Abstract
Aims/Introduction
A higher ratio of triglyceride to high‐density lipoprotein cholesterol (TG/HDL‐C) is considered as the independent risk index of cardiovascular (CV) events. However, cohort studies regarding this correlation are rarely reported, especially in the Chinese population. The aim of present study was to explore the relationship of the TG/HDL‐C ratio with CV risks among Chinese adults during 10‐year follow‐up period.
Materials and Methods
We carried out a prospective study using data obtained from 96,542 individuals in Kailuan, who were grouped through the median value (0.8533) of the TG/HDL‐C ratio. Adverse outcomes mainly referred to major CV events. We used the person‐years incidence and cumulative incidence to predict the morbidity. The risk of CV events was estimated through Cox proportional hazard models.
Results
The mean age of the cohort was 51.5 ± 12.6 years, and 79.6% of participants were men. During a median follow‐up period of 9.75 years, 5,422 major CV events occurred, including 1,312 myocardial infarction cases and 4,228 stroke cases. The cumulative incidence of myocardial infarction, stroke and total CV events was 1.36% (range 1.29–1.43%), 4.38% (range 4.25–4.51%) and 5.62% (range 5.47–5.76%), respectively. Compared with low the TG/HDL‐C ratio (≤0.8533) group, the high TG/HDL‐C ratio (>0.8533) group had higher morbidity of CV events. The hazard ratio of total CV events, stroke and myocardial infarction was 1.19 (95% CI 1.12–1.26), 1.11 (95% CI 1.03–1.18) and 1.50 (95% CI 1.33–1.70), respectively. Furthermore, the TG/HDL‐C ratio and major CV events had a line‐shaped relationship with each other.
Conclusions
Among the Chinese population, a higher TG/HDL‐C ratio is correlated with an increased risk of major CV events.
Keywords: Cardiovascular risks, Chinese, Triglyceride to high‐density lipoprotein cholesterol ratio
This is the first large prospective cohort study regarding the association of the triglyceride to high‐density lipoprotein cholesterol (TG/HDL‐C) ratio with major cardiovascular events among Chinese individuals. Among the Chinese population, an elevated TG/HDL‐C ratio is associated with an increased risk of major cardiovascular events.
Introduction
Despite the advanced diagnosis and treatment strategies, cardiovascular (CV) events are still the main causes of death worldwide. Dyslipidemia is seen as a well‐documented risk indicator for CV disease (CVD)1, which can be reflected by decreased high‐density lipoprotein cholesterol (HDL‐C) content, increased triglycerides (TG) and the predominance of small dense low‐density lipoprotein (sd‐LDL) particles2, 3. Recently, a well‐defined atherogenic dyslipidemia parameter, namely, the TG/HDL‐C ratio, has been thought to be correlated with CV events4, 5, 6. Compared with other individual lipid profiles, the TG/HDL‐C ratio can be a better predictive indicator for insulin resistance (IR)7, 8, 9, obesity10 and coronary heart disease11 by reflecting the complex interactions of lipoprotein metabolism12. Although some studies have shown the relationship between the TG/HDL‐C ratio and CV events, cohort studies regarding this correlation have been rarely reported, especially in the Chinese population.
In the present study, the correlation of the TG/HDL‐C ratio with major CV event risks, including stroke and myocardial infarction (MI), was prospectively examined in a cohort of Chinese adults from the Kailuan population during a 10‐year follow‐up period.
Methods
Population studied
The prospective cohort study was carried out in the Kailuan community, and the comprehensive and functional community was governed through Kailuan Corporation in Tangshan City (Hebei, China). From 2006.6 to 2007.10, Kailuan Corporation organized the physical examination for 101,510 working and retired employees in Kailuan General Hospital and its 10 affiliated hospitals. The detailed information about the characteristics and study design of the Kailuan study population has been described in the previous study13. The medical data were recorded by trained medical personnel and stored in the research database (Oracle 10.2g) that was hosted in the servers at Kailuan General Hospital.
The inclusion criteria for participants were as follows: (i) aged ≥18 years; and (ii) willing to join in the research and sign an informed consent. Among these participants, we excluded those who had incomplete medical data (n = 1,281), who had a history of any malignant cancer (n = 377) or who had a history of stroke and MI (n = 3,310). Finally, a total of 96,542 participants (76,854 ,men, 79.6%) were covered in the research. The research adhered to the Declaration of Helsinki and was approved by the ethics committee of Kailuan General Hospital. All of the participants agreed to sign written informed consent.
