Abstract
Aims/Introduction
We sought to determine if postmenopausal women who develop breast cancer are at increased risk of developing diabetes mellitus.
Materials and Methods
The Taiwan National Health Insurance Research Database was searched from 2001 to 2015 for women aged ≥55 years (postmenopausal) with a diagnosis of primary breast cancer. Participants were age‐matched with women without breast cancer in a 1:5 ratio. Cox proportional hazards analyses were used to examine associations between breast cancer, risk factors and the development of diabetes mellitus.
Results
A total of 4,607 women with primary breast cancer and 23,035 age‐matched controls without breast cancer were included (mean age 58.6 ± 9.1 years). Adjusting for age, income, urbanization, Charlson Comorbidity Index and medical conditions, the risk of diabetes mellitus for participants with breast cancer at 1, 5, 10 and 15 years was 1.70‐, 1.34‐, 1.27‐ and 1.24‐fold higher, respectively, than for participants without breast cancer (adjusted hazard ratio [aHR] 1.70, 95% confidence interval [CI] 1.40–2.05; aHR 1.34, 95% CI 1.17–1.54; aHR 1.27, 95% CI 1.13–1.44; aHR 1.24, 95% CI 1.11–1.40). The risk of diabetes mellitus at 1 year for breast cancer patients receiving hormone therapy was 1.22‐fold higher than in those not receiving hormone therapy (aHR 1.22, 95% CI 0.86–1.74), but without statistical significance.
Conclusions
Postmenopausal women with breast cancer are at increased risk of developing diabetes mellitus, independent of receiving hormone therapy, and should be closely monitored to establish an early diagnosis and therapeutic intervention for improving related outcomes.
Keywords: Breast cancer, Diabetes mellitus, Postmenopausal
Postmenopausal women who develop breast cancer are at higher risk of developing diabetes mellitus than women in the same age group who do not have breast cancer. Women who have received hormonal therapy for breast cancer show no significantly increased risk for developing diabetes mellitus than those who have not received hormonal therapy. These results suggest that postmenopausal women with breast cancer should be closely monitored for the development of diabetes mellitus to establish an early diagnosis and therapeutic intervention for improving related outcomes.

Introduction
Breast cancer is one of the most important malignancies in women worldwide, and the leading cause of cancer‐related death in women1, 2. An increase in the incidence of breast cancer is believed to be due in part to urbanization, and changes in lifestyle and eating habits1, 3. In Taiwan, patients with the highest morbidity from breast cancer are usually 10 years younger than those in Western countries1, 2. In contrast, diabetes mellitus has also become a serious globally health issue, with an increasing incidence of diabetes mellitus and a younger age of onset4. Currently, diabetes mellitus and its associated complications are the fifth leading cause of death among the top 10 major causes of death4.
Diabetes mellitus and various cancers share certain risk factors, including a link between obesity and cancers, such as breast and prostate cancer5. Associations have also been reported between diabetes mellitus, antidiabetic drugs, insulin resistance and breast cancer risk6, 7, 8, 9. For example, Lipscombe et al.8 examined population‐based data from Ontario, Canada, and reported that the risk of diabetes mellitus in postmenopausal women with breast cancer was increased by 1.07‐fold at 2 years after breast cancer diagnosis compared with the risk of diabetes mellitus in women without breast cancer (hazard ratio [HR] 1.21, 95% confidence interval [CI] 1.09–1.35); and the risk was higher in women who received adjuvant treatment. In contrast, Santorelli et al.10 reported that breast cancer patients treated with aromatase inhibitors and tamoxifen do not show an increased frequency of developing diabetes mellitus. In a retrospective case–control study, Dąbrowski et al.7 found that breast cancer was the most common malignancy in women with diabetes mellitus (20.7%). Maskarinec et al.11 investigated breast cancer in American women using a questionnaire, cancer registration database and death registration database, and found that diabetes mellitus was closely associated with breast cancer risk (HR 1.15; 95% CI 0.07–1.23). Two meta‐analyses reported that pre‐existing diabetes mellitus is associated with a poorer prognosis in women who develop breast cancer12, 13. Treatment of women with diabetes mellitus with metformin, rosiglitazone and sitagliptin is shown to reduce the risk of breast cancer14, 15, 16, and metformin might also reduce cervical cancer risk in women with diabetes mellitus 17. Although evidence shows associations between diabetes mellitus and breast cancer, the interplay of factors, such as obesity, insulin resistance, inflammation and oxidative stress, is exceptionally complex and is only beginning to be understood5, 18, 19. However, studies examining the risk of developing diabetes mellitus after a diagnosis of breast cancer are limited. Thus, the purpose of the current study was to investigate the risk over time of postmenopausal women with breast cancer developing diabetes mellitus, and to determine if hormone therapy for breast cancer might affect such risk.
Methods
Data source and participants
The National Health Insurance program in Taiwan integrated all public insurance systems into a single payment program since 1995, and by 2009, the program provided medical insurance for 99% of the Taiwanese population. Subsequently, the National Health Research Institute and Bureau of National Health Insurance established the Taiwan National Health Insurance Research Database (NHIRD20, 21, 22. The NHIRD contains original claims data of >23 million people, 98% of whom are Han Chinese, which is 99.9% of the entire population of Taiwan. The database thus provides a large population‐based resource for epidemiological studies. The NHIRD includes all information on outpatient and inpatient claims data, and all patient information is de‐identified. All clinical diagnoses, procedures and treatments are recorded according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) coding scheme. The present retrospective study was approved by (ethical review board approval) Institution Responsibility Board of National Taiwan University Hospital, and conforms to the provisions of the Declaration of Helsinki.
