Abstract
RATIONALE AND OBJECTIVE:
3D printed anatomic models and surgical guides have been shown to reduce operative time. The purpose of this study was to generate an economic analysis of the cost saving potential of 3D printed anatomic models and surgical guides in orthopedic and maxillofacial surgical applications.
MATERIALS AND METHODS:
A targeted literature search identified operating room cost-per-minute and studies that quantified time saved using 3D printed constructs. Studies that reported operative time differences due to 3D printed anatomic models or surgical guides were reviewed and cataloged. A mean of $62 per operating room minute (range of $22 to $133 per minute) was used as the reference standard for operating room time cost. Different financial scenarios were modeled with the provided cost-per-minute of operating room time (using high, mean, and low values) and mean time saved using 3D printed constructs.
RESULTS:
Seven studies using 3D printed anatomic models in surgical care demonstrated a mean 62 minutes ($3720/case saved from reduced time) of time saved, and 25 studies of 3D printed surgical guides demonstrated a mean 23 minutes time saved ($1488/case saved from reduced time). An estimated 63 models or guides per year (or 1.2/week) were predicted to be the minimum number to breakeven and account for annual fixed costs.
CONCLUSON:
Based on the literature-based financial analyses, medical 3D printing appears to reduce operating room costs secondary to shortening procedure times. While resourceintensive, 3D printed constructs used in patients’ operative care provides considerable downstream value to health systems.
INTRODUCTION
Growing interest in 3D printing applications in medicine and radiology is evidenced by the recent approval of 3D printing-related Current Procedural Terminology (CPT) codes, a proposal that was led by a collaborative effort between the American College of Radiology (ACR) and the Radiologic Society of North America (RSNA) 3D Printing Special Interest Group. Announced in November 2018, the American Medical Association CPT Editorial Panel approved the creation of four category III CPT codes (Table 1), which went into effect July 2019 (1, 2). These codes for 3D printed anatomic models and guides are primarily used as temporary tracking codes to detail usage across the United States to guide future category I code applications (3). Reimbursement for category III codes are determined individually (if at all) by insurers, and thus information on the value of such a service to patients and to a health system could help inform such determinations. Announced in August 2019, a joint ACR-RSNA medical 3D printing clinical data registry is intended to launch in fall 2019 to collect 3D printing data at the point of clinical care to document use and implementation (4). These efforts by professional radiology organizations may lead to radiology departments to initiate or increase the volume of medical 3D printing services.
Table 1.
American Medical Association Category III Current Procedural Codes for 3D printing, which went into effect July 1, 2019. Omitted from ‘Description’ column: each of the above codes should not be reported in conjunction with 76376 or 76377 (3D rendering with interpretation and reporting of CT, magnetic resonance imaging, ultrasound, or other tomographic modality without [76376] or with [76377] postprocessing on an independent workstation). Adapted from reference (2).
| Code | Description |
|---|---|
| 0559T | Anatomic model 3D printed from image data set(s): first individually prepared and processed component of an anatomic structure |
| +0560T | Anatomic model 3D printed from image data set(s): each additional individually prepared and processed component of an anatomic structure (List separately in addition to code for primary procedure) |
| 0561T | Anatomic guide 3D printed from image data set(s): first anatomic guide |
| +0562T | Anatomic guide 3D printed from image data set(s): each additional anatomic guide (List separately in addition to code for primary procedure) |
Other indications suggestive of growing interest in 3D printing in medicine are a rapidly growing number of publications, including a consensus guideline and appropriateness scenarios for medical 3D printing published by the RSNA 3D Printing Special Interest Group (5) along with Radiology Research Alliance 3D Printing Task Force publications (6, 7). Although 3D printing was reported for niche medical applications as early as 1999 (8), the years between 2010 and 2018 saw exponential growth in peer-review publications addressing the topic (6, 7, 9–11). Specifically, a search of Pubmed.gov for “3D printing” revealed 34 results from 1985 to 2009, 109 results from 2010 to 2013, and over 4,700 results for 2014 through July 2019; pairing the “3d printing” search term with “radiology” yields 3, 5, and 333 publications during the same three time periods. This is likely in part due to key intellectual property issued in 1989 expiring in 2009, which in turn made 3D printers more affordable (12).
3D printing has multiple advanced applications, including the manufacture of custom implants, prostheses, and even tissue and organ-like constructs (9). However, 3D printed constructs are most commonly used for surgical guidance and planning via surgical guides and anatomic models, the latter indication for which it is not currently reimbursed by third-party payers. These anatomic models and many guides are primarily derived from computed tomography, magnetic resonance imaging, and volumetric ultrasound images, although data is sometimes supplemented by surface scans and the user’s creative input, e.g., patient photos for trauma cases (13). As part of the surgical process, uses include preoperative planning, intraoperative guidance, medical trainee education, and patient information and consent (6, 7). Used in these ways, it is a direct implementation of precision and personalized medicine (14).
Without historic reimbursement for 3D printed anatomic models, 3D printing has nonetheless been adopted as a novel method to portray volumetric data within hospitals because it has the potential to “pay for itself.” These “payments” include superior confidence of the surgeon or proceduralist, the ability to perform complex procedures that were poorly understood anatomically with 3D visualization (the collective term for viewing a 3D volume in any format on a 2D screen), and financial savings secondary to shorter, more efficient procedures. To date, there are a number of studies that demonstrate operating room time reduction using 3D printed anatomic models and surgical guides (6, 9, 10). Development of a financial model that accounts for surgical time saved due to a 3D printed model or surgical guide and the subsequent cost-savings due to the shorter operating room time may be of interest to healthcare systems and radiology departments that may be considering the implementation of a 3D printing lab or service. Though not a direct tie to reimbursement from the production of these 3D printed constructs, the value of a 3D printing service to a healthcare system, which may or may not be based in a radiology department, may be demonstrated in cost analyses. The purpose of this study was to generate an economic analysis of the downstream cost saving potential of 3D printed anatomic models and surgical guides, using previously reported data on operating room time savings and cost with a focus in orthopedic and maxillofacial surgeries.
