Abstract
Purpose
To compare gait biomechanics throughout stance phase 6 and 12 months following unilateral anterior cruciate ligament reconstruction (ACLR) between ACLR and contralateral limbs and compared to controls.
Methods
Vertical ground reaction force (vGRF), knee flexion angle (KFA), and internal knee extension moment (KEM) were collected bilaterally 6 and 12 months post-ACLR in 30 individuals (50% female, 22±3 years, body mass index [BMI]=23.8±2.2kg/m2) and at a single time point in 30 matched uninjured controls (50% female, 22±4 years, BMI=23.6±2.1kg/m2). Functional analyses of variance were used to evaluate the effects of limb (ACLR, contralateral, and control) and time (6 and 12 months) on biomechanical outcomes throughout stance.
Results
Compared to the uninjured controls, the ACLR group demonstrated bilaterally lesser vGRF (ACLR=9%BW, contralateral=4%BW) during early stance and greater vGRF during mid-stance (ACLR=5%BW, contralateral=4%BW) 6 months post-ACLR. Compared to the uninjured controls, the ACLR group demonstrated bilaterally lesser vGRF (ACLR=10%BW, contralateral=8%BW) during early stance and greater vGRF during mid-stance (ACLR=5%BW, contralateral=5%BW) 12 months post-ACLR. Compared to controls, the ACLR limb demonstrated lesser KFA during early stance at 6 (2.3°) and 12 months post-ACLR (2.0°), and the contralateral limb demonstrated lesser KFA during early stance at 12 months post-ACLR (2.8°). Compared to controls, the ACLR limb demonstrated lesser KEM during early stance at both 6 (0.011BW*height) and 12 months (0.007BW*height) post-ACLR, and the contralateral limb demonstrated lesser KEM during early stance only at 12 months (0.006BW*height).
Conclusion
Walking biomechanics are altered bilaterally following ACLR. During the first 12 months post-ACLR, both the ACLR and contralateral limbs demonstrate biomechanical differences compared to control limbs. Differences between the contralateral and control limbs increase from 6 to 12 months post-ACLR.
Keywords: Vertical ground reaction force, knee flexion angle, knee extension moment, walking biomechanics, anterior cruciate ligament
INTRODUCTION
Individuals with an anterior cruciate ligament (ACL) injury and ACL reconstruction (ACLR) often demonstrate persistent alterations in walking gait biomechanics (1–3), which may contribute to the development of post-traumatic knee osteoarthritis (PTOA) (4–6). Individuals demonstrate a stiffened-knee strategy during walking gait early post-ACLR in the involved limb, characterized by lesser peak knee flexion angle (KFA) and peak internal knee extension moment (KEM) during the first 50% of stance phase (7–9). This stiffened-knee strategy is hypothesized to negatively influence force attenuation at the knee and contribute to the hastened development of PTOA (10, 11). Deleterious alterations in cartilage composition, consistent with PTOA development, have been reported within the first 12 months following ACLR (12); therefore, it is important to evaluate gait at early time points post-ACLR when gait biomechanics are known to associate with early joint tissue metabolism (13) and composition (14, 15). Some studies show individuals achieve symmetrical peak KFA and KEM between limbs between 1 and 2 years following ACLR (16, 17), suggesting symmetrical gait is achieved between limbs over time; however, a longitudinal study is needed to determine if these changes in KFA and KEM differ compared to a matched control group. Without a comparison to a control group, it is difficult to determine if ACLR individuals adopt a more symmetrical gait pattern by adjusting biomechanics in the ACLR limb to more closely resemble those of uninjured controls or if the contralateral limb adopts a biomechanical strategy that resembles the aberrant biomechanics of the ACLR limb. A better understanding of sagittal plane biomechanics post-ACLR is needed as there is no longitudinal study that evaluates sagittal plane biomechanics within the first year post-ACLR in the involved limb with comparisons to both the contralateral limb and uninjured controls (3).
Vertical ground reaction force (vGRF) is a fundamental measure of external load exerted onto the limb during stance (18) and has been associated with joint tissue metabolism (6, 13), femoral cartilage thickness (4, 19) and composition (14, 15), as well as patient-reported outcomes (PROs) post-ACLR (18). Both excessive and insufficient joint loading have been associated with deleterious tissue changes (14, 15) and PROs (18). Previous studies have not separately included vGRF in gait analysis post-ACLR, although changes in vGRF may influence other knee joint moments, such as KEM (3, 18, 20). Additionally, modifying vGRF using real-time biofeedback demonstrated the ability to alter KEM and knee flexion excursion (KFE) during gait in individuals with an ACLR (20), suggesting that cueing a change in vGRF may be used to modify multiple important gait variables. Therefore, a longitudinal analysis of early changes in vGRF is needed to understand how this critical gait variable changes over time, which may be used to inform the development of future interventions. The majority of previous studies (1, 16, 17) have evaluated the peak magnitude of gait biomechanics early in stance when joint tissues are initially loaded after the foot contacts the ground. While these peak biomechanics in early stance are relevant to deleterious changes in joint tissues following ACLR (6, 14), biomechanical changes in mid and late-stance have not been well studied. Determining if differences in gait biomechanics exist throughout the entirety of stance will provide a more complete understanding of other portions of stance phase that may impact aberrant joint loading following ACLR.
Overall, determining bilateral changes in gait biomechanics compared to uninjured controls, throughout stance phase, at 6 and 12 months post-ACLR is a novel approach to better understand early changes in sagittal plane biomechanics. Therefore, the primary purpose of the current study was to evaluate biomechanical variables related to a stiffened-knee strategy (i.e. vGRF, KFA, and KEM) throughout stance phase and compare these variables between involved and contralateral limbs at 6 and 12 months post-ACLR. Our secondary purpose was to separately compare biomechanics from the involved and contralateral limb of the ACLR cohort to an uninjured control cohort at 6 and 12 months post-ACLR. We hypothesized vGRF would increase during early and late stance while decreasing during mid-stance in the involved limb from 6 to 12 months post-ACLR, becoming similar to the vGRF waveforms of the contralateral and control limbs. We hypothesized KFA would increase during the first half of stance and decrease during the second half of stance (close to terminal knee extension), therefore increasing the overall KFE, and become more similar to the contralateral and control limbs. We also hypothesized KEM would increase throughout stance in the involved limb from 6 to 12 months, causing the ACLR limb to more closely resemble the contralateral limb in the ACLR cohort as well as uninjured control limbs.