We searched the household registry data, cancer registry data and NHIRD records of patient data collected from 2000 to 2015 for postmenopausal women aged ≥55 years who were newly diagnosed with primary breast cancer. The age range of ≥55 years was used because it closely corresponds with postmenopausal status23. The index date was defined as the date breast cancer was first diagnosed. Within 1 year before the index date, women diagnosed with another malignancy or primary breast cancer (ICD‐9: 140–173, 175–208, 2330 or ICD‐O‐3: C00–C49, C51–C97, D050–D059), women diagnosed with coding of diabetes mellitus (ICD‐9: 250), women receiving hormone therapy or those diagnosed with metabolic syndrome (ICD‐9: 2777), obesity (ICD‐9: 278), phlebitis, thrombophlebitis, venous thrombosis and thrombosis (ICD‐9: 451–453) were excluded. On the basis of age, participants with breast cancer were matched with those without breast cancer in a 1:5 ratio. Participants who had <1 year of follow up or had recurrent breast cancer were also excluded. Participants were considered to have a comorbidities record (hyperlipidemia, hypertension, cardiovascular diseases/ischemic heart disease, chronic kidney disease, cerebrovascular diseases) if they were diagnosed within 1 year before the index date or at the time of breast cancer diagnosis. The Charlson Comorbidity Index (CCI) was calculated for each patient. For analysis, participants were divided into four subgroups stratified according to the level of urbanization in the Taiwan township where they reside24.
Medications used for hormone therapy included anastrozole, exemestane, goserelin, letrozole, megestrol, tamoxifen and toremifene. Drugs used for chemotherapy included docetaxel, paclitaxel, doxorubicin, epirubicin, cyclophosphamide, fluorouracil, gemcitabine, methotrexate, cisplatin, carboplatin, vinorelbine, mitoxantrone, capecitabine and tegafur.
Statistical analysis
Categorical data are expressed as numbers (n) and percentage (%), and compared using the χ2‐test. Continuous data are expressed as the mean ± standard deviation, and compared using the t‐test. A cause‐specific Cox proportional hazards model accounting for the competing risks of recurrence of breast cancer, presence of new cancer or metastasis and death was used to evaluate the risk for diabetes or breast cancer and associations with hormone therapy over time. Risk factors, such as age, income, urbanization, comorbidities and breast cancer treatment, were also evaluated for associations with diabetes incidence in breast cancer patients. Results are reported as aHR and 95% CI. Risk factors found to be statistically significant in the univariate cause‐specific Cox proportional hazards model were included in the multivariate model. All analyses were two‐sided, and a value of P < 0.05 was considered statistically significant. Statistical analysis was carried out with SAS version 9.4 software (SAS Institute Inc., Cary, NC, USA).
Results
A total of 322,292 women aged ≥55 years were identified in the household registration data of the NHIRD during the study period. Of these, 6,720 had new‐onset breast cancer. A total of 2,113 were excluded, as shown in Figure 1, and thus 4,607 postmenopausal women aged ≥55 years who were diagnosed with primary breast cancer between 2001 and 2013 were included in the analysis. The number of age‐matched control participants without breast cancer was 23,035. The mean age of the participants included in the analysis was 58.6 ± 9.1 years. Demographic and clinical characteristics of participants with and without breast cancer are summarized in Table 1. CCI >1, hyperlipidemia, hypertension, cardiovascular disease and cerebrovascular disease were more prevalent in the breast cancer group. The median follow‐up period in years was longer in the non‐breast cancer group.
Figure 1.
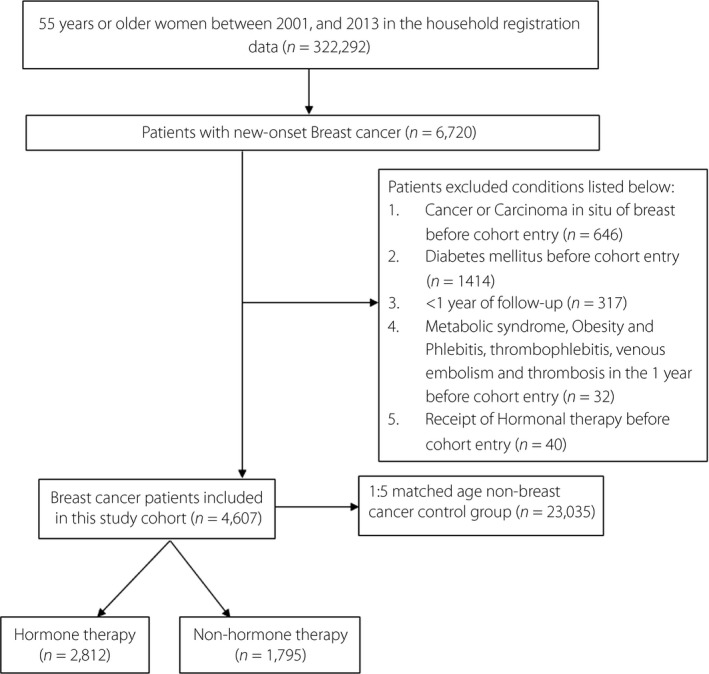
Flow diagram of participant inclusion.
