Abstract
Background:
Few studies have compared clinical characteristics, echocardiographic parameters, exercise capacity, and quality of life between women and men with heart failure with preserved ejection fraction (HFpEF).
Methods and Results:
Subjects in the NIH-sponsored RELAX (N = 216) and NEAT (N = 107) trials completed baseline echocardiography, the Minnesota Living with Heart Failure Questionnaire (MLHFQ), and 6-minute walk test (6MWT). In exploratory analysis, multivariable linear regression models were used to associate clinical and imaging characteristics with baseline 6MWT distance and MLHFQ score in women and men. Our cohort included 158 (49%) men and 165 (51%) women. Men had higher prevalence of atrial arrhythmias, ischemic heart disease, diabetes, anemia, and left ventricular (LV) hypertrophy. 6MWT and MLHFQ score did not differ between sexes. In multivariable analysis, ischemic heart disease, diastolic dysfunction, and exercise capacity predicted MLHFQ score for men, while only age and BMI predicted MLHFQ score for women.
Conclusions:
Men with HFpEF had more co-morbidities and LV hypertrophy than women with HFpEF. In men, quality of life was associated with diastolic dysfunction, ischemic heart disease, and exercise capacity. Further research is needed to identify determinants of quality of life in women with HFpEF.
Keywords: heart failure with preserved ejection fraction, sex differences, women’s health, quality of life
Introduction
Heart failure with preserved ejection fraction (HFpEF) poses a large and growing public health problem.1 Women constitute the majority of HFpEF patients, and women with heart failure are more likely to have HFpEF than HF with reduced ejection fraction (HFrEF).2 Reasons for this disparity may stem from differences in cardiac structure, vascular function, and baseline comorbidities.3 Prior work suggests that, compared to men, women have greater arterial stiffness, more concentric left ventricular (LV) hypertrophy, smaller LV cavities, and worse diastolic function.4 Additionally, recent data suggested sex differences in response to HF therapies, further demonstrating sex differences in HFpEF.5,6 Despite having more favorable clinical outcomes,2,7,8 women with HFpEF may have worse functional status and quality of life.9
Exercise intolerance is a hallmark feature of HFpEF.3 Because no targeted therapies for HFpEF exist, standard treatment goals include improving exercise capacity and quality of life. Despite the female preponderance of HFpEF, women have historically been underrepresented in HF trials, and few studies have examined sex-specific differences in functional and patient-reported outcomes. Here, we combined two HFpEF cohorts to compare clinical characteristics, exercise capacity, and quality of life in men and women with HFpEF. In exploratory analysis, we compared predictors of exercise capacity and quality of life between sexes.
Materials and methods
We pooled subjects in the RELAX (N = 216)10 and NEAT-HFpEF (N = 107)11 trials, two randomized controlled trials conducted by the Heart Failure Clinical Research Network. Three individuals were enrolled in both trials; they were only included in the dataset once using data from RELAX because it was conducted first. Data collection, management, and analysis were performed at the network’s data coordinating center at the Duke Clinical Research Institute. All subjects provided written informed consent.
RELAX evaluated the effect of sildenafil on peak oxygen consumption with cardiopulmonary exercise testing. NEAT-HFpEF assessed the effect of isosorbide mononitrate on exercise capacity as measured by patient-worn accelerometers. Inclusion and exclusion criteria have been published previously and were similar between trials.10,11 Eligibility criteria included New York Heart Association (NYHA) functional class II-IV symptoms with objective evidence of HF, left ventricular ejection fraction ≥50% within 12 months of enrollment, and ability to walk and (in RELAX) complete cardiopulmonary exercise testing.10,11
We compared subjects’ baseline characteristics, exercise capacity, and quality of life (i.e., our analysis is unaffected by study randomization). Subjects completed a baseline medical history and physical examination, transthoracic echocardiogram, six-minute walk test (6MWT), and Minnesota Living with Heart Failure Questionnaire (MLHFQ).10,11 Anemia was defined as hemoglobin <13 g/dL for men and <12 g/dL for women.10,11
Statistical analysis
We compared baseline characteristics by sex using the Student’s t-test or Wilcoxon rank-sum test for continuous variables and the Pearson chi-squared or Fisher exact test for categorical variables as appropriate. Multiple imputation by chained equations was used to impute missing covariate data.
