Abstract
Aims/Introduction
To evaluate the efficacy and safety of once‐weekly (q.w.) extended‐release exenatide after switching from twice‐daily (b.i.d.) exenatide in patients with type 2 diabetes.
Materials and Methods
This was an investigator‐initiated, prospective, single‐arm, multicenter study. Individuals with type 2 diabetes who had been treated with exenatide b.i.d. for at least 3 months were enrolled and switched to exenatide q.w. for 24 weeks. The primary end‐point was change in HbA1c at week 24 to test the glucose‐lowering effect of exenatide q.w. versus exenatide b.i.d.
Results
A total of 58 Japanese individuals with type 2 diabetes completed the study. Glycated hemoglobin was reduced by 0.2% at week 24 (7.2 ± 1.2% vs 7.0 ± 1.2% [56 ± 13 vs 53 ± 13 mmol/mol], 95% confidence interval −0.4 to −0.03%, P < 0.005 for non‐inferiority, P = 0.01 for superiority). Fasting plasma glucose was reduced by 12 mg/dL at week 24 (154 ± 46 vs 142 ± 46 mg/dL, P = 0.02). β‐Cell function assessed by homeostasis model assessment of β‐cell function and C‐peptide index was significantly improved at week 24. The incidence of self‐reported hypoglycemia was reduced, and treatment satisfaction assessed by the Diabetes Treatment Satisfaction Questionnaire and Diabetes Medication Satisfaction Questionnaire was improved at week 24, with no change in body weight. There was no serious adverse event related to the study drug.
Conclusions
Switching from exenatide b.i.d. to exenatide q.w. resulted in a reduction in glycated hemoglobin, fasting plasma glucose and the incidence of hypoglycemia, and improvement in β‐cell function and treatment satisfaction in patients with type 2 diabetes. These findings will be useful for selecting optimal treatment in individuals with type 2 diabetes.
Keywords: Glucagon‐like peptide‐1 receptor agonist, Treatment satisfaction, Type 2 diabetes
In the present study, we report the efficacy and safety of once‐weekly extended‐release exenatide after switching from twice‐daily exenatide in Japanese patients with type 2 diabetes.

Introduction
Diabetes is increasing throughout the world, especially in Asia1, and >90% of diabetes patients are classified as having type 2 diabetes. Patients with type 2 diabetes are at high risk of cardiovascular disease (CVD), as well as microvascular complications2; therefore, optimal management of people with type 2 diabetes is critical to improve CVD outcomes.
In the past two decades, many new antidiabetic medications have been developed and marketed, including incretin‐based drugs; that is, glucagon‐like peptide‐1 (GLP‐1) receptor agonists (GLP‐1RAs) and dipeptidyl peptidase‐4 inhibitors3. In particular, GLP‐1RAs have been shown to possess the benefit of reducing bodyweight in addition to lowering glycated hemoglobin (HbA1c). Treatment with GLP‐1RAs added to standard therapy has been shown to reduce CVD events compared with placebo in recent CVD outcomes trials4–7. However, whether this beneficial effect of GLP‐1RAs on CVD outcomes is a class effect of GLP‐1RAs remains unclear8.
GLP‐1RAs are currently classified into short‐ and long‐acting drugs based on the plasma half‐life of agents9. Differences in pharmacokinetic/pharmacodynamic profiles between short‐ and long‐acting GLP‐1RAs result in important differences in their mechanisms of action. Specifically, short‐acting GLP‐1RAs predominantly reduce postprandial hyperglycemia through suppression of gastric emptying, whereas long‐acting GLP‐1RAs predominantly reduce fasting plasma glucose (FPG) level through enhancing insulin secretion and suppressing glucagon secretion.
Exenatide is a GLP‐1RA that has been developed based on exendin‐410. Uniquely, there are two formulations of exenatide; that is, exenatide twice daily (b.i.d.) formulation, a short‐acting GLP‐1RA, and once‐weekly (q.w.) exenatide extended‐release formulation, a long‐acting GLP‐1RA, which were approved in Japan in 2010 and 2012, respectively. However, there are limited data available as to clinical differences in the efficacy and safety between these two formulations. In the present study, we examined the efficacy and safety of exenatide q.w. switched from exenatide b.i.d. in Japanese patients with type 2 diabetes, including patient‐reported outcomes measures.
