Abstract
Hereditary Leiomyomatosis and Renal Cell Cancer (HLRCC) is an autosomal dominant hereditary cancer syndrome with incomplete penetrance. It is caused by a germline amorphic allele of the FH gene, which encodes the TCA cycle enzyme, fumarate hydratase (FH). HLRCC patients are genetically predisposed to develop skin leiomyomas, uterine fibroids, and the aggressive kidney cancer of type 2 papillary morphology. Loss-of-heterozygocity at the FH locus that cause a complete loss of FH enzymatic function is always detected in these tumor tissues. Molecular pathway elucidation, genomic studies, and systematic genetics screens reported over the last two decades have identified several FH-inactivation driven pathways alterations, as well as rationally conceived treatment strategies that specifically target FH−/− tumor cells. These treatment strategies include ferroptosis induction, oxidative stress promotion, and metabolic alteration. As the fundamental biology of HLRCC continues to be uncovered, these treatment strategies continue to be refined and may one day lead to a strategy to prevent disease onset among HLRCC patients. With a more complete picture of HLRCC biology, the safe translation of experimental treatment strategies into clinical practice is achievable in the foreseeable future.
One gene, two different hereditary disorders
Fumarate hydratase (FH) is a TCA cycle enzyme that catalyzes the hydration of fumarate to form malate. It is a highly conserved protein, with sequence, activity, and functions commonly shared among prokaryotes and eukaryotes including humans.[1] To date, the enzyme is known to carry out two distinct functions: the TCA cycle, the DNA damage response pathway (DDR) (Figure 1).[2] In humans, FH is encoded by the fumarate hydratase gene (FH), located on the q-arm of chromosome 1 (chr1q43). FH inactivating mutations in humans can cause two different diseases: 1. Fumarate hydratase deficiency, a Mendelian inborn error of metabolism, which follows an autosomal recessive inheritance pattern.[3, 4] And 2. Hereditary Leiomyomatosis and Renal Cell Cancer (HLRCC), a hereditary cancer syndrome that follows an autosomal dominant inheritance pattern with incomplete penetrance.[5] The two diseases have very distinct manifestations: The age of onset for fumarate hydratase deficiency is during the neonatal to early infantile periods, with some cases diagnosed at late infantile stages. It is characterized by encephalopathy, failure to thrive, and other neurological abnormalities.[6–9] The age of onset for HLRCC however, is typically around adolescence to adult with penetrance increasing with age.[10] It is characterized by the development of multiple tumor types including skin leiomyomas, uterine fibroids, and HLRCC kidney tumors with morphological and clinical features similar to those of type 2 papillary renal cell carcinoma (PRCC2).[5] In addition to the 3 main tumor types, bladder cancer and Leydig cell tumors of the testis have also been reported in HLRCC patients.[11–13] HLRCC associated skin leiomyomas and uterine fibroids are benign tumors that exhibit a high recurrent rate.[14] A skin leiomyoma can be painful and disfiguring, causing significant hardship to HLRCC patients. Apart from skin leiomyomas and uterine fibroids, HLRCC associated PRCC2 is a very aggressive form of kidney cancer.[15, 16] This review will mainly focus on advances in HLRCC translational researches, and the context as well as the technologies that enabled those discoveries.
Figure 1. Fumarate hydratase (FH) function and the locus of the FH gene.
The FH gene is located at chr1q43. It encodes for the fumarate hydratase enzyme (FH). FH catalyzes the reversible conversion of fumarate to malate in the TCA cycle. FH also takes part in DNA Damage Response (DDR) signaling, whereby FH is recruited to the DNA damage (i.e. DNA double strand break) site, where it catalyzes the conversion of malate to fumarate, which serves as a signal to promote non-homologous end joining (NHEJ).
The discovery of FH as a tumor suppressor gene
Prior to the identification of FH as the tumor suppressor gene associated with HLRCC, there were several anecdotal reports indicating significant overlap between clinical cases of cutaneous leiomyomas and uterine fibroids (a.k.a. uterine leiomyomata).[17–19] This overlap was later recognized as a disorder by the name of Multiple Cutaneous and Uterine Leiomyomata 1 (MCUL1).[19] In 2001, a genome wide screen of 11 MCUL1 families narrowed the region of the genome responsible for MCUL1 to chr1q42.3-q43.[20] This was later refined in 2002 with new patient samples and FH was identified as the tumor suppressor gene associated with MCUL1 and HLRCC.[5, 21] Enzymatic assay confirmed that the mutations ablated FH enzymatic function and that the mutation follows the Knudson’s two hit hypothesis.[5]
FH−/− and the activation of the hypoxia-inducible factor 1-alpha
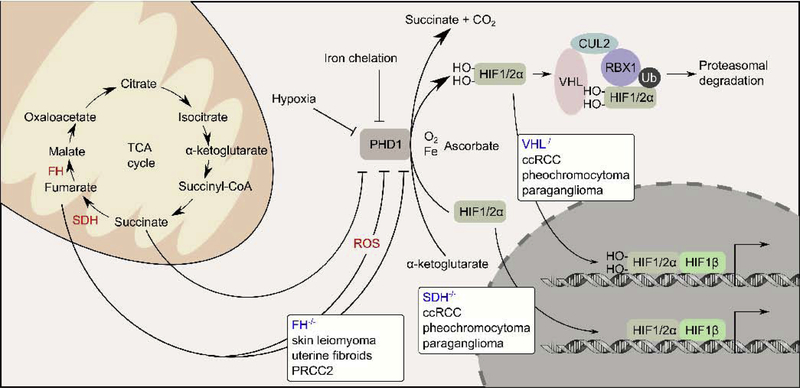
FH was not the first TCA cycle enzyme identified as a tumor suppressor gene. At around the same time of the discovery of FH, the genes encoding the different subunits of succinate dehydrogenase (SDHB, SDHC, SDHD) were identified as tumor suppressor genes associated with a hereditary syndrome known as hereditary pheochromocytoma and/or paraganglioma (HPGL).[22–26] The spectrum of tumors associated with HPGL overlaps with another hereditary cancer syndrome known as the Von Hippel-Lindau (VHL) syndrome, which is associated with the VHL gene loss.[27] Since the molecular mechanism of VHL syndrome was well characterized, it was hypothesized that FH, SDH, and VHL mutations all cause dysregulation to the same pathway. Specifically, VHL gene product is a substrate adapter protein for a CUL2 containing E3 ubiquitin ligase complex that mediates the ubiquitylation of the hydroxylated forms of hypoxia-inducible factors 1A and 2A (HIF1A and HIF2A) (Figure 2).[27] Thus, VHL loss impedes HIF1A and HIF2A ubiquitylation, causing constitutive HIF1A and HIF2A activation, a phenotype known as pseudohypoxia. SDH loss lead to the accumulation of intracellular succinate, which can competitively inhibit prolyl hydroxylase that hydroxylate HIF1A and 2A. As a result, hydroxylation of HIF1A and HIF2A is impaired, leading to pseudohypoxia similar to VHL loss. Since fumarate loss leads to the accumulation of intracellular fumarate, it was hypothesized that the accumulated fumarate could competitively inhibit prolyl hydroxylase in a similar manner as succinate (Figure 2).[28–30] This theory was supported by in vitro enzymatic assays showing that fumarate can competitively inhibit prolyl hydroxylase with a Ki of 6.6μM as compared to succinate with a Ki of 21μM.[28] In a separate study, it was demonstrated through immunohistochemical analysis, that HIF1A staining was only moderate in HLRCC associated fibroids, while the staining was much stronger in HLRCC associated renal tumors.[30] In contrast to HLRCC tumors, SDH mutant HPGL tumors consistently showed high HIF1A expression in the 12 paragangliomas and 4 pheochromocytomas examined.[30] These results point to several inconsistencies as to why FH mutation is more effective at activating HIF1A in fibroids as opposed to renal tumors, and why SDH mutant HPGL tumors show more consistent HIF1A activation.[30]
Figure 2. VHL−/−, SDH−/−, and FH−/− are implicated in pseudohypoxia response.
