochondrial Clearance. Cell metabolism. 24: 402–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roh HC, Tsai LTY, Shao M, et al. 2018. Warming Induces Significant Reprogramming of Beige, but Not Brown, Adipocyte Cellular Identity. Cell metabolism. 27: 1121–1137 e1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen Y, Ikeda K, Yoneshiro T, et al. 2019. Thermal stress induces glycolytic beige fat formation via a myogenic state. Nature. 565: 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang W & Seale P. 2016. Control of brown and beige fat development. Nat Rev Mol Cell Biol. 17: 691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanchez-Gurmaches J & Guertin DA. 2014. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun. 5: 4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang W, Zeve D, Suh JM, et al. 2008. White fat progenitor cells reside in the adipose vasculature. Science. 322: 583–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hassan M, Latif N & Yacoub M. 2012. Adipose tissue: friend or foe? Nat Rev Cardiol. 9: 689–702. [DOI] [PubMed] [Google Scholar]
- 63.Chau YY, Bandiera R, Serrels A, et al. 2014. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol. 16: 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sanchez-Gurmaches J, Hung CM, Sparks CA, et al. 2012. PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell metabolism. 16: 348–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shan T, Liang X, Bi P, et al. 2013. Distinct populations of adipogenic and myogenic Myf5-lineage progenitors in white adipose tissues. J Lipid Res. 54: 2214–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Min SY, Kady J, Nam M, et al. 2016. Human ‘brite/beige’ adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nature medicine. 22: 312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krueger KC, Costa MJ, Du H, et al. 2014. Characterization of Cre recombinase activity for in vivo targeting of adipocyte precursor cells. Stem Cell Reports. 3: 1147–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanchez-Gurmaches J, Hsiao WY & Guertin DA. 2015. Highly selective in vivo labeling of subcutaneous white adipocyte precursors with Prx1-Cre. Stem Cell Reports. 4: 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Billon N, Iannarelli P, Monteiro MC, et al. 2007. The generation of adipocytes by the neural crest. Development. 134: 2283–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xiong C, Xie CQ, Zhang L, et al. 2005. Derivation of adipocytes from human embryonic stem cells. Stem Cells Dev. 14: 671–675. [DOI] [PubMed] [Google Scholar]
- 71.van Harmelen V, Astrom G, Stromberg A, et al. 2007. Differential lipolytic regulation in human embryonic stem cell-derived adipocytes. Obesity (Silver Spring). 15: 846–852. [DOI] [PubMed] [Google Scholar]
- 72.Hannan NR & Wolvetang EJ. 2009. Adipocyte differentiation in human embryonic stem cells transduced with Oct4 shRNA lentivirus. Stem Cells Dev. 18: 653–660. [DOI] [PubMed] [Google Scholar]
- 73.Taura D, Noguchi M, Sone M, et al. 2009. Adipogenic differentiation of human induced pluripotent stem cells: comparison with that of human embryonic stem cells. FEBS Lett. 583: 1029–1033. [DOI] [PubMed] [Google Scholar]
- 74.Kajimura S, Seale P, Kubota K, et al. 2009. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature. 460: 1154–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Svensson PA, Jernas M, Sjoholm K, et al. 2011. Gene expression in human brown adipose tissue. Int J Mol Med. 27: 227–232. [DOI] [PubMed] [Google Scholar]
- 76.Nishio M, Yoneshiro T, Nakahara M, et al. 2012. Production of functional classical brown adipocytes from human pluripotent stem cells using specific hemopoietin cocktail without gene transfer. Cell metabolism. 16: 394–406. [DOI] [PubMed] [Google Scholar]
- 77.Mohsen-Kanson T, Hafner AL, Wdziekonski B, et al. 2014. Differentiation of human induced pluripotent stem cells into brown and white adipocytes: role of Pax3. Stem cells. 32: 1459–1467. [DOI] [PubMed] [Google Scholar]
- 78.Mohsen-Kanson T, Hafner AL, Wdziekonski B, et al. 2013. Expression of cell surface markers during self-renewal and differentiation of human adipose-derived stem cells. Biochem Biophys Res Commun. 430: 871–875. [DOI] [PubMed] [Google Scholar]
- 79.Liu W, Shan T, Yang X, et al. 2013. A heterogeneous lineage origin underlies the phenotypic and molecular differences of white and beige adipocytes. J Cell Sci. 126: 3527–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hafner AL, Contet J, Ravaud C, et al. 2016. Brown-like adipose progenitors derived from human induced pluripotent stem cells: Identification of critical pathways governing their adipogenic capacity. Sci Rep. 6: 32490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zamani N & Brown CW. 2011. Emerging roles for the transforming growth factor-{beta} superfamily in regulating adiposity and energy expenditure. Endocr Rev. 32: 387–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yadav H & Rane SG. 2012. TGF-beta/Smad3 Signaling Regulates Brown Adipocyte Induction in White Adipose Tissue. Frontiers in endocrinology. 3: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang QA, Tao C, Jiang L, et al. 2015. Distinct regulatory mechanisms governing embryonic versus adult adipocyte maturation. Nat Cell Biol. 17: 1099–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guenantin AC, Briand N, Capel E, et al. 2017. Functional Human Beige Adipocytes from Induced Pluripotent Stem Cells. Diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoon JK, Moon RT & Wold B. 2000. The bHLH class protein pMesogenin1 can specify paraxial mesoderm phenotypes. Developmental biology. 222: 376–391. [DOI] [PubMed] [Google Scholar]
- 86.Chan SS, Shi X, Toyama A, et al. 2013. Mesp1 patterns mesoderm into cardiac, hematopoietic, or skeletal myogenic progenitors in a context-dependent manner. Cell Stem Cell. 12: 587–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Darabi R, Gehlbach K, Bachoo RM, et al. 2008. Functional skeletal muscle regeneration from differentiating embryonic stem cells. Nature medicine. 14: 134–143. [DOI] [PubMed] [Google Scholar]
- 88.Su S, Guntur AR, Nguyen DC, et al. 2018. A Renewable Source of Human Beige Adipocytes for Development of Therapies to Treat Metabolic Syndrome. Cell reports. 25: 3215–3228 e3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mahlapuu M, Enerback S & Carlsson P. 2001. Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations. Development. 128: 2397–2406. [DOI] [PubMed] [Google Scholar]
- 90.Vishvanath L, Long JZ, Spiegelman BM, et al. 2017. Do Adipocytes Emerge from Mural Progenitors? Cell Stem Cell. 20: 585–586. [DOI] [PubMed] [Google Scholar]
- 91.Lee MW, Odegaard JI, Mukundan L, et al. 2015. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 160: 74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lizcano F, Vargas D, Gomez A, et al. 2017. Human ADMC-Derived Adipocyte Thermogenic Capacity Is Regulated by IL-4 Receptor. Stem cells international. 2017: 2767916. [DOI] [PMC free article] [PubMed] [Google Scholar]