Abstract
Aims/Introduction
To develop a new non‐invasive risk score for undiagnosed diabetes in Chinese people, and to evaluate the incident diabetes risk in those with high‐risk scores, but no diabetes on initial testing.
Materials and Methods
A total of 2,609 participants with no known diabetes (aged 25–74 years) who underwent oral glucose tolerance tests in Hong Kong (HK) were investigated for independent risk factors of diabetes to develop a categorization point scoring system, the Non‐invasive Diabetes Score (NDS). This NDS was validated in a cross‐sectional study of 2,746 participants in Shaanxi, China. HK participants tested to not have diabetes at baseline were assessed for subsequent incident diabetes rates.
Results
In the HK cohort, hypertension, age and body mass index were the key independent risk factors selected to develop the NDS, with ≥28 out of 50 NDS points considered as high risk. The area under the receiver operating characteristic curve for undiagnosed diabetes was 0.818 and 0.720 for the HK and Shaanxi cohort, respectively. The negative predictive value was 97.4% (HK) and 95.8% (Shaanxi); the number needed to screen to identify one case of diabetes was five (HK) and 11 (Shaanxi), respectively. Among those that tested non‐diabetes at baseline, individuals with NDS ≥28 had a threefold risk of incident diabetes during the subsequent 20.9 years, compared with those with NDS <28 (P < 0.001), with a steeper rise in incident diabetes observed in those with NDS at higher tertiles.
Conclusions
This new three‐component risk score is a user‐friendly tool for diabetes screening, and might inform the subsequent testing interval for high‐risk non‐diabetes individuals.
Keywords: Diabetes, Non‐invasive, Score
We described the development, validation and longitudinal evaluation of a simple diabetes risk score with the fewest number of variables for undiagnosed diabetes in Chinese people. This risk score might help in opportunistic screening of diabetes, and inform subsequent testing interval for high‐risk individuals after initial testing.

Introduction
The prevalence of diabetes is rising globally. One‐quarter of the world's adults with diabetes are in China according to the 8th Diabetes Atlas of the International Diabetes Federation. However, 69.8% of them were previously undiagnosed1. Diabetes complications might have already developed in many newly diagnosed patients2, 3. Although universal screening of diabetes has not been recommended, opportunistic screening as suggested by the World Health Organization might be justified, provided methods with adequate sensitivity and specificity are available, and the health system can implement effective preventive strategies for high‐risk individuals4. Non‐invasive risk assessment might improve cost‐effectiveness of screening strategies by removing a portion of the population from glucose testing, particularly in populous countries5. Diabetes risk scores developed for detecting undiagnosed diabetes based on cross‐sectional studies should serve this purpose6, 7. Although such assessment tools are available, the subsequent rate of incident diabetes among those with high assessment scores, but no diabetes on initial testing, has not been reported.
To develop a user‐friendly diabetes score requiring the minimum number of non‐invasive parameters to facilitate early diabetes detection, we used data from the baseline assessment of the Hong Kong Cardiovascular Risk Factor Prevalence Study (CRISPS; development cohort)8 to develop the Non‐invasive Diabetes Score (NDS), and validate its performance with data from a cohort in Shaanxi Province, China (external validation cohort)9. Furthermore, participants assessed at the baseline visit of CRISPS (CRISPS1) and found to have no diabetes were evaluated for the subsequent development of incident diabetes in relation to their baseline NDS score.
Methods
Participants
Development cohort with prospective follow up (Hong Kong cohort)
The participants were from CRISPS, a long‐term, population‐based, prospective study on the prevalence of cardiovascular risk factors in Hong Kong. In 1995–1996 (CRISPS1)8, 2,895 unrelated Chinese participants were invited randomly by telephone numbers for a detailed assessment. A total of 2,609 participants were included in this analysis after excluding underweight participants with body mass index (BMI) lower than the 2.5th percentile. Participants were contacted for reassessment visits in 2000–2004 (CRISPS2), 2005–2008 (CRISPS3), 2010–2012 (CRISPS4) and 2016–2018 (CRISPS5)10, 11, 12.
External validation cohort (Shaanxi cohort)
This included participants in Shaanxi province, China, who were recruited at one of the centers of the China National Diabetes and Metabolic Disorders Study, a population‐based cross‐sectional study in 2007–2008, using a multistage stratified sampling design, with details described elsewhere9. In short, one midsize city, one developed and one underdeveloped county, plus the provincial capital of Shaanxi were selected. City districts from the cities, rural townships from the counties, followed by street districts from the city districts and rural villages from the townships were selected at random. Participants were interviewed by trained doctors and nurses at local health stations or community clinics with standardized questionnaires including demographic characteristics, family history of diabetes, lifestyle and metabolic risk factors. A total of 2,746 participants of the same age range and similar number as the Hong Kong cohort, with no known diabetes before the screening visit, were included in the present study.