METHODS
Design
Individuals in the ACLR cohort were recruited into a prospective longitudinal study prior to ACL surgery. Participants were recruited within 14 days of ACL injury, and ACLR occurred 31±16 days after ACL injury. Bilateral gait biomechanics were collected at six (201±32 days) and twelve-month (373±19 days) post-ACLR follow-up exams. Control participants were recruited from a convenience sample of uninjured individuals from the community, and gait biomechanics were collected at a single time point. A previous study (18) using a similar functional waveform analysis found vGRF mean differences between symptomatic and asymptomatic individuals with an ACLR that ranged between 2–6% body weight (BW) at different points of stance. Therefore, we estimated 30 individuals would be needed to detect a difference between waveforms if a small effect (d = 0.33; corresponding to a 2%BW mean difference for vGRF) was found for each comparison, assuming similar inter-trial variability as reported in previous research (two tailed alpha= 0.05; 1-ß = 0.8; G*Power, v3.1.9.2) (21). The Institutional Review Board at the University of North Carolina at Chapel Hill approved all methods, and all participants provided written informed consent prior to participation.
ACLR participants
Thirty individuals scheduled for primary unilateral ACLR surgery were consented for the current study during initial presentation at the clinic. Individuals between 18 and 35 years old were included. We excluded individuals who were pregnant, as well as those diagnosed with inflammatory arthritis. We excluded individuals who were in need of a multi-ligament reconstruction, had any other musculoskeletal injury within the past 6 months, or any history of orthopaedic surgery. Individuals with chondral, meniscal, and medial cruciate ligament (MCL) injuries were included. The contralateral limb of all individuals in the ACLR group had no history of injury. All ACL injured individuals underwent arthroscopically assisted single incision ACLR using a patellar tendon autograft performed by one of the three participating surgeons. All participants were referred to a licensed physical therapist or athletic trainer for a supervised, structured rehabilitation regimen that began during the first week following surgery and progressed over the next six months.
Uninjured control participants
Thirty uninjured control individuals were matched to the 30 individuals with an ACLR based on sex, age (±2 years), and body mass index (BMI) (±2 kg/m2). Individuals between 18 and 35 years old were included. We excluded individuals who were pregnant, as well as those diagnosed with inflammatory arthritis. Uninjured control individuals were excluded if they had any history of knee injury, history of lower extremity surgery, or history of any chronic joint pathology (e.g. chronic ankle instability, hip labrum injury, or patellar femoral pain syndrome).
Collection of walking gait biomechanics
For each subject, 25 retroreflective markers were placed on the lower and upper extremities and a rigid cluster of three retroreflective markers were placed over the sacrum (6, 18, 22). Marker positions were collected using a 10-camera three-dimensional motion capture system (Vicon) and post processed with Vicon v1.8.5 motion capture software (Vicon Motion Systems). Participants walked barefoot over two Bertec force plates (40×60cm, FP406010, Bertec Corporation, Columbus, Ohio, United States) embedded in a 6-meter walkway so the entire stance phase for both the right and left limbs could be collected from a single trial (6, 22, 23). Participants were instructed to walk at a self-selected speed (Table 1) described as “comfortably walking over a sidewalk” and to look straight ahead maintaining a constant speed through two sets of infrared timing gates (TF100, Trac Tronix, Lenexa, Kansas) centered over the force plates. Once the participants felt comfortable with the task, five practice trials were performed to determine the average walking speed for the test trials. Participants performed five testing gait trials that were considered acceptable if: 1) Both right and left feet individually made contact with a single force plate for the entirety of stance; 2) A forward gaze was maintained; 3) Consistent gait speed (±5% of the predetermined speed) was maintained; 4) Gait was not visibly altered during the trial (6, 18, 22).
Table 1.
Participant Demographics
| Individuals with anterior cruciate ligament reconstruction | Uninjured controls | |
|---|---|---|
| Sex | 16 (50%) females 16 (50%) males |
16 (50%) females 16 (50%) males |
| Age (years) | 21.6±3.4 | 21.7±3.6 |
| Body Mass Index (kg/m2) | 23.8±2.2 | 23.6±2.1 |
| 6-Month Walking Speed (m/s) | 1.26±0.12 | 1.31±0.16 |
| 12-Month Walking Speed | 1.22±0.14* | |
| Days between reconstruction and 6-month follow-up | 201±32 | |
| Days between reconstruction and 12-month follow-up | 373±19 | |
| Concomitant Lateral Meniscal Tear | n = 22 (76%) | |
| Concomitant Medial Meniscal Tear | n = 7 (24%) | |
| Concomitant Chondral Injury | n = 10 (35%) |
Significantly different from uninjured controls at p ≤ 0.05
Marker trajectories were sampled at 120 Hz and low pass filtered at 10 Hz (4th order recursive Butterworth), while force data were sampled at 1200 Hz and low pass filtered at 10 Hz (4th order recursive Butterworth) (18). Stance was defined as the interval between heel strike (vGRF > 20 N) and toe off (vGRF < 20 N). Biomechanical variables were derived with Visual3D software (C-Motion, Germantown, MD). KFA was calculated as the angle of the shank relative to the thigh using Euler angles (YZX sequence) such that flexion represented a positive value (22). Internal KEM was calculated using an inverse dynamics approach such that a more negative value represented a greater moment. vGRF was normalized to body weight (BW) and KEM was normalized to the product of BW and height (m) (6, 22, 23). vGRF, KFA, and KEM data during stance from each of the five trials were extracted and time-normalized to 101 data points using custom algorithms in MATLAB (MathWorks, Natick, MA, USA)(18). For our analysis, we evaluated bilateral (i.e., involved and contralateral limbs) biomechanics in the ACLR cohort and unilateral biomechanics in the uninjured controls. 54% of the ACLR cohort underwent ACLR surgery on their right limb; therefore, we assessed the right limb for 54% (16 out of 30 participants) of the uninjured control cohort via random assignment, which has been found to be an adequate means of matching limbs for uninjured controls (24).