MATERIALS AND METHODS
Institutional review board approval was not required because only published data (9; 15–44) was used. A previously published report of mean operating room cost-per-minute in the United States (15) was used as the reference standard for operating room cost. Studies demonstrating the effect of operating room time 3D printed anatomic models or surgical guides were generated from a previously published systematic review (9). Authors’ institutional data of 3D printing lab operation costs along with a prior study reported detailed operating room utilization over a one-year period at a large academic institution were also used (16).
Operating Room Costs
Using PubMed.gov and Google Scholar, a literature search was performed to identify studies which describe generalizable cost-per-minute for operating room time in the United States. Search terms included, “operating room cost per minute”, “operating room cost”, and “anesthesia cost per minute.” Preference was given to the most cited articles that were generalizable to several types of surgery, not just a specific specialty or certain procedure. The search yielded a study by Shippert (15), which served as the reference for operating room cost. Overall operating room costs per minute in the United States, while lacking a high level of consensus, were evaluated in 2005 (15) which reported a mean of $62 per minute and a range of $22 - 133 per minute, derived from a survey of 100 hospitals with over 100 patient beds. This was used as the reference standard for operating room cost (Table 2).
Table 2.
Potential cost savings per surgical case from the listed anatomic models and surgical guide studies paired with reference data of mean cost of operating room minutes. Potential cost savings are grouped according to the mean ($62/minute), minimum ($22/minute), and maximum ($133/minute) from the reference study that queried operating room time costs in the United States (15). These mean, minimum, and maximum values per minute of operating room time adjusted for 2019 inflation would increase to $83, $30, and $179 per minute, respectively.
| 3D printed construct | Mean operating room time saved | USD saved per surgical case at $22 per min | USD saved per surgical case at $62 per min | USD saved per surgical case at $133 per min |
|---|---|---|---|---|
| Anatomic model | 62 minutes | $1,364 | $3,844 | $8,246 |
| Cost from 2005 data (15) adjusted for 2019 inflation* | $1,835 | $5,172 | $11,094 | |
| Surgical guide | 23 minutes | $506 | $1,426 | $3,059 |
| Cost from 2005 data (15) adjusted for 2019 inflation* | $681 | $1,918 | $4,115 | |
3D Printing Time Savings
Similarly, in a literature search using PubMed.gov and Google Scholar, a search for “3D printing” and “systematic review” was conducted, specifically looking for studies that included multiple surgical subspecialties and provided specific data on time saved per minute in the queried studies. A previously published systematic review identified studies (9) reported mean time differences of 3D printed constructs used in the surgical treatment of patients as part of the authors’ supplemental data. This single systematic review served as the reference source for prior 3D printing cohorts that reported a difference in operating room time. The 3D printed constructs included anatomic models, surgical guides, and implants. In the current study’s independent review of studies identified from the cited source (9) (no additional studies were identified through an additional literature search), inclusion criteria consisted of a study of a minimum 20 patients, including at least 10 in the 3D printing group and a 10 in a control group, and reporting a mean operating room time or time difference comparison between the two groups. Studies that did not objectively measure and report time differences were excluded. A study investigator (DHB) independently reviewed each study identified by the previous systematic review. The data collected by the investigator in the present study included category of 3D printed construct used in patient care (e.g., anatomic model, surgical guide, or implant), discipline of the study (e.g., oral and maxillofacial focus, orthopedic focus), number of patients, and discrepancies between the data obtained referenced articles by the present study investigator and that originally reported by the systematic review (9). Discrepancies included when the systematic review identified a discrete time savings of an individual scientific study and the present study’s analysis did not agree with these metrics.
Overall, 28 of 230 studies identified from the reference (9) met the present study’s inclusion criteria. Of note, all included studies broadly had either a maxillofacial or orthopedic focus. Thirteen studies were identified for 3D printed anatomic models, 7 of which met inclusion criteria (17–23). Of the 7 studies, 4 are also included in the surgical guide group (17–20). The 6 excluded studies were due to too few patients (fewer than 10 experimental or control subjects) (n=3) (45–47), the number of minutes saved was not objectively measured (n=1) (48), the number of minutes was not reported (n=1), and listing of a duplicate publication (n=1) (17). Thirty-one studies were identified for 3D printed surgical guides, 25 of which met inclusion criteria (17–20, 24–44). Of the 25 studies, 4 studies were also categorized in the anatomic model group (17–20). The 5 studies that were excluded were excluded because they did not measure operative time (only estimated it) (n=3) (48–50), listed a duplicate publication (n=1) (25), or was misidentified as a scientific study when it was a commentary (n=1) (51). 3D printed implant studies were excluded from cost analyses as only 2 of a possible 4 studies would have met inclusion criteria. Of note, the CPT category III 3D printing codes which went into effect July 2019 do not include implantables. Nine errors of operating time metrics in the data of the referenced systematic reviews were noted.
3D Printing Costs
From these data, patients were grouped into different 3D printed construct used in their care (e.g., anatomic models and surgical guides) and different financial scenarios were modeled with the cost-per-minute of operating room time (using high, mean, and low values). The authors’ institutional data and experience were used to produce both variable and fixed costs associated with 3D printing. These included material and labor costs for creating 3D printed models and guides of varying complexity along with an annual operational fee. This consisted of a fixed cost of $150,000/year ($120,000 for salary/percent effort for personnel performing the majority of segmentation and overseeing the print process, $20,000 for a segmentation software license, and $10,000 of facilities operational costs, printer maintenance, and unexpected purchases). Printer depreciation was not included in variable and fixed costs. The $119 and $320 price per model or guide included the variable costs of producing a 3D printed constructs, including the material cost and allocation of time segmenting and printing (separate from the $120,000 salary designation) (Table 3).
Table 3.