The institutional review board or ethics committee of the First Affiliated Hospital of Air Force Medical University and the Faculty of Medicine, University of Hong Kong, reviewed and approved the study. Informed consent was obtained from all participants.
Data collection
In both cohorts, medical history was recorded during each attendance. Anthropometric and biochemical parameters were measured as described in previous publications8, 9. All participants not taking antidiabetic medications underwent a 75‐g oral glucose tolerance test (OGTT) when they attended the assessment in Shaanxi and in Hong Kong, at baseline and reassessment visits. Diabetes was defined according to World Health Organization 1998 criteria: fasting glucose (FG) ≥7 mmol/L or 2‐h post‐OGTT glucose (2hG) ≥11.1 mmol/L13. Central obesity was defined as waist circumference (WC) ≥90 cm in men and >80 cm in women14. Hypertension was defined as blood pressure ≥140/90 mmHg or receiving regular antihypertensive treatment. Dyslipidemia was defined as fasting triglycerides ≥1.69 mmol/L, fasting high‐density lipoprotein cholesterol <1.29 mmol/L in women and <1.04 mmol/L in men, fasting low‐density lipoprotein cholesterol ≥3.4 mmol/L or taking lipid‐lowering agents15. Physical activity was defined as having at least moderate exercise for >30 min per week10.
NDS development and validation
Data from the Hong Kong cohort at CRISPS1 were used to develop NDS. Participants screened to have diabetes were compared with those without diabetes. The minimum possible number of independent non‐invasive risk factors of diabetes was selected, using the Bayesian information criterion (BIC)16 to form a simple model, which was used to develop a categorization point scoring system, the NDS. All participants without known diabetes before CRISPS1 were evaluated for the risk of undiagnosed diabetes with reference to the optimal cut‐off of NDS, and the conclusion drawn was compared with the OGTT results at CRISPS1. We used data from the Shaanxi cohort to validate the score and compare the performance of NDS with the American Diabetes Association (ADA) Diabetes Risk Test17.
Observation of incident diabetes on long‐term follow up based on NDS
Those without diabetes based on OGTT (non‐diabetes) at CRISPS1 were followed for diabetes development until CRISPS‐5. The observed cumulative diabetes incidence of the high‐risk group (NDS score ≥28) was compared with the low‐risk non‐diabetes group (NDS score <28). The NDS high‐risk group was divided into tertiles (28–30; 31–37; ≥38) for further comparisons.
Statistical analysis
All analyses were carried out with SPSS version 24 (SPSS Inc., Chicago, IL, USA) and R v3.0.2 including the “MASS, “bootStepAIC”, “pROC” and “DTComPair” packages (R Foundation for Statistical Computing, Vienna, Austria). Results were presented as the mean ± standard deviation or median with interquartile range, as appropriate. For data not normally distributed, natural logarithmic transformation was applied before analyses. Triglyceride level was the only variable requiring such transformation. Variables were compared between groups by one‐way anova for continuous data, and the χ2‐test for categorical data. Non‐invasive risk factors, including age, BMI, hypertension, physical activity and family history of diabetes, which were significantly associated with diabetes in univariate analysis or of clinical relevance, were selected in the multivariable logistic regression model. We included BMI, but not central obesity, in the model, as WC correlated closely with BMI, and WC measurement was known to have more intra‐ and interobserver variations. The BIC with 100 bootstrap replicates was applied for variable selection. The lowest BIC was identified as the best among the models used. We categorized age at 10‐yearly intervals, and BMI by cut‐offs of obesity classification during the risk score development. Point scoring was assigned to each category according to a regression coefficient‐based scoring algorithm to derive weights for the risk score. The model performance was assessed by using the area under the receiver operating characteristic curve (AUROC) for discrimination18. Calibration was carried out by plotting the observed risk against the predicted risk of undiagnosed diabetes, and evaluated by the Hosmer–Lemeshow goodness of fit test19. The optimal cut‐off point of NDS for identification of undiagnosed diabetes was determined by Youden's index (J), which is the maximum vertical distance from the curve to the chance line on a ROC curve (J = sensitivity + specificity − 1)20. Sensitivity, specificity, positive predictive value and negative predictive value (NPV) were presented at a particular cut‐off point. The number of participants required to screen for identification of one case of diabetes (NNS) was calculated by 1 / absolute risk reduction21. Comparison of AUROCs was carried out by Delong's test. Differences in sensitivity and specificity were compared using the McNemar test. A Kaplan–Meier survival curve was constructed to compare the cumulative incidence of diabetes. The log–rank test was used to compare the incident diabetes between different NDS groups at year 5, 10, 15 and 20.