Statistical analyses
Prior to the primary analysis, demographic variables were compared between the ACLR cohort and uninjured controls as well as between time points in the ACLR cohort (demographics between 6 and 12 months) using independent and paired t-tests, respectively (α ≤ 0.05; SPSS, Version 19.0, IBM Corp., Somers, NY, USA). For our primary analyses, 2 × 2 functional analyses of variance (25) were used to compare our independent variables of limb (involved and contralateral) and time (6 and 12 months) for time-normalized waveforms of each dependent variable (vGRF, KFA, and KEM) in the ACLR cohort. Ensemble averages across all participants and trials were plotted for the involved and contralateral limb at both time points. The functional analyses of variance were performed using the functional data analysis (FDA) package in R statistical software (version 3.4.3) to compute mean differences at each time point, as well as the interaction effect between these differences with a corresponding 95% confidence interval (CI). The magnitude of the effects were considered significant at any percentile of the stance phase where the 95% CI did not cross zero (25). For our secondary analyses, similar functional pairwise comparisons were conducted with the FDA package in R in order to specifically compare 1) the ACLR limbs at 6 months to the uninjured control limbs, 2) the ACLR limbs at 12 months to the uninjured control limbs, 3) the contralateral limbs at 6 months to the uninjured control limbs, and 4) the contralateral limbs at 12 months to the uninjured control limbs (Figures 1, 2, and 3).
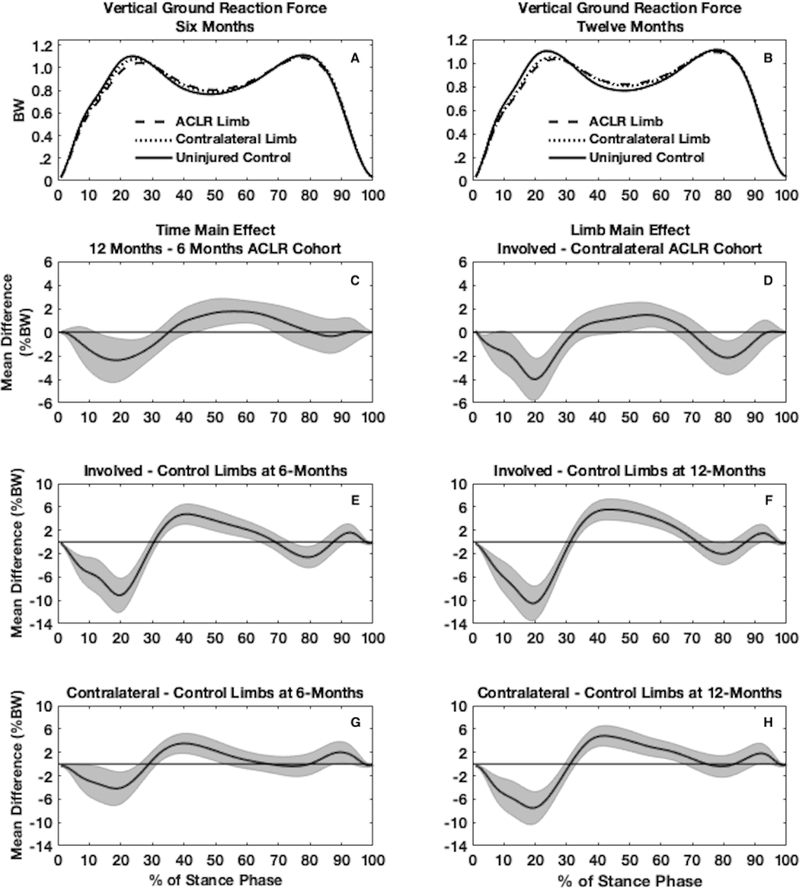
Figure 1.
Waveforms of vertical ground reaction force (vGRF) normalized to body weight (BW) are shown for the involved, contralateral, and uninjured control limbs both at 6 (3A) and 12 months (3B) (one time point for uninjured controls). Main effects for time (1C) and limb (1D) are shown for the functional analysis of variance in individuals with an anterior cruciate ligament reconstruction (ACLR). Pairwise comparisons between involved and control limbs are shown at 6 (1E) and 12 months (1F) post-ACLR as well as between contralateral and control limbs at 6 (1G) and 12 months (1H) post-ACLR.
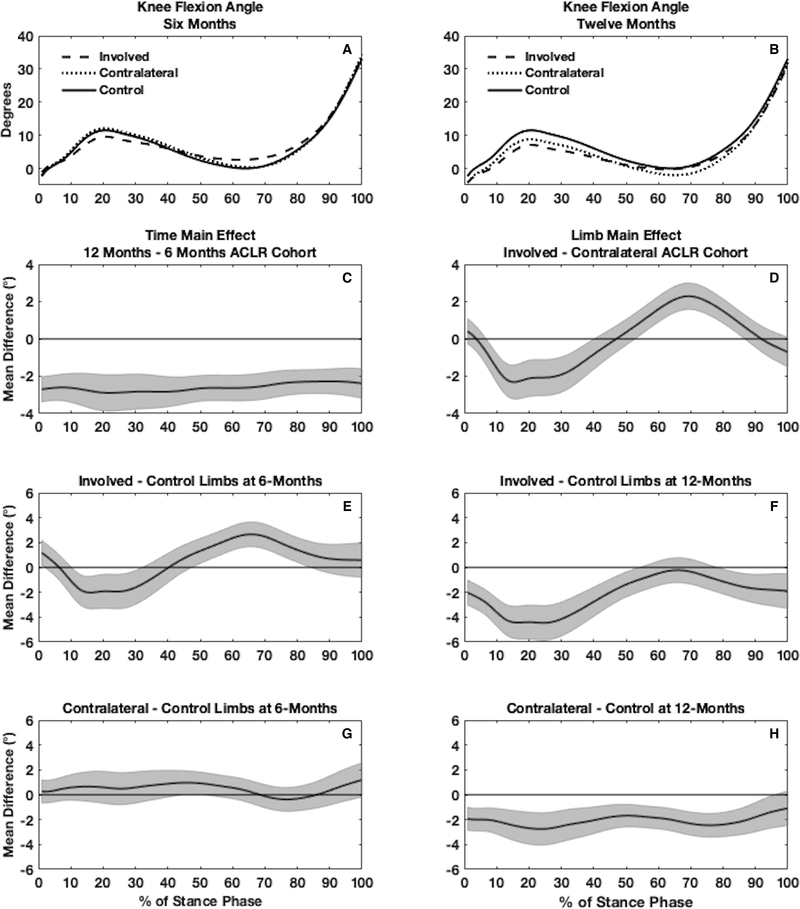
Figure 2.
