Abstract
Recent studies have shown that sodium–glucose cotransporter 2 inhibitors decrease the risk of heart failure in patients with type 2 diabetes. However, the precise mechanisms of action of these drugs are not well understood. In the present study, we evaluated the effect of treatment with tofogliflozin for 6 months on cardiac and vascular endothelial function in 26 patients with type 2 diabetes and heart diseases. Tofogliflozin treatment significantly decreased left ventricular end‐diastolic dimensions and significantly increased flow‐mediated vasodilation. Although E/e′ did not significantly change after treatment, the decrease observed in the E/e′ ratio was significantly correlated with the increase in acetoacetic acid and 3‐hydroxybutyrate levels. These results suggest that sodium–glucose cotransporter 2 inhibitor might improve left ventricular dilatation and vascular endothelial function in patients with type 2 diabetes. Furthermore, it is suggested that the elevation of ketone bodies induced by sodium–glucose cotransporter 2 inhibitors might contribute to a protective effect in left ventricular diastolic dysfunction.
Keywords: Ketone body, Left ventricular diastolic function, Sodium–glucose cotransporter 2 inhibitor
In this study, we evaluated the effect of treatment with tofogliflozin for 6 months on cardiac and vascular endothelial function in 26 patients with type 2 diabetes and heart diseases. The results suggest that sodium–glucose cotransporter 2 inhibitor might improve left ventricular dilatation and vascular endothelial function in patients with type 2 diabetes. Furthermore, it is suggested that the elevation of ketone bodies induced by sodium–glucose cotransporter 2 inhibitors might contribute to a protective effect in left ventricular diastolic dysfunction.

Introduction
Heart failure (HF) is a common and serious comorbidity in patients with type 2 diabetes, and its prevention is crucial1. Recent findings from the large clinical trials showed a significant reduction in hospitalization for HF in patients receiving a sodium–glucose cotransporter 2 (SGLT2) inhibitor compared with those receiving a placebo2, 3. However, the mechanisms involved in the prevention of cardiovascular disease by SGLT2 inhibitors remain unclear, and the effects of SGLT2 inhibitors on cardiac function are not well understood. Furthermore, previous studies showed that diabetes is an independent risk factor for HF with preserved ejection fraction (HFpEF) due to left ventricular diastolic dysfunction4. Therefore, we aimed to evaluate the effects of tofogliflozin, an SGLT2 inhibitor, on cardiac function including left ventricular diastolic dysfunction and vascular endothelial function in patients with type 2 diabetes and heart disease.
Methods
Study design
This was a single‐center, single‐arm, intervention exploratory clinical study. A total of 30 outpatients with type 2 diabetes and a history of heart disease (ischemic heart disease, arrhythmia, valvular heart disease, cardiomyopathy and congenital heart disease) were enrolled. Key exclusion criteria were severe renal disease (estimated glomerular filtration rate <45 mL/min/1.73 m2), low body mass index (<18), past history of cerebral infarction, implantation of a cardiac pacemaker and dose of diuretics changed within the last 3 months. All participants provided written informed consent, and the study was approved by the local ethics committee and carried out in accordance with the Declaration of Helsinki. Eligible participants received 20 mg of tofogliflozin daily for 6 months. The primary outcome of the study was change in cardiac echo parameters and B‐type natriuretic peptide (BNP) after 6 months. The major secondary outcome was change in flow‐mediated vasodilatation (FMD).
Laboratory analysis
Blood samples were collected after 12‐h fasting. We measured the levels of A‐type natriuretic peptide (ANP), BNP, ketone bodies (acetoacetic acid [AcAc] and 3 hydroxybutyrate [3‐OHBA]), leptin, adiponectin and lipid profiles, before and after 6 months of treatment.
