Abstract
Myxomatous mitral valve degeneration (MMVD) is a leading cause of valve repair or replacement secondary to the production of mitral regurgitation, cardiac enlargement, systolic dysfunction, and heart failure. The pathophysiology of MMVD is complex and incompletely understood, but key features include activation and transformation of mitral valve (MV) valvular interstitial cells (VICs) into an active phenotype leading to remodeling of the extracellular matrix and compromise of the structural components of the MV leaflets. Uncovering the mechanisms behind these events offers the potential for therapies to prevent, delay, or reverse MMVD. One such mechanism involves the neurotransmitter serotonin (5HT), which has been linked to development of valvulopathy in a variety of settings, including valvulopathy induced by serotonergic drugs, 5HT-producing carcinoid tumors and development of valvulopathy in laboratory animals exposed to high levels of 5HT. Similar to humans, the domestic dog also experiences naturally-occurring MMVD, and in some breeds of dogs, the lifetime prevalence of MMVD reaches 100%. In dogs, MMVD has been associated with high serum 5HT, increased expression of 5HT-receptors, autocrine production of 5HT within the MV leaflets, and down regulation of 5HT clearance mechanisms. One pathway closely associated with 5HT involves transforming growth factor beta (TGF-β) and the two pathways share a common ability to activate MV VICs in both humans and dogs. Understanding the role of 5HT and TGF-β in MMVD gives rise to potential therapies, such as 5HT receptor (5HT-R) antagonists. The main purposes of this review are to highlight the commonalities between MMVD in humans and dogs, with specific regards to 5HT and TGF-β, and to champion the dog as a relevant and particularly valuable model of human disease that can accelerate development of novel therapies.
Keywords: myxomatous mitral valve degeneration, mitral valve disease, mitral valve repair, mitral valve replacement, serotonin
1. Introduction
Mitral regurgitation (MR) is a commonly encountered and clinically significant valvular heart condition and causes of primary MR, including myxomatous mitral valve degeneration (MMVD), represent an important global healthcare burden [1]. In the developed world, MMVD is the primary indication for surgical correction of severe MR [2]. The pathologic hallmarks of MMVD vary but mainly involve redundant valve tissue, prolapse, and involvement of multiple leaflet segments [3]. Gross findings typically include increased leaflet thickness as a result of extracellular matrix (ECM) remodeling and proteoglycan accumulation, leaflet prolapse or flail, and elongated or ruptured chordae tendineae [3]. The etiology of most cases of MMVD is unknown. In recent years, certain molecular pathways, namely the serotonin (5HT) and transforming growth factor beta (TGF-β) pathways, have been implicated in the etiology of MMVD in humans as well as in animals, such as the domestic dog (Canis familiaris). The similarities of disease between these two species are numerous and offer a unique opportunity for important insight. This review focuses on the comparative epidemiologic, gross, histological, and molecular characteristics of MMVD between humans and dogs, with particular emphasis on the 5HT and TGF-β pathways. These types of comparisons can reveal new therapeutic strategies to combat MMVD many of which could be tested in the dog.
1.1. Definition of myxomatous mitral valve degeneration (MMVD)
MMVD in both humans and dogs is defined by architectural disruption of the histological mitral leaflet layers involving excessive extracellular accumulation of proteoglycans within the spongiosa and fractured and disorganized elastin and collagen fibers within the fibrosa [4, 5]. These changes result in thickening, billowing, prolapse, and flail of the valve leaflets, and elongation and rupture of the chordae tendineae [6].
The extent of any one of these features tends to vary between species. In the dog, the most common occurrence of MMVD involves redundant and thickened valve tissue primarily affecting the entire anterior leaflet, while in humans, the middle posterior leaflet is most commonly affected, however substantial individual variation exists. One important feature of MMVD is mitral valve prolapse (MVP), which has been defined as systolic displacement of any portion of the valve leaflets beyond the plane of the MV annulus and into the left atrium as measured in a long-axis echocardiographic view, typically by > 2mm [7, 8]. One potential source of confusion is that MMVD and MVP are often contemporaneously present but are not synonymous. In humans, myxomatous degeneration can lead to MVP, but not all cases of MVP are due to MMVD. For example, some instances of MVP are characterized more so by superimposed fibrosis than by extensive deposition of ECM within the MV layers [9] while other forms of “syndromic” MVP occur secondary to connective tissue disorders, such as Marfan syndrome or Ehler-Danlos [10]. MVP is also present in valve disease secondary to ventricular deformation due to dilated cardiomyopathy or myocardial infarction: for example, over one-third of patients with ischemic mitral regurgitation (IMR) present with MVP [11]. In contrast, virtually all instances of MVP in the dog are secondary to MMVD (Figure 1). In this review, we only consider MVP in the context of MMVD. Furthermore, in humans, at least 2 main subsets of MMVD are recognized. The first, termed fibroelastic deficiency, is characterized by loss of valve collagen, thin and transparent leaflets, and rupture of one or more thin chordae tendineae that typically affects only single portion of a leaflet (usually the middle scallop of the posterior leaflet) [12]. In the second, termed Barlow’s disease, there is excessive myxoid deposition, leaflet thickening, flail leaflet tips and redundant leaflet tissue, multiple segments of the MV leaflet billow onto the left atrial cavity during ventricular systole, causing MVP, and chordae tendineae are elongated or ruptured [13]. Based on the gross morphological characteristics, which can usually be examined using echocardiography, the Barlow’s form of MMVD in humans is most analogous to MMVD in the dog.
Figure 1.
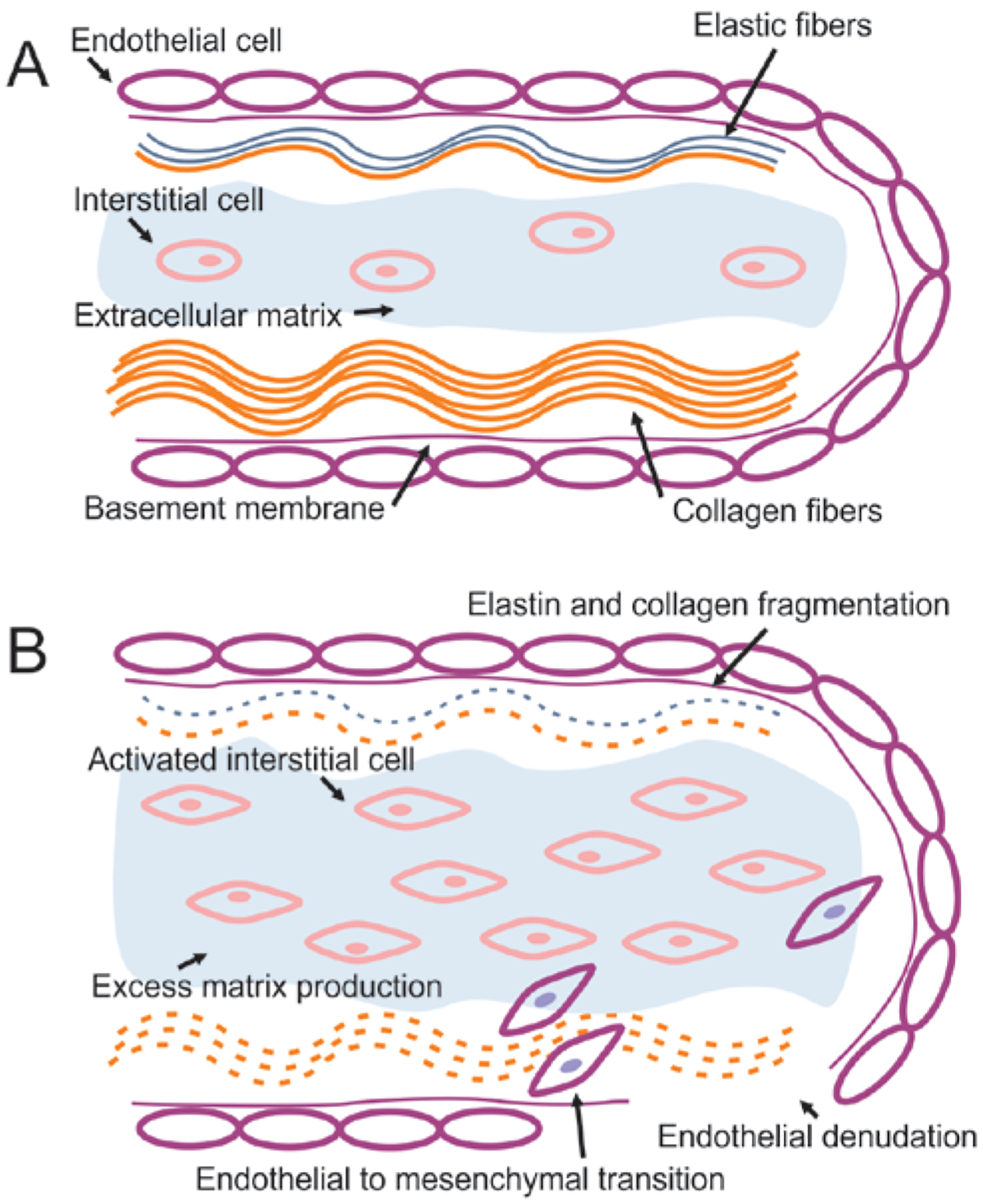
Normal mitral valve structure (A) and mechanism of myxomatous mitral valve disease (MMVD) (B). The normal valve is made up of a layer of endothelial cells surrounding the atrialis layer, which is made up of elastic and collagen fibers, the spongiosa layer, which consists of extracellular matrix (ECM) rich in proteoglycans and the occasional valvular interstitial cell, and the fibrosa, which consists of tightly packed collagen fibers. In valves affected by MMVD, the interstitial cells are activated into a myofibroblast-like phenotype, which is accompanied by excessive deposition of ECM, dissolution and fragmentation of the elastic and collagen fibers of the atrialis and fibrosa, endothelial to mesenchymal cell transformation and migration of endothelial cells into the spongiosa, and denudation of the endothelial cell lining and exposure of the subendothelial collagen.