Waveforms of knee flexion angle (KFA) are shown for the involved, contralateral, and uninjured control limbs both at 6 (2A) and 12 months (2B) (one time point for uninjured controls). Main effects for time (2C) and limb (2D) are shown for the functional analysis of variance in individuals with an anterior cruciate ligament reconstruction (ACLR). Pairwise comparisons between involved and control limbs are shown at 6 (2E) and 12 months (2F) post-ACLR as well as between contralateral and control limbs at 6 (2G) and 12 months (2H) post-ACLR.
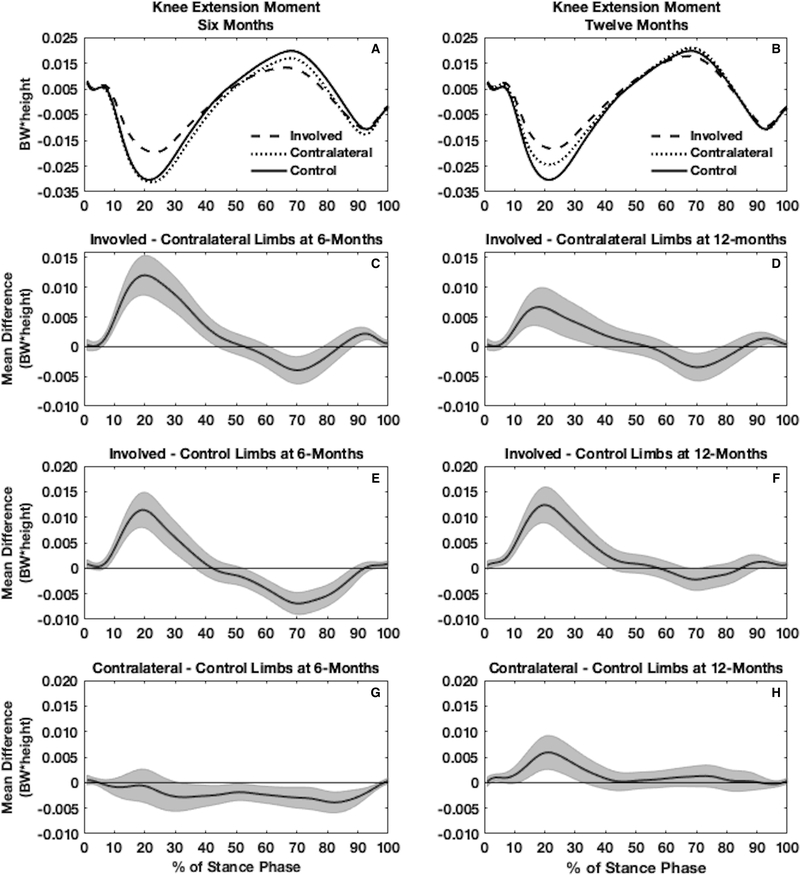
Figure 3.
Waveforms of internal knee extension moment (KEM) normalized to body weight (BW) * height (m) are shown for the involved, contralateral, and uninjured control limbs both at 6 (3A) and 12 months (3B) (one time point for uninjured controls). Planned comparisons between the involved and contralateral limb at 6 months (3C) and 12 months (3D) are shown for the functional analysis of variance in individuals with an anterior cruciate ligament reconstruction (ACLR). Pairwise comparisons between involved and control limbs are shown at 6 (3E) and 12 months (3F) post-ACLR as well as between contralateral and control limbs at 6 (3G) and 12 months (3H) post-ACLR.
RESULTS
Demographics did not differ between groups (Table 1). The peak magnitudes of difference and corresponding effect sizes for the percentages of stance phase that demonstrated differences for each comparison can be found in Table 2 (vGRF and KFA) and Table 3 (KEM). Additionally, we calculated discrete biomechanical variables (peak vGRF, peak KFA, and peak KEM during the first 50% of stance as well as KFE throughout stance), which are included in Supplementary Table 1 (see Supplementary Table 1, discrete biomechanical variable data provided) for easier comparisons to previous studies (1, 16, 17).
Table 2.
Peak Differences and Effect Sizes for Vertical Ground Reaction Force and Knee Flexion Angle Comparisons
| Vertical Ground Reaction Force | Knee Flexion Angle | ||||
|---|---|---|---|---|---|
| Comparisons | Stance Phase (%) | Peak Difference (%BW) (effect size) | Stance Phase (%) | Peak Difference (°) (effect size) | |
| Main Effects | Time (12–6 months) | 13–31 44–69 |
−2 (d=0.21) +2 (d=0.27) |
1–100 | −3.0 (d=0.64) |
| Limb (Involved-contralateral) | 2–29 48–64 76–87 |
−4 (d=0.36) +1 (d=0.22) −2 (d=0.24) |
7–41 53–87 |
−2.3 (d=0.41) +2.3 (d=0.52) |
|
| Involved - Control | 6 Months | 1–30 33–64 74–84 |
−9 (d=0.51) +5 (d=0.44) −3 (d=0.23) |
11–33 48–85 |
−2.0 (d=0.25) +2.7 (d=0.43) |
| 12 Months | 1–30 33–69 77–82 93–94 |
−10 (d=0.56) +5 (d=0.50) −2 (d=0.18) +1 (d=0.17) |
1–55 79–100 |
−4.5 (d=0.51) −2.0 (d=0.22) |
|
| Contralateral - Control | 6 Months | 1–26 32–54 88–93 |
−4 (d=0.24) +4 (d=0.33) +2 (d=0.17) |
46–51 | +1.0 (d=0.16) |
| 12 Months | 1–30 34–68 92–95 |
−8 (d=0.42) +5 (d=0.44) +2 (d=0.17) |
1–96 | −2.8 (d=0.34) | |
%BW = Body weight
Table 3.
Peak Differences and Effect Sizes for Internal Knee Extension Moment Comparisons
| Limb Comparison | Time Point | Stance Phase (%) | Peak Difference (BW*height) (effect size) |
|---|---|---|---|
| Involved – Contralateral (planned comparison) | 6 Months | 7–44 62–79 89–100 |
+0.012 (d=0.58) −0.004 (d=0.27) +0.002 (d=0.33) |
| 12 Months | 7–38 63–79 91–99 |
+0.007 (d=0.34) −0.003 (d=0.24) +0.001 (d=0.23) |
|
| Involved – Control | 6 Months | 8–37 53–89 |
+0.011 (d=0.53) −0.007 (d=0.51) |
| 12 Months | 2–42 68–71 92–100 |
+0.012 (d=0.56) −0.002 (d=0.16) +0.001 (d=0.18) |
|
| Contralateral - Control | 6 Months | 32–97 | −0.004 (d=0.30) |
| 12 Months | 3–7 12–33 |
+0.001 (d=0.24) +0.006 (d=0.28) |
BW = Body weight
Vertical ground reaction force
Involved ACLR vs. Contralateral ACLR
No significant limb x time interaction existed for vGRF in the ACLR cohort. Twelve-month vGRF was lesser than 6-month vGRF in both limbs early during stance (13–31%) and lesser through mid-stance (44–69%; Figure 1C). At both time points the involved limbs demonstrated lesser vGRF compared to contralateral limbs during early (2–29%) and late (76–87%) stance and greater vGRF during mid-stance (48–64%) compared to contralateral limbs (Figure 1D).