Data acquisition
The detailed data of the epidemiological survey and anthropometric measurements were previously published14 The researchers would deliver the questionnaires to collect the participants’ sociodemographic data (e.g., sex, education and age), personal and family medical history (e.g., diabetes, hypertension, CVs) and living habits (e.g., physical activity, smoking status and alcohol status). Smoking status was defined as: daily, occasionally, former and never. Alcohol consumption was defined as a daily consumption of at least 100 mL of moderate pure alcohol (≥50%) per day in recent years. Physical activity was defined as: very active (≥80 min/week), moderately active (1–79 min/week) and inactive (0 min/week) according to the frequency of physical activity in the questionnaires.
After an overnight fast, the blood samples were taken from participants’ antecubital vein and stored in ethylenediaminetetraacetic acid tubes at 07.00–09.00 hours. A Hitachi 7600 auto‐analyzer (Hitachi, Tokyo, Japan) was used to test the biochemical indicators; for instance, fasting plasma glucose (FPG), high‐sensitivity C‐reactive protein, total cholesterol (TC), TG, low‐density lipoprotein cholesterol (LDL‐C) and high‐density lipoprotein cholesterol (HDL‐C). FPG was measured through hexokinase method (BioSino Bio‐Technology & Science Inc., Beijing, China). TG, TC, HDL‐C and LDL‐C content were measured through an enzymatic method (Mind Bioengineering Co. Ltd, Shanghai, China; interassay coefficient of variation <10%). All of the biochemical indicators were measured and analyzed in our central laboratory at Kailuan General Hospital.
After completing the physical examination in 2006–2007, the participants were followed until 31 December 2016. The end‐point events were the first occurrences of the defined adverse outcomes during the follow‐up period.
Adverse outcomes
Adverse outcomes referred to stroke (e.g., intracerebral hemorrhage, subarachnoid hemorrhage and ischemic stroke), non‐fatal MI and so on. The first occurrences of those major CV events were our study outcomes, which should conform to the diagnosis criteria of the World Health Organization15, 16. Total CV events were expressed as the sum of the first occurrences of major CV events. The professionally trained doctors would collect and record the study outcomes every 6 months. The specific data of major CV events were gathered from personal interviews every 2 years, and confirmed through the medical records and claims data from medical insurance. The relevant data were collected from Kailuan General Hospital and its 10 affiliated hospitals.
Then, the collected information would be further validated by the Arbitration Committee for Clinical Outcomes and Data Safety Monitoring Board.
Statistical analysis
The participants were assigned to two groups according to the median value (0.8533) of the TG/HDL‐C ratio: the high TG/HDL‐C ratio (>0.8533) group and low TG/HDL‐C ratio (≤0.8533) group. The baseline characteristics were described and compared between groups. Continuous variables were expressed as the median (quartile) for abnormal distribution and expressed as the mean ± standard deviation for normal distribution. Continuous variables between groups were compared through non‐parametric tests and one‐way anova for abnormal and normal distribution data, respectively. Categorical variables were presented as the number (percentage), and then compared through the χ2‐test.
The adverse event rate was presented by person‐years incidence, as well as cumulative incidence. The cumulative incidence between groups was compared by the log–rank test. In order to explain the relationship between the TG/HDL‐C ratio and major CV events, multivariate Cox proportional hazards analysis was applicable for predefined outcomes. Furthermore, natural cubic spline functions were able to evaluate the correlation of the TG/HDL‐C ratio with CV events. There was statistical significance only when P < 0.05 (two‐tailed). Statistical analysis was made by SPSS System version 21.0 (SPSS Inc., Chicago, IL, USA) and open source statistical software package R (version 3.20).
Results
Baseline characteristics
On exclusion, a total of 96,542 participants (95.11% of the original cohort) were eventually included for the cohort study. The mean age (standard deviation) was 51.5 ± 12.6 years, including 76,854 men (79.6%). Table 1 shows the clinical data of the whole study population and different groups divided through median value (0.8533) of the TG/HDL‐C ratio. Compared with the low TG/HDL‐C ratio group, the high TG/HDL‐C ratio group had a dramatically lower educational degree and HDL‐C levels. Additionally, the values for body mass index, heart rate, blood pressure, TG, FPG, TC, LDL‐C and high‐sensitivity C‐reactive protein, and the percentage of hypertension, diabetes, current smokers, current drinkers, antidiabetic drug therapy and antihypertensive drug therapy in the high TG/HDL‐C ratio group were statistically higher than those of the low ratio group (P < 0.05; Table 1).