Methods
Study design
This was an investigator‐initiated, prospective, single‐arm, multicenter study. Individuals with type 2 diabetes who had been treated with exenatide b.i.d. for at least 3 months were enrolled and switched to exenatide q.w. for 24 weeks. Inclusion criteria were adult patients with type 2 diabetes who had been treated with exenatide b.i.d. (either 10 or 20 μg daily) ± concomitant antidiabetic medication for at least 3 months. Exclusion criteria were patients with: (i) type 1 diabetes; (ii) allergy to exenatide; (iii) pregnancy or breast‐feeding; and (iv) severe renal dysfunction. The patients were switched from exenatide b.i.d. to exenatide q.w. at week 0 after a 4‐week observation period without changing any other antidiabetic medication. They were then asked to attend the outpatient clinic at week 4, 8, 16 and 24. Attending physicians were allowed to change their antidiabetic medication to avoid hypoglycemia during the study period. The primary end‐point of the study was change in HbA1c at week 24, to test non‐inferiority of the glucose‐lowering effect of exenatide q.w. versus exenatide b.i.d. This study was approved by the ethics committee of Keio University School of Medicine, and registered as trial numbers: UMIN000016390 and jRCTs031180320.
Study participants
The disposition of the study participants is shown in Figure 1. Initially, 62 patients agreed to participate in the study. All were Japanese. Of them, three withdrew consent before the study, and 59 were switched from exenatide b.i.d. to exenatide q.w. (2 mg once‐weekly). One patient discontinued the study drug because of constipation, and 58 completed the study. Safety was assessed in all 59 patients treated with exenatide q.w., while efficacy was assessed in the 58 patients who completed the study (full analysis set), whose characteristics are shown in Table 1.
Figure 1.
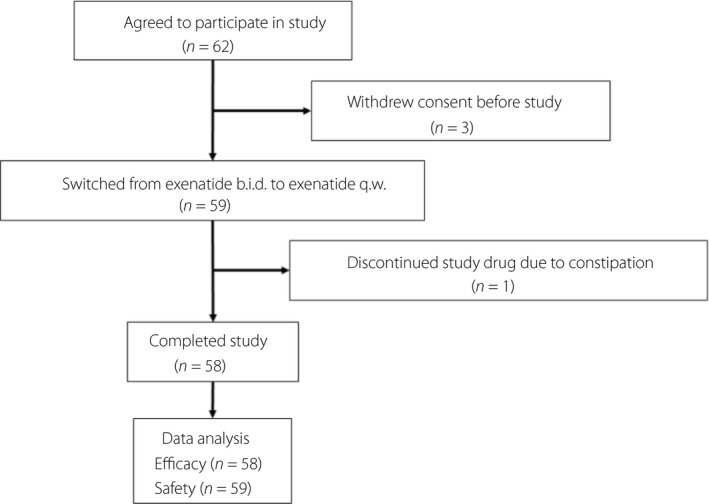
Disposition of study participants. b.i.d., twice‐daily; q.w., once‐weekly.
Table 1.
Characteristics of study participants
| n (male/female) | 58 (43/15) |
| Age (years) | 55 ± 12 |
| Height (m) | 1.66 ± 0.08 |
| Weight (kg) | 86.2 ± 28.2 |
| BMI (kg/m2) | 31.1 ± 9.1 |
| Duration of diabetes (years) | 11 ± 7 |
| FPG (mg/dL) | 154 ± 46 |
| HbA1c (%) | 7.2 ± 1.2 |
| HbA1c (mmol/mol) | 56 ± 13 |
| eGFR (mL/min/1.73 m2) | 76.4 ± 21.0 |
| Hypertension (%) | 74.1 |
| Dyslipidemia (%) | 72.4 |
| CVD (%) | 19.0 |
| Concomitant antidiabetic medication | |
| None (%) | 19.0 |
| SU (%) | 50.0 |
| Metformin (%) | 65.5 |
| TZD (%) | 17.2 |
| AGI (%) | 6.9 |
| SGLT2 inhibitor (%) | 5.2 |
AGI, α‐glucosidase inhibitor; BMI, body mass index; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; SGLT2, sodium–glucose cotransporter 2; SU, sulfonylurea; TZD, thiazolidinedione.
Measurements
Anthropometric characteristics including bodyweight, waist circumference, blood pressure and heart rate were measured, and blood and urine samples were obtained after an overnight fast, at baseline and week 24. Bodyweight, blood pressure, heart rate, and random blood and urine samples were also obtained at weeks 4, 8 and 16. HbA1c was measured by HPLC and expressed as the National Glycohemoglobin Standardization Program value. Glycated albumin and 1,5‐anhydro‐D‐glucitol were measured by enzymatic methods. Insulin, C‐peptide and intact proinsulin were measured by chemiluminescent immunoassays. Glucagon was measured by radioimmunoassay. High‐sensitivity C‐reactive protein was measured by nephelometry. Adiponectin was measured by latex agglutination turbidimetry. Urinary 8‐hydroxy‐2´‐deoxyguanosine and 8‐isoprostaglandin F2α were measured by enzyme‐linked immunosorbent assays. Serum aspartate aminotransferase, alanine aminotransferase, creatinine, uric acid, total cholesterol, high‐density lipoprotein cholesterol, triglyceride, free fatty acids, amylase and lipase were also measured at each visit. Low‐density lipoprotein cholesterol (mg/dL) was calculated as total cholesterol (mg/dL) − high‐density lipoprotein cholesterol (mg/dL) − triglyceride (mg/dL) / 5.