Costs associated with 3D printing derived from authors’ institutional data (DHB and PKW) derived from 10 total 3D printed models or guides, allocated as “simple” or “complex” (definitions in the “Comments” column).
| Variable | Cost (USD) | Cost Descriptor | Comments | ||
|---|---|---|---|---|---|
| 3D printer | $12,000 | Initial investment | Varies considerably from $150 to >$500,000. Examples from this institutional data were from three printers that cost $2,000, $4,000, and $6,000 respectively. | ||
| Segmentation software | $20,000/yr | Fixed annual cost | Commercial 3D printing segmentation software $20,000. The RSNA 3D Printing Special Interest Group recommends using FDA approved software (4). | ||
| Personnel (salary or time allocation) | $120,000/yr (derived from % effort of salary) | Biomedical engineers with radiologist approval in current example. Segmentation can be performed by biomedical engineers, imaging scientists, radiologic technologists, non-radiology physicians, and radiologists. | |||
| “Simple” models or guides, n=6 | $119 (mean of 6 cases; calculated from cost of material and period of allocated time) | Variable cost dependent on volume and provider requests | “Simple” models or guides were defined as anatomic models or guides that consistent of one segmented and printed part, one material, and one color. Example: femoral head model. | ||
| Material cost/case | Segmenting time/case | Interacting with printer and model processing/case | |||
| $5 | 101 min | 55 min | |||
| “Complex” models or guides, n=4 | $320 (mean of 4 cases; calculated from cost of material and period of allocated time) | “Complex” models or guides were defined as anatomic models or guides that consistent of more than segmented and printed part; alternatively more than one material or color. Example: renal mass model consisting of tumor, renal parenchyma, renal artery, renal vein, and collecting system. | |||
| Material cost/case | Segmenting time/case | Interacting with printer and model processing/case | |||
| $31 | 143 min | 149 min | |||
Highlighted column = new in revision
Analyses
Financial analyses included potential cost-savings per surgical case using time saved from the anatomic model and surgical guides studies along with annual utilization analyses. The annual utilization analyses included the gross and net savings if anatomic models and surgical guides were used at a low (5%) and high (20%) rate along with a breakeven analysis that detailed the number of models and guides needed to be produced in an annual period to cover operational costs. The gross savings was defined by the mean cost-savings from the analysis of previous studies multiplied by the number of hours of annual operating room time for the low (5%) or high (20%) utilization rate (i.e., either 5% or 20% of the total annual operating room time). The low (5%) and high (20%) utilization rates were chosen to simulate consistent use in moderate volume procedure or a select case-by-case basis (low utilization) and consistent use in one in a high-volume procedure or consistent use in few moderate procedures (high utilization). The net savings calculation was the same, minus the cost of producing the 3D printed models. Factors such as depreciation, institutional overhead, professional and hospital resources were not accounted for in the simple model.
A model of the minimum number of models to achieve annual operational cost, or the breakeven point, was performed. A breakeven analysis relies on assumptions of linear function of cost and revenue (52). Breakeven point was calculated by the following formula = fixed costs / (cost-savings from 3D printed constructs per case – variable costs of the models or guides. The cost data (previously defined in ‘3D Printing Cost’ section and Table 3) included an annual operating cost of $150,000 and variable cost of producing the 3D printed constructs. For simplicity, the model assumed equal use of the simple and complex guides, at a price of $220 per model or guide (mean of the $119 simple and $320 complex models or guides defined in Table 3). In the breakeven model, an equal incidence of anatomic models and surgical guides was assumed with anatomic models saving a mean 62 minutes of operating room time/case and surgical guides saving a mean 23 minutes of operating room time/case.
Finally, to compare differences between the pooled studies operating room time with 3D printed constructs compared to those without, Student t-test was used to compare the mean operating room time. Statistical significance was set at p<0.05.
RESULTS
The studies included for analyses and detailing the effect of 3D printed anatomic models or surgical guides in patients’ operative care are listed in Table 4. Use of 3D printed anatomic models and surgical guides led to significantly shorter operating room times (mean time saved ranging from 12 to 66 minutes; p-values ranging from <0.001 to 0.04) in all comparisons except for maxillofacial surgical guides, which approach statistical significance (mean time saved 83 minutes; p=05) (Table 4).
Table 4.
Results of literature search initially queried from the referenced systematic review (9) and secondarily reviewed and analyzed in the present study with the inclusion criteria of at least 10 patients where a 3D printed anatomic model or surgical guide was used in operative planning or performance and the presence of a control group with at least 10 patients. The negative values in the “Operative minutes saved” column denote minutes saved while positive values denote added time with the 3D printed construct (references 34, 36, and 44).
| 3D printed anatomic model studies | |||||
|---|---|---|---|---|---|
| Study | Area of focus | Operative minutes saved in experimental groups compared to control group | Patients in experimental group | Patients in control group | |
| 1 | Sieira Gil et al. (17) | Oral and maxillofacial | −41 | 10 | 10 |
| 2 | Xu et al. (18) | Oral and maxillofacial | −17 | 24 | 21 |
| 3 | de Farias et al. (19) | Oral and maxillofacial | −84 | 17 | 20 |
| 4 | Hanasono et al. (20) | Oral and maxillofacial | −102 | 38 | 183 |
| 5 | Zhang et al. (21) | Oral and maxillofacial | −85 | 11 | 24 |
| 6 | Zhang et al. (22) | Orthopedics | −78 | 11 | 11 |
| 7 | Yang et al. (23) | Orthopedics | −28 | 50 | 76 |