Table 1.
Participant characteristics
| Variable | Primary breast cancer | No breast cancer | P‐value |
|---|---|---|---|
| n = 4,607 | n = 23,035 | ||
| Age at diagnosis (years) | |||
| Mean (SD) | 58.6 (9.1) | 58.6 (9.1) | 0.974 |
| 55–65 years | 3,714 (80.6) | 18,562 (80.6) | 0.934 |
| 66–80 years | 782 (17.0) | 3,897 (16.9) | |
| 81 years | 111 (2.4) | 576 (2.5) | |
| Income† | |||
| <1,249 | 1,391 (30.2) | 6,758 (29.3) | <0.001 |
| 1,249–21,899 | 1,389 (30.2) | 7,723 (33.5) | |
| ≥21,900 | 1,827 (39.7) | 8,554 (37.1) | |
| Urbanization‡ | |||
| I (1) | 1,628 (35.3) | 6,714 (29.2) | <0.001 |
| II (2 + 3) | 1,724 (37.4) | 8,738 (37.9) | |
| III (4 + 5) | 557 (12.1) | 3,119 (13.5) | |
| IV (6 + 7) | 207 (4.5) | 1,615 (7.0) | |
| Missing | 491 (10.7) | 2,849 (12.4) | |
| Charlson Comorbidity Index | |||
| Mean (SD) | 0.6 (1.5) | 0.2 (0.6) | <0.001 |
| >1 | 692 (15.0) | 977 (4.2) | <0.001 |
| History of hyperlipidemia | 477 (10.4) | 1,916 (8.3) | <0.001 |
| History of hypertension | 1,177 (25.6) | 4,893 (21.2) | <0.001 |
| History of cardiovascular disease | 301 (6.5) | 1,311 (5.7) | 0.026 |
| History of chronic kidney disease | 25 (0.5) | 133 (0.6) | 0.775 |
| History of cerebrovascular disease | 155 (3.4) | 630 (2.7) | 0.019 |
| Median follow‐up period, years (IQR) | 1.1 (0.2–2.7) | 6.9 (3.9–10.6) | <0.001 |
Data are presented as mean ± standard deviation (SD), number (percentage) or median (interquartile range [IQR]). †Patients were divided into three subgroups according to the 33.3 and 66.6 percentile of income. ‡Patients were divided into four subgroups according to the level of urbanization where they resided: I (1): highly urbanized cities; II (2 + 3): moderately urbanized cities plus boom towns; III (4 + 5): general cities and towns plus aging towns; IV (6 + 7): suburbanized cities and towns plus remote towns.
Among all participants with primary breast cancer, the mean age was 59.0 ± 9.2 years, and 2,812 received hormone therapy (61.0%). Significant differences were observed in age (P < 0.001), CCI >1 (P < 0.001), prevalence of hyperlipidemia (P = 0.014), prevalence of hypertension (P < 0.001), receiving surgery (P < 0.001) and chemotherapy (P < 0.001) treatment within 1 year after breast cancer diagnosis between participants who received hormone therapy and those who did not (Table 2). Treatment for breast cancer, such as surgery and chemotherapy, was not associated with diabetes incidence in the breast cancer study group, and no interaction effect was shown between hormone therapy and diabetes (data not shown).
Table 2.
Characteristics of women with breast cancer stratified by receiving hormone therapy
| Variable | Hormone therapy | No hormone therapy | P‐value |
|---|---|---|---|
| n = 2,812 | n = 1,795 | ||
| Age group | |||
| Mean (SD) | 59.0 (9.2) | 57.8 (8.8) | <0.001 |
| 55–65 years | 2,216 (78.8) | 1,498 (83.4) | <0.001 |
| 66–80 years | 522 (18.6) | 260 (14.5) | |
| ≥81 years | 74 (2.6) | 37 (2.1) | |
| Income | |||
| <1,249 | 826 (29.4) | 565 (31.5) | 0.299 |
| 1,249–21,899 | 853 (30.3) | 536 (29.9) | |
| ≥21,900 | 1,133 (40.3) | 694 (38.7) | |
| Urbanization† | |||
| I (1) | 994 (35.4) | 634 (35.3) | 0.877 |
| II (2 + 3) | 1,046 (37.2) | 678 (37.8) | |
| III (4 + 5) | 336 (12.0) | 221 (12.3) | |
| IV (6 + 7) | 133 (4.7) | 74 (4.1) | |
| Missing | 303 (10.8) | 188 (10.5) | |
| Cancer stage | |||
| 1 | 767 (35.3) | 356 (32.5) | 0.087 |
| 2 | 896 (41.3) | 448 (41.0) | |
| 3 | 392 (18.1) | 236 (21.6) | |
| 4 | 116 (5.3) | 54 (4.9) | |
| Charlson Comorbidity Index | |||
| Mean (SD) | 0.7 (1.6) | 0.5 (1.2) | <0.001 |
| >1 | 446 (15.9) | 246 (13.7) | 0.046 |
| History of hyperlipidemia | 316 (11.2) | 161 (9.0) | 0.014 |
| History of hypertension | 773 (27.5) | 404 (22.5) | <0.001 |
| History of cardiovascular disease | 186 (6.6) | 115 (6.4) | 0.781 |
| History of chronic kidney disease | 15 (0.5) | 10 (0.6) | 0.915 |
| History of cerebrovascular disease | 100 (3.6) | 55 (3.1) | 0.366 |
| Received treatment within 1 year after index date | |||
| Surgery | 2,710 (96.4) | 1,293 (72.0) | <0.001 |
| Chemotherapy | 1,732 (61.6) | 848 (47.2) | <0.001 |
| Median follow‐up period, years (IQR) | 3.1 (0.1–6.8) | 4.1 (1.0–8.5) | <0.001 |
Data are presented as mean ± standard deviation (SD), number (percentage) or median (interquartile range [IQR]). †Patients were divided into four subgroups according to the level of urbanization where they resided: I (1): highly urbanized cities; II (2 + 3): moderately urbanized cities plus boom towns; III (4 + 5): general cities and towns plus aging towns; IV (6 + 7): suburbanized cities and towns plus remote towns.