Table 1.
Baseline characteristics of 96,542 participants of the Kailuan Study
| Characteristics | All participants | TG/HDL‐C ratio | P‐value | |
|---|---|---|---|---|
| Low ≤0.8533 | High >0.8533 | |||
| n (%) | 96,542 | 48,567 (50.3) | 47,975 (49.7) | – |
| Age (years) | 51.5 ± 12.6 | 51.4 ± 13.1 | 51.6 ± 12.0 | 0.154 |
| Male, n (%) | 76,854 (79.6) | 37,140 (76.5) | 39,714 (82.8) | <0.001 |
| Education ≥high school, n (%) | 18,803 (19.5) | 9,650 (19.9) | 9,153 (19.1) | 0.002 |
| Heart rate (b.p.m.) | 73.8 ± 10.2 | 73.2 ± 10.1 | 74.4 ± 10.2 | <0.001 |
| BMI (kg/m2) | 25.0 ± 3.5 | 24.1 ± 3.3 | 26.0 ± 3.4 | <0.001 |
| SBP (mmHg) | 130.7 ± 20.9 | 128.3 ± 20.8 | 133.1 ± 20.7 | <0.001 |
| DBP (mmHg) | 83.4 ± 11.8 | 81.7 ± 11.5 | 85.1 ± 11.8 | <0.001 |
| FPG (mmol/L) | 5.47 ± 1.67 | 5.27 ± 1.40 | 5.67 ± 1.87 | <0.001 |
| HDL‐C (mmol/L) | 1.55 ± 0.40 | 1.68 ± 0.41 | 1.41 ± 0.35 | <0.001 |
| LDL‐C (mmol/L) | 2.34 ± 0.91 | 2.31 ± 0.90 | 2.38 ± 0.93 | <0.001 |
| TC (mmol/L) | 4.94 ± 1.14 | 4.90 ± 1.00 | 4.99 ± 1.27 | <0.001 |
| TG (mmol/L) | 1.27 (0.89–1.92) | 0.90 (0.70–1.12) | 1.92 (1.48–2.78) | <0.001 |
| hs‐CRP (mmol/L) | 0.80 (0.30–2.18) | 0.70 (0.25–1.94) | 0.94 (0.37–2.40) | <0.001 |
| Current drinker, n (%) | 16,804 (17.4) | 8,197 (16.9) | 8607 (17.9) | 0.005 |
| Current smoker, n (%) | 32,164 (33.3) | 15,060 (31.0) | 17,104 (35.7) | <0.001 |
| Physical activity, n (%) | 14,037 (14.5) | 6,994 (14.4) | 7,043 (14.7) | 0.218 |
| Hypertension, n (%) | 41,790 (43.3) | 18,224 (37.5) | 23,566 (49.1) | <0.001 |
| Diabetes, n (%) | 8,728 (9.0) | 2,950 (6.1) | 5,778 (12.0) | <0.001 |
| Antidiabetic drug therapy, n (%) | 2,066 (2.14) | 755 (1.55) | 1,311 (2.73) | <0.001 |
| Antihypertensive drug therapy, n (%) | 8,753 (9.07) | 3,270 (6.73) | 5,483 (11.43) | <0.001 |
Continuous variables were described by mean ± standard deviation (normal distribution)/median (quartile) (abnormal distribution); categorical variables were presented by number (percentage). The ranges in parentheses represence median (quartile). BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL‐C, high‐density lipoprotein cholesterol; hs‐CRP, high sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride.
Incidence rate of adverse outcomes
Table 2 shows the incidence rate of predefined outcomes classified through the TG/HDL‐C ratio. During the mean follow‐up period of 9.75 years, 5,422 major CV events were identified, namely, 1,312 cases of MI and 4,228 cases of stroke. The incidence rate of total CV events, stroke and MI was 576.02, 445.97 and 136.45 per 100,000 person‐years, respectively. As shown in Table 2, a high TG/HDL‐C ratio showed a higher cumulative incidence by comparison with those with a low TG/HDL‐C ratio. The cumulative incidence of total CV events, stroke and MI in the high TG/HDL‐C ratio group was 3.23 (range 3.12–3.34), 2.46 (range 2.36–2.55) and 0.85 (range 0.79–0.91), respectively (Table 2).