Cardiac echography and FMD of brachial artery
Echocardiography was carried out at baseline and after 6 months of treatment. We measured left ventricular end‐diastolic dimension, left ventricular diameter at end‐systole, ejection fraction, deceleration time, left atrial dimension and mitral E/e′ average ratio. Peak early diastolic tissue velocity (E′) was measured from the septal and lateral aspects of the mitral annulus. FMD was carried out using UNEXEF 38G (UNEX, Nagoya, Japan)5. All studies were carried out in the morning, after overnight fasting, in a quiet, dark, air‐conditioned room (constant temperature of 22–25°C). After resting for 15 min, the pressure cuff was placed on the forearm to capture baseline images of brachial artery using high‐resolution ultrasound. Then, the cuff was inflated and kept at 50 mmHg above the systolic blood pressure to occlude the brachial artery. The cuff was released 5 min later, and the image of brachial artery was captured continuously. The diameters of the brachial artery on the rest and the maximum diameter at post‐hyperemia were used to calculate percentage FMD (%FMD).
Statistical analysis
Changes in each of the measured parameters before and after 6 months of treatment were evaluated using the paired t‐test or Wilcoxon signed‐rank test. To evaluate the association among ketones, glycated hemoglobin (HbA1c) and change in hematocrit (Ht) and changes in cardiac parameters, a correlation analysis was carried out. All analyses were carried out using JMP software, version 10.0.0 (SAS Institute, Cary, NC, USA). All P‐values were two‐tailed, and P < 0.05 was considered to show statistical significance. All confidence intervals were calculated at the 95% level. Data were expressed as the mean ± standard deviation.
Results
After withdrawal of consent from one patient and administrative problems with three patients, a total of 26 patients participated in the study and received tofogliflozin for 6 months. The baseline characteristics for the 26 patients are shown in Table 1.
Table 1.
Baseline characteristics
| Age (years) | 62 ± 8 |
| Male (%) | 96.5 |
| Current smoker | 28.5 |
| Bodyweight (kg) | 76.3 ± 16.1 |
| BMI (kg/m2) | 27.8 ± 4.1 |
| Systolic blood pressure (mmHg) | 138 ± 26 |
| Diastolic blood pressure (mmHg) | 83 ± 15 |
| Past history | |
| Diabetes duration (years) | 12.7 ± 8.6 |
| Hypertension (%) | 89.6 |
| Retinopathy (%) | 30.2 |
| Medical history | |
| Insulin (%) | 31.0 |
| ACE/ARB (%) | 78.9 |
| β‐Blockers (%) | 75.9 |
| Diuretics (%) | 38.0 |
| Statins (%) | 82.8 |
| History of heart disease | |
| Ischemic heart disease (%) | 80.8 |
| PCI (%) | 50.0 |
| CABG (%) | 23.0 |
| Atrial fibration (%) | 15.4 |
| Cardiomyopathy (%) | 11.5 |
| Valvular heart disease (%) | 3.8 |
| Congenital heart disease (%) | 3.8 |
ACE, angiotensin‐converting enzyme; ARB, angiotensin‐receptor blocker; BMI, body mass index; CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention.
In the current study, left ventricular end‐diastolic dimension significantly decreased from 52.3 ± 7.2 mm to 50.5 ± 7.7 mm (P = 0.011). BNP level, ANP level and left ventricular diameter at end‐systole also decreased; however, the decrease was not statistically significant. Ejection fraction and E/e′ were not significantly changed by tofoglifrozin treatment. After 6 months, %FMD significantly increased (Table 2).
Table 2.