Patients were asked to carry out self‐monitoring of blood glucose (SMBG) before meals, 1 h after meals and at bedtime on 2 days at baseline and week 24. The incidence of hypoglycemia in the past 4 weeks was assessed by a questionnaire at each visit. Hypoglycemia was defined as having hypoglycemic symptoms with or without confirming capillary glucose level <70 mg/dL. Severe hypoglycemia was defined as a hypoglycemic event that requires assistance from another person to treat. Patients were also asked to fill out the following questionnaires: Diabetes Treatment Satisfaction Questionnaire11, Diabetes Medication Satisfaction Questionnaire12 and Gastrointestinal Symptom Rating Scale13, 14 at baseline and week 24, as previously reported15.
Body mass index was calculated as bodyweight (kg) divided by height squared (m2). The C‐peptide index was calculated as fasting serum C‐peptide (ng/mL) divided by FPG (mg/dL) × 100. Homeostasis model assessment of insulin resistance (HOMA‐IR) and HOMA of β‐cell function (HOMA‐β) were calculated using the HOMA2 calculator (https://www.dtu.ox.ac.uk/homacalculator/).
Statistical analysis
The sample size required to test the primary end‐point was calculated based on the following estimates; standard deviation (SD) of HbA1c 0.8%, one‐sided significance level 0.025, power 80%, non‐inferiority margin 0.4% and dropout rate 15%.
Data are presented as the mean ± SD in the text and tables, and mean ± standard error in the figures. Non‐normally distributed data are presented as median and interquartile range. The paired t‐test or Wilcoxon test was used to assess changes in parameters between baseline and week 24, whereas the unpaired t‐test or Mann–Whitney U test was used to assess differences between two groups. All analyses were carried out using the Statistical Package for the Social Sciences (version 24; SPSS, Chicago, IL, USA), and P < 0.05 was considered statistically significant.
Results
Glycemic control
Changes in glycemic parameters during the study are shown in Table 2. Of 58 participants who completed the study, concomitant antidiabetic medication was changed for three participants during the study; dose reduction of sulfonylurea for two participants, dose reduction of thiazolidinedione for one participant and discontinuation of sulfonylurea for one participant, on the decision of the attending physician. After switching from exenatide b.i.d. to exenatide q.w., HbA1c was reduced by 0.2% at week 24 (7.2 ± 1.2% vs 7.0 ± 1.2% [56 ± 13 vs 53 ± 13 mmol/mol], 95% confidence interval [95% CI] −0.4 to −0.03%, P < 0.005 for non‐inferiority and P = 0.01 for superiority; Table 2; Figure 2). A significant reduction in HbA1c at week 24 was also observed in a subgroup of participants who were treated with a 20 μg daily dose of exenatide b.i.d. before switching to exenatide q.w. (7.3 ± 1.2% vs 7.1 ± 1.1% [57 ± 13 vs 54 ± 12 mmol/mol], P = 0.04).
Table 2.