In exploratory analysis, we used generalized linear regression models to test associations between candidate predictors and 6MWT distance and MLHFQ score by sex. Variables were log2-transformed where appropriate. We stratified analyses by sex (i.e., separate models were derived for men and women) rather than evaluating interactions with sex because the cohort was small relative to the number of degrees of freedom required to explore sex interactions in multivariable modeling. The univariable associations with candidate predictors (listed in Supplemental Tables 1–2) were assessed by sex after adjusting for clinical trial. For multivariable models, all candidate predictors were initially considered for inclusion, and backward variable selection with an inclusion criterion of P<0.10 was used to select key predictors for use in parsimonious multivariable models. All statistical analyses were performed in SAS 9.4 (SAS Institute; Cary, NC). P-values less than 0.05 indicate statistical significance.
Results
Baseline characteristics
The study cohort included 158 (48.9%) men and 165 (51.1%) women. All but 1 patient in each group had NYHA class II-III HF symptoms. As shown in Table 1, most patients were white and obese, with similar body mass index (BMI) between men and women (33.6 kg/m2 vs. 33.3 kg/m2, P=0.57). Men were more likely to have atrial arrhythmias (55.1% vs. 38.2%, P=0.002), ischemic heart disease (55.1% vs. 37.6%, P=0.002), diabetes (51.9% vs. 32.1%, P<0.001), and anemia (42.3% vs. 26.7%, P=0.003). Medication use was similar between sexes. Men were more likely than women to have concentric LV hypertrophy as measured by a relative wall thickness (i.e., the sum of septal and posterior wall thickness divided by LV end-diastolic diameter [LVEDD]) >0.41 (62.4% vs. 43.5%, P<0.001) and greater body surface area-indexed LV mass (88 g/m2 vs. 72 g/m2, P<0.001). 6MWT distance and MLHFQ score did not differ between men and women (Figure 1a–1b).
Table 1.
Characteristics of the study population by sex.
| Characteristic | Men (N=158) | Women (N=165) | P-value+ |
|---|---|---|---|
| Demographics | |||
| Age, years: mean (SD) | 69 (9.4) | 68 (10.3) | 0.39 |
| Self-reported white race: n (%) | 149 (94.3%) | 143 (86.7%) | 0.02 |
| Clinical Assessment | |||
| Body mass index, kg/m2: median (Q1, Q3) | 33.6 (29.1, 40.0) | 33.3 (28.9, 40.0) | 0.57 |
| New York Heart Association functional classification: n (%) | 0.30 | ||
| II | 82 (51.9%) | 76 (46.1%) | |
| III | 75 (47.5%) | 88 (53.3%) | |
| Systolic blood pressure, mmHg: median (Q1, Q3) | 126 (116, 138) | 126 (113, 141) | 0.63 |
| Heart rate, beats/min: mean (SD) | 70 (12.1) | 70 (12.0) | 0.81 |
| Peripheral edema, trace+: n/N (%) | 98 / 157 (62.4%) | 92 / 165 (55.8%) | 0.22 |
| Medical History | |||
| Hypertension: n (%) | 138 (87.3%) | 142 (86.1%) | 0.74 |
| Atrial fibrillation or flutter: n (%) | 87 (55.1%) | 63 (38.2%) | 0.002 |
| Ischemic heart disease: n (%) | 87 (55.1%) | 62 (37.6%) | 0.002 |
| Diabetes mellitus: n (%) | 82 (51.9%) | 53 (32.1%) | <.001 |