Changes in laboratory and cardiac ultrasound parameters
| Baseline | After 6 months | P‐value | |
|---|---|---|---|
| Bodyweight (kg) | 76.3 ± 16.1 | 72.9 ± 13.6 | 0.0005* |
| BMI | 27.8 ± 4.1 | 26.6 ± 3.5 | 0.0003* |
| SBP (mmHg) | 138 ± 26 | 137 ± 24 | 0.9317 |
| DBP (mmHg) | 83 ± 15 | 78 ± 12 | 0.0957 |
| FPG (mg/dL) | 158 ± 53 | 136 ± 26 | 0.055 |
| HbA1c (%) | 8.1 ± 1.1 | 7.2 ± 0.8 | <0.0001* |
| IRI (IU/mL) | 14.8 ± 13.1 | 10.8 ± 9.0 | 0.090 |
| Hemoglobin (g/dL) | 14.7 ± 0.9 | 15.5 ± 1.6 | 0.004* |
| Hematocrit (%) | 44.3 ± 2.7 | 47.2 ± 4.4 | 0.001* |
| AST (IU/L) | 26.5 ± 10.8 | 22.4 ± 7.6 | 0.046* |
| ALT (IU/L) | 28.9 ± 15.3 | 21.9 ± 9.4 | 0.005* |
| ɣGTP (IU/L) | 52.0 ± 29.4 | 40.2 ± 29.4 | 0.013* |
| eGFR (mL/min/1.73 m2) | 67.9 ± 17.7 | 66.9 ± 17.8 | 0.574 |
| Na (mEq/L) | 140.0 ± 1.9 | 139.5 ± 2.2 | 0.065 |
| K (mEq/L) | 4.3 ± 0.4 | 4.4 ± 0.5 | 0.360 |
| Ca (mg/dL) | 9.4 ± 0.4 | 9.4 ± 0.3 | 0.474 |
| P (mg/dL) | 3.3 ± 0.4 | 3.4 ± 0.5 | 0.519 |
| UA (mg/dL) | 5.8 ± 0.8 | 5.2 ± 1.0 | 0.003* |
| Triglyceride (mg/dL) | 222 ± 153 | 176 ± 127 | 0.062 |
| HDL‐C (mg/dL) | 41 ± 8 | 47 ± 10 | 0.0003* |
| LDL‐C (mg/dL) | 94 ± 31 | 92 ± 25 | 0.650 |
| MDA‐LDL (mg/dL) | 137.1 ± 61.4 | 132.2 ± 52.8 | 0.622 |
| AcAc (mmol/L) | 45.3 ± 29.5 | 58.2 ± 56.0 | 0.215 |
| 3‐OHBA (mmol/L) | 87.1 ± 87.1 | 107.3 ± 81.3 | 0.365 |
| Adiponectin (mg/mL) | 7.6 ± 8.8 | 8.2 ± 6.3 | 0.740 |
| Leptin (mg/dL) | 11.4 ± 7.2 | 10.8 ± 4.5 | 0.671 |
| Leptin/adiponectin ratio | 2.48 ± 1.87 | 1.92 ± 1.44 | 0.047 |
| ANP (pg/mL) | 58.9 ± 50.7 | 45.7 ± 41.6 | 0.324 |
| BNP (pg/mL) | 56.9 ± 65.4 | 50.4 ± 54.4 | 0.384 |
| Urinary albumin excretion (mg/gCr) | 168.8 ± 279.6 | 322.7 ± 760.4 | 0.139 |
| Urinary β2 microglobulin excretion (mg/gCr) | 3.3 ± 6.2 | 7.3 ± 10.6 | 0.081 |
| Flow‐mediated vasodilatation (%) | 5.1 ± 1.8 | 6.8 ± 2.0 | 0.019* |
| Ultrasound cardiography | |||
| LVDd (mm) | 52.3 ± 7.2 | 50.6 ± 7.7 | 0.015* |
| LVDs (mm) | 37.8 ± 8.9 | 36.4 ± 9.5 | 0.183 |
| LAD (mm) | 40.6 ± 5.3 | 40.3 ± 4.6 | 0.706 |
| Ejection fraction (%) | 51.6 ± 13.2 | 52.2 ± 12.9 | 0.519 |
| E/e′ | 10.0 ± 2.8 | 9.9 ± 3.1 | 0.601 |
| DcT (s) | 211 ± 46 | 206 ± 56 | 0.594 |
Statistically significant. 3‐OHBA, 3 hydroxybutyrate; ɣGTP, gamma glutamyl transpeptitase; AcAc, acetoacetic acid; ALT, alanine aminotransferase; ANP, A‐type natriuretic peptide; AST, aspartate aminotransferase; BMI, body mass index; BNP, B‐type natriuretic peptide; Ca, calcium; DBP, diastolic blood pressure; DcT, deceleration time; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; IRI, immunoreactive insulin; LAD, left atrial dimension; LDL‐C, low‐density lipoprotein cholesterol; LVDd, left ventricular end‐diastolic dimension; LVDs, left ventricular diameter at end‐systole; MDA‐LDL, malondialdehyde‐modified low‐density lipoprotein; SBP, systolic blood pressure; UA, uric acid.