In participants with breast cancer, the incidence of diabetes mellitus at 1, 5, 10 and 15 years after diagnosis was 92.6, 49.5, 41.9 and 39.9/1,000 person‐years, respectively. In control participants without breast cancer, the incidence of diabetes mellitus at 1, 5, 10 and 15 years after the index date was 53.6, 27.2, 24.5 and 24.0/1,000 person‐years, respectively (Table 3). After adjustment for age, income, urbanization, CCI and medical conditions (hyperlipidemia, hypertension, cardiovascular disease, cerebrovascular disease), the risk of diabetes mellitus in participants with breast cancer at 1, 5, 10 and 15 years after diagnosis of breast cancer was 1.70‐, 1.34‐, 1.27‐ and 1.24‐fold higher, respectively, than in participants without breast cancer (aHR 1.70, 95% CI 1.40–2.05; aHR 1.34, 95% CI 1.17–1.54; aHR 1.27, 95% CI 1.13–1.44; aHR 1.24, 95% CI 1.11–1.40). The aHR for the occurrence of diabetes mellitus in participants with breast cancer over a period of 15 years is shown in Figure 2.
Table 3.
Cumulative incidence and Cox regression analysis of type 2 diabetes over time in participants with and without breast cancer
| Variable | Incidence/1,000 person‐years | Crude HR (95% CI) | Adjusted HR† (95% CI) | |
|---|---|---|---|---|
| Breast cancer | No breast cancer | Breast cancer vs no breast cancer | Breast cancer vs no breast cancer | |
| Year 1 | 92.6 | 53.6 | 1.85 (1.53–2.23)*** | 1.70 (1.40–2.05)*** |
| Year 2 | 67.3 | 37.0 | 1.55 (1.31–1.83)*** | 1.44 (1.22–1.71)*** |
| Year 3 | 57.5 | 31.8 | 1.44 (1.24–1.68)*** | 1.35 (1.16–1.57)** |
| Year 4 | 52.9 | 28.7 | 1.45 (1.26–1.67)*** | 1.36 (1.18–1.57)*** |
| Year 5 | 49.5 | 27.2 | 1.42 (1.24–1.63)*** | 1.34 (1.17–1.54)*** |
| Year 6 | 47.1 | 26.5 | 1.39 (1.22–1.59)*** | 1.32 (1.15–1.50)*** |
| Year 7 | 45.4 | 25.6 | 1.38 (1.22–1.57)*** | 1.31 (1.15–1.49)*** |
| Year 8 | 44.4 | 25.2 | 1.38 (1.22–1.56)*** | 1.31 (1.16–1.49)*** |
| Year 9 | 43.2 | 24.8 | 1.37 (1.21–1.54)*** | 1.30 (1.15–1.47)*** |
| Year 10 | 41.9 | 24.5 | 1.34 (1.19–1.51)*** | 1.27 (1.13–1.44)*** |
| Year 11 | 41.4 | 24.3 | 1.34 (1.19–1.50)*** | 1.27 (1.13–1.43)*** |
| Year 12 | 40.9 | 24.2 | 1.33 (1.18–1.49)*** | 1.27 (1.12–1.42)** |
| Year 13 | 40.5 | 24.0 | 1.32 (1.18–1.49)*** | 1.26 (1.12–1.42)** |
| Year 14 | 40.1 | 24.0 | 1.31 (1.17–1.47)*** | 1.25 (1.11–1.40)** |
| Year 15 | 39.9 | 24.0 | 1.31 (1.16–1.47)*** | 1.24 (1.11–1.40)** |
Censored cases were defined as the follows: (i) recurrence of breast cancer (surgery/chemotherapy/hormone therapy for breast cancer at 1 year after the index date); (ii) the presence of new cancer or metastasis; (iii) death; and (iv) end of observation (follow up for 1–10 years after the index date). †Model adjusted for age, income, urbanization, Charlson Comorbidity Index, and history of hyperlipidemia, hypertension, cardiovascular disease and cerebrovascular disease. CI, confidence interval; HR, hazard ratio.**P < 0.05; ***P < 0.01
Figure 2.