| Chronic kidney disease, stage 3+: n/N (%) | 78 / 157 (49.7%) | 92 / 165 (55.8%) | 0.28 |
| Chronic obstructive pulmonary disease: n (%) | 35 (22.2%) | 23 (13.9%) | 0.06 |
| Anemia: n/N (%) | 66 / 156 (42.3%) | 44 / 165 (26.7%) | 0.003 |
| Medications at Enrollment | |||
| Loop diuretic: n (%) | 122 (77.2%) | 113 (68.5%) | 0.08 |
| Beta-blocker: n (%) | 124 (78.5%) | 115 (69.7%) | 0.07 |
| Angiotensin converting enzyme inhibitor or angiotensin II receptor blocker: n (%) | 110 (69.6%) | 110 (66.7%) | 0.57 |
| Aldosterone antagonist: n (%) | 21 (13.3%) | 28 (17.0%) | 0.36 |
| Calcium channel blocker: n (%) | 51 (32.3%) | 49 (29.7%) | 0.62 |
| Echocardiographic Data | |||
| Left ventricular ejection fraction, %: mean (SD) [N] | 60 (7.6) [156] | 63 (7.9) [164] | 0.004 |
| Left ventricular end diastolic dimension, cm: mean (SD) [N] | 4.9 (0.67) [124] | 4.6 (0.51) [143] | <.001 |
| Left ventricular mass / body surface area, g/m2: median (Q1, Q3) [N] | 88 (67, 109) [123] | 72 (60, 84) [138] | <.001 |
| Left ventricular mass / body surface area >= 95 g/m2 (women) or 115 g/m2 (men): n/N (%) | 22 / 123 (17.9%) | 17 / 138 (12.3%) | 0.21 |
| Relative wall thickness > 0.41: n/N (%) | 79 / 123 (64.2%) | 60 / 138 (43.5%) | <.001 |
| Left atrial volume index, mL/m2: median (Q1, Q3) [N] | 44 (33, 61) [103] | 40 (31, 52) [134] | 0.03 |
| Medial e′, m/s: median (Q1, Q3) [N] | 0.06 (0.05, 0.08) [144] | 0.06 (0.05, 0.08) [155] | 0.32 |
| Medial E/e′: median (Q1, Q3) [N] | 14 (11, 20) [137] | 15 (11, 20) [152] | 0.38 |
| Pulmonary artery systolic pressure, mmHg: median (Q1, Q3) [N] | 40 (32, 49) [78] | 36 (30, 49) [105] | 0.54 |
| Laboratory Biomarkers | |||
| NT-proBNP, pg/mL: median (Q1, Q3) [N] | 622 (214, 1333) [156] | 446 (141, 1187) [164] | 0.11 |
| Outcomes | |||
| Six-minute walk distance, m: mean (SD) [N] | 311 (115.2) [158] | 299 (112.2) [164] | 0.32 |
| MLHFQ total score ^: mean (SD) [N] | 46 (23.6) [148] | 44 (22.3) [151] | 0.61 |
| Physical dimension score (range 0-40): median (Q1, Q3) [N] | 22 (15, 30) [152] | 24 (14, 30) [162] | 0.55 |
| Emotional dimension score (range 0-25): median (Q1, Q3) [N] | 8 (3, 14) [157] | 9 (5, 15) [163] | 0.12 |
Values are displayed as median (interquartile range) [N], mean (SD) [N], or n/N (%) when data are missing in a given cell.
Continuous variables reported as median (interquartile range) were compared using the Wilcoxon rank-sum test. Continuous variables reported as mean (SD) were compared using the Student’s t-test.
Higher scores on the Minnesota Living with Heart Failure Questionnaire correspond to worse health status.
61/507 (57.0%) subjects in NEAT and 104/216 (48.1%) subjects in RELAX were female (P=0.13).
NT-proBNP=N-terminal pro b-type natriuretic peptide; MLHFQ = Minnesota Living with Heart Failure Questionnaire
Figure 1.
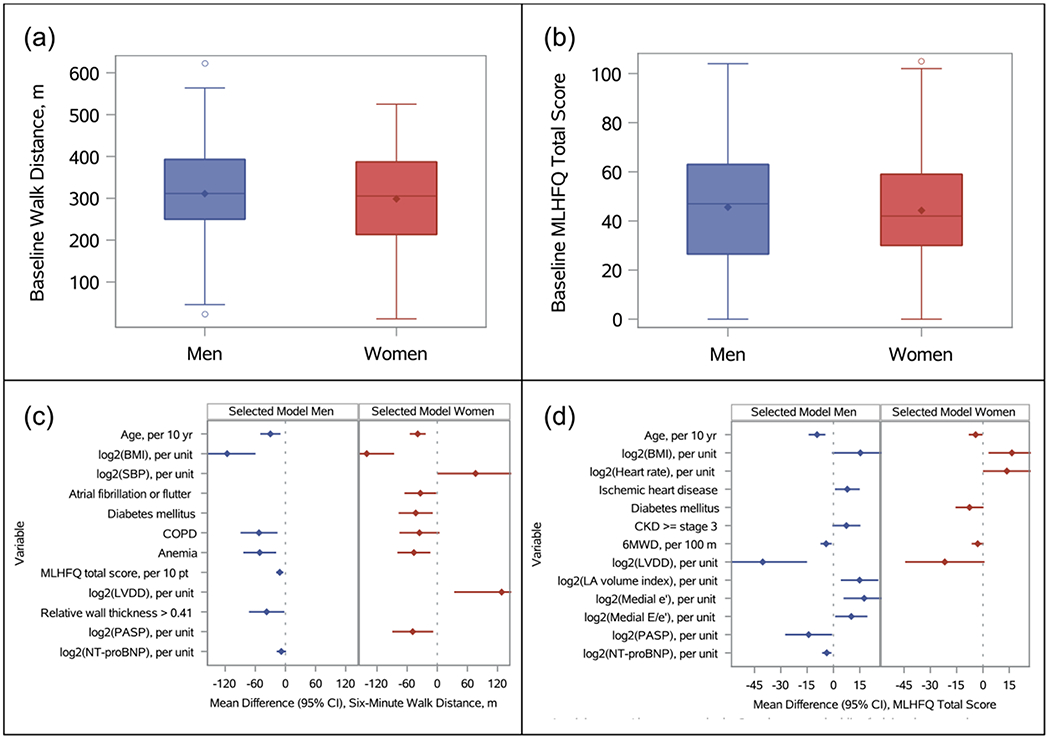
Baseline six-minute walk test distance (a) and Minnesota Living with Heart Failure Questionnaire total score (b) for men and women, and predictors of these measures from backward-selected regression models (c and d).