Table 2.
Incidence rate of adverse outcomes stratified by the triglyceride to high‐density lipoprotein cholesterol ratio
| Group | n | No. CV events | Cumulative incidence (95% CI) | Per 100,000 person‐years |
|---|---|---|---|---|
| Total CV events | ||||
| Total | 96,542 | 5,422 | 5.62 (5.47–5.76) | 576.02 |
| Low TG/HDL‐C ratio group | 48,567 | 2,307 | 2.39 (2.29–2.49) | 485.20 |
| High TG/HDL‐C ratio group | 47,975 | 3,115 | 3.23 (3.12–3.34) | 668.00 |
| P‐value for log–rank test | – | – | <0.001 | – |
| Stroke | ||||
| Total | 96,542 | 4,228 | 4.38 (4.25–4.51) | 445.97 |
| Low TG/HDL‐C ratio group | 48,567 | 1,856 | 1.92 (1.84–2.01) | 388.76 |
| High TG/HDL‐C ratio group | 47,975 | 2,372 | 2.46 (2.36–2.55) | 504.51 |
| P‐value for log–rank test | – | – | <0.001 | – |
| MI | ||||
| Total | 96,542 | 1,312 | 1.36 (1.29–1.43) | 136.45 |
| Low TG/HDL‐C ratio group | 48,567 | 493 | 0.51 (0.47–0.56) | 101.92 |
| High TG/HDL‐C ratio group | 47,975 | 819 | 0.85 (0.79–0.91) | 171.57 |
| P‐value for log–rank test | – | – | <0.001 | – |
CI, confidence interval; CV, cardiovascular; MI, myocardial infarction; TG/HDL‐C, triglyceride to high‐density lipoprotein cholesterol.
Correlation of the TG/HDL‐C ratio with CV events
Table 3 shows the correlation of the TG/HDL‐C ratio with CV events through the Cox proportional hazard regression model. According to unadjusted Cox proportional hazard regression analysis, an elevated TG/HDL‐C ratio had a correlation with elevated risks of MI, stroke and total CV events (model 1). In model 2, the participants’ sex and age were further adjusted; compared with the participants with a low TG/HDL‐C ratio, those with a high TG/HDL‐C ratio had an obvious correlation with higher major CV risks, whose hazard ratio was 1.73 (95% confidence interval [CI] 1.55–1.94), 1.32 (95% CI 1.24–1.41) and 1.41 (95% CI 1.33–1.48), respectively. Multivariate factors, such as smoking status, education, hypertension and diabetes, were further adjusted in model 3; then the hazard ratio of MI, stroke and total CV events was 1.19 (95% CI 1.12–1.26), 1.11 (95% CI 1.03–1.18) and 1.50 (95% CI 1.33–1.70), respectively.
Table 3.
Hazard ratios of major cardiovascular events
| Group | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Total CV events | |||
| Low TG/HDL‐C ratio group | 1 | 1 | 1 |
| High TG/HDL‐C ratio group | 1.37 (1.30–1.45) | 1.41 (1.33–1.48) | 1.19 (1.12–1.26) |
| Stroke | |||
| Low TG/HDL‐C ratio group | 1 | 1 | 1 |
| High TG/HDL‐C ratio group | 1.30 (1.22–1.38) | 1.32 (1.24–1.41) | 1.11 (1.03–1.18) |
| MI | |||
| Low TG/HDL‐C ratio group | 1 | 1 | 1 |
| High TG/HDL‐C ratio group | 1.68 (1.51–1.88) | 1.73 (1.55–1.94) | 1.50 (1.33–1.70) |
Model 1 was stratified for the triglyceride to high‐density lipoprotein cholesterol (TG‐HDL‐C) ratio; model 2 was further adjusted for age and sex; model 3 further adjusted for smoking status, alcohol consumption, education, physical exercise, diabetes, hypertension, total cholesterol, low‐density lipoprotein cholesterol, heart rate, high sensitivity C‐reactive protein, body mass index, antidiabetic drug therapy, antihypertensive drug therapy. The values in parentheses represented by HR (95% CI). CV, cardiovascular; MI, myocardial infarction.