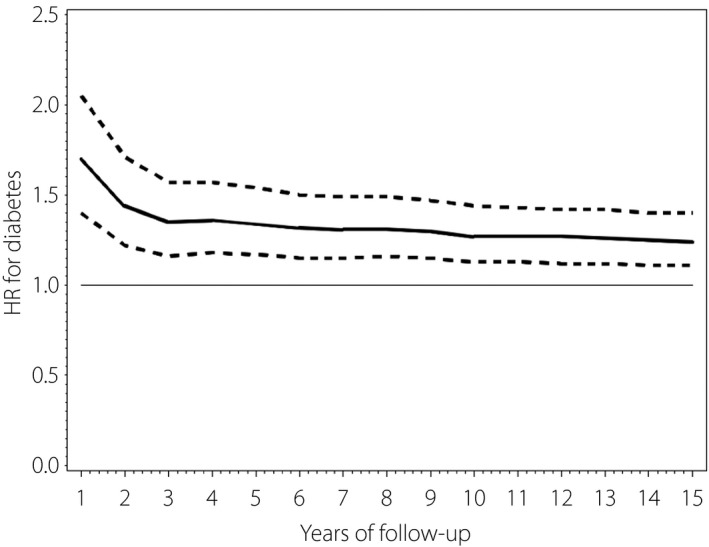
Adjusted hazard ratio (solid line) and 95% confidence intervals (dashed line) for the incidence of diabetes, breast cancer versus no breast cancer, over time. HR, hazard ratio.
The incidence of diabetes mellitus in participants with breast cancer stratified by receiving hormone therapy or not is summarized in Table 4. In participants who received hormone therapy, the incidence of diabetes mellitus at 1, 5, 10 and 15 years after diagnosis was 106.2, 79.0, 71.7 and 69.9/1,000 person‐years, respectively, and in participants who did not receive hormone therapy was 75.1, 36.3, 31.7 and 30.3/1,000 person‐years, respectively. After adjustment for age, income, cancer stage, CCI and medical conditions (hyperlipidemia, hypertension, cardiovascular disease, cerebrovascular disease), the risk of diabetes mellitus at 1 year in breast cancer participants receiving hormone therapy was 1.22‐fold higher than in those who did not receive hormone therapy (aHR 1.22, 95% CI 0.86–1.74), but without statistical significance. The aHR for the occurrence of diabetes mellitus in participants with breast cancer who received hormone therapy over a period of 15 years is shown in Figure 3.
Table 4.
Cumulative incidence and Cox regression analysis of type 2 diabetes over time in participants with breast cancer stratified by receipt of hormone therapy
| Variable | Incidence/1,000 person‐years | Crude HR (95% CI) | Adjusted HR† (95% CI) | |
|---|---|---|---|---|
| Hormonal therapy | No hormonal therapy | Hormonal therapy vs no hormonal therapy | Hormonal therapy vs no hormonal therapy | |
| Year 1 | 106.2 | 75.1 | 1.24 (0.88–1.75) | 1.22 (0.86–1.74) |
| Year 2 | 89.2 | 49.9 | 1.06 (0.77–1.45) | 1.05 (0.76–1.45) |
| Year 3 | 84.2 | 41.4 | 1.05 (0.77–1.42) | 1.05 (0.77–1.44) |
| Year 4 | 80.5 | 38.8 | 1.02 (0.76–1.36) | 1.03 (0.76–1.39) |
| Year 5 | 79.0 | 36.3 | 1.05 (0.79–1.40) | 1.08 (0.81–1.45) |
| Year 6 | 76.9 | 34.9 | 1.04 (0.78–1.37) | 1.07 (0.80–1.42) |
| Year 7 | 75.4 | 33.8 | 1.03 (0.78–1.36) | 1.05 (0.79–1.40) |
| Year 8 | 73.7 | 33.6 | 0.99 (0.75–1.31) | 1.01 (0.77–1.34) |
| Year 9 | 72.4 | 33.0 | 0.98 (0.74–1.28) | 1.00 (0.75–1.32) |
| Year 10 | 71.7 | 31.7 | 0.99 (0.75–1.30) | 1.01 (0.77–1.34) |
| Year 11 | 70.9 | 31.6 | 0.98 (0.74–1.28) | 0.99 (0.75–1.30) |
| Year 12 | 70.5 | 31.2 | 0.99 (0.75–1.29) | 1.00 (0.76–1.32) |
| Year 13 | 70.3 | 30.9 | 1.00 (0.76–1.31) | 1.01 (0.77–1.33) |
| Year 14 | 70.0 | 30.5 | 1.00 (0.76–1.31) | 1.01 (0.77–1.33) |
| Year 15 | 69.9 | 30.3 | 1.00 (0.76–1.31) | 1.01 (0.77–1.33) |
Censored cases were defined as follows: (i) recurrence of breast cancer (surgery for breast cancer at 1 year after the index date); (ii) the presence of new cancer or metastasis; (iii) death; and (iv) end of observation (follow up for 1–10 years after the index date). †Model adjusted for age, income, cancer stage, Charlson Comorbidity Index, and history of hyperlipidemia, hypertension and cardiovascular disease. CI, confidence interval; HR, hazard ratio.
Figure 3.