1.2. Epidemiology of MMVD
In humans, the estimated prevalence of MMVD in the global population is 2–3% with 15% of cases eventually requiring valve surgery [14]. The age-related nature of degenerative conditions such as MMVD coupled with the increasing life span likely contributes to this burden even as rheumatic causes of MR decline in the developed world [15]. In the dog, MMVD is the most common heart disease and is associated with increasing age and certain dog breeds [16–18]. The domestic dog has its origins between 20,000 and 40,000 years ago arising from 1 or more wolf species of the Canis family [19]. Since that time, selective breeding of the dog has resulted in an extremely wide phenotypic array of different dog sizes, conformation, and susceptibility to disease such that the highest occurrence of MMVD is in small and medium-sized chondrodystrophic dog breeds <15 kg of body weight. The prevalence of MMVD in this population is remarkably high with prevalence between 30 and 70% in dogs over the age of 10 years, and lifetime incidence of MMVD in some of these breeds is close to 100% [17, 20]. The effect on mortality is substantial. Heart failure is the third most common cause of death or humane euthanasia comprising 4.9% of all dog mortality [21]. The clinicopathological aspects of MMVD including the clinical, gross, and histological features are similar across dogs.
Certain breeds of dogs exhibit extraordinarily high risk of MMVD [22]. One example is the Cavalier King Charles Spaniel (CKCS) breed, in which death due to heart disease comprises over 30% of all causes of death [21]. The CKCS is a small-sized breed of approximately 9 kg of body weight that originated in England during the reign of King Charles II [23]. Beginning at the age of 3 years, the yearly incidence of MMVD in the CKCS is approximately 7% and rises to as high as 15% in subsequent years, such that virtually all dogs exhibit disease by the age of 10 years [23]. The contemporary CKCS breed standard is based on only a few foundational dogs, which is the likely cause of the high incidence of disease. One study[24] into the heritability of MMVD in the CKCS identified two loci associated with early onset of MMVD, but these results have not been reproduced in other studies [25].
The clinical progression of MMVD has been termed “unrelentingly although variably progressive” [26]. The clinical consequences of severe MMVD include progressive MR, eccentric cardiac hypertrophy, systolic dysfunction, and if left uncorrected, left-sided congestive heart failure (CHF). MMVD is also associated with tricuspid valve degeneration in about 30% of canine MMVD cases [27]. Cardiac mortality in the 6 years following diagnosis of MMVD in the dog is 11% [28]. Unlike in humans, surgical repair in dogs with MR, while associated with improved outcomes, is expensive and not widely available [29]. Thus, the vast majority of dogs with severe MR and CHF secondary to MMVD receive only medical therapy with diuretics, ACE inhibitors, aldosterone receptor blockers, and positive inotropes [30]. The clinical course of spontaneous MMVD in the dog that starts with mild asymptomatic MR with variable progression to moderate MR with LV eccentric hypertrophy and left atrial enlargement, then to severe MR with clinical signs of CHF, occurs within the relatively short adult lifespan of the dog (~3–6 years) as compared to humans. As such, the MMVD in the dog represents a good model in which to better understand the pathophysiology of MMVD as well as to evaluate potential treatments to delay progression. Important pathological features of spontaneous MMVD in the dog and humans will be further discussed.
1.3. Comparative Anatomy and Pathology
The MV apparatus is subject to substantial and repeated mechanical stress, including tension when the valve is closed in systole, flexure during the opening of the valve in diastole, and shear stress across its surface from blood flow [31]. Heart valves open and close more than 3 billion times in an average adult human lifetime, and of the 4 valves, the MV experiences the greatest transvalvular pressure gradient. During systole, the MV leaflets experience high degrees of strain with rapid stretching, a plateau phase with a constant strain state, and upon full closure, an extreme increase in stiffness to prevent further leaflet deformation and prevention of MR that is caused by straightening of collagen fibers within the leaflets [32]. The gross and histological structures of the human [33] and canine [5, 34] valve leaflets are similar and designed to withstand the repeated stress through a combination of a high degree of structural integrity and absorption of forces by inner layers of the MV leaflets.
At the gross level the MV is closely related to the fibrous skeleton of the heart, which is made up of fibrocartilagenous tissue and includes the left and right fibrous trigones and much of the MV annulus [35]. The MV annulus is non-planar and saddle-shaped, which reduces tension on the tendinous chords and valve leaflets [36, 37]. Other features of the MV apparatus in both dogs and humans include the continuity of the anterior MV leaflet with the aortic valve cusps at the aortic root and the presence of an extensive branching network of tendinous chords connecting to an anterior and posterior papillary muscle [38]. The histological structure of the MV leaflet is also similar in humans and dogs consisting of inner layers of highly organized ECM and connective tissue elements (Figure 1A) [39, 40]. The two innermost regions of the valve are particularly important and include the spongiosa with its proteoglycan-rich ECM and the fibrosa with its collagen network that extends into the tendinous chords. Residing within the valve are valve interstitial cell (VICs), which arise from mesenchymal differentiation of endothelial cells that are present during embryonic development of the heart valve [41]. VICs play an important role in both healthy conditions as well as during MMVD by regulating the composition of the spongiosa and fibrosa. The ECM and structural proteins within the MV leaflet are a dynamic network that is constantly being modified by the resident VICs in order to achieve a balance between synthesis and degradation in the ECM, which helps maintain normal structure and function. The proteoglycan-rich composition of the spongiosa helps mitigate the mechanical forces experienced by the valve and gives the leaflet its compressive properties that absorb high forces during leaflet coaptation. At the same time, the high density of collagen and elastin in the layers surrounding the spongiosa provides the leaflets with the necessary tensile strength needed during coaptation while the leaflets are closed [42, 43]. Surrounding the valve’s innermost layers an outer layer of loosely arranged elastic and fibrous fibers and surface covering of valve endothelial cells (VECs) create a barrier between the leaflets and blood. The VECs are attached to the ECM by a basement membrane and capable of responding to mechanical stimulus and sheer stress and actively communicating with the ECM and VICs through various signaling mechanisms [44].
The hallmark features of the Barlow’s form of MMVD in both humans and dogs include leaflet thickening, development of nodules along the leaflet edge, rupture of chordae tendineaae, expansion of the spongiosa, and collagen fiber loss and disorganization in the fibrosa (Figure 1B) [6, 18]. A key step in these changes is the activation of VICs, which transdifferentiate into a population of cells that presents phenotypic characteristics of several different cell types, including fibroblasts, smooth muscle cells, and myofibroblasts [45–47]. The role of activated VICs in cardiac valve remodeling, including fibrosis and calcification, is well established [48]. Stimuli for VIC activation include mechanical stress, injury to the leaflet endothelial surface, and signaling molecules, such as 5HT and TGF-β, among others [49]. Presumably, a degree of constitutive VIC activation occurs in response to normal wear and aging to repair and upkeep the leaflets [50], however MMVD is characterized by an abundance of activated VICs, as well as endothelial-to-meschymal transformation and migration of VECs into the inner valve layers. Activated VICs mediate excessive production of ECM, expansion of the spongiosa, and fragmentation and disruption of elastin and collagen that results in valve incompetence and MR. VIC activation is also present in secondary valve adaptation in IMR, and may mediate a compensatory increase in MV leaflet surface area to improve valve coaptation when left ventricle geometry has been disrupted by dilated or ischemic cardiomyopathy [51]. The molecular features of VIC and VEC activation and remodeling include a wide variety of signaling molecules including but not limited to 5HT, TGF-β, lipopolysaccharide [52], vascular endothelial growth factor [46], matrix metalloproteinases (MMPs) [53, 54], and tissue inhibitors of metalloproteinases (TIMPs) [55, 56]. Over the past decade, the role of 5HT and TGF-β in activation of VICs and MMVD has been a subject of considerable interest.
2. Serotonin and TGF-β Signaling Pathways
5HT and TGF-β pathways play a significant role in the activation of human and canine MV VICs [57–60], and a better understanding of these pathways could improve our understanding of the pathogenesis of MMVD. The nature of the 5HT and TGF-β pathways and important similarities between human and canine MMVD is further described.
Serotonin is a monoamine neurotransmitter produced by serotonergic neurons in the central nervous system as well as by enterochromaffin cells in the gastrointestinal tract. It is synthesized through the hydroxylation of tryptophan, mediated by tryptophan hydroxylase-1 (TPH1), which is the limiting step in this reaction. In the periphery, 5HT is produced and stored mainly in gastrointestinal tract enterochromaffin cells and any 5HT released into circulation is rapidly taken up by platelets or other cells through the 5HT-reuptake transporter (SERT). Thus, SERT is a key protein that regulates the amount of available 5HT in the extracellular space by controlling the uptake and subsequent storage or metabolism of circulating 5HT. Platelets uptake and store large amounts of 5HT in their dense granules, and subsequently release 5HT in response to endothelial damage, immune complex formation, thrombin, collagen, and ischemia, amongst other stimuli, and mediate local vasoconstriction, platelet aggregation, and hemostasis. Serotonin internalized by SERT is subsequently metabolized by monoamine oxidase A (MAO-A) to form 5-hydroxyindole acetic acid (5-HIAA), which is then secreted into the urine.
Serotonin mediates its biological effects on cells by interacting with specific membrane bound receptors (5HT-Rs) [61]. At least 14 distinct 5HT-Rs have been characterized with the majority belonging to the G-protein coupled receptor superfamily. The type 2 subfamily of 5HT-Rs (5HT-R2) including 5HT-R2A, 5HT-R2B, and 5HT-R2C has been previously associated with heart disease [62, 63]. 5HT-R2 signaling activates phospholipase C and increases intracellular calcium and stimulates protein kinase C. Of particular note is 5HT-R2B, which induces Ras activation and Src phosphorylation that mediates tyrosine kinase signaling pathways via extracellular signal-regulated kinase 1 (ERK1) and 2 (ERK2) (Figure 2) and induces cell cycle progression, mitogenesis, and activation of MV VICs [62, 64].
Figure 2.