Changes in glycemic parameters, bodyweight, hypoglycemia and treatment satisfaction assessed by the Diabetes Treatment Satisfaction Questionnaire
| Parameter | Baseline | Week 24 | P‐value |
|---|---|---|---|
| HbA1c (%) | 7.2 ± 1.2 | 7.0 ± 1.2 | 0.01 |
| HbA1c (mmol/mol) | 56 ± 13 | 53 ± 13 | |
| Proportion of patients with HbA1c <7% | 48.3% | 48.3% | 1.00 |
| FPG (mg/dL) | 154 ± 46 | 142 ± 46 | 0.02 |
| GA (%) | 16.9 ± 3.3 | 16.6 ± 3.6 | 0.12 |
| 1,5‐AG (μg/mL) | 9.1 ± 6.2 | 8.9 ± 6.5 | 0.55 |
| Mean preprandial SMBG (mg/dL) | 142 ± 44 | 133 ± 38 | 0.02 |
| Mean postprandial SMBG (mg/dL) | 162 ± 51 | 187 ± 50 | <0.001 |
| SD SMBG (mg/dL) | 35 ± 17 | 40 ± 16 | 0.02 |
| CV SMBG (%) | 22.7 ± 7.8 | 24.9 ± 7.5 | 0.08 |
| Insulin (mU/L) | 13.5 ± 11.5 | 14.1 ± 10.4 | 0.55 |
| HOMA‐IR | 3.6 (2.0–6.6) | 3.8 (2.4–7.5) | 0.97 |
| HOMA‐β (%) | 43.6 (25.6–76.7) | 52.0 (34.2–102.9) | 0.002 |
| C‐peptide (ng/mL) | 3.40 ± 2.04 | 3.51 ± 1.81 | 0.54 |
| C‐peptide index | 2.32 ± 1.47 | 2.66 ± 1.68 | 0.04 |
| Proinsulin (pmol/L) | 12.2 ± 13.2 | 11.6 ± 9.0 | 0.59 |
| Proinsulin/insulin ratio | 0.157 ± 0.098 | 0.156 ± 0.091 | 0.88 |
| Glucagon (pg/mL) | 139 ± 27 | 149 ± 37 | 0.01 |
| Weight (kg) | 86.2 ± 28.2 | 86.1 ± 27.9 | 0.66 |
| Hypoglycemia (events per person month) | 0.40 ± 1.03 | 0.04 ± 0.13 | 0.007 |
| DTSQ | |||
| Q2 (hyperglycemia) | 3.1 ± 1.6 | 2.5 ± 1.9 | 0.03 |
| Q3 (hypoglycemia) | 0.8 ± 1.2 | 1.1 ± 1.6 | 0.31 |
| Total score (sum of Q1, 4–8) | 22.8 ± 6.0 | 26.8 ± 7.0 | 0.001 |
Range of each score (minimum to maximum): Diabetes Treatment Satisfaction Questionnaire (DTSQ): question 2 (Q2) and question 3 (Q3) 0–6, total score 0–36. 1,5‐AG, 1,5‐anhydro‐D‐glucitol; CV, coefficient of variation; FPG, fasting plasma glucose; GA, glycated albumin; HbA1c, glycated hemoglobin; HOMA‐IR, homeostasis model assessment of insulin resistance; SD, standard deviation; SMBG, self‐monitoring of blood glucose.
Figure 2.
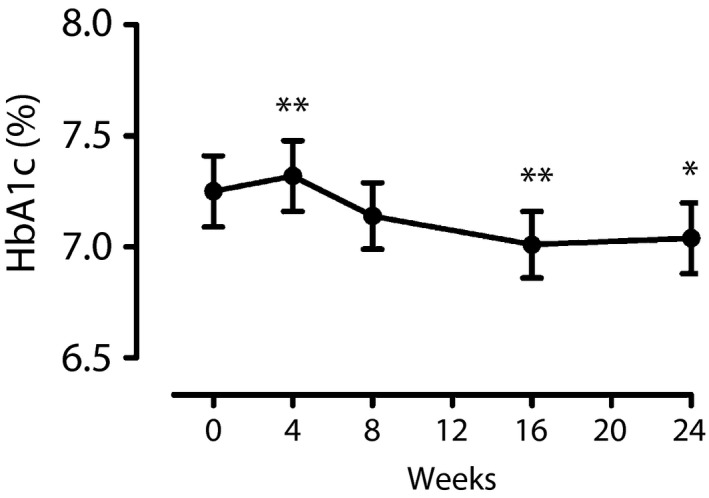
Change in glycated hemoglobin (HbA1c) during the study. *P < 0.05 and **P < 0.01 versus baseline (week 0).
Other glycemic parameters
Fasting plasma glucose level was reduced by 12 mg/dL at week 24 (154 ± 46 vs 142 ± 46 mg/dL, P = 0.02; Table 2; Figure S1). The proportion of patients who achieved HbA1c <7% (<53 mmol/mol) was not significantly changed at week 24 (48.3% vs 48.3%, P = 1.0). There was no significant change in glycated albumin or 1,5‐anhydro‐D‐glucitol at week 24 (Table 2; Figure S1).
The change in daily glycemic profile based on SMBG is shown in Figure 3. At week 24, capillary glucose levels at 1 h post‐breakfast and 1 h post‐dinner were significantly increased (150 ± 45 vs 190 ± 57 mg/dL, P < 0.001, and 148 ± 56 vs 186 ± 56 mg/dL, respectively, P < 0.001), whereas the capillary glucose level at pre‐lunch was significantly reduced (142 ± 52 vs 129 ± 46 mg/dL, P = 0.04). The overall mean preprandial glucose level was significantly reduced, whereas the mean postprandial glucose level was increased at week 24 (Table 2). The SD, but not the coefficient of variation, of SMBG was significantly increased at week 24.
Figure 3.