Under normoxia conditions, HIF1A and HIF2A are hydroxylated by prolyl hydroxylase (PHD1), a reaction that requires molecular oxygen, iron, and α-ketoglutarate as co-factors. VHL is a substrate adapter protein that mediates the ubiquitylation of the hydroxylated forms of HIF1A and HIF2A by a cullin 2 (CUL2) containing E3-ubiquitin ligase complex. The ubiquitylated HIF1A and HIF2A are targeted for proteasomal degradation. VHL−/− causes the hydroxylated forms of HIF1A and HIF2A to accumulate, allowing them to activate target genes under normaxia condition, a phenomenon known as pseudohypoxia. VHL−/− causes ccRCC, pheochromocytoma, and paraganglioma. Like VHL−/−, SDH−/− also causes ccRCC, pheochromocytoma, and paraganglioma. In SDH−/− tumors, succinate accumulates and acts as a competitive inhibitor of PHD1, causing the accumulation and activation of HIF1A and HIF2A, and pseudohypoxia. Unlike VHL−/− and SDH−/−, FH−/− causes a different spectrum of tumors (skin leiomyoma, uterine fibroids, and PRCC2). Several studies demonstrated that fumarate accumulates following FH inactivation and can drive pseudohypoxia by either competitively inhibiting PHD1 or by generating reactive oxygen species, which in turn inhibits PHD1.
To investigate tumorigenesis events that take place following biallelic FH inactivation, Pollard and co-workers developed a mouse model of Fh1 (mouse homolog of human FH) knockout.[31] Unlike humans with FH deficiency, Fh1−/− mice died in early embryogenesis. Thus, to investigate kidney tumorigenesis following Fh1 knockout, they conditionally knocked out Fh1 in the kidney using Ksp1.3/Cre. The Fh1fl/fl Ksp1.3/Cre animals appeared normal until 8 months of age when they present renal failure due to the development of renal cysts, possibly an indication of premalignant hyperproliferative lesions. None of the mice developed kidney tumors and immunohistochemical analyses showed an increase in nuclear HIF1A staining, and a moderate increase in nuclear HIF2A staining in the Fh1 knockout renal cyst.[31] This differential activation of HIF1A vs HIF2A was later demonstrated as a consequence of altered cellular iron homeostasis.[32]
Apart from the direct inhibition of prolyl hydroxylase by fumarate, it was also showed that FH inactivation increases cellular reactive oxygen species (ROS), which in turn drives constitutive HIF activation (Figure 2).[33] Other mechanisms may also contribute to the observed constitutive HIF activation. Those mechanisms will be outlined wherever the relevant signaling pathways is mentioned within this review. It is important to note that conditional Fh1 and Hif1a double knockout mice have been created, and the addition of the Hif1a knockout exacerbates the renal cyst development, suggesting that Hif1a activation is likely a compensatory mechanism and not a tumor promoting event.[31]
Genomic studies revealed new FH-inactivation driven pathway alterations
Genomic studies on HLRCC tumors begin with gene expression profiling of FH mutant and FH wild type uterine fibroids.[34] The study revealed that FH mutant fibroids over-expressed genes involved in carbohydrate metabolism, iron homeostasis, and oxidoreductases. Through the gene expression profiling analyses, a 7 gene classifier, consisting of LDHA, NQO1, LAMA2, BNIP3, MYO15B, CDKN1C and COL6A2, was able to accurately predict FH mutation status in fibroids, signifying that FH inactivation alters gene expression profile in a specific way. Array Comparative Genomic Hybridization (aCGH) revealed chromosomal aberration with gains in chr2, chr7, and chr17, and losses in chr3q12.3-q21.1, chr14, chr18, and chrX in HLRCC associated renal tumors.[35]
Similarly, gene expression profile analyses were performed to evaluate gene expression changes following Fh1 knockout in the kidney tissue of mice. The study found many similar gene expression alterations among the Fh1 knockout mouse kidney tissues, human HLRCC tissues, and sporadic PRCC2 tissues. The study concluded that the observed gene expression changes were most likely due to HIF1A activation.[36]
One of the apparent anomalies within the HIF1A activation theory of HLRCC biology has been the differing spectrums of tumor arise in HLRCC as compared to other disorders associated with the disruption to the HIF1A signaling pathway. To bring this anomaly to light, our group has utilized parametric gene set enrichment analyses to retrospectively measure HIF1A signature enrichment in legacy data derived from sporadic PRCC2, HLRCC, ccRCC, paraganglioma, and pheochromocytoma. As expected, ccRCC, paraganglioma, and pheochromocytoma tumors that were known to harbor either VHL or SDH mutations were enriched for HIF1A signature, but HLRCC and the morphologically similar sporadic PRCC2 tumors did not show the enrichment of the HIF1A signature.[37] Subsequent analyses revealed the persistent upregulation of aldose ketose reductase family 1 member B10 (AKR1B10) in both HLRCC and sporadic PRCC2 tumors and the AKR1B10 overexpression is driven by the activation of the NRF2 transcription factor. This feature unifies HLRCC and sporadic PRCC2. Moreover, the coordinated increased expression of AKR1B10 together with other better known NRF2 targets genes, including NQO1 and TXNRD1, were also seen in the legacy microarray data derived from HLRCC associated fibroids.[37] These computational findings were empirically validated, revealing that fumarate accumulated following FH inactivation, which in turn activates NRF2 by covalently modifying C151 and C288 of Kelch Like ECH Associated Protein 1 (KEAP1), the bonafide negative regulator of NRF2, leading to sustained NRF2 activation.[37] Mechanistically, KEAP1 is a substrate adaptor protein that mediates NRF2 ubiquitylation by a Cullin 3 (CUL3) containing E3-ubiquitin ligase complex.[38, 39] KEAP1 functions as a cellular electrophile sensor whereby electrophiles can form covalent adducts with its surface cysteine residues.[40, 41] These covalent modifications can disrupt KEAP1-NRF2 interaction and prevent NRF2 ubiquitylation. Consequently, newly synthesized NRF2 become available to carry out its transcriptional functions. Thus, covalent modification of C151 and C288 of KEAP1 activates NRF2. Since in HLRCC cells the fumarate accumulation is permanent, the NRF2 activation is constitutive (Figure 3).[37] Around the same time, a mouse model study involving the development of an Fh1/Hif1a double knockout mouse model also identified that Fh1 inactivation in mice drives an Nrf2 activation phenotype, reinforcing that NRF2 activation is one of the main cellular alterations following FH inactivation.[42]