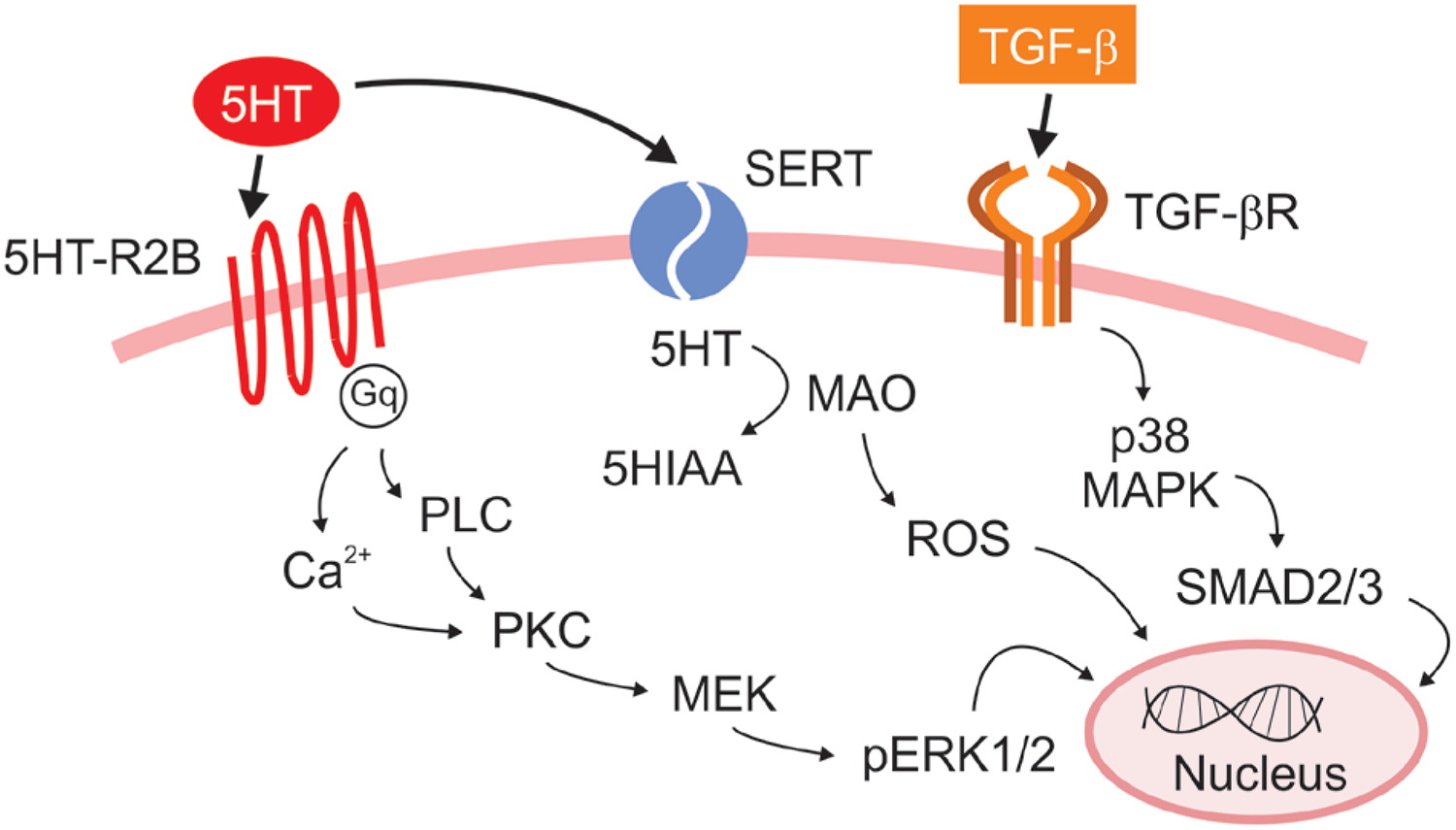
Proposed mechanism of serotonin (5HT) and transforming growth factor beta (TGF-β) in myxomatous mitral valve disease (MMVD).
The TGF-β pathway shares several points of crosstalk with the 5HT pathway (Figure 2). In mammalian species, the TGF superfamily consists of 33 structurally related cytokines, with 3 archetypal isoforms (TGF-β 1, 2, and 3) as well as 20 different bone morphogenic proteins (BMPs) [65]. The TGF system regulates a wide range of cell responses including cell proliferation, differentiation, migration, and apoptosis [66, 67] in a variety of cell types, including VICs. Receptor binding triggers phosphorylation of secondary messenger molecules within the Smad family, which translocate to the nucleus to regulate transcriptional activity. A role for 5HT and TGF-β has been increasingly understood in both human and canine DMVD.
3. Role of 5HT and TGF-β in Valve Disease
A connection between 5HT, TGF-β, and MMVD is supported by a variety of findings, including the capacity of 5HT to activate of human and canine VICs [68–70], increased transcription and expression of 5HT and TGF-β pathway components in affected MV tissue [54, 58, 71–74], increased local and circulating 5HT concentration in dogs with MMVD [74–77], inducement of valve lesions secondary to exogenous 5HT [78], 5HT-producing tumors [79, 80], or serotonergic drugs [81], and finally the ability of 5HT-R antagonists to block VIC activation and mitigate histological changes of MMVD in experimental models [59, 64].
3.1. Activation of VICs by 5HT and Mechanical Stress
5HT and mechanical stress trigger activation and differentiation of VICs into an activated phenotype. Activation of VICs is characterized by increased phosphorylation of mitogenic pathways, such as the ERK1/2 pathway (Figure 2), expression of smooth muscle actin, and increased production of ECM and MMPs. As previously mentioned, heart valves are subject to high degrees of repetitive stress, and both 5HT and TGF-β help mediate response to this stress. MVD patients often have long-standing hypertension, which increases the risk of both primary and secondary MR [82] and contributes to increased strain on the valves. Previous studies utilized a stretch bioreactor to study the role of stress and 5HT on VIC activation in a variety of species and MMVD models. In a series of studies, porcine aortic valve leaflets were subjected to physiologic and pathologic levels of cyclic strain and demonstrated activation of VICs, increased expression of smooth muscle actin, MMPs, 5HT-R2B, and 5HT-R2A, and increased production of ECM proteoglycans [83–85]. This response was heightened in the presence of 5HT stimulation or SERT blockade [86]. In other studies, canine MV leaflets were exposed to either cyclic or static strain and demonstrated significantly increased activation of VICs and expression of smooth muscle actin, MMPs, and glycosaminoglycans, and showed MV capacity for local production of 5HT mediated by TPH1 [87, 88]. Finally, human MV tissue subjected to biomechanical stress demonstrated increased 5HT signaling, resulting in increased 5HTR2A and 5HTR2B expression and activation of VICs [59]. Experimental models showing VIC response to mechanical stress are particularly important in the study of secondary valve diseases like IMR, in which tethering resulting from LV deformation is believed to be the main catalyst for MV remodeling. The mechanisms resulting in VIC activation in secondary MR likely vary by etiology (i.e. ischemic vs. overload); a prior study in sheep with IMR demonstrated that VIC activation and expression of TGF-β were augmented in sheep with ischemia and mechanical tethering of the chordae tendineae in comparison with tethering alone [51]. Increased circulating [89] and interstitial [90] 5HT have been reported after cardiac ischemia and may contribute to MV remodeling in IMR.
3.2. Upregulation of 5HT and TGF-β Pathways in MMVD
5HT and TGF-β pathways are upregulated in MMVD as shown by increased transcriptional activity of various components of the 5HT and TGF-β pathways in various genomic expression studies of human and canine MMVD. These studies demonstrate many similarities between humans and dogs with MMVD with regards to 5HT and TGF-β. In studies of human valve tissue, transcriptional activity of 5HT-R2B was increased 28.4-fold vs. controls, and incubation with 5HT or TGF-β revealed a dose-dependent increase in cell activation and markers of cell proliferation, and collagen and proteoglycan synthesis [59, 64, 91]. Studies in dogs [54, 70, 73, 92, 93] similarly revealed upregulated transcriptional activity of 5HT-R2B in affected MV tissue, which was also associated with increased protein expression of 5HT-R2B and VIC activation. In surgically excised myxomatous human valves, TPH1 was increased three-fold compared to non-diseased control tissue [88]. In dogs, local production of 5HT is evidenced by 3 to 5-fold increases in TPH1, as well as detectable 5HT levels in the culture media and valve tissue of valves undergoing cyclic strain [70, 87, 88, 94].
Multiple mechanisms including inflammation, mechanical forces and angiotensin-II result in TGF-β secretion. Increased TGF-β signaling is associated with a variety of different MV diseases including those associated with fibrillin-1 mutations, X-linked MMVD, MVP, and IMR [95, 96]. A prior case report has described a patient with MMVD as well as myxomatous degeneration of the tricuspid and aortic valves who was found to carry two mutations in the TGFB2 gene [97]. In human and canine MMVD valves, TGF-β signaling, protein expression, and immunohistochemical staining for latent TGF-β receptors is increased [54, 58, 70, 71], and TGF-β level correlated with leaflet thickness in human MMVD [98], which suggests an implication of TGF-β in the progressive nature of MMVD. Human and canine MMVD samples also demonstrated up regulation of TGF-β–related BMP signaling molecules [54, 85, 99]. Angiotensin II receptor blockers reduced TGF-β-dependent VIC activation and ECM production in cultured human VICs obtained from surgical specimens [100]. In a sheep model of IMR, TGF-β downregulation with losartan was associated with a reduction of fibrosis and MV thickness, while the compensatory increase in MV area was preserved [101].
3.3. Myocardial, Valvular, and Circulating 5HT in MMCD in Dogs
Two potential sources of increased 5HT signaling in MMVD include circulating 5HT or autocrine production of 5HT. In one study, dogs with MMVD had an approximately 50% higher 5HT serum concentration compared with control dogs [75]. There are potential important interbreed differences: CKCS, a breed with extremely high prevalence of MMVD, are reported to have particularly increased serum 5HT levels [75, 76, 102, 103]. The relationship between circulating 5HT and MMVD appears complex. Serum and plasma 5HT levels are higher in the early stages of the disease as compared to the later stages and 5HT levels are inversely correlated with the heart size in dogs with MMVD [76]. Free 5HT in circulation is rapidly taken up by platelets and the vast majority of circulating 5HT is stored in platelet dense granules as opposed to free in the plasma [74, 77]. Therefore, serum 5HT likely represents a small amount of plasma 5HT with a majority of serum 5HT secondary to platelet-release. Scanning electron microscopy studies of affected canine and human MMVD MV leaflets revealed denuded endothelium, exposure of subendothelial collagen, and expression of inflammatory molecules that could attract platelets and subsequent 5HT release [104]. 5HT in platelet rich plasma was higher in healthy CKCS, as well as both CKCS and mixed breed dogs with MMVD vs. healthy mixed breed controls [74]. Elevated 5HT in dogs may therefore be related to platelet activation and release of its granules. Previous studies have shown that the degree of MR in dogs is correlated to platelet dysfunction [105, 106]. Of interest, the CKCS breed is also associated with an inherited thrombocytopenia and giant platelet disorder affecting approximately half of all CKCS dogs [107, 108]. Scanning electron microscopy of the giant platelets from these dogs revealed the presence of dense granules and other ultrastructural features similar to normal sized platelets. [107] In addition to having macrothrombocytes, the platelet population in CKCS dogs with MMVD also contains at least one flow cytometry scatterplot subpopulation with a higher percentage of surface-bound 5HT-positive expression and activation, but the exact relationship of platelet morphology, ultrastructure, and function to MMVD requires further study [77]. A previous study in rats reported that platelet lysate containing 5HT-activated cardiac myofibroblasts, led to overproduction of ECM, and provided proof of concept that platelet-derived 5HT was capable of mediating cardiac cell activation [109]. Previous studies have also studied 5HT concentration in left ventricular and MV tissue [74]. Concentration of 5HT in MV tissue from dogs with MMVD was 9-fold greater than in healthy dogs and 13.5-fold greater than in dogs with other types of cardiac disease. Left ventricular 5HT concentration from dogs with MMVD was similarly increased.