| Overall (n=7) mean (median); p-value | −62 (−78); p<0.001 | 23 (17) | 49 (21) | ||
| Oral and maxillofacial (n=5) mean (median); p-value | −66 (−84); p=0.003 | 20 (17) | 52 (21) | ||
| Orthopedics (n=2) mean (median); p-value | −53 (−53); p=0.04 | 31 (31) | 44 (44) | ||
| 3D printed surgical guide studies | |||||
| Study | Area of focus | Operative minutes saved in experimental groups compared to control group | Patients in experimental group | Patients in control group | |
| 1 | Sieira Gil et al.* (17) | Oral and maxillofacial | −42 | 10 | 10 |
| 2 | Xu et al.* (18) | Oral and maxillofacial | −17 | 24 | 21 |
| 3 | Hanasono et al.* (20) | Oral and maxillofacial | −102 | 38 | 183 |
| 4 | Zhang et al.* (22) | Orthopedics | −78 | 11 | 11 |
| 5 | Toto et al. (24) | Oral and maxillofacial | −172.7 | 25 | 12 |
| 6 | Hsu et al. (25) | Orthopedics | −12 | 42 | 29 |
| 7 | Chareancholvanich et al. (26) | Orthopedics | −5.1 | 40 | 40 |
| 8 | Abane et al. (27) | Orthopedics | −6.3 | 59 | 67 |
| 9 | Barrack et al. (28) | Orthopedics | −11 | 100 | 100 |
| 10 | Barrett et al. (29) | Orthopedics | −5.2 | 66 | 86 |
| 11 | Boonen et al. (30) | Orthopedics | −10 | 39 | 40 |
| 12 | Boonen et al. (31) | Orthopedics | −5 | 90 | 90 |
| 13 | Ferrara et al. (32) | Orthopedics | −22.3 | 15 | 15 |
| 14 | Gan et al. (33) | Orthopedics | −15 | 35 | 35 |
| 15 | Hamilton et al. (34) | Orthopedics | 4.3 | 26 | 26 |
| 16 | Kassab and Pietrzak (35) | Orthopedics | −16.7 | 270 | 595 |
| 17 | Kerens et al. (36) | Orthopedics | 5 | 30 | 30 |
| 18 | Nankivell et al. (37) | Orthopedics | −4 | 40 | 45 |
| 19 | Noble et al. (38) | Orthopedics | −6.7 | 19 | 15 |
| 20 | Nunley et al. (39) | Orthopedics | −12.1 | 57 | 57 |
| 21 | Pfitzner et al. (40) | Orthopedics | −15.5 | 60 | 30 |
| 22 | Pietsch et al. (41) | Orthopedics | −12 | 40 | 40 |
| 23 | Rathod et al. (42) | Orthopedics | −18 | 15 | 14 |
| 24 | Renson et al. (43) | Orthopedics | −8.9 | 71 | 60 |
| 25 | Roh et al. (44) | Orthopedics | 12.8 | 50 | 50 |
| Overall (n=26) mean (median); p-value | −23 (−12); p=0.006 | 51 (40) | 68 (40) | ||
| Oral and maxillofacial (n=4) mean (median); p-value | −83 (−72); p=0.05 | 24 (25) | 57 (17) | ||
| Orthopedics (n=21) mean (median); p-value | −12 (−10); p=0.004 | 56 (40) | 70 (40) | ||
= studies are included both in the anatomic model and surgical guide groups
Table 5 details the potential cost-savings per surgical case from the overall mean data of the anatomic model and surgical guide studies, whereas Figure 1 plots potential cost-savings for each identified study. Data for surgical departments utilizing specific time periods was derived from a reference study that detailed total number of surgical cases and total number of hours from a single institution (16). Oral and maxillofacial and orthopedic surgical data were used, as they were the only two disciplines of studies included, as noted in Table 4. There were 509 oral and maxillofacial surgical cases using 1489 hours of operating room time and 807 orthopedic surgical cases using 2,649 hours of operating room time. Table 5 details the potential gross and net savings over a year period if anatomic models or guides were used in maxillofacial or orthopedic surgical procedures at a 5% or 20% rate. At the mean $62 of operating room time per minute, these net savings range from $19,384 to $129,589 and $77,536 to $518,358 for low (5%) and high (20%) utilization rates, respectively. Figure 2 details the breakeven points needed to maintain an annual fixed operation cost of $150,000 along with associated model or guide costs.
Table 5.
One-year projected gross and net savings by using 3D printed anatomic models and surgical guides in patient care for maxillofacial and orthopedic indications. Data are group for both “low” and “high” use of 3D printed anatomic models or surgical guides. A low utilization rate was defined as using 3D printed constructs in 5% of the surgical cases and a high utilization rate was defined as use in 20% of cases. “Simple” and “complex” models are derived from authors’ institutional data and defined in Table 3.
| Gross Savings Chart – Annual total gross savings at low utilization (5% of cases) / high utilization (20% of cases) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 3D printed construct - specialty | Total hours saved/year | Savings at $62 USD per min | Savings at $22 USD per min | Savings at USD $133 per min | ||||
| Low (5%) | High (20%) | Low (5%) | High (20%) | Low (5%) | High (20%) | Low (5%) | High (20%) | |
| Anatomic models – oral and maxillofacial | 28 hours / 112 hours | $104,160 / $416,640 | $36,960 / $147,840 | $223,440 / $893,760 | ||||
| Surgical guide – oral and maxillofacial | 35 hours / 141 hours | $130,944 / $523,776 | $46,464 / $185,856 | $280,869 / $1,123,584 | ||||
| Anatomic models – orthopedics | 36 hours / 143 hours | $132,618 / $530,472 | $47,058 / $188,232 | $284,486 / $1,137,943 | ||||
| Surgical guides – orthopedics | 8 hours / 32 hours | $29,760 / $119,040 | $10,560 / $42,240 | $63,840 / $255,360 | ||||
| Net Savings Chart – Annual total net savings at low utilization (5% of cases) / high utilization (20% of cases) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Technique cost | Total cost | Anesthesia cost/min | Anatomic models – oral and maxillofacial | Surgical guide – oral and maxillofacial | Anatomic models – orthopedics | Surgical guides – orthopedics | ||||
| Low (5%) | High (20%) | Low (5%) | High (20%) | Low (5%) | High (20%) | Low (5%) | High (20%) | |||
| $119 per “simple” model or guide |
Low use (5% of cases) = $3,029) High use (20% of cases) = $12,114 |
Savings at: $62/min Savings at: $22/min Savings at: $133/min |
$101,131 / $404,526 $33,931/ $135,726 $220,411/ $881,646 |
$127,915 / $511,662 $43,435 / $173,742 $277,867 / $1,111,470 |
$129,589 / $518,358 $44,029 / $176,118 $281,457 / $1,125,828 |
$26,731/ $106,926 $7,531/ $30,126 $60,811/ $243,246 |
||||
| $320 per “complex” model or guide |
Low use (5% of cases) $8,144) High use (20% of cases)= $32,576 |
Savings at: $62/min Savings at: $22/min Savings at: $133/min |
$96,016 / $384,064 $28,816 / $115,264 $215,296 / $861,184 |
$122,800 / $491,200 $38,320 / $153,280 $272,752 / $1,091,008 |
$124,474 / $497,896 $38,914 / $155,656 $276,341 / $1,105,367 |
$21,616/ $86,464 $2,416 / $9,664 $55,696 / $222,784 |
||||
Figure 1.