Results
In the Hong Kong cohort at CRISPS1, 222 (8.5%) of the 2,609 participants (age 45.7 ± 12.5 years) were screened to have diabetes by OGTT. Table 1 shows the clinical characteristics of those with screened diabetes, compared with those without. Hypertension, age, BMI, family history of diabetes and physical activity were independently associated with diabetes, as assessed by multivariate logistic regression analysis, which also included two other non‐laboratory‐based risk factors – smoking and history of cardiovascular diseases. The best simple model included just age, BMI and hypertension, after variable selection by the BIC (Table S1). Table 2 shows the point scoring system of NDS. The AUROC of NDS for undiagnosed diabetes was 0.818 (95% confidence interval [CI] 0.792–0.845), and the P‐value for the Hosmer–Lemeshow goodness of fit was 0.137, showing good discrimination and calibration (Figure S1). The optimal cut‐off of NDS, as evaluated by the Youden's index, was 28. Participants with NDS ≥28 out of 50 were considered to be at high risk of having diabetes. Applying this optimal cut‐off, NPV was 97.4% and NNS was 5.
Table 1.
Baseline characteristics of participants of the baseline visit of the Hong Kong Cardiovascular Risk Factor Prevalence Study cohort with and without screened diabetes
| No DM | Undiagnosed DM | P‐value | |
|---|---|---|---|
| n | 2,387 | 222 | – |
| Men (%) | 49.0 | 49.1 | 0.971 |
| Age (years) | 44.7 ± 12.2 | 56.0 ± 11.2 | <0.001* |
| Smoking status (%) | |||
| Never | 74.9 | 72.1 | 0.523 |
| Former | 6.4 | 8.1 | |
| Current | 18.7 | 19.8 | |
| Physical activity (%) | 42.0 | 34.7 | 0.035* |
| Family history of DM (%) | 17.1 | 18.5 | 0.592 |
| BMI (kg/m2) | 24.1 ± 3.42 | 26.9 ± 3.29 | <0.001* |
| WC, cm | |||
| Men | 82.9 ± 8.99 | 90.8 ± 9.02 | <0.001* |
| Women | 74.8 ± 8.86 | 85.4 ± 8.59 | <0.001* |
| Central obesity (%) | 22.9 | 64.4 | <0.001* |
| Systolic BP (mmHg) | 118 ± 18 | 137 ± 22 | <0.001* |
| Diastolic BP (mmHg) | 74 ± 11 | 82 ± 11 | <0.001* |
| FG (mmol/L) | 5.09 ± 0.47 | 8.07 ± 3.0 | <0.001* |
| 2‐h glucose (mmol/L) | 6.18 ± 1.67 | 15.0 ± 5.11 | <0.001* |
| TG† (mmol/L) | 1.00 (0.70–1.42) | 1.50 (1.07–2.30) | <0.001* |
| HDL‐C (mmol/L) | 1.26 ± 0.32 | 1.09 ± 0.30 | <0.001* |
| LDL‐C (mmol/L) | 3.25 ± 0.88 | 3.54 ± 0.87 | <0.001* |
| Hypertension (%) | 15.0 | 51.8 | <0.001* |
| Dyslipidemia (%) | 67.5 | 88.3 | <0.001* |
| History of CVD (%) | 2.1 | 5.9 | 0.001* |
Total n = 2,609. Data presented as mean ± standard deviation or median (25th–75th percentile). *Statistically significant. †Log‐transformed before analysis. Screened diabetes: fasting glucose (FG) ≥7 mmol/L or 2‐h post oral glucose tolerance test glucose ≥11.1 mmol/L. Undiagnosed diabetes: participants without a known history of diabetes, but screened to have diabetes by oral glucose tolerance test at the baseline visit of the Hong Kong Cardiovascular Risk Factor Prevalence Study. BMI, body mass index; BP, blood pressure; CVD, cardiovascular disease; DM, diabetes mellitus; HDL‐C, high‐density lipoprotein cholesterol; HT, hypertension; LDL‐C, low‐density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides; WC, waist circumference.
Table 2.
Risk scores assigned to the variables of the thee‐component Non‐invasive Diabetes Score derived from the baseline visit of the Hong Kong Cardiovascular Risk Factor Prevalence Study cohort
| Clinical parameters | n | Adjusted OR† (95% CI) | P‐value | NDS |
|---|---|---|---|---|
| Age (years) | <0.001* | |||
| 25–34 | 557 | 1 | – | 0 |
| 35–44 | 829 | 2.21 (1.04–4.67) | 0.039* | 8 |
| 45–54 | 533 | 3.30 (1.57–6.94) | 0.002* | 13 |
| 55–64 | 412 | 7.59 (3.65–15.7) | <0.001* | 20 |
| 65–74 | 278 | 8.31 (3.89–17.7) | <0.001* | 22 |
| BMI (kg/m2) | <0.001* | |||
| 18.0–19.9 | 238 | 0.57 (0.17–1.96) | 0.372 | −6 |
| 20.0–22.9 | 747 | 1 | – | 0 |
| 23.0–24.9 | 641 | 2.41 (1.39–4.18) | 0.002* | 9 |
| 25.0–27.4 | 529 | 3.52 (2.07–5.98) | <0.001* | 14 |
| 27.5–29.9 | 278 | 5.64 (3.22–5.98) | <0.001* | 18 |
| ≥30 | 176 | 6.38 (3.48–11.7) | <0.001* | 20 |
| Hypertension (yes) | 473 | 2.20 (1.58–3.07) | <0.001* | 8 |
Total n = 2,609. *Statistically significant. Max score = 50. †The reduced model with the lowest Bayesian information criterion was identified as the best among the models used. BMI, Body Mass Index; CI, confidence interval; NDS, Non‐invasive Diabetes Score; OR, odds ratio.