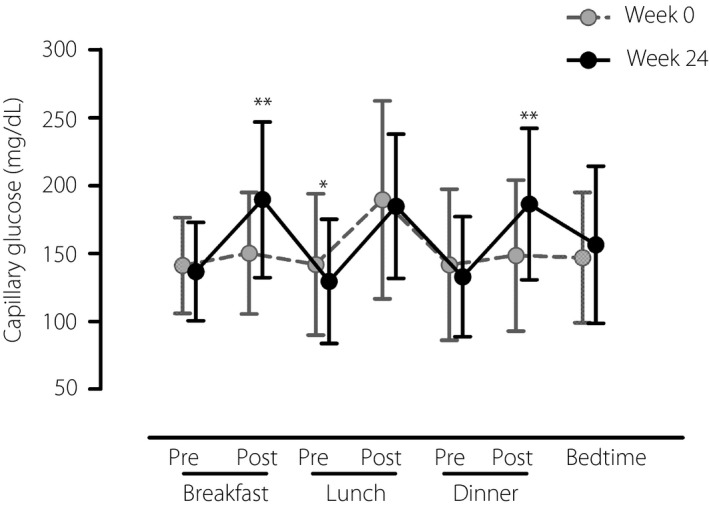
Change in daily glycemic profile assessed by self‐monitoring of blood glucose. *P < 0.05 and **P < 0.01 versus baseline (week 0).
Although fasting plasma insulin and serum C‐peptide levels were not significantly changed at week 24, HOMA‐β and C‐peptide index were significantly increased at week 24 (Table 2). Neither fasting proinsulin nor the proinsulin‐to‐insulin ratio was significantly changed during the study, whereas the fasting serum glucagon level was significantly increased at week 24 (Table 2).
Weight, hypoglycemia and treatment satisfaction
There was no significant change in bodyweight at week 24 (Table 2). The incidence of hypoglycemia was significantly reduced at week 24 (Table 2; Figure S2). There was no case of severe hypoglycemia during the study. Treatment satisfaction assessed by the Diabetes Treatment Satisfaction Questionnaire and Diabetes Medication Satisfaction Questionnaire was significantly improved at week 24 (Table 2; Table S1).
Other parameters and adverse events
Changes in other biochemical parameters, and inflammatory and oxidative stress markers are shown in Table S1. Serum aspartate aminotransferase and alanine aminotransferase levels were slightly, but significantly, increased at week 24, whereas there was no case of an increase in aspartate aminotransferase and/or alanine aminotransferase level to greater than threefold the upper limit of normal. Serum amylase and lipase were increased by ~7% and ~25%, respectively, at week 24 (Table S1; Figure S3). There was no case of acute pancreatitis. Gastrointestinal symptoms assessed by the Gastrointestinal Symptom Rating Scale did not significantly change during the study (Table S1).
There was no significant change in waist circumference or adiponectin level at week 24, whereas high‐density lipoprotein cholesterol was significantly increased (Table S1). Although no significant change was observed in either C‐reactive protein or urinary 8‐hydroxy‐2´‐deoxyguanosine level at week 24, urinary 8‐isoprostaglandin F2α level was significantly increased at week 24 (Table S1). Overall, there were no serious adverse events related to the study drug during the study period.
Comparison of patients in whom HbA1c improved and did not improve
Finally, we compared the clinical parameters between patients in whom HbA1c improved by ≥0.5% after switching to exenatide q.w. and those in whom it did not, to explore the predictors of improvement of HbA1c after switching from exenatide b.i.d. to exenatide q.w. Patients with a HbA1c reduction ≥0.5% showed higher body mass index and HbA1c at baseline, and significant weight loss at week 24 compared with those without (−1.1 ± 2.4 vs 0.4 ± 2.4 kg, P = 0.02; Table S2).
Discussion
In the present study, switching from exenatide b.i.d., a short‐acting GLP‐1RA, to exenatide q.w., a long‐acting GLP‐1RA, resulted in the reduction of FPG and HbA1c levels without increasing the incidence of hypoglycemia, and improved treatment satisfaction in Japanese patients with type 2 diabetes. A recent meta‐analysis of CVD outcomes trials has shown a CVD protective effect of GLP‐1RAs8, and the updated American Diabetes Association/European Association for the Study of Diabetes guidelines for the management of type 2 diabetes recommend the use of GLP‐1RAs for patients with type 2 diabetes and CVD16; however, heterogeneity of the CVD protective effect among the approved GLP‐1RAs has also been suggested8, 17.