Cost-savings from operative room time saved plotted for the individual studies defined in Table 4, including 3D printed anatomic models (a) and surgical guides (b) used in patients’ operative care compared to a control group. The $22, $62, and $133 USD/minute are collective data from the reference study reporting range of operating room time/minute in the United States (14). Note that standard deviation is not presented via error bars due to scale and overlap of the data points.
Figure 2.
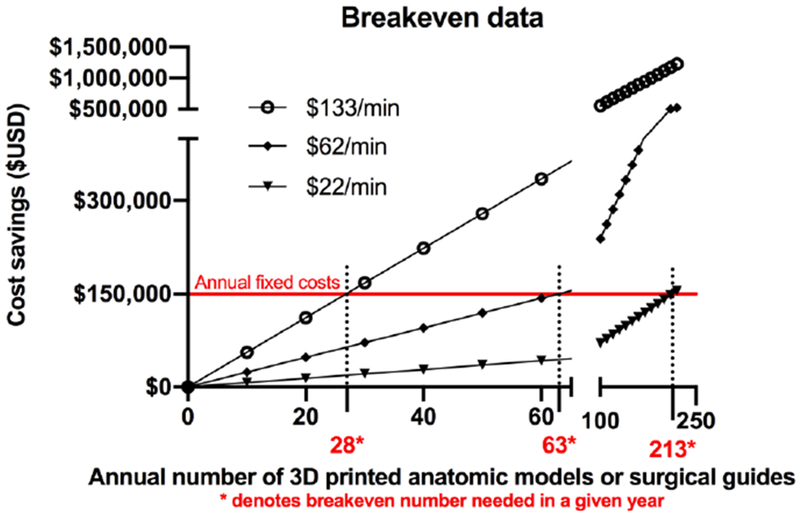
Breakeven point of 3D printed anatomic models and surgical guides. The breakeven point for 3D printed constructs used in patient care at an operating room cost at $62/minute is 63 models or guides/year (1.2 models or guides/week). Breakeven point was calculated by the following formula = fixed costs / (cost-savings from 3D printed constructs per case – variable costs of the models or guides. The fixed and variable cost data is presented in Table 3.
DISCUSSION
Our literature-based financial analyses demonstrate that cost-savings of 3D printed anatomic models and surgical guides can be substantial and financially feasible at relatively low volumes. Specifically, it shows that a volume of approximately 63 models or guides a year, or 1.2 3D printed constructs per week, can potentially cover operational costs needed to maintain a 3D printing lab. One driver of cost-savings for medical modeling is the generation of 3D anatomic models and surgical guides that enhance surgical planning and execution, and in doing so, reduces operating room time. While prospective data are needed to better structure and define the financial value of 3D printing, it is important to benchmark the literature to date that does describe the value, potential cost-savings, and effect on patient outcome with 3D printed anatomic models used for preoperative planning. The present study highlights that potential cost-savings can be substantial.
The current study relied on a previously published systematic review (9) that queried studies through December 2015. This identified only maxillofacial and orthopedic studies that fit the present studies inclusion criteria. The use of 3D printed anatomic models, surgical guides, and implants are indeed skewed with higher levels of evidence with maxillofacial and orthopedic focuses. This is best reflected in a recent systematic review that profiled all published randomized controlled trials in 3D printing (53). These total 21 in number, and all have either an oral and maxillofacial or orthopedic surgery focus. The distribution of these 21 studies includes 11 surgical guide studies, 7 anatomic model studies, and 3 implant studies, similar to the present study’s identification of 25 surgical guide studies, 7 anatomic model studies, and the 2 implant studies that were excluded from analyses. Of note, none of the queried randomized trials were performed in the United States. Another study summarizes 92 different international clinical trials from varying registries and in different stages of completion. China leads in the number of 3D printing-related clinical trials with 42 (46% of the total; 42/92) followed by the United States with 13 (14% of the total; 14/92) (54). Further reflecting the orthopedic and oral and maxillofacial predominance, orthopedic surgery has the largest proportion of registered trial (25 trials; 27% of the total; 13/92), followed by dentistry (13 trials; 14% of the total; 25/92) and oral and maxillofacial surgery (10 trials; 11% of the total; 10/92) (54).
However, studies in different disciplines demonstrating the effect of 3D printed constructs in procedural time have been reported since the referenced systematic review’s December 2015 literature search (9), including studies focusing in vascular surgery (55), interventional cardiology (56), and congenital heart disease (57). Torres and De Luccia (55) reported a cohort of patients undergoing elective abdominal aortic aneurysm repair where they prospectively changed their practice and deployed endovascular stents in an anatomic model preoperatively in 25 patients and compared it to a historical control of 30 patients the year prior to employing anatomic models. The patients with preoperative rehearsal on anatomic models had significantly less operative and fluoroscopic time along with a significantly lower volume of contrast needed during the procedure. Specifically, the operative time was a mean 85 minutes less compared to the control group. Similarly, Obasare et al. (56) changed their practice from primarily profiling cardiac anatomy with echocardiography to cardiac CT and anatomic models before left atrial appendage closure procedure. In comparison to the control group (9 patients), their anatomic model group (13 patients), had a significantly shorter procedural time (mean 37 minutes shorter), anesthesia time, and fluoroscopy time along with significantly fewer peri-device leak events. Ryan et al. (57) reported a cohort of 33 pediatric patients with congenital heart disease and other indications for cardiothoracic surgeries that had their preoperative planning facilitated by anatomic model. They compared those 33 patients to a historical matched cohort of 113 cases and showed a mean 9 minute reduction in operating room time, although this did not achieve statistical significance. Although the data in the present study all had orthopedic or maxillofacial focus that was not intentional but merely reflective of the available data, the reported mean operating room time saved of 85 minutes in endovascular aortic surgery (55), 37 minutes in interventional cardiology procedure (56), and 9 minutes in cardiothoracic surgery (57) would results in cost-savings when extrapolated to the present study’s financial model. Using the mean $62/minute, the cost-savings would be $5,270, $2,294, and $558, respectively. These single study metrics of mean procedural time differences require further studies, each in their respective surgical or procedural discipline.