Involved ACLR vs. Uninjured Controls
At 6 months (Figure 1E) the involved limbs in the ACLR cohort demonstrated lesser vGRF compared to uninjured control limbs during early (1–30%) and late (74–84%) stance and greater vGRF in mid-stance (33–64%). At 12 months (Figure 1F), the involved limbs demonstrated lesser vGRF during early (1–30%) and late (77–82%) stance and greater vGRF during mid-stance (33–69%) and at the very end of stance (93–94%).
Contralateral ACLR vs. Uninjured Controls
At 6 months (Figure 1G), the contralateral limbs in the ACLR cohort demonstrated lesser vGRF compared to uninjured control limbs during early stance (1–26%) and greater vGRF during mid (32–54%) and late stance (88–93%). At 12 months (Figure 1H), the contralateral limbs in the ACLR cohort demonstrated lesser vGRF compared to uninjured control limbs during early stance (1–30%) and greater vGRF during mid (34–68%) and late stance (92–95%).
Knee flexion angle
Involved ACLR vs. Contralateral ACLR
There was no significant limb by time interaction for KFA in the ACLR cohort. Twelve month KFA was lower throughout the entire stance phase (1–100%) compared to 6 months (Figure 2C). Involved limb KFA was lesser compared to the contralateral limbs in the first half of stance (7–41%) and greater during the second half of stance (53–87%), indicating lesser knee flexion excursion throughout stance in the involved limbs compared to the contralateral limbs (Figure 2D).
Involved ACLR vs. Uninjured Controls
At 6 months following ACLR, involved limbs in the ACLR cohort demonstrated lesser KFA compared to uninjured control limbs during the first half of stance (11–33%) and greater KFA through the second half of stance (48–85%), indicating lesser knee flexion excursion throughout stance in the involved limb compared to the contralateral limb (Figure 2E). At 12 months following ACLR, involved limbs demonstrated lesser KFA compared to uninjured control limbs throughout the majority of stance phase (1–55%, 79–100%; Figure 2F).
Contralateral ACLR vs. Uninjured Controls
At 6 months following ACLR, contralateral limbs in the ACLR cohort demonstrated greater KFA compared to uninjured control limbs near mid-stance (46–51%; Figure 2G). At 12 months following ACLR, contralateral limbs demonstrated lesser KFA compared to uninjured control limbs throughout most of stance phase (1–96%; Figure 2H).
Internal knee extension moment
Involved vs. Contralateral ACLR
There was a significant limb x time interaction for KEM in the ACLR cohort, indicating that while the involved limb KEM was smaller (less negative) than the contralateral limb at both 6 and 12 months, the difference between limbs was significantly larger at 6 compared to 12 months. At 6 months (Figure 3C), involved limbs demonstrated lesser KEM compared to the contralateral limbs in early stance (7–44%) and the end of stance phase (89–100%), while demonstrating greater KEM during mid to late stance (62–79%). Similarly, at 12 months (Figure 3D), involved limbs demonstrated lesser KEM compared to the contralateral limbs in early stance (7–38%) and the end of stance phase (91–99%), while demonstrating greater KEM during mid to late stance (63–79%).
Involved ACLR vs. Uninjured Controls
At 6 months following ACLR, the involved limbs demonstrated lesser KEM compared to uninjured control limbs in the first half of stance (8–37%) and greater KEM in the second half of stance (53–89%; Figure 3E). At 12 months following ACLR, the involved limbs demonstrated a lesser KEM compared to uninjured controls limbs in the first half of stance (2–42%), greater KEM during mid to late stance (68–71%) and lesser KEM at the very end of stance (92–100%, Figure 3F).
Contralateral ACLR vs. Uninjured Controls
At 6 months following ACLR, the contralateral limbs of the ACLR cohort demonstrated greater KEM compared to uninjured control limbs during mid and late stance (32–97%; Figure 3G). At 12 months following ACLR, the contralateral limbs demonstrated lesser KEM compared to uninjured control limbs in early stance (3–7%, 12–33%; Figure 3H).
Post Hoc Analysis
Walking speed was not significantly different between 6 and 12 months within individuals with an ACLR (t29=1.96, p=0.06) or between uninjured controls and ACLR individuals at 6 months (t58=−1.13, p=0.26). However the ACLR group walked significantly slower than uninjured controls at 12 months post-ACLR (t58=−2.18, p=0.03; Table 1). Walking speed between uninjured controls and individuals with an ACLR at 12 months was significantly different because individuals with an ACLR slightly decreased their walking speed between 6 and 12 months post-ACLR. Since the FDA does not lend itself to inclusion of covariates such as a change in gait speed, we conducted a post-hoc analysis to determine how the change in gait speed from 6 to 12 months following ACLR influenced the change in discrete biomechanical variables (peak vGRF, peak KFA, and peak KEM in the first 50% of stance, as well as KFE throughout stance phase; Supplementary Table 1) from 6 to 12 months in both limbs (involved and contralateral) of the ACLR cohort. First we calculated percent change scores (Equation 1) for walking speed as well as each biomechanical variable. Next, we conducted separate univariate linear regression analyses in the involved and contralateral limb using the change in self-selected gait speed (Δspeed) as the predictor variable and the change in each biomechanical variable (ΔvGRF, ΔKFA, ΔKFE, and ΔKEM) as the criterion variables for each regression equation.
| (Equation 1) |
Overall, ΔvGRF, ΔKFA, ΔKFE, and ΔKEM in the involved limb were not significantly influenced by Δspeed from 6 to 12 months post-ACLR (R2=0.001–0.054, P>0.05; Supplementary Table 1) In the contralateral limb, ΔvGRF (R2=0.325, P=0.001) and ΔKEM (R2=0.286, P=0.002) significantly associated with Δspeed from 6 to 12 months post-ACLR (Supplementary Table 1). ΔKFA (R2=0.126, P=0.054) and ΔKEM (R2=0.052, P=0.224) did not significantly associate with Δspeed from 6 to 12 months post-ACLR (Supplementary Table 1).