It has been proposed that there are different mechanisms of action between short‐ and long‐acting GLP‐1RAs9. Short‐acting GLP‐1RAs primarily reduce postprandial hyperglycemia through suppression of gastric emptying, whereas long‐acting GLP‐1RAs primarily reduce FPG level through enhancing β‐cell function. In the present study, we found that the preprandial glucose level was reduced after switching from exenatide b.i.d. to exenatide q.w., whereas the postprandial glucose level was rather increased, in line with the previous observation18, 19, 20. Although the pre‐breakfast SMBG value was not significantly reduced at week 24, in contrast to FPG measured in the clinic or hospital after overnight fast, this might be due to the different timing of meals and SMBG in daily life, as seen in another study21. We also found that HOMA‐β and C‐peptide index, indices of β‐cell function, were increased at week 24, showing the improvement of β‐cell function by switching to exenatide q.w., whereas the proinsulin‐to‐insulin ratio did not change. In contrast, the fasting glucagon level was increased at week 24 in the present study. The reason for this is unclear, but the assay method might affect the results, and the recently developed sandwich enzyme‐linked immunosorbent assay, which is more specific for glucagon22 should be used in future studies.
Postprandial hyperglycemia and glycemic variability (GV) are known risk factors for developing CVD23. After switching to exenatide q.w., the postprandial glucose level and SD of SMBG increased, although the increase in the coefficient of variation of SMBG was not statistically significant. The reduction in HbA1c, but not glycated albumin or 1,5‐anhydro‐D‐glucitol, which reflect GV more sensitively than does HbA1c, also might be explained by an increase in GV after switching. Also, as urinary 8‐isoprostaglandin F2α, an oxidative stress marker, but not 8‐hydroxy‐2´‐deoxyguanosine, was significantly increased at week 24, it can be assumed that there is the possibility that greater GV after switching to exenatide q.w. might promote atherosclerosis through production of oxidative stress. In fact, treatment with exenatide q.w. failed to show reduction in CVD events compared with placebo in the Exenatide Study of Cardiovascular Event Lowering (EXSCEL) study24, although other long‐acting GLP‐1RAs showed improvement of CVD outcomes4, 5, 6, 7. Therefore, it remains unclear whether GV affects CVD outcomes under treatment with GLP‐1RAs, and further investigation is required to clarify the effects of GV on CVD outcomes under treatment with long‐acting GLP‐1RAs.
In addition to their glucose‐lowering effect, effects on weight and the incidence of hypoglycemia are also important components of antidiabetic medication16. It has been reported that treatment with exenatide b.i.d. induced greater weight loss compared with exenatide q.w.19. In the present study, we showed that switching from exenatide b.i.d. to q.w. maintained weight loss induced by exenatide b.i.d., consistent with a previous study20. Although the incidence of hypoglycemia was based on a questionnaire to the patients, and hypoglycemic events were not necessarily confirmed by SMBG values in the present study, the reduction in the incidence of hypoglycemia in this study might have been at least partially due to the dose reduction in concomitant medications, especially sulfonylurea, during the study. Nevertheless, we cannot completely exclude the possibility that asymptomatic hypoglycemia might have increased during the study, and further analysis using continuous glucose monitoring is planned in a subgroup of this study.
Treatment satisfaction was significantly improved after switching to exenatide q.w., which was likely due to the reduced number of injections. Despite the fact that 1‐h post‐breakfast and post‐dinner glucose levels were increased after switching to exenatide q.w., the question 2 score regarding the burden of hyperglycemia in Diabetes Treatment Satisfaction Questionnaire was improved. The reason for this is unclear, but we speculate that the improvement of the preprandial glucose level and HbA1c with dose reduction in concomitant antidiabetic medications in some cases might have a greater impact on patients compared with postprandial glycemic excursion.
It is of note that the score of symptoms (side‐effects) in the Diabetes Medication Satisfaction Questionnaire was increased after switching, and within the domain, the only significant increase was in the question regarding “unwanted weight gain” (1.8 ± 3.5 vs 3.9 ± 5.0 [score range 0–20], P = 0.002). Gastrointestinal symptoms assessed by the Gastrointestinal Symptom Rating Scale did not change during the study, suggesting that the increase in the symptom domain score was unlikely to be due to changes in gastrointestinal symptoms.
An increase in serum amylase and lipase levels after initiation of GLP‐1RAs has been reported25, 26. However, the mechanisms by which GLP‐1RAs increase these pancreatic enzymes remain unclear27. It has been reported that an increase in these enzymes did not predict the development of acute pancreatitis25, 26. The present study showed that serum amylase and lipase levels were increased after switching from exenatide b.i.d. to q.w.; however, the use of exenatide q.w. did not result in an increase in the risk of acute pancreatitis in the EXSCEL study24 as well as the present study, and the clinical significance of the elevated pancreatic enzymes in the present study remains unclear.