Limitations to the current study include using different variables from prior studies at different time points and theoretical design of the scenarios. The varied number of sources may limit the generalizability of the data; however, the models and concepts used in the present study may serve as a template to report cost-savings when 3D printing is used in a cohort of patients compared to a control group. As previously mentioned, a published systematic review (9) was used as the reference for studies and its literature search is dated, through December 2015, and new studies in disciplines other than maxillofacial and orthopedics have published studies demonstrated a time saved benefit (55–57). Performing an updated systematic review of the 3D printing literature is certainly warranted, but that was outside the primary purpose of the present study. The present study did not systematically evaluate patient characteristics or specific surgical indications, limiting its generalizable to specific orthopedic and maxillofacial applications; however, our underlying purpose was to create a general financial analysis and the dichotomization of the cohorts limited only to orthopedic and maxillofacial applications was unintentional. Rather, we aimed to provide those interested in establishing a 3D printing lab or increasing their volume, especially given the recently implemented category III CPT codes, financial understanding through our theoretical cost analyses. The current study is limited to orthopedic and maxillofacial applications. 3D printed anatomic models have shown value in cardiovascular, urologic, and other applications and future studies detailing studies that quantify operating room time saved may benefit from similar economic analyses. Furthermore, the present study dichotomized 3D printed constructs into anatomic models or surgical guides, and several studies qualified as both. Detailed patterns of the operative patterns were not objectively evaluated in the present study and cost metrics from individual cohorts was not incorporated into the analysis; anecdotally, the latter was infrequently available. The mean operating room time of $62/min reflected national survey data collected from 2005 (15). Although the Shippert had the underlying purpose to determine the mean operating room time in the United States to serve as a metric of potential per minute costs savings, the author used three common aesthetic surgical procedures to CPT codes for the model of operating room cost. Although data based on aesthetic general surgical procedures may limit the generalizability to other surgical procedures, this reference study was the most cited study of operating room cost (from Google Scholar data) according to our literature search. Although adjustment for inflation is only partially presented (Table 2), operating room cost may vary substantially from region to region; more recent data from a 2018 publication (58) estimated operating room cost in California from fiscal year 2014 was $36 to $37/min. The calculated $150,000 annual operational cost did not account for initial investment in starting a laboratory, which may vary substantially across 3D printing laboratories. Operational costs would include the cost of at least one printer, which would contribute to fixed costs as straight-line depreciation across its useful life, assumed at 5-7 years depending upon the quality of the printer. The cost of initial purchasing of a printer, allotting laboratory space, training, hiring or allocating personnel to perform the technical component of producing the model are among the current challenges to radiology departments implementing 3D printing services (Table 3) (6, 7). If these initial investments are accounted for in operational costs, the breakeven point would be higher. In the current study, we did not include an estimate as the formation of the authors’ 3D printing lab included repurposing space and resources and a true initial investment could not be accurately estimated. The cost and variety of printing simple and complex models and guides also may have substantial variability among different practices. Segmenting and fabricating models can be a timely expenditure, and lack of reimbursement is partly what has kept 3D printing from expanding clinically.
In conclusion, the potential cost-savings for using 3D printed models and surgical guides for patients’ operative care can be substantial. The present study used previously published data (9; 15–44) to illustrate the potential value 3D printing can offer in terms of reducing the number of operating room minutes, using studies published with maxillofacial and orthopedic focuses. Studies to validate these analyses using single and multiple institutional data are needed given the heterogeneity of sources used for analysis. Nevertheless, the present study’s literature-based financial analyses demonstrate surrogates of value and financial feasibility of 3D printing in preoperative planning and saving intraoperative time.
Acknowledgments
Funding and Disclosures: DHB receives salary support from National Institutes of Health TOP-TIER grant T32-EB021955. JAW is a co-inventor of patent application: “Methods and Devices For Three-Dimensional Printing Or Additive Manufacturing Of Bioactive Medical Devices”, Application number US14822275; this is not discussed in the current manuscript. FJR is the Medical Director of Imagia Cybernetics. All other authors claim no conflicts of interest or disclosures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.New ACR-Sponsored CPT Codes Approved by the AMA. American College of Radiology Website. Available at: https://www.acr.org/Advocacy-and-Economics/Advocacy-News/Advocacy-News-Issues/In-the-November-2-2018-Issue/New-ACR-Sponsored-CPT-Codes-Approved-by-the-AMA Accessed August 20, 2019.
- 2.Most recent changes to the CPT®) Category III Codes document. American Medical Association Website. Available at: https://www.ama-assn.org/system/files/2019-03/cpt-category3-codes-long-descriptors.pdf Accessed August 20, 2019.
- 3.Hirsch JA, Leslie-Mazwi TM, Nicola GN, et al. Current procedural terminology; a primer. J Neurointerv Surg 2015;7:309–312. [DOI] [PubMed] [Google Scholar]
- 4.RSNA and ACR to Collaborate on Landmark Medical 3D Printing Registry. Radiological Society of North America Website. Available at: https://www.rsna.org/en/news/2019/August/3D-Printing-Registry Accessed August 20, 2019.