Table S2 summarizes the characteristics of participants from the Hong Kong cohort at CRISPS1 versus the Shaanxi cohort. More of the Hong Kong participants were physically active (P = 0.004), had a family history of diabetes (P < 0.001), higher BMI (P = 0.03) and dyslipidemia (P < 0.001); fewer were active smokers (P < 0.001), had a history of cardiovascular diseases (P = 0.018), central obesity (P < 0.001) or hypertension (P < 0.001). All Hong Kong participants lived in an urban area compared with 51.5% in the Shaanxi cohort (P < 0.001). NDS was validated in the Shaanxi cohort, in which 198 (7.2%) of 2,746 participants (age 45.1 ± 12.3 years), who had no history of diabetes, were screened to have diabetes. Evaluation of the performance of NDS in this cohort showed a good discrimination, with an AUROC of 0.720 (95% CI 0.685–0.756) and calibration (Hosmer–Lemeshow goodness of fit test P = 0.230; Figure S2). The NPV and NNS were 95.8% and 10.4, respectively. The performance of an established risk score including seven variables, the ADA Diabetes Risk Test, was evaluated in the same Shaanxi cohort. Compared with the NDS, the ADA Diabetes Risk Test showed a suboptimal discrimination with an AUROC of 0.697 (95% CI 0.660–0.734) and unsatisfactory calibration (Hosmer–Lemeshow goodness of fit test P < 0.05; Table 3). When applied in the Hong Kong cohort, the AUROC of the ADA Diabetes Risk Test was 0.802 (95% CI 0.774–0.829).
Table 3.
Performance of the Non‐invasive Diabetes Score and American Diabetes Association Diabetes Risk Test in the Shaanxi cohort
| Risk score | Optimal cut‐off/maximum points | No. risk factors | AUROC (95% CI) | Sensitivity (%) | Specificity (%) | NPV (%) | PPV (%) | NNS | Calibration statistic† |
|---|---|---|---|---|---|---|---|---|---|
| ADA Diabetes Risk Test | 5/10 | 7 (Age, sex, GDM, FhxDM, HT, physical active, BMI) | 0.697 (0.660–0.734) | 53.8 | 73.1 | 95.3 | 13.5 | 11.4 | 0.047 |
| NDS | 28/50 | 3 (HT, BMI, age) | 0.720 (0.685–0.756) | 60.8 | 69.7 | 95.8 | 13.5 | 10.4 | 0.230 |
†Hosmer–Lemeshow goodness of fit test, P < 0.05 suggests that the model does not fit the data. AUROC, area under the curve of the receiver operating characteristic; BMI, body mass index; CI, confidence interval; FhxDM, family history of diabetes; HT, hypertension; NDS, Non‐invasive Diabetes Score; NNS, number needed to screen; NPV, negative predictive value; PPV, positive predictive value.
Of 1,984 participants who were confirmed non‐diabetes by OGTT at CRISPS1 and returned for follow‐up visits, 488 were NDS high risk, using the same optimal cut‐off of NDS ≥28. During a median follow‐up period of 20.9 years (interquartile range 12.8, 22.3), incident diabetes was documented in 389 of 1,984 participants (19.6%), with 153 being NDS high risk. Table S3 summarizes the clinical and biochemical characteristics of the participants deemed NDS high risk compared with low risk at CRISP1. Figure 1 shows the observed diabetes incidence in participants with different baseline NDS risk scores. The cumulative diabetes incidence was more than tripled in the NDS high‐risk group (NDS ≥28) at each time point (all P < 0.001, log–rank test) compared with the low‐risk group (NDS <28). Among the high‐risk individuals, those at higher tertiles had a steeper rise in incident diabetes compared with those at the lowest tertile (28–30). Individuals in the highest tertile of the high‐risk group (NDS ≥38) had a more than sixfold (log–rank test P < 0.001) rate of incident diabetes by the 5th year when compared with those with a baseline NDS <28.
Figure 1.