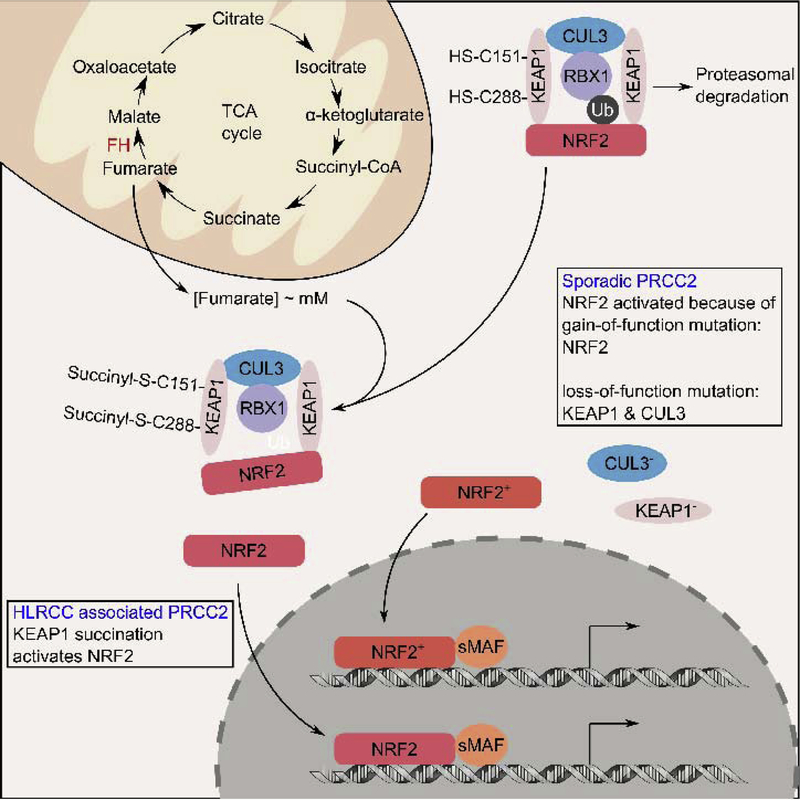
Figure 3. Hereditary and sporadic PRCC2 converge at constitutive NRF2 activation.
In normal cells, two molecules of KEAP1 mediate NRF2 ubiquitylation by a cullin 3 (CUL3) containing E3-ubiquitin ligase complex, marking it for proteasomal degradation. In hereditary PRCC2, FH inactivation causes intracellular fumarate levels to accumulate at millimolar (mM) concentrations. The accumulated fumarate can covalently modify cysteine 151 (C151) and cysteine 288 (C288) on KEAP1, rendering it unable to mediate NRF2 ubiquitylation. This allows newly synthesized NRF2 to accumulate and translocate into the nucleus where it dimerizes with one of the small MAF proteins to promote target gene expression. Since FH inactivation in hereditary PRCC2 is permanent, the resulting NRF2 activation is constitutive. Sporadic PRCC2 rarely harbor FH mutation, but still exhibit constitutive NRF2 activation. Studies have shown that constitutive NRF2 activation in sporadic PRCC2 can be driven by either somatic gain-of-function NRF2 mutations or somatic loss-of-function mutations to KEAP1, CUL3.
Unlike other subtypes of kidney cancer whereby the hereditary and sporadic forms of the tumors harbor mutations to the same tumor suppressor and oncogenes, the sporadic counterpart of HLRCC rarely harbors an FH mutation.[43] Thus, how NRF2 becomes activated in sporadic PRCC2 was an obvious scientific question following the discovery that sustained NRF2 activation is a unifying feature of HLRCC and PRCC2.[37] The answer to this question came to light when our group utilized exome sequencing on carefully curated cases of PRCC2 and found that those tumor cases harbored gain-of-function NRF2 mutations and loss-of-function mutations to CUL3.[44] Functional studies confirmed the functional changes of the identified NRF2 and CUL3 mutations.[44] Subsequently, The Cancer Genome Atlas (TCGA) reported in a larger cohort that mutations that drive sustained NRF2 activation, including NRF2 gain-of-function, KEAP1 loss-of-function, and CUL3 loss-of-function, were significantly enriched in sporadic PRCC2 cases, confirming our initial exome sequencing findings.[45]
The succinated proteome
The identified covalent modification of cysteine residues on proteins by fumarate is a post-translational modification known as succination.[46] Consequently, in HLRCC cells, the high level of fumarate will drive succination of a large number of proteins, thus representing a new frontier in HLRCC research. Antibodies that recognize 2-succinyl-cysteine (2-SC) have been developed and used to detect protein succination in various tissues. Since the discovery of protein succination in HLRCC, immunohistochemical staining of 2-SC emerged as a reliable way to distinguish HLRCC associated tumors from other sporadic cases.[47] The antibody also allows proteome wide identification of succinated proteins through immunoprecipitation followed by shotgun tandem mass spectrometry techniques. One such effort identified a total of 94 succinated proteins from Fh1−/− mice. Notably, they performed a detailed characterization of the TCA cycle enzyme mitochondrial aconitase 2 (ACO2). This enzyme was identified as a succinated protein, which resulted in the inhibition of its enzymatic function.[48] A similar effort was conducted on clinical tumor samples where a total of 60 proteins were identified to have at least a single succinated cysteine.[49] The succination of a number of these proteins was shown to have functional consequences. The study also indicated that a vast majority of these proteins are involved in redox signaling. While comprehensive, these large-scale proteomic efforts are likely to present sampling bias stemmed from tissue specific gene expression differences, distinguishing proteins with high homologies, and the inherent difficulties of teasing out post-translational modifications of proteins that carry elements with diverse isotopic masses. For example, the identification of an overrepresentation of proteins involved in redox signaling in HLRCC tumor samples is likely a consequence of NRF2 activation in those tumors since many redox signaling protein encoding genes are direct NRF2 targets. Another study utilized a clickable chemotype mimic, fumarate-alkyne, to react and pull-down proteins amenable to covalent modification by fumarate.[50] Since the fumarate-alkyne is inherently more reactive than fumarate, the identification of cysteine residues controllable by succination in HLRCC tumors (FH-regulated cysteine residues) was further selected by out competing the fumarate-alkyne bound proteins with physiological concentrations of fumarate in HLRCC tumors (low millimolar concentration) prior to the pull down. This chemical biology approach identified 684 high-confidence FH-regulated cysteines. Further analyses revealed that fumarate reactive cysteine residues are preferably flanked by acidic amino acids, like glutamate and aspartate, and the paradoxical finding that succination is more permissive in a more acidic pH where the sulfhydryl group of cysteine is likely to be protonated.[50]