3.4. Serotonergic Drugs, 5HT-producing Tumors, and Experimental Models
The role of increased 5HT signaling in valve disease is further supported by development of valve injury secondary to 5HT-producing tumors, such as in carcinoid disease, as well as by toxicity of serotonergic drugs. Carcinoid tumors involve enterochromaffin cells in the gastrointestinal tract and release large amounts of hormone-like molecules, including 5HT, into the blood stream. Carcinoid heart disease, present in about 40% of patients with carcinoid tumors, is associated with thickening and regurgitation in the pulmonary and tricuspid valves [110] and, less commonly, in the mitral and aortic valves [111]. Importantly, it has been suggested that carcinoid involvement of the left-sided valves is associated with tumors that produce high levels of circulating 5HT [112]; in these cases distinct “carcinoid” plaques are present on the surface of the valves. Histology of these plaques on the valve surface reveals activated VICs and deposition of glycosaminoglycans within the ECM that resembles MMVD [111, 112]. Unlike in MMVD, which presents with weakening of the chordae tendineae, carcinoid tumors are associated with thickened and fused chordae tendineae, resembling rheumatic valve disease [111]. The histological differences in diseased valves associated with carcinoid tumors compared with the presentation of MMVD may reflect the involvement of other molecules secreted by carcinoid tumors, which include prostaglandins, histamine and bradykinin. Valvulopathy is also associated with a various serotonergic drugs, including methysergide, an ergot alkaloid for migraine headaches, fenfluramine-phenteramine and benfluorex for appetite suppression, pergolide for treatment of Parkinson’s disease, and methylenedioxy-N-methylamphetamine (MDMA), a recreational drug known as “ecstasy”, amongst others [81]. A common feature amongst these types of drugs is activation of 5HT-R2B [113]. MV disease associated with serotonergic drugs was often found in patients with pre-existing MMVD, likely compounding the effect of increased 5HT signaling. Finally, animal models exposed to increased levels of 5HT develop valve lesion with features similar to MMVD [114–117]. Specifically, rats chronically injected with 5HT had an increased prevalence of valve injury compared to wild type, and showed phenotypic activation of VICs [114, 116]. Rabbits fed a high 5HT-containing diet experienced increased plasma 5HT and urinary 5HT metabolite concentrations, mxyomatous thickening of the aortic mitral and tricuspid valve leaflets, and development of echocardiographic valvular regurgitation [117]. Activation of mitogenic and proliferative systems related to angiotensin II, TGF-β, and 5HT leads to accelerated MMVD lesions in mice and sheep valves [118–120]. Collectively, these findings lead to the hypothesis that blockade of 5HT and subsequent suppression of this and other pathways, such as TGF-β, might represent a potential treatment for MMVD.
3.5. Antagonism of 5HT-R2B as a Potential Treatment for Valvular Disease
The link between 5HT and MMVD presents potential for novel treatments, such as 5HT-R2B antagonists. Studies in cell culture and animal models have repeatedly demonstrated that 5HT-R antagonists mitigate VIC activation and the subsequent valvular histological changes associated with MMVD [59, 64, 94, 113, 121, 122]. In one study, the specific 5HT-R2B inhibitor LY272015, prevented activation of canine MV VICs in the presence of 5HT concentrations ranging from 10−6 to 10−8 M (Figure 3).[59] In a study of cultured MVs from sheep subjected to cycle strain, treatment with a 5HT2B/2C or TPH inhibitor reduced markers of VIC activation and matrix catabolic enzymes, such as MMP1, MMP13, and cathepsin K [94]. In mice with MMVD due to chronic administration of angiotensin II or nordexfenfluramine, antagonism of 5HT-R2B reduced histological lesions in the valve leaflets (Figure 4) [59, 121]. Thus, a hypothesis that 5HT-2R antagonism, and in particular 5HT-R2B antagonism, can mitigate MMVD is supported by extensive preclinical data. In addition to targeting of the 5HT-R, the uptake of 5HT, which is mediated by SERT, represents another potential therapeutic avenue.
Figure 3.
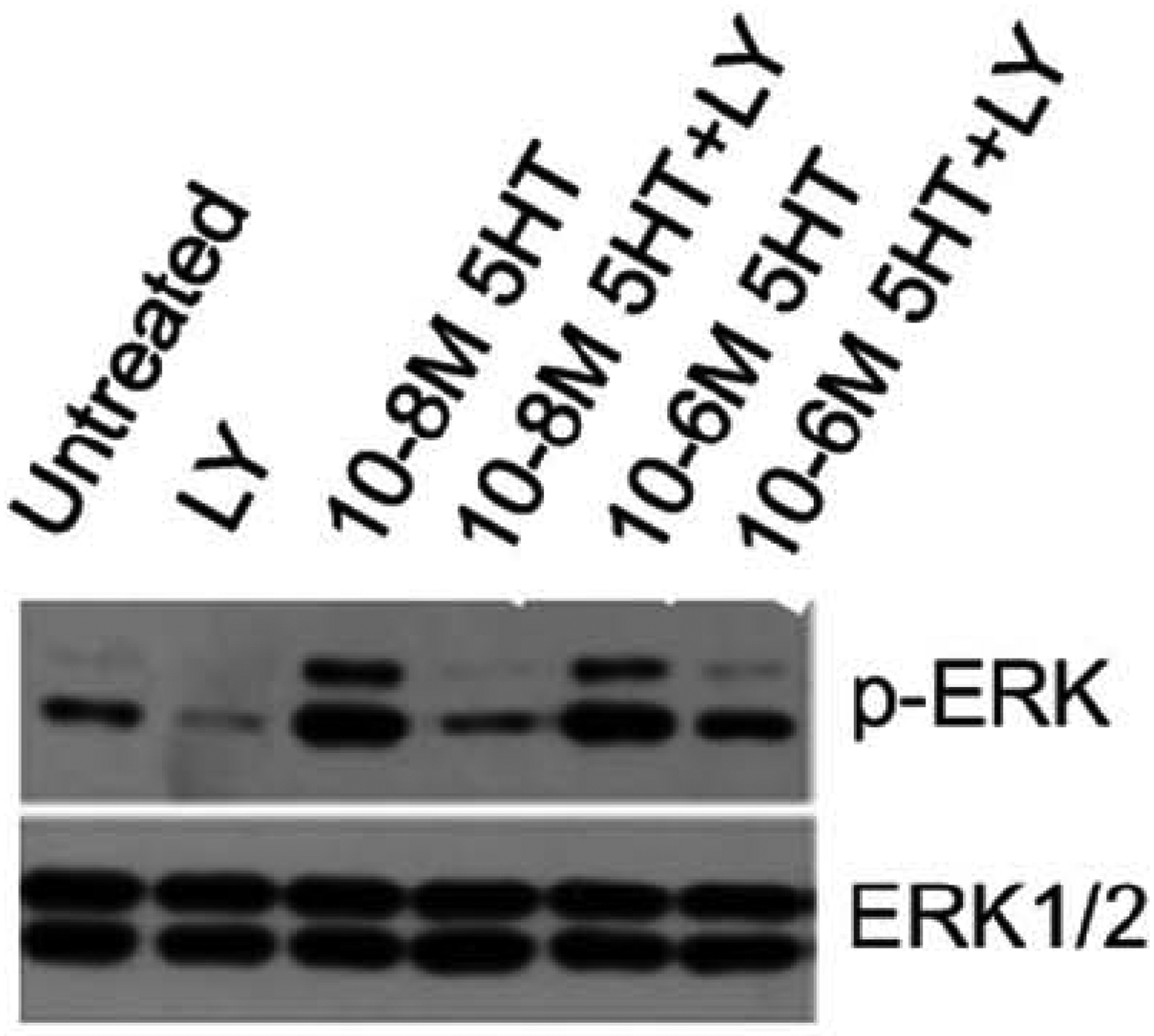
Isolated canine mitral valve valvular interstitial cells (VICs) treated with serotonin (5HT) and vehicle or 5HT and the specific 5HT-receptor subtype 2B antagonist LV272015 (LY) reveals LY reduced phosphorylation of ERK1/2, which is a marker of VIC activation. Adapted from Driesbaugh et al [59].
Figure 4.