- 5.Chepelev L, Wake N, Ryan J, et al. Radiological Society of North America (RSNA) 3D printing Special Interest Group (SIG): guidelines for medical 3D printing and appropriateness for clinical scenarios. 3D Print Med 2018; 4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballard DH, Trace AP, Ali S, et al. Clinical Applications of 3D Printing: Primer for Radiologists. Acad Radiol 2018;25:52–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodgdon T, Danrad R, Patel MJ, et al. Logistics of Three-dimensional Printing: Primer for Radiologists. Acad Radiol 2018;25:40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Urso PS, Barker TM, Earwaker WJ, et al. Stereolithographic biomodelling in craniomaxillofacial surgery: a prospective trial. J Craniomaxillofac Surg 1999;27:30–37. [DOI] [PubMed] [Google Scholar]
- 9.Tack P, Victor J, Gemmel P, Armenians L. 3D-printing techniques in a medical setting: a systematic literature review. Biomed Eng Online 2016; 15:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martelli N, Serrano C, van den Brink H, et al. Advantages and disadvantages of 3-dimensional printing in surgery: A systematic review. Surgery 2016; 159:1485–1500. [DOI] [PubMed] [Google Scholar]
- 11.George E, Liacouras P, Rybicki FJ, Mitsouras D. Measuring and Establishing the Accuracy and Reproducibility of 3D Printed Medical Models. Radiographics 2017;37:1424–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crump SS. Apparatus and method for creating three-dimensional objects. US Patent 5,121,329; October 30, 1989. [Google Scholar]
- 13.Rybicki FJ. Medical 3D printing and the physician-artist. Lancet 2018;391:651–652. [DOI] [PubMed] [Google Scholar]
- 14.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med 2015;372:793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shippert RD. A Study of Time-Dependent Operating Room Fees and How to save $100 000 by Using Time-Saving Products. The American Journal of Cosmetic Surgery 2005;22:25–34. [Google Scholar]
- 16.Resnick AS, Corrigan D, Mullen JL, Kaiser LR. Surgeon contribution to hospital bottom line: not all are created equal. Ann Surg 2005;242:530–537; discussion 537-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sieira Gil R, Roig AM, Obispo CA, Morla A, Pages CM, Perez JL. Surgical planning and microvascular reconstruction of the mandible with a fibular flap using computer-aided design, rapid prototype modelling, and precontoured titanium reconstruction plates: a prospective study. Br J Oral Maxillofac Surg 2015;53:49–53. [DOI] [PubMed] [Google Scholar]
- 18.Xu H, Zhang C, Shim YH, Li H, Cao D. Combined use of rapid-prototyping model and surgical guide in correction of mandibular asymmetry malformation patients with normal occlusal relationship. J Craniofac Surg 2015:26:418–421. [DOI] [PubMed] [Google Scholar]
- 19.de Farias TP, Dias FL, Galvão MS, Boasquevisque E, Pastl AC, Albuquerque Sousa B. Use of prototyping in preoperative planning for patients with head and neck tumors. Head Neck 2014;36:1773–1782. [DOI] [PubMed] [Google Scholar]
- 20.Hanasono MM, Skoracki RJ. Computer-assisted design and rapid prototype modeling in microvascular mandible reconstruction. Laryngoscope 2013;123:597–604. [DOI] [PubMed] [Google Scholar]
- 21.Zhang S, Liu X, Xu Y, et al. Application of rapid prototyping for temporomandibular joint reconstruction. J Oral Maxillofac Surg 2011;69:432–438. [DOI] [PubMed] [Google Scholar]
- 22.Zhang YZ, Chen B, Lu S, et al. Preliminary application of computer-assisted patient-specific acetabular navigational template for total hip arthroplasty in adult single development dysplasia of the hip. Int J Med Robot 2011;7:469–474. [DOI] [PubMed] [Google Scholar]
- 23.Yang M, Li C, Li Y, et al. Application of 3D rapid prototyping technology in posterior corrective surgery for Lenke 1 adolescent idiopathic scoliosis patients. Medicine (Baltimore) 2015;94:e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toto JM, Chang El, Agag R, Devarajan K, Patel SA, Topham NS. Improved operative efficiency of free fibula flap mandible reconstruction with patient-specific, computer-guided preoperative planning. Head Neck 2015;37:1660–1664. [DOI] [PubMed] [Google Scholar]
- 25.Hsu AR, Davis WH, Cohen BE, Jones CP, Ellington JK, Anderson RB. Radiographic Outcomes of Preoperative CT Scan-Derived Patient-Specific Total Ankle Arthroplasty. Foot Ankle Int 2015;36:1163–1169. [DOI] [PubMed] [Google Scholar]
- 26.Chareancholvanich K, Narkbunnam R, Pomrattanamaneewong C. A prospective randomised controlled study of patient-specific cutting guides compared with conventional instrumentation in total knee replacement. Bone Joint J 2013;95-B:354–359. [DOI] [PubMed] [Google Scholar]
- 27.Abane L, Anract P, Boisgard S, Descamps S, Courpied JP, Hamadouche M. A comparison of patient-specific and conventional instrumentation for total knee arthroplasty: a multicentre randomised controlled trial. Bone Joint J 2015;97-B:56–63. [DOI] [PubMed] [Google Scholar]
- 28.Barrack RL, Ruh EL, Williams BM, Ford AD, Foreman K, Nunley RM. Patient specific cutting blocks are currently of no proven value. J Bone Joint Surg Br 2012;94:95–99. [DOI] [PubMed] [Google Scholar]
- 29.Barrett W, Hoeffel D, Dalury D, Mason JB, Murphy J, Himden S. In-vivo alignment comparing patient specific instrumentation with both conventional and computer assisted surgery (CAS) instrumentation in total knee arthroplasty. J Arthroplasty 2014;29:343–347. [DOI] [PubMed] [Google Scholar]
- 30.Boonen B, Schotanus MGM, Kort NP. Preliminary experience with the patient-specific templating total knee arthroplasty. Acta Orthop 2012;83:387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boonen B, Schotanus MGM, Kerens B, van der Weegen W, van Drumpt R a M, Kort NP. Intraoperative results and radiological outcome of conventional and patient-specific surgery in total knee arthroplasty: a multicentre, randomised controlled trial. Knee Surg Sports Traumatol Arthrosc 2013;21:2206–2212. [DOI] [PubMed] [Google Scholar]