DISCUSSION
The current study shows that walking gait biomechanics in the involved and contralateral limbs become more symmetrical from 6 to 12 months following ACLR; however, both limbs become more disparate compared to uninjured controls. Our study demonstrated between-limb biomechanical differences, at 6 and 12 months post-ACLR, as well as differences compared to uninjured controls at various parts of stance. The involved and contralateral limbs of the ACLR cohort demonstrated lesser vGRF during early stance and greater vGRF during mid-stance compared to uninjured controls. These data suggest vGRF became more symmetrical between limbs in ACLR patients from 6 to 12 months, yet the apparent improvement in vGRF symmetry seems to be due to alterations in contralateral vGRF rather than alterations in vGRF of the involved limb. Consistent with a stiffened-knee strategy, involved ACLR limbs demonstrated lesser KFE throughout stance at 6 and 12 months compared to the contralateral and uninjured control limbs (Figure 2). The involved and contralateral limbs of the ACLR cohort demonstrated less KFE throughout stance overtime, suggesting a potential bilateral adoption of a stiffened-knee strategy. Lesser KEM is generated in the involved limb compared to the contralateral limb during early stance and at the end of stance phase at both 6 and 12 months (Figure 3). Additionally, KEM is lower bilaterally in the first half of stance in the ACLR cohort compared to uninjured controls at 12 months (Figure 3). Therefore, improvement of gait symmetry in the first 12 months post ACLR should be interpreted with caution; the contralateral limb may be adopting strategies similar to the ACLR limb, and both involved and contralateral might differ from controls. Future interventions are needed to address gait alterations made throughout stance in both the involved and contralateral limb following ACLR.
Linking Gait Alterations to PTOA
Twelve months post-ACLR, individuals demonstrated less dynamic vGRF throughout stance, meaning lesser vGRF during early stance and greater vGRF at mid-stance, bilaterally compared to uninjured controls. A similar gait strategy has been reported in individuals experiencing experimental knee pain (26) as well as ACLR individuals who are classified as experiencing clinically significant symptoms based on previously defined KOOS cutoff scores (18). The current study demonstrated differences at peak magnitudes during the first 50% of stance in vGRF, KFA, and KEM, similar to other studies that have evaluated biomechanics in individuals with ACLR (1, 16, 17). Evaluation of peak vGRF, KFA, and KEM continues to be important in gait analysis; however, the influence vGRF, KFA, and KEM during mid and late stance may have on the development of PTOA or changes in long-term joint health remains unknown. While speculative, differences in knee kinematics during mid or late stance may alter tibiofemoral contact areas during stance, which may lead to differences in how loads are distributed across the joint. Differences in late stance may influence swing phase kinematics, which in turn may alter joint loading at heel strike. Future studies should identify if the differences found in mid to late stance in vGRF, KFA, and KEM are relevant to PROs as well as measures of joint tissue health. The optimal magnitude of joint loading throughout stance to promote joint tissue health post-ACLR remains unknown, yet our data suggests individuals do not normalize gait bilaterally within the first 12 months following unilateral ACLR without specialized retraining. Future research is needed in order to restore optimal gait biomechanics early post-ACLR, as deleterious changes to articular cartilage occur within the first year following ACLR (12).
Mechanisms and Consequences of Bilateral Changes
It remains unclear what mechanisms cause bilateral changes following ACLR; however, slower walking speed (27, 28) may, to some extent, influence walking gait biomechanics. In the current study, the ACLR cohort demonstrated a relatively small decrease in walking speed from 6 (1.26 m/s) to 12 months (1.22 m/s), and 12-month walking speed in the ACLR cohort (1.22 m/s) was significantly slower compared to uninjured controls (1.31 m/s). Our post-hoc analyses suggest that a change in biomechanics in the involved limbs were not significantly influenced by change in gait speed from 6 to 12 months post-ACLR (Supplementary Table 1). Conversely, the small change in gait speed from 6 to 12 months explained approximately 32% and 29% of the variance in the change in peak vGRF and peak KEM (Supplementary Table 1), respectively. These post hoc analyses suggest a change in walking speed between 6 and 12 months following ACLR is meaningful and should be considered to maximize the external validity of future studies evaluating longitudinal changes in gait biomechanics following ACLR. These findings also suggest approximately 70% of the change in peak vGRF and peak KEM was due to factors other than a change in gait speed (e.g. neuromuscular compensations of lower extremity movement). Previous studies have demonstrated that slower walking speed at both 6 and 12 months following ACLR associates with worse cartilage composition measured with magnetic resonance imaging (MRI) T1rho relaxation times at 12 months following ACLR (28), and a decrease in walking speed of 0.12 m/s over a 12-month period increases risk of developing KOA by 8% (27). While our study did not control gait speed in order to evaluate gait biomechanics at gait speeds most relevant to daily life, manipulating gait speed is one of the multiple methods that could be utilized to alter gait biomechanics. Future gait retraining interventions may cue changes in gait speed as a means of manipulating gait biomechanics following ACLR.
Quadriceps avoidance, associated with less knee flexion excursion and KEM, may be another mechanism influencing gait biomechanics following ACLR throughout stance (22, 29). Following ACLR, some individuals may demonstrate quadriceps dysfunction, which is likely a combination of neurological (30) and muscular deficiencies (31). Less quadriceps strength associates with worse function (32, 33) and altered loading strategies during walking (34). Results from the current study suggest a stiffened-knee strategy may be adopted bilaterally during the first year following ACLR. Deleterious changes in cartilage composition occur not only in the involved limb but also in the contralateral limb 6 months following ACLR (35). These compositional changes may occur due to bilateral adaptations in gait, including characteristics of a stiffened-knee strategy seen in the current study.