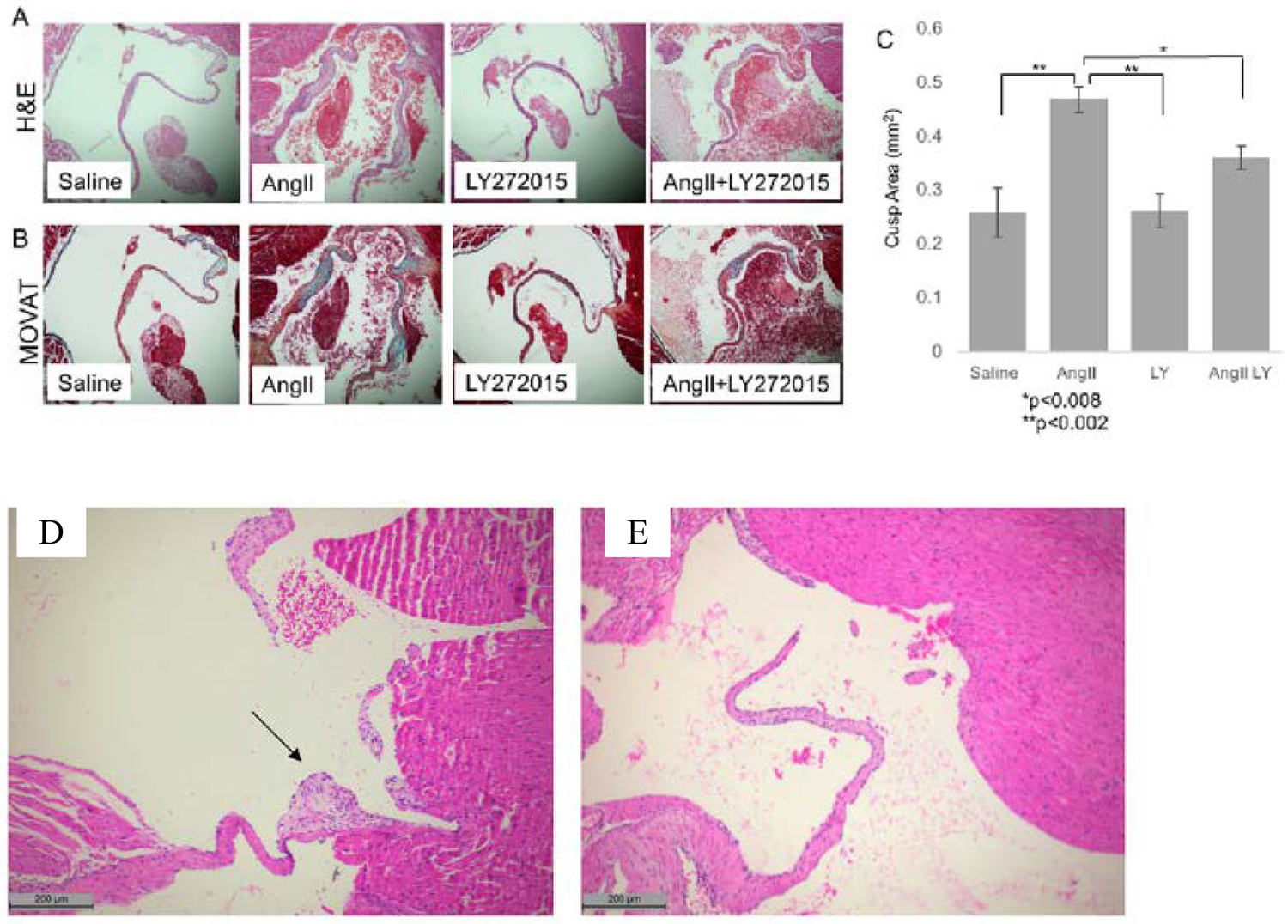
Blockade of mitral valve changes by antagonism of the serotonin 2B receptor (5HT-2BR) in a mouse model of myxomatous mitral valve disease (MMVD) created by angiotensin II (AngII). Representative H&E (A) and modified Movat pentachrome (B) staining of cross sections of the mitral valve from mice treated for 28 days with saline or AngII with or without treatment with the 5HT-R2B antagonist LY272015 (LY). Quantitative analysis of average valve leaflet area for each treatment group indicated a reduction of valve area in AngII mice treated with LY as compared to positive control (C). Representative H&E staining of mitral valve from mice exposed to nordexfenfluramine for 28 days showing myxomatous leaflet nodules (D) and prevention of valve lesions in 5HT-R2B KO mice (D). (A-C) from Driesbaugh et al [59]; (D-E) from Ayme-Dietrich et al [121].
3.6. Role of SERT in Valvular Disease
As previously mentioned, in cardiac and valve tissue, internalization and subsequent metabolism of 5HT is achieved through SERT and MAO-A, respectively. The role of 5HT and SERT in embryological heart valve development, specifically the endothelial-mesenchymal transformation of the endocardial cushions, has been reviewed [123, 124]. Increasingly, the role of SERT in MMVD is a subject of interest. In the mature heart, SERT continues to regulate VIC activity and composition of the ECM by controlling extracellular concentrations of 5HT [124]. SERT deficiency presumably increases extracellular 5HT, which through 5HT-R2B and related TGF-β pathways, induce VIC differentiation and proliferation. In SERT-KO mice, valvular fibrosis develops as a result of sustained interaction between 5HT and 5HT-R2B [125, 126].
Expression of SERT is altered in dogs with spontaneously occurring MMVD [70, 127] as well as in pig disease models [128]. Specifically, MMVD tissue from dogs exhibits decreased SERT expression and decreased SERT protein levels, particularly in advanced stages of disease [70, 127]. Polymorphisms in the SERT gene have been described in humans and dogs. One polymorphism in humans involves 14 short (s allele) or 16 long (l allele) repeat sequences in the promoter region of the SERT gene, resulting in SERT mRNA molecules of either 484 or 528 base pairs [129]. The s allele is associated with decreased SERT expression compared to the l allele and differences in carrier status have been studied in a variety of diseases including idiopathic pulmonary hypertension [130], irritable bowel syndrome [131], and psychiatric disorders [132], indicating the wide ranging effects of disruption to 5HT metabolism. In humans with MV MMVD, the ll genotype was associated with surgical repair at a younger age in men and a lower SERT expression, suggesting a reduced capacity for 5HT uptake and metabolism of extracellular 5HT [133]. Dogs lack the specific insertion/deletion polymorphism that is present in humans, however, at least 6 SNPs in the canine SERT gene have been identified, including three that on the basis of structural protein modeling were predicted to be damaging to protein structure and function [134].
4. Discussion
There are no medical treatments available for MMVD and surgical intervention is the only option for patients that develop severe MR. The ability to prevent, slow, or reduce progression of MMVD through medical means that address the fundamental pathophysiology would treat MMVD in a manner that surgery cannot achieve. It is increasingly clear that the pathophysiology of MMVD in humans and dogs with respect to 5HT and TGF-β pathways is strikingly similar as are the molecular, histological, and gross changes typical of MMVD they induce. At its core, upregulation of 5HT signaling through 5HT-R, and in particular, 5HT-R2B, leads to activation of VICs and the subsequent remodeling of the ECM and fibrosa and loss of gross structural integrity. The 5HT and TGF-β systems are a likely common pathway leading to myxomatous valve change in response to a variety of injurious causes. Despite the mounting promising literature on the potential of 5HT-R2B to prevent the development and progression of MMVD, it is important to note that due to the scarcity of specific inhibitors available the long-term central and peripheral effects of inhibiting 5HT-R2B remain to be defined. 5HT-R2B receptors are expressed in cerebellar Purkinje cells and may mediate the central effects of 5HT-R2B inhibition, including motor and sleep-wake cycle modifications reported in rats treated with the selective antagonist SB-215505 [135]. Additionally, acute treatment of mice with the 5HT-R2B selective antagonist RS127445 resulted in selective schizophrenic-like behavior including social interaction deficit and reduction of startle response to acoustic stimuli [136]. In the liver, 5HT-R2B receptors are necessary for tissue regeneration following hepatectomy in mice [137]. A similarly protective role of 5HT-R2B within cardiac valve pathological remodeling cannot be excluded.
The dog, and in particular the CKCS breed, represents a model to further study 5HT and TGF-β mechanisms and to test novel therapies. One main purpose of this review is to highlight the potential value of spontaneous MMVD in the dog as model for human disease. Specifically, the relatively rapid progression of MMVD coupled with the short life span of the dog creates an opportunity to test the effect of 5HT-R2B antagonism and other therapeutics on echocardiographic valve morphology and severity of MR, indirect measures of disease severity such as circulating cardiac biomarkers like N-terminal pro-B-type natriuretic peptide, as well as central and peripheral side effects. Surgical repair or replacement of the MV in privately-owned dogs is not widely available or performed, and many dogs develop severe MR and eventually suffer from congestion and systolic dysfunction [30]. Thus, novel therapeutic strategies can be tested across a range of clinically important events, ranging from the onset of disease through progression of MR, development of systolic dysfunction and CHF, and ultimately, cardiac mortality. In addition, therapy has been increasingly asked to address outcomes relating to symptoms or quality of life. These “patient-centered outcomes” are amenable to study in privately owned dogs through the use of previously validated survey instruments that are completed by the dog owner [138]. For these many reasons, study in dogs with MMVD has certain advantages over experimental laboratory animal models. The advantages and limitations of experimentally induced animal models, such as those involving use of mice, have been previously reviewed [139–141]. Briefly, advantages include the many physiological similarities between the mouse and humans, relative ease of housing and use, and availability of genetically altered strains that facilitate use as a model species. Disadvantages include the many instances in which mice respond to interventions very differently than humans, differing phenotypes of disease versus their human comparisons, concerns regarding animal welfare and biomedical testing, and the inability to know which lines of evidence will faithfully translate to similar findings in humans. Thus, data from large animals with spontaneous disease can help accelerate clinical studies in humans in ways that experimental mouse models cannot.
As presented, many different lines of evidence support the importance of 5HT and TGF-β in MMVD, but the particular source of increased 5HT is still unknown. Candidates include platelet-derived 5HT, autocrine 5HT production within the valve or heart tissue, and increased 5HT levels secondary to deficient uptake and metabolism. Future studies to address these questions could lead to a wider array of therapeutics than just 5HT-R antagonists. Further discovery and characterization of various SERT polymorphisms in the dog represent an area of potential importance given the close association of particular SERT polymorphisms in humans with need for early MV surgery.
While there are many gross, histological, molecular, transcriptional, and translational similarities between MMVD in humans and the dogs, the two conditions are not entirely homologous. Currently, a single phenotype of MMVD is considered in the dog and is most analogous to the form of MMVD in humans described as Barlow’s disease. Studies should strive to specify and describe the MMVD phenotype when comparing human valves to valves from dogs.
In conclusion, MMVD in the domestic dog represents an underutilized model of human disease. Many lines of evidence point toward the 5HT and TGF-β pathways as playing an important role in MMVD, and novel therapeutic strategies are at a point in time ready for testing. Such studies can further our understanding of the pathophysiology of MMVD and accelerate the discovery of new therapies for humans with MMVD.
Funding:
This work was supported by the National Institutes of Health [HL131872 to R.J.L. and G.F; HL122805 to G.F; T32-HL007854 to A.P.K.].
Abbreviations
- 5-HIAA
5-Hydroxyindole Acetic Acid
- 5HT
Serotonin
- 5HT-R
Serotonin Receptor
- BMP
Bone Morphogenic Protein
- CHF
Congestive Heart Failure
- CKCS
Cavalier King Charles Spaniel
- ECM
Extracellular Matrix
- ERK
Extracellular Signal-Regulated Kinase
- MAO-A
Monoamine Oxidase
- MDMA
Methylenedioxy-N-methylamphetamine
- MMP
Matrix Metalloproteinase
- MMVD
Myxomatous Mitral Valve Degeneration
- MVP
Mitral Vave Prolapse
- SERT
Serotonin-Reuptake Transporter
- TGF-B
Transforming Growth Factor Beta
- THP1
Tryptophan Hydroxylase-1
- TIMP
Tissue Inhibitor of Metalloproteinase
- VEC
Valve Endothelial Cell
- VIV
Valve Interstitial Cell
Footnotes
Conflict of Interest: None declared.