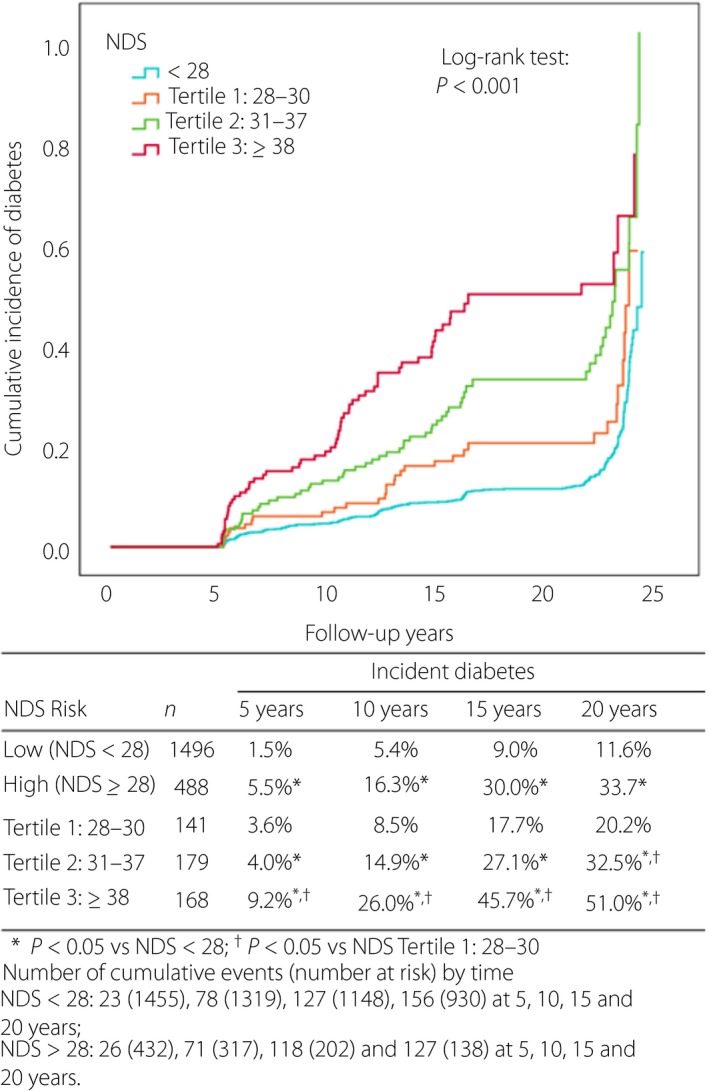
Post‐assessment cumulative diabetes incidence in participants with high‐risk Non‐invasive Diabetes Score (NDS; divided in tertiles) who screened negative for diabetes at the baseline visit of the Hong Kong Cardiovascular Risk Factor Prevalence Study compared with participants with low‐risk NDS as the reference.
Discussion
We developed a new non‐invasive diabetes risk score for undiagnosed diabetes, NDS, consisting of just three variables (age, BMI and hypertension), based on a cross‐sectional study in Hong Kong. We validated the NDS in a geographically distinct Chinese cohort and found that it had a good performance in identifying undiagnosed diabetes in both cohorts, especially regarding NPV and NNS, making it a potentially useful tool in diabetes screening before glucose testing. Furthermore, from our long‐term prospective CRISPS cohort, we observed that among those tested to have no diabetes at CRISP1, NDS high‐risk participants had triple the rate of incident diabetes at any time point during follow up. We also noted that increasingly higher baseline NDS scores were associated with earlier development of diabetes on subsequent follow up. The present findings suggest that this three‐component diabetes risk score can contribute to early diagnosis of diabetes and provide reference information on reassessment planning.
Type 2 diabetes is often asymptomatic at onset, but can cause serious chronic complications that can be preventable if the disease is treated early, through the detection of undiagnosed diabetes. A diabetes screening tool for undiagnosed diabetes would be more useful if it could screen out a large volume of low‐risk cases with the minimal number of false negatives. NDS has high NPVs in both the Hong Kong and Shaanxi cohorts. When applied in Hong Kong at CRISPS1, 70.5% of the participants had a low risk of undiagnosed diabetes (NDS <28) and did not require laboratory testing for diabetes. With a NPV of 97.4%, just 49 individuals in the Hong Kong cohort were false negatives. Hence, NDS should be a screening tool with good potential, which warrants further validation in other populations.
The three variables required by NDS are the commonest independent risk factors included in published diabetes risk models22. Bodyweight and height can be self‐measured with good reliability and less variation compared with WC23. As elevated blood pressure and fasting hyperglycemia are both components of the metabolic syndrome, it is not surprising that having hypertension is associated with a higher chance of having diabetes10. Promoting early diagnosis and treatment of both will help reducing the high mortality from cardiovascular diseases as well24. Although hypertension might be frequently undiagnosed in remote areas, such limitation can be overcome by training community pharmacists or village health volunteers to measure blood pressure and refer high‐risk individuals for glucose testing. Furthermore, home blood pressure monitors are becoming widely available and affordable, and can help identify hypertension in the community.