Implications for gait rehabilitation
Following ACLR, differences exist in vGRF, KFA, and KEM throughout stance compared to uninjured controls (29). It is likely biomechanical alterations are influenced partly by quadriceps dysfunction (32); however, studies have shown that regaining quadriceps strength alone does not alter walking gait biomechanics in individuals with KOA (36, 37). In order to alter walking gait biomechanics, it may be necessary to intervene with gait retraining early following ACLR. A recent study demonstrated real-time biofeedback that cued individuals with an ACLR to increase the first vGRF peak by 5% during gait resulted in greater knee flexion excursion and peak KEM in the first 50% of stance (20). A similar study has used sensory feedback to target knee adduction moment in individuals with KOA (38). While these early studies are promising, it remains unclear if cueing an increase in the first peak of vGRF will translate to a decrease in vGRF in mid-stance, greater knee flexion excursion, and greater KEM during early and mid-stance. Results from the current study suggest gait-retraining should not solely focus on improvement of inter-limb symmetry, but also seek to retrain gait bilaterally in an effort to ensure both the ACLR and contralateral limb demonstrate gait biomechanics that more closely resemble uninjured controls. Future studies will also need to determine the retention and transfer of gait retraining to real-world situations.
Limitations
Our study is the first to evaluate bilateral gait biomechanics post-ACLR, compared to uninjured controls, throughout the stance phase, at multiple time points (6 and 12 months). However, the following limitations may inform future research. While the current study evaluated participants over time, we did not track specific information regarding rehabilitation. All participants were prescribed physical therapy following ACLR, but it was not standardized and all participants were not treated at the same clinic. We did not longitudinally assess gait biomechanics in uninjured controls; however, gait biomechanics in uninjured individuals using 3-deminsional motion capture is known to be reliable over time (39). We focused the current study on biomechanical outcomes associated with a stiffened-knee strategy; future work may evaluate other variables such as frontal plane knee moment, a commonly measured variable in individuals with idiopathic knee osteoarthritis, or other biomechanical variables that are known to change due to knee pain or injury. We currently do not understand the mechanisms that lead to biomechanical alterations at 6 months post-ACLR, and future studies should seek to evaluate the factors influencing gait alterations at 6 months post-ACLR.
Conclusions
Overall, the involved ACLR limb demonstrated lesser vGRF during early and late stance, greater vGRF during mid stance, lesser KFE throughout stance, and lesser KEM compared to the contralateral limb and uninjured controls. At 12 months post-ACLR the contralateral limb demonstrated lesser vGRF during early stance and greater vGRF at mid-stance and lesser KFE compared to 6 months post-ACLR.
Supplementary Material
Acknowledgements and Funding
The current study was funded by grants from: 1) National Athletic Trainers Association Research and Education Foundation (New Investigator Research Grant Award [#14NewInv001]); 2) North Carolina Translational and Clinical Sciences (TraCS) Institute Planning Grant, National Institutes of Health National Institute of Arthritis & Musculoskeletal and Skin Diseases (1R03AR066840-01A1).
Footnotes
Conflict of Interest: The results of the present study do not constitute endorsement by ACSM. The results of this study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. There are no professional relationships with companies or manufacturers who will benefit from the results of the present study to disclose.
REFERENCES
- 1.Hart HF, Culvenor AG, Collins NJ et al. Knee kinematics and joint moments during gait following anterior cruciate ligament reconstruction: a systematic review and meta-analysis. British journal of sports medicine. 2016;50(10):597–612. [DOI] [PubMed] [Google Scholar]
- 2.Kaur M, Ribeiro DC, Theis JC, Webster KE and Sole G. Movement Patterns of the Knee During Gait Following ACL Reconstruction: A Systematic Review and Meta-Analysis. Sports medicine (Auckland, N.Z.). 2016;46(12):1869–95. [DOI] [PubMed] [Google Scholar]
- 3.Slater LV, Hart JM, Kelly AR and Kuenze CM. Progressive Changes in Walking Kinematics and Kinetics After Anterior Cruciate Ligament Injury and Reconstruction: A Review and Meta-Analysis. Journal of athletic training. 2017;52(9):847–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erhart-Hledik JC, Favre J and Andriacchi TP. New insight in the relationship between regional patterns of knee cartilage thickness, osteoarthritis disease severity, and gait mechanics. Journal of biomechanics. 2015;48(14):3868–75. [DOI] [PubMed] [Google Scholar]
- 5.Wellsandt E, Gardinier ES, Manal K, Axe MJ, Buchanan TS and Snyder-Mackler L. Decreased Knee Joint Loading Associated With Early Knee Osteoarthritis After Anterior Cruciate Ligament Injury. American Journal of Sports Medicine. 2016;44(1):143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pietrosimone B, Blackburn JT, Harkey MS et al. Greater Mechanical Loading During Walking Is Associated With Less Collagen Turnover in Individuals With Anterior Cruciate Ligament Reconstruction. The American journal of sports medicine. 2016;44(2):425–32. [DOI] [PubMed] [Google Scholar]
- 7.Webster KE, Wittwer JE, O’Brien J and Feller JA. Gait patterns after anterior cruciate ligament reconstruction are related to graft type. The American journal of sports medicine. 2005;33(2):247–54. [DOI] [PubMed] [Google Scholar]
- 8.Webster KE, Feller JA and Wittwer JE. Longitudinal changes in knee joint biomechanics during level walking following anterior cruciate ligament reconstruction surgery. Gait Posture. 2012;36(2):167–71. [DOI] [PubMed] [Google Scholar]
- 9.Roewer BD, Di Stasi SL and Snyder-Mackler L. Quadriceps strength and weight acceptance strategies continue to improve two years after anterior cruciate ligament reconstruction. Journal of biomechanics. 2011;44(10):1948–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andriacchi TP and Dyrby CO. Interactions between kinematics and loading during walking for the normal and ACL deficient knee. Journal of biomechanics. 2005;38(2):293–8. [DOI] [PubMed] [Google Scholar]
- 11.Andriacchi TP and Mundermann A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Current opinion in rheumatology. 2006;18(5):514–8. [DOI] [PubMed] [Google Scholar]