The proteome wide identification of succination sites allows for the development of a succination site prediction algorithm that may led to in silico prediction of succinated proteins. One initial study examined the different features of succinated sites.[51] These features included: Gene Ontology, hydrophobicity of the cysteine flanking peptide, amino acid composition flanking the cysteine residue, secondary protein structure, accessibility of the sulfur atoms, and the pKa of the sulfhydryl group. The Gene Ontology analyses showed a significant enrichment of mitochondrial-associated terms, indicating that proteins residing within the mitochondria are more likely to be succinated, possibly due to higher fumarate concentration within the compartment. Apart from Gene Ontology, annotating proteins based on proteins’ domains identified two significantly enriched domains: an RNA recognition motif, and a nucleotide-binding alpha-beta plait. Recently, we reported that iron regulatory proteins (IRPs), RNA binding proteins that regulate the translation of iron signaling proteins, are succinated in HLRCC accordingly. The succination alters cellular iron homeostasis in HLRCC and induces a pro-proliferative signaling in HLRCC cells.[52] Among the features analyzed in the study, only two features (Composition of the flanking amino acids and accessibility of the sulfur atom) were deemed predictive of succination sites. Other than that, hydrophobicity of the cysteine flanking peptide was found to be significantly lowered in peptide containing succination target. However, the feature was deemed not predictive as the signal to noise ratio was rather high for the feature. While this study did not find pKa of the sulfhydryl group to be predictive of reactivity to fumarate, protonation of fumarate in an acidic environment was shown to encourage 2-SC formation. To date, a reliable algorithm to predict 2-SC site remains elusive. Increased availability of proteomic data will likely drive the development of such an algorithm in the near future.
Iron signaling perturbation in HLRCC
FH inactivation induced changes in cellular metabolism and HIF activation inspired investigation into the effects of FH inactivation on cellular iron homeostasis. Tong and co-workers demonstrated that FH inactivation decreased the intracellular labile iron pool, which resulted in IRPs repression of HIF2A translation.[32] Specifically, they showed that the absence of FH inactivates AMP-activated protein kinase (AMPK) which in turn suppresses divalent metal transporter 1 (DMT1) expression (Figure 4). Since DMT1 is essential in transporting iron into the cytosol, decreased expression will lead to a lowered labile iron pool and increased IRPs levels. The IRPs can then bind to the iron regulatory element (IRE) located in the 5’ UTR of the HIF2A transcript and repress its translation (Figure 4). The study went on to demonstrate that siRNA mediated repression of HIF1A in HLRCC cells reduced its invasion activity. This suggested that elevation of HIF1A plays a role in HLRCC tumor progression.[32] Interestingly, this differential HIF1A vs HIF2A activation is in discordance with the differential HIF1A vs HIF2A activation in ccRCC, which frequently harbors VHL loss-of-function mutations that lead to sustained HIF activation. In ccRCC, HIF2A is the driver of oncogenic potential, whereby reconstitution of HIF2A into VHL rescued ccRCC cells alone is sufficient to restore tumorigenic potential.[53] Accordingly, aggressive ccRCC often harbors HIF1A loss-of-function mutations that lead to selective activation of HIF2A alone, suggesting a tumor suppressive role of HIF1A in ccRCC.[54] Another contradicting finding comes from evidence emerging from conditional Fh1−/−/Hif1a−/− double knockout mice, whereby the addition of Hif1a−/− exacerbated the development of the premalignant lesions, suggesting a potential tumor suppressive role of HIF1A in HLRCC.[42]
Figure 4. Iron homeostasis disruption in HLRCC.
Fumarate accumulates at millimolar (mM) concentration in HLRCC cells. The accumulated fumarate drives constitutive NRF2 activation, which promoter the transcription of ferritin light (FTL) and ferritin heavy (FTH1) genes. Fumarate also inhibits iron regulatory proteins (IRPs) ability to repress FTL and FTH1 mRNA translation, resulting in high intracellular ferritin level. This high intracellular ferritin can sequester free iron, resulting in a drop in labile iron pool. The high ferritin level also indirectly activates FOXM1 transcription factor, which drive a sustained proliferative signaling. Iron is an essential element in many cellular processes. HLRCC cells rely on ferritinophagy to release ferritin stored iron. Fumarate was also shown to indirectly inhibit AMP kinase (AMPK), which in turn indirectly inhibit divalent metal transporter 1 (DMT1) expression. This prevents the efflux of iron from endosome into the cytoplasm, further reduces the labile iron pool. The low labile iron pool, causes IRPs to repress HIF2A translation, resulting in specific activation of HIF1A. Red colored arrow indicates that the pathway is upregulated in HLRCC cells. Blue colored arrow indicates that the pathway is down regulated in HLRCC cells. Green colored arrow indicates that the pathway is required in HLRCC cells. Dotted line indicates indirect mechanism. Solid line indicates that direct mechanism has been reported.
The effort to catalog the succinated proteome has identified ACO2 as a succination target.[48] ACO2 belongs to the same family of proteins as IRPs. Thus, investigation into the succination of IRPs was performed.[52] The study focused on IRP2, the dominant member of the IRPs, and identified 14 potential succination sites. Succination of IRP2 represses its ability to mediate translational repression on ferritin light (FTL) and ferritin heavy (FTH1) mRNA, resulting in increased FTL and FTH1 translation. Importantly, NRF2 is constitutively active in HLRCC cells, and both FTL and FTH1 are bonafide NRF2 target genes. Thus, NRF2 activation together with inhibition of IRP2 translational repression activity results in increased ferritin protein levels in HLRCC, which in turn drives pro-proliferative signaling by activating the Forkhead Box M1 (FOXM1) transcription factor (Figure 4).[52] It is important to note that the increased ferritin levels may lead to over sequestration of free iron, resulting in lowered intracellular labile iron pool, which may lead to HIF1A activation, inhibition of iron sulfur containing metabolic enzymes, and methyl transferases that require iron as a cofactor. The high sequestration of iron by ferritin also suggest that HLRCC cells may be highly dependent on ferritinophagy to release the stored iron for cellular utilization (Figure 4). These various effects of high cellular ferritin remain to be explored.