References
- 1.Nkomo VT, et al. , Burden of valvular heart diseases: a population-based study. Lancet, 2006. 368(9540): p. 1005–11. [DOI] [PubMed] [Google Scholar]
- 2.Iung B, et al. , A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J, 2003. 24(13): p. 1231–43. [DOI] [PubMed] [Google Scholar]
- 3.Marechaux S, et al. , Functional anatomy and pathophysiologic principles in mitral regurgitation: Non-invasive assessment. Prog Cardiovasc Dis, 2017. 60(3): p. 289–304. [DOI] [PubMed] [Google Scholar]
- 4.Rippe J, et al. , Primary myxomatous degeneration of cardiac valves. Clinical, pathological, haemodynamic, and echocardiographic profile. Br Heart J, 1980. 44(6): p. 621–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox PR, Pathology of myxomatous mitral valve disease in the dog. J Vet Cardiol, 2012. 14(1): p. 103–26. [DOI] [PubMed] [Google Scholar]
- 6.Pomerance A and Whitney JC, Heart valve changes common to man and dog: a comparative study. Cardiovasc.Res, 1970. 4(1): p. 61–66. [DOI] [PubMed] [Google Scholar]
- 7.Lancellotti P, et al. , Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging, 2013. 14(7): p. 611–44. [DOI] [PubMed] [Google Scholar]
- 8.Levine RA, et al. , Reconsideration of echocardiographic standards for mitral valve prolapse: lack of association between leaflet displacement isolated to the apical four chamber view and independent echocardiographic evidence of abnormality. J Am Coll Cardiol, 1988. 11(5): p. 1010–9. [DOI] [PubMed] [Google Scholar]
- 9.Roberts WC, et al. , Gross and histological features of excised portions of posterior mitral leaflet in patients having operative repair of mitral valve prolapse and comments on the concept of missing (= ruptured) chordae tendineae. J Am Coll Cardiol, 2014. 63(16): p. 1667–74. [DOI] [PubMed] [Google Scholar]
- 10.Delling FN and Vasan RS, Epidemiology and pathophysiology of mitral valve prolapse: new insights into disease progression, genetics, and molecular basis. Circulation, 2014. 129(21): p. 2158–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nappi F, et al. , Ischemic mitral valve prolapse. Journal of Thoracic Disease, 2016. 8(12): p. 3752–3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anyanwu AC and Adams DH, Etiologic classification of degenerative mitral valve disease: Barlow’s disease and fibroelastic deficiency. Semin Thorac Cardiovasc Surg, 2007. 19(2): p. 90–6. [DOI] [PubMed] [Google Scholar]
- 13.Barlow JB and Pocock WA, Billowing, floppy, prolapsed or flail mitral valves? Am J Cardiol, 1985. 55(4): p. 501–2. [DOI] [PubMed] [Google Scholar]
- 14.Freed LA, et al. , Prevalence and clinical outcome of mitral-valve prolapse. N.Engl.J Med, 1999. 341(1): p. 1–7. [DOI] [PubMed] [Google Scholar]
- 15.Iung B and Vahanian A, Epidemiology of valvular heart disease in the adult. Nat Rev Cardiol, 2011. 8(3): p. 162–172. [DOI] [PubMed] [Google Scholar]
- 16.Borgarelli M and Buchanan JW, Historical review, epidemiology and natural history of degenerative mitral valve disease. J Vet Cardiol, 2012. 14(1): p. 93–101. [DOI] [PubMed] [Google Scholar]
- 17.Detweiler DK and Patterson DF, The prevalence and types of cardiovascuar disease in dogs. Ann NY Acad Sci, 1965. 127: p. 481–516. [DOI] [PubMed] [Google Scholar]
- 18.Whitney JC, Observations on the effect of age on the severity of heart valve lesions in the dog. J Small Anim Pract, 1974. 15(8): p. 511–522. [DOI] [PubMed] [Google Scholar]
- 19.Botigue LR, et al. , Ancient European dog genomes reveal continuity since the Early Neolithic. Nat Commun, 2017. 8: p. 16082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parker HG and Kilroy-Glynn P, Myxomatous mitral valve disease in dogs: does size matter? J Vet Cardiol, 2012. 14(1): p. 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis TW, et al. , Longevity and mortality in Kennel Club registered dog breeds in the UK in 2014. Canine Genet Epidemiol, 2018. 5: p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egenvall A, Bonnett BN, and Haggstrom J, Heart disease as a cause of death in insured Swedish dogs younger than 10 years of age. J Vet Intern Med, 2006. 20(4): p. 894–903. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen HD, Lorentzen KA, and Kristensen BO, Echocardiographic mitral valve prolapse in cavalier King Charles spaniels: epidemiology and prognostic signficance for regurgitation. Vet Rec, 1999. 144: p. 315–320. [DOI] [PubMed] [Google Scholar]
- 24.Madsen MB, et al. , Identification of 2 loci associated with development of myxomatous mitral valve disease in Cavalier King Charles Spaniels. J Hered, 2011. 102 Suppl 1:S62–7.: p. S62–S67. [DOI] [PubMed] [Google Scholar]
- 25.Meurs KM, et al. , Evaluation of genes associated with human myxomatous mitral valve disease in dogs with familial myxomatous mitral valve degeneration. Vet J, 2018. 232: p. 16–19. [DOI] [PubMed] [Google Scholar]
- 26.Enriquez-Sarano M and Michelena HI, Mitral Regurgitation in the 21st Century. Prog Cardiovasc Dis, 2017. 60(3): p. 285–288. [DOI] [PubMed] [Google Scholar]
- 27.Atkins C, et al. , Guidelines for the Diagnosis and Treatment of Canine Chronic Valvular Heart Disease. Journal of Veterinary Internal Medicine, 2009. 23(6): p. 1142–1150. [DOI] [PubMed] [Google Scholar]
- 28.Borgarelli M, et al. , Survival characteristics and prognostic variables of dogs with preclinical chronic degenerative mitral valve disease attributable to myxomatous degeneration. J Vet Intern Med, 2012. 26(1): p. 69–75. [DOI] [PubMed] [Google Scholar]
- 29.Uechi M, et al. , Mitral valve repair under cardiopulmonary bypass in small-breed dogs: 48 cases (2006–2009). J Am Vet Med Assoc, 2012. 240(10): p. 1194–201. [DOI] [PubMed] [Google Scholar]
- 30.Keene BW, et al. , ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J Vet Intern Med, 2019. 33(3): p. 1127–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merryman WD, et al. , Correlation between heart valve interstitial cell stiffness and transvalvular pressure: implications for collagen biosynthesis. Am J Physiol Heart Circ Physiol, 2006. 290(1): p. H224–31. [DOI] [PubMed] [Google Scholar]
- 32.Sacks MS, et al. , Surface strains in the anterior leaflet of the functioning mitral valve. Ann Biomed Eng, 2002. 30(10): p. 1281–90. [DOI] [PubMed] [Google Scholar]
- 33.McCarthy KP, Ring L, and Rana BS, Anatomy of the mitral valve: understanding the mitral valve complex in mitral regurgitation. Eur J Echocardiogr, 2010. 11(10): p. i3–9. [DOI] [PubMed] [Google Scholar]
- 34.Markby G, et al. , Myxomatous Degeneration of the Canine Mitral Valve: From Gross Changes to Molecular Events. J Comp Pathol, 2017. 156(4): p. 371–383. [DOI] [PubMed] [Google Scholar]
- 35.Saremi F, et al. , Fibrous Skeleton of the Heart: Anatomic Overview and Evaluation of Pathologic Conditions with CT and MR Imaging. Radiographics, 2017. 37(5): p. 1330–1351. [DOI] [PubMed] [Google Scholar]
- 36.Jimenez JH, et al. , Effects of a saddle shaped annulus on mitral valve function and chordal force distribution: an in vitro study. Ann Biomed Eng, 2003. 31(10): p. 1171–81. [DOI] [PubMed] [Google Scholar]
- 37.Padala M, et al. , Saddle shape of the mitral annulus reduces systolic strains on the P2 segment of the posterior mitral leaflet. Ann Thorac Surg, 2009. 88(5): p. 1499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Connell PS, Han RI, and Grande-Allen KJ, Differentiating the aging of the mitral valve from human and canine myxomatous degeneration. J Vet Cardiol, 2012. 14(1): p. 31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aupperle H and Disatian S, Pathology, protein expression and signaling in myxomatous mitral valve degeneration: comparison of dogs and humans. J Vet Cardiol, 2012. 14(1): p. 59–71. [DOI] [PubMed] [Google Scholar]
- 40.Hinton RB and Yutzey KE, Heart valve structure and function in development and disease. Annu Rev Physiol, 2011. 73: p. 29–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Combs MD and Yutzey KE, Heart valve development: regulatory networks in development and disease. Circ Res, 2009. 105(5): p. 408–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stephens EH, Chu CK, and Grande-Allen KJ, Valve proteoglycan content and glycosaminoglycan fine structure are unique to microstructure, mechanical load and age: Relevance to an age-specific tissue-engineered heart valve. Acta Biomater, 2008. 4(5): p. 1148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ayoub S, et al. , Heart Valve Biomechanics and Underlying Mechanobiology. Compr Physiol, 2016. 6(4): p. 1743–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pagnozzi LA and Butcher JT, Mechanotransduction Mechanisms in Mitral Valve Physiology and Disease Pathogenesis. Front Cardiovasc Med, 2017. 4: p. 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu MM, et al. , Culture and characterisation of canine mitral valve interstitial and endothelial cells. Vet J, 2015. 204(1): p. 32–9. [DOI] [PubMed] [Google Scholar]
- 46.Liu AC, Joag VR, and Gotlieb AI, The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol, 2007. 171(5): p. 1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rutkovskiy A, et al. , Valve Interstitial Cells: The Key to Understanding the Pathophysiology of Heart Valve Calcification. J Am Heart Assoc, 2017. 6(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu AC, Joag VR, and Gotlieb AI, The Emerging Role of Valve Interstitial Cell Phenotypes in Regulating Heart Valve Pathobiology. The American Journal of Pathology, 2007. 171(5): p. 1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rabkin-Aikawa E, et al. , Dynamic and reversible changes of interstitial cell phenotype during remodeling of cardiac valves. J Heart Valve Dis, 2004. 13(5): p. 841–847. [PubMed] [Google Scholar]