There was a positive correlation between the rate of change of E/e′, a marker of diastolic function6, and the rate of change of acetoacetic acid and 3‐OHBA (Figure 1). Furthermore, in patients with elevated 3‐OHBA, E/e′ significantly decreased after 6 months (baseline 10.51 ± 2.24 vs after 6 months 9.00 ± 3.00, P = 0.006, n = 13), whereas E/e′ did not change in patients without elevated 3‐OHBA (baseline 9.54 ± 3.35 vs after 6 months 10.75 ± 2.97, n = 13). Rate of change of deceleration time, which is also a marker of diastolic function, was also associated with the rate of change of 3‐OHBA. Conversely, the rate of change of HbA1c and Ht were not associated with any cardiac echo parameters (Table 3). There was no significant association between the rate change of %FMD and E/e′ (r = −0.043, P = 0.8391).
Figure 1.
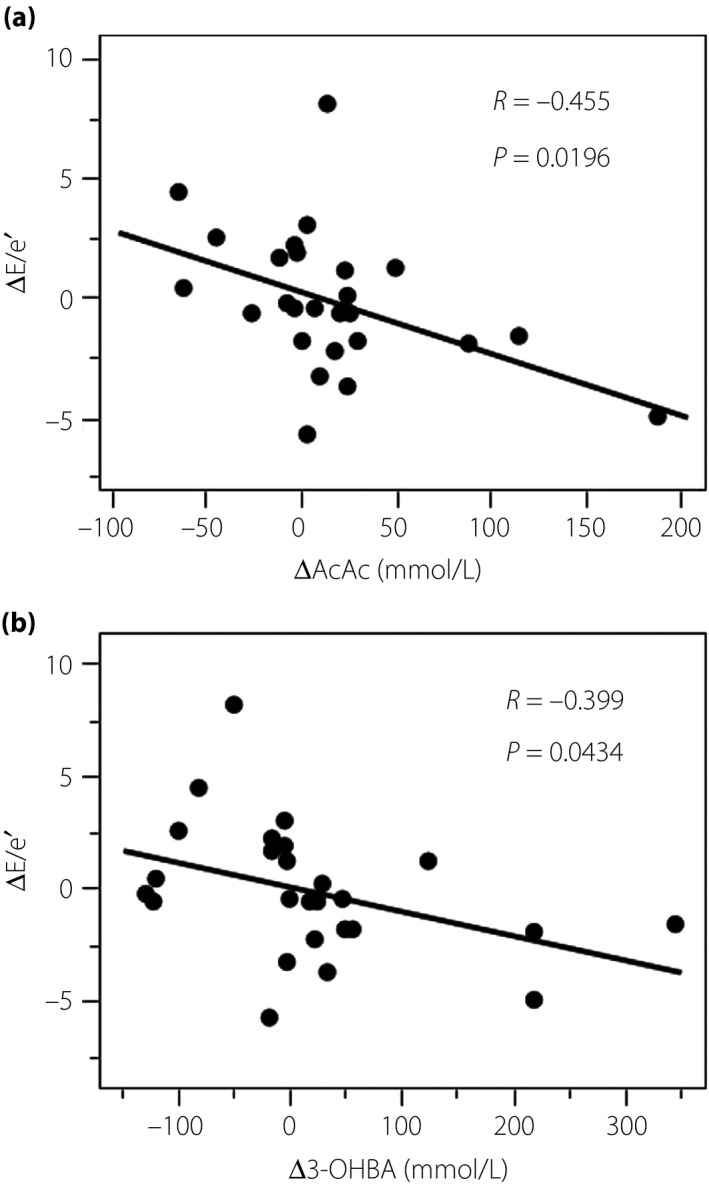
Correlation between change in E/e′ (ΔE/e′) and (a) change in acetoacetic acid (ΔAcAc) and (b) change in 3‐hydroxybutyrate (Δ3‐OHBA).
Table 3.