Finally, we found that the patients whose HbA1c improved after switching to exenatide q.w. were more obese and had higher HbA1c levels at baseline. They also showed greater weight loss after switching compared with those without improvement of HbA1c, suggesting the importance of continuous efforts for lifestyle modification to promote greater HbA1c reduction with long‐acting GLP‐1RA therapy.
There were limitations to the present study. First, the study was single‐armed and the two drugs were not directly compared. However, the present study was carried in a usual care setting and the results can be applied to real‐world clinical practice. It also should be noted that it was difficult to use a cross‐over design for ethical reasons because of the long‐lasting mode of action of exenatide q.w. (~10 weeks), necessitating a prolonged washout period. Second, as the participants were all Japanese, the results might not be applicable to other ethnicities.
In conclusion, switching from exenatide b.i.d. to exenatide q.w. resulted in reductions in HbA1c, FPG and the incidence of self‐reported hypoglycemia, and improvement of β‐cell function and treatment satisfaction in patients with type 2 diabetes. These findings will be useful for selecting optimal treatment for individuals with type 2 diabetes.
Disclosure
The authors declare no conflict of interest.
Supporting information
Figure S1 | Changes in (a) fasting plasma glucose (FPG), (b) glycated albumin (GA) and (c) 1,5‐anhydro‐D‐glucitol (1,5‐AG) during the study. *P < 0.05 and **P < 0.01 versus baseline (week 0). Note that blood sampling at week 4, 8 and 16 was not restricted to fasting.
Figure S2 | Change in incidence of hypoglycemia during study. *P < 0.05 and **P < 0.01 versus baseline (week 0).
Figure S3 | Changes in (a) serum amylase and (b) serum lipase during the study. *P < 0.05 and **P < 0.01 versus baseline (week 0).
Table S1 | Changes in other parameters, treatment satisfaction (Diabetes Medication Satisfaction Questionnaire) and gastrointestinal symptoms (Gastrointestinal Symptom Rating Score).
Table S2 | Comparison between participants in whom glycated hemoglobin improved by ≥0.5% and those in whom glycated hemoglobin did not.
Table S3 | Twin‐exenatide study investigators.
Acknowledgments
We thank Dr Wendy Gray for editing the manuscript. We also thank the investigators of the Twin‐exenatide Study Group (Table S3) and the study participants for their contribution to the study. This study was supported by funding from AstraZeneca. The sponsor of this study had no access to the original data and no role in writing the manuscript. This study was presented at the 55th annual meeting of the European Association for the Study of Diabetes.
J Diabetes Investig 2020; 11: 382–388
Clinical Trial Registry
Japan Clinical Trials Registry
UMIN000016390 and jRCTs031180320
References
- 1. International Diabetes Federation . IDF Diabetes Atlas, 8th edn Brussels, Belgium: International Diabetes Federation, 2017. [Google Scholar]
- 2. Rawshani A, Rawshani A, Franzén S, et al Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med 2017; 376: 1407–1418. [DOI] [PubMed] [Google Scholar]
- 3. Drucker DJ, Nauck MA. The incretin system: glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors in type 2 diabetes. Lancet 2006; 368: 1696–1705. [DOI] [PubMed] [Google Scholar]
- 4. Marso SP, Bain SC, Consoli A, et al Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834–1844. [DOI] [PubMed] [Google Scholar]
- 5. Marso SP, Daniels GH, Brown‐Frandsen K, et al Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hernandez AF, Green JB, Janmohamed S, et al Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double‐blind, randomised placebo‐controlled trial. Lancet 2018; 392: 1519–1529. [DOI] [PubMed] [Google Scholar]
- 7. Gerstein HC, Colhoun HM, Dagenais GR, et al Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double‐blind, randomised placebo‐controlled trial. Lancet 2019; 394: 121–130. [DOI] [PubMed] [Google Scholar]
- 8. Bethel MA, Patel RA, Merrill P, et al Cardiovascular outcomes with glucagon‐like peptide‐1 receptor agonists in patients with type 2 diabetes: a meta‐analysis. Lancet Diabetes Endocrinol 2018; 6: 105–113. [DOI] [PubMed] [Google Scholar]
- 9. Meier JJ. GLP‐1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 2012; 8: 728–742. [DOI] [PubMed] [Google Scholar]
- 10. Knop FK, Bronden A, Vilsboll T. Exenatide: pharmacokinetics, clinical use, and future directions. Expert Opin Pharmacother 2017; 18: 555–571. [DOI] [PubMed] [Google Scholar]