After adjusting the multiple covariates, natural cubic spline analysis was used to verify the line‐shaped relationship between the TG/HDL‐C ratio and adverse reactions. Furthermore, the TG/HDL‐C ratio showed a line‐shaped association with major CV events, while the hazard ratio was increased by a higher TG/HDL‐C ratio (Figures 1, 2, 3).
Figure 1.
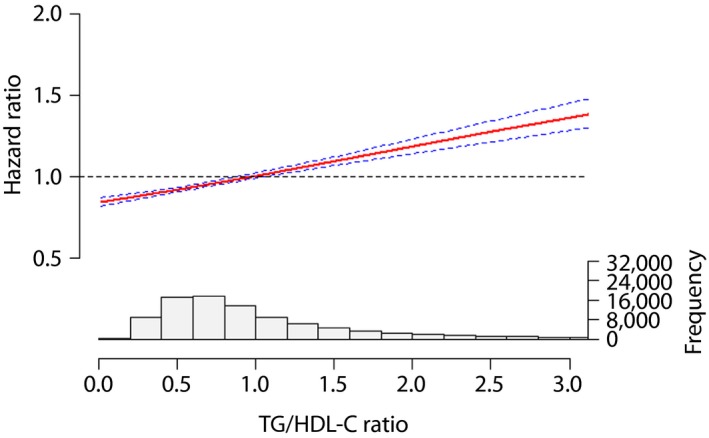
The association between the triglyceride to high‐density lipoprotein cholesterol (TG/HDL‐C) ratio and total cardiovascular events.
Figure 2.
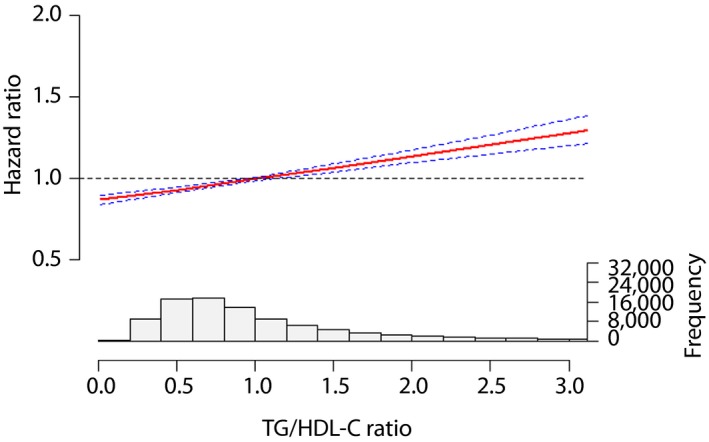
The association between the triglyceride to high‐density lipoprotein cholesterol (TG/HDL‐C) ratio and stroke.
Figure 3.
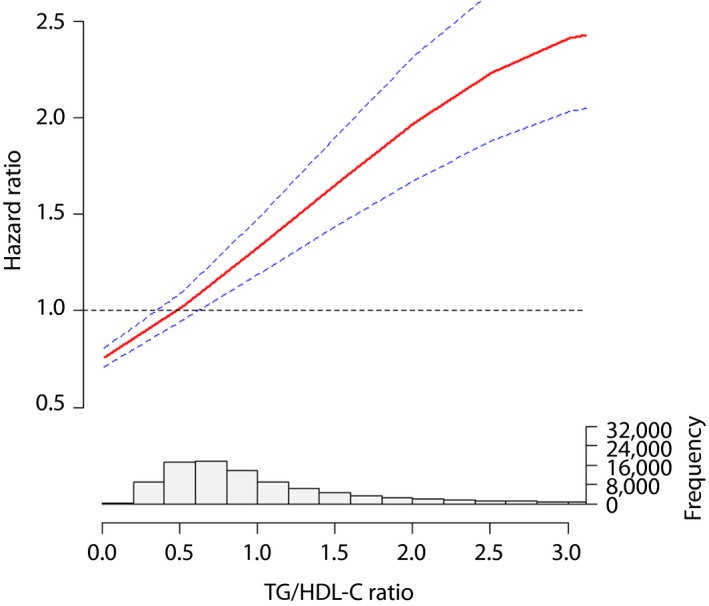
The association between the triglyceride to high‐density lipoprotein cholesterol (TG/HDL‐C) ratio and total myocardial infarction.