Molecular targets of HLRCC
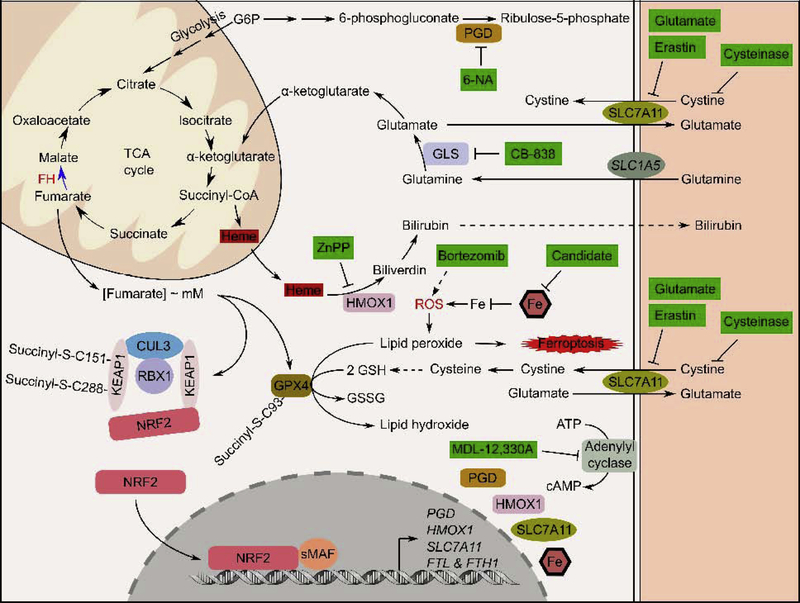
As outlined in the earlier sections, biallelic FH inactivation imparts very significant changes to the transcriptome and the proteome. Since all cancer cells and only cancer cells in HLRCC patients harbor biallelic FH inactivation, biological changes driven by FH inactivation provide unique opportunities for targeted therapy. Prior to the molecular characterization of HLRCC tumors, targeted therapies of this aggressive tumor type were largely based on the metabolic changes impart by FH inactivation. Particularly FH inactivation increases cellular dependency on glycolysis for ATP production. At the same time, this metabolic shift also leads to an increase in production of reactive oxygen species (ROS). Accordingly, HLRCC cells are more sensitive to the proteasome inhibitor, bortezomib, which can increase cellular ROS level and push the cells to undergo apoptotic cell death (Figure 5).[55, 56] Other metabolic based treatment strategies involved the utilization of 2-deoxy glucose to block glycolysis. However, such therapies are not well tolerated and did not result in observable tumor regression.[57] Following the development of the pseudohypoxia hypothesis, antiangiogenic therapy (i.e Sunitinib), that has proven efficacy in treating other pseudohypoxic tumors, were experimented but did not result in a desirable outcome.[58] From in vitro experiments pathway agnostic approaches to target the FH-inactivated cells were also tested. One such effort stemmed from in silico reconstruction of stoichiometric metabolism models. Using the constructed model, it was predicted that FH-inactivated cells will not survive in the absence of heme oxygenases (HMOX1 and HMOX2) which are overexpressed in FH-inactivated cells.[59] Specifically, the study showed that in FHinactivated cells, metabolic rearrangement occurs, whereby carbon sources from glutaminolysis and glycolysis are channeled into heme biosynthesis as succinyl-coA. The synthesized heme is then degraded by HMOX1 to form bilirubin which is excreted out of the cancer cells (Figure 5). [59] As such, heme biosynthesis and degradation serve to complete carbohydrate catabolism and glutaminolysis, while continually supplying the cells with NADH and biosynthetic precursors when the TCA cycle is truncated upon FH inactivation. Inhibiting HMOX1 and heme biosynthesis stifled the cells’ ability to complete carbohydrate and glutamine metabolism and resulted in conditional cell death (Figure 5).[59]
Figure 5. Various therapy intervention strategies for HLRCC.
HLRCC research over the past two decades have identified various ways to selectively target kill HLRCC cells. Green colored boxes indicate compounds and their mechanisms of action. Solid line indicates that the direct mechanism have been reported. Dotted line indicates that the direct mechanism is not known.
The availability of gene expression data from HLRCC cell lines has allowed for the prediction of drug sensitivity using a machine learning approach. We have utilized legacy NCI-60 gene expression data coupled with dose response information on each of the 60 cell lines towards different drugs/compounds with well-defined mechanisms of action to develop predictive classifiers using the established k-tsp algorithm.[60, 61] The effort identified that FH-inactivation sensitizes cells to ferroptosis, an iron dependent cell death mechanism. Mechanistic studies revealed the attenuation of glutathione peroxidase 4 (GPX4), the regulatory node of ferroptotic cell death, activity in FH-inactivated cells.[62] Specifically, C94 of GPX4 is succinated in FH-inactivated cells and the succinated form of GPX4 has reduced activity (Figure 5). Compounds that induce ferroptosis work by either directly or indirectly (by suppressing glutathione production) inhibiting GPX4 which leads to the accumulation of lipid peroxides and causes the eventual oxidative death of the cell.[62] Importantly, sensitivity to ferroptosis presents a means to selectively kill HLRCC cells with little to no apparent adverse reaction. Once such strategy involves the use of the synthetic enzyme, cysteinase, that degrades and depletes circulatory cystine and cysteine.[63] Cysteinase inhibits GPX4 activity by depriving the HLRCC cancer cells of cysteine required for glutathione biosynthesis, and therefore, depriving GPX4 of the co-factor it needs to carry out its function (Figure 5). Importantly, cysteinase treatment has no detectable side effects because normal cells can synthesize cysteine through the transsulfuration pathway, while the transsulfuration pathway is not active in cancer cells.[63] Additionally, ferroptosis is an immunogenic cell death mechanism.[64] Thus, ferroptosis induction may generate secondary cancer killing effects through immunostimulation. Preclinical studies are currently ongoing to evaluate the efficacy of cysteinase in HLRCC treatment.