We categorized BMI by cut‐offs of obesity classification during the risk score development. In line with other screening recommendations, we followed the Asian BMI cut‐off for overweight at 23 kg/m2.17, 25 For BMI <23, cut‐offs were set at the 2.5th and 10th percentile. The relative importance of BMI and age as diabetes risk factors was evidenced by their contribution to the scoring points (Table 2). Age is the prerequisite variable according to the ADA, which recommends that even without other risk factors, screening should start from the age of 45 years and be repeated every 3 years if negative. Although this recommendation appears extremely simple, the high volume of subsequent glucose testing generated might become a barrier for its application, particularly in aging populations. We previously found that the number for glucose testing was smaller if the ADA Diabetes Risk Test, the alternative method that included a few other risk factors, was used for diabetes screening in Hong Kong26. However, this test appeared to have a suboptimal performance in discrimination and calibration compared with the NDS when evaluated in the Shaanxi cohort (Table 3). Although the NDS was based on data in Hong Kong Chinese individuals, it had a good performance in the geographically distinct validation cohort from Shaanxi, where the environmental factors including diet pattern, rural‐urban distribution and lifestyles were different, suggesting its potential application in other Chinese populations.
It is shown that currently available diabetes risk assessment tools are of limited use27, and there is a lack of systematic approaches on identification of high‐risk individuals. Diabetes risk scores can be developed from cross‐sectional or longitudinal cohorts. The clinical application of these scores might also be different. While the former aims to identify undiagnosed diabetes and works for diabetes screening, the latter predicts diabetes development and facilitates diabetes prevention. The Finnish Diabetes risk Score (FINDRISC) was originally developed to predict incident diabetes28, but it had also been validated by cross‐sectional studies for detection of undiagnosed diabetes using different cut‐off points29, 30. The information on the rate of subsequent development of diabetes among individuals with a high‐risk NDS score, but found to have no diabetes at initial testing, might help toward the planning of diabetes testing interval after the first assessment. The current testing interval of 3 years, as suggested by the ADA, has been based largely on the strategic balance of minimizing false positives leading to unnecessary testing, and ensuring false negatives are retested before complications developed31. Individuals at the highest tertile of the NDS high‐risk group (NDS ≥38) in the present study had a sixfold risk of observed incident diabetes when compared with those with low risk (NDS <28; Figure 1). Reducing the testing interval to 2 years or even 1 year might be warranted in this group of patients.
OGTT was used in both cohorts to screen for diabetes. We and other studies have shown that 2hG diagnosed more diabetes than FG in Chinese individuals9, 11. The NDS considered individuals with diabetes diagnosed by both FG and 2hG criteria, making it a more reliable screening tool for undiagnosed diabetes. However, the present study had the limitation of not including glycated hemoglobin in the diagnosis of diabetes, as it was only available in a subgroup in both cohorts. Nevertheless, a recent study showed that glycated hemoglobin added only 0.5% to the prevalence of diabetes defined by OGTT in the Chinese population1. Furthermore, FG and 2hG are considered more relevant to the pathophysiology of diabetes, and effectiveness of diabetes prevention through lifestyle or pharmacological interventions is shown in studies among individuals with impaired glucose tolerance identified by 2hG32, 33, 34. Applying diabetes prevention interventions to the NDS‐defined high‐risk non‐diabetes individuals appears to be an attractive direction that warrants further investigations. The present study had a few limitations. As in other prospective observational studies, we had participants lost to follow up in the CRISPS cohort, with more defaulters having NDS ≥28 (45.1% vs 24.6% of those with reassessment, P < 0.001). Thus, the risk of incident diabetes in NDS high‐risk participants might have been underestimated. However, the development and validation of NDS should not be affected. Using data at CRISPS1 to develop the NDS allowed us to observe the long‐term incident diabetes risk. However, it also resulted in some drawbacks. The definition of physical activity used at CRISPS1, for instance, was different from those in common use nowadays, such as that based on the International Physical Activity Questionnaire, used in our ongoing research35. Nevertheless, physical activity was not selected for the final model by the BIC (Table S1), and would not affect the performance of the NDS. In contrast, the dietary pattern, environmental condition and lifestyle in Hong Kong and Shaanxi are expected to have undergone changes since 1995 and 2007, respectively. Nevertheless, this NDS risk score is likely to remain applicable for screening of undiagnosed diabetes in a current population, as suggested by the observation that despite the two studies being carried out >10 years apart in two geographically distinct regions in China, the performance of the NDS was good in the Shaanxi cohort. Notably, the NDS does not involve behavioral or lifestyle risk factors, which might be more affected by socioeconomic changes over the years.