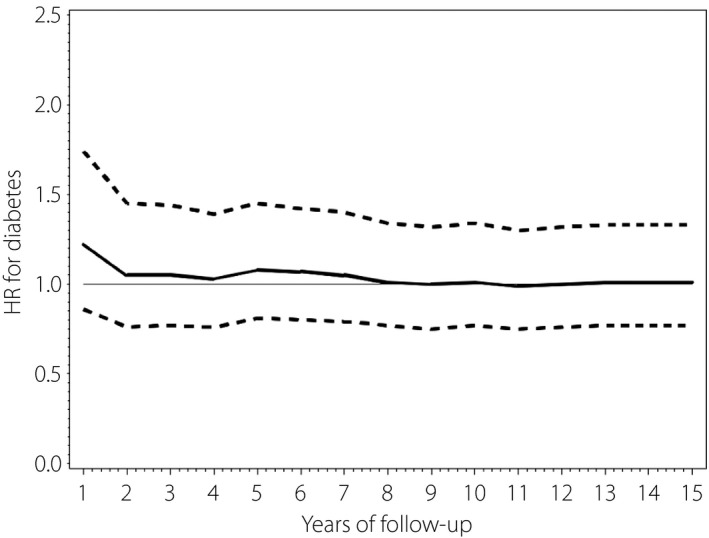
Adjusted hazard ratio (solid line) and 95% confidence intervals (dashed line) for the incidence of diabetes in the breast cancer cohort, receipt of hormonal therapy versus non‐hormonal therapy, over time. HR, hazard ratio.
Discussion
The results of the present large population‐based study showed that the risk of diabetes mellitus in women with breast cancer is increased beginning in the first year after diagnosis when compared with the risk of diabetes mellitus in women without breast cancer. The risk of diabetes mellitus in breast cancer patients remains increased up to 10 years after diagnosis (length of current analysis), although it gradually decreases over time. In addition, women with breast cancer who have received hormone therapy are at increased risk of diabetes mellitus at 6 and 10 years after diagnosis compared with those with breast cancer who have not received hormone therapy, but without statistical significance.
Many studies have examined the effect of pre‐existing diabetes mellitus on the prognosis of women who develop breast cancer. In a previous study in Taiwan by Chen et al.25, who also used NHIRD data, diabetes mellitus was an independent predictor of lower breast cancer‐specific survival and overall survival rates in women with early‐stage breast cancer. A recent case–control study found that obesity and diabetes mellitus increased the risk of breast cancer in women and colorectal cancer in men7. That study also found that hemoglobin A1c level ≥8.5% and insulin treatment (dose‐dependent and time‐dependent) were associated with an increased risk of malignancy, whereas metformin use lowered the risk of malignancy, but the duration of diabetes mellitus, comorbidities, smoking and aspirin use were not associated with risk of malignancy. An epidemiological study carried out in Taiwan found an increase in the incidence of breast cancer from 1997 to 2013, but at the same time found a decline in the 5‐year mortality rate for women with CCI >1 (1997, 5‐year mortality 39.1%; 2008, 5‐year mortality 21.1%)26.
Meta‐analyses have shown that diabetes mellitus is a risk factor for the development of breast cancer27, and that diabetes mellitus is associated with a poorer prognosis in women who develop breast cancer12, 13. The most recent meta‐analysis was published in 2016, including 17 studies and 48,315 women with breast cancer; the results showed that the pooled aHR for poorest overall survival was 1.51, and 1.28 for poorest disease‐free survival in patients with diabetes mellitus in comparison with those without diabetes mellitus 13. In addition, Tsilidis et al.28 carried out an “umbrella review” of meta‐analyses of observational studies examining the relationship of diabetes mellitus and cancer, and suggested that only a minority of studies reporting a link between diabetes mellitus and various cancers had “robust” supporting evidence without bias. Of the different cancers examined, only the associations between diabetes mellitus and the risk of developing various cancers, including breast cancer, were nominally significant.
Generally, exogenous insulin use is associated with an elevated risk of cancer29. However, certain antidiabetic drugs might reduce the risk of breast cancer14, 15, 16. Tseng et al.15 found that rosiglitazone significantly reduced the risk of breast cancer in Taiwanese women with diabetes mellitus, and the risk was further reduced in women who also took metformin. Biologically, the change of insulin resistance in women with breast cancer might hint to the pivotal role of insulin resistance in developing diabetes among women with breast cancer in the present study. In another study, the same author reported that metformin alone reduced the risk of cervical cancer in Taiwanese women with diabetes mellitus 17. Although many studies have examined the effect of diabetes mellitus on cancer risk, few have examined the effect of cancer on developing diabetes mellitus. Lipscombe et al.8 compared the data of 24,976 breast cancer survivors with those of 124,880 non‐cancer controls; the risk of diabetes mellitus in women with breast cancer compared with the risk in the controls began to increase at 2 years after diagnosis (HR 1.07, 95% CI 1.02–1.12), rising to HR 1.21 (95% CI 1.09–1.35) at 10 years. In women who received adjuvant chemotherapy, the risk was the highest during the first 2 years after diagnosis (HR 1.24, 95% CI 1.12–1.38), and subsequently declined over time. In contrast, a 2016 study using the Surveillance, Epidemiology and End Results database reported that aromatase inhibitors and tamoxifen do not increase diabetes mellitus risk in postmenopausal women with stage I–III breast cancer in the 2 years after initiation of treatment10.