Functional genomic screenings for therapeutic targets
Genetic screens utilizing pool viral libraries coupled with massively parallel sequencing offers a quick and systematic mean to identify therapeutic targets. In the past, short hairpin RNA (shRNA) libraries have been used in synthetic lethality screens to identify therapeutic targets that selectively kill cancer cells with a specific tumor suppressor gene loss while leaving normal cells unharmed. To date, there have been two reported synthetic lethality screens to identify genes, when knocked down, are synthetic lethal with FH inactivation. The first study utilized a pooled shRNA library consisting of 55,000 shRNA targeting 10,000 genes in the genome.[65] The study utilized HEK293 cells transduced with the library at low titer (MOI=3). The positively transduced cells were then divided into two groups, with one group being transfected with FH targeting siRNA, while another was transfected with non-targeting control siRNA. Following the identification of under-represented shRNA species using parallel sequencing, adenylate cyclase was identified as the synthetic lethal target of FH inactivated cells.[65] A more recent study utilized a lentiviral shRNA library consisting of shRNA targeting 341 metabolic related genes.[66] The screen identified the synthetic lethal combination of the pentose phosphate enzyme, phosphogluconate dehydrogenase (PGD) knockdown with FH-inactivation.[66] Mechanistically, PGD inhibition blocks glycolysis, suppresses reductive carboxylation of glutamine, and increases the NADP+/NADPH ratio to disrupt redox homeostasis (Figure 5). Small molecule based PGD inhibitors, 6-aminonicotinamide (6-AN), and 6-phosphoric acid mono-[(5R,4R)-5-hydroxycarbamoyl-2,2-dimethyl [1,3]dioxolan-4-ylmethyl] ester (PAME) showed selective killing of FH-inactivated cells in vitro (Figure 5). Activation of PGD targeting shRNA also resulted in tumor regression in vivo. It is important to note that PGD loss-of-function mutation has been previously identified in the human population and is associated with occasional mild hemolytic anemia, suggesting that PGD inhibition should be well tolerated in human.[67] However, existing PGD inhibitors lack specificity. For example, 6-AN caused neurological toxicity in early clinical trials, presumably due to the drug’s direct action on the central nervous system.[68] Thus, there remains a need to develop more specific PGD inhibitors or 6-AN variants that do not cross the blood brain barrier into the central nervous system.
Advances and innovation in CRISPR technology has given rise to various robust systems for genome wide genetic screens. Earlier CRISPR screens include loss-of-function screens that defined the genes’ essentiality.[69] More recently, CRISPR screens have been expanded to include the identified gene’s involvement in a specific signaling pathway using a reporter system,[70, 71] to gene activation and gene knockdown screens using CRISPR interference (CRISPRi) and CRISPR activation (CRISPRa) technology.[72, 73] CRISPR based screens have several advantages over RNA interference-based screen. Importantly, shRNA-based systems rely on endogenous RNAi machineries to achieve knockdown. Competition between endogenous and exogenous substrates for the RNAi machineries may lead to non-specific toxicity. An excellent review that outlined a detailed comparison between CRISPR-based and RNAi-based screens is available.[74] Although direct CRISPR screening for HLRCC therapeutic targets has not been reported, one study has utilized CRISPR based screening to identify therapeutic targets for mouse Keap1−/− lung cancer.[75] This is an important study because a significant subset of lung cancer tumors harbor either somatic loss-of-function mutations to KEAP1 or somatic gain-of-function mutation to NRF2.[76, 77] Collectively, these mutations led to constitutive NRF2 activation which is correlated with poor prognosis. The study utilized a small sgRNA library consisting of 65 sgRNA targeting 17 NRF2 target genes and found that tumors with sustained NRF2 activation is dependent on glutaminolysis for cellular bioenergetics, whereby inhibition of the glutaminase, GLS, could selectively kill cancer cells with sustained NRF2 activation (Figure 5).[75] This is relevant to HLRCC because (as it was outlined in the earlier section), FH inactivation drives sustained NRF2 activation, a phenotype that unifies HLRCC with its sporadic counterpart, PRCC2.[37] Accordingly, HLRCC cell growth is highly dependent on glutamine in vitro. Interestingly, other targets identified in the study include FTL and HMOX1.[75] These agree with other studies that showed genetic suppression of either FTL or HMOX1 can selectively kill FH−/− cells (Figure 5).[52, 59] Thus, pharmacological suppression of those proteins represents a treatment strategy worth exploring.
Concluding remarks
HLRCC is a hereditary cancer syndrome that predisposes afflicted individuals to skin leiomyomas, uterine fibroids, type 2 papillary renal cell carcinoma, bladder cancer (rare), and Leydig cell cancers (rare). HLRCC patients carry a germline amorphic FH allele. Loss-of-heterozygosity is always detected in the tumor tissues indicating bialleic FH loss as the tumor initiating event. Molecular genetics and multi omics studies have unveiled the unique biology of HLRCC. The most direct effect of FH loss is intracellular fumarate accumulation. It is measured that fumarate accumulates to millimolar concentration in HLRCC cells, and at this high concentration, fumarate directly alters various cellular signaling pathways including: increased HIF1A activity, increased NRF2 activity, and attenuated cellular iron homeostasis. Although direct mechanisms remain unclear, FH loss was also shown to reduce AMPK activity and drive a metabolic shift to aerobic glycolysis. It is apparent that our understanding of HLRCC biology remains incomplete, and the knowledge gaps impedes direct translation of these molecular understandings into clinical intervention strategies. However, these knowledges have provided the much-needed explanations to several treatment strategies identified through computational modeling and functional genomic screenings. Continually advancing knowledge in HLRCC biology is also critical to the development of a cancer prevention strategy for HLRCC patients.
To date, much of HLRCC research has been focusing on the aggressive PRCC2. The higher penetrance skin leiomyoma represents another important HLRCC manifestation that deserves much attention. HLRCC patients are frequently diagnosed at the onset of skin leiomyoma. With better understanding of the cancer biology, cancer chemoprevention could be started at the point of diagnosis to prevent the onset of the aggressive PRCC2. The unique biology of HLRCC makes these goals achievable in the foreseeable future.