In conclusion, we have developed a three‐component non‐invasive diabetes risk score, simple enough for self‐assessment, to identify individuals with undiagnosed diabetes. The same score using the same cut‐off also shed light on the risk subsequent diabetes development. The use of this score might help in the early diagnosis of diabetes, at least amongst Chinese adults.
Disclosure
The authors declare no conflict of interest.
Supporting information
Figure S1 | Calibration plot of observed risk against risk predicted by the Non‐invasive Diabetes Score (NDS) at the baseline visit of the Hong Kong Cardiovascular Risk Factor Prevalence Study (CRISPS1) in the Hong Kong cohort.
Figure S2 | Calibration plot of observed risk against risk predicted by the Non‐invasive Diabetes Score (NDS) in the Shaanxi cohort.
Table S1 | Results of Bayesian information criterion of the multivariable stepwise logistic regression models for risk factors associated with undiagnosed diabetes at the baseline visit of the Hong Kong Cardiovascular Risk Factor Prevalence Study in the Hong Kong cohort (n = 2,609).
Table S2 | Descriptive statistics of participants at the baseline visit of the Hong Kong Cardiovascular Risk Factor Prevalence Study and the Shaanxi cohorts.
Table S3 | Clinical and biochemical characteristics of the Non‐invasive Diabetes Score high‐risk and non‐invasive diabetes score low‐risk participants at the baseline visit of the Hong Kong Cardiovascular Risk Factor Prevalence Study in the Hong Kong cohort (n = 1,984).
Acknowledgments
This study was funded by the Ng Teng Fong Charitable Foundation. The study sponsor was not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
J Diabetes Investig 2020; 11: 341–348
References
- 1. Wang L, Gao P, Zhang M, et al Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA 2017; 317: 2515–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Turner R, Holman R, Cull C, et al Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 3. Spijkerman AM, Dekker JM, Nijpels G, et al Microvascular complications at time of diagnosis of type 2 diabetes are similar among diabetic patients detected by targeted screening and patients newly diagnosed in general practice: the hoorn screening study. Diabetes Care 2003; 26: 2604–2608. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization. Chronic Respiratory Diseases and Arthritis Team . Screening for type 2 diabetes: report of a World Health Organization and International Diabetes Federation meeting. 2003. Available from: https://apps.who.int/iris/handle/10665/68614 Accessed August 13, 2019.
- 5. Khan U, Bogue C, Ungar WJ, et al Cost‐effectiveness analysis of different imaging strategies for diagnosis of haemophilic arthropathy. Haemophilia 2010; 16: 322–332. [DOI] [PubMed] [Google Scholar]
- 6. Zhou X, Qiao Q, Ji L, et al Nonlaboratory‐based risk assessment algorithm for undiagnosed type 2 diabetes developed on a nation‐wide diabetes survey. Diabetes Care 2013; 36: 3944–3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bang H, Edwards AM, Bomback AS, et al Development and validation of a patient self‐assessment score for diabetes risk. Ann Intern Med 2009; 151: 775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Janus ED, Watt NM, Lam KS, et al The prevalence of diabetes, association with cardiovascular risk factors and implications of diagnostic criteria (ADA 1997 and WHO 1998) in a 1996 community‐based population study in Hong Kong Chinese. Hong Kong Cardiovascular Risk Factor Steering Committee. American Diabetes Association. Diabet Med 2000; 17: 741–745. [DOI] [PubMed] [Google Scholar]
- 9. Yang W, Lu J, Weng J, et al Prevalence of diabetes among men and women in China. N Engl J Med 2010; 362: 1090–1101. [DOI] [PubMed] [Google Scholar]
- 10. Cheung BM, Wat NM, Tso AW, et al Association between raised blood pressure and dysglycemia in Hong Kong Chinese. Diabetes Care 2008; 31: 1889–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Woo YC, Cheung BM, Yeung CY, et al Cardiometabolic risk profile of participants with prediabetes diagnosed by HbA1c criteria in an urban Hong Kong Chinese population over 40 years of age. Diabet Med 2015; 32: 1207–1211. [DOI] [PubMed] [Google Scholar]
- 12. Woo YC, Lee CH, Fong CH, et al Serum fibroblast growth factor 21 is a superior biomarker to other adipokines in predicting incident diabetes. Clin Endocrinol 2017; 86: 37–43. [DOI] [PubMed] [Google Scholar]
- 13. Alberti KG, Definition Zimmet PZ. diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 15: 539–553. [DOI] [PubMed] [Google Scholar]
- 14. Alberti KG, Zimmet P, Shaw J, Group IDFETFC . The metabolic syndrome – a new worldwide definition. Lancet 2005; 366: 1059–1062. [DOI] [PubMed] [Google Scholar]
- 15. Expert Panel on Detection Evaluation, and Treatment of High Blood Cholesterol in Adults . Executive summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001; 285: 2486–2497. [DOI] [PubMed] [Google Scholar]