As diabetes mellitus has a notable increasing trend among Asian individuals, especially among young and middle‐aged adults30, early detection or prevention of diabetes mellitus is essential, because the macro‐ and microvascular complications associated with diabetes mellitus will influence quality of life in these patients. Diabetes develops at a younger age in Asian populations, arising from early β‐cell dysfunction and insulin resistance, causing many patients to require insulin and increasing the likelihood of diabetic complications31. Asian women comprise the largest group of diabetes mellitus patients with diabetes‐related kidney disease30, so it becomes even more important to understand the association between breast cancer incidence and development of diabetes, as we have investigated in the present study. Although breast cancer incidence is surprisingly similar between Asian and Western women32, Asian women with breast cancer are younger in comparison with women in Western countries, and diabetes mellitus was also associated with a shorter survival time after a diagnosis of breast cancer in China and Korea33. In China, the age distribution for breast cancer included one age peak at 45–49 years, which was unlike the two age peaks shown in Chinese Americans, whose age of onset was remarkably younger, which might be due to environmental influence34. The results of the aforementioned studies further emphasize the need to screen for diabetes mellitus regularly among young and middle‐aged women with breast cancer, especially in Asian regions. Although more research is required to explain interethnic differences in Asian and Chinese Han women (as in the present study)31, urgent and concerted actions are required to raise awareness, facilitate early diagnosis and encourage preventive strategies to combat these growing disease burdens. The present study is the first to raise the important issue of close monitoring and early diagnosis in patients after new‐onset breast cancer diagnosis. This realization is not only epidemiologically important, but is also clinically relevant. In contrast, prior meta‐analysis already found that women with breast cancer and pre‐existing diabetes mellitus have an increased risk of mortality. Therefore, as the associations and outcomes between breast cancer and diabetes mellitus are recognized to be closely associated and clinically important, further study of breast cancer data in middle‐aged post‐menopausal women newly diagnosed with breast cancer is essential to fully understand the risk and consequences of diabetes mellitus in this population.
Although the present study included a large number of women with breast cancer and age‐matched controls, certain limitations must be considered when interpreting the results. As with all population‐based studies, cause‐and‐effect relationships cannot be determined in the present study. In addition, Lipscombe et al.8 raised the issue that adjuvant chemotherapy could have a significant impact on the incidence of diabetes, especially during the first 2 years after diagnosis. We still had no sufficient information about adjuvant chemotherapy in the present study after we reviewed the relevant database. The National Insurance database does not include lifestyle and family history, which precluded using it in the analysis. Such information might have been a useful addition in our evaluation, as changes in lifestyle and diet are shown to decrease breast cancer risk35, and high physical activity levels can also decrease the risk of breast and colon cancer, diabetes mellitus, ischemic heart disease, and ischemic stroke36. The duration of hormone therapy use was not investigated, nor were we able to analyze the relationships of specific hormone therapies, as the numbers of patients using each different therapy were too small and duration of treatment was too short to allow meaningful analysis. Similarly, we were not able to investigate specific chemotherapy regimens, including steroid containing medication. In addition, we did not study breast cancer subtypes; however, no associations have been shown between different breast cancer subtypes and diabetes mellitus in postmenopausal women9. Finally, the cohort in the present study comprised a majority of women of Chinese Han ancestry, and the results might not be generalizable to other ethnic groups. Clearly, as the relationship and outcomes between breast cancer and diabetes mellitus are closely related and clinically important12, additional study of breast cancer data is required to corroborate the results of the present study and to further evaluate the risk of diabetes mellitus in middle‐aged post‐menopausal women with long life expectancy.
In conclusion, postmenopausal women who develop breast cancer are at higher risk of developing diabetes mellitus than women in the same age group who do not have breast cancer. Women who have received hormonal therapy for breast cancer did not show a significantly increased risk for developing diabetes mellitus in comparison with those who have not received hormonal therapy. These results suggest that postmenopausal women with breast cancer should be closely monitored for the development of diabetes mellitus to establish an early diagnosis and therapeutic intervention for improving related outcomes.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
The study is based on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health, Taiwan, and managed by the National Health Research Institutes. The interpretation and reporting of these data are the sole responsibility of the authors.