Acknowledgement
The author is supported by NIEHS R21ES027920, NCI R01-CA226920-01A1. He is a member of the University of Arizona Cancer Center (P30CA023074) and The Southwest Environmental Health Sciences Center (P30ES006694).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Akiba T, Hiraga K, and Tuboi S, Intracellular distribution of fumarase in various animals. J Biochem, 1984. 96(1): p. 189–95. [DOI] [PubMed] [Google Scholar]
- 2.Yogev O, et al. , Fumarase: a mitochondrial metabolic enzyme and a cytosolic/nuclear component of the DNA damage response. PLoS Biol, 2010. 8(3): p. e1000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerrigan JF, et al. , Fumaric aciduria: clinical and imaging features. Ann Neurol, 2000. 47(5): p. 583–8. [PubMed] [Google Scholar]
- 4.Mroch AR, Laudenschlager M, and Flanagan JD, Detection of a novel FH whole gene deletion in the propositus leading to subsequent prenatal diagnosis in a sibship with fumarase deficiency. Am J Med Genet A, 2012. 158A(1): p. 155–8. [DOI] [PubMed] [Google Scholar]
- 5.Tomlinson IP, et al. , Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet, 2002. 30(4): p. 406–10. [DOI] [PubMed] [Google Scholar]
- 6.Chan MMY, et al. , Cascade Fumarate Hydratase mutation screening allows early detection of kidney tumour: a case report. BMC Med Genet, 2017. 18(1): p. 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gellera C, et al. , Fumarase deficiency is an autosomal recessive encephalopathy affecting both the mitochondrial and the cytosolic enzymes. Neurology, 1990. 40(3 Pt 1): p. 495–9. [DOI] [PubMed] [Google Scholar]
- 8.Petrova-Benedict R, et al. , Deficient fumarase activity in an infant with fumaricacidemia and its distribution between the different forms of the enzyme seen on isoelectric focusing. Am J Hum Genet, 1987. 40(3): p. 257–66. [PMC free article] [PubMed] [Google Scholar]
- 9.Whelan DT, Hill RE, and McClorry S, Fumaric aciduria: a new organic aciduria, associated with mental retardation and speech impairment. Clin Chim Acta, 1983. 132(3): p. 301–8. [DOI] [PubMed] [Google Scholar]
- 10.Wong MH, et al. , Potential genetic anticipation in hereditary leiomyomatosis-renal cell cancer (HLRCC). Fam Cancer, 2014. 13(2): p. 281–9. [DOI] [PubMed] [Google Scholar]
- 11.Carvajal-Carmona LG, et al. , Adult leydig cell tumors of the testis caused by germline fumarate hydratase mutations. J Clin Endocrinol Metab, 2006. 91(8): p. 3071–5. [DOI] [PubMed] [Google Scholar]
- 12.Kopp RP, et al. , Utility of prospective pathologic evaluation to inform clinical genetic testing for hereditary leiomyomatosis and renal cell carcinoma. Cancer, 2017. 123(13): p. 2452–2458. [DOI] [PubMed] [Google Scholar]
- 13.Lehtonen HJ, et al. , Increased risk of cancer in patients with fumarate hydratase germline mutation. J Med Genet, 2006. 43(6): p. 523–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher WC and Helwig EB, Leiomyomas of the Skin. Arch Dermatol, 1963. 88: p. 510–20. [DOI] [PubMed] [Google Scholar]
- 15.Menko FH, et al. , Hereditary leiomyomatosis and renal cell cancer (HLRCC): renal cancer risk, surveillance and treatment. Fam Cancer, 2014. 13(4): p. 637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park I, et al. , Long-term response of metastatic hereditary leiomyomatosis and renal cell carcinoma syndrome associated renal cell carcinoma to bevacizumab plus erlotinib after temsirolimus and axitinib treatment failures. BMC Urol, 2019. 19(1): p. 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kloepfer HW, et al. , Hereditary multiple leiomyoma of the skin. Am J Hum Genet, 1958. 10(1): p. 48–52. [PMC free article] [PubMed] [Google Scholar]
- 18.Rudner EJ, Schwartz OD, and Grekin JN, Multiple Cutaneous Leiomyoma in Identical Twins. Arch Dermatol, 1964. 90: p. 81–2. [DOI] [PubMed] [Google Scholar]
- 19.Alam NA, et al. , Localization of a gene (MCUL1) for multiple cutaneous leiomyomata and uterine fibroids to chromosome 1q42.3-q43. Am J Hum Genet, 2001. 68(5): p. 1264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Launonen V, et al. , Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc Natl Acad Sci U S A, 2001. 98(6): p. 3387–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alam NA, et al. , Genetic and functional analyses of FH mutations in multiple cutaneous and uterine leiomyomatosis, hereditary leiomyomatosis and renal cancer, and fumarate hydratase deficiency. Hum Mol Genet, 2003. 12(11): p. 1241–52. [DOI] [PubMed] [Google Scholar]
- 22.Astuti D, et al. , Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet, 2001. 69(1): p. 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baysal BE, et al. , Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science, 2000. 287(5454): p. 848–51. [DOI] [PubMed] [Google Scholar]
- 24.Bourgeron T, et al. , Mutation of a nuclear succinate dehydrogenase gene results in mitochondrial respiratory chain deficiency. Nat Genet, 1995. 11(2): p. 144–9. [DOI] [PubMed] [Google Scholar]
- 25.Niemann S and Muller U, Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat Genet, 2000. 26(3): p. 268–70. [DOI] [PubMed] [Google Scholar]
- 26.Niemann S, Steinberger D, and Muller U, PGL3, a third, not maternally imprinted locus in autosomal dominant paraganglioma. Neurogenetics, 1999. 2(3): p. 167–70. [DOI] [PubMed] [Google Scholar]
- 27.Maxwell PH, et al. , The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature, 1999. 399(6733): p. 271–5. [DOI] [PubMed] [Google Scholar]
- 28.Isaacs JS, et al. , HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell, 2005. 8(2): p. 143–53. [DOI] [PubMed] [Google Scholar]
- 29.Pollard P, et al. , Evidence of increased microvessel density and activation of the hypoxia pathway in tumours from the hereditary leiomyomatosis and renal cell cancer syndrome. J Pathol, 2005. 205(1): p. 41–9. [DOI] [PubMed] [Google Scholar]
- 30.Pollard PJ, et al. , Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet, 2005. 14(15): p. 2231–9. [DOI] [PubMed] [Google Scholar]
- 31.Pollard PJ, et al. , Targeted inactivation of fh1 causes proliferative renal cyst development and activation of the hypoxia pathway. Cancer Cell, 2007. 11(4): p. 311–9. [DOI] [PubMed] [Google Scholar]
- 32.Tong WH, et al. , The glycolytic shift in fumarate-hydratase-deficient kidney cancer lowers AMPK levels, increases anabolic propensities and lowers cellular iron levels. Cancer Cell, 2011. 20(3): p. 315–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sudarshan S, et al. , Fumarate hydratase deficiency in renal cancer induces glycolytic addiction and hypoxia-inducible transcription factor 1alpha stabilization by glucose-dependent generation of reactive oxygen species. Mol Cell Biol, 2009. 29(15): p. 4080–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vanharanta S, et al. , Distinct expression profile in fumarate-hydratase-deficient uterine fibroids. Hum Mol Genet, 2006. 15(1): p. 97–103. [DOI] [PubMed] [Google Scholar]
- 35.Koski TA, et al. , Array comparative genomic hybridization identifies a distinct DNA copy number profile in renal cell cancer associated with hereditary leiomyomatosis and renal cell cancer. Genes Chromosomes Cancer, 2009. 48(7): p. 544–51. [DOI] [PubMed] [Google Scholar]
- 36.Ashrafian H, et al. , Expression profiling in progressive stages of fumarate-hydratase deficiency: the contribution of metabolic changes to tumorigenesis. Cancer Res, 2010. 70(22): p. 9153–65. [DOI] [PubMed] [Google Scholar]
- 37.Ooi A, et al. , An antioxidant response phenotype shared between hereditary and sporadic type 2 papillary renal cell carcinoma. Cancer Cell, 2011. 20(4): p. 511–23. [DOI] [PubMed] [Google Scholar]