Correlation between change in glycated hemoglobin, ketone bodies and hematocrit, and change in cardiac parameters
| ΔHbA1c | ΔAcAc | Δ3OHBA | ΔHt | |||||
|---|---|---|---|---|---|---|---|---|
| r | P‐value | r | P‐value | r | P‐value | r | P‐value | |
| ΔLVDd | 0.285 | 0.158 | 0.014 | 0.9445 | 0.051 | 0.8023 | −0.041 | 0.8441 |
| ΔLVDs | 0.234 | 0.2493 | 0.177 | 0.3866 | 0.291 | 0.1488 | −0.338 | 0.0913 |
| ΔEF | 0.164 | 0.4222 | 0.375 | 0.059 | 0.224 | 0.2704 | −0.325 | 0.1048 |
| ΔE/e′ | 0.031 | 0.8828 | −0.455 | 0.0196 | −0.399 | 0.0434 | 0.022 | 0.9159 |
| ΔLAD | −0.412 | 0.0397 | 0.114 | 0.5885 | 0.364 | 0.074 | −0.2724 | 0.1877 |
| ΔDcT | −0.060 | 0.7751 | 0.352 | 0.0848 | 0.617 | 0.001 | −0.221 | 0.2888 |
| ΔANP | 0.057 | 0.7878 | 0.148 | 0.4792 | 0.193 | 0.3545 | −0.235 | 0.2588 |
| ΔBNP | −0.2 | 0.3373 | 0.125 | 0.5514 | 0.298 | 0.1482 | −0.266 | 0.1996 |
| Δ%FMD | 0.237 | 0.2523 | −0.124 | 0.4982 | −0.026 | 0.9013 | −0.207 | 0.3215 |
ΔAcAc, acetoacetic acid; ΔANP, change in A‐type natriuretic peptide; ΔBNP, change in B‐type natriuretic peptide; ΔDcT, change in deceleration time; ΔE/e′, change in E/e′; ΔEF, change in ejection fraction; Δ%FMD, change in percentage of flow‐mediated vasodilatation; ΔLAD, change in left atrial dimension; ΔLVDd, change in left ventricular end‐diastolic dimension; ΔLVDs, change in left ventricular diameter at end‐systole.
None of the patients withdrew as a result of to serious adverse effects, such as urinary tract or genital infection, ketoacidosis, ischemic stroke, or hypovolemia, and none had a severe hypoglycemic attack.
Discussion
In the current study, we showed that treatment for 6 months with tofogliflozin significantly decreased left ventricular end‐diastolic dimension, suggesting that SGLT2 inhibitors might ameliorate cardiac load, particularly volume load. SGLT2 inhibitors reduce the systemic volume load, as they stimulate osmotic diuresis and sodium excretion.
A recent report showed that patients with E/e′ of diabetes and HF were improved by SGLT2 inhibitor7. However, the mechanism is still unclear. In the present study, decrease in E/e′ was associated with an increase in ketone bodies. To the best of our knowledge, this is the first report showing an association between ketone body increase by SGLT2 inhibitor and left ventricular diastolic function improvement. A previous report suggested that in hyperketonemia induced by SGLT2 inhibitors, the use of β‐hydroxybutyrate as an energy source by the myocardium might be associated with a reduction in cardiovascular events8. Indeed, it was reported using a mouse model that the failing heart shifts to use ketone bodies as a significant fuel source9. Another experimental study showed that a lack of ketone body metabolism in cardiomyocytes promoted cardiac pathological remodeling10. Taken together, it is suggested that tofogliflozin could have preventive effects on HFpEF through elevation of ketone body levels. Therefore, a future clinical study in which the primary outcome is the incidence of HFpEF is required to confirm these findings.
Endothelial dysfunction was recently shown to play a key role in the pathogenesis of HFpEF. Indeed, a clinical observational study showed that decreased %FMD is associated with the incidence of HFpEF11. In the present study, %FMD was significantly improved by tofogliflozin treatment in patients with average HbA1c levels of >8.0%. Kumashiro et al. also reported that other SGLT2 inhibitors restored endothelial dysfunction, particularly in patients with diabetes who had high HbA1c levels5. Therefore, at least in patients with diabetes with poor glycemic control, SGLT2 inhibitors could improve endothelial function, which might be one of mechanisms used to prevent HF. However, change of %FMD was not associated with change of E/e′ in the present study. It is possible that for the evaluation of endothelial function of small vessels, for example, the peripheral arterial tonometry ratio12 might be more suitable, as coronary microcirculation impairment participates in left ventricular diastolic dysfunction13.