Discussion
As far as we know, this is the first prospective community‐based cohort study to be carried out to explain the correlation of the TG/HDL‐C ratio with major CV events among the large Chinese population. In the present study, it was shown that the participants with a higher TG/HDL‐C ratio had evidently elevated major CV risks. Our research findings are beneficial to identify the association of the TG/HDL‐C ratio with major CV events in the Chinese population, and to screen out the high‐risk general population. In this way, the government could establish the corresponding prevention strategies for high‐risk individuals.
The identification of a simple, accurate and inexpensive indicator for predicting CVD patient's prognosis has become a global focus. Previous studies have shown the relationship between the TG/HDL‐C ratio and CV events. As reported by Turak et al.4, a higher TG/HDL‐C ratio is associated with more major adverse CV events in essential hypertensive patients. A case–control study showed an obvious correlation of a high TG/HDL‐C ratio with increased risks of MI6. In addition, studies from the general population in Asia Pacific and Europe showed that the TG/HDL‐C ratio can be a predictive index for the risk of CVD17, 18. Similarly, the present study showed that among 96,542 Chinese participants, the major CV risks for participants with a high TG/HDL‐C ratio were elevated by 26% by comparison with those with a low TG/HDL‐C ratio. Therefore, the present results shows that the TG/HDL‐C ratio can become a critical predictive value for the risk of CVD.
Natural cubic spline analysis was applied to further explore the association of the TG/HDL‐C ratio with major CV events. Through the adjustment for multiple covariates, the results showed a line‐shaped curve association with increased risks of major CV events at a higher TG/HDL‐C ratio. Wu et al.19 believe a higher TG/HDL‐C ratio has a relationship with elevated CV mortality during peritoneal dialysis. However, whether the TG/HDL‐C ratio becomes a widely used clinical biochemical indicator remains to be supported by more studies. After all, the population we studied comes from the northern areas of China, which cannot provide a strong basis for the entire Chinese population. Furthermore, as LDL‐C is a major cause of atherosclerosis and CVD, receiver operating characteristic curve analysis was carried out to investigate the possibilities of the TG/HDL‐C ratio and LDL‐C to diagnose CV events. Receiver operating characteristic analysis results showed that the TG/HDL‐C ratio has a higher predictive value than LDL‐C, and the area under the curve of the TG/HDL‐C ratio is 0.553 (range 0.545–0.560).
In the present study, the demographic data of baseline showed that compared with those of the low ratio group, the high TG/HDL‐C ratio group had statistically higher traditional risk factors, such as heart rate, diastolic blood pressure, systolic blood pressure, body mass index, FPG, TC, TG, LDL‐C and high‐sensitivity C‐reactive protein; meanwhile, HDL‐C was statistically lower. We also found that other covariates, such as smoking status, alcohol status, education, and the percentage of hypertension and diabetes, were significantly different between groups. At the same time, the results showed that the participants with a high TG/HDL‐C ratio also conformed to the diagnostic criteria for metabolic syndrome20, which is consistent with the study carried out by Bittner et al.21 This means that a high TG/HDL‐C ratio is often accompanied by more CV risk factors. Therefore, considering the TG/HDL‐C ratio is more valuable in the high‐risk subgroup.
The mechanism of the TG/HDL‐C ratio with CV events remains unclear, but there is some speculation. First, the TG/HDL‐C ratio is a potent atherogenic indicator and has a remarkable association with the estimate of IR22, 23, 24. It is well‐known that IR, as a CV risk factor, makes great contributions to the development of CV events25, 26. Second, oxidation and inflammation factors can be used to predict the risks of CVD. Decreased HDL‐C content will reduce the ability of anti‐oxidation and anti‐inflammation; furthermore, the TG/HDL‐C ratio has an obvious relationship with the concentrations of sd‐LDL particles, which more readily cause oxidative damage27, 28. Third, impaired IR and dyslipidemia have been proven to be positively associated with decreased pancreatic β‐cell function, which might accelerate the occurrence of CVD19.