- 11. Saisho Y. Use of diabetes treatment satisfaction questionnaire in diabetes care: importance of patient‐reported outcomes. Int J Environ Res Public Health 2018; 15: E947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ishii H, Iwase M, Seino H, et al Assessment of quality of life in patients with type 2 diabetes mellitus before and after starting biphasic insulin aspart 30 (BIAsp 30) therapy: IMPROVE study in Japan. Curr Med Res Opin 2011; 27: 643–650. [DOI] [PubMed] [Google Scholar]
- 13. Kulich KR, Madisch A, Pacini F, et al Reliability and validity of the Gastrointestinal Symptom Rating Scale (GSRS) and Quality of Life in Reflux and Dyspepsia (QOLRAD) questionnaire in dyspepsia: a six‐country study. Health Qual Life Outcomes 2008; 6: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suzuki H, Masaoka T, Sakai G, et al Improvement of gastrointestinal quality of life scores in cases of Helicobacter pylori‐positive functional dyspepsia after successful eradication therapy. J Gastroenterol Hepatol 2005; 20: 1652–1660. [DOI] [PubMed] [Google Scholar]
- 15. Tanaka K, Saisho Y, Kawai T, et al Efficacy and safety of liraglutide monotherapy compared with metformin in Japanese overweight/obese patients with type 2 diabetes. Endocr J 2015; 62: 399–409. [DOI] [PubMed] [Google Scholar]
- 16. Davies MJ, D'Alessio DA, Fradkin J, et al Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41: 2669–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taylor SI. GLP‐1 receptor agonists: differentiation within the class. Lancet Diabetes Endocrinol 2018; 6: 83–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Drucker DJ, Buse JB, Taylor K, et al Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open‐label, non‐inferiority study. Lancet 2008; 372: 1240–1250. [DOI] [PubMed] [Google Scholar]
- 19. Ji L, Onishi Y, Ahn CW, et al Efficacy and safety of exenatide once‐weekly vs exenatide twice‐daily in Asian patients with type 2 diabetes mellitus. J Diabetes Investig 2013; 4: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Onishi Y, Koshiyama H, Imaoka T, et al Safety of exenatide once weekly for 52 weeks in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig 2013; 4: 182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosenstock J, Cheng A, Ritzel R, et al More similarities than differences testing insulin glargine 300 units/mL versus insulin degludec 100 units/mL in insulin‐naive type 2 diabetes: the randomized head‐to‐head BRIGHT trial. Diabetes Care 2018; 41: 2147–2154. [DOI] [PubMed] [Google Scholar]
- 22. Matsuo T, Miyagawa J, Kusunoki Y, et al Postabsorptive hyperglucagonemia in patients with type 2 diabetes mellitus analyzed with a novel enzyme‐linked immunosorbent assay. J Diabetes Investig 2016; 7: 324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saisho Y. Glycemic variability and oxidative stress: a link between diabetes and cardiovascular disease? Int J Mol Sci 2014; 15: 18381–18406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holman RR, Bethel MA, Mentz RJ, et al Effects of once‐weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017; 377: 1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Steinberg WM, Rosenstock J, Wadden TA, et al Impact of liraglutide on amylase, lipase, and acute pancreatitis in participants with overweight/obesity and normoglycemia, prediabetes, or type 2 diabetes: secondary analyses of pooled data from the SCALE clinical development program. Diabetes Care 2017; 40: 839–848. [DOI] [PubMed] [Google Scholar]
- 26. Steinberg WM, Buse JB, Ghorbani MLM, et al Amylase, lipase, and acute pancreatitis in people with type 2 diabetes treated with liraglutide: results from the LEADER randomized trial. Diabetes Care 2017; 40: 966–972. [DOI] [PubMed] [Google Scholar]
- 27. Saisho Y. Incretin‐based therapy and pancreatitis: accumulating evidence and unresolved questions. Ann Transl Med 2018; 6: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Changes in (a) fasting plasma glucose (FPG), (b) glycated albumin (GA) and (c) 1,5‐anhydro‐D‐glucitol (1,5‐AG) during the study. *P < 0.05 and **P < 0.01 versus baseline (week 0). Note that blood sampling at week 4, 8 and 16 was not restricted to fasting.
Figure S2 | Change in incidence of hypoglycemia during study. *P < 0.05 and **P < 0.01 versus baseline (week 0).
Figure S3 | Changes in (a) serum amylase and (b) serum lipase during the study. *P < 0.05 and **P < 0.01 versus baseline (week 0).
Table S1 | Changes in other parameters, treatment satisfaction (Diabetes Medication Satisfaction Questionnaire) and gastrointestinal symptoms (Gastrointestinal Symptom Rating Score).
Table S2 | Comparison between participants in whom glycated hemoglobin improved by ≥0.5% and those in whom glycated hemoglobin did not.
Table S3 | Twin‐exenatide study investigators.