- 32.Ferrara F, Cipriani A, Magarelli N, et al. Implant positioning in TKA: comparison between conventional and patient-specific instrumentation. Orthopedics 2015;38:e271–280. [DOI] [PubMed] [Google Scholar]
- 33.Gan Y, Ding J, Xu Y, Hou C. Accuracy and efficacy of osteotomy in total knee arthroplasty with patient-specific navigational template. Int J Clin Exp Med 2015;8:12192–12201. [PMC free article] [PubMed] [Google Scholar]
- 34.Hamilton WG, Parks NL, Saxena A. Patient-specific instrumentation does not shorten surgical time: a prospective, randomized trial. J Arthroplasty 2013;28:96–100. [DOI] [PubMed] [Google Scholar]
- 35.Kassab S, Pietrzak WS. Patient-specific positioning guides versus manual instrumentation for total knee arthroplasty: an intraoperative comparison. J Surg Orthop Adv 2014;23:140–146. [DOI] [PubMed] [Google Scholar]
- 36.Kerens B, Schotanus MGM, Boonen B, Kort NP. No radiographic difference between patient-specific guiding and conventional Oxford UKA surgery. Knee Surg Sports Traumatol Arthrosc 2015;23:1324–1329. [DOI] [PubMed] [Google Scholar]
- 37.Nankivell M, West G, Pourgiezis N. Operative efficiency and accuracy of patient-specific cutting guides in total knee replacement. ANZ J Surg 2015:85:452–455. [DOI] [PubMed] [Google Scholar]
- 38.Noble JW, Moore CA, Liu N. The value of patient-matched instrumentation in total knee arthroplasty. J Arthroplasty 2012;27:153–155. [DOI] [PubMed] [Google Scholar]
- 39.Nunley RM, Ellison BS, Ruh EL, et al. Are patient-specific cutting blocks cost-effective for total knee arthroplasty? Clin Orthop Relat Res 2012;470:889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfitzner T, Abdel MP, von Roth P, Perka C, Hommel H. Small improvements in mechanical axis alignment achieved with MRI versus CT-based patient-specific instruments in TKA: a randomized clinical trial. Clin Orthop Relat Res 2014;472:2913–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pietsch M, Djahani O, Zweiger C, et al. Custom-fit minimally invasive total knee arthroplasty: effect on blood loss and early clinical outcomes. Knee Surg Sports Traumatol Arthrosc 2013;21:2234–2240. [DOI] [PubMed] [Google Scholar]
- 42.Rathod PA, Deshmukh AJ, Cushner FD. Reducing blood loss in bilateral total knee arthroplasty with patient-specific instrumentation. Orthop Clin North Am 2015;46:343–350, ix. [DOI] [PubMed] [Google Scholar]
- 43.Renson L, Poilvache P, Van den Wyngaert H. Improved alignment and operating room efficiency with patient-specific instrumentation for TKA. Knee 2014;21:1216–1220. [DOI] [PubMed] [Google Scholar]
- 44.Roh YW, Kim TW, Lee S, Seong SC, Lee MC. Is TKA using patient-specific instruments comparable to conventional TKA? A randomized controlled study of one system. Clin Orthop Relat Res 2013;471:3988–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lethaus B, Poort L, Bockmann R, Smeets R, Tolba R, Kessler P. Additive manufacturing for microvascular reconstruction of the mandible in 20 patients. J Craniomaxillofac Surg 2012;40:43–46. [DOI] [PubMed] [Google Scholar]
- 46.Weinstock P, Prabhu SP, Flynn K, Orbach DB, Smith E. Optimizing cerebrovascular surgical and endovascular procedures in children via personalized 3D printing. J Neurosurg Pediatr 2015;16:584–589. [DOI] [PubMed] [Google Scholar]
- 47.Izatt MT, Thorpe PLPJ, Thompson RG, et al. The use of physical biomodelling in complex spinal surgery. Eur Spine J 2007;16:1507–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kunz M, Rudan JF, Xenoyannis GL, Ellis RE. Computer-assisted hip resurfacing using individualized drill templates. J Arthroplasty 2010;25:600–606. [DOI] [PubMed] [Google Scholar]
- 49.Hananouchi T, Saito M, Koyama T, et al. Tailor-made surgical guide based on rapid prototyping technique for cup insertion in total hip arthroplasty. Int J Med Robot 2009;5:164–169. [DOI] [PubMed] [Google Scholar]
- 50.Zinser M, Zoeller J. Computer-Designed Splints for Surgical Transfer of 3D Orthognathic Planning. Facial Plast Surg 2015;31:474–490. [DOI] [PubMed] [Google Scholar]
- 51.Mihalko WM. Patient-Specific Cutting Guides Were Not Better Than Conventional Instrumentation for Total Knee Arthroplasty. J Bone Joint Surg Am 2015;97:1891. [DOI] [PubMed] [Google Scholar]
- 52.Goggans TP. Break-even analysis with curvilinear functions. The Accounting Review 1965;40:867–871. [Google Scholar]
- 53.Diment LE, Thompson MS, Bergmann JHM. Clinical efficacy and effectiveness of 3D printing: a systematic review. BMJ Open 2017;7:e016891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Witowski J, Sitkowski M, Zuzak T, et al. From ideas to long-term studies: 3D printing clinical trials review. Int J Comput Assist Radiol Surg 2018;13:1473–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Torres 10, De Luccia N. A simulator for training in endovascular aneurysm repair: The use of three dimensional printers. Eur J Vase Endovasc Surg 2017;54:247–253. [DOI] [PubMed] [Google Scholar]
- 56.Obasare E, Mainigi SK, Morris DL, et al. CT based 3D printing is superior to transesophageal echocardiography for pre-procedure planning in left atrial appendage device closure. Int J Cardiovasc Imaging 2018;34:821–831. [DOI] [PubMed] [Google Scholar]
- 57.Ryan J, Plasencia J, Richardson R, et al. 3D printing for congenital heart disease: a single site’s initial three-year experience. 3D Print Med 2018;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Childers CP, Maggard-Gibbons M. Understanding Costs of Care in the Operating Room. JAMA Surg 2018;153:el76233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Consumer Price Index Inflation Calculator. U.S. Bureau of Labor Statistics; Available at: https://data.bls.gov/cgi-bin/cpicalc.pl Accessed: August 20, 2019. [Google Scholar]