As above‐mentioned, the participants with a high TG/HDL‐C ratio generally had other metabolic risk factors and met the diagnostic criteria for metabolic syndrome, indicating that they were likely to have a common metabolic soil. The risk stratification of the general population can possibly be achieved by the TG/HDL‐C ratio, whereas the other metabolic risk factors can be detected. If individuals with a high TG/HDL‐C ratio are accompanied by metabolic syndromes, the following goals should be achieved: (i) carrying out physical activity (150 min per week at least); (ii) regulating lifestyle; and (iii) reducing bodyweight (7% of bodyweight)29. The pharmacological interventions should be taken into consideration if the above aims are not achieved. A large‐scale observational study has reported that fibrates can obviously increase HDL‐C levels and decrease TG levels30.
The advantages of the present were the prospective design, long follow‐up time, large research population and so on. However, the current study still had some weaknesses. First, as mentioned previously, the participants of the present study come from an occupational population in north China, and more men are represented than women, so the generalizability of the research findings might be limited for the whole Chinese population. Second, the baseline information is just used to assess the effect on adverse outcomes, which cannot reflect the changes of the variables of the follow‐up period. Consequently, different variations on the predefined outcomes have not been assessed.
Among the general Chinese population, the incidence rate of major CV events in the participants with a high TG/HDL‐C ratio was significantly higher than those with a low TG/HDL‐C ratio. Our research findings show that the TG/HDL‐C ratio can be the independent risk factor for CVD, namely, the risk of major CV events might be elevated with a higher TG/HDL‐C ratio. Among the general population, the TG/HDL‐C ratio should be used for risk‐stratification and be considered as a significant index for adverse reactions in the long term. The present results might help the public policy‐makers to formulate a strategy for individuals at high risk of major CV events in the Chinese population.
Disclosure
The authors declare no conflict of interest.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (No. 81870312).
J Diabetes Investig 2020; 11: 475–481
Contributor Information
Shouling Wu, Email: drwusl@163.com.
Youren Chen, Email: 13902779840@139.com.
References
- 1. Pearson TA. Understanding the impact of hyperlipidemia treatment on medical expenditures for cardiovascular disease. Med Care 2017; 55: 1–3. [DOI] [PubMed] [Google Scholar]
- 2. Yang S, Du Y, Li X, et al Triglyceride to high‐density lipoprotein cholesterol ratio and cardiovascular events in diabetics with coronary artery disease. Am J Med Sci 2017; 354: 117–124. [DOI] [PubMed] [Google Scholar]
- 3. Wu L, Parhofer KG. Diabetic dyslipidemia. Metabolism 2014; 63: 1469–1479. [DOI] [PubMed] [Google Scholar]
- 4. Turak O, Afsar B, Ozcan F, et al The role of plasma triglyceride/high‐density lipoprotein cholesterol ratio to predict new cardiovascular events in essential hypertensive patients. J Clin Hypertens 2016; 18: 772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miller M, Stone NJ, Ballantyne C, et al Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2011; 123: 2292–2333. [DOI] [PubMed] [Google Scholar]
- 6. Gaziano JM, Hennekens CH, O'Donnell CJ, et al Fasting triglycerides, high‐density lipoprotein, and risk of myocardial infarction. Circulation 1997; 96: 2520–2525. [DOI] [PubMed] [Google Scholar]
- 7. McLaughlin T, Abbasi F, Cheal K, et al Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med 2003; 139: 802–809. [DOI] [PubMed] [Google Scholar]
- 8. McLaughlin T, Reaven G, Abbasi F, et al Is there a simple way to identify insulin‐resistant individuals at increased risk of cardiovascular disease? Am J Cardiol 2005; 96: 399–404. [DOI] [PubMed] [Google Scholar]
- 9. Kim‐Dorner SJ, Deuster PA, Zeno SA, et al Should triglycerides and the triglycerides to high‐density lipoprotein cholesterol ratio be used as surrogates for insulin resistance? Metabolism 2010; 59: 299–304. [DOI] [PubMed] [Google Scholar]
- 10. Karelis AD, Pasternyk SM, Messier L, et al Relationship between insulin sensitivity and the triglyceride‐HDL‐C ratio in overweight and obese postmenopausal women: a MONET study. Appl Physiol Nutr Metab 2007; 32: 1089–1096. [DOI] [PubMed] [Google Scholar]