Analysis of the Empagliflozin, Cardiovascular Outcomes and Mortality in Type2 diabetes trial showed that elevation of hemoglobin and Ht strongly contributed to reduced cardiovascular death14. A recent study showed that improved left ventricular diastolic function by canagliflozin is associated with elevation of Ht in patients with diabetes without cardiovascular diseases15. However, in the present study, a change in E/e′ was not associated with a change of Ht in patients with diabetes and cardiovascular diseases. The present study had a small sample size; therefore, it might not be possible to find an association between Ht and left ventricular dysfunction. It is also possible that the mechanisms involved in the preventive effects of SGLT2 inhibitors on left ventricular diastolic function might differ according to the stage of cardiac injury.
This study had several limitations. This was a small, single‐center, single‐arm study carried out over a relatively short period of time. Therefore, it was unable to show the precise mechanisms involved in the cardiac protective effects of SGLT2 inhibitors.
In conclusion, the present study showed that tofogliflozin treatment decreased left ventricular volume and restored vascular endothelial function. Furthermore, elevation of 3‐OHBG and acetoacetic acid was associated with a decrease in E/e′, suggesting that elevation of ketone bodies by tofogliflozin might contribute to improved left ventricular diastolic function.
Disclosure
The authors declare no conflict of interest.
Acknowledgment
This study was funded by the Japanese Circulation Foundation.
J Diabetes Investig 2020; 11: 400–404
Clinical Trial Registry
University Hospital Medical Information Network
UMIN000018344
References
- 1. Rosengren A, Edqvist J, Rawshani A, et al Excess risk of hospitalisation for heart failure among people with type 2 diabetes. Diabetologia 2018; 61: 2300–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zinman B, Wanner C, Lachin JM, et al Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 3. Neal B, Perkovic V, Mahaffey KW, et al Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 4. Lindman BR, Davila‐Roman VG, Mann DL, et al Cardiovascular phenotype in HFpEF patients with or without diabetes: a RELAX trial ancillary study. J Am Coll Cardiol 2014; 64: 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shigiyama F, Kumashiro N, Miyagi M, et al Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early‐stage type 2 diabetes mellitus: DEFENCE study. Cardiovasc Diabetol 2017; 16: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nagueh SF, Smiseth OA, Appleton CP, et al Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016; 17: 1321–1360. [DOI] [PubMed] [Google Scholar]
- 7. Soga F, Tanaka H, Tatsumi K, et al Impact of dapagliflozin on left ventricular diastolic function of patients with type 2 diabetic mellitus with chronic heart failure. Cardiovasc Diabetol 2018; 17: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferrannini E, Mark M, Mayoux E. CV Protection in the EMPA‐REG OUTCOME Trial: a “Thrifty Substrate” Hypothesis. Diabetes Care 2016; 39: 1108–1114. [DOI] [PubMed] [Google Scholar]
- 9. Aubert G, Martin OJ, Horton JL, et al The failing heart relies on ketone bodies as a fuel. Circulation 2016; 133: 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schugar RC, Moll AR, André d'Avignon D, et al Cardiomyocyte‐specific deficiency of ketone body metabolism promotes accelerated pathological remodeling. Mol Metab 2014; 13: 754–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kishimoto S, Kajikawa M, Maruhashi T, et al Endothelial dysfunction and abnormal vascular structure are simultaneously present in patients with heart failure with preserved ejection fraction. Int J Cardiol 2017; 231: 181–187. [DOI] [PubMed] [Google Scholar]
- 12. Hamburg NM, Palmisano J, Larson MG, et al Relation of brachial and digital measures of vascular function in the community: the Framingham heart study. Hypertension 2011; 57: 390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dryer K, Gajjar M, Narang N, et al Coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol 2018; 314: H1033–H1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Inzucchi SE, Zinman B, Fitchett D, et al How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA‐REG OUTCOME trial. Diabetes Care 2018; 41: 356–363. [DOI] [PubMed] [Google Scholar]
- 15. Matsutani D, Sakamoto M, Kayama Y, et al Effect of canagliflozin on left ventricular diastolic function in patients with type 2 diabetes. Cardiovasc Diabetol 2018; 17: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]