J Diabetes Investig 2020; 11: 490–498
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–E386. [DOI] [PubMed] [Google Scholar]
- 2. Ho ML, Hsiao YH, Su SY, et al Mortality of breast cancer in Taiwan, 1971–2010: temporal changes and an age‐period‐cohort analysis. J Obstet Gynaecol 2015; 35: 60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berkemeyer S, Lemke D, Hense HW. Incidence and mortality trends in German women with breast cancer using age, period and cohort 1999 to 2008. PLoS One 2016; 11: e0150723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maruthur NM. The growing prevalence of type 2 diabetes: increased incidence or improved survival? Curr Diabetes Rep 2013; 13: 786–794. [DOI] [PubMed] [Google Scholar]
- 5. Allott EH, Hursting SD. Obesity and cancer: mechanistic insights from transdisciplinary studies. Endocr Relat Cancer 2015; 22: R365–R386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sonnenblick A, Agbor‐Tarh D, Bradbury I, et al Impact of diabetes, insulin, and metformin use on the outcome of patients with human epidermal growth factor receptor 2‐positive primary breast cancer: analysis from the ALTTO phase III randomized trial. J Clin Oncol 2017; 35: 1421–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dąbrowski M, Szymańska‐Garbacz E, Miszczyszyn Z, et al Risk factors for cancer development in type 2 diabetes: a retrospective case‐control study. BMC Cancer 2016; 16: 785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lipscombe LL, Chan WW, Yun L, et al Incidence of diabetes among postmenopausal breast cancer survivors. Diabetologia 2013; 56: 476–483. [DOI] [PubMed] [Google Scholar]
- 9. Bronsveld HK, Jensen V, Vahl P, et al Diabetes and breast cancer subtypes. PLoS One 2017; 12: e0170084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Santorelli ML, Hirshfield KM, Steinberg MB, et al Hormonal therapy for breast cancer and diabetes incidence among postmenopausal women. Ann Epidemiol 2016; 26: 436–440. [DOI] [PubMed] [Google Scholar]
- 11. Maskarinec G, Jacobs S, Park SY, et al Type 2 diabetes, obesity, and breast cancer risk: the multiethnic cohort. Cancer Epidemiol Biomarkers Prev 2017; 26: 854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peairs KS, Barone BB, Snyder CF, et al Diabetes mellitus and breast cancer outcomes: a systematic review and meta‐analysis. J Clin Oncol 2011; 29: 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao XB, Ren GS. Diabetes mellitus and prognosis in women with breast cancer: a systematic review and meta‐analysis. Medicine (Baltimore) 2016; 95: e5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tseng CH. Sitagliptin may reduce breast cancer risk in women with type 2 diabetes. Clin Breast Cancer 2017; 17: 211–218. [DOI] [PubMed] [Google Scholar]
- 15. Tseng CH. Rosiglitazone reduces breast cancer risk in Taiwanese female patients with type 2 diabetes mellitus. Oncotarget 2017; 8: 3042–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hatoum D, McGowan EM. Recent advances in the use of metformin: can treating diabetes prevent breast cancer? Biomed Res Int 2015; 2015: 548436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tseng CH. Metformin use and cervical cancer risk in female patients with type 2 diabetes. Oncotarget 2016; 7: 59548–59555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferroni P, Riondino S, Buonomo O, et al Type 2 diabetes and breast cancer: the interplay between impaired glucose metabolism and oxidant stress. Oxid Med Cell Longev 2015; 2015: 183928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rose DP, Gracheck PJ, Vona‐Davis L. The interactions of obesity, inflammation and insulin resistance in breast cancer. Cancers (Basel) 2015; 7: 2147–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheng SH, Chiang TL. The effect of universal health insurance on health care utilization in Taiwan. Results from a natural experiment. JAMA 1997; 278: 89–93. [DOI] [PubMed] [Google Scholar]
- 21. Chiang CL, Chen PC, Huang LY, et al Impact of universal health coverage on urban‐rural inequity in psychiatric service utilisation for patients with first admission for psychosis: a 10‐year nationwide population‐based study in Taiwan. BMJ Open 2016; 6: e010802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taiwan National Health Insurance Research Database. Available from: https://nhird.nhri.org.tw/en/ Accessed June 23, 2017.
- 23. Stanford JL, Hartge P, Brinton LA, et al Factors influencing the age at natural menopause. J Chronic Dis 1987; 40: 995–1002. [DOI] [PubMed] [Google Scholar]
- 24. Liu CY, Hung YT, Chuang YL, et al [Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey]. J Health Manage 2006; 4: 1–22. [Article in Chinese] [Google Scholar]
- 25. Chen WW, Shao YY, Shau WY, et al The impact of diabetes mellitus on prognosis of early breast cancer in Asia. Oncologist 2012; 17: 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu FC, Lin HT, Kuo CF, et al Epidemiology and survival outcome of breast cancer in a nationwide study. Oncotarget 2017; 8: 16939–16950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liao S, Li J, Wei W, et al Association between diabetes mellitus and breast cancer risk: a meta‐analysis of the literature. Asian Pac J Cancer Prev 2011; 12: 1061–1065. [PubMed] [Google Scholar]
- 28. Tsilidis KK, Kasimis JC, Lopez DS, et al Type 2 diabetes and cancer: umbrella review of meta‐analyses of observational studies. BMJ 2015; 350: g7607. [DOI] [PubMed] [Google Scholar]
- 29. Janghorbani M, Dehghani M, Salehi‐Marzijarani M. Systematic review and metaanalysis of insulin therapy and risk of cancer. Horm Cancer 2012; 3: 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chan JC, Malik V, Kadowaki T, et al Diabetes in Asia: epidemiology, risk factors and pathophysiology. JAMA 2009; 301: 2129–2140. [DOI] [PubMed] [Google Scholar]
- 31. Ma RCW, Chan JCN. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann NY Acad Sci 2013; 1281: 64–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sung H, Rosenberg PS, Chen WQ, et al Female breast cancer incidence among Asian and Western populations: more similar than expected. J Natl Cancer Inst 2015; 107: djv107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leong SP, Shen ZZ, Liu TJ, et al Is breast cancer the same disease in Asian and Western countries? World J Surg 2010; 34: 2308–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen C, Sun S, Yuan JP, et al Characteristics of breast cancer in Central China, literature review and comparison with USA. Breast 2016; 30: 208–213. [DOI] [PubMed] [Google Scholar]
- 35. Harvie M, Howell A, Evans DG. Can diet and lifestyle prevent breast cancer: what is the evidence? Am Soc Clin Oncol Educ Book 2015; e66–e73. [DOI] [PubMed] [Google Scholar]
- 36. Kyu HH, Bachman VF, Alexander LT, et al Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose‐response meta‐analysis for the Global Burden of Disease Study 2013. BMJ 2016; 354: i3857. [DOI] [PMC free article] [PubMed] [Google Scholar]