- 16. Sakamoto Y, Ishiguro M, Kitagawa G. Akaike Information Statistics. Tokyo/Dordrecht: KTK Scientific Publishers/D. Reidel Publishing, 1986. [Google Scholar]
- 17. American Diabetes Association . 2. Classification and diagnosis of diabetes: standards of medical care in diabetes‐2018. Diabetes Care 2018; 41: S13–S27. [DOI] [PubMed] [Google Scholar]
- 18. D'Agostino RB Sr, Pencina MJ, Massaro JM, et al Cardiovascular disease risk assessment: insights from Framingham. Global Heart 2013; 8: 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hosmer DWLS. Applied Logistic Regression, 2nd edn New York, NY: Wiley, 2000. [Google Scholar]
- 20. Perkins NJ, Schisterman EF. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol 2006; 163: 670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rembold CM. Number needed to screen: development of a statistic for disease screening. BMJ 1998; 317: 307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Collins GS, Mallett S, Omar O, et al Developing risk prediction models for type 2 diabetes: a systematic review of methodology and reporting. BMC Med 2011; 9: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nadas J, Putz Z, Kolev G, et al Intraobserver and interobserver variability of measuring waist circumference. Med Sci Monit 2008; 14: 15–18. [PubMed] [Google Scholar]
- 24. Prabhakaran D, Anand S, Watkins D, et al Cardiovascular, respiratory, and related disorders: key messages from disease control priorities, 3rd edition. Lancet 2018; 391: 1224–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Consultation WHOE . Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363: 157–163.‐ [DOI] [PubMed] [Google Scholar]
- 26. Woo YC, Lee CH, Fong CHY, et al Validation of the diabetes screening tools proposed by the American Diabetes Association in an aging Chinese population. PLoS ONE 2017; 12: e0184840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dhippayom T, Chaiyakunapruk N, Krass I. How diabetes risk assessment tools are implemented in practice: a systematic review. Diabetes Res Clin Pract 2014; 104: 329–342. [DOI] [PubMed] [Google Scholar]
- 28. Lindstrom J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care 2003; 26: 725–731. [DOI] [PubMed] [Google Scholar]
- 29. Zhang L, Zhang Z, Zhang Y, et al Evaluation of Finnish Diabetes Risk Score in screening undiagnosed diabetes and prediabetes among U.S. adults by gender and race: NHANES 1999‐2010. PLoS ONE 2014; 9: e97865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Salinero‐Fort MA, Burgos‐Lunar C, Lahoz C, et al Performance of the Finnish Diabetes Risk Score and a Simplified Finnish Diabetes Risk Score in a Community‐Based, Cross‐Sectional Programme for Screening of Undiagnosed Type 2 Diabetes Mellitus and Dysglycaemia in Madrid, Spain: The SPREDIA‐2 Study. PLoS ONE 2016; 11: e0158489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johnson SL, Tabaei BP, Herman WH. The efficacy and cost of alternative strategies for systematic screening for type 2 diabetes in the U.S. population 45‐74 years of age. Diabetes Care 2005; 28: 307–311. [DOI] [PubMed] [Google Scholar]
- 32. Knowler WC, Barrett‐Connor E, Fowler SE, et al Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lindstrom J, Ilanne‐Parikka P, Peltonen M, et al Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow‐up of the Finnish Diabetes Prevention Study. Lancet 2006; 368: 1673–1679. [DOI] [PubMed] [Google Scholar]
- 34. Li G, Zhang P, Wang J, et al The long‐term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20‐year follow‐up study. Lancet 2008; 371: 1783–1789. [DOI] [PubMed] [Google Scholar]
- 35. Lee PH, Yu YY, McDowell I, et al Performance of the international physical activity questionnaire (short form) in subgroups of the Hong Kong chinese population. Int J Behav Nutr Phys Act 2011; 8: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Calibration plot of observed risk against risk predicted by the Non‐invasive Diabetes Score (NDS) at the baseline visit of the Hong Kong Cardiovascular Risk Factor Prevalence Study (CRISPS1) in the Hong Kong cohort.
Figure S2 | Calibration plot of observed risk against risk predicted by the Non‐invasive Diabetes Score (NDS) in the Shaanxi cohort.
Table S1 | Results of Bayesian information criterion of the multivariable stepwise logistic regression models for risk factors associated with undiagnosed diabetes at the baseline visit of the Hong Kong Cardiovascular Risk Factor Prevalence Study in the Hong Kong cohort (n = 2,609).
Table S2 | Descriptive statistics of participants at the baseline visit of the Hong Kong Cardiovascular Risk Factor Prevalence Study and the Shaanxi cohorts.
Table S3 | Clinical and biochemical characteristics of the Non‐invasive Diabetes Score high‐risk and non‐invasive diabetes score low‐risk participants at the baseline visit of the Hong Kong Cardiovascular Risk Factor Prevalence Study in the Hong Kong cohort (n = 1,984).