- 50.Stephens EH, et al. , Mitral valvular interstitial cell responses to substrate stiffness depend on age and anatomic region. Acta Biomater, 2011. 7(1): p. 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dal-Bianco JP, et al. , Active Adaptation of the Tethered Mitral Valve. Circulation, 2009. 120(4): p. 334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Babu AN, et al. , Lipopolysaccharide stimulation of human aortic valve interstitial cells activates inflammation and osteogenesis. Ann Thorac Surg, 2008. 86(1): p. 71–6. [DOI] [PubMed] [Google Scholar]
- 53.Togashi M, et al. , Role of matrix metalloproteinases and their tissue inhibitor of metalloproteinases in myxomatous change of cardiac floppy valves. Pathol Int, 2007. 57(5): p. 251–9. [DOI] [PubMed] [Google Scholar]
- 54.Oyama MA and Chittur SV, Genomic expression patterns of mitral valve tissues from dogs with degenerative mitral valve disease. Am J Vet Res, 2006. 67(8): p. 1307–1318. [DOI] [PubMed] [Google Scholar]
- 55.Aupperle H, et al. , An immunohistochemical study of the role of matrix metalloproteinases and their tissue inhibitors in chronic mitral valvular disease (valvular endocardiosis) in dogs. Vet J., 2009. 180(1): p. 88–94. [DOI] [PubMed] [Google Scholar]
- 56.Aupperle H, et al. , Expression of genes encoding matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) in normal and diseased canine mitral valves. J Comp Pathol, 2009. 140(4): p. 271–277. [DOI] [PubMed] [Google Scholar]
- 57.Orton EC, Lacerda CM, and MacLea HB, Signaling pathways in mitral valve degeneration. J Vet Cardiol, 2012. 14(1): p. 7–17. [DOI] [PubMed] [Google Scholar]
- 58.Hagler MA, et al. , TGF-beta signalling and reactive oxygen species drive fibrosis and matrix remodelling in myxomatous mitral valves. Cardiovasc Res, 2013. 99(1): p. 175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Driesbaugh KH, et al. , Serotonin receptor 2B signaling with interstitial cell activation and leaflet remodeling in degenerative mitral regurgitation. J Mol Cell Cardiol, 2018. 115: p. 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goldberg E, et al. , Serotonin and catecholamines in the development and progression of heart valve diseases. Cardiovasc Res, 2017. 113(8): p. 849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McCorvy JD and Roth BL, Structure and function of serotonin G protein-coupled receptors. Pharmacol Ther, 2015. 150: p. 129–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hutcheson JD, et al. , Serotonin receptors and heart valve disease-It was meant 2B. Pharmacol Ther, 2011. 132(2): p. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu J, et al. , Serotonin mechanisms in heart valve disease II: the 5-HT2 receptor and its signaling pathway in aortic valve interstitial cells. Am J Pathol, 2002. 161(6): p. 2209–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Connolly JM, et al. , Fenfluramine disrupts the mitral valve interstitial cell response to serotonin. Am J Pathol, 2009. 175(3): p. 988–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu J, Jackson-Weaver O, and Xu J, The TGFbeta superfamily in cardiac dysfunction. Acta Biochim Biophys Sin (Shanghai), 2018. 50(4): p. 323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Azhar M, et al. , Transforming growth factor beta in cardiovascular development and function. Cytokine Growth Factor Rev, 2003. 14(5): p. 391–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goumans MJ, Liu Z, and ten Dijke P, TGF-beta signaling in vascular biology and dysfunction. Cell Res, 2009. 19(1): p. 116–27. [DOI] [PubMed] [Google Scholar]
- 68.Cushing MC, Liao JT, and Anseth KS, Activation of valvular interstitial cells is mediated by transforming growth factor-beta1 interactions with matrix molecules. Matrix Biol, 2005. 24(6): p. 428–437. [DOI] [PubMed] [Google Scholar]
- 69.Liu AC and Gotlieb AI, Transforming growth factor-beta regulates in vitro heart valve repair by activated valve interstitial cells. Am J Pathol, 2008. 173(5): p. 1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Disatian S and Orton EC, Autocrine serotonin and transforming growth factor 31 signaling mediates spontaneous myxomatous mitral valve disease. J Heart Valve Dis, 2009. 18(1): p. 44–51. [PubMed] [Google Scholar]
- 71.Aupperle H, et al. , Expression of transforming growth factor-beta1, -beta2 and -beta3 in normal and diseased canine mitral valves. J Comp Pathol, 2008. 139(2–3): p. 97–107. [DOI] [PubMed] [Google Scholar]
- 72.Moesgaard SG, et al. , Matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs) and transforming growth factor-beta (TGF-beta) in advanced canine myxomatous mitral valve disease. Res Vet Sci, 2014. 97(3): p. 560–7. [DOI] [PubMed] [Google Scholar]
- 73.Cremer SE, et al. , Alpha-smooth muscle actin and serotonin receptors 2A and 2B in dogs with myxomatous mitral valve disease. Res Vet Sci, 2015. 100: p. 197–206. [DOI] [PubMed] [Google Scholar]
- 74.Cremer SE, et al. , Serotonin concentrations in platelets, plasma, mitral valve leaflet, and left ventricular myocardial tissue in dogs with myxomatous mitral valve disease. J Vet Intern Med, 2014. 28(5): p. 1534–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arndt JW, et al. , Serum serotonin concentrations in dogs with degenerative mitral valve disease. J Vet Intern Med, 2009. 23(6): p. 1208–1213. [DOI] [PubMed] [Google Scholar]
- 76.Ljungvall I, et al. , Serum serotonin concentration is associated with severity of myxomatous mitral valve disease in dogs. J Vet Intern Med, 2013. 27(5): p. 7. [DOI] [PubMed] [Google Scholar]
- 77.Cremer SE, et al. , Plasma and serum serotonin concentrations and surface-bound platelet serotonin expression in Cavalier King Charles Spaniels with myxomatous mitral valve disease. Am J Vet Res, 2015. 76(6): p. 520–31. [DOI] [PubMed] [Google Scholar]
- 78.Droogmans S, et al. , Dose dependency and reversibility of serotonin-induced valvular heart disease in rats. Cardiovasc Toxicol, 2009. 9(3): p. 134–141. [DOI] [PubMed] [Google Scholar]
- 79.Bhattacharyya S, et al. , Circulating plasma and platelet 5-hydroxytryptamine in carcinoid heart disease: a pilot study. J Heart Valve Dis, 2013. 22(3): p. 400–7. [PubMed] [Google Scholar]
- 80.Robiolio PA, et al. , Carcinoid heart disease. Correlation of high serotonin levels with valvular abnormalities detected by cardiac catheterization and echocardiography. Circulation, 1995. 92(4): p. 790–795. [DOI] [PubMed] [Google Scholar]
- 81.Rothman RB and Baumann MH, Serotonergic drugs and valvular heart disease. Expert.Opin.Drug Saf, 2009. 8(3): p. 317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sheikh A, et al. , Elevated blood pressure and risk of mitral regurgitation: A longitudinal cohort study of 5.5 million United Kingdom adults. PLOS Medicine, 2017. 14(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Balachandran K, et al. , Elevated cyclic stretch and serotonin result in altered aortic valve remodeling via a mechanosensitive 5-HT(2A) receptor-dependent pathway. Cardiovasc Pathol, 2012. 21(3): p. 206–13. [DOI] [PubMed] [Google Scholar]
- 84.Balachandran K, et al. , Elevated cyclic stretch alters matrix remodeling in aortic valve cusps: implications for degenerative aortic valve disease. Am J Physiol Heart Circ.Physiol, 2009. 296(3): p. H756–H764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Balachandran K, et al. , Elevated cyclic stretch induces aortic valve calcification in a bone morphogenic protein-dependent manner. Am J Pathol, 2010. 177(1): p. 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Balachandran K, et al. , Aortic valve cyclic stretch causes increased remodeling activity and enhanced serotonin receptor responsiveness. Ann Thorac Surg, 2011. 92(1): p. 147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lacerda CM, et al. , Static and cyclic tensile strain induce myxomatous effector proteins and serotonin in canine mitral valves. J Vet Cardiol, 2012. 14(1): p. 223–230. [DOI] [PubMed] [Google Scholar]
- 88.Disatian S, Lacerda C, and Orton EC, Tryptophan hydroxylase 1 expression is increased in phenotype-altered canine and human degenerative myxomatous mitral valves. J Heart Valve Dis, 2010. 19(1): p. 71–78. [PubMed] [Google Scholar]
- 89.Figueras J, et al. , Comparison of Plasma Serotonin Levels in Patients With Variant Angina Pectoris Versus Healed Myocardial Infarction. The American Journal of Cardiology, 2005. 96(2): p. 204–207. [DOI] [PubMed] [Google Scholar]
- 90.Shimizu Y, et al. , The role of serotonin in ischemic cellular damage and the infarct size-reducing effect of sarpogrelate, a 5-hydroxytryptamine-2 receptor blocker, in rabbit hearts. Journal of the American College of Cardiology, 2002. 40(7): p. 1347–1355. [DOI] [PubMed] [Google Scholar]
- 91.Obayashi K, et al. , Effects of transforming growth factor-beta3 and matrix metalloproteinase-3 on the pathogenesis of chronic mitral valvular disease in dogs. Am J Vet Res, 2011. 72(2): p. 194–202. [DOI] [PubMed] [Google Scholar]
- 92.Lu CC, et al. , Gene network and canonical pathway analysis in canine myxomatous mitral valve disease: a microarray study. Vet J, 2015. 204(1): p. 23–31. [DOI] [PubMed] [Google Scholar]
- 93.Markby GR, et al. , Comparative trancriptomic profiling and gene expression for myxomatous mitral valve disease in the dog and human. Vet Sci, 2017. 4(34). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lacerda CM, et al. , Local serotonin mediates cyclic strain-induced phenotype transformation, matrix degradation, and glycosaminoglycan synthesis in cultured sheep mitral valves. Am J Physiol Heart Circ.Physiol, 2012. 302(10): p. H1983–H1990. [DOI] [PubMed] [Google Scholar]
- 95.Rizzo S, et al. , TGF-beta1 pathway activation and adherens junction molecular pattern in nonsyndromic mitral valve prolapse. Cardiovasc Pathol, 2015. 24(6): p. 359–67. [DOI] [PubMed] [Google Scholar]