- 38.Itoh K, et al. , Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev, 1999. 13(1): p. 76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kobayashi A, et al. , Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol, 2004. 24(16): p. 7130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang DD and Hannink M, Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol, 2003. 23(22): p. 8137–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wakabayashi N, et al. , Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci U S A, 2004. 101(7): p. 2040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adam J, et al. , Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer Cell, 2011. 20(4): p. 524–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linehan WM, Genetic basis of kidney cancer: role of genomics for the development of disease-based therapeutics. Genome Res, 2012. 22(11): p. 2089–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ooi A, et al. , CUL3 and NRF2 mutations confer an NRF2 activation phenotype in a sporadic form of papillary renal cell carcinoma. Cancer Res, 2013. 73(7): p. 2044–51. [DOI] [PubMed] [Google Scholar]
- 45.Cancer Genome Atlas Research, N., et al. , Comprehensive Molecular Characterization of Papillary Renal-Cell Carcinoma. N Engl J Med, 2016. 374(2): p. 135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blatnik M, Thorpe SR, and Baynes JW, Succination of proteins by fumarate: mechanism of inactivation of glyceraldehyde-3-phosphate dehydrogenase in diabetes. Ann N Y Acad Sci, 2008. 1126: p. 272–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bardella C, et al. , Aberrant succination of proteins in fumarate hydratase-deficient mice and HLRCC patients is a robust biomarker of mutation status. J Pathol, 2011. 225(1): p. 4–11. [DOI] [PubMed] [Google Scholar]
- 48.Merkley ED, et al. , The succinated proteome. Mass Spectrom Rev, 2014. 33(2): p. 98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang M, et al. , The Succinated Proteome of FH-Mutant Tumours. Metabolites, 2014. 4(3): p. 640–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kulkarni RA, et al. , A chemoproteomic portrait of the oncometabolite fumarate. Nat Chem Biol, 2019. 15(4): p. 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miglio G, et al. , A computational analysis of S-(2-succino)cysteine sites in proteins. Biochim Biophys Acta, 2016. 1864(2): p. 211–8. [DOI] [PubMed] [Google Scholar]
- 52.Kerins MJ, et al. , Fumarate Mediates a Chronic Proliferative Signal in Fumarate Hydratase-Inactivated Cancer Cells by Increasing Transcription and Translation of Ferritin Genes. Mol Cell Biol, 2017. 37(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kondo K, et al. , Inhibition of HIF2alpha is sufficient to suppress pVHL-defective tumor growth. PLoS Biol, 2003. 1(3): p. E83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen C, et al. , Genetic and functional studies implicate HIF1alpha as a 14q kidney cancer suppressor gene. Cancer Discov, 2011. 1(3): p. 222–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perrier-Trudova V, et al. , Fumarate Hydratase-deficient Cell Line NCCFH1 as a New In Vitro Model of Hereditary Papillary Renal Cell Carcinoma Type 2. Anticancer Res, 2015. 35(12): p. 6639–53. [PubMed] [Google Scholar]
- 56.Sourbier C, et al. , Increasing reactive oxygen species as a therapeutic approach to treat hereditary leiomyomatosis and renal cell carcinoma. Cell Cycle, 2010. 9(20): p. 4183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamasaki T, et al. , Exploring a glycolytic inhibitor for the treatment of an FH-deficient type-2 papillary RCC. Nat Rev Urol, 2011. 8(3): p. 165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Velasco G, et al. , Sequential treatments in hereditary leiomyomatosis and renal cell carcinoma (HLRCC): Case report and review of the literature. Can Urol Assoc J, 2015. 9(3–4): p. E243–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frezza C, et al. , Haem oxygenase is synthetically lethal with the tumour suppressor fumarate hydratase. Nature, 2011. 477(7363): p. 225–8. [DOI] [PubMed] [Google Scholar]
- 60.Kerins MJ, et al. , Fumarate hydratase inactivation in hereditary leiomyomatosis and renal cell cancer is synthetic lethal with ferroptosis induction. Cancer Sci, 2018. 109(9): p. 2757–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tan AC, et al. , Simple decision rules for classifying human cancers from gene expression profiles. Bioinformatics, 2005. 21(20): p. 3896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang WS, et al. , Regulation of ferroptotic cancer cell death by GPX4. Cell, 2014. 156(1–2): p. 317–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cramer SL, et al. , Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat Med, 2017. 23(1): p. 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garg AD and Agostinis P, Cell death and immunity in cancer: From danger signals to mimicry of pathogen defense responses. Immunol Rev, 2017. 280(1): p. 126–148. [DOI] [PubMed] [Google Scholar]
- 65.Boettcher M, et al. , High throughput synthetic lethality screen reveals a tumorigenic role of adenylate cyclase in fumarate hydratase-deficient cancer cells. BMC Genomics, 2014. 15: p. 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun Y, et al. , Functional Genomics Reveals Synthetic Lethality between Phosphogluconate Dehydrogenase and Oxidative Phosphorylation. Cell Rep, 2019. 26(2): p. 469–482 e5. [DOI] [PubMed] [Google Scholar]
- 67.Parr CW and Fitch LI, Inherited quantitative variations of human phosphogluconate dehydrogenase. Ann Hum Genet, 1967. 30(4): p. 339–53. [DOI] [PubMed] [Google Scholar]
- 68.Herter FP, et al. , Clinical experience with 6-aminonicotinamide. Cancer Res, 1961. 21: p. 31–7. [PubMed] [Google Scholar]
- 69.Wang T, et al. , Genetic screens in human cells using the CRISPR-Cas9 system. Science, 2014. 343(6166): p. 80–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Doll S, et al. , ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol, 2017. 13(1): p. 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kerins MJ, et al. , Genome-Wide CRISPR Screen Reveals Autophagy Disruption as the Convergence Mechanism That Regulates the NRF2 Transcription Factor. Mol Cell Biol, 2019. 39(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kampmann M, et al. , Next-generation libraries for robust RNA interference-based genome-wide screens. Proc Natl Acad Sci U S A, 2015. 112(26): p. E3384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Horlbeck MA, et al. , Compact and highly active next-generation libraries for CRISPR-mediated gene repression and activation. Elife, 2016. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kampmann M, CRISPRi and CRISPRa Screens in Mammalian Cells for Precision Biology and Medicine. ACS Chem Biol, 2018. 13(2): p. 406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Romero R, et al. , Keap1 loss promotes Kras-driven lung cancer and results in dependence on glutaminolysis. Nat Med, 2017. 23(11): p. 1362–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cancer Genome Atlas Research, N., Comprehensive genomic characterization of squamous cell lung cancers. Nature, 2012. 489(7417): p. 519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kerins MJ and Ooi A, A catalogue of somatic NRF2 gain-of-function mutations in cancer. Sci Rep, 2018. 8(1): p. 12846. [DOI] [PMC free article] [PubMed] [Google Scholar]