- 12.Theologis AA, Haughom B, Liang F et al. Comparison of T1rho relaxation times between ACL-reconstructed knees and contralateral uninjured knees. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2014;22(2):298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pietrosimone B, Loeser RF, Blackburn JT et al. Biochemical markers of cartilage metabolism are associated with walking biomechanics 6-months following anterior cruciate ligament reconstruction. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2017;35(10):2288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfeiffer SJ, Spang J, Nissman D et al. Gait Mechanics and T1rho MRI of Tibiofemoral Cartilage 6 Months after ACL Reconstruction. Medicine and science in sports and exercise. 2019;51(4):630–9. [DOI] [PubMed] [Google Scholar]
- 15.Teng HL, Wu D, Su F et al. Gait Characteristics Associated With a Greater Increase in Medial Knee Cartilage T1rho and T2 Relaxation Times in Patients Undergoing Anterior Cruciate Ligament Reconstruction. The American journal of sports medicine. 2017;45(14):3262–71. [DOI] [PubMed] [Google Scholar]
- 16.Capin JJ, Khandha A, Zarzycki R et al. Gait mechanics and tibiofemoral loading in men of the ACL-SPORTS randomized control trial. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2018;36(9):2364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capin JJ, Zarzycki R, Ito N et al. Gait Mechanics in Women of the ACL-SPORTS Randomized Control Trial: Interlimb Symmetry Improves Over Time Regardless of Treatment Group. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pietrosimone B, Seeley MK, Johnston C, Pfeiffer SJ, Spang JT and Blackburn JT. Walking Ground Reaction Force Post-ACL Reconstruction: Analysis of Time and Symptoms. Medicine and science in sports and exercise. 2019;51(2):246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pamukoff DN, Montgomery MM, Holmes SC, Moffit TJ, Garcia SA and Vakula MN. Association between gait mechanics and ultrasonographic measures of femoral cartilage thickness in individuals with ACL reconstruction. Gait Posture. 2018;65:221–7. [DOI] [PubMed] [Google Scholar]
- 20.Luc-Harkey BA, Franz JR, Blackburn JT, Padua DA, Hackney AC and Pietrosimone B. Real-time biofeedback can increase and decrease vertical ground reaction force, knee flexion excursion, and knee extension moment during walking in individuals with anterior cruciate ligament reconstruction. Journal of biomechanics. 2018;76:94–102. [DOI] [PubMed] [Google Scholar]
- 21.Faul F, Erdfelder E, Lang AG and Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior research methods. 2007;39(2):175–91. [DOI] [PubMed] [Google Scholar]
- 22.Luc-Harkey BA, Harkey MS, Stanley LE, Blackburn JT, Padua DA and Pietrosimone B. Sagittal plane kinematics predict kinetics during walking gait in individuals with anterior cruciate ligament reconstruction. Clinical biomechanics (Bristol, Avon). 2016;39:9–13. [DOI] [PubMed] [Google Scholar]
- 23.Pietrosimone B, Loeser RF, Blackburn JT et al. Biochemical markers of cartilage metabolism are associated with walking biomechanics six-months following anterior cruciate ligament reconstruction. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kowalski E, Catelli DS and Lamontagne M. Side does not matter in healthy young and older individuals - Examining the importance of how we match limbs during gait studies. Gait Posture. 2019;67:133–6. [DOI] [PubMed] [Google Scholar]
- 25.Park J, Seeley MK, Francom D, Reese CS and Hopkins JT. Functional vs. Traditional Analysis in Biomechanical Gait Data: An Alternative Statistical Approach. Journal of human kinetics. 2017;60:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seeley MK, Park J, King D and Hopkins JT. A novel experimental knee-pain model affects perceived pain and movement biomechanics. Journal of athletic training. 2013;48(3):337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herzog MM, Driban JB, Cattano NM et al. Risk of Knee Osteoarthritis Over 24 Months in Individuals Who Decrease Walking Speed During a 12-Month Period: Data from the Osteoarthritis Initiative. The Journal of rheumatology. 2017;44(8):1265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfeiffer S, Harkey MS, Stanley LE et al. Associations Between Slower Walking Speed and T1rho Magnetic Resonance Imaging of Femoral Cartilage Following Anterior Cruciate Ligament Reconstruction. Arthritis Care Res (Hoboken). 2018;70(8):1132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Stasi SL, Logerstedt D, Gardinier ES and Snyder-Mackler L. Gait patterns differ between ACL-reconstructed athletes who pass return-to-sport criteria and those who fail. The American journal of sports medicine. 2013;41(6):1310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luc-Harkey BA, Harkey MS, Pamukoff DN et al. Greater intracortical inhibition associates with lower quadriceps voluntary activation in individuals with ACL reconstruction. Experimental brain research. 2017;235(4):1129–37. [DOI] [PubMed] [Google Scholar]
- 31.Pietrosimone BG, Lepley AS, Ericksen HM, Gribble PA and Levine J. Quadriceps strength and corticospinal excitability as predictors of disability after anterior cruciate ligament reconstruction. Journal of sport rehabilitation. 2013;22(1):1–6. [DOI] [PubMed] [Google Scholar]
- 32.Pietrosimone B, Lepley AS, Harkey MS et al. Quadriceps Strength Predicts Self-reported Function Post-ACL Reconstruction. Medicine and science in sports and exercise. 2016;48(9):1671–7. [DOI] [PubMed] [Google Scholar]
- 33.Davis HC, Troy Blackburn J, Ryan ED et al. Quadriceps rate of torque development and disability in individuals with anterior cruciate ligament reconstruction. Clinical biomechanics (Bristol, Avon). 2017;46:52–6. [DOI] [PubMed] [Google Scholar]
- 34.Blackburn JT, Pietrosimone B, Harkey MS, Luc BA and Pamukoff DN. Quadriceps Function and Gait Kinetics after Anterior Cruciate Ligament Reconstruction. Medicine and science in sports and exercise. 2016;48(9):1664–70. [DOI] [PubMed] [Google Scholar]
- 35.Pedoia V, Su F, Amano K et al. Analysis of the articular cartilage T1rho and T2 relaxation times changes after ACL reconstruction in injured and contralateral knees and relationships with bone shape. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2017;35(3):707–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeVita P, Aaboe J, Bartholdy C, Leonardis JM, Bliddal H and Henriksen M. Quadriceps-strengthening exercise and quadriceps and knee biomechanics during walking in knee osteoarthritis: A two-centre randomized controlled trial. Clinical biomechanics (Bristol, Avon). 2018;59:199–206. [DOI] [PubMed] [Google Scholar]
- 37.Davis HC, Luc-Harkey BA, Seeley MK, Troy Blackburn J and Pietrosimone B. Sagittal plane walking biomechanics in individuals with knee osteoarthritis after quadriceps strengthening. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2019;27(5):771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erhart-Hledik JC, Asay JL, Clancy C, Chu CR and Andriacchi TP. Effects of active feedback gait retraining to produce a medial weight transfer at the foot in subjects with symptomatic medial knee osteoarthritis. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2017;35(10):2251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaufman K, Miller E, Kingsbury T et al. Reliability of 3D gait data across multiple laboratories. Gait Posture. 2016;49:375–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.