- 96.Hulin A, et al. , Emerging pathogenic mechanisms in human myxomatous mitral valve: lessons from past and novel data. Cardiovasc Pathol, 2013. 22(4): p. 245–50. [DOI] [PubMed] [Google Scholar]
- 97.Disha K, et al. , Transforming Growth Factor Beta-2 Mutations in Barlow’s Disease and Aortic Dilatation. Ann Thorac Surg, 2017. 104(1): p. e19–e21. [DOI] [PubMed] [Google Scholar]
- 98.Hulin A, et al. , Metallothionein-dependent up-regulation of TGF-beta2 participates in the remodelling of the myxomatous mitral valve. Cardiovasc Res, 2012. 93(3): p. 480–9. [DOI] [PubMed] [Google Scholar]
- 99.Sainger R, et al. , Human myxomatous mitral valve prolapse: role of bone morphogenetic protein 4 in valvular interstitial cell activation. J Cell Physiol, 2012. 227(6): p. 2595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Geirsson A, et al. , Modulation of transforming growth factor-beta signaling and extracellular matrix production in myxomatous mitral valves by angiotensin II receptor blockers. Circulation, 2012. 126(11 Suppl 1): p. S189–97. [DOI] [PubMed] [Google Scholar]
- 101.Bartko PE, et al. , Effect of Losartan on Mitral Valve Changes After Myocardial Infarction. J Am Coll Cardiol, 2017. 70(10): p. 1232–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hoglund K, et al. , Interbreed variation in serum serotonin (5-hydroxytryptamine) concentration in healthy dogs. J Vet Cardiol, 2018. 20(4): p. 244–253. [DOI] [PubMed] [Google Scholar]
- 103.Roels E, et al. , Evaluation of chemokines CXCL8 and CCL2, serotonin, and vascular endothelial growth factor serum concentrations in healthy dogs from seven breeds with variable predisposition for canine idiopathic pulmonary fibrosis. Res Vet Sci, 2015. 101: p. 57–62. [DOI] [PubMed] [Google Scholar]
- 104.Corcoran BM, et al. , Identification of surface morphologic changes in the mitral valve leaflets and chordae tendineae of dogs with myxomatous degeneration. Am J Vet Res, 2004. 65(2): p. 198–206. [DOI] [PubMed] [Google Scholar]
- 105.Tong LJ, et al. , Platelet function and activation in Cavalier King Charles Spaniels with subclinical chronic valvular heart disease. Am J Vet Res, 2016. 77(8): p. 860–868. [DOI] [PubMed] [Google Scholar]
- 106.Tarnow I, et al. , Dogs with heart diseases causing turbulent high-velocity blood flow have changes in platelet function and von Willebrand factor multimer distribution. J.Vet.Intern.Med, 2005. 19(4): p. 515–522. [DOI] [PubMed] [Google Scholar]
- 107.Cowan SM, et al. , Giant platelet disorder in the Cavalier King Charles Spaniel. Exp.Hematol, 2004. 32(4): p. 344–350. [DOI] [PubMed] [Google Scholar]
- 108.Singh MK and Lamb WA, Idiopathic thrombocytopenia in Cavalier King Charles Spaniels. Aust Vet J, 2005. 83(11): p. 700–3. [DOI] [PubMed] [Google Scholar]
- 109.Yabanoglu S, et al. , Platelet derived serotonin drives the activation of rat cardiac fibroblasts by 5-HT2A receptors. J Mol Cell Cardiol, 2009. 46(4): p. 518–525. [DOI] [PubMed] [Google Scholar]
- 110.Gustafsson BI, et al. , Carcinoid heart disease. Int J Cardiol, 2008. 129(3): p. 318–324. [DOI] [PubMed] [Google Scholar]
- 111.Pellikka PA, et al. , Carcinoid heart disease. Clinical and echocardiographic spectrum in 74 patients. Circulation, 1993. 87(4): p. 1188–1196. [DOI] [PubMed] [Google Scholar]
- 112.Simula DV, et al. , Surgical pathology of carcinoid heart disease: a study of 139 valves from 75 patients spanning 20 years. Mayo Clin.Proc, 2002. 77(2): p. 139–147. [DOI] [PubMed] [Google Scholar]
- 113.Dawson P and Moffatt JD, Cardiovascular toxicity of novel psychoactive drugs: lessons from the past. Prog Neuropsychopharmacol Biol Psychiatry, 2012. 39(2): p. 244–52. [DOI] [PubMed] [Google Scholar]
- 114.Elangbam CS, et al. , 5-hydroxytryptamine (5HT)-induced valvulopathy: compositional valvular alterations are associated with 5HT2B receptor and 5HT transporter transcript changes in Sprague-Dawley rats. Exp.Toxicol.Pathol, 2008. 60(4–5): p. 253–262. [DOI] [PubMed] [Google Scholar]
- 115.Gustafsson BI, et al. , Long-term serotonin administration induces heart valve disease in rats. Circulation, 2005. 111(12): p. 1517–1522. [DOI] [PubMed] [Google Scholar]
- 116.Droogmans S, et al. , In vivo model of drug-induced valvular heart disease in rats: pergolide-induced valvular heart disease demonstrated with echocardiography and correlation with pathology. Eur.Heart J, 2007. 28(17): p. 2156–2162. [DOI] [PubMed] [Google Scholar]
- 117.Lancellotti P, et al. , High-dose oral intake of serotonin induces valvular heart disease in rabbits. Int J Cardiol, 2015. 197: p. 72–5. [DOI] [PubMed] [Google Scholar]
- 118.Perez J, et al. , Elevated Serotonin Interacts with Angiotensin-II to Result in Altered Valve Interstitial Cell Contractility and Remodeling. Cardiovasc Eng Technol, 2018. 9(2): p. 168–180. [DOI] [PubMed] [Google Scholar]
- 119.Jian B, et al. , Progression of aortic valve stenosis: TGF-beta1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann.Thorac.Surg, 2003. 75(2): p. 457–465. [DOI] [PubMed] [Google Scholar]
- 120.Jian B, et al. , Serotonin mechanisms in heart valve disease I: serotonin-induced up-regulation of transforming growth factor-beta1 via G-protein signal transduction in aortic valve interstitial cells. Am J Pathol, 2002. 161(6): p. 2111–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ayme-Dietrich E, et al. , The role of 5-HT2B receptors in mitral valvulopathy: bone marrow mobilization of endothelial progenitors. Br J Pharmacol, 2017. 174(22): p. 4123–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hutcheson JD, et al. , 5-HT(2B) antagonism arrests non-canonical TGF-beta1-induced valvular myofibroblast differentiation. J Mol Cell Cardiol, 2012. 53(5): p. 707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pavone LM, et al. , Heart valve cardiomyocytes of mouse embryos express the serotonin transporter SERT. Biochem.Biophys.Res Commun, 2008. 377(2): p. 419–422. [DOI] [PubMed] [Google Scholar]
- 124.Pavone LM and Norris RA, Distinct signaling pathways activated by “extracellular” and “intracellular” serotonin in heart valve development and disease. Cell Biochem Biophys, 2013. 67(3): p. 819–28. [DOI] [PubMed] [Google Scholar]
- 125.Mekontso-Dessap A, et al. , Deficiency of the 5-hydroxytryptamine transporter gene leads to cardiac fibrosis and valvulopathy in mice. Circulation, 2006. 113(1): p. 81–89. [DOI] [PubMed] [Google Scholar]
- 126.Pavone LM, et al. , Serotonin transporter gene deficiency is associated with sudden death of newborn mice through activation of TGF-beta1 signalling. J Mol Cell Cardiol, 2009. 47(5): p. 691–7. [DOI] [PubMed] [Google Scholar]
- 127.Scruggs SM, Disatian S, and Orton EC, Serotonin transmembrane transporter is down-regulated in late-stage canine degenerative mitral valve disease. J Vet Cardiol, 2010. 12(3): p. 163–9. [DOI] [PubMed] [Google Scholar]
- 128.Cremer SE, et al. , Serotonin markers show altered transcription levels in an experimental pig model of mitral regurgitation. Vet J, 2015. 203(2): p. 192–8. [DOI] [PubMed] [Google Scholar]
- 129.Heils A, et al. , Allelic variation of human serotonin transporter gene expression. J Neurochem, 1996. 66(6): p. 2621–2624. [DOI] [PubMed] [Google Scholar]
- 130.Jiao YR, et al. , 5-HTT, BMPR2, EDN1, ENG, KCNA5 gene polymorphisms and susceptibility to pulmonary arterial hypertension: A meta-analysis. Gene, 2019. 680: p. 34–42. [DOI] [PubMed] [Google Scholar]
- 131.Jin DC, et al. , Regulation of the serotonin transporter in the pathogenesis of irritable bowel syndrome. World J Gastroenterol, 2016. 22(36): p. 8137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.de Medeiros Alves V, et al. , Genetic Polymorphisms Might Predict Suicide Attempts in Mental Disorder Patients: A Systematic Review And Meta-Analysis. CNS Neurol Disord Drug Targets, 2015. 14(7): p. 820–7. [DOI] [PubMed] [Google Scholar]
- 133.Driesbaugh KH, et al. , The association of the 5HT transporter polymorphism LL-genotype with non-syndromic mitral valve prolapse requiring surgery at a younger age. J Arterio Thromb Vasc Biol, 2017. 37: p. A247. [Google Scholar]
- 134.Lee CM, et al. , Polymorphism in the serotonin transporter protein gene in Maltese dogs with degenerative mitral valve disease. J Vet Sci, 2018. 19(1): p. 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kantor S, et al. , Increased wakefulness, motor activity and decreased theta activity after blockade of the 5-HT2Breceptor by the subtype-selective antagonist SB-215505. British Journal of Pharmacology, 2004. 142(8): p. 1332–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pitychoutis PM, et al. , Mice Lacking the Serotonin Htr2B Receptor Gene Present an Antipsychotic-Sensitive Schizophrenic-Like Phenotype. Neuropsychopharmacology, 2015. 40(12): p. 2764–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lesurtel M, Platelet-Derived Serotonin Mediates Liver Regeneration. Science, 2006. 312(5770): p. 104–107. [DOI] [PubMed] [Google Scholar]
- 138.Freeman LM, et al. , Development and evaluation of a questionnaire for assessing health-related quality of life in dogs with cardiac disease. Journal of the American Veterinary Medical Association, 2005. 226(11): p. 1864–1868. [DOI] [PubMed] [Google Scholar]
- 139.Perlman RL, Mouse models of human disease: An evolutionary perspective. Evol Med Public Health, 2016. 2016(1): p. 170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Elsea SH and Lucas RE, The mousetrap: what we can learn when the mouse model does not mimic the human disease. ILAR J, 2002. 43(2): p. 66–79. [DOI] [PubMed] [Google Scholar]
- 141.Young NS, Mouse medicine and human biology. Semin Hematol, 2013. 50(2): p. 88–91. [DOI] [PubMed] [Google Scholar]