Predictors of exercise capacity
Results of a multivariable model for predicting 6MWT distance are presented in Figure 1c. Supplemental Table 1 lists univariable associations for all candidate predictors. Age, BMI, and anemia predicted 6MWT distance for both men and women. Atrial arrhythmias and diabetes independently predicted 6MWT distance for women. Left atrial (LA) volume index, medial e’, and medial E/e’ were not associated with 6MWT distance in either sex. Higher pulmonary artery systolic pressure (PASP) was independently associated with lower 6MWT distance in women. N-terminal pro-B-type natriuretic peptide (NT-proBNP) level was significantly associated with exercise capacity for both sexes in univariable analysis but not after multivariable adjustment for co-morbidities and echocardiographic parameters.
Predictors of quality of life
Results of a multivariable model for MLHFQ score are shown in Figure 1d. BMI was negatively associated with quality of life in women. Ischemic heart disease predicted worse quality of life in men. Several echocardiographic parameters were independently predictive of MLHFQ score for men, including LVEDD, LA volume index, and medial E/e’. In univariable and multivariable analyses (Figure 1c–1d, Supplemental Tables 1–2), greater 6MWT distance was associated with more favorable MLHFQ score for men but not women.
Discussion
In this pooled secondary analysis of two prospective HFpEF trials, we found baseline differences in clinical and echocardiographic parameters between women and men with HFpEF. In contrast with prior HFpEF studies,4,7,8 women and men had similar age and BMI, while men had more co-morbidities. Also in contrast to prior reports,4 we found that women had less ventricular hypertrophy.
While baseline 6MWT distance and HF-related quality of life were similar between sexes, we found sex differences in predictors of these measures. For example, we found that the relationship between exercise limitation and quality of life may differ between men and women with HFpEF. This finding is relevant given a recent analysis of sex differences in HFrEF which found women had worse quality of life and disability compared to men, despite having fewer co-morbidities and better survival.12 Prior work suggested a strong association between exercise capacity in HFpEF and quality of life.13 In our study, 6MWT distance was associated with quality of life for men but not women, aligning with a study which found quality of life was more strongly associated with NYHA class and natriuretic peptide levels in men.9 This potential sex difference is important to recognize because prior studies and HF guidelines have implicitly assumed that improving exercise capacity will improve quality of life.
Reasons for the apparently attenuated association between exercise capacity and quality of life in women are currently unknown. Unmeasured psychosocial factors may contribute. Sex differences in perception of disease severity and its impact on physical function have been proposed. For example, women with HFpEF appear to have higher rates of depression than men,14 and depression has been shown to impact patient perceptions of HF severity and quality of life independent of objective disease severity.15 Lack of data on co-morbid depression is a limitation of this analysis. Further, it is possible that social determinants of health (e.g., economic circumstances, caregiver burden, sexism) may differentially affect women and men with HF. It is striking that few factors predicted quality of life in women and that the multivariable model for HF-related quality of life performed worse in women vs. men (R2 0.178 vs. 0.38). Further research is needed to understand these factors with the ultimate goal of optimizing patients’ overall quality of life.
Our findings should be interpreted in the context of limitations. The sample size, while similar to recent HFpEF trials, was small relative to the number of candidate predictors, limiting power. By fitting separate models for men and women, we cannot directly compare differences in estimated associations with candidate predictors. Inclusion criteria in RELAX and NEAT-HFpEF included ability to walk/exercise, potentially limiting generalizability. Data on comorbidities beyond those routinely ascertained in HFpEF trials were not available. Finally, our study population was predominantly white and therefore our findings may not be generalizable to other racial or ethnic minorities with HFpEF.
Conclusions
In this pooled HFpEF cohort from the RELAX and NEAT-HFpEF clinical trials, men with HFpEF had more co-morbidities and LV hypertrophy than women. Ischemic heart disease, echocardiographic parameters of diastolic function, and exercise capacity predicted quality of life in men, whereas none of these factors was significantly associated with quality of life in women. Further research is needed to identify determinants of quality of life in women with HFpEF.