- 11. Kim JS, Kim W, Woo JS, et al The predictive role of serum triglyceride to high‐density lipoprotein cholesterol ratio according to renal function in patients with acute myocardial infarction. PLoS ONE 2016; 11: e165484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Millan J, Pinto X, Munoz A, et al Lipoprotein ratios: physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag 2009; 5: 757–765. [PMC free article] [PubMed] [Google Scholar]
- 13. Liu H, Wu S, Li Y, et al Body mass index and mortality in patients with type 2 diabetes mellitus: a prospective cohort study of 11,449 participants. J Diabetes Complications 2017; 31: 328–333. [DOI] [PubMed] [Google Scholar]
- 14. Chen Z, Wu S, Huang J, et al Metabolic syndrome increases cardiovascular risk in a population with prediabetes: a prospective study in a cohort of Chinese adults. J Diabetes Investig 2019; 10: 673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rose GAB. Cardiovascular survey methods. WHO Monograph Series No. 58. Geneva, Switzerland: World Health Organization, 1982. [PubMed] [Google Scholar]
- 16. Stroke–1989 . Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on stroke and other cerebrovascular Disorders. Stroke 1989; 20: 1407–1431. [DOI] [PubMed] [Google Scholar]
- 17. Barzi F, Patel A, Woodward M, et al A comparison of lipid variables as predictors of cardiovascular disease in the Asia Pacific region. Ann Epidemiol 2005; 15: 405–413. [DOI] [PubMed] [Google Scholar]
- 18. Salazar MR, Carbajal HA, Espeche WG, et al Identifying cardiovascular disease risk and outcome: use of the plasma triglyceride/high‐density lipoprotein cholesterol concentration ratio versus metabolic syndrome criteria. J Intern Med 2013; 273: 595–601. [DOI] [PubMed] [Google Scholar]
- 19. Wu H, Xiong L, Xu Q, et al Higher serum triglyceride to high‐density lipoprotein cholesterol ratio was associated with increased cardiovascular mortality in female patients on peritoneal dialysis. Nutr Metab Cardiovasc Dis 2015; 25: 749–755. [DOI] [PubMed] [Google Scholar]
- 20. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults . Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): final report. Circulation 2002; 106: 3143–3421. [PubMed] [Google Scholar]
- 21. Bittner V, Johnson BD, Zineh I, et al The triglyceride/high‐density lipoprotein cholesterol ratio predicts all‐cause mortality in women with suspected myocardial ischemia: a report from the women's ischemia syndrome evaluation (wise). Am Heart J 2009; 157: 548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kannel WB, Vasan RS, Keyes MJ, et al Usefulness of the triglyceride‐high‐density lipoprotein versus the cholesterol‐high‐density lipoprotein ratio for predicting insulin resistance and cardiometabolic risk (from the Framingham Offspring Cohort). Am J Cardiol 2008; 101: 497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Giannini C, Santoro N, Caprio S, et al The triglyceride‐to‐HDL cholesterol ratio: association with insulin resistance in obese youths of different ethnic backgrounds. Diabetes Care 2011; 34: 1869–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salazar MR, Carbajal HA, Espeche WG, et al Relation among the plasma triglyceride/high‐density lipoprotein cholesterol concentration ratio, insulin resistance, and associated cardio‐metabolic risk factors in men and women. Am J Cardiol 2012; 109: 1749–1753. [DOI] [PubMed] [Google Scholar]
- 25. Robins SJ, Lyass A, Zachariah JP, et al Insulin resistance and the relationship of a dyslipidemia to coronary heart disease: the Framingham Heart Study. Arterioscler Thromb Vasc Biol 2011; 31: 1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Briel M, Ferreira‐Gonzalez I, You JJ, et al Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta‐regression analysis. BMJ 2009; 338: b92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoogeveen RC, Gaubatz JW, Sun W, et al Small dense low‐density lipoprotein‐cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study. Arterioscler Thromb Vasc Biol 2014; 34: 1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tenjin N, Shinji K, Yuya Y, et al Elevated small dense low‐density lipoprotein cholesterol as a predictor for future cardiovascular events in patients with stable coronary artery disease. J Atheroscler Thromb 2014; 21: 755–767. [DOI] [PubMed] [Google Scholar]
- 29. Diabetes prevention program (DPP): Type 2 diabetes and prediabetes [Internet]. Bethesda, MD: National Diabetes Information Clearinghouse, 2013. [Google Scholar]
- 30. Flory JH, Ellenberg S, Szapary PO, et al Antidiabetic action of bezafibrate in a large observational database. Diabetes Care 2009; 32: 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]