Supplementary Material
Acknowledgments
Sources of Funding: Both the RELAX and NEAT-HFpEF trials were funded by grants from the National Heart, Lung, and Blood Institute.
Abbreviations
- HF
Heart failure
- HFpEF
Heart failure with preserved ejection fraction
- HFrEF
Heart failure with reduced ejection fraction
- LV
Left ventricle
- LVEDD
Left ventricular end-diastolic diameter
- MLHFQ
Minnesota Living with Heart Failure Questionnaire
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- NYHA
New York Heart Association
- 6MWT
Six-minute walk test
- LA
left atrium
- PASP
pulmonary artery systolic pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial Registration: NCT02053493 (https://clinicaltrials.gov/ct2/show/NCT02053493); NCT00763867 (https://clinicaltrials.gov/ct2/show/NCT00763867)
Disclosures: None
In multivariable models for six-minute walk test distance, R2 = 0.307 for men, and R2 = 0.383 for women. In multivariable models for Minnesota Living with Heart Failure Questionnaire score, R2 = 0.38 for men, and R2 = 0.178 for women.
References
- 1.Steinberg BA, Zhao X, Heidenreich PA, et al. Trends in Patients Hospitalized With Heart Failure and Preserved Left Ventricular Ejection Fraction. Circulation. 2012;126(1):65–75. [DOI] [PubMed] [Google Scholar]
- 2.Deswal A, Bozkurt B. Comparison of Morbidity in Women Versus Men With Heart Failure and Preserved Ejection Fraction. The American journal of cardiology. 2006;97(8):1228–1231. [DOI] [PubMed] [Google Scholar]
- 3.Beale AL, Meyer P, Marwick TH, Lam C, Kaye DM. Sex Differences in Cardiovascular Pathophysiology. Circulation. 2018;138(2):198–205. [DOI] [PubMed] [Google Scholar]
- 4.Gori M, Lam CS, Gupta DK, et al. Sex-specific cardiovascular structure and function in heart failure with preserved ejection fraction. Eur J Heart Fail. 2014;16(5):535–542. [DOI] [PubMed] [Google Scholar]
- 5.Solomon SD, McMurray JJV, Anand IS, et al. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2019;381(17):1609–1620. [DOI] [PubMed] [Google Scholar]
- 6.Merrill M, Sweitzer NK, Lindenfeld J, Kao DP. Sex Differences in Outcomes and Responses to Spironolactone in Heart Failure With Preserved Ejection Fraction: A Secondary Analysis of TOPCAT Trial. JACC Heart Fail. 2019;7(3):228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam CS, Carson PE, Anand IS, et al. Sex differences in clinical characteristics and outcomes in elderly patients with heart failure and preserved ejection fraction: the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ Heart Fail. 2012;5(5):571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stolfo D, Uijl A, Vedin O, et al. Sex-Based Differences in Heart Failure Across the Ejection Fraction Spectrum: Phenotyping, and Prognostic and Therapeutic Implications. JACC Heart Fail. 2019;7(6):505–515. [DOI] [PubMed] [Google Scholar]
- 9.Faxén UL, Hage C, Donal E, Daubert JC, Linde C, Lund LH. Patient reported outcome in HFpEF: Sex-specific differences in quality of life and association with outcome. Int J Cardiol. 2018;267:128–132. [DOI] [PubMed] [Google Scholar]
- 10.Redfield MM, Chen HH, Borlaug BA, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309(12):1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Redfield MM, Anstrom KJ, Levine JA, et al. Isosorbide Mononitrate in Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2015;373(24):2314–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dewan P, Rørth R, Jhund PS, et al. Differential Impact of Heart Failure With Reduced Ejection Fraction on Men and Women. J Am Coll Cardiol. 2019;73(1):29–40. [DOI] [PubMed] [Google Scholar]
- 13.Napier R, McNulty SE, Eton DT, Redfield MM, AbouEzzeddine O, Dunlay SM. Comparing Measures to Assess Health-Related Quality of Life in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2018;6(7):552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamo CE, Heitner JF, Pfeffer MA, et al. Baseline distribution of participants with depression and impaired quality of life in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial. Circ Heart Fail. 2015;8(2):268–277. [DOI] [PubMed] [Google Scholar]
- 15.Gottlieb SS, Kop WJ, Ellis SJ, et al. Relation of depression to severity of illness in heart failure (from Heart Failure And a Controlled Trial Investigating Outcomes of Exercise Training [HF-ACTION]). Am J Cardiol. 2009;103(9):1285–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


